Abstract
AIM
To clarify the extent to which medical comorbidities and goals-of-care decisions influence death among individuals with childhood-onset hydrocephalus.
METHOD
This was a retrospective cohort study of 1705 individuals (759 males, 946 females, mean age 11y 5mo, SD 6y 6mo, range 0–37y 7mo at last follow-up) with childhood-onset hydrocephalus, of whom 88 (5.2%) were deceased. Existing medical records, death records, and publicly available internet sources were analyzed. We estimated hazard ratios for putative risk factors through Cox regression based upon 10 529 person-years of data and quantitatively and qualitatively analyzed the circumstances surrounding each death.
RESULTS
Mortality did not differ statistically by demographic factors, although higher proportions of non-White and Hispanic individuals were deceased. Most deaths were related to medical comorbidities rather than hydrocephalus itself. Of the 14 deaths directly related to hydrocephalus, seven were caused by shunt complications and four occurred after decisions to forgo treatment, apparently in response to poor outcomes predicted by the medical team. Half the deaths were preceded by shifts to comfort-based care; however, these decisions appeared to substantially change the patient’s clinical trajectory only half the time.
INTERPRETATION
Children are more likely to die with, rather than from, hydrocephalus. Our results emphasize the complexities of medical decision-making and the influence of clinicians in guiding these choices.
Hydrocephalus is a condition characterized by accumulation of cerebrospinal fluid (CSF) within the brain’s ventricular system, which over time can compress and injure the surrounding brain. Hydrocephalus can develop at any age and is estimated to affect 1 in 1000 infants during their initial birth hospitalization.1 Multifactorial in nature, hydrocephalus can be a consequence of hemorrhage, developmental malformations, trauma, or infection, among many other conditions.2 Hydrocephalus can cause many symptoms depending on the age of the child, ranging from pauses in breathing to vomiting and increasing lethargy. Hydrocephalus is usually progressive and, if untreated, may be fatal.3
Pediatric-onset hydrocephalus carries the reputation of high morbidity4 and mortality,5 yet affected children commonly have additional medical comorbidities, further elevating the risk of death in this patient population. Only limited information is available about whether individuals are more likely to die as a direct result of hydrocephalus or as a consequence of accompanying medical conditions.
Also unknown is the extent to which predicted outcome influences mortality in individuals with hydrocephalus. Parents of affected children are often counselled that hydrocephalus is a life-threatening condition that will adversely affect their child’s development.6 This has the potential to act as a self-fulfilling prophecy: hearing that hydrocephalus is associated with high morbidity and mortality, some families may shift goals of care to ensure their child’s comfort rather than to prolong life. However, these comfort-based decisions may not have much influence upon the trajectory of a dying process that was primarily driven by other medical reasons.
To investigate mortality in this patient population, we used existing medical records, imaging, official state death records, and publicly available internet material. We assessed a cohort of individuals diagnosed at a children’s hospital with pediatric-onset, non-tumor-associated hydrocephalus. We first assessed numbers of deaths and risks associated with demographic factors and with hydrocephalus cause. We next determined whether death was more commonly due to hydrocephalus itself or to a medical comorbidity. Finally, we examined the extent to which death was influenced by medical decisions that reflected a shift in goals of care. For children whose deaths were directly related to hydrocephalus, we examined the extent to which medical decisions were influenced by perceived prognosis.
METHOD
Study design, study population, and data sources
This retrospective cohort study assessed children diagnosed with or treated for hydrocephalus at a regional medical center. Individuals with hydrocephalus were identified through the Clinical Data Repository (https://www.seattlechildrens.org/research/resources/bear/clinical-data-repository), a proprietary tool developed by Research Informatics and Genospace and funded by Seattle Children’s Research Institute. The Clinical Data Repository contains demographic information and International Classification of Diseases, 9th Revision (ICD-9) and International Classification of Diseases, 10th Revision (ICD-10) codes associated with outpatient clinic and emergency department visits and inpatient stays. At the time that it was accessed for this study, the Clinical Data Repository contained data from January 2009 through February 2018 (though for some patients the initial diagnosis of hydrocephalus had been made earlier). With the approval of the Seattle Children’s Hospital Institutional Review Board, patients were provisionally identified on the basis of several ICD-9 diagnosis and procedure codes (Appendix S1, online supporting information). Medical records were accessed through May 2020, and hydrocephalus was verified through review of patients’ existing clinical notes and imaging studies. For children identified as deceased, additional data were obtained from official death records from the Washington State Department of Health, supplemented by publicly available internet-based sources (online obituaries, blogs, fundraising requests, and social media posts).
Inclusion and exclusion criteria
We defined hydrocephalus according to the standard definition of progressive ventricular dilation. As such, we reviewed medical records and imaging studies to confirm ongoing ventricular expansion. We excluded children with stable ventriculomegaly due solely to structural brain differences (e.g. colpocephaly in the setting of agenesis of the corpus callosum) or a purely ex vacuo process. In patients who also had acute intracranial hemorrhage or cerebral edema, we reviewed serial scans to confirm that progressive ventricular dilatation was also present. Patients were also excluded if insufficient clinical information was available to confirm progressive ventricular dilation, or if they were 21 years of age or older at the time of diagnosis. After provisionally identifying 2372 individuals in the Clinical Data Repository, we excluded 136 who did not have progressive hydrocephalus, 82 in whom hydrocephalus could not be verified because of insufficient records, and seven who were 21 years of age or older at time of diagnosis. We also excluded 442 individuals whose hydrocephalus was due to a CSF obstruction from a brain tumor, leaving a cohort of 1705 individuals with confirmed childhood-onset, non-tumor-associated hydrocephalus available for analysis.
Clinical data collected
From existing medical records, we recorded basic demographic information, date of diagnosis of hydrocephalus (recorded as date of birth if diagnosed prenatally), mortality status, and date of last contact with patient family (or death, when applicable). We assigned patients to a hydrocephalus subtype based upon presumed cause (posthemorrhagic [germinal matrix hemorrhage or otherwise], myelomeningocele, primary brain malformation, neoplasm, infection, trauma, skeletal dysplasia/craniofacial condition, other genetic condition, vascular anomaly, ischemia, or other/unknown). We recorded the date and nature of any surgical interventions for hydrocephalus. We also noted major additional medical comorbidities, defined as acute and chronic illnesses separate from hydrocephalus, such as chronic lung disease associated with preterm birth, or physical disability related to myelomeningocele. When applicable, we noted date and place of death (hospital, hospice, community).
Causes and circumstances of death
From medical records and official death records, supplemented by information received from medical professionals directly involved in these patients’ care, we recorded cause of death and assessed whether this was directly related to hydrocephalus (e.g. shunt failure), to a comorbidity (e.g. respiratory failure from preterm birth), or was unrelated (e.g. motor vehicle accident). Using our best judgement, we rated whether the death was expected (e.g. the inevitable consequence of a longstanding medical condition), somewhat expected (e.g. recognized complication of a medical condition), somewhat unexpected (e.g. acute medical event directly related to a chronic medical condition), or unexpected (e.g. accident or rare surgical complication). We reviewed medical records in detail to determine whether the family or care team had explicitly articulated a desire to focus on the affected individual’s comfort rather than to pursue life-prolonging measures before terminal events. We also recorded whether these decisions directly preceded death and whether they were a primary driver of death (e.g. cessation of cardiorespiratory monitoring or lab tests would not be considered a primary driver, but withholding of intravenous hydration or tube-delivered nutritional support would). When medical decisions such as compassionate extubation were made before death but the illness itself was deemed to be imminently and unequivocally terminal (i.e. death expected within hours even with full support), or if the child met criteria for brain death at the time goals-of-care-related decisions were made, we considered the cause of death to be the illness itself.
Statistical analysis
All analyses were performed in Stata version 14 (StataCorp, College Station, TX, USA). Descriptive analyses are presented as numbers, means with standard deviations, and proportions. We calculated hazard ratios through Cox models that contained the variables of interest (sex, ethnicity, hydrocephalus subtype) and risks expressed as hazard ratio (95% confidence interval [CI]). Variables were first assessed in a series of univariate analyses, and variables of particular interest were then included in a multivariable model. For all models, we included the adjustment variables ‘age at diagnosis’ and ‘time since diagnosis’ based on the a priori assumption that both would be associated with mortality status and hydrocephalus subtype. We tested whether the potential adjustment variables sex and ethnicity were associated with mortality status and the variables of interest. Finding they were not, we did not include these as adjustment variables in the Cox models. To account for bias introduced by children who were diagnosed before the study period began, we considered time at risk to begin at the start of the study period (for children diagnosed earlier), date of diagnosis (if within the study period), or at the date of entry into our hospital system (if diagnosis had been made at an outside institution on an unknown date). Children were censored at death or at last contact with the family. For Kaplan–Meier curves based upon hydrocephalus cause, we assessed for differences between curves via a logrank test, with 0.05 set as the threshold for statistical significance. When a variable of interest was missing, the individual was excluded from that analysis.
RESULTS
Epidemiology and risk factors for increased mortality
We reviewed records of 1705 individuals (759 males, 946 females) verified to have childhood-onset, non-tumor-associated hydrocephalus, for whom an average of 9 years 10 months of follow-up data were available (SD 6y 6mo, range 0–37y 7mo), corresponding to 10 529 person-years of time within the study period. Among our cohort, 88 (5.2%) were deceased. Mean age at death was 7 years 1 month (SD 6y 11mo, range 0–21y), occurring on average 4 years 11 months (SD 6y 3mo, range 0–21y) after diagnosis. Basic clinical characteristics of patients with hydrocephalus, deceased and not deceased, are shown in Table 1.
Table 1:
Clinical characteristics of patients with hydrocephalus, by mortality status
| Deceased (n=88) |
Not deceased (n=1617) |
|||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Male | 47 | (53.4) | 899 | (55.6) |
| Ethnicity | ||||
| White | 44 | (52.4) | 926 | (62.2) |
| Black | 5 | (6.0) | 96 | (6.5) |
| Asian | 2 | (2.4) | 69 | (4.6) |
| Native | 5 | (6.0) | 52 | (3.5) |
| Mixed/other | 28 | (33.3) | 345 | (23.2) |
| Hispanic | 23 | (28.1) | 314 | (20.8) |
| Age at diagnosis, y:mo, mean, SD (range) | 2:2, 4:5 (0–18:6) |
1:4, 3:7 (0–20:11) |
||
| Age at death or last follow-up, y:mo, mean, SD (range) | 7:1, 6:11 (0–21:0) |
11:7, 6:5 (0–37:7) |
||
| Hydrocephalus subtype | ||||
| Malformation/congenital | 27 | (31.8) | 416 | (26.6) |
| Posthemorrhagic | 26 | (30.6) | 399 | (25.5) |
| Spina bifida/myelomeningocele | 7 | (8.2) | 337 | (21.6) |
| Craniofacial/skeletal dysplasia | 2 | (2.4) | 100 | (6.4) |
| Postinfectious | 4 | (4.7) | 78 | (5.0) |
| Trauma | 6 | (7.1) | 71 | (4.6) |
| Multifactorial/other | 13 | (15.3) | 161 | (10.3) |
| Hydrocephalus-related surgery, any | 55 | (63.2) | 1375 | (85.4) |
| Permanent shunt | 45 | (81.8)a | 1299 | (90.8)a |
| ETV/CPC | 1 | (1.8)a | 54 | (3.9)a |
| Cyst fenestration | 1 | (1.8)a | 8 | (0.6)a |
| Temporary drain only | 8 | (14.6)a | 38 | (2.8)a |
| Other surgery type | 0 | (0.0)a | 20 | (1.3)a |
Proportion of those who underwent surgery. ETV, endoscopic third ventriculostomy; CPC, choroid plexus cauterization.
The overall mortality rate in our cohort was 8.3 deaths per 1000 person-years (95% CI: 6.7–10.2). Kaplan–Meier curves (Fig. 1) showed statistically significant differences in survival between hydrocephalus subtypes (p=0.02); and adjusted analyses confirmed two- to three-fold higher risks of death in several subtypes (Table 2). In a multivariable analysis (Table 3), we detected no difference in mortality on the basis of sex. Risk of death was higher among non-White and Hispanic children, though these results did not achieve statistical significance (hazard ratio: 1.3, 95% CI: 0.8–2.1; hazard ratio: 1.5, 95% CI: 0.9–2.6 for non-White and Hispanic ethnicity respectively.)
Figure 1:
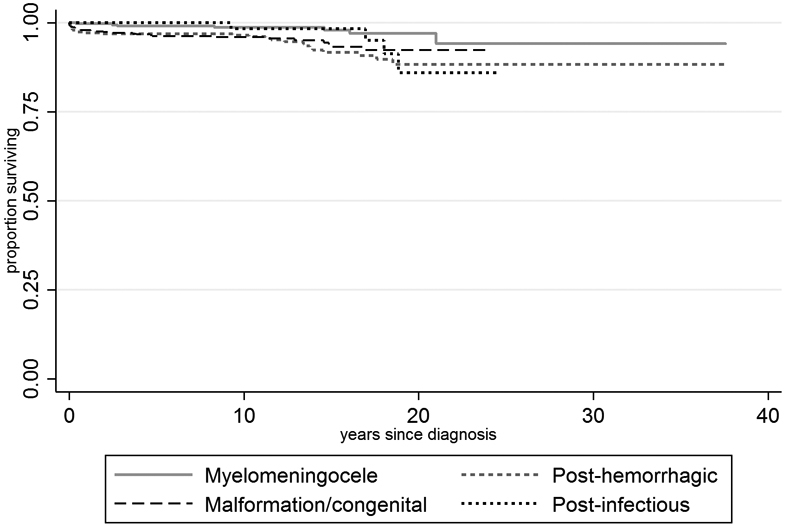
Survival by hydrocephalus subtype.
Table 2:
Adjusted risk of death by demographic factors and hydrocephalus subtype, univariate analyses
| aHRa | 95% CI | |
|---|---|---|
| Male | 0.85 | (0.56–1.31) |
| Ethnicityb | ||
| White | 1.00 | (ref) |
| Black | 1.05 | (0.42–2.66) |
| Asian | 0.60 | (0.15–2.49) |
| Native | 1.54 | (0.55–4.30) |
| Hispanic (Y/N) | 1.55 | (0.96–2.52) |
| Hydrocephalus subtype | ||
| Spina bifida | 1.00 | (ref) |
| Malformation/congenital | 2.24 | (0.97–5.20) |
| Posthemorrhagic | 2.77 | (1.20–6.37) |
| Craniofacial/skeletal dysplasia | 0.65 | (0.13–3.20) |
| Postinfectious | 1.62 | (0.46–5.71) |
| Trauma | 2.28 | (0.74–7.02) |
| Multifactorial/other | 2.34 | (0.87–6.32) |
| Hydrocephalus surgery | ||
| Any hydrocephalus surgery | 0.49 | (0.31–0.77) |
| Temporary drain placement onlyc | 3.70 | (1.69–8.13) |
Adjusted for age at diagnosis and time since diagnosis.
Excludes other/mixed; White/Black/Asian/Native are exclusive categories, Hispanic is not.
Compared to surgery intended to be definitive (ventriculoperitoneal shunt, endoscopic third ventriculostomy/choroid plexus coagulation, cyst fenestration). aHR, adjusted hazard ratio; CI, confidence interval.
Table 3:
Adjusted risk of death, multivariable analysis
| aHRa | 95% CI | |
|---|---|---|
| Male | 1.01 | (0.63–1.60) |
| Non-White | 1.30 | (0.79–2.14) |
| Hispanic | 1.54 | (0.90–2.65) |
| Hydrocephalus subtype | ||
| Spina bifida | 1.00 | (ref) |
| Malformation/congenital | 2.14 | (0.86–5.32) |
| Posthemorrhagic | 2.64 | (1.07–6.51) |
| Any hydrocephalus surgery | 0.48 | (0.29–0.79) |
Adjusted for age at diagnosis and time since diagnosis. aHR, adjusted hazard ratio; CI, confidence interval.
Causes and circumstances of death
We analyzed the causes and circumstances of death for all deceased patients. Death occurred in a hospital setting in 58 (68.2%) patients. Among the 24 who died in non-hospital settings, two were in long-term care facilities and eight were receiving hospice care at home. We were able to determine specific causes of death in 86 of 88 patients. All deaths were due to medical issues; none resulted from accidents. Death was related to medical comorbidities rather than elevated intracranial pressure in 65 patients (76%), including respiratory complications of preterm birth, sepsis, and sudden unexplained death with epilepsy, among many others (Table S1, online supporting information). Death was directly related to elevated intracranial pressure in 21 children (24%), but in seven, the immediate cause was acute hemorrhage due to the primary disease process superimposed upon hydrocephalus.
Circumstances of the terminal illness were documented in 84 patients. The clinical situation leading to death was assessed as mostly or fully expected in 36 and mostly or fully unexpected in 48. Among 76 deceased patients whose medical records documented discussions about goals of care, 37 deaths were preceded by medical decisions that specifically prioritized patient comfort over prolongation of life, although in only 20 was the trajectory of the dying process judged to be substantially altered by those decisions (for example, withholding of enteral nutrition or forgoing of ventriculoperitoneal shunt placement in a patient with progressive hydrocephalus).
Deaths directly related to hydrocephalus
For only 14 children did hydrocephalus itself play a major role in death. Seven children (10% of deaths with an established cause) died of shunt-related complications. This included six children with shunt failure, all of whom had a rapid progression to brain death. Each of their families maintained full medical support until death was declared on clinical grounds. One infant born preterm with a treatment-resistant shunt infection (among other comorbidities) died after compassionate extubation.
For the remaining seven children, death was substantially influenced by preceding discussions about prognosis and goals of care. Three children with severe accompanying illnesses underwent surgical CSF diversion procedures, but care was subsequently redirected to comfort measures. For four newborn infants with severe congenital hydrocephalus (discovered unexpectedly at birth in two and shortly before birth in a third), CSF diversion was not performed. Goals-of-care discussions that influenced these decisions appeared to focus upon survivability, quality of life, and – particularly for infants – neurodevelopmental outcome (Table S2, online supporting information).
DISCUSSION
In this retrospective investigation of mortality in children with hydrocephalus, we show that affected individuals are more likely to die from medical comorbidities than from hydrocephalus itself, and only half the deaths directly related to hydrocephalus were caused by shunt complications. The other deaths directly related to hydrocephalus were driven to varying degrees by medical decisions made in response to perceptions of clinical and neurodevelopmental outcome.
Overall mortality
The estimated mortality in our cohort falls within the very broad published range of 1% to 48%.4,5,7-11 In prior studies, the highest proportion of deaths were observed in the longest-followed patient cohorts. This is partly because of the increased amount of time at risk, but also likely reflects recent improvements over time in shunt designs, surgical techniques, and prevention of infection.5 Among our patients, most deaths were due to comorbidities; less than a third of deaths were shunt-related, which falls within the published range of 9% to 43%.5,8,12
Not surprisingly, hydrocephalus etiology was strongly associated with mortality. Compared to children with hydrocephalus in the setting of myelomeningocele, the risk of death was markedly higher in patients with prenatal-onset and posthemorrhagic hydrocephalus, who would be expected to be more medically fragile.
After taking other risk factors into account, non-White and Hispanic ethnicity were independently associated with 30% and 50% higher risks of death respectively. Although these results did not attain statistical significance, they are striking enough to warrant further exploration. Higher risk of death in non-White and Hispanic children could reflect cultural differences in approaches to end-of-life care, implicit biases among healthcare workers leading to less aggressive medical treatment, or delays in care because of language barriers (notably, half the children who died of catastrophic shunt failure came from non-English-speaking families).
Surgical treatment is associated with a 50% reduced risk of death overall, although the risk of death was four-fold higher (compared to no surgery) when surgery was limited to temporary drain placement. We presume these results primarily reflect the underlying cause of the hydrocephalus and medical decisions made as a result.
Mortality and medical decision-making
When goals of care were documented in medical records, we found that before death, care was redirected to focus on a child’s comfort rather than on prolonging life approximately half the time. These findings are in line with other recent investigations of the influence of medical decision-making upon pediatric mortality in critically ill children. In a cohort of 149 Swiss children who died from cardiac, neurological, or oncological conditions, decisions to withdraw life-sustaining treatment preceded death in 84%.13 Among patients in a cardiac intensive care unit14 and pediatric intensive care unit,15 49% and 74% of deaths were preceded by such decisions.
Mortality due to hydrocephalus itself
The complexities of medical decision-making are highlighted by children whose deaths were directly linked to hydrocephalus. For six children with longstanding hydrocephalus who experienced rapid, catastrophic shunt failure, goals-of-care-related decisions did not play a role. Instead, each child received full medical support at the request of their family until brain death was declared. Notably, these children were otherwise reasonably typically developing, although they had physical, sensory, intellectual, and communication disabilities that ranged from mild to profound.
For five newborn infants with severe congenital hydrocephalus, neurosurgical intervention was not performed (four infants), or care was quickly redirected after temporary CSF diversion (one infant). Two of these infants were otherwise typically developing and two had medical conditions that would generally be considered treatable. For these newborn infants, records document that the medical team’s perception, conveyed to families, was that these infants’ neurodevelopmental potential was extremely poor. How these individuals might have done had they received maximal medical support is unknowable. However, our results underscore the perception of severe congenital hydrocephalus as a universally devastating condition, despite published outcomes data that suggests a more optimistic prognosis for at least some affected children.3,6,12,16-20
Strengths and limitations
Our study is limited by its retrospective nature. However, available medical records and imaging allowed us to establish subtypes of hydrocephalus and comorbidities with certainty. Moreover, the highly detailed nature of the medical documentation illuminated how children’s medical situations evolved over time, and how medical decisions were made.
Another limitation of this study is that its cohort was drawn from a tertiary medical center providing specialized care to children from a wide geographic region, which could bias it towards inclusion of more severely affected individuals with a higher mortality risk. However, care for children with hydrocephalus is usually concentrated at larger centers such as ours,21 so our overall results may therefore apply to many pediatric patients with hydrocephalus. However, factors that influence medical decisions and the extent to which families participate in those decisions may be more variable by location; these results may therefore be less generalizable. In our analyses, we were able to mitigate the potential bias introduced by older patients who were diagnosed with hydrocephalus before the study period began, but our study design cannot account for members of the cohort who were lost to follow-up by moving from the region. However, we have no reason to suspect that loss to follow-up would be differentially associated with mortality risk, and so our inability to account for all patients would be unlikely to substantially bias our results.
Conclusions
Children are more likely to die with rather than of hydrocephalus. Among the minority of deaths directly related to hydrocephalus itself, several were caused by catastrophic shunt failure; however, almost as many were driven by decisions not to treat the hydrocephalus, influenced by a perception of ‘poor prognosis’ conveyed by members of the medical team. Our results further underscore the complexities of medical decision-making, the influence of clinicians in guiding these difficult choices, and the need for long-term studies that assess outcomes in ways that are relevant to patients and families.
Supplementary Material
ICD-9 and ICD-10 diagnosis and procedure codes used to identify individuals with hydrocephalus
Table S1: Cause of death in children with non-tumor-associated hydrocephalus, when death was not directly related to hydrocephalus
Table S2: Deaths directly related to hydrocephalus
What this paper adds.
Children with hydrocephalus are more likely to die from other conditions, rather than directly from hydrocephalus.
Hydrocephalus-related deaths involve shunt failure and decisions to forgo shunt placement.
Among children with hydrocephalus, more non-White and Hispanic individuals were deceased than other ethnicities.
Acknowledgements
HT is grateful for the assistance of Jennifer Phillips for obtaining data from the Clinical Data Repository database. She also appreciates the assistance of Emily Loter (American Society for Clinical Pathology) and the King County Medical Examiner’s office for providing detailed information about causes of death.
This publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2 TR002317. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATION
- CSF
Cerebrospinal fluid
Footnotes
Supporting information
The following additional material may be found online:
REFERENCES
- 1.Jeng S, Gupta N, Wrensch M, Zhao S, Wu YW. Prevalence of congenital hydrocephalus in California, 1991–2000. Pediatr Neurol 2011; 45: 67–71. [DOI] [PubMed] [Google Scholar]
- 2.Tully HM, Dobyns WB. Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet 2014; 57: 359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurence KM, Coates S. The natural history of hydrocephalus. Detailed analysis of 182 unoperated cases. Arch Dis Child 1962; 37: 345–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppe-Hirsch E, Laroussinie F, Brunet L, et al. Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst 1998; 14: 97–9. [DOI] [PubMed] [Google Scholar]
- 5.Gmeiner M, Wagner H, Zacherl C, et al. Long-term mortality rates in pediatric hydrocephalus – a retrospective single-center study. Childs Nerv Syst 2017; 33: 101–9. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni AV. Quality of life in childhood hydrocephalus: a review. Childs Nerv Syst 2010; 26: 737–43. [DOI] [PubMed] [Google Scholar]
- 7.Casey AT, Kimmings EJ, Kleinlugtebeld AD, Taylor WA, Harkness WF, Hayward RD. The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg 1997; 27: 63–70. [DOI] [PubMed] [Google Scholar]
- 8.Tuli S, Tuli J, Drake J, Spears J. Predictors of death in pediatric patients requiring cerebrospinal fluid shunts. J Neurosurg 2004; 100(5 Suppl Pediatrics): 442–6. [DOI] [PubMed] [Google Scholar]
- 9.Kao CL, Yang TF, Wong TT, et al. The outcome of shunted hydrocephalic children. Zhonghua Yi Xue Za Zhi (Taipei) 2001; 64: 47–53. [PubMed] [Google Scholar]
- 10.Paulsen AH, Lundar T, Lindegaard KF. Twenty-year outcome in young adults with childhood hydrocephalus: assessment of surgical outcome, work participation, and health-related quality of life. J Neurosurg Pediatr 2010; 6: 527–35. [DOI] [PubMed] [Google Scholar]
- 11.Heinsbergen I, Rotteveel J, Roeleveld N, Grotenhuis A. Outcome in shunted hydrocephalic children. Eur J Paediatr Neurol 2002; 6: 99–107. [DOI] [PubMed] [Google Scholar]
- 12.Paulsen AH, Lundar T, Lindegaard KF. Pediatric hydrocephalus: 40-year outcomes in 128 hydrocephalic patients treated with shunts during childhood. Assessment of surgical outcome, work participation, and health-related quality of life. J Neurosurg Pediatr 2015; 16: 633–41. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann K, Cignacco E, Engberg S, et al. Patterns of paediatric end-of-life care: a chart review across different care settings in Switzerland. BMC Pediatr 2018; 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polito A, Garisto C, Pezzella C, Iacoella C, Cogo PE. Modes of death in a pediatric cardiac ICU. Pediatr Crit Care Med 2016; 17: 406–10. [DOI] [PubMed] [Google Scholar]
- 15.Moore P, Kerridge I, Gillis J, Jacobe S, Isaacs D. Withdrawal and limitation of life-sustaining treatments in a paediatric intensive care unit and review of the literature. J Paediatr Child Health 2008; 44: 404–8. [DOI] [PubMed] [Google Scholar]
- 16.Adle-Biassette H, Saugier-Veber P, Fallet-Bianco C, et al. Neuropathological review of 138 cases genetically tested for X-linked hydrocephalus: evidence for closely related clinical entities of unknown molecular bases. Acta Neuropathol 2013; 126: 427–42. [DOI] [PubMed] [Google Scholar]
- 17.Vinchon M, Rekate H, Kulkarni AV. Pediatric hydrocephalus outcomes: a review. Fluids Barriers CNS 2012; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacy M, Pyykkonen BA, Hunter SJ, et al. Intellectual functioning in children with early shunted posthemorrhagic hydrocephalus. Pediatr Neurosurg 2008; 44: 376–81. [DOI] [PubMed] [Google Scholar]
- 19.Dalen K, Bruaroy S, Wentzel-Larsen T, Laegreid LM. Intelligence in children with hydrocephalus, aged 4–15 years: a population-based, controlled study. Neuropediatrics 2008; 39: 146–50. [DOI] [PubMed] [Google Scholar]
- 20.Kutscher A, Nestler U, Bernhard MK, et al. Adult long-term health-related quality of life of congenital hydrocephalus patients. J Neurosurg Pediatr 2015; 16: 621–5. [DOI] [PubMed] [Google Scholar]
- 21.Dewan MC, Baticulon RE, Rattani A, Johnston JM, Warf BC, Harkness W. Pediatric neurosurgical workforce, access to care, equipment and training needs worldwide. Neurosurg Focus 2018; 45: E13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD-9 and ICD-10 diagnosis and procedure codes used to identify individuals with hydrocephalus
Table S1: Cause of death in children with non-tumor-associated hydrocephalus, when death was not directly related to hydrocephalus
Table S2: Deaths directly related to hydrocephalus


