Abstract
Background:
Volatile anesthetic exposure during development leads to long-term cognitive deficits in rats which are dependent on age and sex. Female rats are protected relative to male rats for the same exposure on postnatal day 7. Here we test our hypothesis that androgens can modulate chloride co-transporter expression to alter the susceptibility to neurotoxicity from GABAergic drugs using female rats with exogenous testosterone exposure.
Methods:
Female rats were injected with testosterone (100ug/animal) or vehicle on postnatal days 1–6. On postnatal day 7, the animals were randomized to either isoflurane exposure or sham. Spatial memory was assessed with the Barnes maze starting on postnatal day 41. Western blots were run from testosterone treated postnatal day 7 animals to measure levels of chloride co-transporters NKCC1 and KCC2.
Results:
Exogenous testosterone modulated isoflurane anesthetic neurotoxicity in female rats based on poor performance in the probe trial of the Barnes Maze. By contrast, females with vehicle and isoflurane exposure were able to differentiate the goal position. These behavioral differences corresponded to differences in the protein levels of NKCC1 and KCC2 after exogenous testosterone exposure, with NKCC1 increasing (p<0.001) and KCC2 decreasing (p=0.003) relative to female controls.
Conclusions:
The expression of chloride co-transporters, NKCC1 and KCC2, is altered by testosterone in female rats and corresponds to a cognitive deficit after isoflurane exposure. This confirms the role of androgens in perinatal anesthetic neurotoxicity and supports our hypothesis that the developing GABAergic system plays a critical role in the underlying mechanism.
Keywords: anesthesia, neurotoxicity, developmental neurotoxicity, sex differences, NKCC1, KCC2
Introduction
Susceptibility to cognitive deficits following early life anesthesia exposure depends on age and sex of the animals at the time of exposure. Although the mechanism remains unknown, a leading hypothesis is that gamma-aminobutyric acid (GABA)-ergic drugs, such as anesthetic agents, cause deleterious effects on neurodevelopment during this critical period of GABAergic development. From the third trimester through the perinatal period, the GABA system is maturing in the central nervous system. In the mature state, GABAA activation results in hyperpolarization of the membrane potential and inhibition of post-synaptic potentiation, while immature GABAA activation is excitatory in effect. This functional switch from excitatory to inhibitory is thought to occur through a change in the intracellular chloride gradient to which the GABAA receptor is permeable when activated1,2. The chloride gradient is determined by the relative expression and activity of two chloride co-transporters, basolateral sodium-potassium-chloride symporter (NKCC1) and chloride-potassium symporter 5 (KCC2)1. NKCC1 is highly expressed in immature neurons and is responsible for shunting chloride into cells, while KCC2 is more highly expressed in mature animals and shunts chloride extracellularly functioning to inhibit post-synaptic potentials with activation. Given the action of many anesthetic agents to modulate the GABAA receptor, it is possible that exposure to these agents in this early developmental period may have important physiologic effects on acute and developmental processes.
Our lab has previously identified sex specific differences in the susceptibility to neurotoxicity from volatile anesthesia; male rats are susceptible at postnatal day 7 while female rats are not3,4. However, females are at risk earlier in development at postnatal day 43,4. This window of susceptibility is correlated with the relative expression of two chloride co-transporters (NKCC1 and KCC2) and can be overcome in males by inhibiting NKCC1 with bumetanide.
We recently reported that pharmacologic blockade of the androgen receptor with flutamide prevented susceptibility in male rats and altered expression of the chloride co-transporters NKCC1 and KCC25. Both co-transporters’ protein levels moved from the male pattern to the normal female pattern (females have lower NKCC1 and higher KCC2 levels at postnatal day 7). In a different study we found that gonadectomized male rats in the susceptible period are also protected from this same neurotoxic insult, presumably through disruption of androgen production and signaling from the testes6.
To further investigate the role of androgens on susceptibility to anesthetic neurotoxicity and their effect on the development of the GABAergic system, we hypothesized that androgen signaling modulates the expression of chloride co-transporters which are critical for the function of the GABAergic system and can alter the temporal window of neurotoxic susceptibility to GABAergic agents. Here we test this hypothesis by studying female rats treated with exogenous testosterone followed by volatile anesthesia exposure after the susceptible period is normally closed on postnatal day 7.
Methods
All procedures were carried out in compliance with ARRIVE guidelines according to the University of California, San Francisco Institutional Animal Care Use Committee guidelines and with approval of our institutional review committee (AN176902–03B, 9/17/18).
Animals
Sprague Dawley rats were purchased on postnatal day 1 rats from Charles River (South San Francisco, CA) and arrived with a foster dam per 10 animals. Experiments were performed with female rats treated on days 1–6 with either vehicle (0.05mL of sesame oil, Fisher Scientific, Waltham, MA) or testosterone propionate (100ug/animal in 0.05mL) (Spectrum Pharmaceuticals, Gardena, CA) administered by subcutaneous injection on their dorsum with a 30g needle. On postnatal day 7, animals were randomized to receive isoflurane or sham and reunited with dam after return of righting reflex (Figure 1A). A subset of experiments (western blots, enzyme-linked immunosorbent assay [ELISA], and secondary sex characteristics) also included male control rats as an additional comparison. For experiments utilizing male controls, the males were injected identically with vehicle.
Figure 1. Testosterone is sufficient to cause a spatial memory deficit after neonatal Isoflurane exposure.
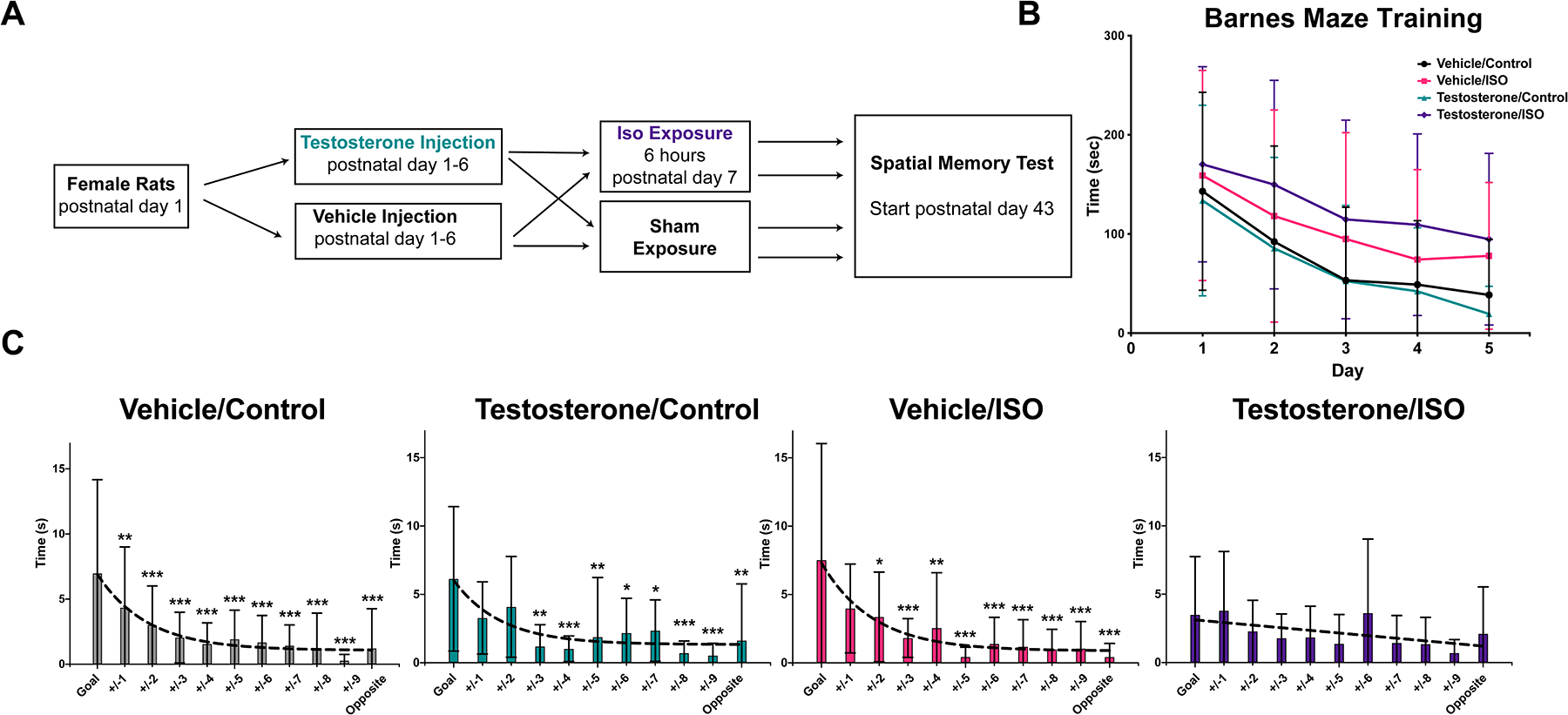
A. Experimental design
B. Barnes Maze training
One trial per day, showed improvement in the latency to find the escape box over five days of training (two-way ANOVA Fday(4,220)=19.72, p<0.001;) with no differences by group (Fgroup(3,55)=2.757, p=0.051).
C. Probe trial with time spent investigating positions equidistant around the maze.
A Dunnett’s multiple comparison test compared the time spent at the goal vs. equidistant positions away from the goal which revealed good discrimination in groups vehicle/control, testosterone/control, and vehicle/isoflurane, but not the testosterone/isoflurane group which had no significantly different positions. Curve fit F-test similarly found one phase decay patterns for all the groups except the testosterone/isoflurane which resembled a straight line.
* p<0.05, ** p<0.01, ***p<0.001.
iso, isoflurane
Rats were weaned at postnatal day 21 and co-housed in groups of three by sex. Cages contained a single red plastic tube 15cm in length and 8cm in diameter for limited enrichment given previous findings that enrichment can influence behavioral performance7,8. Food and water were available ad libitum. Day/night light cycle was reversed in the housed room and in the behavior testing room.
Isoflurane exposure
Animals were exposed to isoflurane as previously described3,5. Briefly, isoflurane was administered in a custom-built chamber in a step-down protocol over six hours starting at 2%, decreasing to 1.4% and then 0.8% every two hours. The carrier gas was a mixture of humidified air and oxygen (approximately 45% oxygen). A carbon dioxide absorber (Litholyme, Allied Healthcare, St. Louis, MO) was present during exposure. Isoflurane, oxygen, and carbon dioxide were monitored with a Datex-Ohmeda gas analyzer (West Bloomfield, MI). Temperature was regulated with a heat pad (Thermo Haake, Waltham MA), and monitored every 15 minutes by an infrared thermometer. Recorded parameters (isoflurane, oxygen and carbon dioxide concentrations and average animal temperature) during exposure are reported in the supplementary material (Supplemental Digital content 1: Figures showing physiologic variables averaged across all anesthetic exposures). There was a single mortality during isoflurane exposure of 33 animals in total exposed.
Barnes Maze
On postnatal day 41, animals began the training phase of the Barnes maze, conducted as previously described3,5. Testing was performed between 08.00 and 17.00 h. Visual cues were placed on all four walls for orientation. 70% ethanol was used to clean the maze between trials. The position of the goal was randomized among individuals with an equal distribution maintained for each experimental group to control for a regional preference on the maze table. Animal testing order was randomized among groups and kept consistent over the testing. Learning trials were conducted on five consecutive days in which the animal searched for the hidden escape chamber at a fixed location for up to 4 minutes. Latency to the goal was measured with a camera (Basler aca1280, Basler Inc, Exton, PA) and tracking software (Ethovision XT 11.5, Noldus Information Technology, Inc, Leesburg, VA). One week after the final training day, a probe trial was conducted in which the goal platform was removed and the animal’s movements and the time investigating holes was recorded over 90 seconds. Investigators were blinded to the animal groups during testing. Four animals were excluded from the probe trial analysis as they were unable to find the escape box on the last day of training (vehicle/control n=1, testosterone/isoflurane n=2, vehicle/isoflurane n=1).
Western blots
A cohort of 24 animals was injected with vehicle or testosterone on postnatal days 1–6, then left undisturbed with their dams until weaning. On postnatal day 7, the animals were briefly exposed to isoflurane to render them unconscious and following decapitation their brains were immediately removed and placed in ice-cold phosphate buffered saline. Lysates for western blot were collected from freshly dissected frontal cortex of rat brains suspended in radioimmunoprecipitation assay (RIPA) buffer, homogenized by steel bead and tissue homogenizer (Qiagen Sciences, Germantown, MD ). Protein concentrations were determined by ND-1000 spectrophotometry (Nanodrop Technologies, Wilmington DE). Western blots were run, as previously described3,5. NKCC1 and KCC2 blots were run in parallel (different gels and blots, same samples). Primary antibodies were NKCC1 1:1000 (#14581 Cell signaling Technologies; Danvers MA), rabbit anti-KCC2 1:1000 (#07–432 Millipore; Hayward CA), rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) 1:2000 (#1440 Cell Signaling Technology). Secondary antibody was goat anti-rabbit HRP 1:1000 (#A16104 Life Technologies, Carlsbad CA). The NKCC1 was blot was cut along the 75 kDa ladder and developed with chemiluminescent substrate Supersignal West Femto (Fisher Scientific, Waltham, MA, NKCC1 only). GAPDH and KCC2 blot was developed with Supersignal West Pico Plus (Fisher Scientific, Waltham, MA). Images were obtained with a ChemiDoc Touch imager (Biorad, Hercules, CA ) and densitometry calculated using Image Lab 6.0 (Biorad, Hercules, CA ) software. Values were normalized to GAPDH, and then to a single control female.
Enzyme-linked immunosorbent assay
The testosterone ELISA (EIA-1559, DRG International, Inc, Springfield, NJ) was conducted according to the manufacturer’s instructions and as previously described3. Blood (approximately 1.5ml) was obtained during cardiac perfusion at postnatal day 7 and postnatal day 41. Blood was centrifuged at 15xg for 30 min at 4°C. Plasma was then aliquoted and stored at −20°C until assay. Densitometry was performed with plate reader FLUOstar Optima (BMG Labtech, Cary, NC). A standard curve was constructed using Prism 9.0 software (Graphpad Software, San Diego, CA).
Tissue preservation/immunohistochemistry
At 12 weeks of age, after completion of behavior testing, animals were briefly anesthetized with isoflurane then pericardially perfused with ice cold phosphate buffered saline then 4% paraformaldehyde. Brains were immediately removed, and tissue was fixed in 4% paraformaldehyde overnight. After sinking in 30% sucrose, brains were sliced at 60 microns on a freezing microtome.
For the parvalbumin study, 8 brains from each group were randomly selected for analysis. Immunohistochemistry was accomplished by staining all brain slices, 360 microns apart in 12 well plates as previously described3,5. 10% goat serum for 1 h at room temperature was used for blocking followed by primary antibody incubation of mouse anti-parvalbumin 1:1000 (#MAB1572, Millipore, Burlington, MA) overnight at 4° C with gentle agitation. Secondary Goat anti-mouse 488 1:1000 (#ab150113, Abcam, Cambridge, MA) was incubated for 1 hr. Sections were floated on Superfrost slides (Fisher Scientific, Waltham, MA) and coverslipped with aqua-poly mount (Polysciences, Inc., Warrington, PA).
Imaging and cell counting
The entire slide was scanned using a Cytation 5 machine (BioTek, Winooski, VT) with a 4x objective and GFP filter set. All images were processed identically (acquisition settings- LED:10, Integration Time 682, Gain 15.6; automated image deconvolution; automated image stitching) and stored at high resolution for image analysis. Using Image J software (NIH, Bethesda, MD), hippocampal regions of interest were outlined, and the area recorded. Using the counting function, parvalbumin positive cells were manually counted and normalized to give density. Counting was completed by a single blinded individual.
Statistical analysis
Initial planning was for 15 animals per group based on previous studies3,5,8. A power calculation based on these studies found that for an alpha of 0.05 and effect size of 0.98 to 1.5 depending on given test, a group size of 8 to 13 animals per group would give a power of 0.8. Thus, inclusion of 15 animals per group provided a small cushion for loss due to mortality or inability to complete a task during behavior assessment. An error at randomization placed an additional 5 animals in the testosterone/isoflurane group, which left the testosterone/control and vehicle/isoflurane groups with 3 and 2 fewer animals, respectively, than anticipated but still within our predicted power. An additional mortality in the vehicle/isoflurane group occurred during isoflurane exposure. The final group totals were, therefore, vehicle/control n=15, testosterone/control n=12, vehicle/isoflurane n=12 and testosterone/isoflurane n=20. The western blot and ELISA studies utilized only 24 and 26 animals, respectively, given the limitations in the gel size and plate size. A single animal from the female+testosterone group was eliminated from the ELISA analysis due to a value higher than the range of sensitivity, as well as being identified as an outlier by ROUT method, Q=1%.
The statistical analysis was conducted using Prism 9.0 software (Graphpad Software, San Diego, CA). All data sets were tested for normality with a Shapiro-Wilk test. For normal datasets appropriate parametric tests were used, while for non-normal datasets, non-parametric tests were used.
The learning phase of the Barnes maze was assessed with a two-way repeated measures analysis of variance (ANOVA). The probe trial was assessed within each group by comparing the time spent exploring the goal position to equidistant positions (e.g., +/−1 hole, +/−2 hole etc.) using a Dunnett’s multiple comparison test. Comparison across groups was achieved as previously described5 using a curve fit analysis with the extra sum of squares F-test. This allowed either a linear or one phase-decay function to be fitted to the exploration pattern. A value p <0.05 was interpreted to favor the decay function over the straight line, and, if the experimental group’s exploration curves followed different functions, this was interpreted to mean that the group’s exploration was different.
For the western blot analysis, normalized densitometry values were subjected to Shapiro-Wilk test for normality and then one-way ANOVA. A Tukey’s multiple comparison post-hoc test was then completed to compare groups. For the ELISA analysis, the Shapiro-Wilk test showed non-normal distributions for female and male postnatal animals, and Kruskal-Wallis followed by post-hoc Dunnett’s multiple comparison tests were applied to the datasets. Finally, analysis of the parvalbumin staining experiment was performed with two-way ANOVAs on each region studied, followed by post-hoc multiple comparison testing with Sidak’s test.
Results
In the Barnes Maze training trials, all groups regardless of treatment demonstrated decreased latency to find the goal hole over the course of 5 days (two-way ANOVA Fday(4,220)=19.72, p<0.001; Fgroup(3,55)=2.757, p=0.051; Finteraction(12,220)=0.2587; p=0.994) (Figure 1B). Seven days after the final training session, the probe trial was completed which found successful discrimination (significantly more exploration time) between the goal and equidistant averaged positions around the maze by Dunnett’s test for vehicle/control (+/−1, 2, 3, 4, 5, 6, 7, 8, 9, opposite), testosterone/control (+/−3, 4, 5, 6, 7, 8, 9,opposite), and vehicle/isoflurane (+/−2, 3, 4, 5, 6, 7, 8, 9,opposite) groups (Figure 1C). There were no differences in exploration time from the goal to any other position in the testosterone/isoflurane group. The pattern of each groups’ exploration times was subjected to a curve fitting analysis, comparing a straight line with a one phase decay using the extra sum-of-squares F test. For the vehicle/control, vehicle/isoflurane and testosterone/control groups, the curve fitting favored a one phase decay (Fvehicle/control(1,151)=12.89, p<0.001; Fvehicle/isoflurane(1,107)=5.935, p=0.017; Ftestosterone/control (1,118)=7.736, p=0.006) (Figure 1C). By contrast, the testosterone/isoflurane group favored a straight line, illustrating the lack of discrimination of the goal, and fundamentally separating this group’s performance from the control groups (Ftestosterone/isoflurane(1,195)=1.603, p=0.21).
Previously we have reported changes in protein levels of the chloride co-transporters NKCC1 and KCC2 in the brains of postnatal day 7 animals which corresponded with group susceptibility and could be altered by the androgen receptor antagonist flutamide, in males. To determine if the expression of these transporters could be altered after testosterone exposure in females, we performed western blots from lysates of the frontal cortex of brains from animals exposed to 6 days of testosterone (female+testosterone) or vehicle (female+vehicle), sacrificed at postnatal day 7. In addition, we included control male rats (male+vehicle) for comparison. NKCC1 was increased in female+testosterone relative to female+vehicle (p<0.001) and male+vehicle (p=0.0005) animals, but there was no difference between female+vehicle and male+vehicle (p=0.287; one-way ANOVA FNKCC1(2,21)=28.28, p<0.0001) groups (Figure 2A). KCC2 was lower in the female+testosterone group than in female+vehicle group (p=0.003), and the same as in the male+vehicle group (p=0.999); male+vehicle was also lower than female+vehicle (p=0.017; one-way ANOVA FKCC2(2,21)=8.083, p=0.025) groups (Figure 2B). These results suggest that testosterone is a potent modulator of NKCC1 and KCC2 chloride co-transporter protein expression and repeats our finding that higher levels of NKCC1 and lower levels of KCC2 correspond with vulnerability to isoflurane neurotoxicity.
Figure 2. Testosterone induces changes in protein levels of NKCC1 and KCC2.
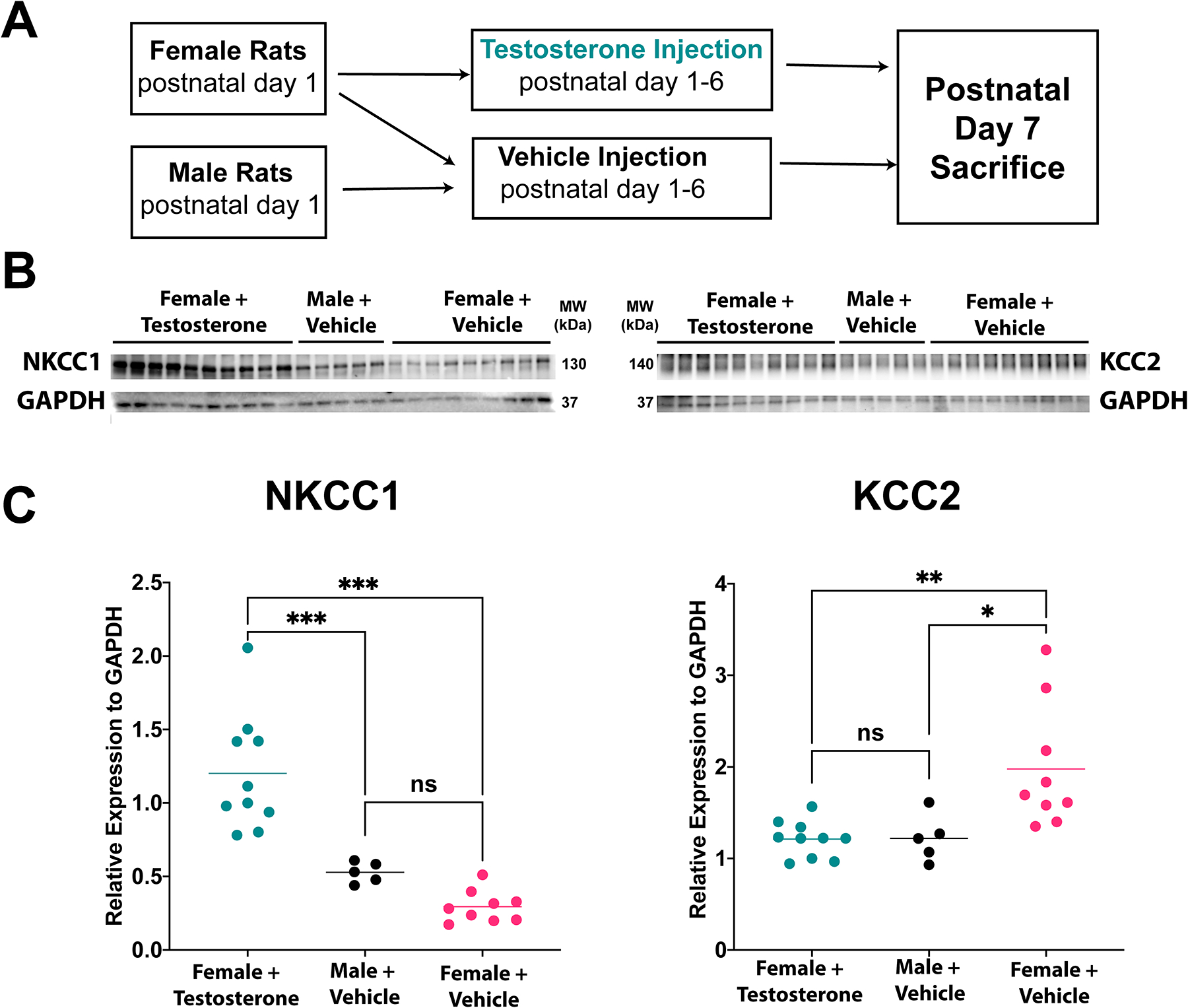
A. Experimental design of western blot experiment
B. Western blots for the chloride transporters NKCC1 and KCC2, with GAPDH as loading control. C. Quantification of densitometry of western blots.
Female+testosterone group had significantly higher NKCC1 protein compared to male+vehicle and female+vehicle groups. KCC2 levels were lower in the female+testosterone group compared to the female+vehicle group, but no different from the male+vehicle group.
*p<0.05, **p<0.01, ***p<0.001
kDa, kilodalton; ns, not significant
It was noted that the external genitalia of the female rats exposed to testosterone were qualitatively different from the vehicle controls, with the clitoral tissue of the testosterone treated females more closely resembling the male phallus than female controls (Figure 3A), and consistent with other protocols of testosterone injection at this age9. To quantify the concentration of testosterone at postnatal day 7 after 6 days of injections, a testosterone ELISA was performed on blood collected after sacrifice on postnatal day 7. The female rats treated with exogenous testosterone had much higher testosterone levels than vehicle treated females, which were all below the level of detection (averagetestosterone=4.49ng/ml, SD=0.966 vs. averagevehicle=0ng/ml, SD=0.0; Dunn’s multiple comparison p<0.001) (Figure 3B). For comparison, vehicle treated males were also analyzed and had lower testosterone levels than testosterone treated females (averagemale=0.082ng/ml, SD=0.124; Dunn’s multiple comparison p=0.016). In a group of rats, serum testosterone was also measured at the time of the initiation of behavioral testing. By postnatal day 41, the levels of testosterone in the female treated group had fallen to levels not statistically different from vehicle treated females (averagetestosterone=0.665ng/ml, SD=0.146 vs. averagefemale=0.861ng/ml, SD=0.116; Sidak’s multiple comparison p=0.327), which were both less than testosterone levels in vehicle treated males (averagemale= 1.531ng/ml, SD=0.646; Sidak’s female+testosterone vs. male p<0.0001, female+vehicle vs. male p=0.0014). A graphical representation of these data is presented in the supplementary material (Supplemental Digital Content 2: Figure showing serum testosterone levels in young adult animals).
Figure 3. Effects of exogenous testosterone on female rats.
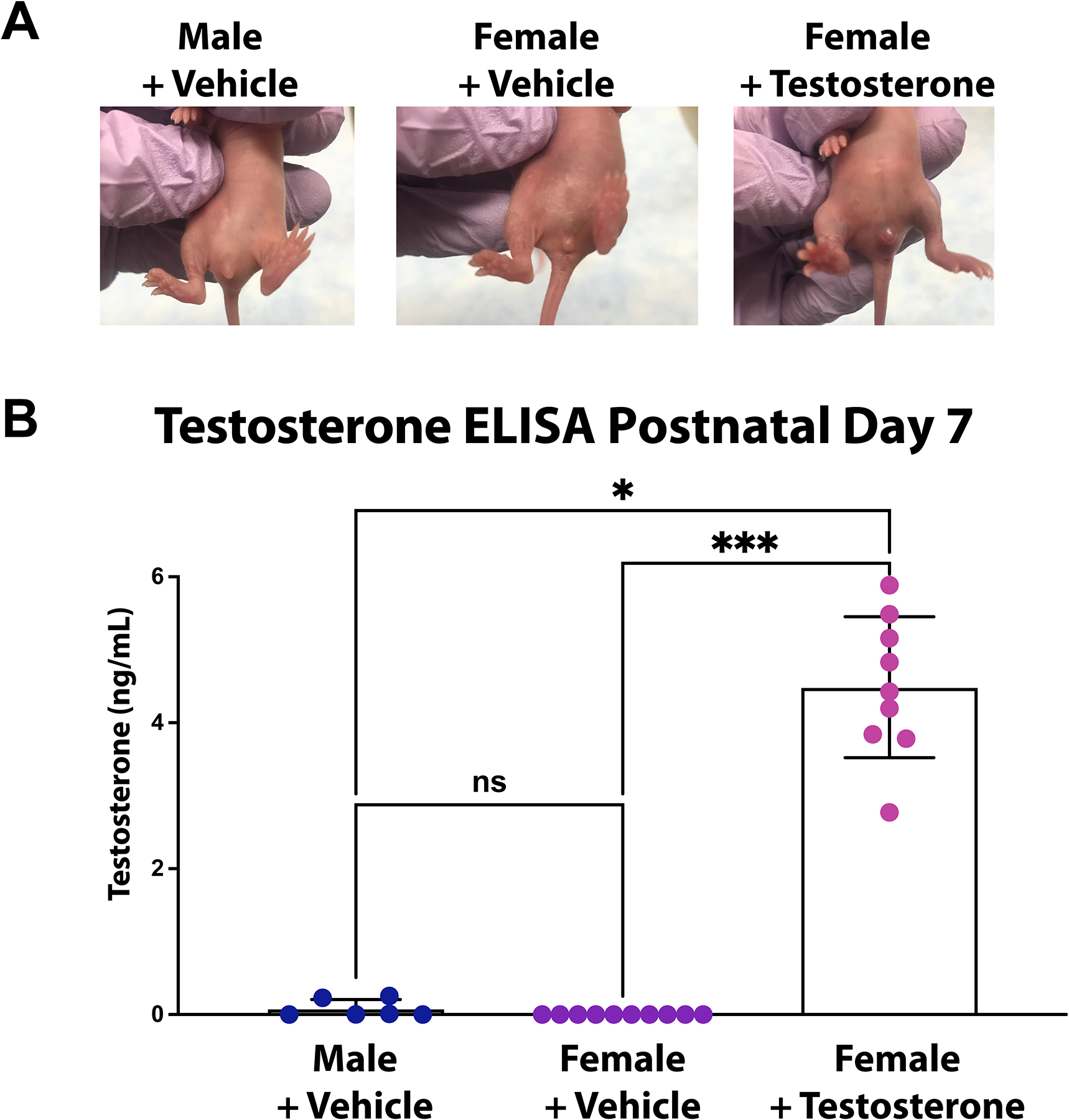
A. Images of postnatal day 7 rats show the secondary sex characteristics of the different treatments with those in the female+testosterone group having significant changes to the external genitalia.
B. Testosterone ELISA performed on plasma from P7 rats showed significantly increased levels of testosterone in the female+testosterone group compared to male+vehicle and female+vehicle groups.
*p<0.05, ***p<0.001
ns, not significant
Unpublished work by our lab and others suggests that GABAergic interneurons, specifically the parvalbumin positive interneuron population, might play a critical role in the development of the neurotoxic deficit from isoflurane10,11. We sought to quantify the number of parvalbumin interneurons in specific regions of the hippocampus, a region critically important for spatial memory, after the animals had completed behavioral testing (12 weeks of age). With the same four experimental groups, we found a significant effect of isoflurane on the number of parvalbumin interneurons in the dentate gyrus (two-way ANOVA: Fisoflurane(1,28)=14.33, p=0.036, CA3 Fisoflurane(1,28)=12.65, p=0.048, CA1 Fisoflurane(1,28)=13.87, p=0.040) (Figure 4). However, testosterone had no effect on the numbers of parvalbumin cells counted in these regions (dentate gyrus Ftestosterone(1,28)=2.483, p=0.368, CA3 Ftestosterone(1,28)=2.677, p=0.351, CA1 Ftestosterone (1,28)=1.614, p=0.469), nor was there an effect by interaction (dentate gyrus Finteraction(1,28)=0.148, p=0.824, CA3 Finteraction(1,28)=1.100, p=0.548, CA1 Finteraction(1,28)=0.348, p=0.736). These results suggest that the isoflurane-induced decrease of parvalbumin cells cannot fully explain the observed cognitive deficit. Alternative cell types and processes that are altered after exposure to isoflurane during brain development may play a larger role in the testosterone-sensitive outcomes reported here.
Figure 4. Fewer Parvalbumin interneurons found in the hippocampus after neonatal isoflurane exposure.
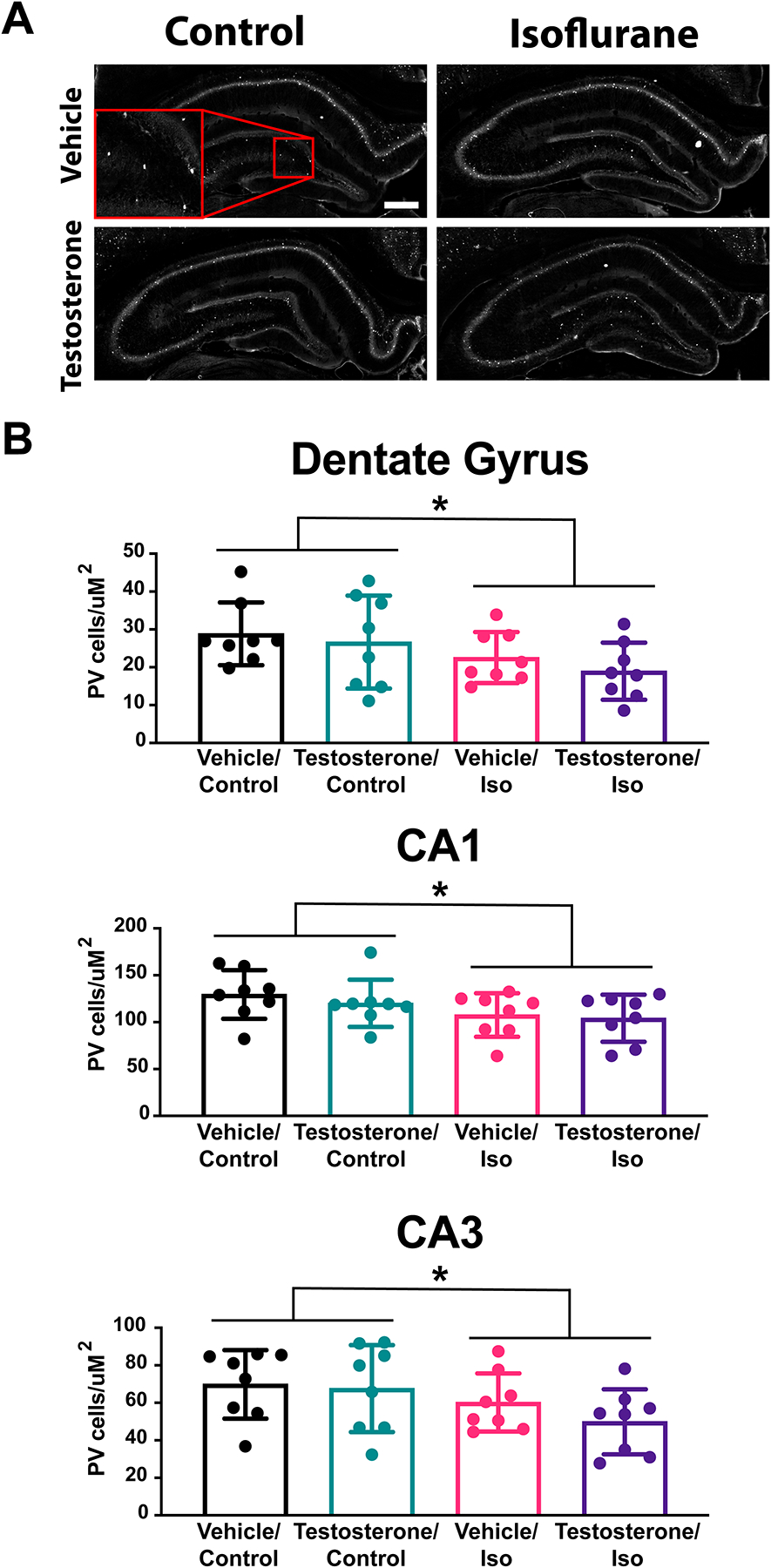
A. Representative sections of the hippocampus from composite images of parvalbumin immuno-labeled brains.
B. Average cell density of parvalbumin positive cells by hippocampal region showed a general effect of isoflurane leading to decreased cell density by two-way ANOVA: Fdentate gyrus(1,28)=14.33, p=0.036, FCA3(1,28)=12.65, p=0.048, FCA1(1,28)=13.87, p=0.040). However, there was no difference between groups by multiple comparison test.
Scale bar 100μm
*p<0.05
Discussion
These studies show that exogenous testosterone administered to female rats can alter the susceptibility to volatile anesthetic neurotoxicity as measured by a spatial memory task. Testosterone also increases the protein level of the immature chloride co-transporter protein NKCC1 relative to controls and decreases the mature chloride co-transporter KCC2. This pattern is consistent with previous work and may mechanistically underlie the observed susceptibility changes. Our investigation into the GABAergic interneuron populations of parvalbumin cells within regions of the hippocampus could not explain the behavioral findings, although interestingly there is an effect of isoflurane exposure on these populations lasting into adulthood.
The Barnes Maze task, which has two phases, showed no differences in the learning phase among groups which is consistent with prior experiments3,5,8 and suggests that the encoding of short-term spatial memory is not significantly affected by the treatment. However, the ability to recall the position of the escape box after a one-week delay was significantly altered by exposure of females to testosterone and isoflurane. It is also notable that we replicated our previous finding that females (without testosterone) exposed to isoflurane on postnatal day 7 do not develop a spatial memory deficit in the Barnes Maze3.
The current findings are consistent with our previous studies comparing males with females, gonadectomized males, or flutamide-treated males which have all shown susceptibilities to volatile anesthetic that correspond with increased NKCC1 and decreased KCC23,5. The balance of expression of these co-transporters has important implications for chloride homeostasis in development and there is ample evidence for sex differences in maturation of this system12–15. Excitatory GABAergic action during normal development has been shown to be critical for neuronal migration16, network formation17 and synapse maturation18. It is possible that any or all of these could be disrupted with excessive GABAergic activity during development. Pharmacologic blockade of NKCC1 with the diuretic bumetanide has demonstrated the functional importance of NKCC1 in development in a variety of circumstances19. We previously showed that bumetanide can protect male rats from postnatal day 7 isoflurane exposure5. Taken together, these experiments build on a developing story of sex hormone-regulated, GABAergic-mediated neural development and its role in anesthetic neurotoxicity.
Testosterone’s role in masculinizing the developing brain has been known for almost a century20. Exogenous estrogens have also long been observed to cause similar masculinization21,22. Since then, it is widely understood that aromatase in the central nervous system can convert testosterone to estrogen which then exerts its masculinizing effects (for review see23). In our previous studies, in which we blocked androgen receptors with flutamide prior to isoflurane exposure, male rats were protected from anesthetic neurotoxicity suggesting an important role for androgen receptors in our model5. The results in the current study are consistent with our prior work, but do not exclude the possibility that the exogenous testosterone we administered may be aromatized to estrogen to mediate the change in neurotoxic susceptibility. Together, our studies highlight the importance of androgens in regulating the development of the GABAergic system. Furthermore, our studies are also consistent with others showing that exogenous estrogen can decrease KCC2 protein in male rats24 and that blocking estrogen synthesis with formestane can protect against sevoflurane induced electrophysiologic abnormalities in male rats25. Altogether these studies affirm the importance of androgens in modulating the development of the GABAergic system.
Parvalbumin interneurons are the largest population of GABAergic neurons and have been shown to play critical roles in developmental plasticity and learning and memory26,27. Their populations in different brain regions are also significantly reduced after neonatal volatile anesthetic exposure10,11. While we found a decrease after isoflurane exposure on postnatal day 7 in the overall density of parvalbumin neurons in all three regions of the hippocampus studied, we did not see a difference between those treated with vehicle versus exogenous testosterone despite differences in behavioral outcome. While others have reported that hippocampal parvalbumin neurons are critical for spatial memory28, in our study it appears that the reduced density in the hippocampus of female rats exposed to vehicle and isoflurane was sufficient to successfully navigate the Barnes maze. In contrast, the testosterone and isoflurane exposed animals, despite no difference in the density of parvalbumin cells, did have a spatial memory deficit suggesting there may be additional (unknown) cellular deficiencies which could account for the differences in behavior between these groups. Another possibility is that any differences in the density of parvalbumin cells at the time of memory testing in the vehicle/isoflurane and testosterone/isoflurane groups could have normalized by the time the animals were sacrificed as the numbers of parvalbumin positive neurons change from puberty to adulthood in rodents29. In any case, this isoflurane-induced change in parvalbumin hippocampal density cannot solely account for the long-term cognitive outcome observed in females with exogenous testosterone and isoflurane exposure, but it may be a contributor with other factors to this phenotype.
The dose of exogenous testosterone used in this study resulted in a supraphysiologic exposure, even by comparison against male controls. However, while sufficient to cause changes to external genitalia and alter the protein levels of chloride co-transporters in the brain, the exposure was transient and, by the time of behavioral testing at postnatal 41, testosterone levels in females that were previously treated with testosterone had returned to the levels of control females, and both had significantly lower testosterone levels than the male controls, as expected. Thus, direct hormonal effects were limited to the perinatal period and were unlikely to influence the outcome of the behavior studies in young adulthood. Still, these results should be considered within the confines of the experimental construct, and generalization should be done with caution.
We do not have direct electrophysiologic evidence that GABA excitation is at the root of the susceptibility. However, the consistent demonstration of a specific memory deficit and alterations in chloride co-transporter expression following isoflurane exposure strongly support the hypothesis. Care must be given when extrapolating these findings to humans, especially given the method of volatile anesthetic administration in these studies, in which, because of the number of animals and size of animals, we do not individually intubate and ventilate each animal. However, while this type of exposure can result in increased carbonemia, this alone cannot account for the memory deficit30.
In summary, our results demonstrate the importance of sex and age factors in defining susceptible periods of anesthetic neurotoxicity during early development. The broader implication of sex differences in GABAergic development and the vulnerability of the young brain could have important consequences in humans. While sex hormones have long been known to be important regulators of neurodevelopment, how androgens exert their modulatory activity on the developing brain remains an important and exciting question in neuroscience and justifies future preclinical and clinical study.
Supplementary Material
Acknowledgements:
The authors would like to thank and acknowledge Jason Leong for technical assistance with western blots, dissections, and ELISAs.
Funding Statement:
Funding was provided by Ruth L. Kirschstein National Research Service Award T32 GM08440 (GAC), Foundation for Anesthesia Education and Research (FAER) MRTG-08–15-2020-Chinn (GAC), and National Institutes of Health Grant R01 GM112831 (JWS)
Footnotes
Conflict of Interest: The authors declare no competing interests.
References:
- 1.Ben-Ari Y Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe M, Fukuda A. Development and regulation of chloride homeostasis in the central nervous system. Front Cell Neurosci. 2015;9:371. doi: 10.3389/fncel.2015.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki Russell JM, Chinn GA, Maharjan D, Eichbaum Y, Sall JW. Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Br J Anaesth. 2019;122(4):490–499. doi: 10.1016/j.bja.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee BH, Chan JT, Kraeva E, Peterson K, Sall JW. Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology. 2014;83:9–17. doi: 10.1016/j.neuropharm.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinn GA, Sasaki Russell JM, Yabut NA, Maharjan D, Sall JW. Androgenic Modulation of the Chloride Transporter NKCC1 Contributes to Age-dependent Isoflurane Neurotoxicity in Male Rats. Anesthesiology. 2020;133:852–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki Russell JM, Hagelstein M, Lee BH, Sall JW. Anesthesia-induced Recognition Deficit is Improved in Postnatally Gonadectomized Male Rats. J Neurosurg Anesthesiol. 2019;Sept 9(ePublish Ahead of print). doi:doi: 10.1097/ANA.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih J, May LDV, Gonzalez HE, et al. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116(3):586–602. doi: 10.1097/ALN.0b013e318247564d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinn GA, Sasaki Russell JM, Banh ET, Lee SC, Sall JW. Voluntary Exercise Rescues the Spatial Memory Deficit Associated With Early Life Isoflurane Exposure in Male Rats. Anesth Analg. 2019;129(5):1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mong JA, Glaser E, McCarthy MM. Gonadal Steroids Promote Glial Differentiation and Alter Neuronal Morphology in the Developing Hypothalamus in a Regionally Specific Manner. J Neurosci. 1999;19(4):1464 LP–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji M, Wang Z, Sun X, et al. Repeated Neonatal Sevoflurane Exposure-Induced Developmental Delays of Parvalbumin Interneurons and Cognitive Impairments Are Reversed by Environmental Enrichment. Mol Neurobiol. 2017;54(5):3759–3770. doi: 10.1007/s12035-016-9943-x [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Chen J, Cai G, et al. Exposure to Sevoflurane Affects the Development of Parvalbumin Interneurons in the Main Olfactory Bulb in Mice. Front Neuroanat. 2016;10:72. doi: 10.3389/fnana.2016.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuñez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67(14):1879–1890. doi: 10.1002/dneu.20567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80(2):99–113. doi: 10.1016/j.eplepsyres.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM. Sex Differences in the Chloride Cotransporters, NKCC1 and KCC2, in the Developing Hypothalamus. J Neuroendocrinol. 2007;19(4):302–308. doi: 10.1111/j.1365-2826.2007.01530.x [DOI] [PubMed] [Google Scholar]
- 15.Murguía-Castillo J, Beas-Zárate C, Rivera-Cervantes MC, Feria-Velasco AI, Ureña-Guerrero ME. NKCC1 and KCC2 protein expression is sexually dimorphic in the hippocampus and entorhinal cortex of neonatal rats. Neurosci Lett. 2013;552:52–57. doi: 10.1016/j.neulet.2013.07.038 [DOI] [PubMed] [Google Scholar]
- 16.Bortone D, Polleux F. KCC2 Expression Promotes the Termination of Cortical Interneuron Migration in a Voltage-Sensitive Calcium-Dependent Manner. Neuron. 2009;62(1):53–71. doi: 10.1016/j.neuron.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherubini E, Griguoli M, Safiulina V, Lagostena L. The Depolarizing Action of GABA Controls Early Network Activity in the Developing Hippocampus. Mol Neurobiol. 2011;43(2):97–106. doi: 10.1007/s12035-010-8147-z [DOI] [PubMed] [Google Scholar]
- 18.Akerman CJ, Cline HT. Depolarizing GABAergic Conductances Regulate the Balance of Excitation to Inhibition in the Developing Retinotectal Circuit In Vivo; J Neurosci. 2006;26(19):5117 LP–5130. doi: 10.1523/JNEUROSCI.0319-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kharod SC, Kang SK, Kadam SD. Off-Label Use of Bumetanide for Brain Disorders: An Overview . Front Neurosci . 2019;13:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeiffer CA. Sexual differences of the hypophyses and their determination by the gonads. Am J Anat. 1936;58(1):195–225. doi: 10.1002/aja.1000580112 [DOI] [Google Scholar]
- 21.Gorski RA. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am J Physiol Content. 1963;205(5):842–844. doi: 10.1152/ajplegacy.1963.205.5.842 [DOI] [PubMed] [Google Scholar]
- 22.Sheridan PJ, Sar M, Stumpf WE. Interaction of Exogenous Steroids in the Developing Rat Brain1. Endocrinology. 1974;95(6):1749–1753. doi: 10.1210/endo-95-6-1749 [DOI] [PubMed] [Google Scholar]
- 23.Shay DA, Vieira-Potter VJ, Rosenfeld CS. Sexually Dimorphic Effects of Aromatase on Neurobehavioral Responses . Front Mol Neurosci . 2018;11:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184(2):1003–1009. doi: 10.1016/S0014-4886(03)00387-X [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Yang B, Ju L, et al. The Estradiol Synthesis Inhibitor Formestane Diminishes the Ability of Sevoflurane to Induce Neurodevelopmental Abnormalities in Male Rats . Front Syst Neurosci . 2020;14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science (80- ). 2002;298(5596):1248–1251. doi: 10.1126/science.1072699 [DOI] [PubMed] [Google Scholar]
- 27.Ferguson BR, Gao WJ. Pv interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits. 2018;12:37. doi: 10.3389/fncir.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray AJ, Sauer J-F, Riedel G, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14(3):297–299. doi: 10.1038/nn.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu YC, Du X, van den Buuse M, Hill RA. Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: A role for estradiol. Psychoneuroendocrinology. 2014;45:167–178. doi: 10.1016/j.psyneuen.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 30.Stratmann G, Sall JW, May LDV, Loepke AW, Lee MT. Beyond anesthetic properties: The effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function. Anesth Analg. 2010;110(2):431–437. doi: 10.1213/ane.0b013e3181af8015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


