Abstract
Background:
Our previous studies demonstrated the presence of interleukin (IL-5) receptor alpha chain (IL-5Rα, CD125) on neutrophils in a murine model of influenza and in the lung fluid of children with severe asthma.
Objective:
The current studies were performed to further examine the functional characteristics and the effects of clinical factors and inflammatory variables on neutrophil surface IL-5Rα abundance in lung fluid and blood.
Methods:
IL-5Rα expression was quantified by flow cytometry performed on purified neutrophils from blood and bronchoalveolar lavage fluid samples obtained from healthy controls and asthmatics. Expression was further confirmed by immunohistochemistry. Functional signaling through the IL-5Rα was evaluated by measurement of IL-5-inducible modulation of neutrophil surface CD62L and IL5Rα expression.
Results:
IL-5Rα were consistently present but at variable magnitude on blood and lung neutrophils. Expression on lung neutrophils was significantly higher than on blood cells where their expression was higher in the presence of airway pathogens, especially with respiratory viruses. Increased receptor expression occurred in response to the translocation of preformed receptors from intracellular stores. Receptors were functional as demonstrated by IL-5-mediated down-regulation of CD62L and the feed-forward upregulation of reception expression.
Conclusion:
In addition to expression on eosinophils and basophils, the IL-5Rα is consistently and abundantly expressed on the surface of blood and especially airspace neutrophils. These observations support the concept that some of the efficacy of IL5/IL-5R-targeting biologics observed in asthma may reflect their ability to target neutrophilic airspace inflammation.
Keywords: interleukin 5, CD125, neutrophil, asthma
Introduction:
IL-5 is a canonical type 2 inflammatory cytokine whose receptor, IL-5R, is a heterodimer consisting of an IL-5-binding protein (IL-5Rα or CD125) and a ß-chain (CD131) that is shared with IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF). IL-5R expression is most widely recognized on eosinophils, along with expression on B cells and basophils 1, 2. In recent experiments we unexpectedly found that >98% of the lung neutrophils in mice infected with influenza A express CD125 3, 4. The finding that neutrophils can express CD125 had previously been noted on neutrophils in the lungs of dust mite sensitized mice 5, in the lungs of horses with heaves (an asthma-like condition)6 and in the lungs of mice after severe hemorrhagic shock and tissue trauma 7, as well as on human neutrophils in the setting of mycobacterial infection and sepsis 8, 9 but was not previously reported in the setting of human lung disease. This prompted us to examine CD125 expression on neutrophils and we demonstrated its presence on neutrophils isolated from blood and bronchoalveolar lavage (BAL) in a sample of children with asthma 10.
We performed the current investigations to further explore the pattern of CD125 expression on blood and lung airspace neutrophils in subjects with asthma. Studies were performed to assess inflammatory and cellular mechanisms which regulate surface CD125 expression on neutrophils. We explored whether the expression of CD125 on lung neutrophils was related to the airspace cytokine/chemokine milieu, the presence of pathogens, and the magnitude of allergen-sensitization. We also performed mechanistic experiments to test whether these receptors were functional and capable of transducing an activation signal. The findings presented here demonstrate that functional IL-5 receptors are expressed on the surface of blood and airspace neutrophils. This finding supports the novel clinical implication that targeting the IL-5/IL-5R pathway via treatment with biologics in patients with severe asthma may have some complementary anti-neutrophil actions in the airspace, in addition to the well-recognized antieosinophil effects that likely confer the majority of clinical benefit.
Methods
Subjects.
Neutrophil CD125 expression was examined in two cohorts. Children with severe asthma and inadequate symptom control despite guidelines-directed controller treatment underwent a clinically-indicated diagnostic bronchoscopy with BAL as previously reported 11, 12. Further demographic and clinical information are presented in eTable 1. Importantly, children with current viral respiratory infection, history of lower respiratory infection within 2 months, or recent exacerbations treated with systemic corticosteroids were excluded. In the adult studies, blood was obtained from healthy controls (n=23) and type 2 mucosal inflammatory disease subgroups including allergic rhinitis (AR, n=21), eosinophilic chronic rhinosinusitis (E-CRS, n=4), and asthma (n=9). AR subjects had relevant symptoms on exposures to aeroallergens and were skin prick test positive (≥3 mm) to at least one aeroallergen. E-CRS subjects had nasal polyposis on endoscopic exam with eosinophilia defined by presence of ≥10 eosinophils/400x high-powered field on tissue samples obtained at previous endoscopic sinus surgery 13. Adult asthmatic subjects had physician-diagnosed asthma and were not utilizing systemic corticosteroids or biologic therapies. Participants provided written informed consent and children provided oral assent under protocols approved by the University of Virginia Investigational Review Board (IRB#14457 (adults), IRB#19180 (children)).
Cell preparation.
For airspace samples, BAL fluid was centrifuged and cell pellets obtained. Expression of CD125 on airspace neutrophils was compared to blood neutrophils obtained simultaneously. Neutrophils were enriched (>98% pure) from peripheral blood via dextran sedimentation followed by positive selection using magnetic affinity beads (anti-CD16 antibody; Miltenyi, Auburn, CA) to remove contaminating eosinophils. Eosinophils (>95% pure) were obtained from the negative selection fraction.
CD125 expression.
Cells were blocked with mouse IgG (Lampire Biological, Pipersville, PA) and Fc block (BD Pharmingen; Sparks, MD), washed and stained with Live/Dead fixable violet (ThermoFisher Scientific, Waltham, MA), PerCPCy5.5 conjugated anti-CD16 or anti-CD66b (BD Bioscience, San Jose, CA), APC-conjugated anti-Siglec 8 (BioLegend, San Diego, CA) and PE-conjugated anti-CD125 (BD Bioscience) or mouse isotype control. To examine total (surface and intracellular) CD125 expression, neutrophils were permeabilized (Intracellular Fix&Perm kit; eBioscience, San Diego, CA). Flow cytometry was performed including live/dead viability gate (for BAL fluid), doublet exclusion gate, and isotype controls (eFigure1A-C). Neutrophils were identified as CD45+SSChighCD66b+ Siglec8− and eosinophils were identified as CD45+SSChighCD66b+Siglec8+. Fluorescence minus one (FMO) staining was used to set gates. Although some basophils can express low levels of Siglec8, these would be expected to be SSClow and so were excluded. Initially, staining with multiple clones of CD125 antibody was performed in a subset of samples, with most consistent results obtained with the BD Pharmingen clone that was then used for the remainder of experiments (eFigure 1D). Staining with both CD16 and CD66b antibodies to identify granulocytes was performed in a subset of samples, with similar CD125 staining patterns demonstrated (eFigure 1E).
Immunohistochemistry.
Enriched neutrophil and eosinophil fractions were cytospun and fixed in 2% paraformaldehyde. After blocking with 1% bovine serum albumin, 10% goat serum (Sigma Aldrich), and 1 μg/ml Fc Block, slides were labeled with anti-IL-5Ra primary antibody (5 μg/ml; R&D Systems; Minneapolis, MN) or isotype control for 16 hrs at 4°C and then secondary allophycocyanin (APC)-conjugated goat anti-mouse IgG (1:200, Life Technologies). Nuclei were stained with 100 ng DAPI (4’, 6-diamidino-2-phenylindole, Sigma Aldrich). Samples were washed and aqueous mounted with VectaMount AQ (Vector Laboratories; Burlington, CA).
IL-5 Receptor Activation.
Upregulation of surface CD125 was assessed after stimulating neutrophils for 30 min with either IL-5 (30 ng/mL), N-formyl methionine-leucyl-phenylalanine (fMLP; 10 μM) with cytochalasin b (1 μg/mL), or phorbol myristate acetate (PMA; 50 ng/mL) and ionomycin (1 μg/ml) (Sigma Aldrich, St. Louis, MO). Downregulation of surface CD62L expression after stimulation with IL-5 (10-100 ng/mL) for 15 min was assessed via flow cytometry using PE-conjugated anti-CD62L (BioLegend).
BAL granulocyte pattern, pathogen recovery and cytokine/chemokine levels.
BAL cell pellets were cytospun and underwent Wright staining. Granulocyte phenotypes were assigned as neutrophilic (≥6% neutrophils), eosinophilic (≥1% eosinophils), mixed granulocytic, or pauci-granulocytic as described 11. BAL absolute neutrophil and eosinophil counts were defined as the log10 total BAL cell count multiplied by the percentage neutrophils or eosinophils. BAL fluid was submitted to the Clinical Microbiology Laboratory for bacterial culture and for PCR for respiratory viral pathogens (Luminex xTAG reagents, LiquiChip). BAL samples from a subset of subjects were 5-fold concentrated and analyzed using a 26-plex chemokine/cytokine bead assay (Bio-Rad, Hercules, CA).
Quantitative PCR.
qPCR was performed on 200 ng of isolated total RNA to quantify relative expression of transcripts for transmembrane (TM) and secreted isoforms of CD125 within blood neutrophils 14. qPCR was performed using 200 μM each of a common forward primer annealing to exon 10 (5’-GCAGCAGTGAGCTCCATGTG-3’) and isoform-specific reverse primers (transmembrane: 5’-AGGGCTTGTGTTCATCATTTCC-3’; soluble: 5’-TGGATGTTATCTCCTTTATCTTGAGAA-3’), generating PCR products of 89 and 95 bp, respectively (Integrated DNA Technologies, Inc., Coralville, IA)14, 15. Data were analyzed as the difference in cycle threshold (CT) of each transcript compared to the housekeeping gene ß-actin (ΔCT).
Principal component analysis (PCA).
Principal component analysis was a precursor step to reduce the number of variables into factors which accounted for the greatest amount of variance in the dataset. Factors were selected based on Eigenvectors >1 with a correlation matrix, and Varimax rotation. We did the analysis with individual BAL cytokine values and with cytokines grouped into five pre-defined arrays, T2 (IL-4, IL-5, IL-9, IL-13, CCL5, CCL11), T1/T17 (interferon-γ, IL-17, IL-6, IL-12), macrophage (tumor necrosis factor-α, IL-12, CCL2 (MCP)-1, CCL3 (MIP-1α), CCL4 (MIP-1β), IL-1β, IL-6), neutrophil (CXCL8, CXCL10, G-CSF), and anti-inflammatory (IL-1 receptor antagonist, IL-10).
Statistical Analyses.
Scaled data that did not fit a Gaussian distribution were log10 transformed to allow un-paired and paired t-tests between the BAL and blood compartments. Absolute BAL neutrophil surface and total CD125 abundance was analyzed as the product of the total neutrophil count and the percent CD125 positive neutrophils. Dispersion of BAL and blood neutrophil surface CD125 abundance were shown with scatter and violin plots, wherein the width of the violin plot depicts the probability density of the data at different values, smoothed by a kernel density function. Absolute BAL neutrophil CD125 surface abundance was also compared in dichotomous clinical outcome variables pre-identified through PCA with factor loads ≥.50 or ≤−.50 with a t-test for variables with normal distributions and the Mann Whitney U test for variables with non-normal distributions. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS (Version 26, IBM Corp, Armonk, NY) and GraphPad Prism 8 (La Jolla, CA).
Results
BAL and blood neutrophils express CD125.
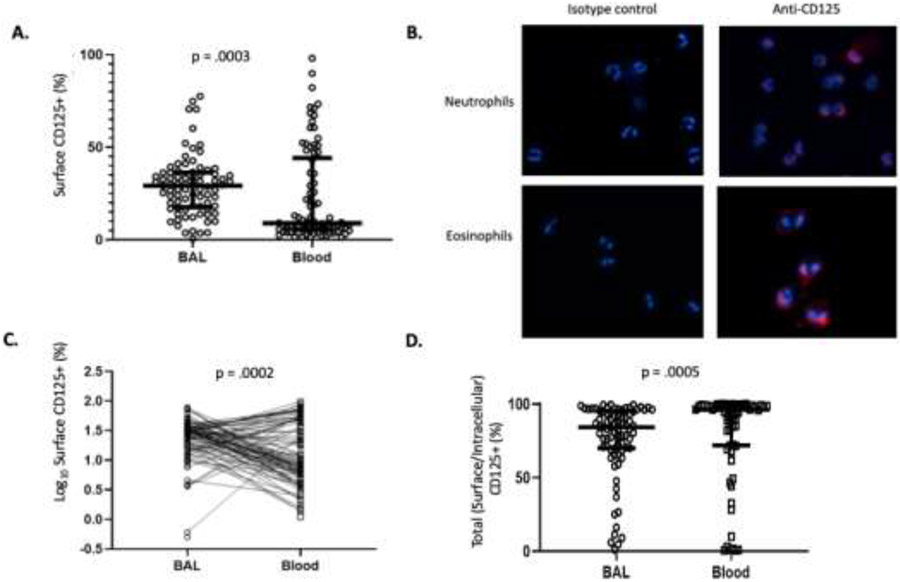
We first sought to confirm our previously published finding of CD125 expression on blood and lung neutrophils 10 in a larger cohort of asthmatic children. Eighty-two children (33 with problematic wheeze <5 years of age and 49 with severe asthma ages 5 to 22 years) underwent bronchoscopy and BAL with measurement of BAL and blood neutrophil CD125 expression by flow cytometry. Neutrophil CD125 surface abundance was calculated as the product of the total neutrophil count and the percent CD125 positive neutrophils and represents the number of CD125 positive neutrophils in the blood/BAL fluid compartments. These studies confirmed that CD125 is consistently expressed on both BAL and purified blood neutrophils (Figure 1A). To further demonstrate expression of CD125 on blood neutrophils and to visualize the pattern of expression, we performed confirmatory IHC on neutrophil samples and, as a positive control, on eosinophils on a subset of samples. A subset of neutrophils (identified by their characteristic polymorphic nuclei) displayed varying degrees of surface CD125 expression, while eosinophils (identified by their characteristic bilobed nuclei) consistently demonstrated diffuse surface CD125 expression (Figure 1B). Surface CD125 abundance was significantly higher in neutrophils purified from BAL than it was in neutrophils from blood (Figure 1A, C). When compared between paired samples, although CD125 expression could be higher and lower for individual cases, the paired t-test demonstrated significantly higher values in BAL samples (p=0.0002). We examined total cellular expression of CD125 in permeabilized BAL and blood neutrophils (comprising intracellular and surface compartments) and demonstrated expression by virtually all neutrophils (Figure 1D), indeed suggesting presence of an intracellular pool.
Figure 1. Comparison of blood and lung neutrophil CD125 expression.
A. Scatter plot of surface neutrophil CD125 abundance in the BAL and blood of children with severe asthma. B. Immunohistochemistry of non-permeabilized blood neutrophils (top) and eosinophils (bottom) examined for surface CD125 (right panels). Also shown are isotype controls (left panels). C. Scatter plot showing paired relationships between BAL and blood neutrophil surface CD125. D. Scatter plot of total (surface and intracellular) neutrophil CD125 abundance in the BAL and blood of children with severe asthma
Surface CD125 expression by circulating neutrophils in type 2 inflammatory states.
To determine whether expression of CD125 on circulating neutrophils was informed by the presence of allergic/type 2 inflammation, we enrolled adult subjects including healthy controls (n=21) and subjects with AR (n=23), eosinophilic CRS (E-CRS) (n=4), and asthma (n=9). These studies again confirmed that CD125 is consistently expressed on blood neutrophils and that this expression is variable, ranging from 0.73 to 90.0% positive (median 14.9%) (eFigure 2). The wide variability in expression extended to these other T2 diagnostic categories and no significant differences in expression were detected between control and AR groups; the sample size for the E-CRS and adult asthma groups was underpowered to allow for comparisons to be made.
Neutrophil CD125 surface abundance is related to clinical variables.
Absolute BAL neutrophil CD125 surface abundance was compared in relationship to clinical variables (Table 1). Neutrophil surface CD125 abundance was significantly greater in preschool compared to school-age and non-allergen sensitized compared to allergen sensitized children. Prevalent granulocyte patterns in BAL included isolated eosinophilia (10%), isolated neutrophilia (26%), mixed granulocytic (12%), and pauci-granulocytic (52%). Children with BAL fluid neutrophilia and mixed granulocytes had significantly greater neutrophil surface CD125 abundance compared to children with isolated eosinophilia and pauci-granulocytic BAL (Figure 2A).
Table 1.
Log10 BAL neutrophil CD125 surface abundance compared in subjects with presence or absence of binary clinical variables
| Log10 BAL neutrophil CD125 Surface Abundance |
|||
|---|---|---|---|
| Clinical Variable | Clinical Variable Present |
Clinical Variable Absent |
P value |
| Preschool age | 2.62 ± 1.02 | 1.96 ± .88 | .007 |
| Male sex | 2.39 ± 1.06 | 2.13 ± .91 | ns |
| Minority race | 1.86 ± 1.00 | 2.46 ± .97 | ns |
| Obese or overweight | 2.14 ± 1.19 | 2.25 ± .92 | ns |
| Premature birth <35 weeks | 2.19 ± .93 | 2.32 ± 1.04 | ns |
| Airway malacia | 2.45 ± .80 | 2.25 ± 1.05 | ns |
| Laryngeal cleft | 2.33 ± .95 | 2.27 ± 1.03 | ns |
| High-dose ICS treatment | 2.37 ± 1.10 | 2.13 ± .81 | ns |
| Blood neutrophils >6000 cells/μl | 2.43 ± 1.22 | 2.04 ± .95 | ns |
| Blood eosinophils >300 cells/μl | 2.31 ± 1.10 | 2.23 ± .95 | ns |
| Serum IgE >130 IU/mL | 2.11 ± 1.13 | 2.40 ± .91 | ns |
| Any positive allergen sIgE test | 2.27 ± 1.08 | 2.24 ± .89 | ns |
| >3 positive allergen sIgE tests | 2.08 ± 1.17 | 2.33 ± .90 | ns |
| Positive food allergen sIgE | 1.92 ± .94 | 2.32 ± .99 | ns |
| Positive inhalant allergen sIgE | 2.34 ± 1.07 | 2.12 ± .93 | ns |
| Positive foods and inhalant allergen sIgE | 1.77 ± 1.00 | 2.39 ± .94 | .03 |
| hs-CRP > 0.25 mg/L | 2.35 ± 1.07 | 2.17 ± .87 | ns |
| FEV1 < 80% predicted | 2.13 ± .95 | 2.14 ± 1.07 | ns |
| FEV1/FVC <0.90 | 1.98 ± .88 | 2.24 ± 1.13 | ns |
| BAL eosinophilia (≥ 1% eos) | 2.47 ± 1.19 | 2.23 ± .94 | ns |
| BAL neutrophilia (≥ 6% neutrophils) | 2.95 ± .73 | 1.68 ± .82 | .0001 |
| Positive BAL RV PCR | 2.93 ± .81 | 2.02 ± .96 | .0007 |
| Positive non-enterovirus PCR | 3.60 ± .37 | 2.18 ± .96 | .002 |
| Positive bacterial pathogen culture | 2.64 ± .94 | 2.20 ± 1.01 | ns |
| Positive virus PCR and bacterial culture | 3.27 ± .77 | 2.18 ± .97 | .01 |
| Ciliary motion present | 2.29 ± .99 | 2.37 ± 1.19 | ns |
| LLM index ≥1 | 2.50 ± .91 | 2.24 ± 1.02 | ns |
| BAL amylase >48 (50th percentile) | 2.23 ± .98 | 1.82 ±1.04 | ns |
Abbreviations: hs-CRP, high sensitivity C-reactive protein; ICS, inhaled corticosteroid; IgE, immunoglobulin E; sIgE, allergen specific IgE; IU, international unit; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BAL, bronchoalveolar lavage; PCR, polymerase chain reaction; RV, rhinovirus; LLM, lipid-laden macrophage
Figure 2. Lung neutrophil CD125 expression.
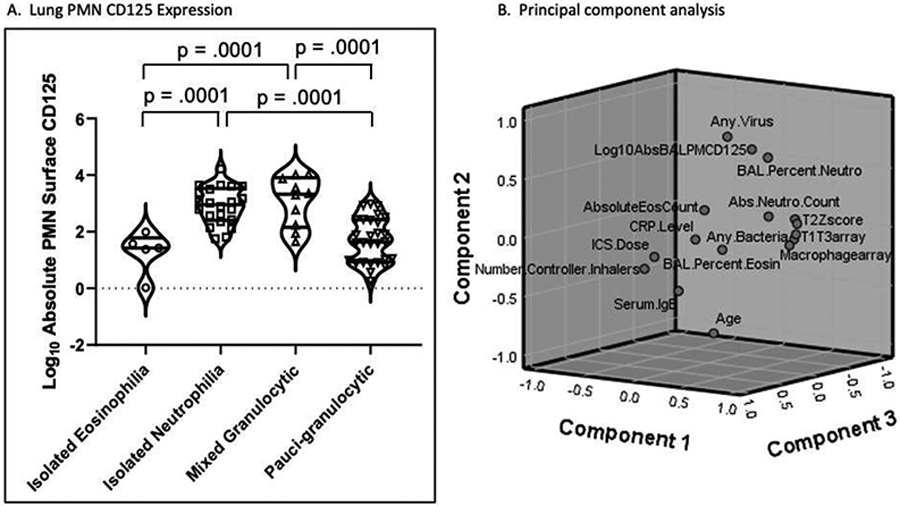
A. Violin plots showing the log10 BAL neutrophil (PMN) surface CD125 abundance compared according to four categorical BAL granulocyte patterns. B. Component plot in three dimensions utilizing rotated factor loads based on the correlation of clinical outcome variables.
PCA identified six factors which accounted for 77% of the total variance in the dataset (eTable 2). Log10 BAL absolute neutrophil surface CD125 abundance loaded highest (.67) in factor 2 and correlated positively with recovery of BAL viral transcripts (.85) and BAL neutrophil % (.62) but inversely with age (−.79). No association was observed with either any individual cytokine or with any inflammatory (T1/T17, T2, macrophage, neutrophil, or anti-inflammatory) signature. A three-dimensional rotated component plot (Figure 2B) shows that BAL neutrophil surface CD125 abundance is closely correlated with detection of any virus and BAL neutrophil %, but is distant from BAL eosinophil % and advancing age.
BAL neutrophil CD125 surface abundance was compared in the presence or absence of clinical outcomes selected according to high factor loads with PCA (Figure 3). We found that log10 neutrophil surface CD125 abundance was significantly higher in children with any BAL viral transcript (p=.0001) including BAL rhinovirus (RV) transcripts (p=.0007) but varied inversely with advancing age (p=.004) by linear regression. This was driven in large part by higher prevalence of virus in the younger children. We found no difference in CD125 abundance in children with and without BAL eosinophilia.
Figure 3. Influences on lung neutrophil CD125 expression.
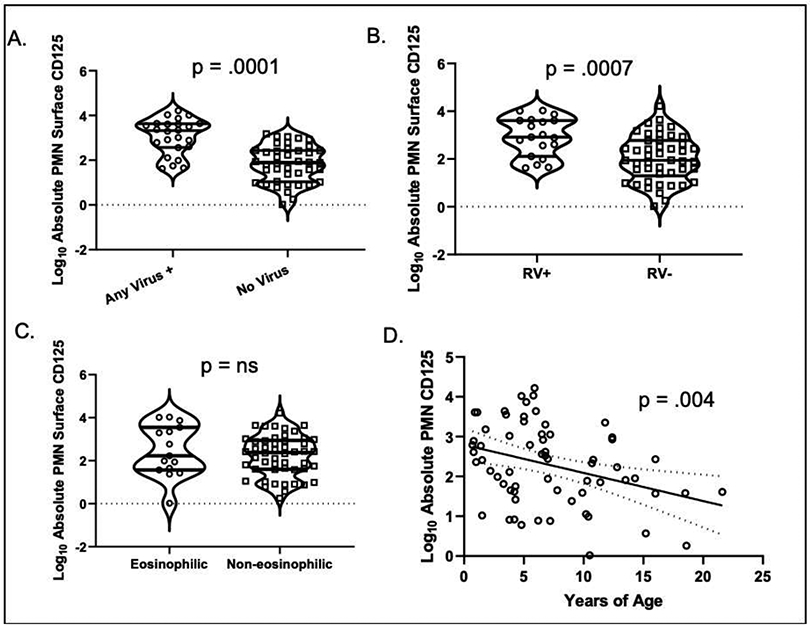
Violin plot of BAL neutrophil CD125 surface abundance in children A. with/without viral transcripts in BAL; B. with/without rhinovirus transcripts in BAL; C. with/without BAL eosinophilia. D. Scatter plot with advancing age and BAL neutrophil CD125 abundance.
CD125 surface expression is not transcriptionally regulated.
We next explored molecular mechanisms that could underlie the highly variable surface expression demonstrated on neutrophils. We initially posited that expression of surface CD125 could reflect the post-transcriptional modification of the mRNA from a secreted to a transmembrane form of the receptor. We performed qPCR of purified blood neutrophils, utilizing a common forward primer for CD125 and reverse primers specific for the secretory and transmembrane isoforms 14. To our surprise, transcripts expressing the transmembrane domain were only observed in 3/6 adult subjects (eTable 3). In contrast, secretory CD125 transcripts were observed in all 6 subjects and with higher levels of expression. This argues against the variability in surface receptor expression being transcriptionally regulated. An alternative mechanism for the highly variable expression of CD125 on blood neutrophils would involve the transit of CD125 from a pool of transmembrane proteins expressed intracellularly (such as within a secretory granule) that is rapidly exported to the surface with activation.
Functional consequences of IL-5 receptor engagement.
To test the hypothesis that CD125 could be exported to the surface with activation, neutrophils from adult subjects were stimulated for 15 min with IL-5 (30 ng/mL) or as positive controls, fMLP/cytochalasin b or PMA/ionomycin. These studies demonstrated the ability of neutrophil activation to rapidly increase surface CD125 expression (Figure 4A). To further establish functional consequences of IL-5 binding, we investigated the rapid shedding of L-selectin (CD62L) that occurs by proteolytic cleavage after neutrophil activation 16. These studies demonstrated that CD62L is significantly downregulated following stimulation with 100 ng/mL of IL-5 (p<0.05) (Figure 4B).
Figure 4. Activation of neutrophils.
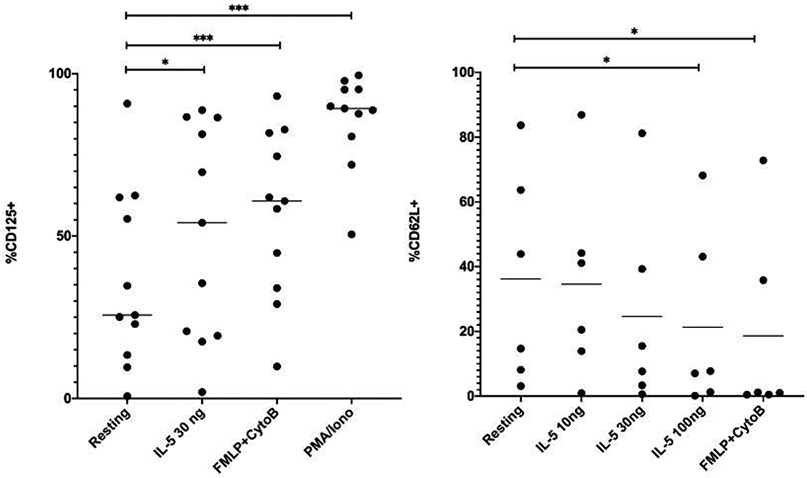
A. Neutrophils were stimulated with IL-5, fMLP/cytochalasin b, or PMA/ionomycin for 15 min and surface expression of CD125 quantified by flow cytometry. B. Neutrophils were stimulated with IL-5 or fMLP/cytochalasin b and surface expression of CD62L quantified via flow cytometry. *p<0.05, ***p<0.01.
Discussion
The expression of interleukin-5 receptors is conventionally considered to be exclusively limited to eosinophils, some basophils, and B1 B lymphocytes 17. However, our experiments with influenza A-infected mice led to the observation that >98% of lung neutrophils expressed these receptors 3, 4. Although unexpected, this tendency of neutrophils to express CD125 had previously been described in animals with allergic inflammation 5, 6, as well as on human neutrophils in the setting of acute infection or trauma 8, 9. That this finding has been noted by multiple groups using diverse animal and human models, and that the finding was replicated in our laboratory using multiple clones of monoclonal antibodies as well as by a variety of techniques (flow cytometry, immunohistochemistry, qPCR) argues strongly against this being artifactual or related to laboratory technique. We were therefore eager to further explore this ability of neutrophils to express CD125, define factors influencing its expression, and, most importantly, determine if these receptors are functional. The current studies unambiguously established the expression of IL-5Rα (CD125) on neutrophils as demonstrated by both flow cytometry and IHC (Figure 1). While we were concerned that our data could have been confounded by the presence of contaminating eosinophils or basophils, the fact these granulocytes were exclusively neutrophils was established by the combination of their high SSC, expression of CD16 or CD66b and absence of Siglec 8 expression as well as by their characteristic polymorphonuclear staining patterns on IHC.
These data demonstrated wide variability of CD125 expression which led us to investigate mechanisms that could influence their expression. We addressed whether this could reflect the presence of a type 2high inflammatory state. This concept was driven by studies that demonstrated the tendency of neutrophils derived from allergic and asthmatic subjects to express other features more associated with eosinophils, including receptors for cysteinyl leukotrienes, IL-9, and VLA-4 6, 18-20. However, no differences were observed when we enrolled adult healthy control and subjects with AR, along with smaller cohorts of subjects with eosinophilic-CRS and asthma (eFigure 2). In our previous study of asthmatic children 10, we identified higher levels of CD125 in lung as compared to blood neutrophils, a finding which was confirmed in the larger cohort of treatment-refractory asthmatic children recruited for this study (Figure 1). We addressed whether there were distinctive inflammatory or cellular features to which we could ascribe the observed highly variable receptor expression. Using PCA, we found that absolute BAL neutrophil surface CD125 abundance is closely correlated to the presence of respiratory viral transcripts and varies inversely with advancing age (Figure 2 and 3). We therefore reasoned that CD125 expression corresponds to a non-specific response of the neutrophil to any inflammatory process driving their recruitment into the lung (such as the presence of a bacterial or viral pathogen in the airspace)21. In support of this concept, high CD125 was consistently a feature of neutrophil-associated severe asthma but was not a feature of the rare neutrophils present in the lungs of children with either eosinophilic or pauci-granulocytic asthma (Figure 2).
We reasoned that upregulated surface CD125 could result either from de novo synthesis of mRNA transcripts having a transmembrane domain or the translocation of previously formed receptors from an intracellular locus. Paradoxically, our qPCR data demonstrated that a secretory transcript was the predominant isoform present in neutrophils and that in many individuals the transmembrane form was not being actively transcribed (eTable 3). This would argue that the transmembrane receptor was primarily synthesized during hematopoiesis and that mature protein was constitutively present in an intracellular locus, such as the membrane of the secretory granule, and available for upregulation with activation. Consistent with this reasoning, we quantified expression of CD125 in permeabilized neutrophils (reflecting intracellular and surface expression) and demonstrated CD125 expression in close to 100% of neutrophils (Figure 1D). The fact these intracellular pools contained transmembrane receptors was demonstrated by their rapid translocation to the cell surface after stimulation (Figure 4A). Although we cannot categorically exclude receptor stabilization as a mechanism for IL-5-mediated IL-5Rα upregulation, the time frame for this effect (15 min) is more consistent with receptor translocation.
Previous work has demonstrated the ability of IL-5Rα engagement to lead to suppression of neutrophil respiratory burst and STAT5 phosphorylation on differentiated human neutrophil-like cells 10. To further investigate the functional capacity of these receptors, we investigated the rapid down-regulation of surface CD62L expression on neutrophils in response to IL-5 as a highly sensitive indicator of activation (Figure 4B). In addition, expression of functional receptors was demonstrated as the rapid upregulation of IL-5 receptors, themselves (Figure 4A).
The ability to target interleukin (IL)-5 with anti-IL-5 or anti-IL-5 receptor (IL-5R) monoclonal antibodies has led to the recent development of multiple novel treatments for severe asthma 22-24. The indication for treating asthma with these biologics requires increased blood eosinophil number, based upon the rationale that they will target eosinophils in the lung and this removal of eosinophils is sufficient to reduce the frequency of asthma exacerbations and improve lung function 25, 26. However, it is apparent from published studies in adults as well as in our studies in children 11 that lung granulocyte patterns in severe asthma are heterogeneous and that neutrophils alone or in combination with eosinophils are often found in the airspaces 27-30. Furthermore, the correlation between blood and lung lavage eosinophilia is often remarkably poor, and patients selected for treatment on the basis of blood eosinophilia may not in fact display prominent airway eosinophilia 31. These observations suggest that the beneficial effect of IL-5/IL-5-targeting biologics in patients with eosinophilic asthma (as typically defined by a blood eosinophil count of >300 cells/ μl) may not be explained by their effects on lung eosinophils alone and that they may also ameliorate neutrophilic inflammation, at least in part. This concept is supported by pooled analyses that suggest some benefit in asthmatic patients with a lower eosinophil count, particularly those with comorbid type 2 inflammatory conditions32.
The ability of an IL-5-targeting biologic to mitigate neutrophilic inflammation would be contingent on the receptor having functional capability. However, it is important to point out that an anti-IL-5R biologic could eliminate CD125-expressing neutrophils via antibody-dependent cellular cytotoxicity, even if it were a “decoy” (non-functional) surface receptor. It also remains possible that neutrophils are actively transcribing (and secreting) a soluble receptor which could serve as an anti-inflammatory “sink” by binding free IL-5. Although the safety profile of IL-5/IL-5R biologics is now reasonably well-established after several years of use in both research and clinical settings, it is noteworthy that treatment with an anti-IL-5 receptor antibody was associated with a slight decrease in blood neutrophil number, consistent with the expression of these receptors on a modest percent of blood neutrophils 33. It is possible that a greater decrease in neutrophils would be noted in the lungs, given the higher expression of these receptors in the BAL in our studies, or during periods of acute infection/inflammation, given upregulation of CD125 expression in response to neutrophil activation 9, 10.
In summary, these studies demonstrate that the expression of IL-5 receptors on granulocytes is not limited to eosinophils and that these receptors are widely expressed on blood and airway neutrophils. Receptor expression is widely variable and reflects the universal presence of these receptors in an intracellular milieu where it is available to rapidly transit to the surface with activation. These are functional receptors in response to IL-5 binding. Together, these observations raise the possibility that IL-5/IL-5R-targeting biologics may have the ability to target neutrophilic inflammation, in addition to their already well-recognized effects on eosinophilic inflammation11, 27-30.
Supplementary Material
Funding Sources:
Supported by NIH UO1 AI123337, R21 AI151496, UG1 HL139126, and UO1 AI100799, the National Natural Science Foundation of China (81700890); Shandong Provincial Key Research and Development Program, China (2019GSF108227); the American Lung Association/American Academy of Asthma, Allergy and Immunology Allergic Respiratory Diseases Award (ML); University of Virginia Ivy Foundation; and by an investigator-initiated medical school grant from Astra Zeneca.
Abbreviations:
- APC
allophycocyanin
- AR
allergic rhinitis
- BAL
bronchoalveolar lavage
- CRS
chronic rhinosinusitis
- CT
cycle threshold
- DAPI
4’, 6-diamidino-2-phenylindole
- E-CRS
eosinophilic CRS
- FEV1
forced expiratory volume in 1 second
- fMLP
formyl methionine leucyl phenylalanine
- FVC
forced vital capacity
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- ICS
inhaled corticosteroid
- IHC
immunohistochemistry
- IL
interleukin
- LLM
lipid-laden macrophage
- PCA
principal component analysis
- PMA
phorbol myristate acetate
- qPCR
quantitative polymerase chain reaction
- R
receptor
- RV
rhinovirus
- STAT
signal transducer and activator of transcription
Footnotes
Conflict of Interest: Dr. Larry Borish was the recipient of an investigator-initiated medical school grant from Astra Zeneca which funded parts of these studies. All funds were transmitted to the University of Virginia. None of the other authors have relevant COIs to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotsimbos AT, Hamid Q. IL-5 and IL-5 receptor in asthma. Mem Inst Oswaldo Cruz. 1997;92 Suppl 2:75–91. [DOI] [PubMed] [Google Scholar]
- 2.Wright AKA, Weston C, Rana BMJ, Brightling CE, Cousins DJ. Human group 2 innate lymphoid cells do not express the IL-5 receptor. J Allergy Clin Immunol. 2017;140:1430–1433 e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorski SA, Hahn YS, Braciale TJ. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS Pathog. 2013;9:e1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorski SA, Braciale TJ. IL-5 produced by natural helper cells suppresses neutrophil function during influenza infection. (P4239). J Immunol. 2013;190:19. [Google Scholar]
- 5.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewachi O, Joubert P, Hamid Q, Lavoie JP. Expression of interleukin (IL)-5 and IL-9 receptors on neutrophils of horses with heaves. Vet Immunol Immunopathol. 2006;109:31–36. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Guardado J, Hoffman R, et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14:e1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. [DOI] [PubMed] [Google Scholar]
- 9.Linch SN, Danielson ET, Kelly AM, Tamakawa RA, Lee JJ, Gold JA. Interleukin 5 is protective during sepsis in an eosinophil-independent manner. Am J Respir Crit Care Med. 2012;186:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorski SA, Lawrence MG, Hinkelman A, et al. Expression of IL-5 receptor alpha by murine and human lung neutrophils. PLoS One. 2019;14:e0221113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teague WG, Lawrence MG, Shirley DT, et al. Lung Lavage Granulocyte Patterns and Clinical Phenotypes in Children with Severe, Therapy-Resistant Asthma. J Allergy Clin Immunol Pract. 2019;7:1803–1812 e1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinke JW, Lawrence MG, Teague WG, Braciale TJ, Patrie JT, Borish L. Bronchoalveolar lavage cytokine patterns in children with severe neutrophilic and paucigranulocytic asthma. J Allergy Clin Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne SC, Borish L, Steinke JW. Genetics and phenotyping in chronic sinusitis. J Allergy Clin Immunol. 2011;128:710–720; quiz 721-712. [DOI] [PubMed] [Google Scholar]
- 14.Wilson TM, Maric I, Shukla J, et al. IL-5 receptor alpha levels in patients with marked eosinophilia or mastocytosis. J Allergy Clin Immunol. 2011;128:1086–1092 e1081-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez C, Vandesompele J, Vandenbroucke I, et al. Quantitative real time polymerase chain reaction for measurement of human interleukin-5 receptor alpha spliced isoforms mRNA. BMC Biotechnol. 2003;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann BS, Chung KF. Blood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapy. Respir Res. 2006;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geijsen N, Koenderman L, Coffer PJ. Specificity in cytokine signal transduction: lessons learned from the IL-3/IL-5/GM-CSF receptor family. Cytokine Growth Factor Rev. 2001;12:19–25. [DOI] [PubMed] [Google Scholar]
- 18.Abdelilah S, Latifa K, Esra N, et al. Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J Immunol. 2001;166:2768–2774. [DOI] [PubMed] [Google Scholar]
- 19.Cheung DS, Ehlenbach SJ, Kitchens RT, et al. Cutting edge: CD49d+ neutrophils induce FcepsilonRI expression on lung dendritic cells in a mouse model of postviral asthma. J Immunol. 2010;185:4983–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polak D, Hafner C, Briza P, et al. A novel role for neutrophils in IgE-mediated allergy: Evidence for antigen presentation in late-phase reactions. J Allergy Clin Immunol. 2019;143:1143–1152 e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch v J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Asthma EP, Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. [DOI] [PubMed] [Google Scholar]
- 23.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. [DOI] [PubMed] [Google Scholar]
- 24.Guilbert TW, Bacharier LB, Fitzpatrick AM. Severe asthma in children. J Allergy Clin Immunol Pract. 2014;2:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romeo MJ, Agrawal R, Pomes A, Woodfolk JA. A molecular perspective on TH2-promoting cytokine receptors in patients with allergic disease. J Allergy Clin Immunol. 2014;133:952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–1096. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156:737–743. [DOI] [PubMed] [Google Scholar]
- 28.Hauk PJ, Krawiec M, Murphy J, et al. Neutrophilic airway inflammation and association with bacterial lipopolysaccharide in children with asthma and wheezing. Pediatr Pulmonol. 2008;43:916–923. [DOI] [PubMed] [Google Scholar]
- 29.Moore WC, Hastie AT, Li X, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–1563 e1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson CK, Adams A, Nagakumar P, et al. Intraepithelial neutrophils in pediatric severe asthma are associated with better lung function. J Allergy Clin Immunol. 2017;139:1819–1829 e1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy. 2013;68:402–406. [DOI] [PubMed] [Google Scholar]
- 32.Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–1244 e1232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.