Abstract
Background:
Deaths involving illicitly manufactured fentanyl (IMF) have increased since 2013 in the United States. Little research has examined individuals using IMF. This study aims to explore the characteristics of US adults who used IMF, heroin, or misused prescription opioids and examine the associations between demographic, clinical, psychosocial characteristics and IMF use.
Methods:
A convenience sample of adults aged ≥ 18 years being assessed for substance use disorder (SUD) treatment was collected between January-December 2019 using the Addiction Severity Index-Multimedia Version instrument. We used a multivariable logistic regression model to examine the associations between demographic, clinical, psychosocial characteristics and IMF use.
Results:
Adults reporting IMF as their primary lifetime substance use problem also reported using other substances—most often alcohol or heroin—both in the past 30 days and during their lifetime. Characteristics associated with increased odds of reporting IMF as the primary lifetime substance use problem included age 18–24 years (adjusted odds ratio (aOR) = 1.68; 95% confidence interval (CI) = 1.18–2.38) versus 45–54 years, non-Hispanic Black persons (aOR = 1.44; 95% CI = 1.11–1.85) versus non-Hispanic White persons, being assessed in Northeast (aOR = 15.46; 95% CI = 8.67–27.56) versus West, and having a history of at least one lifetime overdose (1 overdose (aOR = 1.91; 95% CI = 1.49–2.44); 2 overdoses (aOR = 1.95; 95% CI = 1.48–2.58); 3 or more overdoses (aOR = 2.27; 95% CI = 1.82–2.82)).
Conclusions:
These findings provide new insights into this high-risk population and help identify strategies to address increasing overdose death rates involving IMF. Opportunities for intervention include expanding naloxone distribution and harm reduction programs and connecting individuals with nonfatal overdoses to SUD treatment.
Keywords: Adults assessed for substance use disorder treatment, Illicitly manufactured fentanyl, Heroin, Prescription opioid misuse
1. Introduction
The opioid overdose epidemic in the United States continues to impact individuals, families, and communities across the country. An estimated 92,452 overdose deaths occurred in 2020, a 30% increase compared to 2019. The increase was driven by continued increases in overdose deaths involving synthetic opioids such as illicitly manufactured fentanyl and fentanyl analogs (referred to as IMF hereafter) (Ahmad et al., 2021). Data from CDC’s State Unintentional Drug Overdose Reporting System (SUDORS) underscore the dominant role IMF plays in the overdose crisis – with recent research from 24 states and the District of Columbia during January–June 2019 indicating that nearly 80% of overdose deaths involved one or more opioids and that IMF was involved in approximately 75% of these opioid-involved overdose deaths (O’Donnell et al., 2020).
Increases in synthetic opioid overdose deaths are closely linked to the rapid proliferation of IMF into the illicit drug supply since 2013 (Gladden et al., 2016). IMF enters the drug supply primarily as a heroin or cocaine adulterant (Ciccarone, 2017; DiSalvo et al., 2021; Mars et al., 2019; O’Donnell et al., 2017). Although previous research indicates that many individuals used IMF unintentionally (Amlani et al., 2015; Arfken et al., 2017; Kenney et al., 2018; Peiper et al., 2019), an increasing number of individuals are able to suspect IMF adulteration of their illicit drugs (Ciccarone et al., 2017; Mars et al., 2018; Morales et al., 2019). Furthermore, as IMF has become more available and awareness has increased, many individuals prefer and purposefully seek IMF (Chandra et al., 2021; Ciccarone et al., 2017; Kenney et al., 2018; Morales et al., 2019). Although research has examined the role of IMF in overdose deaths, including analyses by demographic factors, there is very little research examining individuals using IMF and the patterns of IMF use outside of small geographically limited studies (Arfken et al., 2017; Buresh et al., 2019; Chandra et al., 2021). This is largely due to an absence of questions related to IMF in traditional substance use surveillance systems such as the National Survey on Drug Use and Health, the Treatment Episode Data Set, and claims-based databases.
The National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO) provides in-depth information on substance use patterns, demographic information, and biopsychosocial challenges faced by individuals being assessed for substance use disorder (SUD) treatment. In 2017, NAVIPPRO added questions related to IMF. These new questions enable the first examination of the substance use patterns and unique challenges faced by individuals using IMF among a geographically diverse population of individuals being assessed for SUD treatment. Whether individuals who report IMF use as their primary lifetime substance use problem have distinct characteristics as compared to those who report heroin use or prescription opioid misuse as their primary lifetime substance use problem is also unknown. This paper aims to: 1) explore the characteristics of adults assessed for SUD treatment in the United States who reported IMF use, heroin use, or prescription opioid misuse as their primary lifetime substance use problem in 2019; and 2) examine the associations between demographic, clinical, psychosocial characteristics and IMF use.
2. Methods
2.1. Data
We used data collected between January and December 2019 from 399 treatment centers located in 37 states throughout the United States using the Addiction Severity Index-Multimedia Version (ASI-MV), an instrument integral to NAVIPPRO (Vosburg et al., 2020). The ASI-MV is a validated, self-administered, computerized structured clinical assessment tool which captures data on a convenience sample of individuals assessed for substance use problems for clinical treatment planning and triage purposes (Butler et al., 2001, 2008; Hendriks et al., 1989; Kosten et al., 1983; McLellan et al., 1992; Vosburg et al., 2020). Since individuals can be administered the ASI-MV multiple times (Butler et al., 2018), the unit of analysis was each clinical assessment instead of unique individuals.
The ASI-MV measures the severity ratings of problems an individual may have in one or more of the following seven biopsychosocial domains for which they may need treatment or assistance: medical, employment, legal, family, psychiatric, alcohol, and drug use (Vosburg et al., 2020). Interpretation of the biopsychosocial domain problem severity ratings are as follows: 0–1, no problem; 2–3, slight problem; 4–5, moderate problem; 6–7, severe problem; and 8–9, extreme problem. Moderate to extreme problem is defined as a score of 5–9 on a scale of 0–9, suggesting that the individual probably needs treatment or assistance in that area. In addition, the ASI-MV captures detailed information on lifetime and past 30-day use of illicit drugs such as cocaine, heroin, and IMF (IMF in ASI-MV includes illegal fentanyl and carfentanil, sometimes combined with other drugs such as heroin or cocaine), use and misuse of prescription drugs (e.g., opioids, stimulants), tobacco, and alcohol. Prescription opioid misuse is any use of an opioid not considered “use as prescribed.” “Use as prescribed” requires that an individual satisfy these conditions: 1) they have a current pain problem and are taking a prescribed opioid medication for pain; 2) they obtain the medication only from their own prescription; and 3) they do not use the medication via an alternate route of administration. Prescription stimulant misuse is any use that is not considered “use as prescribed,” which, for stimulants, requires that conditions (2) and (3) of the prior definition are satisfied. Prescription stimulant misuse is also assigned if a respondent indicates having used the medication “not in a way prescribed by your doctor to treat a diagnosed attention deficit or hyperactivity disorder.” The ASI-MV only asks about past 30-day prescription opioid misuse and prescription stimulant misuse. Thus, we developed new algorithms to define lifetime prescription opioid misuse and prescription stimulant misuse. Lifetime prescription opioid/stimulant misuse is defined as when individuals either reported 1) the age of first nonmedical use of prescription opioids/stimulants or 2) reported prescription opioid/stimulant nonmedical use in the past 30 days (Appendix 1).
NAVIPPRO defined the primary lifetime substance use problem as the primary or most serious problem individuals reported among the substances they used in their lifetime (only one substance could be selected). The ASI-MV does not include a variable indicating misuse of prescription opioids as the primary lifetime substance use problem. Thus, we defined a new algorithm for this variable which requires that individuals reported prescription opioid use as their primary lifetime substance use problem AND they either reported the age of first nonmedical use of prescription opioids, or reported prescription opioid nonmedical use in the past 30 days (Appendix 2).
2.2. Study design
This is an observational, cross-sectional study, including adults aged ≥ 18 years being assessed for SUD treatment. Demographic, moderate to extreme severity problems (scores between 5 and 9) experienced across seven biopsychosocial domains, and routes of drug administration were obtained from the ASI-MV among treatment assessments indicating a primary lifetime substance use problem of IMF, heroin, prescription opioid misuse, or other substances* (primary lifetime substance use problems were mutually exclusive). This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Appendix 3).
Routes of drug administration included swallowing, snorting/sniffing, smoking, injecting (in skin or muscle, or in vein), or other route of use. Routes of drug administration were not mutually exclusive; thus, the sum of response categories may be greater than 100% (Vosburg et al., 2020).
Official medications for SUD program (previously referred to medication-assisted therapy program in the ASI-MV) included a methadone maintenance program, buprenorphine or Suboxone® treatment, or Vivitrol® or naltrexone treatment for alcohol or drugs.
Within three categories of primary lifetime substance use problems– 1) IMF, 2) heroin, and 3) prescription opioid misuse—we calculated the percentage of assessments reporting lifetime and past 30-day use of other substances. We assessed lifetime use of alcohol, cannabis (marijuana, hashish, or a prescription cannabinoid product such as Marinol® or Cesamet®), cocaine, heroin, IMF, and prescription sedatives/tranquilizers/sleeping pills, as well as lifetime misuse of prescription opioids, and prescription stimulants. We examined past 30-day use of alcohol, cannabis, cocaine, heroin, IMF, prescription sedatives/tranquilizers/sleeping pills, and illicit stimulants (i.e., illegal methamphetamine), as well as past 30-day misuse of prescription opioids, and prescription stimulants.
We also examined the percentage of assessments within each primary opioid substance use problem that reported lifetime overdose on any drug and past-year overdose on heroin or other opioids. Lifetime overdose was defined as ever overdosing on any drugs seriously enough that the individual needed someone else’s help to recover (i.e., they could not just sleep it off). Past-year overdose was defined similarly but only included overdoses on heroin or other opioids in the past 12 months. The question for past-year overdose was only asked among respondents who either 1) used at least one prescription opioid product in the past 30 days, or 2) did not respond to product-specific questions about prescription opioid use in the past 30 days. We categorized overdose history as 0, 1, 2, and ≥ 3 times.
Finally, we examined demographic and clinical predictors of IMF use as the primary lifetime substance use problem.
2.3. Statistical analyses
We compared the differences in baseline characteristics using an analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables among the three comparison groups (IMF, heroin, or prescription opioid misuse as the primary lifetime substance use problem).
We used a multivariable logistic regression model to examine the associations between demographic, clinical, psychosocial characteristics and IMF use as the primary lifetime substance use problem. We conducted a number of sensitivity analyses. First, we examined past 30-day use of IMF as the outcome, adjusting for covariates mentioned earlier as well as other lifetime substance use (i.e., alcohol, heroin, cannabis, cocaine or crack, and prescription sedatives/tranquilizers/sleeping pills) and lifetime substance misuse (including prescription opioids, and prescription stimulants). We defined past 30-day use of IMF for the sensitivity analysis as IMF use with or without any other substance use in the 30 days prior to the date of an individual’s ASI-MV treatment assessment. The reference group was those who used any substance** except for IMF in the same period. Second, we conducted a sensitivity analysis restricted to assessments reporting IMF use as the primary lifetime substance use problem and IMF use in the past 30 days. Lastly, we conducted a sensitivity analysis to examine the robustness of our findings at the individual level, rather than the assessment level. For the 6.8% of patients with multiple assessments, we used the last assessment if multiple assessments were performed on the same day (given more complete data), and the first assessment for individuals with multiple assessments on different days. Missing variables, occurring in less than 10% of cases for any individual variable, were excluded from analyses (Dong and Peng, 2013). Variance Inflation Factors (VIF) were used to check multicollinearity of the variables in the multivariable logistic regression model. No multicollinearity was detected (O’brien, 2007).
A p value of < 0.05 indicated statistical significance. All analyses were conducted using SAS (version 7.1; SAS Institute, Cary, NC).
3. Results
3.1. Characteristics of adults assessed for SUD treatment reporting IMF use as their primary lifetime substance use problem
Among the 44,040 assessments of adults assessed for SUD treatment in the United States in 2019, 685 (1.6%) indicated IMF as the primary lifetime substance use problem (“IMF group”), 5559 (12.6%) indicated heroin as the primary lifetime substance use problem (“heroin group”), and 2135 (4.8%) indicated prescription opioid misuse as the primary lifetime substance use problem (“prescription opioid misuse group”) (Table 1). The remaining 81% of assessments indicated another substance* as the primary lifetime substance use problem.
Table 1.
Demographic characteristics among ASI-MV assessments reporting illicitly manufactured fentanyl use, heroin use, or prescription opioid misuse as the primary lifetime substance use problem in 2019 (N = 8379).
| Unit of analysis: adult treatment assessment | Illicitly Manufactured Fentanyl N = 685 | Heroin N = 5559 | Prescription Opioid Misusea N = 2135 | P value |
|---|---|---|---|---|
|
| ||||
| Sex | < 0.0001 | |||
| Male | 459 (67.0%) | 3517 (63.3%) | 1049 (49.1%) | |
| Female | 226 (33.0%) | 2042 (36.7%) | 1086 (50.9%) | |
| Age (years) | < 0.0001 | |||
| 18–24 | 83 (12.1%) | 597 (10.7%) | 142 (6.7%) | |
| 25–34 | 308 (45.0%) | 2876 (51.7%) | 958 (44.9%) | |
| 35–44 | 182 (26.6%) | 1352 (24.3%) | 654 (30.6%) | |
| 45–54 | 73 (10.7%) | 481 (8.7%) | 262 (12.3%) | |
| ≥ 55 | 39 (5.7%) | 253 (4.6%) | 119 (5.6%) | |
| Race/ethnicity | < 0.0001 | |||
| Non-Hispanic White | 503 (73.4%) | 4219 (75.9%) | 1793 (84.0%) | |
| Non-Hispanic Black | 109 (15.9%) | 458 (8.2%) | 127 (6.0%) | |
| Non-Hispanic American Indian or Alaska Native | 7 (1.0%) | 88 (1.6%) | 44 (2.1%) | |
| Non-Hispanic Other | 20 (2.9%) | 218 (3.9%) | 74 (3.5%) | |
| Hispanic | 46 (6.7%) | 576 (10.4%) | 97 (4.5%) | |
| Education Level | 0.07 | |||
| Less than high school | 31 (4.5%) | 195 (3.5%) | 74 (3.5%) | |
| High school | 453 (66.1%) | 3751 (67.5%) | 1372 (64.3%) | |
| Some college | 160 (23.4%) | 1337 (24.1%) | 575 (26.9%) | |
| 4 years of college or more | 41 (6.0%) | 276 (5.0%) | 114 (5.3%) | |
| Employment Status (past 3 years) | < 0.0001 | |||
| Full-time | 270 (39.4%) | 2185 (39.3%) | 848 (39.7%) | |
| Part-time | 155 (22.6%) | 1206 (21.7%) | 502 (23.5%) | |
| Otherb | 98 (14.3%) | 854 (15.4%) | 471 (22.1%) | |
| Unemployed | 162 (23.7%) | 1314 (23.6%) | 314 (14.7%) | |
| Urban-Rural Status (ASI-MV site) | < 0.0001 | |||
| Metropolitan | 550 (80.3%) | 4115 (74.0%) | 1364 (63.9%) | |
| Micropolitan | 64 (9.3%) | 601 (10.8%) | 373 (17.5%) | |
| Rural | 71 (10.4%) | 843 (15.2%) | 398 (18.6%) | |
| United States Census Region (ASI-MV site) | < 0.0001 | |||
| Northeast | 230 (33.6%) | 874 (15.7%) | 44 (2.1%) | |
| Midwest | 106 (15.5%) | 921 (16.6%) | 467 (21.9%) | |
| South | 334 (48.8%) | 3140 (56.5%) | 1463 (68.52%) | |
| West | 15 (2.2%) | 624 (11.2%) | 161 (7.5%) | |
| Moderate to Extreme Severity Problems by Biopsychosocial Domain | ||||
| Medical | 176 (25.7%) | 1329 (23.9%) | 805 (37.7%) | < 0.0001 |
| Employment | 203 (29.6%) | 1470 (26.4%) | 504 (23.6%) | 0.003 |
| Legal | 125 (18.3%) | 1129 (20.3%) | 393 (18.4%) | 0.11 |
| Family | 130 (19.0%) | 1017 (18.3%) | 504 (23.6%) | < 0.0001 |
| Psychiatric | 204 (29.8%) | 1626 (29.3%) | 858 (40.2%) | < 0.0001 |
| Alcohol | 126 (18.4%) | 679 (12.2%) | 245 (11.5%) | < 0.0001 |
| Drug | 626 (91.4%) | 4684 (84.3%) | 1800 (84.3%) | < 0.0001 |
| Route of Use for Primary Substance | NA | |||
| Swallowed | 37 (5.4%) | 188 (3.4%) | 1468 (68.8%) | |
| Snorted/Sniffed | 335 (48.9%) | 2446 (44.0%) | 1092 (51.1%) | |
| Smoked | 90 (13.1%) | 1151 (20.7%) | 164 (7.7%) | |
| Injected | 376 (54.9%) | 4033 (72.5%) | 569 (26.7%) | |
| Insurance type | < 0.0001 | |||
| Medicare/Medicaidc | 336 (49.1%) | 2374 (42.7%) | 709 (33.2%) | |
| Medicare only | 27 (3.9%) | 65 (1.2%) | 7 (0.3%) | |
| Self-pay | 43 (6.3%) | 450 (8.1%) | 245 (11.5%) | |
| Uninsured/Exhausted benefits | 69 (10.1%) | 391 (7.0%) | 140 (6.6%) | |
| Commercial payer | 8 (1.2%) | 38 (0.7%) | 44 (2.1%) | |
| Otherd | 202 (29.5%) | 2241 (40.3%) | 990 (46.4%) | |
| Ever stopped using the major or primary substance for at least a month in their lifetime | 489 (71.4%) | 3817 (68.7%) | 1284 (60.1%) | < 0.0001 |
| Past 30 days in controlled environment | < 0.0001 | |||
| Inpatient controlled environmente | 235 (34.3%) | 1211 (21.8%) | 340 (15.9%) | |
| Not in an inpatient controlled environment (includes jail/prison) | 450 (65.7%) | 4348 (78.2%) | 1795 (84.1%) | |
| No income in the past 30 days (%) | 505 (73.7%) | 3813 (68.6%) | 1205 (56.4%) | < 0.0001 |
| Attended outpatient treatment or counseling for alcohol or drug problems, past 30 days (%) | 203 (29.6%) | 1513 (27.2%) | 595 (27.9%) | 0.39 |
| Mean days attending outpatient treatment or counselling for alcohol or drug problems, past 30 days (median) | 11.6 (7) | 13.0 (8) | 9.6 (5) | < 0.0001f |
| Attended self-help meetings in the past 30 days (%)g | 268 (39.1%) | 2028 (36.5%) | 715 (33.5%) | 0.01 |
| Mean days attending self-help meetings in the past 30 days (median) | 13.8 (10) | 12.3 (10) | 9.6 (7) | < 0.0001 |
| Received treatment as part of an official medications for substance use disorders program in the past 30 days (%) | 226 (33.0%) | 1763 (31.7%) | 863 (40.4%) | < 0.0001 |
| Mean days receiving treatment as part of an official medications for substance use disorders program in the past 30 days (median) | 18.0 (20) | 20.5 (30) | 19.4 (29) | 0.003f |
Prescription Opioid Misuse is a combination of misuse of methadone or buprenorphine and other opioids or pain medications like OxyContin, Oxycodone, Vicodin, or Percocet.
Other: Student or Homemaker, Military service, retired or disabled, in a prison or a hospital
“Medicare/Medicaid” option is a mix of those who had dual eligibility as well as those who had Medicaid only.
Other: includes New Mexico Behavioral Health Services Division (BHSD), access to recovery, methamphetamine initiative, New Mexico Children’s Code (NMCC), New Mexico Probation and Pre-trial (NMPP), Temporary Assistance for Needy Families Substance Abuse Services (TANF SA Services), Income Support Division/New Mexico Works (ISD/NM Works), New Mexico Total Community Approach (TCA), and unassigned.
Inpatient controlled environment: Inpatient alcohol or drug treatment, inpatient medical treatment, or inpatient psychiatric treatment
P-value is for the difference of the mean.
self-help meetings include Alcoholics Anonymous, Narcotics Anonymous or Cocaine Anonymous, etc.
Most baseline characteristics were statistically different among the three groups (all p-values < 0.05), except for education level, attendance at outpatient treatment or counseling for alcohol or drug problems in the past 30 days, and moderate to extreme severity problems in the legal domain. Across the three groups, the IMF group showed the highest percentage of males (67.0%), younger people (18–24 years) (12.1%), non-Hispanic Black persons (15.9%), people assessed for treatment in a metropolitan site (80.3%), people assessed for treatment in the Northeast (33.6%), people having Medicare/Medicaid (49.1%), people who were uninsured (10.1%), and people having Medicare only (3.9%) (Table 1).
The IMF group also had the highest percentage of assessments reporting moderate to extreme severity problems in the employment (29.6%), alcohol (18.4%), and drug biopsychosocial domains (91.4%). Notably, the percentage of assessments reporting moderate to extreme severity problems in the drug domain was > 84% for all three groups. The three most common routes of drug administration for the IMF group to use IMF were injecting, snorting/sniffing, and smoking (54.9%, 48.9%, and 13.1%, respectively) while the three most common routes for the prescription opioid misuse group to misuse prescription opioids were swallowing, snorting/sniffing and injecting (68.8%, 51.1%, and 26.7%, respectively).
One third (34.3%) of the IMF group reported receiving inpatient alcohol or drug treatment, inpatient medical treatment, or inpatient psychiatric treatment in the past 30 days (Table 1). The percentage among the heroin and prescription opioid misuse groups were 21.8% and 15.9%, respectively. Nearly 40% of the IMF group reported attending self-help meetings such as Alcoholics Anonymous or Narcotics Anonymous in the past 30 days (39.1%), while the heroin group and prescription opioid misuse groups reported 36.5% and 33.5%, respectively. Moreover, approximately one-third of the IMF (33.0%) and heroin (31.7%) groups reported receiving medications for SUD in the past 30 days, while the prescription opioid misuse group reported 40.4%.
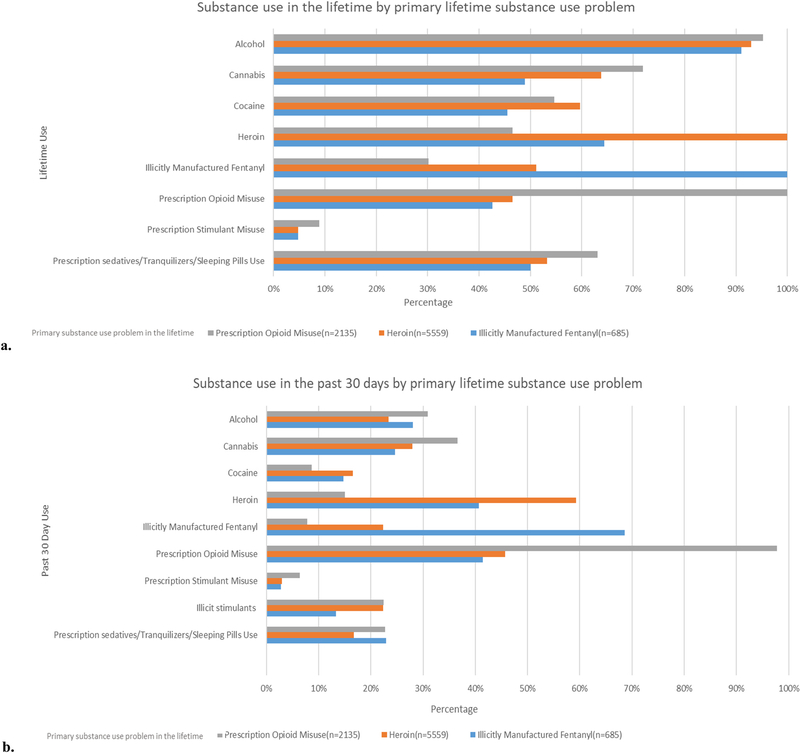
Use of other substances was common in all groups, both in the past 30 days and across the lifetime. Among the IMF group, the percentage of assessments reporting lifetime substance use was highest for alcohol (91.1%), followed by heroin (64.4%), prescription sedatives/tranquilizers/sleeping pills use (50.1%), cannabis (48.9%), cocaine (45.5%), prescription opioid misuse (42.6%), and prescription stimulant misuse (4.8%) (Fig. 1). The percentage of assessments reporting past 30-day substance use among the IMF group followed similar patterns and was highest for prescription opioid misuse (41.5%), followed by use of heroin (40.7%), alcohol (28.0%), cannabis (24.7%), prescription sedatives/tranquilizers/sleeping pills use (22.9%), cocaine (14.7%), illicit stimulants (13.3%), and prescription stimulant misuse (2.8%).
Fig. 1.
Comparison of a) lifetime and b) past-30-day substance use among individuals reporting illicitly manufactured fentanyl use, heroin use, or prescription opioid misuse as their primary lifetime substance use problem.
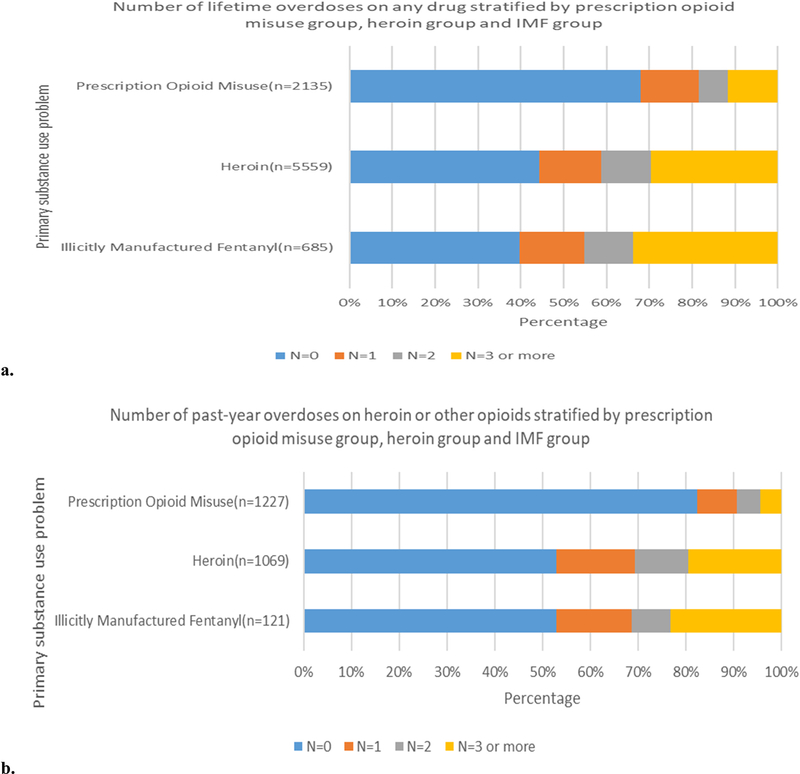
Fig. 2 depicts the percentage of assessments reporting lifetime overdose on any drug and past-year overdose on heroin or other opioids by primary opioid substance use problem. The percentage of assessments reporting 3 or more lifetime overdoses on any drug was greatest for the IMF group (33.7%), followed by the heroin group (29.5%) and the prescription opioid misuse group (11.7%). Similarly, the IMF group had the greatest percentage of assessments reporting 3 or more overdoses on heroin or other opioids in the past year (23.1%), followed by the heroin group (19.6%) and the prescription opioid misuse group (4.4%).
Fig. 2.
a) Lifetime overdose on any drug and b) past-year overdose on heroin or other opioids among individuals reporting illicitly manufactured fentanyl use, heroin use, or prescription opioid misuse as their primary lifetime substance use problem, Note: Among the original sample, 0.15% of assessments for illicitly manufactured fentanyl, 0.14% of assessments for heroin, 0.28% of assessments for misuse of prescription opioids did not answer the question about past-year overdose on heroin or other opioids. Eighty-two percent (82.2%) of assessments for illicit fentanyl, 80.6% of assessments for heroin, and 42.3% of assessments for misuse of prescription opioids were not presented to individuals about the past year overdose question dur to the skip logic (i.e., variable existed but was not presented due to logic skip). Therefore, we excluded those missing observations.
3.2. Factors associated with reporting IMF use as the primary lifetime substance use problem
Factors statistically associated with increased odds of reporting IMF use as the primary lifetime substance use problem included: being younger [18–24 years (adjusted odds ratio (aOR) = 1.68; 95% confidence interval (CI) = 1.18–2.38) versus 45–54 years], non-Hispanic Black persons (aOR = 1.44; 95% CI = 1.11–1.85) versus non-Hispanic White persons, assessed in the Northeast (aOR = 15.46; 95% CI = 8.67–27.56), Midwest (aOR = 5.30; 95% CI = 2.95–9.50), or South (aOR = 4.09; 95% CI = 2.36–7.11) versus the West, moderate to extreme severity problems for the drug domain (aOR = 3.79; 95% CI = 2.83–5.06) versus lower severity problems in the drug domain, having only Medicare (aOR = 2.76; 95% CI = 1.70–4.47) or being uninsured/having exhausted benefits (aOR = 1.42; 95% CI = 1.06–1.90) versus having Medicare/Medicaid, assessed in a metropolitan site (aOR = 1.52; 95% CI = 1.14–2.04) versus rural site, stayed in inpatient controlled environment in the past 30 days (aOR = 1.52; 95% CI = 1.25–1.84), received medications for SUD in the past 30 days (aOR = 1.53; 95% CI = 1.27–1.85), and having a lifetime history of overdose on any drug (1 overdose (aOR = 1.91; 95% CI = 1.49–2.44), 2 overdoses (aOR = 1.95; 95% CI = 1.48–2.58) and 3 or more overdoses (aOR = 2.27; 95% CI = 1.82–2.82)) (Table 2).
Table 2.
Multivariable logistic regression model examining predictors of reporting illicitly manufactured fentanyl use as the primary substance use problem versus reporting any other primary substance use problem in the lifetime (N = 29,758).
| Unadjusted Odds Ratio (95% Confidence Interval) | Adjusted Odds Ratio (95% Confidence Interval) | |
|---|---|---|
|
| ||
| Sex | ||
| Female | 0.82 (0.70, 0.97) | 0.85 (0.71, 1.01) |
| Male | Reference | Reference |
| Age (years) | ||
| 18–24 | 1.19 (0.86, 1.65) | 1.68 (1.18, 2.38) |
| 25–34 | 1.42 (1.09, 1.85) | 1.36 (1.02, 1.81) |
| 35–44 | 1.24 (0.93, 1.64) | 1.22 (0.91, 1.65) |
| ≥ 55 | 0.72 (0.45, 1.14) | 0.78 (0.48, 1.25) |
| 45–54 | Reference | Reference |
| Race/Ethnicity | ||
| Non-Hispanic Black | 1.10 (0.88, 1.37) | 1.44 (1.11, 1.85) |
| Non-Hispanic American Indian or Alaska Native | 0.25 (0.12, 0.53) | 0.58 (0.27, 1.24) |
| Non-Hispanic other | 0.62 (0.40, 0.97) | 0.75 (0.47, 1.19) |
| Hispanic | 0.68 (0.50, 0.92) | 0.98 (0.71, 1.36) |
| Non-Hispanic White | Reference | Reference |
| Education Level | ||
| Less than high school | 1.24 (0.86, 1.79) | 1.22 (0.83, 1.79) |
| Some college | 0.98 (0.82, 1.18) | 1.10 (0.90, 1.33) |
| 4 years of college or more | 0.84 (0.60, 1.18) | 1.14 (0.80, 1.64) |
| High school | Reference | Reference |
| United States Census Region (ASI-MV site) | ||
| Northeast | 53.93 (31.34, 92.80) | 15.46 (8.67, 27.56) |
| Midwest | 5.75 (3.29, 10.07) | 5.30 (2.95, 9.50) |
| South | 6.10 (3.57, 10.43) | 4.09 (2.36, 7.11) |
| West | Reference | Reference |
| Moderate to Extreme Domain Severity Rating | ||
| Medical | 1.05 (0.88, 1.26) | 1.13 (0.92, 1.38) |
| Employment | 1.41 (1.19, 1.66) | 1.10 (0.91, 1.33) |
| Alcohol | 0.66 (0.54, 0.81) | 0.52 (0.41, 0.64) |
| Drug | 7.27 (5.54, 9.55) | 3.79 (2.83, 5.06) |
| Legal | 0.74 (0.60, 0.90) | 0.82 (0.66, 1.01) |
| Family | 0.97 (0.79, 1.17) | 1.05 (0.83, 1.32) |
| Psychiatric | 0.81 (0.69, 0.96) | 0.75 (0.61, 0.91) |
| Route of Use for Any Substance in the Lifetime | ||
| Injection drug use with or without also reporting swallowed, snorted, or smoked | 2.64 (2.26, 3.10) | 1.10 (0.90, 1.33) |
| Swallowed, snorted, or smoked without reporting any injection drug use | Reference | Reference |
| Insurance type | ||
| Medicare only | 3.10 (2.05, 4.69) | 2.76 (1.70, 4.47) |
| Self-pay | 0.16 (0.12, 0.23) | 0.70 (0.49, 0.99) |
| Uninsured/Exhausted benefits | 0.92 (0.71, 1.20) | 1.42 (1.06, 1.90) |
| Commercial payer | 0.34 (0.16, 0.71) | 0.97 (0.44, 2.12) |
| Othera | 0.41 (0.34, 0.49) | 0.78 (0.63, 0.97) |
| Medicare/Medicaidb | Reference | Reference |
| Urban-Rural Status (ASI-MV site) | ||
| Metropolitan | 2.12 (1.63, 2.75) | 1.52 (1.14, 2.04) |
| Micropolitan | 0.69 (0.48, 0.99) | 0.85 (0.58, 1.24) |
| Rural | Reference | Reference |
| In inpatient controlled environment during the past 30 days c | ||
| Yes | 2.29 (1.95, 2.69) | 1.52 (1.25, 1.84) |
| No | Reference | Reference |
| Any income in the past 30 days | ||
| Yes | 0.49 (0.42, 0.59) | 0.86 (0.71, 1.05) |
| No | Reference | Reference |
| Received treatment as part of an official medications for substance use disorders program in the past 30 days (%) | ||
| Yes | 3.10 (2.63, 3.65) | 1.53 (1.27, 1.85) |
| No | Reference | Reference |
| Attended any outpatient treatment or counseling for alcohol or drug problems in the past 30 days | ||
| Yes | 1.39 (1.17, 1.64) | 1.19 (0.98, 1.46) |
| No | Reference | Reference |
| Attended self-help meeting in the past 30 days d | ||
| Yes | 1.12 (0.96, 1.31) | 0.84 (0.69, 1.01) |
| No | Reference | Reference |
| Lifetime overdoses on any drug | ||
| 1 lifetime overdose | 2.82 (2.24, 3.57) | 1.91 (1.49, 2.44) |
| 2 lifetime overdoses | 3.41 (2.63, 4.41) | 1.95 (1.48, 2.58) |
| 3 or more lifetime overdoses | 5.17 (4.31, 6.20) | 2.27 (1.82, 2.82) |
| None | Reference | Reference |
Includes New Mexico Behavioral Health Services Division (BHSD), access to recovery, methamphetamine initiative, New Mexico Children’s Code (NMCC), New Mexico Probation and Pre-trial (NMPP), Temporary Assistance for Needy Families Substance Abuse Services (TANF SA Services), Income Support Division/New Mexico Works (ISD/NM Works), New Mexico Total Community Approach (TCA), and unassigned.
“Medicare/Medicaid” option is a mix of those who had dual eligibility as well as those who had Medicaid only.
Inpatient controlled environment: Inpatient alcohol or drug treatment, inpatient medical treatment, or inpatient psychiatric treatment
Self-help meetings include Alcoholics Anonymous, Narcotics Anonymous or Cocaine Anonymous and etc.
Factors statistically associated with decreased odds of reporting IMF use as the primary lifetime substance use problem included moderate to extreme severity problems for the alcohol domain (aOR = 0.52; 95% CI = 0.41–0.64), and psychiatric domain (aOR = 0.75; 95% CI = 0.61–0.91) versus lower severity problems in the corresponding domains, and self-pay as insurance type (aOR = 0.70; 95% CI = 0.49–0.99) versus those with Medicare/Medicaid (Table 2).
3.3. Factors associated with reporting IMF use in the past 30 days
In the sensitivity analysis examining characteristics associated with IMF use in the past 30 days, most results remained consistent with the main analysis (Table 3).
Table 3.
Multivariable logistic regression model examining predictors of reporting illicitly manufactured fentanyl use in the past 30 days versus reporting any other substance use in the past 30 days (N = 28,138).a
| Unadjusted Odds Ratio (95% Confidence Interval) | Adjusted Odds Ratio (95% Confidence Interval) | |
|---|---|---|
|
| ||
| Sex | ||
| Female | 1.05 (0.96, 1.15) | 0.91 (0.82,1.01) |
| Male | Reference | Reference |
| Age (years) | ||
| 18–24 | 1.31 (1.09, 1.58) | 1.94 (1.56, 2.41) |
| 25–34 | 1.80 (1.54, 2.11) | 1.35 (1.12, 1.61) |
| 35–44 | 1.45 (1.23, 1.71) | 1.18 (0.97,1.42) |
| ≥ 55 | 0.67 (0.50, 0.89) | 0.76 (0.56,1.04) |
| 45–54 | Reference | Reference |
| Alcohol use in the lifetime | ||
| Yes | 0.65 (0.58, 0.72) | 0.98 (0.86, 1.11) |
| No | Reference | Reference |
| Heroin use in the lifetime | ||
| Yes | 19.63 (17.29, 22.29) | 4.43 (3.82, 5.15) |
| No | Reference | Reference |
| Prescription opioid misuse in the lifetime | ||
| Yes | 6.18 (5.65, 6.77) | 2.26 (2.02, 2.54) |
| No | Reference | Reference |
| Cannabis use in the lifetime | ||
| Yes | 0.87 (0.80, 0.96) | 0.69 (0.60, 0.79) |
| No | Reference | Reference |
| Cocaine or crack use in the lifetime | ||
| Yes | 3.30 (3.01, 3.62) | 1.23 (1.09, 1.39) |
| No | Reference | Reference |
| Prescription stimulant misuse in the lifetime | ||
| Yes | 2.74 (2.37, 3.17) | 1.57 (1.32, 1.86) |
| No | Reference | Reference |
| Prescription sedatives/tranquilizers/sleeping pills use in the lifetime | ||
| Yes | 4.00 (3.65, 4.37) | 1.43 (1.28, 1.61) |
| No | Reference | Reference |
| Race/Ethnicity | ||
| Non-Hispanic Black | 0.60 (0.52, 0.69) | 1.30 (1.08, 1.55) |
| Non-Hispanic American Indian or Alaska Native | 0.31 (0.21, 0.45) | 0.67 (0.45, 0.99) |
| Non-Hispanic other | 0.72 (0.58, 0.89) | 0.88 (0.69, 1.12) |
| Hispanic | 0.49 (0.41, 0.58) | 0.89 (0.73, 1.09) |
| Non-Hispanic White | Reference | Reference |
| Education Level | ||
| Less than high school | 1.15 (0.93, 1.42) | 0.91 (0.72, 1.16) |
| Some college | 0.93 (0.84, 1.02) | 0.94 (0.73, 1.21) |
| 4 years of college or more | 0.59 (0.49, 0.72) | 0.95 (0.69, 1.30) |
| High school | Reference | Reference |
| United States Census Region (ASI-MV site) | ||
| Northeast | 12.00 (9.58, 15.04) | 3.20 (2.43, 4.22) |
| Midwest | 3.00 (2.42, 3.72) | 1.95 (1.52, 2.52) |
| South | 3.05 (2.50, 3.72) | 2.09 (1.67, 2.63) |
| West | Reference | Reference |
| Moderate to Extreme Domain Severity Rating | ||
| Medical | 1.63 (1.49, 1.79) | 1.12 (1.00, 1.25) |
| Employment | 2.23 (2.03, 2.44) | 1.29 (1.15, 1.44) |
| Alcohol | 0.82 (0.73, 0.91) | 0.68 (0.59, 0.77) |
| Drug | 12.04 (10.32, 14.06) | 3.34 (2.82, 3.95) |
| Legal | 1.10 (0.99, 1.22) | 0.84 (0.74, 0.95) |
| Family | 1.81 (1.64, 1.99) | 1.21 (1.07, 1.37) |
| Psychiatric | 1.41 (1.30, 1.54) | 0.83 (0.74, 0.94) |
| Route of Use for Any Substance in the Lifetime | ||
| Injection drug use with or without also reporting swallowed, snorted, or smoked | 5.79 (5.28, 6.36) | 1.30 (1.16, 1.47) |
| Swallowed, snorted, or smoked without reporting any injection drug use | Reference | Reference |
| Insurance type | ||
| Medicare only | 2.28 (1.61, 3.23) | 1.96 (1.31, 2.96) |
| Self-pay | 0.12 (0.10, 0.15) | 0.47 (0.38, 0.58) |
| Uninsured/Exhausted benefits | 0.92 (0.78, 1.08) | 0.97 (0.80, 1.18) |
| Commercial payer | 0.15 (0.09, 0.27) | 0.43 (0.24, 0.79) |
| Otherb | 0.70 (0.64, 0.77) | 0.84 (0.74, 0.95) |
| Medicare/Medicaidc | Reference | Reference |
| Urban-Rural Status (ASI-MV site) | ||
| Metropolitan | 1.02 (0.91, 1.16) | 1.18 (1.02, 1.37) |
| Micropolitan | 0.45 (0.38, 0.53) | 0.69 (0.57, 0.84) |
| Rural | Reference | Reference |
| In inpatient controlled environment during the past 30 days d | ||
| Yes | 2.08 (1.88, 2.29) | 1.34 (1.18, 1.51) |
| No | Reference | Reference |
| Any income in the past 30 days | ||
| Yes | 0.55 (0.50, 0.60) | 1.11 (1.00, 1.23) |
| No | Reference | Reference |
| Received treatment as part of an official medications for substance use disorders program in the past 30 days (%) | ||
| Yes | 2.53 (2.29, 2.79) | 0.86 (0.76, 0.97) |
| No | Reference | Reference |
| Attended any outpatient treatment or counseling for alcohol or drug problems in the past 30 days | ||
| Yes | 1.32 (1.20, 1.46) | 0.90 (0.80, 1.03) |
| No | Reference | Reference |
| Attended self-help meeting in the past 30 days e | ||
| Yes | 1.08 (0.98, 1.19) | 0.66 (0.59, 0.74) |
| No | Reference | Reference |
| Lifetime overdoses on any drug | ||
| 1 lifetime overdose | 3.88 (3.41, 4.42) | 1.59 (1.37, 1.83) |
| 2 lifetime overdoses | 5.39 (4.68, 6.21) | 1.90 (1.62, 2.23) |
| 3 or more lifetime overdoses | 7.12 (6.40, 7.91) | 2.09 (1.84, 2.37) |
| None | Reference | Reference |
The sample size for this sensitivity analysis is different from the main analysis because the outcome variable in the sensitivity analysis (illicitly manufactured fentanyl use in the past 30 days) had different missing values than the outcome variable in the main analysis.
Includes New Mexico Behavioral Health Services Division (BHSD), access to recovery, methamphetamine initiative, New Mexico Children’s Code (NMCC), New Mexico Probation and Pre-trial (NMPP), Temporary Assistance for Needy Families Substance Abuse Services (TANF SA Services), Income Support Division/New Mexico Works (ISD/NM Works), New Mexico Total Community Approach (TCA), and unassigned.
“Medicare/Medicaid” option is a mix of those who had dual eligibility as well as those who had Medicaid only.
Inpatient controlled environment: Inpatient alcohol or drug treatment, inpatient medical treatment, or inpatient psychiatric treatment.
Self-help meetings include Alcoholics Anonymous, Narcotics Anonymous or Cocaine Anonymous and etc.
Notably, injection drug use (aOR = 1.30; 95% CI = 1.16–1.47) and lifetime substance use variables, including use of heroin (aOR = 4.43; 95% CI = 3.82–5.15), misuse of prescription opioids (aOR = 2.26; 95% CI = 2.02–2.54), use of cocaine or crack (aOR = 1.23; 95% CI = 1.09–1.39), misuse of prescription stimulants (aOR = 1.57; 95% CI = 1.32–1.86), or use of prescription sedatives/tranquilizers/sleeping pills (aOR=1.43; 95% CI = 1.28–1.61) were associated with increased odds of reporting IMF use in the past 30 days. However, lifetime cannabis use was associated with a 31% decreased odds of reporting IMF use in the past 30 days (Table 3).
Moreover, attendance at self-help meetings in the past 30 days was associated with a 34% decreased odds of reporting IMF use in the past 30 days (aOR = 0.66; 95% CI = 0.59–0.74). Lastly, obtaining medications for SUD in the past 30 days was associated with a 14% decreased odds of reporting IMF use in the past 30 days (aOR = 0.86; 95% CI = 0.76–0.97) (Table 3).
Results of the other two sensitivity analyses were consistent with the main analysis (Supplemental Table 1&2).
4. Discussion
This study is the first to compare the demographic, psychosocial, and substance use characteristics of adults assessed for SUD treatment who reported IMF use to those who reported heroin or prescription opioid misuse in the United States. We found that adults reporting IMF as their primary lifetime substance use problem also reported using other substances—most often alcohol, heroin (both in the past 30 days and during their lifetime), or prescription opioid misuse (in the past 30 days). In addition, adults with moderate to extreme drug problems were more likely to report IMF use. Furthermore, adults with any lifetime heroin use, prescription opioid misuse, cocaine or crack use, prescription stimulant misuse, or use of prescription sedatives/tranquilizers/sleeping pills were more likely to report using IMF in the past 30 days. These findings highlight the complex nature of polysubstance use among individuals who use IMF and reflect the severity of their substance use. Importantly, these findings can be used to inform the development of innovative prevention, treatment, and response strategies to address IMF use and related harms. For example, ensuring access to syringe services programs and the use of fentanyl test strips (FTS) in community-based venues are potential harm reduction strategies (CDC, 2021; Goldman et al., 2019; Peiper et al., 2019). Together, these efforts could motivate people who use drugs to take steps to change their drug use behaviors and reduce their risk for health harms (CDC, 2021; Peiper et al., 2019).
The finding that non-Hispanic Black persons more commonly reported IMF as their primary lifetime substance use problem is consistent with increases in overdose mortality among this population. A recent study found an 18-fold increase in mortality due to synthetic opioids other than methadone, such as IMF, among non-Hispanic Black persons from 2013 to 2017, the largest change among racial/ethnic groups over that time period (CDC, 2019; SAMHSA, 2020). These findings underscore the importance of designing tailored overdose prevention strategies to address IMF use and related harms among this population, to reduce stigma, and to improve access and linkage to evidence-based treatment for SUD.
We also found that the odds of IMF use as the primary lifetime substance use problem and IMF use in the past 30 days were highest among adults assessed in the Northeast. This is consistent with current trends in overdose deaths involving IMF and availability of IMF in illicit drug markets in the Northeast (DEA, 2020; Mattson et al., 2021). Further, consistent with the continued expansion of IMF into illicit drug markets throughout the United States (DEA, 2020), drug overdose death rates involving synthetic opioids (primarily IMF) increased not only in the Northeast, but also in the South and West from 2017 to 2018 (CDC, 2020). Continued surveillance of the IMF supply and use patterns coupled with expansion of innovative overdose prevention and response strategies are needed to address rising rates of IMF-related overdoses.
Our analysis also found that socioeconomic factors were associated with IMF use. For example, a moderate to extreme employment problem was associated with higher odds of reporting IMF use in the past 30 days. Additionally, being uninsured was associated with higher odds of reporting IMF use as the primary lifetime substance use problem. These findings are consistent with prior research on prescription opioid misuse (Han et al., 2017) as well as heroin use (Jones et al., 2015) and underscore the impact that policies related to insurance coverage, employment support services, and job skills training may have among people who have SUD (Oh et al., 2020).
We found that those with a history of at least one lifetime overdose on any drug, especially with 3 or more lifetime overdoses, were more likely to report IMF use as their primary lifetime substance use problem or to use IMF in the past 30 days. These results are concerning and consistent with other studies (Carroll et al., 2017; Gunn et al., 2021). One qualitative study found that respondents aged 18–25 years regarded nonfatal overdose as a primary risk related to IMF use. Most did not believe experiencing a nonfatal overdose would translate into concerns about future fatal overdose (Gunn et al., 2021). However, due to the high potency of IMF and unpredictability in the illicit drug supply (DEA, 2020; Gill et al., 2019), individuals exposed to IMF are at extremely high risk for fatal overdose (Carroll et al., 2017; Chandra et al., 2021). Therefore, nonfatal overdose represents a key opportunity to identify patients who may benefit from early interventions to reduce subsequent overdoses involving IMF (O’Donnell et al., 2020; Suffoletto and Zeigler, 2020). Interventions including linking individuals in the emergency department for nonfatal overdoses to SUD treatment and harm reduction programs, and expanding naloxone distribution and overdose prevention education, as well as ensuring that adequate doses of naloxone are available, could prevent individuals from dying from an overdose (O’Donnell et al., 2020).
A particularly concerning finding, but consistent with prior research on opioid use disorder (Jones and McCance-Katz, 2019), was that fewer than 40% of assessments reporting IMF, heroin, or misuse of prescription opioids as their primary lifetime substance use problem indicated they attended outpatient treatment or counselling for alcohol or drug problems, attended self-help meetings, or received medications for SUD in the past 30 days. However, our finding that receiving medications for SUD in the past 30 days was associated with reduced odds of past 30-day IMF use is encouraging and demonstrates the value of receiving treatment. Many barriers to receiving medications for SUD have been identified, including stigma, lack of long-term insurance benefits, inadequate public funding of medications for SUD, unequal geographic distribution of and limited numbers of DATA-waived providers, and lack of information on where medications for SUD can be obtained (Andrilla et al., 2019; Knudsen et al., 2011; Knudsen et al., 2010; Rosenblatt et al., 2015; Van Boekel et al., 2013). Taken together, these findings highlight the importance of linkage to and coverage for evidence-based treatment for opioid and other SUD and prioritizing public health educational campaigns to increase awareness about effective treatment and where to get it, and to encourage and normalize help-seeking behaviors.
Finally, we found that lifetime cannabis use was associated with reduced odds of reporting IMF use in the past 30 days. This finding is similar to a recent analysis indicating that participants on opioid agonist therapies using cannabis had a substantially lower risk of being exposed to IMF in Canada (Socías et al., 2021). While there is no rigorous evidence that cannabis works to treat opioid use disorder, these findings may warrant further study to explore how cannabis use may be associated with illicit opioid use.
There are several strengths to our study. We are the first to examine IMF use in a large, geographically diverse dataset of adults assessed for SUD treatment, and the first to compare adults assessed for SUD treatment reporting IMF use to those who use heroin or misuse prescription opioids. Additionally, the clinical assessment tool ASI-MV includes specific questions on drug use, routes, amounts used, and images of substances, which are rarely found in other datasets. Our findings may help identify more targeted prevention, treatment, and response strategies.
Our study results are subject to limitations. First, some people who use drugs may not know they used IMF, as IMF can be mixed with other illicit drugs unknown to people who use drugs (Griswold et al., 2018). Thus, our study only represents those who reported knowingly using IMF. We do not have information on whether these self-reports of IMF were confirmed with FTS, nor do we collect information on whether the respondent deliberately sought out IMF or whether they believed they were usually sold/given IMF. Individuals reporting IMF use in our study could be those who deliberately sought IMF out, those who believe that they ended up taking IMF (e.g., mixed with heroin or cocaine), or those who confirmed the presence of IMF with FTS (Ciccarone et al., 2017; Morales et al., 2019). Second, the NAVIPPRO dataset is a convenience sample of individuals being assessed for SUD treatment and is not nationally representative. Thus, our results may not be generalizable to all adults being assessed for SUD treatment or to adults who use substances but are not assessed for SUD treatment. Third, these findings may not be generalizable to people who experience fatal overdoses, as we cannot track most people over time to identify long-term outcomes. Fourth, most of the individuals were not asked the past-year overdose question (i.e., logic skip) since they selected “None” for past 30-day use on each prescription opioid product screen. However, it is important to note that the logic skip for the past-year overdose question does not factor in responses related to past 30-day heroin use. Therefore, we may underestimate the number of past-year overdoses on heroin or other opioids. Finally, there are potential reporting and recall biases due to the self-reported nature of our data. For example, we lack diagnosis codes to confirm an overdose.
5. Conclusion
Younger adults, non-Hispanic Black persons, those assessed in Northeast treatment sites, adults with moderate to extreme severity drug problems, and those with a history of at least one lifetime overdose were more likely to report IMF use as their primary lifetime substance use problem, compared to any other primary lifetime substance use problem. Innovative evidence-based prevention, treatment, and response strategies can prioritize these disproportionately affected populations when addressing increasing overdose death rates involving IMF. Additionally, it is vital to identify opportunities to intervene before fatal IMF-related overdoses occur, such as expanding naloxone distribution and harm reduction programs as well as connecting individuals presenting to the emergency department for nonfatal overdoses to SUD treatment and harm reduction programs.
*Other substances assessed in the ASI-MV tool include tobacco, alcohol, cannabis, cocaine, prescription or illicit stimulants, prescription sedatives/tranquilizers/sleeping pills use, barbiturates, hallucinogens, inhalants, ecstasy, Gamma-hydroxybutyrate, ketamine, K2 (spice, synthetic cannabis), bath salts, rohypnol, over-the-counter medications, other (unspecified) drugs and none.
**Any substances as the reference group assessed in the ASI-MV tool include tobacco, alcohol, cannabis, cocaine, illicit stimulants, heroin, prescription opioid misuse, prescription stimulant misuse, prescription sedatives/tranquilizers/sleeping pills use, barbiturates, hallucinogens, inhalants, ecstasy, Gamma-hydroxybutyrate, ketamine, K2 (spice, synthetic cannabis), bath salts, rohypnol, over-the-counter medications, and other (unspecified) drugs.
Supplementary Material
Acknowledgements
Thank you to Kun Zhang, Jody Green, Akadia Kacha-Ochana for their consultations on the dataset.
Abbreviations:
- IMF
illicitly manufactured fentanyl
- NAVIPPRO
National Addictions Vigilance Intervention and Prevention Program
- ASI-MV
Addiction Severity Index-Multimedia Version
- STROBE
Strengthening the Reporting of Observational studies in Epidemiology
- ANOVA
analysis of variance
- aOR
adjusted odds ratio
- CI
confidence interval
- SUD
substance use disorder
- SUDORS
State Unintentional Drug Overdose Reporting System
- VIF
Variance Inflation Factors
Footnotes
Financial disclosures
The authors have no relevant financial relationships interest to disclose.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.drugalcdep.2021.109160.
References
- Ahmad FB, Rossen LM, Sutton P, 2021. Provisional drug overdose death counts. Natl. Cent. Health Stat [Google Scholar]
- Amlani A, McKee G, Khamis N, Raghukumar G, Tsang E, Buxton JA, 2015. Why the FUSS (Fentanyl Urine Screen Study)? A cross-sectional survey to characterize an emerging threat to people who use drugs in British Columbia. Can. Harm. Reduct. J 12 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrilla CHA, Moore TE, Patterson DG, Larson EH, 2019. Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5–year update. J. Rural Health 35 (1), 108–112. [DOI] [PubMed] [Google Scholar]
- Arfken CL, Suchanek J, Greenwald MK, 2017. Characterizing fentanyl use in methadone- maintained clients. J. Subst. Abuse Treat. 75, 17–21. [DOI] [PubMed] [Google Scholar]
- Buresh M, Genberg BL, Astemborski J, Kirk GD, Mehta SH, 2019. Recent fentanyl use among people who inject drugs: results from a rapid assessment in Baltimore, Maryland. Int. J. Drug Policy 74, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Black RA, Fleming AB, 2018. Relative abuse of crush-resistant prescription opioid tablets via alternative oral modes of administration. Pain Med. 19 (8), 1613–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Licari A, Cassidy TA, Lioy K, Dickinson J, Brownstein JS, Benneyan JC, Green TC, Katz N, 2008. National addictions vigilance intervention and prevention program (NAVIPPROTM): a real-time, product-specific, public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol. Drug Saf. 17 (12), 1142–1154. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Goldman RJ, Newman FJ, Beckley KE, Trottier D, Cacciola JS, 2001. Initial validation of a computer-administered addiction severity index. ASI–MV. Psychol. Addict. Behav. 15 (1), 4–12. [DOI] [PubMed] [Google Scholar]
- Carroll JJ, Marshall BD, Rich JD, Green TC, 2017. Exposure to fentanyl-contaminated heroin and overdose risk among illicit opioid users in Rhode Island: a mixed methods study. Int. J. Drug Policy 46, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published November 1, 2019. From <https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillancereport.pdf> (Accessed 1st May 2021). [Google Scholar]
- Centers for Disease Control and Prevention, 2020. Synthetic Opioid Overdose Data. Published November 1, from <https://www.cdc.gov/drugoverdose/deaths/synthetic/index.html> (Accessed 1st May 2021).
- Centers for Disease Control and Prevention, 2021, Federal Grantees May Now Use Funds to Purchase Fentanyl Test Strips. Published April 7. Accessed [May 1st, 2021] from <https://www.cdc.gov/media/releases/2021/p0407-Fentanyl-Test-Strips.html> (Accessed 1st May 2021).
- Chandra DK, Altice FL, Copenhaver MM, Zhou X, Didomizio E, Shrestha R, 2021. Purposeful fentanyl use and associated factors among opioid-dependent people who inject drugs. Subst. Use Misus 56 (7), 979–987. [DOI] [PubMed] [Google Scholar]
- Ciccarone D, 2017. Fentanyl in the US heroin supply: A rapidly changing risk environment. [DOI] [PMC free article] [PubMed]
- Ciccarone D, Ondocsin J, Mars SG, 2017. Heroin uncertainties: Exploring users’ perceptions of fentanyl-adulterated and-substituted ‘heroin’. Int. J. Drug Policy 46, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEA, 2020. drug enforcement administration NDTA National Drug threat assessment. Published March 1, 2021. From <https://www.dea.gov/sites/default/files/2021–02/DIR-008->21%202020%20National%20Drug%20Threat%20Assessment_WEB.pdf (Accessed 1st May 2021).
- DiSalvo P, Cooper G, Tsao J, Romeo M, Laskowski LK, Chesney G, Su MK, 2021. Fentanyl-contaminated cocaine outbreak with laboratory confirmation in New York City in 2019. Am. J. Emerg. Med. 40, 103–105. [DOI] [PubMed] [Google Scholar]
- Dong Y, Peng C-YJ, 2013. Principled missing data methods for researchers. SpringerPlus 2 (1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H, Kelly E, Henderson G, 2019. How the complex pharmacology of the fentanyls contributes to their lethality. Addiction 114 (9), 1524–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden RM, Martinez P, Seth P, 2016. Fentanyl law enforcement submissions and increases in synthetic opioid–involved overdose deaths—27 states, 2013–2014. Morb. Mortal. Wkly. Rep. 65 (33), 837–843. [DOI] [PubMed] [Google Scholar]
- Goldman JE, Waye KM, Periera KA, Krieger MS, Yedinak JL, Marshall BD, 2019. Perspectives on rapid fentanyl test strips as a harm reduction practice among young adults who use drugs: a qualitative study. Harm. Reduct. J. 16 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MK, Chai PR, Krotulski AJ, Friscia M, Chapman B, Boyer EW, Logan BK, Babu KM, 2018. Self-identification of nonpharmaceutical fentanyl exposure following heroin overdose. Clin. Toxicol. 56 (1), 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn CM, Maschke A, Harris M, Schoenberger SF, Sampath S, Walley AY, Bagley SM, 2021. Age-based preferences for risk communication in the fentanyl era: ‘a lot of people keep seeing other people die and that’s not enough for them’. Addiction 116 (6), 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM, 2017. Prescription opioid use, misuse, and use disorders in US adults: 2015 National Survey on Drug Use and Health. Ann. Int. Med. 167 (5), 293–301. [DOI] [PubMed] [Google Scholar]
- Hendriks VM, Kaplan CD, Van Limbeek J, Geerlings P, 1989. The Addiction Severity Index: reliability and validity in a Dutch addict population. J. Subst. Abuse Treat. 6 (2), 133–141. [DOI] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM, Bohm MK, 2015. Vital signs: demographic and substance use trends among heroin users—United States, 2002–2013. MMWR Morb. Mortal. Wkly. Rep. 64 (26), 719–725. [PMC free article] [PubMed] [Google Scholar]
- Jones CM, McCance-Katz EF, 2019. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 197, 78–82. [DOI] [PubMed] [Google Scholar]
- Kenney SR, Anderson BJ, Conti MT, Bailey GL, Stein MD, 2018. Expected and actual fentanyl exposure among persons seeking opioid withdrawal management. J. Subst. Abuse Treat. 86, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Roman PM, 2011. Adoption and implementation of medications in addiction treatment programs. J. Addict. Med. 5 (1), 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM, Oser CB, 2010. Facilitating factors and barriers to the use of medications in publicly funded addiction treatment organizations. J. Addict. Med. 4 (2), 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD, 1983. Concurrent validity of the addiction severity index. J. Nerv. Ment. Dis. 171, 606–610. [DOI] [PubMed] [Google Scholar]
- Mars SG, Ondocsin J, Ciccarone D, 2018. Sold as heroin: perceptions and use of an evolving drug in Baltimore, MD. J. Psychoact. Drugs 50 (2), 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Rosenblum D, Ciccarone D, 2019. Illicit fentanyls in the opioid street market: desired or imposed? Addiction 114 (5), 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL, 2021. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013– 2019. Morb. Mortal. Wkly. Rep. 70 (6), 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The fifth edition of the Addiction Severity Index. J. Subst. Abuse Treat. 9 (3), 199–213. [DOI] [PubMed] [Google Scholar]
- Morales KB, Park JN, Glick JL, Rouhani S, Green TC, Sherman SG, 2019. Preference for drugs containing fentanyl from a cross-sectional survey of people who use illicit opioids in three United States cities. Drug Alcohol Depend. 204, 107547. [DOI] [PubMed] [Google Scholar]
- Oh S, DiNitto DM, Powers DA, 2020. Spillover effects of job skills training on substance misuse among low-income youths with employment barriers: a longitudinal cohort study. Am. J. Public Health 110 (6), 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’brien RM, 2007. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 41 (5), 673–690. [Google Scholar]
- O’Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL, 2020.. Vital signs: Characteristics of drug overdose deaths involving opioids and stimulants—24 states and the District of Columbia, January–June 2019. Morb. Mortal. Wkly. Rep. 69 (35), 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell JK, Halpin J, Mattson CL, Goldberger BA, Gladden RM, 2017. Deaths involving fentanyl, fentanyl analogs, and U-47700–10 states, July–December 2016. MMWR Morb. Mortal. Wkly. Rep 63 (43), 1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper NC, Clarke SD, Vincent LB, Ciccarone D, Kral AH, Zibbell JE, 2019. Fentanyl test strips as an opioid overdose prevention strategy: findings from a syringe services program in the Southeastern United States. Int. J. Drug Policy 63, 122–128. [DOI] [PubMed] [Google Scholar]
- Rosenblatt RA, Andrilla CHA, Catlin M, Larson EH, 2015. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann. Fam. Med. 13 (1), 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socías ME, Choi J, Lake S, Wood E, Valleriani J, Hayashi K, Kerr T, Milloy M-J, 2021. Cannabis use is associated with reduced risk of exposure to fentanyl among people on opioid agonist therapy during a community-wide overdose crisis. Drug Alcohol Depend. 219, 108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffoletto B, Zeigler A, 2020. Risk and protective factors for repeated overdose after opioid overdose survival. Drug Alcohol Depend. 209, 107890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Rockville MD, 2020. The opioid crisis and the black/african american population: an urgent issue. Working Paper. <https://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP20-05-02-001_508%20Final.pdf>. [Google Scholar]
- Van Boekel LC, Brouwers EP, Van Weeghel J, Garretsen HF, 2013. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 131 (1–2), 23–35. [DOI] [PubMed] [Google Scholar]
- Vosburg SK, Beaumont J, Dailey-Govoni ST, Butler SF, Green JL, 2020. Evaluation of abuse and route of administration of extended-release tapentadol among treatment- seeking individuals, as captured by the addiction severity index–multimedia version (ASI-MV). Pain Med. 21 (9), 1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.