Abstract
The discovery of cytokines and their role in immune and inflammatory disease led to the development a plethora of targeted biologic therapies. Later, efforts to understand mechanisms of cytokine signal transduction led to the discovery of Janus kinases (JAKs), which themselves were quickly identified as therapeutic targets. It has been a decade since the first JAK inhibitors (jakinibs) were approved and now there are nine jakinibs approved for treatment of rheumatologic, dermatologic, hematologic, and gastrointestinal indications along with emergency authorization for Covid-19. In this review, we will summarize relevant discoveries that led to first generation jakinibs and review their efficacy and safety demonstrated in pivotal clinical studies. We will discuss the next generation of more selective jakinibs, along with agents that target kinase families beyond JAKs. Finally, we will reflect on both the opportunities and challenges ahead as we enter the second decade of the clinical use of jakinibs.
Keywords: Cytokines, JAK, Autoimmunity, Inflammation, Kinase Inhibitors, COVID-19
Introduction
Cytokines serve as critical means of intercellular communication among immune and other cells, controlling many aspects of normal immune responses. However, their aberrant production, results in the loss of immune homeostasis and exaggerated immune responses that underlie pathologies from autoimmunity to “cytokine storm.”
The introduction of targeted anti-cytokine biologic (protein) therapies at the end of the 20th century led to dramatic improvements in our ability to treat many diseases including rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), psoriasis (PsO), atopic dermatitis (AD), and inflammatory bowel disease (IBD). Their clinical success was largely driven by our understanding of the cells and molecules that drive these pathologies to produce effective, generally safe, molecules that inhibit relevant inflammatory pathways. The development of biologic disease-modifying anti-rheumatic drugs (bMARDs) — namely, engineered proteins, capable of selectively binding cytokines or their cognate receptors that include Tumor Necrosis Factor (TNF), Interleukin (IL)-1, IL-4/IL-13, IL-6, IL-17, and IL-12/23 — was the capstone of years of research.
Many, but not all, of the cytokines that drive immunopathology act via type I or type II cytokine receptors. Cytokine binding to these receptors activates phosphotransferases (kinases) associated with the intracellular portion of these receptors. These kinases belong to a small family termed Janus kinase (JAKs), comprising four members: JAK1, JAK2, JAK3 and TYK2. Different receptors are coupled with different JAKs working in pairs, either in a heterodimeric or homodimeric complex. Following receptor engagement, the JAKs phosphorylate themselves and tyrosine residues on the receptor chains that recruit the Signal Transducers and Activator of Transcription (STAT) family of DNA binding proteins. These factors are phosphorylated by the JAKs resulting in their dimerization, translocation to the nucleus, and subsequent regulation of gene expression.
A series of mutant cell lines revealed the essential functions of JAKs in cytokine signaling,1 but identification of JAK3 mutations in patients with severe combined immunodeficiency (SCID) revealed in vivo criticality,2 as did various knockout mice. These insights led to the proposition that JAK inhibitors could represent a new class of immunomodulatory drugs.3 Recognition of JAK2 gain-of-function mutations in myeloproliferative neoplasms4 provided further evidence of JAK inhibition as an attractive therapeutic option. TYK2 mutations in humans result in primary immunodeficiency5 and TYK2 variants are linked to a wide range of autoimmune diseases. Specifically, TYK2 protein-coding variants (e.g. rs34536443, P1104A) provide protection against multiple sclerosis, RA, PsO, and systemic lupus erythematosus (SLE).5, 6
The development of jakinibs has accelerated over the past decade with the subsequent approval of multiple agents and more candidates under investigation in various diseases. In this review, we will briefly discuss the efficacy of the approved jakinibs and their adverse events (AEs). Though these jakinibs are relatively specific for JAK family kinases, several inhibit multiple members of the family; other jakinibs demonstrate improved in vitro selectivity, which is influenced by a number of factors that will be discussed. We will also discuss the role of this class of drugs in the management of Coronavirus disease 2019 (Covid-19) patients.
Efficacy of approved jakinibs
The group of inhibitory molecules initially developed exert their effect by blocking the ATP binding pocket of the JAK catalytic domain. Although these compounds are relatively selective with limited off-target effects compared to other kinases, these jakinibs block the activity of multiple JAKs both in vitro and in vivo. Nonetheless, the promising results of these jakinibs in a wide-range of preclinical disease models quickly led to investigation in multiple clinical settings and for diverse pathologies; regulatory agencies have now approved nine jakinibs (Table 1).
Table 1:
Approved Jakinibs and their current indications
| Drug | Target | Indication |
|---|---|---|
| Tofacitinib | JAK1/JAK2/JAK3 | RA, PsA, UC, polyarticular course JIA |
| Baricitinib | JAK1/JAK2 | RA, Covid-19 (EUA) |
| Upadacitinib | JAK1 | RA |
| Filgotinib | JAK1 | RA (approved in EU, Japan) |
| Peficitinib | Multiple JAKs | RA (approved in Japan, Korea) |
| Ruxolitinib | JAK1/JAK2 | MPN, acute GvHD |
| Fedratinib | JAK2/FLT3/RET/BRD4 | MPN |
| Delgocitinib (Topical) | Multiple JAKs | AD (approved in Japan) |
| Oclacitinib | Multiple JAKs | AD (dogs) |
RA, rheumatoid arthritis; PsA, psoriatic arthritis; UC, ulcerative colitis; JIA, juvenile idiopathic arthritis; MPN, myeloproliferative neoplasms; GvHD, graft-versus-host disease; AD, atopic dermatitis
The first jakinib to enter clinical trials was tofacitinib, which inhibits JAK1/JAK2/JAK3, initially for renal transplantation; however, ruxolitinib, a JAK1/JAK2 inhibitor, was the first agent to receive approval for myeloproliferative neoplasms (MPN) that often exhibit gain-of-function (GOF) JAK2 mutations. Ruxolitinib is also approved for steroid refractory acute graft-versus-host disease (GvHD).
Tofacitinib was the first jakinib to be approved for a rheumatologic disease, specifically for patients with RA with an incomplete response to a conventional synthetic DMARD (csDMARD) either as monotherapy or in combination with a csDMARD . 7, 8, 9, 10, 11 Subsequently, tofacitinib has been approved for PsA12, 13 and ulcerative colitis (UC).14 Tofacitinib has been approved for the treatment of polyarticular course juvenile idiopathic arthritis (JIA) in the US.
Baricitinib, a JAK1/JAK2 inhibitor, is approved for patients with RA in the United States limited to 2 mg QD in combination with a csDMARD or as monotherapy in patients with RA refractory to a bDMARD based on phase 3 trial results. In many other countries, both 2 and 4 mg are approved in csDMARD incomplete responders. 15, 16, 17, 18 Baricitinib is also approved for treatment of AD in Europe.
Peficitinib, a pan JAK inhibitor, is approved in Japan, S. Korea, and Taiwan for the treatment of RA in csDMARD incomplete responders.19, 20 Tofacitinib, baricitinib, and peficitinib have shown clinical, functional, and radiographic efficacy after failure of a csDMARD, prior to a bDMARD, or after a bDMARD. Broadly speaking, with the exception of peficitinib, jakinibs have been documented to be more effective than MTX.7, 18
Selective targeting of JAK1 should block the broadest cytokine profile compared to other JAKs while avoiding negative impact on hematopoiesis by sparing erythropoietin (EPO), thrombopoietin (TPO), and granulocyte colony stimulating factor (G-CSF) signaling. Two jakinibs with relative JAK1 selectivity have been approved in RA: upadacitinib in many countries including the United States, the European Union (EU), Canada, and Japan, and filgotinib in the EU and Japan. Phase 3 trials demonstrated clinical, functional, and radiographic efficacy both in combination with a csDMARD or as monotherapy in patients with incomplete response to a csDMARD or a bDMARD.21, 22, 23, 24, 25, 26, 27 Upadacitinib monotherapy has also been shown to be more effective than MTX in MTX naïve patients with RA.24 Both have been referred to as “second generation jakinibs” as they both preferentially inhibit JAK1 in vitro but their clinical efficacy and safety, to a large extent, is similar to pan jakinibs in RA, although anemia and herpes zoster is seen less with filgotinib.
The clinical responses of jakinibs are more rapid than bDMARDs, with an earlier clinical effect shown as early as the first week of therapy as well as more rapid and deeper pain alleviation. Of clinical interest, tofacitinib, baricitinib, upadacitinib and filgotinib were all at least as effective as a bDMARD in MTX incomplete responders, which suggests that after an incomplete response to a csDMARD, one can utilize a jakinib prior to a bDMARD. Additionally, if a patient does not respond adequately to a jakinib (upadacitinib), they may respond to a TNF inhibitor (adalimumab).17, 25, 26, 28, 29
Whether more selectivity of JAK inhibition will lead to different outcomes remains unclear and there is currently a lack of well-designed clinical studies evaluating the efficacy or safety of one jakinib compared to another. Determination of selectivity is not simple and is dependent upon the nature of the assay, whether the assay is done in whole blood and exactly what is being measured. Evaluation of the in vitro cellular selectivity of baricitinib, tofacitinib, upadacitinib and filgotinib have shown that: all four agents inhibit IL-6 and IFNα signaling; upadacitinib and tofacitinib were more potent inhibitors of JAK1/3-dependent cytokines (IL-2, IL-4, IL-15, and IL-21) than baricitinib and filgotinib; baricitinib, tofacitinib, and upadacitinib but not filgotinib inhibited JAK2/2 and JAK2/TYK2 cytokines IL-3, GM-CSF, and G-CSF but to varying degrees.30, 31 Upadacitinib was the most potent inhibitor of JAK2/2-dependent cytokines IL-3 and GM-CSF, followed by baricitinib and tofacitinib; filgotinib was the least potent JAK2 inhibitor. Tofacitinib was the most potent inhibitor of JAK2/TYK2-dependent cytokine G-CSF, followed by upadacitinib and baricitinib.31 A common measure of JAK inhibition is the assessment of cytokine-induced STAT phosphorylation and a nuanced aspect of the assay is exactly what STAT is being measured; measurement of different STATs can give different answers. Thus, interpretation of selectivity appears to also be influenced by the cytokine and STAT being assessed. Most receptors use JAKs in pairs, but the importance of one JAK versus the other in signaling is still not fully understood and will require more basic research and likely more specific agents. Perhaps most important in terms of safety and efficacy is that no jakinib mediates sustained reduction in cell signaling throughout the dosing interval – a high degree of JAK inhibition is very transient. This is likely to be relevant for side effects.
Nonetheless, while the clinical relevance of these differences is unclear, jakinibs all appear to have similar efficacy and safety with the exception of fewer cases of anemia and herpes zoster with filgotinib.
Ongoing trials are investigating the efficacy of jakinibs in a wide range of additional indications. Phase 3 studies involving patients with active ankylosing spondylitis (AS) demonstrated promising efficacy and improvement with tofacitinib32 and upadacitinib.33 Efficacy of upadacitinib in PsA has also recently been reported with the 30 mg upadacitinib group was superior to adalimumab.34, 35 Upadacitinib has been evaluated in dose ranging trials assessing efficacy in Crohn’s disease (CD) and UC and has shown efficacy in both.36, 37 Upadacitinib was found to be more effective than placebo in AD38 and phase 3 trials are ongoing.
Although there were positive results with filgotinib in phase 2 studies of PsA, AS, CD, and UC, these clinical development programs have been halted subsequent to the complete response letter from the FDA for filgotinib denying approval at present.39, 40, 41
An open label trial of subjects with refractory dermatomyositis receiving tofacitinib and a trial of tofacitinib in combination with glucocorticoids in subjects with amyopathic dermatomyositis-associated interstitial lung disease both suggested efficacy and reasonable safety profiles.42, 43 In a compassionate use program for refractory juvenile dermatomyositis patients, preliminary data indicated clinically significant improvements with baricitinib treatment.44
Other indications being investigated include alopecia areata (AA), SLE, interferonopathies including Down syndrome, relapsing giant cell arteritis, GvHD, myasthenia gravis, and vitiligo (Table 2).
Table 2:
Selected ongoing clinical trials of first generation Jakinibs
Trials for cancer and/or malignancies have been excluded
AA, alopecia areata; AD, atopic dermatitis; GvHD, graft-versus-host disease; JIA, juvenile idiopathic arthritis; MPN, myeloproliferative neoplasms; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; UC, ulcerative colitis
Delgocitinib (JTE-052) is a topical jakinib approved in Japan for AD and oclacitinib, a JAK1/JAK2/JAK3 inhibitor, is approved for canine allergic dermatitis.
Safety of approved jakinibs
Jakinibs have been confirmed to be reasonably safe with an AE profile that is similar to bDMARDs, although the specific AEs are somewhat different (Table 3). Many of the AEs seen with jakinibs could have been predicted based on their mechanism of action (MOA) while others were not. Common side effects are infection, including serious and opportunistic infections and herpes zoster (HZ).45, 46, 47 Vaccination with recombinant adjuvanted HZ subunit is generally safe in patients with autoimmune disease, although flares are not uncommon.48, 49 Investigation of the serological response to the recombinant vaccine, in RA patients treated with jakinibs suggests satisfactory antibody responses and acceptable tolerability.50 By contrast to HZ, the rate of influenza AEs in a RA clinical program were comparable in the tofacitinib, adalimumab, MTX, and placebo treated groups.51
Table 3:
Major side effects of jakinibs
| Infection |
| Serious and opportunistic infections |
| Herpes Zoster |
| Hematologic |
| Anemia |
| Leukopenia |
| Thrombocytopenia |
| Venous Thrombosis? |
| Hyperlipidemia |
Cytopenias such as neutropenia and anemia are also common AEs, likely due to JAK2 inhibition. Upadacitinib, a relatively JAK1-selective molecule, has similar effects as pan-jakinibs on anemia, whereas filgotinib appears to produce less anemia. Jakinibs can also result in lymphopenia, in particular reduction in NK cells, related to JAK3 inhibition. Jakinib administration has been associated with elevated serum lipids due to reduced cholesterol ester catabolism, increasing cholesterol levels toward those in healthy volunteers and improvement in markers of antiatherogenic HDL function.52 The FDA requested a long-term safety study post approval of tofacitinib in 2012, evaluating the safety of tofacitinib at 5 mg and 10 mg twice daily versus a TNF inhibitor (TNFi), all on MTX in subjects with rheumatoid arthritis (RA) 50 years of age or older and who had at least one additional cardiovascular (CV) risk factor (ORAL-Surveillance). The co-primary endpoints were non-inferiority of the combined tofacitinib doses (5 and 10 mg BID) compared to the TNFi (etanercept or adalimumab) regarding (1) major adverse cardiovascular events (MACE) and (2) malignancies. Non-inferiority was defined by the upper bound of the confidence interval not being greater than 1.8 (lower bound not calculated). The top-line results have been reported. Although the incidence rates for both endpoints for both mechanisms were lower than 1.2 per 100/pt-yrs with differences between tofacitinib and TNFi being 0.25-.35, the prespecified non-inferiority criteria were not met for the primary comparison of the combined tofacitinib doses to TNFi. As seen in the general RA population, for tofacitinib, the most frequently reported MACE was myocardial infarction, and the most frequently reported malignancy was lung cancer. Of note, most of the events with both mechanisms occurred in subjects with a higher prevalence of known risk factors for MACE and malignancy (e.g., older age, smoking) and one can only conclude that there is probably a slight advantage of TNFi over tofacitinib for these events but cannot conclude that tofacitinib increases these events. (https://www.fda.gov/drugs/drug-safety-and-availability/initial-safety-trial-results-find-increased-risk-serious-heart-related-problems-and-cancer-arthritis?utm_medium=email&utm_source=govdelivery accessed on 2-24-2020).53
Concern of increased incidence of pulmonary embolism and venous and arterial thrombosis (VTE) was originally raised with baricitinib. During the placebo-controlled portion of the phase 3 studies, there was an imbalance in the development of VTEs, occurring only in the 4 mg group and not in the 2 mg or placebo group. However, in a report of over eight years of follow-up, the incidence rate of VTE was comparable between 2 and 4 mg and similar to what has been observed in RA patients treated with other MOAs.46 No disparity was seen in the clinical trials of tofacitinib34 and upadacitinib54. Other reports have shown there is no evidence of short-term risk of MACE or VTEs in RA patients initiating jakinibs.55 However, the situation has been confused because of the results of ORAL-Surveillance. Prior to completion of the study, data was released suggesting that, while there was not a statistically significant risk of the development of VTE between the tofacitinib 5 mg BID and TNF inhibitors for VTE and pulmonary embolism, there was one for tofacitinib 10 mg BID and a numerical difference favoring TNF inhibitors for 5 mg BID. For that reason, the EMA placed severe restrictions on the use of tofacitinib while the FDA strengthened its guidance. In a systemic review and meta-analysis of nine RCTs, risk of thromboembolism was increased in patients treated with jakinibs compared to placebo; baricitinib was the only jakinib that showed statistically significant increased thrombosis risk when compared to ruxolitinib, tofacitinib, and upadacitinib.56 To complicate matters further, there has been no clear mechanism that explains an increased risk of VTE with jakinibs, and thus, this is an area that requires further exploration.
Regarding the risk of developing malignancies in RA, patients treated with jakinibs revealed higher rates of malignancy in the treated groups versus controls in a meta-analysis of 36 trials, but these rates did not reach statistical significance.57
A long-term safety study of tofacitinib integrating data from phase 1, 2, 3b/4, and long-term extension studies in adult patients with RA (N = 7061) found AEs were stable over time and that incident rates were consistent with previous reports. No new safety risks were observed over the span of 9.5 years of cumulative tofacitinib exposure, and rates of safety events were comparable to bDMARDs and other jakinibs.58
Filgotinib, but not other jakinibs, produced defects in spermatogenesis in rodent models which prompted conduction of a safety study in male patients investigating whether the 200 mg dose will have similar effects in humans. The top-line results were reported: 8.3% patients on placebo and 6.7% patients on filgotinib had a 50% or more decline in sperm concentration at week 13. The study is ongoing and results as to whether the decrease will persist or affect more patients on filgotinib over time is still pending. Assessed April 23, 2021 https://www.globenewswire.com/news-release/2021/03/04/2186756/0/en/GALAPAGOS-REPORTS-PRIMARY-ENDPOINT-FOR-THE-ONGOING-FILGOTINIB-MANTA-AND-MANTA-RAy-SAFETY-STUDIES.html
In summary, multiple trials support the conclusion that in RA, jakinibs are at least equivalent or superior to TNF inhibitors when used with csDMARD in inducing a rapid response, observing a reduction in pain in as early as one week. Jakinibs have safety profiles generally comparable to biologics with the exceptions of increased HZ and the hematologic effects but perhaps with a slightly higher risk of MACE and malignancy in older patients with a history of smoking.
Next generation jakinibs
The success of jakinibs has motivated the development of yet more jakinibs (Table 4). CTP-543, a deuterium-modified form of ruxolitinib, has received breakthrough and fast track designation by the FDA and is currently being assessed in phase 2 and 3 studies of AA. Deuterated compounds have the potential advantage of improved pharmacokinetics with lower rates of metabolism, hence a longer half-life.
Table 4:
Ongoing clinical trials of selective jakinibs
| Agent | Specificity | Disease |
|---|---|---|
| Upadacitinib | JAK1 |
AD: NCT03569293, NCT03738397, NCT03568318, NCT03661138, NCT04195698, NCT03607422, NCT04666675 Hiradenitis Suppurativa: NCT04430855 Spondyloarthritis: NCT04169373 PsA: NCT03104374, NCT03104400 AS: NCT03178487 SLE: NCT03978520, NCT04451772 UC: NCT03653026, NCT03006068, NCT02819635, CD: NCT03345836, NCT03345823, NCT02782663, NCT02365649 Takayasu Arteritis: NCT04161898 Giant Cell Arteritis: NCT03725202 |
| Filgotinib | JAK1 |
RA: NCT02065700, NCT03025308 PsA: NCT03320876, NCT04115839, NCT04115748 CD: NCT02914600, NCT02914561, NCT03077412 AS: NCT04483700, NCT04483687 UC: NCT02914535 IBD: NCT03201445 Noninfectious Uveitis: NCT03207815 |
| Abrocitinib | JAK1 | AD: NCT04345367, NCT03422822, NCT03915496 |
| Itacitinib | JAK1 |
Acute GvHD:
NCT03846479 Chronic GvHD: NCT04200365, NCT03584516, NCT04446182 Prophylaxis for GvHD and Immune Effector Cell Therapy (Prevention of Cytokine Release Syndrome): NCT04071366, NCT04339101 Asthma: NCT04129931 MPN/Myelofibrosis: NCT04640025, NCT03144687, NCT01633372, NCT04629508 Non-Small Cell Lung Cancer: NCT03425006, NCT02917993 |
| ARQ-252 | JAK1 | Hand Eczema: NCT04378569 |
| BMS-986165 | TYK2 |
SLE: NCT03252587, NCT03920267 Lupus Nephritis: NCT03943147 CD: NCT03599622 UC: NCT03934216, NCT04613518 PsA: NCT03881059 Plaque Psoriasis: NCT03624127, NCT03611751, NCT04167462 Psoriasis: NCT04036435, NCT03924427 |
| Brepocitinib (PF-06700841) |
TYK2/ JAK1 |
Psoriasis: NCT03850483 Acne Inversa: NCT04092452 Hidradenitis suppurativa: NCT04092452 NSV: NCT03715829 PsA: NCT03963401 SLE: NCT03845517 CD: NCT03395184 UC: NCT02958865 |
| Ritlecitinib | JAK3/Tec kinases |
RA: NCT02969044 CD: NCT03395184 UC: NCT02958865 NSV: NCT03715829 AA: NCT03732807, NCT04006457, NCT04517864 |
| Cerdulatinib* | JAK1/JAK2/Syk | Vitiligo: NCT04103060 |
| Momelotinib | JAK1/JAK2/ACVR1 | MPN: NCT04173494 |
| Pacritinib* | JAK2/IRAK1 |
GvHD:
NCT02891603 MPN: NCT03645824, NCT03165734 Covid-19: NCT04404361 |
| Gusacitinib* (ASN002) | JAK1/JAK2/JAK3/TYK2/Syk |
Chronic Hand Dermatitis:
NCT03728504 AD: NCT03531957 |
Trials for cancer and/or malignancies have been excluded
AA, alopecia areata; AD, atopic dermatitis; AS, ankylosing spondylitis; CD, Crohn’s Disease; GvHD, graft-versus-host disease; IBD, inflammatory bowel disease; JIA, juvenile idiopathic arthritis; MPN, myeloproliferative neoplasms; NSV, non-segmental vitiligo; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; UC, ulcerative colitis
Another JAK1-selective jakinib, abrocitinib, has been evaluated in phase 3 trials in AD and plaque psoriasis,59, 60 showing efficacy in both diseases. AEs included Herpes simplex and HZ infection, appendicitis, pancreatitis, and IBD. No cases of VTEs, malignancies, MACE, or deaths were observed. Decreased platelets were noted with no decrease in hemoglobin. The JAK1-selective inhibitor, itacitinib, is currently being assessed in multiple clinical trials for oncologic indications as well as for chronic and acute GvHD, including GvHD prophylaxis. ARQ-252 is a selective topical JAK1 inhibitor, and a phase 1/2b trial for adults with chronic hand eczema is underway.
Inhibition of TYK2 would be expected to impact type I and type III IFNs, IL-10 family cytokines, IL-12, and IL-23, but not other cytokines. The success of monoclonal antibodies targeting IL-12 and IL-23 with acceptable AEs along with TYK2 coding variants being associated with reduced incidence of autoimmunity 61, 62 support the potential efficacy of TYK2 inhibition. Deucravacitinib (BMS-986165) has shown efficacy in psoriasis trial 63 and significantly greater American College of Rheumatology (ACR)20 responses in PsA compared to placebo. No anemia, cytopenias, serious infections, HZ, opportunistic infections, or thrombotic events were observed.64 Trials are ongoing in SLE, including lupus nephritis, CD and UC. Unlike other jakinibs, deucravacitinib binds to the kinase-like domain.65 Since the kinase-like domain is a relatively unique feature of JAKs, in principle, this strategy could provide increased kinome selectivity.
PF-06826647 is a TYK2-selective jakinib 66 currently in trials for psoriasis, UC, and hidradenitis suppurativa; brepocitinib is a TYK2/JAK1 inhibitor that was assessed in a phase 2 trial of psoriasis where clinical responses were seen with all doses.67 Ongoing investigations with brepocitinib with either oral administration or topical application include trials in AD, SLE, CD, UC, AA, hidradenitis suppurativa, and vitiligo.
Though the goal of JAK1- and TYK2-selective inhibitor use is to minimize JAK2 signaling interference and subsequent cytopenias, specifically targeting JAK2 can offer a treatment advantage for myeloproliferative neoplasms and potentially other hematological malignancies. Fedratinib is a selective JAK2 inhibitor approved for primary and secondary myelofibrosis. An important AE observed with fedratinib is Wernicke’s encephalopathy in patients taking the highest daily dosage (500 mg).68 Fedratinib exerts off-target inhibitory activity against bromodomain-containing protein 4 (BRD4) and is effective regardless of JAK2 mutational status. Phase 3 trials are ongoing to assess fedratinib’s effects on long-term safety, efficacy, and overall survival.69
Gandotinib is a JAK2-selective inhibitor that has potential increased potency for the JAK2V617F mutant kinase and has shown efficacy in MPN.70, although there are currently no ongoing clinical investigations of gandotinib.
JAK3’s action is seemingly most restricted within the JAK family because of its exclusive association with the common γ-chain (γc), a shared subunit used by IL-2, IL-4, IL-7, IL-19, IL-15 and IL-21. Mutations of either the γc (encoded by IL2RG) or JAK3 lead to complete absence of signaling of these cytokines and the consequent development of severe combined immunodeficiency disease (SCID) characterized by depletion of T, B, and natural killer (NK) cells but with no other defects. Thus, JAK3 has been identified as a potential target to treat various inflammatory and autoimmune diseases.
Developed to target the kinase domain of JAK3, decernotinib (VX-509) showed selective in vitro inhibition of JAK3 over the other JAKs and efficacy in RA.71, 72 However, a CYP3A4-mediated drug-drug interaction was also described, potentially limiting decernotinib’s use; development of this molecule has currently been halted.73
Beyond just JAKs
Despite the effort to search for drugs with greater specificity, there may be advantages in efficacy in targeting JAKs along with kinases involved in other signaling pathways (Table 4).
Ritlecitinib is an irreversible covalent inhibitor that binds to a distinct cysteine residue in the JAK3 catalytic domain, which other JAK family members lack. However, TEC family kinases possess this cysteine residue in their kinase catalytic domains. TEC family consists of five members: Bruton’s tyrosine kinase (BTK), bone marrow tyrosine kinase on chromosome X (BMX), interleukin 2-inducible T cell kinase (ITK), resting lymphocyte kinase (RLK), and tec protein tyrosine kinase (Tec). These kinases are important mediators of signaling for T cell antigen receptors, B cell antigen receptors (BCR), and chemokine receptors. Thus, ritlecitinib targets more than just type I/II cytokine receptor signaling. Ritlecitinib was found to be superior to placebo in a phase 2 study in RA patients with inadequate response to MTX.74 AEs included lymphopenia and infection such as herpes simplex. Ritlecitinib is currently in phase 2 trials for CD, UC, AA, and vitiligo.
The spleen tyrosine kinase (SYK) is highly expressed in hematopoietic cells and plays important and varied roles in immune cell signaling from BCR and FcR to lectins and integrins. Thus, simultaneous targeting of SYK along with JAKs could have utility in the treatment of immune and inflammatory disease. Gusacitinib (ASN002) is an oral JAK/SYK inhibitor that showed clinical efficacy in a phase 2 trial in AD associated with decreased expression of inflammatory biomarkers.75 Another JAK/SYK inhibitor, cerdulatinib (PRT062070), has been tested in several trials for leukemia and lymphoma, and a topical formulation is being tested in phase 2 trials in patients with vitiligo.
Momelotinib is a JAK1/JAK2 inhibitor as well as an activin receptor-like kinase (ACVR1) inhibitor. Because ACVR1 is a receptor for bone morphogenic proteins, momelotinib is undergoing testing in myelofibrosis.76 Pacritinib is a JAK2 and interleukin-1 receptor-associated kinase 1 (IRAK1) inhibitor also being evaluated in myelofibrosis. Blocking IL-1 and toll receptor signaling might offer some interesting opportunities for autoimmune disease treatment.
Jakinibs in Covid-19
The emergence of a pandemic due to novel coronavirus SARS-CoV-2 with its associated severe systemic immune hyperactivation has motivated the search for novel therapeutics to treat Covid-19 cytokine release syndrome, also termed cytokine storm. The extreme elevated levels of multiple cytokines are associated with pulmonary and endothelial disease, myocardial damage, and mortality. Consequently, the search for effective drugs to manage Covid-19 has focused on modulation of cytokines. While treatment of Covid-19 with dexamethasone has shown efficacy in the RECOVERY trial, use of biologics has not consistently demonstrated efficacy.
Many of the cytokines elevated in Covid-19 including IL-2, IL-6, IL-12, IFN-γ, and GM-CSF signal via JAKs. For this reason, jakinibs were considered potential therapeutic candidates. However, an obvious argument against the use of jakinibs is the risk of increased infection. While jakinibs generally have a reasonable degree of kinome selectivity, baricitinib additionally inhibits the numb-associated kinase (NAK) family, AAK1 and cyclin G-associated kinase (GAK) 77, 78 involved in viral entry of SARS-CoV-2. Several small trials have shown efficacy of ruxolitinib in the context of severe SARS-CoV-2.79, 80
Baricitinib has shown utility in small trials in Covid-19.81, 82 However, Adaptive Covid-19 Treatment Trial (ACTT)-283 is a double-blind, randomized, placebo-controlled trial testing remdesivir plus 4 mg daily baricitinib or remdesivir plus placebo. This study enrolled 1033 hospitalized adults with Covid-19 in 67 sites in 8 countries, assessing the outcomes of time to recovery and clinical status at day 15. The results showed that baricitinib and remdesivir were safe and superior to remdesivir alone for patients with Covid-19 pneumonia. The observed benefit of combination treatment was most evident in patients receiving supplemental oxygen, high-flow oxygen, or noninvasive ventilation. Overall, patients receiving baricitinib had a median time to recovery of 7 days compared to 8 days for control patients, whereas the time to recovery for patients receiving oxygen or noninvasive ventilation was 10 days for baricitinib plus remdesivir versus 18 days for remdesivir and placebo. The benefit of the combination treatment was less evident in patients not requiring oxygen or in patients requiring mechanical oxygenation or extracorporeal membrane oxygenation. Importantly and perhaps surprisingly, serious AEs, including infection, were less frequent in the combination group than in the control group.
ACTT-4, the fourth adaptive Covid-19 treatment trial, randomized patients to either remdesivir, intravenous dexamethasone, and placebo tablets or remdesivir, baricitinib tablets, and an IV placebo. The goal was to assess which combination was more effective at preventing adults hospitalized with COVID-19 on supplemental oxygen from progressing to requiring mechanical ventilation or death. Upon review of the current data, the data safety monitoring board determined that it is unlikely that the study will show a significant difference between these two arms even if the trial continued to full enrollment of 1,500 participants. As a result, enrollment in the trial was closed after slightly more than 1,000 participants. Thus, at present, the two strategies appear to be equivalent (https://www.nih.gov/news-events/news-releases/nih-closes-enrollment-trial-comparing-covid-19-treatment-regimens).
An important outstanding question is the relevance of baricitinib’s potential anti-viral activity via inhibition of the NAK family. The COV-BARRIER trial examined addition of baricitinib versus placebo to standard of care. The results have been announced but not yet been published. The primary endpoints were progression to non-invasive or invasive mechanical ventilation or death, which was not statistically significant in the baricitinib group; although, reduction in mortality was seen without additional adverse events (https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-results-phase-3-cov-barrier-study).
Many other trials with a variety of jakinibs are underway and will hopefully assess the utility of baricitinib versus other jakinibs. Beyond Covid-19, the data beg the question as to whether jakinibs have broad use in sepsis and acute respiratory distress syndrome (ARDS).
Conclusions and challenges ahead
The year 2011 witnessed the approval of the first jakinib, ruxolitinib. Since this time, it has been remarkable to see the rapid development of multiple jakinibs and their approval for use of a broad range of indications, crossing multiple clinical specialties. We now know that jakinibs are generally as safe and effective as biologics, albeit with some differences in safety, and exert their effect more rapidly. Not surprisingly, they are being tested in a plethora of disorders from genetic interferonopathies to Covid-19. Despite the impressive advances, many questions remain.
In RA, should jakinibs be used prior to MTX, assuming equal patient access and affordability? Tofacitinib, baricitinib and upadacitinib have been shown to be more efficacious than MTX, but there are some differences in safety and tolerability - some of which favor MTX while others favor jakinibs. Should jakinibs be employed prior to bDMARDs considering that each jakinib has been shown to be at least as effective as bDMARDs and have the advantage of oral administration with rapid benefit? It has been shown with each of the jakinibs in RA that they are more effective in combination with a csDMARD although monotherapy is effective in many patients. If a jakinib is added to a csDMARD and the patient achieves sustained control of their disease, should the jakinib or csDMARD be tapered or discontinued first? Many patients are initially treated with glucocorticoids (GC) as “bridge therapy,” but in many, the “bridge” lasts for a prolonged time. Can jakinibs decrease the total exposure to GC and thus prevent their significant toxicity? Importantly, what is the real risk of VTE, MACE and malignancy with use of a jakinib versus another MOA? As discussed above, issues surrounding specificity are not straightforward; do different in vitro specificities, measured by various assays, correlate with differences in efficacy and safety amongst the jakinibs? We still don’t know but well-designed head-to-head trials will need to be performed to answer all these questions. Many of the same questions lack answers in PsA and AS, such as initial versus later use, monotherapy versus combination therapy, and treatment with a second jakinib after failure of the first. The GC question is not as relevant; however, we do not know the efficacy of jakinibs compared to non-TNF bDMARDs, such as the inhibitors of IL-17 or IL-23.
The use of jakinibs in combination with other inhibitors, whether intracellular or bDMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine, is currently not recommended. However, it is conceivable that there are combinations which are more efficacious and/or safer; obviously, this too will need to be assessed in the setting of a rigorous randomized control trials – again, not a trivial undertaking. Mechanistic studies have been performed that elucidate relevant aspects jakinib action in terms of efficacy;84–87 however, more work is required to understand how transient blockade of signaling by jakinibs versus sustained blockade of cytokines by biologics impacts relevant cell populations and immunopathogenesis.
In disorders such as lupus nephritis or vasculitis, aggressive approaches are warranted. Use of jakinibs have the potential for reducing toxicities of other agents, but as was clear in transplant trials, they also have the potential for over-immunosuppression.88 We now know that jakinibs have utility in Covid-19 cytokine storm; remarkably, these agents are safe in patients with severe, life-threatening infectious disease. However, it is by no means clear that we have identified the right dose and the right combination of agents. Also remarkable is that a parenteral jakinib has yet to be generated. An advantage of this class of drugs is their rapid onset of action and short half-life, but it is not clear that in severely ill patients that the current doses and formulations are optimal to interdict inflammatory responses quickly and effectively.
The utility of jakinibs in Covid-19 cytokine storm suggests that these agents may be useful in additional clinical scenarios. Cytokine storm associated with chimeric antigen receptor (CAR) T cell therapies is one possible setting. A concern of course would be that first generation jakinibs might be toxic for the engineered T cells; however, this is a setting in which a selective JAK2 inhibitor might be advantageous. Similarly, in autoimmune disease associated with checkpoint blockade inhibitors, jakinibs might also have utility in dampening immune responses.89 On one hand, jakinibs are being studied as therapy for a number of cancers – many of which are driven by a cytokine/STAT signature.90 On the other hand, jakinibs have the capacity to block IFNs and limit the ability of immune cells to kill cancer cells. However, there is an additional wrinkle here – by attenuating the action of IFNs, jakinibs have the capacity to reverse T cell exhaustion and promote elimination of tumors.91
These musings are certainly not an exhaustive list of challenges and opportunities in the jakinib arena. We present a few ideas of issues that will need to be addressed by thoughtful clinical studies, the need for which will likely increase as generic forms of jakinibs become available and jakinibs are more accessible to more patients. Considering a decade in, especially for those of us in the specialty, the advances in the jakinib field have been astonishing; however, we hope we have made the case that, in many respects, we are still at the beginning of what needs to be understood.
Figure 1:
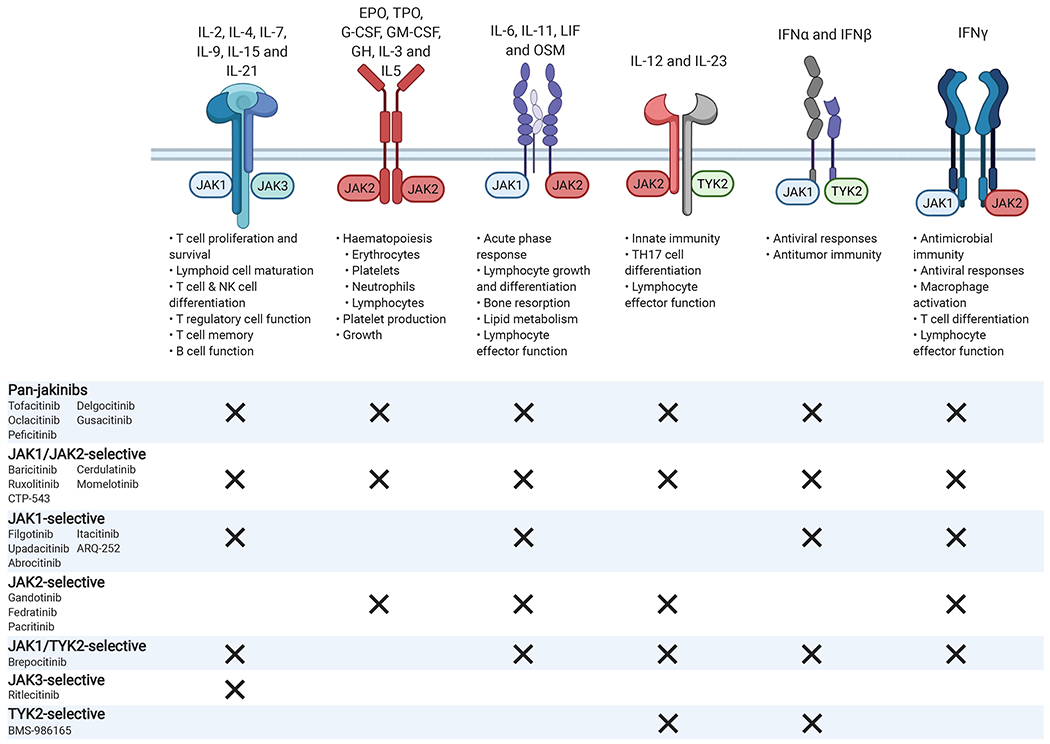
Type I and type II cytokine receptors associate with different members of the Janus kinase family (JAK1, JAK2, JAK3, and TYK2) in order to transduce intracellular signals. Selective blockade of specific JAK molecules should inhibit specific biologic actions while allowing other JAK-dependent cytokines to signal normally. For example, by selectively inhibiting JAK1, the adverse events related to JAK2 inhibition such as anemia and neutropenia should be avoided. JAK3 mediated signaling, which is associated exclusively with the common γ-chain receptor, should also be unaffected, sparing T, B, and NK (natural killer) cell function. JAK, Janus kinase; TYK, tyrosine kinase; IL, interleukin; EPO, erythropoietin; TPO, thrombopoietin; granulocyte colony-stimulating factor (G-CSF); GM-CSF, granulocyte–macrophage colony-stimulating factor; GH, growth hormone; OSM, oncostatin M; IFN, interferon. Created with BioRender.com
Figure 2:
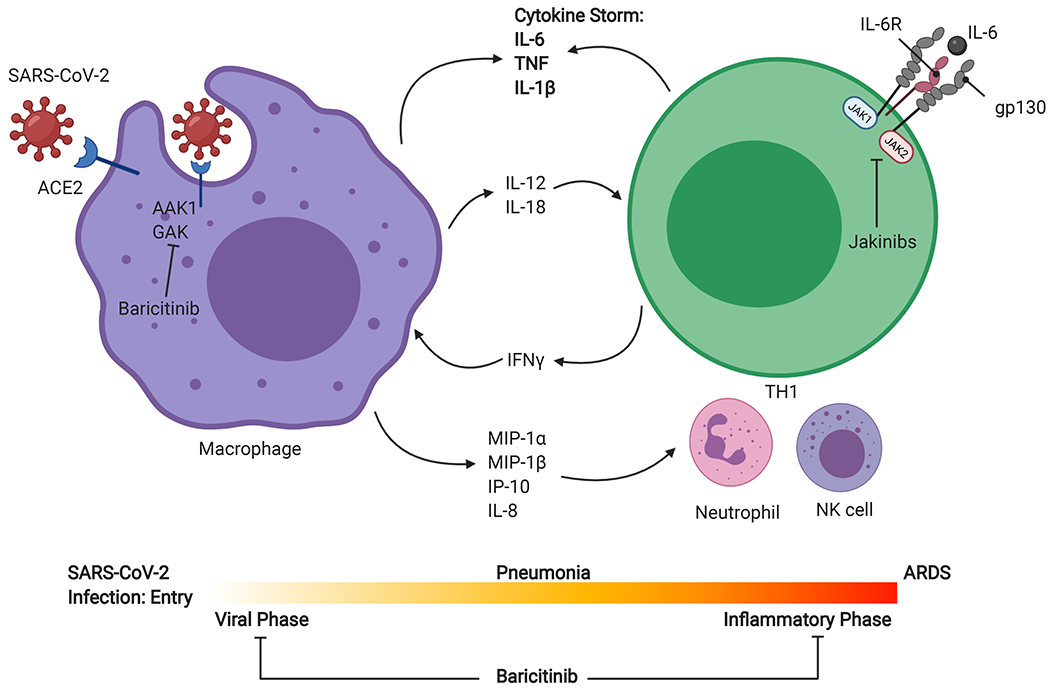
SARS-CoV-2 entry is mediated by ACE2 (Angiotensin converting enzyme 2), a receptor widely expressed in the lungs, heart, vasculature, kidneys, and gastrointestinal tract. Primary site of infection is alveolar epithelial cells in the lungs, and rapid replication of virus can lead to a hyperimmune response. Macrophage activation and chemokine release for neutrophil, TH1, and NK cell recruitment can lead to a massive cytokine release responsible for the clinical evolution to acute respiratory distress syndrome (ARDS). Jakinibs such as baricitinib can potentially block viral entry by inhibiting numb-associated kinase family (NAK) proteins AAK1 and cyclin G-associated kinase (GAK). During the inflammatory phase, many of the cytokines elevated in Covid-19 (IL-6, IL-12, and IFNγ) signal via JAKs, and therefore, jakinibs are being considered as potential therapeutics in severe SARS-CoV-2. JAK, Janus kinase; TYK, tyrosine kinase; IL, interleukin; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; IP, interferon γ-induced protein. Created with BioRender.com
Disclosures:
This work was supported by NIAMS Intramural Research Program
John J. O’Shea and Massimo Gadina have a Collaborative Research and Development Agreement (CRADA) with Pfizer.
Roy Fleischmann received consulting fees from: AbbVie; Pfizer; Lilly and Gilead.
Abbreviations
- JAK
janus kinase
- Jakinib
JAK inhibitor
- STAT
signal transducers and activator of transcription
- IFN
interferon
- GOF
gain of function
- RA
rheumatoid arthritis
- PsO
psoriasis
- PsA
psoriatic arthritis
- AS
ankylosing spondylitis
- IBD
inflammatory bowel disease
- UC
ulcerative colitis
- CD
Crohn’s disease
- JIA
juvenile idiopathic arthritis
- TNF
tumor necrosis factor
- SLE
systemic lupus erythematosus
- MPN
myeloproliferative neoplasms
- GvHD
graft-versus-host disease
- AD
atopic dermatitis
- AA
alopecia areata
- AE
adverse events
- MTX
methotrexate
- EPO
erythropoietin
- TPO
thrombopoietin
- G-CSF
granulocyte colony stimulating factor
- MOA
mechanism of action
- HZ
herpes zoster
- MACE
major adverse cardiovascular events
- BRD4
bromodomain-containing protein 4
- SYK
spleen tyrosine kinase
- BTK
Bruton’s tyrosine kinase
- TEC
tec protein tyrosine kinase
- BMX
bone marrow tyrosine kinase on chromosome X
- ITK
interleukin 2-inducible T cell kinase
- BCR
B cell antigen receptors
- CC
case control study
- RCT
randomized control trial
- ICU
intensive care unit
- NOS
nitric oxide synthase
- ARDS
acute respiratory distress syndrome
- SARS-CoV2
Severe Acute Respiratory Syndrome Corona Virus 2
- Covid-19
Coronavirus Disease 2019
- NAK
numb-associated kinases
- AAK1
AP2-associated protein kinase 1
- GAK
cyclin G-associated kinase
- GC
glucocorticoids
- CAR
chimeric antigen receptor
References:
- 1.Darnell JEK, I. M, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. June 1994;264:1415–21. [DOI] [PubMed] [Google Scholar]
- 2.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. November 1995;270:797–800. [DOI] [PubMed] [Google Scholar]
- 3.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. October 2003;302:875–8. [DOI] [PubMed] [Google Scholar]
- 4.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. April 2005;352:1779–90. [DOI] [PubMed] [Google Scholar]
- 5.Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramírez-Alejo N, Kilic SS, et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. September 2015;212:1641–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hainzl E, Stockinger S, Rauch I, Heider S, Berry D, Lassnig C, et al. Intestinal Epithelial Cell Tyrosine Kinase 2 Transduces IL-22 Signals To Protect from Acute Colitis. J Immunol. November 2015;195:5011–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. June 19 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. August 9 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 9.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. August 20 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 10.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. August 9 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 11.Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. February 9 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 12.Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. N Engl J Med. October 19 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 13.Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N Engl J Med. October 19 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Su C, Panes J. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. August 3 2017;377:496–7. [DOI] [PubMed] [Google Scholar]
- 15.Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in Patients with Refractory Rheumatoid Arthritis. N Engl J Med. March 31 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 16.Dougados M, van der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. January 2017;76:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. February 16 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
- 18.Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, Methotrexate, or Combination in Patients With Rheumatoid Arthritis and No or Limited Prior Disease-Modifying Antirheumatic Drug Treatment. Arthritis Rheumatol. March 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, Takeuchi T, Tanaka S, Kawakami A, Iwasaki M, Song YW, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3). Ann Rheum Dis. October 2019;78:1320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi T, Tanaka Y, Tanaka S, Kawakami A, Iwasaki M, Katayama K, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. October 2019;78:1305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. June 23 2018;391:2513–24. [DOI] [PubMed] [Google Scholar]
- 22.Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. June 23 2018;391:2503–12. [DOI] [PubMed] [Google Scholar]
- 23.Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 06 2019;393:2303–11. [DOI] [PubMed] [Google Scholar]
- 24.van Vollenhoven R, Takeuchi T, Pangan AL, Friedman A, Mohamed MF, Chen S, et al. Efficacy and Safety of Upadacitinib Monotherapy in Methotrexate-Naive Patients With Moderately-to-Severely Active Rheumatoid Arthritis (SELECT-EARLY): A Multicenter, Multi-Country, Randomized, Double-Blind, Active Comparator-Controlled Trial. Arthritis Rheumatol. October 2020;72:1607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib Versus Placebo or Adalimumab in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate: Results of a Phase III, Double-Blind, Randomized Controlled Trial. Arthritis Rheumatol. 11 2019;71:1788–800. [DOI] [PubMed] [Google Scholar]
- 26.Westhovens R, Rigby WFC, van der Heijde D, Ching DWT, Stohl W, Kay J, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis. January 15 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese MC, Kalunian K, Gottenberg JE, Mozaffarian N, Bartok B, Matzkies F, et al. Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial. JAMA. 07 2019;322:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. July 29 2017;390:457–68. [DOI] [PubMed] [Google Scholar]
- 29.Fleischmann RM, Blanco R, Hall S, Thomson GTD, Van den Bosch FE, Zerbini C, et al. Switching between Janus kinase inhibitor upadacitinib and adalimumab following insufficient response: efficacy and safety in patients with rheumatoid arthritis. Ann Rheum Dis. November 4 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traves PG, Murray B, Campigotto F, Galien R, Meng A, Di Paolo JA. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. March 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McInnes IB, Byers NL, Higgs RE, Lee J, Macias WL, Na S, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 08 2019;21:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deodhar A, Sliwinska-Stanczyk P, Xu H, Baraliakos X, Gensler L, Fleishaker D, et al. Tofacitinib for the Treatment of Adult Patients with Ankylosing Spondylitis: Primary Analysis of a Phase 3, Randomized, Double-blind, Placebo-controlled Study [abstract]. Arthritis Rheumatol. 2020;72: [Google Scholar]
- 33.van der Heijde D, Song IH, Pangan AL, Deodhar A, van den Bosch F, Maksymowych WP, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 12 2019;394:2108–17. [DOI] [PubMed] [Google Scholar]
- 34.Mease PJ, Lertratanakul A, Anderson JK, Papp K, Van den Bosch F, Tsuji S, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. December 3 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McInnes IB, Anderson JK, Magrey M, Merola JF, Liu Y, Kishimoto M, et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N Engl J Med. April 1 2021;384:1227–39. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Feagan BG, Loftus EV, Peyrin-Biroulet L, Van Assche G, D’Haens G, et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients With Crohn’s Disease. Gastroenterology. June 2020;158:2123–38.e8. [DOI] [PubMed] [Google Scholar]
- 37.Sandborn WJ, Ghosh S, Panes J, Schreiber S, D’Haens G, Tanida S, et al. Efficacy of Upadacitinib in a Randomized Trial of Patients With Active Ulcerative Colitis. Gastroenterology. June 2020;158:2139–49.e14. [DOI] [PubMed] [Google Scholar]
- 38.Guttman-Yassky E, Thaçi D, Pangan AL, Hong HC, Papp KA, Reich K, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 03 2020;145:877–84. [DOI] [PubMed] [Google Scholar]
- 39.Mease P, Coates LC, Helliwell PS, Stanislavchuk M, Rychlewska-Hanczewska A, Dudek A, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 12 2018;392:2367–77. [DOI] [PubMed] [Google Scholar]
- 40.van der Heijde D, Baraliakos X, Gensler LS, Maksymowych WP, Tseluyko V, Nadashkevich O, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 12 2018;392:2378–87. [DOI] [PubMed] [Google Scholar]
- 41.Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 01 2017;389:266–75. [DOI] [PubMed] [Google Scholar]
- 42.Paik J, Albayda J, Tiniakou E, Purwin G, Koenig A, Christopher-Stine L. Long Term Open Label Extension of Study of Tofacitinib in Refractory Dermatomyositis [abstract]. Arthritis Rheumatol. 2020;72: [Google Scholar]
- 43.Chen Z, Wang X, Ye S. Tofacitinib in Amyopathic Dermatomyositis-Associated Interstitial Lung Disease. N Engl J Med. 07 2019;381:291–93. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, Dill S, O’Brien M, Vian L, Li X, Manukyan M, et al. Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann Rheum Dis. August 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. April 5 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genovese MC, Smolen JS, Takeuchi T, Burmester GR, Deberdt W, Schlichting D, et al. Safety profile of baracitinib for the treatment of rheumatoid arthritis up to 8.4 years: an updated integrated safety analysis [abstract]. Annals of the Rheumatic Diseases. 2020;79:642–43. [Google Scholar]
- 47.Cohen SB, Van Vollenhoven R, Curtis JR, Calabrese L, Zerbini C, Tanaka Y, et al. Safety profile of upadacitinib up to 3 years of exposure in patients with rheumatoid arthritis [abstract]. Annals of the Rheumatic Diseases. 2020;79:319–20. [Google Scholar]
- 48.Stevens E, Weinblatt ME, Massarotti E, Griffin F, Emani S, Desai S. Safety of the Zoster Vaccine Recombinant Adjuvanted in Rheumatoid Arthritis and Other Systemic Rheumatic Disease Patients: A Single Center’s Experience With 400 Patients. ACR Open Rheumatol. June 2020;2:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenfant T, Jin Y, Kirchner E, Hajj-Ali RA, Calabrese LH, Calabrese C. Safety of Recombinant Zoster Vaccine: a Retrospective Study of 622 Rheumatology Patients. Rheumatology (Oxford). February 9 2021; [DOI] [PubMed] [Google Scholar]
- 50.Källmark H, Gullstrand B, Nagel J, Einarsson J, Jönsson G, Kahn F, et al. Immunogenicity of Adjuvanted Herpes Zoster Subunit Vaccine in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors and Controls: Preliminary Results [abstract]. Arthritis Rheumatol. 2020;72: [Google Scholar]
- 51.Winthrop K, Yndestad A, Henrohn D, Jo H, Marsal S, Galindo M, et al. Influenza Adverse Events in Patients with Rheumatoid Arthritis in the Tofacitinib Clinical Program [abstract]. Arthritis Rheumatol. 2020;72: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charles-Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. March 2015;67:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 08 2019;78:1048–54. [DOI] [PubMed] [Google Scholar]
- 54.Cohen SB, van Vollenhoven RF, Winthrop KL, Zerbini CAF, Tanaka Y, Bessette L, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. October 28 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malaurie M, Constantin A, Degboé Y, Ruyssen-Witrand A, Barnetche T. Short-term Risk of Major Adverse Cardiovascular Events or Venous Thrombo-embolic Events in Patients with Rheumatoid Arthritis Initiating a Janus Kinase Inhibitor: A Meta-analysis of Randomised Controlled Trials [abstract]. Arthritis Rheumatol. 2019;71: [Google Scholar]
- 56.Bilal J, Riaz I, Sadiq M, Salick M, Nomaan Y, Iqbal N, et al. Risk of Thromboembolism with Janus Kinase Inhibitors: A Systematic Review and Meta-Analysis of Randomized Placebo Controlled Trials [abstract]. Arthritis Rheumatol. 2019;71: [Google Scholar]
- 57.Lopez-Olivo MA, Tayar J, Zamora N, Pratt G, M. S-A. Malignancies and Serious Infections in Randomized Controlled Trials of Janus Kinase Inhibitors in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2018;70: [Google Scholar]
- 58.Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. October 2020;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 07 2020;396:255–66. [DOI] [PubMed] [Google Scholar]
- 60.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. August 2020;156:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sigurdsson S, Nordmark G, Göring HH, Lindroos K, Wiman AC, Sturfelt G, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. March 2005;76:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cunninghame Graham DS, Morris DL, Bhangale TR, Criswell LA, Syvänen AC, Rönnblom L, et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. October 2011;7:e1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papp K, Gordon K, Thaçi D, Morita A, Gooderham M, Foley P, et al. Phase 2 Trial of Selective Tyrosine Kinase 2 Inhibition in Psoriasis. N Engl J Med. 10 2018;379:1313–21. [DOI] [PubMed] [Google Scholar]
- 64.Mease P, Deodhar A, van der Heijde D, Behrens F, Kivitz A, Kim J, et al. Efficacy and Safety of Deucravacitinib (BMS-986165), an Oral, Selective Tyrosine Kinase 2 Inhibitor, in Patients with Active Psoriatic Arthritis: Results from a Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial [abstract]. Arthritis Rheumatol. 2020;72: [Google Scholar]
- 65.Burke JR, Cheng L, Gillooly KM, Strnad J, Zupa-Fernandez A, Catlett IM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 07 2019;11: [DOI] [PubMed] [Google Scholar]
- 66.Gerstenberger BS, Ambler C, Arnold EP, Banker ME, Brown MF, Clark JD, et al. Discovery of Tyrosine Kinase 2 (TYK2) Inhibitor (PF-06826647) for the Treatment of Autoimmune Diseases. J Med Chem. November 25 2020;63:13561–77. [DOI] [PubMed] [Google Scholar]
- 67.Forman SB, Pariser DM, Poulin Y, Vincent MS, Gilbert SA, Kieras EM, et al. TYK2/JAK1 Inhibitor PF-06700841 in Patients with Plaque Psoriasis: Phase IIa, Randomized, Double-Blind, Placebo-Controlled Trial. J Invest Dermatol. December 2020;140:2359–70.e5. [DOI] [PubMed] [Google Scholar]
- 68.Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, et al. Safety and Efficacy of Fedratinib in Patients With Primary or Secondary Myelofibrosis: A Randomized Clinical Trial. JAMA Oncol. August 2015;1:643–51. [DOI] [PubMed] [Google Scholar]
- 69.Talpaz M, Kiladjian JJ. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia. July 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berdeja J, Palandri F, Baer MR, Quick D, Kiladjian JJ, Martinelli G, et al. Phase 2 study of gandotinib (LY2784544) in patients with myeloproliferative neoplasms. Leuk Res. 08 2018;71:82–88. [DOI] [PubMed] [Google Scholar]
- 71.Fleischmann RM, Damjanov NS, Kivitz AJ, Legedza A, Hoock T, Kinnman N. A randomized, double-blind, placebo-controlled, twelve-week, dose-ranging study of decernotinib, an oral selective JAK-3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol. February 2015;67:334–43. [DOI] [PubMed] [Google Scholar]
- 72.Genovese MC, van Vollenhoven RF, Pacheco-Tena C, Zhang Y, Kinnman N. VX-509 (Decernotinib), an Oral Selective JAK-3 Inhibitor, in Combination With Methotrexate in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. January 2016;68:46–55. [DOI] [PubMed] [Google Scholar]
- 73.Zetterberg C, Maltais F, Laitinen L, Liao S, Tsao H, Chakilam A, et al. VX-509 (Decernotinib)-Mediated CYP3A Time-Dependent Inhibition: An Aldehyde Oxidase Metabolite as a Perpetrator of Drug-Drug Interactions. Drug Metab Dispos. 08 2016;44:1286–95. [DOI] [PubMed] [Google Scholar]
- 74.Robinson MF, Damjanov N, Stamenkovic B, Radunovic G, Kivitz A, Cox L, et al. Efficacy and Safety of PF-06651600 (Ritlecitinib), a Novel JAK3/TEC Inhibitor, in Patients With Moderate-to-Severe Rheumatoid Arthritis and an Inadequate Response to Methotrexate. Arthritis Rheumatol. October 2020;72:1621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bissonnette R, Maari C, Forman S, Bhatia N, Lee M, Fowler J, et al. The oral Janus kinase/spleen tyrosine kinase inhibitor ASN002 demonstrates efficacy and improves associated systemic inflammation in patients with moderate-to-severe atopic dermatitis: results from a randomized double-blind placebo-controlled study. Br J Dermatol. 10 2019;181:733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. February 2018;5:e73–e81. [DOI] [PubMed] [Google Scholar]
- 77.Sorrell FJ, Szklarz M, Abdul Azeez KR, Elkins JM, Knapp S. Family-wide Structural Analysis of Human Numb-Associated Protein Kinases. Structure. March 2016;24:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eberl HC, Werner T, Reinhard FB, Lehmann S, Thomson D, Chen P, et al. Chemical proteomics reveals target selectivity of clinical Jak inhibitors in human primary cells. Sci Rep. October 2019;9:14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. July 2020;146:137–46.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giudice V, Pagliano P, Vatrella A, Masullo A, Poto S, Polverino BM, et al. Combination of Ruxolitinib and Eculizumab for Treatment of Severe SARS-CoV-2-Related Acute Respiratory Distress Syndrome: A Controlled Study. Front Pharmacol. 2020;11:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cantini F, Niccoli L, Nannini C, Matarrese D, Natale MED, Lotti P, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 10 2020;81:647–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stebbing J, Sanchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. November 13 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. December 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis. January 2016;75:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGarry T, Orr C, Wade S, Biniecka M, Wade S, Gallagher L, et al. JAK/STAT Blockade Alters Synovial Bioenergetics, Mitochondrial Function, and Proinflammatory Mediators in Rheumatoid Arthritis. Arthritis Rheumatol. December 2018;70:1959–70. [DOI] [PubMed] [Google Scholar]
- 86.Hanlon MM, Rakovich T, Cunningham CC, Ansboro S, Veale DJ, Fearon U, et al. STAT3 Mediates the Differential Effects of Oncostatin M and TNFalpha on RA Synovial Fibroblast and Endothelial Cell Function. Front Immunol. 2019;10:2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marzaioli V, Canavan M, Floudas A, Wade SC, Low C, Veale DJ, et al. Monocyte-Derived Dendritic Cell Differentiation in Inflammatory Arthritis Is Regulated by the JAK/STAT Axis via NADPH Oxidase Regulation. Front Immunol. 2020;11:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Busque S, Leventhal J, Brennan DC, Steinberg S, Klintmalm G, Shah T, et al. Calcineurin-inhibitor-free immunosuppression based on the JAK inhibitor CP-690,550: a pilot study in de novo kidney allograft recipients. Am J Transplant. August 2009;9:1936–45. [DOI] [PubMed] [Google Scholar]
- 89.Murray K, Floudas A, Murray C, Fabre A, Crown J, Fearon U, et al. First use of tofacitinib to treat an immune checkpoint inhibitor-induced arthritis. BMJ Case Rep. February 4 2021;14: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan LN, Murakami MA, Robinson ME, Caeser R, Sadras T, Lee J, et al. Signalling input from divergent pathways subverts B cell transformation. Nature. 07 2020;583:845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. December 2016;167:1540–54.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]


