Abstract
Neural implants enable bidirectional communications with nervous tissue and have demonstrated tremendous potential in research and clinical applications. To obtain high fidelity and stable information exchange, we need to minimize the undesired host responses and achieve intimate neuron-device interaction. This paper highlights the key bio-integrative strategies aimed at seamless integration through intelligent device designs to minimize the immune responses, as well as incorporate bioactive elements to actively modulate cellular reactions. These approaches span from surface modification and bioactive agent delivery, to biomorphic and biohybrid designs. Many of these strategies have shown effectiveness in functional outcome measures, others are exploratory but with fascinating potentials. The combination of bio-integrative strategies may synergistically promote the next generation of neural interfaces.
Keywords: biointegration, Neural interface, neural device integration, Bioactive coatings, Drug Delivery, biohybrid, biomorphic
Graphical Abstract
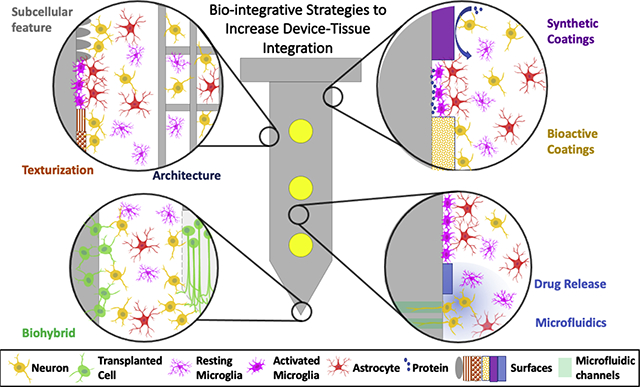
1. Introduction
The research and development of implantable neural interfacing devices has experienced exponential growth in recent years. These devices are placed within the neural tissue to measure electrophysiological and neurochemical signals or to modulate neural activities via methods including but not limited to electrical, optical, chemical, magnetic, and ultrasound stimulation. Advancements in neural interface devices have not only greatly expanded the toolboxes for neuroscience research, but also enabled diagnosis, treatment, and assistive technologies to potentially benefit millions of patients suffering from neurological disorders and injuries [1], [2]. Currently, the functionality of the implantable components of neural devices is far from optimum due to poor integration between the artificial implant and the neural tissue. Implantation inevitably causes vascular and cellular damage, which triggers a cascade of host tissue responses including the immediate adsorption of blood and brain proteins, damage to nearby neurons, activation and polarization of microglia, and monocyte recruitment, followed by the activation of astrocytes and NG2 glia. With the presence of the foreign body, the acute inflammatory responses evolve into a persistent chronic response, characterized by glial scar formation, demyelination, axonal degeneration, and neuronal loss, all of which can compromise the information exchange at the neural tissue-device interface [3]. To achieve seamless device-tissue integration, efforts need to be made to 1) reduce the insertion damage, 2) to minimize the foreign body responses and promote functional connection with neurons through the use of bio integrative strategies. While the insertion damage may be minimized by reducing implant size [4] and optimizing insertion methods [4], [5], this opinion paper focuses on bio-integrative design strategies aimed at optimizing the device factors such as implant size, shape, surface that aggravate the foreign body response (FBR) and/or incorporating bioactive components to actively modulate the cellular response.
2. Recent Advances in Bio-integrative Designs
2.1. Biomimetic and bioactive coatings
Surface chemistry plays a critical role in implant pathology. Protein adsorption occurs within seconds of implantation followed by inflammatory cell adhesion. As these initial responses occur at the surface of implants, surface modification is an intuitive strategy to control the inflammatory response. Different materials have been investigated for surface coatings, ranging from synthetic to biological, with varying degrees of success in mitigating the FBR, promoting neuron adhesion to electrodes, and improving functional outcomes [6].
2.1.1. Synthetic materials
Anti-fouling
Anti-fouling coatings inhibit nonspecific protein adsorption and prevent inflammatory cell attachment. Zwitterionic polymers present superior antifouling properties compared to conventional hydrophilic polymers due to the abundance of the water-binding ions, charge neutrality, and minimum immunogenicity. Remarkably, subcutaneously implanted zwitterionic polymer hydrogel completely escaped the FBR [7]. One type of zwitterionic polymer, polysulfobetaine methacrylate (PSBMA) has been covalently grafted onto the neural probe surface via photoiniferter mediated polymerization and reduced microglia end-feet spreading on the probe surface immediately post-implant [8]. When PSBMA is co-deposited with polydopamine via catechol chemistry on neural probe surfaces, decreased inflammatory gliosis was observed at one week post implant [9].
Conductive polymers (CPs)
Conductive polymers (CPs) have been extensively investigated as neural electrode coatings due to their outstanding low impedance and high charge injection properties. Incorporation of functional molecules via doping, blending, or covalent functionalization may further improve tissue integration[10]–[12]. Poly(3,4-ethylenedioxythiophene) (PEDOT) doped with negatively charged carbon nanotubes presents a nanofibrous morphology (Figure 1a) that promotes cellular process ingrowth as indicated by increased impedance in vivo but stable recording and higher stimulation efficiency (Figure 1b–d) compared to uncoated smooth metal sites assessed for 12 weeks [13]–[15]. Finally, conductive polymers and their composites are softer and more flexible than traditional electrode materials, minimizing the mechanical mismatch induced inflammation [16], [17].
Figure 1.
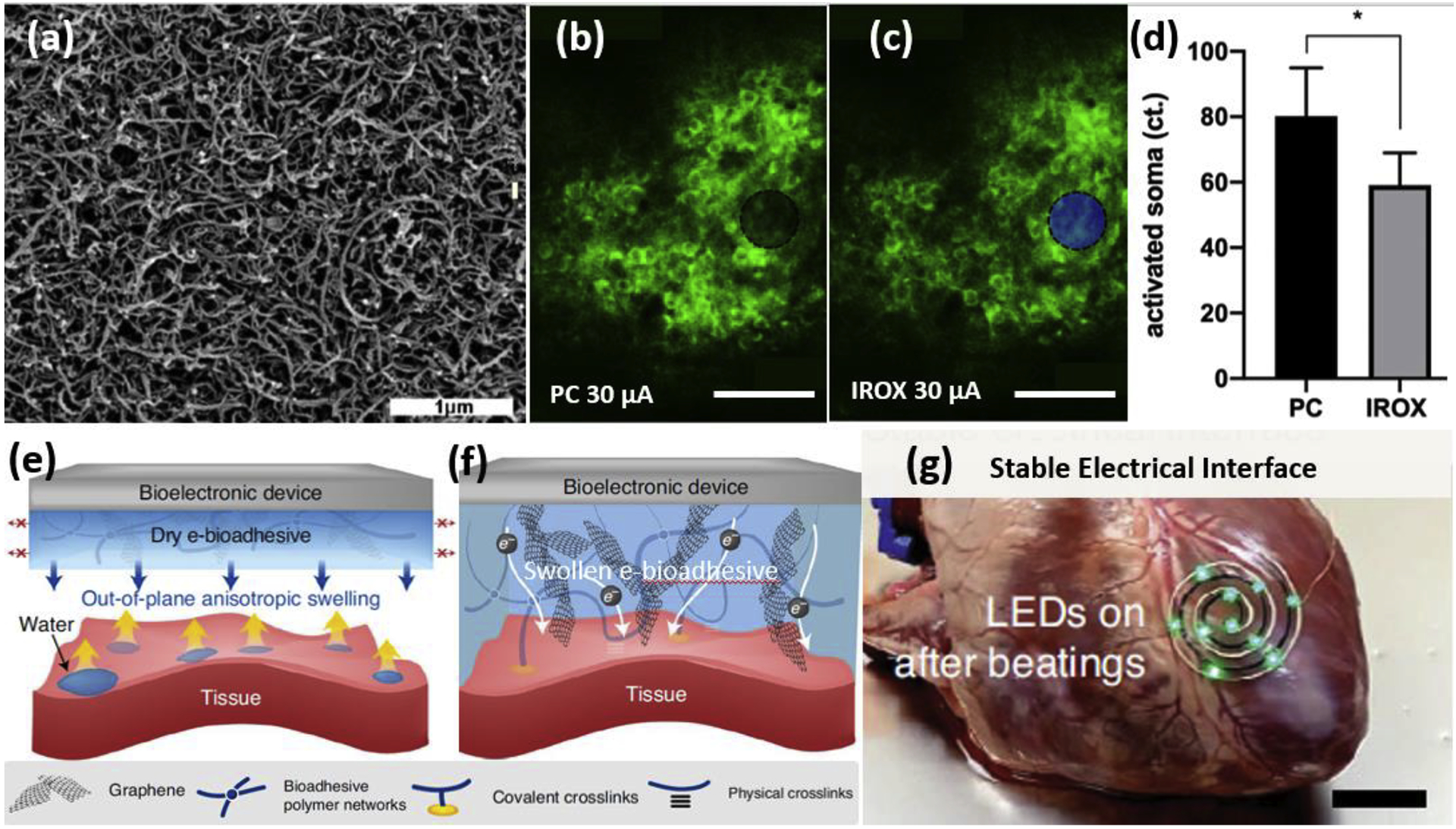
Examples of synthetic coating materials on neural interfaces from recent literature. (a) SEM image of electrochemically deposited PEDOT/CNT on an electrode recording site showing nanofibrous and porous morphology. Adapted with permission from [14]. (b)-(d) Two-photon microscopy investigation of neural elements evoked by a 30 μA biphasic current-controlled stimuli from a PEDOT/CNT (PC) (black) and iridium oxide (blue) coated microelectrodes, respectively [15]. Electrode locations are denoted by black and blue disks, respectively. (d) Quantification of the number of activated neuronal soma as a result of the stimulation. *p<0.05. (e)-(f) Mechanism of the e-bioadhesive interface [18]. (e) The graphene nanocomposite poly (vinyl alcohol) hydrogel-based e-bioadhesive provides anisotropic out-of-plane swelling and removes water upon contact with the wet tissue interface through functionalized carboxylic acids groups forming hydrogen bonds with the tissue under gentle pressure within 5s. Red arrows with crosses in (e) indicate limited in-plane swelling. (f) The NHS ester can further form covalent crosslinks with primary amines on the tissue surface. After adhering to the tissue surface, the e-bioadhesive interface becomes a thin layer of a graphene nanocomposite hydrogel with high water content, softness, stretchability, and conductivity. The adhesion can be promptly reversed with a triggering solution (sodium bicarbonate and glutathione) to achieve on-demand removal. (g) Circuits with LEDs were attached to a beating ex vivo porcine heart with the e-bioadhesive, showing a stable electrical interface [18]. Scale bars: (a) 1μm, (b)(c)50μm, (g) 20 mm.
Conductive polymers or other conductive materials may be combined with hydrogels to form conductive hydrogels, which present several desired attributes of neural electrode coating, including neural tissue-mimicking mechanical modulus, hydrophilicity, and high ionic and/or electrical conductivity. While most of the hydrogels are not adherent to wet tissue, an e-bioadhesive conductive hydrogel has been reported to enable rapid, robust, and reversible integration of bioelectronic devices on dynamic wet tissue [18]. Once the dried composite hydrogel is placed in contact with the wet tissue, their carboxylic acid groups rapidly remove interfacial water and strong adhesion is subsequently formed through covalent and non-covalent bonds (Figure 1e&f). The robust performance of this e-bioadhesive interface has been demonstrated on a beating porcine heart and a rat sciatic nerve, showcasing a stable tissue-device integration for up to 14 days (Figure 1g).
2.1.2. Biologically derived materials
While synthetic materials showed promising potentials in reducing the FBR, biologically derived materials have also been applied as coatings to disguise the artificial implant or actively modulate the cellular interaction with the implants. Extracellular matrix (ECM) proteins like laminin, fibronectin, or their peptide fragments have been attached to the neural implants to reduce FBR [19], [20] or promote neuronal attachment. A recent study compared a collagen-based hemostatic material to astrocyte secreted ECM matrix [19]. In vivo implantation resulted in reduced astrocyte activation at 4 weeks only from the astrocyte matrix coating, but neither coatings reduced inflammatory markers nor improved neuronal survival. Since both coatings were applied via physical adsorption without covalent bind or crosslinking, the benefit may have been diminished due to coating desorption or degradation. Utilizing micro-transfer-molding, a collagen coating has been made to encapsulate subdural electrocorticography (ECoG) arrays. Without covalent surface attachment or crosslinking, the collagen film partially degraded in 4 weeks in vivo [20]. It is important to note that the ECM proteins are subjected to enzymatic degradation in vivo and the degradation products may recruit inflammatory cells and counteract their intended function.
Cell surface receptor proteins
Cell surface receptor proteins, compared to ECM proteins, mediate more specific cellular interactions. Neuronal adhesion molecule L1 has been covalently immobilized onto a silicon-based electrode surface and shown to specifically promote neuronal attachment and survival while inhibiting microglia attachment and astrogliosis in vivo [21]–[23]. Importantly, L1 coating greatly improved single-unit recording yield in mice over 16 weeks of implantation demonstrating the great potential of this biological coating for further use in larger animal models and ultimately clinical translation [22].
Polysaccharides
Polysaccharides are well suited for neural tissue engineering due to their hydrophilicity and low immunogenicity. Hyaluronic acid (HA), an abundant polysaccharide in the brain tissue with anti-inflammatory properties, is combined with polypyrrole (PPy) to form electrode coatings [24]. The PPy/HA coating attenuated the inflammatory response while maintaining stable electrical performance for three weeks. Antibacterial chitosan has also been fabricated as a hydrogel coating for neural electrodes in acute in vivo electrophysiology [25]. The vast pool of polysaccharides with anticoagulative, anti-inflammation, or neurite promoting functions have yet to be further explored as biointegrative coatings. While biologically derived molecules have unique bioactivities that enable more precise control of the cellular responses, they are usually fragile and susceptible to denaturing in non-physiological conditions during storage or degradation in vivo. Research aimed at improving the binding efficiency and stability of immobilized biomolecules is imperative [26].
Although each material type has its individual merits, combining them may receive a synergistic benefit. For example, a zwitterionic polymer coating may be decorated with multiple types of bioactive molecules to form a multifunctional coating that can both minimize non-specific protein adsorption and provide unique biological cues to either repel or attract certain types of cells.
2.2. Drug Delivery
Biochemical and molecular studies have identified multiple therapeutic targets for minimizing undesired host responses and improving neural interface function [27]–[29]. Guided by these studies, systemic administration of anti-inflammatory, antioxidant, and neuroprotective agents have resulted in effective reduction of inflammation, oxidative stress, and neuronal loss near the electrodes [30], [31], and even improved chronic recording quality [32]. Since systemic administration bears the risk of side effects [33], the next logical step is to locally deliver therapeutics.
Drug-eluting coatings
Drug-eluting coatings is one way to achieve localized drug delivery. Although some acute effects have been observed, drug load is limited in such coatings and the release rate is difficult to control [34]. One alternative strategy is to immobilize the drug on the implant surface, in the scenario where the drug can function without being consumed by the cells. Superoxide dismutase mimics have been attached to the implant surfaces to catalytically convert the harmful reactive oxygen species into less harmful products, thereby reducing the degree of the inflammatory response and material damage for up to 1 week in vivo [35].
Electrically controlled drug delivery
Electrically controlled drug delivery from conducting polymer electrode coating has been explored to deliver anti-inflammatory drugs from the electrode site to reduce inflammation and increase neuronal survival [36]. The electrical control allows the timing, location, and dose of delivery to be precise and on-demand. To increase drug loading capacity, mesoporous nanoparticle drug reservoirs can be introduced as a dopant for PEDOT electrode coatings and the functionality has been verified in vivo [37]. Similarly, poly(lactic-co-glycolic acid) microspheres can be preloaded with drugs and co-deposited with PPy onto electrodes and validated with in vitro experiments [38]. Even with these nano and micro-reservoirs, this method of drug delivery is better suited for fine-tuning the local environment of the microelectrode sites at discrete time points.
Sustained drug delivery
Sustained drug delivery can be achieved by microfluidics with refillable reservoirs. Significant advances have been made in microfluidic systems from miniaturization and improving flexibility and insertion method, to wireless and multimodal capacity [39]. These advances can be utilized for improving the device-tissue integration. One study proposed a dual-layer microchannel system integrated with neural probes for repeated infusion of anti-inflammatory factors through a hydrogel-filled channel for in vivo implantation up to 2 weeks [40]. Another acute in vivo study has equipped a peripheral nerve cuff with microfluidic channels which can focally release lysing agents to remove connective tissue separating the electrodes from nerve fibers, and then deliver neurotrophic factors to promote axonal sprouting of the exposed nerve fibers onto the microfluidic channels where the stimulation or recording electrodes are embedded [41].
RNAs and DNAs delivery
RNAs and DNAs delivery could greatly expand our ability to modulate the cellular response. A gene-embedded optoelectronic array has enabled spatiotemporal electroporation directly from the electrode for gene delivery for up to 1 week in vivo [42]. Such an approach could be used to drive the expression of brain-derived neurotrophic factor from mesenchymal cells lining the cochlear peri-lymphatic canals to promote neurite regeneration towards cochlear implant electrodes [42], [43]. Integrated microfluidics may also enable the delivery of vectors to modulate cellular production or change cell type completely (e.g. turning a glial scar into functional neurons [44]) at the implant-tissue interface [45].
2.3. Biomorphic Design
Designing electrode devices to closely mimic the tissue mechanics and architecture has been a common approach for improving device-tissue integration. Electrode surfaces can be patterned with micro-cone structures matching cellular features of neurons to promote their direct attachment and growth while reducing glia attachments for up to 6 weeks in vivo [46]. In addition to the elaborate microstructures, randomly-nanotextured surfaces can also promote neurite attachment and growth, enhance neuronal survival and reduce gliosis in vivo for up to 3 months [26], [49].
Ultrasmall and ultra-flexible implant designs have shown remarkable success in reducing the FBR [50]. The Lieber lab fabricated neuron-like electronics (NeuE) mimicking the shape, structure, and softness of neuronal somas and axons. These probes demonstrated very stable single-unit recording from rodent brains with no signs of glial scarring or neuronal degradation for up to 3 months [47].
Inspired by twining plants, a peripheral neural interface was fabricated with the capability of self-climbing around the peripheral nerve, driven by the body temperature. These stretchable serpentine wire meshes were integrated onto flexible shape memory substrates and reconfigured into a 3D helix, which reduced nerve injury associated with mechanical and geometrical mismatch [48].
2.4. Biohybrid design
Cells and tissues may be pre-integrated onto the device and serve as a bridge to promote a biofriendly interface and/or a stable neuron/electrode connection. Although the idea of biohybrid design has been proposed for several decades, the progress has been limited (see review [53]). Recent advances in microtechnology, tissue engineering, and cell therapy are poised to accelerate the progress of the biohybrid approach in the near future.
Previous cell seeding studies demonstrated feasibility and evidence of FBR reduction, but they also highlight the need for additional scaffolds or carriers to improve the survival of the transplanted cells. Multilayered constructs (Figure 3a) consisting of conductive hydrogels and biomolecule ligands have been proposed to provide a supportive 3D environment for cell survival and growth at the confined interface [51], [54]. After coating this cell integrated conducting hydrogel layer on a platinum macroelectrode, this living electrode design demonstrates good cell viability and ECM production. Significant challenges still remain on maintaining cell viability, controlling differentiation, preventing proliferation, and achieving synaptic connection with the host cells.
Figure 3.
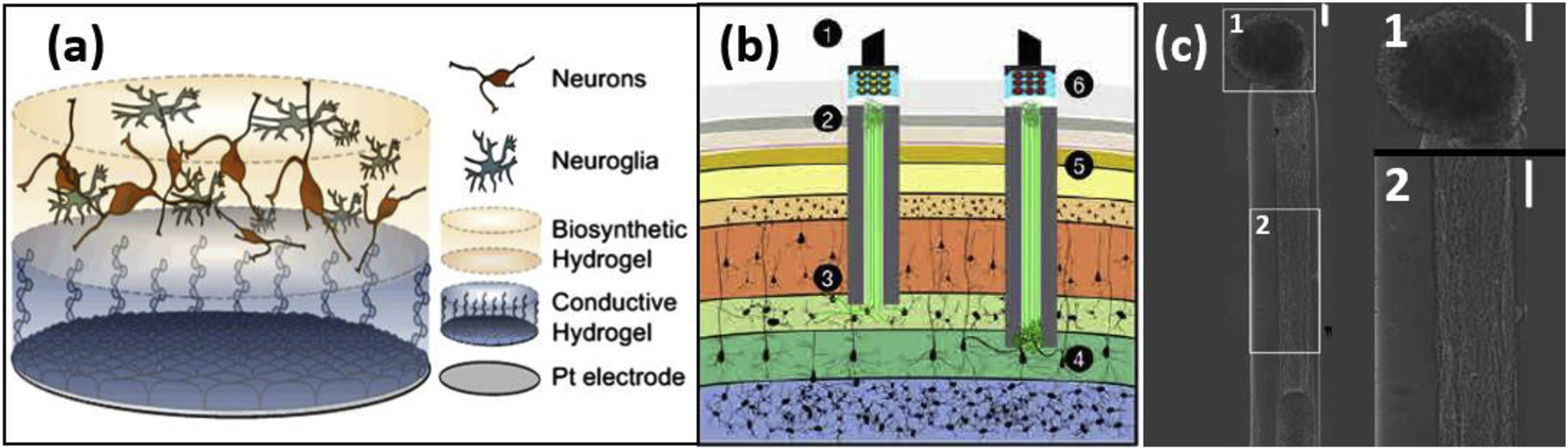
Examples of neural electrodes with biohybrid designs from recent literature. (a) Schematic representation of synaptic interfaces between an electrode and the target tissue produced by integrating living neural cells into tissue-engineered hydrogel coatings. Bottom, Pt electrode site. Middle, conductive hydrogel coating and top, neural network encapsulated within the degradable hydrogel. Adapted from [51] with Creative Commons license. (b)-(c) Engineered living axon-based electrode [52]. (b) Optogenetically active micro tissue engineered neural network (μTENNs) as transplantable input/output channels. Inputs: An LED array (1) optically stimulates a unidirectional, channelrhodopsin-positive μTENN (2) to activate layer IV neurons (3). Outputs: Layer V neurons (4) synapse a bidirectional μTENN (5); relayed neuronal activity is recorded by a photodiode array on the brain surface (6). Axon-based living electrodes enables synaptic specificity, biological multiplexing, and stability. (c) Aggregate μTENNs exhibit robust axonal growth and controllable architecture, with discrete regions of cell bodies (c.1) and neuritic projections (c.2). Scale bar: (c) 100μm.
The Cullen lab developed axon-based living electrodes, micro tissue engineered neural networks (μTENN) [52] (Figure 3b&c). Neurons are seeded at one end of the agarose-collagen hydrogel microcolumn structure while their axons project through the micro conduit towards the other end. The in vitro grown axon construct was implanted into the brain for a month and formed functional synaptic connections with the host neurons and achieved bi-directional communication. Since only biological components remain inside the tissue, the chronic FBR may be eliminated. Combined with optogenetic manipulations, the μTENN may be capable of cell type specific synaptic targeting and biological multiplexing.
In addition to incorporating whole cells and tissues, functional organelles or artificial cells made with a biosynthetic approach may also be incorporated on electrode devices. These organelles or synthetic cells may be engineered to transduce a variety of functional signals, with more robust survival and lower risk of an adaptive immune response.
3. Conclusions
To promote tissue-device integration, we look for answers from nature to take advantage of the evolutionarily optimized design principles. Bio-integrative designs range from the relatively simple surface coating and drug delivery strategies to biomorphic architecture and incorporation of living components (Figure 4). Some strategies have shown in vivo effectiveness with stable functional outcome for months, others are still exploratory but also shown fascinating potentials from in vitro experiments. It is important to realize that as the complexity increases, the chance of failure grows, requiring additional considerations for device design and testing. Each approach has its advantages and limitations and should be carefully selected or combined based on the needs of specific applications. Combining different domains of knowledge and bringing in new tools will lead us to the next generation of seamless neural interfaces.
Figure 4.
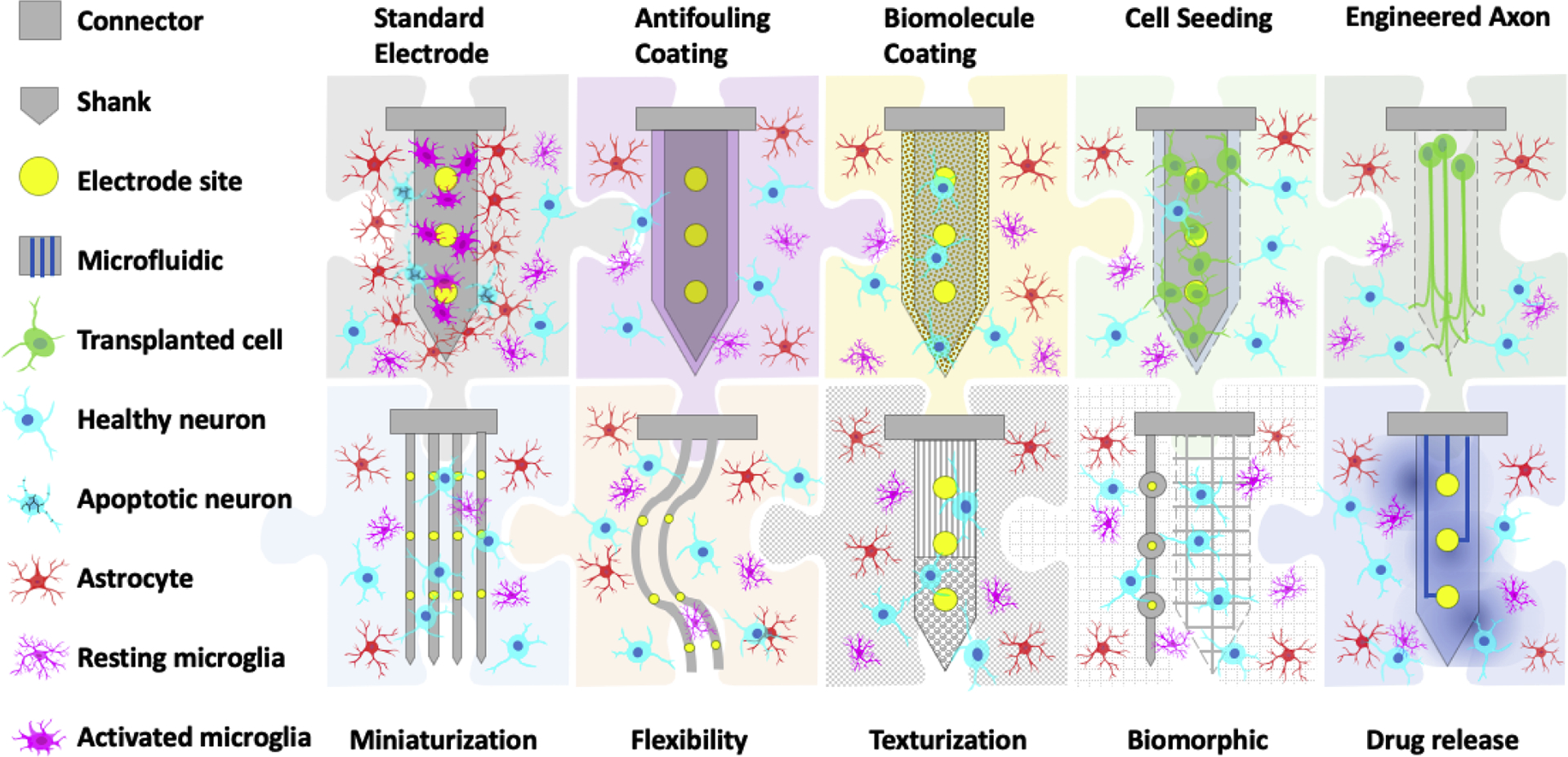
Bio-integrative design strategies that are aimed at eliminating the device factors that aggravate foreign body response and/or incorporating bioactive components to actively modulate the cellular response. These approaches span from anti-fouling coatings, bioactive surface treatments, releasing of therapeutic agents, mimicking tissue mechanics, cellular size, and structure, to the attachment of living cells and tissues. These strategies used alone or in combination will provide a roadmap for future developments to achieve seamless device-tissue interaction.
Figure 2.
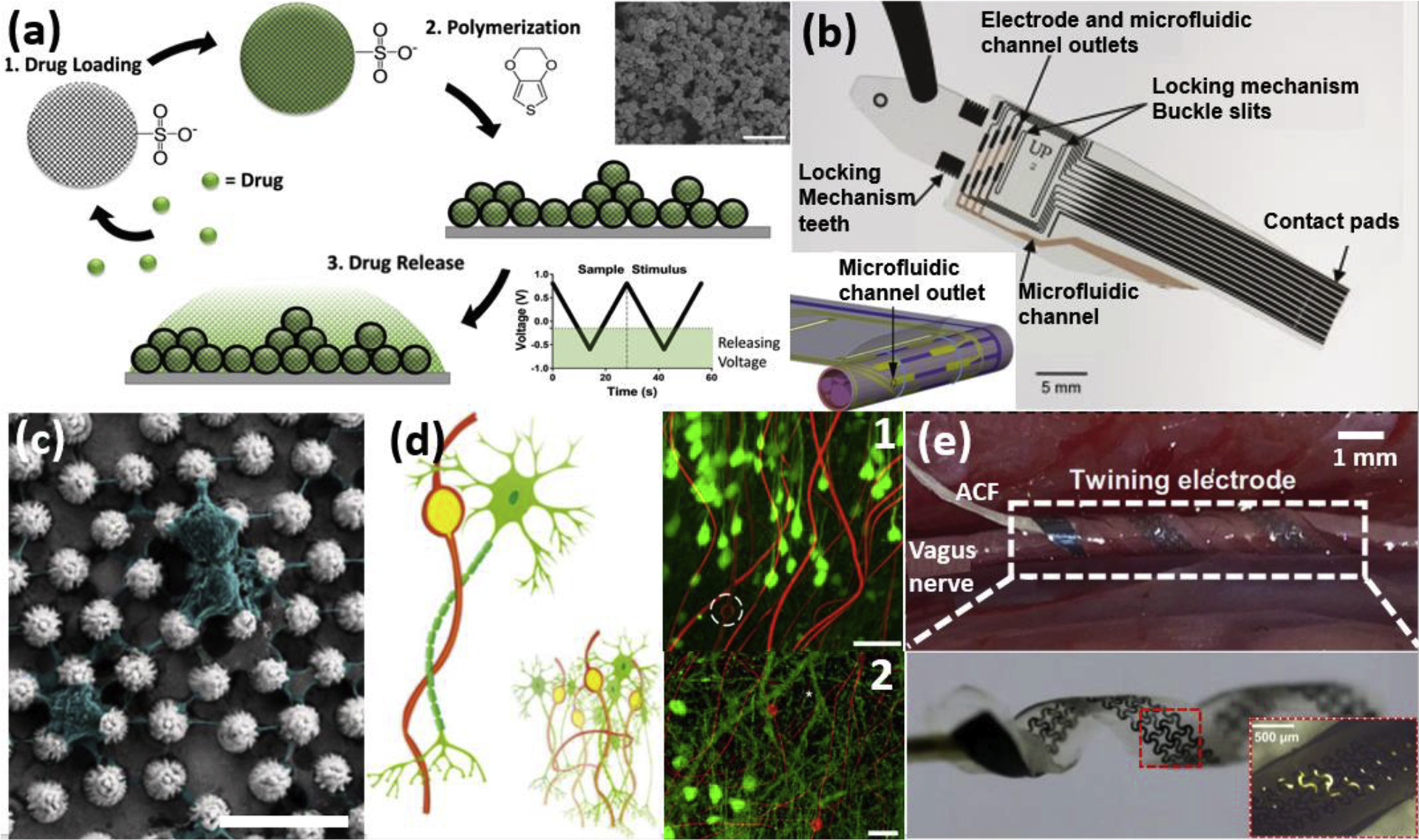
Examples of neural electrodes with drug delivery functionality and biomorphic designs from recent literature. (a) Schematics of drug loading into sulfonated silica particle (SNP) and electrically triggered drug-releasing PEDOT/SNP. Drugs are loaded into the porous nanoparticles via sonication. PEDOT/SNP films (inset shows SEM image) are polymerized under constant current, and a drug can be released by applying triangular voltage pulses via cyclic voltammetry. Adapted with permission from [37]. (b) A fabricated Parylene-C lyse-and-attract cuff electrode (LACE) with integrated microfluidic channels. A red photoresist layer is shown in the channels for visibility. Inset shows a rendered image of the LACE installed on the nerve. Adapted with permission from [41]. (c) SEM images of hippocampal neurons cultured on a Pt microcone-array-based (MA) film; neurons and neurites are shown in pseudo colors. Adapted from [46] with Creative Commons license. (d) Schematics showing the structural similarity between neuron-like electronics (NeuE) and neurons from the subcellular level to the network level (inset). Neurons, green; electrodes and interconnects, yellow; polymer layers, red. (d.1) High-resolution images of the 3D reconstructed interface between neurons (green) and NeuE (red) at 6 weeks post-implantation. Electrodes are indicated by white dashed circles. (d.2) A close-up 3D neural interface of the smaller NeuE in additional independent samples near the dentate gyrus (DG) at 2 weeks post-injection. White asterisks indicate dendritic branches[47]. (e) The climbing-inspired shape-memory twining electrode implanted on a rabbit vagus nerve. (e, top) The twining electrode (inner diameter ~1mm) can conformally contact the vagus nerve. (e, bottom) It can phase transition from the temporarily flattened state to the twined state (inner diameter of ~1 mm) as the temperature becomes body temperature (inset shows the zoom-in of the mesh serpentine electrode design) [48]. ACF, anisotropic conductive film. Scale bar: (c)10μm, (e)500μm. Scale bar: (a)1μm, (b)5mm (c)10 μm, (d.1) 50 μm, (d.2) 20 μm, (e. top) 1mm, (e. bottom)500 μm.
Highlights:
Bio-integrative strategies promote neural tissue-device integration
Surface modifications camouflage the surface and promote neuron attachment
Drug delivery dampens the local inflammation and encourages axonal ingrowth
Matching the tissue mechanics and architecture enhances device integration
Biohybrids incorporating live cell and tissue components offer new opportunities
Acknowledgments:
This work is supported by National Institute of Health 1R01 NS089688, R01NS110564, U01 NS113279, R01NS102725, R21 DA049592-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Reference
- [1].Chaudhary U, Birbaumer N, and Ramos-Murguialday A, “Brain–computer interfaces for communication and rehabilitation,” Nat. Rev. Neurol, vol. 12, no. 9, Art. no. 9, September. 2016, doi: 10.1038/nrneurol.2016.113. [DOI] [PubMed] [Google Scholar]
- [2].Chen R, Canales A, and Anikeeva P, “Neural recording and modulation technologies,” Nat. Rev. Mater, vol. 2, no. 2, Art. no. 2, January. 2017, doi: 10.1038/natrevmats.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Michelson NJ et al. , “Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: new emphasis on the biological interface,” J. Neural Eng, vol. 15, no. 3, p. 033001, 2018, doi: 10.1088/1741-2552/aa9dae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luan L et al. , “Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration,” Sci. Adv, vol. 3, no. 2, p. e1601966, February. 2017, doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fiáth R et al. , “Slow insertion of silicon probes improves the quality of acute neuronal recordings,” Sci. Rep, vol. 9, no. 1, p. 111, January. 2019, doi: 10.1038/s41598-018-36816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wellman SM et al. , “A Materials Roadmap to Functional Neural Interface Design,” Adv. Funct. Mater, p. n/a–n/a, doi: 10.1002/adfm.201701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang L et al. , “Zwitterionic hydrogels implanted in mice resist the foreign-body reaction,” Nat. Biotechnol, vol. 31, no. 6, Art. no. 6, June. 2013, doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- [8].Yang Q, Wu B, Eles JR, Vazquez AL, Kozai TDY, and Cui XT, “Zwitterionic Polymer Coating Suppresses Microglial Encapsulation to Neural Implants In Vitro and In Vivo,” Adv. Biosyst, vol. n/a, no. n/a, p. 1900287, doi: 10.1002/adbi.201900287. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First examination of the antifouling effect of pure zwitterionic polymer coating on neural implant showing reduced microglia endfeet spreading on implant surface revealed by 2 photon in vivo imaging
- [9].Golabchi A, Wu B, Cao B, Bettinger CJ, and Cui XT, “Zwitterionic polymer/polydopamine coating reduce acute inflammatory tissue responses to neural implants,” Biomaterials, vol. 225, p. 119519, December. 2019, doi: 10.1016/j.biomaterials.2019.119519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cui X et al. , “Surface modification of neural recording electrodes with conducting polymer/biomolecule blends,” J. Biomed. Mater. Res, vol. 56, no. 2, pp. 261–272, 2001, doi: . [DOI] [PubMed] [Google Scholar]
- [11].Cui X and Martin DC, “Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays,” Sens. Actuators B Chem, vol. 89, no. 1, pp. 92–102, March. 2003, doi: 10.1016/S0925-4005(02)00448-3. [DOI] [Google Scholar]
- [12].Wu B, Cao B, Taylor IM, Woeppel K, and Cui XT, “Facile Synthesis of a 3,4-Ethylene-Dioxythiophene (EDOT) Derivative for Ease of Bio-Functionalization of the Conducting Polymer PEDOT,” Front. Chem, vol. 7, 2019, doi: 10.3389/fchem.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alba NA, Du ZJ, Catt KA, Kozai TDY, and Cui XT, “In Vivo Electrochemical Analysis of a PEDOT/MWCNT Neural Electrode Coating,” Biosensors, vol. 5, no. 4, pp. 618–646, October. 2015, doi: 10.3390/bios5040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kozai TDY et al. , “Chronic In Vivo Evaluation of PEDOT/CNT for Stable Neural Recordings,” IEEE Trans. Biomed. Eng, vol. 63, no. 1, pp. 111–119, January. 2016, doi: 10.1109/TBME.2015.2445713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng S, “Multimodal Investigation of the Efficiency and Stability of Microstimulation using Electrodes Coated with PEDOT /CNT and Iridium Oxide,” Doctoral Thesis, University of Pittsburgh, 2021. [Google Scholar]
- [16].Goding JA, Gilmour AD, Aregueta-Robles UA, Hasan EA, and Green RA, “Living Bioelectronics: Strategies for Developing an Effective Long-Term Implant with Functional Neural Connections,” Adv. Funct. Mater, vol. 28, no. 12, p. 1702969, 2018, doi: 10.1002/adfm.201702969. [DOI] [Google Scholar]
- [17].Zheng X et al. , “Soft Conducting Elastomer for Peripheral Nerve Interface,” Adv. Healthc. Mater, vol. 8, no. 9, p. 1801311, 2019, doi: 10.1002/adhm.201801311. [DOI] [PubMed] [Google Scholar]
- [18].Deng J et al. , “Electrical bioadhesive interface for bioelectronics,” Nat. Mater, pp. 1–8, September. 2020, doi: 10.1038/s41563-020-00814-2. [DOI] [PubMed] [Google Scholar]; •• This paper demonstrated a graphene nanocomposite based bioadhesive layer which provided a reversible, stable and seamless integration with dynamic wet tissue, without comprising the functionality of the stimulating or the recording electrodes.
- [19].Oakes RS, Polei MD, Skousen JL, and Tresco PA, “An astrocyte derived extracellular matrix coating reduces astrogliosis surrounding chronically implanted microelectrode arrays in rat cortex,” Biomaterials, vol. 154, pp. 1–11, February. 2018, doi: 10.1016/j.biomaterials.2017.10.001. [DOI] [PubMed] [Google Scholar]
- [20].Vitale F et al. , “Biomimetic extracellular matrix coatings improve the chronic biocompatibility of microfabricated subdural microelectrode arrays,” PLOS ONE, vol. 13, no. 11, p. e0206137, November. 2018, doi: 10.1371/journal.pone.0206137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eles JR et al. , “Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy,” Biomaterials, vol. 113, pp. 279–292, January. 2017, doi: 10.1016/j.biomaterials.2016.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Golabchi A, Woeppel KM, Li X, Lagenaur CF, and Cui XT, “Neuroadhesive protein coating improves the chronic performance of neuroelectronics in mouse brain,” Biosens. Bioelectron, vol. 155, p. 112096, May 2020, doi: 10.1016/j.bios.2020.112096. [DOI] [PMC free article] [PubMed] [Google Scholar]; • L1 protein coated microelectrodes demonstrated enhanced neurite outgrowth, reduced microglial activation and stable chronic recording for 16 weeks. Biomimetic coating improved overall biointegration at the device-tissue interface.
- [23].Azemi E, Lagenaur CF, and Cui XT, “The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface,” Biomaterials, vol. 32, no. 3, pp. 681–692, January. 2011, doi: 10.1016/j.biomaterials.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee JY, Khaing ZZ, Siegel JJ, and Schmidt CE, “Surface modification of neural electrodes with a pyrrole-hyaluronic acid conjugate to attenuate reactive astrogliosis in vivo,” RSC Adv, vol. 5, no. 49, pp. 39228–39231, 2015, doi: 10.1039/C5RA03294F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rauhala OJ et al. , “Chitosan-Based, Biocompatible, Solution Processable Films for In Vivo Localization of Neural Interface Devices,” Adv. Mater. Technol, vol. 5, no. 3, p. 1900663, 2020, doi: 10.1002/admt.201900663. [DOI] [Google Scholar]
- [26].Woeppel KM, Zheng XS, and Cui XT, “Enhancing surface immobilization of bioactive molecules via a silica nanoparticle based coating,” J. Mater. Chem. B, vol. 6, no. 19, pp. 3058–3067, 2018, doi: 10.1039/C8TB00408K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kozai TDY et al. , “Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response,” Biomaterials, vol. 35, no. 36, pp. 9620–9634, December. 2014, doi: 10.1016/j.biomaterials.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bedell HW, Schaub NJ, Capadona JR, and Ereifej ES, “Differential expression of genes involved in the acute innate immune response to intracortical microelectrodes,” Acta Biomater, vol. 102, pp. 205–219, January. 2020, doi: 10.1016/j.actbio.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bennett C, Álvarez-Ciara A, Franklin M, Dietrich WD, and Prasad A, “The complement cascade at the Utah microelectrode-tissue interface,” Biomaterials, vol. 268, p. 120583, January. 2021, doi: 10.1016/j.biomaterials.2020.120583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dubaniewicz MT et al. , “Inhibition of Na+/H+ exchanger modulates microglial activation and scar formation following microelectrode implantation,” J. Neural Eng, 2021, doi: 10.1088/1741-2552/abe8f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Haley RM, Zuckerman ST, Dakhlallah H, Capadona JR, von Recum HA, and Ereifej ES, “Resveratrol Delivery from Implanted Cyclodextrin Polymers Provides Sustained Antioxidant Effect on Implanted Neural Probes,” Int. J. Mol. Sci, vol. 21, no. 10, Art. no. 10, January. 2020, doi: 10.3390/ijms21103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Golabchi A et al. , “Melatonin improves quality and longevity of chronic neural recording,” Biomaterials, vol. 180, pp. 225–239, October. 2018, doi: 10.1016/j.biomaterials.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Potter-Baker KA et al. , “Implications of chronic daily anti-oxidant administration on the inflammatory response to intracortical microelectrodes,” J. Neural Eng, vol. 12, no. 4, p. 046002, May 2015, doi: 10.1088/1741-2560/12/4/046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Potter KA, Jorfi M, Householder KT, Foster EJ, Weder C, and Capadona JR, “Curcumin-releasing mechanically adaptive intracortical implants improve the proximal neuronal density and blood–brain barrier stability,” Acta Biomater, vol. 10, no. 5, pp. 2209–2222, May 2014, doi: 10.1016/j.actbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- [35].Zheng XS et al. , “A superoxide scavenging coating for improving tissue response to neural implants,” Acta Biomater, vol. 99, pp. 72–83, November. 2019, doi: 10.1016/j.actbio.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kolarcik CL et al. , “Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion,” J. Neural Eng, vol. 12, no. 1, p. 016008, February. 2015, doi: 10.1088/1741-2560/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Woeppel KM, Zheng XS, Schulte ZM, Rosi NL, and Cui XT, “Nanoparticle Doped PEDOT for Enhanced Electrode Coatings and Drug Delivery,” Adv. Healthc. Mater, vol. 8, no. 21, p. 1900622, 2019, doi: 10.1002/adhm.201900622. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This work showcased the use of porous sulfonated silica nanoparticles for loading drug volume which can be released upon application of cyclic voltage sweeps in vitro and in vivo.
- [38].Antensteiner M, Khorrami M, Fallahianbijan F, Borhan A, and Abidian MR, “Conducting Polymer Microcups for Organic Bioelectronics and Drug Delivery Applications,” Adv. Mater, vol. 29, no. 39, p. 1702576, 2017, doi: 10.1002/adma.201702576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Frank JA et al. , “In Vivo Photopharmacology Enabled by Multifunctional Fibers,” ACS Chem. Neurosci, vol. 11, no. 22, pp. 3802–3813, November. 2020, doi: 10.1021/acschemneuro.0c00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Frey L, Bandaru P, Zhang YS, O’Kelly K, Khademhosseini A, and Shin SR, “A Dual-Layered Microfluidic System for Long-Term Controlled In Situ Delivery of Multiple Anti-Inflammatory Factors for Chronic Neural Applications,” Adv. Funct. Mater, vol. 28, no. 12, p. 1702009, 2018, doi: 10.1002/adfm.201702009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Elyahoodayan S, Larson C, Cobo AM, Meng E, and Song D, “Acute In Vivo Testing of a Polymer Cuff Electrode with Integrated Microfluidic Channels for Stimulation, Recording, and Drug Delivery on Rat Sciatic Nerve,” J. Neurosci. Methods, p. 108634, February. 2020, doi: 10.1016/j.jneumeth.2020.108634. [DOI] [PubMed] [Google Scholar]; • A cuff electrode was integrated with a microfluidic system which focally released lysing agents to separate nerve fibers from electrode sites. This system can be utilized for release of neurotropic factors to promote desired nerve growth.
- [42].Huang W-C et al. , “Gene-Embedded Nanostructural Biotic–Abiotic Optoelectrode Arrays Applied for Synchronous Brain Optogenetics and Neural Signal Recording,” ACS Appl. Mater. Interfaces, vol. 11, no. 12, pp. 11270–11282, March. 2019, doi: 10.1021/acsami.9b03264. [DOI] [PubMed] [Google Scholar]
- [43].Pinyon JL, Klugmann M, Lovell NH, and Housley GD, “Dual-Plasmid Bionic Array-Directed Gene Electrotransfer in HEK293 Cells and Cochlear Mesenchymal Cells Probes Transgene Expression and Cell Fate,” Hum. Gene Ther, vol. 30, no. 2, pp. 211–224, July. 2018, doi: 10.1089/hum.2018.062. [DOI] [PubMed] [Google Scholar]
- [44].Wu Z et al. , “Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington’s disease,” Nat. Commun, vol. 11, no. 1, Art. no. 1, February. 2020, doi: 10.1038/s41467-020-14855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Winter BM, Daniels SR, Salatino JW, and Purcell EK, “Genetic Modulation at the Neural Microelectrode Interface: Methods and Applications,” Micromachines, vol. 9, no. 10, Art. no. 10, October. 2018, doi: 10.3390/mi9100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen H, Wang L, Lu Y, and Du X, “Bioinspired microcone-array-based living biointerfaces: enhancing the anti-inflammatory effect and neuronal network formation,” Microsyst. Nanoeng, vol. 6, no. 1, Art. no. 1, July. 2020, doi: 10.1038/s41378-020-0172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Chen et al introduced rough morphologies of micro cones which help with neurite outgrowth and viability around implant, along with reduced astrocytic encapsulation. The cones resembled the cellular features of neurons which is hypothesized to promote the increased neuronal viability.
- [47].Yang X et al. , “Bioinspired neuron-like electronics,” Nat. Mater, p. 1, February. 2019, doi: 10.1038/s41563-019-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Electrodes mimicking the neuron structure, softness and size were fabricated which demonstrated excellent integration with neural tissue, indicated by the uniform distribution of neuronal and glial populations around implant site. Stable single-unit recording was also observed.
- [48].Zhang Y et al. , “Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording,” Sci. Adv, vol. 5, no. 4, p. eaaw1066, April. 2019, doi: 10.1126/sciadv.aaw1066. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Biocompatible twining electrode were fabricated, using shape memory polymers, which self-climb on nerves, driven by body temperature. Highly conformable, these electrodes reduce nerve injury associated with mechanical mismatch.
- [49].Kim Y et al. , “Nano-Architectural Approaches for Improved Intracortical Interface Technologies,” Front. Neurosci, vol. 12, 2018, doi: 10.3389/fnins.2018.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Comprehensive review discussing the different fabrication techniques used for introducing nanoarchitecture to neural interface devices, the effect of texturization on electrode-tissue interface and the underlying mechanisms.
- [50].Luan L et al. , “Recent Advances in Electrical Neural Interface Engineering: Minimal Invasiveness, Longevity, and Scalability,” Neuron, vol. 108, no. 2, pp. 302–321, October. 2020, doi: 10.1016/j.neuron.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Aregueta-Robles UA, Woolley AJ, Poole-Warren LA, Lovell NH, and Green RA, “Organic electrode coatings for next-generation neural interfaces,” Front. Neuroengineering, vol. 7, 2014, doi: 10.3389/fneng.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Adewole DO et al. , “Development of optically controlled ‘living electrodes’ with long-projecting axon tracts for a synaptic brain-machine interface,” Sci. Adv, vol. 7, no. 4, p. eaay5347, January. 2021, doi: 10.1126/sciadv.aay5347. [DOI] [PubMed] [Google Scholar]; •• This work showcases the advancements in the microtissue engineered neural network (μTENN) system to incorporate transplanted living cortical neurons and axonal tracts which can be optically stimulated to interact with host neural circuitries in the rat cortex.
- [53].Rochford AE, Carnicer-Lombarte A, Curto VF, Malliaras GG, and Barone DG, “When Bio Meets Technology: Biohybrid Neural Interfaces,” Adv. Mater, vol. 32, no. 15, p. 1903182, 2020, doi: 10.1002/adma.201903182. [DOI] [PubMed] [Google Scholar]
- [54].Goding J et al. , “A living electrode construct for incorporation of cells into bionic devices,” MRS Commun, vol. 7, no. 3, pp. 487–495, September. 2017, doi: 10.1557/mrc.2017.44. [DOI] [Google Scholar]


