Abstract
Background:
Emotional eating has emerged as a contributing factor to overeating, potentially leading to obesity or disordered eating behaviors. However, the underlying biological mechanisms related to emotional eating remain unclear. The present study examined emotional, hormonal, and neural alterations elicited by an acute laboratory stressor in individuals with and without emotional eating.
Methods:
Emotional (n=13) and non-emotional eaters (n=15) completed two main study visits, one week apart: one visit included a Stress version and the other a No-stress version of the Maastricht Acute Stress Task (MAST). Immediately pre- and post-MAST, blood was drawn for serum cortisol and participants rated their anxiety level. After the MAST, participants completed a Food Incentive Delay (FID) task during functional magnetic resonance imaging (fMRI), followed by an ad libitum snack period.
Results:
Emotional eaters exhibited elevated anxiety (p=0.037) and cortisol (p=0.001) in response to the Stress MAST. There were no changes in anxiety or cortisol among non-emotional eaters in response to the Stress MAST or in either group in response to the No-stress MAST. In response to the Stress MAST, emotional eaters exhibited reduced activation during anticipation of food reward in mesolimbic reward regions (caudate: p=0.014, nucleus accumbens: p=0.022, putamen: p=0.013), compared to non-emotional eaters. Groups did not differ in snack consumption.
Conclusions:
These data indicate disrupted neuroendocrine and neural responsivity to psychosocial stress amongst otherwise-healthy emotional eaters, who demonstrated hyperactive HPA-axis response coupled with hypoactivation in reward circuitry. Differential responsivity to stress may represent a risk factor in the development of maladaptive eating behaviors.
Keywords: emotional eating, eating behaviors, stress, cortisol, fMRI, food reward
1. Introduction
Obesity and eating disorders remain highly prevalent in the United States (Hales et al., 2020; Hudson et al., 2007) and are associated with a high mortality rate and comorbidity with psychiatric and medical conditions (Foreyt et al., 1996). A better understanding of the risk factors underlying obesity and subtypes of eating disorders is vital for the reduction of these medical morbidities. Emotional eating, a tendency to eat in response to negative emotions or emotional distress, has often been linked to obesity (Faith et al., 1997; Geliebter and Aversa, 2003), anorexia nervosa binge-purge type, bulimia nervosa (Ricca et al., 2012), and binge eating disorder (Masheb and Grilo, 2006; Pinaquy et al., 2003). Moreover, emotional eating has been increasingly suggested as an important psychopathological dimension that contributes to overeating (Cornelis et al., 2014).
Several theories have been proposed to explain the mechanisms behind the engagement in emotional eating under the influence of stress or negative emotions, collectively suggesting that maladaptive emotion regulation strategies contribute to overeating in emotional eaters. Learning theory, proposed by Kaplan and Kaplan (1957), viewed overeating as a learned behavior that can reduce anxiety. On the other hand, Heatherton and Baumeister (1991) argued that some people have high and aversive self-perception, finding it burdensome to be aware of themselves. This state is often accompanied by emotional distress, which motivates these individuals to engage in binging or emotional eating as a way to escape from such unpleasant feelings. Another model focuses on eating as an emotional coping mechanism or attentional distraction from negative emotions (Deroost and Cserjési, 2018; Spoor et al., 2007). Additionally, recent findings have linked heightened anxiety responses during stress or negative mood induction to eating disorder symptomatology in clinical populations, including anorexia nervosa and bulimia nervosa (Monteleone et al., 2020a; Monteleone et al., 2020b; Wildes et al., 2012). While several mechanisms explaining emotional eating have been introduced, relatively little is known about biological factors underlying this behavioral phenotype and putative risk factors for disordered eating.
One of the proposed biological mechanisms that is associated with eating behavior is hypothalamic-pituitary-adrenal (HPA) axis reactivity (Dallman et al., 2004a; Dallman et al., 2004b). Findings in clinical populations indicate an association between heightened HPA-axis reactivity to stress and binge eating disorder (Gluck et al., 2004), bulimia nervosa (Koo-Loeb et al., 1998), and obesity (Mårin et al., 1992), with these clinical populations exhibiting elevated cortisol levels following a stressor, compared to healthy controls. However, other studies have reported blunted cortisol responsivity to a stressor in anorexia nervosa and bulimia nervosa (Ginty et al., 2012; Het et al., 2015; Monteleone et al., 2020a) which persists following recovery (Het et al., 2020). In these chronically-ill populations, stress-induced cortisol responses appear to be unrelated to self-rated hunger in anorexia nervosa (Monteleone et al., 2020a) or positively related to desire to binge in binge eating disorder (Rosenberg et al., 2013), although direct examination of relationships between HPA-axis function in response to stress and actual, observed eating behavior in individuals with eating disorders has not been reported (Monteleone et al., 2018).
There have also been contrasting results regarding the relationship between emotional eating behaviors and HPA-axis reactivity in healthy individuals. Earlier findings found an association between stress reactivity and food consumption in healthy individuals after an acute stressor (Epel et al., 2001; Newman et al., 2007), with high cortisol reactors consuming more calories and choosing more sweet food compared to low reactors, suggesting a stress-induced sympathetic dysregulation in high cortisol reactors. Raspopow et al. (2010) found more pronounced cortisol level increases in emotional eaters compared to non-emotional eaters after an acute stressor, and Klatzkin and colleagues reported greater cortisol responsivity to mental stress among young healthy women who endorsed heightened perceived stress, in comparison to those with lower perceived stress (Klatzkin et al., 2019). On the other hand, van Strien et al. (2013b) reported no significant differences in cortisol between emotional and non-emotional eaters, although emotional eating was a significant moderator of the relationship between cortisol reactivity and food consumption across the whole group. These results indicate a role of emotional eating in the relationship between HPA-axis reactivity and eating behaviors, but more studies are needed to understand this association.
In addition to HPA-axis reactivity, central nervous system networks involved in reward processing represent a biological mechanism underlying emotional eating. Many studies focus on the striatum, due to its established function in reward processing and learning (Schultz, 2016). For example, some studies suggest that the hyperactivation of food reward circuitry in emotional eaters leads to increased risk for overeating and binge eating. This view is supported by a positive association between emotional eating and activation in reward-related brain areas (e.g., amygdala and insula) in response to food stimuli (van Bloemendaal et al., 2015; Wood et al., 2016). Furthermore, Loxton and Tipman (2017) found a positive association between reward sensitivity and food addiction symptoms, with emotional eating as a mediator. On the other hand, some data suggest an attenuated activation in the reward-related brain areas (e.g., putamen, caudate, and thalamus) in emotional eaters in response to food receipt (Bohon, 2014), supporting hypoactivation of food reward circuitry in emotional eaters, which may lead to overeating as a compensatory behavior. While these studies have examined the brain activation in emotional eaters, they were primarily conducted in a neutral state (i.e., in absence of acute stress or negative mood induction). Only one study examined the relationship between emotional eating and brain reward circuitry in individuals under the influence of a music-induced negative mood, which suggested greater activation of reward-related brain regions in an induced negative mood condition in women with emotional eating (Bohon et al., 2009). Thus, there is a significant gap in understanding potential aberrant reactivity of the HPA-axis and reward circuitry in response to psychosocial stress amongst individuals who exhibit emotional eating.
In the present study, our objective was to examine the relationship between emotional eating status and its associated neuroendocrine and neural alterations under acute stress. Specifically, in a group of healthy men and women, we assessed differences between emotional and non-emotional eaters in the effect of an acute psychosocial stress task (vs. a No-stress control task) on the following multiple systemic levels (a) HPA-axis reactivity (i.e., cortisol response), (b) neural activation in reward circuitry during anticipation and outcome phases of a (visual) food incentive delay task and (c) eating behavior (i.e., snack intake). We hypothesized that stress induction would differentially impact emotional and non-emotional eaters, such that emotional eaters, compared to non-emotional eaters, would exhibit hyperactive cortisol response, display aberrant neural activation in response to anticipation and receipt of food reward in reward-related regions (nucleus accumbens, caudate, putamen, and amygdala), and consume more snack calories.
2. Material and Methods
2.1. Participants
As part of a larger study on the relationship between stress and brain response to food-related reward in depression, healthy men and women (n=40), 21-45 years of age with BMI between 19 and 45 kg/m2 (upper limit chosen to ensure subject comfort during the scanning procedures), were recruited from online advertisements. Exclusion criteria included: any history of substance abuse; history of or current psychiatric disorders; current psychotropic medications use; mental retardation; endocrine disorders; diabetes; cardiovascular disease; treatment with weight loss medications; glucocorticoids; steroids; contraindications to MRI; history of neurological disease; current suicidal ideation; traumatic brain injury; for females, pregnancy or breastfeeding, current use of hormonal birth control (e.g., pills, patches, and intrauterine devices), and past amenorrhea greater than three months. Participants with Dutch Eating Behavior Questionnaire (DEBQ; Van Strien et al., 1986) emotional eating subscale scores below 1.62 or above 2.46 (corresponding to the 33rd and 66th percentiles of the sample) were included in the current analyses, yielding 30 participants. Of these, two participants did not complete both study visits. Therefore, complete data were obtained and are reported from 28 participants (14 females, 14 males): 13 emotional eaters (EE) and 15 non-emotional eaters (NE). The mean age of the sample was 28.29 years (SD = 5.47) and the mean body mass index (BMI) was 25.8 (SD = 4.75). The sample was 57.1% Caucasian, 21.4% African American, 17.9% Asian, and 3.6% other race. Participants were paid up to $425 for completing all parts of the study. All study procedures were approved by the Partners Healthcare Institutional Review Board.
2.2. Procedures
Participants completed three in-person visits on different days. Participants were pre-screened by phone by a trained research assistant to determine initial eligibility. The first visit was a screening visit for pre-screened eligible participants to determine study eligibility. The second and third visits were experimental sessions consisting of the stress induction, blood draws, neuroimaging session, and an ad libitum snack period.
2.2.1. Screening visit
During the screening visit, participants were oriented to study expectations and provided written informed consent. A trained clinical interviewer with over 20 years of experience administered the Mood Episode, Mood Differential, Psychosis, and Eating Disorders modules of the Structured Clinical Interview for Diagnoses (SCID-IV) for DSM-IV-TR (Spitzer et al., 2002) to rule out major psychiatric disorders. Participants completed the Beck Depression Inventory (BDI-II) (Beck et al., 1996), and anthropometric measurements (height, weight) and a blood draw (for hematocrit level) were obtained.
2.2.2. Main visits
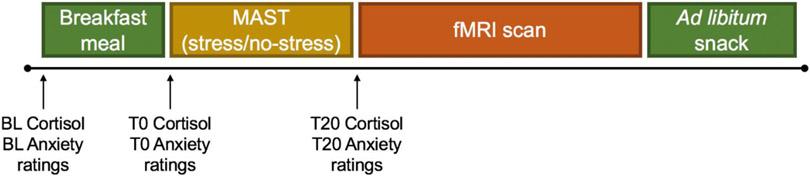
Eligible participants completed two main visits (1 week apart; see Figure 1). Visits were identical except for the version of the Maastricht Acute Stress Task (MAST) completed (see below). For female participants, both main visits were scheduled within the follicular phase of their menstrual cycle (i.e., day 1-12 in their cycle), determined by a self-report tracking questionnaire, to reduce the potential impact of circulating gonadal hormones on primary outcomes. All sessions were conducted between 0800 and 1300 h, following a 12 h overnight fast. A nurse inserted an IV catheter with a saline lock into the antecubital vein for serial blood sampling at three timepoints, collected in tandem with hunger and mood ratings (see details below). Following a fasting baseline blood draw, participants consumed a breakfast meal standardized for micro- and macronutrient content for 15 minutes and were advised to consume everything if possible. The meal contained 30% of their recommended daily caloric intake (varying according to each participant’s basal metabolic rate and physical activity level, measured by the Harris-Benedict equation (Harris and Benedict, 1919); with 18% calories from protein, 23% calories from fat, and 59% calories from carbohydrates). T0 blood draw was completed immediately following the breakfast session.
Figure 1. Schematic depiction of the main visit protocol.
Fasting baseline (BL) blood draw for cortisol and anxiety ratings were collected around 8:30 am, followed by a 15-minute breakfast meal. Next, T0 cortisol and anxiety ratings were completed, after which the MAST (Maastricht Acute Stress Task; stress or no-stress version) was administered, followed by the fMRI (functional magnetic resonance imaging) scan and ad libitum snack period.
Participants then completed either the Stress or No-stress version of the MAST (Smeets et al., 2012), with order (Stress, No-stress) counterbalanced across visits. During the MAST, participants were introduced to a female experimenter posing as a doctor who told them that they would complete a water and math task. For both visits, the MAST began with a 5 min instruction/preparation phase followed immediately by a 10 min phase involving hand immersion trials alternating with arithmetic trials. During the Stress visit, participants were instructed to perform five cold water hand immersion trials (these varied in duration from 60 sec to 90 sec), with the water temperature held between 0° and 2°C. In between the cold-water hand immersion trials, participants completed arithmetic trials (which varied in duration between 30 sec and 90 sec) during which they were asked to count backwards as quickly and accurately as possible from 2,043 in intervals of 17. If a mistake was made, participants were instructed to start again from 2,043. During the procedure, participants were told that they were being videotaped using a webcam mounted to the computer in front of them, to assess for facial expressions of pain. In reality, the camera was not recording. Following the final cold-water hand immersion trial, participants were told that their performance was poor and that they would need to repeat the task later during the visit. This manipulation was used to induce sustained levels of stress throughout the visit.
During the No-stress visit, there was no mention of videotaping and the water was lukewarm (35°-37°C). In between warm water hand immersion trials, participants were instructed to count up consecutively from 1-25, at their own pace, starting over when they reached 25. The experimenter stayed in the room to ensure compliance but gave no feedback on performance. The study staff member playing the role of the experimenter was kept constant for each subject across stress and No-stress visits.
Following MAST procedures, T20 blood draw was completed, and participants were then escorted to the MRI scan room. Participants underwent the 80-minute fMRI scanning session involving a food reward paradigm and were told that they could win actual snacks if they perform well in the task. Following the MRI session, participants were escorted to a quiet room and were allowed ad libitum access to preselected snack foods for 30 min. They were left alone during the snack period and were not aware that their snack intake was being recorded. At the end of the visit, participants were fully debriefed.
2.3. Measures
2.3.1. Emotional eating
During the No-stress visit, participants completed the 13-item emotional eating subscale of the DEBQ to assess the desire to eat in response to various negative emotions (e.g., “Do you have the desire to eat when you are irritated?”). All items are rated on a 5-point scale with responses that range from 1 (‘Never’) to 5 (‘Very Often’). Previous population-based studies based on samples with demographics similar to the current study have reported mean Emotional Eating subscale scores of 1.21 to 2.67 (Koenders and van Strien, 2011). The mean values of the scores on this subscale were used to determine emotional eating group status, based on upper and lower tertiles as described above. In the current sample, this subscale exhibited high reliability (Cronbach’s α = 0.97).
2.3.2. Anthropometry
Height was measured at the screening visit using a stadiometer. Weight was measured using the same scale at each main visit. Body mass index (BMI) was calculated for each visit and averaged across visits.
2.3.3. Physical activity
Participants completed selected questions from the Paffenbarger Physical Activity Questionnaire (Paffenbarger et al., 1993). The two questions, “For the last month, about how often have you taken part in moderate / very hard physical activity?” assessed participants’ engagement in monthly physical activities. For both questions, options ranged from 1 to 5 where “1” indicated “More than 4 times a week” and “5” indicated “Rarely or never”. Scores on the Paffenbarger were used to calculate the basal metabolic rate for determining individual caloric intake during the breakfast meal.
2.3.4. Cortisol sampling
Three blood draws were taken in the morning, with timepoints selected based on maximal response to the MAST as reported by Smeets and colleauges (Smeets et al., 2012). A baseline (fasting) draw was obtained 15 minutes after angio catheter insertion. Time 0 and Time 20 blood samples were collected immediately prior to and following the MAST, respectively. Blood samples were cold centrifuged, aliquoted, and stored at −80°C in plastic tubes containing a 10-mg/ml solution of PMSF (phenylmethanesulfonylfluoride) in methanol until assayed. Serum samples were assayed by LabCorp (Raritan, NJ) using electrochemiluminescence immunoassay (ECLIA) on a Roche Cobas analyzer; intra-assay CV 1.0-1.7%; inter-assay CV 1.4-2.2%.
2.3.5. Mood & appetite visual analog scales (VAS)
Ratings of appetite and mood were measured using an electronic visual analogue scale (VAS) system (Whybrow et al., 2006). VAS ratings of appetite (i.e., “How hungry do you feel?”) were measured upon arrival to obtain a baseline hunger level. Mood ratings were made upon arrival and immediately before (T0) and after (T20) the MAST. The mood VAS questions asked how nervous participants were at that moment. For all VAS scales, a line anchored by 0 (“not at all”) and 100 (“never been more”) was displayed, and participants placed a vertical mark on the line to make their rating.
2.3.6. fMRI paradigm
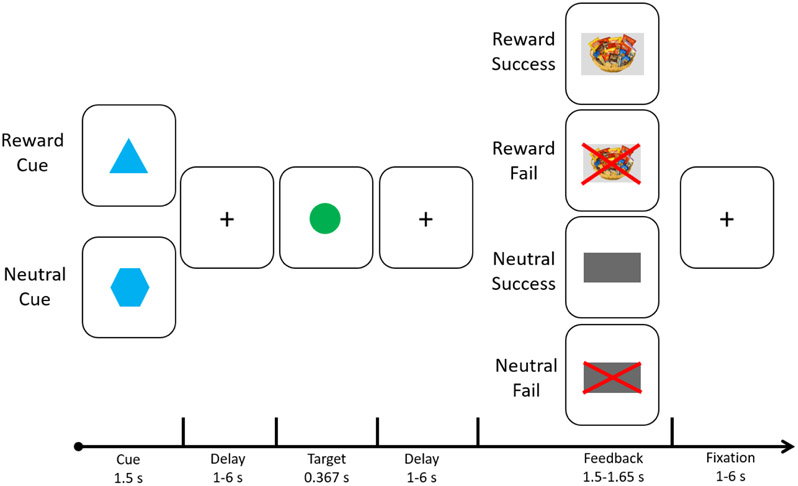
The Food Incentive Delay (FID) task, employed by Simon et al. (2014), was used to elicit neural responses during anticipation and receipt of food reward (see Figure 2). Participants completed 3 runs of the FID task: two runs consisted of 33 trials, and one run consisted of 34 trials. This yielded a total of 100 trials, of which 60 trials assessed responses to reward cues and 40 trials assessed responses to neutral cues (pseudorandomly ordered within each run). During each trial, subjects were shown either a reward cue (a triangle) or a neutral cue (a hexagon) for 1.5 sec. Next, they fixated on a crosshair while waiting for a variable duration (anticipation; 1-6 sec) until a circle-shaped target was presented (target; 0.367 sec). When the target appeared, subjects were instructed to press a button as quickly as possible. After an additional delay of variable duration (1-6 sec), a visual cue indicating success or failure was presented (outcome; 1.5-1.65 sec). For reward trials, success corresponded to presentation of a snack basket, signifying that the participant had won snacks on that trial. Failure was indicated by a snack basket with a red “X” overlaid on it, signifying that they had not won snacks. For neutral trials, success was indicated by a large gray rectangle, whereas failure was indicated by a large red “X” overlaid on the same gray rectangle. Participants were told that snacks they won during the FID task would be available for consumption immediately after the scanning session.
Figure 2. Food Incentive Delay (FID) Task Design.
During the FID, each trial began with a cue indicating the participant would have the opportunity to earn a food reward (reward trials; denoted by a blue triangle) or not have the opportunity to earn a food reward (no incentive trials; denoted by a blue hexagon). Following a variable delay of 1-6 seconds, a target appeared (green circle) prompting the participant to press a button as quickly as possible (as previously trained during a practice trial). This was followed by another variable delay of 1-6 seconds, after which feedback was provided indicating whether the button press occurred within the time limit (success) or not (fail). Trials were separated by an intertrial interval of 1-6 seconds.
During the anatomical scan that preceded the FID, participants were trained on the task, tested for explicit cue comprehension, and completed a practice version. Target duration for each run was individually determined based on reaction time (RT) collected during the prior run (for run 1, it was based on the RT during the practice session) and set such that participants would succeed on approximately 66% of the trials.
2.3.7. fMRI data acquisition
Data were acquired on a Siemens Skyra 3T scanner (Siemens Healthineers, Munich, Germany) equipped with a 32-channel head coil. A multi-band sequence was used to collect 794 oblique-axial echo planar imaging (EPI) volumes during the FID task (multiband acceleration=5; TR=1250 ms; TE=33 ms; flip angle=30°; slice thickness=2mm; number of slices=75; field of view=196 x 196mm). Images were collected in the oblique-axial plane (approximately −30° relative to AC-PC) to minimize susceptibility artifacts. Before EPI data acquisition, a magnetic (B0) fieldmap (magnitude and phase images with the same slice prescription and resolution as the functional volumes) was collected to enable fieldmap correction. A T1-weighted 3-dimensional spoiled gradient scan was also acquired (TR=2300 ms, TE=2.95 ms, flip angle=9°, voxel size=1 x 1 x 1.2mm3, number of sagittal slices=176) for coregistration to functional volumes.
2.3.8. Snack intake
During the screening visit, participants selected their five preferred snacks from the following items: Fig Newtons, potato chips, peanuts, yogurt-covered raisins, M & M’s, Lorna Doone cookies, Doritos, almonds, mini blueberry muffins, Hershey chocolate bar, Chips Ahoy cookies, Cheez-It crackers, peanut butter crackers, fruit snacks, and Dipps peanut butter granola bar. Participants were instructed that these five items would then be offered during the main study visits. During the ad libitum snack period at each main study visit, participants were presented with a selection of their five preferred snacks, portions of each snack providing 600 kcal (total of 3,000 kcal from all five snacks). After the snack period, total calories consumed were calculated by Center for Clinical Investigation (CCI) dietary staff.
2.4. Statistical methods
Sample size was based on an a priori power analysis conducted with η2 values reported by Bohon and colleagues (Bohon et al., 2009), which ranged from 0.3 to 0.53 (corresponding to effect size f = 0.65 to 1.06) and 0.37 to 0.6 (corresponding to effect size f = 0.77 to 1.22) for the interaction between emotional eating status and mood condition for anticipatory and consummatory reward phases, respectively. Thus, for the current study, for the anticipatory reward phase, to achieve 95% power to detect an interaction effect size (f) of at least 0.65 between emotional eating status and stress condition using repeated measures ANOVA at α = 0.05 requires a minimum of 14 subjects per group. For the consummatory reward phase, to achieve 95% power to detect an interaction effect size (f) of at least 0.77 between emotional eating status and stress condition using repeated measures ANOVA at α = 0.05 requires a minimum of 12 subjects per group.
The fMRI data were preprocessed using SPM12 (Wellcome Trust Centre for Neuroimaging www.fil.ion.ucl.ac.uk/spm). First, the initial five EPI volumes were discarded from each dataset to allow for T1 equilibration. Standard preprocessing procedures included realignment and geometric unwrapping using magnitude and phase images from the fieldmap, slice timing correction, EPI coregistration to the T1 image, normalization to the MNI (Montreal Neurological Institute) 152 space with resampling to 2 mm isotropic using 4th degree B-spline interpolation, and smoothing with a 6 mm full-width-half-maximum (FWHM) Gaussian kernel. The ART toolbox (Artifact Detection Tools; www.nitrc.org/projects/artifact_detect) was used to detect outliers in the global mean image time series (threshold: 3.5 S.D.) and motion (threshold: 0.8 mm, measured as scan-to-scan movement; see below).
Statistical analysis of fMRI data focused on blood oxygen level-dependent (BOLD) responses during the anticipation and receipt phases of the FID task. For single-subject analyses, reward and neutral anticipation and receipt represented the four primary conditions of interest modeled using a general linear model, along with conditions representing target presentation, reward/neutral failures, and error trials (when the participant either responded prior to target presentation or did not respond at all to the target presentation). To do so, regressors were specified for the following eight conditions: reward cue, neutral cue, reward success, reward failure, neutral success, neutral failure, and errors. The reward cue and neutral cue regressors represented the reward/neutral anticipation phase, with onsets set at the start cue representing reward/neutral and duration lasting 2.5 seconds. The reward/neutral success/failure regressors represented the reward/neutral receipt/lack of receipt phase with onsets set at the start of the cue representing success/failure and duration lasting 1.5 seconds. The target duration was set at 0.367 seconds. Error trials included onsets/durations for each phase (anticipation, target, outcome). Regressors were convolved with the canonical hemodynamic response function. Global mean signal and motion outliers, along with motion statistics representing linear (x, y, z) and rotational (roll, pitch, yaw) motion, detected using ART, were entered as nuisance regressors. Following GLM estimation, two primary contrasts of interest were computed: Reward Anticipation vs. Neutral Anticipation; Reward Success vs. Neutral Success). For analyses regarding the relationship between activation in reward circuitry and emotional eating status, degree of functional response (beta estimates, β) was determined for each contrast and each subject within anatomically-defined a priori regions of interest (ROIs). Predefined ROIs for a priori hypotheses were the caudate, nucleus accumbens (NAcc), putamen, and amygdala. Anatomical borders of hypothesized regions were defined using a manually segmented Montreal Neurological Institute-152 brain (Makris et al., 2006; Makris et al., 2016; Makris et al., 2013) and implemented as overlays on the SPM12 canonical brain using the Wake Forest University PickAtlas (Maldjian et al., 2003) toolbox for SPM. Beta estimates were extracted using the REX toolbox (Whitfield-Gabrieli, 2009) and exported to SPSS software (version 26; SPSS, Inc., Chicago, IL) for further analysis.
All further analyses were carried out in SPSS version 26. Demographic data, DEBQ scores, and baseline characteristics were assessed using Fisher’s Exact Tests, χ2, and independent samples t-tests. Cortisol levels and subjective mood ratings were analyzed using a 2 (Group: NE/EE) × 2 (Visit: No-stress/Stress) χ 2 (Time: T0/T20) repeated measures ANOVA. The relationship between subjective anxiety ratings and cortisol change in response to acute stress across the whole sample was assessed using Pearson correlation. FID beta estimates and kcal consumed during snack intake were analyzed using 2 (Group: NE/EE) × 2 (Visit: No-stress/Stress) repeated measures ANOVAs. Exploratory analyses using Pearson correlations examined relationships between change in VAS nervous ratings (T0 and T20) at the Stress visit and: 1) percentage change in cortisol levels (from T0 to T20) at the Stress visit, and 2) FID beta estimates at the Stress visit. Between-group differences in Pearson correlations were interrogated using Fisher r-to-Z transformations. Alpha was set at 0.05.
3. Results
3.1. Demographic and baseline appetite characteristics
Direct comparison between EE and NE confirmed significantly higher emotional eating in EE as measured by the DEBQ, t(26) = −9.37, p < 0.001 (see Table 1). EE and NE groups did not differ in demographic characteristics [age (p = 0.77); sex (p = 0.26); BMI (p = 0.70)], depression (p = 0.61) or recent physical activity levels [moderate (p = 0.57); very hard (p = 0.56)]. Groups did not differ in appetite or pre-stress meal intake characteristics during main visits [baseline hunger level (p = 0.60 and p = 0.44 for No-stress and Stress visits, respectively); percentage of breakfast meal consumed (p = 0.57; p = 0.77)].
Table 1.
Demographic and Baseline Variables
| Group | |||||
|---|---|---|---|---|---|
| Variable | Emotional Eaters (n=13) |
Non-emotional Eaters (n=15) |
Between-Group Comparisons |
||
| Mean | SD | Mean | SD | ||
| Age (years) | 28.6 | 5.8 | 28.0 | 5.4 | t(26)=0.29, p=0.77 |
| BMI | 26.2 | 3.4 | 25.5 | 5.8 | t(26)=0.40, p=0.70 |
| Emotional Eating (DEBQ) | 3.0 | 0.7 | 1.3 | 0.2 | t(26)=9.37, p<0.001 |
| Depression (BDI) | 0.4 | 1.0 | 0.6 | 1.2 | t(26)=−0.52, p=0.61 |
| Baseline hunger (VAS) | |||||
| No-stress | 55.5 | 26.1 | 60.9 | 26.3 | t(26)=−0.54, p=0.60 |
| Stress | 61.0 | 26.7 | 52.3 | 30.9 | t(26)=0.78, p=0.44 |
| Breakfast consumed (%) | |||||
| No-stress | 83.1 | 20.3 | 78.9 | 18.5 | t(26)=0.57, p=0.57 |
| Stress | 83.0 | 22.0 | 80.6 | 21.1 | t(26)=0.29, p=0.77 |
| n | % | n | % | ||
| Sex | χ2=1.29, p=0.26 | ||||
| Female | 8 | 28.6 | 6 | 21.4 | |
| Male | 5 | 17.9 | 9 | 32.1 | |
| Physical activity (moderate) | p=0.99a | ||||
| >4 times per week | 2 | 7.1 | 2 | 7.1 | |
| 2-4 times per week | 8 | 28.6 | 9 | 32.0 | |
| Once a week | 2 | 7.1 | 2 | 7.1 | |
| 2-3 times per month | 1 | 3.6 | 0 | 0.0 | |
| Rarely or never | 0 | 0.0 | 2 | 7.1 | |
| Physical activity (very hard) | p=0.95a | ||||
| >4 times per week | 3 | 10.7 | 1 | 3.6 | |
| 2-4 times per week | 3 | 10.7 | 5 | 17.9 | |
| Once a week | 2 | 7.1 | 5 | 17.9 | |
| 2-3 times per month | 3 | 10.7 | 3 | 10.7 | |
| Rarely or never | 2 | 7.1 | 1 | 3.6 | |
Fisher’s Exact Test p value
BMI = Body mass index
DEBQ = Dutch Eating Behavior Questionnaire
BDI = Beck Depression Inventory
VAS = Visual analogue scale
3.2. Subjective anxiety ratings in response to acute stress
There was a significant Group × Visit × Time interaction on ratings of anxiety [F(1,26) = 9.36, p = 0.005, η2 = 0.27] (see Figure 3). Furthermore, there was a significant Group × Visit interaction [F(1,26) = 7.62, p = 0.010, η2 = 0.23], Visit × Time interaction [F(1,26) = 11.24, p = 0.002, η2 = 0.30], and main effect of Visit [F(1,26) = 4.78, p = 0.038, η2 = 0.16] on ratings of anxiety. Post-hoc analyses revealed that EE showed a significant increase in ratings of anxiety from T0 to T20 [F(1,12) = 5.51, p = 0.037, η2 = 0.32], while NE did not exhibit significant changes in the ratings (p = 0.09) during the Stress visit. There were no significant changes in ratings of anxiety from T0 to T20 among either group during the No-stress visit (EE: p = 0.13; NE: p = 0.51). Furthermore, EE exhibited significantly higher T20 anxiety levels compared to NE during the Stress visit [F(1,26) = 8.44, p = 0.007, η2 = 0.25] but not during the No-stress visit (p = 0.33). There were no significant differences in T0 anxiety levels either during the Stress visit (p = 0.25) or the No-stress visit (p = 0.28). Collectively, these affective rating findings confirmed that EE showed larger stress-induced increases in anxiety relative to the NE, who showed slight but non-significant increases in anxiety at the Stress visit.
Figure 3. Effect of Stress on Anxiety Ratings in Emotional and Non-Emotional Eaters.
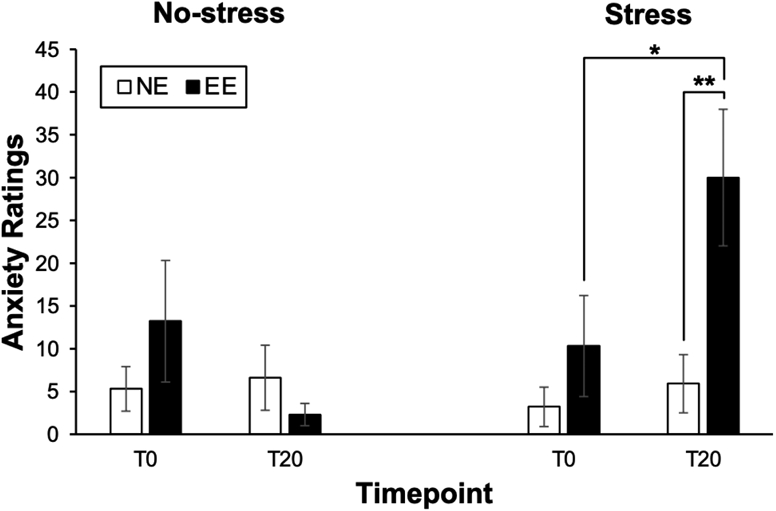
Mean (±SEM) anxiety ratings before (T0) and after (T20) the Stress or No-stress control task for emotional eaters (EE) compared to non-emotional eaters (NE). *p<0.05, **p<0.01.
3.3. Cortisol levels at baseline and reactivity to acute stress
There was a significant Group × Visit × Time interaction on cortisol levels [F(1,26) = 4.42, p = 0.045, η2 = 0.15] (see Figure 4). Furthermore, there was a significant Visit × Time interaction [F(1,26) = 18.00, p < 0.001, η2 = 0.41] and main effect of Time [F(1,26) = 14.61, p = 0.001, η2 = 0.002] on cortisol levels. Post-hoc analyses revealed that during the Stress visit, there was a significant increase in cortisol from T0 to T20 [F(1,12) = 19.28, p = 0.001, η2 = 0.62]. On the other hand, cortisol levels increased from T0 to T20 but did not reach statistical significance [F(1,14) = 4.54, p = 0.051, η2 = 0.25]. There were no significant changes in cortisol from T0 to T20 among EE or NE during the No-stress visit. There were no significant differences in T0 or T20 cortisol levels between EE and NE either during the Stress visit (EE: p = 0.76; NE: p = 0.12) or the No-stress visit (EE: p = 0.62; NE: p = 0.68). Collectively, these cortisol findings suggested that the MAST elicited stress-induced increases in cortisol levels in EE but not in NE.
Figure 4. Effect of Stress on Serum Cortisol in Emotional and Non-Emotional Eaters.
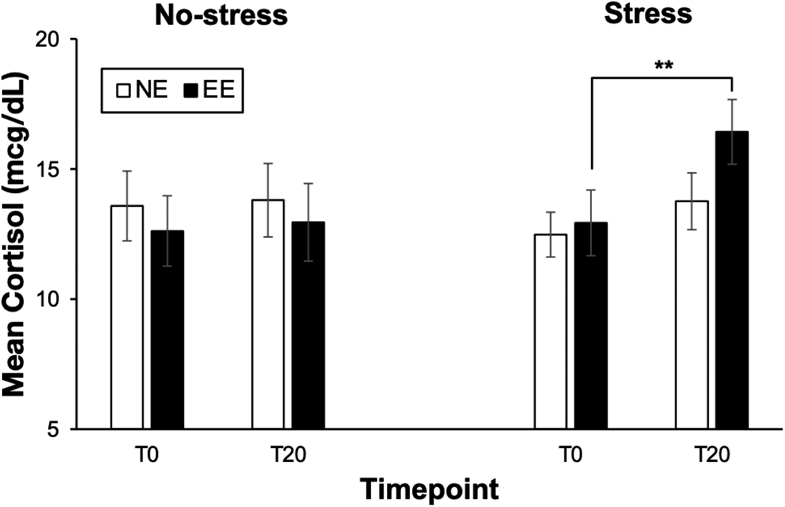
Mean (±SEM) serum cortisol levels before (T0) and after (T20) the Stress or No-stress control task for emotional eaters (EE) compared to non-emotional eaters (NE). **p<0.01.
3.4. Exploratory analysis of relationships between subjective anxiety ratings and cortisol reactivity to acute stress
As expected, across groups, change in cortisol from T0 to T20 (expressed as a percentage) was positively related to change in self-reported anxiety (defined as VAS ratings of nervousness from T0 to T20) during the Stress visit (r = 0.38, p = 0.048; Figure 5). Examined within each group, cortisol changes were positively related to self-reported anxiety in NE (r = 0.53, p = 0.04) but was not significant in EE (r = 0.24, p = 0.42). The associations did not differ between groups (Fisher Z = 0.81, p = 0.21).
Figure 5. Relationship between Anxiety Ratings and Serum Cortisol in Response to Stress in Emotional and Non-Emotional Eaters.
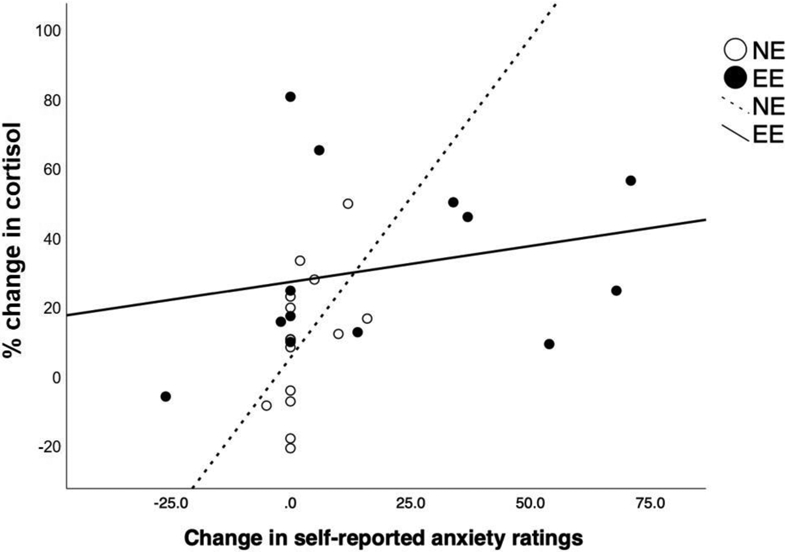
Positive correlation between absolute change in self-reported anxiety [change in visual analogue scale (VAS) nervousness ratings from T0 to T20] and percentage change in cortisol (from T0 to T20) during the Stress visit.
3.5. Brain activation during food reward anticipation following acute stress
A 2 (Group) × 2 (Visit) repeated measures ANOVA indicated no significant main effects of Group or Visit on neural activation during food reward anticipation in a priori regions of interest. However, there were significant Group × Visit interactions in the caudate [F(1,26) = 6.99, p = 0.014, η2 = 0.21], NAcc [F(1,26) = 5.63, p = 0.025, η2 = 0.18], and putamen [F(1,26) = 6.99, p = 0.014, η2 = 0.21] in response to anticipation of food reward versus neutral cue (see Figure 6). No Group × Visit interactions were found in the amygdala [F(1,26) = 4.20, p = 0.051, η2 = 0.14]. Simple effects analyses revealed a significantly reduced activation in caudate [F(1,26) = 6.89, p = 0.014, η2 = 0.21], NAcc [F(1,26) = 5.91, p = 0.022, η2 = 0.18], and putamen [F(1,26) = 7.19, p = 0.013, η2 = 0.21] during anticipation of food reward (versus neutral cue) in EE compared to NE during the Stress visit. There were no significant differences between groups in activation in these regions during the No-stress visit (p = 0.10 to 0.58). Collectively, these neural findings confirmed that, following an acute stress task, EE exhibited weaker activation in the striatal areas when anticipating food reward.
Figure 6. Effect of Stress on Activation during Food Reward Anticipation in Emotional and Non-Emotional Eaters.
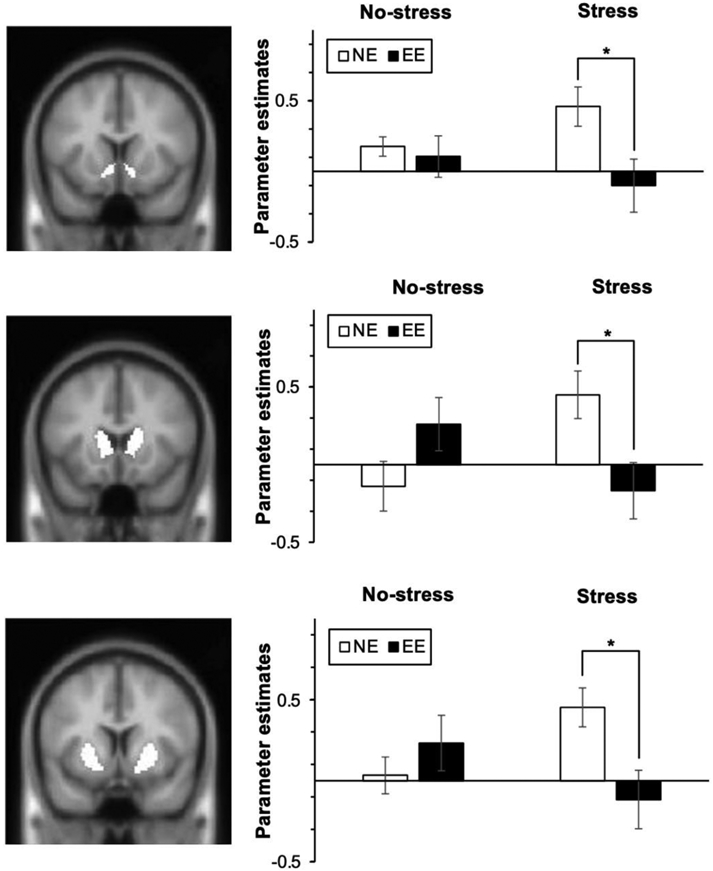
Food reward-related anticipatory activation in emotional eaters (EE) and non-emotional eaters (NE) in response to stress vs. no-stress. Relative to the No-stress visit, during the Stress visit, the EE group exhibited significantly lower activation during anticipation of food reward in the (A) nucleus accumbens, (B) caudate, and (C) putamen. Coronal slices (left panel) showing anticipatory reward activity [Anticipation of food reward vs. Anticipation of neutral] in reward regions are shown for the interaction between Group and Visit. Parameter estimates extracted from each region (right panel). *p<0.05.
3.6. Brain activation during food reward receipt in response to acute stress
A 2 (Group × 2 (Visit) repeated measures ANOVA revealed no significant main effects of Group or Visit and no Visit × Group interaction effect on the neural activation in response to receipt of food reward versus neutral cue.
3.7. Exploratory analysis of relationships between VAS ratings and brain activation during food reward anticipation following acute stress
Exploratory analyses examined associations between VAS ratings of anxiety and brain activation. During the Stress visit, change in anxiety (VAS nervousness, T0 to T20) was negatively correlated with brain activation during food reward anticipation in the NAcc (r = −0.55, p = 0.003; Figure 7A) and caudate (r = −0.46, p = 0.015; Figure 7B). Examined within each group, in both EE and NE, anxiety ratings were negative related to the activation in the caudate (EE: r = −0.46; NE: r = −0.14) and the NAcc (EE: r = −0.60; NE: r = −0.10). These correlations did not differ between groups (NAcc: Fisher Z = 1.37, p = 0.09; caudate: Fisher Z = 0.82, p = 0.21).
Figure 7. Relationship between Anxiety Ratings and Activation during Food Reward Anticipation in Response to Stress in Emotional and Non-Emotional Eaters.
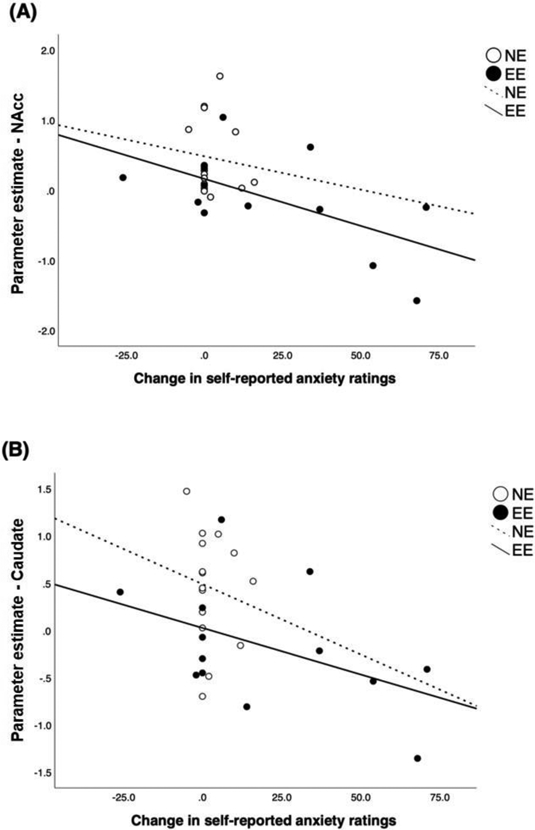
Negative correlation between absolute change in self-reported anxiety [change in visual analogue scale (VAS) nervousness ratings from T0 to T20] and brain activation during the food reward anticipation in (A) the nucleus accumbens (NAcc) and (B) caudate during the Stress visit.
3.8. Snack intake
A 2 (Group) × 2 (Visit) repeated measures ANOVA revealed no significant main effects of Visit [F(1,26) = 0.95, p = 0.34, η2 = 0.035], Group [F(1,26) = 0.61, p = 0.44, η2 = 0.023], or Visit × Group interaction [F(1,26) = 0.01, p = 0.93, η2 < 0.01] on caloric intake during the ad libitum snack period.
4. Discussion
The overarching goal of the present study was to investigate the effect of stress on food intake, cortisol reactivity, and the striatal response to food rewards among healthy individuals classified as emotional eaters. Results showed that emotional eaters exhibited elevated cortisol and anxiety levels in response to an acute psychosocial stressor, compared to a control (no-stress) state, while non-emotional eaters did not show such elevation. Among both groups, change in self-reported anxiety levels was positively correlated with change in cortisol level during the Stress visit. Furthermore, in the stress condition, emotional eaters demonstrated significantly weaker activation in caudate, nucleus accumbens, and putamen when anticipating food reward compared to non-emotional eaters, while no difference was found in the control condition. Brain activation in the NAcc and caudate during food reward anticipation was inversely associated with stress-induced anxiety, with relationships qualitatively stronger in the emotional eating group. Contrary to our hypothesis, ad libitum snack consumption following stress induction did not vary according to emotional eating status.
The findings of elevated cortisol level and heightened feelings of anxiety under stress amongst emotional eaters are consistent with a prior report by Raspopow et al. (2010) in which emotional eaters presented with more pronounced changes in cortisol following an acute stressor compared to non-emotional eaters. High cortisol reactivity has often been linked to increased food consumption in healthy adults (Epel et al., 2001; George et al., 2010), suggesting a potential neurobiological link between stress response systems and human eating behavior, although others report opposing directionality for the relationship between HPA-axis response and food intake (Wierenga et al., 2018). Furthermore, Herhaus et al. (2020) found that high cortisol reactivity and maladaptive emotional coping were associated with increased food intake in individuals with obesity, suggesting that cortisol reactivity may serve as a neuroendocrine marker of vulnerability to stress-induced eating in obesity. These previous findings and the results of the present study indicate that individuals who engage in emotional eating are physiologically sensitive to stress such that encountering a psychosocial stressor induces acute HPA-axis hyperactivity to cope with the stressor and may contribute to the maintenance of emotional eating behaviors.
The striatum has a distinct function in reward processing and reward learning (Schultz, 2016), and is sensitive to states of high physiological stress. Our data, indicating that emotional eaters exhibited lower activation of the striatum during the anticipation of food reward compared to non-emotional eaters specifically in response to psychosocial stress, are consistent with preclinical and clinical studies showing that while acute stress increased dopaminergic firing and neural activation to monetary rewards in the striatum, chronic stress attenuated activation in the reward-related regions (Kumar et al., 2014; Valenti et al., 2012). Although we did not measure self-reported chronic stress levels in our sample, we speculate that emotional eaters may experience heightened levels of chronic stress, which, in the setting of an acute laboratory stressor, has an additive effect to further attenuate reward activation.
Additionally, our findings are in line with those of previous studies reporting that stress attenuates reward sensitivity to food cues in healthy individuals (Born et al., 2010), individuals with obesity (Wang et al., 2002), and patients with bulimia nervosa (Jimerson et al., 1992), providing converging evidence for reward deficit in the context of stress induction across various eating phenotypes. These results are in line with the theory of reward deficiency syndrome, which posits that when the brain reward system malfunctions, this leads to multiple pleasure-seeking behaviors including glucose binging (Blum et al., 2000). From this perspective, reduced striatal activation to food reward in response to stress may trigger compensatory behaviors such as emotional eating in an attempt to normalize striatal function. Furthermore, Wonderlich et al. (2018) found that individuals with greater stress-induced decreases in activation in ventromedial prefrontal cortex and amygdala to visual food cues exhibited greater increases in negative affect prior to binge eating, suggesting the role of decreased neural activities under stress in the trajectory of negative mood to binge eating. Hence, emotional eaters may utilize emotional eating behavior as a means to compensate for the decreased activation of reward circuits when they experience distress.
Our findings appear to contradict results of Bohon et al. (2009), who reported greater activation of left caudate nucleus and pallidum in response to receipt of food in female emotional eaters under negative mood induction. Important differences in methodology are worth noting here; specifically, Bohon and colleagues (2009) induced negative mood using music and used an fMRI food reward paradigm involving gustatory stimuli (chocolate milkshake). These methods are in contrast with the present study, which included both males and females, used induction of psychosocial stress via the MAST which incorporates social, cognitive, and sensory challenges, and measured brain activation during a food incentive delay task with visual stimuli. More notably, emotional eaters included in Bohon et al. (2009) displayed significantly greater levels of depression than their non-emotional eating group, and had a mean BDI score (13.56) near the cutoff for mild depression and standard deviation over 7, indicating at least some EE subjects scored in the mild or moderate range of depression. This clinically relevant level of depression in their EE group is important to consider with respect to their results, which are consistent with prior findings of elevated neural responsivity to cues predicting reward in limbic regions (Ubl et al., 2015) and hyperactivation in cortical regions during anticipation of monetary rewards (Dichter et al., 2012), relative to healthy controls. By comparison, in our study, both EE and NE groups had mean BDI scores of <1 (Table 1), and group means did not differ significantly from each other. We propose that underlying mood dysfunction (even subclinical) in Bohon et al.’s EE subject could at least partially explain the opposing findings between our studies. In fact, the authors acknowledged the potential impact of depressed mood on their EE findings, noting “it is unclear whether differences in neural activation between groups are a product of emotional eating or result from depressive symptoms or negative affect” (p. 219). These differences in sample characteristics and methodology may explain the discrepancy between the present outcomes and those of Bohon and colleagues. Future studies examining neurobehavioral response to stress among individuals with emotional eating with and without depression would aid in elucidating the impact of mood on reward function in EE.
Contrary to prior research indicating that stress alters food intake patterns toward high calorie snack foods as well as increased food consumption among stress eaters (Epel et al., 2004; Zellner et al., 2006), in the present study, emotional eating status and psychosocial stress induction did not significantly impact snack intake. This discrepancy may be due to several contributing factors. First, food consumption within a lab setting may not adequately generalize to the everyday conditions under which stress-induced eating occurs. Participants might have been influenced by performance expectations and social desirability bias to the extent that they did not engage in food intake in the same way that they might in a real-world setting. In contrast, studies that utilized Ecological Momentary Assessment have provided evidence that individuals with high emotional and stress eating behaviors exhibit increased food intake as measured in naturalistic settings (Reichenberger et al., 2018; Reichenberger et al., 2020). Second, the temporal delay between completion of the stress (or control) task and the ad libitum snack period (approximately 2 hours) might have influenced snack food intake such that the effect of the stressor may have dissipated by the time subjects were provided access to snack food. This possibility seems plausible considering that Epel et al. (2004) and Zellner et al. (2006) presented subjects with a stressor and snacks simultaneously, which allowed an investigation of the immediate effect of stress on food intake. Third, it was suggested by Frayn et al. (2018) that while many emotional eaters overeat and exhibit weight gain, some rely on additional compensatory behaviors (other than eating) in an effort to regulate their overconsumption and to maintain their weight. Considering that the sample in the present study were primarily healthy individuals with BMIs spanning healthy weight to overweight categories, with no other medical nor psychological comorbidities, it is possible that they engaged in other coping strategies during the study visits such that stress did not result in increased snack intake.
Finally, there has been an ongoing debate regarding the validity of self-reported emotional eating in the context of actual changes in food intake under stress and negative mood induction (Bongers and Jansen, 2016; Evers et al., 2018), based on conflicting findings: some have found that EE scores do not predict laboratory-based (Adriaanse et al., 2011) or naturalistic (Boh et al., 2016) consumption, while others reported that high (vs. low) emotional eating is associated with increased food intake following sad or stressful mood manipulation (van Strien et al., 2013a; van Strien et al., 2012). Our null results with respect to stress-induced snack food intake in EE vs. NE groups could be interpreted as support for the former perspective. However, given the reasons stated in the preceding paragraph, and clear evidence from objective, biological measures (cortisol, brain activity) indicating distinct patterns of responsivity in EE vs. NE groups, we would argue for the validity of DEBQ-measured emotional eating in the context of our study. At a more nuanced level, we acknowledge the possibility that constructs defined by self-report questionnaires do not fully capture the complex and heterogenous factors underlying emotional state and food intake. Additional studies incorporating multifactorial measurement of emotional eating along with objective assessment of food cue reactivity and intake are needed to explore whether modification of these factors impacts food intake in individuals with emotional eating in response to psychosocial stress.
4.1. Strengths and limitations
The present study was the first to integrate fMRI, cortisol sampling, and a robust psychosocial stressor to examine differences in individuals with varying levels of emotional eating, which provided an avenue to address both neural and neuroendocrine alterations under stress. Nonetheless, we acknowledge limitations to this study. First, there was a relatively constrained distribution of emotional eating scores resulting from the small sample size. The mean emotional eating scores of emotional eaters ranged from 2.46 to 4.85 with most subjects scoring below 3.46. The highest possible mean score for this measure was 5. Although tertile split was applied to examine differences between opposing ends of the spectrum of emotional eating (excluding those with moderate EE scores), the utilization of a larger sample size with a quartile or quintile split would maximize our ability to utilize a “deviant subsample” approach in investigating the effect of stress according to low and high EE scores amongst healthy individuals. Relatedly, as our goal was not to understand trends in otherwise-healthy individuals, the constrained range of emotional eating behavior does not necessarily reflect levels of EE that would be observed in clinical populations. Second, snack consumption was quantified using the total caloric intake, a relatively gross measure of food intake, and explicit ratings of snack pleasantness and palatability were not measured. Examining variations in macro- and micronutrient composition, variations in snack choices, and relative reward value ratings would provide a broader understanding of the effect of stress on food selection and intake in emotional eaters. Third, sex-specific patterns were not analyzed due to small sample size. As there exist changes in food consumption in response to stress in males and females (Weinstein et al., 1997), future studies should conduct the study with a bigger sample to control for sex effect. Fourth, unlike several studies that employed fMRI paradigm in which an actual gustatory stimulus, such as milkshake, was delivered to the subjects in the MRI scanner (Bohon et al., 2009; Stice et al., 2008), our paradigm used visual images of snacks to examine the subjects’ brain activation during (indirect) receipt of food reward, more akin to receipt of cues indicating they would be able to consume snacks in the near future, rather than direct receipt. Implementing a paradigm involving immediate delivery of feedback in the form of rewarding gustatory stimuli may reveal distinct results. Fifth, the experimental protocol was implemented in the morning, potentially reducing the ability to detect maximal differences in EE and NE groups in cortisol responding given typical diurnal cortisol patterns (Kudielka et al., 2009). Finally, the NE group exhibited a much less robust response to the MAST, as measured by self-report and cortisol levels, relative to the EE group. Indeed, only 40% of individuals in the NE group were classified as cortisol responders (>15.5% increase in cortisol; (Miller et al., 2013)), compared to 70% of the EE group. However, post-hoc analyses of cortisol responder status failed to reveal a significant interaction between responder status and EE status (data not shown). It is challenging to discern whether the attenuated response indicates disrupted physiology amongst non-emotional eaters, or whether this pattern of response is within the range of normal variation for healthy individuals. Further investigations using larger samples of individuals which span the range in emotional eating and focus on the physiological mechanisms of the stress response in non-emotional eaters are warranted.
5. Conclusion
The present study offers new insight into the neurobiological factors associated with emotional eating in healthy individuals, which may represent a risk factor in the development of maladaptive eating behaviors, eating disorders, or obesity. Our data demonstrate that psychosocial stress has a differential effect on hormonal and neural pathways in emotional and non-emotional eaters, with hyperactivity of the HPA-axis and hypoactivation during anticipation of food reward amongst otherwise-healthy emotional eaters. These findings provide evidence of aberrant pathways underlying emotional eating and highlight stress reduction techniques as a potential therapeutic target for those at risk for developing clinically significant emotional eating behaviors.
Supplementary Material
Acknowledgements
We would like to thank W. Scott Hoge, Ph.D. for assistance with MRI sequence parameters.
Funding Source
This work was supported the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK104772; LMH, PI). The project described was also supported by the NIH National Center for Advancing Translational Science (1UL1TR001102 and 1UL1TR002541-01).
Footnotes
Declaration of Competing Interest
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, and Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics. Dr. Goldstein is on the scientific advisory board and has an equity interest in Cala Health, a neuromodulation company. This relationship is under the management of Mass General Brigham Healthcare systems policies regarding potential conflicts. However, there is no conflict of interest for this study. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. The other authors have no financial disclosures.
Ethical Statement
All study procedures were approved by the Partners Healthcare Institutional Review Board (protocol #2015P002603). All participants provided written informed consent prior to completing study activities. Data will be made available by written request to and approval of the Corresponding Author; a formal data sharing agreement will be required for data sharing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriaanse MA, de Ridder DT, Evers C, 2011. Emotional eating: eating when emotional or emotional about eating? Psychol Health 26, 23–39. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G, 1996. Manual for Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJH, Comings DE, 2000. The Reward Deficiency Syndrome: A Biogenetic Model for the Diagnosis and Treatment of Impulsive, Addictive and Compulsive Behaviors. Journal of Psychoactive Drugs 32, 1–112. [DOI] [PubMed] [Google Scholar]
- Boh B, Jansen A, Clijsters I, Nederkoorn C, Lemmens L, Spanakis G, Roefs A, 2016. Indulgent thinking? Ecological momentary assessment of overweight and healthy-weight participants' cognitions and emotions. Behav Res Ther 87, 196–206. [DOI] [PubMed] [Google Scholar]
- Bohon C, 2014. Greater emotional eating scores associated with reduced frontolimbic activation to palatable taste in adolescents. Obesity 22, 1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C, Stice E, Spoor S, 2009. Female emotional eaters show abnormalities in consummatory and anticipatory food reward: A functional magnetic resonance imaging study. International Journal of Eating Disorders 42, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers P, Jansen A, 2016. Emotional Eating Is Not What You Think It Is and Emotional Eating Scales Do Not Measure What You Think They Measure. Front Psychol 7, 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born JM, Lemmens SGT, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, Westerterp-Plantenga MS, 2010. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. International Journal of Obesity 34, 172–181. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Rimm EB, Curhan GC, Kraft P, Hunter DJ, Hu FB, van Dam RM, 2014. Obesity susceptibility loci and uncontrolled eating, emotional eating and cognitive restraint behaviors in men and women. Obesity 22, E135–E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F, 2004a. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci 1018, 141–150. [DOI] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF, 2004b. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology 145, 2633–2638. [DOI] [PubMed] [Google Scholar]
- Deroost N, Cserjési R, 2018. Attentional avoidance of emotional information in emotional eating. Psychiatry Research 269, 172–177. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, Smoski MJ, 2012. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord 136, 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R, 2004. Are stress eaters at risk for the metabolic syndrome? Annals of the New York Academy of Sciences 1032, 208–210. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K, 2001. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26, 37–49. [DOI] [PubMed] [Google Scholar]
- Evers C, Dingemans A, Junghans AF, Boeve A, 2018. Feeling bad or feeling good, does emotion affect your consumption of food? A meta-analysis of the experimental evidence. Neurosci Biobehav Rev 92, 195–208. [DOI] [PubMed] [Google Scholar]
- Faith MS, Allison DB, Geliebter A, 1997. Emotional eating and obesity: theoretical considerations and practical recommendations. [Google Scholar]
- Foreyt JP, Poston WSC II, Goodrick GK, 1996. Future directions in obesity and eating disorders. Addictive Behaviors 21, 767–778. [DOI] [PubMed] [Google Scholar]
- Frayn M, Livshits S, Knäuper B, 2018. Emotional eating and weight regulation: a qualitative study of compensatory behaviors and concerns. Journal of eating disorders 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter A, Aversa A, 2003. Emotional eating in overweight, normal weight, and underweight individuals. Eating behaviors 3, 341–347. [DOI] [PubMed] [Google Scholar]
- George SA, Khan S, Briggs H, Abelson JL, 2010. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 35, 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Phillips AC, Higgs S, Heaney JL, Carroll D, 2012. Disordered eating behaviour is associated with blunted cortisol and cardiovascular reactions to acute psychological stress. Psychoneuroendocrinology 37, 715–724. [DOI] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Hung J, Yahav E, 2004. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosomatic Medicine 66, 876–881. [DOI] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL, 2020. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. [PubMed] [Google Scholar]
- Harris JA, Benedict FG, 1919. A biometric study of basal metabolism in man. Carnegie institution of Washington. [Google Scholar]
- Heatherton TF, Baumeister RF, 1991. Binge eating as escape from self-awareness. Psychological bulletin 110, 86. [DOI] [PubMed] [Google Scholar]
- Herhaus B, Ullmann E, Chrousos G, Petrowski K, 2020. High/low cortisol reactivity and food intake in people with obesity and healthy weight. Translational psychiatry 10, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Het S, Vocks S, Wolf JM, Hammelstein P, Herpertz S, Wolf OT, 2015. Blunted neuroendocrine stress reactivity in young women with eating disorders. J Psychosom Res 78, 260–267. [DOI] [PubMed] [Google Scholar]
- Het S, Vocks S, Wolf JM, Herpertz S, Wolf OT, 2020. Treatment-Resistant Blunted HPA Activity, but Reversible Cardiovascular Stress Reactivity in Young Women With Eating Disorders. Front Psychiatry 11, 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC, 2007. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological psychiatry 61, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimerson DC, Lesem MD, Kaye WH, Brewerton TD, 1992. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Archives of General Psychiatry 49, 132–138. [DOI] [PubMed] [Google Scholar]
- Kaplan HI, Kaplan HS, 1957. The psychosomatic concept of obesity. Journal of Nervous and Mental Disease. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Baldassaro A, Rashid S, 2019. Physiological responses to acute stress and the drive to eat: The impact of perceived life stress. Appetite 133, 393–399. [DOI] [PubMed] [Google Scholar]
- Koenders PG, van Strien T, 2011. Emotional Eating, Rather Than Lifestyle Behavior, Drives Weight Gain in a Prospective Study in 1562 Employees. Journal of Occupational and Environmental Medicine 53, 1287–1293. [DOI] [PubMed] [Google Scholar]
- Koo-Loeb JH, Pedersen C, Girdler SS, 1998. Blunted cardiovascular and catecholamine stress reactivity in women with bulimia nervosa. Psychiatry Research 80, 13–27. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S, 2009. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34, 2–18. [DOI] [PubMed] [Google Scholar]
- Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer F, Greve D, Pizzagalli DA, 2014. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience 266, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxton NJ, Tipman RJ, 2017. Reward sensitivity and food addiction in women. Appetite 115, 28–35. [DOI] [PubMed] [Google Scholar]
- Makris, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ, 2006. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia research 83, 155–171. [DOI] [PubMed] [Google Scholar]
- Makris, Rathi Y, Mouradian P, Bonmassar G, Papadimitriou G, Ing WI, Yeterian EH, Kubicki M, Eskandar EN, Wald LL, 2016. Variability and anatomical specificity of the orbitofrontothalamic fibers of passage in the ventral capsule/ventral striatum (VC/VS): precision care for patient-specific tractography-guided targeting of deep brain stimulation (DBS) in obsessive compulsive disorder (OCD). Brain imaging and behavior 10, 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, Swaab DF, van der Kouwe A, Abbs B, Boriel D, Handa RJ, Tobet S, Goldstein JM, 2013. Volumetric parcellation methodology of the human hypothalamus in neuroimaging: normative data and sex differences. NeuroImage 69, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mårin P, Darin N, Amemiya T, Andersson B, Jern S, Björntorp P, 1992. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism 41, 882–886. [DOI] [PubMed] [Google Scholar]
- Masheb RM, Grilo CM, 2006. Emotional overeating and its associations with eating disorder psychopathology among overweight patients with Binge eating disorder. International Journal of Eating Disorders 39, 141–146. [DOI] [PubMed] [Google Scholar]
- Miller R, Plessow F, Kirschbaum C, Stalder T, 2013. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom Med 75, 832–840. [DOI] [PubMed] [Google Scholar]
- Monteleone AM, Cascino G, Ruzzi V, Pellegrino F, Carfagno M, Raia M, Del Giorno C, Monteleone P, Maj M, 2020a. Multiple levels assessment of the RDoC "system for social process" in Eating Disorders: Biological, emotional and cognitive responses to the Trier Social Stress Test. J Psychiatr Res 130, 160–166. [DOI] [PubMed] [Google Scholar]
- Monteleone AM, Ruzzi V, Patriciello G, Cascino G, Pellegrino F, Vece A, Monteleone P, Maj M, 2020b. Emotional reactivity and eating disorder related attitudes in response to the trier social stress test: An experimental study in people with anorexia nervosa and with bulimia nervosa. J Affect Disord 274, 23–30. [DOI] [PubMed] [Google Scholar]
- Monteleone AM, Treasure J, Kan C, Cardi V, 2018. Reactivity to interpersonal stress in patients with eating disorders: A systematic review and meta-analysis of studies using an experimental paradigm. Neurosci Biobehav Rev 87, 133–150. [DOI] [PubMed] [Google Scholar]
- Newman E, O’Connor DB, Conner M, 2007. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology 32, 125–132. [DOI] [PubMed] [Google Scholar]
- Paffenbarger R, Blair S, Lee I-M, Hyde R, 1993. Measurement of physical activity to assess health effects in free-living populations. Medicine & Science in Sports & Exercise 25, 60–70. [DOI] [PubMed] [Google Scholar]
- Pinaquy S, Chabrol H, Simon C, Louvet JP, Barbe P, 2003. Emotional eating, alexithymia, and binge-eating disorder in obese women. Obesity research 11, 195–201. [DOI] [PubMed] [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H, 2010. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: influence of anger and shame. Hormones and Behavior 58, 677–684. [DOI] [PubMed] [Google Scholar]
- Reichenberger J, Kuppens P, Liedlgruber M, Wilhelm FH, Tiefengrabner M, Ginzinger S, Blechert J, 2018. No haste, more taste: An EMA study of the effects of stress, negative and positive emotions on eating behavior. Biological Psychology 131, 54–62. [DOI] [PubMed] [Google Scholar]
- Reichenberger J, Pannicke B, Arend A-K, Petrowski K, Blechert J, 2020. Does stress eat away at you or make you eat? EMA measures of stress predict day to day food craving and perceived food intake as a function of trait stress-eating. Psychology & Health, 1–19. [DOI] [PubMed] [Google Scholar]
- Ricca V, Castellini G, Fioravanti G, Sauro CL, Rotella F, Ravaldi C, Lazzeretti L, Faravelli C, 2012. Emotional eating in anorexia nervosa and bulimia nervosa. Comprehensive psychiatry 53, 245–251. [DOI] [PubMed] [Google Scholar]
- Rosenberg N, Bloch M, Ben Avi I, Rouach V, Schreiber S, Stern N, Greenman Y, 2013. Cortisol response and desire to binge following psychological stress: comparison between obese subjects with and without binge eating disorder. Psychiatry Res 208, 156–161. [DOI] [PubMed] [Google Scholar]
- Schultz W, 2016. Reward functions of the basal ganglia. Journal of neural transmission 123, 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Skunde M, Hamze Sinno M, Brockmeyer T, Herpertz SC, Bendszus M, Herzog W, Friederich H-C, 2014. Impaired Cross-Talk between Mesolimbic Food Reward Processing and Metabolic Signaling Predicts Body Mass Index. Frontiers in Behavioral Neuroscience 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Cornelisse S, Quaedflieg CW, Meyer T, Jelicic M, Merckelbach H, 2012. Introducing the Maastricht Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology 37, 1998–2008. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Gibbon ME, Skodol AE, Williams JB, First MB, 2002. DSM-IV-TR casebook: A learning companion to the diagnostic and statistical manual of mental disorders, text rev. American Psychiatric Publishing, Inc. [Google Scholar]
- Spoor ST, Bekker MH, Van Strien T, van Heck GL, 2007. Relations between negative affect, coping, and emotional eating. Appetite 48, 368–376. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM, 2008. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of abnormal psychology 117, 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl B, Kuehner C, Kirsch P, Ruttorf M, Flor H, Diener C, 2015. Neural reward processing in individuals remitted from major depression. Psychol Med 45, 3549–3558. [DOI] [PubMed] [Google Scholar]
- Valenti O, Gill KM, Grace AA, 2012. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. European journal of neuroscience 35, 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bloemendaal L, Veltman DJ, ten Kulve JS, Drent ML, Barkhof F, Diamant M, IJzerman RG, 2015. Emotional eating is associated with increased brain responses to food-cues and reduced sensitivity to GLP-1 receptor activation. Obesity 23, 2075–2082. [DOI] [PubMed] [Google Scholar]
- van Strien T, Cebolla A, Etchemendy E, Gutierrez-Maldonado J, Ferrer-Garcia M, Botella C, Banos R, 2013a. Emotional eating and food intake after sadness and joy. Appetite 66, 20–25. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JE, Bergers GP, Defares PB, 1986. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International journal of eating disorders 5, 295–315. [Google Scholar]
- van Strien T, Herman CP, Anschutz DJ, Engels RC, de Weerth C, 2012. Moderation of distress-induced eating by emotional eating scores. Appetite 58, 277–284. [DOI] [PubMed] [Google Scholar]
- van Strien T, Roelofs K, de Weerth C, 2013b. Cortisol reactivity and distress-induced emotional eating. Psychoneuroendocrinology 38, 677–684. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Fowler JS, 2002. The role of dopamine in motivation for food in humans: implications for obesity. Expert opinion on therapeutic targets 6, 601–609. [DOI] [PubMed] [Google Scholar]
- Weinstein SE, Shide DJ, Rolls BJ, 1997. Changes in Food Intake in Response to Stress in Men and Women: Psychological Factors. Appetite 28, 7–18. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, 2009. Region of interest extraction (REX) toolbox. Boston, MA: 497. [Google Scholar]
- Whybrow S, Stephen J, Stubbs R, 2006. The evaluation of an electronic visual analogue scale system for appetite and mood. European journal of clinical nutrition 60, 558–560. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Lavender JM, Hays CC, 2018. The potential of calibrated fMRI in the understanding of stress in eating disorders. Neurobiol Stress 9, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD, Bright AC, Dapelo MM, Psychol MC, 2012. Emotion and eating disorder symptoms in patients with anorexia nervosa: an experimental study. Int J Eat Disord 45, 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich JA, Breithaupt L, Thompson JC, Crosby RD, Engel SG, Fischer S, 2018. The impact of neural responses to food cues following stress on trajectories of negative and positive affect and binge eating in daily life. Journal of psychiatric research 102, 14–22. [DOI] [PubMed] [Google Scholar]
- Wood SM, Schembre SM, He Q, Engelmann JM, Ames SL, Bechara A, 2016. Emotional eating and routine restraint scores are associated with activity in brain regions involved in urge and self-control. Physiology & behavior 165, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A, 2006. Food selection changes under stress. Physiology & behavior 87, 789–793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.