Abstract
The ability of ten polyphenolic antioxidants to prevent CuO nanoparticle (NPCuO) and H2O2-mediated DNA damage and cytotoxicity was investigated. Five of the polyphenols (MEPCA, PREGA, MEGA, ECG, and EGCG) prevent NPCuO/H2O2-mediated DNA damage (IC50 values of 7.5–800 μM), three have no effect (PCA, VA, and EC), and two (GA and EGC) result in increased DNA damage. Most polyphenols had similar antioxidant/prooxidant activity in the presence of NPCuO or free copper ions. Electron paramagnetic resonance (EPR) spectroscopy of reactive oxygen species (ROS) generated by NPCuO/H2O2 in the presence of representative polyphenols correlate with results of DNA damage studies: in the presence of NPCuO/H2O2, MEPCA prevents ROS formation, VA has no effect on ROS levels, and EGC increases ROS levels. EPR results with CuO nanoparticles washed to remove dissolved copper in solution (wCuO) in the presence of H2O2/ascorbate suggest that MEPCA prevents ROS formation on the nanoparticle surface in addition to preventing ROS formation from dissolved copper. In mouse fibroblast (L929) cells, combining NPCuO with H2O2 results in significantly greater cytotoxicity than observed for either component alone. After 3 h incubation with MEPCA or MEGA, the viability loss in L929 cells induced by NPCuO/H2O2 challenge was significantly rescued at physiologically relevant polyphenol levels (1 μM). These studies show that polyphenols can protect DNA and inhibit cytotoxicity generated by NPCuO under oxidative stress conditions.
Keywords: polyphenol, CuO nanoparticles, DNA damage, reactive oxygen species, cell death prevention, prooxidant
Graphical Abstract
1. Introduction
Copper oxide nanoparticles (NPCuO) are widely used in consumer products such as cosmetics (Borkow, 2014), electronics (Son et al., 2009), sensors (X. Zhang et al., 2008), wood preservation (Evans et al., 2008), antifouling paints (Detty et al., 2014), and antibacterial textiles (Ren et al., 2009; Suresh et al., 2013). The physicochemical properties of these nanoparticles raise concerns about risks to human health (Karlsson et al., 2008a). However, the evidence is limited for acute toxicity from nanoparticles at realistic doses, especially at low concentrations that may cause oxidative stress and adverse long-term health effects. Toxicity from nanoparticles is generally different than from the constituent ions due to differences in nanoparticle uptake/pharmacokinetics as well as surface dependent biochemical properties (Angelé-Martínez et al., 2017; Klaine et al., 2020). Therefore, considerable efforts have been placed on identifying the potential toxicity of nanoparticles to cells and organisms (Bondarenko et al., 2013; Karlsson et al., 2008a; Karlsson et al., 2009; Oberdorster et al., 2005; Rim et al., 2013; Stone et al., 2007).
To reduce the toxic effects of NPCuO, one can directly modify the nanoparticle surface by coating it with an inert shell, by adding ligands to the NPCuO surface, or by altering methods of synthesis (Barua et al., 2013; Jo et al., 2012; Kanninen et al., 2008; C. C. Li and Chang, 2004; Studer et al., 2010). Since NPCuO toxicity is due to effects of reactive oxygen species (ROS) generated both from dissolved copper from the nanoparticles and to ROS generated directly on the nanoparticle surface (Aruoja et al., 2009; Bondarenko et al., 2013; Heinlaan et al., 2008; Isani et al., 2013; Jo et al., 2012; Karlsson et al., 2008a; Karlsson et al., 2009; Karlsson et al., 2008b; Kasemets et al., 2009; Midander et al., 2009; Misra et al., 2012; Mortimer et al., 2010; Nel et al., 2006; Shi et al., 2012), another possible strategy to prevent NPCuO toxicity is to use radical scavenging antioxidants to prevent the ROS damage, as reported in similar ROS-generating systems. Sulforaphane, an isothiocyanate with anticancer properties (Juge et al., 2007; Y. Zhang et al., 1994) (Figure 1A) found primarily in green vegetables (Juge et al., 2007; Liang et al., 2007; Liang et al., 2006), reduces ROS generation and increases cell viability in mouse embryonic fibroblast (BALB 3T3) cells exposed to NPCuO (Akthar et al., 2012). Treatment of NPCuO-exposed cells (HEp-2; 5 h) with resveratrol (100 μM) resulted an 80% reduction in 8-isoprostane levels, a marker for oxidative stress, compared to cells without resveratrol treatment (Fahmy and Cormier, 2009). More recently, rats treated with curcumin (200 mg/kg; Figure 1A) and NPCuO (250 mg/kg) showed improved renal toxicity markers, including lower creatinine and blood urea-nitrogen levels, compared to rats treated with only NPCuO (250 mg/kg) (Elkhateeb et al., 2020). Ameliorating NPCuO toxicity is potentially important since NPCuO and copper nanoparticles are being examined as breast cancer treatments (Kamble et al., 2016; Mariadoss et al., 2020), and dietary polyphenols could thus affect both the therapeutic response in tumors and side-effects in normal tissue.
Figure 1.
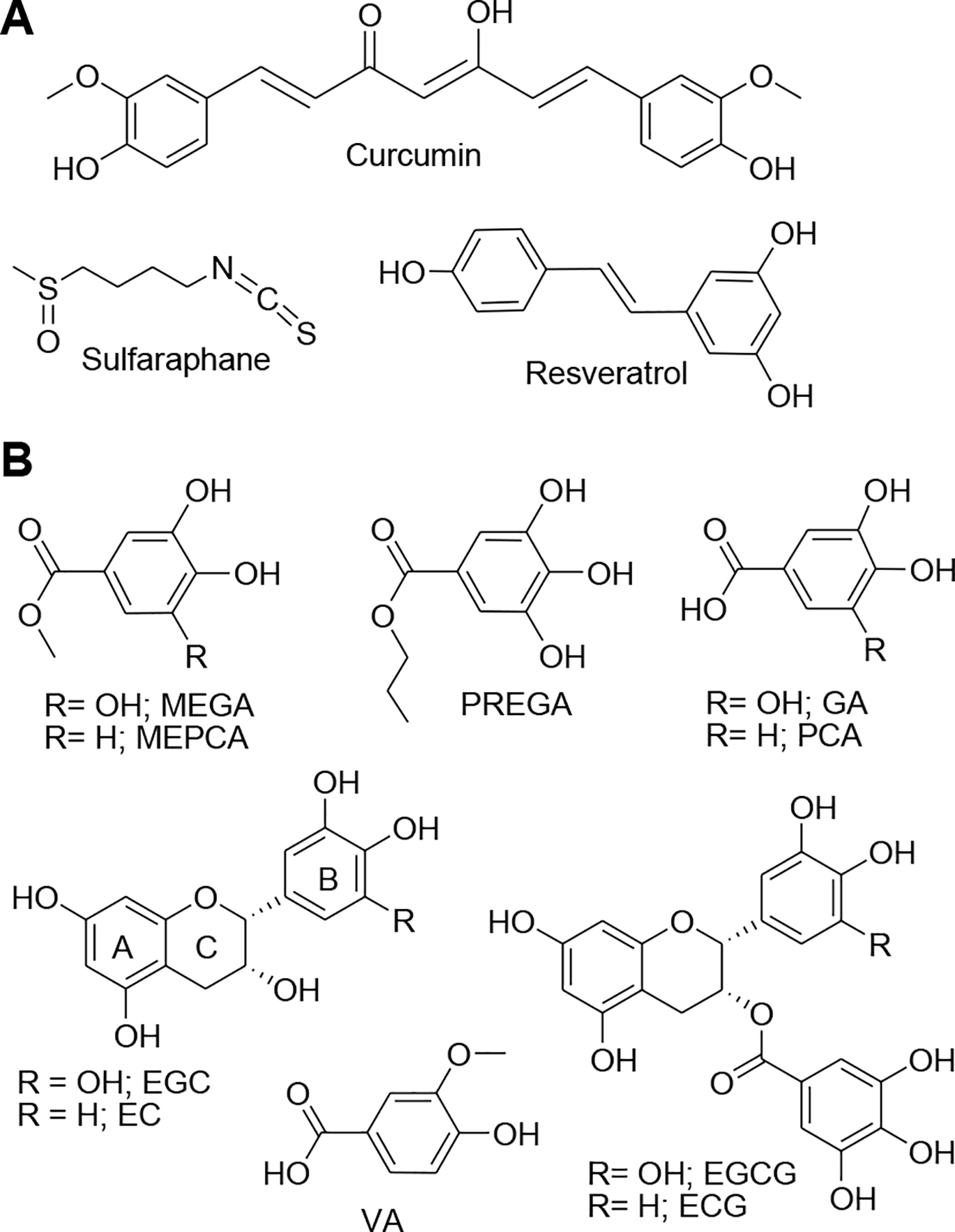
A) Molecular structures of sulforaphane, curcumin, and resveratrol used to prevent nanoparticle toxicity, and B) polyphenolic compounds evaluated for their abilities to prevent NPCuO-mediated damage in this study.
Polyphenols, such as those shown in Figure 1B, are the most common dietary antioxidants, with an average daily intake around 1 g/day in humans (Scalbert and Williamson, 2000). Their antioxidant properties can protect DNA, lipids, and proteins from oxidative damage in cells, rodents, and humans (Asensi et al., 2011; Babich et al., 2011; Blokhina et al., 2003; Haslam, 1996). Epicatechin (EC) protects plasmid DNA from γ-irradiation (Nair and Salvi, 2008), and propyl gallate (PREGA), ellagic, gallic, and tannic acids protect human lymphocytes from DNA damage and lipid peroxidation induced by food mutagens or hydrogen peroxide (C. H. Chen et al., 2007). In general, the antioxidant and radical scavenging ability of gallols (polyphenolic compounds with three adjacent OH groups on an aromatic ring; e.g., gallic acid) is greater than analogous catechols (with two adjacent OH groups; e.g., protocatechuic acid) (Perron et al., 2011; Reis et al., 2010; H. C. Wang and Brumaghim, 2011). Other polyphenol structural modifications also correlate with antioxidant activity; for example, the ROS scavenging activity of protocatechuic acid (PCA) and its esters increases as the length of the alkylated ester chain increases (methyl, ethyl, and propyl) (Reis et al., 2010). Polyphenol antioxidants can scavenge ROS produced in normal cellular processes and protect cells from oxidative damage, so they are widely studied for their ability to prevent development of diseases such as cancer and heart disease (Dall’Asta et al., 2015; Ding et al., 2013; Oak et al., 2018; Z. Wang et al., 2012).
Herein, we systematically investigate ten polyphenols for their effects on NPCuO-mediated DNA damage. For representative polyphenols, activity observed in the DNA gel electrophoresis experiments were also correlated with ROS levels as determined using electron paramagnetic (EPR) spectroscopy. The most effective polyphenols for preventing NPCuO-mediated DNA damage, MEPCA and MEGA, were then examined for their ability to prevent NPCuO/H2O2-induced oxidative damage in mouse fibroblast (L929) cells. The ability of polyphenols to modulate NPCuO toxicity is an important aspect of these dietary antioxidants as incorporation of NPCuO into consumer materials increases and the medicinal effects of NPCuO are explored.
2. Materials and Methods
2.1. General
Water was purified using a Barnstead NANOpure DIamond Life Science water deionization system (Barnstead International). 3-(N-Morpholino)propanesulfonic acid (MOPS, Alfa Aesar), CuSO4 (Acros Organics), L-ascorbic acid (30% v/v in water, Alfa Aesar), Chelex 100 resin (Sigma-Aldrich), hydrogen peroxide (H2O2, Alfa Aesar), methyl 3,4-dihydroxybenzoate (MEPCA, Alfa Aesar), methyl 3,4,5-trihydroxybenzoate (MEGA, Alfa Aesar), n-propyl gallate (PREGA, Acros), gallic acid (GA, TCI America), protocatechuic acid (PCA, Frontier Scientific), (−)-epigallotechin (EGC, Alfa Aesar), (−)-epcatechin (EC, MP Biomedicals), (−)-epigallotechin-3-gallate (EGCG, Alfa Aesar), (−)-epicatechin-3-gallate (ECG, Frontier Scientific), vanillic acid (VA, Frontier Scientific), and copper(II) oxide (CuO) nanoparticles (50% weight, Alfa Aesar) were used as received. Cu2+, polyphenol, and H2O2 solutions were prepared prior to each experiment and used immediately. Microcentrifuge tubes were rinsed in 1 M HCl, rinsed in deionized water, and dried prior to use. Buffered solutions were treated with Chelex resin (2 g / 80 mL buffer) for 24 h prior to use.
Mouse fibroblast (L929) cells were purchased from the American Type Culture Collection (ATCC). Dulbecco’s modified Eagle’s medium (DMEM), Eagle’s minimum essential medium (EMEM), glutamine, and fetal bovine serum (FBS) were purchased from Corning. A CellTiter 96® Aqueous One Solution Cell Proliferation MTS assay kit was purchased from Promega.
2.2. Preparation of CuO nanoparticle suspensions and copper solutions
In addition to commercial CuO nanoparticle suspensions (NPCuO), washed CuO nanoparticles (wCuO), and CuO nanoparticle leachate after removal of dissolved Cu ions (lCuO) were used in this study. wCuO were prepared by separating NPCuO from the suspensions using a bench-top centrifuge at 14,000 rpm (30,074 g RCF) for 45 min, removing the supernatant via pipette, and then re-dispersing the NPCuO pellet in deionized water. This centrifugation and re-dispersion process was repeated 6 times. Removal of dissolved copper from the supernatant of NPCuO suspensions to yield lCuO solutions were performed as previously described (Angelé-Martínez et al., 2017). Dissolved copper solutions were prepared using CuCl2. The concentrations of Cu2+ solution used for the cell studies were based on the measured dissolved copper concentration from the NPCuO supernatant prior to copper removal (Angelé-Martínez et al., 2017). All NPCuO, wCuO, lCuO, and copper suspensions/solutions were freshly prepared prior to each experiment and used immediately.
2.3. Transfection, amplification, and purification of plasmid DNA
Plasmid DNA (pBSSK) was purified from E. coli strain DH1 using a PerfectPrep Spin kit (Fisher). The plasmid DNA was dialyzed at 4 °C against EDTA (1 mM) and NaCl (50 mM) for 24 h and then against NaCl (130 mM) for 24 h to remove metal ions from the DNA. For all experiments, the absorbance ratios for DNA solutions were A250/A260 ≤ 0.95 and A260/A280 ≥ 1.8.
2.4. DNA damage assays
To evaluate the activity of polyphenols samples for the DNA damage assays, reactions were prepared by adding polyphenol (0.50 – 800 μM) to samples containing NPCuO (500 μM), NaCl (130 mM), MOPS (pH 7, 10 mM), and ethanol (10 mM, as a radical scavenger) in 10 μL of total volume. This mixture was allowed to stand at room temperature for 5 min and plasmid DNA (0.1 pM in 130 mM NaCl) was added. After 5 min, H2O2 (50 μM) was added to initiate DNA damage. After 150 min, the reaction was stopped by adding EDTA (200 mM, 0.5 μL) and loading dye (2 μL) to give a final volume of 12.5 μL.
Gel electrophoresis was run on a 1% agarose gel in TAE buffer for 60 min at 140 V and 255 mA to separate the nicked and supercoiled forms of the plasmid DNA. Gels were then stained for 5 min using ethidium bromide and washed for an additional 10 min with deionized water before imaging under UV light. The intensities of the damaged and undamaged DNA gel bands were quantified using UVIproMW software (Jencons Scientific Inc.). Ethidium stains supercoiled DNA less efficiently than nicked DNA, so supercoiled DNA band intensities were multiplied by 1.24 prior to comparison (Hertzberg and Dervan, 1982). Intensities of the nicked and supercoiled bands were normalized for each lane so that % nicked + % supercoiled = 100%. Sample gel images (Figures S1–S10) and tables of gel data (Tables S1–S10) are provided in the Supplementary Material.
2.5. Electron paramagnetic resonance (EPR) studies
EPR spectra were measured on a Bruker EMX spectrometer using a quartz flat cell at room temperature using 2,2-diphenyl-1-picrylhydrazyl (DPPH) as a standard (g = 2.0036 (Mani et al., 2004)) centered at 3500 with a sweep width of 100 G. The modulation amplitude was 0.50–1.00 G, time and conversion constants were 81.92 s, and microwave power and frequency were 20.02 mW and 9.752 GHz, respectively. Samples (500 μL total volume) were prepared in MOPS buffer (10 mM, pH 7), containing NPCuO (300 μM), ascorbate (375 μM), H2O2 (22.5 mM), polyphenol (600 μM), and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as a spin trap, and measured in less than 5 minutes. In all cases, H2O2 was added last to initiate the reaction. Complete EPR data for NPCuO generation of ROS with ascorbic acid and H2O2 are provided in Figures S12 and S13.
2.6. Dynamic light scattering (DLS) size measurements of NPCuO and wCuO
DLS measurements were performed with a Brookhaven NanoBrook Omni particle sizer and zeta potential analyzer system at 640 nm and 589 nm wavelengths. After loading the sample in the cuvette, the sample was left to sit for 5–10 min to allow any turbulence to dissipate before spectral acquisition. A detection angle of 90° was chosen for all size measurements. EMEM was used for particle characterization studies. Unless otherwise stated, EMEM suspensions were supplemented with 10% FBS, L-glutamine (2 mM), non-essential amino acids, streptomycin (100 μg/mL), and penicillin G (100 UI/mL). Aliquots were taken from NPCuO or wCuO stock suspensions and mixed with 3 mL of either EMEM or sterile deionized water. The solutions were mixed well and incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air for 24 h. The test suspensions were mixed gently prior to DLS measurements. DLS measurements of NPCuO and wCuO in water and EMEM are presented in Table S11 and Figure S14 in the Supplementary Material.
2.7. Cell culturing procedures
Mouse fibroblast cells (L929) were cultured in 75 cm2 tissue-culture flasks in presence of EMEM at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. EMEM was supplemented with 10% FBS, L-glutamine (2 mM), non-essential amino acids, streptomycin (100 μg/mL), and penicillin G (100 UI/mL). At 95% confluence, cells were harvested using 0.25% trypsin and were seeded at a concentration of 1.5×104 cells/well in 96-well plates. Cells were allowed to attach the surface for 24 h prior to treatment. The appropriate dilutions of wCuO and NPCuO suspensions were then sonicated using a sonicator bath at room temperature for 10 min to avoid nanoparticle aggregation prior to administration. Cells not exposed to nanoparticles served as controls in each experiment. Selection of final concentrations of 15.625–625 μM dose range for both wCuO and NPCuO nanoparticles was based on a previous report on copper oxide nanoparticle interactions with lung epithelial cells (Z. Wang et al., 2012). When cells reached sub-confluence, they were pretreated for 24 h with or without H2O2 and the indicated concentrations of wCuO, NPCuO, lCuO, or copper solution. Following treatment, the culture supernatant was removed, and cells were washed three times with phosphate-buffered saline (PBS) before determining cell viability.
To study the effects of MEPCA and MEGA addition, 1.5×104 cells/well were incubated with various concentrations (1–400 μM) of MEPCA or MEGA at 37 °C in a humidified atmosphere of 5% CO2 and 95% air for 3 h. Next, a mixture of NPCuO (153.5 μM) and H2O2 (80 μM) was added. After 24 h incubation, the culture supernatant was removed, and cells were washed three times with phosphate buffered saline (PBS) before determining cell viability. Cell viability results with NPCuO/H2O2, with and without polyphenol addition, are provided in Figures S15–S22 and Tables S12–S19 in the Supplementary Material.
2.8. Cell viability measurements
After L929 cell treatment with wCuO, NPCuO, lCuO, or copper solutions with or without H2O2, MEPCA, or MEGA, MTS assays were performed according to the manufacturer’s protocol, and the plate was read at 490 nm optical density to measure the absorbance of the formazan product using a microplate reader (Thermo Scientific Multiskan™ FC). Since some polyphenols can interfere with formazan formation in the MTS assay (Akter et al., 2019; Wang, P. et al., 2010;), we tested MEGA and MEPCA in this assay prior to the cell studies to ensure that no spurious signals were observed due to polyphenol addition. Viability of untreated cells was normalized to 100% to calculate the viability of the cells treated with different concentrations of NPCuO and polyphenol compounds. Cell viability data for these experiments are presented in the Supplementary Material (Tables S11–S18 and Figures S11–S18).
2.9. Statistical analysis
For all studies, data are expressed as means of at least three independent trials with error bars representing standard deviations. Statistically significant differences within and between the groups were evaluated by performing ANOVA and 2-tailed unpaired t-tests. Results with p values of ≤ 0.05 were considered to be statistically significant. To determine EC50/IC50 values, a graph of percent non-viable cells was plotted with respect to NPCuO, wCuO, lCuO, dissolved copper, H2O2, MEPCA, or MEGA concentrations on a semi-log plot and fit to a sigmoidal dose-response curve using OriginPro 8.1 software. EC50 values were calculated by fitting all points of four trials with a single curve.
3. Results
3.1. Activity of polyphenols with NPCuO/H2O2–mediated DNA damage
To evaluate polyphenol effects on NPCuO/H2O2-mediated DNA damage, plasmid gel electrophoresis assays were performed. Focusing on damage prevention using a single biomolecule (DNA) allows a more complete mechanistic examination of biologically relevant oxidative damage prevention than can be obtained in a cellular environment. Since gel electrophoresis readily separates damaged (nicked) from undamaged (supercoiled) plasmid DNA, sample treatment is straightforward and assessment time is short. After staining, intensities of the damaged and undamaged plasmid DNA bands are quantified, so that polyphenol antioxidant abilities can be directly determined and compared. Treating plasmid DNA with NPCuO (500 μM) and H2O2 (50 μM) for 150 min at pH 7 results in ≥ 85% DNA damage. These conditions result in an average of one strand break per plasmid to facilitate DNA damage quantification, and this DNA damage is used as the positive control. As reported, the manufacturer-added NPCuO dispersant has little effect on observed NPCuO/H2O2 DNA damage effects (Angelé-Martínez et al., 2017).
Once initial DNA damaging conditions were established, ten polyphenol compounds were evaluated for their ability to prevent NPCuO/H2O2-mediated DNA damage. Hydrogen peroxide alone and MEPCA (800 μM) alone do not damage DNA (Figure 2, lanes 2 and 3, respectively); however, more than 90% of plasmid DNA is nicked (top band) when treated with NPCuO (500 μM) and H2O2 (50 μM; lane 4). In lanes 5 to 15, DNA treated with NPCuO/H2O2 and increasing concentrations of MEPCA (0.5–800 μM) show decreased DNA damage, as indicated by the increasing intensity of supercoiled DNA (bottom band). Percent DNA damage inhibition plotted with respect to MEPCA concentration results in a best-fit, sigmoidal dose-response curve (Figure 2B) used to determine the MEPCA concentration at which 50% of DNA damage is inhibited (IC50 value). The IC50 value of 7.5 ± 0.5 μM for MEPCA indicates that it is a potent antioxidant, even at low micromolar concentrations. Blood concentrations of dietary polyphenols are typically between 0.3–10 μM (Reddy et al., 2005; Scalbert and Williamson, 2000; Sugisawa and Umegaki, 2002; van het Hof et al., 1998; Yamamoto et al., 2003; Yang et al., 1998), so MEPCA shows significant antioxidant effects at biologically relevant concentrations.
Figure 2.
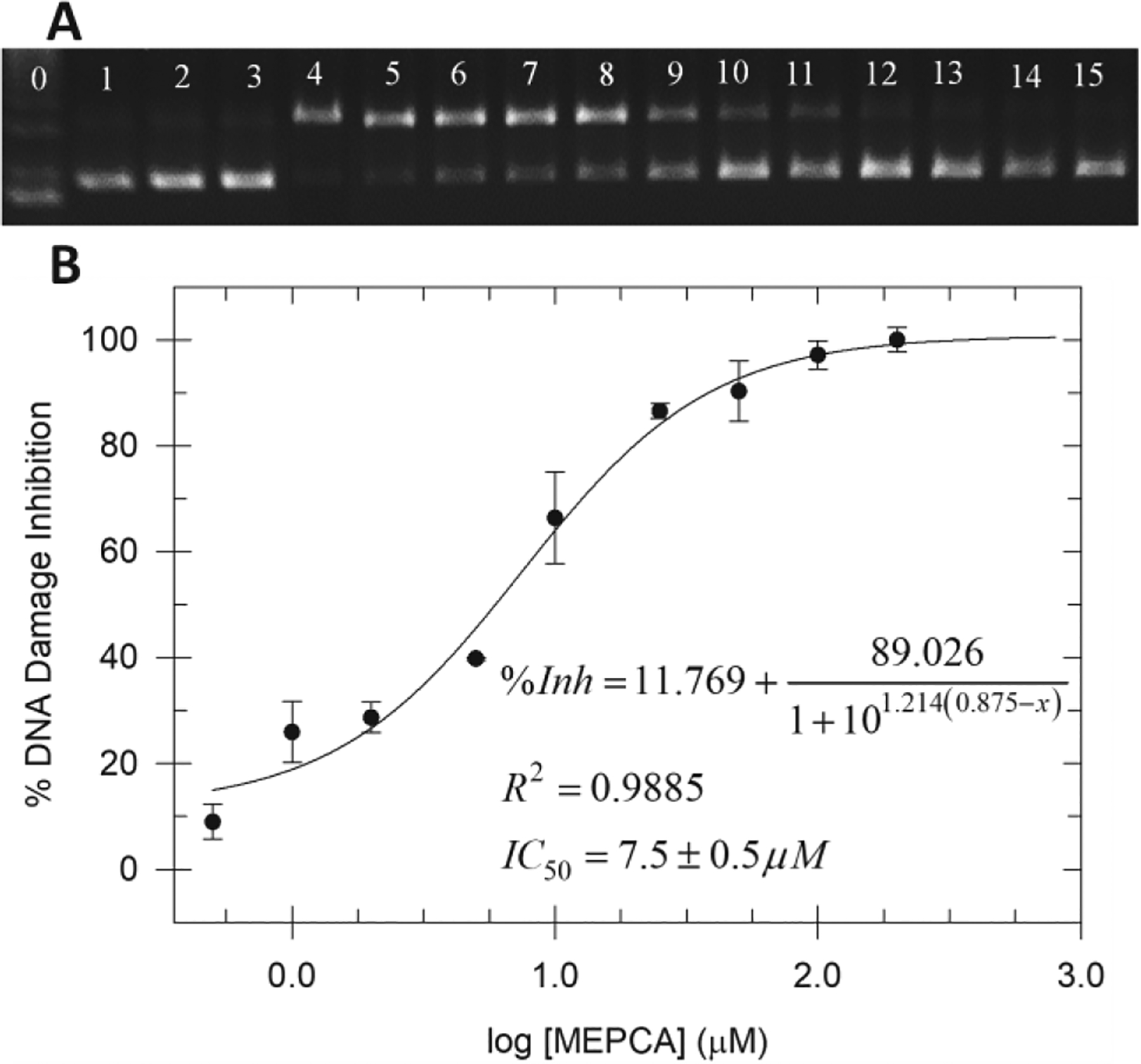
A) Gel electrophoresis image of plasmid DNA treated with NPCuO (500 μM), H2O2 (50 μM), and MEPCA (0.5–800 μM) at pH 7 (MOPS buffer) for 150 min. Lane 0: 1 kb molecular weight ladder; lane 1: plasmid (p); lane 2: p + H2O2 (50 μM); lane 3: p + MEPCA (800 μM); lane 4: p + NPCuO (500 μM) + H2O2 (50 μM); lanes 5–15: p + NPCuO (500 μM) + and H2O2 (50 μM) + increasing concentrations of MEPCA (0.5, 1, 2, 5, 10, 25, 50, 100, 200, 400, and 800 μM, respectively). Damaged (nicked) plasmid DNA is in the top band, undamaged (supercoiled) DNA is in the bottom band, and the brightest band in lane 0, just below the undamaged DNA band, is the 3 kb DNA marker. B) Percentage of DNA damage inhibition with respect to MEPCA concentration in the presence of NPCuO and H2O2 fit with a sigmoidal dose-response curve.
Table 1 summarizes the results obtained for each of the polyphenol compounds evaluated using this DNA damage assay (all gel electrophoresis results are provided in Figures S1–10 and Tables S1–S10 of the Supplementary Material). Four of these polyphenols, MEPCA, propyl gallate, epicatechin gallate, and MEGA prevent NPCuO/H2O2-mediated damage. EGCG prevents a maximum of ~50% DNA damage only at very high concentrations (~800 μM). PCA, VA, and EC show no activity. Little correlation is observed between polyphenol DNA damage prevention ability (IC50 value) and polyphenol oxidation potential (Perron et al., 2008) (R2 = 0.42) or the pKa of the first phenolic hydrogen pKa (Perron and Brumaghim, 2009) (R2 = 0.43; Supplementary Material, Figure S11).
Table 1.
Polyphenol affects on DNA damage by NPCuO or Cu+ and H2O2.
| Polyphenol | IC50a (μM) with NPCuO/H2O2 | IC50 (μM) with Cu+/H2O2b |
|---|---|---|
| MEPCA | 7.5 ± 0.5 | 8.24 ± 0.03 |
| PREGA | 112 ± 19 | 125.90 ± 0.02 (prooxidant 0.2–10 μM)c |
| ECG | 120 ± 22 | 53.04 ± 0.02 (prooxidant 0.1–4 μM)c |
| MEGA | 185 ± 8 | 102.3 ± 0.1 (prooxidant 0.2–10 μM)c |
| EGCG | ~800d | 225.9 ± 0.1 |
| PCA | No activity | ~480 |
| VA | No activity | No activity |
| EC | No activity | Prooxidant only 0.2–500 μM |
| GA | Prooxidant | Prooxidant 4–10 μM; damage prevention ≥ 300 μMc,d |
| EGC | Prooxidant | Prooxidant only 0.2–1000 μM |
Concentration that inhibits 50% DNA damage
Cu+ concentration = 6 μM; H2O2 concentration = 50 μM (Perron et al., 2011)
Polyphenols are prooxidants at lower concentrations and antioxidants at higher concentrations
A complete IC50 curve was not obtained due to concentration limitations
In contrast, adding EGC alone to plasmid DNA (Figure 3, lane 3) results in a significant amount of damaged DNA after 150 min. With NPCuO/H2O2 and EGC addition (lanes 5–15), DNA is increasingly damaged as EGC concentration increases until it is so damaged with strand breaks at multiple sites that only a diffuse band at lower molecular weight is observed. Thus, EGC acts as a prooxidant to enhance DNA damage caused by NPCuO/H2O2. GA also enhances NPCuO/H2O2-mediated DNA damage but to a lesser degree than EGC (Table 1).
Figure 3.
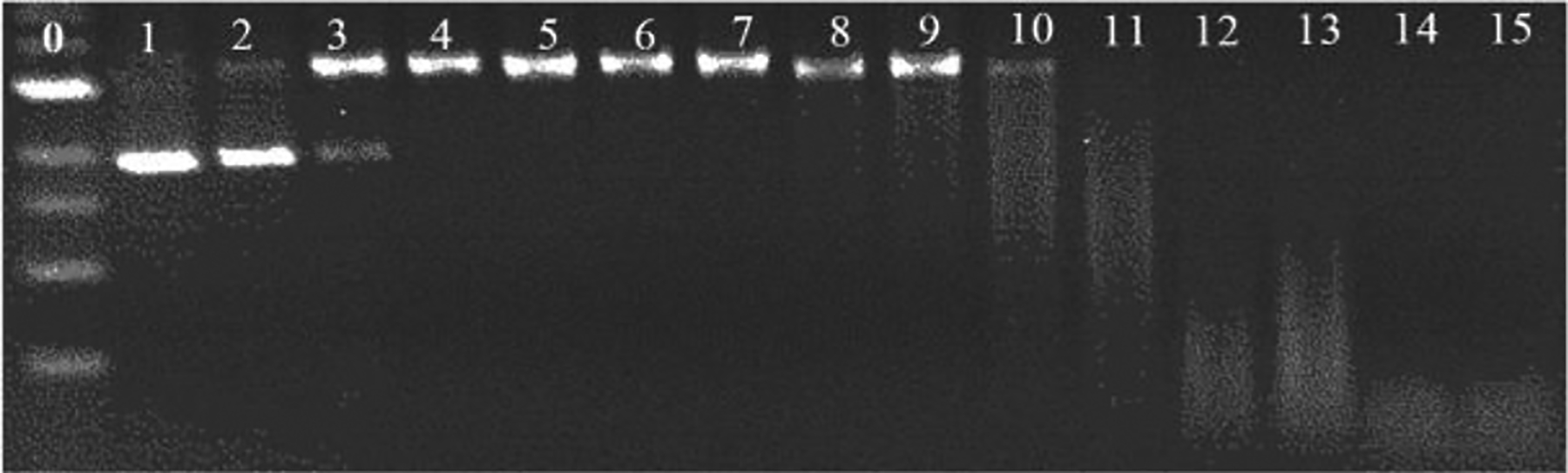
Gel electrophoresis image of plasmid DNA treated with NPCuO (500 μM), H2O2 (50 μM), and EGC (0.5 – 800 μM) at pH 7 (MOPS buffer) for 150 min. Lane 0: 1 kb molecular weight ladder (with bands labeled in Figure S10); lane 1: plasmid (p); lane 2: p + H2O2 (50 μM); lane 3: p + EGC (800 μM); lane 4: p + NPCuO (500 μM) + H2O2 (50 μM); lanes 5–15: p + NPCuO (500 μM) + H2O2 (50 μM) + increasing concentrations of EGC (0.5, 1, 2, 5, 10, 25, 50, 100, 200, 400, and 800 μM, respectively). Damaged (nicked) plasmid DNA is in the top band, undamaged (supercoiled) DNA is in the bottom band, and the diffuse bands at lower molecular weights indicate significant DNA damage with many strand breaks per plasmid.
3.2. ROS detection using EPR spectroscopy
To more directly ascertain the ROS formed by NPCuO/H2O2 under various conditions and to determine the effects of polyphenol addition on these ROS, EPR spectroscopy studies were performed. NPCuO/H2O2 conditions generate primarily 1O2, O2•−, and •OH, likely by different mechanisms (Cu+ reduction of hydrogen peroxide, electron transfer from NPCuO conduction band, polyphenol oxidation, etc.) (Angelé-Martínez et al., 2017; Bandara et al., 2005; Bondarenko et al., 2012; W. Chen et al., 2012; Gunawan et al., 2011; Hong et al., 2013; Jose et al., 2011; Y. Li et al., 2012; Pascholiano et al., 2008; Qin and Li, 2011; Shi et al., 2012; Song et al., 2016; Srikanth et al., 2015). Due to the short lifetime of these ROS, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was used as a spin trap in these experiments, since it can form distinguishable, long-lived adducts with •OH and O2•− (Supplementary Material, Figure S12). The spin trap 2,2,5,5-tertramethyl-1-pyrroline-N-oxide (TEMP) was also used because it forms an adduct with 1O2 (TEMPO; Supplementary Material, Figure S13) that can be distinguished from •OH and O2•− adducts (Alia et al., 2001; Fufezan et al., 2002). Using DMPO and TEMP allows trapping of generated ROS to determine the relative amounts formed under different conditions. These EPR experiments were conducted with representative polyphenols that have differing effects on NPCuO/H2O2-mediated DNA damage: MEPCA as an antioxidant, VA with no effect, and EGC as a prooxidant.
The room temperature EPR spectrum of NPCuO/H2O2 shows a DMPO-OH adduct resonance derived from •OH generation (Figure 4A). However, the EPR spectra of the superoxide salt K2O under the same conditions also results in only DMPO-OH adduct resonances. Thus, although •OH adduct is the only ROS detected under NPCuO/H2O2 conditions, O2•− may also form and decompose rapidly to generate •OH (Angelé-Martínez et al., 2017).
Figure 4.
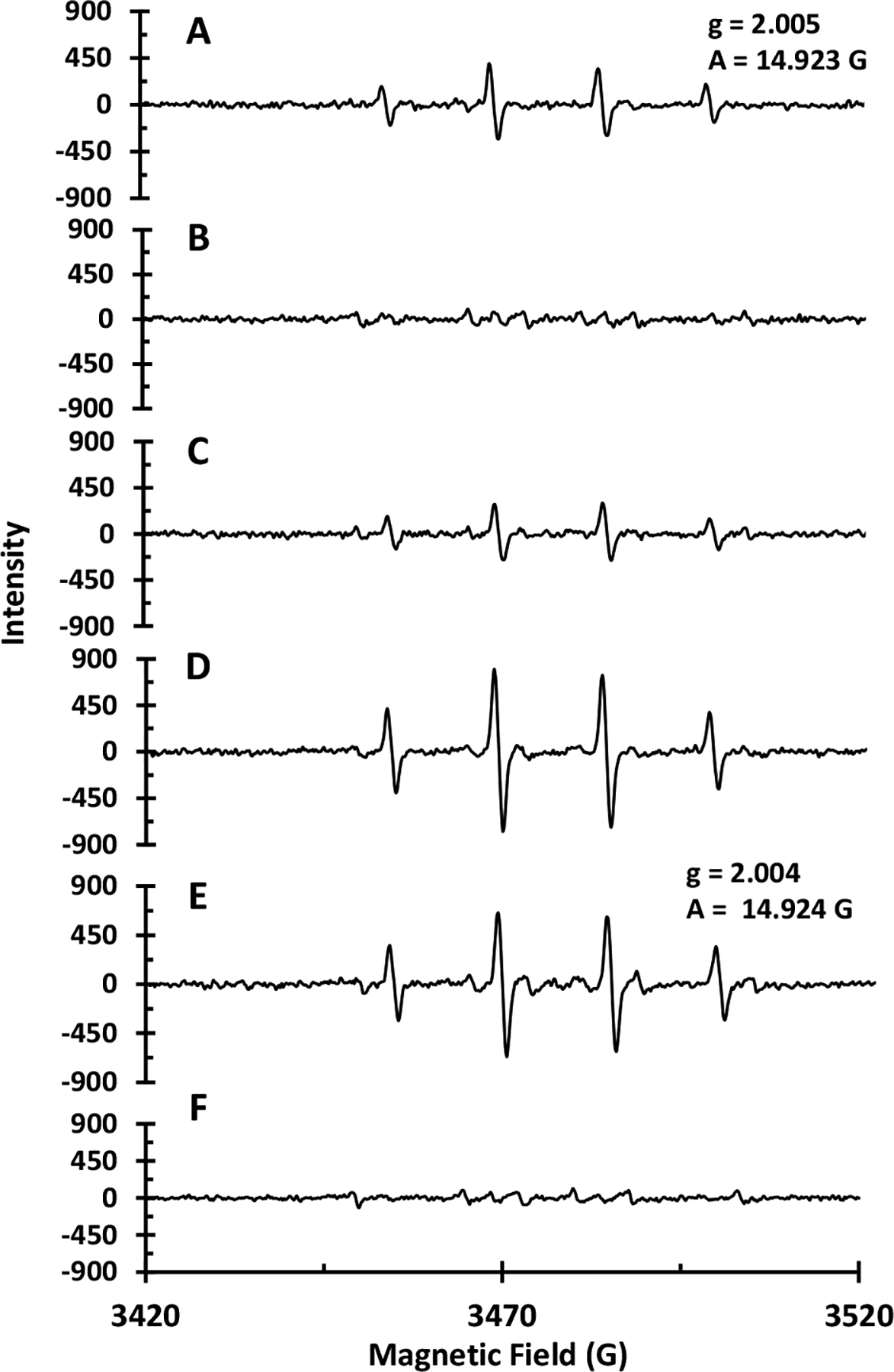
EPR spectra of A) NPCuO (300 μM) and H2O2 (22.5 mM); B) NPCuO (300 μM), H2O2 (22.5 mM), and MEPCA (600 μM); C) NPCuO (300 μM), H2O2 (22.5 mM), and VA (600 μM); D) NPCuO (300 μM), H2O2 (22.5 mM), and EGC (600 μM); E) wCuO (300 μM) and H2O2 (22.5 mM); and (F) wCuO (300 μM), H2O2 (22.5 mM), and MEPCA (600 μM). All spectra were collected at room temperature and pH 7 (MOPS, 10 mM) with DMPO (30 mM) as a spin trap.
Addition of MEPCA, the polyphenol compound that most effectively prevents NPCuO-mediated DNA damage, to a mixture of NPCuO and H2O2 almost completely suppresses the DMPO-OH resonance intensity (Figure 4B). The remaining weak resonance observed upon MEPCA addition(Figure 4B) is due to superoxide generation and decomposition into DMPO-OOH, which in turn decomposes into DMPO-OH (Finkelstein et al., 1979).
In contrast, the EPR spectrum showed very little effect on the observed DMPO-OH resonance intensity upon VA addition (Figure 4C), whereas the EPR spectrum in the presence of the prooxidant EGC (Figure 4D) shows a DMPO-OH resonance with a two-fold increase over that of NPCuO/H2O2 alone (Figure 4A). Generation of •OH under these conditions directly follows the trend observed for polyphenol prevention of NPCuO-mediated DNA damage, suggesting that in vitro polyphenol antioxidant and prooxidant activity with NPCuO/H2O2 can be evaluated accurately by both DNA gel electrophoresis and by measuring the intensity of the DMPO-OH resonance using EPR spectroscopy.
Since copper chelation by polyphenols is well known (Galleano et al., 2010), to determine whether these polyphenol compounds prevent or promote ROS formation by interacting with dissolved copper or with the nanoparticle surface, additional EPR experiments were carried out with washed CuO nanoparticles (wCuO). Thoroughly washing the nanoparticles before suspending them in aqueous solution results in a supernatant with less than 2.5 μM dissolved copper (from the original 5 mM stock), as measured using the bathocuproine method (Angelé-Martínez et al.). By removing dissolved copper from the NPCuO suspension supernatant, any ROS generated are likely due to H2O2 reaction with the nanoparticle surface, and polyphenol prevention of this ROS generation will likely be due to surface interactions (Angelé-Martínez et al., 2017).
In the EPR spectrum of wCuO/H2O2 (Figure 4E), the DMPO-OH resonance is again observed, with an intensity slightly higher than that observed for the non-washed NPCuO/H2O2 (Figure 4A). Upon MEPCA addition, this DMPO-OH resonance is completely suppressed, but a remaining weak resonance is observed (Figure 4F) that is similar to the resonance observed upon MEPCA addition to NPCuO/H2O2. This resonance has been assigned previously to O2•− and •OOH (Finkelstein et al., 1979; Hu and Jiang, 1996), indicating that MEPCA suppresses •OH, O2•−, and •OOH formation both at the nanoparticle surface and from dissolved copper in solution.
Prevention of NPCuO cytotoxicity requires that polyphenol compounds are antioxidants in the reducing environment of the cytoplasm and nucleus (Akthar et al., 2012; Bulcke and Dringen, 2015; Jing et al., 2015; Sharifi et al., 2012; Soliman et al., 2013; Zou et al., 2013). Thus, the ability of MEPCA to prevent hydroxyl radical production by NPCuO/H2O2 was also examined in the presence of ascorbate, a reducing agent that can reduce Cu2+ to hydroxyl-radical-forming Cu+) (Kadiiska and Mason, 2002; Perron et al., 2011). The EPR spectrum of NPCuO with H2O2 and 1.25 equivalents of ascorbate per copper ion shows the DMPO-OH adduct resonance as well as an additional resonance from a second ROS (Figure 5A). These additional resonances are similar to the weak resonances observed in Figures 4B and 4F and are consistent with superoxide formation (Finkelstein et al., 1979; Hu and Jiang, 1996) with subsequent decomposition into the hydroperoxide adduct (DMPO-OOH) and then to DMPO-OH (Finkelstein et al., 1979; Noda et al., 2008; Yamaguchi et al., 2000). The ascorbyl radical signal is not observed under these conditions.
Figure 5.
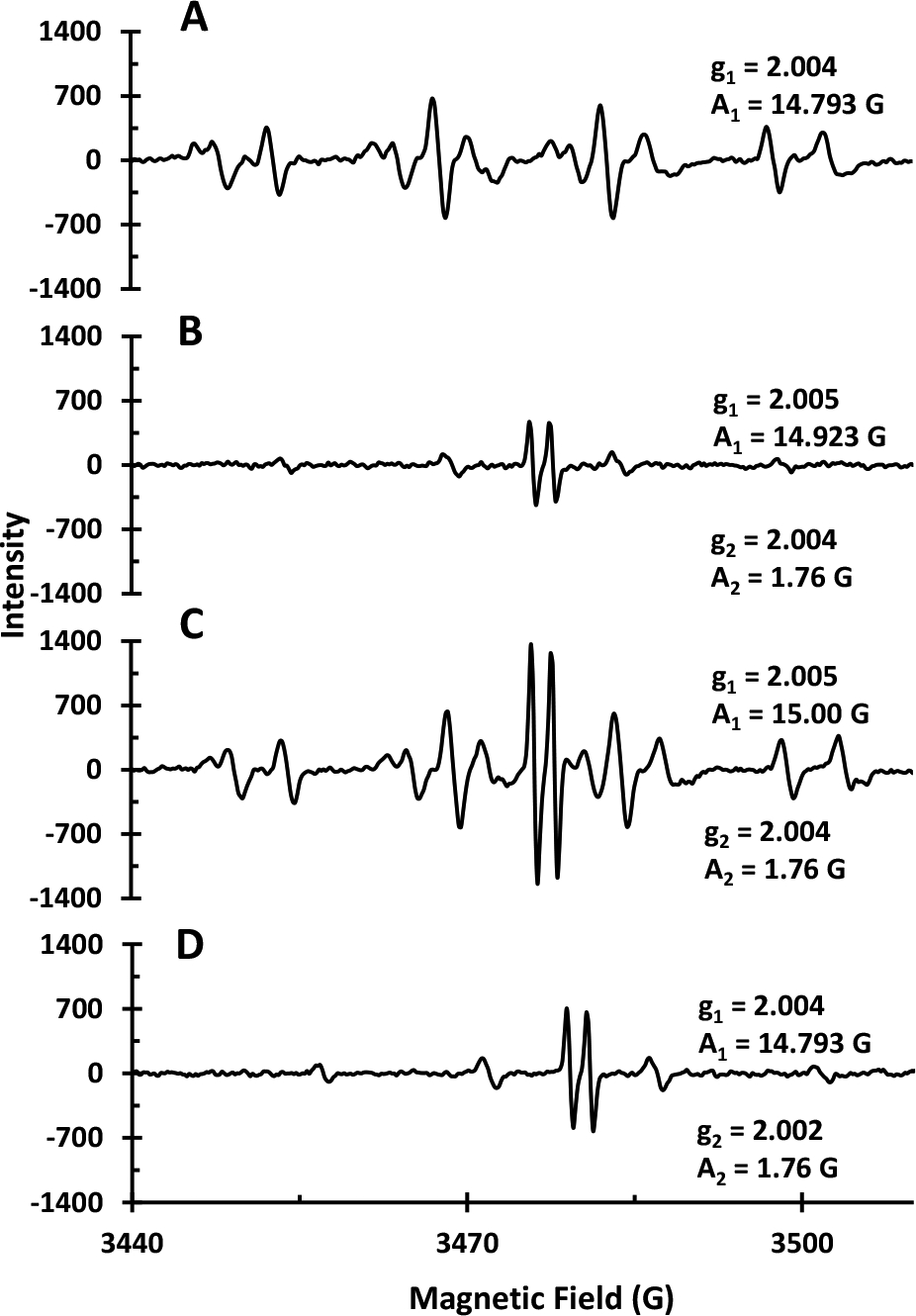
EPR spectra of: A) NPCuO (300 μM), ascorbate (375 μM), and H2O2 (22.5 mM); B) NPCuO (300 μM), ascorbate (375 μM), H2O2 (22.5 mM) , and MEPCA (600 μM); C) wCuO (300 μM), ascorbate (375 μM), and H2O2 (22.5 mM); and D) wCuO (300 μM), ascorbate (375 μM), H2O2 (22.5 mM), and MEPCA (600 μM). All spectra were acquired at room temperature and pH 7 (MOPS, 10 mM) with DMPO (30 mM) as a spin trap.
Addition of MEPCA to the NPCuO/H2O2/ascorbate solution has a significant effect on ROS generation (Figure 5B). The DMPO-OH resonance intensity is reduced by approximately 7-fold from the spectrum without MEPCA (Figure 5A), and no DMPO-O2•− resonance is observed. In addition, a new resonance from ascorbyl radical is observed in the spectrum as a doublet centered at g = 2.004 (Barbehenn et al., 2003; Boatright and Crum, 2016; Mouithys-Mickalad et al., 1998; Pietri et al., 1990; Warren and Mayer, 2008).
The EPR spectrum of wCuO/H2O2/ascorbate shows resonances for ascorbyl radical, DMPO-OH, and DMPO-O2 (Figure 5C). The intensities of the DMPO-OH and DMPO-O2 resonances are similar to those observed in the NPCuO/H2O2/ascorbate sample (Figure 5A), but the ascorbyl radical resonance is only observed in the presence of wCuO, not NPCuO. MEPCA addition to the wCuO/H2O2/ascorbate system (Figure 5D) reduces the intensity of the DMPO-OH by 5-fold, the ascorbyl radical by 2-fold, and the DMPO-O2 resonance to zero compared to samples without MEPCA.
The same EPR experiments were repeated using the TEMP spin trap, but no 1O2 was detected. However, resonances similar to those reported for DMPO-O2 are observed in the presence of NPCuO/H2O2 and NPCuO/H2O2/ascorbate (Figure S21). Thus, from these EPR results, MEPCA clearly suppresses •OH and O2•− generation at the NPCuO surface or from dissolved copper with or without addition of ascorbate. Suppression of •OH and O2•− formation by MEPCA demonstrates its ability to quench different ROS, either in the presence of the reducing agent ascorbate, on the surface of the nanoparticles, or by the dissolved ions from NPCuO.
3.3. Investigating NPCuO/H2O2-mediated L929 cell death
Human lung and skin fibroblast cells play a pivotal role in many diseases including lung cancer, skin cancer, pulmonary hypertension, and pulmonary fibrosis and are often used in cell toxicology studies (Kalluri and Zeisberg, 2005). Mouse fibroblasts (L929) cell cultures were used to assess the toxicity of NPCuO at different doses (15.63–625 μM) after 24 h exposure. Cell viability decreased in a dose-dependent manner following NPCuO exposure (Figure 6A). At low concentrations (<15.63 μM), NPCuO treatment resulted in insignificant toxicity to L929 cells. For higher NPCuO exposures (31–313 μM), cell viability decreased with increasing concentration, falling to 0.2% of control at ≥ 313 μM. The effective concentration of NPCuO required to kill 50% of cells (EC50) was calculated as 154 ± 15 μM (Table S12 and Figure S15, Supplementary Material).
Figure 6.
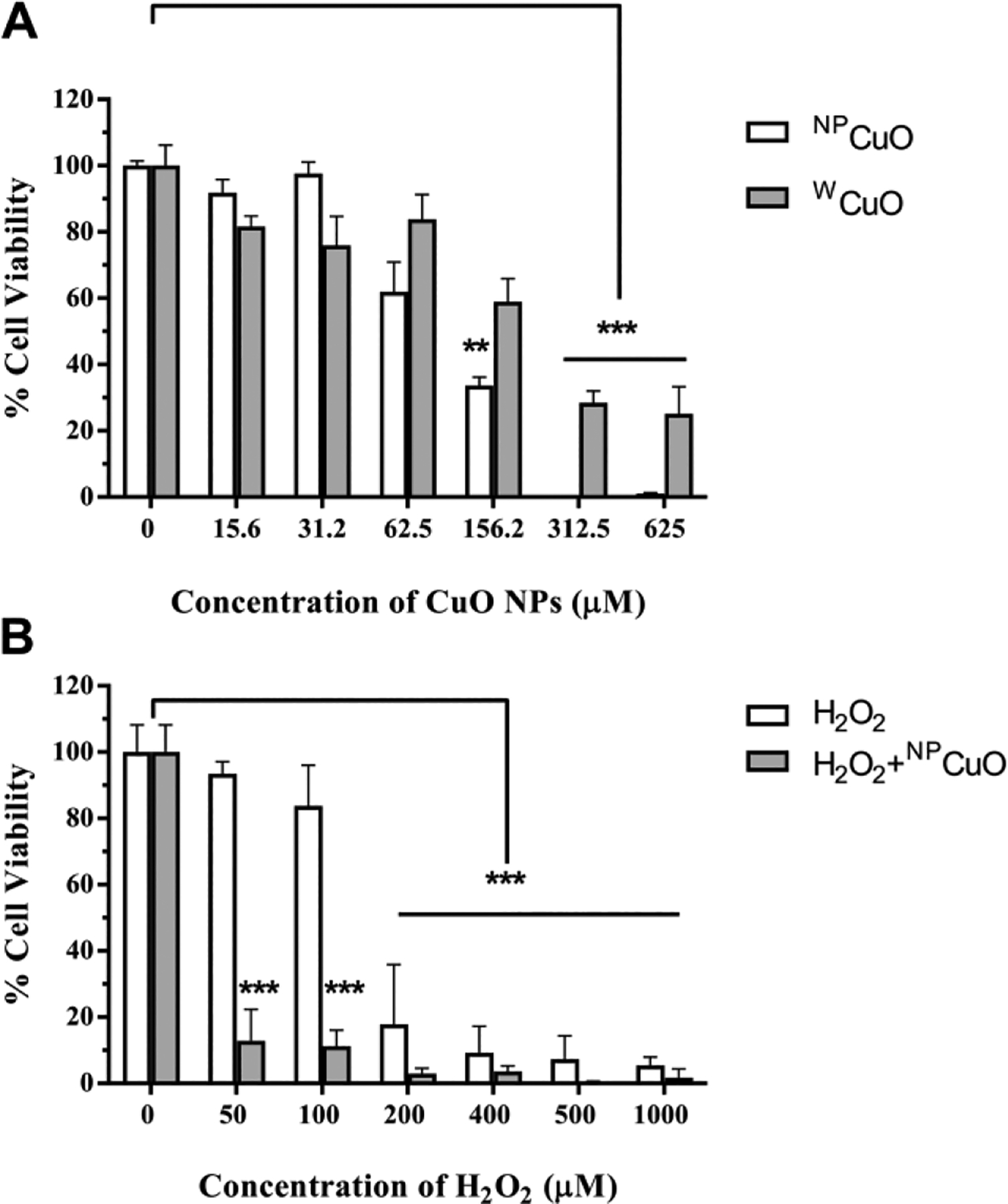
L929 cell viability measured with the MTS assay after exposure to different conditions: cell viability after exposure to A) different concentrations of NPCuO or wCuO nanoparticle suspensions for 24 h for NPCuO or wCuO concentrations from 15.6–625 μM and B) cell viability after 24 h exposure to NPCuO (62.5 μM) and varying H2O2 concentrations (50–1000 μM). Data are expressed as means ± standard deviations (n = 4); **p-value <0.01 and ***p-value <0.001.
wCuO toxicity to L929 cells was also examined at 16–625 μM after 24 h (Figure 6A). Similar to NPCuO, cell viability upon wCuO exposure is concentration dependent, with an EC50 value of 170 ± 15 μM for wCuO (Table S13 and Figure S16, Supplementary Material). While cell viability was lower at every concentration for the washed nanoparticles than the unwashed particles, the overall IC50 value was just 10% higher for the unwashed particles (170 ± 15 μM vs. 154 ± 15 μM), and within the margin of error. Since the washing reduced the dissolved copper concentration by 2000-fold (5 mM to 2.5 μM, Angelé-Martínez et al.), the dissolved copper ions from solution must not play a significant role in toxicity, especially for the washed particles..
To confirm the similarity of NPCuO and wCuO particle sizes and their stability in cell medium, both types of nanoparticles were characterized. TEM images showed that NPCuO are roughly spherical, with a diameter of 50–60 nm (Angelé-Martínez et al., 2017). To test NPCuO stability in water and EMEM cell culture medium, a suspension was incubated in cell culture medium at 37 °C in a humidified atmosphere of 5% CO2 and 95% air for 24 h. The mean hydrodynamic diameter (weighted by intensity) of NPCuO in EMEM cell culture medium and in water measured by DLS is ~139 ± 2 nm and ~175 ± 14 nm, respectively (Table S19 and Figure S20, Supplementary Material). Similarly, the mean hydrodynamic diameter (weighted by intensity) of wCuO in EMEM cell culture medium and in water measured by DLS is ~154 ± 11 nm and ~186 ± 13 nm, respectively (Table S11 and Figure S14, Supplementary Material). The size distributions of NPCuO and wCuO suspended in sterile, deionized water and EMEM cell culture medium, as measured by DLS analysis, showed larger values than the particle size measured by TEM. This indicates that the NPCuO and wCuO form aggregates in water and EMEM cell culture medium that are ~2 to 3 times larger than the primary particle sizes. NPCuO and wCuO appear to be moderately dispersed in water and EMEM cell culture medium; however, NPCuO and wCuO in EMEM medium formed smaller aggregates compared to NPCuO and wCuO in water. Proteins in the medium are known to form a corona on the surface of the nanoparticles which can affect aggregation and how they interact with cells (Monopoli et al., 2012). In addition, the composition of the medium may affect the rate and degree of copper dissolution from both types of nanoparticles. The washed particles had similar diameters to the unwashed particles both in deionized water and EMEM medium (slightly larger size for the washed particles but close to the margin of error), which suggests similar cellular uptake would be expected with and without washing.
Since dissolved copper can decrease cell viability by causing DNA damage and cell death (Aruoma et al., 1991) and by directly altering the expression of apoptotic genes (Chan et al., 2008), we tested whether copper released from NPCuO into the cell media contributes to L929 cytotoxicity. Release of copper ions from the surface of NPCuO was demonstrated when NPCuO were suspended in DMEM, and this released copper is responsible for some of the nanoparticles’ toxic effects (Midander et al., 2009). To examine the contribution of dissolved copper on NPCuO toxicity in our system, cells were treated with CuCl2 solutions (0.17–6.6 μM), and L929 cell viability assays were performed to compare the cell-damaging effects of dissolved copper in the NPCuO supernatant with those of CuCl2 solutions at comparable dissolved copper concentrations, as calculated based on our previous work (Angelé-Martínez et al., 2017). Less than 30% of non-viable cells were observed when L929 cells were incubated with different concentrations of CuCl2 solutions for 24 h (Table S14 and Figure S17, Supplementary Material). Our data suggest that even if copper ions were released from NPCuO into the cell culture media, they do not significantly contribute to cytotoxicity or the oxidative damage associated with NPCuO nanoparticle exposure. This result is consistent with previous findings demonstrating that dissolved copper from NPCuO is insufficient to produce mortality in zebrafish (Griffitt et al., 2007).
We also investigated the effect of the organic dispersant in the supernatant of NPCuO suspensions. This is the supernatant after the first centrifugation to separate the nanoparticles from the dissolved copper, but with dissolved copper removed (lCuO). High concentrations of lCuO (12 mM) showed less than 30% non-viable L929 cells (70% viable) (Table S15 and Figure S18, Supplementary Material). Thus, at the much lower concentrations used in our experiments (typically 62.5 μM) the effect of this dispersant should be negligible.
L929 cell viability in the presence of H2O2 was also examined after 24 h. As shown in Figure 6B, increased cell killing results from H2O2 addition up to 1000 μM, at which less than 0.8% of treated cells survive. The EC50 value for H2O2 alone was measured to be 152 ± 9 μM (Table S16 and Figure S19, Supplementary Material). When NPCuO and H2O2 were combined, L929 cell survival also decreased in a dose-dependent manner (Figure 6B), and cells are killed much more effectively by NPCuO/H2O2 than by H2O2 or NPCuO alone. In fact, NPCuO or H2O2 alone at 50 μM concentration causes no change in cell viability, but the combination of NPCuO/H2O2 at 50 μM each cause almost 90% cell death. These results demonstrate that NPCuO/H2O2 system is highly toxic to L929 cells, and this combination causes greater-than-additive cell killing compared to either NPCuO or H2O2 alone.
3.4. Effect of MEPCA and MEGA on NPCuO/H2O2-mediated L929 cell death
Since MEPCA was the only polyphenol compound to prevent NPCuO-mediated DNA damage at blood polyphenol concentrations (0.3–10 μM (Reddy et al., 2005; Scalbert and Williamson, 2000; Sugisawa and Umegaki, 2002; van het Hof et al., 1998; Yamamoto et al., 2003; Yang et al., 1998); MEPCA IC50 = 7.5 μM), we tested this polyphenol as well as its gallol analog, MEGA, for their ability to inhibit NPCuO/H2O2-mediated L929 cell death (Tables S18 and S19 and Figures S21 and S22 in the Supplementary Material, respectively). MEGA has no effect on L929 viability at these concentrations (Schlickmann et al., 2017), and protocatechuic acid and its esters show no tocxicity to L929 cells up to 50 μM (Daré et al., 2020). Upon treatment with NPCuO/H2O2 (153.5 and 80 μM, respectively), only 17% of cells were viable. MEPCA or MEGA addition (1 μM) reduced NPCuO/H2O2 cytotoxicity, resulting in 81% and 71% cell viability, respectively (Figure 7A). Increasing polyphenol concentrations to 10 μM decreases this viability rescue, and above 50 μM, MEPCA or MEGA treatment shows no significant rescue of NPCuO/H2O2 toxicity.
Figure 7.
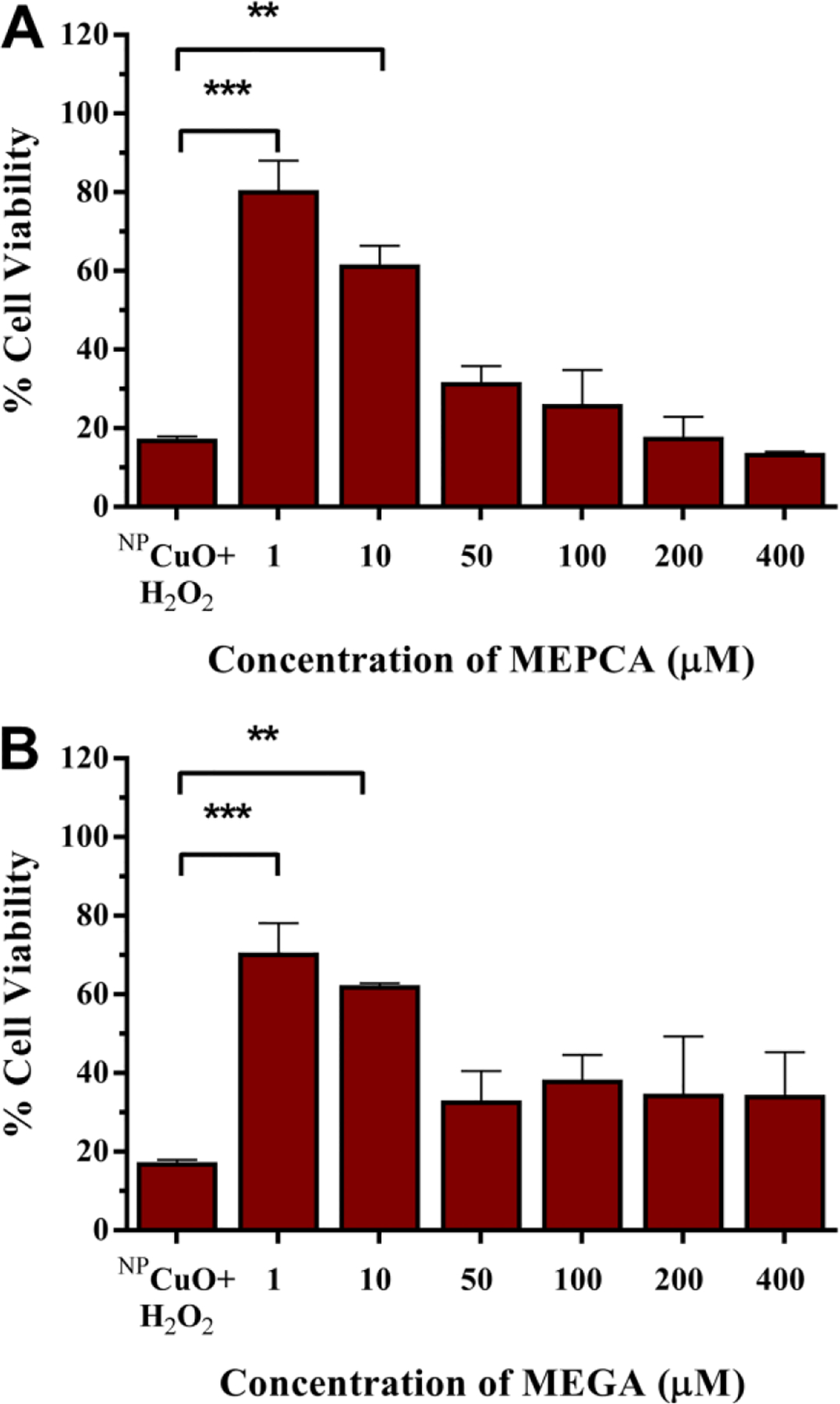
L929 cell viability measured by MTS assay after exposure to NPCuO/H2O2 (153.5 μM and 80 μM, respectively) and A) MEPCA (1–400 μM) and B) MEGA (1–400 μM). Data expressed as means ± standard deviations (n = 4); **p-value <0.01, and ***p-value <0.001.
4. Discussion
4.1. Polyphenol effects on NPCuO/H2O2–mediated DNA damage and ROS generation
Plasmid DNA damage assays evaluate both antioxidant and prooxidant polyphenol activity, and both behaviors were observed in these studies. The catechol-containing MEPCA is the most effective polyphenol for prevention of NPCuO-mediated DNA damage, with an IC50 value within the physiological range of blood polyphenol concentrations (0.3–10 μM) (Reddy et al., 2005; Sugisawa and Umegaki, 2002; van het Hof et al., 1998; Yamamoto et al., 2003; Yang et al., 1998). Catechol-containing polyphenols more effectively prevent DNA damage than their gallol analogs. The more potent ECG differs from EGCG only in the presence of a catechol group on the B-ring (Figure 1) instead of the gallol group in EGCG, indicating that the presence of a catechol group confers greater antioxidant behavior. Similarly, the carboxylic-acid-containing GA is a prooxidant under these conditions, whereas its methyl ester PCA has no activity. In addition, gallol-containing GA and EGC are prooxidants, promoting DNA damage. In bacteria, gallol-containing polyphenols are observed to promote DNA damage, induce apoptosis, and inhibit growth (Chuang et al., 2010; Hanasaki et al., 1994; Heim et al., 2002; Liu et al., 2013; Long et al., 2010; Nemeikaite-Ceniene et al., 2005; Yamashita et al., 1999; Yoshino et al., 1999). Carboxylic acid functional groups on the phenol ring also leads to lower polyphenol DNA damage prevention ability. MEPCA, the methyl ester of the carboxylate-containing PCA, is the most effective polyphenol tested, whereas PCA does not prevent NPCuO/H2O2-mediated DNA damage. Likewise, the methyl ester MEGA inhibits DNA damage from NPCuO/H2O2, but the corresponding carboxylate GA increases DNA damage under similar conditions.
The antioxidant and prooxidant activity of the polyphenols with NPCuO/H2O2-mediated DNA damage differs somewhat from that observed for Cu+-mediated DNA damage (Table 1). Some differences are expected due to varying DNA assay times (150 min for NPCuO and 30 min for Cu+) and H2O2 concentrations (400 μM for NPCuO and 50 μM for Cu+). In addition, ascorbate is added to reduce Cu2+ to Cu+ for the copper-mediated DNA damage studies but not for the NPCuO-mediated DNA damage assays. Nonetheless, MEPCA, PREGA, ECG, MEGA, and EGCG exhibit primarily antioxidant effects under both NPCuO/H2O2- and Cu+/H2O2-mediated DNA damage conditions, although PREGA, ECG, and MEGA show slight prooxidant effects at lower concentrations (0.1–10 μM) in the Cu+/H2O2 system (Table 1). MEPCA and PREGA have the same IC50 values under both DNA-damaging conditions, but 2- to 3-fold higher concentrations of ECG, MEGA, and EGCG are required to prevent the same amount of DNA damage by NPCuO/H2O2 or Cu+/H2O2.
In the presence of Cu2+, EGCG, ECG, EGC, and catechin promote copper-mediated DNA damage (Furukawa et al., 2003). Resveratrol also causes slight •OH-mediated DNA damage in the presence of Cu2+ that is enhanced upon H2O2 addition (Burkitt and Duncan, 2000). Thus, polyphenol effects on NPCuO/H2O2-mediated DNA damage (Table 1) differ somewhat from their effects on either Cu+- or Cu2+-mediated DNA damage. Since DNA-damaging NPCuO/H2O2 effects result from ROS generated by both dissolved copper and on the nanoparticle surface (Angelé-Martínez et al., 2017), differences in antioxidant or prooxidant activity between NPCuO- and copper-mediated DNA damage may be a consequence of different mechanisms of ROS generation at the NPCuO surface or differing interactions between the polyphenols and dissolved copper compared to the nanoparticle surface.
Although interactions of polyphenols with NPCuO are not well investigated, density functional theory calculations of Cu+ binding to flavonoid compounds in the gas phase demonstrate the importance of the catechol group on the B-ring for copper binding; in the absence of a carbonyl group on the C-ring (Figure 1) the preferred Cu+ binding site is between the deprotonated phenolic oxygens on the B ring (Kazazic et al., 2006). UV spectra of flavones and flavonols change upon Cu2+ addition and return to the original spectra after addition of Cu2+ chelators EDTA or DTPA, demonstrating the metal chelation ability of the catechol group in the B-ring and suggesting that polyphenol-copper binding affects DNA damage (Brown et al., 1998). VA has no effect on NPCuO-mediated DNA damage, likely because its lone phenolic group reduces metal binding ability (Khokhar and Owusu-Apenten, 2003; Melidou et al., 2005) so that it cannot effectively bind copper in solution or on the nanoparticle surface.
Similar to trends observed for NPCuO/H2O2-mediated DNA damage, catechol-containing polyphenols more effectively prevent Cu+-mediated DNA damage than gallol-containing polyphenols (Perron et al., 2011). Under copper-mediated DNA damage conditions, this catechol-antioxidant activity is explained by the formation of a stable Cu2+ complex with high affinity constant that does not reduce to hydroxyl-radical-generating Cu+ (Reaction 1) (Bhattacharya and Patel, 1985; Perron et al., 2011). The prooxidant activity of dopamine, EC, ECG, EGC, GA, MEGA, and PREGA in Cu+-mediated DNA damage assays is explained by the formation of a polyphenol-Cu2+ complex, but it this case, the complex then undergoes intramolecular electron transfer, yielding an unstable Cu+ complex that dissociates to make Cu+ available for •OH generation (Perron et al., 2011). Thus, copper binding, either in solution or at the nanoparticle surface may be responsible for both the observed antioxidant and prooxidant effects. In addition, ROS other than •OH radical are generated by NPCuO surface reactions and possible electron transfer from the NPCuO conduction band (Angelé-Martínez et al., 2017), and these differences in ROS generation may also contribute to differences between polyphenol effects on NPCuO/H2O2- and Cu+/H2O2-medated DNA damage.
| [1] |
4.2. ROS detection using EPR spectroscopy
EPR results with NPCuO/H2O2 and NPCuO/H2O2/ascorbate indicate that MEPCA prevents generation of •OH and O2•− radicals. Because this reactivity is observed with both NPCuO/H2O2 and wCuO/H2O2, these results suggest that MEPCA interacts with the copper ions on the surface of the nanoparticle. A similar effect was observed upon adding a series of polyphenols to ZnO samples irradiated with UV light; H2O2 generation was reduced compared to non-polyphenol-treated nanoparticles (Markham et al., 1954).
Mechanisms for •OH generation by NPCuO include reduction of H2O2 by electron transfer from the NPCuO conduction band (Reaction 2) or by electron transfer to Cu2+ to form hydroxyl-radical-generating Cu+ (Reaction 3) (Pascholiano et al., 2008). Generation of O2•− also has been postulated to occur by electron transfer from the NPCuO conduction band to O2, where the limiting step is O2 adsorption on the nanoparticle surface (Reaction 4) (Bandara et al., 2005; Pascholiano et al., 2008; Shi et al., 2012), or from reduced Cu+ (Reaction 5) (Rowley and Halliwell, 1983). Once O2•− is generated, •OH may form through the Cu2+-assisted Haber-Weiss process (Reaction 6). As corroborated by EPR results, the ability of EGC to promote DNA damage by NPCuO/H2O2 results from its ability to increase •OH (or preliminary O2•−) generation under these conditions. Polyphenol effects on ROS generation by NPCuO/H2O2 and NPCuO/H2O2/ascorbate do not correlate with their first-phenolic-hydrogen pKa (Perron and Brumaghim, 2009) or their oxidation potentials (Perron et al., 2008), indicating complexity in both radical generation and copper/ NPCuO-polyphenol interactions. The strong correlation between EPR results and polyphenol antioxidant/prooxidant activity suggests that this method can be used to screen polyphenols for their affects on NPCuO-mediated DNA damage.
| [2] |
| [3] |
| [4] |
| [5] |
| [6] |
4.3. Cell viability analysis
Our results demonstrate that NPCuO/H2O2 induces cytotoxicity in L929 cells significantly more than either component alone. This cytotoxicity is likely due to production of intracellular ROS by NPCuO under hydrogen-peroxide induced oxidative stress. Both MEPCA and MEGA significantly reduce NPCuO/H2O2 cytotoxicity at 1 μM, a concentration within the physiological polyphenol range of 0.3–10 μM in blood (Reddy et al., 2005; Scalbert and Williamson, 2000; Sugisawa and Umegaki, 2002; van het Hof et al., 1998; Yamamoto et al., 2003; Yang et al., 1998). The IC50 measured for NPCuO/H2O2-mediated DNA damage prevention assay is significantly higher than 1 μM (7.5 for MEPCA and 185 for MEGA); however, these cellular and DNA damage assays are not directly comparable because NPCuO and the polyphenols must be taken up and trafficked in cells, unlike in the DNA assays. In addition, MEPCA and MEGA can interact with cells through multiple pathways aside from direct DNA damage inhibition (Reddy et al., 2005; Sugisawa and Umegaki, 2002; van het Hof et al., 1998; Yamamoto et al., 2003; Yang et al., 1998).
Our results are qualitatively consistent with other studies showing antioxidants can rescue cells exposed to copper or NPCuO. For example, addition of sulphoraphane (6 μM) to mouse BALB 3T3 cells exposed to 10 μg/mL NPCuO reduced cytotoxicity (34% to 63% viable cells) and also reduced glutathione oxidative stress biomarkers (Akthar et al., 2012). Similarly, addition of the polyphenol resveratrol at high concentrations (100 μM) partially reversed NPCuO-induced cell killing in Hep-2 airway epithelial cells (33% to 58% viable cells) and reduced cellular oxidative stress biomarkers (Fahmy and Cormier, 2009). Our results are not directly comparable to these prior cell studies because we used different cell lines and included H2O2 in the challenge, a common source of oxidative stress that is known to produce ROS in the presence of copper (Reaction 1). Under these conditions, we have demonstrated that the MEPCA and MEGA polyphenols have a substantially larger effect at lower concentrations (1 μM increases cell viability by 64% and 54% for MEPCA and MEGA, respectively). Overall, the results show that hydrogen peroxide significantly increases NPCuO cytotoxicity and that the polyphenols MEPCA and MEGA protect against NPCuO/H2O2-mediated DNA damage and cytotoxicity.
5. Conclusions
The activity of polyphenols to affect NPCuO-mediated DNA in the presence of H2O2 ranges from potent antioxidant activity (MEPCA), to no activity (PCA), to potent prooxidant activity (EGC). Polyphenol effects on NPCuO/H2O2-mediated and Cu+-mediated DNA damage are substantially similar. NPCuO-generated ROS were monitored by EPR spectroscopy and correlate with DNA assay results for the three observed polyphenol effects (antioxidant, no activity and prooxidant), indicating that this method can be used as a screening tool for polyphenol prevention of DNA damage by NPCuO and H2O2. In addition, we found that combining H2O2 with NPCuO significantly increases cytotoxicity compared to either component alone.
Our work demonstrates that two polyphenols, MEPCA and MEGA, prevent NPCuO/H2O2-mediated L929 cytotoxicity at physiologically relevant (1 μM) concentrations. The differences in behavior observed for polyphenol prevention or promotion of NPCuO/H2O2–mediated DNA damage and polyphenol prevention of NPCuO/H2O2 cytotoxicity highlight the need for additional investigation into polyphenol-nanoparticle interactions as well as their biological effects to identify polyphenols that prevent NPCuO toxicity under oxidative stress conditions.
Supplementary Material
Research Highlights.
Polyphenol antioxidants were tested for their effects on DNA damage and cytotoxicity by CuO nanoparticles (NPCuO) and hydrogen peroxide
Half of the ten tested polyphenols prevent NPCuO/H2O2-mediated DNA damage, but two increase DNA damage
Levels of reactive oxygen species generated by NPCuO/H2O2 with polyphenols correlate with their DNA damaging effects
Combining NPCuO and H2O2 is significantly more cytotoxic than either component alone
Polyphenols MEPCA and MEGA prevent NPCuO/H2O2-induced cell death in mouse fibroblast cells
Acknowledgements
We thank the National Institutes of Health (NIH-NIBIB 1R15EB014560) for financial support. Electron microscopy characterization was supported The South Carolina Bioengineering Center of Regeneration and Formation of Tissues (BioCRAFT) center funded under NIGMS of the National Institutes of Health, award number 5P20GM103444-07. C.A.M. thanks the Department of Science of the Government of Costa Rica for a graduate fellowship.
Abbreviations
- NPCuO
copper oxide nanoparticles
- wCuO
washed copper oxide nanoparticles
- lCuO
leachate of copper oxide nanoparticles
- DMEM
dulbecco’s modified Eagle’s medium
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- EC
epicatechin
- ECG
epicatechin gallate
- EGC
epigallocatechin
- EGCG
epigallocatechin gallate
- EPR
electron paramagnetic resonance spectroscopy
- FBS
fetal bovine serum
- GA
gallic acid
- L929
mouse fibroblast (L929) cells
- MEGA
methyl-3,4,5-trihydroxybenzoate
- MEPCA
methyl-4,5-dihydroxybenzoate
- MOPS
3-(N-morpholino)propanesulfonic acid
- PCA
protocatechuic acid
- PREGA
propyl gallate
- ROS
reactive oxygen species
- TEMP
2,2,5,5-tertramethyl-1-pyrroline-N-oxide
- TEMPO
singlet oxygen adduct of TEMP
- VA
vanillic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Transparency document
The transparency document associated with this article can be found online.
Conflicts of interest
The authors have no conflicts of interest to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A: Supplementary Material
All data presented in this work, including DNA gel electrophoresis data and IC50 plots, cell viability data and EC50 plots, data from dynamic light scattering measurements, and electron paramagnetic resonance spectra are provided in the associated Supplementary Material.
References
- Akter S, Addepalli R, Netzel ME, Tinggi U, Fletcher MT, Sultanbawa Y, Osborne SA, 2019. Antioxidant-rich extracts of Terminalia ferdinandiana interfere with estimation of cell viability. Antioxidants 8, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akthar MJ, Ahamed M, Fareed M, Airokayan SA, Kumar S, 2012. Potective effect of sulphoraphane against oxidative stress mediated toxicity induced by CuO nanoparticles in mouse embryonic fibroblasts BALB 3T3. J. Toxicol. Sci 37, 139–148. [DOI] [PubMed] [Google Scholar]
- Alia A, Mohanty P, Matysik J, 2001. Effect of proline on the production of singlet oxygen. Amino Acids 21, 195–200. [DOI] [PubMed] [Google Scholar]
- Angelé-Martínez C, Nguyen KVT, Ameer FS, Anker JN, Brumaghim JL, 2017. Reactive oxygen species generation by copper (II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 11, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoja V, Dubourguier HC, Kasemets K, Kahru A, 2009. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ. 407, 1461–1468. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M, 1991. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J 273 601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensi M, Ortega A, Mena S, Feddi F, Estrela JM, 2011. Natural polyphenols in cancer therapy Crit. Rev. Clin. Lab. Sci, 48, 197–216. [DOI] [PubMed] [Google Scholar]
- Babich H, Schuck AG, Weisburg JH, Zuckerbraun HL, 2011. Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol 2011, 467305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara J, Guasaquillo I, Bowen P, Soare L, Jardim WF, Kiwi J, 2005. Photocatalytic storing of O2 as H2O2 mediated by high surface area CuO. Evidence for a reductive-oxidative interfacial mechanism. Langmuir 21, 8554–8559. [DOI] [PubMed] [Google Scholar]
- Barbehenn RV, Poopat U, Spencer B, 2003. Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem. Mol. Biol 33, 125–130. [DOI] [PubMed] [Google Scholar]
- Barua S, Das G, Aidew L, Buragohain AK, Karak N, 2013. Copper-copper oxide coated nanofirillar cellulose: A promising biomaterial. RSC Adv. 3, 14997–15004. [Google Scholar]
- Bhattacharya PK, Patel VK, 1985. Effect of substitution on the catecholate ring on ternary complex stability. Proc. Indian Acad. Sci 94, 495–500. [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV, 2003. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot 91, 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright WL, Crum AD, 2016. Redox cycling and generation of reactive oxygen species in commercial infant formulas. Food Chem. 196, 189–195. [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Ivask A, Kakinen A, Kahru A, 2012. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut 169, 81–89. [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A, 2013. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol 87, 1181–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G, 2014. Using copper to improve the well-being of the skin. Curr. Chem. Biol 8, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Khodr H, Hider RC, Rice-Evans CA, 1998. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J 330, 1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulcke F, Dringen R, 2015. Copper oxide nanoparticles stimulate glycolytic flux and increase the cellular contents of glutathione and metallothioneins in cultured astrocytes. Neurochem. Res 40, 15–26. [DOI] [PubMed] [Google Scholar]
- Burkitt MJ, Duncan J, 2000. Effects of trans-resveratrol on copper-dependent hydroxyl-radical formation and DNA damage: Evidence for hydroxyl-radical scavenging and a novel, glutathione-sparing mechanism of action. Arch. Biochem. Biophys 381, 253–263. [DOI] [PubMed] [Google Scholar]
- Chan HW, Liu T, Verdile G, Bishop G, Haasl RJ, Smith MA, Perry G, Martins RN, Atwood CS, 2008. Copper induces apoptosis of neuroblastoma cells via post-translational regulation of the expression of Bcl-2-family proteins and the tx mouse is a better model of hepatic than brain Cu toxicicity. Int. J. Clin. Exp. Med 1, 76–88. [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Liu TZ, Wong CH, Lu FJ, Chen SC, 2007. The efficacy of protective effects of tannic acid, gallic acid, ellagic acid, and propyl gallate against hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells. Mol. Nutr. Food Res 51, 962–968. [DOI] [PubMed] [Google Scholar]
- Chen W, Hong L, Liu AL, Liu JQ, Lin XH, Xia XH, 2012. Enhanced chemiluminescence of the luminol-hydrogen peroxide system by colloidal cupric oxide nanoparticles as peroxidase mimic. Talanta 99, 643–648. [DOI] [PubMed] [Google Scholar]
- Chuang CY, Liu HC, Wu LC, Chen CY, Chang JT, Hsu SL, 2010. Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species-dependent ataxia telangiectasia mutated-p53 activation pathway. J. Agric. Food Chem 58, 2943–2951. [DOI] [PubMed] [Google Scholar]
- Dall’Asta M, Bayle M, Neasta J, Scazzina F, Bruni R, Cros G, Del Rio D, Oiry C, 2015. Protection of pancreatic β-cell function by dietary polyphenols. Phytochem. Rev 14, 933–959. [Google Scholar]
- Daré RG, Oliveira MM, Truiti MCT, Nakamura CV, Ximenes VF, Lautenschlager SOS, 2020. Abilities of protocatechuic acid and its alkyl esters, ethyl and heptyl protocatechuates, to counteract UVB-induced oxidative injuries and photoaging in fibroblasts L929 cell line. J. Photochem. Photobiol B203, 111771. [DOI] [PubMed] [Google Scholar]
- Detty MR, Ciriminna R, Bright FV, Pagliaro M, 2014. Environmentally benign sol–gel antifouling and foul-releasing coatings. Acc. Chem. Res 47, 678–687. [DOI] [PubMed] [Google Scholar]
- Ding Y, Yao H, Yao Y, Fai LY, Zhang Z, 2013. Protection of dietary polyphenols against oral cancer. Nutrients 5, 2173–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhateeb SA, Ibrahim TR, El-Shal AS, Hamid OIA, 2020. Ameliorative role of curcumin on copper oxide nanoparticles-mediated renal toxicity in rats: An investigation of molecular mechanisms. J. Biochem. Mol. Toxicol 34, e22593. [DOI] [PubMed] [Google Scholar]
- Evans P, Matsunaga H, Kiguchi M, 2008. Large-scale application of nanotechnology for wood protection. Nat. Nanotechnol 3, 577. [DOI] [PubMed] [Google Scholar]
- Fahmy B, Cormier SA, 2009. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. in Vitro 23, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E, Rosen GM, Rauckman EJ, Paxton J, 1979. Spin trapping of superoxide. Mol. Pharmacol 16, 676–685. [PubMed] [Google Scholar]
- Fufezan C, Rutherford AW, Krieger-Liszkay A, 2002. Singlet oxygen production in herbicide-treated photosystem II. FEBS Lett. 532, 407–410. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S, 2003. (−)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem. Pharmacol 66, 1769–1778. [DOI] [PubMed] [Google Scholar]
- Galleano M, Verstraeten SV, Oteiza PI, Fraga CG, 2010. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys 501, 23–30. [DOI] [PubMed] [Google Scholar]
- Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS, 2007. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol 41, 8178–8186. [DOI] [PubMed] [Google Scholar]
- Gunawan C, Teoh WY, Marquis CP, Amal R, 2011. Cytotoxic origin of copper(II) oxide nanoparticles: Comparative studies with micron-sized particles, leachate, and metal salts. ACS Nano 5, 7214–7425. [DOI] [PubMed] [Google Scholar]
- Hanasaki Y, Ogawa S, Fukui S, 1994. The correlation between active oxygen scavenging and antioxidative effects of flavonols. Free. Radic. Biol. Med 16, 845–850. [DOI] [PubMed] [Google Scholar]
- Haslam E, 1996. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod 59, 205–215. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ, 2002. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem 13, 572–584. [DOI] [PubMed] [Google Scholar]
- Heinlaan M, Ivask A, Blinova I, Dubourguier H-C, Kahru A, 2008. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71, 1308–1316. [DOI] [PubMed] [Google Scholar]
- Hertzberg RP, Dervan PB, 1982. Cleavage of double helical DNA by methidium-propyl-EDTA-iron(II). J. Am. Chem. Soc 104, 313–315. [Google Scholar]
- Hong L, Liu AL, Li GW, Chen W, Lin XH, 2013. Chemiluminescent cholesterol sensor based on peroxidase-like activity of cupric oxide nanoparticles. Biosens. Bioelectron 43, 1–5. [DOI] [PubMed] [Google Scholar]
- Hu Y-Z, Jiang L-J, 1996. J. Photochem. Photobiol B 33, 51–59. [Google Scholar]
- Isani G, Falcioni ML, Barucca G, Sekar D, Andreani G, Carpene E, Falcioni G, 2013. Comparative toxicity of CuO nanoparticles and CuSO4 in rainbow trout. Ecotoxicol. Environ. Saf 97, 40–46. [DOI] [PubMed] [Google Scholar]
- Jing X, Park JH, Peters TM, Thorne PS, 2015. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air-liquid interface compared with in vivo assessment. Toxicol. in Vitro 29, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Choi JW, Lee SH, Hong SW, 2012. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods. J. Hazard. Mater 227–228, 301–318. [DOI] [PubMed] [Google Scholar]
- Jose GP, Santra S, Mandal SK, Sengupta TK, 2011. Singlet oxygen mediated DNA degradation by copper nanoparticles: Potential towards cytotoxic effect on cancer cells. J. Nanobiotechnol 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M, 2007. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci 64, 1105–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska MB, Mason RP, 2002. In vivo copper-mediated free radical production: An ESR spin-trapping study. Spectrochim. Acta A Mol. Biomol. Spectrosc 58, 1227–1239. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M, 2005. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–402. [DOI] [PubMed] [Google Scholar]
- Kamble S, Utage B, Mogle P, Kamble R, Hese S, Dawane B, Gacche R, 2016. Evaluation of curcumin capped copper nanoparticles as possible inhibitors of human breast cancer cells and angiogenesis: A comparative study with native curcumin. AAPS Pharm. Sci. Tech 17, 1030–1041. [DOI] [PubMed] [Google Scholar]
- Kanninen P, Johans C, Merta J, Kontturi K, 2008. Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid. Interface Sci 318, 88–95. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Cronholm P, Gustafsson J, Möller L, 2008a. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol 21, 1726–1732. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Holgersson A, Moller L, 2008b. Mechanisms related to the genotoxicity of particles in the subway and from other sources. Chem. Res. Toxicol 21, 726–731. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Gustafsson J, Cronholm P, Moller L, 2009. Size-dependent toxicity of metal oxide particles--a comparison between nano- and micrometer size. Toxicol. Lett 188, 112–118. [DOI] [PubMed] [Google Scholar]
- Kasemets K, Ivask A, Dubourguier H-C, Kahru A, 2009. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. in Vitro 23, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Kazazic SP, Butkovic V, Srzic D, Klasinc L, 2006. Gas-phase ligation of Fe+ and Cu+ ions with some flavonoids. J. Agric. Food Chem 54, 8391–8396. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Owusu-Apenten RK, 2003. Iron binding characteristics of polyphenol compounds: Some tentative structure-activity relations. Food Chem. 81, 133–140. [Google Scholar]
- Klaine SJ, Burbage JT, Millhouse PW, Uzair U, Anker JN, 2020. Toxicology of Magnetic Nanoparticles. In: Anker J, Mefford O, Biomedical Applications of Magnetic Particles, CRC Press, Boca Raton, Chapter 12. [Google Scholar]
- Li CC, Chang MH, 2004. Colloidal stability of CuO nanoparticles in alkanes via oleate modifications. Mater. Lett 58, 3903–3907. [Google Scholar]
- Li Y, Zhang W, Niu J, Chen Y, 2012. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 6, 5164–5173. [DOI] [PubMed] [Google Scholar]
- Liang H, Li C, Yuan Q, Vriesekoop F, 2007. Separation and purification of sulforaphane from broccoli seeds by solid phase extraction and preparative high-performance liquid chromatography. J. Agric. Food Chem 55, 8047–8053. [DOI] [PubMed] [Google Scholar]
- Liang H, Yuan QP, Dong HR, Liu YM, 2006. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J. Food Comp. Anal 19, 473–476. [Google Scholar]
- Liu X, Li J, Wang Y, Li T, Zhao J, Zhang C, 2013. Green tea polyphenols function as prooxidants to inhibit Pseudomonas aeruginosa and induce the expression of oxidative stress-related genes. Folia Microbiol. 58, 211–217. [DOI] [PubMed] [Google Scholar]
- Long LH, Hoi A, Halliwell B, 2010. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch. Biochem. Biophys 501, 162–169. [DOI] [PubMed] [Google Scholar]
- Mani RG, Smet JH, von Klitzing K, Narayanamurti V, Johnson WB, Umansky V, 2004. Demonstration of a 1/4-cycle phase shift in the radiation-induced oscillatory magnetoresistance in GaAs/AlGaAs devices. Phys. Rev. Lett 92, 146801–146805. [DOI] [PubMed] [Google Scholar]
- Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, Venkatachalam K, Wang M-H, 2020. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol 164, 2073–2084. [DOI] [PubMed] [Google Scholar]
- Markham MC, Hannan MC, Evans SW, 1954. Factors influencing the oxidation of phenols, catalyzed by zinc oxide and light. J. Am. Chem. Soc 76, 820–823. [Google Scholar]
- Melidou M, Riganakos K, Galaris D, 2005. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation. Free Radic. Biol. Med 39, 1591–1600. [DOI] [PubMed] [Google Scholar]
- Midander K, Cronholm P, Karlsson HL, Elihn K, Möller L, Leygraf C, Wallinder IO, 2009. Surface characteristics, copper release, and toxicity of nano-and micrometer-sized copper and copper (II) oxide particles: A cross-disciplinary study. Small 5, 389–399. [DOI] [PubMed] [Google Scholar]
- Misra SK, Dybowska A, Berhanu D, Luoma SN, Valsami-Jones E, 2012. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ 438, 225–232. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Aberg CS, A., Dawson KA, 2012. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol 7, 779–786. [DOI] [PubMed] [Google Scholar]
- Mortimer M, Kasemets K, Kahru A, 2010. Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila. Toxicology 269, 182–189. [DOI] [PubMed] [Google Scholar]
- Mouithys-Mickalad A, Deby C, Deby-Dupont G, Lamy M, 1998. An electron spin resonance (ESR) study on the mechanism of ascorbyl radical production by metal-binding proteins. BioMetals 11, 81–88. [DOI] [PubMed] [Google Scholar]
- Nair CK, Salvi VP, 2008. Protection of DNA from gamma-radiation induced strand breaks by Epicatechin. Mutat. Res 650, 48–54. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Maedler L, Li N, 2006. Toxic potential of materials at the nanolevel. Sci.Total Environ 311, 622–662. [DOI] [PubMed] [Google Scholar]
- Nemeikaite-Ceniene A, Imbrasaite A, Sergediene E, Cenas N, 2005. Quantitative structure-activity relationships in prooxidant cytotoxicity of polyphenols: Role of potential of phenoxyl radical/phenol redox couple. Arch. Biochem. Biophys 441, 182–190. [DOI] [PubMed] [Google Scholar]
- Noda Y, Murakami S, Mankura M, Mori A, 2008. Inhibitory effect of fermented papaya preparation on hydroxyl radical generation from methylguanidine. J. Clin. Biochem. Nutr 43, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak M-H, Auger C, Belcastro E, Park S-H, Lee H-H, Schini-Kerth VB, 2018. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med 122, 161–170. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J, 2005. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect 113, 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascholiano M, Guedes NC, Jardim W, Mielczarski E, Mielczarski JA, Bowen P, Kiwi J, 2008. Inactivation of E. coli mediated by high surface area CuO accelerated by light irradiation >360 nm. J. Photochem. Photobiol. A 199, 105–111. [Google Scholar]
- Perron NR, Brumaghim JL, 2009. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys 53, 75–100. [DOI] [PubMed] [Google Scholar]
- Perron NR, Garcia CR, Pinzon JR, Chaur MN, Brumaghim JL, 2011. Antioxidant and prooxidant effects of polyphenol compounds on copper-mediated DNA damage. J. Inorg. Biochem 105, 745–753. [DOI] [PubMed] [Google Scholar]
- Perron NR, Hodges JN, Jenkins M, Brumaghim JL, 2008. Predicting how polyphenol antioxidants prevent DNA damage by binding to iron. Inorg. Chem 47, 6153–6161. [DOI] [PubMed] [Google Scholar]
- Pietri S, Culcasi M, Stella L, Cozzone PJ, 1990. Ascorbyl free radical as a reliable indicator of free-radical-mediated myocardial ischemic and post-ischemic injury. A real-time continuous-flow ESR study. Eur. J. Biochem 193, 845–854. [DOI] [PubMed] [Google Scholar]
- Qin W, Li X, 2011. A theoretical study on the catalytic effect of nanoparticle confined in carbon nanotube. Chem. Phys. Lett 502, 96–100. [Google Scholar]
- Reddy VC, Vidya Sagar GV, Sreeramulu D, Venu L, Raghunath M, 2005. Addition of milk does not alter the antioxidant activity of black tea. Ann. Nutr. Metab 49, 189–195. [DOI] [PubMed] [Google Scholar]
- Reis B, Martins M, Barreto B, Milhazes N, Garrido EM, Silva P, Garrido J, Borges F, 2010. Structure-property-activity relationship of phenolic acids and derivatives. Protocatechuic acid alkyl esters. J. Agric. Food Chem 58, 6986–6993. [DOI] [PubMed] [Google Scholar]
- Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP, 2009. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 33, 587–590. [DOI] [PubMed] [Google Scholar]
- Rim KT, Song SW, Kim HY, 2013. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 4, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley DA, Halliwell B, 1983. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals in the presence of copper salts: A physiologically significant reaction? Arch. Biochem. Biophys 225, 279–284. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Williamson G, 2000. Dietary Intake and Bioavailability of Polyphenols. J. Nutr 130, 2073S–2085S. [DOI] [PubMed] [Google Scholar]
- Schlickmann F, de Souza P, Boeing T, Mariano L, Steimbach V, Krueger C, da Silva LM, de Andrade SF, Cechinel-Filho V, 2017. Chemical composition and diuretic, natriuretic and kaliuretic effects of extracts of Mimosa bimucronata (DC.) Kuntze leaves and its majority constituent methyl gallate in rats. J. Pharm. Pharmacol 69, 1615–1624. [DOI] [PubMed] [Google Scholar]
- Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M, 2012. Toxicity of nanomaterials. Chem. Soc. Rev 41, 2323–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Kwon HS, Peng Z, Elder A, Yang H, 2012. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 6, 2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman MM, Attia HF, Hussein MM, Mohamed EH, Ismail TA, 2013. Protective effect Of N-acetylcystiene against titanium dioxide nanoparticles modulated immune responses in male albino rats. Am. J. Immunol 9, 148–158. [Google Scholar]
- Son DI, You CH, Kim TW, 2009. Structural, optical, and electronic properties of colloidal CuO nanparticles formed by using a collo-thermal synthesis process. Appl. Surf. Sci 255, 8794–8797. [Google Scholar]
- Song G, Hou W, Gao Y, Wang Y, Lin L, Zhang Z, Niu Q, Ma R, Mu L, Wang H, 2016. Effects of CuO nanoparticles on Lemna minor. Bot. Stud 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth K, Pereira E, Duarte AC, Rao JV, 2015. Evaluation of cytotoxicity, morphological alterations and oxidative stress in Chinook salmon cells exposed to copper oxide nanoparticles. Protoplasma 253, 873–884. [DOI] [PubMed] [Google Scholar]
- Stone V, Johnston H, Clift MJ, 2007. Air pollution, ultrafine and nanoparticle toxicology: Cellular and molecular interactions. IEEE Trans. Nanobioscience 6, 331–340. [DOI] [PubMed] [Google Scholar]
- Studer AM, Limbach LK, Van Duc L, Krumeich F, Athanassiou EK, Gerber LC, Moch H, Stark WJ, 2010. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett 197, 169–174. [DOI] [PubMed] [Google Scholar]
- Sugisawa A, Umegaki K, 2002. Physiological concentrations of (−)-epigallocatechin-3-O-gallate (EGCg) prevent chromosomal damage induced by reactive oxygen species in WIL2-NS cells. J. Nutr 132, 1836–1839. [DOI] [PubMed] [Google Scholar]
- Suresh AK, Pelletier DA, Doktycz MJ, 2013. Relating nanomaterial properties and microbial toxicity. Nanoscale 5, 463–474. [DOI] [PubMed] [Google Scholar]
- van het Hof KH, Kivits GA, Weststrate JA, Tijburg LB, 1998. Bioavailability of catechins from tea: The effect of milk. Eur. J. Clin. Nutr 52, 356–359. [DOI] [PubMed] [Google Scholar]
- Wang HC, Brumaghim JL, 2011. Polyphenol compounds as antioxidants for disease prevention: Reactive oxygen species scavenging, enzyme regulation, and metal chelation mechanisms in E. coli and human cells. in: Andreescu S, Hepel M, Oxidative Stress: Diagnostics, Prevention, and Therapy, vol. 1083, ACS Symposium Series, American Chemical Society, Washington, DC, pp. 99–175. [Google Scholar]
- Wang P, Henning SM, Heber D, 2010. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE, 5, e10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li N, Zhao J, White JC, Qu P, Xing B, 2012. CuO nanoparticle interaction with human epithelial cells: Cellular uptake, location, export, and genotoxicity. Chem. Res. Toxicol 25, 1512–1521. [DOI] [PubMed] [Google Scholar]
- Warren JJ, Mayer JM, 2008. Surprisingly long-lived ascorbyl radicals in acetonitrile: Concerted proton-electron transfer reactions and thermochemistry. J. Am. Chem. Soc 130, 7546–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F, Saito M, Ariga T, Yoshimura Y, Nakazawa H, 2000. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem 48, 2320–2325. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hsu S, Lewis J, Wataha J, Dickinson D, Singh B, Bollag WB, Lockwood P, Ueta E, Osaki T, Schuster G, 2003. Green tea polyphenol causes differential oxidative environments in tumor versus normal epithelial cells. J. Pharmacol. Exp. Ther 307, 230–236. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Tanemura H, Kawanishi S, 1999. Mechanism of oxidative DNA damage induced by quercetin in the presence of Cu(II). Mutat. Res 425, 107–115. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP, 1998. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomarkers Prev 7, 351–354. [PubMed] [Google Scholar]
- Yoshino M, Haneda M, Naruse M, Murakami K, 1999. Prooxidant activity of flavonoids: copper-dependent strand breaks and the formation of 8-hydroxy-2’-deoxyguanosine in DNA. Mol. Genet. Metab 68, 468–472. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang G, Zhang W, Hu N, Wu H, Fang B, 2008. Seed-mediated growth method for epitaxial array of CuO nanowires on surface of Cu nanostructures and its application as a glucose sensor. J. Phys. Chem C 112, 8856–8862. [Google Scholar]
- Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P, 1994. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 91, 3147–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou XY, Xu B, Yu CP, Zhang HW, 2013. Imbalance between oxidative and antioxidative systems: Toward an understanding of visible light-induced titanium dioxide nanoparticles toxicity. Chemosphere 93, 2451–2457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


