Abstract
Background:
Although fentanyl has gained widespread prominence, there remains a lack of knowledge on the opioid synthetic agonist, particularly related to sex effects. Therefore, we conducted behavioral tests in female and male rats to measure drug abuse-related responses to fentanyl hypothesizing sex-specific responses.
Methods:
Using female and male rats, we measured the effects of acute or repeated administration of fentanyl (20 μg/kg) on locomotor activity (LMA) and behavioral sensitization in an open field test. We further measured contextual-reward and associated locomotor activity during training in a conditioned place preference (CPP) paradigm using a low (4 μg/kg) or high (16 μg/kg) dose of fentanyl. Vaginal lavage samples were collected from female rats in the CPP study, and the estrous phase was determined based on the cytological characterization.
Results:
Female, but not male, rats showed elevated LMA in response to acute fentanyl and behavioral sensitization to repeated administration of fentanyl. Fentanyl produced significant CPP in both sexes, but it was more potent in males. Finally, our secondary investigation of the estrous cycle on fentanyl-CPP suggests that non-estrus estrous phases, likely reflecting high estradiol, may predict the degree of fentanyl preference in females.
Conclusions:
Fentanyl was more potent and/or effective to produce LMA and LMA sensitization in females but more potent to produce CPP in males. Furthermore, the role of sex in fentanyl responses varied across endpoints, and sex differences in LMA were not predictive of sex differences in CPP.
Keywords: Conditioned Place Preference, Contextual Reward, Opioid, Sensitization, Male, Female
Introduction
Opioid Use Disorder (OUD) affects more than 2 million people in the United States alone, and this number has been steadily rising in recent years due to the opioid epidemic (Wei et al., 2019). Fentanyl, a synthetic opioid and potent mu-opioid receptor agonist, became a commonly abused drug in the so-called “third wave” of the opioid epidemic, in part because of its contamination with other drugs of abuse and its unintentional consumption. Along with other synthetic opioids, Fentanyl has been a significant contributor to the marked rise in overdose deaths between 2010–2015 (O’Donnell et al., 2017; Rudd et al., 2016). Although epidemiological data suggests no recent growth in opioid-related overdose deaths for 2017–2018, synthetic opioid overdose deaths increased by 10% during this period (Wilson et al., 2020). Additionally, the current novel coronavirus pandemic has caused a sudden increase in opioid use and overdose deaths, particularly from illicit fentanyl use (American Medical Association, 2020; Lambert, 2020). There is a growing concern that the stress and isolation of this pandemic will precipitate a substantial increase in the abuse of fentanyl and OUD (Becker and Fiellin, 2020).
Of those with OUD, women display higher rates of prescription overdose deaths (Unick et al., 2013), and recently, a greater rate of growth in opioid-related deaths compared to men (Mazure and Fiellin, 2018) was also reported. Despite evidence that the prevalence of OUD between sexes is equalizing (Becker and Chartoff, 2018; Mazure and Fiellin, 2018), there is an abundance of clinical and pre-clinical research that suggests opioids and other drugs of abuse have distinct biologically based sex effects (Craft 2008; Huhn et al., 2018; Serdarevic et al, 2017; Strang et al., 2020). Most research using rat models of drug abuse indicates that females are generally more responsive to drugs of abuse (Cicero et al., 2020; Becker and Koob, 2016; Collins et al., 2016). This includes evidence indicating sex differences in various phases of opioid addiction (Lynch et al., 2002; Lynch, 2006; Harp et al., 2020). For example, preclinical studies show that females consume greater levels of heroin and morphine compared to males under extended access conditions and acquire drug self-administration at a faster rate, working harder and binging for longer times (Lynch et al., 2002; Lynch, 2006). Lynch and colleagues recently showed that female rats self-administering more fentanyl and responded at higher levels during subsequent extinction testing (Bakhti-Suroosh et al., 2021). Also, female rats have been shown to have a higher demand for fentanyl or a food-reinforcer than males, but in a food versus fentanyl choice task, males show higher fentanyl choice compared to females, highlighting the need for more studies on fentanyl in females and males (Townsend et al., 2019). Therefore, it is important to understand the effects of sex on fentanyl responses and the mechanism(s) underpinning these sex-related differences.
In research investigating sex as the primary variable of interest, sex hormones have been identified as important mechanisms for driving physiology and behavior. Estradiol, the predominant estrogen in the female reproductive cycle, has been posited as a key sex hormone in the regulation of drug reward (Bertz et al., 2016) and motivated behavior (Uban et al., 2011). Additionally, evidence from gonadectomized rats suggests an activation role for estradiol enabling females to be more vulnerable to addiction-related behaviors (Harp et al., 2020). Although more work needs to be done to elucidate the contribution of estradiol in drug-seeking behavior, there is a growing body of research suggesting the role of estradiol in enhancing dopamine-mediated reward circuitry (Kokane and Perrotti, 2020; Calipari et al., 2017; Vandegrift et al., 2017; Peterson et al., 2015).
Before fentanyl became a common illicit drug, it had a history of use as a selective mu-opioid receptor agonist in early animal models of opioid reward and withdrawal (Bruiinzeel et al., 2006; Awasaki et al., 1997). Many publications were able to show a contextual preference for fentanyl via the Conditioned Place Preference (CPP) paradigm (Rech et al., 2010; Tzschentke, 2007; Vitale et al., 2003; Finlay and Jakubovic, 1998; Reid et al., 1989; Mucha and Herz, 1985). Drug-associated CPP is commonly used to measure the motivational effects of a drug-paired context, whether rewarding or aversive (Napier et al., 2013). Briefly, drug-associated CPP uses the principles of Pavlovian conditioning to pair a drug treatment with a neutral stimulus, typically a small chamber with salient contextual stimuli (e.g., tactile and visual). Drug preference in the CPP paradigm is measured by time voluntarily spent in the drug-paired context versus another accessible neutral context, usually paired with a vehicle-control. The CPP paradigm allows researchers to measure the salience of drug reward integration with a neutral context that serves as a conditioned stimulus, rather than a direct model of drug self-administration. Although it does not model voluntary drug-taking, CPP is nonetheless a powerful predictive model of drug-related reward (Karami and Zarrindast, 2008; Robinson and Berridge, 2000), and the CPP paradigm provides a second measure of drug response via behavioral sensitization to fentanyl-induced locomotor effects observed during conditioning.
Behavioral sensitization to drug-induced activity is a well-established animal model used in the substance abuse research field that is observed following repeated drug administrations as an increase in LMA or stereotypic behaviors. Drug-induced behavioral sensitization relies on neural sensitization and neuroplasticity within the reward circuitry. Indeed, there is considerable overlap in circuitry, neurotransmitters, and receptor systems that underlie behavioral sensitization and drug-seeking behaviors observed in self-administration models (Steketee and Kalivas, 2011). Similar to psychostimulants, such as cocaine, central nervous system depressants, including fentanyl, also induce robust behavioral sensitization (Bryant et al., 2009; Trujillo et al., 2004). However, the effects of sex on fentanyl-induced behavioral sensitization have not been studied previously.
Here, we used an Open Field assay and LMA during training days of a CPP assay to characterize acute response and behavioral sensitization to fentanyl. In addition, we measured drug-related reward via a contextual preference in the CPP paradigm. Concurrent with our experiments, we also collected estrous cycle data of female rats for indirect measurement of the impact of sex hormones on behavior.
Methods
The Wayne State University Institutional Animal Care and Use Committee approved these procedures, and we followed the relevant guidelines in the Guide for the Care and Use of Laboratory Animals (2011). Female (n = 72) and male (n = 48) Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) were ordered at 10 weeks of age. All animals were pair-housed by sex upon arrival and then allowed to acclimate for one week in a climate-controlled vivarium with sex-specific rooms under a reverse light/dark cycle (lights off at 6:00 and lights on at 18:00). Rats had ad libitum access to food and water. After a week of acclimation, animals were handled and weighed daily for a week before beginning the experiments. All procedures were performed during the animal’s active phase (between 10:00 and 16:00) under dim red light to preserve the reverse light/dark cycle and minimize disruption of circadian rhythms. Experimental groups were pre-determined randomly before the beginning of experiments, and cage mates were always assigned to the same experimental group. On study days, they were transported from animal quarters to the behavioral study laboratory. All animals were given at least 30 minutes to acclimate to the behavioral suite before proceeding with any procedures. The behavioral suite was designed to minimize noise and provide a separate staging and testing area, uniform surroundings, controlled temperature and humidity, appropriate lighting, and an overhead camera for digital recording with connections to a computer in an adjacent room.
For the behavioral assays, the testing of male and female cohorts did not overlap. Female rats were brought into the behavioral room only after the conclusion of testing all male rats for each behavioral assay. The behavioral apparatuses were cleaned between each animal and test with 70% ethanol, and the entire behavioral testing room was thoroughly cleaned between male and female rat testing. Sterile saline (0.9% NaCl, preservative-free) was purchased from VetOne (Boise, Idaho). The National Institute for Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD) generously provided fentanyl hydrochloride free of charge. A stock solution of fentanyl was prepared in sterile saline and stored at 4°C. Each day, fentanyl doses were made in sterile saline and allowed to reach room temperature before use. All injections were given subcutaneously (S.C.) with a 27-gauge needle at 1 ml/kg within the scruff of the neck.
Custom-made Plexiglas behavioral apparatuses (Formtech Plastics, Oak Park, MI) were used for the Open Field study (70 cm length x 30 cm width) and for the CPP study with the addition of Plexiglas wall inserts to create 2 chambers (each 35 cm length x 30 cm width). For each assay, we placed four apparatuses side-by-side, with removable wire covers placed on top. During the tests, animal behavior was digitally recorded by overhead cameras and tracked using Ethovision software (Noldus, Leesburg, VA) to determine locomotor activity via distance traveled, and preference via time spent in each chamber.
Open Field Assay
For the Open Field study, we divided rats into three groups before testing (Fig.1A): saline-control, acute fentanyl, and chronic fentanyl (n= 8/group for males and females). Rats in the control group received daily sterile saline injections on all eight days. Rats in the acute fentanyl group received a daily saline injection for seven days and a single fentanyl injection (20 μg/kg) on the eighth day. Rats in the chronic fentanyl group received a daily fentanyl injection (20 μg/kg) for eight consecutive days. This dose of fentanyl was similar to published research (Mucha and Herz, 1985). On days 1 and 8 of the experiment, LMA in the open-field arena was measured for a 15-minute habituation period followed by a 30-minute post-injection period inside the arenas. For injections during behavioral testing, rats were removed after the habituation period, injected with appropriate drugs, and immediately returned to the open field arenas for the post-injection period observations. Injections on days 2–7 were given in the home-cage at the same time of day as behavioral testing on days 1 and 8 (starting ~11:00 daily). For normalization of LMA to the control group, we divided each LMA value by the mean LMA of the control group for its corresponding sex.
Figure 1. Fentanyl (FTY) differentially affects locomotor activity (LMA) in female and male rats.
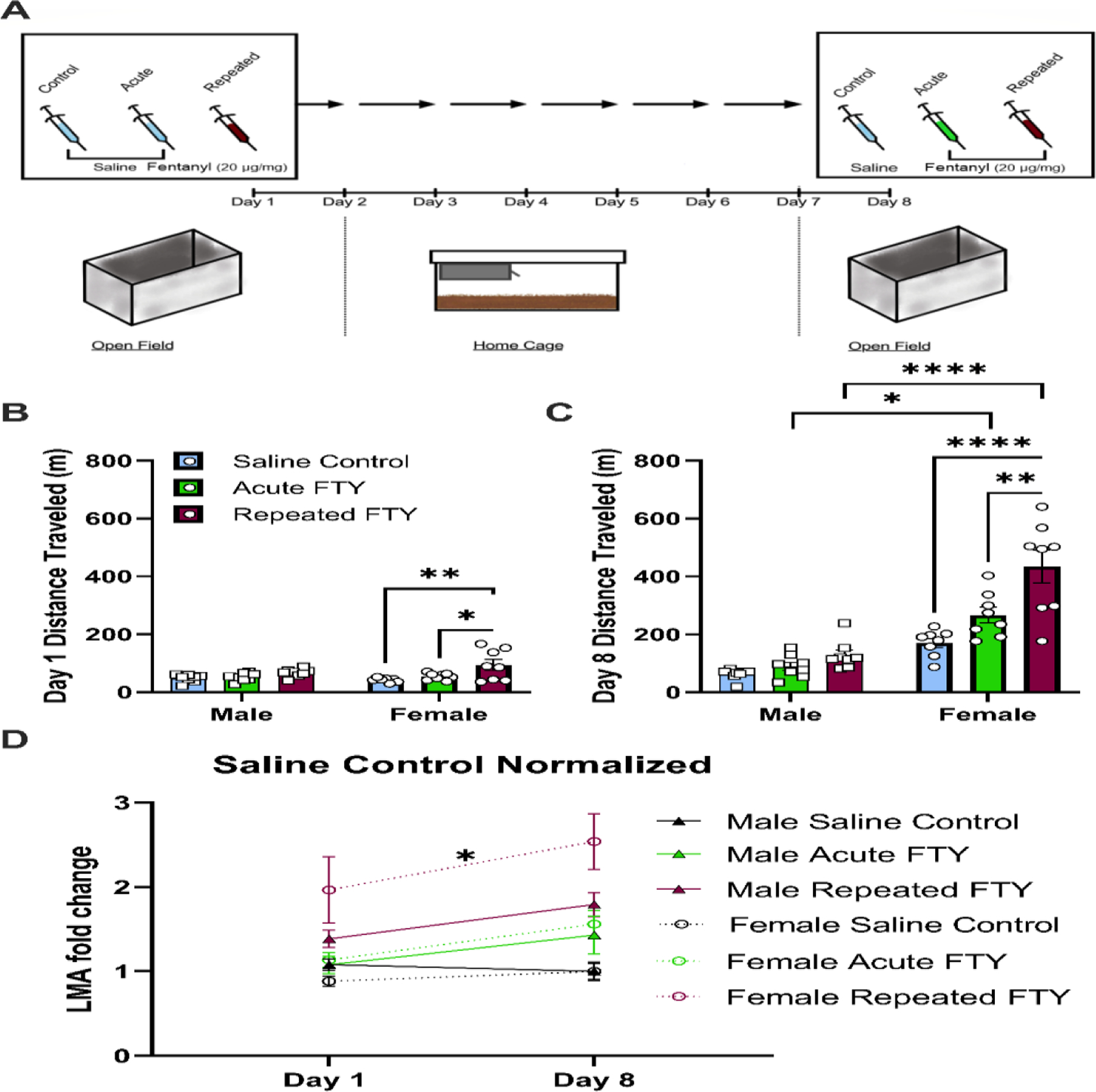
(A) A schematic of the timeline of the open field experiment. Rats were first tested for LMA following saline or fentanyl (20 μg/kg) subcutaneous injection. After 1 week of daily injections, LMA was tested in rats given repeated fentanyl, acute fentanyl (i.e., rats given saline through days 1–7 and fentanyl on day 8 only), or saline-control. (B) Only female rats, given fentanyl injection on day 1 had elevated LMA compared to saline-control or acute fentanyl that received saline on day 1 (*p < 0.05). No significant changes were observed in male rats on day 1. (C) After 8 days of repeated fentanyl administration, female rats showed elevated LMA compared to saline-control (****p < 0.0001) or to acute fentanyl (**p < 0.01). Additionally, female rats given acute and chronic fentanyl had significantly more LMA than males of the same treatment groups (***p < 0.001 and ****p < 0.0001, respectively). (D) Only female rats showed a time-dependent increase in locomotor activity for rats given fentanyl daily for 8 days (chronic) when data are normalized to respective group saline-control data (*p < 0.05). Graphs display means ± SEM, with points on bar charts representing individual values.
Conditioned Place Preference
For the CPP study, we used three treatment groups for male (n = 24 (n=8/group)) and female (n = 48 (n=16/group)) rats: saline-control (vehicle), low dose (4 μg/kg), and high dose (16 μg/kg) fentanyl. These doses of fentanyl were chosen based on published work (Mucha and Herz, 1985). We placed rats in the CPP apparatus for testing immediately after injection, with every other apparatus rotated 180° to avoid a side bias for the room. The CPP apparatuses had 2 chambers separated by an exchangeable piece of plastic that created a wall or an arch-style opening/door. Context A had a wire mesh floor and black walls with diagonal white stripes. Context B had a smooth black floor and white walls with black dots. The black/white balance of the walls was even. On the pretest day (day 1), we placed a black wall with an arch-style opening between both sides of the apparatus to allow free exploration for 20 minutes between Context A and Context B in a drug-free state. To determine a preference, we measured the total duration on each side compared to the total test duration (1200 s). The less-preferred side from the pretest was assigned to the drug treatment condition to remove side bias as a potential confound to a drug effect. On conditioning days (days 2–9), we placed a black wall in the apparatus to enclose the rats in only one context. On the first day of conditioning, we gave all rats a control injection of saline before placing the animal in the control-paired context for 20 minutes. The following day, we gave rats the drug treatment (saline for controls and fentanyl 4 or 16 μg/kg for the experimental groups) and placed them in the drug-paired context for 20 minutes. We repeated this process of alternating days of contextually paired saline and drug administration 3 more times until the rats received 4 pairings of each treatment/context, for a total of 8 conditioning days. The test day (day 10) followed the same schedule as the pretest day (day 1) in a drug-free state. We replaced the separating wall with the arch-style opening in the CPP apparatus, and we recorded the duration of time each rat spent in each context.
Characterization of Estrous Cycle
To understand how the estrous cycle might affect behavior in female rats, daily vaginal lavage samples were collected. Male rats did not undergo a sham procedure, and we acknowledge this as an experimental limitation. For each experimental day during the CPP and Open Field experiments, and for the three days leading up to each experiment, vaginal lavage samples were collected from female rats for later cytological characterization of estrous staging and corresponding indirect measure of estradiol levels. The vaginal lavage procedure was performed per published methods (Cora et al., 2015; Paccola et al., 2013; Marcondes et al., 2002). Briefly, we used smooth glass pipettes to flush deionized water into the vaginal canal, then collected the sample on a microscope slide where it was stained with Cresyl Violet (Sigma Aldrich, St. Louis, MO) for later cellular characterization. Vaginal lavages were performed 30 minutes after the end of behavioral testing or drug administration for that day. The vaginal lavage samples were analyzed for estrous cycle phases following published methods (McLean et al., 2012; Cora et al., 2015) using a two-rater system. The raters were blinded to group assignment during estrous cycle rating. Discrepancies in coding were resolved by a third-rater, and inter-rater reliability was good with a significant Pearson r between raters (r = 0.9197, p < 0.0001). In the interest of appropriate power for analysis, classifications of estrous cycle phases were then clustered into two groups: estrus and non-estrus (proestrus, metestrus, and diestrus), as described previously (Kerstetter et al., 2008; Peterson et al., 2014; Nicholas et al., 2019; Tan et al., 2019; Bakhti-Suroosh et al., 2021). This grouping provided a high estradiol condition, where non-estrus phases included diestrus and metestrus with rising/surging levels and proestrus with peaking levels of estradiol, and a low estradiol condition, where the estrus phase has the lowest level of estradiol during the estrous cycle (Zachry et al., 2021).
Data Analysis
Place preference and locomotor activity digital recordings were analyzed with Ethovision XT 14 video tracking software (Noldus Information Technology, Wageningen, Netherlands). Data were statistically analyzed, and graphs were created using Prism 9.2.0 (GraphPad Software, San Diego, CA). Before running statistical tests, we determined homoscedasticity and normality of distribution for each of our datasets. Raw data are reported in all cases, and in some cases analyzed data are additionally provided, including normalizing LMA data and expressing CPP as a change in preference. For multivariate, mixed-factor ANOVAs, we used Sidak’s Multiple Comparison Test (α = 0.05) for the post hoc comparison of groups. Post hoc analyses for multiple comparisons examined the effects of sex, treatment (drug/dose), and time (day) on behavioral measures. An independent 2-way ANOVA was used to analyze changes in preference data for the 2 sub-groups of the estrous cycle (i.e., ‘estrus’ or ‘non-estrus’) for the female rats receiving low or high dose fentanyl in the CPP study, and Fisher’s LSD was used for post hoc analysis. In some cases, we performed post hoc analysis of multiple comparisons in the absence of an interaction or main factor effect, for example when a priori hypotheses related to sex effects are expected. These Protected F post hoc analyses are not meant to support sex differences but rather show qualitatively different patterns of fentanyl-induced behaviors between females and males, which was the primary hypothesis of the study.
Results
Acute and repeated effects of fentanyl on locomotor activity in female and male rats in an open field
In the light of literature suggesting elevated locomotion and behavioral sensitization to opioids in rats (Bryant et al., 2009; Trujillo et al., 2004), we compared female and male rats for the locomotor response to single and repeated doses of fentanyl using an open field assay as illustrated in Fig. 1A. A 3-way ANOVA showed an interaction by treatment, day, and sex (F2,41 = 5.599, p = 0.0071) and all 2-way interactions and 1-way factors were significantly different (p < 0.01). For visual clarity and to analyze multiple comparisons at day 1 or day 8, we presented the data by day in Fig. 1B and 1C and analyzed them by 2-way ANOVA. On the first day of drug administration, a main effect for drug treatment (F2, 41 = 8.801, p = 0.0007) was observed, but no interaction of sex effect was observed. Based on the a priori hypothesis that acute fentanyl administration would increase locomotor activity, we accepted the multiple comparisons that show female rats given fentanyl on day 1 showed significantly more LMA than females in either saline control group (i.e., saline-control (p < 0.01) or acute fentanyl group that did not receive fentanyl yet (p < 0.05)), whereas males given fentanyl did not show any LMA changes compared to their male saline-controls (Fig. 1B). As shown in Fig. 1C, on day 8, after one week of repeated injections, female and male rats were again tested for LMA. We found an interaction between treatment and sex (F2, 42 = 6.380, p = 0.0038), and main effect for drug treatment (F2, 42 = 17.46, p < 0.0001) and sex (F2, 42 = 73.82, p < 0.0001). Post hoc analysis of day 8 data revealed that female rats in the repeated fentanyl group showed significantly higher LMA than those in the saline-control or the acute fentanyl group (p < 0.0001 or p < 0.01, respectively). Female rats also show more LMA activity than male rats in their respective treatment groups, i.e., acute fentanyl (p < 0.05) or repeated fentanyl (p < 0.0001). Next, as shown in Fig. S1, we analyzed changes within groups across days 1 to 8 using a 2-way ANOVA and found an interaction between group and time (F5, 42 = 25.51, p < 0.0001) and main effects for time (F1, 42 = 170.3, p < 0.0001) and group (F5, 42 = 20.79, p < 0.0001). Multiple comparisons revealed that all the female groups, including saline-controls, significantly increased in LMA from day 1 to day 8 (p < 0.0001), and males did not differ between days in any group. Therefore, to accurately compare males and females across days, we normalized male and female LMA to the mean of their respective saline-control groups, by dividing each LMA value by the mean LMA of the saline group for each corresponding sex. As shown in Fig. 1D, after normalizing to saline-control, while we did not find an interaction between group and day, we found a main effect for time (F1, 39 = 12.66, p < 0.001) and treatment (F5, 42 = 1.400, p < 0.0001) using a mixed-effects model. This normalization and post hoc analysis for multiple comparisons revealed that only female rats given repeated fentanyl showed a significant increase in LMA from day 1 to day 8 (p < 0.05), indicative of behavioral sensitization.
Effects of fentanyl on locomotor activity in female and male rats during the CPP training paradigm
Building upon our Open Field data suggesting sexually dimorphic LMA response to fentanyl, we conducted a CPP test to measure drug-context-paired locomotion and contextual reward for fentanyl between sexes (data shown in Fig. 3). Additionally, for the eight days of contextual training (i.e., conditioning), we measured LMA immediately after drug administration. A 3-way ANOVA showed an interaction by treatment, day, and sex (F2, 64 = 3.363, p = 0.041) but only treatment by day for 2-way interactions (F2, 64 = 4.553, p = 0.0142) and treatment (F2, 64 = 6.183, p = 0.0035) and day (F1, 64 = 34.28, p < 0.0001) as main factors were significantly different with sex not being a significant source of variance. Nonetheless, based on a priori hypotheses we presented the data by sex in Fig. 2A and 2B and analyzed them by 2-way repeated-measures ANOVA. In females, we found an interaction between time and drug treatment (F14, 301 = 7.015, p < 0.0001) as well as a main effect for drug treatment (F2, 43 = 6.373, p = 0.0038) and time (F7, 301 = 11.23, p < 0.0001). We observed increased LMA in response to high dose (16 μg/kg) fentanyl on day 3, day 5, day 7, and day 9 (Fig. 2A) in females. Post hoc testing across days showed that this effect of high dose fentanyl on day 3 was significantly different from day 9 (p = 0.0113), but no other high dose fentanyl treatment days were significantly different from one another. Also, every high dose fentanyl treatment day was significantly different from every saline treatment day (p < 0.05), and no differences were observed across the saline treatment days in females. Post hoc testing among groups on each treatment day revealed significant effects on day 3 between saline and high dose fentanyl groups (p = 0.0264), on day 5 between saline and high dose fentanyl groups (p < 0.0001), and between low dose and high dose fentanyl groups (p = 0.0029), on day 7 between saline and high dose fentanyl groups (p < 0.0001) and between low dose and high dose fentanyl groups (p = 0.0003), on day 9 between saline and high dose fentanyl groups (p < 0.0001) and between low dose and high dose fentanyl groups (p = 0.0001). No significant post hoc effects were observed for the low dose (4 μg/kg) fentanyl or saline groups in females. This drug treatment or interaction effect was not observed in males (Fig. 2B). However, male rats did show a time effect during CPP training (F7, 147 = 9.316, p < 0.0001). Post hoc testing across days showed that only day 3 and day 9 of low dose fentanyl administrations were significantly different for males (p = 0.0158). Post hoc testing among groups on each treatment day revealed a significant difference on day 9 between saline and high dose fentanyl groups (p = 0.0498).
Figure 3. Fentanyl (FTY) produced significant conditioned place preference (CPP) in both sexes, but it was more potent in males.
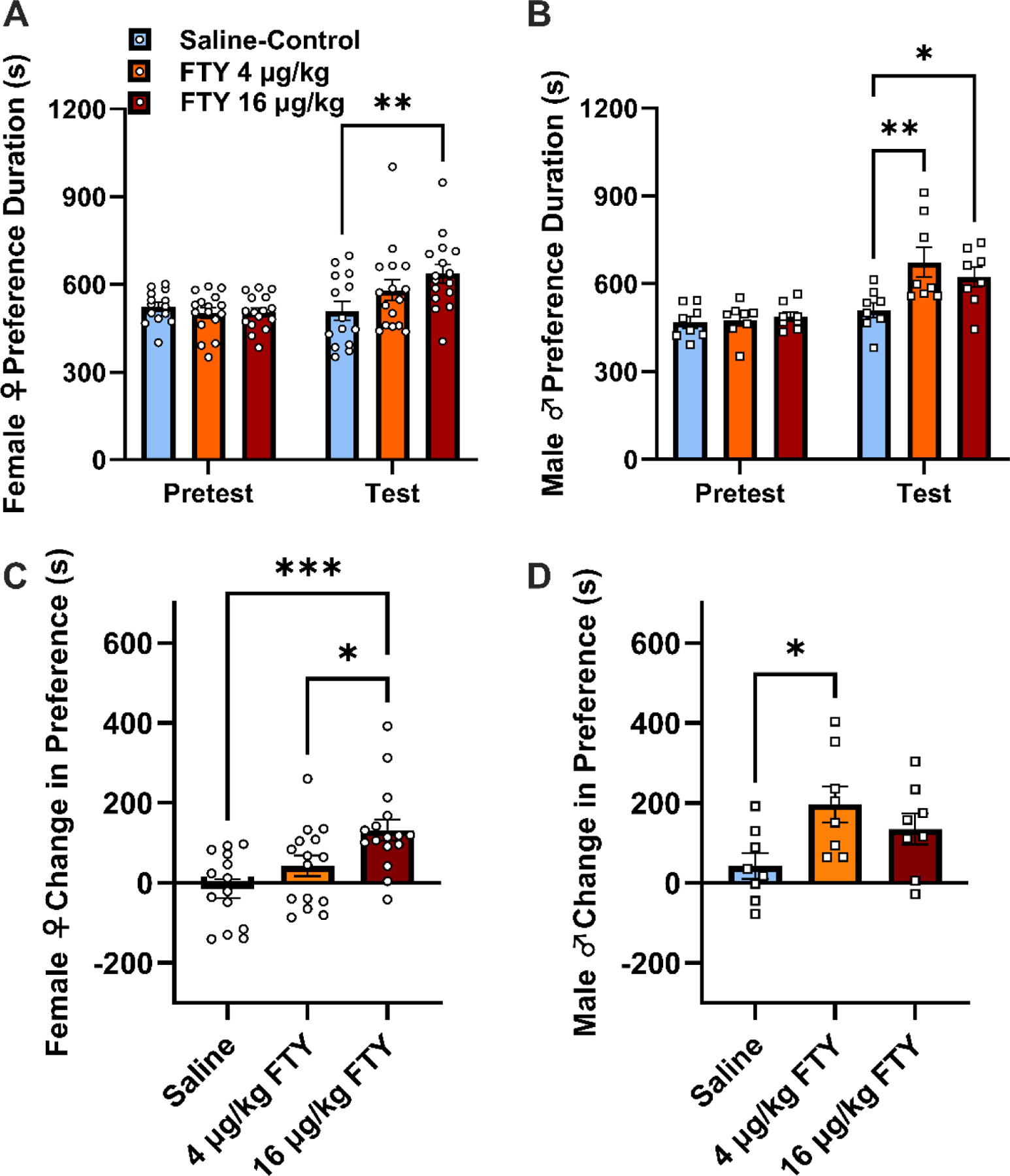
(A) On Test day, female rats given high dose (16 μg/kg) fentanyl were significantly different from the saline-control group (**p < 0.01). (B) Males preferred both low (4 μg/kg) and high doses of fentanyl-paired contexts compared to a saline-control group on Test day (**p < 0.01, *p < 0.05). (C) When data are expressed as change in preference from Pretest to Test day, high dose fentanyl was significantly different from saline or low dose fentanyl (***p < 0.001, *p < 0.05). (D) A significant difference between low dose fentanyl and saline was observed for male rats (*p < 0.05). Bar graphs display means ± SEM, with points representing individual values.
Figure 2. Locomotor activity (LMA) response to fentanyl (FTY) is different between females and males in the acute response and behavioral sensitization over repeated administration to fentanyl in the conditioned place preference paradigm.
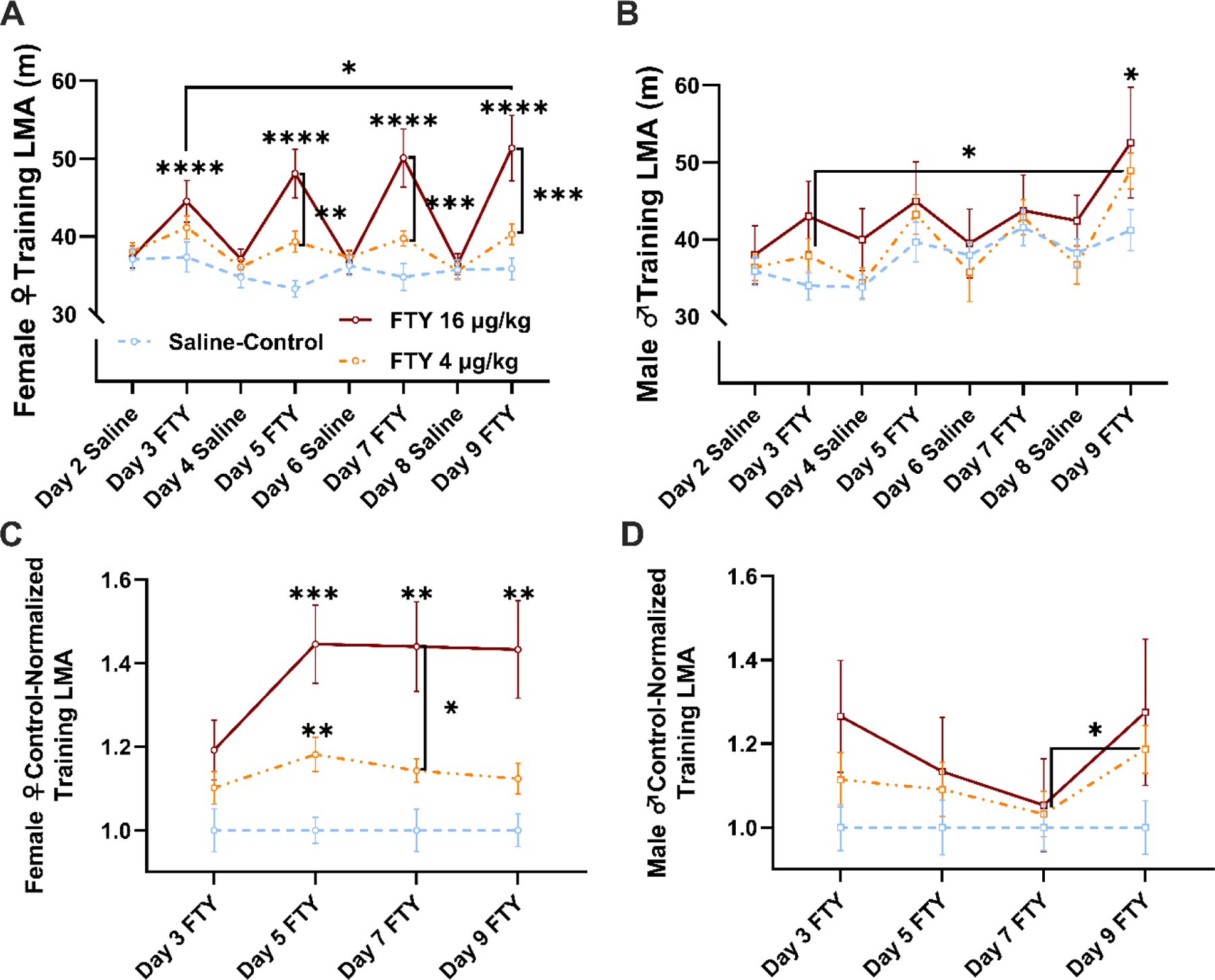
(A) In alternating days of no-drug/drug conditioning (i.e., intermittent administration), female rats given high dose (16 μg/kg) fentanyl showed a significantly elevated LMA response compared to saline (****p < 0.0001) or compared to low dose (4 μg/kg) fentanyl (**p < 0.01, ***p < 0.001). The LMA returned to baseline on days when drug groups were given saline conditioning. A sensitized response was observed in females given high dose fentanyl when comparing day 3 fentanyl with day 9 fentanyl (*p < 0.05). (B) Male rats did not show an acute response to fentanyl, but did show a significant difference between saline and high dose fentanyl on day 9 (*p < 0.05). Males also showed sensitization to the low dose fentanyl comparing day 3 with day 9, but this may have been due to an increased LMA response over time in all male groups. (C) Female rats did not show a significant difference in LMA between drug days when the data were normalized to the saline-control, but significant differences were observed between low dose fentanyl and saline on day 5 (*p < 0.01), between saline and high dose fentanyl on days 5, 7, or 9 (***p < 0.001, **p < 0.01), and between low dose and high dose fentanyl on day 7 (*p < 0.05). (D) Male rats given 4 μg/kg fentanyl showed a significant increase in LMA between day 7 and day 9 of conditioning when the data were normalized to the saline-control. Points on the line graphs are means ± SEM.
When LMA was isolated exclusively for drug treatment days, no interaction in treatment and time was observed for either sex. Females showed a main effect for treatment (F2, 45 = 11.95, p < 0.001), and male rats showed a main effect for time (F2.239, 47.03 = 14.47, p < 0.001). In females, a treatment effect was observed on each day between saline and high dose fentanyl (p < 0.05), between saline and low dose fentanyl on day 5 or day 7 (p < 0.05), and between low dose and high dose fentanyl on day 7 (p = 0.0469). In males, a time-dependent increase in LMA was observed within the low dose (4 μg/kg) fentanyl group as well as the saline group (p < 0.05), but not the high dose (16 μg/kg) fentanyl group (Fig. S2B). For saline, day 3 was different from day 5, day 7, or day 9 (p < 0.05), and for low dose fentanyl, day 3 was different from day 5, day 7, or day 9, and day 9 was also different from day 5 or day 7 (p < 0.05).
To control for the increase in the control group, we normalized drug groups with respect to the saline control for both sexes and performed 2-way repeated-measures ANOVAs (Fig. 2C and 2D). We found a time by treatment interaction only in females (F6, 129 = 2.215, p = 0.0456) and females also had significant main effects of time (F2.768,119 = 3.699, p = 0.0161) and treatment (F2, 43 = 11.82, p < 0.0001), but males only showed a main effect of time (F2.262, 47.50 = 4.894, p = 0.0092). Post hoc analysis in this Protected F value case showed that female rats did not have a time-dependent increase in LMA between any two drug conditioning days, but did show significant differences among groups on select treatment days (Fig. 2C). Significant effects were observed on day 5 between saline and low dose fentanyl groups (p = 0.0046) and between saline and high dose fentanyl groups (p = 0.0008), on day 7 between saline and high dose fentanyl groups (p = 0.0038) and between low dose and high dose fentanyl groups (p = 0.0469), and on day 9 between saline and high dose fentanyl groups (p = 0.0072). Comparatively, post hoc testing showed that only male rats in the low dose fentanyl group had a significant increase in LMA between days 7 and 9 (Fig. 2D. p = 0.0332), and no significant effects were observed among groups on any treatment day.
Effects of fentanyl on conditioned place preference in female and male rats
We next measured contextual reward for fentanyl between sexes using the CPP paradigm. A 3-way ANOVA of all preference data failed to show an interaction by treatment (0, 4, or 16 μg/mg fentanyl), day (pretest vs. test), and sex, but a 2-way interaction between treatment and day was found (F2, 64 = 6.750, p = 0.0022) and treatment (F2, 64 = 4.040, p = 0.0223) and day (F1, 64 = 37.74, p < 0.0001) as main factors were significantly different with sex not being a significant source of variance. Nonetheless, based on an a priori hypotheses we presented the data by sex in Fig. 3A and 3B and analyzed them by 2-way repeated-measures ANOVA to qualitatively compare effects by sex. The preference data revealed a day by treatment interaction on contextual preference for fentanyl for female (F2, 43 = 4.939, p = 0.0117) and male rats (F2, 21 = 3.939, p = 0.0353) and main effects of time in female rats (F1, 43 = 11.91, p = 0.0013) and treatment (F2, 21 = 3.727, p = 0.0412) and time (F1, 21 = 30.44, p < 0.0001) in male rats. Post hoc analysis revealed that female rats showed a preference for the high dose (16 μg/kg) fentanyl paired context on test day compared to the saline control group (Fig. 3A, p = 0.0031). In comparison, male rats given the low (4 μg/kg) or high dose of fentanyl demonstrated a significant preference for the drug paired side compared to saline-control (Fig. 3B, p = 0.0014 or p = 0.037, respectively).
We also expressed our data as a change in preference. A 2-way ANOVA failed to show an interaction between sex and treatment, but main effects of sex (F1, 63 = 7.418, p = 0.0083) and treatment (F2, 63 = 8.316, p = 0.0006) were observed. Post hoc analysis showed that a significant difference was observed between the female and male groups at the low dose fentanyl context (p < 0.0171). In line with presenting data by sex, we analyzed the female and male change in preference data independently using 1-way ANOVA (Fig. 3C and 3D). For females, a significant main effect was observed (Fig. 3C, F2, 42 = 8.403, p = 0.0009), and post hoc testing revealed significant differences between saline and high dose fentanyl (p = 0.007) and between low dose and high dose fentanyl (p = 0.0479). For males, a significant main effect was found (Fig. 3D, F2, 21 = 3.939, p = 0.0353), and post hoc testing showed significant differences between saline and low dose fentanyl (p = 0.0326).
Association of contextual reward for fentanyl and estrous cycle phase at the onset of conditioning to fentanyl in female rats
Using vaginal lavage samples taken daily during our experiments, we analyzed behavior based on cytological classifications of the estrous cycle. In our analysis, we categorized the four phases of the rat estrous cycle into estrus and non-estrus phases based on their purported levels of estradiol (i.e., lower circulating estradiol in estrus) (Miller and Takahashi, 2013; Peterson et al., 2014; Staley and Scharfman, 2015). Using samples taken no more than two hours after relevant behaviors, we examined phases of the estrous cycle as a factor in behavioral measures for the Open Field and CPP tests (Fig. S3 and Fig 4, respectively). In the Open Field test, using a 2-way ANOVA we did not observe an interaction between treatment and estrous phase on LMA for the first drug day (Fig. S3A) or the last drug day (Fig. S3B). For the CPP test, using a 2-way ANOVA we did not find an interaction between treatment and estrous phase on LMA (Fig. S3C) or on preference at test day (Fig. S3D). However, based on findings that initial fentanyl exposure conditions persistently impact vulnerability to drug-seeking in females, but not males, (Bakhti-Suroosh et al., 2021) and given our a priori hypothesis that elevated estradiol in females during the first fentanyl exposure may enhance the development of a drug-context preference, we examined the effect of the estrus phase on first fentanyl exposure in the low or high dose fentanyl groups (Fig. 4). Using a 2-way ANOVA, a main effect of estrous phase (estrus vs. non-estrus) was observed (F1, 27 = 4.427, p = 0.0448) despite not obtaining an interaction between estrous phase and treatment (4 or 16 μg/mg fentanyl) or a main effect of treatment. Post hoc analysis found that females in non-estrus phases (i.e., including proestrus with high estradiol) during the first exposure to high dose fentanyl had a greater change in preference in the drug context in the CPP test (p = 0.0372) compared to female rats in estrus phase (i.e., low estradiol).
Figure 4. Estrous cycle phase during the first treatment of high dose fentanyl (FTY) predicts later fentanyl preference in the conditioned place preference (CPP) paradigm.
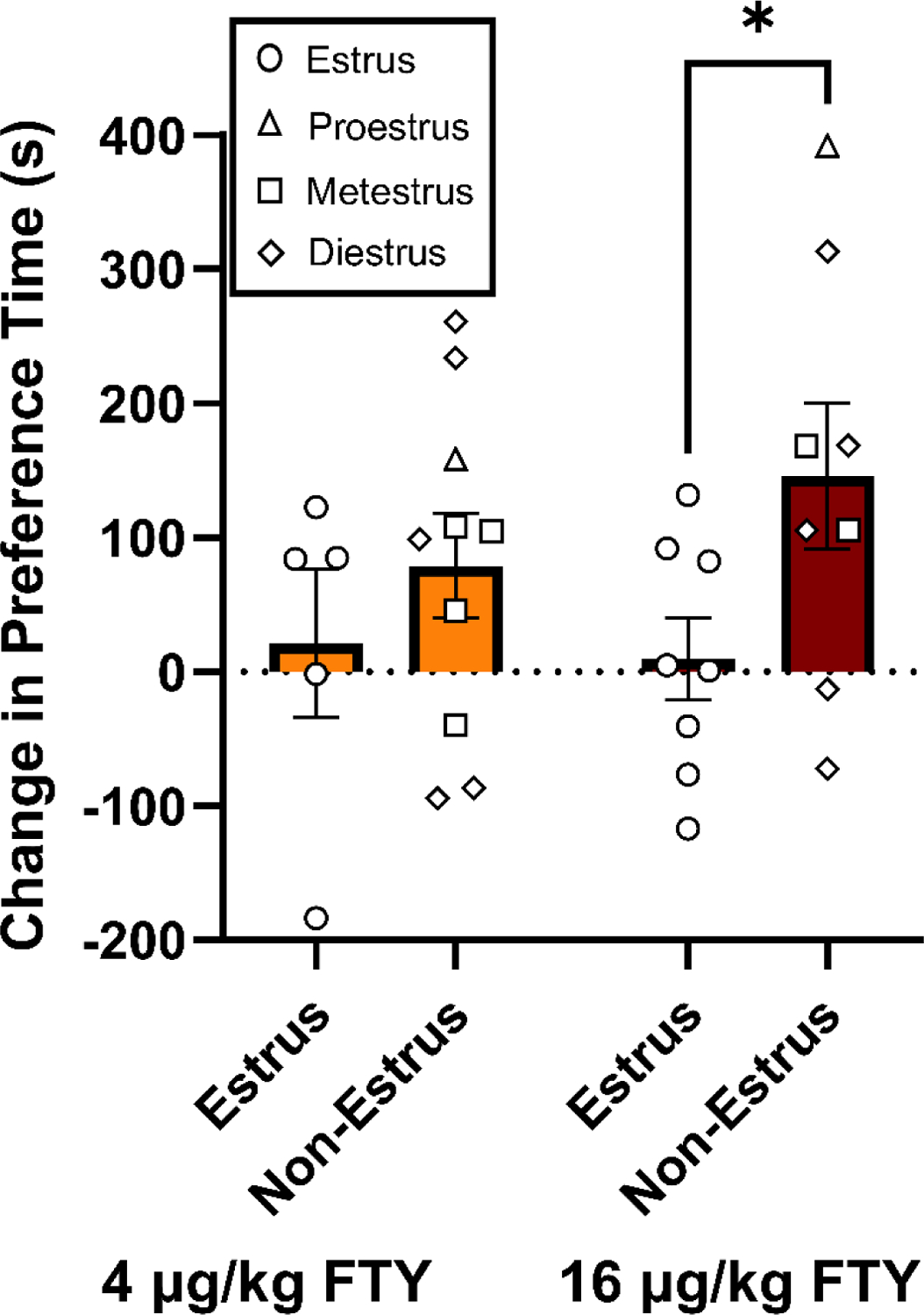
(A) Among the female rats administered low dose (4 μg/kg) or high dose (16 μg/kg) fentanyl-paired context, estrous phase (i.e., estrus vs. non-estrus) at initial fentanyl exposure during conditioning was predictive of subsequent fentanyl preference as a main factor. Post hoc analysis showed that the change in preference for the fentanyl-paired context in the CPP paradigm was significantly different between estrus and non-estrus groups in females given high dose fentanyl (*p < 0.05). Bar graphs display means ± SEM, with unique symbols representing individual estrous phases of females.
Discussion
We use 3 independent drug measures (i.e., LMA in Open Field, LMA in CPP, and contextual reward in CPP) to show sex-related differences in fentanyl response, and our data are consistent with the few published reports showing sex differences to fentanyl in animals and humans (Bryant et al., 2020; Townsend and Banks, 2019; Klein et al., 1997). The primary findings of our research illustrate that the behavioral response to fentanyl is regulated, in part, by sex. Specifically, we present three main findings for sex-related effects: 1) initial exposure to the fentanyl doses tested in this study produced enhanced LMA in females but not males, indicating that acute fentanyl was either more potent or more effective to produce LMA in females than males, 2) behavioral sensitization to fentanyl-induced LMA in both open field and CPP to repeated fentanyl was observed in females but not males, and 3) fentanyl produced significant CPP in both sexes, but it was more potent in males. Together, these data support the previous research suggesting that sex regulates the behavioral response to opioids and potentially the rewarding, and at least the contextually rewarding, effects of opioids (Lynch et al., 2002; Lynch, 2006; Harp et al., 2020). A secondary finding of our research suggests that the estrous cycle phase in female rats can predict the degree of preference for a fentanyl-paired context, with high estradiol during a non-estrus phase at the onset of drug conditioning being a predictor. Our findings present a general congruence between clinical and pre-clinical literature that could be developed for a better understanding of sex effects for the treatment of OUD.
In the current study, we show that female rats progressively increase in LMA in response to repeated fentanyl, with a more robust effect for a daily rather than alternating fentanyl administration. This incremental increase in locomotion after drug administration, also known as behavioral sensitization, replicates previous work with other drugs of abuse and has been suggested to model aspects of substance abuse and be a proxy-measure of drug reward given the neuroplasticity that occurs within the reward circuitry and neurotransmitter systems (Niikura et al., 2013; Vanderschuren et al., 2000). Indeed, some animal research suggests that males are more likely to escalate drug-taking of opioids (Mavrikaki et al., 2017). However, clinical and preclinical data indicate that females, more than males, show rapid drug-taking escalation, also known as “telescoping” (Becker and Chartoff, 2018). This clinical observation is consistent with our CPP training data, which shows an increase in female LMA by the second drug day and remaining stable for the remainder of the drug training days. Although these data support clinical and pre-clinical findings, behavioral sensitization does not closely model drug-taking or seeking behaviors; and there is a growing body of evidence showing sex effects in rat self-administration models (Smith et al., 2020; Carroll and Smethells, 2015; Lynch, 2006).
Our fentanyl preference data from the CPP assay suggests that male rats engage in drug-context-seeking behavior at a lower dose of fentanyl. Although both the sexes showed a preference for high dose fentanyl context, specifically in CPP-Test data, only male rats preferred the low dose fentanyl context as well, suggesting a more sensitive reward profile for fentanyl among male rats. This was a surprising finding given our open field and CPP training data showing more overall locomotor activation to fentanyl in females compared to males. Assuming that time spent in the drug-paired context reflects a measure of drug-context seeking, this data supports the idea that male rats are more sensitive to seeking fentanyl. Our preference data are different from recent work showing sex differences in fentanyl self-administration. Banks, Townsend, and colleagues have shown that while female rats display greater fentanyl and food choice behavior compared to males in a single-choice paradigm, male rats had higher fentanyl responding compared to female rats in a fentanyl vs. food choice paradigm (Townsend et al., 2019), but no neural mechanism was explored. In addition to hormone-related or genetic-based sex differences, pharmacokinetic differences between females and males have been raised as a potential explanation for a sexually dimorphic response to fentanyl, and these differences could be attributed to the drug delivery method, whereby differences in body fat content could alter the absorption of subcutaneous opioids (Ohtsuka et al., 2007). Cunningham and colleagues have also shown that male rats self-administrating fentanyl have profound effects on rat brain innate immune targets (Cisneros and Cunningham, 2021; Ezeomah et al., 2020); however, this effect and mechanism was not studied in females. Therefore, we are currently investigating mechanisms that underlie the sex effects caused by non-contingent delivery or self-administration of fentanyl.
In the open field and CPP assays, we found a robust sex effect of acutely elevated locomotor response to fentanyl in females compared to male rats. A potential explanation for the observed sex differences is in opioid pharmacology, regional neuroanatomy, and the influence of estradiol on opioid-induced behaviors. For example, preclinical evidence suggests that gonadal hormones can alter the affinity and density of mu-opioid receptors, as well as alter endogenous opioid (e.g., dynorphin and enkephalin) levels (Becker and Chartoff, 2018). Specifically, female rats with high circulating estradiol, such as those in proestrus, show a greater density of mu-opioid receptors in addiction-related brain regions, such as the striatum (Cruz et al., 2015). Indeed, elevated estrogen has been positively associated with locomotor and reward response to opioids (Mirbaha et al., 2009). Yet, there is some evidence that experimentally elevating estradiol can also attenuate the self-administration of heroin (Smith et al., 2020; Smith et al., 2021), however, the exact mechanism for this behavioral modulation remains unknown. More studies are needed to understand the hormonal influence on opioid behaviors, particularly in response to fentanyl because it is a major misassumption that mu-opioid receptor agonists are similar in pharmacokinetics and pharmacodynamics. Fentanyl is ~100 times more potent than morphine (Comer and Cahill, 2019), but the research community knows a fraction about it comparatively. For example, naturally occurring (opiates) and semisynthetic opioids, such as morphine and oxycodone, respectively, have been shown to produce robust pro-inflammatory effects in the brain (Wang et al., 2012; Fan et al., 2018; Osmanlioglu et al., 2020). These neuroinflammatory effects are thought to modulate pain and addiction-related behaviors (Cahill and Taylor, 2017; Kohno et al., 2020); however, fentanyl is devoid of pro-inflammatory response and activates anti-inflammatory mechanisms instead (Molina-Martinez et al., 2014; Kwon et al., 2019).
As a secondary analysis, we collected vaginal lavage samples for histological analysis and cytological characterization of the estrous cycle. Although our open field data were under-powered when divided into groups based on the estrous phase, we show that drug exposure during ‘non-estrus phases’ (i.e., proestrus, metestrus, or diestrus), which typically reflect higher estradiol levels than estrus (Zachry et al., 2021), is predictive of a greater increase in fentanyl place preference in the CPP assay for females. This result is not entirely surprising, because estradiol has previously been shown to augment reward signaling in response to drugs of abuse (Calipari et al., 2017, Vandegrift et al., 2017). As such, we hypothesized that rats in non-estrus phases are primed by estradiol to have a more salient drug conditioning upon first exposure. Despite our preliminary findings, there is some evidence that females in proestrus, when estradiol is highest, will self-administer less opioids (Lacy et al., 2020; Lacy et al. 2016), which may be affected by behavioral changes that occur during proestrus that often focus the female’s attention on partner-seeking instead of drug-seeking activities. However, analyzing proestrus alone is impeded by the relatively short proestrus time window, lasting less than a quarter of the overall cycle duration (Cora et al., 2015). In future studies, we aim to investigate the interaction between sex hormones and fentanyl response to test the causal role of estradiol in the development of drug reward.
The work presented in this study supports the hypothesis that sex influences addiction-related behavioral responses to fentanyl. We showed that female rats had a higher initial (acute) locomotor response to fentanyl, whereas males showed a gradual increase in locomotor response to fentanyl and a place preference for a lower dose of fentanyl. We also present preliminary data using estrous phase classification to suggest that estradiol may play a key role in sexually dimorphic responses to fentanyl, particularly at the onset of drug exposure and drug/context-related learning. Although these data support clinical and preclinical work on sex differences in opioid response, determining the mechanisms that drive these behaviors and others, including fentanyl self-administration to measure drug-taking, remains an important area for future research. Investigating the interaction between gonadal hormones and mu-opioid receptor pharmacology in reward circuitry remains a priority in this area of research.
Supplementary Material
(A) All the female treatment groups showed a significant increase in LMA from day 1 to day 8. Line graph points display means ± SEM (****p < 0.0001 when comparing day 1 vs. day 8 for each respective group).
(A) In female rats, LMA during drug context training days showed no time-dependent changes in LMA for any treatment group, but significant differences were observed between groups on specific days. The low dose (4 μg/kg) fentanyl group was significantly different from saline on days 5 or 7 (*p < 0.05), between high dose (16 μg/kg) fentanyl group was significantly different from saline on all treatment days (*p < 0.05, ***p< 0.001, or **p < 0.01), and between low dose and high dose fentanyl on day 7 (*p < 0.05). (B) In male rats, LMA during drug-context training days was not different between groups on specific treatment days; however, significant changes across days for saline-control was observed between day 3 and days 5, 7, or 9 (*p < 0.05) and across days for low dose fentanyl between day 3 and days 5, 7, or 9 and between day 9 and days 5 or 7 (*p < 0.05). Line graphs display means ± SEM.
(A) There was no effect for the estrous phase (i.e., estrus vs. non-estrus) on LMA on the first day of Open Field testing. (B) After 8 days of repeated injections, there was no effect for the estrous phase on LMA. (C) The estrous phase also had no effect on LMA during the test day of the CPP paradigm. (D) Preference for the fentanyl-associated context, when measured on test day, was not affected by estrous phase. Bar graphs display means ± SEM, with points representing individual values.
Highlights.
Female, but not male, rats show elevated LMA in response to acute fentanyl.
Female, but not male, rats develop behavioral sensitization to repeated fentanyl.
Female rats only preferred the context paired with high fentanyl dose.
Male rats preferred contexts associated with low or high fentanyl doses.
Estrous phase may predict the degree of fentanyl preference in females.
Acknowledgments
We thank the NIDA Drug Supply Program for generously providing fentanyl hydrochloride. We thank Mr. Jamaine Atkins and Mr. Sean Halloran, who participated in the NIDA Summer Research Internship Program and conducted pilot experiments related to this study. This work was supported by NIH R01-DA-042057 to S.A.P. The Wayne State Translational Neuroscience Program provided funding for A.D.G. Finally, we thank the reviewers of this manuscript for their thorough reviews, constructive comments and edits, and suggested phrases.
1. Role of Funding Source –
Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Biomedical Financial Interests and Potential Conflicts of Interest
All authors declare no conflicts of interest.
Conflict of Interest – No conflict declared
References
- American Medical Association, 2020. Issue Brief: Reports of Increases in Opioid-and Other Drug-Related Overdose and Other Concerns during COVID Pandemic *Updated. 2021. [Google Scholar]
- Awasaki Y, Nishida N, Sasaki S, Sato S, 1997. Dopamine D 1 antagonist SCH23390 attenuates self-administration of both cocaine and fentanyl in rats. Environmental toxicology and pharmacology. 3, 115–122. [DOI] [PubMed] [Google Scholar]
- Bakhti-Suroosh A, Towers EB, Lynch WJ. 2021. A buprenorphine-validated rat model of opioid use disorder optimized to study sex differences in vulnerability to relapse. Psychopharmacology. 238(4):1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Chartoff E, 2018. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 44, 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA, 2020. When Epidemics Collide: Coronavirus Disease 2019 (COVID-19) and the Opioid Crisis. Annals of internal medicine. 173, 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacological reviews. 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz J, Jackson E, Barron D, Woods J, 2016. Effects of sex and remifentanil dose on rats’ acquisition of responding for a remifentanil-conditioned reinforcer. Behavioural Pharmacology. 27, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M, 2006. Severe Deficit in Brain Reward Function Associated with Fentanyl Withdrawal in Rats. Biological Psychiatry. 59, 477–480. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Healy AF, Ruan QT, Coehlo MA, Lustig E, Yazdani N, Luttik KP, Tran T, Swancy I, Brewin LW, Chen MM, Szumlinski KK, 2021. Sex‐dependent effects of an Hnrnph1 mutation on fentanyl addiction‐relevant behaviors but not antinociception in mice. Genes, brain and behavior. 20, e12711. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, Fanselow MS, 2009. Pavlovian conditioning of multiple opioid-like responses in mice. Drug Alcohol Depend. 103, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han M, Nestler EJ, 2017. Dopaminergic dynamics underlying sex-specific cocaine reward. Nature communications. 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Taylor AM, 2017. Neuroinflammation-a co-occurring phenomenon linking chronic pain and opioid dependence. Curr Opin Behav Sci. 13:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Smethells JR, 2016. Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments; Front Psychiatry. 6, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER, 2000. Gender Differences in the Reinforcing Properties of Morphine. Pharmacology, Biochemistry and Behavior. 65, 91–96. [DOI] [PubMed] [Google Scholar]
- Cisneros IE, Cunningham KA, 2021. Self-administered fentanyl profoundly impacts rat brain innate immune targets. Neuropsychopharmacology. 46, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Reed B, Zhang Y, Kreek MJ, 2016. Sex differences in responsiveness to the prescription opioid oxycodone in mice. Pharmacology, Biochemistry and Behavior. 148. [DOI] [PubMed] [Google Scholar]
- Comer SD, Cahill CM, 2019. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci Biobehav Rev. 106:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals, National Academies Press, (U. S.), Institute for Laboratory Animal Research, (U. S.), 2011. Guide for the Care and use of Laboratory Animals. National Academies Press, Washington, D.C. [Google Scholar]
- Cora MC, Kooistra L, Travlos G, 2015. Vaginal Cytology of the Laboratory Rat and Mouse. Toxicologic Pathology. 43, 776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, 2008. Sex Differences in Analgesic, Reinforcing, Discriminative, and Motoric Effects of Opioids. Experimental and Clinical Psychopharmacology. 16, 376–385. [DOI] [PubMed] [Google Scholar]
- Cruz W, Pereira L, Cezar L, Camarini R, Felicio L, Bernardi M, Teodorov E, 2015. Role of steroid hormones and morphine treatment in the modulation of opioid receptor gene expression in brain structures in the female rat. SpringerPlus. 4, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeomah C, Cunningham KA, Stutz SJ, Fox RG, Bukreyeva N, Dineley KT, Paessler S, Cisneros IE, 2020. Fentanyl self-administration impacts brain immune responses in male Sprague-Dawley rats. Brain Behav Immun. 87, 725–738. [DOI] [PubMed] [Google Scholar]
- Fan R, Schrott LM, Arnold T, Snelling S, Rao M, Graham D, Cornelius A, Korneeva NL, 2018. Chronic oxycodone induces axonal degeneration in rat brain. BMC Neurosci. 19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Jakubovic A, Phillips AG, Fibiger HC, 1988. Fentanyl-induced conditional place preference: lack of associated conditional neurochemical events. Psychopharmacology. 96, 534–540. [DOI] [PubMed] [Google Scholar]
- Harp SJ, Martini M, Lynch WJ, Rissman EF. 2020. Sexual Differentiation and Substance Use: A Mini-Review. Endocrinology. 161(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Berry MS, Dunn KE, 2018. Systematic review of sex-based differences in opioid-based effects. International Review of Psychiatry: Opioid Epidemic. 30, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami M, Zarrindast MR, 2008. Morphine sex-dependently induced place conditioning in adult Wistar rats. European Journal of Pharmacology. 582, 78–87. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE, 2008. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 198, 63–75. [DOI] [PubMed] [Google Scholar]
- Klein LC, Popke EJ, Grunberg NE, 1997. Sex differences in effects of predictable and unpredictable footshock on fentanyl self-administration in rats. Experimental and clinical psychopharmacology. 5, 99–106. [DOI] [PubMed] [Google Scholar]
- Kohno M, Link J, Dennis LE, McCready H, Huckans M, Hoffman WF, Loftis JM, 2019. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol Biochem Behav. 179:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokane SS, Perrotti LI, 2020. Sex Differences and the Role of Estradiol in Mesolimbic Reward Circuits and Vulnerability to Cocaine and Opiate Addiction. Frontiers in behavioral neuroscience. 14, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Hwang SM, Jang JS, Ryu BY, Kang BY, Kang SS, Lee JJ, 2019. Effects of a Preoperative Transdermal Fentanyl Patch on Proinflammatory Cytokine and Pain Levels During the Postoperative Period: A Randomized Controlled Trial. Surg Laparosc Endosc Percutan Tech. 29:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Austin BP, Strickland JC, 2020. The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addiction biology. 25, e12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy R, Strickland J, Feinstein M, Robinson A, Smith M, 2016. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology. 233, 3201–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DG, 2020. Opioids and the COVID-19 pandemic: does chronic opioid use or misuse increase clinical vulnerability? British journal of anaesthesia: BJA. 125, e382–e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. 2002. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 164(2):121–137. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, 2006. Sex Differences in Vulnerability to Drug Self-Administration. Exp Clin Psychopharmacol. 14, 34–41. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC, 2001. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 74, 435–440. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E, 2017. Oxycodone self-administration in male and female rats. Psychopharmacology. 234, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM, Fiellin DA, 2018. Women and opioids: something different is happening here. The Lancet. 392, 9–11. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA 2012. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 5;(67):e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Takahashi JS, 2013. Central circadian control of female reproductive function. Frontiers in endocrinology. 5, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H, Tabaeizadeh M, Shaterian-Mohammadi H, Tahsili-Fahadan P, Dehpour AR, 2009. Estrogen pretreatment modulates morphine-induced conditioned place preference in ovariectomized mice. Pharmacology, Biochemistry and Behavior. 92, 399–403. [DOI] [PubMed] [Google Scholar]
- Molina-Martinez LM, Gonzalez-Espinosa C, Cruz SL, 2014. Dissociation of immunosuppressive and nociceptive effects of fentanyl, but not morphine, after repeated administration in mice: fentanyl-induced sensitization to LPS. Brain Behav Immun. 42:60–4. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A, 1985. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology. 86, 274–280. [DOI] [PubMed] [Google Scholar]
- Napier TC, Herrold AA, de Wit H, 2013. Using conditioned place preference to identify relapse prevention medications. Neuroscience and biobehavioral reviews. 37, 2081–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You Z, McCarthy MM, Shaham Y, Ikemoto S, 2019. Incubation of Cocaine Craving After Intermittent-Access Self-administration: Sex Differences and Estrous Cycle. Biological Psychiatry. 85, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y, 2013. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacology, Biochemistry and Behavior. 110, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell JK, Halpin J, Mattson CL, Goldberger BA, Gladden RM, 2017. Deaths Involving Fentanyl, Fentanyl Analogs, and U-47700 — 10 States, July–December 2016. MMWR. Morbidity and mortality weekly report. 66, 1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka H, Fujita K, Kobayashi H, 2007. Pharmacokinetics of Fentanyl in Male and Female Rats after Intravenous Administration. Arzneimittelforschung. 57, 260–263. [DOI] [PubMed] [Google Scholar]
- Osmanlioglu HO, Yildirim MK, Akyuva Y, Yildizhan K, Naziroglu M, 2020. Morphine Induces Apoptosis, Inflammation, and Mitochondrial Oxidative Stress via Activation of TRPM2 Channel and Nitric Oxide Signaling Pathways in the Hippocampus. Mol Neurobiol. 57(8):3376–3389. [DOI] [PubMed] [Google Scholar]
- Paccola CC, Resende CG, Stumpp T, Miraglia SM, Cipriano I 2013. The Rat Estrous Cycle Revisited: A Quantitative and Qualitative Analysis. Anim Reprod. 10, 4, 677–683. [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ, 2014. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology. 231(13):2661–70. [DOI] [PubMed] [Google Scholar]
- Peterson B, Mermelstein P, Meisel R, 2015. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 220, 2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech RH, Briggs SL, Mokler DJ, 2010. Fentanyl and spiradoline interactions in a place-conditioning black-white shuttle-box. Pharmaceuticals. 4, 101–116. [Google Scholar]
- Reid LD, Marglin SH, Mattie ME, Hubbell CL, 1989. Measuring morphine’s capacity to establish a place preference. Pharmacology, biochemistry and behavior. 33, 765–775. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2000. The psychology and neurobiology of addiction: an incentive–sensitization view. Addiction. 95, 91–117. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015. MMWR. Morbidity and mortality weekly report. 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Serdarevic M, Striley CW, Cottler LB, 2017. Sex differences in prescription opioid use. Current opinion in psychiatry. 30, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ethridge SB, Gibson AN, Schmidt KT, Sharp JL, 2020. The Effects of Artificially Induced Proestrus on Heroin Intake: A Critical Role for Estradiol. Exp Clin Psychopharmacology. [DOI] [PMC free article] [PubMed]
- Smith MA, Ethridge SB, Pearson T, Zhang H, Marcus MM, Ballard SL, Casimir AT, Potter KM, Schmidt KT, Sharp JL, Robinson AM, 2021. Modulation of heroin intake by ovarian hormones in gonadectomized and intact female rats. Psychopharmacology. 238, 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K, Scharfman H, 2005. A woman’s prerogative. Nat Neurosci. 8, 697–699. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW, 2011. Drug Wanting: Behavioral Sensitization and Relapse to Drug-Seeking Behavior. Pharmacological reviews. 63, 348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BD, Tyndall M, Walsh SL, 2020. 514698. Nature Reviews Disease Primers. 6, 1–28. [DOI] [PubMed] [Google Scholar]
- Tan S, Xue S, Behnood-Rod A, Chellian R, Wilson R, Knight P, Panunzio S, Lyons H, Febo M, Bruijnzeel AW, 2019. Sex differences in the reward deficit and somatic signs associated with precipitated nicotine withdrawal in rats. Neuropharmacology. 160, 107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML, 2019. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology. 44, 2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Kubota KS, Warmoth KP, 2004. Continuous administration of opioids produces locomotor sensitization. Pharmacology, biochemistry and behavior. 79, 661–669. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, 2007. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction biology. 12, 227–462. [DOI] [PubMed] [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LAM, 2011. Estradiol Modulates Effort-Based Decision Making in Female Rats. Neuropsychopharmacology. 37, 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick GJ, Rosenblum D, Mars S, Ciccarone D, 2013. Intertwined Epidemics: National Demographic Trends in Hospitalizations for Heroin- and Opioid-Related Overdoses, 1993–2009. PloS one. 8, e54496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW, 2017. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PloS one. 12, e0187698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren Louk J. M. J, Kalivas PW, 2000. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 151, 99–120. [DOI] [PubMed] [Google Scholar]
- Vitale MA, Chen D, Kanarek RB, 2003. Chronic access to a sucrose solution enhances the development of conditioned place preferences for fentanyl and amphetamine in male Long–Evans rats. Pharmacology, biochemistry and behavior. 74, 529–539. [DOI] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H, 2012. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci.109:6325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YJ, Chen C, Fillingim R, Schmidt SO, Winterstein AG, 2019. Trends in prescription opioid use and dose trajectories before opioid use disorder or overdose in US adults from 2006 to 2016: A cross-sectional study. PLoS medicine. 16, e1002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith Herschel 4., Davis NL, 2020. Drug and Opioid-Involved Overdose Deaths - United States, 2017–2018. MMWR. Morbidity and mortality weekly report. 69, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachry JE, Nolan SO, Brady LJ, Kelly SJ, Siciliano CA, Calipari ES. 2021. Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology. 46(3):491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) All the female treatment groups showed a significant increase in LMA from day 1 to day 8. Line graph points display means ± SEM (****p < 0.0001 when comparing day 1 vs. day 8 for each respective group).
(A) In female rats, LMA during drug context training days showed no time-dependent changes in LMA for any treatment group, but significant differences were observed between groups on specific days. The low dose (4 μg/kg) fentanyl group was significantly different from saline on days 5 or 7 (*p < 0.05), between high dose (16 μg/kg) fentanyl group was significantly different from saline on all treatment days (*p < 0.05, ***p< 0.001, or **p < 0.01), and between low dose and high dose fentanyl on day 7 (*p < 0.05). (B) In male rats, LMA during drug-context training days was not different between groups on specific treatment days; however, significant changes across days for saline-control was observed between day 3 and days 5, 7, or 9 (*p < 0.05) and across days for low dose fentanyl between day 3 and days 5, 7, or 9 and between day 9 and days 5 or 7 (*p < 0.05). Line graphs display means ± SEM.
(A) There was no effect for the estrous phase (i.e., estrus vs. non-estrus) on LMA on the first day of Open Field testing. (B) After 8 days of repeated injections, there was no effect for the estrous phase on LMA. (C) The estrous phase also had no effect on LMA during the test day of the CPP paradigm. (D) Preference for the fentanyl-associated context, when measured on test day, was not affected by estrous phase. Bar graphs display means ± SEM, with points representing individual values.


