Summary
Background
Biologics directed against the T-helper (Th)-17 pathway have been approved for several inflammatory diseases. Interleukin (IL)-17 is involved in anti-Candida host defense, and clinical trials suggested increased candidiasis incidence during IL-17 inhibitor therapy. We describe the worldwide epidemiology of candidiasis during Th17 inhibitor therapy, and immunological mechanisms involved in candidiasis susceptibility.
Methods
A comprehensive analysis of multiple independent sources reporting Candida adverse events during biologics inhibiting the Th17 pathway was performed. Association between Th17 inhibitors and candidiasis was assessed using safety reports of (1) WHO and (2) EMA, (3) a population-based prescriptions registry, and (4) a psoriasis cohort. In a cohort of psoriasis patients experiencing candidiasis during Th17 inhibitors, Candida killing by immune cells and serum inflammatory proteome were analyzed.
Findings
A strong association between IL-17 inhibitors and candidiasis (ROR 10·20) was found in the WHO database, particularly for cutaneous (ROR 12·28), oropharyngeal (ROR 19·18), and esophageal candidiasis (ROR 21·20). Risk was higher relative to TNF-α inhibitors (4–10-fold, depending on candidiasis type), confirmed by EMA reports (16–33-fold), prescriptions registry (2–42-fold), and a psoriasis cohort (3–25-fold). After start of IL-17 inhibitors, patients’ risk of candidiasis requiring antifungals increased 2–16 fold. In the psoriasis cohort, 58% of IL-17 treatment episodes were associated with candidiasis. In Th17 inhibitor recipients, proteins involved in anti-Candida immunity and Candida killing by mononuclear leukocytes were impaired.
Interpretation
IL-17 inhibitors are associated with an increased risk of oropharyngeal, esophageal, and cutaneous candidiasis, posing a significant disease burden for IL-17 inhibitor recipients.
Funding
RadboudUMC.
Keywords: Candidiasis, IL-17 inhibitors, IL-12/23 inhibitors, Drug safety, Pharmacovigilance, ustekinumab, secukinumab, ixekizumab
Research in context.
Evidence before this study
We searched Pubmed for published research articles on Candida adverse events during Th17 inhibitor therapy in May 2016. We have used the search terms ‘’candidiasis’’, Candida infection’’, ‘’ustekinumab’’, ‘’guselkumab’’, ‘’secukinumab’’, ‘’ixekizumab’’, ‘’brodalumab’’, ‘’IL-17 inhibitors’’, anti-IL17 monoclonal antibodies’’, ‘’IL12/23 inhibitors’’, and ‘’anti-IL12/23 monoclonal antibodies’’. All articles found described data from pre-authorization clinical trials. Publications of all major phase 2 and 3 clinical trials and pooled safety analyses of IL-17 inhibitors (secukinumab, ixekizumab, brodalumab), and IL-12/23 inhibitors (IL-12/23p40 (ustekinumab), IL-23p19 (guselkumab)) were studied. Studies reporting on Candida adverse events during the trial were included. Candidiasis was reported in 4.0- 6.5% of patients treated with brodalumab, in 1.7-4.7% of patients treated with secukinumab, and in 3.3-3.6% of patients treated with ixekizumab. A pooled safety analysis of 10 phase 2 and 3 clinical studies of secukinumab showed a skin/mucosal candidiasis incidence rate of 3.55 per 100 subject years for secukinumab 300 mg and of 1.85 per 100 subject years for secukinumab 150 mg. No mention of Candida adverse events was made in clinical trial publications of ustekinumab and guselkumab.
Added value of this study
To our knowledge, our global pharmacovigilance study of multiple independent sources is the first to describe the epidemiology of Candida adverse events during ustekinumab, secukinumab, ixekizumab and brodalumab treatment in the real world. To put results in a broader perspective, TNF-α inhibitors served as a reference group for all epidemiological substudies. We found that treatment with IL-17 inhibitors is associated with an increased risk of candidiasis, especially oropharyngeal and esophageal candidiasis. Risk of IL-12/23 inhibitors is comparable to the risk of TNF-α inhibitors. Furthermore, we provide a first insight into the immunological mechanisms involved in candidiasis susceptibility in Th17 inhibitor recipients who developed candidiasis during therapy. We found an impaired Candida killing by mononuclear leukocytes, and targeted serum proteomics of inflammatory factors involved in host defense displayed nine significantly downregulated proteins.
Implications of all the available evidence
Oropharyngeal and esophageal candidiasis pose a significant disease burden in IL-17 inhibitor recipients. This suggests that close monitoring of IL-17 inhibitor recipients for candidiasis is advisable, and antifungal prophylaxis may be considered for patients with recurrent or chronic candidiasis. Future studies may identify additional predisposing host factors amenable for screening of anti-IL17 candidates, to guide antifungal prophylaxis or anti-Candida vaccination (currently in phase 2 studies) in the future.
Alt-text: Unlabelled box
Introduction
Biologics inhibiting the T-helper (Th)-17 pathway have been approved for plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis,1, 2, 3, 4, 5, 6, 7, 8 and are currently under investigation for rheumatoid arthritis.9 This group of biologics include IL-17 inhibitors (secukinumab, ixekizumab, brodalumab), and IL-12/23 inhibitors (IL-12/23p40 (ustekinumab), IL-23p19 (guselkumab)).
Interleukin (IL)-17 is essential in the host defense against mucocutaneous candidiasis, inducing neutrophil chemotaxis and antimicrobial peptide activity.10,11 Patients with inborn defects in IL-17 immunity display severe and chronic skin and mucosal candidiasis.12, 13, 14 Mucocutaneous candidiasis as complication of IL-17 inhibitors has been described in several clinical trials.15, 16, 17 Candidiasis was reported in 6·5% of patients treated with brodalumab,16 4·7% with secukinumab,18 and 3·6% with ixekizumab.19 In a pooled safety analysis of ten clinical trials, a skin/mucosal candidiasis incidence rate of 3·55 per 100 person-years was found for secukinumab (300 mg), compared to 1·37 for etanercept.15 A recent trial of the new IL-17 inhibitor bimekizumab reported oropharyngeal candidiasis in 15% of patients, versus 1% for ustekinumab.20
Here, we describe the real-world epidemiology of candidiasis in Th17 inhibitor recipients by a comprehensive analysis of multiple independent sources worldwide, including the World Health Organization (WHO) and European Medicines Agency's (EMA) adverse drug reaction databases, combined with a population-based drug prescriptions registry, and a psoriasis cohort. Furthermore, we provide insights into immunological mechanisms involved in candidiasis susceptibility.
Methods
WHO
The WHO global database of individual case safety reports (ICSR), Vigibase®, contains all ICSR of national pharmacovigilance centers of 131 member countries of the WHO Programme for International Drug Monitoring.21,22 All ICSR available in the database on January 2nd, 2018 containing a MedDRA (Medical Dictionary for Regulatory Activities) term possibly codifying for Candida infection and a WHO-Drug term codifying for secukinumab, ixekizumab, ustekinumab, etanercept, adalimumab, or infliximab, listed as the suspected drug by the reporter, were extracted by a data scientist at the Uppsala Monitoring Centre.23,24 The MedDRA terms were classified along anatomical site and probability (proven, probable, or possible) of candidiasis (eTable 1).
EMA
EMA assesses pre-authorization clinical trials before approving new drugs to the market. European public assessment reports (EPAR), contain these clinical trial safety sets.25 Post-authorization, EMA receives confidential periodic safety update reports (PSUR) from companies’ safety databases. PSUR contain serious adverse events (SAE) from clinical trials, company-initiated and sponsored post-authorization safety studies, and spontaneously reported adverse drug reactions (ADR). Candida adverse events (AE) and ADR were collected from the PSUR for secukinumab, ixekizumab, infliximab, etanercept, and adalimumab, using the categorization of MedDRA terms along anatomical site and probability (eTable 1). The numbers of patients in post-authorization clinical trials and estimates of the person-years of exposure during post-authorization use were extracted from PSUR, and used for incidence proportion (clinical trials) and event rate (ER) (commercial use) calculations (eMethods).
Population-based drug prescriptions registry (PHARMO)
The PHARMO population-based drug prescriptions registry is a network of electronic healthcare databases combining data from primary and secondary healthcare in the Netherlands, and covers a catchment area representing 4·2 million residents.26 Prescriptions of Th17 (ustekinumab, secukinumab, ixekizumab), TNF-α inhibitors (infliximab, etanercept, adalimumab), and concomitant drugs were obtained from 1998 (first TNF-α inhibitor authorization) to 2018 (eTable 2). Antifungal prescriptions were classified along anatomical site of candidiasis (eTable 3) and divided into single and chronic events (eMethods). Comparisons were made with psoriasis patients without biologics and healthy controls (patients without psoriasis and without biologics) (eMethods).
Psoriasis Cohort
All patients treated for plaque psoriasis with biologics at the outpatient dermatology ward at the Radboudumc university centre between January and November, 2018 were included. Patients were treated according to Dutch and European guidelines. Patients had previously signed informed consent for the prospective BioCAPTURE registry.27 For additional immunology studies, the study protocol was approved by the medical ethics review committee, and written informed consent was provided by all participants.
At any time during treatment, patients were asked for current symptoms or previous episodes of candidiasis. For all episodes, results of microbiological cultures, endoscopy, pathology, and reports on physical examination and treatment response were collected (eMethods). All candidiasis episodes were classified along anatomical site and probability (eTable 4). For each type of Candida infection, only one episode was counted per patient, to prevent recollection bias.
For immunological investigations, psoriasis patients with a history of candidiasis during Th17 inhibitors (ustekinumab, guselkumab, ixekizumab, brodalumab) served as index cases (eMethods), those without candidiasis and healthy persons served as independent control groups. Blood samples were collected for isolation and incubation of mononuclear and polymorphonuclear leukocytes with live C. albicans as described previously, and killing of Candida cells was assessed after 24h.28 Targeted inflammation proteomics analysis was performed in serum by Olink Proteomics (Uppsala, Sweden). Circulating concentrations of 92 unique proteins involved in inflammation were measured by real-time PCR using proximity extension assay (PEA) technology.29
Statistical analysis
Normally distributed variables were tested using independent-samples T tests, non-normal distribution with non-parametric alternatives. Pearson Chi-squared test was used for distribution of categorical variables. Incidence proportions, exposure-adjusted incidence and event rates, and risk ratios (RR) were compared using Fisher's exact test with mid-p method. All hypotheses tests were 2-sided (level of significance a priori: 0·05). IBM SPSS v25; SAS v9·4, SAS Enterprise Guide v8·3 (SAS), R v3·52 with Limma package were used. Source specific analyses: see eMethods.
For the WHO database, reporting odds ratios (ROR) were calculated as a measure of disproportionality representing the associations between biologic use and reports on candidiasis.30,31 The odds for candidiasis was defined as the likelihood of Candida infection, expressed as a proportion of the likelihood that any other adverse event in the WHO database will occur. The ROR represents the odds that Candida infection will occur during use of a specified biologic, compared to the odds of Candida infection occurring during any other drug use in the WHO database (eMethods). ROR >1 indicates more frequent reporting of a given ADR than any other drug in the database. To assess the risk of candidiasis for Th17 compared to TNF-α inhibitors, ROR were also calculated using only TNF-α inhibitors as the reference group, rather than all other drugs in the database.
EMA safety reports (PSUR) have not captured patient exposure durations for post-authorization clinical trials. Therefore, incidence proportions were calculated for SAE. Exposure-adjusted ER were calculated for ADR during commercial post-authorization use.
For the population-based prescriptions registry (PHARMO), the event rate (ER) of antifungal prescriptions was compared between biologics, psoriasis controls, and healthy controls. In addition, before-and-after comparisons within patients were performed comparing the rate of antifungal prescriptions during biologic use to the period prior to biologic use. Analyses were done for biologics prescribed for any indication and for a subgroup of psoriasis patients. Mixed Poisson regression models were used to correct for repeated measures, since patients can be included in more than one biologic cohort. All models were corrected for age, sex, concomitant use of antibiotics, and immunosuppressive drugs.
For the psoriasis cohort, incidence proportions and exposure-adjusted incidence rates per 100 patient-years of exposure were calculated. For a detailed description of calculations and models: see eMethods.
Role of the funding source
The funding sources did not have any role in study design, data collection, data analysis, interpretation, or writing of the report.
Results
WHO
The WHO database contained 16,343,451 ICSR. Candidiasis was reported in 50,353 ICSR, of which 33,735 were classified as proven or probable. Anti-IL17 was reported as the suspected drug in 427 reports (356 proven/probable candidiasis), anti-IL12/23 in 88 (63 proven/probable), and anti-TNFα in 6454 (3514 proven/probable). Patient characteristics of ICSR classified as proven/probable candidiasis are provided in eTable 5.
Anti-IL17 (any indication) showed a strong association with candidiasis (ROR 10·20; 95%CI 9·18, 11·33), as shown in Table 1. Median latency time between start of biologic and reported candidiasis was 84 days for anti-IL17 (p=0·002) and 189 days for anti-IL12/23 (p=0·80), compared to 204 days for anti-TNFα (eTable 5). Associations were strongest for cutaneous (ROR 12·28; 95%CI, 9·02, 16·71), oropharyngeal (ROR 19·18; 95%CI, 16·41, 22·41), and esophageal candidiasis (ROR 21·20; 95%CI, 15·43, 29·13). Candidiasis RR for anti-IL17 compared to anti-TNFα was 3·94 (95%CI; 2·86, 5·41) for cutaneous, 10·54 (8·90, 12·48) for oropharyngeal, and 10·27 (7·28, 14·49) for esophageal candidiasis.
Table 1.
Reporting odds ratios of proven and probable Candida infections for biologics for all indications, calculated from WHO VigiBase
| Reference group for all indications |
Risk relative to full database (N = 16,343,451)a |
Risk relative to TNF-α inhibitors (N = 887,002) |
||||
|---|---|---|---|---|---|---|
| Index group Biologics for all indications |
Anti-IL17 (N = 17,398) |
Anti-IL12/23 (N = 17,398) |
Anti-TNFα (N = 887,002) |
Anti-IL17 (N = 17,398) |
Anti-IL12/23 (N = 17,398) |
|
| Proven or probable Candida infectionb | no. Candida ICSR | no. Candida ICSR ROR (95% CI)c | no. Candida ICSR ROR (95% CI) | no. Candida ICSR ROR (95% CI) | no. Candida ICSR ROR (95% CI) | no. Candida ICSR ROR (95% CI) |
| All Candida infections | 33,735 | 356 10.20 (9.18-11.33) |
63 1.76 (1.37-2.25) |
3514 2.03 (1.96-2.10) |
356 5.26 (4.71-5.87) |
63 0.91 (0.71-1.17) |
| Mucocutaneous candidiasisd | 12,910 | 232 17.39 (15.26-19.82) |
37 2.70 (1.96-3.73) |
1496 2.29 (2.17-2.41) |
232 8.01 (6.97-9.20) |
37 1.26 (0.91-1.75) |
| Cutaneous candidiasis | 3,182 | 41 12.28 (9.02-16.71) |
19 5.64 (3.59-8.86) |
532 3.50 (3.19-3.84) |
41 3.94 (2.86-5.41) |
19 1.82 (1.15-2.88) |
| Oropharyngeal candidiasis | 8,211 | 163 19.18 (16.41-22.41) |
12 1.37 (0.78-2.42) |
797 1.87 (1.74-2.02) |
163 10.54 (8.90-12.48) |
12 0.77 (0.43-1.36) |
| Esophageal candidiasis | 1,769 | 39 21.20 (15.43-29.13) |
6 3.19 (1.43-7.12) |
194 2.15 (1.85-2.49) |
39 10.27 (7.28-14.49) |
6 1.58 (0.70-3.55) |
| Onychomycosis | 1,821 | 12 6.23 (3.53-10.99) |
4 2.07 (0.77-5.51) |
467 6.01 (5.41-6.68) |
12 1.31 (0.74-2.32) |
4 0.44 (0.16-1.17) |
| Vulvovaginal candidiasis | 5,603 | 30 5.06 (3.53-7.24) |
10 1.68 (0.90-3.12) |
662 2.34 (2.15-2.53) |
30 2.31 (1.60-3.33) |
10 0.77 (0.41-1.44) |
| Candidemia and deep-seated candidiasis | 1,678 | 3 1.68 (0.54-5.22) |
1 N/A |
49 0.52 (0.39-0.70) |
3 3.12 (0.97-10.01) |
1 N/A |
N indicates the number of individual case safety reports (ICSR) in the WHO database. ICSR for individual drugs were anti-IL12/23, ustekinumab, 17,398; Anti-IL17, secukinumab, 15,768; ixekizumab, 1,633; Anti-TNFα, etanercept, 403,764; adalimumab, 365,322; infliximab, 127,623.
Proven or probable Candida infection as defined in supplement S1.1.
ROR (95% CI) denotes the reporting odds ratio with its 95% confidence interval.
Mucocutaneous candidiasis denotes cutaneous, oropharyngeal, and esophageal candidiasis.
For vulvovaginal candidiasis, ROR for anti-IL17 was 5·06 (95%CI; 3·53, 7·24); the RR compared to anti-TNFα was 2·31 (95%CI; 1·60, 3·33). For onychomycosis, ROR was 6·23 (95%CI; 3·53, 10·99); while risk was comparable to anti-TNFα. There was no statistically significant association of anti-IL17 with candidemia or deep-seated candidiasis (ROR 1·68; 95%CI, 0·54, 5·22). Associations for separate biologics and candidiasis probabilities are shown in eTable 6. Associations of anti-IL12/23 and anti-TNFα with mucocutaneous (cutaneous, oropharyngeal, and esophageal) candidiasis were significant, with ROR ranging from 1·37-5·64 for anti-IL12/23 and 1·87-3·50 for anti-TNFα (Table 1). In psoriasis patients only, all associations with mucocutaneous candidiasis remained strong for anti-IL17 but were no longer significant for anti-IL12/23 and anti-TNFα biologics (eTable 7). Anti-IL17 was not associated with infection in general, since associations with herpes virus and bacterial skin infections were moderate and comparable to anti-TNFα (eTable 8).
EMA
In the EMA pre-authorization clinical trial safety sets of anti-IL17 (EPAR), Candida infections most frequently reported were oral and esophageal candidiasis. Candidiasis incidence rate was 3·55 per 100 person-years of exposure for secukinumab (300mg) and 4·90 for ixekizumab, compared to 1·37 for etanercept and 1·0 for placebo (eTable 9). Mucocutaneous candidiasis SAE in post-authorization clinical trials (PSUR) were significantly more frequent for anti-IL17 compared to anti-TNFα (RR 2·68; 95%CI; 1·29, 5·57, p=0·01) (eTable 10).
Exposure-adjusted candidiasis ER, based on ADR during post-authorization use, are shown in Table 2. Candidiasis ER was significantly higher for anti-IL17 compared to anti-TNFα (RR 16·61; 95%CI; 15·31, 18·03, p<0·001). The highest RR were found for oropharyngeal (RR 33·01, 95%CI; 28·89, 37·72, p<0·001) and esophageal candidiasis (RR 28·9, 95%CI; 22·35, 37·37, p=0·04). No increased risks compared to anti-TNFα was found for onychomycosis, vulvovaginal and invasive candidiasis.
Table 2.
Event rates of Candida adverse drug reactions (ADR) during commerical post-authorization use, extracted from periodic safety update reports (PSUR) from EMA
| Biologics in commercial use |
||
|---|---|---|
| IL-17 inhibitors (Exposure = 193,535 PY)a | TNF-α inhibitors (Exposure = 16,420,245 PY) | |
| Adverse drug reactions attributed to Candida infections | no. Candida ADRER (95% CI)b RR (95% CI)c |
no. Candida ADRER (95% CI) |
| All Candida infections | 686 0.35 (0.33-0.38) RR 16.61 (15.31-18.03) p<0.001d |
3,504 0.02 (0.02-0.02) |
| Mucocutaneous candidiasise | 465 0.24 (0.22-0.26) RR 28.44 (25.61-31.59) p<0.001 |
1,387 0.008 (0.008-0.009) |
| Cutaneous candidiasis | 87 0.05 (0.04-0.06) RR 19.07 (15.12-24.07) p=0.06 |
387 0.002 (0.002-0.003) |
| Oropharyngeal candidiasis | 300 0.16 (0.14-0.17) RR 33.01 (28.89-37.72) p<0.001 |
771 0.005 (0.004-0.005) |
| Esophageal candidiasis | 78 0.04 (0.03-0.05) RR 28.90 (22.35-37.37) p=0.04 |
229 0.001 (0.001-0.002) |
| Onychomycosis | 7 0.004 (0.002-0.007) RR 1.57 (0.74-3.31) p=0.95 |
379 0.002 (0.002-0.003) |
| Vulvovaginal candidiasis | 60 0.03 (0.02-0.04) RR 6.67 (5.13-8.68) p=0.09 |
763 0.005 (0.004-0.005) |
| Invasive candidiasis | 0 0 (<0.001-<0.001) N/A p= 0.99 |
82 <0.001 (<0.001-0.001) |
Exposure denotes the total amount of person-years (PY) of exposure to the biologic class in commercial use worldwide. Individual drugs were anti-IL17, secukinumab, ixekizumab; Anti-TNFα, etanercept, adalimumab, infliximab.
ER (95% CI) denotes the event rate per 100 patient years of exposure and its 95% confidence interval.
RR (95% CI) denotes the risk ratio relative to TNF-α inhibitors and its 95% confidence interval.
P values were calculated in comparison with TNF-α inhibitors using a two-tailed Fisher's exact test with mid-p method.
Mucocutaneous candidiasis denotes cutaneous, oropharyngeal, and esophageal candidiasis.
Population-based drug prescriptions registry (PHARMO)
A total of 5,733 patients with biologics for any indication (anti-IL17, 215; anti-IL12/23, 351; anti-TNFα, 5,167), 33,051 healthy controls and 33,415 psoriasis patients without biologics were included (eTable 11). Only significant differences are described.
Compared to anti-TNFα, candidiasis ER (all single or chronic events) for anti-IL17 was higher (RR 1·99; 95%CI 1·27, 3·11, p=0·003) and most pronounced for cutaneous (RR 1·94; 95%CI 1·11, 3·42, p=0·02) and oropharyngeal candidiasis (RR 9·59; 95%CI 1·70, 53·94, p=0·01), as shown in Table 3 (patient characteristics provided in eTable 12). Chronic candidiasis ER (all chronic events) was 5·17-fold higher (95%CI 3·13, 8·51, p<0·001), especially for chronic cutaneous (RR 2·54; 95%CI 1·20, 5·41, p=0·02) and chronic oropharyngeal candidiasis (RR 42·20, 95%CI 12·26, 145·29, p<0·001). For anti-IL12/23 (eTable 14), candidiasis risk was equal to that of anti-TNFα, except for chronic oropharyngeal candidiasis (RR 13·16; 95%CI 1·10, 158·85, p=0·04).
Table 3.
Event rates and risk ratios of candidiasis in the population-based drug prescriptions registry (PHARMO).
| All patients (any indication) with IL-17 inhibitor therapy(N = 215)a(Exposure = 231.2 PY)(Treatment episodes = 246) | All patients (any indication) with TNF-α inhibitor therapy(N = 5,167)(Exposure = 12,212.7 PY)(Treatment episodes = 8,390) | Healthy controlsb(N = 33,051)(Years of follow-up in database = 380,212.2 PY) | |
|---|---|---|---|
| Candida infections incidence ratec | no. patients IR (95% CI)d |
no. patients IRRRe |
no. patientsIRRR |
| All Candida infections | 31 IR 13.4 |
706 IR 5.8 RR 2.4 |
15,257 IR 4.0 RR 3.4 |
| Candida infections event ratec | no. events ER (95% CI) |
no. events ER (95% CI) RR (95% CI) |
no. events ER (95% CI) RR (95% CI) |
| All Candida infections | 42 ER 20.96 (13.85-31.71) |
1205 ER 11.07 (10.10-12.14) RR 1.99 (1.27-3.11) p=0.003f |
22,799 ER 6.46 (6.28-6.64) RR 2.46 (1.46-4.16) p<0.001 |
| Cutaneous candidiasis | 24 ER 10.66 (6.67-17.05) |
652 ER 5.56 (5.16-6.11) RR 1.94 (1.11-3.42) p=0.02 |
12,929 ER 3.62 (3.52-3.72) RR 2.98 (1.74-5.12) p<0.001 |
| Oropharyngeal candidiasis | 5 ER 4.50 (1.27-16.02) |
65 ER 0.62 (0.43-0.90) RR 9.59 (1.70-53.94) p=0.01 |
703 ER 0.24 (0.21-0.28) RR 4.13 (0.56-30.52) p=0.17 |
| Onychomycosis | 1 ER 0.44 (0.06-3.13) |
46 ER 0.38 (0.28-0.51) RR 0.97 (0.14-6.78) p=0.98 |
902 ER 0.24 (0.22-0.25) RR 2.24 (0.31-15.99) p=0.42 |
| Vulvovaginal candidiasis Vulvovaginal candidiasis |
5 ER 2.23 (0.75-6.60) |
243 ER 2.31 (1.90-2.81) RR 0.90 (0.28-2.95) p=0.87 |
5,840 ER 1.53 (1.45-1.62) RR 1.44 (0.33-6.28) P=0.63 |
| All chronic Candida infectionsg | 35 ER 10.63 (5.79-19.52) |
272 ER 2.46 (2.10-2.88) RR 5.17 (3.13-8.51) p<0.001 |
4,377 ER 1.21 (1.12-1.26) RR 9.60 (4.96-18.58) p<0.001 |
| Cutaneous candidiasis | 21 ER 8.06 (4.61-14.11) |
160 ER 1.35 (1.14-1.60) RR 2.54 (1.20-5.41) p=0.02 |
2,917 ER 0.81 (0.77-0.84) RR 11.33 (4.15-30.94) p<0.001 |
| Oropharyngeal candidiasis | 4 ER 1.99 (0.60-6.60) |
12 ER 0.10 (0.06-0.18) RR 42.20 (12.26-145.29) p<0.001 |
106 ER 0.029 (0.02-0.04) RR 67.87 (9.57-481.11) p<0.001 |
| Onychomycosis | 0 N/A |
13 N/A |
214 N/A |
| Vulvovaginal candidiasis | 4 ER 0.83 (0.13-5.49) |
46 ER 0.44 (0.30-0.66) RR 1.63 (0.42-6.33) p=0.48 |
758 ER 0.20 (0.18-0.22) RR 12.94 (1.25-134.13) p=0.03 |
N indicates the number of patients in the PHARMO database. Exposure denotes the total amount of person-years (PY) of exposure. Individual drugs were anti-IL17, secukinumab and ixekizumab. Anti-TNFα, etanercept, adalimumab, and infliximab. Treatment episodes indicate the number of treatment episodes for all patients in the cohort, a patient can have more than one treatment episode with the same or with another biologic in the same cohort.
Patients without a calcipotriol/betamethasone prescription and without a biologic prescription during registration in PHARMO database.
Candida infections denote the sum of all single and chronic infections. Single infections are defined as ≥ 28 days between calculated end date and new prescription date for the same type of Candida infection. Chronic infections are defined as <10 days between calculated end date and new prescription date for the same type of Candida infection. For onychomycosis and cutaneous candidiasis prescription end dates could not be calculated. Events of cutaneous candidiasis were counted as separate events when there were ≥100 days between prescription dates, preventing standard 90 days’ prescription renewals to count as separate events. Events with <100 days between prescription dates were seen as chronic events. Separate events of onychomycosis were defined as ≥365 days between prescription dates, since these infections often require months of treatment and treatment is often non or not completely successful. Events with <365 days between prescription dates were seen as chronic events.
IR (95% CI) denotes the incidence rate per 100 patient years of exposure, ER (95% CI) denotes the event rate per 100 patient years of exposure and its 95% confidence interval. The raw analysis was used for IR and ER calculations.
RR (95% CI) denotes the risk ratio of IL-17 inhibitor therapy relative to TNF-α inhibitor therapy or relative to the outpatient pharmacy background population and its 95% confidence interval. The RR for the IR are the unadjusted estimates, the RR for the ER are the adjusted estimates derived from a mixed Poisson regression model.
P values were derived from a mixed Poisson regression model, p values are given in comparison to all patients (any indication) with IL-17 inhibitor therapy.
All chronic Candida infections denote the sum of all chronic Candida infections. Chronic infections are defined as <10 days between prescriptions for the same type of Candida infection.
Comparison of anti-IL17 to healthy controls (Table 3) showed similar results (candidiasis RR 2·46; 95%CI 1·46, 4·16, p<0·001; chronic RR 9·60; 95%CI 4·96, 18·58, p<0·001, cutaneous candidiasis RR 2·98, 95%CI 1·74, 5·12, p<0·001; chronic cutaneous RR 11·33; 95%CI 4·15, 30·94, p<0·001, oropharyngeal RR 4·13; 95%CI 0·56, 30·52, p=0·17; chronic oropharyngeal RR 67·87; 95%CI 9·57, 481·11, p<0·001).
In the psoriasis subpopulation, candidiasis risks for anti-IL17 (vs. anti-TNFα or vs. psoriasis patients without biologics, eTable 13) were similar to those for patients with anti-IL17 prescribed for all indications. For psoriasis patients with anti-IL12/23 or anti-TNFα, candidiasis risks were equal to those of psoriasis patients without biologics (eTable 15).
In before/after start of biologics comparisons within patients, risk of oropharyngeal candidiasis increased 16·10-fold (95%CI, 2·38, 108·87, p=0·004) and chronic oropharyngeal candidiasis 11·74-fold (95%CI, 1·98, 82·11, p=0·01) after starting anti-IL17, compared to the period prior to biologics (eTable 18). After start of anti-IL12/23 or anti-TNFα treatment, oropharyngeal candidiasis risk did not increase (eTable 18).
Psoriasis cohort
A total of 548 biologic treatment episodes in 300 patients were studied (baseline characteristics provided in eTable 20). During anti-IL17 use, 38 candidiasis events were reported in 66 treatment episodes (58%; RR relative to anti-TNFα 4; 95%CI 3, 5, p<0·001), 49 events in 142 anti-IL12/23 episodes (34·5%; RR relative to anti-TNFα 2·2; 95%CI 1·6, 3·2, p<0·001), and 52 in 340 anti-TNFα episodes (15·3%) (Table 4).
Table 4.
Incidence proportions of biologic treatment episodes associated with candidiasis of the psoriasis patients cohort.
| Monoclonal antibodies for psoriasis |
|||
|---|---|---|---|
| Anti-IL17Treatment episodes = 66a | Anti-IL12/23Treatment episodes = 142 | Anti-TNFαTreatment episodes = 340 | |
| Candida infection | no. Candida episodesb% (95% CI)c RR (95% CI)d |
no. Candida episodes% (95% CI) RR (95% CI) |
no. Candida episodes% (95% CI) |
| All Candida infections | 38 58% (46-69) RR 4(3-5) p<0.001e |
49 34.5% (27.2-42.7) RR 2.2 (1.6-3.2) p<0.001 |
52 15.3% (11.8-19.5) |
| Mucocutaneous candidiasisf | 28 42% (31-55) RR 2 (2-4) p<0.001 |
33 23.2% (17.0-30.9) RR 2.8 (1.8-4.5) p<0.001 |
28 8.2% (5.7-11.7) |
| Cutaneous candidiasis | 15 23% (14-34) RR 4 (2-6) p<0.001 |
24 16.9% (11.6-24.0) RR 2.6 (1.5-4.5) p<0.001 |
22 6.5% (4.3-9.7) |
| Oropharyngeal candidiasis | 8 12% (6-22) RR 8 (3-24) p<0.001 |
9 6.3% (3.2-11.8) RR 4.3 (1.5-12.6) p=0.008 |
5 1.5% (0.5-3.5) |
| Esophageal candidiasis | 5 8% (3-17) RR 26 (3-217) p<0.001 |
0 N/A |
1 0.3% (0.0-1.8) |
| Onychomycosis | 4 6% (2-15) RR 1 (0.4-4) p=0.70 |
14 9.9% (5.9-16.0) RR 2.0 (1.0-3.9) p=0.06 |
17 5.0% (3.1-7.9) |
| Vulvovaginal candidiasis | 6 9% (4-19) RR 4 (2-13) p=0.01 |
2 1.4% (0.1-5.3) RR 0.7 (0.1-3.3) p=0.68 |
7 2.1% (0.9-4.3) |
Treatment episodes denotes the number of biologic treatment episodes per biologic class. Treatment episodes for the individual drugs were anti-IL12/23, ustekinumab, 140; guselkumab, 2; Anti-IL17, secukinumab, 41; ixekizumab, 24; brodalumab 1; Anti-TNFα, etanercept, 146; adalimumab, 174; infliximab, 20.
For each anatomical site of candidiasis, only the first occurrence was counted.
% (95% CI) denotes the proportion as % of the number of treatment episodes, with its 95% confidence interval.
RR (95% CI) denotes the risk ratio relative to TNF-α inhibitors and its 95% confidence interval.
P values were calculated in comparison with TNF-α inhibitors using a two-tailed Fisher's exact test with mid-p method.
Mucocutaneous candidiasis denotes cutaneous, oropharyngeal, and esophageal candidiasis.
For anti-IL17, cutaneous candidiasis occurred in 23% of treatment episodes, versus 6·5% for anti-TNFα (RR 4; 95%CI 2, 6, p<0·001), oropharyngeal candidiasis in 12% of episodes (anti-TNFα 1·5%, RR 8, 95%CI 3, 24, p<0·001), esophageal candidiasis in 8% (anti-TNFα 0·3%; RR 26, 95%CI 3, 217, p<0·001), and vulvovaginal candidiasis in 9% (anti-TNFα 2·1%, RR 4, 95%CI 2, 13, p=0·01). Only the percentage of treatment episodes associated with onychomycosis was not significantly higher for anti-IL17 compared to anti-TNFα. For anti-IL12/23, the percentage of treatment episodes associated with candidiasis was only significantly higher for cutaneous (16·9%, RR 2·6, 95%CI 1·5, 4·5, p<0·001) and oropharyngeal candidiasis (6·3%, RR 4·3, 95%CI 1·5, 12·6, p=0·008) compared to anti-TNFα.
When expressed as incidence rates per 100 person-years of exposure, RR compared to anti-TNFα ranged from 4 (95%CI; 1, 12, p=0·03) to 84·3 (95%CI, 10, 721, p<0·001) for anti-IL17 (depending on Candida type) and from 0·9 (95%CI, 0·2, 4·1, p=0·90) to 5·4 (95%CI, 1·8, 16·2, p=0·002) for anti-IL12/23 (eTable 21). Five of 41 secukinumab patients (12·2%) required chronic suppressive antifungal therapy, whereas no suppressive therapy was prescribed to anti-TNFα or anti-IL12/23 recipients.
All candidiasis episodes were typical in appearance although some patients suffered from a combination of onychomycosis, cutaneous candidiasis and oropharyngeal/esophageal candidiasis. Many of the candidiasis infections were dificult to treat, and recurrences frequently occured.32
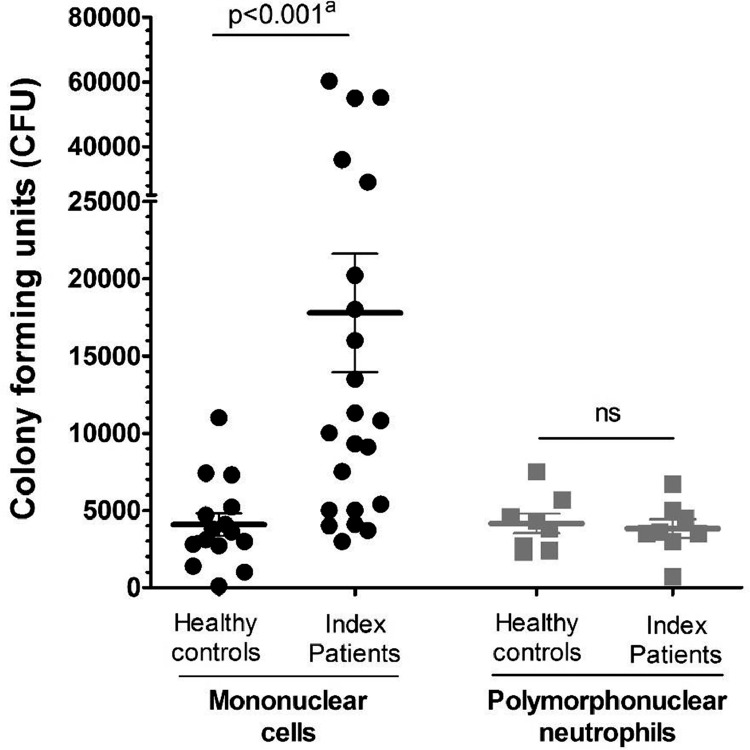
A C. albicans killing assay was performed in blood samples of 15 healthy controls and 22 psoriasis patients with a history of candidiasis during anti-Th17 (eFigure 1). There was no difference in Candida survival in neutrophils between anti-Th17 recipients and healthy controls (Figure 1). Candida survival in mononuclear leukocytes, however, was 4-fold greater (killing, 97%, 95%CI 95, 99, p<0·001) in anti-Th17 recipients compared to healthy controls (99%, 95%CI 99, 100).
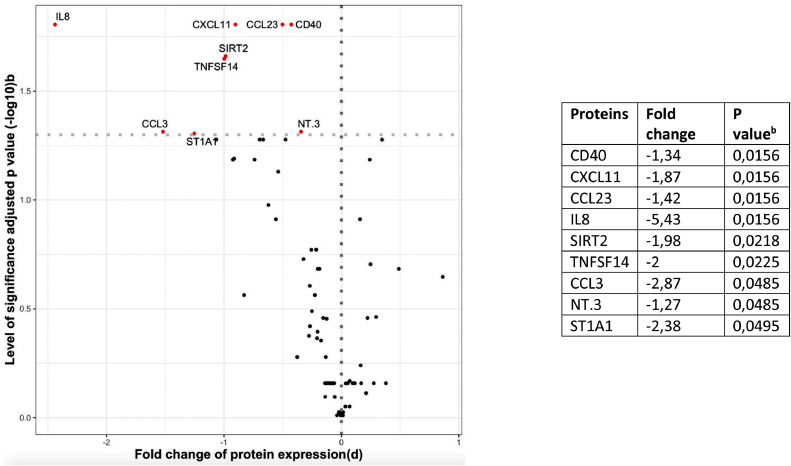
Figure 1.
Immunology studies in psoriasis patients with candidiasis during Th17 inhibitor therapy
a. Candida outgrowth in white blood cells, expressed as colony-forming units (CFU).
b. Differential expression of serum proteins from psoriasis patients using Th17 inhibitor therapy with and without candidiasis.
Serum inflammatory proteome was compared between psoriasis patients with and without a history of candidiasis during Th17 inhibitor therapy (eTable 22 and 23). Nine proteins were significantly downregulated in patients with candidiasis during Th17 inhibitor therapy (Figure 1). IL-8 was 5·43-fold reduced, ST1A1 2·38-fold, CCL3 2·87-fold, TNFSF14 2·00-fold, SIRT2 1·98-fold, CXCL11 1·87-fold, CCL23 1·42-fold, CD40 1·34-fold, and NT3 was 1·27-fold reduced. No differences related to sex or age were detected.
Discussion
The present study shows that IL-17 inhibitors are associated with an increased risk of candidiasis, based on data from multiple independent sources worldwide. Risk relative to TNF-α inhibitors was, depending on Candida type, 4- to 10-fold higher in the WHO database, 16- to 33-fold higher in EMA safety reports, 2- to 42-fold higher in a population-based antifungal drug prescriptions registry, and 3- to 25-fold higher in a prospective psoriasis cohort.
The global WHO database of individual case safety reports revealed a strong association between IL-17 inhibitors and candidiasis (ROR 10·20), in particular for cutaneous (ROR 12·28), oropharyngeal (ROR 19·18), and esophageal candidiasis (ROR 21·20). The median latency time between biologic therapy onset and reported Candida infection was 84 days for anti-IL17, compared to 204 days for anti-TNFα (p=0·002), suggesting a causal relationship. In EMA post-authorization reports, the RR of anti-IL17 compared to anti-TNFα was 16·61 for all Candida infections, 33·01 for oropharyngeal, and 28·90 for esophageal candidiasis.
In a population-based drug prescriptions registry (PHARMO), candidiasis ER of anti-IL17 was increased 1·99-fold compared to anti-TNFα, and especially high for oropharyngeal (RR 9·59) and cutaneous candidiasis (RR 1·94). The risk for chronic manifestations of cutaneous (RR 2·54) or oropharyngeal candidiasis (RR 42·20) was even more pronounced. In a before/after anti-IL17 treatment comparison within patients, risk of oropharyngeal candidiasis increased 16·10-fold after starting therapy and 11·74-fold for chronic oropharyngeal candidiasis, compared to the period prior to biologics.
In our psoriasis patients cohort, 58% of anti-IL17 treatment episodes was associated with ≥1 candidiasis episode, compared to 15% of anti-TNFα treatment episodes (cutaneous candidiasis, RR 3·5; oropharyngeal, RR 8·2; esophageal, RR 25·8; p<0·001). Of patients receiving IL-17 inhibitor secukinumab, 12% required chronic suppressive antifungal therapy, versus none in anti-IL12/23 or anti-TNFα groups. The association of anti-IL12/23 with candidiasis in the WHO, EMA, and population-based drug prescriptions registry data sets was moderate but comparable to that of anti-TNFα. The consistent observation that IL-17 inhibitors strongly increase the risk for oropharyngeal, esophageal, and cutaneous candidiasis, but not for invasive and moderately for vulvovaginal candidiasis, is in agreement with findings in patients with IL-17 immunodeficiencies.12, 13, 14 Since IL-17’s main function is recruitment of neutrophils to the site of Candida infection, IL-17 inhibition primarily has effects on skin and mucosal Candida infections and not on disseminated infections, as neutrophils are present in the circulation. IL-17 is less important in host defense against vulvovaginal candidiasis, where major mechanisms of pathogenesis include local estrogen level, imbalances in local microbiome, and IL-1β-induced hyperinflammation. Defects in IL-12/23 immunity cause a milder candidiasis phenotype.
To gain insight into the immunological background, we showed that, compared to healthy controls, Candida killing by mononuclear leukocytes from Th17 inhibitor recipients with a history of candidiasis was impaired. In these subjects, targeted serum proteomics of inflammatory factors involved in host defense displayed nine significantly downregulated proteins compared with Th17 inhibitor recipients without candidiasis, suggesting an individual host susceptibility trait in combination with a direct effect of IL-17 inhibition.33 Indeed, most of these proteins are pro-inflammatory cytokines, chemokines, or growth factors important for anti-Candida host defense, including neutrophil chemotactic factor IL-8, cluster of differentiation 40 (CD40), C-X-C motif chemokine 11 (CXCL11), C-C motif chemokine 3 (CCL3) and the CCL23 chemokine.34, 35, 36, 37, 38, 39, 40, 41
The main strength of this study is the comprehensive worldwide analysis of multiple independent sources reporting Candida adverse events during anti-Th17 therapy. In addition, candidiasis risk was shown to be increased relative to anti-TNFα therapy, thereby placing results in a broader perspective. The WHO global database of spontaneous reports and EMA's safety reports were combined with a population-based prescriptions registry and the psoriasis cohort. Analyses of antifungal prescriptions (PHARMO) were corrected for confounding and repeated measures. Additionally, the before/after comparisons within biologic recipients provide adjustment for unknown confounding. Furthermore, we demonstrated a downregulation of anti-Candida host defense mechanisms in candidiasis patients during anti-Th17 therapy.
Potential weaknesses of the study include reporting bias and potential confounding that we could not control for in every substudy. Spontaneous safety reports originate from a variety of sources, which may lead to variability in assessment of drug-relatedness of events. In addition, candidiasis ER calculated from EMA's safety reports potentially represent an underestimate, as these are based on spontaneous reporting. In contrast, risks relative to other biologics for candidiasis as calculated from WHO and EMA data sets are expected to be independent of underreporting. Importantly, EMA's clinical trial safety reports only include serious adverse events attributed to candidiasis, hence excluding non-serious candidiasis. In addition, patients with a history of recurrent mucocutaneous candidiasis had been excluded from pivotal clinical trials across the clinical development program of ustekinumab and guselkumab. WHO and EMA safety reports depend on the rigidity of underlying clinical diagnoses. As diagnoses of cutaneous candidiasis and dermatophyte infections may overlap, and the MedDRA terms reported are numerous, we prespecified all potential MedDRA terms (eTable 1). Sensitivity analyses of WHO and EMA safety reports showed that excluding terms that may have reported dermatophyte infections did not weaken our conclusions. In contrast, further excluding potential dermatophytosis reports, the risks voor cutaneous candidiasis (anti-IL12/23; WHO) and nail candidiasis (anti-IL17; EMA) were higher (eTable 25 and eTable 26).
The event rates found in the population-based prescriptions registry may be underestimated, as the use of over-the-counter antifungals in unknown. However, assuming that the fraction of over-the-counter versus prescription-based antifungal use may remain stable, this does not affect the relative risks between biologic classes.
Since four different data sources were used to assess candidiasis incidence in the real world, definitions of candidiasis types and methods of analysis varried. The fact that multiple independent data sources and analyses led to a consistent conclusion, however, supports our conclusions.
Concomitant immunosuppressive medication may be a potential confounder. Concomitant immunosuppressive medication was more prevalent among patients with anti-TNFα (33·72%, versus 7·61% for anti-IL17 (p<0·001) and 19·05% for anti-IL12/23 (p=0·03)) and may be, at least in part, attributing to the associations with mucocutaneous candidiasis in the WHO database. In addition, the underlying diseases in the anti-TNFα group (rheumatoid arthritis, 36·2%; inflammatory bowel disease, 15·3% as opposed to psoriasis, 8·7%) may have contributed to the risk of candidiasis (eTable 5). In a subset of psoriasis patients, all associations with mucocutaneous candidiasis remained strong for anti-IL17, but were no longer significant for anti-TNFα (eTable 7). In the population-based drug prescriptions registry, adjustment for these potential confounders showed an independent effect of anti-IL17 on candidiasis.
The Candida killing in Th17 inhibitor recipients with candidiasis during therapy was compared to healthy controls, while ideally we would also have included a control group of psoriasis patients who did not experience candidiasis during therapy, in order to give a definite answer to the cause of the impaired Candida killing capacity.
Interestingly, we found a higher candidiasis incidence for anti-IL17 than previously described in clinical trials (3·6 – 6·5%).16,18,19 In a retrospective cohort study, 2·9% of patients on secukinumab and 6·6% on ixekizumab developed candidiasis.42 In the prospective BioCAPTURE cohort study, we found fungal infections in 13% of patients receiving secukinumab, with an event rate of 21·6 per 100 person-years, predominantly oropharyngeal candidiasis (ER 11.9).43 Likewise, in a retrospective cohort study 12·5% of patients reported oral candidiasis during secukinumab treatment.44
In conclusion, treatment with IL-17 inhibitors is associated with an increased risk of candidiasis, especially oropharyngeal and esophageal candidiasis, as demonstrated by the WHO database, EMA's safety reports, a population-based drug prescriptions registry, and a psoriasis patients cohort, and confirmed by immunological tests showing impaired anti-Candida host defense mechanisms. Recurrences of oropharyngeal and esophageal candidiasis are frequent, and chronic suppressive antifungal therapy is often needed to enable continuation of IL-17 inhibitor therapy. These infections pose a significant disease burden in anti-IL17 recipients. This suggests that close monitoring of IL-17 inhibitor recipients for oropharyngeal and esophageal candidiasis is advisable, and antifungal prophylaxis may be considered for patients with recurrent or chronic candidiasis. Future studies may identify additional predisposing host factors amenable for screening of anti-IL17 candidates, to guide antifungal prophylaxis or anti-Candida vaccination (currently in phase 2 studies) in the future.
Contributors
BJK conceived the study. BJK, EDJ, MGN, LD and JvdR designed the study. LD collected the data. LD, MB, VM, CB, VK, MGN, and HR analysed the data. FH, RH, HG, JvdR verified the data. LD drafted the first version of the manuscript. All authors saw the draft of the manuscript, contributed to data interpretation, and provided input on writing. All authors critically reviewed the manuscript and had the final responsibility to submit for publication.
Data availability statement
The authors do not have ownership of WHO, EMA and PHARMO datasets and these can therefore not be publicly released by the authors. However, access to those datasets can be requested at these institutes in order to carry out a study, a research or an evaluation of public interest. Since the BioCAPTURE registry is an ongoing database used for future research, data from the psoriasis patients cohort is not yet publicly accessible.
Declaration of Competing Interest
J.M.P.A. van den Reek carried out clinical trials for AbbVie, Celgene and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, Leo Pharma and Eli Lilly and reimbursement for attending a symposium from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud university medical centre Nijmegen, the Netherlands.
E.M.G.J. de Jong received grants from ZonMw, AbbVie, Janssen, Novartis, Pfizer, and Leo Pharma outside the submitted work. E.M.G.J. de Jong received research grants from the independent research fund of the Department of Dermatology, Radboud University Medical Center, Nijmegen, the Netherlands and acts as a consultant and/or paid speaker for and/or participating in research sponsored by AbbVie, Janssen, Novartis, Eli Lilly and Company, Celgene, and Leo Pharma.
Acknowledgements
We thank the Uppsala Monitoring Centre and the Dutch Medicines Evaluation Board, Marcel Kwa at the Dutch Medicines Evaluation Board, Camilla Westerberg and Tomas Bergvall at the Uppsala Monitoring Centre for their collaboration in this study. The information obtained from VigiBase does not represent the opinion of the WHO or the Uppsala Monitoring Centre. This study was funded by RadboudUMC. MGN was supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100266.
Appendix. Supplementary materials
References
- 1.Deodhar A, van der Heijde D, Gensler LS, Kim TH, Maksymowych WP, Ostergaard M, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020;395(10217):53–64. doi: 10.1016/S0140-6736(19)32971-X. [DOI] [PubMed] [Google Scholar]
- 2.Deodhar A, Blanco R, Dokoupilova E, Hall S, Kameda H, Kivitz AJ, et al. Secukinumab improves signs and symptoms of non-radiographic axial spondyloarthritis: primary results of a randomized controlled phase III study. Arthritis Rheumatol. 2020 doi: 10.1002/art.41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb AB, Kubanov A, van Doorn M, Sullivan J, Papp KA, You R, et al. Sustained efficacy of secukinumab in patients with moderate-to-severe palmoplantar psoriasis: 2.5-year results from GESTURE, a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2020;182(4):889–899. doi: 10.1111/bjd.18331. [DOI] [PubMed] [Google Scholar]
- 4.McInnes IB, Behrens F, Mease PJ, Kavanaugh A, Ritchlin C, Nash P, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395(10235):1496–1505. doi: 10.1016/S0140-6736(20)30564-X. [DOI] [PubMed] [Google Scholar]
- 5.Mease PJ, Smolen JS, Behrens F, Nash P, Liu Leage S, Li L, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–131. doi: 10.1136/annrheumdis-2019-215386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blauvelt A, Lomaga M, Burge R, Zhu B, Shen W, Shrom D, et al. Greater cumulative benefits from ixekizumab versus ustekinumab treatment over 52 weeks for patients with moderate-to-severe psoriasis in a randomized, double-blinded phase 3b clinical trial. J Dermatolog Treat. 2020;31(2):141–146. doi: 10.1080/09546634.2019.1587146. [DOI] [PubMed] [Google Scholar]
- 7.Bagel J, Blauvelt A, Nia J, Hashim P, Patekar M, de Vera A, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY) J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helliwell PS, Gladman DD, Chakravarty SD, Kafka S, Karyekar CS, You Y, et al. Effects of ustekinumab on spondylitis-associated endpoints in TNFi-naive active psoriatic arthritis patients with physician-reported spondylitis: pooled results from two phase 3, randomised, controlled trials. RMD Open. 2020;6(1) doi: 10.1136/rmdopen-2019-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Hou SY, Zhao S, Hou LX, Jiao T, Xu NN, et al. Meta-analysis of IL-17 inhibitors in two populations of rheumatoid arthritis patients: biologic-naive or tumor necrosis factor inhibitor inadequate responders. Clin Rheumatol. 2019;38(10):2747–2756. doi: 10.1007/s10067-019-04608-z. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6(1):67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 11.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15(10):630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 12.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365(1):54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 14.Okada S, Puel A, Casanova JL, Kobayashi M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin Transl Immunology. 2016;5(12):e114. doi: 10.1038/cti.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83–98. doi: 10.1016/j.jaad.2016.03.024. e4. [DOI] [PubMed] [Google Scholar]
- 16.Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 17.Mease P, Roussou E, Burmester GR, Goupille P, Gottlieb A, Moriarty SR, et al. Safety of ixekizumab in patients with psoriatic arthritis: results from a pooled analysis of three clinical trials. Arthritis Care Res (Hoboken) 2019;71(3):367–378. doi: 10.1002/acr.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 19.Gordon KB, Colombel JF, Hardin DS. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2016;375(21):2102. doi: 10.1056/NEJMc1610828. [DOI] [PubMed] [Google Scholar]
- 20.Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498. doi: 10.1016/S0140-6736(21)00125-2. [DOI] [PubMed] [Google Scholar]
- 21.Lindquist M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Information Journal. 2008;42(5):409–419. [Google Scholar]
- 22.World Health Organization. Uppsala Monitoring Center, Vigibase® 2019 [Available from: https://www.who-umc.org/
- 23.Medical Dictionary for Regulatory Activities (MedDRA). 2019 [Available from: https://www.meddra.org/. [DOI] [PubMed]
- 24.World Health Organization, WHODrug Global. 2019 [Available from: https://www.who-umc.org/whodrug/whodrug-portfolio/whodrug-global/.
- 25.European Medicines Agency. 2019 [Available from: https://www.ema.europa.eu/en.
- 26.PHARMO Database Network. 2019 [Available from: https://www.pharmo.nl/.
- 27.van den Reek JM, Zweegers J, Kievit W, Otero ME, van Lumig PP, Driessen RJ, et al. Happy' drug survival of adalimumab, etanercept and ustekinumab in psoriasis in daily practice care: results from the BioCAPTURE network. Br J Dermatol. 2014;171(5):1189–1196. doi: 10.1111/bjd.13087. [DOI] [PubMed] [Google Scholar]
- 28.Vonk AG, Netea MG, Kullberg BJ. Phagocytosis and intracellular killing of Candida albicans by murine polymorphonuclear neutrophils. Methods Mol Biol. 2012;845:277–287. doi: 10.1007/978-1-61779-539-8_18. [DOI] [PubMed] [Google Scholar]
- 29.Olink. Validation document inflammation panel 2019 [Available from: www.olink.com/downloads.
- 30.Noren GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22(1):57–69. doi: 10.1177/0962280211403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–523. doi: 10.1002/pds.1001. [DOI] [PubMed] [Google Scholar]
- 32.Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177(1):47–62. doi: 10.1111/bjd.15015. [DOI] [PubMed] [Google Scholar]
- 33.Iyoda M, Shibata T, Kawaguchi M, Hizawa N, Yamaoka T, Kokubu F, et al. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta. Am J Physiol Renal Physiol. 2010;298(3):F779–F787. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- 34.Dongari-Bagtzoglou A, Wen K, Lamster IB. Candida albicans triggers interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol Immunol. 1999;14(6):364–370. doi: 10.1034/j.1399-302x.1999.140606.x. [DOI] [PubMed] [Google Scholar]
- 35.Dongari-Bagtzoglou A, Kashleva H. Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb Pathog. 2003;34(4):169–177. doi: 10.1016/s0882-4010(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 36.Netea MG, Meer JW, Verschueren I, Kullberg BJ. CD40/CD40 ligand interactions in the host defense against disseminated Candida albicans infection: the role of macrophage-derived nitric oxide. Eur J Immunol. 2002;32(5):1455–1463. doi: 10.1002/1521-4141(200205)32:5<1455::AID-IMMU1455>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74(3):448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 38.Valdivia-Silva J, Medina-Tamayo J, Garcia-Zepeda EA. Chemokine-derived peptides: novel antimicrobial and antineoplasic agents. Int J Mol Sci. 2015;16(6):12958–12985. doi: 10.3390/ijms160612958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167(2):623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 40.Yuan X, Hua X, Wilhelmus KR. Proinflammatory chemokines during Candida albicans keratitis. Exp Eye Res. 2010;90(3):413–419. doi: 10.1016/j.exer.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swamydas M, Break TJ, Lionakis MS. Mononuclear phagocyte-mediated antifungal immunity: the role of chemotactic receptors and ligands. Cell Mol Life Sci. 2015;72(11):2157–2175. doi: 10.1007/s00018-015-1858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baez-Gutierrez N, Rodriguez-Ramallo H. Candidiasis in patients treated with interleukin-17 inhibitors. Indian J Pharmacol. 2021;53(3):244–245. doi: 10.4103/ijp.ijp_1178_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Reek J, van Vugt LJ, van Doorn MBA, van der Kraaij GE, de Kort WJA, Lucker GPH, et al. Initial results of secukinumab drug survival in patients with psoriasis: a multicentre daily practice cohort study. Acta Derm Venereol. 2018;98(7):648–654. doi: 10.2340/00015555-2900. [DOI] [PubMed] [Google Scholar]
- 44.Esra Ağaoğlu HKE, Ersoy Acer. Secukinumab treatment in psoriasis: a single center experience. Turkderm. 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have ownership of WHO, EMA and PHARMO datasets and these can therefore not be publicly released by the authors. However, access to those datasets can be requested at these institutes in order to carry out a study, a research or an evaluation of public interest. Since the BioCAPTURE registry is an ongoing database used for future research, data from the psoriasis patients cohort is not yet publicly accessible.