Abstract
Objective:
The objective was to design a questionnaire to identify daily CI use habits and barriers to daily CI use and to administer this questionnaire to adult CI users. We hypothesized that recipients who reported a greater number of barriers to daily CI use would show lower daily CI use.
Study design:
Questionnaire
Setting:
Academic medical center
Patients:
100 adult CI recipients
Main Outcome Measures:
Questionnaire responses and amount of CI use per day as measured from the CI software
Results:
The Cochlear Implant Use Questionnaire (CIUQ) was created and responses were obtained from 100 participants. The CIUQ yielded an average overall score of 23 (range = 3–54) out of 100; responses were variable, and CI recipients experienced different barriers to using their CI processor. The CIUQ overall score was significantly correlated with recipients’ daily CI use (hours/day) (rs = - 0.561, p < 0.0001, 95% confidence interval [−0.694, −0.391]), which provides evidence of construct validity. Responses were immediately useful for identifying and overcoming barriers to consistent CI use with our study participants.
Conclusions:
Increasing evidence suggests that daily CI use is correlated with speech recognition outcomes. To optimize outcomes, clinicians should consider implementing this questionnaire to identify and overcome barriers to consistent, full-time CI processor use.
Keywords: cochlear implant, data logging, outcomes, questionnaire
INTRODUCTION
Cochlear implant (CI) devices have successfully improved speech recognition and communication abilities for many years, yet recipients continue to demonstrate high variability in outcomes. Understanding this variability continues to be of interest clinically and in the CI literature as a clearer understanding of this variability can support interventions to optimize outcomes for CI users. Historically, major factors of consideration have included duration of deafness, etiology, age, spiral ganglion cell count, electrode position, programming, electrode type and manufacturer, surgical technique, aural rehabilitation, etc. More recently, daily CI use, or the average number of hours a recipient uses their external CI processor per day, has been added to the list of factors thought to contribute to variability in speech recognition outcomes1–8.
Data logging has been a feature in hearing aids for over a decade, but it is a newer feature to CIs (first released with the Cochlear Nucleus 6 in 2013). Data logging in the CI software allows for objective calculation of the number of hours per day that the CI processor is on and connected to the internal device. Unlike hearing aids, removal of the CI processor leads to loss of access to spoken communication for most. Despite this, there is significant variability in average daily CI use in the adult population, ranging from 0 to 24 hours per day with an average of about 10 hours per day4,5,8. Clinical recommendations are to use the CI processor “all day” or “during all waking hours,” but these data suggest that adult recipients are wearing their devices much less. In addition to these findings, it is also of significance to audiologists, surgeons, healthcare payers, and perhaps the general public that CI recipients are making use of and receiving benefit from an expensive, surgically implanted device. Without use of the external processor, the surgically implanted device is rendered useless.
Several groups have already studied average daily CI use with data logging in pediatric CI recipients and have concluded that consistent use of the external CI processor optimizes speech and language outcomes1,6,7,9–12. Two studies have shown a similar trend in adult CI recipients. Schvartz-Leyzac and colleagues (2019) and Holder and colleagues (2019) demonstrated a moderate to strong correlation (rs = 0.43 – 0.61) between average daily CI use and speech recognition outcomes in adult recipients. This correlational data suggest that daily CI use may account for a significant portion of the variability in speech recognition outcomes in adult CI recipients.
Given the variability in daily CI use and the emerging literature suggesting this variability is related to speech recognition outcomes, daily CI use is of important clinical interest. Identifying reasons people use or do not use their CI and barriers to using it more is critical to understanding and addressing this variability. To our knowledge, a questionnaire aimed at assessing habits and barriers to daily CI use does not yet exist. The Cochlear Implant Management Skills assessment13 is perhaps the most closely related; however, this questionnaire focuses on physical handling and care of implant devices and does not probe other potential explanations for inconsistent CI processor use. Therefore, we created a questionnaire to probe daily routines and barriers to daily CI use in a quantitative and qualitative manner. Formulation of the questionnaire was based on the Information-Motivation-Behavioral skills (IMB) model of adherence14.
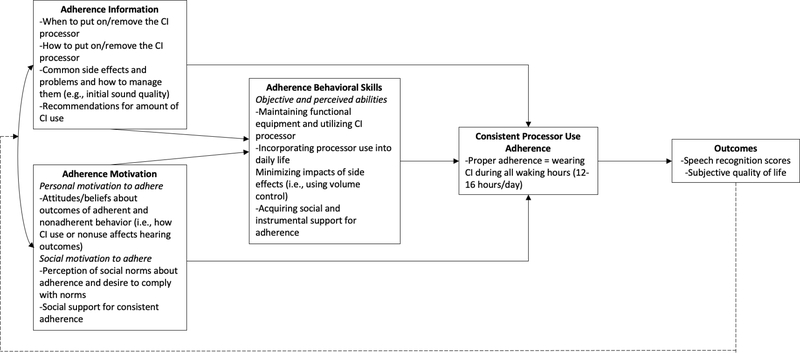
The IMB is a theory-based model for identifying factors that contribute to successful medication adherence15,16, which we applied to understand CI device use adherence (Figure 1). The IMB model of adherence asserts that adherence behavior is a function of the extent to which the patient is informed about the recommendation, motivated to adhere, and has the requisite skills and self-efficacy to adhere to the recommendation15–18. Adherence information includes accurate knowledge about wearing the CI processor (i.e., how and when to put the processor on and take it off), potential side effects of wearing the processor (i.e., headaches, fatigue, loudness, ear pain), and accurate theories that support consistent adherence (as opposed to inaccurate heuristics such as “I only have to wear my processor when I want to hear.”)15–18. Adherence motivation refers to the patient’s personal and social motivation to adhere. Personal motivation to adhere is consistent with a patient’s attitudes about adherence and is rooted in one’s beliefs that wearing the CI processor is helpful and not wearing the CI processor would produce undesirable outcomes. Social motivation to adhere reflects one’s experience of social norms regarding adherence and their social support for adherence. Lastly, adherence behavioral skills include one’s objective and perceived abilities to manage functional CI equipment in different situations despite difficulties14. Applications of IMB models have resulted in successful, data-driven interventions for improving medication adherence for patients with HIV and diabetes17,19,20.
Figure 1.
An IMB model of cochlear implant processor use adherence, adapted from Fisher et al. 14, Amico et al. 15, and Mayberry & Osborn 16. Solid lines indicate effects between IMB components and desired adherence, and the dashed line shows a feedback loop in which the outcomes affect future adherence information and motivation.
The purpose of the current study was to: 1) design a questionnaire aimed at identifying daily CI use habits and barriers to daily CI use using the IMB model; and 2) administer this questionnaire to adult CI users with varying degrees of daily CI use to determine construct validity. We hypothesized that recipients who reported a greater number of barriers to daily CI use would show lower daily CI use.
METHODS
The design and methods of this study were approved by the Institutional Review Board (IRB# 200807). Participants were recruited from our medical center’s CI patient pool. Participants were invited via email to complete online informed consent for the study team to access their CI programming software and electronic medical record and finish the questionnaire online via REDCap21. Participants completed the questionnaire via REDCap independently on their personal devices outside of the clinic. Responses were obtained May 2020 through October 2020. We collected data from consenting participants’ medical record or CI programming software retrospectively. In total, 100 adult CI recipients provided responses to the questionnaire (Table 1). The mean age of the sample was 61.6 years (SD = 15.9) and ranged from 18 to 87 years. Exclusion criteria included less than 18 years of age, prelingual onset of deafness, and incomplete questionnaire response.
Table 1.
Participant characteristics; CI = cochlear implant.
| Participants | N = 100 |
| Sex | Male = 55, Female = 45 |
| Age (years) | Mean = 61.6, SD = 15.9, Range = 18–87 |
| CI Manufacturer | Advanced Bionics = 29, Cochlear = 39, Med-El = 12 |
| Hearing Device Configuration | Bilateral CI = 27, Bimodal = 58, Unilateral CI = 15 |
| Living Situation | Alone = 15, With Someone = 85 |
| Employment | Retired = 58, Full-time = 31, Part-time = 11 |
| Average data logging from software (hours/day) | Mean = 10.4, SD = 3.7, Range = 0.5–15.2 |
| Average participant reported CI use (hours/day) | Mean = 13.0, SD = 3.3, Range = 2.5–24 |
Questionnaire Design
First, clinical audiologists were asked to provide a list of most commonly reported barriers to CI use. Responses were compiled from six clinical audiologists. Items were then created based off of these responses and mapped onto the IMB model. Additional items that aligned with the IMB model constructs were also added following consultation with IMB model expert (author LSM). The questionnaire was piloted with ten CI recipients. Pilot participants were asked to provide feedback on the questions to ensure that the questions were clear, and they were also asked to suggest additional barriers to CI use that we had not previously considered. Following this pilot, the wording of two questions was amended, but no questions were added or deleted. This process established the face validity and content validity for the measure. The finalized items were compiled to form the Cochlear Implant Use Questionnaire (CIUQ). The CIUQ and accompanying instructions had a Flesch readability score of 68.7 (standard/average) and a Flesch-Kincaid grade level of 5.9.
The CIUQ consists of two sections. The first section probes the following: employment status, living situation, time of day they put the CI on, time of day they take the CI off, activities for which they remove their CI, number of hours per day they think they wear their CI, their surgeon/audiologist’s recommendation for how often they should wear their CI processor, and any additional information they would like to share about their daily CI use habits. The second section contains quantitative questions that probe specific barriers to daily CI use using a five point scale in which the choices consist of: never, rarely, sometimes, often, or always. The participants were instructed to, “think about your daily life with your cochlear implant(s) and answer how often each statement applies to your feelings and experiences.” The questions covered the following categories: equipment management, motivation to hear, social support, social norms, listening fatigue, hearing benefit, sound quality, hearing configuration, ear/head pain, and alternate forms of communication. See Table 2 and Appendix for specific questions. The quantitative question responses were assigned a value from 0–4 and reverse scored when necessary. Responses were added together for a total between 0 and 100 such that a higher total corresponded to a greater number of reported barriers to CI use.
Table 2.
Cochlear Implant Use Questionnaire items (N = 100). Percentage refers to the percent of participants who provided a response other than “never” (or “always” if the item was reverse scored) to each item. Asterisk indicates that the item was reverse scored such that a higher number is consistent with greater barrier to cochlear implant use.
| Questionnaire Item | IMB Model Distinction | Participants reporting as a barrier (%) | Average score, 0 = “never” to 4 = “always” (Mean ± SD) |
|---|---|---|---|
|
| |||
| 1. When my cochlear implant processor battery dies, I have a backup battery with me.* | Behavioral Skills | 36% | 0.7 ± 1.1 |
| 2. It is important that I hear my best at all times.* | Motivation | 32% | 0.4 ± 0.6 |
| 3. When I take my cochlear implant processor off, I enjoy the silence. | Motivation | 93% | 2.2 ± 1.1 |
| 4. I take my cochlear implant processor off when I am home alone. | Information | 75% | 1.5 ± 1.2 |
| 5. I get so exhausted from listening that I want to take my cochlear implant processor off. | Motivation | 68% | 1.3 ± 1.1 |
| 6. When sounds are annoying, I take my cochlear implant processor off. | Behavioral Skills | 76% | 1.5 ± 1.0 |
| 7. If I am sick or do not feel well, I do not like to wear my cochlear implant processor. | Motivation | 78% | 1.6 ± 1.2 |
| 8. I do not see the purpose of wearing my cochlear implant processor because it does not benefit my hearing ability. | Motivation | 14% | 0.2 ± 0.6 |
| 9. My cochlear implant processor or processor parts are broken. | Behavioral Skills | 72% | 0.7 ± 0.9 |
| 10. I remove my cochlear implant processor because it is too loud to wear comfortably. | Information | 37% | 0.5 ± 0.7 |
| 11. The sound quality of my cochlear implant discourages me from wearing it. | Motivation | 59% | 0.6 ± 1.0 |
| 12. I can hear and communicate effectively without my cochlear implant processor. | Motivation | 43% | 0.8 ± 1.1 |
| 13. I tend to remove my cochlear implant processor when I am not communicating. | Information | 37% | 0.7 ± 1.0 |
| 14. It is hard for me to put my cochlear implant processor on. | Behavioral Skills | 18% | 0.2 ± 0.6 |
| 15. I forget to put my cochlear implant processor on. | Behavioral Skills | 37% | 0.5 ± 0.7 |
| 16. It is important that I maximize my results with my cochlear implant.* | Motivation | 21% | 0.4 ± 0.8 |
| 17. I take breaks from wearing my cochlear implant processor because my ear hurts. | Behavioral Skills | 48% | 0.8 ± 0.9 |
| 18. My cochlear implant processor falls off of my ear. | Behavioral Skills | 79% | 1.4 ± 1.0 |
| 19. I look forward to putting my cochlear implant processor on in the morning.* | Motivation | 56% | 1.0 ± 1.1 |
| 20. If I forget to wear my cochlear implant processor, my friends or family members will ask me why I’m not wearing it.* | Motivation | 78% | 2.5 ± 1.6 |
| 21. I take off my cochlear implant processor to avoid getting it wet while exercising or working outside during the summer. | Behavioral Skills | 87% | 2.1 ± 1.3 |
| 22. I don’t wear my cochlear implant processor because I’m afraid of what people might think or say about it. | Motivation | 8% | 0.1 ± 0.5 |
| 23. I use alternate forms of communication (Ex. ASL, writing). | Motivation | 47% | 0.8 ± 0.9 |
| 24. My friends and family members think it is important that I wear my cochlear implant processor.* | Motivation | 10% | 0.3 ± 0.8 |
| 25. Wearing my cochlear implant processor gives me a headache. | Information | 23% | 0.7 ± 0.8 |
Construct validity refers to the extent to which a test or tool actually measures what it intends to measure22. We evaluated the construct validity of the quantitative portion of the CIUQ using a correlation analysis between total questionnaire score and CI use assessed with data logging. A significant negative correlation between these two measures would provide evidence of construct validity. If the CIUQ is valid, patients who report a low number of barriers to CI use should, in theory, wear their CI processor more consistently (higher data logging), and patients who report a high number of barriers to CI use should, in theory, wear their CI processor less consistently (lower data logging).
Demographic and Data Logging Data Collection
We collected participants’ age, sex, hours of CI use per day, and listening configuration (i.e., unilateral, bilateral, bimodal). Data logging data were extracted from the CI programming software. The data logging value closest to the time of questionnaire completion was recorded for each participant. Audiology reports were also reviewed to ensure data logging accuracy for patients utilizing more than one processor. Specifically, if patients used more than one processor, the data logging from each processor was added together. Data logging information could not be included for patients utilizing equipment that did not support data logging such as bilaterally initialized Advanced Bionics Naida CI users, Advanced Bionics Harmony and Neptune users, Cochlear Nucleus 5 users, and MED-EL Rondo and Opus 2 users. 78 of the 100 participants had data logging information available.
RESULTS
Cochlear Implant Use Questionnaire Items
The average total score for the quantitative section of the CIUQ was 23.3 (SD = 11.3), and total scores ranged from 3 to 54 (possible range = 0 to 100). Table 2 shows the percentage of respondents who provided a response other than “never” for the barriers (or “always” if the item was reverse scored) for each item and the mean and standard deviation for responses to each question.
Device Use Habits Questions
Recipients were asked about their employment status. Responses were as follows: 31 respondents worked full-time, 11 respondents worked part-time, and 58 respondents were retired. A Kruskal-Wallis test showed no difference total questionnaire score for employment status (H(3) = 0.155, p = 0.925, r = 0.138). 15 respondents reported that they lived alone, while 85 reported that they lived with someone. Mann Whitney test showed no difference in total questionnaire score between living situation (U = 389, p = 0.168, r = 0.248).
Respondents were asked to recall their surgeon or audiologist’s recommendation for how often they should wear their CI processor. Free responses were categorized into three categories: 1) don’t remember or no recommendation was made, 2) response was inconsistent with current recommendations (i.e., “5 hours per day,” “as much as I want to”), or 3) all waking hours or all day. 43 respondents (43%) reported that they did not remember or no recommendation was made. 14 respondents (14%) provided a response inconsistent with current recommendations. 43 respondents (43%) reported being told to wear it all day or all waking hours.
When asked if they remove their processor for certain activities, 67 respondents (67%) reported that they did. Commonly reported activities for which respondents removed their processor included: sleep/nap, showering, exercise, working outside (heat/sweat), to enjoy silence, and noisy environments (mowing lawn, woodworking).
Construct Validity
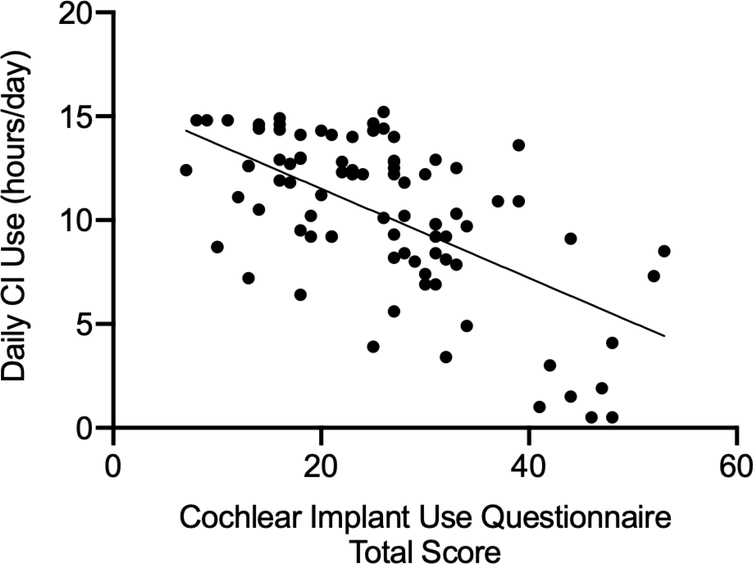
To assess construct validity, Spearman’s rho correlation between the total questionnaire score and the data logging data from the CI software was computed. A significant negative correlation would indicate presence of construct validity. Spearman’s correlation between these two measures yielded a large, significant effect size (rs = - 0.561, p < 0.0001, 95% confidence interval [−0.694, −0.391])23 suggesting that the questionnaire is valid for its intended purpose (Figure 2).
Figure 2.
The correlation (rs = −0.561, p<.0001) between the total score from the questionnaire and the participants’ daily CI use (data logging values mined from the CI software) is shown. This figure demonstrates construct validity of the Cochlear Implant Use Questionnaire (CIUQ).
Subjective vs. Objective Data logging
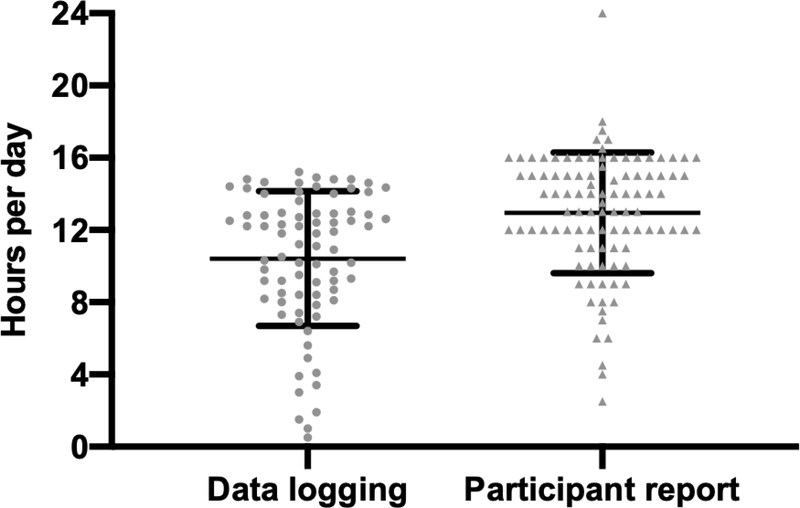
Respondents were asked to report how many hours per day they wear their CI processor. On average, they reported 13.0 hours per day (n = 100, SD = 3.3, range = 2.5 – 24). Objective data logging collected from the software indicated a mean of 10.4 hours per day (n = 78, SD = 3.7, range = 0.5 – 15.2). This difference of 2.6 hours was significant (W = 2387, p < 0.0001, r = 0.857) (Figure 3).
Figure 3.
Figure compares subjective and objective daily cochlear implant (CI) use. Individual data are shown for average daily CI use collected from the CI software (data logging) and from a question (participant report) on the Cochlear Implant Use Questionnaire (CIUQ).
DISCUSSION
The purpose of this study was to design a questionnaire aimed at identifying daily CI use habits and barriers to daily CI use and to administer the questionnaire to adult CI users to determine the construct validity of the questionnaire. We developed items based on the IMB model and found them to be items acceptable to CI users. The resulting scale, the CIUQ, had an average overall score of 23.3 and a range of 3 to 54 indicating that responses were quite variable, and CI recipients experience different barriers to using their CI processor. The five statements yielding the highest average response were as follows: “I take off my cochlear implant processor to avoid getting it wet such as while exercising or working outside during the summer;” “If I forget to wear my cochlear implant processor, my friends or family members will ask me why I’m not wearing it;” “When I take my cochlear implant processor(s) off, I enjoy the silence;” “If I am sick or do not feel well, I do not like to wear my cochlear implant processor(s);” “I take my cochlear implant processor off when I am home alone.” The CIUQ showed evidence of construct validity via a significant, large correlation between total score and the recipients’ daily CI use mined from the CI software suggesting that the questionnaire is a valid tool to use for understanding the underlying drivers of daily CI use. It should be noted that incomplete questionnaire responses were excluded which may be a potential source of bias. Those unable to complete all questions may represent a unique patient population to which the conclusions found in this study may not be applied.
Recipients were asked about their employment and living status (alone or with someone). No significant difference in total questionnaire score was found for these responses; however, two of the most frequently reported questions were related to living/social status (“If I forget to wear my cochlear implant processor, my friends or family members will ask me why I’m not wearing it” and “I take my cochlear implant processor off when I am home alone.”). These findings suggest that CI recipients may need additional counseling and/or support from family members or friends to ensure that they are wearing their CI during all waking hours. Previous studies of adherence to medical recommendations such as diabetes have also shown that social support contributed to adherence24,25.
Respondents were asked to recall their surgeon or audiologist’s recommendation for how many hours per day they should be wearing their CI processor. Nearly half of the respondents (43%) reported that they were never provided a recommendation, or they could not recall a recommendation. While we do not yet have a data-driven recommendation for exactly how long recipients should wear their CI processor daily, our clinicians recommend wearing their CI processor all the time except when showering or sleeping. This finding coupled with an average data logging value of 10.4 suggests that the importance of use during all waking hours (~15 hours per day) is not being communicated effectively. Clinicians may wish to provide this recommendation in writing and/or reiterate this recommendation at follow-up visits to improve patient retention of this recommendation.
67% of respondents reported removing their CI processor for certain activities such as exercising, working outside, napping, enjoying silence, or when environmental noise is too loud. Removing the processor for these activities contributes to lower daily average CI use. CI recipients may be unaware of potential solutions that could be implemented to keep them on the air during these activities. The CIUQ may allow clinicians to identify and address these activities via accessories such as a waterproof case to use while exercising if the patient is concerned about sweat harming the processor or a remote control to reduce the volume when environmental noise is too loud. During the collection of these data, anecdotally, we noticed that patients had forgotten about some of the solutions available to them because they had not been reviewed since their initial order form was submitted. They had coped with some of the challenges they experience by just removing their processor rather than potentially utilizing an available accessory. We found that the questions directly posed in the CIUQ helped bring these challenges to light, when otherwise they may have not been shared.
Respondents reported that they wore their CI processor 13 hours per day on average compared to 10.4 hours per day measured by data logging in the CI software. 10.4 hours per day is in line with previous average data logging reports in adult CI users4,5,8. This finding suggests that CI recipients overestimate how consistently they wear their CI processor, which is in agreement with previous reports in hearing aid users26–28. Subjective versus objective daily CI use has not been previously compared in the literature to our knowledge. Given this finding, audiologists may wish to review data logging with their patients at follow-up visits to allow patients to accurately monitor their daily use. CI manufacturers have begun to implement data logging in patient-accessible phone applications. Currently only “time in speech” is reported in such applications, but perhaps future iterations could give patients access to average CI use per day to allow them to monitor their own usage similar to a fitness tracker.
During data collection, we anecdotally noted several ways the CIUQ was able to identify fixable barriers to more consistent CI use for patients. One respondent, when asked about headaches in item number 25, reported that she removed her processor due to pain between the external and internal magnet. This challenge was easily resolved by reducing the external magnet strength. Another patient reported that her only rechargeable battery lasted 4 hours per charge, so if it dies at work, she didn’t have a replacement. She had not asked about a replacement battery because she could not afford it, but the questionnaire prompted us to identify the challenge and seek new batteries for her through her insurance. Yet another respondent reported that she often removes her processor because she is afraid it will fall off and get damaged. Item 18 allowed us to address this challenge by ordering her an accessory to support retention of the external processor. In these three examples, the respondents had been implanted for over a year, and they had completed at least five CI appointments with their audiologist; however, only when they completed this questionnaire were these concerns brought to light. Clinicians may consider administering the CIUQ to recipients with low data logging to explore potential barriers to CI use that may be driving inconsistent processor use. In our experience, we were able to uncover otherwise unknown barriers, which were easily addressed, to support patients’ consistent processor use.
CONCLUSION
The CIUQ is a newly developed tool to measure CI use habits and barriers to daily CI use. It is quick and easy to administer, and it shows evidence of construct validity via a significant correlation with daily CI use. Increasing evidence suggests that daily CI use is correlated with speech recognition outcomes. In order to optimize outcomes, clinicians should consider implementing this questionnaire to identify and overcome barriers to consistent, full-time CI processor use.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors would like to thank Rayah Kirby for her assistance with administering the questionnaire. The authors would also like to express sincere gratitude to the cochlear implant surgeons and audiologists involved in caring for the participants enrolled in our study. This research was supported by the National Institutes of Health via NIH/NIDCD R01-DC13117. This study was also supported by the National Institutes of Health National Center for Advancing Translational Sciences UL1 TR000445 through the use of REDCap.
Footnotes
CONFLICT(S) OF INTEREST TO DECLARE:
René Gifford: Advisory board for Advanced Bionics, Cochlear, and Frequency Therapeutics
Jourdan Holder: Speaking honoraria from Cochlear
INSTITUTIONAL REVIEW BOARD APPROVAL:
Vanderbilt University IRB Approval: 200807
REFERENCES
- 1.Glaubitz C, Liebscher T, Hoppe U. Impact of CI use and CI fitting on speech production in very early cochlear-implanted infants. HNO. September 2020. doi: 10.1007/s00106-020-00942-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sparreboom M, Beynon AJ, Snik AFM, Mylanus EAM. The effect of device use after sequential bilateral cochlear implantation in children: An electrophysiological approach. Int J Pediatr Otorhinolaryngol. 2016;86:161–166. doi: 10.1016/j.ijporl.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Wiseman KKB, Warner-Czyz AD. Inconsistent device use in pediatric cochlear implant users: Prevalence and risk factors. Cochlear Implants Int. 2018;19(3):131–141. doi: 10.1080/14670100.2017 [DOI] [PubMed] [Google Scholar]
- 4.Schvartz-Leyzac KC, Conrad CA, Zwolan TA. Datalogging Statistics and Speech Recognition During the First Year of Use in Adult Cochlear Implant Recipients. Otol Neurotol. 2019;40(7):e686–e693. doi: 10.1097/MAO.0000000000002248 [DOI] [PubMed] [Google Scholar]
- 5.Holder JT, Dwyer N, Gifford R. Duration of processor use per day is significantly correlated with speech recognition abilities in adults with cochlear implants. Otol Neurotol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Easwar V, Sanfilippo J, Papsin B, Gordon K. Impact of Consistency in Daily Device Use on Speech Perception Abilities in Children with Cochlear Implants: Datalogging Evidence. J Am Acad Audiol. 2018;29(9):835–846. doi: 10.3766/jaaa.17051 [DOI] [PubMed] [Google Scholar]
- 7.Busch T, Vermeulen A, Langereis M, Vanpoucke F, van Wieringen A. Cochlear Implant Data Logs Predict Children’s Receptive Vocabulary. Ear Hear. 2020;41(4):733–746. doi: 10.1097/AUD.0000000000000818 [DOI] [PubMed] [Google Scholar]
- 8.Busch T, Vanpoucke F, van Wieringen A. Auditory Environment Across the Life Span of Cochlear Implant Users: Insights From Data Logging. J Speech Lang Hear Res. 2017;60(5):1362. doi: 10.1044/2016_JSLHR-H-16-0162 [DOI] [PubMed] [Google Scholar]
- 9.Easwar V, Sanfilippo J, Papsin B, Gordon K. Factors affecting daily cochlear implant use in children: Datalogging evidence. J Am Acad Audiol. 2016;27(10):824–838. doi: 10.3766/jaaa.15138 [DOI] [PubMed] [Google Scholar]
- 10.Guerzoni L, Cuda D. Speech processor data logging helps in predicting early linguistic outcomes in implanted children. Int J Pediatr Otorhinolaryngol. 2017;101:81–86. doi: 10.1016/j.ijporl.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 11.Gagnon EB, Eskridge H, Brown KD. Pediatric cochlear implant wear time and early language development. Cochlear Implants Int. 2020;21(2):92–97. doi: 10.1080/14670100.2019.1670487 [DOI] [PubMed] [Google Scholar]
- 12.Gagnon EB, Eskridge H, Brown KD, Park LR. The Impact of Cumulative Cochlear Implant Wear Time on Spoken Language Outcomes at Age 3 Years. J Speech Lang Hear Res. March 2021:1–7. doi: 10.1044/2020_JSLHR-20-00567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett RJ, Jayakody DMP, Eikelboom RH, Atlas MD. Self-reported cochlear implant management skills: development and validation of the self-administered Cochlear Implant Management Skills (CIMS-self) survey. Clin Otolaryngol. 2017;42(1):164–171. doi: 10.1111/coa.12713 [DOI] [PubMed] [Google Scholar]
- 14.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Heal Psychol. 2006. doi: 10.1037/0278-6133.25.4.462 [DOI] [PubMed] [Google Scholar]
- 15.Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the Information, Motivation and Behavioral Skills model of antiretroviral therapy adherence. AIDS Care - Psychol Socio-Medical Asp AIDS/HIV. 2005. doi: 10.1080/09540120500038058 [DOI] [PubMed] [Google Scholar]
- 16.Mayberry LS, Osborn CY. Empirical Validation of the Information–Motivation–Behavioral Skills Model of Diabetes Medication Adherence: A Framework for Intervention. Diabetes Care. 2014;37(5):1246–1253. doi: 10.2337/dc13-1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amico KR, Barta W, Konkle-Parker DJ, et al. The information-motivation-behavioral skills model of ART adherence in a deep south HIV+ clinic sample. AIDS Behav. 2009. doi: 10.1007/s10461-007-9311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starace F, Massa A, Amico KR, Fisher JD. Adherence to antiretroviral therapy: An empirical test of the information-motivation-behavioral skills model. Heal Psychol. 2006. doi: 10.1037/0278-6133.25.2.153 [DOI] [PubMed] [Google Scholar]
- 19.Mannheimer SB, Morse E, Matts JP, et al. Sustained benefit from a long-term antiretroviral adherence intervention: Results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006. doi: 10.1097/01.qai.0000245887.58886.ac [DOI] [PubMed] [Google Scholar]
- 20.Nelson LA, Greevy RA, Spieker A, et al. Effects of a Tailored Text Messaging Intervention Among Diverse Adults With Type 2 Diabetes: Evidence From the 15-Month REACH Randomized Controlled Trial. Diabetes Care. November 2020:dc200961. doi: 10.2337/dc20-0961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messick S Validity of psychological assessment: Validation of inferences from persons’ responses and performances as scientific inquiry into score meaning. Am Psychol. 1995. doi: 10.1037/0003-066X.50.9.741 [DOI] [Google Scholar]
- 23.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 24.Sherbourne CD, Hays RD, Ordway L, DiMatteo MR, Kravitz RL. Antecedents of adherence to medical recommendations: Results from the medical outcomes study. J Behav Med. 1992;15(5):447–468. doi: 10.1007/BF00844941 [DOI] [PubMed] [Google Scholar]
- 25.Mayberry LS, Berg CA, Greevy RA, Wallston KA. Assessing helpful and harmful family and friend involvement in adults’ type 2 diabetes self-management. Patient Educ Couns. 2019;102(7):1380–1388. doi: 10.1016/j.pec.2019.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laplante-Lévesque A, Nielsen C, Jensen LD, Naylor G. Patterns of hearing aid usage predict hearing aid use amount (data logged and self-reported) and overreport. J Am Acad Audiol. 2014. doi: 10.3766/jaaa.25.2.7 [DOI] [PubMed] [Google Scholar]
- 27.Walker EA, Spratford M, Moeller MP, et al. Predictors of Hearing Aid Use Time in Children With Mild-to-Severe Hearing Loss. Lang Speech Hear Serv Sch. 2013. doi: 10.1044/0161-1461(2012/12-0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz K, Olson WA, Twohig MP, Preston E, Blaiser K, White KR. Pediatric hearing aid use: Parent-reported challenges. Ear Hear. 2015. doi: 10.1097/AUD.0000000000000111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.