Abstract
Devices that can record or modulate neural activity are essential tools in clinical diagnostics and monitoring, basic research, and consumer electronics. Realizing stable functional interfaces between manmade electronics and biological tissues is a longstanding challenge that requires device and material innovations to meet stringent safety and longevity requirements and to improve functionality. Compared to conventional materials, nanocarbons and carbides offer a number of specific advantages for neuroelectronics that can enable advances in functionality and performance. Here, we review the latest emerging trends in neuroelectronic interfaces based on nanocarbons and carbides, with a specific emphasis on technologies developed for use in vivo. We highlight specific applications where the ability to tune fundamental material properties at the nanoscale enables interfaces that can safely and precisely interact with neural circuits at unprecedented spatial and temporal scales, ranging from single synapses to the whole human body.
Keywords: nanomaterials, 2D materials, neuroengineering, neural interfaces, bioelectronics, MXene, graphene
Introduction
Since Luigi Galvani’s first observations of bioelectricity in the 1790s, the library of tools to map and modulate neural functions has greatly expanded. Many of these technologies, such as neuromodulation devices for epilepsy and Parkinson’s disease, have reached maturity for translation and are now routinely used in clinical practice. Others, such as implantable microelectrode arrays, have enabled breakthrough discoveries on fundamental neural processes, as well as advanced neuroprosthetics for restoring function. Finally, skin-mounted and wearable interfaces are increasingly being adopted in the consumer market.
Despite the rapid pace of technology development, significant challenges remain in realizing safe, stable, and functional interfaces between manmade electronics and biological tissues. For recording and stimulation in the brain and peripheral nerves, the push towards miniaturization, high spatial resolution, and minimal invasiveness conflicts with the performance requirements of low interface impedance and effective charge transfer. In wearable sensors, high conformability, flexibility, and resistance to cyclic deformations are key to realizing devices that are safe, functional, and comfortable for long-term use. Finally, incorporating multiple interfacing modalities within a single platform introduces a host of challenges in materials, design, and manufacturing. Conventional neuroelectronic materials, such as metals and silicon (Si), are inadequate for matching the electrical, mechanical, and chemical properties of neural tissues, and meeting the performance requirements of the next generation of neural interfaces.
In the last decade, intense research efforts have focused on new materials and fabrication approaches to address these critical challenges. Nanocarbons and carbides, in particular, have emerged as exceptionally promising candidates for safe neuroelectronic interfaces with a broad range of functionalities across multiple scales. Nanocarbons, including carbon nanotubes (CNTs), various forms of graphene, and nanodiamond, have unique properties that make them particularly well-suited for neuroelectronic applications, including high electrical conductivity, high surface area, flexibility, and surface functionalization [1]**. MXenes, a recently discovered class of two dimensional (2D) carbides and nitrides, share many of the same advantages as the nanocarbon allotropes, while also offering highly scalable top-down synthesis and liquid-phase processability endowed by their hydrophilic nature [2]. Furthermore, recent evidence of the in vivo biocompatibility of nanocarbon and MXene interfaces at the neuronal cell, immune system, brain, and systemic levels supports the potential of these materials for future translation [3–10].
In this review we cover the latest advances in neuroelectronic technologies based on nanocarbons and carbides, with a specific focus on applications where these materials have shown promise in vivo (Figure 1). We discuss how the molecular properties translate into favorable device performance, and highlight various synthesis and processing methods compatible with the manufacturing of neuroelectronics (Figure 2).
Figure 1.
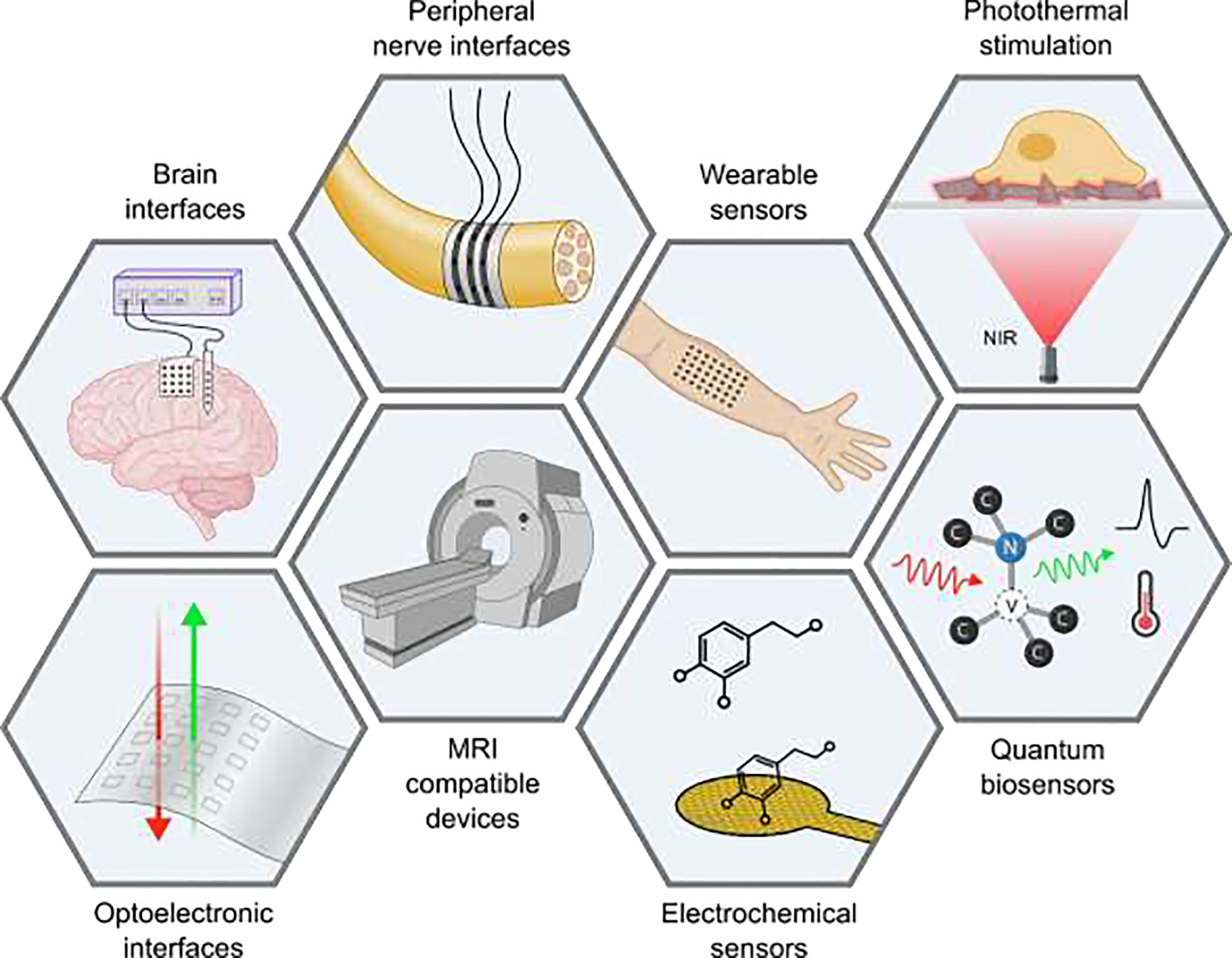
Schematic of the key emerging applications of nanocarbons and MXenes for in vivo neuroelectronics.
Figure 2.
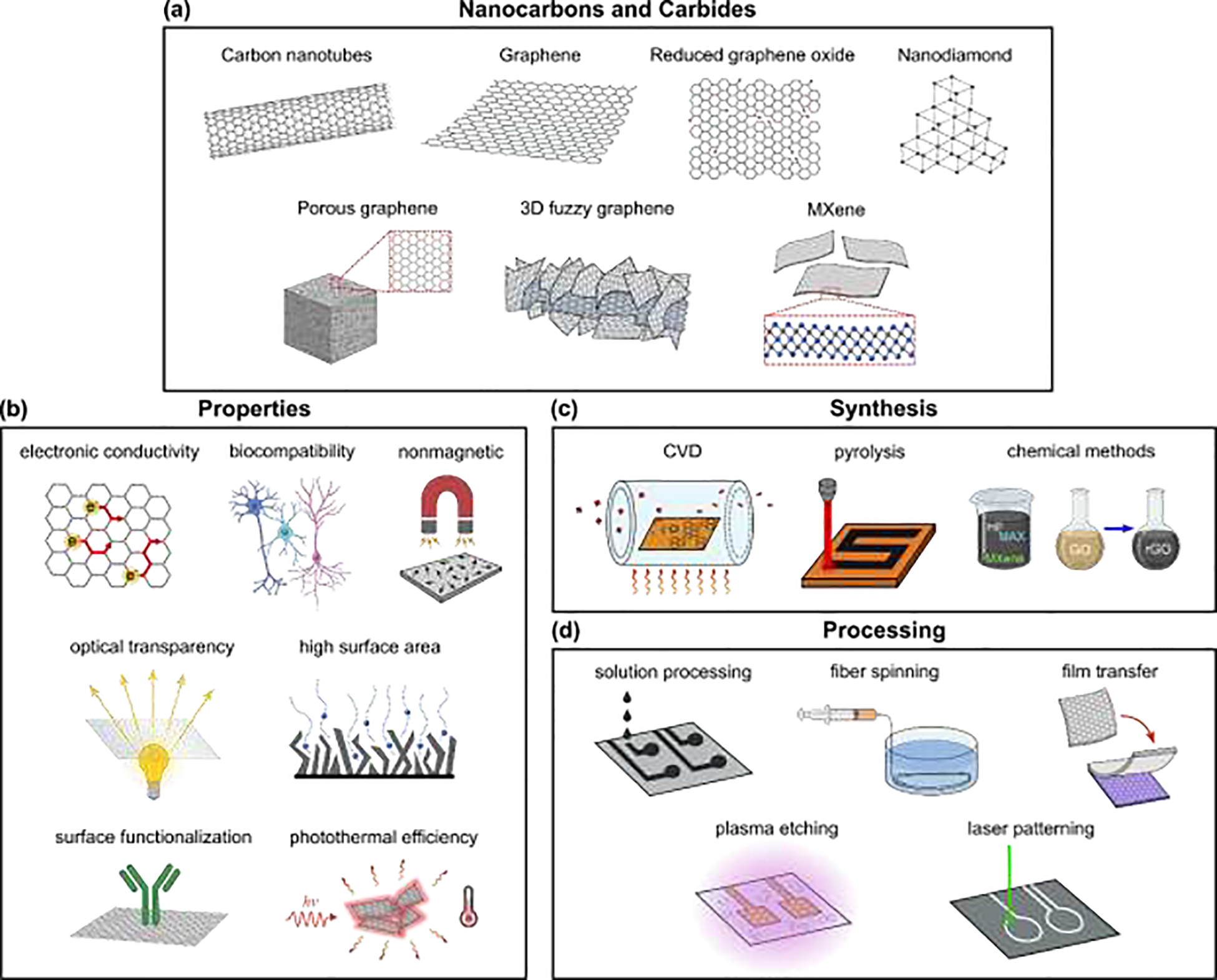
Schematic diagram illustrating the main classes of nanocarbons and carbides, together with their advantages, synthesis, and processing methods. (a) Schematics of the nanocarbons and carbides adopted in neuroelectronic applications in vivo discussed in this review. The term “MXene” refers to the broad family of 2D MXenes, with numerous carbide formulations. (b) Key properties of these materials, which drive performance and enable incorporating multiple interaction modalities within single devices. (c) Primary synthesis methods, and (d) main fabrication processes for neuroelectronic interfaces based on the nanocarbons and MXenes shown in (a).
Implantable interfaces for brain recording and stimulation
Nanocarbons and carbides have numerous properties that make them ideal for implantable neural interfaces. Their nanostructured topology results in a high volumetric surface area. Coupled with their high electronic conductivity and capacitance, these enhanced interfacial features act to promote charge transfer and signal transduction between the electrodes and the neural circuits. From a performance standpoint, this translates into safer and more efficient charge injection during stimulation, as well as improved signal quality for microscale recording electrodes. Key early demonstrations involved carbon nanotube coatings on tungsten and stainless steel microwires [11]. Since then, the library of nanocarbons proposed in neuroelectronic interfaces has greatly expanded. Recently, reduced graphene oxide (rGO) and platinum (Pt) black coatings electrodeposited on planar Pt microelectrodes resulted in >60x impedance reduction, allowing detection of neuronal spikes chronically in epileptic mice [12]. A major limitation of coatings, however, is the poor adhesion with the underlying substrate, which can lead to degradation and delamination. Alternative strategies include the direct synthesis of nanocarbons on supporting polymeric, semiconducting, or metallic substrates, or the assembly and patterning of free-standing films followed by selective polymer encapsulation. Recently, it was shown that porous graphene micro-electrocorticography (μECoG) arrays can be directly formed by laser pyrolysis of polyimide films [13,14]. These high-surface area electrodes show low impedance and high charge injection capacity (CIC), allowing mapping cortical dynamics at high-resolution [13] and delivering neural stimulation in vivo [14].
Many nanocarbon allotropes are synthesized through high-temperature chemical vapor deposition (CVD). These processes are incompatible with common flexible polymer substrates and require several post-processing steps to transfer and pattern films. In addition to direct laser patterning, solution processing offers another scalable and high-throughput manufacturing route. Due to abundant surface terminations, Ti3C2 MXene is hydrophilic and processable in additive-free water-based dispersions. Ti3C2 MXene films with electrical conductivity of up to 20,000 S/cm have been recently produced, surpassing the conductivity of all other solution-processed 2D materials [15,16]. Recently, solution processing of Ti3C2 MXene coupled with conventional microfabrication has been shown to be a feasible route to form neural microelectrode arrays on 10 μm-thick parylene substrates. Both μECoG and laminar microelectrode arrays from Ti3C2 MXene show 4x reduced impedance and susceptibility to noise interference, as well as improved sensitivity and signal-to-noise ratio (SNR) for recording neuronal spiking activity compared to size-matched Au electrodes [17] [18]**. In another demonstration, Ti3C2 MXene-based electrodes were shown to possess lower impedance and >10x enhanced CIC and charge storage capacity (CSC) compared to size-matched Pt electrodes, and they were used for neural recording in swine and neural microstimulation in rats [19]**.
Another emerging nanocarbon for high resolution implantable neural interfaces is boron or nitrogen-doped nanocrystalline diamond (NCD). Nitrogen-doped ultrananocrystalline diamond (N-UNCD) has been proposed in high-density arrays of 3D pillar electrodes for localized stimulation in high-acuity retinal prostheses [20] and in hybrid carbon fiber electrodes with N-UNCD selectively deposited at the tip for neural stimulation, high-quality single-unit neural recording, and neurotransmitter detection [21].
In addition to enhancing signal transduction capabilities, neural interfaces incorporating nanocarbons can have high mechanical flexibility, resulting from the high aspect ratios of nanomaterials, which is essential to minimize the brain inflammatory response and ensure implant longevity. For example, highly flexible and stretchable μECoG arrays of densely entangled CNTs exhibit excellent chronic recording performance and elicit minimal tissue reaction [5]. In this report, the CNT-based devices remained intact after two months of implantation, while comparison Pt electrodes showed damage and delamination due to the reduced compliance and stretchability of the Pt films compared to the CNT films. Beyond planar cortical arrays, highly flexible microwire electrodes can target deeper brain structures while also minimizing tissue damage. CNTs can be spun into thin and flexible fiber microwires (~10–100 μm in diameter) using either dry- or wet-spinning from liquid crystalline dispersions, yielding low-impedance, high-CIC electrodes [22,23]. Microwire electrodes can also be fabricated by wet-spinning liquid crystalline dispersions of graphene oxide (LCGO), resulting in graphene fibers with highly aligned graphene sheets in a porous, high surface area structure [24]. LCGO microwire electrodes, with a Pt coating on one side acting as current collector, show high SNR in single-unit recordings and high CIC [24]. While these strategies allow fabricating ultra-thin and flexible electrodes, a notable challenge is the insertion of these devices into the target brain structure without buckling. A variety of strategies relying on chemical, mechanical, fluidic, or magnetic approaches have been proposed to address this issue [25].
Field effect transistors (FETs) have also been proposed as alternatives to passive recording electrodes. FET arrays can incorporate active multiplexing to achieve higher density of spatial sampling and reduce wiring. Graphene solution-gated FETs (gSGFETs) leverage the high flexibility, electrochemical inertness, DC stability, and high carrier mobility of pristine CVD graphene [26–28]. Recent works have shown that gSGFETs can detect low-frequency infraslow brain activity (<0.1 Hz), which is precluded from traditional microelectrodes because their high impedance at low frequencies results in a voltage divider effect that degrades low-frequency components of the signal [26]*. Switchless multiplexing of gSGFETs with an amplitude modulation scheme allows high spatial sampling and wireless recordings with flexible μECoG arrays [27]** [28], while significantly simplifying the fabrication scheme compared to flexible FETs formed from ultrathin silicon layers [29]. Graphene FETs have also been combined with scanning photocurrent microscopy for high temporal resolution mapping of individual synapses and spines in cell cultures by leveraging the ultrafast photocurrent response of graphene [30].
Peripheral nerve interfaces
Bioelectronic technologies for interfacing with peripheral nerves are commonly used in electrodiagnostic and rehabilitation medicine, as well as for treating a variety of neurological, inflammatory, and organ-specific conditions [31]. Soft and flexible microscale interfaces based on nanocarbons and MXenes are well-suited for interfacing with curved peripheral circuits at scales ranging from single axons to the entire nerve bundle surface, while minimizing geometric and mechanical mismatches that prevent current state-of-the-art peripheral nerve interfaces from providing robust, chronic interfaces in vivo. Parylene-insulated CNT fibers and yarns with diameters ranging from 12 [32] to 500 μm [33] have been implanted as recording and stimulating microwires in the zebra finch hypoglossal nerves [32] and in the rat tibial nerves [33]. The combined high flexibility and tensile strength of CNT microwires allows different implantation strategies, ranging from threading the microwires through a nanoclip affixed to the nerve, to direct needle-assisted insertion. Planar electrodes from surfactant-stabilized aqueous dispersions of CNTs cast on polydimethylsiloxane (PDMS), with impedances ~7 to 80x smaller than that of comparison Au electrodes on PDMS, have also been proposed for stimulating the central nerve cord of a horse leech [34].
Graphene oxide (GO) is stable in aqueous suspensions and is ideal for high-throughput fabrication on larger scales. In a recent demonstration, nanoribbons were formed via direct ink printing in which GO flakes self-assembled within in a sodium alginate-matrix solution. The GO nanoribbons act as nanoscale fluidic channels with high ionic conductivity and have been used for recording and stimulation of sciatic nerves in bullfrogs [35]*. Furthermore, carboxylic GO-composited polypyrrole/poly-L-lactic acid films have shown efficacy as conductive conduits to bridge 10-mm sciatic nerve defects in rats and promote nerve injury repair through electrical stimulation [36].
Multimodal mapping technologies
Transparent optoelectronic interfaces
Devices that allow combining multiple interfacing modalities can provide new insights into the dynamics of neural circuits underlying function, behavior, and disease. In particular, optical methods of recording and modulating the activity of neurons and other cells have distinct advantages, such as selective targeting of specific cell types at finely tuned spatial scales.
Nanocarbons have emerged as ideal materials for transparent electronics in multimodal studies combining optical and electrical mapping (Figure 1). Graphene, with its 2D structure and high optical transmittance combined with high conductivity, has gained particular attention for these applications. From the first pioneering works demonstrating flexible μECoG arrays from HNO3 doped-single or multilayer graphene [37,38], a number of transparent optoelectronic interfaces have been developed and adopted in basic neuroscience studies. Transparent graphene arrays have been used for artifact-free electrophysiology combined with ~1 mm deep 2-photon imaging and optogenetic stimulation [39], for probing the mechanisms of therapeutic electrical stimulation via optical imaging [40], and for studying epileptic seizure dynamics using simultaneous electrophysiology and wide-field epifluorescence imaging [41].
Despite these exciting advances, the interface impedance of graphene electrodes is too high to realize high-density mapping with single-cell resolution, even with processing optimization and chemical doping. One strategy to overcome this limitation is to electrochemically deposit Pt nanoparticles on the surface of the graphene contacts [42]. This approach yielded ~100x reduction in the electrode impedance, though at a slight cost to optical transparency [42]. Beyond graphene, CNTs have also been adopted in optoelectronic neural interfaces. Compared to CVD-grown graphene, transparent CNTs films have a lower interface impedance and higher degree of resistance to bending and stretching. Transparent μECoG arrays from CNT films have been used for simultaneous electrophysiology, optical imaging, and optogenetic stimulation in rodent models of traumatic brain injury, where both the brain and the device were subjected to rapid deformations [43]*.
Electrochemical sensors
Chemical species such as neurotransmitters, hormones, and metabolic substrates/products play key roles in mediating and modulating neural functions. Devices that integrate electrophysiological recording with electrochemical sensing enable investigating the interplay between chemical and electrophysiological signaling in neural circuits. Traditionally, the detection of neurotransmitters and other brain analytes has relied on 7–10 μm carbon fibers in glass pipette electrodes. These devices have significant drawbacks, such as the mechanical fragility of carbon fibers, their susceptibility to biofouling, and the compromise between impedance and surface area that precludes the ability to record at single unit resolution. Nanocarbons are well suited for in vivo electrochemical sensing due to their high surface area, fast charge transfer kinetics, electrocatalytic nature, and high resistance to biofouling [44]. For example, electrodeposited rGO/Pt on a laminar electrode probe enabled simultaneous glutamate detection and single neuron recordings by functionalization with glutamate oxidase [12]. Nanodiamond is also gaining attention for electrochemical sensing due to its wide potential window, low baseline current, and improved stability and longevity in vivo compared to carbon fibers [45]. Boron-doped diamond-coated tungsten electrodes have been used for in vivo monitoring of dopamine (DA) and adenosine in the human brain, and for long-term monitoring in a swine model. In the chronic setting, diamond-based electrodes show enhanced stability during prolonged fast-scanning cyclic voltammetry and robustness against tip degradation [45]*. N-UNCD selectively deposited at the tip of carbon fiber electrodes has also been shown to be highly sensitive and selective to DA, in addition to offering neural recording and stimulation capabilities [21]. In another demonstration, CNT fibers assembled into ultra-flexible helical bundles were individually functionalized for simultaneous detection of different analytes in vivo, including H2O2, prostate-specific antigens (PSAs), and glucose [46]**. In the same structure, sensing terminals could be distributed along the helical bundle length to produce a μm-scale spatial gradient map of a single analyte in tissues [46]**. This platform demonstrated the versatility of CNTs for electrochemical sensing and could easily be adapted for sensing of neurochemicals in the brain.
MRI compatible electrodes
MRI is an invaluable tool for post-operatively localizing deep brain stimulation (DBS) leads and electrodes implanted for epilepsy evaluation. Combining structural and functional (fMRI) brain mapping with high-resolution electrophysiological recordings or DBS stimulation is also extremely valuable for basic neuroscience research. Traditionally, DBS and other implantable brain electrodes are made of Pt or Pt-Ir alloys. These materials have a large magnetic susceptibility mismatch with tissues, resulting in artifacts or signal loss around the electrodes [47]. Nanocarbons and carbides, by comparison, have magnetic susceptibility much closer to that of native tissues. This property can be leveraged to realize electrodes that are not only safe for use during MRI, but also do not induce imaging artifacts and distortions. CNT disk electrodes, with magnetic susceptibility of −5.9 to −8.1 ppm, have been shown to produce no distortions in MR images because of their close match to native tissues (−9.0 ppm), in contrast to Pt-Ir alloys (~240 ppm) [48]. Similarly, soft, flexible CNT fiber electrodes are virtually artifact-free when implanted into a rat brain and imaged in a 7.0 T MRI scanner [49]. Graphene fiber electrodes fabricated from GO suspensions have also been used for simultaneous DBS and artifact-free fMRI in the subthalamic nuclei of Parkinsonian rats [50]*. This multimodal paradigm, made possible by artifact-free nanocarbon electrodes, can serve as a useful tool for studying the effects of electrical stimulation on brain network activity in vivo to investigate mechanisms of DBS [50]*. Ti3C2 MXene electrodes, with magnetic susceptibility of 0.21 ppm, were also shown to enable artifact-free MRI imaging, and they were additionally compatible with computed tomography (CT) imaging [19]**.
Wearable sensors
Skin-mounted electrodes for monitoring a number of biometric variables are common across many areas of healthcare for disease prevention, diagnosis, and treatment. These devices are also gaining traction in consumer electronics, such as in smartwatches and wearable biosensors for videogaming and fitness tracking. Conventional electrodes for epidermal electrophysiology include “wet” Ag/AgCl and dry (i.e., gel-free) metal electrodes. In wet electrodes, the conductive gel used to lower the interface impedance and improve the electrode-skin contact can cause irritation and skin breakdown, as well as signal instability in long-term recordings. Despite these drawbacks, wet electrodes are the mainstay in scalp electroencephalography (EEG) for neurodiagnostics and monitoring, surface electromyography (sEMG) for diagnosing nerve and neuromuscular disorders, and electrooculography (EOG) for evaluating retinal pathologies. Dry metal electrodes overcome the issues caused by gels, but are typically stiff, prone to noise and motion artifacts, and offer poor spatial resolution. Nanocarbons and carbides assembled into flexible, conformal, and stretchable structures offer great promise for dry, low-impedance, high-fidelity skin sensors with potential to be integrated into many types of wearable sensor technologies. Graphene electronic tattoos, fabricated by sandwiching a CVD-grown graphene monolayer between liquid bandage and polymethyl methacrylate substrates, have been used for human EOG with angular resolution of up to 4° [51]. The limited stretchability of graphene, however, is an issue in wearable applications where the sensors are required to follow skin deformations and sustain repeated cycles of application and removal. To overcome this challenge, a mechanically-robust structure comprising graphitized fibers electrospun onto monolayer graphene and semi-embedded into soft elastomers has been demonstrated for EEG and sEMG with stability to repeated skin placement [52]. Recently, rGO has also been widely explored in wearable applications. Some examples include planar multichannel rGO electrodes embedded into PDMS for EMG, EOG, and EEG recordings and hand gesture recognition [53], rGO flakes bridged by silver nanowires (AgNWs) for EEG [54], and rGO-nylon membranes for EEG and sEMG [55]. In these structures, the integration of rGO with PDMS, AgNWs, and nylon membranes serves to increase the device stability under bending strains and reduce cracking. MXenes also show great potential for large-scale epidermal electronics due to their higher conductivity compared to rGO and facile, scalable processing from aqueous dispersions. Ti3C2 MXene multichannel electrode arrays have been recently proposed for high-density, high-resolution, low-noise sEMG [56]*. These arrays demonstrated 100- to 1000-fold lower impedance, which translated to higher SNR and spatiotemporal resolution than commercially available gelled electrodes for finely mapping sEMG across small muscle groups during resisted flexion. In another demonstration, Ti3C2 MXene-based electrodes produced in a range of geometries showed excellent performance for gel-free recording of sEMG, EEG, EOG, and electrocardiography (ECG) [19]**. Ti3C2 MXene has also been inkjet-printed into a flexible electrode patch for sweat analysis and cytokine detection [57]. Direct inkjet-printing of MXene can be easily adapted for specific neuroelectronic applications.
Novel transduction modalities
In addition to traditional electrophysiological recording and stimulation, and more recent approaches for functional optical mapping and manipulation, nanocarbons are advancing novel tools for interrogating neural structures at unprecedented temporal resolution and spatial scales. Specifically, nanoscale transducers based on nanocarbons are enabling novel approaches for contactless, nongenetic control of local neural activity by leveraging the high bidirectional conversion efficiency of optical, magnetic and acoustic stimuli into biological signals. Photothermal stimulation via nanoparticle-mediated light-to-heat conversion is emerging as an alternative to optogenetics for remote neuromodulation, as it allows subcellular-scale precision without requiring genetic modifications. Three-dimensional fuzzy graphene (NT-3DFG), consisting of 2D mono- and few-layer graphene flakes CVD-grown out-of-plane on 1D Si nanowires, has recently been proposed as a nanoagent for photothermal stimulation due to its high photothermal conversion efficiency, broadband absorption spectra, and ease of peptide functionalization to promote cell adhesion and aqueous stability [58]*. NT-3DFG has been demonstrated for effective activation of rat dorsal root ganglion neurons and 3D cortical neural spheroids via photothermal stimulation at 10 to 200x lower laser power densities compared to Au and Si nanoparticles [58]*. Ti3C2 MXene exhibits peak absorption in the near-infrared (NIR) range, which is beneficial for biomedical applications as the radiation penetration in the tissue is higher. Recently, Ti3C2 has been demonstrated to be a suitable material for NIR photothermal neuromodulation at subcellular scale, both as single-flake as well as in film form [59].
Quantum biosensors rely on nitrogen-vacancy (NV) complexes in diamond for optically transducing magnetic, electrical, and thermal cellular signaling [60]. The naturally-occurring system of the nitrogen atom, neighboring vacancy-type defect, and three adjacent carbon atoms possesses a tetrahedral (C3v) symmetry, while an additional trapped electron gives the system a total electronic spin of S = 1 and a desirable electronic structure for quantum sensing. The electronic spin levels of the NV center are highly sensitive to magnetic fields and thermal fluctuations and can be interrogated via the optically detected magnetic resonance (ODMR) technique, which involves laser pump initialization and fluorescence readout. Through the Zeeman effect, NV sensors can detect external magnetic fields transduced as resonance frequency shifts in the NV fluorescence spectrum. A NV magnetometer, based on a single-crystal diamond chip grown via CVD, has recorded single-neuron action potentials on the exterior of marine fanworms [61]. Intracellular thermometry has also been shown with colloidal suspensions of nanodiamonds (~170 nm diameter and NV densities ranging from 500 [62] to 900 NVs/particle [63]*) dispersed within mouse primary cortical neurons [62] and C. elegans in vivo [63]*. ODMR from the NV centers provides intracellular temperature maps [62] as well as temperature dynamics of chemically stimulated worm thermogenesis [63]*.
Conclusion
Intense research efforts from the materials science and neuroengineering communities have pushed nanocarbons and carbides to the forefront for enabling high-resolution, multifunctional neuroelectronic interfaces. The unique combination of nanostructured topology, high surface area, electronic conductivity, and capacitance allows establishing bidirectional communication with neural circuits through electrical recording and stimulation, while the enhanced flexibility and strength endows the devices with softness and compliance matching the native tissue. Additional advantages include optical transparency, low magnetic susceptibility, electrocatalytic behavior, and photothermal efficiency, which can be leveraged to integrate additional functionalities into multifunctional platforms, such as optical and MR imaging, electrochemical sensing, and photothermal stimulation. The recent works highlighted in this review and summarized in Table 1 demonstrate the capabilities of nanocarbons and carbides to transduce and modulate neural signals in vivo across multiple modalities and scales. To fully evaluate the feasibility and potential of these nanomaterials for clinical translation, several challenges must still be addressed including: 1) establishing the safety and long-term stability of the devices in vivo over periods of months to years in a range of relevant animal models, 2) further increasing the resolution and density to monitor and control large networks with sub-cellular resolution, 3) scaling up the materials synthesis, processing, and manufacturing pipelines to meet demand.
Table 1.
Overview of the emerging neuroelectronic technologies based on nanocarbon or carbide materials for in vivo use.
| Technology | Device type | Materials | Functionality | Spatial scale | Ref. |
|---|---|---|---|---|---|
| Implantable brain interfaces | Depth array | rGO & Pt black coating | Recording & glutamate sensing | 25 μm | [12] |
| ECoG array | Porous graphene (pyrolyzed) | Recording & stimulation | 250 | [13,14] | |
| ECoG & depth arrays | Ti3C2 MXene | Recording | 25–50 μm | [17,18]** | |
| ECoG arrays | Recording & stimulation | 500 μm | [19]** | ||
| Microwires | Carbon fiber & N-UNCD | Recording, stimulation, & dopamine sensing | 10 μm | [21] | |
| ECoG array | CNTs | Recording | 50 μm | [5] | |
| Microwires | Recording & stimulation | 53 μm-88 μm | [22] | ||
| Microwires | Recording | 12 μm | [23] | ||
| Microwires | rGO | Recording | 20 – 40 μm | [24] | |
| FET cortical & depth arrays | CVD graphene | Recording | 50 – 100 μm | [26]* | |
| FET cortical array | [27]**,[28] | ||||
| FET array | Synaptic recording | 40 μm | [30] | ||
| Retinal electrodes | Planar 3D pillar array | N-UNCD | Stimulation | 80 μm | [20] |
| Peripheral nerve interfaces | Microwires | CNTs | Recording & stimulation | 12 μm | [32] |
| Microwires | Recording & stimulation | 500 μm | [33] | ||
| Planar electrode | Stimulation | 160 μm | [34] | ||
| Nanofluidic channels | GO | Recording & stimulation | 3 mm | [35]* | |
| Cuff electrode | Carboxylic GO | Stimulation | 2 mm | [36] | |
| Optoelectronic interfaces | ECoG arrays | CVD graphene | Recording, optical stimulation and imaging | 100 μm | [39] |
| Stimulation & optical imaging | 100–200 μm | [40] | |||
| Recording & optical imaging | 50 μm | [41] | |||
| CVD graphene & Pt nanoparticles | Recording & optical imaging | 100 μm | [42] | ||
| CNTs | Recording, optical stimulation and imaging | 100 μm | [43]* | ||
| Electrochemical sensors | Fiber bundle | CNTs | Recording & multiple species sensing | 50 μm | [46]** |
| Microwires | Boron-doped diamond | Dopamine sensing | 50 μm | [45] | |
| MRI-compatible interfaces | Depth array | CNTs | Stimulation | 3 mm | [48] |
| Microwires | Recording & MR imaging | 5–20 μm | [49] | ||
| Microwires | rGO | Recording & MR imaging | 75 μm | [50]* | |
| Wearable sensors | Planar electrode | CVD graphene | EOG | ~5–10 mm | [51] |
| Planar electrode | EEG, EMG | 10 – 20 mm | [52] | ||
| Electrode array | rGO | EEG, EOG, EMG | 10 mm | [53] | |
| Planar electrode | EEG | ~1 cm | [54] | ||
| Planar electrode | EEG, EMG | ~1–5 cm | [55] | ||
| Electrode array | Ti3C2 MXene | EMG | 1.6 mm | [56]* | |
| Electrode array | EMG, EEG, ECG, EOG | 3 mm | [19]** | ||
| Contactless, nongenetic neuromodulation | Microfibers | NT-3DFG | Photothermal stimulation | 1.4 μm | [58]* |
| Single nanoflakes | Ti3C2 MXene | ~10 μm | [59] | ||
| Quantum biosensors | Single-crystal diamond chip | Diamond NV centers | Recording | 4 mm x 13 μm NV thickness | [61] |
| Thermometry | 170 nm | [62] | |||
| Single nanodiamonds | Nanodiamond NV centers | 168 nm | [63]* |
Acknowledgments
The authors would like to acknowledge the support of the National Institutes of Health (K12HD073945, R21NS106434, R01NS121219). Portions of Figure 1 and Figure 2 were created with BioRender.com.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest statement
Nothing declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1. Rastogi SK, Kalmykov A, Johnson N, Cohen-Karni T: Bioelectronics with nanocarbons. J Mater Chem B 2018, 22:649–652. ** This article is an extensive and comprehensive review on properties, safety, and emerging applications of nanocarbons in the broad context of bioelectronics beyond the nervous system, spanning from in vitro tissue engineering platforms, to implantable devices for in vivo electrophysiology.
- 2.Shuck CE, Sarycheva A, Anayee M, Levitt A, Zhu Y, Uzun S, Balitskiy V, Zahorodna V, Gogotsi O, Gogotsi Y: Scalable Synthesis of Ti3C2Tx MXene. Adv Eng Mater 2020, 22:1901241. [Google Scholar]
- 3.Rahmati M, Mozafari M: Biological response to carbon-family nanomaterials: Interactions at the nano-bio interface. Front Bioeng Biotechnol 2019, 7:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan JS, Orecchioni M, Vitale F, Coco JA, Duret G, Antonucci S, Pamulapati SS, Taylor LW, Dewey OS, Di Sante M, et al. : Biocompatibility studies of macroscopic fibers made from carbon nanotubes: Implications for carbon nanotube macrostructures in biomedical applications. Carbon N Y 2021, 173:462–476. [Google Scholar]
- 5.Pavone L, Moyanova S, Mastroiacovo F, Fazi L, Busceti C, Gaglione A, Martinello K, Fucile S, Bucci D, Prioriello A, et al. : Chronic neural interfacing with cerebral cortex using single-walled carbon nanotube-polymer grids. J Neural Eng 2020, 17. [DOI] [PubMed] [Google Scholar]
- 6.Rastogi SK, Raghavan G, Yang G, Cohen-Karni T: Effect of Graphene on Nonneuronal and Neuronal Cell Viability and Stress. Nano Lett 2017, 17:3297–3301. [DOI] [PubMed] [Google Scholar]
- 7.Gazzi A, Fusco L, Orecchioni M, Ferrari S, Franzoni G, Yan JS, Rieckher M, Peng G, Lucherelli MA, Vacchi IA, et al. : Graphene, other carbon nanomaterials and the immune system: toward nanoimmunity-by-design. J Phys Mater 2020, 3. [Google Scholar]
- 8.Nguyen D, Valet M, Dégardin J, Boucherit L, Illa X, de la Cruz J, del Corro E, Bousquet J, Garrido JA, Hébert C, et al. : Novel Graphene Electrode for Retinal Implants: An in vivo Biocompatibility Study. Front Neurosci 2021, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soleymaniha M, Shahbazi M-A, Rafieerad AR, Maleki A, Amiri A: Promoting Role of MXene Nanosheets in Biomedical Sciences: Therapeutic and Biosensing Innovations. Adv Healthc Mater 2019, 8. [DOI] [PubMed] [Google Scholar]
- 10.Unal MA, Bayrakdar F, Fusco L, Besbinar O, Shuck CE, Yalcin S, Erken MT, Ozkul A, Gurcan C, Panatli O, et al. : 2D MXenes with antiviral and immunomodulatory properties: a pilot study against SARS-CoV-2. Nano Today 2021, doi: 10.1016/j.nantod.2021.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW: Carbon nanotube coating improves neuronal recordings. Nat Nanotechnol 2008, 3:434–439. [DOI] [PubMed] [Google Scholar]
- 12.Xiao G, Song Y, Zhang Y, Xing Y, Xu S, Lu Z, Wang M, Cai X: Cellular-Scale Microelectrode Arrays to Monitor Movement-Related Neuron Activities in the Epileptic Hippocampus of Awake Mice. IEEE Trans Biomed Eng 2021, 68:19–25. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Lu Y, Kuzum D: High-Density Porous Graphene Arrays Enable Detection and Analysis of Propagating Cortical Waves and Spirals. Sci Rep 2018, 8:17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Lyu H, Richardson AG, Lucas TH, Kuzum D: Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing. Sci Rep 2016, 6:33526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathis T, Maleski K, Goad A, Sarycheva A, Anayee M, Foucher AC, Stach E, Gogotsi Y, Mathis C, Mathis TS, et al. : Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ChemRxiv 2020, doi: 10.26434/chemrxiv.12805280.v1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang CJ, Anasori B, Seral-Ascaso A, Park S-H, McEvoy N, Shmeliov A, Duesberg GS, Coleman JN, Gogotsi Y, Nicolosi V: Transparent, Flexible, and Conductive 2D Titanium Carbide (MXene) Films with High Volumetric Capacitance. Adv Mater 2017, 29:1702678. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll N, Maleski K, Richardson AG, Murphy B, Anasori B, Lucas TH, Gogotsi Y, Vitale F: Fabrication of Ti3C2 MXene Microelectrode Arrays for In Vivo Neural Recording. J Vis Exp 2020, 15:1–9. [DOI] [PubMed] [Google Scholar]
- 18. Driscoll N, Richardson AG, Maleski K, Anasori B, Adewole O, Lelyukh P, Escobedo L, Cullen DK, Lucas TH, Gogotsi Y, et al. : Two-Dimensional Ti3C2 MXene for High-Resolution Neural Interfaces. ACS Nano 2018, 12:10419–10429. ** This work presents the first example of a MXene-based sensor for in vivo electrophysiology, showing that Ti3C2 MXene is a promising candidate for low-impedance, high-performance recording electrodes. Solution processing methods were used to produce the electrodes, demonstrating a unique advantage of water-soluble MXenes, and the biocompatibility of Ti3C2 MXene to neurons was also reported.
- 19. Driscoll N, Erickson B, Murphy BB, Richardson AG, Robbins G, Apollo N V, Mathis T, Hantanasirisakul K, Bagga P, Gullbrand SE, et al. : MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci Transl Med 2021, 13:eabf8629. ** This work established a simple and low-cost method to fabricate Ti3C2 MXene-based electrodes, named “MXtrodes” in a range of sizes and geometries for epidermal and implantable sensing and stimulation. Arrays of mm-scale MXtrodes were demonstrated for gel-free epidermal recording of EEG, ECG, EMG, and EOG and ~500 μm MXtrodes were demonstrated for ECoG recording in the swine brain and microstimulation of rat cortex. This study also established the compatibility of Ti3C2 MXene-based electrodes with both MRI and CT imaging.
- 20.Stamp MEM, Tong W, Ganesan K, Prawer S, Ibbotson MR, Garrett DJ: 3D Diamond Electrode Array for High-Acuity Stimulation in Neural Tissue. ACS Appl Bio Mater 2020, 3:1544–1552. [DOI] [PubMed] [Google Scholar]
- 21.Hejazi MA, Tong W, Stacey A, Soto-Breceda A, Ibbotson MR, Yunzab M, Maturana MI, Almasi A, Jung YJ, Sun S, et al. : Hybrid diamond/ carbon fiber microelectrodes enable multimodal electrical/chemical neural interfacing. Biomaterials 2020, 230:119648. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez NT, Buschbeck E, Miller S, Le AD, Gupta VK, Ruhunage C, Vilinsky I, Ma Y: Carbon Nanotube Fibers for Neural Recording and Stimulation. ACS Appl Bio Mater 2020, 3:6478–6487. [DOI] [PubMed] [Google Scholar]
- 23.Vitale F, Vercosa DG, Rodriguez AV, Pamulapati SS, Seibt F, Lewis E, Yan JS, Badhiwala K, Adnan M, Royer-Carfagni G: Fluidic microactuation of flexible electrodes for neural recording. Nano Lett 2018, 18:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K, Frewin CL, Esrafilzadeh D, Yu C, Wang C, Pancrazio JJ, Romero-Ortega M, Jalili R, Wallace G: High-Performance Graphene-Fiber-Based Neural Recording Microelectrodes. Adv Mater 2019, 31:1–10. [DOI] [PubMed] [Google Scholar]
- 25.Apollo NV, Murphy B, Prezelski K, Driscoll N, Richardson AG, Lucas TH, Vitale F: Gels, jets, mosquitoes, and magnets: a review of implantation strategies for soft neural probes. J Neural Eng 2020, 17:041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masvidal-Codina E, Illa X, Dasilva M, Calia AB, Dragojević T, Vidal-Rosas EE, Prats-Alfonso E, Martínez-Aguilar J, De la Cruz JM, Garcia-Cortadella R, et al. : High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat Mater 2019, 18:280–288. * This study demonstrated key advantages of graphene SGFETs over traditional microelectrodes for recording infraslow brain activity by mapping cortical spreading depressions both across the cortical surface and spanning the depth of rat cortex.
- 27. Garcia-Cortadella R, Schäfer N, Cisneros-Fernandez J, Ré L, Illa X, Schwesig G, Moya A, Santiago S, Guirado G, Villa R, et al. : Switchless multiplexing of graphene active sensor arrays for brain mapping. Nano Lett 2020, 20:3528–3537. ** This manuscript presented a novel approach for multiplexing graphene SGFETs that eliminates the need for on-site switches, significantly reducing fabrication complexity while enabling sensor arrays with high spatial sampling.
- 28.Garcia-Cortadella R, Schwesig G, Jeschke C, Illa X, Gray AL, Savage S, Stamatidou E, Schiessl I, Masvidal-Codina E, Kostarelos K, et al. : Graphene active sensor arrays for long-term and wireless mapping of wide frequency band epicortical brain activity. Nat Commun 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco J a, Kim Y-S, Avrin AE, Tiruvadi VR, Hwang S-W, Vanleer AC, et al. : Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci 2011, 14:1599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R, Shi M, Brewer B, Yang L, Zhang Y, Webb DJ, Li D, Xu YQ: Ultrasensitive Graphene Optoelectronic Probes for Recording Electrical Activities of Individual Synapses. Nano Lett 2018, 18:5702–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitale F, Litt B: Bioelectronics: the promise of leveraging the body’s circuitry to treat disease. Bioelectron Med 2018, 1:3–7. [Google Scholar]
- 32.Lissandrello CA, Gillis WF, Shen J, Pearre BW, Vitale F, Pasquali M, Holinski BJ, Chew DJ, White AE, Gardner TJ: A micro-scale printable nanoclip for electrical stimulation and recording in small nerves. J Neural Eng 2017, 14:36006–36012. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Su JYY, Guo JYY, Zhang XHH, Li RHH, Chai XYY, Chen Y, Zhang DGG, Wang JGG, Sui XHH, et al. : Spatiotemporal characteristics of neural activity in tibial nerves with carbon nanotube yarn electrodes. J Neurosci Methods 2019, 328:1–10. [DOI] [PubMed] [Google Scholar]
- 34.Terkan K, Zurita F, Jamal Khalaf T, Rinklin P, Teshima T, Kohl T, Wolfrum B: Soft peripheral nerve interface made from carbon nanotubes embedded in silicone. APL Mater 2020, 8. [Google Scholar]
- 35. Zhang M, Guo R, Chen K, Wang Y, Niu J, Guo Y, Zhang Y, Yin Z, Xia K, Zhou B, et al. : Microribbons composed of directionally self-assembled nanoflakes as highly stretchable ionic neural electrodes. Proc Natl Acad Sci 2020, 117. * This work is notable for utilizing GO microribbons as an ionic conductor instead of electronic. The nanoribbons can spontaneously transform into helical springs upon water stimulation, becoming a soft and stretchable ionic neural electrode with superior ionic conductivity that did not diminish under physical deformation and performed better than the Pt wire control electrodes.
- 36.Chen X, Liu C, Huang Z, Pu X, Shang L, Yin G, Xue C: Preparation of carboxylic graphene oxide-composited polypyrrole conduits and their effect on sciatic nerve repair under electrical stimulation. J Biomed Mater Res - Part A 2019, 107:2784–2795. [DOI] [PubMed] [Google Scholar]
- 37.Kuzum D, Takano H, Shim E, Reed JC, Juul H, Richardson AG, de Vries J, Bink H, Dichter MA, Lucas TH, et al. : Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat Commun 2014, 5:5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park D-W, Schendel AA, Mikael S, Brodnick SK, Richner TJ, Ness JP, Hayat MR, Atry F, Frye ST, Pashaie R, et al. : Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat Commun 2014, 5:5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thunemann M, Lu Y, Liu X, Kılıç K, Desjardins M, Vandenberghe M, Sadegh S, Saisan PA, Cheng Q, Weldy KL, et al. : Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays. Nat Commun 2018, 9:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park D-W, Ness JP, Brodnick SK, Esquibel C, Novello J, Atry F, Baek D-H, Kim H, Bong J, Swanson KI, et al. : Electrical Neural Stimulation and Simultaneous in Vivo Monitoring with Transparent Graphene Electrode Arrays Implanted in GCaMP6f Mice. ACS Nano 2018, 12:148–157. [DOI] [PubMed] [Google Scholar]
- 41.Driscoll N, Rosch R, Murphy BB, Ashourvan A, Vishnubhotla R, Dickens OO, Johnson ATC, Davis KA, Litt B, Bassett DS, et al. : Multimodal in vivo recording using transparent graphene microelectrodes illuminates spatiotemporal seizure dynamics at the microscale. Commun Biol 2021, 4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Y, Liu X, Hattori R, Ren C, Zhang X, Komiyama T, Kuzum D: Ultralow Impedance Graphene Microelectrodes with High Optical Transparency for Simultaneous Deep Two-Photon Imaging in Transgenic Mice. Adv Funct Mater 2018, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang J, Liu X, Xu W, Luo W, Li M, Chu F, Xu L, Cao A, Guan J-S, Tang S, et al. : Stretchable transparent electrode array for simultaneous electrical and optical interrogation of neural circuits in vivo. Nano Lett 2018, doi: 10.1021/acs.nanolett.8b00087. * This report presents the first example of a transparent and stretchable electrode array for combining electrophysiology with calcium imaging and optogenetic stimulation by leveraging thin-film networks of CNTs. The stretchability, which is not achievable for transparent electronics using CVD graphene, allows these arrays to leveraged for real-time recording of traumatic brain injury, where the array and brain are subjected to rapid deformation.
- 44.Tan C, Robbins EM, Wu B, Cui XT: Recent Advances in In Vivo Neurochemical Monitoring. Micromachines 2021, 12:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennet KE, Tomshine JR, Min HK, Manciu FS, Marsh MP, Paek SB, Settell ML, Nicolai EN, Blaha CD, Kouzani AZ, et al. : A diamond-based electrode for detection of neurochemicals in the human brain. Front Hum Neurosci 2016, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang L, Xie S, Wang Z, Liu F, Yang Y, Tang C, Wu X, Liu P, Li Y, Saiyin H, et al. : Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat Biomed Eng 2020, 4:159–171. ** This manuscript presented functionalized CNT fiber bundles capable of simultaneously monitoring multiple biomarkers in vivo, as well as monitoring the spatial distribution of a single biomarker along the length of the fiber. By integrating these multifunctional sensors with wireless data transmission, they present a critical new tool for studying the contributions of specific biomolecules to disease states.
- 47.Schenck JF: The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys 1996, 23:815–850. [DOI] [PubMed] [Google Scholar]
- 48.Chen G, Dodson B, Johnson F, Hancu I, Fiveland E, Zhang W, Galligan C, Puleo C, Davis RC, Ashe J, et al. : Tissue-susceptibility matched carbon nanotube electrodes for magnetic resonance imaging. J Magn Reson 2018, 295. [DOI] [PubMed] [Google Scholar]
- 49.Lu L, Fu X, Liew Y, Zhang Y, Zhao S, Xu Z, Zhao J, Li D, Li Q, Stanley GB, et al. : Soft and MRI Compatible Neural Electrodes from Carbon Nanotube Fibers. Nano Lett 2019, 19:1577–1586. [DOI] [PubMed] [Google Scholar]
- 50. Zhao S, Li G, Tong C, Chen W, Wang P, Dai J, Fu X, Xu Z, Liu X, Lu L, et al. : Full activation pattern mapping by simultaneous deep brain stimulation and fMRI with graphene fiber electrodes. Nat Commun 2020, 11. * This pioneering study demonstrated the utility of MRI-compatible graphene fiber electrodes to enable simultaneous fMRI and DBS. This multimodal paradigm is a powerful technique for examining the modulatory effects of electrical stimulation on brain-wide network activity to understand therapeutic mechanisms of DBS.
- 51.Ameri SK, Kim M, Kuang IA, Perera WK, Alshiekh M, Jeong H, Topcu U, Akinwande D, Lu N: Imperceptible electrooculography graphene sensor system for human–robot interface. npj 2D Mater Appl 2018, 2. [Google Scholar]
- 52.Qiu J, Yu T, Zhang W, Zhao Z, Zhang Y, Ye G, Zhao Y, Du X, Liu X, Yang L, et al. : A Bioinspired, Durable, and Nondisposable Transparent Graphene Skin Electrode for Electrophysiological Signal Detection. ACS Mater Lett 2020, 2. [Google Scholar]
- 53.Li Z, Guo W, Huang Y, Zhu K, Yi H, Wu H: On-skin graphene electrodes for large area electrophysiological monitoring and human-machine interfaces. Carbon N Y 2020, 164:164–170. [Google Scholar]
- 54.Qiao Y, Wang Y, Jian J, Li M, Jiang G, Li X, Deng G, Ji S, Wei Y, Pang Y, et al. : Multifunctional and high-performance electronic skin based on silver nanowires bridging graphene. Carbon N Y 2020, 156:253–260. [Google Scholar]
- 55.Das PS, Park SH, Baik KY, Lee JW, Park JY: Thermally reduced graphene oxide-nylon membrane based epidermal sensor using vacuum filtration for wearable electrophysiological signals and human motion monitoring. Carbon N Y 2020, 158:386–393. [Google Scholar]
- 56. Murphy BB, Mulcahey PJ, Driscoll N, Richardson AG, Robbins GT, Apollo NV., Maleski K, Lucas TH, Gogotsi Y, Dillingham T, et al. : A Gel-Free Ti3C2Tx-Based Electrode Array for High-Density, High-Resolution Surface Electromyography. Adv Mater Technol 2020, 5. * This paper is the first realization of Ti3C2 MXene as gel-free epidermal sensors for obtaining high-density sEMG maps with low interfacial impedance and high skin conformability. This demonstrates Ti3C2 MXene to be a promising material for high-performance, wearable electrode applications.
- 57.Saleh A, Wustoni S, Bihar E, El-Demellawi JK, Zhang Y, Hama A, Druet V, Yudhanto A, Lubineau G, Alshareef HN, et al. : Inkjet-printed Ti3C2Tx MXene electrodes for multimodal cutaneous biosensing. J Phys Mater 2020, 3:044004. [Google Scholar]
- 58. Rastogi SK, Garg R, Scopelliti MG, Pinto BI, Hartung JE, Kim S, Murphey CGE, Johnson N, Roman DS, Bezanilla F, et al. : Remote nongenetic optical modulation of neuronal activity using fuzzy graphene. Proc Natl Acad Sci U S A 2020, 117:13339–13349. * This manuscript demonstrated the photothermal stimulation of rat neurons in 2D dorsal root ganglion (DRG) neurons and 3D cortical neural spheroids using nanowire-templated three-dimensional fuzzy graphene (NT-3DFG). Fuzzy graphene has high photothermal efficiency, requiring lower input laser energies, and provides a nongenetic, subcellular alternative to optogenetic and magnetogenetic techniques for neuronal modulation.
- 59.Wang Y, Garg R, Hartung JE, Goad A, Patel DA, Vitale F, Gold MS, Gogotsi Y, Cohen-Karni T: Ti3C2Tx MXene Flakes for Optical Control of Neuronal Electrical Activity. ACS Nano 2021, doi: 10.1021/acsnano.1c04431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrini G, Moreva E, Bernardi E, Traina P, Tomagra G, Carabelli V, Pietro Degiovanni I, Genovese M: Is a Quantum Biosensing Revolution Approaching? Perspectives in NV-Assisted Current and Thermal Biosensing in Living Cells. Adv Quantum Technol 2020, 3. [Google Scholar]
- 61.Barry JF, Turner MJ, Schloss JM, Glenn DR, Song Y, Lukin MD, Park H, Walsworth RL: Optical magnetic detection of single-neuron action potentials using quantum defects in diamond. Proc Natl Acad Sci 2016, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simpson DA, Morrisroe E, McCoey JM, Lombard AH, Mendis DC, Treussart F, Hall LT, Petrou S, Hollenberg LCL: Non-Neurotoxic Nanodiamond Probes for Intraneuronal Temperature Mapping. ACS Nano 2017, 11. [DOI] [PubMed] [Google Scholar]
- 63. Fujiwara M, Sun S, Dohms A, Nishimura Y, Suto K, Takezawa Y, Oshimi K, Zhao L, Sadzak N, Umehara Y, et al. : Real-time nanodiamond thermometry probing in vivo thermogenic responses. Sci Adv 2020, 6. * This experiment was the first demonstration of intracellular NV-based thermometry inside complex, multicellular organisms (C. elegans). It presents nanodiamonds as potential sensing platforms that can overcome the many technical challenges inherent to in vivo studies, such as the complex movement and structure of living organisms.


