Abstract
Although traditionally seen as regulators of hematopoiesis, colony-stimulating factors (CSFs) have emerged as important players in the nervous system, both in health and disease. This review summarizes the cellular sources, patterns of expression and physiological roles of the macrophage (CSF-1, IL-34), granulocyte-macrophage (GM-CSF) and granulocyte (G-CSF) colony stimulating factors within the nervous system, with a particular focus on their actions on microglia. CSF-1 and IL-34, via the CSF-1R, are required for the development, proliferation and maintenance of essentially all CNS microglia in a temporal and regional specific manner. In contrast, in steady state, GM-CSF and G-CSF are mainly involved in regulation of microglial function. The alterations in expression of these growth factors and their receptors, that have been reported in several neurological diseases, are described and the outcomes of their therapeutic targeting in mouse models and humans are discussed.
Keywords: CSF-1, IL-34, CSF-1R, GM-CSF, G-CSF, microglia
1. Introduction:
The colony stimulating factors (CSFs) were named due to their ability to stimulate the formation of colonies of mature cells from single bone marrow progenitor cells in semi solid cultures. CSF-1, or macrophage CSF (M-CSF), stimulates macrophage colony formation, CSF-2, or granulocyte-macrophage CSF (GM-CSF), stimulates development of colonies comprised of granulocytes and macrophages, CSF-3, or granulocyte CSF (G-CSF), causes the formation of granulocytic colonies, interleukin-3 (IL-3), or multi-CSF, stimulates formation of colonies of mixed hematopoietic cells and interleukin-5 (IL-5), stimulates eosinophilic colony formation. They are known primarily for their regulation of myeloid cells (reviewed in [1]). CSF-1, together with the more recently discovered interleukin-34 (IL-34), signal via the CSF-1 receptor (CSF-1R), to regulate tissue macrophage and osteoclast development and maintenance (reviewed in [2–5]). In the steady state, both circulating and locally expressed CSF-1 play important roles in maintaining tissue macrophage densities [6–8]. CSF-1 action on macrophages is primarily trophic and anti-inflammatory. CSF-1 induced miRNA-21 suppresses the expression of inflammatory mediators and enhances anti-inflammatory marker expression [9]. While GM-CSF is not important for steady-state hematopoiesis, it supports the development and functions of alveolar macrophages [10, 11]. In contrast, gene targeting in mice showed that G-CSF is important for the steady state neutrophil production [12].
CSF-1 is readily detectable in the circulation [6, 13], whereas expression of IL-34 is quite low [14]. Circulating GM-CSF and G-CSF are normally barely detectable, but are increased in response to various inflammatory and other stimuli (reviewed in [15, 16]). CSF-1, IL-34, CSF-2 and CSF-3 have significant roles in the nervous system and are the main focus of this review.
The receptors for CSF-1, GM-CSF and G-CSF differ significantly in terms of structure, mechanism of ligand binding and signaling (Fig. 1). The CSF-1R is a class III receptor tyrosine kinase that is activated by transphosphorylation following dimeric ligand-induced receptor dimerization (reviewed in [2]) (Fig. 1A). CSF-1 and IL-34 are both homodimeric cytokines with limited (~10%) primary sequence homology but sharing a similar three-dimensional structure (reviewed in [2]). The binding sites for both CSF-1 and IL-34 overlap [17] and they can effectively compete one with the other for CSF-1R binding [18, 19]. There are 8 receptor intracellular domain tyrosines that are phosphorylated in the response to ligand binding to create binding sites for different downstream signaling molecules [2]. As the differences between CSF-1 and IL-34 signaling are slight and their tissue and cell type expression patterns tend not to overlap ([19–22], regulation by these ligands in development and in the adult is, for the most part, spatially [21, 23, 24] and temporally [21, 25] distinct.
Figure 1. CSF-1, GM-CSF and G-CSF receptors and their main downstream signaling pathways.
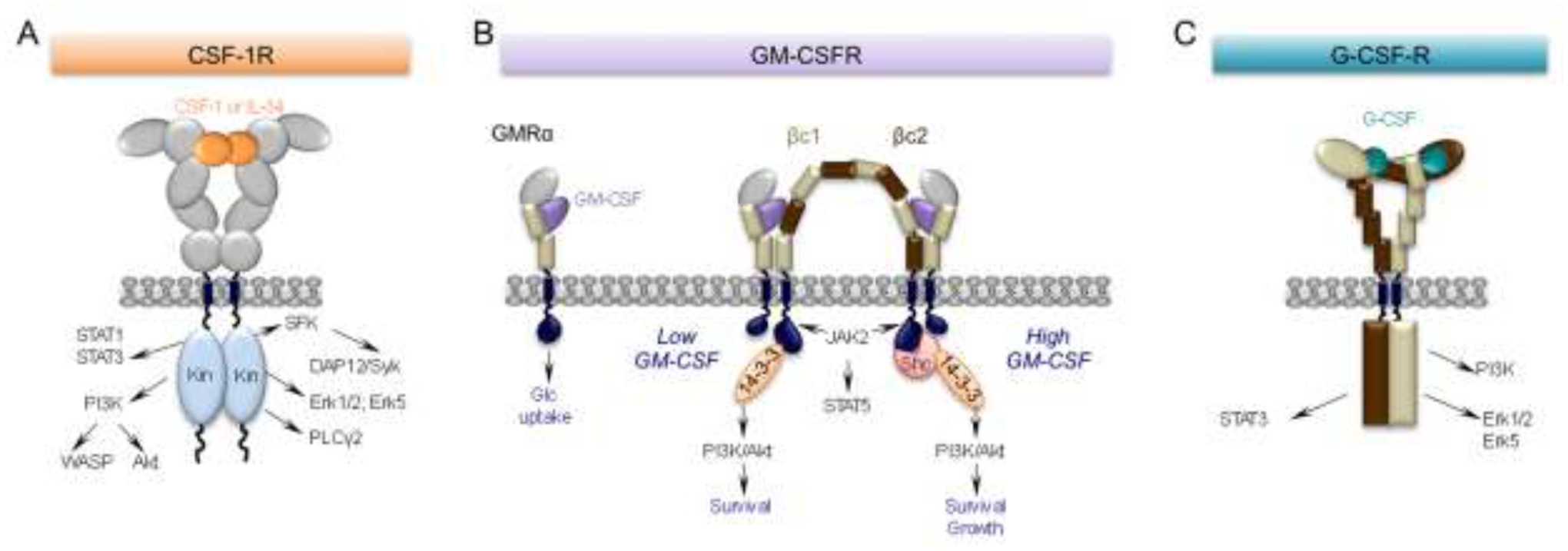
(A) The CSF-1R is a homodimeric tyrosine kinase that binds homodimers of CSF-1 or IL-34. The extracellular domain consists of five immunoglobulin (Ig)-like domains. Ligand binding triggers downstream tyrosine phosphorylation, initiating a cascade of signaling events resulting in cell differentiation, survival, proliferation, migration and suppression of inflammation (reviewed in [2]). (B) Monomeric GM-CSF binds with relatively low affinity (nM range) to a “wrench-like” structure formed by the N-terminal Ig domain and the 2 fibronectin type III domains of the GMRα chain (GMRα). Even in the absence of the common β subunit (βc), this interaction is sufficient to elicit a biological response, i.e. glucose uptake [37]. In cells also expressing βc, the GM-CSF/GMRα complexes further associate with the β chains, creating a hexameric high-affinity receptor (100pM range) with the depicted structure. These complexes associate laterally through the GMRα subunit to form a dodecameric complex that is responsible for signaling (reviewed in [16]). Mutually exclusive phosphorylation events at βc residues, S585 or Y577, recruit either the 14–3-3 adaptor protein at low concentrations of GM-CSF (S585) or, in the setting of high GM-CSF concentrations, Shc (Y577), mediating a molecular switch between survival and survival and growth, respectively. (C) G-CSF is monomeric. The G-CSF receptor has six extracellular domains (D1-D6). D1 is an N-terminal Ig-like domain and D2-D6 are fibronectin type III domains. These are followed by a transmembrane domain and an intracellular domain without intrinsic kinase activity. D2 and D3 form the cytokine receptor homologous (CRH) module involved in ligand binding, while D4-D6 facilitate dimerization of the cytoplasmic regions. The signaling unit is a 2:2 receptor:ligand complex, in which each G-CSF monomer binds one receptor through the CRH module and the second receptor through the Ig domain (reviewed in [40]). In neurons, ligand binding activates the Erk family and enhances neuronal survival while activation of the PI3-K/Akt and STAT3 signaling pathways and prevents apoptotic cell death, by inhibiting activation of caspases and by increasing anti-apoptotic protein members such as Bcl-xL (reviewed in [42]).
GM-CSF is a monomeric cytokine belonging to the β common (βc) family of cytokines that also includes IL-3 and IL-5 [26–28]. Their receptors each possess unique alpha subunits that associate with the βc as their signaling subunit (reviewed in [16]). GM-CSF binds the GM-CSF receptor α chain (GMRα) with low affinity (Kd ~5 nM, [29]), but the binding becomes high affinity (Kd ~100 pM) in the presence of the βc) [30–32]. The structures of the GM-CSF receptor (GM-CSFR) subunit complexes have been studied in detail ([33–36]). GM-CSF binding initiates the assembly of a hexameric (2GM-CSF:2GMRα:2βc) complex (Fig. 1B). Through lateral association, these hexameric receptor-ligand complexes transition to a dodecameric signaling complex, leading to the activation of the JAK2-STAT5A/B and PI3K/Akt pathways [33, 35] (reviewed in [16]) (Fig. 1B). In cells lacking the βc, the GM-CSF:GMRα complex alone has been reported to mediate tyrosine kinase-independent signaling, leading to increased glucose transport [37].
G-CSF is monomeric cytokine that binds to a single, membrane-spanning protein, the G-CSF receptor (G-CSFR). The G-CSF:G-CSFR complex forms a 2:2 stoichiometry signaling unit in which each G-CSF monomer binds one receptor chain through the cytokine receptor homologous (CRH) module and the second receptor through the Ig domain [38–40] (Fig. 1C). Downstream signaling is via the JAK/STAT3, PI3K/AKT and MAPK/ERK pathways (reviewed in [41, 42]).
In steady state, CSF-1 and IL-34 are expressed in brain in a largely non-overlapping manner by neuronal cells [20–22]. Transcripts for CSF-1 have also been reported in astrocytes, oligodendrocytes and microglia [43, 44]. While CSF-1 circulates at 4.5 ng/ml [13], the circulating concentration of IL-34 is quite low (52 pg/ml, [14]). It is presently unknown whether IL-34 can cross the blood-brain barrier (BBB) and, at normal physiological concentrations, CSF-1 fails to cross (Chitu, V. & Stanley, E.R. unpublished). In contrast, in steady state, both GM-CSF and G-CSF cross the BBB [45, 46].
Within the central nervous system (CNS), functional receptors for CSF-1, GM-CSF and G-CSF are expressed on microglia and some neural lineage cells [22, 47–70] (Fig. 2) and balanced signaling through all three receptors is important for normal CNS homeostasis [62] (Biundo, F., Chitu, V. & Stanley, E.R. manuscript in preparation). Their roles in the nervous system homeostasis and disease are considered for each separately.
Figure 2. Cellular sources of CSF-1, IL-34, GM-CSF and G-CSF and their effects on target cells in the nervous system.
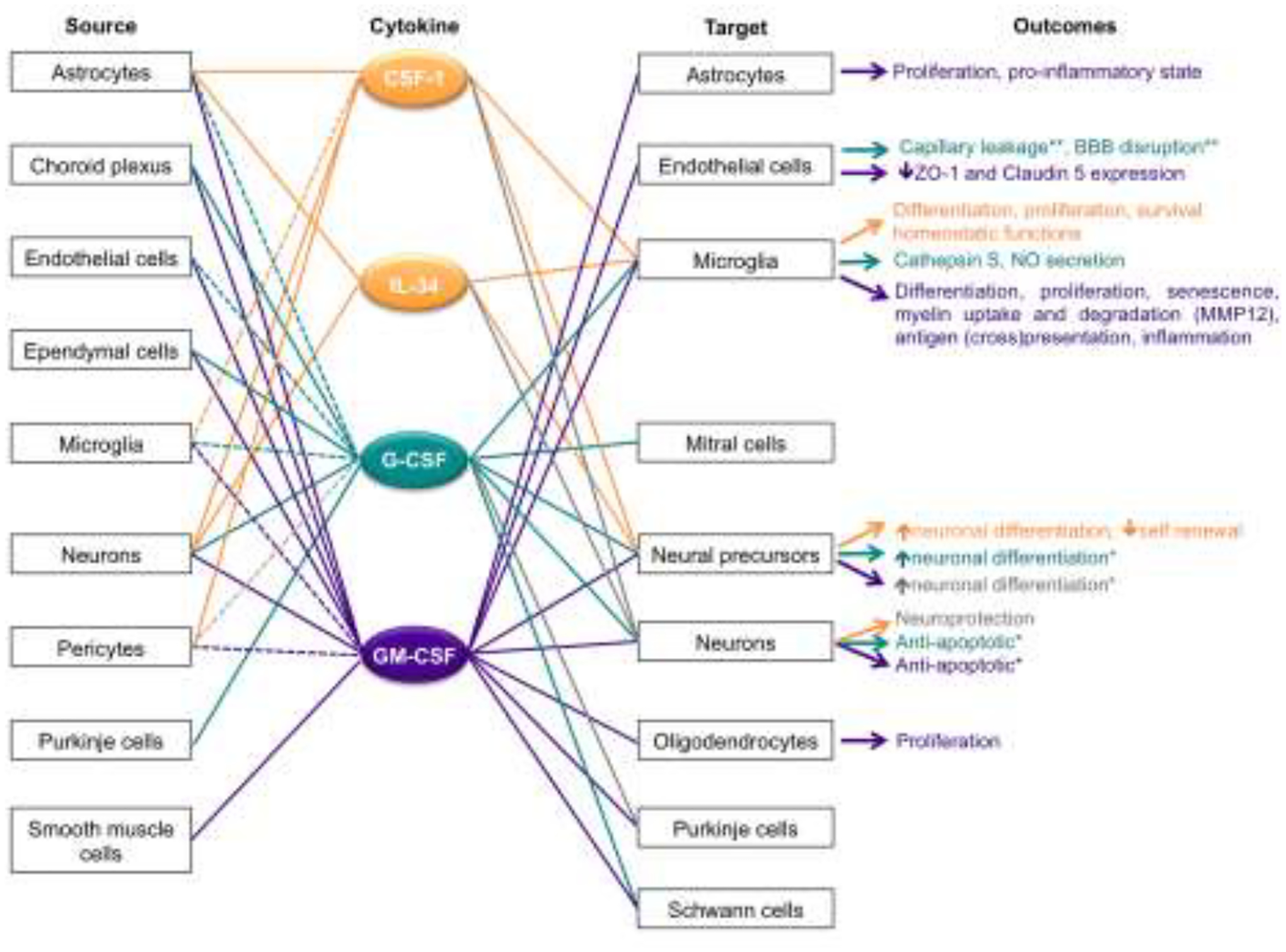
The relationships between individual cytokines and their source, target and the outcome of target activation, are color coded. Dashed lines indicate that cytokine expression requires an activation signal. Grey lines and font indicate controversial, or species-specific findings. *, The effects might be indirect, as other cell types expressing the cytokine receptor were present in the culture system. **, Observed following administration in vivo.
2. CSF-1R and its ligands
2.1. Expression of the CSF-1R in the CNS
The CSF-1R is expressed on all microglia, parenchymal and non-parenchymal macrophages (BAMs) [47, 48, 71]. CSF-1R expression has also been reported in neural precursors, where it may be important in neuronal differentiation during development [22]. The expression of the CSF-1R in mature neurons is controversial (reviewed in [4]). While several studies report that CSF-1R expression defines a subpopulation of pyramidal projection neurons [49] and promotes the survival of injured neurons [50], others have failed to detect expression in adult neurons (reviewed in [4]) [51]). In this review, we have primarily focused on the action of the CSF-1 ligands on microglia.
2.2. Expression of the CSF-1R ligands in the CNS
Both outside and within the brain, CSF-1 and IL-34 mRNAs are differentially expressed [19, 22] and there is no compensatory increase in tissue IL-34 mRNA expression in CSF-1-deficient mice [19]. In the brain, CSF-1 and IL-34 are primarily expressed by neurons in a non-overlapping manner except in the CA3 region of the hippocampus where they co-localize [22] (Fig. 2). CSF-1 mRNA is mainly expressed in the deep cortical layers, cerebellum and spinal cord, whereas IL-34 mRNA is expressed throughout the forebrain (neocortex, olfactory bulb, striatal cells, ependymal cells and choroid plexus) [20–22]. Generally, CSF-1 is preferentially expressed in cerebellum and white matter, whereas IL-34 is preferentially expressed in forebrain and grey matter [19, 23, 24]. Interestingly, the non-overlapping pattern of expression of CSF-1 and IL-34 observed in the adult brain is also observed during embryogenesis [22] and early post-natal development [22, 24]. During embryogenesis, expression of CSF-1 mRNA increases gradually, while IL-34 levels are low [19, 22]. Postnatally, associated with microglial expansion, IL-34 RNA levels increase dramatically relative to CSF-1 mRNA, then decrease in the adult [19, 20, 22, 72]. Together these expression studies suggest complementary, non-redundant regulation by CSF-1 and IL-34 in the brain.
2.3. CSF-1R and its ligands in the nervous system at steady state
2.3.1. Role in the regulation of the development and maintenance of microglia and brain macrophages
The derivation of microglia and meningeal, perivascular and some choroid plexus macrophages from early erythro-myeloid progenitors (EMPs) in the yolk sac is dependent on CSF-1R [73, 74] (reviewed in [75]). CSF-1R expression was reported to first occur at E8 in EMPs [76] and at E9 in EMP-derived A2 progenitors [77]. With the establishment of the embryonic circulatory system, the A2 progenitors migrate to the neural tube and populate the developing brain and the spinal cord at ~E9.5, where they give rise to microglia [73, 77–79], meningeal, and perivascular macrophages [80]. While both Csf1r+/+ and Csf1r−/− progenitors seed the brain rudiment by E10.5, at E12.5 the Csf1r−/− progenitors are greatly reduced compared to their wild-type counterparts, indicating that the CSF-1R provides crucial survival/proliferation signals [73, 81]. The development of the BBB at ~E11.5 ensures virtually no contribution of monocytes derived from hematopoietic stem cells to the establishment of parenchymal microglia [73].
The profound depletion of microglia in adult Csf1r-deficient mice demonstrates that CSF-1R signaling is required for microglial development [73]. CSF-1 deficiency results in a moderate decrease in microglial numbers in adult mice [82], whereas IL-34 deficiency confers a more substantial reduction [20, 21]. However, analysis of microglial precursors and microglia in yolk sac and brain rudiments during embryonic development and in newborn mice revealed no difference between wild type and IL-34 deficient mice [21], suggesting that IL-34 is not involved in the development and seeding of microglial precursors. Furthermore, using function-blocking, anti-CSF-1R ligand antibodies, it was shown that CSF-1 alone is required for microglial colonization and maintenance in embryonic brain, while IL-34 begins to be required postnatally [24]. In addition, experiments involving the genetic ablation of either Csf1 or Il34 in neural lineage cells suggest that, after precursor seeding, the development and maintenance of cerebellar microglia is uniquely dependent on CSF-1, while forebrain microglia require IL-34 [23]. Interestingly, non-overlapping regulation by CSF-1 and IL-34 occurs even within the forebrain, where CSF-1 regulates white matter microglia and IL-34 regulates grey matter microglia [24, 83]. Other studies show that within the developing brain CSF-1R signaling also plays a central role in the establishment of microglial processes [82, 84] and migration [85].
2.3.2. Role of CSF-1R in the maintenance of adult microglia
Coupled proliferation and apoptosis cause mouse microglia to turn over 1–6 times per lifetime [86, 87] and estimates in humans indicate that cortical microglia turn over approximately once per lifetime [88]. In adult mice, the almost complete elimination of microglia by treatment with CSF-1R inhibitors demonstrates that the adult microglial population is uniquely dependent on the CSF-1R for survival and proliferation [89]. After acute depletion in mice, repopulated microglia are solely derived from the proliferation of residual microglia [89, 90]. Studies involving cyclic elimination and recovery of microglia following treatment with a CSF-1R inhibitor [91] or inducible genetic deletion of the Csf1r [92] suggest that the potential for microglia to repopulate is limited. Further, microglia from aged mice fail to proliferate, suggesting that the repopulation ability might be lost with aging [93].
2.3.3. Role in the regulation of neuronal lineage cells
Developmental studies in Csf1r−/− mice revealed a role for CSF-1R signaling in suppressing the expansion forebrain neural progenitor cells (NPC), enhancing their differentiation and promoting their survival and the survival of their early neuronal progeny (reviewed in [4]). Supporting a direct role of the CSF-1R in regulating these processes, CSF-1 or IL-34 each suppressed the self-renewal, but not the proliferation of cultured, purified CSF-1R-expressing NPC and, in clonal differentiation assays, increased the percentage of neuronal colonies, without affecting the percentage of astrocytic or oligodendrocytic colonies. In addition, conditional deletion of the Csf1r in NPC using Csf1rfl/fl;Nestin-Cre/+ mice yielded mice with normal cortical microglia densities at E18.5 and P20, but higher perinatal lethality, smaller brain size and enhanced forebrain progenitor cell proliferation and apoptosis [22]. These studies indicate direct regulation of NPC via the CSF-1R during development. Interestingly, the more efficient stimulation of neuronal differentiation in vitro by IL-34, compared with CSF-1, prompted the identification of a second receptor for IL-34, RPTP-ζ, that is also expressed on NPC [94](reviewed in [4]).
2.4. Physiological Roles of the CSF-1R
Studies of the physiological roles of the CSF-1R in microglia have been limited. As microglia are dependent on the CSF-1R for survival, targeted inactivation of the Csf1r [73], or administration of CSF-1R inhibitors [95] leads to microglial death. In addition, investigations of the effects of systemic administration of CSF-1 in healthy mice are compromised by the lack of BBB penetrance. Csf1r heterozygosity does not lead to depletion of microglia, offering a unique opportunity to determine how reduced CSF-1R signaling impacts microglial function. Studies carried out so far indicate that reduced CSF-1R signaling causes a dyshomeostatic microglial phenotype [62, 96–98]. However, they have primarily characterized microglia isolated from aged Csf1r+/− mice with ongoing neuropathology, thereby documenting both causative (CSF-1R-related) and reactive (pathology related) changes (see 2.6.1 below). To address the physiological roles of CSF-1R signaling, studies before the onset of pathology in this model are warranted.
2.5. Role in aging
Microglia undergo major functional changes during aging that are associated with altered gene expression [99–101]. These include their expansion, acquiring a dystrophic morphology and hyperresponsiveness to inflammatory stimuli, reductions in phagocytosis and motility (reviewed in [102, 103]). In an attempt to rejuvenate microglia in aged mice, 24-month-old mice were treated for 14 days with the CSF-1R inhibitor, PLX5622, eliminating ~70% of microglia. Analysis was commenced 28 days after cessation of inhibitor treatment, when dystrophic microglia were replaced by newly generated ones. The treatment normalized microglia and dendritic spine densities, improved neurogenesis and was accompanied by full-rescue of the aged-induced deficits in long-term potentiation and spatial memory [104]. In another study [105], microglial depletion and repopulation in 6–18 months old mice was reported to reverse age-associated lysosome enlargement and the accumulation of lipofuscin, but produced less significant changes in the age-induced transcriptomic changes in the whole brain tissue and failed to rescue the hyper-responsiveness to LPS challenge, suggesting that the aged brain microenvironment promotes the priming of repopulated microglia. Furthermore, a combination of chronic CSF-1R inhibitor treatment and environmental enrichment has been shown to attenuate metabolic decline in middle-aged female mice, presumably by reducing inflammation in the hypothalamus through depletion of microglia and/or by modulating adipose tissue macrophages [106]. Investigations using tissue-specific deletion of microglia and macrophages should help delineate the contribution of the central and peripheral CSF-1R-dependent phagocytes to aging-related metabolic decline.
2.6. CSF-1R in neurological disease
2.6.1. CSF-1R deficiency diseases: BANDDOS and CRL
The initial identification of dominantly inherited monallelic CSF1R mutations in patients with adult onset leukodystrophy [107] and of homozygous CSF1R mutations in pediatric patients with brain malformations and osteopetrosis [108] has stimulated interest in investigating the involvement of CSF-1R in human developmental and neurodegenerative disease and the identification of animal models that permit the investigation of underlying mechanisms. The two diseases associated with CSF-1R deficiency, Brain Abnormalities, Neurodegeneration, and Dysosteosclerosis (BANDDOS, OMIM #618476) and CSF1R-related leukoencephalopathy (CRL, OMIM #221820), as well as the animal models, have been the subject of a recent review [109] and are only briefly covered here.
BANDDOS is an autosomal recessive disorder caused by bi-allelic mutations in the CSF1R gene. The mutations involved are primarily dominant inactivating missense mutations within the region encoding the intracellular tyrosine kinase domain. Patients exhibit epilepsy, structural brain anomalies, including ventriculomegaly and cerebellar atrophy, as well as leukodystrophy. The disease is variably associated with osteopetrosis. BANDDOS patients that are homozygous for amorphic CSF1R alleles exhibit a marked osteosclerosis and early onset, possessing most of the phenotypic characteristics reported for Csf1r-nullizygous mice [108, 110, 111], whereas homozygous carriers of hypomorphic mutations have no perceptible, or mild, osteosclerosis (reviewed in [109]). These data suggest a CSF1R gene dosage effect on the severity of BANDDOS.
CRL is an autosomal dominant disease associated with cognitive impairment, psychiatric disorders, motor dysfunction and seizures, with typical onset between 10–60 years of age [112]. Based on the observation that a CRL mutation caused CSF1R haploinsufficiency [113], a Csf1r+/− mouse model was developed that faithfully models the human disease [114] (reviewed in [109]). Subsequently, Biundo et al. demonstrated that Csf1r monallelic deletion in microglia was sufficient to cause all aspects of the disease, supporting the idea that CRL is a primary microgliopathy [97]. Indeed, characterization of young and aged Csf1r+/− mice revealed an early elevation in microglial density [114] and provided evidence for microglia dyshomeostasis that was associated with an early loss of presynaptic markers and disruption of the extracellular matrix (ECM) [98], as well as with a transcriptomic profile indicative of pro-oxidant and demyelinating functions [62]. Treatment of the Csf1r+/− mice for 2 months from 6 months of age with the CSF-1R inhibitor, PLX5622, eliminated > 90% of microglia and prevented synaptic changes [98]. A lower, non-depleting dose of PLX65622 (that reduced microglia density by ~ 25%) was sufficient to prevent the development of early spatial memory deficits in Csf1r heterozygous mice [98]. However, the effects of long-term or cessation of treatment were not investigated. Studies focusing on the molecular mechanisms involved revealed that the microgliosis in Csf1r+/− mice was not associated with a compensatory increased expression of the CSF-1R ligands and identified GM-CSF as a direct mediator of microglia expansion and a significant contributor to the oxidative stress and demyelination associated with disease [62, 114]. G-CSF expression was also elevated in Csf1r+/− mouse brains and shown to contribute to microglial activation and disease progression in a non-overlapping manner with GM-CSF (Biundo, F., Chitu, V. & Stanley, E.R., in preparation). Relevant to the human disease, there is evidence that GM-CSF and G-CSF may also contribute to the development of dyshomeostatic microglia in CRL patients [62, 96]. Overall, these studies suggest that balanced regulation by CSF-1R, GM-CSF and G-CSF is necessary for microglial homeostasis and identify new therapeutic targets for CRL.
2.6.2. CSF-1R in inflammatory demyelinating diseases
Studies of remyelination, following lysolecithin-induced white matter demyelination, demonstrate the importance of CSF-1 in preserving an anti-inflammatory phenotype in microglia, as well as indirectly suppressing astrocyte activation, thus preventing axonal damage [115, 116]. Consistent with this, removal of CSF-1 in the twitcher mouse, a model of globoid cell leukodystrophy (GCL), exacerbated the progressive demyelination and neurological symptoms. Mechanistically, lack of CSF-1 resulted in decreased microglia/macrophage numbers, increased myelin debris and reduced recruitment of oligodendrocyte precursor cells, suggesting that CSF-1-activated phagocytes fulfill functions that are crucial for remyelination [117]. In contrast, in connexin 32-deficient mice, a model of Charcot–Marie–Tooth disease type 1X (CMT1X), MCP-1 produced by Schwann cells and CSF-1 secreted from endoneurial cells together support the expansion of monocyte-derived macrophages and microglia that cause myelin damage [118–121]. Oral administration of the CSF-1R inhibitor, PLX5622, for a 6-month period immediately prior to the development of degenerative changes at 18 months, decreased macrophage numbers in the mice by ~70%, ameliorated nerve structural changes and preserved muscle strength [122]. Thus, depending on the cellular mechanisms involved, inhibition of the CSF-1 signaling in demyelinating diseases may have beneficial effects in some settings, but not in others.
2.6.3. CSF-1R in autoimmune demyelination
Studies in autopsied brain tissue from patients with multiple sclerosis (MS) showed that, despite the tremendous increase in macrophages/microglia within lesions, the percentage of CSF-1 and CSF-1R expressing cells was significantly decreased in active and demyelinated lesions compared to control case white matter [123]. Consistent with this, monocytes and macrophages are also decreased in the cerebrospinal fluid of MS patients [124]. The role of CSF-1R signaling in demyelination has been extensively studied in the cuprizone-induced model of MS in which inhibition of CSF-1R signaling prevents microgliosis and reduces demyelination and the destruction of nerve fibers [125–128]. Furthermore, direct injection of CSF-1 in the healthy corpus calosum was sufficient to drive demyelination [128]. However, it should be noted that cuprizone-induced demyelinating lesions lack the T cell infiltration characteristic of MS [129] and therefore they do not take into account the contribution of the autoimmune component of the disease, which is better reflected in experimental autoimmune encephalomyelitis (EAE). CSF-1 levels were elevated in the spinal cord of a mouse model of EAE with specific upregulation in spinal cord neurons and white, but not grey matter, astrocytes [130]. Furthermore, lentivirus-mediated overexpression of CSF-1 in neurons led to focal microgliosis and demyelination that mirrored EAE pathology [130]. In contrast, another EAE study shows that intrathecal delivery of either CSF-1 or IL-34 at the first signs of EAE in mice attenuates demyelination and prevents the progression of EAE symptoms. Mechanistically, CSF-1R activation promoted the expansion of CD11c+ microglia and decreased T cell infiltration, without altering the recruitment of myeloid cells to the spinal cord [131]. However, ablation of microglia/macrophages during the symptomatic phase of EAE, using the dual specificity (CSF-1R and c-kit) inhibitor, PLX3397, has been reported to improve animal mobility and myelination while withdrawal of the treatment caused the re-emergence of symptoms [132]. Administration of a more specific CSF-1R inhibitor, BLZ945, at the near-maximal disease stage, resulted in depletion of spinal-cord, but not cortical microglia, and did not produce a difference in the clinical score [126]. Further work is needed to understand how the CSF-1R regulates microglia at different stages of EAE and its relevance to human disease.
2.6.4. Neurodegenerative disease
2.6.4.1. Alzheimer’s disease
Alzheimer’s disease is the most common human neurodegenerative disease. It is characterized by the extracellular deposition of amyloid beta (Aβ) and intraneuronal accumulation of fibrillar tangles of abnormally phosphorylated Tau (pTau) protein in the brain [133, 134]. Studies using autopsied brain samples showed that, compared to age-matched non-demented controls, the expression of CSF-1R and CSF-1 transcripts is increased in the temporal gyri of AD patients, but not in their cerebelli, while the expression of IL-34 is decreased selectively in the inferior temporal gyri of AD patients [47, 135]. Similarly, an increase in Csf1r expression was reported in the AβPPV717F mouse model of AD [136], indicating that CSF-1R signaling is activated in AD. However, investigation of the role of CSF-1R activation in the regulation of microglia function in AD has produced controversial results. For example, stimulation of primary human microglia with either IL-34 or CSF-1 was reported to downregulate genes associated with lysosomal function and Aβ removal [135], but also to stimulate the phagocytosis of Aβ in vitro [137]. Administration of CSF-1R inhibitors to mouse models of AD and AD tauopathy has produced variable effects on Aβ deposition [138–144], Tau pathology and phosphorylation [145–147] that might depend on the timing of administration and on the extent of microglial depletion achieved. Nevertheless, most preclinical studies indicate that inhibition of CSF-1R signaling ameliorates inflammation and improves cognitive function (reviewed in Han, J., Chitu, V., Stanley, E.R., Wszolek, Z.K., Karrenbauer, V.D. and Harris, R.A., in preparation). Surprisingly, activation of CSF-1R signaling has also been reported to improve cognitive function in mouse models of AD (reviewed in [4]). Thus, either activation of CSF-1R signaling inducing a trophic state in microglia, or removal of activated microglia using CSF-1R inhibitors, might ultimately achieve the same goal of preventing neuronal loss in AD.
2.6.4.2. Parkinson’s disease
Parkinson’s disease (PD) is a movement disorder characterized by progressive degeneration of dopaminergic neurons in the substantia nigra (SN) of the midbrain. Since microglial functions are tightly controlled by neuronal activity and neurotransmitters [83, 148] it is possible that the death of dopaminergic neurons may cause microglia to lose their physiological functions and develop a pathological phenotype. Indeed, it is generally accepted that neuroinflammation plays an important role in the pathogenesis of PD [149]. The CSF-1R pathway is activated in both human PD and pre-clinical models of PD as evidenced by the increased expression of CSF1 in the SN of PD patients and by the increase of both Csf1r and Csf1 expression in the striatum during the acute phase of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced toxicity in mice [150]. Furthermore, the same study shows that MPTP toxicity triggered the phosphorylation of CSF-1R at tyrosine residue 721 [150], an event that mediates the activation of the PI3K/AKT pathway and contributes to macrophage survival [2], but also to the suppression of inflammatory activation [9]. In preclinical studies, inhibition of CSF-1R signaling has produced mixed results, depending on the timing of inhibitor administration. When applied after the neurotoxic insult, CSF-1R inhibitor treatments that either depleted microglia [151] or just prevented their expansion [150] dampened the inflammatory response and contributed to the amelioration of motor dysfunction. In contrast, elimination of microglia before the neurotoxic insult aggravated neurotoxicity [152]. These data suggest that targeting microglia through inhibition of CSF-1R signaling might be beneficial in symptomatic PD.
2.6.4.3. Huntington’s disease
Huntington’s disease (HD) is another devastating neurodegenerative disorder in which early microglial activation plays an important role [153–155]. Studies in R6/2 mice, a rapidly progressing model of HD, showed that a 5-week CSF-1R inhibitor treatment, that effectively depleted the microglia, resulted in functional improvement over the untreated group, while having no effect in wild type control mice [155]. Consistent with the depletion of microglia, the treatment also ameliorated the transcriptomic changes indicative of dysregulated interferon gamma activity in the striatum and prevented the degradation of perineural nets in the somatosensory and motor areas of the cortex [155]. However, to date there is no information regarding the expression of CSF1R or its ligands in the human disease.
2.6.4.4. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease involving oxidative stress. Although ALS is not initiated by inflammatory responses, neuroinflammation, widespread microglial activation and infiltration of monocytes in the peripheral nerves are prominent pathological features of ALS [156, 157]. Immunoreactivity for both CSF-1 and IL-34 is increased in sciatic nerves from ALS patients and in SOD1G93A rats, suggesting that a paracrine mechanism of endoneurial macrophage expansion, driven by reactive Schwann cell-expressed CSF-1 and IL-34, contributes to peripheral nerve pathology [158]. Consistent with this, administration of CSF-1 exacerbates disease progression in SOD1G37R mice by enhancing microglial/macrophage activation [159]. In contrast, inhibitor studies in SOD1G93A mice suggest that targeting the CSF-1R may slow the disease progression, by suppressing both central and peripheral immunity [160]. An open-label phase 2 clinical trial, investigating the safety and tolerability of the CSF-1R inhibitor, BLZ945, in patients with ALS, is ongoing (ClinicalTrials.gov, identifier: NCT04066244).
2.6.4.5. Prion disease
Activation of the innate immune system also contributes to the neurodegenerative pathology of prion disease. Studies in mice infected with scrapie agents demonstrated an increased expression of CSF-1 protein and transcripts [161]. Further evidence for a role of CSF-1R activation in the expansion of microglia in prion disease comes from a study showing that central inhibition of IL-34 reduces microglial proliferation in ME7 prion mice [162]. However, ablation of microglia, using the CSF-1R inhibitor, PLX5622, did not prevent prion-induced degeneration of retinal photoreceptor cells, suggesting that microglia were not required for prion-mediated damage [163]. In fact, elimination of microglia led to faster onset of photoreceptor damage and exacerbated prion pathology, suggesting that the activation of microglia contributes to the host defense against prion disease [163, 164].
2.6.5. Psychiatric and affective disorders
2.6.5.1. Catatonia
Increased innate immune activation, including an increase in mononuclear phagocytes that are also dysfunctional, is a common finding in psychotic and affective disorders (Hughes 2021). Low-grade inflammation has been shown to contribute to catatonia, a psychomotor syndrome observed across neuropsychiatric diseases that could be alleviated by short-term (5 weeks) depletion of microglia in mice using a CSF-1R inhibitor [165]. However, a subsequent study, employing two consecutive rounds of microglial depletion separated by a recovery period, suggested that the improvements might be transient. It further showed that microglia surviving CSF-1R inhibition acquire a highly activated phenotype and the capacity to phagocytose live oligodendrocytes [166]. These data are consistent with the dual role of CSF-1R signaling in promoting the survival and suppressing the inflammatory activities of macrophages [2, 9], and suggest that microglia adapting to low CSF-1R activity acquire a tissue-damaging phenotype.
2.6.5.2. Depression and anxiety
Examination of CSF-1R expression in postmortem samples of cortex, cerebellum and spleen obtained from patients with major depressive disorder (MDD), schizophrenia (SZ) and bipolar disorder (BD), revealed decreased CSF1R expression in the spleens, but not in the brains, of patients with MDD and SZ [167], leading to the suggestion that dysregulation of a spleen-brain axis may play a role in these disorders. However, in another study, CSF-1 mRNA was upregulated in the dorsal medial prefrontal cortex (PFC) of patients with MDD [168]. Consistent with this, studies in Thy1-GFP mice exposed to chronic unpredictable stress showed that stress induces activation of the CSF-1R pathway by increasing the expression of CSF-1 in the PFC. Microglia isolated from the PFC exhibited increased expression of CSF-1R and increased phagocytosis of neuronal elements that correlated with reduced dendritic spine density in Layer I of the PFC. Viral-mediated knock-down of neuronal CSF-1 in the medial PFC prevented anxiety and depressive-like behaviors [168]. In contrast, other studies examining the effects of intraperitoneal administration of CSF-1 show anti-depressive effects in chronically stressed mice [169–172] and suggest that the anti-depressive actions of CSF-1 are mediated by direct restoration of microglial homeostasis in the hippocampus of the stressed animals [170, 171]. The mechanisms by which both central inhibition and peripheral administration of CSF-1 mitigate depression need to be addressed.
2.6.6. Stroke
After stroke, microglia contribute both to the early neuroinflammatory response and to neurorestorative events [173]. Serum levels of IL-34 are increased in patients with ischemic stroke and directly correlate both with the infarct size and poor functional outcome at 3 months post-infarct [174], suggesting that CSF-1R signaling might be detrimental in stroke. However, inhibition of CSF-1R signaling in mice with transient middle cerebral artery occlusion (MCAo), by treatment with PLX5622, did not affect infarct volume, or the level of translocator protein (TSPO), a measure of neuroinflammation, in the ipsilateral hemisphere [175]. Within the infarct area, PLX5622 treatment reduced the expression of homeostatic microglia markers but did not change the total number of Iba1+ cells [175], an observation that explains how PLX5622 ultimately impaired functional recovery. Other studies, in which either CSF-1R inhibitors (PLX3397 and PLX5622) or an antibody directed to CSF-1R, AFS98, resulted in increased neuroinflammation after ischemia [176–179], support these observations. Overall, these data suggest that CSF-1R activity in the brain is necessary to support homeostatic microglial functions after ischemic stroke.
2.6.7. Chronic pain
Human imaging studies and preclinical models of neuropathic pain suggest that following nerve injury, cellular and molecular interactions of macrophages and microglia with spinal dorsal horn neurons contribute to the establishment of chronic pain [180, 181]. One of the molecular mechanisms involved is activation of the CSF-1/CSF-1R/DAP12 pathway [181]. Peripheral nerve injury induces the upregulation of CSF-1, but not of IL-34, in injured sensory neurons and the upregulation of CSF-1R expression in microglia [182, 183]. Activation of the CSF-1R pathway contributes both to microglia proliferation and mechanical hypersensitivity [182, 183]. Interestingly, the adaptor protein DAP12 was required for the induction of pain, but not of microglia proliferation by CSF-1 [182]. Further mechanistic investigations showed that the CSF-1/CSF-1R axis increases excitatory drive to excitatory neurons and decreases excitatory drive to inhibitory neurons via BDNF -dependent and -independent mechanisms, respectively [184]. Both genetic and pharmacological inhibition of CSF-1R signaling were shown to alleviate pain while intrathecal administration of CSF-1 induced mechanical allodynia [130, 182, 183, 185]. Similar results were obtained in models of pain caused by ischemia/reperfusion, lumbar disc degeneration and psychosocial stress [130, 186–188], indicating that CSF-1R signaling might be targeted to alleviate chronic pain.
2.6.8. Seizures
Studies in mouse models indicate that seizures induce the infiltration of monocytes into the hippocampus, as well as the CSF-1R-dependent proliferation of microglia and their activation, which contributes to neuronal death [189]. In silico analysis using the Causal Reasoning Analytical Framework for Target discovery (CRAFT) computational approach, that identifies cell membrane receptors with a direction-specified influence over disease-related gene expression profiles, has identified CSF-1R as a potential therapeutic target in epilepsy [190]. In a mouse model of acquired temporal lobe epilepsy (TLE), treatment with the CSF-1R inhibitor, PLX3397, reverted the gene expression profile of a gene module associated with TLE, and enriched in microglial markers, towards a healthy profile. Notably, this module was conserved in human TLE [190]. Furthermore, systemic treatment with PLX3397 decreased seizure frequency in two preclinical models of TLE (intraperitoneal administration of pilocarpine in Crl:NMRI(Han)-FR outbred mice, or hippocampal administration of kainic acid in C57/BL6 mice). In contrast, in acute seizure models, CSF-1R inhibitor treatment has been reported to have either no anti-seizure effects [190] or to exacerbate seizures [83, 191]. These data suggest that at steady-state microglia protect neurons from overexcitation, but that once microglial dyshomeostasis is induced by repeated seizures, they contribute to pathology and this dyshomeostatic state can be reversed by attenuating CSF-1R signaling. However, tamoxifen-induced ablation of Csf1r in Cx3Cr1-expressing mononuclear phagocytes was reported to exacerbate both acute and chronic seizures [192]. The reasons for the discrepant findings regarding the role of CSF-1R signaling in chronic seizures should be addressed.
2.7. CSF-1R in glioblastomas
The presence of tumor associated macrophages (TAMs) is associated with poor prognosis and correlates with resistance to chemotherapy, and in mouse models TAMs can be targeted by inhibiting CSF-1R [193]. Glioblastoma is the most common and aggressive primary adult brain tumor and is treated initially by surgical intervention. The most common postoperative standard-of-care therapy, involving whole brain ionizing irradiation, often combined with chemotherapy, is of transient benefit. Glioblastoma cell lines secrete CSF-1 [194]. CSF-1R blockade in a mouse model of glioblastoma multiforme (GBM) significantly increased survival, regressed established tumors and slowed intracranial growth of patient-derived glioma xenografts. Interestingly, CSF-1R inhibition depleted normal microglia, but not TAMs, which were “re-educated” to an anti-tumor phenotype by the locally produced GM-CSF and interferon-γ [195]. Apart from eliminating microglia, CSF-1R inhibition also reduced two side effects of whole brain irradiation, dendritic spine loss and cognitive disability [196]. However, the tumors recurred in >50% CSF-1R inhibitor treated mice, driven by macrophage-derived insulin-like growth factor-1 (IGF-1) and tumor cell IGF-1 receptor (IGF-1R), that elevated the tumor PI3K pathway activity, suggesting that a combination of IGF-1R or PI3K blockade with CSF-1R inhibition may suppress glioma resistance to CSF-1R inhibition [197]. Recent studies have shown substantially enhanced survival in preclinical models in which CSF-1R inhibitor treatment is combined with radiotherapy [198]. The effectiveness of CSF-1R inhibitors, in combination with either standard-of-care therapy (NCT01790503), or immune checkpoint inhibitors (NCT02829723) is currently under clinical evaluation.
3. GM-CSF
3.1. GM-CSF and GM-CSFR in the central nervous system at steady-state
GM-CSF readily crosses the BBB [45]. However, in mice at steady-state, the level of circulating GM-CSF is below the detection limit (<5pg/ml) [199]. In humans, studies suggest a gradual increase in circulating levels starting from ≤6 pg/ml in neonates and early childhood [200–202], to 24±11pg/ml in young adults [203] and reaching 337±163 pg/ml in aged adults, in which low concentrations of GM-CSF (18±9 pg/ml) can also be detected in the cerebrospinal fluid (CSF) [204, 205]. The expression of both GM-CSF protein and transcripts in brain tissue is barely detectable [62, 114, 206, 207], a finding that is difficult to reconcile with the widespread expression of both GM-CSF and GMRα documented histologically [52, 70]. Given the low concentration of circulating GM-CSF, one possible explanation, backed by in vitro studies showing that a low concentration (0.1 pg/ml) of GM-CSF was sufficient to support the survival of primary human eosinophils in culture [208], is that GM-CSF might be biologically active at very low concentrations in vivo. Alternatively, it might signal in a paracrine fashion, through focal production and utilization.
Irrespective of the manner and amounts in which it is produced, GM-CSF signaling has an important role in the functioning of the nervous system. Studies in GM-CSF null and heterozygous mice demonstrate a role in cognitive function [62, 209]. Furthermore, CNS administration of GM-CSF decreases food intake [210] and is somnogenic [211] in rats. Exogenous GM-CSF stimulates melatonin release by the pineal gland [212] and promotes both non-REM and REM sleep, the latter by stimulating the release of somatostatin by the hypothalamus [211]. Conversely, intracerebroventricular administration of an anti-GM-CSF antibody inhibits pregnancy-enhanced sleep [213]. In addition, expression studies in mouse embryos reveal dynamic regulation of both GM-CSF and GM-CSFR expression from embryonic day 13 (E13) to postnatal day 1 (P1) [214]. Although not quantitative, the data indicate that in the developing brain, both GM-CSF and GMRα levels are regulated in a synchronized fashion peaking at E13-E14 and decreasing thereafter. GM-CSF might be produced intrathecally, as its levels in the cerebrospinal fluid (CSF) peaked at E14-E15, while circulating levels gradually decreased between E13-P1. Similarly, in the human embryonic brain both GM-CSF and GM-CSFR transcripts are expressed, starting at 10 weeks of gestation, and the immunoreactivity for the respective protein products is preserved in the adult, at least in the spinal cord [200].
Increased GM-CSF production may negatively impact pre- and post- natal neurodevelopment. Elevated levels of GM-CSF in infants with neonatal encephalopathy negatively correlate with survival and brain function [215]. Additionally, emerging evidence suggests a role for GM-CSF in autism. GM-CSF can cross the placenta [216] and increased levels of GM-CSF were found in mid-gestation sera of mothers of children with autism spectrum disorder and intellectual disability [217]. Furthermore, Li et al [218] found a two-fold increase in GM-CSF in the frontal cortex of autistic compared to non-autistic children. These data prompt further exploration of the role of GM-CSF in brain development.
3.2. Sources of GM-CSF in the CNS
The expression of GM-CSF is rapidly induced in vivo in response to acute tissue injury, including ischemia/reperfusion [52], traumatic brain injury [219] and demyelinating disease [207, 220–222]. Brain levels of GM-CSF are also elevated in neurodegenerative conditions, including AD, vascular dementia [204], multisystem atrophy, spinocerebellar ataxia [223] and CRL [62].
In vivo, immunohistochemical analysis detects its expression at steady-state in subpopulations of CNS neurons, astrocytes, choroid plexus and ependymal cells [70]. In vitro, GM-CSF is constitutively produced by human astrocytes [224, 225], brain microvascular smooth muscle and endothelial cells [226]. In cell culture systems, rapid and strong (5–200-fold) GM-CSF expression can be induced in a variety of cell types including neuronal precursors [227], astrocytes [224, 228–232], endothelial cells [232, 233], pericytes [232, 234] and microglia [231, 235, 236], by various inflammatory (LPS, IL-1β, TNF-α, MSP) and lipid (sphingosine-1-phosphate and lysophosphatidylcholine) mediators, or by neurotoxins [237] (Fig. 2).
3.3. GM-CSF target cells in the CNS
Human GMRα is broadly expressed by cortical neurons and Purkinje cells [52]. Similarly in rats, expression of GMRα has been detected in both embryonic [53] and adult subventricular and subgranular zone neural progenitor cells [54], and immunoreactivity for both GMRα and βc subunits was reported in Purkinje cells, cortical, hippocampal and olfactory bulb neurons [52]. Gene expression studies in mouse brains show a widespread distribution of Csf2ra transcripts, while transcripts for Csf2rb are largely restricted to microglia (Chitu, V., Lui, Y., Zheng, D. and Stanley, E.R., unpublished observations). This observation is supported by reporter gene expression studies showing that in adult mice robust Csf2rb promoter activity is sparsely detected only in the hilus of the hippocampus, in basal amygdala, thalamus and in the supramamillary bodies [55]. However, since the GMRα subunit binds GM-CSF and can induce cellular responses without engaging the kinase signaling cascade [37] (Fig. 1), this observation does not preclude widespread activity of GM-CSF in CNS neurons via the α subunit.
High affinity functional GM-CSFRs are expressed on primary rat oligodendrocytes and addition of GM-CSF to oligodendrocyte cultures stimulated proliferation over a concentration range of 0.025-100pM [56]. In contrast, there is controversy regarding the expression of functional GM-CSFRs in astrocytes. While several studies failed to detect ligand binding in cultured rat astrocytes [56], or immunoreactivity for GM-CSF receptor subunits in mouse astrocytes [52], others demonstrated the presence of GMRα and βc subunits transcripts in mouse [57, 58] and macaque [59] astrocytes. Functional receptor expression was indicated by the ability of exogenous GM-CSF to potentiate the LPS-induced production of IL-6 by astrocytes [58] and to induce the proliferation of macaque primary astrocytes in a concentration- and GMRα subunit- dependent manner [59].
Steady-state GM-CSFR expression in microglia has been the subject of similar controversy. The absence of ligand binding sites and of GMRα immunoreactivity has been reported by different groups [52, 56]. Nevertheless, Csf2ra and Csf2rb transcripts encoding the GMRα and βc have been reported in cultured neonatal microglia [60], microglia acutely isolated from neonatal [58] and adult [62] mice, as well as in human microglia [61]. Immunoreactivity for GMRα was demonstrated in cultured neonatal microglia [60]. Furthermore, addition of GM-CSF to microglia cultures elicits a plethora of biological effects including, but not limited to, cell proliferation [93, 238–241], changes in morphology [239, 242], stimulation of myelin phagocytosis [243] and antigen presentation [244, 245]. Furthermore, GM-CSF triggers microglia proliferation in situ [246] and, conversely, genetic targeting of Csf2rb in Cx3Cr1-expressing phagocytes attenuates microgliosis in a mouse model of leukodystrophy [62].
Together, these data indicate that GM-CSF signals in both neural and non-neural lineage cells in the CNS.
3.4. Regulation of Microglia by GM-CSF
3.4.1. Control of microglial proliferation by GM-CSF
Addition of GM-CSF promotes the proliferation of primary microglia isolated from human [247] and rodent [238–241] embryonic or perinatal brains. In human microglia, the proliferative actions of GM-CSF are mediated by a pathway involving the Src-family kinase, Hck, as well as PI3K and Erk1/2 [247]. However, microglia isolated from aged (24-month-old) rat brains do not proliferate in response to GM-CSF [93]. Furthermore, while out of many hematopoietic growth factors tested in vitro (including IL-1α, IL-3, G-CSF, GM-CSF and CSF-1), GM-CSF is the most powerful mitogen [238], neonatal rat microglia propagated in GM-CSF rapidly become senescent as a result of progressive loss of their longest telomeres [248]. These data suggest that injuries that induce the upregulation of GM-CSF in vivo might contribute to the rapid expansion of microglia but also accelerate their senescence. More recent studies identify IRF8, an IFNγ-responsive transcription factor, as a suppressor of the GM-CSF proliferative responses in microglia [249]. IRF8-deficient mice have increased densities of microglia exhibiting marked reductions in branching and surface area and altered expression of myeloid cell markers [250].
3.4.2. Regulation of microglial differentiation and function by GM-CSF
Because isolated rodent microglia do not survive in vitro in the absence of growth factors, most studies involve comparisons of cells obtained by propagating non-adherent microglial precursors (obtained from mixed neonatal glial cultures) in either GM-CSF or CSF-1 for several days. These neonatal microglial precursors are akin to circulating monocytes, having lost the granulocytic potential but not yet being committed to a macrophage or dendritic cell phenotype [251]. Thus, the morphological and functional differences reported in their mature progeny obtained after exposure to different growth factors might also reflect a differentiation bias. Indeed, consistent with the role of GM-CSF in promoting dendritic cell differentiation from monocytes [252], GM-CSF differentiated microglia can induce antigen-specific T cell responses, while CSF-1 differentiated microglia are unable to do so [244]. Furthermore, in combination with the TLR-9 agonist, CpG-ODN, GM-CSF increases antigen cross-presentation by acutely isolated mouse adult microglia [253]. Phenotypic characterization of GM-CSF-differentiated microglia revealed that they express higher levels of gene products involved in antigen processing and presentation, including MHC class II proteins and cathepsins L and F [245], as well as of dendritic cell surface markers, including CD11c and Dec-205 (CD205) [251]. CD205 plays an important role in antigen uptake, presentation and cross presentation to T cells and, depending on the presence or absence of additional agonistic signals, can promote activation or cause anergy [254]. Interestingly, CD205 was also suggested to act as a receptor that mediates the uptake and presentation of apoptotic and necrotic self-antigens [255]. While at steady-state brain-resident microglia do not express CD205 [256], it would be of interest to examine whether upregulation of GM-CSF under pathological conditions can induce CD205 expression in vivo with subsequent (self) antigen presentation and how this might contribute to disease pathology.
GM-CSF has been reported to increase FcγR-mediated phagocytosis in primary mouse microglia [257, 258]. The mechanism remains to be established, as the reported upregulation of the inhibitory FcγRIIb mRNA, combined with a lack of significant effect on the expression of the activating FcγRI mRNA [257], is rather counterintuitive. Differentiation in the presence of GM-CSF upregulates the expression of inducible nitric oxide synthase, NOS2, and of the costimulatory ligand, CD86 [258]. In addition, GM-CSF potentiates inflammatory responses, priming the PMA-stimulated oxidative burst [259] and facilitating the production of nitric oxide [60, 260], nitrite [261] and of inflammatory cytokines (IL-1β, IL-6, TNF-α) in response to LPS [60]. Potentiation of LPS responses involves the upregulation of LPS receptors TLR4 and CD14 through pathways involving Erk1/2 and p38 MAP kinases, respectively, thus enhancing LPS-induced nuclear translocation of NFκB [60].
Since GM-CSF not only facilitates inflammatory activation but also expands microglia, it is conceivable that, if uncontrolled, these combined activities could increase the production of inflammatory mediators up to toxic levels in vivo, ultimately leading to neural tissue damage. Relevant to this, GM-CSF-induced proliferation of microglia is subjected to endocrine control, being suppressed by somatostatin [262] and glucocorticoids [242].
3.4.3. Role of Csf2 in phagocyte-induced demyelination and neurotoxicity
A first indication that GM-CSF might participate in myelin clearance or destruction by microglia came from the work of Marion Smith, who showed that microglia cultured in the presence of GM-CSF showed slightly increased cholesterol ester production from opsonized myelin but significantly greater (154%) increase from untreated myelin [243]. Subsequent studies showed that GM-CSF differentiated microglia strongly upregulate matrix metalloproteinase 12 (MMP-12) [245] that contributes to demyelination in vivo [263, 264]. Antibody depletion of astrocyte conditioned medium demonstrated that GM-CSF is the major mediator of activation of microglia towards an oligotoxic phenotype [265].
GM-CSF expression is elevated in leukodystrophies [62, 221] and genetic targeting of Csf2 in mice with demyelinating disease has been shown to attenuate the loss of white matter, microgliosis, and the expression of Cystatin 7 and MMP-12 in microglia [62]. In contrast, overexpression of GM-CSF in Th cells lead to development of neurological deficits reminiscent of atypical EAE associated with extensive invasion of leukocytes (T cells, granulocytes and monocytes), microgliosis, astrogliosis, differentiation of ROS-producing macrophages and demyelination in the brain [266], while global overexpression of constitutively activated βc in mice under the control of the PGK1 promoter leads to brainstem white matter necrosis [267]. In addition, circulating GM-CSF is elevated in patients with immune effector cell-associated neurotoxicity syndrome and animal studies using a neutralizing antibody indicate that it may play a role in neurotoxicity [268].
Thus, apart from its multifactorial contribution to automimmune demyelination, discussed in section 3.7.1., GM-CSF can directly promote neurotoxicity and myelin degradation by mononuclear phagocytes in vivo.
3.5. Regulation of the neuronal lineage by GM-CSF
Intravenously administered GM-CSF crosses the BBB [45] and increases cell proliferation in adult rat subventricular zone and dentate gyrus [269], suggesting a neurogenic activity. However, in vitro studies examining the effect of GM-CSF on NPCs have produced conflicting results, with both inhibition [53] and enhancement of the expression of neuronal markers [54], being reported. Regardless of its role in neuronal differentiation, GM-CSF was shown to have anti-apoptotic actions in staurosporine-treated NPCs [53, 270], an effect that was mediated by the JAK/STAT5/Bcl-2 pathway [270] and counteracted by antibodies to GM-CSF Rα subunit [53]. The anti-apoptotic effects of GM-CSF in cultures extend to sympathetic cervical ganglia neurons, which express both GMRα and βc [271], as well as to primary cortical neurons [52]. Interestingly, while GM-CSF protected differentiated neurons against apoptosis induced by NGF withdrawal, its addition to cultures of undifferentiated neurons promoted process outgrowth without affecting survival, indicating that depending on the cellular context, GM-CSF may activate different signaling pathways. In primary embryonic cortical neurons, GM-CSF induces the activation of multiple signaling pathways including Jak2/STAT3, Erk1/2, Erk5 and PDK1/Akt [52]. Out of these, the pathways contributing to its anti-apoptotic effects include the MEK1/2 kinase cascade [271] and the PDK/Akt1 pathway [52] (Fig.1).
GM-CSF also exerts anti-apoptotic effects in vivo. Intra-ocular administration of GM-CSF in RCS rats, a model of retinitis pigmentosa, attenuates photoreceptor death and the expression of apoptotic markers, while at the same time increasing the retinal expression of BCL-2 and neurotrophins (CNTF, BDNF and GDNF) through a pathway involving the activation of Src and STAT3 [272]. It is not clear whether these anti-apoptotic effects are mediated through direct action of GM-CSF on photoreceptor cells, or indirectly, by modulation of microglial function. Relevant actions in microglia include increasing microglial phagocytosis of photoreceptor outer segments, thus compensating for MerTK dysfunction, and by increasing their ability to produce neurotrophic factors. In another study [273] a combination of GM-CSF and IL-3 has been reported to increase the expression of neurotrophic factors, IGF1 and HGF, in microglia and to suppress apoptosis in a model of acute dopaminergic degeneration in vivo. Together, these data suggest that, in environments where substantial neuronal cell death occurs, the actions of GM-CSF on microglia and/or neurons result in neuroprotection.
Other studies indicate that GM-CSF regulates neuronal activity. In hippocampal slice cultures, GM-CSF induced Ca2+ increase [269] and long-lasting disturbances of gamma oscillations [246]. While the former effect might reflect direct signaling in neurons, the latter was mediated by a rapid expansion of microglia without inflammatory activation [246]. GM-CSF was also shown to affect neurotransmitter production and metabolism. Addition of GM-CSF to cultures of primary septal neurons elevated choline acetyltransferase activity in a dose-dependent manner. The same effect was observed in a cholinergic hybridoma cell line, suggesting direct trophic effects on cholinergic neurons [274]. Peripheral administration of GM-CSF in mice reduced the levels of glutamic acid, GABA, norepinephrine and serotonin in the hypothalamus, but not in the hippocampus. The effect of GM-CSF was blocked by IL-1 receptor antagonist, indicating the involvement of IL-1 as a secondary mediator, potentially produced by central or peripheral immune cells [275].
3.6. Physiological Roles of GM-CSF
3.6.1. Role in cognition
The observation that GMRα is constitutively expressed by neurons in multiple regions of the adult rodent brain [52] prompted an investigation of GM-CSF in brain function. Csf2 heterozygosity was sufficient to cause cognitive deficits in aged mice and ataxic behavior in females [62]. Genetic ablation of Csf2 caused various hippocampal and amygdala-dependent deficits in spatial and fear memory, without affecting motor function, inherent anxiety and pain threshold levels of young mice [209]. These deficits of Csf2-null mice were accompanied by excessive pruning of the dendritic trees, reduced spine densities and lower percentages of mature spines in neurons from the CA1 and DG areas of the hippocampus, but did not cause impairments in long-term potentiation. However, the acute manipulation of GMRα expression levels in the adult hippocampus, using adenovirus-mediated overexpression or knock down, failed to produce strong effects on learning and memory, suggesting that GM-CSF might regulate the development of neuronal networks and/or that chronic perturbation of GM-CSF signaling is necessary to produce a physiological change.
3.6.2. Regulation of CNS responses to infection
Systemic LPS challenge leads to the acute activation of both astrocytes and microglia. Based on in vitro cell culture studies with primary mouse astrocytes and BV2 microglia, Kano et al., have proposed a mechanism of coordinated microglia-astrocyte activation that involves the upregulation of TNF-α and Il-1β in microglia leading to production of GM-CSF and CCL2 by astrocytes that fuels further microglial activation [276]. However, it is unclear whether GM-CSF contributes to microglial activation following systemic immune challenge in vivo, as other studies failed to detect significant upregulation of brain GM-CSF in the acute phase of the response to systemic LPS administration or to H1N5 infection, both of which induce parkinsonian pathology [277, 278]. Ong et al., [277] found a specific elevation of GM-CSF in the substantia nigra occurring 6 months after LPS challenge and speculate that this delayed upregulation might be part of a neuroprotective response against LPS-induced dopaminergic cell degeneration. Other studies [278] suggest that this elevation may be due to the increased production of dopamine itself in substantia nigra, rather than a protective response. Addition of dopamine to substantia nigra cultures induces GM-CSF production. Furthermore, in mice with H1N5 infections, the timing of elevation of GM-CSF in the brainstem (by 10 days post-infection) and in substantia nigra (by 60 days post-infection), coincides with the peak increase and recovery of dopamine levels in these regions, respectively [278]. In fact, the delayed elevation of GM-CSF in LPS-injected mice also occurs on the background of increased tyrosine hydroxylase specific activity [277]. Further investigations are needed to establish the functional significance of this delayed elevation of GM-CSF following systemic immune activation.
Studies in patients with COVID-19 show a specific and positive association of circulating GM-CSF levels with disease severity [279]. Since many patients recovering from SARS-CoV-2 infection develop long-lasting neurological symptoms [280] that might be associated with CNS autoimmunity [281], it would be of interest to investigate how GM-CSF contributes to SARS-CoV-2 neuropathology.
3.6.3. Regulation of neuro-endocrine communication
Various infections and systemic inflammation activate the hypothalamic-pituitary-adrenal axis and inhibit the pituitary-gonadal axis [282, 283]. Several studies suggest that GM-CSF might be implicated in both responses. Systemic administration of GM-CSF rapidly increased the circulating levels of both ACTH and corticosterone in rats, and prolonged administration was reported to increase the proliferation of both corticotrophs and adrenal cortex cells, potentially leading to chronic activation of the pituitary-adrenal axis [212]. Addition of GM-CSF to hypothalamic tissue explants inhibits the release of LHRH via a mechanism involving increased release of GABA [283].
3.6.4. Regulation of neuro-immune interactions
Short-term pretreatment of hematopoietic stem cells (HSCs) with GM-CSF facilitates cathecolamine-induced migration and MMP expression by increasing the expression of dopamine and adrenergic receptors [284]. Thus, GM-CSF may participate in the regulation of hematopoietic cell function by neurotransmitters.
3.7. Role of GM-CSF in nervous system disease and damage
3.7.1. GM-CSF in autoimmune demyelination
3.7.1.1. Multiple sclerosis
MS is a chronic autoimmune disease of the CNS characterized by infiltration of leukocytes, followed by tissue damage and neuronal dysfunction. Greater numbers of GM-CSF-expressing T cells in the periphery have been found in patients with MS, in which a significant proportion of brain-infiltrating T cells also express GM-CSF [285–288]. Other sources of GM-CSF include microglia and macrophages present in MS lesions [289] and, possibly, endothelial cells activated by disruption of blood flow due to stenoses in the extracranial veins draining the CNS, a frequently occurring feature in MS patients [290]. The expression of GMRα is also increased in macrophages/microglia of the spinal chords of MS patients [291], in their brain lesions [288, 289] and in lesional brain astrocytes [288]. In vitro studies using human primary cells indicate that GM-CSF enhances the production of TNF-α by monocytes and monocyte migration across the blood-brain barrier [289]. In addition, GM-CSF enhanced the production of IL-6 and ROS by monocyte-derived macrophages and promoted a phenotypic change characterized by the increased expression of both pro-and anti-inflammatory markers that was phenotypically similar to what is observed in active MS lesions [289]. Interestingly, these responses did not occur in primary human microglia cultured in GM-CSF for a comparable period. Together, these data suggest that GM-CSF participates in autoimmune demyelination in humans by promoting leukocyte infiltration in the brain and by activating monocyte-derived macrophages. These data, together with the mechanistic investigation of the role of GM-CSF in autoimmune demyelination in mice (summarized below), prompted the initiation of a clinical trial in MS that addressed the safety of MOR103, a humanized monoclonal antibody to GM-CSF. The antibody was well tolerated and MRI reports showed no new lesions in trial subjects after 10 weeks of treatment [292]. Additional clinical trials are necessary to assess its efficacy.
GM-CSF is elevated in the cerebrospinal fluid of patients suffering from MOG-IgG+ idiopathic inflammatory CNS disease and Aqp4-IgG+ neuromyelitis optica spectrum disorder [293], suggesting that it may be involved in the pathology of other autoimmune demyelinating diseases.
3.7.1.2. Experimental autoimmune encephalitis
The mechanism through which GM-CSF contributes to autoimmune CNS demyelination has been extensively studied in mice with EAE induced by immunization with encephalitogenic proteins or peptides derived from myelin (e.g. proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG) or myelin basic protein (MBP)) or by passive transfer of myelin-reactive T lymphocytes [294]. Csf2 deficiency renders several strains of mice (NOD/Lt, B10.PL and C57/BL6) resistant to the development of spinal cord pathology and ascending paralysis, that are characteristic of classical EAE [207, 295–297], while treatment with GM-CSF exacerbates disease [295, 298].
Adoptive transfer studies show that GM-CSF production by the encephalitogenic T cells, but not by the host tissues, is necessary for the development of EAE. Csf2-deficient encephalitogenic T cells retain the capacity to infiltrate the CNS, become activated and produce cytokines. However, they fail to activate microglia in the CNS and to promote the infiltration of peripheral leukocytes [207]. Using Csf2rb−/− mouse chimeras, it was shown that activation of the hematopoietic cell compartment, but not of resident microglia, by GM-CSF, was essential for EAE development [297]. These studies indicate that GM-CSF is required to establish a CNS microenvironment favorable to sustained autoimmune reactions. Two positive feed-back loops, that allow reciprocal upregulation of IL-1β and GM-CSF and of IL-23 and GM-CSF, are important components of this microenvironment. GM-CSF stimulates the production of IL-1β by monocytes which, in turn, promotes the expression of GM-CSF by endothelial and T helper (Th) cells [299–301]. Meningeal mast cells are another recently identified source of IL-1β relevant to the pathology of EAE [302]. The second positive feed-back loop, involving the reciprocal upregulation of IL-23 and GM-CSF, occurs between GM-CSF-secreting T cells and IL-23-producing antigen-presenting cells [303]. Indeed, fate-mapping studies show that IL-23 in encephalitogenic Th cells is essential for GM-CSF expression, susceptibility to EAE and the infiltration of monocytes into the CNS [300]. The released GM-CSF is important for inducing a competent antigen-presenting phenotype in monocytes that migrate across the blood-brain barrier [299] and possibly for activating meningeal macrophages.
GM-CSF production by encephalitogenic T cells is negatively regulated by IL-9 and IFN-β, both of which were reported to act indirectly by modulating the activities of dendritic cells and monocytes, respectively [301, 304]. In addition, in PLP-induced EAE, IFNγ suppresses GM-CSF production by CD4+ T lymphocytes.
Antibody-mediated blockade of GMRα in mouse models of chronic (C57BL/6, MOG35–55-induced) or remitting-relapsing (SJL/J, MOG139–151-induced) EAE, starting at the peak of disease, ameliorated the severity of disease by reducing inflammatory infiltrates and decreasing myelin-specific T cell responses to the disease-inducing epitope and limiting epitope spreading [305]. In addition, lineage-specific targeting of Csf2rb revealed that GM-CSF signaling in CCR2+ Ly6Chi monocytes, but not in dendritic cells, neutrophils or microglia, was essential for EAE [306]. Targeting of Csf2rb in astrocytes was also reported to ameliorate EAE by preventing the activation of proinflammatory pathways [307]. Furthermore, a disease–associated astrocyte population expressing a gene signature associated with activation of GM-CSF signaling has also been identified in MS patients [307].
However, the requirement for GM-CSF is not universal across EAE models or mouse strains. In SJL mice, the requirement for GM-CSF was alleviated under conditions that promoted the expression of monocyte-attracting chemokines in the CNS (IL12p70 primed T cells/PLP-driven EAE) [296]. In contrast, GM-CSF was essential for forms of EAE that involve the recruitment of neutrophils, such as the development of brain inflammation in the atypical EAE of C3HeB/FeJ mice [308] and the IL-23-primed T cell/PLP-driven EAE in SJL mice, which is reminiscent of fulminant MS and neuromyelitis optica [296]. As transgenic overexpression of GM-CSF in vivo induces severe brain pathology, while the spinal cord is less affected [266], these data suggest that GM-CSF has a unique role in brain inflammation.
3.7.2. GM-CSF in neurodegenerative diseases
3.7.2.1. Alzheimer’s disease
Early studies showed that GM-CSF is produced intrathecally in patients with AD and vascular dementia [204] and proposed a contribution to pathology by induction of microglial activation and of Aβ1–42 production [309]. Furthermore, monocytes from AD patients were reported to overexpress CSF2RB, a feature that, combined with the ability of GM-CSF to increase vascular permeability, could promote their infiltration in the brain [310]. However, studies in mouse models of AD indicate that the activation of phagocytes by GM-CSF might be beneficial in AD. GM-CSF was reported to increase the phagocytosis of Aβ in brain slices [311] and, when administered in vivo, to reduce Aβ load and improve cognition [312, 313]. A randomized, double-blind, placebo-controlled trial showed that recombinant GM-CSF (Sargamostim) improved cognition and decreased neurodegeneration in AD patients [314].
3.7.2.2. Parkinson’s disease
Reports documenting the expression of GMRα in tyrosine hydroxylase positive neurons and the anti-apoptotic effects of GM-CSF in neurons prompted the examination of its utility as a neuroprotective agent in PD. In mouse models of PD, GM-CSF administration improved dopaminergic neuron survival and locomotor function [315, 316]. Furthermore, in combination with IL-3, GM-CSF provided strong protection against 6-OHDA-induced parkinsonism in rats, by promoting the production of neuroprotective factors, IGF-1 and HGF, by microglia and the expression of the anti-apoptotic protein, Bcl-xL, in dopaminergic neurons [273]. Another mechanism through which GM-CSF protects against parkinsonism involves the induction of regulatory T cells (Treg) which, in turn, suppress inflammation and decrease reactive microgliosis [317, 318]. A small 12-month clinical trial indicated that, when administered to PD patients, using a low-dose discontinuous regimen, Sargamostim was well tolerated, enhanced Treg function and prevented disease progression [319]. Recently, lipid nanoparticles containing GM-CSF mRNA have been developed and validated in preclinical PD models [318].
3.7.3. Psychiatric and affective disorders
Circulating GM-CSF is elevated in major depression [320] and in anti-psychotic naïve patients with first episode psychosis, where it decreases following antipsychotic treatment [321]. In addition, a role for GM-CSF in regulation of the reward circuitry has been suggested [322]. Involvement of GM-CSF in psychiatric and affective disorders was investigated in rodent models. Systemic administration of GM-CSF alleviates stress- and inflammation- induced depression in mice by preventing microglial loss and the upregulation of Indoleamine 2, 3-dioxygenase 1, respectively [323, 324]. In contrast, intrahippocampal administration of GM-CSF in unstressed rats led to behavioral deficits reminiscent of schizophrenia (hyperlocomotion, social interaction and pre-pulse inhibition deficits) that were mediated by a dramatic increase in microglia activation [325]. These data indicate that fluctuations in GM-CSF levels may contribute to psychiatric and affective disorders by perturbing microglia activity.
3.7.4. Stroke
Studies in rodent models of stroke showed that GM-CSF has positive effects by inhibiting neuronal apoptosis and decreasing infarct size, as well as stimulating vascular collateral growth, the recruitment of macrophages to the penumbra area and neuroplasticity [52, 326–330]. Furthermore, administration of GM-CSF might augment immune function and prevent stroke-associated pneumonia [331]. However, although stroke patients have higher plasma levels of GM-CSF than healthy controls, no relationship was found with the clinical outcome [332].
3.7.5. Neuronal injury and pain
GM-CSF is rapidly upregulated in models of neuronal injury, including facial nerve axotomy [333] and spinal cord injury [334], and has pronociceptive actions in damaged tissues [58, 335–337]. It is unlikely that GM-CSF induces sensitization through direct activation, since isolated dorsal root ganglia nociceptors do not express detectable levels of Csf2rb [338]. Indeed, recent studies [338] indicate that GM-CSF activates nociceptors indirectly, through its actions on glial cells. Consistent with this, studies in Csf2rb-deficient mice showed that GM-CSF receptor signaling is not involved in steady-state nociception [58].
The effects of GM-CSF on tissue recovery are variable. In spinal cord injury, administration of GM-CSF promoted the production of BDNF and an early recovery of motor function [339]. However, following sciatic nerve crush GM-CSF did not improve myelin clearance or improve locomotor recovery [340].
3.7.6. Seizures
Early studies show that stereotactic injection of GM-CSF in rat hippocampi initiates spontaneous epileptiform discharges through a mechanism involving reactive microglia [341]. Furthermore, chronic exposure of organotypic hippocampal slice cultures to GM-CSF induced microglia expansion and long-lasting disturbances of neuronal gamma oscillations, such as slowing and neural hyperexcitability, that were attenuated by depletion of microglia [246]. These data suggest that prolonged elevation of GM-CSF in the brain, following infection or trauma, may contribute to epileptic seizure development via microglial activation.
3.8. Role of GM-CSF in cancer
GM-CSF is overexpressed in human astrocytoma and glioma and inversely correlates with the survival of glioma patients [342]. Comparative studies of different grade human gliomas and astrocytomas revealed that high clinical grade gliomas expressed high levels of GM-CSF as well as GMRα and βc subunits, while low grade tumors were weakly positive or negative [343]. GM-CSF promotes monocytic infiltration, angiogenesis and tumor growth in experimental glioma [342, 344] and supports the growth and survival of human glioma and leptomeningeal carcinoma in an autocrine fashion [343, 345]. In addition, plasma levels of GM-CSF directly correlate with cancer treatment-related cognitive impairment [346]. Together, these data suggest that GM-CSF might be a therapeutic target in both neurological and non-neurological malignancies.
4. G-CSF
4.1. G-CSF and G-CSF receptor (G-CSFR) in the nervous system at steady-state
Although G-CSF was originally described as a hematopoietic growth factor [12], more recent data support its involvement in regulating diverse functions in the nervous system. In humans, the concentration of circulating G-CSF was reported to decrease 3-fold from birth to 46± 33 pg/mL in the early neonatal period, further decreasing to ~25 pg/ml in the late neonatal period and adulthood [347–349]. In vivo, microdialysis in mice indicates that the hippocampal G-CSF levels are also reduced with age [350]. Interestingly, although in rodents G-CSF was reported to cross the BBB slowly and continuously [46], studies in HIV patients without cognitive impairment show that the level of G-CSF in the cerebrospinal fluid (CSF) was ~15 times lower than in plasma (4.4pg/ml vs 64.3pg/ml), indicating that, at least in humans, the transport of G-CSF through the BBB is limited [351]. Conversely, elevation of G-CSF in CSF compared to plasma, suggestive of intrathecal production, was detected in other acute [352] and chronic [353] conditions. Thus, the peripheral and CNS levels of G-CSF can vary independently.
4.2. Sources of G-CSF and target cells in the CNS
G-CSF can be produced by a plethora of cells, including monocytes, mesothelial cells, fibroblasts, endothelial cells and stromal cells, while the G-CSFR is detected mainly in hematopoietic (neutrophilic granulocytes and their precursors, monocytes, platelets) and endothelial cells [65, 67, 354–357]. In the rat peripheral nervous system, G-CSFR was found in neurons of the dorsal root ganglia and Schwann cells of the sciatic nerve [63, 69]. In the CNS, G-CSFR immunoreactivity was documented throughout the brain, with particularly high immunopositivity in cortical layer II and V neurons, subpopulations of neurons located in the hippocampus and the subventricular zone, mitral cells of the olfactory bulb, Purkinje cells, deep cerebellar and brainstem nuclei, the substantia nigra and the nucleus accumbens, as well as in spinal cord motoneurons [64–69] (Fig. 2). In rat, the expression of G-CSF was detected in all brain regions in which the receptor was found. Similarly, in human brain samples, G-CSF and G-CSFR immunoreactivity were detected ubiquitously, but not uniformly, in neurons throughout the CNS and in ependymal and choroid plexus cells [65, 70]; G-CSFR was also detected in the spinal cord motoneurons [69].
Interestingly, while one study shows complete colocalization of the ligand and receptor in rat cortical neurons [65], another shows localization of G-CSFR in the soma of medium spiny neurons and a clear peri-neuronal location of the ligand [67]. Furthermore, human Purkinje cells display G-CSF but not G-CSFR immunoreactivity [70]. These studies suggest that G-CSF signaling in neurons involves both autocrine and paracrine signaling mechanisms.
Constitutive expression of G-CSF was reported in human astrocytes in situ [70] and in astrocytoma cell lines [358]. However, in rodents there is no compelling evidence of astrocytic or microglial expression of G-CSF at steady-state. Neither the co-localization of G-CSF with astrocytic markers nor the presence of Csf3 transcripts in laser-captured astrocytes could be demonstrated in rats at steady-state [65]. Nevertheless, strong induction of G-CSF expression was reported in IL-1-stimulated astrocytoma cell lines and in microglia and endothelial cells stimulated by lipopolysaccharide (LPS) [358–360]. Induction of G-CSF expression in pericytes was also reported by some authors [234] and contradicted by others [232, 233].
Somewhat unexpectedly, single cell transcriptome profiling experiments in mice detect Csf3r transcripts mainly in cerebral microglia and macrophages, while the expression in other cell types, including neurons, is sporadic at best (databases available at https://portals.broadinstitute.org/single_cell/study/aging-mouse-brain, http://dropviz.org/, and www.microgliasinglecell.com) [361–363]. These studies also fail to detect Csf3 transcripts in the brain. The reasons for the discrepancies between immunohistochemical and transcriptional profiling data remain to be addressed.
4.3. Regulation of neural lineage cells and microglia by G-CSF
Administration of recombinant G-CSF was reported to increase the number of microglia and phagocytosis in vivo [238, 364, 365]. However, the proliferative response might be regulated indirectly, as G-CSF has no effect on microglial growth in vitro [238]. G-CSF has also been shown to induce the production of factors that promote neuronal excitability by cultured BV2 microglia [366].
G-CSF exhibited neurotrophic effects on primary cholinergic neurons in vitro [367] and in vivo promoted neuronal survival after focal cerebral ischemia [64, 65, 67, 368]. In addition, G-CSF was shown to stimulate neuronal differentiation in hippocampal cultures [65]. Since many of these experiments involve mixed neuronal/glial cultures it is possible that the effects of G-CSF on neuronal cells in these cultures are mediated through its actions on microglia.
4.4. Physiological roles of G-CSF
Studies involving genetic targeting of Csf3, or modulation of G-CSF bioavailability, in rodents revealed that G-CSF regulates different behavioral and neuronal functions. Csf3-null mice exhibited impaired memory and motor deficits, reduction of adult neurogenesis in the dentate gyrus area of the hippocampus, a long-term potentiation deficit and decreased dendritic complexity of hippocampal neurons [369]. Systemic administration of G-CSF and/or its central neutralization in vivo established its role as regulator of motivation, reward and cognitive flexibility, by inducing dopamine release in the mesolimbic system, as well as in cocaine addiction extinction [68, 370, 371]. In addition, G-CSF has been found to regulate the neuroendocrine system by promoting the secretion of ACTH from the pituitary–adrenal axis and of melatonin from the pineal gland [212, 372].
Intravenous administration of G-CSF to children with cerebral palsy produced neurodevelopmental improvement [373]. However, at least in susceptible individuals, elevation of G-CSF may cause neuronal dysfunction. Posterior reversible leukoencephalopathy syndrome is a rare, acute and transient encephalopathy that can develop following the administration of recombinant human G-CSF (Filgrastim) to both adult and pediatric patients [374, 375] and can be fatal [376]. The proposed mechanism involves G-CSF-induced neutrophil mobilization and activation with subsequent temporary disruption of BBB permeability [375], a hypothesis reinforced by reports of G-CSF-induced capillary leakage syndrome [377]. It would be of interest to investigate the factors that predispose to these rare adverse reactions to G-CSF.
4.5. Role of G-CSF in nervous system disease and damage
4.5.1. Autoimmune demyelination
4.5.1.1. G-CSF in MS
CSF3 transcripts were found to be elevated in tissue samples from MS patients with acute/active lesions, compared to chronic/silent lesions [378]. A pathogenic role of G-CSF-recruited neutrophils in autoimmune demyelinating pathologies is strongly suggested by the severe clinical episodes occurring in patients with MS or neuromyelitis optica treated with Filgrastim [379, 380].
4.5.1.2. G-CSF in EAE
Analysis of the mechanisms underlying the detrimental effects of Filgrastim in humans has been carried out in EAE. During the preclinical stage of EAE, activated IL-17-secreting γδT (γδT17) cells and Th17 cells induce the expression of neutrophil-activating factors, including G-CSF, leading to a significant increase of neutrophils in the bone marrow, serum, and spleen [381, 382]. Neutrophils represent a significant percentage of CNS-infiltrating cells prior to disease onset and relapse [383, 384]. The severity of the symptoms, the number of circulating neutrophils and monocytes, and the percentage of neutrophils, monocytes and dendritic cells in the spinal cord were significantly reduced in Csf3r-deficient mice, suggesting that the G-CSF-mediated recruitment of neutrophils is critical for MS [381, 382].
Despite the association between G-CSF and EAE pathogenesis, G-CSF administration yielded conflicting results, exacerbating or improving the clinical score based on the stage in which it was administered. Verda et al. showed that G-CSF administered for 10 days after EAE induction with PLP139–151 exacerbated the clinical score, increasing the peak of severity, and administration after the peak exacerbated the clinical score observed during the relapsing phase [385]. Conversely, when G-CSF was administered before, or at the onset of the clinical symptoms, it provided a significant protection from MOG35-55- or MBP-induced EAE, leading to clinical score improvement, reduced cerebellar demyelination and reduced cerebellar T cell infiltration [378, 386].
4.5.2. Neurodegenerative diseases
4.5.2.1. Alzheimer’s disease
Serum concentrations of G-CSF were found to be decreased in a small cohort of patients affected by AD [349, 387]. The significance of this finding is unclear, given that one study found an inverse correlation between G-CSF levels and the CSF levels of Aβ1−42 [349], while another found a direct association with disease severity [387]. However, the intrathecal levels of G-CSF were increased in AD patients (~80 ± 8pg/mL), compared to a control group without dementia (50± 8pg/mL) or a group with fronto-temporal dementia (51± 5pg/mL) [388]. The role of G-CSF in AD was tested in several rodent models in which G-CSF administration prevented the cognitive deficits, decreased Aβ deposition in the hippocampus and entorhinal cortex and augmented total microglial activity, while at the same time suppressing both central and peripheral inflammation and stimulating hippocampal neurogenesis [364, 389–391]. In addition, bone marrow mesenchymal stem cells mobilized in response to G-CSF were shown to infiltrate the brain and contribute to neuroregeneration in a mouse model of AD [389, 392]. Also, G-CSF was shown to attenuate Aβ toxicity through the enhancement of the activity of the Aβ-degrading enzyme, neprilysin, in neurons [393]. Furthermore, systemic administration of G-CSF and SCF was shown to augment bone marrow-derived macrophages in the brains of APP/PS1 mice, suggesting that these cells might also contribute to the reduction of Aβ deposits [394]. However, a clinical trial for Filgrastim did not produce significant cognitive improvements in a small cohort of patients affected by AD (ClinicalTrials.gov Identifier: NCT01617577) [364].
4.5.2.2. Parkinson’s disease
Systemic administration of G-CSF in rodents before, or immediately after, induction of parkinsonism protected against histological and functional damage [66, 67, 395], through a mechanism that might involve neuronal Erk1/2 activation [67], suppression of neuroinflammation and of BDNF depletion [395]. Furthermore, administration of G-CSF after the onset of motor dysfunction promoted the recovery of striatal dopamine levels, reduced striatal microgliosis and improved locomotor function [396–398], providing proof of principle that G-CSF is neuroprotective in PD. In addition, administration of pegylated G-CSF (Pegfilgrastim) [399] following MPTP-toxicity in mice was reported to induce the expression of metastasis-associated protein 1 (MTA1), an upstream regulator of tyrosine hydroxylase that is substantially downregulated in the substantia nigra of patients with PD [400]. A phase I clinical trial of low-dose Filgrastim in patients with early PD alleviated disease deterioration, presumably through attenuation of dopaminergic neuron degeneration [401].
4.5.2.3. Amyotrophic lateral sclerosis
In patients with ALS, the intrathecal concentration of G-CSF is elevated and within the spinal cord its expression is increased in reactive astrocytes, while the expression of the G-CSFR in motoneurons is decreased [402, 403]. Pre-clinical studies in SOD1(G93A) transgenic mice showed that systemic administration of G-CSF or its overexpression in the CNS improved motor performance [69, 404]. The proposed mechanisms include direct neuroprotection [69], restoration of microglia function [405], and restoration of transcriptomic deregulation in motoneurons [406]. Notably, studies utilizing pegfilgrastim show that the neuroprotective and pro-survival effects of G-CSF are male gender-specific and related to its ability to suppress the increase in ROS occurring in the spinal cords of male, but not female mice [407, 408]. Despite these encouraging results in pre-clinical studies, most clinical trials indicate no benefit of G-CSF administration to ALS patients [409–411] and one study [410] suggests that it may accelerate progression in females.
4.5.2.4. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)
CADASIL is characterized by the progressive degeneration of vascular smooth muscle cells of the small arteries in the brain, leading to recurrent ischemic stroke, vascular dementia and death. Administration of G-CSF and SCF in a mouse model of CADASIL improved cognitive function, attenuated the degeneration of vascular smooth muscle cells and the loss of cerebral capillary endothelial cells, increased cerebral blood vascular density and promoted neurogenesis. These data suggest that SCF and G-CSF might be of therapeutic value in CADASIL [412].
4.5.3. Stroke
Several studies have reported the efficacy of G-CSF in reducing infarct size and improving functional recovery in rodent models of cerebral ischemia [413–415]. When administered peripherally, G-CSF limited the cortical damage and the motor deficits caused by cerebral ischemia. Moreover, in response to ischemia, both ligand and receptor are expressed in neurons adjacent to the damaged brain area, suggesting autocrine G-CSF protective signaling in response to neural injury [65, 416]. Multiple mechanisms for the protective effects of G-CSF have been suggested, including inhibition of apoptosis, immunomodulation and stimulation of neurogenesis and angiogenesis [64, 65, 369, 417–419]. These effects have been attributed to direct action on neural cells or to the recruitment of hematopoietic stem cells to the damaged tissue. The anti-apoptotic function is mediated by the activation of JAK2/STAT3 signaling [64, 65, 368, 420]. In the chronic stage of stroke, G-CSF has been shown to promote motor recovery when co-administered with SCF. The two factors synergistically activated NF-kB via PI3K/Akt and promoted tissue repair, blood vessel generation and neurite outgrowth of the ipsilateral hemisphere affected by stroke, and induced axonal sprouting from the contralateral hemisphere towards the affected hemisphere [421].
4.5.4. Traumatic brain injury
A retrospective study showed that plasma G-CSF levels are rapidly elevated in traumatic brain injury (TBI) patients and decline after 14 days in those with mild, but not severe TBI, in which higher circulating levels correlate with good outcome at 6 months post-injury [422]. Because of its abilities to attenuate chronic neuroinflammation, promote the production of BDNF, stimulate neurogenesis in the hippocampus and mobilize bone marrow mesenchymal cells that can integrate into the brain and differentiate into neurons, administration of G-CSF has been proposed as a particularly effective strategy for preventing the long-term complications of TBI, including dementia (reviewed in [423]). Acute phase administration of G-CSF to mouse models of TBI has produced mixed results [424, 425]. However, repeated treatment with G-CSF and stem cell factor (SCF), initiated in the chronic phase (3 months post-TBI), was reported to improve neurological function by enhancing corticospinal tract sprouting into the denervated side of the cervical spinal cord, ameliorating microglia degeneration and re-balancing synaptic pruning in the hippocampus [426].
4.5.5. Pain
G-CSF signaling has been shown to play a significant role in different models of pain. In tumor-related bone pain, G-CSF can induce significant dose-dependent hyperalgesia by stimulating Erk and STAT3 upon binding to the G-CSFR present on nerve fibers and dorsal root ganglia [335]. In an experimental model of peripheral nerve injury, G-CSF was described as a main factor controlling the peripheral production of pronociceptive cytokines, such as TNFα and IL-1β, and driving the infiltration of inflammatory cells into the injured area [427, 428]. In a dextran sulfate sodium-induced colitis model, G-CSF promoted nerve sensitization in the spinal cord through G-CSFR-mediated activation of a Cathepsin S-CX3CR1-inducible NOS pathway in microglia [366]. Activated microglia are known to release an array of factors, including cytokines (such as TNF-α, IL-1β and IL-6), which modulate neuronal central sensitization and nerve injury-induced persistent pain (reviewed in [429]). In arthritis, Lee et al. have characterized the contribution of G-CSF signaling to the development of inflammatory and arthritic pain via a pathway involving the activation of cyclooxygenase-2 [430]. However, others have shown that G-CSF can attenuate neuropathic pain caused by spinal cord and peripheral nerve injury by promoting the recruitment of opioid-releasing leukocytes to the injured area, or by suppressing microglial activation and the release of pro-inflammatory cytokines in the dorsal horn [431–433]. Consistent with this, in rodent models of diabetic neuropathy, G-CSF exerts beneficial effects by attenuating the damage to peripheral nerves and brain endothelial cells [63, 434–436]. Further studies are needed to clarify the effects of G-CSF administration on neuropathic pain in different settings.
5. Conclusions
Colony stimulating factors have emerged as important regulators in the nervous system. Although some of these cytokines can cross the BBB, there is evidence that they are locally produced within the CNS at steady state, where they may act in a paracrine/autocrine manner. While the expression of their receptors in the neural lineage is at times controversial, it is clear that they regulate the development, survival and/or function of microglia. CSF-1 and IL-34, via the CSF-1R, are required for the development, proliferation and maintenance of essentially all CNS microglia in a temporal and regional specific manner and usually suppress inflammatory activation. In contrast, in steady state, GM-CSF and G-CSF are mainly involved in regulation of microglial function. Upregulation of CSF-1, GM-CSF and G-CSF and/or their receptors has been documented in several neurological diseases and conditions and, in some cases, preclinical therapeutic intervention has been successful (Fig. 3). However, in other instances, microglia depletion using CSF-1R inhibitors and modulation of their activation by G-CSF, have produced both beneficial and damaging effects depending on the stage of disease, emphasizing the need for appropriately timed preclinical studies.
Figure 3. Involvement of CSF-1R, GM-CSF and G-CSF in neurological disease and effects of pharmacological and genetic intervention.
(A) Animal model studies. Mixed effects, indicating that different results have been reported when the intervention was applied in different experimental settings (e.g. in acute or chronic stages of disease, in prophylactic versus therapeutic protocols, or when using different inhibitors). See the main text for details. (B) Human trials. AD, Alzheimer’s disease; ALS, Amyotrophic lateral sclerosis; CADASIL, Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CMT1X, Charcot–Marie–Tooth disease type 1X; CRL, CSF-1R-related leukoencephalopathy; GCL, globoid cell leukodystrophy; HD, Huntington’s disease; MS, multiple sclerosis; PD, Parkinson’s disease; TBI, traumatic brain injury.
Highlights.
CSFs regulate the development, survival and/or function of microglia
CSF-1R signaling is required for the development and maintenance of CNS microglia
Studies in Csf1r+/− mice suggest a role for the CSF-1R in microglial homeostasis
At steady-state, GM-CSF and G-CSF mainly regulate microglial functions
CSFs and their receptors are therapeutic targets in several neurological diseases
Acknowledgements
This work was supported by grants from the National Institutes of Health: Grant R01NS091519 (to ERS), U54 HD090260 (support for the Rose F. Kennedy IDDRC) and the P30CA013330 NCI Cancer Center Grant. We also thank David and Ruth Levine for their support. The funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the review and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interests: none.
References
- [1].Metcalf D, Hematopoietic cytokines, Blood 111(2) (2008) 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stanley ER, Chitu V, CSF-1 receptor signaling in myeloid cells, Cold Spring Harb Perspect Biol 6(6) (2014) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chitu V, Caescu CI, Stanley ER, Lennartsson J, Ronnstrand L, Heldin C-H, PDGF Receptor Family, in: Wheeler DL, Yarden Y (Eds.), The Receptor Tyrosine Kinases: Family and Subfamilies, Springer Science, New York, 2015, pp. 373–538. [Google Scholar]
- [4].Chitu V, Gokhan S, Nandi S, Mehler MF, Stanley ER, Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System, Trends Neurosci 39(6) (2016) 378–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chitu V, Stanley ER, Regulation of Embryonic and Postnatal Development by the CSF-1 Receptor, Curr Top Dev Biol 123 (2017) 229–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bartocci A, Mastrogiannis DS, Migliorati G, Stockert RJ, Wolkoff AW, Stanley ER, Macrophages specifically regulate the concentration of their own growth factor in the circulation, Proc.Natl.Acad.Sci.USA 84 (1987) 6179–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER, Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse, Development 120(6) (1994) 1357–72. [DOI] [PubMed] [Google Scholar]
- [8].Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER, Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis, Blood 98(1) (2001) 74–84. [DOI] [PubMed] [Google Scholar]
- [9].Caescu CI, Guo X, Tesfa L, Bhagat TD, Verma A, Zheng D, Stanley ER, Colony stimulating factor-1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA-21, Blood 125(8) (2015) e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. , Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis, Science 264(5159) (1994) 713–6. [DOI] [PubMed] [Google Scholar]
- [11].Shibata Y, Berclaz P-Y, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC, GM-CSF regulates alveolar macrophage differentiation and innate immunity int he lung through Pu.1, Immunity (2001) Re-submitted. [DOI] [PubMed] [Google Scholar]
- [12].Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR, Mice Lacking Granulocyte-Colony-Stimulating Factor Have Chronic Neutropenia, Granulocyte and Macrophage Progenitor-Cell Deficiency, and Impaired Neutrophil Mobilization, Blood 84(6) (1994) 1737–1746. [PubMed] [Google Scholar]
- [13].Janowska-Wieczorek A, Belch AR, Jacobs A, Bowen D, Padua RA, Paietta E, Stanley ER, Increased circulating colony-stimulating factor-1 in patients with preleukemia, leukemia, and lymphoid malignancies, Blood 77(8) (1991) 1796–1803. [PubMed] [Google Scholar]
- [14].Wang H, Cao J, Lai X, Serum Interleukin-34 Levels Are Elevated in Patients with Systemic Lupus Erythematosus, Molecules (Basel, Switzerland) 22(1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hamilton JA, GM-CSF in inflammation, J Exp Med 217(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dougan M, Dranoff G, Dougan SK, GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation, Immunity 50(4) (2019) 796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Felix J, Elegheert J, Gutsche I, Shkumatov AV, Wen YR, Bracke N, Pannecoucke E, Vandenberghe I, Devreese B, Svergun DI, Pauwels E, Vergauwen B, Savvides SN, Human IL-34 and CSF-1 Establish Structurally Similar Extracellular Assemblies with Their Common Hematopoietic Receptor, Structure 21(4) (2013) 528–539. [DOI] [PubMed] [Google Scholar]
- [18].Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT, Discovery of a cytokine and its receptor by functional screening of the extracellular proteome, Science 320(5877) (2008) 807–11. [DOI] [PubMed] [Google Scholar]
- [19].Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, Lin H, Stanley ER, Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells, J Leukoc Biol 88(3) (2010) 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M, IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia, Nat Immunol 13(8) (2012) 753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, Becher B, Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia, Immunity 37(6) (2012) 1050–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, Stanley ER, The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation, Dev Biol 367(2) (2012) 100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kana V, Desland FA, Casanova-Acebes M, Ayata P, Badimon A, Nabel E, Yamamuro K, Sneeboer M, Tan IL, Flanigan ME, Rose SA, Chang C, Leader A, Le Bourhis H, Sweet ES, Tung N, Wroblewska A, Lavin Y, See P, Baccarini A, Ginhoux F, Chitu V, Stanley ER, Russo SJ, Yue Z, Brown BD, Joyner AL, De Witte LD, Morishita H, Schaefer A, Merad M, CSF-1 controls cerebellar microglia and is required for motor function and social interaction, J Exp Med 216(10) (2019) 2265–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Easley-Neal C, Foreman O, Sharma N, Zarrin AA, Weimer RM, CSF1R Ligands IL-34 and CSF1 Are Differentially Required for Microglia Development and Maintenance in White and Gray Matter Brain Regions, Front Immunol 10 (2019) 2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Bugatti M, Ulland TK, Vermi W, Gilfillan S, Colonna M, Nonredundant roles of keratinocyte-derived IL-34 and neutrophil-derived CSF1 in Langerhans cell renewal in the steady state and during inflammation, Eur J Immunol 46(3) (2016) 552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rozwarski DA, Diederichs K, Hecht R, Boone T, Karplus PA, Refined crystal structure and mutagenesis of human granulocyte-macrophage colony-stimulating factor, Proteins 26 (1996) 304–313. [DOI] [PubMed] [Google Scholar]
- [27].Feng Y, Klein BK, McWherter CA, Three-dimensional solution structure and backbone dynamics of a variant of human interleukin-3, J Mol Biol 259(3) (1996) 524–41. [DOI] [PubMed] [Google Scholar]
- [28].Milburn MV, Hassell AM, Lambert MH, Jordan SR, Proudfoot AE, Graber P, Wells TN, A novel dimer configuration revealed by the crystal structure at 2.4 A resolution of human interleukin-5, Nature 363(6425) (1993) 172–6. [DOI] [PubMed] [Google Scholar]
- [29].Gearing DP, King JA, Gough NM, Nicola NA, Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor, EMBO J 8(12) (1989) 3667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hayashida K, Kitamura T, Gorman DM, Arai K-I, Yokota T, Miyajima A, Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): Reconstitution of a high-affinity GM-CSF receptor, Proc.Natl.Acad.Sci.USA 87 (1990) 9655–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kitamura T, Hayashida K, Sakamaki K, Yokota T, Arai K, Miyajima A, Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): Evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor, Proc.Natl.Acad.Sci.USA 88 (1991) 5082–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kitamura T, Sato N, Arai K, Miyajima A, Expression Cloning of the Human IL-3 Receptor cDNA Reveals a Shared Beta Subunit for the Human IL-3 and GM-CSF Receptors, Cell 66 (1991) 1165–1174. [DOI] [PubMed] [Google Scholar]
- [33].Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, Ramshaw H, Woodcock JM, Xu Y, Guthridge M, McKinstry WJ, Lopez AF, Parker MW, The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation, Cell 134(3) (2008) 496–507. [DOI] [PubMed] [Google Scholar]
- [34].Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW, The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling, Immunol Rev 250(1) (2012) 277–302. [DOI] [PubMed] [Google Scholar]
- [35].Broughton SE, Hercus TR, Nero TL, Dottore M, McClure BJ, Dhagat U, Taing H, Gorman MA, King-Scott J, Lopez AF, Parker MW, Conformational Changes in the GM-CSF Receptor Suggest a Molecular Mechanism for Affinity Conversion and Receptor Signaling, Structure 24(8) (2016) 1271–1281. [DOI] [PubMed] [Google Scholar]
- [36].Broughton SE, Hercus TR, Nero TL, Kan WL, Barry EF, Dottore M, Cheung Tung Shing KS, Morton CJ, Dhagat U, Hardy MP, Wilson NJ, Downton MT, Schieber C, Hughes TP, Lopez AF, Parker MW, A dual role for the N-terminal domain of the IL-3 receptor in cell signalling, Nat Commun 9(1) (2018) 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ding DX-H, Rivas CI, Heaney ML, Raines MA, Vera JC, Golde DW, The a subunit of the human granulocyte-macrophage colony-stimulating factor receptor signals for glucose transport via a phosphorylation-independent pathway, Proc.Natl.Acad.Sci.USA 91 (1994) 2537–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Horan TP, Martin F, Simonet L, Arakawa T, Philo JS, Dimerization of granulocyte-colony stimulating factor receptor: The ig plus CRH construct of granulocyte-colony stimulating factor receptor forms a 2:2 complex with a ligand, J.Biochem.(Tokyo) 121 (1997) 370–375. [DOI] [PubMed] [Google Scholar]
- [39].Tamada T, Honjo E, Maeda Y, Okamoto T, Ishibashi M, Tokunaga M, Kuroki R, Homodimeric cross-over structure of the human granulocyte colony-stimulating factor (GCSF) receptor signaling complex, Proc Natl Acad Sci U S A 103(9) (2006) 3135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Layton JE, Hall NE, The interaction of G-CSF with its receptor, Front Biosci 11 (2006) 3181–9. [DOI] [PubMed] [Google Scholar]
- [41].Dwivedi P, Greis KD, Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies, Exp Hematol 46 (2017) 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Solaroglu I, Jadhav V, Zhang JH, Neuroprotective effect of granulocyte-colony stimulating factor, Front Biosci 12 (2007) 712–24. [DOI] [PubMed] [Google Scholar]
- [43].Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA, A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function, J Neurosci 28(1) (2008) 264–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S, Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq, Science 347(6226) (2015) 1138–42. [DOI] [PubMed] [Google Scholar]
- [45].McLay RN, Kimura M, Banks WA, Kastin AJ, Granulocyte-macrophage colony-stimulating factor crosses the blood--brain and blood--spinal cord barriers, Brain 120 (Pt 11) (1997) 2083–91. [DOI] [PubMed] [Google Scholar]
- [46].Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, Kessler JA, Groothuis DR, Hematopoietic growth factors pass through the blood-brain barrier in intact rats, Exp Neurol 204(2) (2007) 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Akiyama H, Nishimura T, Kondo H, Ikeda K, Hayashi Y, McGeer PL, Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis, Brain Res 639(1) (1994) 171–4. [DOI] [PubMed] [Google Scholar]
- [48].Raivich G, Haas S, Werner A, Klein MA, Kloss C, Kreutzberg GW, Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: A quantitative immunofluorescence study using confocal laser microscopy, J.Comp.Neurol 395 (1998) 342–358. [DOI] [PubMed] [Google Scholar]
- [49].Clare AJ, Day RC, Empson RM, Hughes SM, Transcriptome Profiling of Layer 5 Intratelencephalic Projection Neurons From the Mature Mouse Motor Cortex, Front Mol Neurosci 11 (2018) 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Luo J, Elwood F, Britschgi M, Villeda S, Zhang H, Ding Z, Zhu L, Alabsi H, Getachew R, Narasimhan R, Wabl R, Fainberg N, James ML, Wong G, Relton J, Gambhir SS, Pollard JW, Wyss-Coray T, Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival, J Exp Med 210(1) (2013) 157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Grabert K, Sehgal A, Irvine KM, Wollscheid-Lengeling E, Ozdemir DD, Stables J, Luke GA, Ryan MD, Adamson A, Humphreys NE, Sandrock CJ, Rojo R, Verkasalo VA, Mueller W, Hohenstein P, Pettit AR, Pridans C, Hume DA, A Transgenic Line That Reports CSF1R Protein Expression Provides a Definitive Marker for the Mouse Mononuclear Phagocyte System, J Immunol 205(11) (2020) 3154–3166. [DOI] [PubMed] [Google Scholar]
- [52].Schabitz WR, Kruger C, Pitzer C, Weber D, Laage R, Gassler N, Aronowski J, Mier W, Kirsch F, Dittgen T, Bach A, Sommer C, Schneider A, A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF), Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 28(1) (2008) 29–43. [DOI] [PubMed] [Google Scholar]
- [53].Kim JK, Choi BH, Park HC, Park SR, Kim YS, Yoon SH, Park HS, Kim EY, Ha Y, Effects of GM-CSF on the neural progenitor cells, Neuroreport 15(14) (2004) 2161–5. [DOI] [PubMed] [Google Scholar]
- [54].Kruger C, Laage R, Pitzer C, Schabitz WR, Schneider A, The hematopoietic factor GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes neuronal differentiation of adult neural stem cells in vitro, BMC Neurosci 8 (2007) 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen S, He L, Huang AJY, Boehringer R, Robert V, Wintzer ME, Polygalov D, Weitemier AZ, Tao Y, Gu M, Middleton SJ, Namiki K, Hama H, Therreau L, Chevaleyre V, Hioki H, Miyawaki A, Piskorowski RA, McHugh TJ, A hypothalamic novelty signal modulates hippocampal memory, Nature 586(7828) (2020) 270–274. [DOI] [PubMed] [Google Scholar]
- [56].Baldwin GC, Benveniste EN, Chung GY, Gasson JC, Golde DW, Identification and characterization of a high-affinity granulocyte-macrophage colony-stimulating factor receptor on primary rat oligodendrocytes, Blood 82(11) (1993) 3279–82. [PubMed] [Google Scholar]
- [57].Sawada M, Itoh Y, Suzumura A, Marunouchi T, Expression of cytokine receptors in cultured neuronal and glial cells, Neurosci Lett 160(2) (1993) 131–4. [DOI] [PubMed] [Google Scholar]
- [58].Nicol LSC, Thornton P, Hatcher JP, Glover CP, Webster CI, Burrell M, Hammett K, Jones CA, Sleeman MA, Billinton A, Chessell I, Central inhibition of granulocyte-macrophage colony-stimulating factor is analgesic in experimental neuropathic pain, Pain (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guillemin G, Boussin FD, Le Grand R, Croitoru J, Coffigny H, Dormont D, Granulocyte macrophage colony stimulating factor stimulates in vitro proliferation of astrocytes derived from simian mature brains, Glia 16(1) (1996) 71–80. [DOI] [PubMed] [Google Scholar]
- [60].Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, Mizuno T, Suzumura A, GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia, J Neuroinflammation 9 (2012) 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Morabito S, Miyoshi E, Michael N, Swarup V, Integrative genomics approach identifies conserved transcriptomic networks in Alzheimer’s disease, Hum Mol Genet 29(17) (2020) 2899–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chitu V, Biundo F, Shlager GGL, Park ES, Wang P, Gulinello ME, Gokhan S, Ketchum HC, Saha K, DeTure MA, Dickson DW, Wszolek ZK, Zheng D, Croxford AL, Becher B, Sun D, Mehler MF, Stanley ER, Microglial Homeostasis Requires Balanced CSF-1/CSF-2 Receptor Signaling, Cell Rep 30(9) (2020) 3004–3019 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim KS, Song YS, Jin J, Joe JH, So BI, Park JY, Fang CH, Kim MJ, Cho YH, Hwang S, Ro YS, Kim H, Ahn YH, Sung HJ, Sung JJ, Park SH, Lipton SA, Granulocyte-colony stimulating factor as a treatment for diabetic neuropathy in rat, Mol Cell Endocrinol 414 (2015) 64–72. [DOI] [PubMed] [Google Scholar]
- [64].Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, Sommer C, Schwab S, Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia, Stroke 34(3) (2003) 745–51. [DOI] [PubMed] [Google Scholar]
- [65].Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR, The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis, J Clin Invest 115(8) (2005) 2083–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Meuer K, Pitzer C, Teismann P, Kruger C, Goricke B, Laage R, Lingor P, Peters K, Schlachetzki JC, Kobayashi K, Dietz GP, Weber D, Ferger B, Schabitz WR, Bach A, Schulz JB, Bahr M, Schneider A, Weishaupt JH, Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson’s disease, J Neurochem 97(3) (2006) 675–86. [DOI] [PubMed] [Google Scholar]
- [67].Huang HY, Lin SZ, Kuo JS, Chen WF, Wang MJ, G-CSF protects dopaminergic neurons from 6-OHDA-induced toxicity via the ERK pathway, Neurobiol Aging 28(8) (2007) 1258–69. [DOI] [PubMed] [Google Scholar]
- [68].Calipari ES, Godino A, Peck EG, Salery M, Mervosh NL, Landry JA, Russo SJ, Hurd YL, Nestler EJ, Kiraly DD, Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine, Nat Commun 9(1) (2018) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Pitzer C, Kruger C, Plaas C, Kirsch F, Dittgen T, Muller R, Laage R, Kastner S, Suess S, Spoelgen R, Henriques A, Ehrenreich H, Schabitz WR, Bach A, Schneider A, Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis, Brain 131(Pt 12) (2008) 3335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ridwan S, Bauer H, Frauenknecht K, von Pein H, Sommer CJ, Distribution of granulocyte-monocyte colony-stimulating factor and its receptor alpha-subunit in the adult human brain with specific reference to Alzheimer’s disease, J Neural Transm 119(11) (2012) 1389–406. [DOI] [PubMed] [Google Scholar]
- [71].Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B, High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease, Immunity 48(2) (2018) 380–395 e6. [DOI] [PubMed] [Google Scholar]
- [72].Nikodemova M, Kimyon RS, De I, Small AL, Collier LS, Watters JJ, Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week, J Neuroimmunol 278 (2015) 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M, Fate mapping analysis reveals that adult microglia derive from primitive macrophages, Science 330(6005) (2010) 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Munro DAD, Bradford BM, Mariani SA, Hampton DW, Vink CS, Chandran S, Hume DA, Pridans C, Priller J, CNS macrophages differentially rely on an intronic Csf1r enhancer for their development, Development 147(23) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Prinz M, Erny D, Hagemeyer N, Ontogeny and homeostasis of CNS myeloid cells, Nat Immunol 18(4) (2017) 385–392. [DOI] [PubMed] [Google Scholar]
- [76].Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR, Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors, Nature 518(7540) (2015) 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M, Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways, Nat Neurosci (2013). [DOI] [PubMed] [Google Scholar]
- [78].Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F, A lineage of myeloid cells independent of Myb and hematopoietic stem cells, Science 336(6077) (2012) 86–90. [DOI] [PubMed] [Google Scholar]
- [79].Hagemeyer N, Kierdorf K, Frenzel K, Xue J, Ringelhan M, Abdullah Z, Godin I, Wieghofer P, Costa Jordao MJ, Ulas T, Yorgancioglu G, Rosenbauer F, Knolle PA, Heikenwalder M, Schultze JL, Prinz M, Transcriptome-based profiling of yolk sac-derived macrophages reveals a role for Irf8 in macrophage maturation, EMBO J (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M, Origin, fate and dynamics of macrophages at central nervous system interfaces, Nat Immunol 17(7) (2016) 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F, Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages, J Exp Med 209(6) (2012) 1167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wegiel J, Wisniewski HM, Dziewiatkowski J, Tarnawski M, Kozielski R, Trenkner E, Wiktor-Jedrzejczak W, Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice, Brain Res 804(1) (1998) 135–9. [DOI] [PubMed] [Google Scholar]
- [83].Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, Hwang P, Chan AT, Graves SM, Uweru JO, Ledderose C, Kutlu MG, Wheeler MA, Kahan A, Ishikawa M, Wang YC, Loh YE, Jiang JX, Surmeier DJ, Robson SC, Junger WG, Sebra R, Calipari ES, Kenny PJ, Eyo UB, Colonna M, Quintana FJ, Wake H, Gradinaru V, Schaefer A, Negative feedback control of neuronal activity by microglia, Nature 586(7829) (2020) 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sasaki A, Yokoo H, Naito M, Kaizu C, Shultz LD, Nakazato Y, Effects of macrophage-colony-stimulating factor deficiency on the maturation of microglia and brain macrophages and on their expression of scavenger receptor, Neuropathology 20(2) (2000) 134–142. [DOI] [PubMed] [Google Scholar]
- [85].Lelli A, Gervais A, Colin C, Cheret C, Ruiz de Almodovar C, Carmeliet P, Krause KH, Boillee S, Mallat M, The NADPH oxidase Nox2 regulates VEGFR1/CSF-1R-mediated microglial chemotaxis and promotes early postnatal infiltration of phagocytes in the subventricular zone of the mouse cerebral cortex, Glia 61(9) (2013) 1542–55. [DOI] [PubMed] [Google Scholar]
- [86].Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnar Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D, Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain, Cell Rep 18(2) (2017) 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Datta Sagar, M., Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grun D, Ronneberger O, Prinz M, A new fate mapping system reveals context-dependent random or clonal expansion of microglia, Nat Neurosci 20(6) (2017) 793–803. [DOI] [PubMed] [Google Scholar]
- [88].Reu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, Frisen J, The Lifespan and Turnover of Microglia in the Human Brain, Cell Rep 20(4) (2017) 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Huang Y, Xu Z, Xiong S, Sun F, Qin G, Hu G, Wang J, Zhao L, Liang YX, Wu T, Lu Z, Humayun MS, So KF, Pan Y, Li N, Yuan TF, Rao Y, Peng B, Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion, Nat Neurosci 21(4) (2018) 530–540. [DOI] [PubMed] [Google Scholar]
- [90].Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Muller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S, Waisman A, Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System, Immunity 43(1) (2015) 92–106. [DOI] [PubMed] [Google Scholar]
- [91].Najafi AR, Crapser J, Jiang S, Ng W, Mortazavi A, West BL, Green KN, A limited capacity for microglial repopulation in the adult brain, Glia 66(11) (2018) 2385–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cronk JC, Filiano AJ, Louveau A, Marin I, Marsh R, Ji E, Goldman DH, Smirnov I, Geraci N, Acton S, Overall CC, Kipnis J, Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia, J Exp Med 215(6) (2018) 1627–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rozovsky I, Finch CE, Morgan TE, Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation, Neurobiol Aging 19(1) (1998) 97–103. [DOI] [PubMed] [Google Scholar]
- [94].Nandi S, Cioce M, Yeung YG, Nieves E, Tesfa L, Lin H, Hsu AW, Halenbeck R, Cheng HY, Gokhan S, Mehler MF, Stanley ER, Receptor-type protein-tyrosine phosphatase zeta is a functional receptor for interleukin-34, J Biol Chem 288(30) (2013) 21972–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN, Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain, Neuron 82(2) (2014) 380–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kempthorne L, Yoon H, Madore C, Smith S, Wszolek ZK, Rademakers R, Kim J, Butovsky O, Dickson DW, Loss of homeostatic microglial phenotype in CSF1R-related Leukoencephalopathy, Acta Neuropathol Commun 8(1) (2020) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Biundo F, Chitu V, Shlager GGL, Park ES, Gulinello ME, Saha K, Ketchum HC, Fernandes C, Gokhan S, Mehler MF, Stanley ER, Microglial reduction of colony stimulating factor-1 receptor expression is sufficient to confer adult onset leukodystrophy, Glia 69 (2021) 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Arreola MA, Soni N, Crapser JD, Hohsfield LA, Elmore MRP, Matheos DP, Wood MA, Swarup V, Mortazavi A, Green KN, Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R(+/−) mouse model of ALSP, which can be rescued via CSF1R inhibitors, Sci Adv 7(35) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW, Microglial brain region-dependent diversity and selective regional sensitivities to aging, Nat Neurosci 19(3) (2016) 504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Galatro TF, Holtman IR, Lerario AM, Vainchtein ID, Brouwer N, Sola PR, Veras MM, Pereira TF, Leite REP, Moller T, Wes PD, Sogayar MC, Laman JD, den Dunnen W, Pasqualucci CA, Oba-Shinjo SM, Boddeke E, Marie SKN, Eggen BJL, Transcriptomic analysis of purified human cortical microglia reveals age-associated changes, Nat Neurosci 20(8) (2017) 1162–1171. [DOI] [PubMed] [Google Scholar]
- [101].Soreq L, U.K.B.E. Consortium, C. North American Brain Expression, Rose J, Soreq E, Hardy J, Trabzuni D, Cookson MR, Smith C, Ryten M, Patani R, Ule J, Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging, Cell Rep 18(2) (2017) 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mosher KI, Wyss-Coray T, Microglial dysfunction in brain aging and Alzheimer’s disease, Biochem Pharmacol 88(4) (2014) 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Norden DM, Muccigrosso MM, Godbout JP, Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease, Neuropharmacology 96(Pt A) (2015) 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Elmore MRP, Hohsfield LA, Kramar EA, Soreq L, Lee RJ, Pham ST, Najafi AR, Spangenberg EE, Wood MA, West BL, Green KN, Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice, Aging Cell 17(6) (2018) e12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].O’Neil SM, Witcher KG, McKim DB, Godbout JP, Forced turnover of aged microglia induces an intermediate phenotype but does not rebalance CNS environmental cues driving priming to immune challenge, Acta Neuropathol Commun 6(1) (2018) 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ali S, Mansour AG, Huang W, Queen NJ, Mo X, Anderson JM, Hassan QN 2nd, Patel RS, Wilkins RK, Caligiuri MA, Cao L, CSF1R inhibitor PLX5622 and environmental enrichment additively improve metabolic outcomes in middle-aged female mice, Aging (Albany NY) 12(3) (2020) 2101–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, DeJesus-Hernandez M, Adamson J, Kouri N, Sundal C, Shuster EA, Aasly J, MacKenzie J, Roeber S, Kretzschmar HA, Boeve BF, Knopman DS, Petersen RC, Cairns NJ, Ghetti B, Spina S, Garbern J, Tselis AC, Uitti R, Das P, Van Gerpen JA, Meschia JF, Levy S, Broderick DF, Graff-Radford N, Ross OA, Miller BB, Swerdlow RH, Dickson DW, Wszolek ZK, Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids, Nat Genet 44(2) (2011) 200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Monies D, Maddirevula S, Kurdi W, Alanazy MH, Alkhalidi H, Al-Owain M, Sulaiman RA, Faqeih E, Goljan E, Ibrahim N, Abdulwahab F, Hashem M, Abouelhoda M, Shaheen R, Arold ST, Alkuraya FS, Autozygosity reveals recessive mutations and novel mechanisms in dominant genes: implications in variant interpretation, Genet Med (2017). [DOI] [PubMed] [Google Scholar]
- [109].Chitu V, Gokhan S, Stanley ER, Modeling CSF-1 receptor deficiency diseases - how close are we?, Febs J (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Oosterhof N, Chang IJ, Karimiani EG, Kuil LE, Jensen DM, Daza R, Young E, Astle L, van der Linde HC, Shivaram GM, Demmers J, Latimer CS, Keene CD, Loter E, Maroofian R, van Ham TJ, Hevner RF, Bennett JT, Homozygous Mutations in CSF1R Cause a Pediatric-Onset Leukoencephalopathy and Can Result in Congenital Absence of Microglia, Am J Hum Genet 104(5) (2019) 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER, Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects, Blood 99(1) (2002) 111–20. [DOI] [PubMed] [Google Scholar]
- [112].Konno T, Yoshida K, Mizuno T, Kawarai T, Tada M, Nozaki H, Ikeda SI, Nishizawa M, Onodera O, Wszolek ZK, Ikeuchi T, Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation, Eur J Neurol 24(1) (2017) 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Konno T, Tada M, Tada M, Koyama A, Nozaki H, Harigaya Y, Nishimiya J, Matsunaga A, Yoshikura N, Ishihara K, Arakawa M, Isami A, Okazaki K, Yokoo H, Itoh K, Yoneda M, Kawamura M, Inuzuka T, Takahashi H, Nishizawa M, Onodera O, Kakita A, Ikeuchi T, Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS, Neurology 82(2) (2014) 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chitu V, Gokhan S, Gulinello M, Branch CA, Patil M, Basu R, Stoddart C, Mehler MF, Stanley ER, Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP), Neurobiol Dis 74 (2015) 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C, M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination, Nat Neurosci 16(9) (2013) 1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wylot B, Mieczkowski J, Niedziolka S, Kaminska B, Zawadzka M, Csf1 Deficiency Dysregulates Glial Responses to Demyelination and Disturbs CNS White Matter Remyelination, Cells 9(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kondo Y, Adams JM, Vanier MT, Duncan ID, Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy, J Neurosci 31(10) (2011) 3610–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Groh J, Weis J, Zieger H, Stanley ER, Heuer H, Martini R, Colony-stimulating factor-1 mediates macrophage-related neural damage in a model for Charcot-Marie-Tooth disease type 1X, Brain 135(Pt 1) (2012) 88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Groh J, Klein I, Hollmann C, Wettmarshausen J, Klein D, Martini R, CSF-1-activated macrophages are target-directed and essential mediators of Schwann cell dedifferentiation and dysfunction in Cx32-deficient mice, Glia 63(6) (2015) 977–86. [DOI] [PubMed] [Google Scholar]
- [120].Klein D, Patzko A, Schreiber D, van Hauwermeiren A, Baier M, Groh J, West BL, Martini R, Targeting the colony stimulating factor 1 receptor alleviates two forms of Charcot-Marie-Tooth disease in mice, Brain 138(Pt 11) (2015) 3193–205. [DOI] [PubMed] [Google Scholar]
- [121].Groh J, Basu R, Stanley ER, Martini R, Cell-Surface and Secreted Isoforms of CSF-1 Exert Opposing Roles in Macrophage-Mediated Neural Damage in Cx32-Deficient Mice, J Neurosci 36(6) (2016) 1890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yuan X, Klein D, Kerscher S, West BL, Weis J, Katona I, Martini R, Macrophage depletion ameliorates peripheral neuropathy in aging mice, J Neurosci (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Werner K, Bitsch A, Bunkowski S, Hemmerlein B, Bruck W, The relative number of macrophages/microglia expressing macrophage colony-stimulating factor and its receptor decreases in multiple sclerosis lesions, Glia 40(1) (2002) 121–9. [DOI] [PubMed] [Google Scholar]
- [124].Li Z, Liu Y, Jia A, Cui Y, Feng J, Cerebrospinal fluid cells immune landscape in multiple sclerosis, J Transl Med 19(1) (2021) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tahmasebi F, Pasbakhsh P, Mortezaee K, Madadi S, Barati S, Kashani IR, Effect of the CSF1R inhibitor PLX3397 on remyelination of corpus callosum in a cuprizone-induced demyelination mouse model, J Cell Biochem 120(6) (2019) 10576–10586. [DOI] [PubMed] [Google Scholar]
- [126].Beckmann N, Giorgetti E, Neuhaus A, Zurbruegg S, Accart N, Smith P, Perdoux J, Perrot L, Nash M, Desrayaud S, Wipfli P, Frieauff W, Shimshek DR, Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945, Acta Neuropathol Commun 6(1) (2018) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mancini A, Tantucci M, Mazzocchetti P, de Iure A, Durante V, Macchioni L, Giampa C, Alvino A, Gaetani L, Costa C, Tozzi A, Calabresi P, Di Filippo M, Microglial activation and the nitric oxide/cGMP/PKG pathway underlie enhanced neuronal vulnerability to mitochondrial dysfunction in experimental multiple sclerosis, Neurobiol Dis 113 (2018) 97–108. [DOI] [PubMed] [Google Scholar]
- [128].Marzan DE, Brugger-Verdon V, West BL, Liddelow S, Samanta J, Salzer JL, Activated microglia drive demyelination via CSF1R signaling, Glia 69(6) (2021) 1583–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kipp M, Clarner T, Dang J, Copray S, Beyer C, The cuprizone animal model: new insights into an old story, Acta Neuropathol 118(6) (2009) 723–36. [DOI] [PubMed] [Google Scholar]
- [130].Gushchina S, Pryce G, Yip PK, Wu D, Pallier P, Giovannoni G, Baker D, Bo X, Increased expression of colony-stimulating factor-1 in mouse spinal cord with experimental autoimmune encephalomyelitis correlates with microglial activation and neuronal loss, Glia 66(10) (2018) 2108–2125. [DOI] [PubMed] [Google Scholar]
- [131].Wlodarczyk A, Benmamar-Badel A, Cédile O, Jensen KN, Kramer I, Elsborg NB, Owens T, CSF1R Stimulation Promotes Increased Neuroprotection by CD11c+ Microglia in EAE, Front Cell Neurosci 12 (2018) 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Nissen JC, Thompson KK, West BL, Tsirka SE, Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery, Exp Neurol 307 (2018) 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Neddens J, Temmel M, Flunkert S, Kerschbaumer B, Hoeller C, Loeffler T, Niederkofler V, Daum G, Attems J, Hutter-Paier B, Phosphorylation of different tau sites during progression of Alzheimer’s disease, Acta Neuropathol Commun 6(1) (2018) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K, Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry, Acta Neuropathol 112(4) (2006) 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Walker DG, Tang TM, Lue LF, Studies on Colony Stimulating Factor Receptor-1 and Ligands Colony Stimulating Factor-1 and Interleukin-34 in Alzheimer's Disease Brains and Human Microglia, Front Aging Neurosci 9 (2017) 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Murphy GM Jr., Zhao F, Yang L, Cordell B, Expression of macrophage colony-stimulating factor receptor is increased in the AbetaPP(V717F) transgenic mouse model of Alzheimer’s disease, The American journal of pathology 157(3) (2000) 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Smith AM, Gibbons HM, Oldfield RL, Bergin PM, Mee EW, Curtis MA, Faull RL, Dragunow M, M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia, J Neuroinflammation 10 (2013) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Casali BT, MacPherson KP, Reed-Geaghan EG, Landreth GE, Microglia depletion rapidly and reversibly alters amyloid pathology by modification of plaque compaction and morphologies, Neurobiol Dis 142 (2020) 104956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sosna J, Philipp S, Albay R 3rd, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM, Glabe CG, Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease, Mol Neurodegener 13(1) (2018) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Son Y, Jeong YJ, Shin NR, Oh SJ, Nam KR, Choi HD, Choi JY, Lee HJ, Inhibition of Colony-Stimulating Factor 1 Receptor by PLX3397 Prevents Amyloid Beta Pathology and Rescues Dopaminergic Signaling in Aging 5xFAD Mice, Int J Mol Sci 21(15) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, Green KN, Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology, Brain 139(Pt 4) (2016) 1265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA, Zhang Y, Spevak W, Lin J, Phan NY, Habets G, Rymar A, Tsang G, Walters J, Nespi M, Singh P, Broome S, Ibrahim P, Zhang C, Bollag G, West BL, Green KN, Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model, Nat Commun 10(1) (2019) 3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Olmos-Alonso A, Schetters ST, Sri S, Askew K, Mancuso R, Vargas-Caballero M, Holscher C, Perry VH, Gomez-Nicola D, Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology, Brain 139(Pt 3) (2016) 891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, West BL, Green KN, Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice, J Neuroinflammation 12 (2015) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kugler S, Ikezu T, Depletion of microglia and inhibition of exosome synthesis halt tau propagation, Nat Neurosci 18(11) (2015) 1584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Bennett RE, Bryant A, Hu M, Robbins AB, Hopp SC, Hyman BT, Partial reduction of microglia does not affect tau pathology in aged mice, J Neuroinflammation 15(1) (2018) 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Shi Y, Manis M, Long J, Wang K, Sullivan PM, Remolina Serrano J, Hoyle R, Holtzman DM, Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model, J Exp Med 216(11) (2019) 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, Umpierre AD, Zhu J, Bosco DB, Dong H, Wu LJ, Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling, Nat Neurosci 22(11) (2019) 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lecours C, Bordeleau M, Cantin L, Parent M, Paolo TD, Tremblay ME, Microglial Implication in Parkinson’s Disease: Loss of Beneficial Physiological Roles or Gain of Inflammatory Functions?, Front Cell Neurosci 12 (2018) 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Neal ML, Fleming SM, Budge KM, Boyle AM, Kim C, Alam G, Beier EE, Wu LJ, Richardson JR, Pharmacological inhibition of CSF1R by GW2580 reduces microglial proliferation and is protective against neuroinflammation and dopaminergic neurodegeneration, Faseb j 34(1) (2020) 1679–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Oh SJ, Ahn H, Jung KH, Han SJ, Nam KR, Kang KJ, Park JA, Lee KC, Lee YJ, Choi JY, Evaluation of the Neuroprotective Effect of Microglial Depletion by CSF-1R Inhibition in a Parkinson’s Animal Model, Mol Imaging Biol 22(4) (2020) 1031–1042. [DOI] [PubMed] [Google Scholar]
- [152].Yang X, Ren H, Wood K, Li M, Qiu S, Shi FD, Ma C, Liu Q, Depletion of microglia augments the dopaminergic neurotoxicity of MPTP, Faseb j 32(6) (2018) 3336–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A, The Role of Microglia and Astrocytes in Huntington’s Disease, Front Mol Neurosci 12 (2019) 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Politis M, Lahiri N, Niccolini F, Su P, Wu K, Giannetti P, Scahill RI, Turkheimer FE, Tabrizi SJ, Piccini P, Increased central microglial activation associated with peripheral cytokine levels in premanifest Huntington’s disease gene carriers, Neurobiol Dis 83 (2015) 115–21. [DOI] [PubMed] [Google Scholar]
- [155].Crapser JD, Ochaba J, Soni N, Reidling JC, Thompson LM, Green KN, Microglial depletion prevents extracellular matrix changes and striatal volume reduction in a model of Huntington’s disease, Brain 143(1) (2020) 266–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Beers DR, Appel SH, Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies, Lancet Neurol 18(2) (2019) 211–220. [DOI] [PubMed] [Google Scholar]
- [157].Gargiulo S, Anzilotti S, Coda AR, Gramanzini M, Greco A, Panico M, Vinciguerra A, Zannetti A, Vicidomini C, Dolle F, Pignataro G, Quarantelli M, Annunziato L, Brunetti A, Salvatore M, Pappata S, Imaging of brain TSPO expression in a mouse model of amyotrophic lateral sclerosis with (18)F-DPA-714 and micro-PET/CT, Eur J Nucl Med Mol Imaging 43(7) (2016) 1348–59. [DOI] [PubMed] [Google Scholar]
- [158].Trias E, Kovacs M, King PH, Si Y, Kwon Y, Varela V, Ibarburu S, Moura IC, Hermine O, Beckman JS, Barbeito L, Schwann cells orchestrate peripheral nerve inflammation through the expression of CSF1, IL-34, and SCF in amyotrophic lateral sclerosis, Glia 68(6) (2020) 1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Gowing G, Lalancette-Hebert M, Audet JN, Dequen F, Julien JP, Macrophage colony stimulating factor (M-CSF) exacerbates ALS disease in a mouse model through altered responses of microglia expressing mutant superoxide dismutase, Exp Neurol 220(2) (2009) 267–75. [DOI] [PubMed] [Google Scholar]
- [160].Martinez-Muriana A, Mancuso R, Francos-Quijorna I, Olmos-Alonso A, Osta R, Perry VH, Navarro X, Gomez-Nicola D, Lopez-Vales R, CSF1R blockade slows the progression of amyotrophic lateral sclerosis by reducing microgliosis and invasion of macrophages into peripheral nerves, Sci Rep 6 (2016) 25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Chen J, Chen C, Hu C, Liu L, Xia Y, Wang L, Yang W, Wu HY, Zhou W, Xiao K, Shi Q, Wu Y, Chen ZB, Dong XP, IP10, KC and M-CSF Are Remarkably Increased in the Brains from the Various Strains of Experimental Mice Infected with Different Scrapie Agents, Virol Sin 35(5) (2020) 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Obst J, Simon E, Martin-Estebane M, Pipi E, Barkwill LM, Gonzalez-Rivera I, Buchanan F, Prescott AR, Faust D, Fox S, Brownlees J, Taylor D, Perry VH, Nuthall H, Atkinson PJ, Karran E, Routledge C, Gomez-Nicola D, Inhibition of IL-34 Unveils Tissue-Selectivity and Is Sufficient to Reduce Microglial Proliferation in a Model of Chronic Neurodegeneration, Front Immunol 11 (2020) 579000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Striebel JF, Race B, Williams K, Carroll JA, Klingeborn M, Chesebro B, Microglia are not required for prion-induced retinal photoreceptor degeneration, Acta Neuropathol Commun 7(1) (2019) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Carroll JA, Race B, Williams K, Striebel J, Chesebro B, Microglia Are Critical in Host Defense Against Prion Disease, J Virol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Janova H, Arinrad S, Balmuth E, Mitjans M, Hertel J, Habes M, Bittner RA, Pan H, Goebbels S, Begemann M, Gerwig UC, Langner S, Werner HB, Kittel-Schneider S, Homuth G, Davatzikos C, Volzke H, West BL, Reif A, Grabe HJ, Boretius S, Ehrenreich H, Nave KA, Microglia ablation alleviates myelin-associated catatonic signs in mice, J Clin Invest 128(2) (2018) 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Garcia-Agudo LF, Janova H, Sendler LE, Arinrad S, Steixner AA, Hassouna I, Balmuth E, Ronnenberg A, Schopf N, van der Flier FJ, Begemann M, Martens H, Weber MS, Boretius S, Nave KA, Ehrenreich H, Genetically induced brain inflammation by Cnp deletion transiently benefits from microglia depletion, FASEB J 33(7) (2019) 8634–8647. [DOI] [PubMed] [Google Scholar]
- [167].Zhang J, Chang L, Pu Y, Hashimoto K, Abnormal expression of colony stimulating factor 1 receptor (CSF1R) and transcription factor PU.1 (SPI1) in the spleen from patients with major psychiatric disorders: A role of brain-spleen axis, J Affect Disord 272 (2020) 110–115. [DOI] [PubMed] [Google Scholar]
- [168].Wohleb ES, Terwilliger R, Duman CH, Duman RS, Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior, Biol Psychiatry 83(1) (2018) 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Ji J, Xiang H, Lu X, Tan P, Yang R, Ye T, Chen Z, Chen D, He H, Chen J, Ma Y, Huang C, A prophylactic effect of macrophage-colony stimulating factor on chronic stress-induced depression-like behaviors in mice, Neuropharmacology 193 (2021) 108621. [DOI] [PubMed] [Google Scholar]
- [170].Ye T, Wang D, Cai Z, Tong L, Chen Z, Lu J, Lu X, Huang C, Yuan X, Antidepressive properties of macrophage-colony stimulating factor in a mouse model of depression induced by chronic unpredictable stress, Neuropharmacology 172 (2020) 108132. [DOI] [PubMed] [Google Scholar]
- [171].Gong Y, Tong L, Yang R, Hu W, Xu X, Wang W, Wang P, Lu X, Gao M, Wu Y, Xu X, Zhang Y, Chen Z, Huang C, Dynamic changes in hippocampal microglia contribute to depressive-like behavior induced by early social isolation, Neuropharmacology 135 (2018) 223–233. [DOI] [PubMed] [Google Scholar]
- [172].Tong L, Gong Y, Wang P, Hu W, Wang J, Chen Z, Zhang W, Huang C, Microglia Loss Contributes to the Development of Major Depression Induced by Different Types of Chronic Stresses, Neurochemical research 42(10) (2017) 2698–2711. [DOI] [PubMed] [Google Scholar]
- [173].Bai Q, Xue M, Yong VW, Microglia and macrophage phenotypes in intracerebral haemorrhage injury: therapeutic opportunities, Brain 143(5) (2020) 1297–1314. [DOI] [PubMed] [Google Scholar]
- [174].Huang X, Li F, Yang T, Li H, Liu T, Wang Y, Xu M, Yan L, Zhang Y, Wang Y, Fu L, Geng D, Increased serum interleukin-34 levels as a novel diagnostic and prognostic biomarker in patients with acute ischemic stroke, J Neuroimmunol 358 (2021) 577652. [DOI] [PubMed] [Google Scholar]
- [175].Barca C, Kiliaan AJ, Foray C, Wachsmuth L, Hermann S, Faber C, Schaefers M, Wiesmann M, Jacobs AH, Zinnhardt B, A longitudinal PET/MR imaging study of colony stimulating factor-1 receptor-mediated microglia depletion in experimental stroke, J Nucl Med (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Marino Lee S, Hudobenko J, McCullough LD, Chauhan A, Microglia depletion increase brain injury after acute ischemic stroke in aged mice, Exp Neurol 336 (2021) 113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hou B, Jiang C, Wang D, Wang G, Wang Z, Zhu M, Kang Y, Su J, Wei P, Ren H, Ju F, Pharmacological Targeting of CSF1R Inhibits Microglial Proliferation and Aggravates the Progression of Cerebral Ischemic Pathology, Front Cell Neurosci 14 (2020) 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Otxoa-de-Amezaga A, Miro-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, Ruiz-Jaen F, Salas-Perdomo A, Bosch A, Calvo M, Marquez-Kisinousky L, Denes A, Gunzer M, Planas AM, Microglial cell loss after ischemic stroke favors brain neutrophil accumulation, Acta Neuropathol 137(2) (2019) 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Jin WN, Shi SX, Li Z, Li M, Wood K, Gonzales RJ, Liu Q, Depletion of microglia exacerbates postischemic inflammation and brain injury, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37(6) (2017) 2224–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Malcangio M, Role of the immune system in neuropathic pain, Scand J Pain 20(1) (2019) 33–37. [DOI] [PubMed] [Google Scholar]
- [181].Yu X, Basbaum A, Guan Z, Contribution of colony-stimulating factor 1 to neuropathic pain, Pain Rep 6(1) (2021) e883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI, Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain, Nat Neurosci 19(1) (2016) 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Okubo M, Yamanaka H, Kobayashi K, Dai Y, Kanda H, Yagi H, Noguchi K, Macrophage-Colony Stimulating Factor Derived from Injured Primary Afferent Induces Proliferation of Spinal Microglia and Neuropathic Pain in Rats, PLoS One 11(4) (2016) e0153375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Boakye PA, Rancic V, Whitlock KH, Simmons D, Longo FM, Ballanyi K, Smith PA, Receptor-dependence of BDNF Actions in Superficial Dorsal Horn; Relation to Central Sensitization and Actions of Macrophage Colony Stimulating Factor 1 (CSF-1), J Neurophysiol (2019). [DOI] [PubMed] [Google Scholar]
- [185].Lee S, Shi XQ, Fan A, West B, Zhang J, Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain, Molecular pain 14 (2018) 1744806918764979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Tang Y, Liu L, Xu D, Zhang W, Zhang Y, Zhou J, Huang W, Interaction between astrocytic colony stimulating factor and its receptor on microglia mediates central sensitization and behavioral hypersensitivity in chronic post ischemic pain model, Brain Behav Immun 68 (2018) 248–260. [DOI] [PubMed] [Google Scholar]
- [187].Yang G, Chen L, Gao Z, Wang Y, Implication of microglia activation and CSF-1/CSF-1Rpathway in lumbar disc degeneration-related back pain, Molecular pain 14 (2018) 1744806918811238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sawicki CM, Kim JK, Weber MD, Faw TD, McKim DB, Madalena KM, Lerch JK, Basso DM, Humeidan ML, Godbout JP, Sheridan JF, Microglia Promote Increased Pain Behavior through Enhanced Inflammation in the Spinal Cord during Repeated Social Defeat Stress, J Neurosci 39(7) (2019) 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Feng L, Murugan M, Bosco DB, Liu Y, Peng J, Worrell GA, Wang HL, Ta LE, Richardson JR, Shen Y, Wu LJ, Microglial proliferation and monocyte infiltration contribute to microgliosis following status epilepticus, Glia 67(8) (2019) 1434–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Srivastava PK, van Eyll J, Godard P, Mazzuferi M, Delahaye-Duriez A, Van Steenwinckel J, Gressens P, Danis B, Vandenplas C, Foerch P, Leclercq K, Mairet-Coello G, Cardenas A, Vanclef F, Laaniste L, Niespodziany I, Keaney J, Gasser J, Gillet G, Shkura K, Chong SA, Behmoaras J, Kadiu I, Petretto E, Kaminski RM, Johnson MR, A systems-level framework for drug discovery identifies Csf1R as an anti-epileptic drug target, Nat Commun 9(1) (2018) 3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Liu M, Jiang L, Wen M, Ke Y, Tong X, Huang W, Chen R, Microglia depletion exacerbates acute seizures and hippocampal neuronal degeneration in mouse models of epilepsy, Am J Physiol Cell Physiol 319(3) (2020) C605–c610. [DOI] [PubMed] [Google Scholar]
- [192].Wu W, Li Y, Wei Y, Bosco DB, Xie M, Zhao MG, Richardson JR, Wu LJ, Microglial depletion aggravates the severity of acute and chronic seizures in mice, Brain Behav Immun 89 (2020) 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Cassetta L, Pollard JW, Targeting macrophages: therapeutic approaches in cancer, Nat Rev Drug Discov 17(12) (2018) 887–904. [DOI] [PubMed] [Google Scholar]
- [194].Alterman RL, Stanley ER, Colony stimulating factor-1 expression in human glioma, Mol Chem Neuropathol 21(2–3) (1994) 177–188. [DOI] [PubMed] [Google Scholar]
- [195].Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA, CSF-1R inhibition alters macrophage polarization and blocks glioma progression, Nat Med 19(10) (2013) 1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Feng X, Liu S, Chen D, Rosi S, Gupta N, Rescue of cognitive function following fractionated brain irradiation in a novel preclinical glioma model, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, Holland EC, Sutton JC, Joyce JA, The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas, Science 352(6288) (2016) aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Akkari L, Bowman RL, Tessier J, Klemm F, Handgraaf SM, de Groot M, Quail DF, Tillard L, Gadiot J, Huse JT, Brandsma D, Westerga J, Watts C, Joyce JA, Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance, Sci Transl Med 12(552) (2020). [DOI] [PubMed] [Google Scholar]
- [199].Kadar E, Sureda A, Mangues MA, Ingles-Esteve J, Valls A, Garcia J, Serum levels of G-CSF, IL-3, IL-6 and GM-CSF after a single intraperitoneal dose of rhG-CSF in lethally irradiated B6D2F1 mice, Acta Haematol 98(3) (1997) 119–24. [DOI] [PubMed] [Google Scholar]
- [200].Dame JB, Christensen RD, Juul SE, The distribution of granulocyte-macrophage colony-stimulating factor and its receptor in the developing human fetus, Pediatr Res 46(4) (1999) 358–66. [DOI] [PubMed] [Google Scholar]
- [201].O’Hare FM, Watson RW, O’Neill A, Segurado R, Sweetman D, Downey P, Mooney E, Murphy J, Donoghue V, Molloy EJ, Serial cytokine alterations and abnormal neuroimaging in newborn infants with encephalopathy, Acta Paediatr 106(4) (2017) 561–567. [DOI] [PubMed] [Google Scholar]
- [202].Lee KY, Suh BG, Kim JW, Lee W, Kim SY, Kim YY, Lee J, Lim J, Kim M, Kang CS, Han K, Varying expression levels of colony stimulating factor receptors in disease states and different leukocytes, Exp Mol Med 32(4) (2000) 210–5. [DOI] [PubMed] [Google Scholar]
- [203].Frydecka D, Krzystek-Korpacka M, Lubeiro A, Stramecki F, Stanczykiewicz B, Beszlej JA, Piotrowski P, Kotowicz K, Szewczuk-Boguslawska M, Pawlak-Adamska E, Misiak B, Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study, Brain Behav Immun (2018). [DOI] [PubMed] [Google Scholar]
- [204].Tarkowski E, Wallin A, Regland B, Blennow K, Tarkowski A, Local and systemic GM-CSF increase in Alzheimer’s disease and vascular dementia, Acta Neurol Scand 103(3) (2001) 166–74. [DOI] [PubMed] [Google Scholar]
- [205].Tarkowski E, Andreasen N, Tarkowski A, Blennow K, Intrathecal inflammation precedes development of Alzheimer’s disease, J Neurol Neurosurg Psychiatry 74(9) (2003) 1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Niesman IR, Schilling JM, Shapiro LA, Kellerhals SE, Bonds JA, Kleschevnikov AM, Cui W, Voong A, Krajewski S, Ali SS, Roth DM, Patel HH, Patel PM, Head BP, Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice, J Neuroinflammation 11 (2014) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN, GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis, J Immunol 178(1) (2007) 39–48. [DOI] [PubMed] [Google Scholar]
- [208].Esnault S, Malter JS, Minute quantities of granulocyte-macrophage colony-stimulating factor prolong eosinophil survival, J Interferon Cytokine Res 21(2) (2001) 117–24. [DOI] [PubMed] [Google Scholar]
- [209].Krieger M, Both M, Kranig SA, Pitzer C, Klugmann M, Vogt G, Draguhn A, Schneider A, The hematopoietic cytokine granulocyte-macrophage colony stimulating factor is important for cognitive functions, Sci Rep 2 (2012) 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, Seeley RJ, GM-CSF action in the CNS decreases food intake and body weight, J Clin Invest 115(11) (2005) 3035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Kimura M, Kodama T, Aguila MC, Zhang SQ, Inoue S, Granulocyte-macrophage colony-stimulating factor modulates rapid eye movement (REM) sleep and non-REM sleep in rats, J Neurosci 20(14) (2000) 5544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Zylinska K, Komorowski J, Robak T, Mucha S, Stepien H, Effect of granulocyte-macrophage colony stimulating factor and granulocyte colony stimulating factor on melatonin secretion in rats in vivo and in vitro studies, J Neuroimmunol 56(2) (1995) 187–90. [DOI] [PubMed] [Google Scholar]
- [213].Kimura M, Inoue S, Involvement of granulocyte-macrophage colony-stimulating factor (GM-CSF) in pregnancy-enhanced sleep, Psychiatry Clin Neurosci 56(3) (2002) 337–8. [DOI] [PubMed] [Google Scholar]
- [214].Matsumoto A, Hatta T, Ono A, Hashimoto R, Otani H, Stage-specific changes in the levels of granulocyte-macrophage colony-stimulating factor and its receptor in the biological fluid and organ of mouse fetuses, Congenit Anom (Kyoto) 51(4) (2011) 183–6. [DOI] [PubMed] [Google Scholar]
- [215].Sweetman DU, Strickland T, Melo AM, Kelly LA, Onwuneme C, Watson WR, Murphy JFA, Slevin M, Donoghue V, O’Neill A, Molloy EJ, Neonatal Encephalopathy Is Associated With Altered IL-8 and GM-CSF Which Correlates With Outcomes, Front Pediatr 8 (2020) 556216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Gregor H, Egarter C, Levin D, Sternberger B, Heinze G, Leitich H, Reisenberger K, The passage of granulocyte-macrophage colony-stimulating factor across the human placenta perfused in vitro, J Soc Gynecol Investig 6(6) (1999) 307–10. [DOI] [PubMed] [Google Scholar]
- [217].Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, DeLorenze GN, Kharrazi M, Yolken R, Ashwood P, Van de Water J, Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation, Mol Psychiatry 22(2) (2017) 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M, Elevated immune response in the brain of autistic patients, J Neuroimmunol 207(1–2) (2009) 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Sharma R, Rosenberg A, Bennett ER, Laskowitz DT, Acheson SK, A blood-based biomarker panel to risk-stratify mild traumatic brain injury, PLoS One 12(3) (2017) e0173798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Paintlia AS, Gilg AG, Khan M, Singh AK, Barbosa E, Singh I, Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X-ALD: implications for potential therapies, Neurobiol Dis 14(3) (2003) 425–39. [DOI] [PubMed] [Google Scholar]
- [221].Ferrer I, Aubourg P, Pujol A, General aspects and neuropathology of X-linked adrenoleukodystrophy, Brain Pathol 20(4) (2010) 817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Kostic M, Zivkovic N, Cvetanovic A, Stojanovic I, Granulocyte-macrophage colony-stimulating factor as a mediator of autoimmunity in multiple sclerosis, J Neuroimmunol 323 (2018) 1–9. [DOI] [PubMed] [Google Scholar]
- [223].Yamasaki R, Yamaguchi H, Matsushita T, Fujii T, Hiwatashi A, Kira JI, Early strong intrathecal inflammation in cerebellar type multiple system atrophy by cerebrospinal fluid cytokine/chemokine profiles: a case control study, J Neuroinflammation 14(1) (2017) 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Choi SS, Lee HJ, Lim I, Satoh J, Kim SU, Human astrocytes: secretome profiles of cytokines and chemokines, PLoS One 9(4) (2014) e92325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Spampinato SF, Obermeier B, Cotleur A, Love A, Takeshita Y, Sano Y, Kanda T, Ransohoff RM, Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli?, PLoS One 10(7) (2015) e0133392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Hart MN, Fabry Z, Love-Homan L, Keiner J, Sadewasser KL, Moore SA, Brain microvascular smooth muscle and endothelial cells produce granulocyte macrophage colony-stimulating factor and support colony formation of granulocyte-macrophage-like cells, Am J Pathol 141(2) (1992) 421–7. [PMC free article] [PubMed] [Google Scholar]
- [227].Dame JB, Chegini N, Christensen RD, Juul SE, The effect of interleukin-1beta (IL-1beta) and tumor necrosis factor-alpha (TNF-alpha) on granulocyte macrophage-colony stimulating factor (GM-CSF) production by neuronal precursor cells, Eur Cytokine Netw 13(1) (2002) 128–33. [PubMed] [Google Scholar]
- [228].Malipiero UV, Frei K, Fontana A, Production of hemopoietic colony-stimulating factors by astrocytes, J.Immunol 144 (1990) 3816–3821. [PubMed] [Google Scholar]
- [229].Aloisi F, Carè A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C, Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1b and tumor necrosis factor-a, J.Immunol 149 (1992) 2358–2366. [PubMed] [Google Scholar]
- [230].Spampinato MV, Castillo M, Rojas R, Palacios E, Frascheri L, Descartes F, Magnetic resonance imaging findings in substance abuse: alcohol and alcoholism and syndromes associated with alcohol abuse, Top Magn Reson Imaging 16(3) (2005) 223–30. [DOI] [PubMed] [Google Scholar]
- [231].Sheikh AM, Nagai A, Ryu JK, McLarnon JG, Kim SU, Masuda J, Lysophosphatidylcholine induces glial cell activation: role of rho kinase, Glia 57(8) (2009) 898–907. [DOI] [PubMed] [Google Scholar]
- [232].Banks WA, Kovac A, Morofuji Y, Neurovascular unit crosstalk: Pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 38(6) (2018) 1104–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Smyth LCD, Rustenhoven J, Park TI, Schweder P, Jansson D, Heppner PA, O’Carroll SJ, Mee EW, Faull RLM, Curtis M, Dragunow M, Unique and shared inflammatory profiles of human brain endothelia and pericytes, J Neuroinflammation 15(1) (2018) 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Kovac A, Erickson MA, Banks WA, Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide, J Neuroinflammation 8 (2011) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, Huang JY, Zhao XJ, Sun XL, Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway, Aging Cell (2018) e12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Suzuki Y, Funakoshi H, Machide M, Matsumoto K, Nakamura T, Regulation of cell migration and cytokine production by HGF-like protein (HLP) / macrophage stimulating protein (MSP) in primary microglia, Biomed Res 29(2) (2008) 77–84. [DOI] [PubMed] [Google Scholar]
- [237].Henze C, Hartmann A, Lescot T, Hirsch EC, Michel PP, Proliferation of microglial cells induced by 1-methyl-4-phenylpyridinium in mesencephalic cultures results from an astrocyte-dependent mechanism: role of granulocyte macrophage colony-stimulating factor, J Neurochem 95(4) (2005) 1069–77. [DOI] [PubMed] [Google Scholar]
- [238].Giulian D, Ingeman JE, Colony-stimulating factors as promoters of ameboid microglia, J.Neurosci 8 (1988) 4707–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Suzumura A, Marunouchi T, Yamamoto H, Morphological transformation of microglia in vitro, Brain Res 545(1–2) (1991) 301–6. [DOI] [PubMed] [Google Scholar]
- [240].Ganter S, Northoff H, Mannel D, Gebicke-Harter PJ, Growth control of cultured microglia, J Neurosci Res 33(2) (1992) 218–30. [DOI] [PubMed] [Google Scholar]
- [241].Ringheim GE, Mitogenic effects of interleukin-5 on microglia, Neurosci Lett 201(2) (1995) 131–4. [DOI] [PubMed] [Google Scholar]
- [242].Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N, Glucocorticoid- and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids, Glia 20(1) (1997) 23–37. [PubMed] [Google Scholar]
- [243].Smith ME, Phagocytosis of myelin by microglia in vitro, J Neurosci Res 35(5) (1993) 480–7. [DOI] [PubMed] [Google Scholar]
- [244].Fischer HG, Nitzgen B, Germann T, Degitz K, Daubener W, Hadding U, Differentiation driven by granulocyte-macrophage colony-stimulating factor endows microglia with interferon-gamma-independent antigen presentation function, J Neuroimmunol 42(1) (1993) 87–95. [DOI] [PubMed] [Google Scholar]
- [245].Re F, Belyanskaya SL, Riese RJ, Cipriani B, Fischer FR, Granucci F, Ricciardi-Castagnoli P, Brosnan C, Stern LJ, Strominger JL, Santambrogio L, Granulocyte-macrophage colony-stimulating factor induces an expression program in neonatal microglia that primes them for antigen presentation, J Immunol 169(5) (2002) 2264–73. [DOI] [PubMed] [Google Scholar]
- [246].Dikmen HO, Hemmerich M, Lewen A, Hollnagel JO, Chausse B, Kann O, GM-CSF induces noninflammatory proliferation of microglia and disturbs electrical neuronal network rhythms in situ, J Neuroinflammation 17(1) (2020) 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Suh HS, Kim MO, Lee SC, Inhibition of granulocyte-macrophage colony-stimulating factor signaling and microglial proliferation by anti-CD45RO: role of Hck tyrosine kinase and phosphatidylinositol 3-kinase/Akt, J Immunol 174(5) (2005) 2712–9. [DOI] [PubMed] [Google Scholar]
- [248].Flanary BE, Streit WJ, Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes, Glia 45(1) (2004) 75–88. [DOI] [PubMed] [Google Scholar]
- [249].Horiuchi K, Miyamoto T, Takaishi H, Hakozaki A, Kosaki N, Miyauchi Y, Furukawa M, Takito J, Kaneko H, Matsuzaki K, Morioka H, Blobel CP, Toyama Y, Cell surface colony-stimulating factor 1 can be cleaved by TNF-alpha converting enzyme or endocytosed in a clathrin-dependent manner, Journal of Immunology 179(10) (2007) 6715–6724. [DOI] [PubMed] [Google Scholar]
- [250].Minten C, Terry R, Deffrasnes C, King NJ, Campbell IL, IFN regulatory factor 8 is a key constitutive determinant of the morphological and molecular properties of microglia in the CNS, PLoS One 7(11) (2012) e49851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R, Developmental plasticity of CNS microglia, Proc Natl Acad Sci U S A 98(11) (2001) 6295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Inaba K, Steinman RM, Pack MW, Aya H, Inaba M, Sudo T, Wolpe S, Schuler G, Identification of proliferating dendritic cell precursors in mouse blood, J Exp Med 175(5) (1992) 1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Beauvillain C, Donnou S, Jarry U, Scotet M, Gascan H, Delneste Y, Guermonprez P, Jeannin P, Couez D, Neonatal and adult microglia cross-present exogenous antigens, Glia 56(1) (2008) 69–77. [DOI] [PubMed] [Google Scholar]
- [254].Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC, Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo, J Exp Med 194(6) (2001) 769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA, CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self, Mol Immunol 46(6) (2009) 1229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Rezaie P, Corbisiero V, Male D, Transient expression of MIDC-8 in the normal mouse brain, Neurosci Lett 377(3) (2005) 189–94. [DOI] [PubMed] [Google Scholar]
- [257].Quan Y, Moller T, Weinstein JR, Regulation of Fcgamma receptors and immunoglobulin G-mediated phagocytosis in mouse microglia, Neurosci Lett 464(1) (2009) 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Kobashi S, Terashima T, Katagi M, Nakae Y, Okano J, Suzuki Y, Urushitani M, Kojima H, Transplantation of M2-Deviated Microglia Promotes Recovery of Motor Function after Spinal Cord Injury in Mice, Mol Ther 28(1) (2020) 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Tambuyzer BR, Nouwen EJ, Inhibition of microglia multinucleated giant cell formation and induction of differentiation by GM-CSF using a porcine in vitro model, Cytokine 31(4) (2005) 270–9. [DOI] [PubMed] [Google Scholar]
- [260].Duport S, Garthwaite J, Pathological consequences of inducible nitric oxide synthase expression in hippocampal slice cultures, Neuroscience 135(4) (2005) 1155–1166. [DOI] [PubMed] [Google Scholar]
- [261].Fischer HG, Bielinsky AK, Nitzgen B, Daubener W, Hadding U, Functional dichotomy of mouse microglia developed in vitro: differential effects of macrophage and granulocyte/macrophage colony-stimulating factor on cytokine secretion and antitoxoplasmic activity, J Neuroimmunol 45(1–2) (1993) 193–201. [DOI] [PubMed] [Google Scholar]
- [262].Feindt J, Schmidt A, Mentlein R, Receptors and effects of the inhibitory neuropeptide somatostatin in microglial cells, Brain Res Mol Brain Res 60(2) (1998) 228–33. [DOI] [PubMed] [Google Scholar]
- [263].Vos CM, van Haastert ES, de Groot CJ, van der Valk P, de Vries HE, Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions, J Neuroimmunol 138(1–2) (2003) 106–14. [DOI] [PubMed] [Google Scholar]
- [264].Hansmann F, Herder V, Kalkuhl A, Haist V, Zhang N, Schaudien D, Deschl U, Baumgartner W, Ulrich R, Matrix metalloproteinase-12 deficiency ameliorates the clinical course and demyelination in Theiler’s murine encephalomyelitis, Acta Neuropathol 124(1) (2012) 127–42. [DOI] [PubMed] [Google Scholar]
- [265].Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN, Lim R, A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines, J Neurochem 101(2) (2007) 364–76. [DOI] [PubMed] [Google Scholar]
- [266].Spath S, Komuczki J, Hermann M, Pelczar P, Mair F, Schreiner B, Becher B, Dysregulation of the Cytokine GM-CSF Induces Spontaneous Phagocyte Invasion and Immunopathology in the Central Nervous System, Immunity 46(2) (2017) 245–260. [DOI] [PubMed] [Google Scholar]
- [267].D’Andrea RJ, Harrison-Findik D, Butcher CM, Finnie J, Blumbergs P, Bartley P, McCormack M, Jones K, Rowland R, Gonda TJ, Vadas MA, Dysregulated hematopoiesis and a progressive neurological disorder induced by expression of an activated form of the human common beta chain in transgenic mice, J Clin Invest 102(11) (1998) 1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ, Cytokines in CAR T Cell-Associated Neurotoxicity, Front Immunol 11 (2020) 577027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Ishiguro M, Okada A, Asai K, Kojima K, Okada H, Stimulation of neuronal cells by culture supernatant of T lymphocytes triggered by anti-CD3 mAb followed by propagation in the presence of interleukin-2, Microbiology and immunology 60(1) (2016) 47–55. [DOI] [PubMed] [Google Scholar]
- [270].Choi JK, Kim KH, Park H, Park SR, Choi BH, Granulocyte macrophage-colony stimulating factor shows anti-apoptotic activity in neural progenitor cells via JAK/STAT5-Bcl-2 pathway, Apoptosis 16(2) (2011) 127–34. [DOI] [PubMed] [Google Scholar]
- [271].Kannan Y, Moriyama M, Sugano T, Yamate J, Kuwamura M, Kagaya A, Kiso Y, Neurotrophic action of interleukin 3 and granulocyte-macrophage colony-stimulating factor on murine sympathetic neurons, Neuroimmunomodulation 8(3) (2000) 132–41. [DOI] [PubMed] [Google Scholar]
- [272].Schallenberg M, Charalambous P, Thanos S, GM-CSF protects rat photoreceptors from death by activating the SRC-dependent signalling and elevating anti-apoptotic factors and neurotrophins, Graefes Arch Clin Exp Ophthalmol 250(5) (2012) 699–712. [DOI] [PubMed] [Google Scholar]
- [273].Choudhury ME, Sugimoto K, Kubo M, Nagai M, Nomoto M, Takahashi H, Yano H, Tanaka J, A cytokine mixture of GM-CSF and IL-3 that induces a neuroprotective phenotype of microglia leading to amelioration of (6-OHDA)-induced Parkinsonism of rats, Brain Behav 1(1) (2011) 26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Kamegai M, Konishi Y, Tabira T, Trophic effect of granulocyte-macrophage colony-stimulating factor on central cholinergic neurons in vitro, Brain Res. 532 (1990) 323–325. [DOI] [PubMed] [Google Scholar]
- [275].Bianchi M, Clavenna A, Bondiolotti GP, Ferrario P, Panerai AE, GM-CSF affects hypothalamic neurotransmitter levels in mice: involvement of interleukin-1, Neuroreport 8(16) (1997) 3587–90. [DOI] [PubMed] [Google Scholar]
- [276].Kano SI, Choi EY, Dohi E, Agarwal S, Chang DJ, Wilson AM, Lo BD, Rose IVL, Gonzalez S, Imai T, Sawa A, Glutathione S-transferases promote proinflammatory astrocyte-microglia communication during brain inflammation, Sci Signal 12(569) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Ong LK, Briggs GD, Guan L, Dunkley PR, Dickson PW, Peripheral inflammation induces long-term changes in tyrosine hydroxylase activation in the substantia nigra, Neurochemistry international 146 (2021) 105022. [DOI] [PubMed] [Google Scholar]
- [278].Jang H, Boltz D, McClaren J, Pani AK, Smeyne M, Korff A, Webster R, Smeyne RJ, Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice, J Neurosci 32(5) (2012) 1545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, Liew F, Russell CD, Moore SC, Fairfield C, Carter E, Abrams S, Short CE, Thaventhiran T, Bergstrom E, Gardener Z, Ascough S, Chiu C, Docherty AB, Hunt D, Crow YJ, Solomon T, Taylor GP, Turtle L, Harrison EM, Dunning J, Semple MG, Baillie JK, Openshaw PJ, I.C. investigators, Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19, Sci Immunol 6(57) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, DiBiase RM, Jia DT, Balabanov R, Ho SU, Batra A, Liotta EM, Koralnik IJ, Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”, Ann Clin Transl Neurol 8(5) (2021) 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Song E, Bartley CM, Chow RD, Ngo TT, Jiang R, Zamecnik CR, Dandekar R, Loudermilk RP, Dai Y, Liu F, Sunshine S, Liu J, Wu W, Hawes IA, Alvarenga BD, Huynh T, McAlpine L, Rahman NT, Geng B, Chiarella J, Goldman-Israelow B, Vogels CBF, Grubaugh ND, Casanovas-Massana A, Phinney BS, Salemi M, Alexander JR, Gallego JA, Lencz T, Walsh H, Wapniarski AE, Mohanty S, Lucas C, Klein J, Mao T, Oh J, Ring A, Spudich S, Ko AI, Kleinstein SH, Pak J, DeRisi JL, Iwasaki A, Pleasure SJ, Wilson MR, Farhadian SF, Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms, Cell Rep Med 2(5) (2021) 100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Heyner M, Schreier S, Kroger A, The brain-immune cells axis controls tissue specific immunopathology, Cellular & molecular immunology 16(2) (2019) 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Kimura M, Yu WH, Rettori V, McCann SM, Granulocyte-macrophage colony stimulating factor suppresses LHRH release by inhibition of nitric oxide synthase and stimulation of gamma-aminobutyric acid release, Neuroimmunomodulation 4(5–6) (1997) 237–43. [DOI] [PubMed] [Google Scholar]
- [284].Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, Nagler A, Rubinstein M, Lapidot T, Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling, Nat Immunol 8(10) (2007) 1123–31. [DOI] [PubMed] [Google Scholar]
- [285].Hartmann FJ, Khademi M, Aram J, Ammann S, Kockum I, Constantinescu C, Gran B, Piehl F, Olsson T, Codarri L, Becher B, Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells, Nat Commun 5 (2014) 5056. [DOI] [PubMed] [Google Scholar]
- [286].Noster R, Riedel R, Mashreghi MF, Radbruch H, Harms L, Haftmann C, Chang HD, Radbruch A, Zielinski CE, IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells, Sci Transl Med 6(241) (2014) 241ra80. [DOI] [PubMed] [Google Scholar]
- [287].Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, Mari ER, Safavi F, Leist TP, Zhang GX, Rostami A, Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-beta Therapy, J Immunol 194(11) (2015) 5085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Imitola J, Rasouli J, Watanabe F, Mahajan K, Sharan AD, Ciric B, Zhang GX, Rostami A, Elevated expression of granulocyte-macrophage colony-stimulating factor receptor in multiple sclerosis lesions, J Neuroimmunol 317 (2018) 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Vogel DY, Kooij G, Heijnen PD, Breur M, Peferoen LA, van der Valk P, de Vries HE, Amor S, Dijkstra CD, GM-CSF promotes migration of human monocytes across the blood brain barrier, Eur J Immunol 45(6) (2015) 1808–19. [DOI] [PubMed] [Google Scholar]
- [290].Simka M, Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis, Curr Neurovasc Res 6(2) (2009) 132–9. [DOI] [PubMed] [Google Scholar]
- [291].Donatien P, Anand U, Yiangou Y, Sinisi M, Fox M, MacQuillan A, Quick T, Korchev YE, Anand P, Granulocyte-macrophage colony-stimulating factor receptor expression in clinical pain disorder tissues and role in neuronal sensitization, Pain Rep 3(5) (2018) e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Constantinescu CS, Asher A, Fryze W, Kozubski W, Wagner F, Aram J, Tanasescu R, Korolkiewicz RP, Dirnberger-Hertweck M, Steidl S, Libretto SE, Sprenger T, Radue EW, Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis, Neurol Neuroimmunol Neuroinflamm 2(4) (2015) e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Kaneko K, Sato DK, Nakashima I, Ogawa R, Akaishi T, Takai Y, Nishiyama S, Takahashi T, Misu T, Kuroda H, Tanaka S, Nomura K, Hashimoto Y, Callegaro D, Steinman L, Fujihara K, Aoki M, CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: a cross-sectional study and potential therapeutic implications, J Neurol Neurosurg Psychiatry 89(9) (2018) 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Ignatius Arokia Doss PM, Roy AP, Wang A, Anderson AC, Rangachari M, The Non-Obese Diabetic Mouse Strain as a Model to Study CD8(+) T Cell Function in Relapsing and Progressive Multiple Sclerosis, Front Immunol 6 (2015) 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC, Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis, J Exp Med 194(7) (2001) 873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM, IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition, J Exp Med 205(7) (2008) 1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B, RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation, Nat Immunol 12(6) (2011) 560–7. [DOI] [PubMed] [Google Scholar]
- [298].Hesske L, Vincenzetti C, Heikenwalder M, Prinz M, Reith W, Fontana A, Suter T, Induction of inhibitory central nervous system-derived and stimulatory blood-derived dendritic cells suggests a dual role for granulocyte-macrophage colony-stimulating factor in central nervous system inflammation, Brain 133(Pt 6) (2010) 1637–54. [DOI] [PubMed] [Google Scholar]
- [299].Pare A, Mailhot B, Levesque SA, Lacroix S, Involvement of the IL-1 system in experimental autoimmune encephalomyelitis and multiple sclerosis: Breaking the vicious cycle between IL-1beta and GM-CSF, Brain Behav Immun (2016). [DOI] [PubMed] [Google Scholar]
- [300].Komuczki J, Tuzlak S, Friebel E, Hartwig T, Spath S, Rosenstiel P, Waisman A, Opitz L, Oukka M, Schreiner B, Pelczar P, Becher B, Fate-Mapping of GM-CSF Expression Identifies a Discrete Subset of Inflammation-Driving T Helper Cells Regulated by Cytokines IL-23 and IL-1beta, Immunity 50(5) (2019) 1289–1304 e6. [DOI] [PubMed] [Google Scholar]
- [301].Rasouli J, Casella G, Ishikawa LLW, Thome R, Boehm A, Ertel A, Melo-Silva CR, Mari ER, Porazzi P, Zhang W, Xiao D, Sigal LJ, Fortina P, Zhang GX, Rostami A, Ciric B, IFN-beta Acts on Monocytes to Ameliorate CNS Autoimmunity by Inhibiting Proinflammatory Cross-Talk Between Monocytes and Th Cells, Front Immunol 12 (2021) 679498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Russi AE, Walker-Caulfield ME, Guo Y, Lucchinetti CF, Brown MA, Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity, J Autoimmun 73 (2016) 100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A, The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF, Nat Immunol 12(6) (2011) 568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Yoshimura S, Thome R, Konno S, Mari ER, Rasouli J, Hwang D, Boehm A, Li Y, Zhang GX, Ciric B, Rostami A, IL-9 Controls Central Nervous System Autoimmunity by Suppressing GM-CSF Production, J Immunol 204(3) (2020) 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Ifergan I, Davidson TS, Kebir H, Xu D, Palacios-Macapagal D, Cann J, Rodgers JM, Hunter ZN, Pittet CL, Beddow S, Jones CA, Prat A, Sleeman MA, Miller SD, Targeting the GM-CSF receptor for the treatment of CNS autoimmunity, J Autoimmun 84 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, Becher B, The Cytokine GM-CSF Drives the Inflammatory Signature of CCR2+ Monocytes and Licenses Autoimmunity, Immunity 43(3) (2015) 502–14. [DOI] [PubMed] [Google Scholar]
- [307].Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V, Rotem A, Heyman JA, Thaploo S, Sanmarco LM, Ragoussis J, Weitz DA, Petrecca K, Moffitt JR, Becher B, Antel JP, Prat A, Quintana FJ, MAFG-driven astrocytes promote CNS inflammation, Nature 578(7796) (2020) 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Pierson ER, Goverman JM, GM-CSF is not essential for experimental autoimmune encephalomyelitis but promotes brain-targeted disease, JCI insight 2(7) (2017) e92362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Manczak M, Mao P, Nakamura K, Bebbington C, Park B, Reddy PH, Neutralization of granulocyte macrophage colony-stimulating factor decreases amyloid beta 1–42 and suppresses microglial activity in a transgenic mouse model of Alzheimer’s disease, Hum Mol Genet 18(20) (2009) 3876–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Shang S, Yang YM, Zhang H, Tian L, Jiang JS, Dong YB, Zhang K, Li B, Zhao WD, Fang WG, Chen YH, Intracerebral GM-CSF contributes to transendothelial monocyte migration in APP/PS1 Alzheimer’s disease mice, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Daria A, Colombo A, Llovera G, Hampel H, Willem M, Liesz A, Haass C, Tahirovic S, Young microglia restore amyloid plaque clearance of aged microglia, EMBO J 36(5) (2017) 583–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H, GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice, J Alzheimers Dis 21(2) (2010) 507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Jim HS, Boyd TD, Booth-Jones M, Pidala J, Potter H, Granulocyte Macrophage Colony Stimulating Factor Treatment is Associated with Improved Cognition in Cancer Patients, Brain Disord Ther 1(1) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Potter H, Woodcock JH, Boyd TD, Coughlan CM, O’Shaughnessy JR, Borges MT, Thaker AA, Raj BA, Adamszuk K, Scott D, Adame V, Anton P, Chial HJ, Gray H, Daniels J, Stocker ME, Sillau SH, Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease, Alzheimers Dement (N Y) 7(1) (2021) e12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Kim NK, Choi BH, Huang X, Snyder BJ, Bukhari S, Kong TH, Park H, Park HC, Park SR, Ha Y, Granulocyte-macrophage colony-stimulating factor promotes survival of dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced murine Parkinson’s disease model, Eur J Neurosci 29(5) (2009) 891–900. [DOI] [PubMed] [Google Scholar]
- [316].Mangano EN, Peters S, Litteljohn D, So R, Bethune C, Bobyn J, Clarke M, Hayley S, Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson’s disease, Neurobiol Dis 43(1) (2011) 99–112. [DOI] [PubMed] [Google Scholar]
- [317].Kosloski LM, Kosmacek EA, Olson KE, Mosley RL, Gendelman HE, GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice, J Neuroimmunol 265(1–2) (2013) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Olson KE, Namminga KL, Lu Y, Thurston MJ, Schwab AD, de Picciotto S, Tse SW, Walker W, Iacovelli J, Small C, Wipke BT, Mosley RL, Huang E, Gendelman HE, Granulocyte-macrophage colony-stimulating factor mRNA and Neuroprotective Immunity in Parkinson’s disease, Biomaterials 272 (2021) 120786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Olson KE, Namminga KL, Lu Y, Schwab AD, Thurston MJ, Abdelmoaty MM, Kumar V, Wojtkiewicz M, Obaro H, Santamaria P, Mosley RL, Gendelman HE, Safety, tolerability, and immune-biomarker profiling for year-long sargramostim treatment of Parkinson’s disease, EBioMedicine 67 (2021) 103380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Schmidt FM, Lichtblau N, Minkwitz J, Chittka T, Thormann J, Kirkby KC, Sander C, Mergl R, Fasshauer M, Stumvoll M, Holdt LM, Teupser D, Hegerl U, Himmerich H, Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity, J Psychiatr Res 55 (2014) 29–34. [DOI] [PubMed] [Google Scholar]
- [321].Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA Jr., Cordeiro Q, Belangero SI, Gadelha A, Bressan RA, Noto C, Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis, Eur Neuropsychopharmacol 29(3) (2019) 416–431. [DOI] [PubMed] [Google Scholar]
- [322].Bradley KA, Stern ER, Alonso CM, Xie H, Kim-Schulze S, Gabbay V, Relationships between neural activation during a reward task and peripheral cytokine levels in youth with diverse psychiatric symptoms, Brain Behav Immun 80 (2019) 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R, Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis, Mol Psychiatry 19(6) (2014) 699–709. [DOI] [PubMed] [Google Scholar]
- [324].Hemmati S, Sadeghi MA, Mohammad Jafari R, Yousefi-Manesh H, Dehpour AR, The antidepressant effects of GM-CSF are mediated by the reduction of TLR4/NF-kB-induced IDO expression, J Neuroinflammation 16(1) (2019) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Zhu F, Liu Y, Zhao J, Zheng Y, Minocycline alleviates behavioral deficits and inhibits microglial activation induced by intrahippocampal administration of Granulocyte-Macrophage Colony-Stimulating Factor in adult rats, Neuroscience 266 (2014) 275–81. [DOI] [PubMed] [Google Scholar]
- [326].Schneider UC, Schilling L, Schroeck H, Nebe CT, Vajkoczy P, Woitzik J, Granulocyte-macrophage colony-stimulating factor-induced vessel growth restores cerebral blood supply after bilateral carotid artery occlusion, Stroke 38(4) (2007) 1320–8. [DOI] [PubMed] [Google Scholar]
- [327].Todo K, Kitagawa K, Sasaki T, Omura-Matsuoka E, Terasaki Y, Oyama N, Yagita Y, Hori M, Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion, Stroke 39(6) (2008) 1875–82. [DOI] [PubMed] [Google Scholar]
- [328].Sugiyama Y, Yagita Y, Oyama N, Terasaki Y, Omura-Matsuoka E, Sasaki T, Kitagawa K, Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke, Stroke 42(3) (2011) 770–5. [DOI] [PubMed] [Google Scholar]
- [329].Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, Strazzer S, Bresolin N, Bersano A, Growth factors in ischemic stroke, J Cell Mol Med 15(8) (2011) 1645–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Theoret JK, Jadavji NM, Zhang M, Smith PD, Granulocyte macrophage colony-stimulating factor treatment results in recovery of motor function after white matter damage in mice, Eur J Neurosci 43(1) (2016) 17–24. [DOI] [PubMed] [Google Scholar]
- [331].Dames C, Winek K, Beckers Y, Engel O, Meisel A, Meisel C, Immunomodulatory treatment with systemic GM-CSF augments pulmonary immune responses and improves neurological outcome after experimental stroke, J Neuroimmunol 321 (2018) 144–149. [DOI] [PubMed] [Google Scholar]
- [332].Navarro-Sobrino M, Rosell A, Penalba A, Ribo M, Alvarez-Sabin J, Fernandez-Cadenas I, Montaner J, Role of endogenous granulocyte-macrophage colony stimulating factor following stroke and relationship to neurological outcome, Curr Neurovasc Res 6(4) (2009) 246–51. [DOI] [PubMed] [Google Scholar]
- [333].Raivich G, Gehrmann J, Kreutzberg GW, Increase of macrophage colony-stimulating factor and granulocyte- macrophage colony-stimulating factor receptors in the regenerating rat facial nucleus, J Neurosci Res 30(4) (1991) 682–686. [DOI] [PubMed] [Google Scholar]
- [334].Zhou H, Shi Z, Kang Y, Wang Y, Lu L, Pan B, Liu J, Li X, Liu L, Wei Z, Kong X, Feng S, Investigation of candidate long noncoding RNAs and messenger RNAs in the immediate phase of spinal cord injury based on gene expression profiles, Gene 661 (2018) 119–125. [DOI] [PubMed] [Google Scholar]
- [335].Schweizerhof M, Stosser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, Schmelz M, Bali KK, Michalski CW, Brugger S, Dickenson A, Simone DA, Kuner R, Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain, Nat Med 15(7) (2009) 802–7. [DOI] [PubMed] [Google Scholar]
- [336].Cook AD, Pobjoy J, Steidl S, Durr M, Braine EL, Turner AL, Lacey DC, Hamilton JA, Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development, Arthritis Res Ther 14(5) (2012) R199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Bali KK, Venkataramani V, Satagopam VP, Gupta P, Schneider R, Kuner R, Transcriptional mechanisms underlying sensitization of peripheral sensory neurons by granulocyte-/granulocyte-macrophage colony stimulating factors, Molecular pain 9 (2013) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Tewari D, Cook AD, Lee MC, Christensen AD, Croxford A, Becher B, Poole D, Rajasekhar P, Bunnett N, Smith JE, Hamilton JA, McMahon SB, Granulocyte-Macrophage Colony Stimulating Factor As an Indirect Mediator of Nociceptor Activation and Pain, J Neurosci 40(11) (2020) 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Hayashi K, Ohta S, Kawakami Y, Toda M, Activation of dendritic-like cells and neural stem/progenitor cells in injured spinal cord by GM-CSF, Neurosci Res 64(1) (2009) 96–103. [DOI] [PubMed] [Google Scholar]
- [340].Bombeiro AL, Pereira BTN, de Oliveira ALR, Granulocyte-macrophage colony-stimulating factor improves mouse peripheral nerve regeneration following sciatic nerve crush, Eur J Neurosci 48(5) (2018) 2152–2164. [DOI] [PubMed] [Google Scholar]
- [341].Giulian D, Li J, Li X, George J, Rutecki PA, The impact of microglia-derived cytokines upon gliosis in the CNS, Dev Neurosci 16(3–4) (1994) 128–36. [DOI] [PubMed] [Google Scholar]
- [342].Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J, Vinnakota K, Kettenmann H, Kotulska K, Grajkowska W, Kaminska B, Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response, J Pathol 230(3) (2013) 310–21. [DOI] [PubMed] [Google Scholar]
- [343].Revoltella RP, Menicagli M, Campani D, Granulocyte-macrophage colony-stimulating factor as an autocrine survival-growth factor in human gliomas, Cytokine 57(3) (2012) 347–59. [DOI] [PubMed] [Google Scholar]
- [344].Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B, Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas, PLoS One 6(8) (2011) e23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Ansari KI, Bhan A, Saotome M, Tyagi A, De Kumar B, Chen C, Takaku M, Jandial R, Autocrine GM-CSF signaling contributes to growth of HER2+ breast leptomeningeal carcinomatosis, Cancer Res (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Yap NY, Toh YL, Tan CJ, Acharya MM, Chan A, Relationship between cytokines and brain-derived neurotrophic factor (BDNF) in trajectories of cancer-related cognitive impairment, Cytokine 144 (2021) 155556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Ishiguro A, Inoue K, Nakahata T, Nishihira H, Kojima S, Ueda K, Suzuki Y, Shimbo T, Reference intervals for serum granulocyte colony-stimulating factor levels in children, J Pediatr 128(2) (1996) 208–12. [DOI] [PubMed] [Google Scholar]
- [348].Kiriyama R, Chichibu K, Matsuno T, Ohsawa N, Sensitive chemiluminescent immunoassay for human granulocyte colony-stimulating factor (G-CSF) in clinical applications, Clinica chimica acta; international journal of clinical chemistry 220(2) (1993) 201–9. [DOI] [PubMed] [Google Scholar]
- [349].Laske C, Stellos K, Stransky E, Leyhe T, Gawaz M, Decreased plasma levels of granulocyte-colony stimulating factor (G-CSF) in patients with early Alzheimer’s disease, J Alzheimers Dis 17(1) (2009) 115–23. [DOI] [PubMed] [Google Scholar]
- [350].Jin WN, Shi K, He W, Sun JH, Van Kaer L, Shi FD, Liu Q, Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition, Nat Neurosci 24(1) (2021) 61–73. [DOI] [PubMed] [Google Scholar]
- [351].Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D, The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment, Biomed Res Int 2015 (2015) 506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Fukushima K, Ishiguro A, Shimbo T, Transient elevation of granulocyte colony-stimulating factor levels in cerebrospinal fluid at the initial stage of aseptic meningitis in children, Pediatr Res 37(2) (1995) 160–4. [DOI] [PubMed] [Google Scholar]
- [353].Yoshio T, Okamoto H, Kurasawa K, Dei Y, Hirohata S, Minota S, IL-6, IL-8, IP-10, MCP-1 and G-CSF are significantly increased in cerebrospinal fluid but not in sera of patients with central neuropsychiatric lupus erythematosus, Lupus 25(9) (2016) 997–1003. [DOI] [PubMed] [Google Scholar]
- [354].Koeffler HP, Gasson J, Ranyard J, Souza L, Shepard M, Munker R, Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor, Blood 70 (1987) 55–59. [PubMed] [Google Scholar]
- [355].Sieff CA, Niemeyer CM, Mentzer SJ, Faller DV, Interleukin-1, tumor necrosis factor, and the production of colony-stimulating factors by cultured mesenchymal cells, Blood 72 (1988) 1316–1323. [PubMed] [Google Scholar]
- [356].Vellenga E, Rambaldi A, Ernst TJ, Ostapovicz D, Griffin JD, Independent regulation of M-CSF and G-CSF gene expression in human monocytes, Blood 71 (1988) 1529–1532. [PubMed] [Google Scholar]
- [357].Zsebo KM, Wypych J, Yuschenkoff VN, Lu H, Hunt P, Dukes PP, Langley KE, Effects of hematopoietin-1 and Interleukin-1 activities on early hematopoietic cells of the bone marrow, Blood 71 (1988) 962–968. [PubMed] [Google Scholar]
- [358].Tweardy DJ, Mott PL, Glazer EW, Monokine modulation of human astroglial cell production of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor.I.Effects of IL-1a and IL-b, J.Immunol 144 (1990) 2233–2241. [PubMed] [Google Scholar]
- [359].He Y, Taylor N, Yao X, Bhattacharya A, Mouse primary microglia respond differently to LPS and poly(I:C) in vitro, Sci Rep 11(1) (2021) 10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Thomas DM, Francescutti-Verbeem DM, Kuhn DM, Gene expression profile of activated microglia under conditions associated with dopamine neuronal damage, FASEB J 20(3) (2006) 515–7. [DOI] [PubMed] [Google Scholar]
- [361].Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA, Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain, Cell 174(4) (2018) 1015–1030 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA, Stevens B, Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes, Immunity 50(1) (2019) 253–271 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Ximerakis M, Lipnick SL, Innes BT, Simmons SK, Adiconis X, Dionne D, Mayweather BA, Nguyen L, Niziolek Z, Ozek C, Butty VL, Isserlin R, Buchanan SM, Levine SS, Regev A, Bader GD, Levin JZ, Rubin LL, Single-cell transcriptomic profiling of the aging mouse brain, Nat Neurosci 22(10) (2019) 1696–1708. [DOI] [PubMed] [Google Scholar]
- [364].Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, Mori T, Cao C, Arendash GW, Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice, Neuroscience 163(1) (2009) 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Chen CH, Huang SY, Chen NF, Feng CW, Hung HC, Sung CS, Jean YH, Wen ZH, Chen WF, Intrathecal granulocyte colony-stimulating factor modulate glial cell line-derived neurotrophic factor and vascular endothelial growth factor A expression in glial cells after experimental spinal cord ischemia, Neuroscience 242 (2013) 39–52. [DOI] [PubMed] [Google Scholar]
- [366].Basso L, Lapointe TK, Iftinca M, Marsters C, Hollenberg MD, Kurrasch DM, Altier C, Granulocyte-colony-stimulating factor (G-CSF) signaling in spinal microglia drives visceral sensitization following colitis, Proc Natl Acad Sci U S A 114(42) (2017) 11235–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Konishi Y, Chui DH, Hirose H, Kunishita T, Tabira T, Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo, Brain Res 609(1–2) (1993) 29–35. [DOI] [PubMed] [Google Scholar]
- [368].Komine-Kobayashi M, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, Mochizuki H, Mizuno Y, Urabe T, Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 26(3) (2006) 402–13. [DOI] [PubMed] [Google Scholar]
- [369].Diederich K, Sevimli S, Dorr H, Kosters E, Hoppen M, Lewejohann L, Klocke R, Minnerup J, Knecht S, Nikol S, Sachser N, Schneider A, Gorji A, Sommer C, Schabitz WR, The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain: a characterization of G-CSF-deficient mice, J Neurosci 29(37) (2009) 11572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Kutlu MG, Brady LJ, Peck EG, Hofford RS, Yorgason JT, Siciliano CA, Kiraly DD, Calipari ES, Granulocyte Colony Stimulating Factor Enhances Reward Learning through Potentiation of Mesolimbic Dopamine System Function, J Neurosci 38(41) (2018) 8845–8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Hofford RS, Euston TJ, Wilson RS, Meckel KR, Peck EG, Godino A, Landry JA, Calipari ES, Lam TT, Kiraly DD, Granulocyte-Colony Stimulating Factor Reduces Cocaine-Seeking and Downregulates Glutamatergic Synaptic Proteins in Medial Prefrontal Cortex, J Neurosci 41(7) (2021) 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Mucha S, Zylinska K, Pisarek H, Komorowski J, Robak T, Korycka A, Stepien H, Pituitary-adrenocortical responses to the chronic administration of granulocyte colony-stimulating factor in rats, J Neuroimmunol 102(1) (2000) 73–8. [DOI] [PubMed] [Google Scholar]
- [373].Rah WJ, Lee YH, Moon JH, Jun HJ, Kang HR, Koh H, Eom HJ, Lee JY, Lee YJ, Kim JY, Choi YY, Park K, Kim MJ, Kim SH, Neuroregenerative potential of intravenous G-CSF and autologous peripheral blood stem cells in children with cerebral palsy: a randomized, double-blind, cross-over study, J Transl Med 15(1) (2017) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Gumus H, Per H, Kumandas S, Yikilmaz A, Reversible posterior leukoencephalopathy syndrome in childhood: report of nine cases and review of the literature, Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 31(2) (2010) 125–31. [DOI] [PubMed] [Google Scholar]
- [375].Stubgen JP, Posterior reversible encephalopathy syndrome (PRES) after granulocyte-colony stimulating factor (G-CSF) therapy: a report of 2 cases, J Neurol Sci 321(1–2) (2012) 35–8. [DOI] [PubMed] [Google Scholar]
- [376].Okeda R, Kawamoto T, Tanaka E, Shimizu H, An autopsy case of drug-induced diffuse cerebral axonopathic leukoencephalopathy: the pathogenesis in relation to reversible posterior leukoencephalopathy syndrome, Neuropathology 27(4) (2007) 364–70. [DOI] [PubMed] [Google Scholar]
- [377].Deeren DH, Zachee P, Malbrain ML, Granulocyte colony-stimulating factor-induced capillary leak syndrome confirmed by extravascular lung water measurements, Ann Hematol 84(2) (2005) 89–94. [DOI] [PubMed] [Google Scholar]
- [378].Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L, Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis, Nat Med 8(5) (2002) 500–8. [DOI] [PubMed] [Google Scholar]
- [379].Openshaw H, Stuve O, Antel JP, Nash R, Lund BT, Weiner LP, Kashyap A, McSweeney P, Forman S, Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor, Neurology 54(11) (2000) 2147–50. [DOI] [PubMed] [Google Scholar]
- [380].Jacob A, Saadoun S, Kitley J, Leite M, Palace J, Schon F, Papadopoulos MC, Detrimental role of granulocyte-colony stimulating factor in neuromyelitis optica: clinical case and histological evidence, Mult Scler 18(12) (2012) 1801–3. [DOI] [PubMed] [Google Scholar]
- [381].Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM, Neutrophil-related factors as biomarkers in EAE and MS, J Exp Med 212(1) (2015) 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].McGinley AM, Sutton CE, Edwards SC, Leane CM, DeCourcey J, Teijeiro A, Hamilton JA, Boon L, Djouder N, Mills KHG, Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1beta-Producing Myeloid Cells that Promote Pathogenic T Cells, Immunity 52(2) (2020) 342–356 e6. [DOI] [PubMed] [Google Scholar]
- [383].Soulika AM, Lee E, McCauley E, Miers L, Bannerman P, Pleasure D, Initiation and progression of axonopathy in experimental autoimmune encephalomyelitis, J Neurosci 29(47) (2009) 14965–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Christy AL, Walker ME, Hessner MJ, Brown MA, Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE, J Autoimmun 42 (2013) 50–61. [DOI] [PubMed] [Google Scholar]
- [385].Verda L, Luo K, Kim DA, Bronesky D, Kohm AP, Miller SD, Statkute L, Oyama Y, Burt RK, Effect of hematopoietic growth factors on severity of experimental autoimmune encephalomyelitis, Bone Marrow Transplant 38(6) (2006) 453–60. [DOI] [PubMed] [Google Scholar]
- [386].Zavala F, Abad S, Ezine S, Taupin V, Masson A, Bach JF, G-CSF therapy of ongoing experimental allergic encephalomyelitis via chemokine- and cytokine-based immune deviation, J Immunol 168(4) (2002) 2011–9. [DOI] [PubMed] [Google Scholar]
- [387].Barber RC, Edwards MI, Xiao G, Huebinger RM, Diaz-Arrastia R, Wilhelmsen KC, Hall JR, O’Bryant SE, Serum granulocyte colony-stimulating factor and Alzheimer’s disease, Dement Geriatr Cogn Dis Extra 2(1) (2012) 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Taipa R, das Neves SP, Sousa AL, Fernandes J, Pinto C, Correia AP, Santos E, Pinto PS, Carneiro P, Costa P, Santos D, Alonso I, Palha J, Marques F, Cavaco S, Sousa N, Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline, Neurobiol Aging 76 (2019) 125–132. [DOI] [PubMed] [Google Scholar]
- [389].Tsai KJ, Tsai YC, Shen CK, G-CSF rescues the memory impairment of animal models of Alzheimer’s disease, J Exp Med 204(6) (2007) 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Jiang H, Liu CX, Feng JB, Wang P, Zhao CP, Xie ZH, Wang Y, Xu SL, Zheng CY, Bi JZ, Granulocyte colony-stimulating factor attenuates chronic neuroinflammation in the brain of amyloid precursor protein transgenic mice: an Alzheimer’s disease mouse model, J Int Med Res 38(4) (2010) 1305–12. [DOI] [PubMed] [Google Scholar]
- [391].Prakash A, Medhi B, Chopra K, Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-beta induced experimental model of Alzheimer’s disease, Pharmacol Biochem Behav 110 (2013) 46–57. [DOI] [PubMed] [Google Scholar]
- [392].Wu CC, Wang IF, Chiang PM, Wang LC, Shen CJ, Tsai KJ, G-CSF-mobilized Bone Marrow Mesenchymal Stem Cells Replenish Neural Lineages in Alzheimer’s Disease Mice via CXCR4/SDF-1 Chemotaxis, Mol Neurobiol 54(8) (2017) 6198–6212. [DOI] [PubMed] [Google Scholar]
- [393].Doi Y, Takeuchi H, Mizoguchi H, Fukumoto K, Horiuchi H, Jin S, Kawanokuchi J, Parajuli B, Sonobe Y, Mizuno T, Suzumura A, Granulocyte-colony stimulating factor attenuates oligomeric amyloid beta neurotoxicity by activation of neprilysin, PLoS One 9(7) (2014) e103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Li B, Gonzalez-Toledo ME, Piao CS, Gu A, Kelley RE, Zhao LR, Stem cell factor and granulocyte colony-stimulating factor reduce beta-amyloid deposits in the brains of APP/PS1 transgenic mice, Alzheimers Res Ther 3(2) (2011) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Azmy MS, Menze ET, El-Naga RN, Tadros MG, Neuroprotective Effects of Filgrastim in Rotenone-Induced Parkinson’s Disease in Rats: Insights into its Anti-Inflammatory, Neurotrophic, and Antiapoptotic Effects, Mol Neurobiol 55(8) (2018) 6572–6588. [DOI] [PubMed] [Google Scholar]
- [396].McCollum M, Ma Z, Cohen E, Leon R, Tao R, Wu JY, Maharaj D, Wei J, Post-MPTP treatment with granulocyte colony-stimulating factor improves nigrostriatal function in the mouse model of Parkinson’s disease, Mol Neurobiol 41(2–3) (2010) 410–9. [DOI] [PubMed] [Google Scholar]
- [397].Song S, Sava V, Rowe A, Li K, Cao C, Mori T, Sanchez-Ramos J, Granulocyte-colony stimulating factor (G-CSF) enhances recovery in mouse model of Parkinson’s disease, Neurosci Lett 487(2) (2011) 153–7. [DOI] [PubMed] [Google Scholar]
- [398].Yuan J, Xue LX, Ren JP, Granulocyte-colony stimulating factor, a potential candidate for the treatment of Parkinson’s disease, J Neurosurg Sci (2021). [DOI] [PubMed] [Google Scholar]
- [399].Frank T, Klinker F, Falkenburger BH, Laage R, Luhder F, Goricke B, Schneider A, Neurath H, Desel H, Liebetanz D, Bahr M, Weishaupt JH, Pegylated granulocyte colony-stimulating factor conveys long-term neuroprotection and improves functional outcome in a model of Parkinson’s disease, Brain 135(Pt 6) (2012) 1914–25. [DOI] [PubMed] [Google Scholar]
- [400].Kumar AS, Jagadeeshan S, Subramanian A, Chidambaram SB, Surabhi RP, Singhal M, Bhoopalan H, Sekar S, Pitani RS, Duvuru P, Venkatraman G, Rayala SK, Molecular Mechanism of Regulation of MTA1 Expression by Granulocyte Colony-stimulating Factor, J Biol Chem 291(23) (2016) 12310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Tsai ST, Chu SC, Liu SH, Pang CY, Hou TW, Lin SZ, Chen SY, Neuroprotection of Granulocyte Colony-Stimulating Factor for Early Stage Parkinson’s Disease, Cell Transplant 26(3) (2017) 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Tanaka F, Niwa J, Ishigaki S, Katsuno M, Waza M, Yamamoto M, Doyu M, Sobue G, Gene expression profiling toward understanding of ALS pathogenesis, Ann N Y Acad Sci 1086 (2006) 1–10. [DOI] [PubMed] [Google Scholar]
- [403].Chen H, Kankel MW, Su SC, Han SWS, Ofengeim D, Exploring the genetics and non-cell autonomous mechanisms underlying ALS/FTLD, Cell Death Differ 25(4) (2018) 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Henriques A, Pitzer C, Dittgen T, Klugmann M, Dupuis L, Schneider A, CNS-targeted viral delivery of G-CSF in an animal model for ALS: improved efficacy and preservation of the neuromuscular unit, Mol Ther 19(2) (2011) 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Yamasaki R, Tanaka M, Fukunaga M, Tateishi T, Kikuchi H, Motomura K, Matsushita T, Ohyagi Y, Kira J, Restoration of microglial function by granulocyte-colony stimulating factor in ALS model mice, J Neuroimmunol 229(1–2) (2010) 51–62. [DOI] [PubMed] [Google Scholar]
- [406].Henriques A, Kastner S, Chatzikonstantinou E, Pitzer C, Plaas C, Kirsch F, Wafzig O, Kruger C, Spoelgen R, Gonzalez De Aguilar JL, Gretz N, Schneider A, Gene expression changes in spinal motoneurons of the SOD1(G93A) transgenic model for ALS after treatment with G-CSF, Front Cell Neurosci 8 (2014) 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Pollari E, Savchenko E, Jaronen M, Kanninen K, Malm T, Wojciechowski S, Ahtoniemi T, Goldsteins G, Giniatullina R, Giniatullin R, Koistinaho J, Magga J, Granulocyte colony stimulating factor attenuates inflammation in a mouse model of amyotrophic lateral sclerosis, J Neuroinflammation 8 (2011) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Naumenko N, Pollari E, Kurronen A, Giniatullina R, Shakirzyanova A, Magga J, Koistinaho J, Giniatullin R, Gender-Specific Mechanism of Synaptic Impairment and Its Prevention by GCSF in a Mouse Model of ALS, Front Cell Neurosci 5 (2011) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [409].Nefussy B, Artamonov I, Deutsch V, Naparstek E, Nagler A, Drory VE, Recombinant human granulocyte-colony stimulating factor administration for treating amyotrophic lateral sclerosis: A pilot study, Amyotroph Lateral Scler 11(1–2) (2010) 187–93. [DOI] [PubMed] [Google Scholar]
- [410].Amirzagar N, Nafissi S, Tafakhori A, Modabbernia A, Amirzargar A, Ghaffarpour M, Siroos B, Harirchian MH, Granulocyte colony-stimulating factor for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled study of Iranian patients, J Clin Neurol 11(2) (2015) 164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Pocock K, Suresh N, Suradi Y, Dang S, Harvey B, Cao C, Sutherland K, Lin X, Vu TH, Gooch C, An Open-Label, Prospective Study Evaluating the Clinical and Immunological Effects of Higher Dose Granulocyte Colony-Stimulating Factor in ALS, J Clin Neuromuscul Dis 21(3) (2020) 127–134. [DOI] [PubMed] [Google Scholar]
- [412].Liu XY, Gonzalez-Toledo ME, Fagan A, Duan WM, Liu Y, Zhang S, Li B, Piao CS, Nelson L, Zhao LR, Stem cell factor and granulocyte colony-stimulating factor exhibit therapeutic effects in a mouse model of CADASIL, Neurobiol Dis 73 (2015) 189–203. [DOI] [PubMed] [Google Scholar]
- [413].Minnerup J, Heidrich J, Wellmann J, Rogalewski A, Schneider A, Schabitz WR, Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia, Stroke 39(6) (2008) 1855–61. [DOI] [PubMed] [Google Scholar]
- [414].Sevimli S, Diederich K, Strecker JK, Schilling M, Klocke R, Nikol S, Kirsch F, Schneider A, Schabitz WR, Endogenous brain protection by granulocyte-colony stimulating factor after ischemic stroke, Exp Neurol 217(2) (2009) 328–35. [DOI] [PubMed] [Google Scholar]
- [415].Zhang L, Shu XJ, Zhou HY, Liu W, Chen Y, Wang CL, Li Y, Chen QX, Liu LJ, Wang JZ, Protective effect of granulocyte colony-stimulating factor on intracerebral hemorrhage in rat, Neurochemical research 34(7) (2009) 1317–23. [DOI] [PubMed] [Google Scholar]
- [416].Dittgen T, Pitzer C, Plaas C, Kirsch F, Vogt G, Laage R, Schneider A, Granulocyte-colony stimulating factor (G-CSF) improves motor recovery in the rat impactor model for spinal cord injury, PLoS One 7(1) (2012) e29880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].von Aulock S, Diterich I, Hareng L, Hartung T, G-CSF: boosting endogenous production--a new strategy?, Curr Opin Investig Drugs 5(11) (2004) 1148–52. [PubMed] [Google Scholar]
- [418].Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, Lee YS, Lo EH, Kim M, Roh JK, Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia, Brain Res 1058(1–2) (2005) 120–8. [DOI] [PubMed] [Google Scholar]
- [419].Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T, Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells, Circulation 113(5) (2006) 701–10. [DOI] [PubMed] [Google Scholar]
- [420].Solaroglu I, Tsubokawa T, Cahill J, Zhang JH, Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat, Neuroscience 143(4) (2006) 965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Cui L, Duchamp NS, Boston DJ, Ren X, Zhang X, Hu H, Zhao LR, NF-kappaB is involved in brain repair by stem cell factor and granulocyte-colony stimulating factor in chronic stroke, Exp Neurol 263 (2015) 17–27. [DOI] [PubMed] [Google Scholar]
- [422].Huang X, Dai Y, Ma X, Wang S, Xu X, Pei X, Li R, Wang H, Different changes in granulocyte-colony stimulating factor and its correlation with inflammatory biomarkers in patients after traumatic brain injury, Neuroreport 31(4) (2020) 293–299. [DOI] [PubMed] [Google Scholar]
- [423].Corrigan F, Arulsamy A, Teng J, Collins-Praino LE, Pumping the Brakes: Neurotrophic Factors for the Prevention of Cognitive Impairment and Dementia after Traumatic Brain Injury, J Neurotrauma 34(5) (2017) 971–986. [DOI] [PubMed] [Google Scholar]
- [424].Sheibani N, Grabowski EF, Schoenfeld DA, Whalen MJ, Effect of granulocyte colony-stimulating factor on functional and histopathologic outcome after traumatic brain injury in mice, Crit Care Med 32(11) (2004) 2274–8. [DOI] [PubMed] [Google Scholar]
- [425].Song S, Kong X, Acosta S, Sava V, Borlongan C, Sanchez-Ramos J, Granulocyte-colony stimulating factor promotes brain repair following traumatic brain injury by recruitment of microglia and increasing neurotrophic factor expression, Restor Neurol Neurosci 34(3) (2016) 415–31. [DOI] [PubMed] [Google Scholar]
- [426].Qiu X, Ping S, Kyle M, Chin L, Zhao LR, SCF + G-CSF treatment in the chronic phase of severe TBI enhances axonal sprouting in the spinal cord and synaptic pruning in the hippocampus, Acta Neuropathol Commun 9(1) (2021) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [427].Liou JT, Lui PW, Liu FC, Lai YS, Day YJ, Exogenous granulocyte colony-stimulating factor exacerbate pain-related behaviors after peripheral nerve injury, J Neuroimmunol 232(1–2) (2011) 83–93. [DOI] [PubMed] [Google Scholar]
- [428].Carvalho TT, Borghi SM, Pinho-Ribeiro FA, Mizokami SS, Cunha TM, Ferreira SH, Cunha FQ, Casagrande R, Verri WA Jr., Granulocyte-colony stimulating factor (G-CSF)-induced mechanical hyperalgesia in mice: Role for peripheral TNFalpha, IL-1beta and IL-10, Eur J Pharmacol 749 (2015) 62–72. [DOI] [PubMed] [Google Scholar]
- [429].Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR, Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain, Neuron 100(6) (2018) 1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [430].Lee MC, McCubbin JA, Christensen AD, Poole DP, Rajasekhar P, Lieu T, Bunnett NW, Garcia-Caraballo S, Erickson A, Brierley SM, Saleh R, Achuthan A, Fleetwood AJ, Anderson RL, Hamilton JA, Cook AD, G-CSF Receptor Blockade Ameliorates Arthritic Pain and Disease, J Immunol 198(9) (2017) 3565–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [431].Chao PK, Lu KT, Lee YL, Chen JC, Wang HL, Yang YL, Cheng MY, Liao MF, Ro LS, Early systemic granulocyte-colony stimulating factor treatment attenuates neuropathic pain after peripheral nerve injury, PLoS One 7(8) (2012) e43680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [432].Kato K, Koda M, Takahashi H, Sakuma T, Inada T, Kamiya K, Ota M, Maki S, Okawa A, Takahashi K, Yamazaki M, Aramomi M, Hashimoto M, Ikeda O, Mannoji C, Furuya T, Granulocyte colony-stimulating factor attenuates spinal cord injury-induced mechanical allodynia in adult rats, J Neurol Sci 355(1–2) (2015) 79–83. [DOI] [PubMed] [Google Scholar]
- [433].Liao MF, Yeh SR, Lo AL, Chao PK, Lee YL, Hung YH, Lu KT, Ro LS, An early granulocyte colony-stimulating factor treatment attenuates neuropathic pain through activation of mu opioid receptors on the injured nerve, Sci Rep 6 (2016) 25490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [434].Lee KA, Park KT, Yu HM, Jin HY, Baek HS, Park TS, Effect of granulocyte colony-stimulating factor on the peripheral nerves in streptozotocin-induced diabetic rat, Diabetes Metab J 37(4) (2013) 286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [435].Erbas O, Solmaz V, Taskiran D, Granulocyte colony-stimulating factor provides protection against cardiovascular autonomic neuropathy in streptozotocin-induced diabetes in rats, Diabetes Res Clin Pract 107(3) (2015) 377–83. [DOI] [PubMed] [Google Scholar]
- [436].Su J, Zhou H, Tao Y, Guo J, Guo Z, Zhang S, Zhang Y, Huang Y, Tang Y, Dong Q, Hu R, G-CSF protects human brain vascular endothelial cells injury induced by high glucose, free fatty acids and hypoxia through MAPK and Akt signaling, PLoS One 10(4) (2015) e0120707. [DOI] [PMC free article] [PubMed] [Google Scholar]


