Abstract
Sex and age have distinct influences and roles in behavior and immune reactivity; yet, most studies use adult male rodents with little attention to middle age, a time associated with key physiological transitions in both sexes. Thus, this study investigated sex differences during middle age in behavior, immune response to lipopolysaccharide (LPS), and glucose regulation in C57BL/6 mice with GFP-tagged monocytes/microglia. Behaviorally, males performed better in tests of motor function (Open Field [OF], Grip Strength, Sticker Removal, Gait, and Pole tests) and displayed less depressive- and anxiety-like behaviors across multiple mood tests (OF, Elevated Zero Maze, Sucrose Preference, and Swim test). However, females performed better in tests of cognition (Barnes Maze and Novel Object Recognition). Following behavioral assessment, mice were given LPS to characterize sex-dependent inflammagen responses. Females displayed greater sickness behavior in the OF, higher levels of peripheral cytokines, and subtle neuroinflammation in the cortex, striatum, and hippocampus. A separate middle-aged cohort was used for glucose tolerance and insulin sensitivity testing. Both sexes had excessive blood glucose rebound after insulin challenge, but displayed differences following glucose administration, where males had higher baseline glucose and females remained hyperglycemic. This study suggests that during middle-age male mice have better emotional regulation and motor function, but not cognitive ability than females. Further, males are less sensitive than females to the acute effects of LPS peripherally and centrally, but both sexes showed sex-specific impairments in blood glucose regulation. Overall, it appears that middle age is an important transition point with multiple sex differences, some of which are unique to this stage of life.
Keywords: Middle-age, sex, behavior, LPS, inflammation, glucose homeostasis
1. Introduction
Senescence is the progressive deterioration of living cells and systems that, while ubiquitous to all organisms, varies widely in speed depending on species, cell, and system type [1]. One facet of the aging process, neurodegeneration, is difficult to study due to the brain’s susceptibility to lifespan environmental influences and its innate plasticity [2]. As neurons age, they undergo structural and functional alterations resulting in dedifferentiation, a process where highly specialized and segregated neural networks and systems become more simplified [3]. In fact, human imaging studies suggest these age-dependent alterations are especially noticeable during the transition to middle-age (approximately 40–65 years) [4, 5]. Diagnosed emotional disturbances at this age are strong predictors for later development of dementias, illuminating the importance of understanding what is “normal” at middle-age [6, 7]. However, the relationship between cognitive function in middle age with future development of dementia is markedly understudied, with one current longitudinal study underway [8]. This is the case for most age-related research, including in laboratory rodents, that focuses primarily on pediatric development or the elderly, largely ignoring the middle-aged, which, in the United States, makes up a fourth of the population [9].
Sex is an understudied variable in the trajectory of healthy brain aging, but plays an integral role in the organism’s functional development by producing innate differences between males and females from a chromosomal level to global physiology [10]. Despite the importance of sex differences, research lacks a focus on female-based studies and male: female comparison studies [11]. Although many human studies now investigate both genders at the urging of the National Institutes of Health (NIH), laboratory investigations have been slower to address this disparity [12]. Nevertheless, some behavioral studies have identified key sex differences in humans and rodents. For example, adult females reliably exhibit higher anxiety-like behaviors [11, 13–17], while adult males show an advantage in spatial memory, but not in landmark recognition [18–20]. Further, within motor function, adult female rodents are reported to be more active and more coordinated than males [21–23]. However, these studies used young adult rodents (< 4 months old) that consequently exclude comparisons of sex at later stages of life.
Multiple physiological methods are utilized to translate the age of a mouse to that of a human [24]. Although the rate of aging may vary among different rodent strains, a consensus for a middle-age range in a healthy laboratory mouse is 12–15 months [24] and utilizing the natural aging process allows for characterization of differences across a range of variables. The few animal studies investigating behavioral and neurological sex differences [25, 26] have not focused on the middle-age period. As the brain ages, it undergoes significant remodeling and, in females, is further subjected to abrupt hormone alterations during middle age as women undergo pre-menopause and perimenopause [5, 27]. Beginning at middle-age, testosterone also decreases age-dependently, leading to more male-linked neural changes, but this decrease has a gradual trajectory compared to estrogen [28]. These sex steroids are heavily linked to mood and cognitive ability and are required for optimal neural functioning [28–30]. The impact of steroid loss is especially noticeable in mental illness demographics; middle-aged women are more likely to experience mood dysfunction than both age-matched men and other female age groups [31].
Sex has also been linked to immune differences, i.e., females produce more robust immune responses to the same infectious load as males [32], while males experience fewer autoimmune disorders [32, 33]. It is commonly accepted that age decreases one’s adaptive immunity due to difficulty sustaining immune cell populations and function [34, 35]. However, the effect of sex on immunity, particularly innate immunity, in middle-aged and older populations remains unclear. The immune system is controlled by a host of signaling molecules that, within the context of innate immunity, can be labeled as pro-inflammatory or anti-inflammatory [36]. It has been reported that females produce more anti-inflammatory cytokines than males; however, as females reach old age, this production of anti-inflammatory cytokines plateaus or diminishes [34]. This, together with their greater infection response [32], suggests female hyper-responsiveness early in life that may be lost with age, but it is unknown if this difference is still present during the middle age.
Like inflammation, glucose homeostasis is a physiological response to the introduction of exogenous molecules (i.e., food), and its maintenance is also less efficient with age [37]. When blood glucose rises, insulin is released from pancreatic β-cells into the blood stream and acts on various targets to activate glucose uptake and maintain glucose homeostasis [38]. For proper function of this process, β-cells must sense elevated blood glucose and, in turn, produce and release sufficient insulin; further, other tissues must respond to insulin and react, as necessary. Impairments in this process, such as the inability to sense excessive glucose levels or unresponsive target tissues, can lead to insulin resistance, persistent hyperglycemia, and associated complications [38]. Understanding glucose tolerance and insulin sensitivity is a top priority for human health as worldwide diabetes prevalence continues to grow, especially in middle-aged populations [39]. While we recently reported that insulin resistance increases with age in female C57BL/6 mice [40], how this compares to males, especially during the middle-aged period, is unknown. Importantly, dysregulated glucose homeostasis is associated with neurological dysfunction [41]; thus, is important to study this relationship in the middle-aged.
The present study aimed to characterize the sex differences in the often-overlooked middle-aged group, so this stage of life may be better understood and offer a glimpse into sex-dependent aging alterations for the benefit of the growing middle-aged population. First, a variety of behavioral tests were utilized to assess motor function, cognitive ability, and mood between male and female middle-aged mice. Further, LPS was administered as an inflammatory challenge to identify sex-linked differences in sickness behavior and innate immunity. Finally, in a separate cohort of male and female middle-aged mice, glucose tolerance and insulin sensitivity were assessed to understand sex-dependency of the metabolic implications of middle-age.
2. Materials and Methods
2.1. Animals
Age-matched (Cohort 1: 12 months-old and Cohort 2: 14 months-old at the beginning of the experiments) C57BL/6-mice with GFP-tagged monocytes/microglia (CX3CR1-GFP, Jackson Labs, Bar Harbor, ME) were group-housed (4–5/cage) in an environment-controlled room (20–22 °C) and maintained on a 12 h normal light/dark cycle (0700–1900, lights on). Food and water were available ad libitum. All animal experiments were approved in advance by the University of Georgia Institutional Animal Care and Use Committee, and all procedures were in line with the ARRIVE guidelines and latest NIH recommendations.
2.2. Experimental Design
Figure 1 depicts the study timeline for both mouse cohorts used in this study. Cohort 1 (N=19) was aged to 12 months, and a subgroup of male and female mice (n=8/sex) were used for behavioral testing (Figure 1A). Prior to behavioral testing, all mice were handled daily for one week to minimize experimenter-induced stress. In addition, for females, the estrous cycle in was staged daily for 5 days using methods from [42, 43]; briefly, daily vaginal lavages were performed, wet mounted to a slide, and evaluated under a microscope. All females were determined to be in persistent diestrus prior to the start of the study.
Figure 1. Study Timeline.
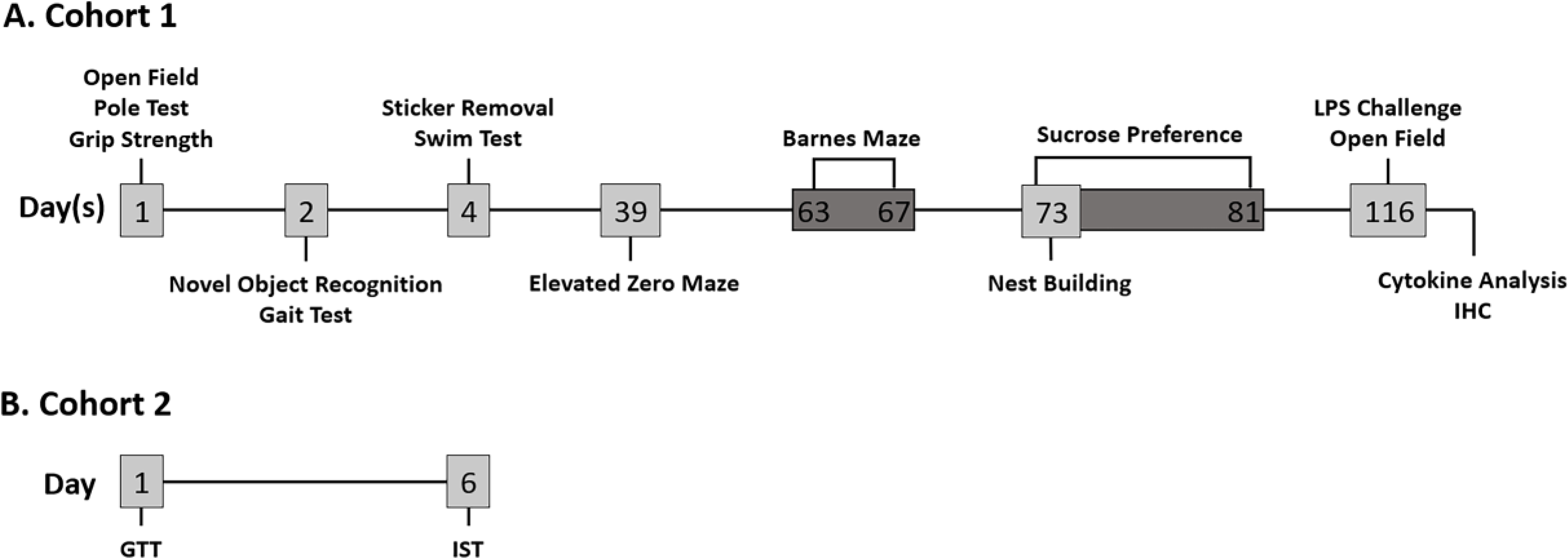
Two separate cohorts underwent testing. (A) Cohort 1 (N=19) was aged to 12 months prior to behavioral testing (n=8/sex). Behavioral tests were spread out over a 3-month span; the majority were performed during month 1 of testing (Open Field, Pole Test, Grip Strength, Novel Object Recognition, Gait Test, Sticker Removal, and Swim Test). The Elevated Zero Maze was performed during month 2, and the Barnes Maze, Nest Building, and Sucrose Preference tests were done during month 3. At 14.5 months of age, these mice, plus the 3 additional mice co-housed/handled with Cohort 1, were randomly given LPS (n = 5/sex) or saline (n = 5 female, 4 male). Four h post-LPS, they underwent a 15-min OF test and were sacrificed 6 h post LPS-treatment. (B) Cohort 2 (N=12; n=6/sex) was aged to 14 months and underwent the Glucose Tolerance Test (GTT) followed by the Insulin Sensitivity Test (IST) 6 days later. Both tests were done after a 3-h fast.
Over the course of 2.5 months, Cohort 1 underwent an established battery of tests in a sequence designed to minimize test-to-test effects and/or stress similar to [40, 44]. All behavioral testing was conducted in designated rooms during the animals’ light cycle (0800–1500 h); mice from a single sex were present during testing and testing environment was thoroughly sanitized to prevent influence of sex. All tests were performed in the same order at similar times for each mouse.
At 14.5 months of age, mice were injected (0700–1000 h) either with LPS (n=5/sex) or saline (n=4–5/sex) four h before beginning a 15-min Open Field (OF) test (1100–1400 h). Six h post LPS injection (1300–1600 h), mice were sacrificed, and blood/tissues were collected.
Cohort 2 consisted of a separate set of 14-month-old male and female mice (N=12; n=6/sex) that were used for the Glucose Tolerance test (GTT; 1000–1200 h) and 6 days later, the Insulin Sensitivity test (IST; 1000–1200 h) as in [40] and as outlined in Figure 1B.
2.3. Motor Function Tests
2.3.1. Pole Test (PT)
The PT was conducted as previously described to assess motor coordination [44]. The PT is accepted as a sensitive measure of bradykinesia as it allows for assessment of basal motor coordination deficits that are not confounded by practice-based motor improvements [45]. Prior to testing, mice were acclimated to testing conditions by placing the mice on the pole and turning them to descend the pole. On the day of the test, mice were placed facing upwards on a gauze-wrapped pole (55 cm tall) and timed on latency to turn (60 s max) and total time to complete the test (120 s max) over 4 trials (5-min intertrial interval). The best and average performance of the trials were analyzed.
2.3.2. Grip Strength Test (GST)
This test was done as previously described in [44]. Briefly, mice were gently placed on a 6 cm × 6 cm wire grid and forepaw grip strength (N) was measured using a strength gauge (Bioseb, France) over 4 trials (1-min intertrial interval); the average and max grip strength were analyzed.
2.3.3. Gait Test (GT)
The GT was used to quantify multiple gait related parameters so that motor function abnormalities may be identified, according to [46] with some modifications. Briefly, a white paper-covered custom runway (82 cm long, 5.5 cm wide with 8 cm high walls) with an escape cage containing home-cage bedding placed at the end of the runway was used. Prior to testing, the front and hind paws of the mice were painted with non-toxic red and black ink (Office Depot, item: #839–994 and #839–967), respectively, and two, timed trials (3-min intertrial interval) were conducted. The number of steps, base width, stride length, inter- and intra-step distance, stride variability, and velocity were evaluated as in [46].
2.3.4. Sticker Removal Task (SRT)
To measure fine motor skills, a small round sticker (Avery 1/4th inch round label) was placed using forceps on the snout of each mouse [47]. Mice were placed in a cage filled 1/4th with bedding from their home-cages and had 90 s to remove the sticker; time to first contact and time to remove the sticker were measured with a stopwatch. Each mouse had 3 trials (5-min intertrial interval); the averages of all trials were analyzed.
2.3.5. Open Field (OF) Test
The OF was performed as in [44] to measure locomotion and anxiety-based parameters. Mice were placed in an opaque square arena (25 cm L × 25 cm W × 30 cm H; Coulbourn Instruments, Whitehall, PA) and recorded from above with Limelight tracking software (Actimetrics, Wilmette, IL) for either 30-min (initial OF) or 15-min (post-LPS OF). Locomotor parameters, including distance traveled and crossings through the zones, were automatically tracked by the software; rearing behavior was manually scored from the recording by an experimenter blind to study conditions [44]. Data were analyzed for the entire test and per 5-min intervals. Time spent in the center zone (12.5 cm L × 12.5 cm W) was measured to assess anxiety-like behavior [44]; data for this measure were analyzed for the first 5-min of the test to correspond to data collected during the Elevated Zero Maze.
2.4. Mood Tests
2.4.1. Elevated Zero Maze (EZM)
The EZM apparatus (Stoelting Co., Wood Dale, IL) is a circular, elevated platform divided into zones (2 walled “closed” arms, 2 open arms) that measures anxiety-like behavior [48]. The maze was illuminated by a minimally anxiogenic red light (8W red bulb) [49]; lighting conditions were measured by an URCERI light meter (30–40 lux in open, 10–15 lux in closed). Mice were placed on the center of an open arm and allowed free maze exploration for 5-min. Time and entries in each zone were recorded and scored by ANY-maze software (Stoelting); 70% of the mouse’s body was required to be in a zone [48].
2.4.2. Swim Test (ST)
The ST was performed as described in [44] to measure depressive-like behavior. Mice were placed individually into a 3 L beaker filled with warm water (30 ± 1 °C) and their behavior was recorded with Limelight tracking software (Actimetrics) for 15-min. Total climbing counts and time spent climbing, mobile, and immobile (both total and per 5-min interval) were scored by a blind observer using the Limelight software.
2.4.3. Sucrose Preference (SP)
The SP test was used to measure depressive-like behavior, specifically anhedonia [50]. Individually housed mice were given access to two bottles of water for 4 days to establish a baseline. Following baseline, mice had access to one bottle of water and one bottle of 1.5% sucrose water for 4 days. Solution intake was measured by daily bottle weighing and bottle position was switched after each measurement to prevent side preference. Sucrose preference (%) was determined using the following equation: [(total sucrose intake / (sucrose + water intake)) × 100] [50].
2.5. Cognition Tests
2.5.1. Novel Object Recognition (NOR)
The NOR assesses short-term recognition memory and novelty detection [51] and was performed as described in [40] with the OF on the previous day serving as a habituation phase for the NOR. During test’s identical phase, mice were placed in the arena with two identical objects (blocks; each with 33.2 cm3 volume) and allowed to freely explore them for 5-min, before returning to their home cage for 1 h. During the novel phase, one identical object was replaced with a similarly sized novel object (ball; 33.5 cm3 volume) and the mice were allowed a 5-min exploration of the novel and identical objects [40]. To control for any object configuration preference, objects were rotated so that four possible configurations were evenly divided between the mice. Utilizing the nose of the mouse as a reference point, the number of approaches to each object and time spent in the object zone (10 cm L × 10 cm W) were scored manually using the Limelight tracking software (Actimetrics) in a blind manner. Only one approach was counted once the mouse entered the object zone, regardless of exploration, and time in the object zone was recorded until the mouse exited the zone [40].
2.5.2. Barnes Maze (BM)
The BM measures long-term spatial memory and learning ability without the need of food restriction [52]. The circular maze (Stoelting) has 20 holes around the circumference with one hole equipped with an escape box located underneath (target hole; TH). The maze is brightly illuminated to promote anxiogenic escape motivation (~1000 lux measured by URCERI light meter). Mice were habituated to the maze one day prior to the acquisition phase. During the acquisition phase (days 1–4), mice were trained to learn and escape into the TH over four 3-min trials; after trial completion, mice remained or were manually placed into the TH for 1-min. For the probe trial, the escape box was removed, and mice freely explored the maze for 90 s [53]. Between mice, the maze was rotated and sanitized with Rescue disinfectant to remove any residual olfactory cues. Videos were recorded and analyzed using ANY-maze software (Stoelting). For the acquisition phase, the average and total errors made, time to reach TH, and distance to TH were analyzed. For probe trial, TH approaches, time to reach TH, errors made prior to TH, and distance traveled to TH were analyzed.
2.5.3. Nest Building
Nest building was assessed prior to the SP test to measure cognitive, particularly hippocampal, function [54], and motor function, as it has been reported that striatal damage leads to nest building deficits, likely through forelimb and orofacial dexterity impairments [55]. Mice naturally build nests to maintain body temperature regardless of sex, and nests built for maternal purposes have a distinct appearance from typical nests [56, 57]. Since the females used were nulliparous and, therefore, not subject to confounding pregnancy hormones at any point in their life, this test was appropriate to assess sex differences in cognitive and motor function. Mice were individually housed with a fresh paper nestlet; pictures of the nests were taken from above at different time points (30, 60, 90, 120, 180, 240, 300, and 360-min, and 24-h) and blindly scored later based on a 4-point scale (1 = untouched, 2 = nestlet partially shredded <50%, 3 = nestlet mostly shredded >50–90%, but no identifiable nest, 4 = nest >90 % completed) as in [54].
2.6. Lipopolysaccharide (LPS) Challenge and OF for sickness behavior
Mice within each sex were randomly selected to receive via an IP injection either saline vehicle (n=4/5; male/female) or 0.3 mg/kg BW LPS (Escherichia coli serotype 0111:B4, Sigma Aldrich; St. Louis, MO) (n= 5/5; male/female) that has previously been found to induce behavioral alterations and neuroinflammation in C57BL/6 mice [44]. Three behaviorally naive mice co-housed with this cohort were used for weight monitoring, LPS administration, and postmortem plasma cytokine analysis, but not for the 15-min OF 4 h post-LPS (n=4/group/sex) to assess sickness behavior due to OF test timing limitations.
2.7. Euthanasia and Sample Collection
Six h post-LPS injection, mice were euthanized, blood was collected into Na citrate tubes (0.109M, 3.2%, B&D, San Jose, CA) for plasma harvesting, and tissues (brain, inguinal lymph nodes, spleen, thymus, liver, kidney, and fats) were weighed and frozen on dry ice. For the brain, a sagittal cut was made and half of the brain was fresh frozen, while the other half was immersion- fixed in 4% paraformaldehyde for 48 h, cryoprotected in 30% sucrose for 72 h, and flash frozen in isopentane as in [58]. All samples were stored at −80° C until analysis.
2.8. Plasma and Brain Cytokine Analysis
A Milliplex Cytokine Panel (EMD Millipore Corporation; Billerica, MA) that measures concentrations of the following: interferon gamma (IFNγ), macrophage inflammatory protein 3 alpha (MIP-3α/CCL20), interleukin (IL)-1β, IL-22, IL-23, IL-27p70, IL-27, IL-15, IL-17A, Il-17/IL-25, IL-17F, IL-33, IL-31, tumor necrosis factor alpha (TNFα) and beta (TNFβ), IL-4, IL-5, IL-28B, IL-10, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), CD40 ligand (CD40L), and IL-2 was used to assess cytokines and chemokines in the plasma and brain (cortex and striatum). Brain sample lysates were prepared as in [59] with slight modifications. Briefly, the brain was sectioned into 500-μm thick slices and 3 punches (1.5 mm diameter) were taken from both the cortex (approximate bregmas 2.58, 2.08, 1.58; [60]) and striatum (approximate bregmas 1.58, 1.08, 0.58; [60]); punches from each region were pooled on a per animal basis and weighed in tubes. Samples were then reconstituted in RIPA buffer without 0.1% SDS, containing Halt Protease/Phosphatase Inhibitor cocktail (5 mg tissue/75 μl; ThermoFisher 78442) on ice, sonicated, and centrifuged for 10-min at 10,000 rcf and 4° C before supernatant (SN) collection. Per manufacturer’s instructions, plasma and brain lysate SN were added to a 96-well plate followed by the addition of premixed, antibody-immobilized beads and incubated with agitation on a shaker at 4° C overnight. Following washes, detection antibodies were added and incubated for 1 h followed by addition of Streptavdidin-Phycoerythrin for 30-min with agitation on a shaker at room temperature. After the final washes, sheath fluid was added, and the plate was run on a MagPix instrument using xPONENT v.4.2 (Luminex Corp., Austin, TX). Data were analyzed with Milliplex Analyst software, v.5.1 (EMD Millipore). Data were extracted based on either a 4- or 5-parameter log curve. A heat map of the plasma results post-statistical analysis was generated using gplots library in R 3.4.2 software [61].
2.9. Immunohistochemistry
Fixed brains were coronally sectioned into 40 μm thick sections and placed in phosphate buffer at 4°C until immunofluorescent staining processing. Free-floating sections containing the striatum (STR; 1:4 series, approximate bregmas 1.34 - −0.34; [60]) and hippocampus (1:6 series, approximate bregmas −1.34 - −3.16; [60]) were immunostained for glial fibrillary acidic protein (GFAP). Briefly, sections were permeabilized with 1% TritonX-100/0.5% Tween20 in PBS for 30 min and washed (3x) before blocking sections in 3% normal goat serum for 30-min at room temperature (RT). Sections were then incubated with the primary antibody (1:500 rabbit anti-GFAP, Dako) diluted in 0.1% TritonX-100 in PBS for 48 h at 4°C. Following primary antibody incubation, sections were thoroughly washed and incubated with the appropriate secondary fluorescent antibody (1:1000 donkey anti-rabbit 594, Invitrogen) for 2 h at RT in the dark, followed by a 5-min nuclear stain incubation (Hoechst 33258, Invitrogen). Following the final washes, sections were mounted to slides, fixed with an ethanol gradient followed by xylene clearing, and coverslipped with VectaMount (Vector Labs). Images were captured using a Zeiss Axioscope A1 microscope, and analysis of GFP+ microglia (green) and GFAP+ astrocytes (red) intensity was done using ImageJ. Regions of interest in the STR included the dorsolateral (DL) and ventromedial (VM) STR and were captured at 5x, whereas the hilus of the hippocampus was captured at 10x. Four mice per LPS-treatment/sex were used for statistical analysis of the fluorescent intensity of each subregion (e.g., DL and VM STR, hilus of hippocampus) using 6 slices/region/mouse.
2.10. Glucose Tolerance (GTT) and Insulin Sensitivity Tests (IST)
A separate cohort of male (n=6) and female (n=6) mice aged 14 months was used for GTT and IST. Mice were fasted for 3 h prior to each test as in [40]. For the GTT, a baseline glucose measurement was taken and then mice were given glucose (2 g/kg BW; Sigma Aldrich) via an oral gavage. Blood glucose levels were measured at 15, 30, 60, 90, and 120-min. After a 6-day rest, mice underwent IST during which mice received insulin (0.5 IU/kg BW IP; Sigma Aldrich) and blood glucose was subsequently measured at 15, 30, 60, 75, 90, 105, and 120-min [40]. For both tests, the blood was collected via the tail vein and measured using a glucose monitor (TRUEtrack, Trividia Health, Inc, Fort Lauderdale, FL; strips lot number: RV5255).
2.11. Statistical Analysis
All statistical analyses were done using Sigmaplot 12.5 (Systat Software, Inc., Chicago, IL) and graphs were generated using GraphPad Prism 5 (San Diego, CA). A two-way repeated measures (RM) ANOVA was used to assess behavior parameters across 5-min intervals for the OF and ST, day-to-day analysis of the BM acquisition phase, and time point analysis of the Nest Building test, GTT, and IST. A two-way ANOVA was used to assess parameters for the entire test and within an interval for the OF and ST, plasma and brain cytokine analysis, and immunohistochemistry. For all ANOVAs, Student-Newman-Keuls (SNK) post-hoc comparisons were run if a main effect or interaction was detected. The PT, GST, GT, SRT, EZM, SP, NOR, and BM probe trial all used t-tests (male vs female) to analyze parameters stated in their respective methods. A p-value of ≤ 0.05 was considered significant.
3. Results
3.1. Weights at 14.5 months old
At the conclusion at the study, no significant differences were observed in overall body or absolute brain, brown fat, or thymus weights between males and females (Table 1). Males had heavier kidneys (t(17)=−2.76, p ≤ 0.05) and livers (t(17)=−3.31, p ≤ 0.01) than females, while females had heavier spleens (t(17)=2.45, p ≤ 0.05) and compiled white fat depots (includes subcutaneous, retroperitoneal, and gonadal fat depots; t(15)=3.68, p ≤ 0.01) (Table 1).
Table 1.
Cohort 1 body and selected organ weights at end of study. Cohort I body and organ weights in grams (g) were assessed for males and females at the end of study (14.5 months). Data are presented as mean ± SEM; n = 9 male, n = 10 female.
| Sex | BW | Brain | Kidneys* | Liver* | Spleen # | White Fat # | Brown Fat | Thymus |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Male | 42.5 ± 1.1 | 0.49 ± 0.01 | 0.56 ± 0.02 | 1.75 ± 0.04 | 0.10 ± 0.01 | 2.15 ± 0.22 | 0.15 ± 0.01 | 0.03 ± 0.002 |
| Female | 42.5 ± 2.1 | 0.49 ± 0.004 | 0.47 ± 0.02 | 1.55 ± 0.05 | 0.15 ± 0.01 | 4.54 ± 0.65 | 0.10 ± 0.02 | 0.03 ± 0.002 |
indicate increases p ≥ 0.05 in weight for males and females, respectively.
3.2. Males display higher levels of coordination, activity, and motor ability than females
Locomotor results from the OF test indicated that, for the entirety of the test, males traveled greater distance (Figure 2A inset; t(14)=2.21, p ≤ 0.05) and made more crossings (t(14)=2.48, p ≤ 0.05; Supplemental Table 1) than females. In line with this increased activity, males tended to rear more than females, and this approached significance (Figure 2B; t(14)=2.08, p = 0.056). Both sexes exhibited habituation as evident by decreases in the distance traveled over time (Figure 2A; F(5,70)=12.18, p ≤ 0.001) and this was significantly affected by sex (Figure 2A; F(1,70)=4.89, p ≤ 0.05). Here, activity (distance travelled) significantly decreased from interval 1 for all intervals in females (Figure 2A; p’s ≤ 0.001) and for intervals 3, 5, and 6 in males (Figure 2A; p’s ≤ 0.05), with the magnitude of the decrease in the males being smaller. Consequently, differences between males and females were observed at intervals 5 (p=0.065) and, especially, 6 (p ≤ 0.01).
Figure 2. Males were more active and displayed greater strength and better coordination in motor function tests.
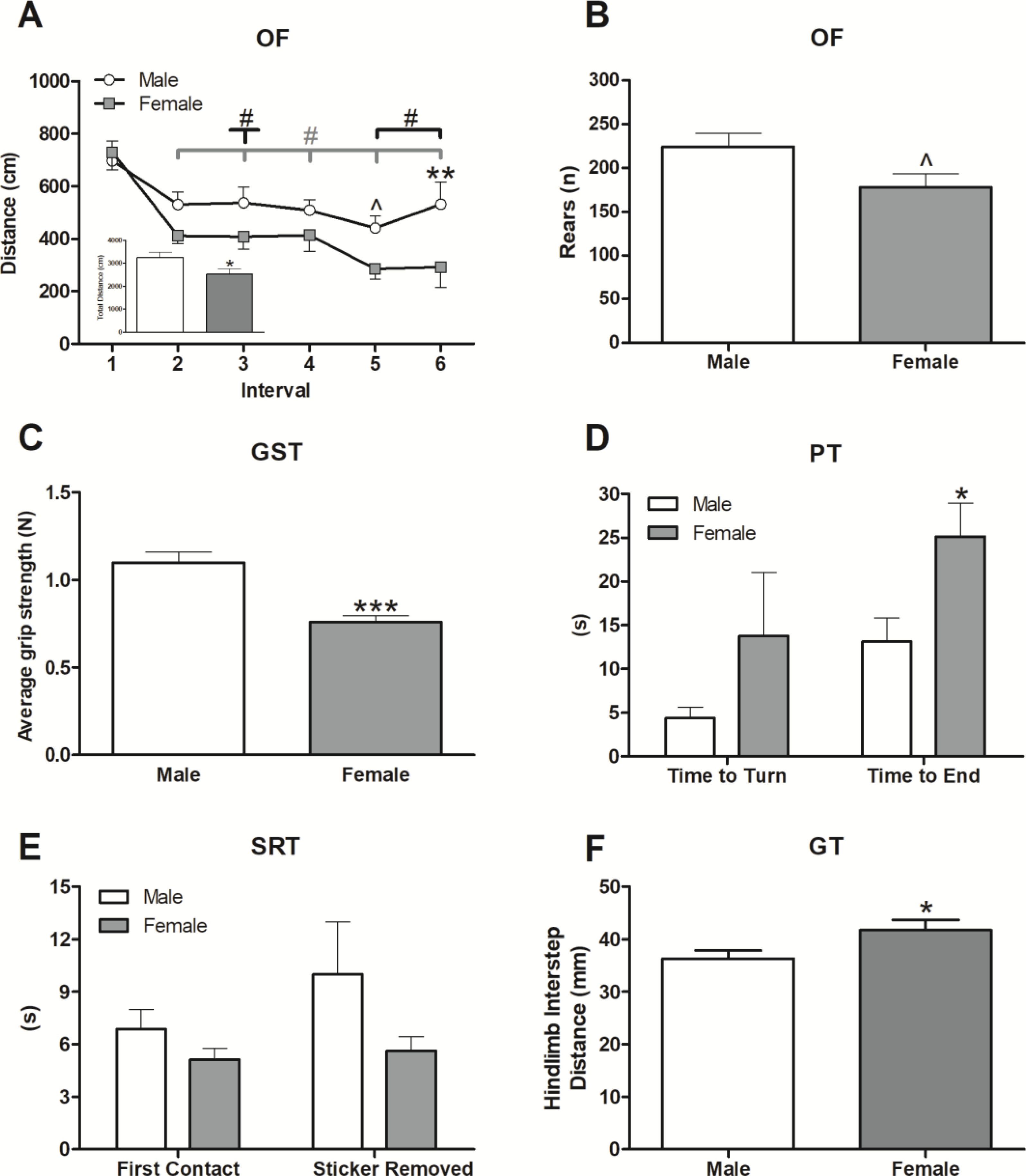
Locomotion was assessed in the Open Field (OF) using the parameters: distance traveled per 5-min interval (A), total distance traveled (inset A), and vertical movement (rears; (B)) throughout the test. Strength was measured with the Grip Strength Test (GST) (C), coordination was evaluated using the Pole Test (PT) (D), fine motor ability was assessed using the Sticker Removal Test (SRT) (E), and gait parameters were analyzed with the gait test (GT) (F). Data are presented as mean ± SEM; n = 8/sex; ^ indicate p ≤ 0.10; *, **, and *** indicate p ≤ 0.05, 0.01 and 0.001, respectively, between sex; # indicates p ≤ 0.05 within sex differences between interval 1 and indicated (line with downward tick) interval.
Males had stronger grip (Figure 2C; t(14)=4.96, p ≤ 0.001) than females, which was expected due to their lower adiposity despite similar body weights (Table 1). The males’ stronger grip coincided with faster pole descent (Figure 2D; t(14)=−2.56, p ≤ 0.05), indicating that pole climbing ability is independent of total body mass and likely demonstrates a sex difference in motor coordination due to differential muscle/fat composition. There were no significant sex differences in ability to turn during the PT (Figure 2D). Fine motor ability, as tested by the SRT (Figure 2E), was unaffected by sex. During gait analysis, there were no significant differences among all measured parameters save for hindlimb interstep distance, which was greater in the females (Figure 2F; t(14)=2.19, p ≤ 0.05). This effect may be due, in part, to increased female adiposity (Table 1).
3.3. Females showed more anxiety-like behaviors than males
The EZM and some parameters of the OF both assess anxiety-like behavior in mice. In the EZM, females spent significantly more time in the closed arms of the maze than they did the open (Figure 3A; t(14)=−5.52, p ≤ 0.001), while males showed no preference. In addition, females had increased latencies to exit the closed arm after entering (Figure 3B; t(11)=−2.30, p ≤ 0.05), further suggesting higher female anxiety-like behavior. A similar effect was observed in the first 5-min of the OF, where a significant effect of location was present, in which female mice spent more time in the periphery than in the center (Figure 3C; F(1,28)=23.52, p ≤ 0.001). Further, within sex, females tended to have more dramatic shifts in the times spent in the periphery (e.g. higher) and center (e.g. lower) than males (Figure 3C and Supplemental Table 1; p’s = 0.07).
Figure 3. Females displayed higher levels of anxiety- and depressive-like behaviors.
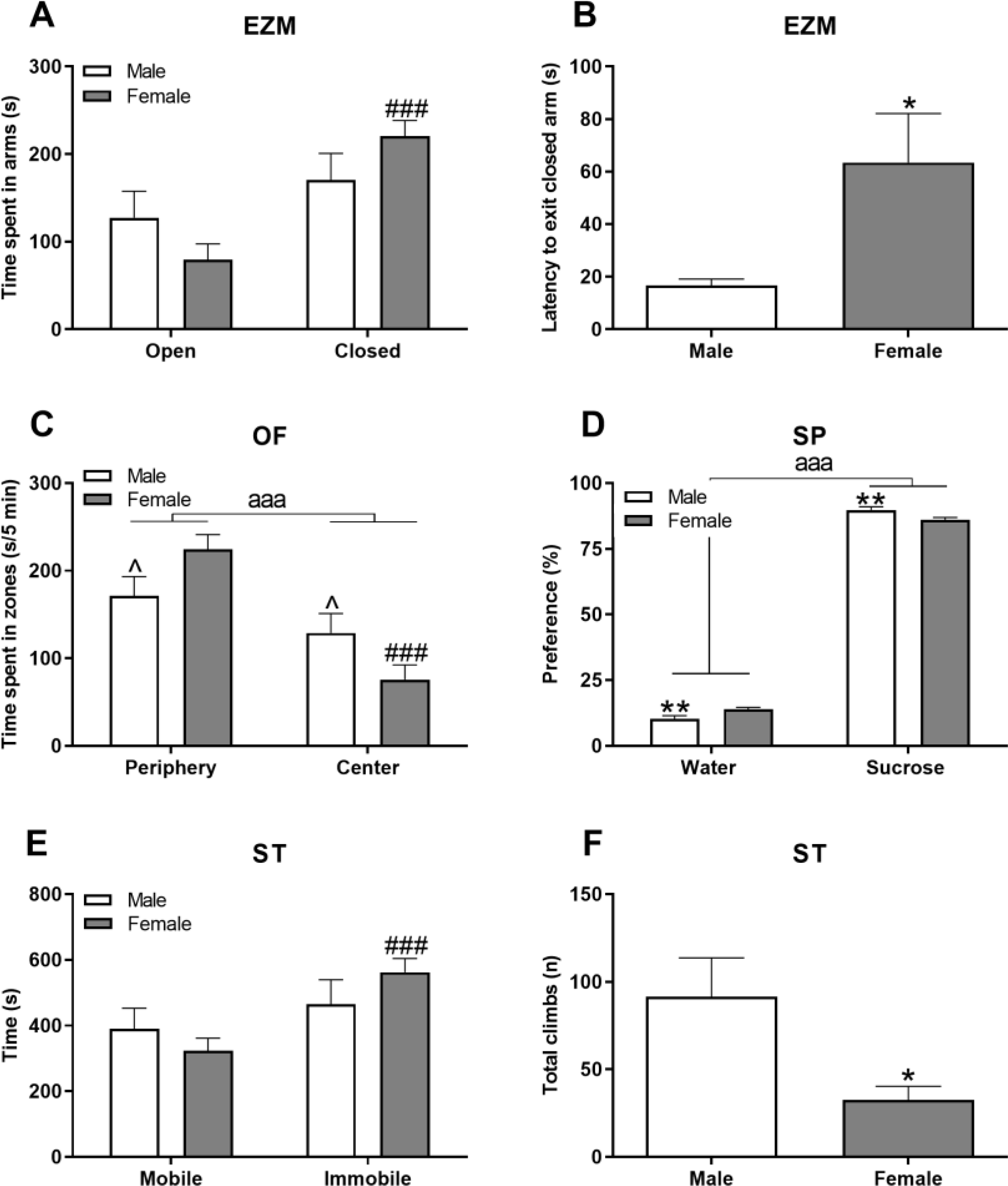
Anxiety was measured in the Elevated Zero Maze (EZM) based on time spent in the different arms of the maze (A) and the latency to exit the closed arm (B). The OF assessed anxiety-like behaviors based on time spent in the center vs periphery of the arena in the first 5-min (C). Depressive-like behavior was assessed with the Sucrose Preference test (SP) for anhedonia (D) and in the Swim Test (ST) by measuring the time spent swimming vs immobile (E) and total number of climbing bouts (F). Data are presented as mean ± SEM; n = 8/sex; *, **, and ^ indicate p ≤ 0.05, 0.01, and 0.10 respectively between sex; ### indicates 0.001 within sex; aaa indicates main sucrose/water or center/periphery effect where p ≤ 0.001.
3.4. Females showed more depressive-like behaviors than males
Both sexes showed a distinct preference for sucrose over water (Figure 3D; F(1,14)=131.48, p ≤ 0.001), and males displayed greater sucrose preference than females (Figure 3D; p ≤ 0.01). The ST also revealed higher levels of depressive-like behavior in females as they spent more time immobile than mobile throughout the test (Figure 3E; t(14)=−4.22, p ≤ 0.001) and this was not observed in males. Further, females made significantly fewer climbing attempts than males over the test duration (Figure 3F; t(14)=2.84, p ≤ 0.05).
3.5. Females performed better in tests of memory and cognition
In the NOR, females approached and tended to spend more time at the novel object more than the familiar object (Figure 4A; approaches t(14)=3.99, p ≤ 0.01 and Figure 4B; time t(14)=−2.04, p=0.06); males showed equal exploration of each object, suggesting middle-aged females detected novelty while males did not. Both sexes completed nest building over the course of 6 h (Figure 4C; F(8,112)=86.75, p ≤ 0.001); there was a slight female advantage early on, as females had higher nest building scores at 30 and 90 min (p’s ≤ 0.05).
Figure 4. Females performed more effectively in multiple cognitive tests.
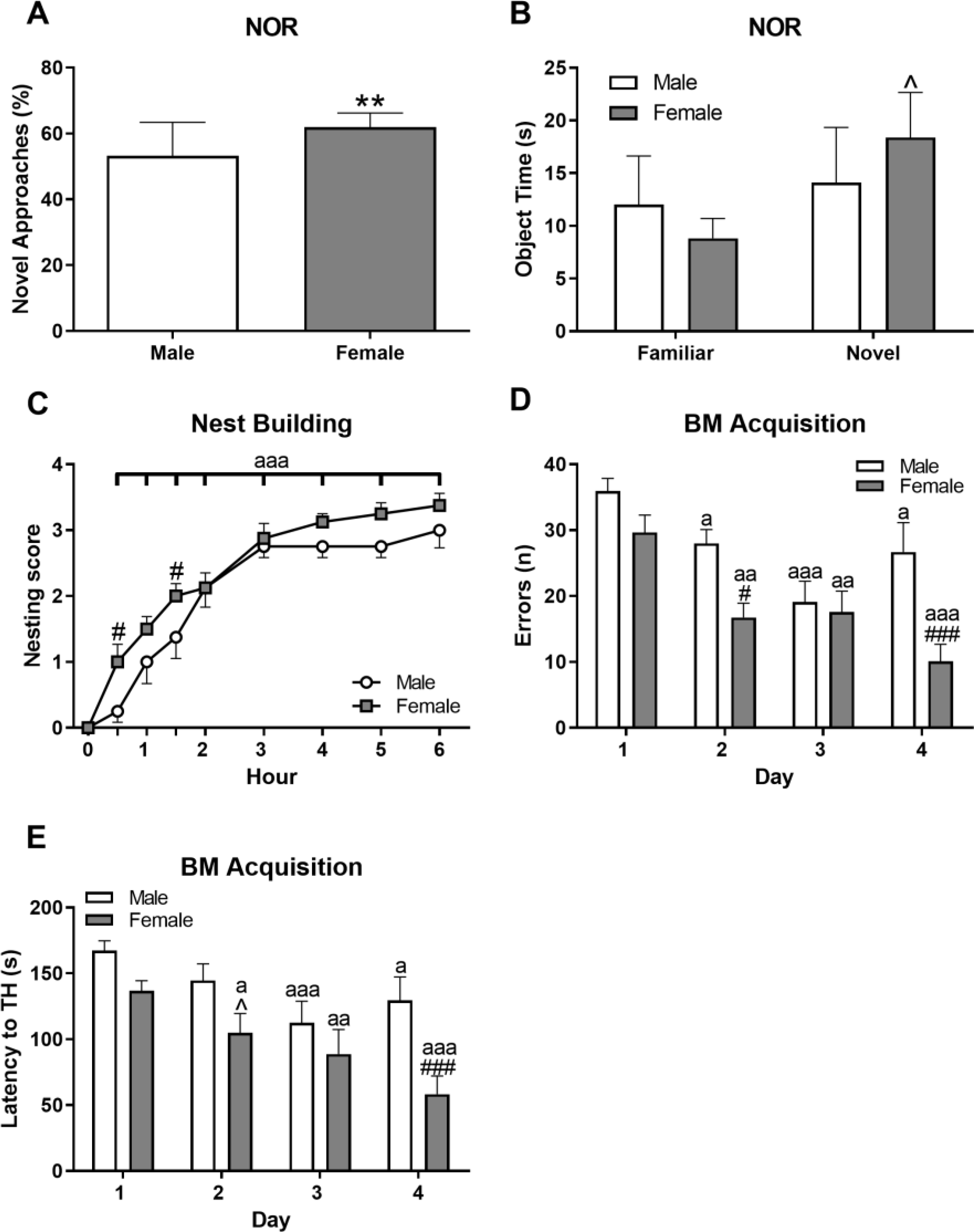
Short term, object-based memory was determined using percent approaches to the novel object (A) and time spent at each objects (B) in a Novel Object Recognition test (NOR). Nesting analysis was used to assess hippocampal and motor function via nest-building ability (C). The Barnes Maze (BM) acquisition phase measured learning ability through day-by-day comparison of latency to target hole (TH; (D)) and errors during the test (E). Data are presented as mean ± SEM; n = 8/sex; ** indicates p ≤ 0.01 between NOR phases; ^ indicates p ≤ 0.10; # and ## indicates p ≤ 0.05 and 0.01, respectively, between sexes; a, aa, and aaa indicate p ≤ 0.05, 0.01 and 0.001, respectively, between day (1) or hour (0) and indicated day or hour.
The BM revealed differences between sexes in learning and long-term memory, especially during the acquisition phase. Using RM-ANOVA, main effects for sex were found in the following parameters: errors (Figure 4D; F(1,41)=9.40, p≤0.01), latency to enter target hole (TH) (Figure 4E; F(1,42)= 6.46, p ≤ 0.05), and distance to TH (F(1,42)=5.98, p≤0.05; data not shown). Additionally, both males and females showed improvements when main effects of day were analyzed (errors, Figure 4D, F(3,41)=15.55, p≤0.001; latency, Figure 4E, F(3,42)=14.82, p ≤ 0.001; and distance traveled, data not shown, F(3,42)=23.29, p≤0.001), although females tended to perform better. In the probe trial, there were no sex differences observed.
3.6. Females had higher plasma cytokine levels following LPS administration concordant with greater sickness behaviors
Sickness behavior was analyzed using the 15-min OF test to identify decreases in movement associated with this state. Post-LPS, there were significant decreases in the number of crossings (Figure 5A; F(1,12)=8.14, p≤0.05) and trending decreases of the traveled distance (F(1,12)=4.19, p = 0.06; Supplemental Table 1). Pairwise comparisons revealed the overall decrease in crossings due to LPS treatment was driven by the LPS response in the females (p ≤ 0.05). Vertical movement (rears) among intervals (Figure 5B) showed effects for LPS treatment (F(1,36)=15.86, p ≤ 0.001) and interval (F(2,36)=7.28, p ≤ 0.01) but not sex × interval, although, within sex, LPS-treated females exhibited significant decreases in number of rears in intervals 2 (p ≤ 0.05) and 3 (p ≤ 0.01).
Figure 5. Females exhibited greater sickness behavior in the OF test post-LPS.
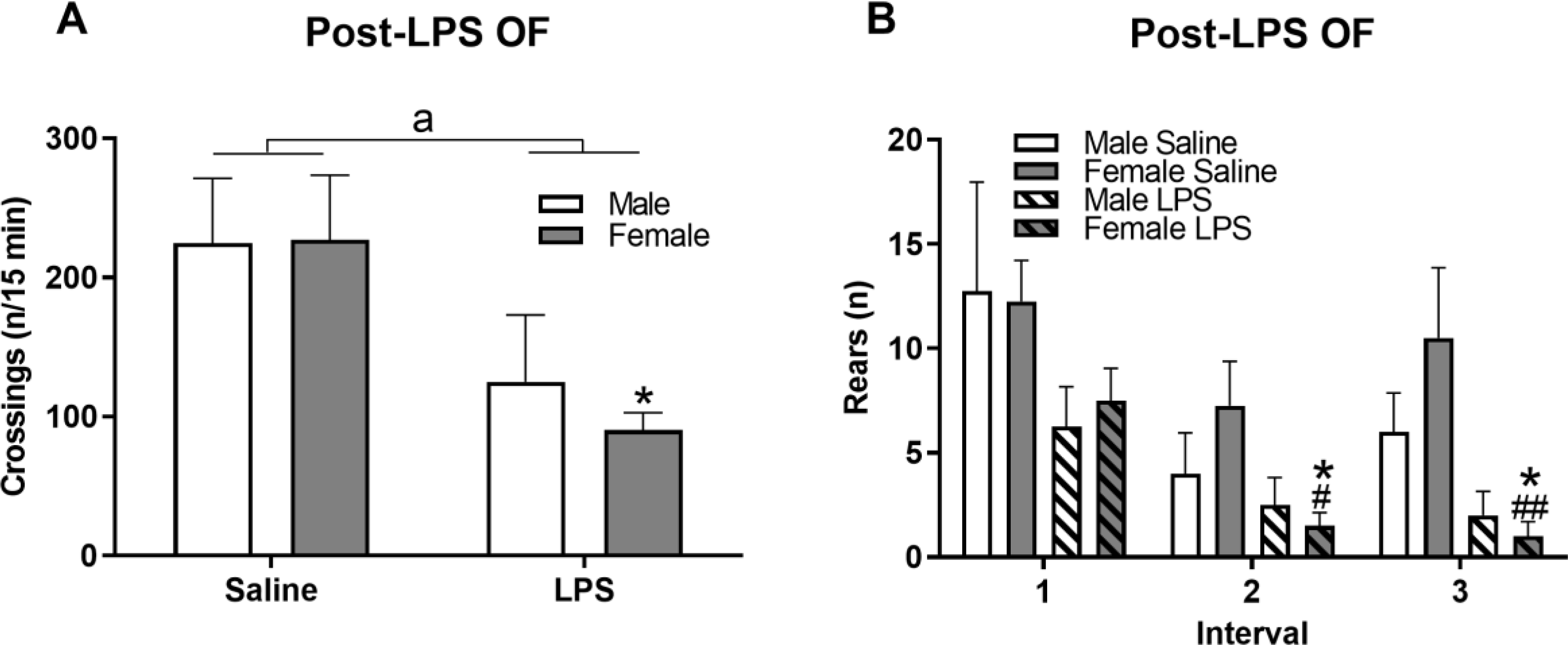
Four h following LPS administration, sickness behavior was assessed via the OF by measuring number of zone crossings made (A) and rearings in each of the 5-min intervals (B). Data are presented as mean ± SEM; n = 4/sex/treatment. a indicates main effect of LPS treatment p ≤ 0.05; * indicates p ≤ 0.05 LPS effect within sex; # and ## indicate p ≤ 0.05 and 0.01, respectively, between interval 1 and indicated interval.
The higher female sickness behavior observed in the OF coincided with increases in circulating pro- and anti-inflammatory cytokines measured after completion of the OF. Peripherally, there were significant increases in multiple plasma cytokines by LPS (Figure 6). As examples, significant main effects of LPS were present as increases in IL-1β (Figure 6A; F(1,15)=8.71, p ≤ 0.01), IL-6 (Figure 6B; F(1,15)=140.75 p ≤ 0.001), TNFα (Figure 6C; F(1,15)=38.55, p < 0.001), IL-10 (Figure 6D; F(1,15)=45.14, p ≤ 0.001), and MIP-3α/CCL20 (Figure 6E; F(1,15)=52.22, p ≤ 0.001) for both sexes. Additionally, main effects of sex were also observed for MIP-3α/CCL20 (Figure 6E; F(1,15)=6.12, p ≤ 0.05) and trended for IL-1β (Figure 6A; F(1,15)=2.98, p = 0.105). For most of the cytokines tested, females had numerically higher concentrations post LPS than males and have been summarized in Figure 6F (heatmap).
Figure 6. Females exhibited greater plasma cytokine response following LPS administration.
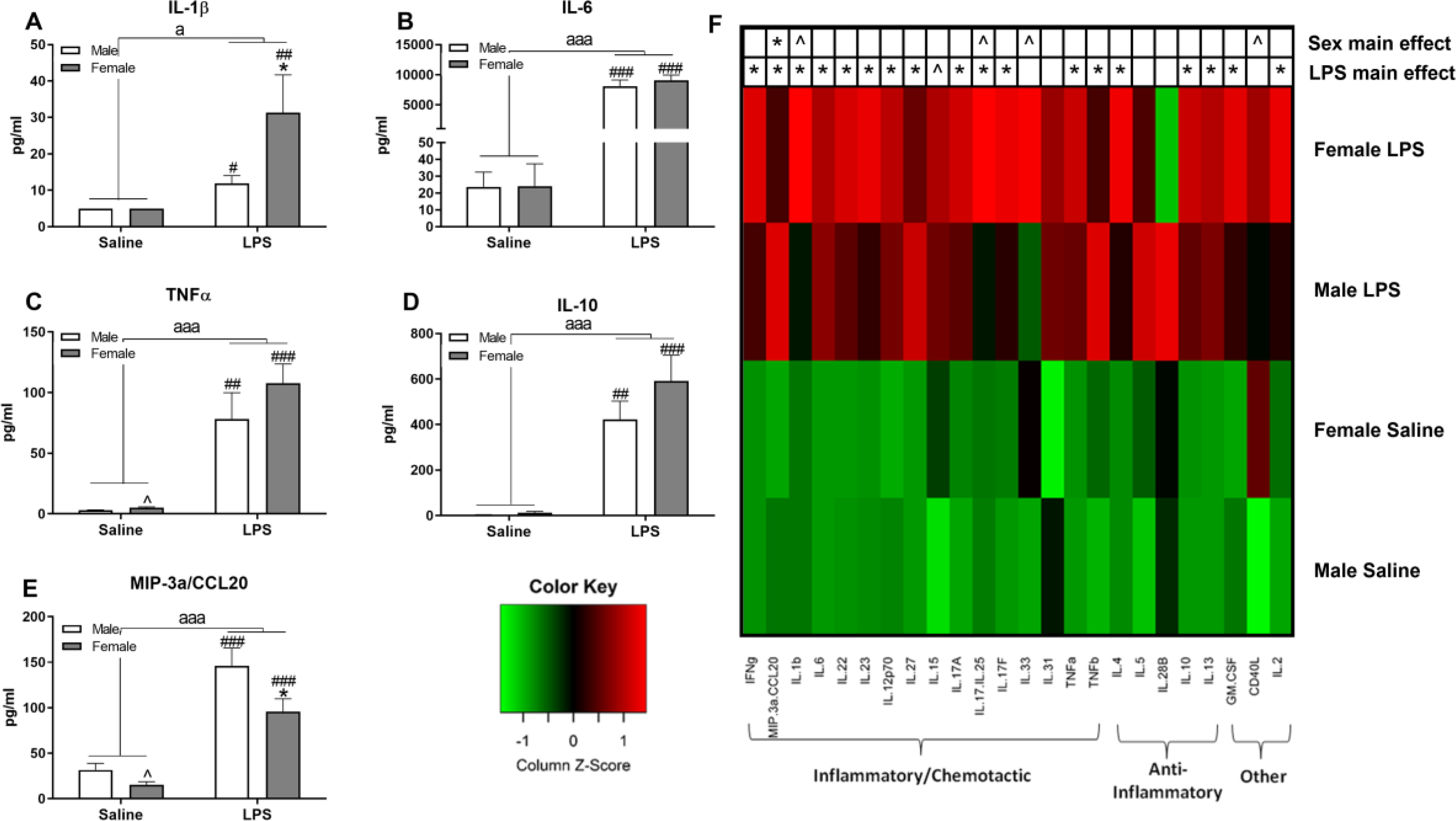
Selected plasma cytokines measured 6 h post-LPS include IL-1β (A), IL-6 (B), TNFα (C), IL-10 (D), and MIP-3α (E). Data are presented as mean ± SEM; LPS: n = 5/sex, Vehicle: n = 4/male, 5/female; a and aaa indicate p ≤ 0.05 and 0.001, respectively, for a main effect of LPS treatment; ^ indicates trend for sex differences within treatment, p ≤ 0.10; * indicates sex differences within LPS treatment, p ≤ 0.05; ## and ### indicate p ≤ 0.01 and 0.001, respectively, within sex. A heatmap was generated to display 24 different measured cytokines (F). Green and red transitions indicate, respectively, decreases and increases from the average cytokine concentration. ^ and * indicate p ≤ 0.10 and 0.05, respectively, for LPS or sex effects as designated on the heatmap.
LPS administration also increased multiple cytokines centrally, albeit these increases were brain region specific. LPS increased brain levels of IL-6 in the cortex (Figure 7A; F(1,14)=142.47, p ≤ 0.001 and striatum (Figure 7B; F(1,14)=96.48, p ≤ 0.001) of both sexes. Further, in the cortex, an interaction (LPS × sex) was present for TNF-α (F(1,14)=6.37, p ≤ 0.05), in which LPS-treated females had higher levels than male counterparts and female controls (Figure 7C; p’s ≤ 0.05). In the striatum, control females had significantly lower TNF-α concentrations compared to males (Figure 7D; t(6)=−3.38, p ≤ 0.05) and there was a numeral increase of TNFα by LPS only in females. A significant interaction was also apparent for cortical anti-inflammatory IL-10 (F(1,14)=6.94, p ≤ 0.05), in which LPS males had significantly lower concentrations than control males and LPS females (Figure 7E; p’s ≤ 0.05). Cortical levels of IL-5 were not affected, but there was a numerical increase in striatal IL-5 in LPS females compared to LPS males (Figure 7H; t(8)=−1.88, p = 0.097).
Figure 7. Selected cytokines were increased in the brain (cortex and striatum), more so in females, following LPS.
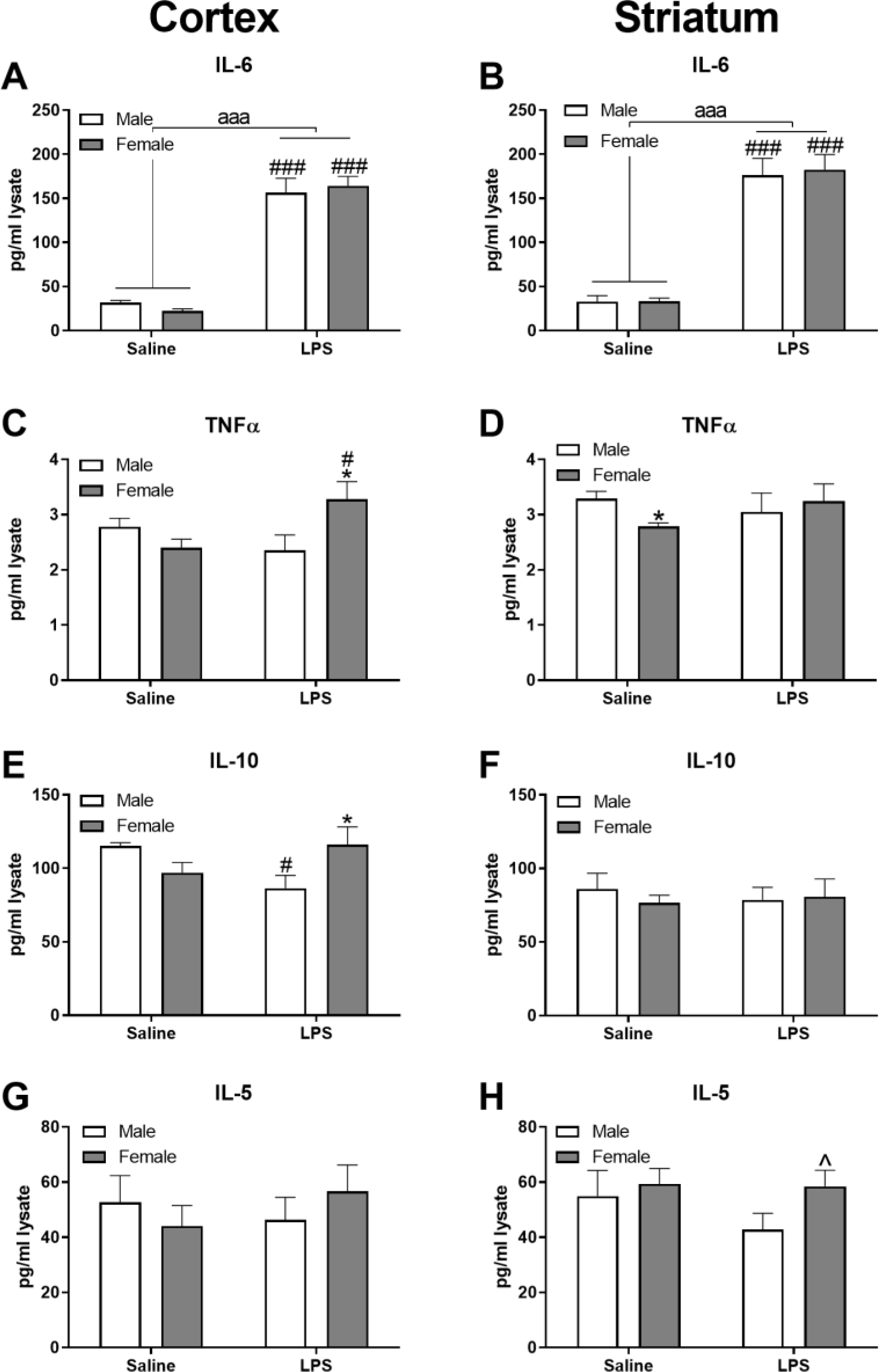
Selected cytokines include IL-6 (A and B), TNFα (C and D), IL-10 (E and F), and IL-5 (G and H) in the cortex and striatum, respectively. Data are presented as mean ± SEM; LPS: n = 5/sex, Vehicle: n = 4/male, 5/female; aaa indicates p ≤ 0.001 for a main effect of LPS treatment; * and ^ indicate p ≤ 0.05 and 0.10, respectively, for sex differences/trends within treatment; #, ##, and ### indicate p ≤ 0.05, 0.01, and 0.001, respectively, within sex.
3.7. Females had higher increases in glial activation 6 h post LPS in the dorsolateral striatum and hippocampus
In the dorsolateral (DL) striatum, trends for sex (F(1,91)=3.65, p=0.06) and an interaction (Figure 8A; sex × LPS, (F(1,91)=3.75, p=0.06)) were observed for GFP (microglia) within LPS such that LPS-treated females had increased GFP signal intensity compared to LPS-treated males (p ≤ 0.01). There was a significant interaction (Figure 8B; sex × LPS, F(1,90)=9.21, p ≤ 0.01) for GFAP (astrocytes), where control females had higher GFAP intensity compared to control males (p ≤ 0.01). There were no significant alterations in GFP or GFAP (Figure 8C and D, respectively) in the ventromedial (VM) striatum. In the hilar region of the hippocampus, a significant effect of treatment was observed for microglia (Figure 8E; F(1, 84)= 6.07, p ≤ 0.05), where LPS increased GFP optical density in females compared to control (p ≤ 0.05). No main effects in hippocampal GFAP signal were present for either treatment or sex. However, a significant interaction was present (Figure 8F; sex × LPS, F(1,84)=4.38, p ≤ 0.05) in which LPS increased GFAP in males compared to control males (p ≤ 0.05) and it did so to a lesser extent in females (p = 0.06). See Figure 8G for representative images of GFP and GFAP labeling in the hippocampal hilus.
Figure 8. Increases in glial activation 6 h post LPS were brain region specific and more robust in females.
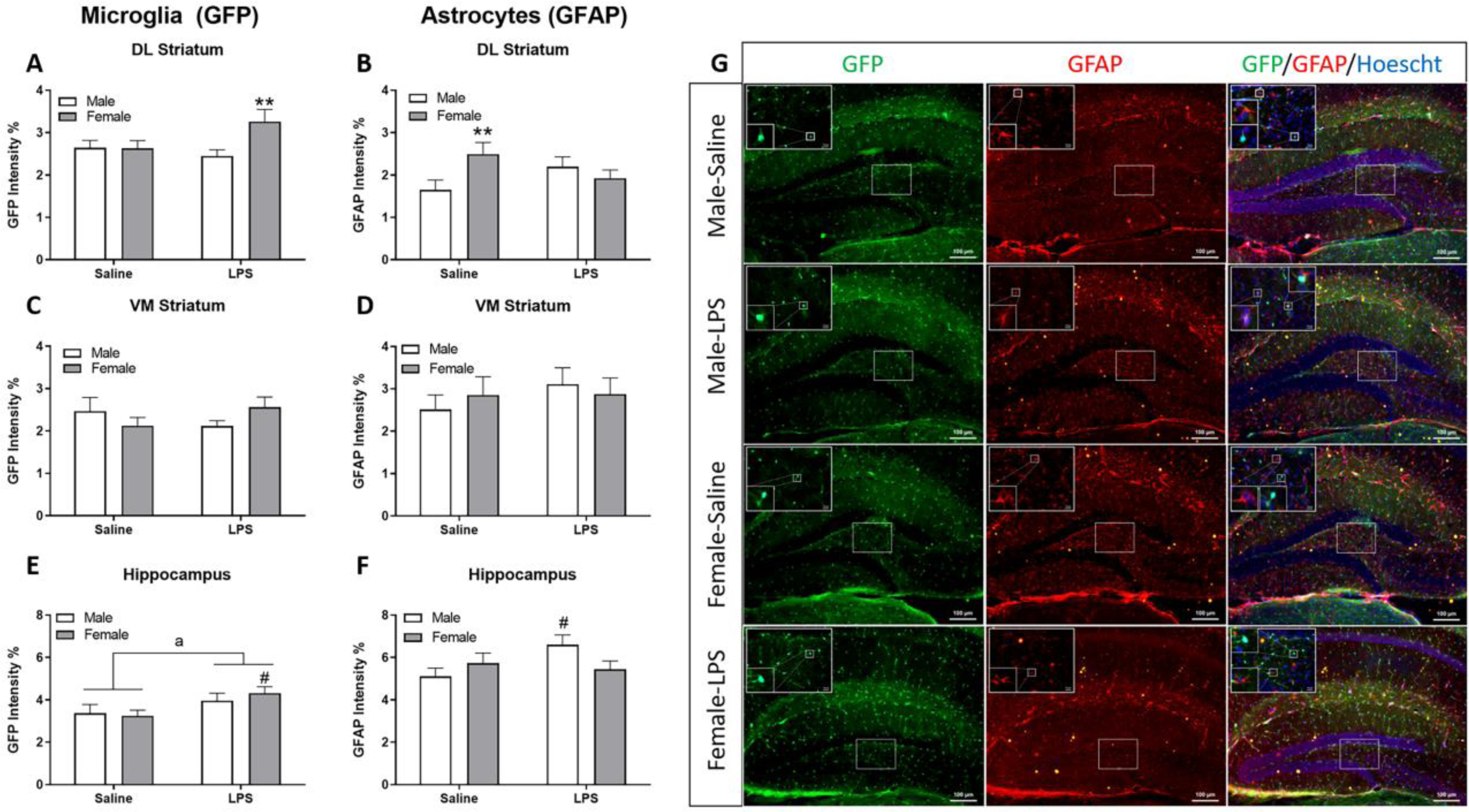
Calculated integrated optical density (as % of total area) for dorsolateral (DL) striatum for GFP (microglia) (A) and GFAP (astrocytes) (B). Similar analysis is represented in the ventromedial (VM) striatum and hilus of the hippocampus for GFP (C, E) and GFAP (D, F), respectively. Representative images of the hippocampus were captured at 10x with insets at 63x for GFP (green), GFAP (red), and nuclear (Hoechst) staining (blue) (G). Data are presented as mean ± SEM of the integrated density of fluorescent intensity of GFP or GFAP; n = 23–24 slices/group. a indicates p ≤ 0.05 for main treatment effect; ** indicates sex difference (p ≤ 0.01) within treatment; # indicates treatment effect (p ≤ 0.05) within sex.
3.8. GTT: Male glucose returned to baseline while female glucose levels remained elevated throughout the entire test
Prior to the beginning of the glucose tolerance test (GTT), males had higher baseline glucose than females (Figure 9A; t(10)=5.538, p ≤ 0.001). Both males and females were hyperglycemic (Figure 9A; F(5,50)= 8.012, p ≤ 0.001) post glucose challenge, with males having elevated glucose levels as compared to baseline at 15, 30 and 60-min (p’s ≤ 0.05), and females being hyperglycemic at every post-challenge time point tested (p’s ≤ 0.01). The greater impairment of glucose tolerance in females is better reflected if data are presented as percent of baseline (Figure 9B); percent baseline blood glucose was increased in males at 15 and 30-min (p’s ≤ 0.05) and at all time points tested in females (p’s ≤ 0.001) (Figure 9B). Further, baseline-adjusted blood glucose remained increased in females compared to males at every time point post 15-min; at 120-min, this effect was significant (Figure 9B; p ≤ 0.05). Due to higher baseline glucose in males, no difference between sexes was observed in the standard AUC (Figure 9A inset), but a numerical increase for females was observed in the baseline-corrected AUC (Figure 9B inset).
Figure 9. Sex differences in blood glucose handling were present during the GTT, but less so during the IST.
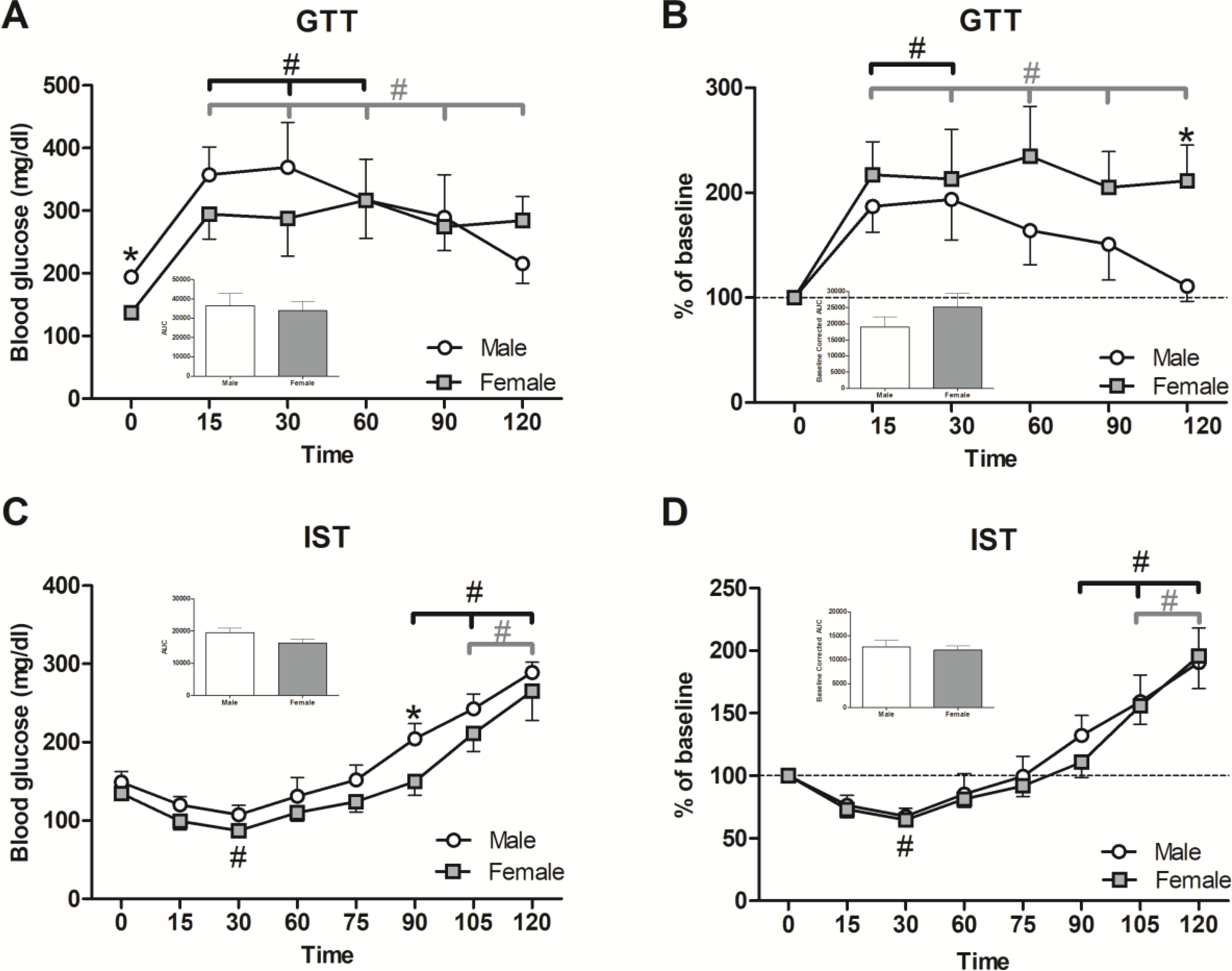
To determine differences in the response to glucose administration, glucose was given by gavage (2 g/kg BW). Blood was tested periodically for glucose levels (A), charted as percent of baseline levels (B), and the areas under the curve (AUC) were calculated (inset A and B). Insulin (0.5 IU/kg/BW) was injected IP to determine insulin sensitivity and blood glucose was again recorded periodically (C), charted as percent of baseline levels (D), and AUCs were calculated (inset C and D). Data are presented as mean ± SEM; n = 6/sex; * indicates p ≤ 0.05 between sex; # indicates p ≤ 0.05 within sex between interval 1 and indicated interval.
3.9. IST: No distinct differences were observed in insulin sensitivity, but both sexes showed excessive compensatory glucose rebound towards the end of the test
Following insulin administration, blood glucose levels decreased in both sexes and then elevated over the course of the test (Figure 9C; F(7,63)=42.128, p ≤ 0.001). Blood glucose levels decreased moderately in both sexes at 30-min (p ≤ 0.05) and the effect was numerically more pronounced in the females. A post-insulin glucose increase was apparent in males at 90-min (Figure 9C: p ≤ 0.05) and in both sexes at 105 and 120-min (p’s ≤ 0.001), such that the glucose elevation was higher than respective baselines; these effects were similar when the results were baseline corrected (Figure 9D). As in the GTT, there were no significant difference in the IST standard and baseline-corrected AUCs (Figure 9C and 9D insets), indicating similar response to insulin in both sexes.
4. Discussion
Aging leads to disturbances in motor function, memory and cognitive abilities, immune system effectiveness, metabolic homeostasis, and emotional regulation [35, 37, 62–64]. The impact of sex on these age-related changes has been understudied, particularly when considering the middle-age period. Several sex-related behavioral, immunological, and metabolic differences in 12–14.5-month-old mice were determined in this study, suggesting that sex influences normal aging at this stage of life more than previously understood. This study found that males maintained superior performance in tests for muscle strength, motor function, and coordination. Females showed higher levels of anxiety- and depression-related behaviors, as well as better performance in cognitive tests. Further, females displayed a more robust innate immune response to LPS, both peripherally and centrally, and higher levels of sickness behavior. Finally, both sexes exhibited rebound hyperglycemia following insulin treatment, but only females showed impaired glucose tolerance during the GTT. The utilization of multiple behavioral and physiological analyses in this study produced a well-rounded, comprehensive characterization of sex differences present in middle-aged mice.
Increased lean mass and muscle is a well-known secondary sex characteristic of males from many species, including humans [65] and mice [66]. Males demonstrate accelerated muscle growth/development and repair muscle fibers more efficiently after injury than females [67]. During aging, while both sexes exhibit decreases in overall muscle mass and have consequently lower levels of strength and coordination, males typically maintain an advantage in physical performance [68, 69]. This study found that middle-aged males had a strength advantage, indicating that a higher level of muscle mass in males is retained during middle age. In addition to superior grip strength, males completed the PT faster than females, indicating better motor coordination. However, while grip strength plays a role in successfully completing the PT, it cannot definitively explain the sex difference found here. Previous research in younger C57BL/6 mice found similar superiority in this test in males, but there are studies where females performed better in other tests of motor function [70]. Further, males were more active than females in the OF when locomotor performance (distance travelled, crossings, and rearings) was measured, and this is in line with another middle-aged study investigating sex differences in motor functions [17]. While most gait parameters were not different between sexes, it is important to distinguish the increase in female hindlimb interstep distance. This effect may be partly attributable to the increased white fat depots of females we observed in this study.
In humans, mood disorders are often associated with adolescence. However, recent research points that anxiety and depression in adults not only reduces quality of life, but can also be predictive of dementia development [6]. Anxiety is considered the most common mental disorder in the US, with a much higher prevalence in women than men throughout lifespan that levels off following the menopausal middle-age stage of life; a similar difference is seen in the occurrence of depression [71, 72]. Middle age is a time of distinct estrogen fluctuations and, eventually, decline in females and consistent, slow loss of testosterone in males, leading to behavioral changes distinct from those in younger and older populations [15, 25, 26]. Here, anxiety-like behavior, specifically thigmotaxis, was measured using the OF [73, 74]. Within the first 5-min of the OF, females displayed increased thigmotaxis compared to males by spending more time in the periphery than center, suggesting higher levels of anxiety-like behavior; a similar trend was seen for the entirety of the OF testing period. Further, throughout the 30-min OF duration, males sustained more locomotor activity, indicating greater exploration and less habituation, which have been linked to, respectively, less sustained anxiety [75] and less initial anxiety [76]. In line with the OF results, females also exhibited increased anxiety-like behavior in the EZM by spending more time in the closed than open arms than males. Females also spent more time in the closed arm after entering than males; this EZM effect further complements the increased OF exploration seen in males that could be linked to less anxiety. These results are in line with a study investigating a middle-age group compared to younger and older female and male groups [15]. It is known that females endure more dramatic changes in hormones than males during middle-age, but reduced androgen secretion in aging males has been found to increase anxiety-like behavior [77]. However, within this study, any potential impact of age-dependent androgen decline was not enough to match the observed female-biased anxiety-like phenotype.
Mobility (mobile vs. immobile) and climbing activity in the ST are useful parameters for assessing depressive-like behavior [78], especially when used in conjunction with other depression-focused tests. Here, females exhibited increased immobility time and less climbing attempts than males, indicating heightened depressive-like behavior. Younger female rodents reportedly spend more time mobile than males, suggesting that younger females have lower levels of depressive-like behavior [78–80]. These results, when combined with the present data, suggest an age-related change in behavior between the sexes, with either females exhibiting more depressive-like behaviors, or males becoming less so, as they reach middle-age. Statistically, while both sexes showed preference for sucrose over water, the SP results indicate greater female anhedonia. Viewed alone, the small difference may not be biologically meaningful, but when combined with the ST results, it does suggest that females had higher levels of depressive-like behavior in this study. Although preference levels are well above those of depression models, this modest difference is still important to note when understanding sex differences in baseline behavior during middle-age is the goal. Given previous data where younger adult female rodents preferred saccharine solutions over males [50, 81, 82], there appears to be a shift due to age in this test as well. Further, demographic data highlights the increased prevalence of depression in middle-aged women [27, 31, 71], and the current results suggest females experience more depressive-related behaviors with the transition to middle-age.
Typically, females are better at remembering local cues and landmarks, while males are more efficient at spatial recognition and orientation related memory in both humans [18, 20, 83] and rodents [19, 84]. The basis for this spatial male advantage is sexually dimorphic mechanisms in the hippocampus, a primary region for consolidating spatial memory [85]. During hippocampal long-term-potentiation (LTP), females, but not males, have membrane-bound estrogen receptors on LTP-related neurons, as well as a higher threshold required to reach LTP and consolidate spatial memory [86]. However, estrogen also has a protective effect on the female hippocampus, in part, by increasing dendritic spine density [34]. Thus, while baseline performance in adult females may be lower in spatial recognition tasks, they may be more resilient to age-related deterioration in these tasks than males [83, 87, 88]; this is consistent with the present results. The nest-building task evaluates an innate learned behavior that is not sex-specific; thus, deficiencies in this task suggest cognitive or motor impairment. Here, females outperformed males early on in the nest-building test, suggesting subtle, but greater cognitive capacity than their counterparts; this cannot be attributed to superior motor skills, as males were better at other motor tasks within this study. Both sexes completed building the nest, indicating that impairments were not substantial to a level that will prevent test completion. Females had better short-term object recognition memory than males as evident by increases in both the number of approaches and time spent at the novel object during the NOR test. Further, in the BM test, females learned the maze at a faster rate during the acquisition phase as evident by locating the TH faster and with less distance and errors. Performance in the NOR, nest building test, and BM is dependent, at least in part, on hippocampal function, as deficits in this brain region are associated with reduced efficiency in these tests [53, 54]. While females in this study were found to be in persistent diestrus, there may be more robust estrogen production centrally than in circulation, allowing cognitive function to be preserved at this age despite the natural drop in overall estrogen production. Alternatively, pre-middle age estrogen might have residual benefits. Additional studies comparing performance of these tests in parallel to estrogen charting across the lifespan are warranted to further solidify the hormonal basis behind these observations.
Adult females reportedly possess smaller infectious loads than males and have higher general immunity, but often show lower levels of inflammatory cytokine response than males, partly due to the effects of estrogen [32, 89]. In fact, several studies have observed increased inflammation following ovariectomy [90, 91]. Since aging itself leads to elevated baseline cytokine levels in both sexes, as well as to increased cytokine release in response to an inflammatory stimulus, the loss of female protection via estrogen may lead to even more dramatic changes in female innate immunity than in males with age [35, 92–94]. The endotoxin LPS stimulates a robust immune response in both humans and rodents and is a prototypical inflammagen [95] used to elicit sickness behavior. Sickness behavior is characterized by decreased food and water intake, lethargy, reduced grooming behavior, decreased social activity, and increases in pro-inflammatory cytokines, such as IL-1β, TNFα, and IL-6 [96]. Here, sex differences in sickness behavior were assessed during acute inflammation by utilizing the OF (15-min) 4 h after administering LPS. This study found that LPS induced sickness behavior (decreased crossings and rears) that affected females more than males and is consistent with previous research in younger mice [97] and human studies [98]. Further, following LPS administration, expected increases in pro-inflammatory mediators were observed primarily peripherally, but also centrally. Of the 24 analytes tested, only 5 remained unaffected in the plasma. Females showed higher and more dramatic increases in circulating plasma cytokine levels following LPS administration, as evident by higher z-scores in the females compared to males (Figure 6). Both sexes demonstrated peripheral increases in IL-1β, IL-6, TNFα, IL-10, and MIP-3a/CCL20. IL-1β mediates fever, initiates sickness behavior, reduces appetite, decreases locomotion, and, centrally, is involved in age-related decline in hippocampal function [99]. However, here, females produced much more peripheral IL-1β post LPS, an effect seen in other studies [100, 101] that coincides with the higher level of sickness behavior during the OF [96]. Similar elevations in IL-6 levels were observed in both sexes post-LPS, although prior research has shown that an exaggerated IL-6 response occurs in young adult males [101]. Further, while TNFα was increased in both sexes, females showed a numerically higher total concentration, an effect opposite to studies investigating younger mice [91, 92]. IL-10, an anti-inflammatory cytokine, is vital for modulating macrophages and for rebalancing the immune response to a homeostatic state. Estrogen helps accelerate the change to homeostatic conditions by inducing IL-10 action [102]. While the present results showed equivalent increases in IL-10 between LPS-challenged males and females, other studies using younger animals have found higher IL-10 levels in females [92, 100]. Chemokine levels of MIP-3a/CCL20 were increased by LPS, and more so in males. Interestingly, it has been shown that this chemokine is strongly regulated by IL-10 [103]; thus, it appears that the post-LPS heightened response of IL-10 may be modulating the proinflammatory MIP-3a/CCL20 response in females. Overall, these data suggest that middle-aged female mice may have decreased estrogen production that is not effectively regulating the inflammation process, thus resulting in a pro-inflammatory cytokine skewing and sickness behavior. In fact, IL-6 and TNFα are increased in ovariectomized rodents following LPS challenge [90]. This suggestion, however, needs to be verified experimentally.
Within the brain, elevated cortical and striatal levels of cytokines were also present in mice treated with LPS, albeit to a lesser extent than in the periphery. Both sexes demonstrated increases in IL-6, an effect that is consistent at the timepoint tested after LPS administration [104]. Additionally, TNFα was significantly increased in the cortex of females following LPS and is consistent with the peripheral data. These effects, in conjunction with the peripheral increases of IL-6 and TNFα, could contribute to the responses observed here and may further drive the exaggeration of sickness behavior [96] observed in females post LPS. IL-10 was only increased in the cortex of LPS females, consistent with the notion that females have higher general immunity to males. It is important to note that the kinetics of LPS response are different in the periphery and CNS. The peak in which LPS induces peripheral inflammatory cytokine secretion generally precedes a central response [105]. Immunohistochemical analysis in the striatum and hippocampus analysis revealed changes in GFP intensity that are associated with microglial activation. More specifically, in the dorsolateral striatum, LPS increased microglial response within females, an effect corresponding with IL-6 elevations from the brain cytokine data. Consequently, these increases may contribute to the locomotor effects associated with sickness behavior [96]. Interestingly, control females had increased GFAP within the striatum, suggesting that middle-aged females, in the absence of inflammagen stimulation, might have increased astrocytic activity. This increased astrocytic activity within this region may also explain the female motor differences in this study, such as decreased OF distance, OF rears, and PT performance. In the hippocampus, there was a significant increase in microglia activation in both sexes, albeit females had it to a higher extent. Further, increased astrocytic GFAP was observed in males post-LPS, an effect not seen in females. Cognitive/memory performance was not tested post-LPS administration. Thus, it is yet to be known how an inflammatory stimulus at this middle-age period could affect the male-biased deficiencies in memory performance observed in this study.
The ability to maintain stable blood glucose levels and exert appropriate insulin and glucagon action is often discussed in the context of diabetes, a potentially life-threatening inability to control blood glucose that has been diagnosed in an estimated 9.7% of the U.S. adult population [39]. The impact of age is especially hard to measure in humans due to very different lifestyles that might impact heavily glucose homeostasis, highlighting a need for more studies of middle-age glucose regulation [106]. Middle-aged mice were tested using the GTT and IST to identify sex differences so that what is “normal” in middle-age can be established and changes in the population may be better understood. This study found during the GTT, females produced glucose levels that remained elevated during the entire 2-hour testing period, while blood glucose in males returned to baseline levels by the end of the test. During the IST, both sexes displayed drops in blood glucose, which were blunted relative to younger mice, i.e., [40]. This sex-independent effect indicates that the elevated glucose levels in females during the GTT were not due to greater insulin resistance. Rather, a persistent elevation in blood glucose following glucose consumption may be due to either impaired insulin secretion or defects in glucose sensing, leading to difficulties in returning glucose to homeostatic levels [107]. In humans, diabetes is generally more prevalent in adult males until middle age. Upon reaching menopause, diabetes incidence in females increases greatly, indicating that estrogen plays a role in glucose regulation [108, 109]. In fact, research on estrogen receptors in murine pancreatic B-cells has found that insulin secretion is regulated via estrogen receptors [110]. Our female mice may be estrogen-deficient and thus, showing deficits in insulin secretion, but this needs to be investigated further. In males, aging and associated androgen loss has been found to increase glucose intolerance, which could account for increased baseline glucose [111].Typically, insulin secretion is pulsatile in response to glucose consumption or to counteract glucagon action, instead of being constantly released [112]. In aging, pancreatic beta cells begin to malfunction, so that lower levels of insulin are released, and the release is constant rather than pulsatile [112]. As the cells become unable to detect changes in blood glucose effectively, blood glucose remains high [112]. Interestingly, while both males and females showed an initial decrease in blood glucose following insulin administration, by the end of the 2-hour test both sexes had blood glucose levels well-above their baselines. The exact reason is unclear; the IST glucose curves in our study most closely resembled an occurrence in humans known as the “Dawn Phenomenon” [113]. In diabetics, this phenomenon is characterized by a morning hyperglycemia despite evening insulin injection and fasting all night; the time scale for these mice is much smaller (2 h), but the hyperglycemia present after a brief period of hypoglycemia following insulin injection reflects a response with similar characteristics [113]. Glucose release in response to hypoglycemia is mediated via epinephrine, glucagon, and corticosterone signaling that is further regulated by insulin [110]. Since insulin has a short half-life of 10 minutes, there may be decreased basal insulin levels in both sexes that fail to recover within the IST’s time frame, leading to excess glucagon activity and hyperglycemia. However, since this study did not measure insulin, glucagon, or other relevant hormones, the exact mechanism need to be elucidated in future studies.
While glucose metabolism may seem distinct from both the immune system and brain function, there is extensive crosstalk between the three. Insulin sensitivity is partially regulated by the pro-inflammatory factors TNFα and IL-6, which are also implicated in neurodegenerative conditions such as Alzheimer’s disease; higher levels of these cytokines inhibit insulin receptor action in both the periphery and the brain due to their ability to easily cross the blood brain barrier [114]. An inability to properly regulate the immune system can lead to adverse consequences in both the brain and in glucose metabolism. In this study, we found that post-LPS, inflammation (IL-6, TNFα) is heightened within the peripheral and central nervous system in middle-age mice. We further found that independent of LPS challenge, middle-age mice showed impairments in blood glucose regulation. These findings suggest that this crosstalk may play an important role during middle-age; however, these mechanisms will need to be elucidated in conjunction further.
5. Conclusion
Through this study and in context to previous studies, sex differences in mice specific to the middle-age period of life have been revealed. Middle-aged males maintain greater strength and motor coordination than age matched females and display greater levels of activity. Males have lower scores in measures of both anxiety and depression, indicating higher female emotionality that is typical in younger mice as well. Females in this age group perform better in short-term object memory and displayed unexpected superiority in learning and spatial memory. Following endotoxin-induced inflammation, females showed more drastic signs of sickness behavior and produced correlative higher levels of pro-inflammatory cytokines and glial activation. Finally, while both sexes responded to insulin challenge initially, they both showed excessive increases in blood glucose at the end of the challenge which suggests dysfunctional compensatory mechanisms; also, females were unable to return to baseline glucose levels following oral glucose challenge. It is important to note that, while this study thoroughly characterizes differences between male and female middle-aged mice, it does not compare this age to mice at other ages within the same study. Additional work is necessary to fully and completely characterize the aging process and provide baseline understanding of sex differences in behavior and metabolic capabilities. Sex has long been neglected in research despite evidence of how much it can impact physiological and behavioral outcomes, and the middle-age period is ignored despite it being a dynamic stage of the aging process. By identifying changes occurring due to sex differences in middle-age, the aging process can continue to be charted and understood to better the treatment and preemptive care given to the rapidly growing aging population.
Supplementary Material
Highlights:
Sex differences in middle-age mice were evaluated for behavior, immune and metabolic responses
Middle-aged males performed better in tests for motor function and mood
Middle-aged females performed better in cognitive and memory tasks
Middle-aged females were more sensitive to the effects of LPS, both peripherally and centrally
Both middle-aged males and females displayed impairments in blood glucose regulation
6. Acknowledgements
We would like to thank James Barber in the College of Veterinary Medicine Cytometry Core Facility at the University of Georgia for his assistance with the multiplex cytokine analysis. AND’s and RAB’s participation in this research was made possible by NIH grant T35OD010433. This project was funded in part by NIH (NIEHS) grant R21ES026383 to NMF.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T, A synopsis on aging-Theories, mechanisms and future prospects, Ageing Res Rev 29 (2016) 90–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wyss-Coray T, Ageing, neurodegeneration and brain rejuvenation, Nature 539(7628) (2016) 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harada CN, Natelson Love MC, Triebel KL, Normal cognitive aging, Clin Geriatr Med 29(4) (2013) 737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ferreira LK, Regina AC, Kovacevic N, Martin Mda G, Santos PP, Carneiro Cde G, Kerr DS, Amaro E Jr., McIntosh AR, Busatto GF, Aging Effects on Whole-Brain Functional Connectivity in Adults Free of Cognitive and Psychiatric Disorders, Cerebral cortex (New York, N.Y. : 1991) 26(9) (2016) 3851–65. [DOI] [PubMed] [Google Scholar]
- [5].Siman-Tov T, Bosak N, Sprecher E, Paz R, Eran A, Aharon-Peretz J, Kahn I, Early Age-Related Functional Connectivity Decline in High-Order Cognitive Networks, Front Aging Neurosci 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh-Manoux A, Akbaraly TN, Marmot M, Melchior M, Ankri J, Sabia S, Ferrie JE, Persistent depressive symptoms and cognitive function in late midlife: the Whitehall II study, The Journal of clinical psychiatry 71(10) (2010) 1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA, Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia, Arch Gen Psychiatry 69(5) (2012) 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ritchie K, Carrière I, Su L, O’Brien JT, Lovestone S, Wells K, Ritchie CW, The midlife cognitive profiles of adults at high risk of late-onset Alzheimer’s disease: The PREVENT study, Alzheimers Dement 13(10) (2017) 1089–1097. [DOI] [PubMed] [Google Scholar]
- [9].U.S. Census Bureau, Current Population Survey, 2016. https://www.census.gov/data/tables/2016/demo/age-and-sex/2016-age-sex-composition.html. (Accessed November 25 2019).
- [10].Cox KH, Bonthuis PJ, Rissman EF, Mouse model systems to study sex chromosome genes and behavior: relevance to humans, Frontiers in neuroendocrinology 35(4) (2014) 405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beery AK, Zucker I, Sex Bias in Neuroscience and Biomedical Research, Neurosci Biobehav Rev 35(3) (2011) 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clayton JA, Collins FS, NIH to balance sex in cell and animal studies, Nature 2014, pp. 282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, Jia R, Zhang X, Liu EQ, Broders H, Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice, Experimental animals 60(2) (2011) 111–23. [DOI] [PubMed] [Google Scholar]
- [14].Archer J, Sex differences in the emotional behaviour of laboratory mice, British journal of psychology (London, England : 1953) 68(1) (1977) 125–31. [DOI] [PubMed] [Google Scholar]
- [15].Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J, Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex, Neuroscience 95(1) (2000) 293–307. [DOI] [PubMed] [Google Scholar]
- [16].Joeyen-Waldorf J, Edgar N, Sibille E, The roles of sex and serotonin transporter levels in age- and stress-related emotionality in mice, Brain research 1286 (2009) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Palanza P, Parmigiani S, How does sex matter? Behavior, stress and animal models of neurobehavioral disorders, Neurosci Biobehav Rev 76(Pt A) (2017) 134–143. [DOI] [PubMed] [Google Scholar]
- [18].Andreano JM, Cahill L, Sex influences on the neurobiology of learning and memory, Learn Mem 16(4) (2009) 248–66. [DOI] [PubMed] [Google Scholar]
- [19].Bettis T, Jacobs LF, Sex differences in object recognition are modulated by object similarity, Behavioural Brain Research 233(2) (2012) 288–292. [DOI] [PubMed] [Google Scholar]
- [20].Rabbitt P, Donlan C, Watson P, McInnes L, Bent N, Unique and interactive effects of depression, age, socioeconomic advantage, and gender on cognitive performance of normal healthy older people, Psychology and aging 10(3) (1995) 307–13. [DOI] [PubMed] [Google Scholar]
- [21].Borbelyova V, Domonkos E, Babickova J, Tothova L, Bosy M, Hodosy J, Celec P, No effect of testosterone on behavior in aged Wistar rats, Aging 8(11) (2016) 2848–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnston AL, File SE, Sex differences in animal tests of anxiety, Physiol Behav 49(2) (1991) 245–50. [DOI] [PubMed] [Google Scholar]
- [23].Tucker LB, Fu AH, McCabe JT, Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research, J Neurotrauma 33(9) (2016) 880–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dutta S, Sengupta P, Men and mice: Relating their ages, Life Sci 152 (2016) 244–8. [DOI] [PubMed] [Google Scholar]
- [25].Guimaraes RA, Asth L, Engelberth RC, Cavalcante Jde S, Soares-Rachetti Vde P, Gavioli EC, Spontaneous failure of the estrous cycle induces anxiogenic-related behaviors in middle-aged female mice, Physiol Behav 147 (2015) 319–23. [DOI] [PubMed] [Google Scholar]
- [26].Moretti M, de Souza AG, de Chaves G, de Andrade VM, Romao PR, Gavioli EC, Boeck CR, Emotional behavior in middle-aged rats: Implications for geriatric psychopathologies, Physiol Behav 102(1) (2011) 115–20. [DOI] [PubMed] [Google Scholar]
- [27].Bale TL, Epperson CN, Sex differences and stress across the lifespan, Nature neuroscience 18(10) (2015) 1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM, Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men, J Clin Endocrinol Metab 87(11) (2002) 5001–7. [DOI] [PubMed] [Google Scholar]
- [29].Dalla C, Shors TJ, Sex differences in learning processes of classical and operant conditioning, Physiol Behav 97(2) (2009) 229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Walf AA, Frye CA, Estradiol reduces anxiety- and depression-like behavior of aged female mice, Physiol Behav 99(2) (2010) 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bebbington P, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, Meltzer H, The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity, International review of psychiatry (Abingdon, England) 15(1–2) (2003) 74–83. [DOI] [PubMed] [Google Scholar]
- [32].Heineman MJ, Faas MM, Bouman A, Sex hormones and the immune response in humans, Human Reproduction Update 11(4) (2005) 411–423. [DOI] [PubMed] [Google Scholar]
- [33].Pennell LM, Galligan CL, Fish EN, Sex affects immunity, Journal of autoimmunity 38(2–3) (2012) J282–91. [DOI] [PubMed] [Google Scholar]
- [34].Barrientos RM, Brunton PJ, Lenz KM, Pyter L, Spencer SJ, Neuroimmunology of the female brain across the lifespan: Plasticity to psychopathology, Brain Behav Immun 79 (2019) 39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fulop T, Witkowski JM, Pawelec G, Alan C, Larbi A, On the immunological theory of aging, Interdiscip Top Gerontol 39 (2014) 163–76. [DOI] [PubMed] [Google Scholar]
- [36].Borish LC, Steinke JW, 2. Cytokines and chemokines, J Allergy Clin Immunol 111(2 Suppl) (2003) S460–75. [DOI] [PubMed] [Google Scholar]
- [37].Chang AM, Halter JB, Aging and insulin secretion, Am J Physiol Endocrinol Metab 284(1) (2003) E7–12. [DOI] [PubMed] [Google Scholar]
- [38].Boland BB, Rhodes CJ, Grimsby JS, The dynamic plasticity of insulin production in beta-cells, Mol Metab 6(9) (2017) 958–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].American Diabetes Association, Economic Costs of Diabetes in the U.S. in 2017, Diabetes Care 41(5) (2018) 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Krishna S, Lin Z, de La Serre CB, Wagner JJ, Harn DH, Pepples LM, Djani DM, Weber MT, Srivastava L, Filipov NM, Time-dependent behavioral, neurochemical, and metabolic dysregulation in female C57BL/6 mice caused by chronic high-fat diet intake, Physiol Behav 157 (2016) 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCrimmon RJ, Ryan CM, Frier BM, Diabetes and cognitive dysfunction, Lancet (London, England) 379(9833) (2012) 2291–9. [DOI] [PubMed] [Google Scholar]
- [42].Byers SL, Wiles MV, Dunn SL, Taft RA, Mouse estrous cycle identification tool and images, PloS one 7(4) (2012) e35538–e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cora MC, Kooistra L, Travlos G, Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears, Toxicologic Pathology 43(6) (2015) 776–793. [DOI] [PubMed] [Google Scholar]
- [44].Krishna S, Dodd CA, Filipov NM, Behavioral and monoamine perturbations in adult male mice with chronic inflammation induced by repeated peripheral lipopolysaccharide administration, Behav Brain Res 302 (2016) 279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Abramow-Newerly W, Lipina T, Abramow-Newerly M, Kim D, Bechard AR, Xie G, Clapcote SJ, Roder JC, Methods to rapidly and accurately screen a large number of ENU mutagenized mice for abnormal motor phenotypes, Amyotroph Lateral Scler 7(2) (2006) 112–8. [DOI] [PubMed] [Google Scholar]
- [46].Mulherkar S, Liu F, Chen Q, Narayanan A, Couvillon AD, Shine HD, Tolias KF, The small GTPase RhoA is required for proper locomotor circuit assembly, PloS one 8(6) (2013) e67015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF, Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein, The Journal of neuroscience : the official journal of the Society for Neuroscience 24(42) (2004) 9434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Braun AA, Skelton MR, Vorhees CV, Williams MT, Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents, Pharmacol Biochem Behav 97(3) (2011) 406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM, Exploration of mice in a black and white test box: validation as a model of anxiety, Pharmacol Biochem Behav 32(3) (1989) 777–85. [DOI] [PubMed] [Google Scholar]
- [50].Eagle AL, Mazei-Robison M, Robison AJ, Sucrose Preference Test to Measure Stress-induced Anhedonia, Bio-protocol 6(11) (2016) e1822. [Google Scholar]
- [51].Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A, Performance of different mouse strains in an object recognition task, Behav Brain Res 147(1–2) (2003) 49–54. [DOI] [PubMed] [Google Scholar]
- [52].Patil SS, Sunyer B, Hoger H, Lubec G, Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze, Behav Brain Res 198(1) (2009) 58–68. [DOI] [PubMed] [Google Scholar]
- [53].Sunyer B, Patil S, Höger H, Luber G, Barnes maze, a useful task to assess spatial reference memory in the mice, Protocol Exchange (2007). [Google Scholar]
- [54].Deacon R, Assessing burrowing, nest construction, and hoarding in mice, J Vis Exp (59) (2012) e2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hofele K, Sedelis M, Auburger GW, Morgan S, Huston JP, Schwarting RKW, Evidence for a Dissociation between MPTP Toxicity and Tyrosinase Activity Based on Congenic Mouse Strain Susceptibility, Experimental Neurology 168(1) (2001) 116–122. [DOI] [PubMed] [Google Scholar]
- [56].Lisk RD, Oestrogen and progesterone synergism and elicitation of maternal nest-building in the mouse (Mus musculus), Animal Behaviour 19(3) (1971) 606–610. [DOI] [PubMed] [Google Scholar]
- [57].Olsson IA, Dahlborn K, Improving housing conditions for laboratory mice: a review of “environmental enrichment”, Lab Anim 36(3) (2002) 243–70. [DOI] [PubMed] [Google Scholar]
- [58].Coban A, Filipov NM, Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice, journal of neurochemistry 100(5) (2007) 1177–1187. [DOI] [PubMed] [Google Scholar]
- [59].Hasegawa-Ishii S, Inaba M, Shimada A, Widespread time-dependent changes in tissue cytokine concentrations in brain regions during the acute phase of endotoxemia in mice, NeuroToxicology 76 (2020) 67–74. [DOI] [PubMed] [Google Scholar]
- [60].Paxinos G, Franklin KBJ, The Mouse Brain in Stereotaxic Coordinates, Elsevier Academic Press; 2004. [Google Scholar]
- [61].Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, gplots: Various R programming tools for plotting data, R package version, 2009, p. 1. [Google Scholar]
- [62].Bettio LEB, Rajendran L, Gil-Mohapel J, The effects of aging in the hippocampus and cognitive decline, Neurosci Biobehav Rev 79 (2017) 66–86. [DOI] [PubMed] [Google Scholar]
- [63].Frontera WR, Physiologic Changes of the Musculoskeletal System with Aging: A Brief Review, Phys Med Rehabil Clin N Am 28(4) (2017) 705–711. [DOI] [PubMed] [Google Scholar]
- [64].Mather M, The Affective Neuroscience of Aging, Annu Rev Psychol 67 (2016) 213–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Isen J, McGue M, Iacono W, Genetic influences on the development of grip strength in adolescence, Am J Phys Anthropol 154(2) (2014) 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Stickland NC, O’Shaughnessy PJ, The influence of male-specific genes on female muscle fiber types: studies on the sex-reversed (Sxr) mouse, J Exp Zool 269(4) (1994) 378–82. [DOI] [PubMed] [Google Scholar]
- [67].Manzano R, Toivonen JM, Calvo AC, Miana-Mena FJ, Zaragoza P, Munoz MJ, Montarras D, Osta R, Sex, fiber-type, and age dependent in vitro proliferation of mouse muscle satellite cells, J Cell Biochem 112(10) (2011) 2825–36. [DOI] [PubMed] [Google Scholar]
- [68].Doherty TJ, The influence of aging and sex on skeletal muscle mass and strength, Curr Opin Clin Nutr Metab Care 4(6) (2001) 503–8. [DOI] [PubMed] [Google Scholar]
- [69].Fearing CM, Melton DW, Lei X, Hancock H, Wang H, Sarwar ZU, Porter L, McHale M, McManus LM, Shireman PK, Increased Adipocyte Area in Injured Muscle With Aging and Impaired Remodeling in Female Mice, J Gerontol A Biol Sci Med Sci 71(8) (2016) 992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI, Sex differences in motor behavior in the MPTP mouse model of Parkinson’s disease, Pharmacol Biochem Behav 95(4) (2010) 466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Faravelli C, Alessandra Scarpato M, Castellini G, Lo Sauro C, Gender differences in depression and anxiety: the role of age, Psychiatry Res 210(3) (2013) 1301–3. [DOI] [PubMed] [Google Scholar]
- [72].Bekker MH, van Mens-Verhulst J, Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment, Gend Med 4 Suppl B (2007) S178–93. [DOI] [PubMed] [Google Scholar]
- [73].Choleris E, Thomas AW, Kavaliers M, Prato FS, A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field, Neurosci Biobehav Rev 25(3) (2001) 235–60. [DOI] [PubMed] [Google Scholar]
- [74].Seibenhener ML, Wooten MC, Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice, J Vis Exp (96) (2015) e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Treit D, Animal models for the study of anti-anxiety agents: a review, Neurosci Biobehav Rev 9(2) (1985) 203–22. [DOI] [PubMed] [Google Scholar]
- [76].Salomons AR, Pinzon NE, Boleij H, Kirchhoff S, Arndt SS, Nordquist RE, Lindemann L, Jaeschke G, Spooren W, Ohl F, Differential effects of diazepam and MPEP on habituation and neuro-behavioural processes in inbred mice, Behav Brain Funct 8 (2012) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Frye CA, Edinger K, Sumida K, Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33(5) (2008) 1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Petit-Demouliere B, Chenu F, Bourin M, Forced swimming test in mice: a review of antidepressant activity, Psychopharmacology 177(3) (2005) 245–55. [DOI] [PubMed] [Google Scholar]
- [79].Alonso SJ, Castellano MA, Afonso D, Rodriguez M, Sex differences in behavioral despair: relationships between behavioral despair and open field activity, Physiol Behav 49(1) (1991) 69–72. [DOI] [PubMed] [Google Scholar]
- [80].Voikar V, Koks S, Vasar E, Rauvala H, Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies, Physiol Behav 72(1–2) (2001) 271–81. [DOI] [PubMed] [Google Scholar]
- [81].Feigin MB, Sclafani A, Sunday SR, Species differences in polysaccharide and sugar taste preferences, Neurosci Biobehav Rev 11(2) (1987) 231–40. [DOI] [PubMed] [Google Scholar]
- [82].Joshi N, Leslie RA, Perrot TS, Analyzing the experiences of adolescent control rats: Effects of the absence of physical or social stimulation on anxiety-like behaviour are dependent on the test, Physiol Behav 179 (2017) 30–41. [DOI] [PubMed] [Google Scholar]
- [83].McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM, Sex differences in cognitive trajectories in clinically normal older adults, Psychology and aging 31(2) (2016) 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J, Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity, Neuroscience 137(2) (2006) 413–23. [DOI] [PubMed] [Google Scholar]
- [85].Wang W, Le AA, Hou B, Lauterborn JC, Cox CD, Levin ER, Lynch G, Gall CM, Memory-Related Synaptic Plasticity Is Sexually Dimorphic in Rodent Hippocampus, The Journal of neuroscience : the official journal of the Society for Neuroscience 38(37) (2018) 7935–7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Go J, Park TS, Han GH, Park HY, Ryu YK, Kim YH, Hwang JH, Choi DH, Noh JR, Hwang DY, Kim S, Oh WK, Lee CH, Kim KS, Piperlongumine decreases cognitive impairment and improves hippocampal function in aged mice, Int J Mol Med 42(4) (2018) 1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A, Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice, Hippocampus 25(3) (2015) 309–28. [DOI] [PubMed] [Google Scholar]
- [88].Xiong XD, Xiong WD, Xiong SS, Chen GH, Age- and Gender-Based Differences in Nest-Building Behavior and Learning and Memory Performance Measured Using a Radial Six-Armed Water Maze in C57BL/6 Mice, Behavioural neurology 2018 (2018) 8728415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Darnall BD, Suarez EC, Sex and gender in psychoneuroimmunology research: past, present and future, Brain Behav Immun 23(5) (2009) 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Iwasa T, Matsuzaki T, Kinouchi R, Gereltsetseg G, Murakami M, Munkhzaya M, Altankhuu T, Kuwahara A, Yasui T, Irahara M, Changes in central and peripheral inflammatory responses to lipopolysaccharide in ovariectomized female rats, Cytokine 65(1) (2014) 65–73. [DOI] [PubMed] [Google Scholar]
- [91].Mabley JG, Horvath EM, Murthy KG, Zsengeller Z, Vaslin A, Benko R, Kollai M, Szabo C, Gender differences in the endotoxin-induced inflammatory and vascular responses: potential role of poly(ADP-ribose) polymerase activation, The Journal of pharmacology and experimental therapeutics 315(2) (2005) 812–20. [DOI] [PubMed] [Google Scholar]
- [92].Cai KC, van Mil S, Murray E, Mallet JF, Matar C, Ismail N, Age and sex differences in immune response following LPS treatment in mice, Brain Behav Immun 58 (2016) 327–337. [DOI] [PubMed] [Google Scholar]
- [93].Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW, Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system, Faseb j 19(10) (2005) 1329–31. [DOI] [PubMed] [Google Scholar]
- [94].Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K, Microglia derived from aging mice exhibit an altered inflammatory profile, Glia 55(4) (2007) 412–24. [DOI] [PubMed] [Google Scholar]
- [95].Cavaillon JM, Exotoxins and endotoxins: Inducers of inflammatory cytokines, Toxicon 149 (2018) 45–53. [DOI] [PubMed] [Google Scholar]
- [96].Dantzer R, Cytokine, Sickness Behavior, and Depression, Immunol Allergy Clin North Am 29(2) (2009) 247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Millett CE, Phillips BE, Saunders EFH, The Sex-specific Effects of LPS on Depressive-like Behavior and Oxidative Stress in the Hippocampus of the Mouse, Neuroscience 399 (2019) 77–88. [DOI] [PubMed] [Google Scholar]
- [98].Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI, Sex Differences in Depressive and Socioemotional Responses to an Inflammatory Challenge: Implications for Sex Differences in Depression, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2015, pp. 1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA, The age-related attenuation in long-term potentiation is associated with microglial activation, J Neurochem 99(4) (2006) 1263–72. [DOI] [PubMed] [Google Scholar]
- [100].Erickson MA, Liang WS, Fernandez EG, Bullock KM, Thysell JA, Banks WA, Genetics and sex influence peripheral and central innate immune responses and blood-brain barrier integrity, PloS one 13(10) (2018) e0205769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Everhardt Queen A, Moerdyk-Schauwecker M, McKee LM, Leamy LJ, Huet YM, Differential Expression of Inflammatory Cytokines and Stress Genes in Male and Female Mice in Response to a Lipopolysaccharide Challenge, PloS one 11(4) (2016) e0152289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP, Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge, Glia 64(2) (2016) 300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A, Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta, The Journal of Immunology 158(3) (1997) 1033–1036. [PubMed] [Google Scholar]
- [104].Beurel E, Jope RS, Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain, Journal of Neuroinflammation 6(1) (2009) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Erickson MA, Banks WA, Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: Multiplex quantification with path analysis, Brain, Behavior, and Immunity 25(8) (2011) 1637–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Unwin N, Shaw J, Zimmet P, Alberti KG, Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention, Diabet Med 19(9) (2002) 708–23. [DOI] [PubMed] [Google Scholar]
- [107].Samuel Varman T., Shulman Gerald I., Mechanisms for Insulin Resistance: Common Threads and Missing Links, Cell 148(5) (2012) 852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Stubbins RE, Najjar K, Holcomb VB, Hong J, Nunez NP, Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance, Diabetes Obes Metab 14(1) (2012) 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Gupte AA, Pownall HJ, Hamilton DJ, Estrogen: an emerging regulator of insulin action and mitochondrial function, J Diabetes Res 2015 (2015) 916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sharma G, Prossnitz ER, Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells, Endocrinology 152(8) (2011) 3030–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kapoor D, Malkin CJ, Channer KS, Jones TH, Androgens, insulin resistance and vascular disease in men, 63(3) (2005) 239–250. [DOI] [PubMed] [Google Scholar]
- [112].Goyal R, Jialal I, Glucose Intolerance, StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- [113].Porcellati F, Lucidi P, Bolli GB, Fanelli CG, Thirty years of research on the dawn phenomenon: lessons to optimize blood glucose control in diabetes, Diabetes Care 36(12) (2013) 3860–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ferreira ST, Clarke JR, Bomfim TR, De Felice FG, Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease, Alzheimers Dement 10(1 Suppl) (2014) S76–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


