STRUCTURED ABSTRACT
Background
The in vivo depletion of recipient and donor T-lymphocytes using anti-thymocyte globulin (ATG) is widely adopted in allogeneic hematopoietic stem cell transplantation (HCT) to reduce the incidence of both graft failure and graft versus host disease (GVHD). However excess toxicity to donor lymphocytes may hamper immune reconstitution, compromising anti-tumour effects and increasing infection. Granulocyte-colony stimulating factor (G-CSF) administered early after HCT may increase ATG-mediated lympho-toxicity.
Objective
Our study objective was to investigate the effect of an interaction between ATG and post-transplant G-CSF on allogeneic transplant outcomes, using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry.
Study Design
We studied patients aged ≥18 years with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) who received thymoglobulin-containing preparative regimens for HLA-matched sibling/unrelated or mismatched unrelated donor HCT from 2010-2018. The effect of planned G-CSF that was started between pre-transplant day 3 and post-transplant day 12 was studied in comparison to transplantations that did not include G-CSF. Cox regression models were built to identify risk factors associated with outcomes 1 year after transplantation.
Results
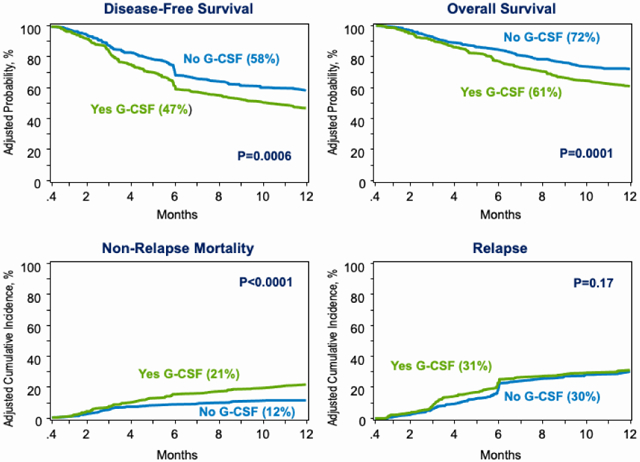
874 patients met study eligibility criteria; 459 (53%) received planned G-CSF. HCTs with planned G-CSF significantly increased risk for non-relapse mortality (HR 2·03, p<0·0001; 21% vs. 12%) compared to HCTs without G-CSF. The 6-month incidence of viral infections was higher with G-CSF (56% vs. 47%, p=0·007), with a particular increase in EBV infections (19% vs. 11%, p=0·002). The observed higher non-relapse mortality with planned G-CSF led to lower overall survival (HR 1·52, p=0·0005; 61% vs. 72%). There was no difference in GVHD risk between treatment groups. We include two subgroup analyses showing our findings held true (i) in patients aged ≥50 years and (ii) in centers where G-CSF was used in some but not all patients.
Conclusion
In allogeneic peripheral blood HCT performed with Thymoglobulin for AML and MDS, G-CSF administered early post-transplant results in a two-fold increase in non-relapse mortality and a 10% absolute decrement in survival. The use of planned G-CSF in the early post-transplant period should be carefully considered on an individual patient basis, weighing any perceived benefits against these risks.
Graphical Abstract
INTRODUCTION
The in vivo depletion of recipient and donor T-lymphocytes using anti-thymocyte globulin (ATG) is a widely adopted approach to reduce both graft rejection and graft-versus-host disease (GVHD) in allogeneic hematopoietic stem cell transplantation (HCT). Randomized control trials have convincingly shown that ATG decreases the incidence of GVHD and improves quality of life but none of the studies have demonstrated a survival advantage.1-5 Four recent meta-analyses evaluating the use of ATG in allogeneic HCT have also reported a significant reduction in both acute and chronic GVHD without a difference in overall survival or non-relapse mortality (NRM)6-9 and an international expert consensus now recommends the inclusion of either thymoglobulin or anti-T lymphocyte globulin with myeloablative conditioning regimens for HLA-matched sibling and HLA-matched or mismatched unrelated donor peripheral blood HCT.10 The use of thymoglobulin or anti-T lymphocyte globulin with reduced intensity conditioning regimens for hematologic malignancy is considered appropriate, but is not uniformly employed due to a potential higher risk of relapse.10
ATG has a long half-life and there is substantial inter-patient variability in its clearance.11 ATG exposure is mediated by dose, timing of administration and patient-related factors such as weight and lymphocyte count at time of ATG infusion.12,13 Pharmacokinetic studies have shown that high ATG exposure post-transplant has an adverse effect on survival in adults with hematologic malignancy.12 In children with myeloid malignancy, high ATG exposure has been shown to decrease CD4+ cell immune reconstitution with higher risk for death related to infections and increased risk for relapse.13
Granulocyte-colony stimulating factor (G-CSF) may be used to hasten hematopoietic recovery after allogeneic HCT. Data from de Koning and colleagues suggest that this practice might sensitize lymphocytes to the cytotoxic effects of residual ATG.14 G-CSF drives myeloid precursor proliferation and differentiation while also functionally activating phagocytosis, at least partially through induction of the IgG receptor FcγRI.15,16 In ex vivo experiments, G-CSF-primed neutrophils display dramatically higher antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) for ATG-coated cells.14 Thus we hypothesize that in the presence of residual post-transplant ATG, G-CSF exaggerates donor lymphocyte clearance, with a net detrimental effect on immune reconstitution similar to that seen with high ATG exposure. To test our hypothesis, we evaluated allogeneic HCT performed with ATG (thymoglobulin)-containing preparative regimens in patients aged ≥18 years with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) and compared outcomes in those who received planned G-CSF to those who did not.
METHODS
Patients
Data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR), a working group of transplant centers who submit data on standardized reporting forms with patients being followed longitudinally. Patients were transplanted in the United States at 76 transplant centers from 2010 to 2018. Included are patients aged ≥18 years with AML in first or second complete remission, or MDS (refractory anaemia, refractory anaemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, refractory anaemia with excess blasts). Eighteen (24%) of 76 centers used planned G-CSF in all patients. Thirty-four (45%) centers used no planned G-CSF in any patients and 24 (31%) centers performed transplants with planned G-CSF in some but not all patients during the study period. Patients received peripheral blood graft from HLA-matched sibling, HLA-matched or 1-locus mismatched unrelated donors. Patients received myeloablative or reduced intensity conditioning regimens and ATG (Thymoglobulin). GVHD prophylaxis included a calcineurin inhibitor with either methotrexate or mycophenolate. Of the 874 eligible patients, 459 patients were recorded as having received planned G-CSF. The first date of G-CSF administration ranged from 3 days before infusion of the graft to 12 days after infusion of the graft. Transplantations that included G-CSF for a clinical indication (prolonged pancytopenia, infection or other specified/unspecified clinical indications) were excluded. Also excluded were transplants using equine-ATG or alemtuzumab. Patients provided written informed consent. The Institutional Review Board of the National Marrow Donor Program study approved the study.
Outcomes
Relapse was the primary end point. Other endpoints studied included hematopoietic recovery, acute and chronic GVHD, NRM, disease-free and overall survival. Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) ≥0·5 x 109/L for three consecutive days. Platelet recovery was defined as achieving 20 x 109/L unsupported for 7 days. Grade II-IV acute and chronic GVHD were graded using previously described criteria.17,18 Relapse was defined as molecular, cytogenetic or morphologic recurrence of disease. Non-relapse mortality was defined as death in remission. Disease-free survival was defined as being alive in remission. Surviving patients were censored at last follow-up and death from any cause was considered an event.
Statistical Analysis
The characteristics of patients who received planned G-CSF and those who did not receive G-CSF were compared using the Chi-square statistic. The incidences of neutrophil and platelet recovery were calculated using the cumulative incidence estimator.19 Multivariate analyses were performed using Cox proportional hazards models20 for acute and chronic GVHD, relapse, NRM, disease-free and overall survival to examine the effect of planned G-CSF versus none with adjustment for age, sex, race, Karnofsky performance status (KPS), HCT co-morbidity score, cytomegalovirus serostatus, body mass index, disease risk index, conditioning regimen, GVHD prophylaxis and transplant period. The start time for all analyses was day+12 from the date of transplantation which allowed us to consider the effect of planned G-CSF on transplant outcomes. A stepwise model building approach was adopted, and variables that attained a p-value ≤0.05 were retained in the final model with the exception of the variable for G-CSF administration which was held in the final model regardless of its level of significance. The incidence of acute and chronic GVHD and the probabilities of relapse, NRM, disease-free and overall survival were calculated from the final Cox model.21,22 The incidence of infections within 100 days and 6-months after transplantation was calculated using the cumulative incidence estimator.19 An effect of transplant center effect on survival was tested using the frailty model.23 Two subset analyses to study the effect of planned G-CSF versus none were performed: 1) patients aged ≥50 years and 2) patients transplanted at the 24 centers that used planned G-CSF in some but not all patients during the study period. All p-values are two-sided and analyses were done using SAS version 9·4 (Cary, NC).
Role of the funding source
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit this article for publication. All authors had full access to the data and the corresponding author had final responsibility for the decision to submit for publication.
RESULTS
Patient, disease and transplant characteristics
Patient, disease and transplant characteristics by treatment group are shown in Table 1. Four hundred and fifty-nine recipients received planned G-CSF for their transplantation and 415 recipients did not receive G-CSF. The characteristics of the patients in the treatment groups were comparable except for age at transplant (18-49 years or 50-80 years) and hematopoietic cell transplant co-morbidity score (≤2 or ≥3). Compared to patients who received planned G-CSF, patients who did not receive G-CSF were more likely to be aged 18-49 years (20% vs. 14%, p=0·02) and report co-morbidity scores ≤2 (51% vs. 43%, p=0·03). The median age of patients who did not receive G-CSF was 62 years compared to 64 years for those who received planned G-CSF. A third of patients had body mass index greater than 30, meeting the Center for Disease Control and Prevention definition of obesity. MDS was the predominant disease type in both groups. The proportion of patients with intermediate and high disease risk index was similar between treatment groups. HLA-matched unrelated donor was the predominant donor type in both treatment groups accounting for approximately 80% of transplantations. However, HLA-matched sibling transplantations were less common in the group that did not receive G-CSF compared the group that received planned G-CSF (7% vs. 13%, p=0·03). An alkylating agent with fludarabine was the predominant preparative regimen. Among recipients of myeloablative regimens, fludarabine/busulfan regimen was more common in the group who received G-CSF. All regimens included thymoglobulin and the median dose was 4·5 mg/kg for both groups. A calcineurin inhibitor with methotrexate was the predominant GVHD prophylaxis in both groups. The median follow-up was 49 months in the group that received planned G-CSF and 50 months in the group that did not receive G-CSF.
Table 1.
Patient disease and transplant characteristics
| Variables | HCT with G-CSF | HCT without G-CSF | P-value |
|---|---|---|---|
| Number | 459 | 415 | |
| Age, years | 0·02 | ||
| 18-49 | 66 (14%) | 85 (20%) | |
| 50-80 | 393 (86%) | 330 (80%) | |
| Sex | 0·32 | ||
| Male | 284 (62%) | 243 (59%) | |
| Female | 175 (38%) | 172 (41%) | |
| Karnofsky Performance Status (KPS) | 0·62 | ||
| 90-100 | 250 (54%) | 237 (57%) | |
| <90 | 201 (44%) | 178 (43%) | |
| Not reported | 8 (2%) | - | |
| Hematopoietic cell transplant co-morbidity | 0·04 | ||
| ≤2 | 200 (43%) | 209 (51%) | |
| ≥3 | 258 (56%) | 205 (49%) | |
| Not reported | 1 (<1%) | 1 (<1%) | |
| Body mass index | 0·27 | ||
| 18-24.9 | 127 (28%) | 99 (24%) | |
| 25 - 29.9 | 176 (38%) | 159 (38%) | |
| 30 - 34.9 | 100 (22%) | 88 (21%) | |
| ≥35 | 55 (12%) | 67 (16%) | |
| Not reported | 1 (<1%) | 2 (<1%) | |
| Disease type | 0·56 | ||
| Acute myeloid leukemia | 207 (45%) | 179 (43%) | |
| Myelodysplastic syndrome | 252 (55%) | 236 (57%) | |
| Disease risk index | 0·94 | ||
| Low | 21 (5%) | 18 (4%) | |
| Intermediate | 232 (51%) | 217 (52%) | |
| High | 196 (43%) | 176 (42%) | |
| Not reported | 10 (2%) | 4 (1%) | |
| Donor type | 0·03 | ||
| HLA-matched sibling | 58 (13%) | 31 (7%) | |
| HLA-matched unrelated | 352 (77%) | 330 (80%) | |
| 1-locus HLA-mismatched unrelated | 49 (11%) | 54 (13%) | |
| Conditioning regimen * | 0·99 | ||
| Myeloablative | |||
| Fludarabine + busulfan + ATG | 145 (32%) | 109 (26%) | |
| Busulfan + cyclophosphamide + ATG | 33 (7%) | 39 (9%) | |
| Total body irradiation + fludarabine + ATG | 18 (4%) | 29 (7%) | |
| Reduced intensity | |||
| Fludarabine + busulfan + ATG | 171 (37%) | 145 (35%) | |
| Fludarabine + melphalan + ATG | 60 (13%) | 65 (16%) | |
| Total body irradiation + fludarabine + ATG | 32 (7%) | 28 (7%) | |
| Graft vs. host disease prophylaxis | 0·13 | ||
| Calcineurin inhibitor + methotrexate | 313 (68%) | 263 (63%) | |
| Calcineurin inhibitor + mycophenolate | 146 (32%) | 152 (37%) | |
| Transplant period | 0·38 | ||
| 2010-2013 | 155 (34%) | 152 (37%) | |
| 2014-2018 | 304 (66%) | 263 (63%) |
Median total ATG dose per kg (IQR) by regimen: HCT with G-CSF: Myeloablative: Fludarabine + busulfan + ATG: 4·0 (4·0, 5·0); Busulfan + cyclophosphamide + ATG: 5·0 (3·5, 5·0); Total body irradiation + fludarabine + ATG: 5·0 (4·5, 6·0). Reduced intensity: Fludarabine + busulfan + ATG: 6·0 (4·0, 6·5); Fludarabine + melphalan + ATG: 4·0 (4·0, 5·0); Total body irradiation + fludarabine + ATG: 3·75 (3·0, 4·5). HCT without G-CSF: Myeloablative: Fludarabine + busulfan + ATG: 4·5 (4·0, 5·0); Busulfan + cyclophosphamide + ATG: 5·0 (4·0, 6·0); Total body irradiation + fludarabine + ATG: 4·5 (4·0, 5·0). Reduced intensity: Fludarabine + busulfan + ATG: 5·0 (4·5, 6·0); Fludarabine + melphalan + ATG: 4·5 (3·5, 5·5); Total body irradiation + fludarabine + ATG: 4·5 (3·5, 4·5).
Abbreviation:
HCT = hematopoietic cell transplant
G-CSF = granulocyte colony stimulating factor
ATG = rabbit derived anti-thymocyte globulin
Patient, disease and transplant characteristics of patients from the 24 centers that performed transplantation with planned G-CSF in some but not all patients during the study period are shown in Supplemental Table 1. The characteristics of the two patient groups in this subset did not differ except fewer recipients of transplants without G-CSF received their graft from HLA-matched sibling (4% vs. 13%), and more received calcineurin inhibitor with methotrexate GVHD prophylaxis regimen (76% vs. 61%) and were transplanted between 2014 and 2018 (63% vs. 56%).
Hematopoietic recovery
The median time to neutrophil recovery in recipients of planned G-CSF was 12 days (Inter-quartile range [IQR] 11-13 days) compared to 15 days (IQR 13-17 days) in untreated patients. However, the corresponding day-28 incidence of neutrophil recovery was 98% (95% CI: 97-99%) and 98% (95% CI: 97-99%), p=0·97. The median time to platelet recovery was 17 days (IQR 14-19 days) compared to 15 days (IQR 13-18 days). The day-100 incidence of platelet recovery was delayed in G-CSF recipients; 93% (95% CI: 90-95%) versus 96% (95% CI: 94-98%), p=0·023.
Acute and chronic GVHD
The results of multivariate analysis are shown in Table 2. The incidence of acute and chronic GVHD was not associated with use of planned G-CSF. Patients with MDS were at higher risk for grade II-IV and grade III-IV acute GVHD. The adjusted day-100 incidence of grade II-IV acute GVHD was 39% (95% CI: 34-43%) and 38% (95% CI: 33-42%) in patients who received planned G-CSF and those who did not receive G-CSF, respectively, p=0·74. Similarly, grade III-IV acute GVHD did not differ between treatment groups. The day-100 incidence of grade III-IV acute GVHD was 14% (95% CI: 11-17%) and 12% (95% CI: 9-15%), p=0·47 in patients who received planned G-CSF and those who did not receive G-CSF. Patients with MDS were also at higher risk for chronic GVHD. The adjusted 1-year incidence of chronic GVHD was 33% (95% CI: 29-37%) 37% (95% CI: 33-42%) in patients who received planned G-CSF and those who did not, respectively, p=0·21.
Table 2.
Effect of G-CSF on GVHD, relapse, non-relapse mortality, disease-free and overall survival
| Outcome | Number Events/Evaluable |
Hazard Ratio (95% confidence interval) |
p-value |
|---|---|---|---|
| Grade 2-4 acute GVHD* | |||
| HCT without G-CSF | 166/403 | 1·00 | |
| HCT with G-CSF | 185/449 | 1·03 (0·83 – 1·27) | 0·80 |
| Grade 3-4 acute GVHD** | |||
| HCT without G-CSF | 56/406 | 1·00 | |
| HCT with G-CSF | 67/440 | 1·14(0·80 – 1·62) | 0·48 |
| Chronic GVHD*** | |||
| HCT without G-CSF | 153/409 | 1·00 | |
| HCT with G-CSF | 142/445 | 0·99 (0·79 – 1·25) | 0·97 |
| Relapse♯ | |||
| HCT without G-CSF | 123/409 | 1·00 | |
| HCT with G-CSF | 139/448 | 1·19 (0·93 – 1·52) | 0·17 |
| Non-relapse mortality╪ | |||
| HCT without G-CSF | 47/409 | 1·00 | |
| HCT with G-CSF | 96/448 | 2·03 (1·43 – 2·88) | <0·0001 |
| Disease-free survival∥ | |||
| HCT without G-CSF | 170/409 | 1·00 | |
| HCT with G-CSF | 235/448 | 1·42(1·16 – 1·73) | 0·0006 |
| Overall survival$ | |||
| HCT without G-CSF | 111/409 | 1·00 | |
| HCT with G-CSF | 173/448 | 1·52(1·20 – 1·94) | 0·0005 |
Abbreviation:
HCT = hematopoietic cell transplant
G-CSF = granulocyte colony stimulating factor
GVHD = graft-versus-host disease
Model adjusted for disease: grade 2-4 acute GVHD was higher with myelodysplastic syndrome (HR 1·44, 95% CI 1·16 – 1·78, p=0·0009)
Model adjusted for disease and KPS: grade 3-4 acute GVHD was higher with myelodysplastic syndrome (HR 1·56, 95% CI 1·09 – 2·26, p=0·0157) and poor performance status (HR 1·65, 95% CI 1·16 – 2·33, p=0·0049)
Model adjusted for disease: chronic GVHD was higher with myelodysplastic syndrome (HR 1·31, 95% CI 1·03 – 1·65, p=0·0252)
Model adjusted for disease risk index, KPS and condition regimen intensity: relapse was higher with intermediate (HR 3·11, 95% CI 0·99 – 9·80, p=0·0528) and high (HR 6·02, 95% CI 1·92 – 18·89, p=0·0021) DRI, poor performance status (HR 1·31, 95% CI 1·02 – 1·67, p=0·0335) and reduced intensity conditioning regimen (HR 1·51, 95% CI 1·16 – 1·96, p=0·0023)
Model adjusted for disease and hematopoietic comorbidity score: non-relapse mortality was higher with myelodysplastic syndrome (HR 1·61, 95% CI 1·15 – 2·26, p=0·0051) and high comorbidity score (HR 1·55, 95% CI 1·11 – 2·16, p=0·0108)
Model adjusted for disease risk index, KPS and condition regimen intensity: disease-free survival was lower with intermediate (HR 1·09, 95% CI 0·63 – 1·88, p=0·76) and high (HR 1·93, 95% CI 1·12 – 3·32, p=0·076) DRI, poor performance status (HR 1·39, 95% CI 1·14 – 1·69, p=0·0011) and reduced intensity conditioning regimen (HR 1·27, 95% CI 1·03 – 1·56, p=0·0233)
Model adjusted for KPS and hematopoietic comorbidity score: survival was lower with poor performance status (HR 1·51, 95% CI 1·19 – 1·90, p=0·0005) and high co-morbidity score (HR 1·30, 95% CI 1·03 – 1·65, p=0·0279).
Relapse and Non-relapse mortality
The risk for relapse did not differ between treatment group after adjustment for disease risk index, KPS and conditioning regimen intensity - the other factors associated with relapse risk (Table 2). Poor performance status, high disease risk index and reduced intensity regimen were associated with higher relapse risk and independent of use of G-CSF. The adjusted 1-year incidence of relapse is shown in Figure 1A.
Figure 1: Relapse and Non-relapse mortality.
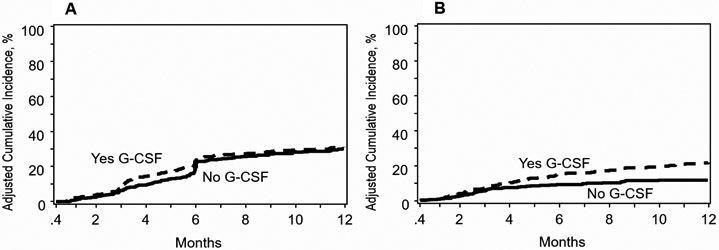
A: Relapse: The 1-year adjusted incidence of relapse was 31% (95% CI 27-35%) after planned G-CSF and 30% (95% CI 26-35%) without G-CSF, p=0·17
B: Non-relapse mortality: The 1-year adjusted incidence of non-relapse mortality was 21% (95% CI 18-25%) after planned G-CSF and 12% (95% CI 9-15%) without G-CSF, p<0·0001
The risk for NRM was two-fold higher in patients who received planned G-CSF after adjustment for HCT co-morbidity score and disease (Table 2). NRM risk was higher for patients with HCT comorbidity score ≥3 and MDS and independent of use of G-CSF. The adjusted 1-year incidence of NRM is shown in Figure 1B. Although it is unlikely the effect of G-CSF given within 12 days after transplantation extends beyond the early post-transplant period we examined for an effect of G-CSF on longer follow-up. The higher risk for NRM with planned G-CSF persists with continued with follow up through 5-years (HR 1·56, 95% CI: 1·17-2·10, p=0·0028).
Disease-free and Overall survival
Disease-free survival was lower in patients who received planned G-CSF after adjustment for KPS, disease risk index and regimen intensity (Table 2). Poor performance status, high disease risk index and reduced intensity regimen were associated with lower disease-free survival and independent of use of G-CSF. The adjusted 1-year incidence of disease-free survival is shown in Figure 2A. Lower disease-free survival with planned G-CSF persists with continued follow up through 5-years (HR 1·25, 95% CI: 1·05-1·48, p=0·0106). Similarly, overall survival was lower in patients who received planned G-CSF after adjustment for KPS, HCT-comorbidity score and disease risk index (Table 2). Poor performance status, HCT-comorbidity score ≥3 and high disease risk index was associated with lower overall survival and independent of use of G-CSF. The adjusted 1-year incidence of overall survival is shown in Figure 2B. Lower overall survival with planned G-CSF persisted with continued follow up through 5-years although the level of significance was marginal (HR 1·19, 95% CI: 0·99-1·44, p=0·0624). We tested for an effect of transplant center on survival using a gamma frailty model and found none (p=0·32). The effect of planned G-CSF versus none on survival with a random frailty was HR 1·52, 95% CI 1·20 – 1·94, p=0·0005 and without a random frailty was HR 1.53, 95% CI 1·20 – 1·95, p=0·0007. The duration of index hospitalization did not differ by treatment group. For recipients of planned G-CSF, median index hospitalization was 16 days (IQR 14 – 20) and for those who did not receive G-CSF, 18 days (IQR 15 – 21).
Figure 2: Disease-free Survival and Overall Survival.
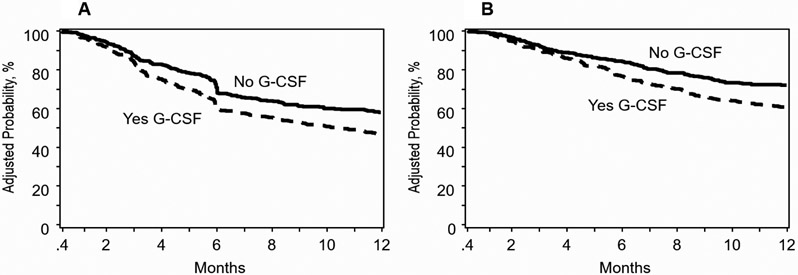
A: Disease-free survival: The 1-year adjusted incidence of disease-free survival was 47% (95% CI 43-52%) after planned G-CSF and 58% (95% CI 53-63%) without G-CSF, p=0·0006
B: Overall survival: The 1-year adjusted incidence of overall survival was 61% (95% CI 56-65%) after planned G-CSF and 72% (95% CI 68-76%) without G-CSF, p=0·0001
Infection
The 3- and 6-month incidences of viral infection were higher after transplantations with planned G-CSF (p=0·007). The 3-month incidence of viral infections after planned G-CSF was 50% (95% CI 45 – 54) compared to 42% (95% CI 38 – 47) for transplants without G-CSF. The corresponding 6-month incidences of viral infections were 56% (95% CI 51 – 60) and 47% (95% CI 42 – 52). Among the viral infections, the incidence of cytomegalovirus infection did not differ by treatment group (Table 3). However, the 3- and 6-month incidence of Epstein-Barr virus (EBV) infection was significantly higher in patients who received planned G-CSF compared to none (Table 3). All other viral infections were grouped together, and polyomavirus was the predominant organism in this group. The 3- and 6-month incidence of all other viral infections was higher with planned G-CSF compared to none (Table 3). There was no significant difference between treatment groups in the incidence of bacterial or fungal infections. The 6-month incidence of bacterial infections after planned G-CSF was 48% (95% CI 43 – 53) compared to 42% (95% CI 38 – 47), p=0·79. The corresponding 6-month incidences of fungal infections were 7% (95% CI 5 – 9) and 7% (95% CI 4 – 9), p=0·89.
Table 3.
Incidence of viral infections
| HCT with G-CSF | HCT without G-CSF | p-value | |
|---|---|---|---|
| CMV | |||
| Within 3-months after HCT | 32·1% (95% CI 28-37) | 28·1% (95% CI 24-33) | 0·196 |
| Within 6 months after HCT | 33·9% (95% CI 30-38) | 29·1% (95% CI 25-34) | 0·123 |
| EBV | |||
| Within 3-months after HCT | 17·7% (95% CI 14-21) | 10·4% (95% CI 8-14) | 0·002 |
| Within 6 months after HCT | 19·1% (95% CI 16-23) | 11·4% (95% CI 9-15) | 0·002 |
| Other* | |||
| Within 3-months after HCT | 22·5% (95% CI 19-27) | 16·0% (95% CI 13-20) | 0·013 |
| Within 6 months after HCT | 26·1% (95% CI 22-30) | 19·2% (95% CI 16-23) | 0·016 |
Abbreviation:
HCT = hematopoietic cell transplant
G-CSF = granulocyte colony stimulating factor
CMV = Cytomegalovirus
EBV = Epstein Barr virus
Other includes adenovirus, herpesviridae and respiratory viruses.
Subset Analyses
A subset analysis was undertaken to ensure our findings held true when the population was limited to those aged ≥50 years (Table 4). Consistent with the main analysis, risks for NRM were higher for transplantations with planned G-CSF and disease-free and overall survival were lower. Risks for acute and chronic GVHD did not differ by treatment group (Table 4). NRM and overall survival models in patients aged ≥50 years were adjusted for HCT-comorbidity score – 232 patients (51%) in the planned G-CSF group had HCT-comorbidity scores ≥3 compared to 172 patients (41%) in the group that did not receive G-CSF.
Table 4.
Effect of G-CSF on GVHD, relapse, non-relapse mortality, disease-free and overall survival in patients aged ≥50 years
| Outcome | Number Events/Evaluable |
Hazard Ratio (95% confidence interval) |
p-value |
|---|---|---|---|
| Grade 2-4 acute GVHD* | |||
| HCT without G-CSF | 138/320 | 1·00 | |
| HCT with G-CSF | 162/385 | 1·03 (0·82 – 1·29) | 0·83 |
| Grade 3-4 acute GVHD** | |||
| HCT without G-CSF | 44/323 | 1·00 | |
| HCT with G-CSF | 64/377 | 1·32 (0·90 – 1·95) | 0·15 |
| Chronic GVHD*** | |||
| HCT without G-CSF | 120/325 | 1·00 | |
| HCT with G-CSF | 122/380 | 1·07 (0·83 – 1·39) | 0·59 |
| Relapse♯ | |||
| HCT without G-CSF | 109/325 | 1·00 | |
| HCT with G-CSF | 124/383 | 1·16 (0·90 – 1·51) | 0·25 |
| Non-relapse mortality╪ | |||
| HCT without G-CSF | 38/325 | 1·00 | |
| HCT with G-CSF | 86/383 | 2·10 (1·43 – 3·08) | 0·0002 |
| Disease-free survival∥ | |||
| HCT without G-CSF | 147/325 | 1·00 | |
| HCT with G-CSF | 210/383 | 1·40 (1·14 – 1·74) | 0·0017 |
| Overall survival$ | |||
| HCT without G-CSF | 87/325 | 1·00 | |
| HCT with G-CSF | 154/383 | 1·48 (1·15 – 1·92) | 0·0024 |
Abbreviation:
HCT = hematopoietic cell transplant
G-CSF = granulocyte colony stimulating factor
GVHD = graft-versus-host disease
Model adjusted for disease
Model adjusted for disease and KPS
Model adjusted for disease
Model adjusted for disease risk index, KPS and condition regimen intensity
Model adjusted for disease and hematopoietic comorbidity score
Model adjusted for disease risk index, KPS and condition regimen intensity
Model adjusted for KPS and hematopoietic comorbidity score
A second subset analysis limited to the 24 centers that transplanted patients with planned G-CSF in some but not all patients during the study period was performed. Consistent with the main analysis, NRM (HR 1·79, 95% CI 1·10 – 2·92, p=0·0187) was higher and disease-free (HR 1·65, 95% CI 1·24 – 2·21, p=0·0007) and overall survival (HR 1·67, 95% CI 1·18 – 2·34, p=0·0035) were lower after transplantations with planned G-CSF. However, contrary to the main analysis, relapse risk was higher in the group that received planned G-CSF (HR 1·66, 95% CI 1·16 – 2·38, p=0·0058). There were no differences in risks for grade II-IV GVHD (HR 0·93, 95% CI 0·68 – 1·26, p=0·64), grade III-IV acute GVHD (HR 1·09, 95% CI 0·65 – 1·80, p=0·75) and chronic GVHD (HR 0·91, 95% CI 0·65 – 1·29, p=0·59).
Causes of Death
We examined the reported causes of death in the first year after HCT. There were 178 of 459 (39%) deaths among patients who received planned G-CSF and 116 of 415 (28%) deaths in patients who did not receive G-CSF. Recurrent disease was the most common cause of death in both treatment groups. Of the 178 deceased patients who received planned G-CSF, 35% died from recurrent disease, 17% from GVHD, 21% from infections, 4% from interstitial pneumonitis, 11% from organ failure and 1% from other causes. Cause of death was not reported for 17 patients. Of the 116 deceased patients who did not receive G-CSF, 52% died from recurrent disease, 14% from GVHD, 16% from infections, 4% from interstitial pneumonitis, 10% from organ failure and 3% from other causes. Cause of death was not reported for 3 patients.
Discussion
De Koning et al. recently showed ex vivo that exposure to G-CSF increased neutrophil-mediated ATG cytotoxicity by 40-fold.14 To our knowledge this interaction had not been previously reported in clinical studies. As thymoglobulin and G-CSF are used widely for allogeneic HCT in adults with myeloid malignancy, we sought to study whether planned G-CSF that was initiated between 3 days prior to transplantation and 12 days after transplantation with thymoglobulin-containing myeloablative or reduced intensity regimens, had an adverse effect on relapse or survival. The most striking finding of our study is the twofold increase in NRM risk and consequently lower overall survival at 1-year when G-CSF was administered at a time when residual thymoglobulin levels are likely to be still above lymphotoxic thresholds. We acknowledge that patients who did not receive G-CSF were relatively younger and more likely to report co-morbidity score ≤2. We addressed any potential bias of age on NRM and survival by performing a subset analysis limited to patients aged 50 years and above, and confirmed that the findings of the main analyses hold. As expected NRM was higher and survival lower for those with co-morbidity scores ≥3 but this effect was independent of the effect of planned G-CSF. We also observed higher rates of EBV reactivation and viral infections other than CMV reactivation in patients who received planned G-CSF.
G-CSF-mobilized peripheral blood has been shown to have reduced T-cell proliferative responses, impaired antiviral functionality of T-cells, and reduced NK cell activity as well as reduced numbers of NK progenitors.24-27 ATG impairs CD4+ T cell recovery for up to 6 months post-transplant and compromises the generation of the naive T cell compartment, reducing T cell responses to infection and increasing the risk for EBV reactivation.28 Here we show that post-transplant exposure to G-CSF amplifies this effect.
We observed a mixed effect of G-CSF on relapse with a higher risk for relapse in a subset of patients who received planned G-CSF, but only in centers where planned G-CSF was not consistently used. This suggests that in those centers, planned G-CSF may have been used preferentially for higher risk patients. When the analysis was restricted to a comparison between centers using a consistent policy of planned G-CSF in all or in none, no effect of G-CSF on relapse was found. Historically there has been concern that G-CSF could promote myeloid leukemic cell proliferation where malignant precursors express G-CSF receptors.15 This concern has largely been assuaged by clinical experience outside of patients with G-CSF receptor mutations or monosomies of chromosome 7.15 Limited data has even suggested some anti-leukemic efficacy of single agent G-CSF in post-HCT leukemic relapse.29,30
A potential advantage to planned G-CSF is shorter duration of index hospitalization which we did not observe in the current analyses. To our knowledge this is the first study that has specifically examined the effects of planned G-CSF for myeloablative and reduced intensity transplants performed using Thymoglobulin. None of the previously published studies examined or took into account a possible interaction between ATG exposure and G-CSF. Our study has several limitations. First, we lack data on CD4+ recovery and are unable to confirm our initial hypothesis that G-CSF in the presence of residual thymoglobulin increases neutrophil-mediated lymphotoxicity with impaired CD4+ cell recovery. An examination of lymphocyte subsets and the pace of immune recovery is of particular interest and should be studied in a prospective manner. Second, other types of antithymocyte globulin and alemtuzumab-containing regimens were excluded a priori as these agents are not commonly used in the United States. As such our findings are limited to thymoglobulin-containing regimens for AML and MDS. Third, the ideal approach to study treatment outcomes is a randomized trial. To our knowledge there are no such trials that are on-going. Fourth, although we performed a carefully controlled analysis adjusting for patient, disease and transplant characteristics, we acknowledge there are unknown and unmeasured factors that were not adjusted for in our analyses. In a subset analysis, we studied outcomes at centers that performed HCTs with planned G-CSF in some but not all patients during the study period and confirmed higher NRM and lower survival with planned G-CSF use. However, based on the available data on patient, disease and transplant characteristics we are unable to explain the choice of planned G-CSF in some patients and not others.
A two-fold increase in NRM and a 10% absolute decrement in overall survival observed with administration of G-CSF beginning 3 days before infusion to 12 days later cannot be ignored. We conclude that in allogeneic peripheral blood transplantation performed using thymoglobulin for AML and MDS, the practice of planned G-CSF in the early post-transplant period must be cautiously considered and any relative benefit with planned G-CSF weighed against the risk of higher non-relapse mortality and lower survival.
Supplementary Material
Highlights.
ATG reduces graft failure and GVHD after HCT but may delay lymphocyte recovery.
G-CSF given early after HCT may increase ATG-mediated lymphotoxicity.
We examine peripheral blood HCT performed with Thymoglobulin for AML and MDS.
Planned post-transplant G-CSF adversely impacts disease-free and overall survival.
G-CSF also doubles non-relapse mortality and increases viral infection.
ACKNOWLEDGEMENTS
Supported primarily by Public Health Service Grant Agreement U24-CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases and contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS). The views expressed in this article do not reflect the official policy or position of the National Institute of Health, Health Resources and Services Administration, or any other agency of the U.S. Government.
Footnotes
FINANCIAL CONFLICT OF INTEREST
The authors declare none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12(5):560–565. [DOI] [PubMed] [Google Scholar]
- 2.Bonifazi F, Solano C, Wolschke C, et al. Acute GVHD prophylaxis plus ATLG after myeloablative allogeneic haemopoietic peripheral blood stem-cell transplantation from HLA-identical siblings in patients with acute myeloid leukaemia in remission: final results of quality of life and long-term outcome analysis of a phase 3 randomised study. Lancet Haematol. 2019;6(2):e89–e99. [DOI] [PubMed] [Google Scholar]
- 3.Finke J, Schmoor C, Bethge WA, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4(6):e293–e301. [DOI] [PubMed] [Google Scholar]
- 4.Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J Clin Oncol. 2017;35(36):4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17(2):164–173. [DOI] [PubMed] [Google Scholar]
- 6.Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A. Efficacy of antithymocyte globulin for allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Leuk Lymphoma. 2017;58(8):1840–1848. [DOI] [PubMed] [Google Scholar]
- 7.Gagelmann N, Ayuk F, Wolschke C, Kroger N. Comparison of Different Rabbit Anti-Thymocyte Globulin Formulations in Allogeneic Stem Cell Transplantation: Systematic Literature Review and Network Meta-Analysis. Biol Blood Marrow Transplant. 2017;23(12):2184–2191. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Reljic T, Hamadani M, Mohty M, Kharfan-Dabaja MA. Antithymocyte globulin for graft-versus-host disease prophylaxis: an updated systematic review and meta-analysis. Bone Marrow Transplant. 2019;54(7):1094–1106. [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Pei R, Su W, Cao J, Lu Y. Meta-analysis of the actions of antithymocyte globulin in patients undergoing allogeneic hematopoietic cell transplantation. Oncotarget. 2017;8(7):10871–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonifazi F, Rubio MT, Bacigalupo A, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transplant. 2020;55(6):1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storek J, Mohty M, Boelens JJ. Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):959–970. [DOI] [PubMed] [Google Scholar]
- 12.Admiraal R, Nierkens S, de Witte MA, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4(4):e183–e191. [DOI] [PubMed] [Google Scholar]
- 13.Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2(5):e194–203. [DOI] [PubMed] [Google Scholar]
- 14.de Koning C, Gabelich JA, Langenhorst J, et al. Filgrastim enhances T-cell clearance by antithymocyte globulin exposure after unrelated cord blood transplantation. Blood Adv. 2018;2(5):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battiwalla M, McCarthy PL. Filgrastim support in allogeneic HSCT for myeloid malignancies: a review of the role of G-CSF and the implications for current practice. Bone Marrow Transplant. 2009;43(5):351–356. [DOI] [PubMed] [Google Scholar]
- 16.Kerst JM, de Haas M, van der Schoot CE, et al. Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood. 1993;82(11):3265–3272. [PubMed] [Google Scholar]
- 17.Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM. Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1989;4(3):247–254. [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 19.Fine JP GR. A proportional hazards model for the sub-distribution of a competing risk. . Journal of the Americal Statistical Association. 1999;94((446)):496–509. [Google Scholar]
- 20.DR C Regression models and life tables. Journal of the Royal Statistical Society. 1972;34(4):187–217. [Google Scholar]
- 21.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18(12):1489–1500. [DOI] [PubMed] [Google Scholar]
- 24.Bunse CE, Borchers S, Varanasi PR, et al. Impaired functionality of antiviral T cells in G-CSF mobilized stem cell donors: implications for the selection of CTL donor. PLoS One. 2013;8(12):e77925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JS, Prosper F, McCullar V. Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cell collections. Blood. 1997;90(8):3098–3105. [PubMed] [Google Scholar]
- 26.Rondelli D, Raspadori D, Anasetti C, et al. Alloantigen presenting capacity, T cell alloreactivity and NK function of G-CSF-mobilized peripheral blood cells. Bone Marrow Transplant. 1998;22(7):631–637. [DOI] [PubMed] [Google Scholar]
- 27.Su YC, Li SC, Hsu CK, et al. G-CSF downregulates natural killer cell-mediated cytotoxicity in donors for hematopoietic SCT. Bone Marrow Transplant. 2012;47(1):73–81. [DOI] [PubMed] [Google Scholar]
- 28.Gooptu M, Kim HT, Chen YB, et al. Effect of Antihuman T Lymphocyte Globulin on Immune Recovery after Myeloablative Allogeneic Stem Cell Transplantation with Matched Unrelated Donors: Analysis of Immune Reconstitution in a Double-Blind Randomized Controlled Trial. Biol Blood Marrow Transplant. 2018;24(11):2216–2223. [DOI] [PubMed] [Google Scholar]
- 29.Carral A, Sanz GF, Sanz MA. Filgrastim for the treatment of leukemia relapse after bone marrow transplantation. Bone Marrow Transplant. 1996;18(4):817–819. [PubMed] [Google Scholar]
- 30.Giralt S, Escudier S, Kantarjian H, et al. Preliminary results of treatment with filgrastim for relapse of leukemia and myelodysplasia after allogeneic bone marrow transplantation. N Engl J Med. 1993;329(11):757–761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


