Abstract
Agro-industrial wastes provide potential sources of carbon for production of fungal enzymes applied for various biotechnological applications. In this study, 23 strains of Aspergillus niger were systematically investigated for their capability on production of carbohydrate-processing enzymes used in industries. The strains were grown on glucose or selected agricultural wastes comprising varied chemical compositions as the sole carbon source. As a control, glucose induced basal activities of amylase, pectinase, and xylanase in only a few strains, while the CMCase, β-glucanase, and invertase activities were detected only when the carbon source was switched to the agro-industrial biomass. According to one-way ANOVA analysis, banana peels containing lignocellulosic components with high pectin and starch contents with its easily digestible nature, were found to be the best carbon source for inducing production of most target enzymes, while the cellulose-rich sugarcane bagasse efficiently promoted maximal levels of β-glucanase and xylanase activities. The starch fiber-rich cassava pulp also effectively supported the activities of amylase and most other enzymes, but at relatively lower levels compared to those obtained with banana peel. The A. niger TL11 strain was considered the most potent strain for production of all target enzymes with the CMCase, xylanase, pectinase, β-glucanase, amylase, and invertase activities of 76.15, 601.59, 160.89, 409.20, 426.73, and 1186.94 U/mL, respectively. The results provide insights into the efficiency of various carbon sources with different chemical compositions on inducing the target enzymes as well as the dissimilarity of A. niger strains on the production of different carbohydrate-processing enzymes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-03086-y.
Keywords: Aspergillus niger, Enzymes, Sugarcane bagasse, Cassava pulp, Banana peel
Introduction
Agricultural-based industries annually generate an abundance of waste residues, which are underutilized economically and they can cause environmental pollution from the disposal process. Currently, these waste residues have become more valuable due to the development of bio-industries that enables various agricultural wastes to be used as alternative carbon sources for microorganisms during enzyme production. Therefore, using these wastes can significantly lower the cost of enzyme manufacturing. For its use as a carbon source for fermentation, it must be considered that each agro-industrial waste has its own unique physical characteristics and chemical compositions. Thus, understanding the effect of these different agricultural wastes on microorganism’s enzyme production profile will be extremely beneficial for the efficient utilization of enzymes in industrial applications. The major component of plant-derived wastes is lignocellulose comprising different proportions of carbohydrate polymers (cellulose and hemicellulose) and aromatic polymer (lignin) along with other minor constituents including pectin, proteins, lipids, extractives, and minerals (van den Brink and de Vries 2011). In addition to lignocellulose, high contents of starch can be found in fruit-derived wastes, such as banana peel, jackfruit seed, and pineapple stems (Kringel et al. 2020), as well as wastes from starch processing such as cassava pulp (Djuma’ali et al. 2011). The diversity of chemical compositions of agro-industrial wastes and the enzymatic characteristics of each microorganism have become the principal factors affecting the variation of the enzyme productivity exhibited by different microbes. For example, utilization of sugarcane bagasse as a sole carbon source for fermentation of six filamentous fungi including Aspergillus japonicus, Fusarium sp., Fusarium. solani, Fusarium oxysporum, Pestalotiopsis sp., and Trichoderma pseudokoningii resulted in the production of various types of enzymes, such as cellulases, xylanases, amylases, pectinases, and laccases (Ferreira et al. 2018). Recently, banana peel has emerged as a promising substrate for enzyme production due to the high pectin and starch contents (Wachirasiri et al. 2009; Orozco et al. 2014). In addition, its soft tissues allow efficient digestion of its components. Banana peels have been used as substrates for the production of amylase by A. niger NCIM 616 (Paladi et al. 2012) and Penicillium sp. (Ponnuswamy et al. 2011), xylanase and pectinase by A. fumigatus MS16 (Zehra et al. 2020), cellulase by Trichoderma viride (Sun et al. 2011), and laccase by A. fumigatus (Vivekanand et al. 2011). Likewise, A. niger MS23 produced multiple enzymes including xylanase, pectinase, amylase, and endoglucanase from solid-state fermentation (SSF) using banana peel as the substrate (Rehman et al. 2015). These showed that banana peel can induce production of many carbohydrate-degrading enzymes potentially valuable for industrial applications. A complete enzyme induction profile of A. niger facilitated by banana peel will most likely promote the use of banana peel as a carbon source for A. niger’s enzyme production in industries.
Aspergillus niger is one of the most powerful fungi in biotechnology applications. It was commonly isolated from soil, leaf litter, and compost. In particular, A. niger is renowned as a high-performance cell factory for industrial production of non-starch polysaccharide-degrading enzymes including cellulases (Akula and Golla 2018), β-glucanases (Shindia et al. 2013), xylanases (Hmida-Sayari et al. 2012; Ajijolakewu et al. 2017), pectinases (Ahmed et al. 2016), and invertases (Romero-Gomez et al. 2000) as well as starch-degrading enzymes including α-amylase (Gupta et al. 2008) and glucoamylase (Izmirlioglu and Demirci 2016; Slivinski et al. 2011). These enzymes are widely used in several industries, such as food, feed, textile, pulp and paper, pharmaceutical, and biorefinery (Manisha 2017; Chukwuma et al. 2020). The enzymes can be employed in the form of crude enzyme mixtures, such as complex lignocellulose-degrading enzyme preparations used to enhance digestibility and nutrition uptake from difficult-to-digest feedstuffs in food, animal feed, and biorefinery industries (Kuhad et al. 2011; Ejaz et al. 2021; Bhardwaj et al. 2019; Satapathy et al. 2020; de Souza and de Oliveira Magalhães 2010). Additionally, enzyme preparations with a single dominated activity can be used in certain target applications. For example, in pulp and paper industry, xylanase is the main enzyme used for the bio-bleaching step while cellulase is used for smoothing fibers, enhancing pulp drainage, and promoting ink removal (Kirk and Jeffries 1996). Pectinase usage led to improvement of the brightness and whiteness and reduction of the yellowness of pulp (Sheoran et al. 2008), while application of amylase resulted in the increased drainage rate of recycled paper pulp and reduced viscosity of the backwater (Kenealy and Jeffries 2003).
According to food safety examinations, several products from A. niger strains are approved with Generally Regarded As Safe (GRAS) status by the US Food and Drug Administration (FDA) (Abarca et al. 2004), and at least 19 food enzymes produced from A. niger have been licensed in the US FDA (Li et al. 2020). Most importantly, A. niger has excellent secretion machinery facilitating enzyme releases into the culture medium (Krijgsheld et al. 2012), making it an ideal microorganism for effective enzyme production using either submerged fermentation (SmF) or solid-state fermentation (SSF) (Li et al. 2020). The secreted enzymes can then be easily collected from the fermentation media. Production of different carbohydrate-degrading enzymes from various strains of A. niger has continually been reported. However, a comparative study among many strains of A. niger on the production of multiple enzymes using various agro-industrial wastes as carbon sources has been very limited. The aim of this work is to systematically study the enzyme production potential and enzyme profiles of 23 strains of A. niger previously isolated from various environments, using different agricultural residues as the sole carbon source for submerged fermentation. The agricultural wastes used include sugarcane bagasse (SB), cassava pulp (CP), and banana peel (BP), each of which contains varying chemical compositions. A high-throughput platform was employed to determine activities of 6 carbohydrate-processing enzymes with potential industrial applications including endoglucanase (CMCase), β-glucanase, xylanase, pectinase, invertase, and amylase. This work provides insights into the effects of chemical compositions in the raw substrates on profiles of carbohydrate-degrading enzymes produced by A. niger, which can be used as a guideline for further biotechnological applications.
Materials and methods
Strains, media, and growth condition
Twenty-three strains of Aspergillus niger were obtained from the Thailand Bioresource Research Center (TBRC; www.tbrcnetwork.org) (Table 1 and Supplementary Fig. 1). The A. niger strains were grown on complete medium (CM) agar containing 15 g/L agar, 1 g/L casamino acid, 5 g/L yeast extract, 1% glucose, 20 mL/L ASPA + N (stock solution 50x: 297.5 g/L NaNO3, 26.1 g/L KCl, and 74.8 g/L KH2PO4 at pH 5.5), 1 mL/L Vishniac solution, and 1 mM MgSO4, at pH 5.5 (Arentshorst et al. 2012). The fungi were incubated at 30 °C for 5 days to produce spores. The spores were harvested by spore harvesting solution [SHS: 0.9% (w/v) NaCl and 0.001% (v/v) Tween80], washed by spore washing solution [SWS: 0.9% (w/v) NaCl], and restored in SWS at 4 ºC.
Table 1.
List of Aspergillus niger strains
| Fungi | New code | Original (TBRC) code | Source |
|---|---|---|---|
| Aspergillus niger | TL01 | TBRC 2372 | Soil |
| TL02 | TBRC 2619 | Soil | |
| TL03 | TBRC 2764 | Soil | |
| TL04 | TBRC 2765 | Soil | |
| TL05 | TBRC 2767 | Soil | |
| TL06 | TBRC 2789 | Sand | |
| TL07 | TBRC 2856 | Soil | |
| TL08 | TBRC 3637 | Soil | |
| TL09 | TBRC 3732 | Soil | |
| TL10 | TBRC 3734 | Soil | |
| TL11 | TBRC 3738 | Dung—deer | |
| TL12 | TBRC 6619 | Soil—hot spring | |
| TL13 | TBRC 6634 | Soil—hot spring | |
| TL14 | TBRC 6642 | Soil—hot spring | |
| TL15 | TBRC 6644 | Soil—hot spring | |
| TL16 | TBRC 6651 | Soil—hot spring | |
| TL17 | TBRC-BCC 17136 | Dung—elephant | |
| TL18 | TBRC-BCC 17391 | Soil | |
| TL19 | TBRC-BCC 17396 | Soil | |
| TL20 | TBRC-BCC 17406 | Soil | |
| TL21 | TBRC-BCC 17469 | Soil | |
| TL22 | TBRC-BCC 17723 | Soil | |
| TL23 | TBRC-BCC 19088 | Soil |
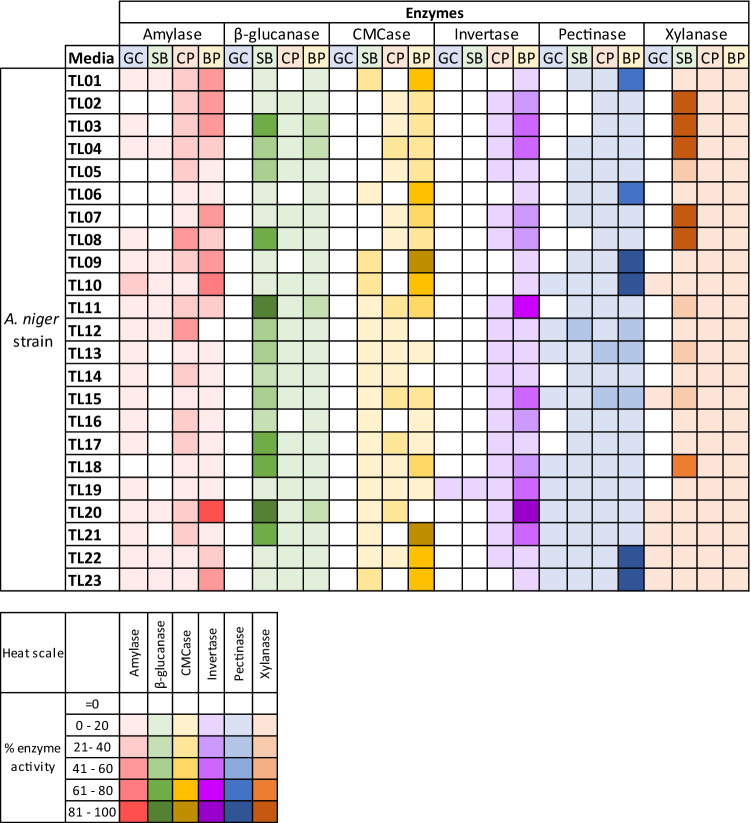
Remark: The phenotypes of the 23 A. niger strains were shown on Supplementary Fig S1
Determination of chemical compositions of agricultural wastes
The contents of cellulose, hemicellulose, and lignin were determined according to the protocol of the Association of Official Analytical Chemists (AOAC; Arlington, VA (1990)) (AOAC 1990), while the starch content was analyzed by the Megazyme total starch assay kit (K-TSTA-100A, Megazyme, Bray, Ireland).
Fermentation in media containing different carbon sources
Sugarcane bagasse was obtained from Mitrphol Sugar Mill (Khon Kaen, Thailand) and pre-treated by liquid hot compress water according to Chotirotsukon et al. (2021). Cassava pulp was bought from Cholcharoen Co., Ltd., Cholburi, Thailand. Ripe banana peel was collected from a food factory (Ang Thong, Thailand), dried in the oven for 3 days, and ground into small sizes. The screening medium (SM) contains 1 g/L casamino acid, 1 g/L yeast extract, 1.2 g/L NaNO3, 0.5 g/L KH2PO4, 0.2 g/L MgSO4.7H2O, and 40 µL/L Vishniac solution, at pH 6.0, supplemented with 5% (w/v) of SB, CP, BP or 20 g/L glucose to obtain SMSB, SMCP, SMBP, or SMGC medium, respectively. The fungal spore was inoculated into 50 mL of SMSB, SMCP, SMBP, or SMGC medium in 250-mL flasks. The cultures were incubated at 30 °C with shaking at 200 rpm for 4 days. The supernatant was collected on day 4 for subsequent determination of enzyme activities and pH (Supplementary Table S1). The mycelium was collected for cell dry weight (CDW) only for SMGC (Supplementary Table S2).
Enzyme assay
Activities of six enzymes were determined with the 3,5-dinitrosalicylic acid (DNS) method (Miller 1959) using a customized high-throughput automated enzyme screening platform (Perkin Elmer, Waltham, MA). The activities of endoglucanase (CMCase), β-glucanase, xylanase, pectinase, amylase, and invertase were assayed using 1% (w/v) carboxymethyl cellulose (CMC), 1% (w/v) β-glucan, 1% (w/v) beechwood xylan, 1% (w/v) citrus peel pectin, 1% (w/v) soluble starch, and 10% (w/v) sucrose, respectively. These substrates were dissolved in 50 mM sodium acetate buffer, pH 5.5. The enzymatic reactions containing 50 µL substrate and 10 µL of A. niger supernatant were incubated at 50 °C for 10 min. After the incubation period, 120 µL of the DNS solution was added to the reaction mixtures. The mixtures were heated up at 95 °C for 10 min and then cooled down at 15 °C for 10 min before the amount of reducing sugars released into the solution were measured by absorbance at 540 nm using a spectrophotometer. The amount of reducing sugar was interpolated from a standard curve of glucose to determine the activities of endoglucanase, β-glucanase, amylase, and invertase. Similarly, standard curves of xylose and D-galacturonic acid were used for determination of xylanase and pectinase activities. One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmole of product per min.
Statistical analysis
The experiments were carried out in triplicates. All results in our study were presented as mean values. Groups of data with differences in mean values were identified by One-way ANOVA analysis of variance using Tukey's range test. The p value less than 0.05 was considered statistically significant.
Results
Chemical composition of agricultural wastes
Differences in chemical compositions of carbon sources can have a profound effect on types and levels of the enzymes produced by microorganisms during fermentation. In this study, each agricultural waste was analyzed for cellulose, hemicellulose, lignin, and starch contents (shown in Table 2) before being employed as a carbon source for A. niger. Among these agricultural wastes, cellulose is a major component found in SB comprising 57.24% of total matter, followed by BP (11.43%) and CP (9.43%). SB contains the highest hemicellulose content at 9.85%, while BP and CP comprise 7.28% and 5.41% of hemicellulose contents, respectively. Lignin occupies the highest fraction in SB at 10.15%, followed by BP at 9.86%, whereas only 7.18% of lignin content was found in CP. As predicted, CP is the best source of starch with a starch content of 50.52%, while BP contains 25.35% starch content. In contrast, a very low content of starch was found in SB. The pectin content in these agricultural wastes was not measured. However, it was reported to range between 0.6 and 0.8% in native SB (Yadav et al. 2015), 7% in CP (Djuma’ali et al. 2011), and 11.8–15.9% in BP (Oberoi et al. 2012; Castillo-Israel et al. 2015). It should be noted that, in this study, SB was pretreated by liquid hot water, which resulted in the enrichment of cellulose with partial removals of hemicellulose and lignin. Compositions of the agricultural residues in this study are in the same range of those previously reported (Djuma’ali et al. 2011; Yu et al. 2013; Orozco et al. 2014; Ladeira Ázar et al. 2020; Silva et al. 2011). The other chemical components not yet analyzed in this work include protein, fat, fiber, and ash.
Table 2.
Chemical compositions of agricultural wastes
| Agricultural wastes | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Starch content (%) |
|---|---|---|---|---|
| Sugarcane bagasse (SB) | 57.24 | 9.85 | 10.15 | 0.06 |
| Cassava pulp (CP) | 9.43 | 5.41 | 7.18 | 50.52 |
| Banana peel (BP) | 11.43 | 7.28 | 9.86 | 25.35 |
The analysis was performed in triplicate with the standard deviations below 5%
Enzyme profiles of A. niger growing on various carbon sources
Glucose
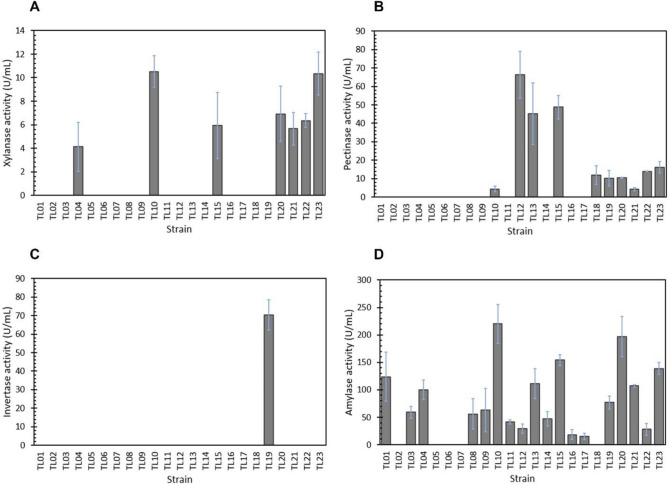
Glucose can induce the production of at least three enzymes (xylanase, pectinase, and amylase) in several strains of A. niger in this study (Fig. 1). Seven strains of A. niger produced xylanase in a low, but detectable, level at 4.11–10.52 U/mL (Fig. 1A). Similarly, pectinase activity was also detected in 8 strains using the medium containing glucose (Fig. 1B). A moderate pectinase activity was detected in the A. niger TL12 strain at 66.34 U/mL, followed by the TL15 (48.77 U/mL) and TL13 (45.17 U/mL) strains, while the rest of A. niger strains produced low levels of pectinase activity in the range of 10.30–16.12 U/mL. A varying range of amylase activity between 15.33 and 219.98 U/ml was induced in 18 strains using the glucose-containing medium with the highest activity found in the TL10 strain. Interestingly, only one strain, A. niger TL19, produced a detectable invertase activity at 70.36 U/mL in the glucose medium (Fig. 1C). No activity of endoglucanase and β-glucanase was detected in the supernatant of these A. niger strains.
Fig. 1.
Profiles of enzyme activities produced by A. niger strains grown on glucose as the carbon source (SMGC medium). A Xylanase activity, B pectinase activity, C invertase activity, and D amylase activity. The enzyme activities were determined in 50 mM sodium acetate buffer, pH 5.5 at 50 °C. The measurements were done in triplicate
Sugarcane bagasse
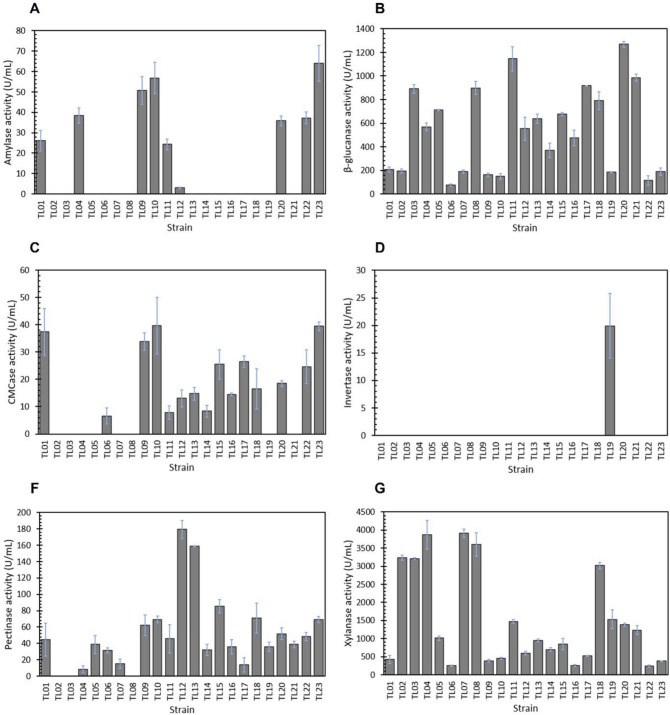
When glucose was replaced by SB as the only carbon source for fermentation, the activity profiles of the 23 A. niger strains changed significantly, especially for endoglucanase and β-glucanase (Fig. 2). SB strongly induced the endoglucanase activity of 15 A. niger strains in the range of 6.53–39.60 U/mL with the highest activity occurring in the TL10 strain (Fig. 2C). The activity of β-glucanase was markedly induced by SB in all 23 strains in the range of 76.95–1268.17 U/mL (Fig. 2B). Among them, the TL20 and TL11 strains showed a remarkably high activity above 1000 U/mL. Similar to β-glucanase, all strains produced a varying level of xylanase activity in the range of 242.94–3910.06 U/mL with the highest activity exhibited by the TL07 strain (Fig. 2F). Additionally, the use of SB increased the activity of pectinase in 19 strains in a range of 15.10–176.26 U/mL. The highest activity was detected in the TL12 strain, displaying 2.7 times higher activity than that observed in the glucose medium (Fig. 2E).
Fig. 2.
Profiles of enzyme activities produced by A. niger strains grown in sugarcane bagasse as the carbon source (SMSB medium). A Amylase activity, B β-glucanase activity, C CMCase activity, D invertase activity, E pectinase activity, and F xylanase activity. The enzyme activities were determined in 50 mM sodium acetate buffer, pH 5.5 at 50 °C. The measurements were done in triplicate
Interestingly, SB acts not only as an inducer but also as an inhibitor of the production of certain enzymes, as the activities of invertase and amylase of several strains were reduced compared to those obtained in the glucose medium. The A. niger TL19 strain was the only one producing invertase in both glucose- and SB-containing media, but the activity was reduced from 70.36 U/mL in the SMGC medium to only 19.89 U/mL in the SMSB medium (Fig. 2D). Additionally, SB inhibited the amylase activity of 18 strains that presented high activity when grown in the SMGC medium. In the SMSB medium, the amylase activity was detected in only 8 strains with a range of 24.29–63.98 U/mL (Fig. 2A). The TL23 strain showed the highest amylase activity at 63.98 U/mL, which was reduced from 138.82 U/mL in the glucose medium. On the other hand, a slight increase in the amylase activity was found in the TL22 strain.
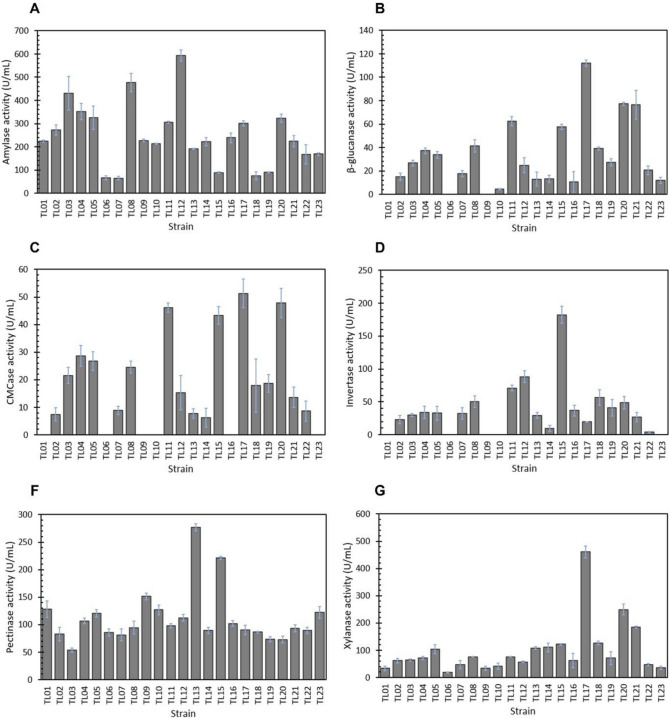
Cassava pulp
Overall, CP strongly induced the expression of all six enzymes tested, especially amylase, pectinase, and invertase (Fig. 3). All 23 strains produced the amylase activity at 64.82–593.37 U/mL with the greatest activity shown by the TL12 strain (Fig. 3A). Interestingly, a slight decrease in amylase activity was observed in the TL10 strain grown in CP (213.27 U/mL) compared to that in the glucose-containing medium (219.98 U/mL). The pectinase activities exhibited by the A. niger strains grown in the SMCP medium were in the range of 53.12–276.97 U/mL, which were substantially higher compared to those observed in glucose- and SB-containing media. The highest pectinase activity was obtained from the TL13 strain (Fig. 3E). The invertase activities were found in all 23 strains of A. niger when grown in CP-containing medium and they tended to increase in most strains when CP was included in the media compared to those observed in SMGC and SMSB media. The activities were in the range of 3.82–182.41 U/mL with the highest activity occurring in fermentation of the TL15 strain. Remarkably, CP inhibited the invertase activity of the TL19 strain, which displayed the activity of only 41.03 U/mL compared to 70.36 U/mL obtained in the SMGC medium. However, its activity in the SMCP medium was still higher than that in the SMSB medium (19.89 U/mL).
Fig. 3.
Profiles of enzyme activities produced by 23 A. niger strains grown on cassava pulp as the carbon source (SMCP medium). A Amylase activity, B β-glucanase activity, C CMCase activity, D invertase activity, E pectinase activity, and F xylanase activity. The enzyme activities were determined in 50 mM sodium acetate buffer, pH 5.5 at 50 °C. The measurements were done in triplicate
With regard to endoglucanase (CMCase) activity after fermentation in the SMCP medium, there were 17 A. niger strains secreting endoglucanase in the range of 7.45–51.28 U/mL (Fig. 2E). Most of these strains showed higher endoglucanase activities than those obtained in SMSB, whereas 3 strains (TL02, TL13, and TL14) showed a relatively low activity level (< 8.63 U/mL) and 6 strains (TL01, TL06, TL09, IL10, TL16, and IL23) showed no detectable activity. The β-glucanase was moderately induced by CP with activities in the range of 4.40–112.16 U/mL in 20 strains (Fig. 2E), but its activity was not found in 3 strains (TL01, TL06, and TL09). The activity of β-glucanase in SMCP was lower than that in SMSB. Likewise, the induction of the xylanase activity in the SMCP medium was weaker than that in the SMSB medium, as the activities of the 23 strains were between 19.10 and 460.78 U/mL. The highest xylanase activity was obtained with the TL17 strain.
Banana peel
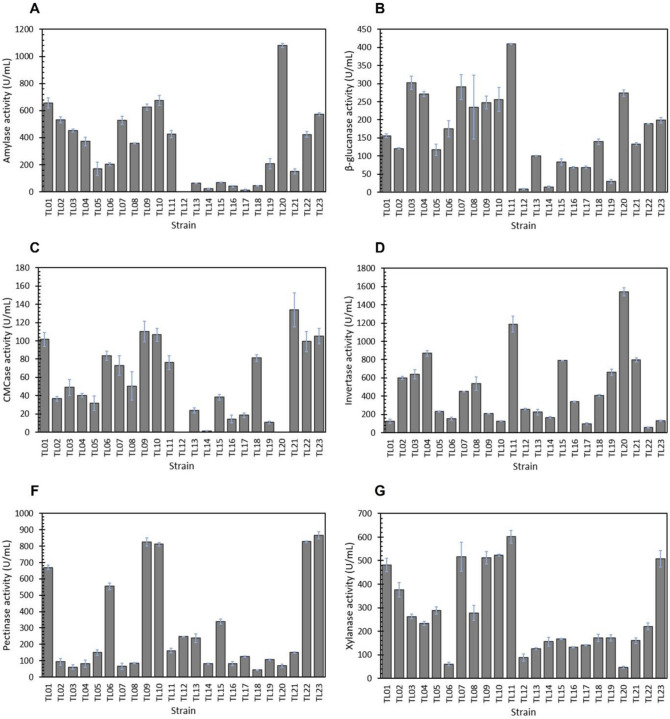
BP contains cellulose, hemicellulose, starch, lignin, and a substantial proportion of pectin. In this study, the use of BP resulted in the induction of all six enzymes in most strains, with the exception of TL12, TL14, and TL20 strains that did not produce certain enzyme activities (Fig. 4). Based on the enzyme activity profiles, BP is considered the best carbon source for the production of invertase, pectinases, endoglucanase, and amylase compared to BG and CP. The invertase activity of 23 strains were in the range of 57.04–1542.66 U/mL (Fig. 4D). The strongest activity was found in the A. niger TL20 strain. Likewise, the pectinase activities were found in the range of 43.79–865.67 U/mL (Fig. 4E). From these 23 strains, 4 strains secreted pectinase with activities over 800 U/mL including the TL23, TL22, TL09, and TL10. In addition, the A. niger grown in SMBP showed a wide range of amylase activity between 11.79 and 1082.52 U/mL, with the highest activity occurring in the TL20 strain (Fig. 4A). Interestingly, no amylase activity was detected from the TL12 strain cultivated in SMBP, while this strain secreted a high amylase activity in SMCP. Both the TL12 and TL20 strains showed no endoglucanase activity in SMBP. Excluding these two strains, BP promoted the endoglucanase activities in 17 strains with the activity range of 10.58–133.90 U/mL (Fig. 4C). In contrast, the endoglucanase activities of the other 4 strains including TL14, TL15, TL17 and TL19 were lower than those obtained in SMCP. BP was also a powerful inducer for β-glucanase and xylanase production with the activity range of 8.09–409.20 U/mL and 46.48–601.59 U/mL, respectively (Fig. 4B and F). The A. niger TL11 strain was found to produce the highest level of β-glucanase and xylanase activities when BP was used as the carbon source. Both enzyme activities obtained in BP were lower than those found in SB-containing medium but higher than those found in CP-containing medium.
Fig. 4.
Profiles of enzyme activities produced by A. niger grown on banana peel as the carbon source (SMBP medium). A Amylase activity, B β-glucanase activity, C CMCase activity, D invertase activity, E pectinase activity, and F xylanase activity. The enzyme activities were determined in 50 mM sodium acetate buffer, pH 5.5 at 50 °C. The measurements were done in triplicate
Discussion
Many studies have shown varying abilities of A. niger strains on the production of various carbohydrate-degrading enzymes. However, those reports mostly focused on only one or a few strains. In this study, a comparative study on enzyme activity profiles of 23 A. niger strains was concurrently and systematically investigated to give a comprehensive picture of enzyme production obtained by submerged fermentation, using three selected agricultural residues (SB, CP, and BP) as carbon sources. These agricultural wastes were chosen for the study because they are major agro-industrial wastes available in Thailand. During fermentation, these wastes acted as both carbon sources and inducers for the production of different polysaccharide-processing enzymes that are highly useful for industrial applications. These enzymes are selected based on the potential of A. niger for the production of these enzymes in industrial processes as demonstrated in various reports (Arnau et al. 2020). The effects of the agricultural wastes on the induced enzyme levels and profiles were shown in this study.
Among the strains tested in this study, the A. niger TL11 strain was suggested as a powerful strain for multi-enzyme production, as it can produce relatively high levels of all targeted enzymes using BP as a sole carbon source. The A. niger TL17 strain was also proposed as an effective strain for the production of the complex non-starch polysaccharide-degrading enzymes comprising CMCase, β-glucanase, and xylanase when either CP or BP was used as a carbon source. Our results suggested that these A. niger strains could provide a crude enzyme mixture that has potential use as an additive for animal feed to enhance animal’s nutrition uptake. Furthermore, the enzyme mixture produced by these selected microbial strains can be effectively utilized for fiber degradation and modification (Kuhad et al. 2011; Graham and Balnave 1995; Lewis et al. 1996). Additionally, the complex non-starch polysaccharide-degrading activities with strong pectinase activity are also advantageous for reducing the viscosity of cassava root mash in bioethanol production by high gravity fermentation (Poonsrisawat et al. 2016) and for facilitating the extraction of starch from root crops (Sriroth et al. 2000; Collares et al. 2012). Our results suggested that the TL09 strain can effectively produce these enzymes and, thus, its use in biofuel and animal feed applications should be encouraged.
Regarding the production of a single dominant enzyme, the A. niger TL07 strain produced a very strong xylanase activity when grown in SMSB, whereas pectinase and β-glucanase were produced at very low levels with no side activity of cellulase, amylase, and invertase. Therefore, this strain can be a potent producer of cellulase-free xylanase for application in various industries including food, animal feed (Harris and Ramalingam 2010), and pulp industries (Kumar 2021). On the other hand, the A. niger TL20 strain produces the highest level of β-glucanase activity when grown in SMSB and should be effectively employed for digestion of feedstuffs and barley gums in feed (Kuhad et al. 2011) and brewing industries (Canal-Llaubères 2010), respectively. Moreover, the A. niger TL20 strain was also highly effective for amylase production, as this strain secreted a relatively high level of amylase activity in every media tested, especially SMBP. Compared to thermophilic amylases from Bacillus origins, the mesophilic fungal amylases derived from A. niger can be utilized in detergent, food, and feed industries (Angelia et al. 2019; Silano et al. 2019; Elyasi Far et al. 2020). Besides amylase and β-glucanase, the TL20 strain also effectively produced invertase when using BP as the carbon source, whereas the TL19 strain is also considered a potent invertase-producing strain especially when cultivated in glucose- or SB-containing media. Therefore, both the TL20 and TL19 stains can play an important role in the production of invert sugars with applications in food, beverage, and pharmaceutical industries (Chaudhary et al. 2015).
The activity levels of target enzymes produced by A. niger in this study were compared to those reported in previous works (Table 3). Overall, the enzyme activities from many of our selected strains were higher or at least comparable to those previously reported. The differences in enzyme induction profiles can be related to the various compositions of the carbon sources as well as to the inherent genetic background of each microbial strain. The high levels of enzyme activities shown in this work suggest that the selected A. niger strains grown in media containing particular agricultural wastes should be immensely favorable for utilization in industrial applications. However, it should be noted that direct comparison on the activity levels may not be straightforward due to variation in enzyme assay reaction set-up, the substrates, the experimental conditions, and fungal strains used in different works.
Table 3.
Enzyme activities from A. niger grown on various carbon sources
| Enzyme | Strain | Carbon source | Enzyme activity (U/mL) | References |
|---|---|---|---|---|
| Amylase | A. niger ANSS-B5 | date waste | 285.60a | Said et al. (2014) |
| A. niger | ragi husk* | 213.00a | Rajan et al. (2019) | |
| A. niger | paddy straw* | 81.60a | Rajan et al. (2019) | |
| A. niger | bagasse* | 111.01a | Rajan et al. (2019) | |
| A. niger TL23 | sugarcane bagasse | 63.98a | This study | |
| A. niger MS23 | banana peel* | 0.23a | Rehman et al. (2015) | |
| A. niger NCIM 616 | banana peel | 23.00a | Paladi et al. (2012) | |
| A. niger TL20 | banana peel | 1082.52a | This study | |
| A. niger TL12 | cassava pulp | 593.37a | This study | |
| A. niger TL10 | Glucose | 219.98a | This study | |
| β-glucanase | A. niger US368 | barley flour | 34.46c | Elgharbi et al. (2013) |
| A. niger TL11 | banana peel | 409.20c | This study | |
| A. niger TL20 | sugarcane bagasse | 1268.17c | This study | |
| A. niger TL17 | cassava pulp | 112.16c | This study | |
| Endo-glucanase | A. niger | coir waste | 8.89b | Mrudula and Murugammal (2011) |
| A. niger | rice husks | 10.90b | Jadhav et al. (2013) | |
| A. niger | wheat bran | 8.90b | Jadhav et al. (2013) | |
| A. niger | banana peel | 11.30b | Jadhav et al. (2013) | |
| A. niger MS23 | banana peel* | 3.91b | Rehman et al. (2015) | |
| A. niger TL21 | banana peel | 133.90b | This study | |
| A. niger TL10 | sugarcane bagasse | 39.60b | This study | |
| A. niger TL17 | cassava pulp | 51.28b | This study | |
| Invertase | A. niger | pineapple peels, potato peels* | 321.40d | Ire et al. (2019) |
| A. niger ANSS-B5 | date waste | 195.56d | Said et al. (2014) | |
| A. niger OSH5 | wheat bran | 9.70d | Al-Hagar et al. (2015) | |
| A. niger OSH5 | rice bran | 5.10d | Al-Hagar et al. (2015) | |
| A. niger OSH5 | oat meal | 7.70d | Al-Hagar et al. (2015) | |
| A. niger OSH5 | sugarcane bagasse | 2.10d | Al-Hagar et al. (2015) | |
| A. niger TL19 | sugarcane bagasse | 19.89d | This study | |
| A. niger TL20 | banana peel | 1542.66d | This study | |
| A. niger TL15 | cassava pulp | 182.41d | This study | |
| A. niger TL19 | Glucose | 70.36d | This study | |
| Pectinase | A. niger NRC1ami | mandarin peel | 76.35e | El Enshasy et al. (2018) |
| A. niger MS23 | banana peel* | 0.40e | Rehman et al. (2015) | |
| A. niger TL23 | banana peel | 865.67e | This study | |
| A. niger TL12 | sugarcane bagasse | 179.26e | This study | |
| A. niger TL13 | cassava pulp | 276.97e | This study | |
| A. niger TL12 | Glucose | 66.34e | This study | |
| Xylanase | A. niger ANL301 | wheat bran | 6.47f | Okafor et al. (2007) |
| A. niger ANL301 | sugarcane pulp | 0.95f | Okafor et al. (2007) | |
| A. niger BCC14405 | wheat bran, rice bran, soybean meal | 89.50 g | Khonzue et al. (2011) | |
| A. niger MS23 | banana peel* | 0.70 g | Rehman et al. (2015) | |
| A. niger TL11 | banana peel | 601.59 h | This study | |
| A. niger TL07 | sugarcane bagasse | 3910.06 h | This study | |
| A. niger TL17 | cassava pulp | 460.78 h | This study | |
| A. niger TL10 | Glucose | 10.52 h | This study |
*SSF; asoluble starch; bCMC; cbarley β-glucan; dsucrose; ecitrus pectin; foat spelt xylan; gbirchwood xylan; hbeechwood xylan
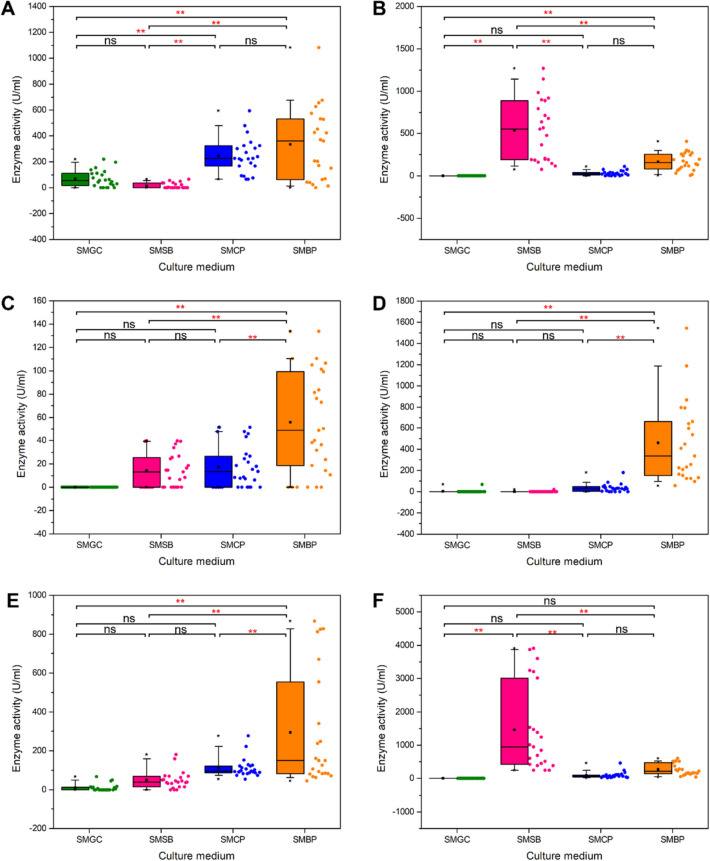
Compositions of the substrates used as carbon sources are shown by many studies to be strongly related to the profiles of induced enzyme activity. Among the various nutrients used in this study (Figs. 5 and 6), glucose was found to allow the production of only some enzymes at relatively basal levels. This agrees well with previous works where glucose was suggested as a metabolic repressor on the production of several carbohydrate-degrading enzymes (Amore et al. 2013). In A. niger, this is due to the repression of xlnR gene promoter by glucose, which resulted in inhibition of these catabolic enzyme activities (Tamayo et al. 2008). On the other hand, most complex carbohydrates do not exert this repression on enzyme expression. Since many agricultural residues contain abundant amounts of complex polysaccharides, using agricultural wastes as carbon sources can be considered advantageous for the production of starch- and non-starch polysaccharide-degrading enzymes. High levels of cellulase and xylanase activities are observed when the fungi were grown on many lignocellulosic wastes including wheat bran, sugarcane bagasse, rice straw, and forest litters (Okafor et al. 2007; Kaushal et al. 2012; Jadhav et al. 2013; El-Morsy et al. 2014; Ferreira et al. 2018). Our work indicates that each agricultural residue can induce a distinct set of enzyme expression. Among the carbon sources tested, BP represents the best substrates for inducing the highest activities of most carbohydrate-degrading enzymes including amylase, CMCase, pectinase (Solís-Pereira et al. 1993), and invertase (Vainstein and Peberdy 1991) (p values < 0.05). The ability to induce expression of various enzymes is thought to correlate with the relatively high overall contents of starch, fibrous, and pectic polysaccharides in BP, even though BP contains lower contents of cellulose and hemicellulose than SB and a lower content of starch than CP. Remarkably, several A. niger strains could produce higher levels of amylase in SMBP than in SMCP, despise the fact that CP has approximately twice as much starch content as BP. Substrates with a high starch content, such as barley flour containing approximately 3 – 5% glucan, can be used for the production of amylase and β-glucanase (Kinner et al. 2011; Zheng et al. 2011). The ability of BP to induce endoglucanase activity from A. niger was also previously reported (Jadhav et al. 2013). This agrees well with our finding that the highest CMCase activity was obtained from the A. niger TL21 strain when grown in BP-containing medium. BP is also the second-most effective substrate for the induction of β-glucanase and xylanase activities. Similarly, several fungal strains showed high levels of secretion of pectinases when grown in media supplementing with pectin as a carbon source (Martos et al. 2009). Rich sources of pectin are mostly found in fruit wastes, such as mandarin peel and banana peel, which contain 22.9% and 15.9% of pectin, respectively (Sandhu et al. 2011; Oberoi et al. 2012). The mandarin peel supported the production of pectinase in A. niger NRC1ami at 76.35 U/mL (El Enshasy et al. 2018). Our study also showed that BP, which is rich in pectin, could cause high levels of pectinase production by several A. niger strains. When the A. niger strains were grown in SB, which represents a common lignocellulosic waste, the highest levels of β-glucanase and xylanase activities (p values < 0.05) with moderate levels of cellulase activity were achieved. On the other hand, SB is not an effective substrate for the induction of pectinase activity. These results can be owing to SB’s high cellulose and xylan contents but low pectin content compared to BP and CP (Djuma’ali et al. 2011; Oberoi et al. 2012; Castillo-Israel et al. 2015; Yadav et al. 2015). Compared to BP and SB, CP can moderately induce most enzymes and it constitutes the 2nd-best substrate for amylase, CMCase, and pectinase productions and the 3rd-best substrate for β-glucanase, invertase, and xylanase productions. Our results showed that CP can induce a significantly higher production of amylase than SB (p-values < 0.05). Its efficiency in enhancing amylase, CMCase, and pectinase activities is correlated to CP’s high levels of starch, cellulose, and pectin contents. In contrast, its minimal ability to induce xylanase activity would reflect its low hemicellulose content.
Fig. 5.
Relative enzyme activities of A. niger grown on various carbon sources. GC stands for glucose, whereas SB, CP, and BP represent sugarcane bagasse, cassava pulp, and banana peel, respectively
Fig. 6.
Box plot of enzyme activities produced by A. niger strains grown in media containing various carbon sources. The data include A amylase, B, β-glucanase, C CMCase, D invertase, E pectinase, and F xylanase. Each box plot displays medians (50th percentile), 5th, 25th, 75th and 95th percentiles. Asterisks indicate significant differences (p values ≤ 0.05) and ns represents not significant
Induction of expression of polysaccharide-degrading enzymes in A. niger was reported to be triggered by small molecules derived from the large-MW polysaccharides. It is hypothesized that, due to its insolubility in water, cellulose cannot directly stimulate the expression of lignocellulose-degrading enzymes (Fang et al. 2008; Liao et al. 2014). On the contrary, small soluble saccharides, such as cellobiose, sophorose, lactose, sorbose, and galactose have been demonstrated to be good inducers for cellulase biosynthesis (Morikawa et al. 1995; Karaffa et al. 2006). Similarly, the xylanase activity can be induced by xylose and xylooligosaccharide (XOS) (Gong et al. 2018). Another point of interest is the similar trends of xylanase and β-glucanase expression by most fungal strains observed under various fermentation conditions in our study. Co-regulation of cellulase and xylanase has been thought to depend on (hemi-)cellulolytic regulator XlnR (Xlr1/Xyr1) (Hasper et al. 2000; Tani et al. 2012; Klaubauf et al. 2014). In contrast, there is no report on co-regulation between cellulase and β-glucanase. Pectinase activity, particularly endo-galacturonase, was induced in the presence of pectin using fruit peels as carbon sources (Dartora et al. 2002; Eze et al. 2014; Ahmed et al. 2016). Compared to plant cell wall-degrading enzymes, amylase was regulated by transcriptional activator AmyR as well as the hydrolysis products of starch including maltose and isomaltose (Murakoshi et al. 2012; van Kuyk et al. 2012; Dojnov et al. 2015). Sucrose and fructose were reported to be more effective inducers than glucose for invertase production by A. niger MTCC 282 (Raju et al. 2016). Similarly, our work showed that invertase activity was mostly absent when SB was used as the substrate. The TL19 strain is the only strain that showed induction of invertase activity in glucose- and SB-containing media. Other studies suggested the effectiveness of fruit wastes containing free sugars, including pineapple peel and date wastes, on induction of invertase (Ire et al. 2019; Said et al. 2014). The generation of these small sugar molecules acting as inducers were related to the basal activities of these catabolic enzymes in the absence of the polysaccharides which can function on releasing these inducer molecules when the complex polysaccharide substrates are available (El-Gogary et al. 1989; Aro et al. 2005; Kubicek et al. 2009; Murakoshi et al. 2012; Gao et al. 2017).
Conclusion
In this study, the comparative study on the effects of agricultural wastes with varied chemical compositions on the production of an array of carbohydrate-processing enzymes by different A. niger strains has been investigated. Different agricultural wastes were found to significantly affect enzyme profiles and activity levels, which also depended on the inherent capability of the fungal strains. The work provides insights into the selection of substrates for enzyme production and warrants further investigation on the relevant genetic background of the strains for improving their capability to produce carbohydrate-processing enzymes for industrial applications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financial supported by National Science and Technology Development Agency (P-18-52705). We thank to Cassava and starch research unit (CSTRU), BIOTEC for analyzing the chemical compositions, Dr. Marisa Raita for providing pretreated sugarcane bagasse, and Dr. Pattanop Kanokratana and Ms. Katewadee Boonyapakron for technical support on a customized high throughput automated enzyme screening platform. The authors would like to thank Dr. Piyanun Harnpicharnchai for manuscript proofreading and comments.
Author contributions
TL and VC conceived and designed the work. TL performed the experiments, analyzed and interpreted the data. BB analyzed and interpreted the data. TL drafted the manuscript. TL and VC critically revised the manuscript for intellectual content. All authors have read and agree to the submission of the manuscript.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abarca ML, Accensi F, Cano J, Cabañes FJ. Taxonomy and significance of black aspergilli. Antonie Van Leeuwenhoek. 2004;86(1):33–49. doi: 10.1023/B:ANTO.0000024907.85688.05. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Zia MA, Hussain MA, Akram Z, Naveed MT, Nowrouzi A. Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J Radiat Res Appl Sci. 2016;9(2):148–154. [Google Scholar]
- Ajijolakewu AK, Leh CP, Wan Abdullah WN, Ck L. Optimization of production conditions for xylanase production by newly isolated strain Aspergillus niger through solid state fermentation of oil palm empty fruit bunches. Biocatal Agric Biotechnol. 2017;11:239–247. [Google Scholar]
- Akula S, Golla N. Optimization of cellulase production by Aspergillus niger isolated from forest soil. Open Biotechnol J. 2018;2018:12. [Google Scholar]
- Al-Hagar OEA, Ahmed AS, Hassan IA. Invertase production by irradiated Aspergillus niger OSH5 Using agricultural wastes as carbon source. Br Microbiol Res J. 2015;6(3):135–146. [Google Scholar]
- Amore A, Giacobbe S, Faraco V. Regulation of cellulase and hemicellulase gene expression in fungi. Curr Genomics. 2013;14(4):230–249. doi: 10.2174/1389202911314040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelia C, Sanjaya A, Aida A, Tanudjaja E, Victor H, Cahyani A, Tan TJ, Pinontoan R. Characterization of alpha-amylase from Aspergillus niger aggregate F isolated from a fermented cassava gatot grown in potato peel waste medium. Microbiol Biotechnol Lett. 2019;47:364–371. [Google Scholar]
- AOAC (1990) Official methods of analysis. 15th edn. In: Association of Official Analytical Chemists, Arlington, VA
- Arentshorst M, Ram AF, Meyer V. Using non-homologous end-joining-deficient strains for functional gene analyses in filamentous fungi. Methods Mol Biol. 2012;835:133–150. doi: 10.1007/978-1-61779-501-5_9. [DOI] [PubMed] [Google Scholar]
- Arnau J, Yaver D, Hjort CM. Strategies and challenges for the development of industrial enzymes using fungal cell factories. In: Nevalainen H, editor. Grand challenges in fungal biotechnology. Cham: Springer International Publishing; 2020. pp. 179–210. [Google Scholar]
- Aro N, Pakula T, Penttilä M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev. 2005;29(4):719–739. doi: 10.1016/j.femsre.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Kumar B, Verma P. A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess. 2019;6(1):40. [Google Scholar]
- Canal-Llaubères RM. 4—enzymes and wine quality. In: Reynolds AG, editor. Managing wine quality. Sawston: Woodhead Publishing; 2010. pp. 93–132. [Google Scholar]
- Castillo-Israel KAT, Baguio SF, Diasanta MDB, Lizardo RCM, Dizon EI, Mejico MIF. Extraction and characterization of pectin from Saba banana [Musa 'saba'(Musa acuminata x Musa balbisiana)] peel wastes: a preliminary study. Int Food Res J. 2015;22(1):202–207. [Google Scholar]
- Chaudhary S, Sagar S, Kumar M, Sengar R, Tomar A (2015) The use of enzymes in food processing: a review. SAJFTE 01
- Chotirotsukon C, Raita M, Yamada M, et al. Sequential fractionation of sugarcane bagasse using liquid hot water and formic acid-catalyzed glycerol-based organosolv with solvent recycling. Bioenergy Res. 2021;14(1):135–152. [Google Scholar]
- Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N. Lignocellulolytic enzymes in biotechnological and iIndustrial processes: a review. Sustainability. 2020;12(18):7282. [Google Scholar]
- Collares RM, Miklasevicius LVS, Bassaco MM, et al. Optimization of enzymatic hydrolysis of cassava to obtain fermentable sugars. J Zhejiang Univ Sci B. 2012;13(7):579–586. doi: 10.1631/jzus.B1100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartora AB, Bertolin TE, Bilibio D, Silveira MM, Costa JA. Evaluation of filamentous fungi and inducers for the production of endo-polygalacturonase by solid state fermentation. Z Naturforsch C J Biosci. 2002;57(7–8):666–670. doi: 10.1515/znc-2002-7-820. [DOI] [PubMed] [Google Scholar]
- de Souza PM, de Oliveira MP. Application of microbial α-amylase in industry—a review. Braz J Microbiol. 2010;41(4):850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuma’ali D, Nonot S, Sumarno S, Dyah P, Wahono S. Cassava pulp as a biofuel feedstock of an enzymatic hydrolysis process. Makara J Technol. 2011;15:2. [Google Scholar]
- Dojnov B, Grujic M, Percevic B, Vujčić Z. Enhancement of amylase production using carbohydrates mixtures from triticale in Aspergillus sp. J Serbian Chem Soc. 2015;80:43–43. [Google Scholar]
- Ejaz U, Sohail M, Ghanemi A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics. 2021;6(3):44. doi: 10.3390/biomimetics6030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Enshasy HA, Elsayed EA, Suhaimi N, Malek RA, Esawy M. Bioprocess optimization for pectinase production using Aspergillus niger in a submerged cultivation system. BMC Biotechnol. 2018;18(1):71. doi: 10.1186/s12896-018-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgharbi F, Hmida-Sayari A, Sahnoun M, Kammoun R, Jlaeil L, Hassairi H, Bejar S. Purification and biochemical characterization of a novel thermostable lichenase from Aspergillus niger US368. Carbohydr Polym. 2013;98(1):967–975. doi: 10.1016/j.carbpol.2013.07.009. [DOI] [PubMed] [Google Scholar]
- El-Gogary S, Leite A, Crivellaro O, Eveleigh DE, el-Dorry H. Mechanism by which cellulose triggers cellobiohydrolase I gene expression in Trichoderma reesei. Proc Natl Acad Sci USA. 1989;86(16):6138–6141. doi: 10.1073/pnas.86.16.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Morsy E-S, Khattab S, Ziada M, Mohesien M. Xylanase, cellulase and protease enzymes production by fungi in rice straw medium under submerged fermentation. Sci J Damietta Faculty Sci. 2014;3:111–119. [Google Scholar]
- Elyasi Far B, Ahmadi Y, Yari Khosroshahi A, Dilmaghani A. Microbial alpha-amylase production: progress, challenges and perspectives. Adv Pharm Bull. 2020;10(3):350–358. doi: 10.34172/apb.2020.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze SO, Ezike T, Nsude C, Chilaka F. Production of pectinases from Aspergillus niger using submerged fermentation with orange peels as carbon source. Sylwan. 2014;158:434–440. [Google Scholar]
- Fang X, Yano S, Inoue H, Sawayama S. Lactose enhances cellulase production by the filamentous fungus Acremonium cellulolyticus. J Biosci Bioeng. 2008;106(2):115–120. doi: 10.1263/jbb.106.115. [DOI] [PubMed] [Google Scholar]
- Ferreira FL, Dall'Antonia CB, Shiga EA, Alvim LJ, Pessoni RAB. Sugarcane bagasse as a source of carbon for enzyme production by filamentous fungi. Hoehnea. 2018;45:134–142. [Google Scholar]
- Gao J, Qian Y, Wang Y, Qu Y, Zhong Y. Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei. Biotechnol Biofuels. 2017;10(1):272. doi: 10.1186/s13068-017-0963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Dai L, Zhang H, Zhang L, Wang L. A highly efficient xylan-utilization system in Aspergillus niger An76: a functional-proteomics study. Front Microbiol. 2018;9:430. doi: 10.3389/fmicb.2018.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H, Balnave D (1995) Dietary enzymes for increasing energy availability. In: Biotechnology in animal feeds and animal feeding, pp 295–309
- Gupta A, Gupta VK, Modi DR, Yadava LP. Production and characterization of α-amylase from Aspergillus niger. Biotechnology. 2008;7(3):551–556. [Google Scholar]
- Harris AD, Ramalingam C. Xylanases and its application in food industry: a review. J Exp Sci. 2010;1(7):1–11. [Google Scholar]
- Hasper AA, Visser J, De Graaff LH. The Aspergillus niger transcriptional activator XlnR, which is involved in the degradation of the polysaccharides xylan and cellulose, also regulates d-xylose reductase gene expression. Mol Microbiol. 2000;36(1):193–200. doi: 10.1046/j.1365-2958.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- Hmida-Sayari A, Taktek S, Elgharbi F, Bejar S. Biochemical characterization, cloning and molecular modeling of a detergent and organic solvent-stable family 11 xylanase from the newly isolated Aspergillus niger US368 strain. Process Biochem. 2012;47(12):1839–1847. [Google Scholar]
- Ire F, Aguguo V, Ezebuiro V. Optimization of invertase from Aspergillus niger grown on low cost agricultural wastes by Response Surface Methodology (RSM) J Adv Microbiol. 2019;14(4):1–15. [Google Scholar]
- Izmirlioglu G, Demirci A. Strain selection and medium optimization for glucoamylase production from industrial potato waste by Aspergillus niger. J Sci Food Agric. 2016;96(8):2788–2795. doi: 10.1002/jsfa.7445. [DOI] [PubMed] [Google Scholar]
- Jadhav AR, Girde AV, More SM, More SB, Khan S. Cellulase production by utilizing agricultural wastes. Res J Agric for Sci. 2013;1:6–9. [Google Scholar]
- Karaffa L, Fekete E, Gamauf C, Szentirmai A, Kubicek CP, Seiboth B. D-galactose induces cellulase gene expression in Hypocrea jecorina at low growth rates. Microbiol (read Engl) 2006;152(Pt 5):1507–1514. doi: 10.1099/mic.0.28719-0. [DOI] [PubMed] [Google Scholar]
- Kaushal R, Sharma N, Tandon D. Cellulase and xylanase production by co-culture of Aspergillus niger and Fusarium oxysporum utilizing forest waste. Turk J Biochem. 2012;37:35–41. [Google Scholar]
- Kenealy WR, Jeffries TW (2003) Enzyme processes for pulp and paper: a review of recent developments. In: Goodell B, Nicholas DD, Schultz TP (eds) Wood deterioration and preservation: advances in our changing world. American Chemical Society, San Diego, California
- Khonzue P, Laothanachareon T, Rattanaphan N, et al. Optimization of xylanase production from Aspergillus niger for biobleaching of eucalyptus pulp. Biosci Biotechnol Biochem. 2011;75(6):1129–1134. doi: 10.1271/bbb.110032. [DOI] [PubMed] [Google Scholar]
- Kinner M, Nitschko S, Sommeregger J, et al. Naked barley-optimized recipe for pure barley bread with sufficient beta-glucan according to the EFSA health claims. J Cereal Sci. 2011;53(2):225–230. doi: 10.1016/j.jcs.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk TK, Jeffries TW (1996) Roles for microbial enzymes in pulp and paper processing. In: Enzymes for pulp and paper processing, vol 655. ACS Symposium Series. American Chemical Society, pp 2–14.
- Klaubauf S, Narang HM, Post H, et al. Similar is not the same: differences in the function of the (hemi-)cellulolytic regulator XlnR (Xlr1/Xyr1) in filamentous fungi. Fungal Genet Biol. 2014;72:73–81. doi: 10.1016/j.fgb.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Krijgsheld P, Altelaar AFM, Post H, Ringrose JH, Müller WH, Heck AJR, Wösten HAB. Spatially resolving the secretome within the mycelium of the cell factory Aspergillus niger. J Proteome Res. 2012;11(5):2807–2818. doi: 10.1021/pr201157b. [DOI] [PubMed] [Google Scholar]
- Kringel DH, Dias ARG, Zavareze EdR, Gandra EA. Fruit wastes as promising sources of starch: extraction, properties, and applications. Starch Stärke. 2020;72(3–4):1900200. [Google Scholar]
- Kubicek CP, Mikus M, Schuster A, Schmoll M, Seiboth B. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol Biofuels. 2009;2(1):19. doi: 10.1186/1754-6834-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhad RC, Gupta R, Singh A. Microbial cellulases and their industrial applications. Enzyme Res. 2011;2011:280696. doi: 10.4061/2011/280696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. Biobleaching: an eco-friendly approach to reduce chemical consumption and pollutants generation. Phys Sci Rev. 2021;6:4. [Google Scholar]
- Ladeira Ázar RIS, Bordignon-Junior SE, Laufer C, Specht J, Ferrier D, Kim D. Effect of lignin content on cellulolytic saccharification of liquid hot water pretreated sugarcane bagasse. Molecules. 2020;25(3):623. doi: 10.3390/molecules25030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GE, Hunt CW, Sanchez WK, Treacher R, Pritchard GT, Feng P. Effect of direct-fed fibrolytic enzymes on the digestive characteristics of a forage-based diet fed to beef steers. J Anim Sci. 1996;74(12):3020–3028. doi: 10.2527/1996.74123020x. [DOI] [PubMed] [Google Scholar]
- Li C, Zhou J, Du G, Chen J, Takahashi S. Developing Aspergillus niger as a cell factory for food enzyme production. Biotechnol Adv. 2020;44:107630. doi: 10.1016/j.biotechadv.2020.107630. [DOI] [PubMed] [Google Scholar]
- Liao H, Li S, Wei Z, Shen Q, Xu Y. Insights into high-efficiency lignocellulolytic enzyme production by Penicillium oxalicum GZ-2 induced by a complex substrate. Biotechnol Biofuels. 2014;7(1):162. doi: 10.1186/s13068-014-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisha YSK. Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresour Technol. 2017;245:1727–1739. doi: 10.1016/j.biortech.2017.05.066. [DOI] [PubMed] [Google Scholar]
- Martos M, Vazquez F, Benassi F. Production of pectinases by A. niger: influence of fermentation conditions. Braz Arch Biol Technol. 2009;2009:52. [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. [Google Scholar]
- Morikawa Y, Ohashi T, Mantani O, Okada H. Cellulase induction by lactose in Trichoderma reesei PC-3-7. Appl Microbiol Biotechnol. 1995;44(1):106–111. [Google Scholar]
- Mrudula S, Murugammal R. Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz J Microbiol. 2011;42:1119–1127. doi: 10.1590/S1517-838220110003000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi Y, Makita T, Kato M, Kobayashi T. Comparison and characterization of α-amylase inducers in Aspergillus nidulans based on nuclear localization of AmyR. Appl Microbiol Biotechnol. 2012;94(6):1629–1635. doi: 10.1007/s00253-012-3874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberoi HS, Sandhu SK, Vadlani PV. Statistical optimization of hydrolysis process for banana peels using cellulolytic and pectinolytic enzymes. Food Bioprod Process. 2012;90(2):257–265. [Google Scholar]
- Okafor UA, Okochi VI, Onyegeme-okerenta BM, Nwodo-Chinedu S. Xylanase production by Aspergillus niger ANL 301 using agro-wastes. Afr J Biotechnol. 2007;6(14):1710–1714. [Google Scholar]
- Orozco RS, Hernández PB, Morales GR, et al. Characterization of lignocellulosic fruit waste as an alternative feedstock for bioethanol production. Bioresour Technol. 2014;9(2):1873–1885. [Google Scholar]
- Paladi RK, Srivastava A, Ramaswamy N, Penna S, D'Souza S. Banana peel as substrate for α-amylase production using Aspergillus niger NCIM 616 and process optimization. Indian J Biotechnol. 2012;11:314–319. [Google Scholar]
- Ponnuswamy V, Devi L, Vincent S. Bio-Processing of banana peel for amylase production by Penicillium sp. Asian J Exp Biol Sci. 2011;2:257–264. [Google Scholar]
- Poonsrisawat A, Wanlapatit S, Wansuksri R, et al. Synergistic effects of cell wall degrading enzymes on rheology of cassava root mash. Process Biochem. 2016;51(12):2104–2111. [Google Scholar]
- Rajan C, Grover S, Biswas R, et al. Amylase production by Aspergillus niger using agroindustrial residues under temperature mediated solid state fermentation. Enliven Microbes Microbial Tech. 2019;6:1. [Google Scholar]
- Raju A-C, Pulipati K, Jetti A. Production of invertase by Aspergillus niger under solid state fermentation using orange fruit peel as substrate. Adv Crop Sci Tech. 2016;4:6. [Google Scholar]
- Rehman S, Aslam H, Ahmad A, Khan SA, Sohail M. Production of plant cell wall degrading enzymes by monoculture and co-culture of Aspergillus niger and Aspergillus terreus under SSF of banana peels. Braz J Microbiol. 2015;45(4):1485–1492. doi: 10.1590/s1517-83822014000400045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Gomez S, Augur C, Viniegra-González G. Invertase production by Aspergillus niger in submerged solid-state fermentation. Biotechnol Lett. 2000;22:1255–1258. [Google Scholar]
- Said A, LeilaKaouther AD, Sadia B. Date wastes as substrate for the production of α-amylase and invertase. Iran J Biotechnol. 2014;12(3):41–49. [Google Scholar]
- Sandhu SK, Oberoi HS, Dhaliwal SS, Babbar N, Kaur U, Nanda D, Kumar D. Ethanol production from Kinnow mandarin (Citrus reticulata) peels via simultaneous saccharification and fermentation using crude enzyme produced by Aspergillus oryzae and the thermotolerant Pichia kudriavzevii strain. Ann Microbiol. 2011;62:655–666. [Google Scholar]
- Satapathy S, Rout JR, Kerry RG, Thatoi H, Sahoo SL. Biochemical prospects of various microbial pectinase and pectin: an approachable concept in pharmaceutical bioprocessing. Front Nutr. 2020;7:117. doi: 10.3389/fnut.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran S, Mandhan RP, Dhiman S, Kumar R, Sharma J. Potential application of alkaline pectinase from Bacillus subtilis SS in pulp and paper industry. Appl Biochem Biotechnol. 2008;149:287–293. doi: 10.1007/s12010-007-8096-9. [DOI] [PubMed] [Google Scholar]
- Shindia AA, Khalaf SA, Yassin MA. Production and partial characterization of β- glucanase from Aspergillus niger JQ1516491 under submerged and solid state fermentation. Asian J Microbiol Biotechnol Environ Sci. 2013;15:459–472. [Google Scholar]
- Silano V, Barat Baviera JM, Bolognesi C, et al. Safety evaluation of the food enzyme alpha-amylase from non-genetically modified Aspergillus niger strain (strain DP-Azb60) EFSA J. 2019;17(5):e05680. doi: 10.2903/j.efsa.2019.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva VFN, Arruda PV, Felipe MGA, Gonçalves AR, Rocha GJM. Fermentation of cellulosic hydrolysates obtained by enzymatic saccharification of sugarcane bagasse pretreated by hydrothermal processing. J Ind Microbiol Biotechnol. 2011;38(7):809–817. doi: 10.1007/s10295-010-0815-5. [DOI] [PubMed] [Google Scholar]
- Slivinski CT, Machado AVL, Iulek J, Ayub RA, Almeida MMd. Biochemical characterisation of a glucoamylase from Aspergillus niger produced by solid-state fermentation. Braz Arch Biol Technol. 2011;54:559–568. [Google Scholar]
- Solís-Pereira S, Favela-Torres E, Viniegra-González G, Gutiérrez-Rojas M. Effects of different carbon sources on the synthesis of pectinase by Aspergillus niger in submerged and solid state fermentations. Appl Microbiol Biotechnol. 1993;39(1):36–41. [Google Scholar]
- Sriroth K, Chollakup R, Chotineeranat S, Piyachomkwan K, Oates CG. Processing of cassava waste for improved biomass utilization. Bioresour Technol. 2000;71(1):63–69. [Google Scholar]
- Sun H, Li J, Zhao P, Peng M. Banana peel: A novel substrate for cellulase production under solid-state fermentation. Afr J Biotechnol. 2011;10:17887–17890. [Google Scholar]
- Tamayo EN, Villanueva A, Hasper AA, de Graaff LH, Ramón D, Orejas M. CreA mediates repression of the regulatory gene xlnR which controls the production of xylanolytic enzymes in Aspergillus nidulans. Fungal Genet Biol. 2008;45(6):984–993. doi: 10.1016/j.fgb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tani S, Kanamasa S, Sumitani J, Arai M, Kawaguchi T. XlnR-independent signaling pathway regulates both cellulase and xylanase genes in response to cellobiose in Aspergillus aculeatus. Curr Genet. 2012;58(2):93–104. doi: 10.1007/s00294-012-0367-5. [DOI] [PubMed] [Google Scholar]
- Vainstein MH, Peberdy JF. Regulation of invertase in Aspergillus nidulans: effect of different carbon sources. J Gen Microbiol. 1991;137(2):315–321. doi: 10.1099/00221287-137-2-315. [DOI] [PubMed] [Google Scholar]
- van den Brink J, de Vries RP. Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol. 2011;91(6):1477–1492. doi: 10.1007/s00253-011-3473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuyk PA, Benen JAE, Wösten HAB, Visser J, de Vries RP. A broader role for AmyR in Aspergillus niger: regulation of the utilisation of D-glucose or D-galactose containing oligo- and polysaccharides. Appl Microbiol Biotechnol. 2012;93(1):285–293. doi: 10.1007/s00253-011-3550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekanand V, Dwivedi P, Pareek N, Singh RP. Banana peel: a potential substrate for laccase production by Aspergillus fumigatus VkJ2.4.5 in solid-state fermentation. Appl Biochem Biotechnol. 2011;165(1):204. doi: 10.1007/s12010-011-9244-9. [DOI] [PubMed] [Google Scholar]
- Wachirasiri P, Julakarangka S, Wanlapa S. The effects of banana peel preparations on the properties of banana peel dietary fibre concentrate. Songklanakarin J Sci Technol. 2009;31:605–611. [Google Scholar]
- Yadav S, Gupta G, Bhatnagar R. A review on composition and properties of bagasse fibers. Int J Sci Eng Res. 2015;6:5. [Google Scholar]
- Yu Q, Zhuang X, Lv S, He M, Zhang Y, Yuan Z, Qi W, Wang Q, Wang W, Tan X. Liquid hot water pretreatment of sugarcane bagasse and its comparison with chemical pretreatment methods for the sugar recovery and structural changes. Bioresour Technol. 2013;129:592–598. doi: 10.1016/j.biortech.2012.11.099. [DOI] [PubMed] [Google Scholar]
- Zehra M, Syed MN, Sohail M. Banana peels: A promising substrate for the coproduction of pectinase and xylanase from Aspergillus fumigatus MS16. Pol J Microbiol. 2020;69(1):19–26. doi: 10.33073/pjm-2020-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Li L, Wang X. Molecular characterization of arabinoxylans from hull-less barley milling fractions. Molecules. 2011;16(4):2743–2753. doi: 10.3390/molecules16042743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.