Abstract
The emerging data supports rhythm control to prevent major adverse cardiac events (MACE) in high-risk patients with atrial fibrillation (AF). Limited data demonstrated rivaroxaban 10 mg combining dronedarone seemed feasible. This study aimed at investigating clinical events in a dronedarone-treated cohort. This exploratory, retrospective chart review was conducted in nonpermanent AF patients receiving dronedarone for ≥ 3 months between 2009/1 and 2016/2. In Taiwan, dronedarone's labeled indication was strict to age ≥ 70 or 65 to 70 years with either hypertension, diabetes, prior stroke, or left atrium >50 mm. We divided all into 4 groups using antithrombotic strategies to evaluate the safety, effectiveness, and MACE endpoints. A total of 689 patients (mean CHA2DS2-VASc score 3.8 ± 1.4) were analyzed: rivaroxaban 10 mg (n = 93, 13.5%), warfarin (n = 89, 12.9%), antiplatelet (n = 331, 48.0%), and none (n = 176, 25.5%). During the follow-up period (mean 946 ± 493.8 days), the rivaroxaban group did not report any stroke or thromboembolism (ishcmeic stroke rate: antiplatelet [0.6%], none [1.1%]; hemorrahgic stroke rate: warfarin [2.2%]; thromboembolism rate: warfarin [2.2%]). There was no significant difference in safety, effectiveness, and MACE endpoints between groups. Also, >104 weeks of dronedarone use was the independent predictor for MACE after adjusting the strategy and other covariates (hazard ratio 0.14 [95% confidence interval 0.04-0.44], P = .001). Our findings warrant concomitant rivaroxaban 10 mg and dronedarone for further investigation. Regardless of antithrombotic strategies, a more extended persistence of dronedarone was associated with fewer MACE.
Keywords: atrial fibrillation, rhythm control, dronedarone, rivaroxaban
Introduction
Atrial fibrillation (AF) is associated with a doubling effect on cardiovascular (CV) death and an increased risk of systemic thromboembolism. AF accounts for approximately 15% of all stroke cases.1,2 Stroke prevention for AF (SPAF) refers to antithrombotic therapy either by antiplatelet or an anticoagulant. Although, anticoagulation became the predominant choice. A higher incidence of stroke, systemic thromboembolism, and bleeding occurred in Asians than non-Asians despite warfarin or nonvitamin K antagonist oral anticoagulants (NOAC) treatment.3,4 Notably, routine rhythm control in previous SPAF studies lacks since the recommendation was only for symptom relief till 2016. 5
AF rhythm control changes a lot during the past decade. The 2020 European Society of Cardiology guideline recommends that rhythm control reduces rather than eliminates the recurrence of AF. 6 Furthermore, antiarrhythmic drugs (AAD) may be considered a temporizing, rather than a curative, treatment.7,8 Various factors may lead to a neutral effect, including inappropriate patient and AAD selection, drug-drug interaction, or mere neglect of SPAF.5,9,10 The ATHENA study demonstrated that dronedarone prevents CV hospitalization and stroke in high CV-risk and nonpermanent AF. 11 The EAST-AFNET 4 study proved that early rhythm control could reduce stroke and CV events. 12 Concomitant use of dronedarone and rivaroxaban 10 mg seems feasible in a retrospective study. 13 However, more clinical data are required to determine their roles. This study aimed to investigate SPAF strategies' outcomes on top of routine rhythm control in a dronedarone-treated population from 2009 to 2016 using an exploratory approach without preset hypothesis and prospectively powered sample size calculation.
Methods
Data Source and Study Population
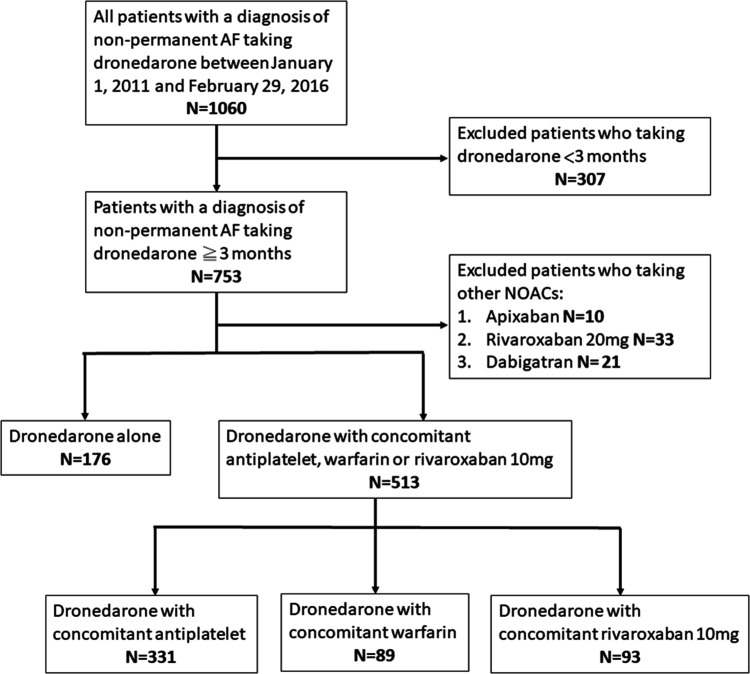
In this multicenter, longitudinal cohort study, all patients with nonpermanent AF, and prescribed dronedarone for rhythm control, were screened between January 2009 and February 2016. The Institutional Review Board (IRB) of Mackay Memorial Hospital approved this study protocol (IRB No. 16MMHIS009), which waived the requirement for informed consent for this retrospective study. All patients were followed up until February 2016 or until death. We only enrolled those receiving dronedarone for more than 3 months. In Taiwan, the labeled indication of dronedarone was limited to nonpermanent AF patients at the age of ≥70, or 65 to 70 years with either hypertension, diabetes, prior stroke, or left atrium >50 mm. The treating physicians made the SPAF choice according to patients’ underlying diseases and consideration on drug-drug interactions. 14 According to individual concomitant agents, the enrolled patients were divided into 4 study groups: rivaroxaban, warfarin, antiplatelet, and none of SPAF.
Regarding the drug-drug interaction and availability in 2009 to 2016, we only enrolled the patient receiving rivaroxaban with 10 mg in this study (Figure 1). The antiplatelets included aspirin 100 mg and clopidogrel 75 mg. The clinical characteristics, risk factors, and echocardiographic parameters of each patient were reviewed from the electronic medical records. The thromboembolic and bleeding factors were assessed for each patient at the time of the treatment selection
Figure 1.
Study flow diagram.
Clinical Outcomes
The primary objective of this study was to determine the decision-making factors for the SPAF strategy and how they relate to safety, effectiveness, and major adverse cardiac event (MACE) endpoints. The safety endpoints (by ISTH definition) were the composite of hemoglobin reduction ≥2 g/dL, blood transfusion ≥2 U PRBC, critical site bleeding, and/or fatal bleeding. The effectiveness endpoints were the composite of new ischemic stroke, hemorrhagic stroke, and/or systemic thromboembolism. MACE was the composite of CV death, myocardial infarction, stroke, and/or systemic thromboembolism. Moreover, CV death was independently evaluated by attending physicians. The secondary objective was to identify predictors of therapeutic response.
Covariates
The following covariates were included: gender, age, permanent pacemaker, CHA2DS2-VASc and HAS-BLED scores, mitral regurgitation, thyroid disease, glomerular filtration rate, and dronedarone period. The CHA2DS2-VASc score (congestive heart failure, hypertension, aged ≥75 years [doubled], diabetes mellitus, prior stroke, transient ischemic attack or thromboembolism [doubled], vascular disease, age of 65-74 years, female) refers to the composite risk of stroke and thromboembolism for each patient.3,15 The HAS-BLED score (uncontrolled hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, the labile international normalized ratio [INR], elderly, drugs/alcohol concomitantly) was used to assess major bleeding risk.3,15 Mitral regurgitation was assessed by mapping jet expansion in the left atrium in 4-chamber views during the systole phase. Also, the persistence of dronedarone during the treatment period was calculated for each patient and was classified into 12‒52, 52.1‒104, and >104 weeks.
Statistical Analysis
Distributions of baseline patient characteristics and safety, effectiveness, and MACE endpoints between groups with different SPAF strategies were evaluated using the Chi-square test for categorical variables, and the Kruskal-Wallis test for continuous variables, as appropriate. The cumulative incidence of safety, effectiveness, and MACE for receiving other concomitant agents was estimated using the Kaplan-Meier method. Differences in safety, effectiveness, and MACE between the 4 study groups were tested using the log-rank test. Besides, multivariate Cox proportional hazard models with the robust sandwich variance estimator were analyzed to assess the 4 SPAF strategies' influence and the covariates associated with safety, effectiveness, and MACE endpoints. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Statistical significance was set at P < .05. All data analyses were performed using IBM SPSS statistics 24.0 for windows (IBM Corp., Chicago, IL, USA). Due to the exploratory nature of this study, no prospectively powered sample size calculation was performed.
Results
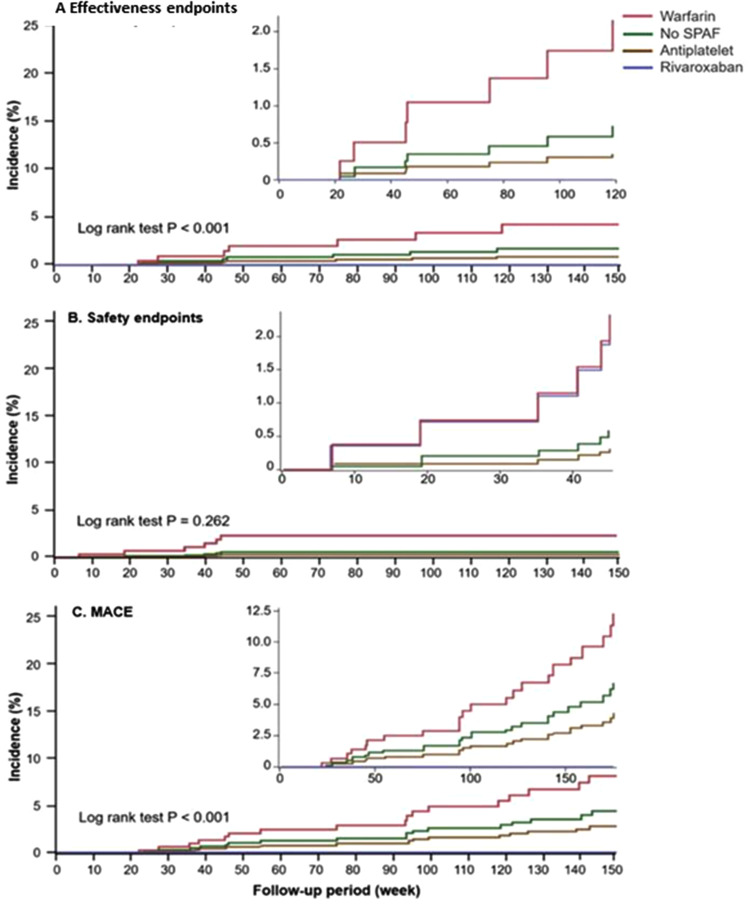
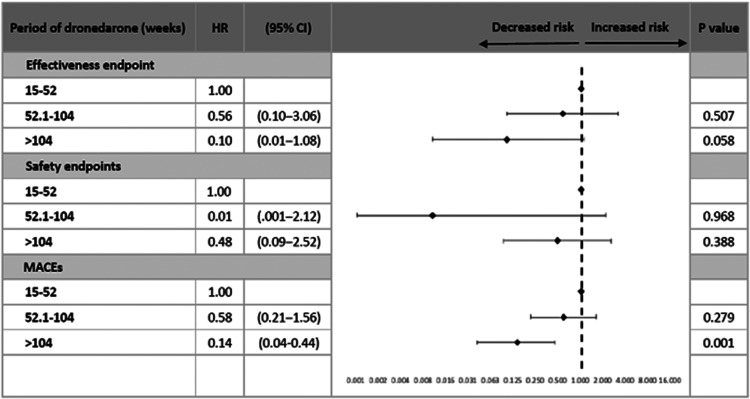
Among 1060 subjects with nonpermanent AF and rhythm control by dronedarone, 689 (mean age 75.8 ± 8.7 years, 58.3% female) were enrolled after excluding those who had incomplete information, NOAC other than rivaroxaban 10 mg, or had received dronedarone for less than 3 months. Of these, there were 89 (12.9% of all subjects) treated with warfarin, 176 (25.5%) with no SPAF, 331 (48.1%) in the antiplatelet group, and 93 (13.5%) in the rivaroxaban group. Coadministration of antiplatelet and anticoagulant was not seen in this cohort. All baseline characteristics were shown in Table 1. The therapeutic range (TTR) for prothrombin time INR 1.5 to 2.5 and 2.0 to 3.0 were 35.0% and 21.1% in the warfarin group, respectively. The mean CHA2DS2-VASc score was 3.8 ± 1.4, and the mean HAS-BLED score was 1.44 ± 0.7. Age, gender distribution, medical histories, CHA2DS2-VASc score, and HAS-BLED score were generally comparable between the 4 study groups, except the presence of pacemaker (P = .046), the periods of dronedarone treatment (P = .048), and follow-up (P < .001). Compared with the other study groups, the warfarin group had a higher proportion of new hemorrhagic stroke (P = .042) and systemic thromboembolism (P = .042), which all led to the results of bleeding at critical sites (P = .042) and fatal bleeding (P = .042) (Table 2). Furthermore, the results of Kaplan-Meier analysis showed the warfarin group had a higher cumulative incidence of efficacy endpoints (P < .001) and MACE (P < .001) than the other 3 study groups except for the safety endpoint (P = .262) (Figure 2A and C). During the follow-up period, the rivaroxaban group did not report any events of efficacy endpoints (P = .092), MACE (P = .063), and nongastrointestinal (GI) safety endpoints (P = .042) despite a numerically higher incidence of minor bleeding (P = .101) and GI bleeding (P = .196) (Table 2 and Figure 2B). Using the log-rank test, the rivaroxaban group showed the best results in regards to the efficacy endpoints (P < .001) and MACE (P < .001) (Figure 2A and C). All the safety endpoints (n = 2, 2.2%) in the rivaroxaban group were GI bleeding-related transfusions. In terms of the proportion, 2 of 3 GI bleeding events resulted in a hemoglobin reduction ≥2 g/dL or transfusion ≥2 U PRBC in the rivaroxaban group, compared with 1 of 3 in the antiplatelet group and 1 of 2 in the no SPAF group (Table 2). By multivariate Cox regression analysis, the model indicated that taking an antiplatelet modestly predicted the safety endpoints (adjusted HR 0.11 [95% CI 0.01‒1.07], P = .058) and MACE (HR 0.34 [95% CI 0.11‒1.02], P = .054) with reference to the anticoagulants (warfarin/rivaroxaban) (Table 3 and Figure 3). Also, the >104 weeks of dronedarone period was independently associated with fewer MACE (HR 0.14 [95% CI 0.04-0.44], P = .042) and modestly efficacy endpoint (HR 0.10 [95% CI 0.01-1.08], P = .058) after multivariate adjustment (Table 3 and Figure 3).
Table 1.
Demographic Data Stratified According to Different SPAF Strategies.
| Characteristic | Warfarin | (N = 89) | No SPAF | (N = 176) | Antiplatelet | (N = 331) | Rivaroxaban | (N = 93) | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Male, n (%) | 30 | (33.7) | 65 | (36.9) | 149 | (45.0) | 43 | (46.2) | .099 |
| Age (years), mean (SD) | 73.7 | (10.1) | 76.2 | (8.8) | 76.2 | (8.2) | 76.1 | (8.4) | .205 |
| Pacemaker, n (%) | 23 | (25.8) | 63 | (35.8) | 97 | (29.3) | 39 | (41.9) | .046 |
| CHA2DS2-VASc, mean (SD) | 3.7 | (1.4) | 3.8 | (1.3) | 3.9 | (1.6) | 3.6 | (1.2) | .490 |
| HAS-BLED, mean (SD) | 1.3 | (0.8) | 1.4 | (0.7) | 1.5 | (0.7) | 1.4 | (0.7) | .251 |
| Mitral regurgitation, n (%) | 51 | (57.3) | 120 | (68.2) | 218 | (65.9) | 58 | (62.4) | .323 |
| Thyroid disease, n (%) | 5 | (5.6) | 4 | (2.3) | 14 | (4.2) | 3 | (3.2) | .534 |
| GFR (mL/min/1.73 m2), mean (SD) | 65.9 | (28.8) | 60.1 | (28.0) | 63.0 | (25.2) | 62.9 | (23.3) | .220 |
| ≤60, n (%) | 35 | (39.3) | 83 | (47.2) | 141 | (42.6) | 35 | (37.6) | .420 |
| Dronedarone period (wk), mean (SD) | 109.1 | (65.8) | 100.1 | (68.7) | 107.1 | (71.6) | 80.3 | (39.8) | .048 |
| 12−52, n (%) | 85 | (25.7) | 57 | (32.4) | 85 | (25.7) | 27 | (29.0) | .123 |
| 52.1−104 | 102 | (30.8) | 51 | (29.0) | 102 | (30.8) | 38 | (40.9) | |
| >104 | 144 | (43.5) | 68 | (38.6) | 144 | (43.5) | 28 | (30.1) | |
| Follow-up period (wk), mean (SD) | 129.1 | (66.3) | 138.5 | (74.1) | 147.1 | (71.4) | 92.6 | (43.3) | <.001 |
Abbreviations: GFR, glomerular filtration rate; SD, standard deviation; SPAF, stroke prevention for atrial fibrillation.
Table 2.
Efficacy, Safety, and MACE Outcomes Stratified According to Different SPAF Strategies.
| Warfarin | (N = 89) | No SPAF | (N = 176) | Antiplatelet | (N = 331) | Rivaroxaban | (N = 93) | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Effectiveness endpoints, n (%) | 3 | (3.4) | 2 | (1.1) | 2 | (0.6) | 0 | (0.0) | .092 |
| New ischemic stroke, n (%) | 0 | (0.0) | 2 | (1.1) | 2 | (0.6) | 0 | (0.0) | .571 |
| New ICH, n (%) | 2 | (2.2) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | .042 |
| Systemic thromboembolism, n (%) | 2 | (2.2) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | .042 |
| Safety endpoints, n (%) | 2 | (2.2) | 1 | (0.6) | 1 | (0.3) | 2 | (2.2) | 0.162 |
| Hb fall ≥ 2 g/dL or transfusion ≥ 2 U PRBC, n (%) |
0 | (0.0) | 1 | (0.6) | 1 | (0.3) | 2 | (2.2) | .177 |
| Critical site bleeding, n (%) | 2 | (2.2) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | .042 |
| Fatal bleeding, n (%) | 2 | (2.2) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | .042 |
| Non-GI bleeding safety endpoints, n (%) | 2 | (2.2) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | .042 |
| GI bleeding, n (%) | 0 | (0.0) | 2 | (1.1) | 3 | (0.9) | 3 | (3.2) | .196 |
| Minor bleeding, n (%) | 1 | (1.1) | 2 | (1.1) | 1 | (0.3) | 3 | (3.2) | .101 |
| HF hospitalization, n (%) | 2 | (2.2) | 6 | (3.4) | 7 | (2.1) | 3 | (3.2) | .814 |
| MACE, n (%) | 6 | (6.7) | 7 | (4.0) | 9 | (2.7) | 0 | (0.0) | .063 |
| CV death, n (%) | 4 | (4.5) | 5 | (2.8) | 6 | (1.8) | 0 | (0.0) | .180 |
Abbreviations: CV, cardiovascular; GI, gastrointestinal; Hb, hemoglobin; HF, heart failure; ICH, intracerebral hemorrhage; MACE, major adverse cardiac event; SPAF, stroke prevention for atrial fibrillation.
Figure 2.
Kaplan-Meier curves on (A) efficacy endpoint (B) safety endpoint (C) MACE in AF patients receiving different SPAF strategies. AF indicates atrial fibrillation; MACE, major adverse cardiac event; SPAF, stroke prevention for atrial fibrillation.
Table 3.
Multivariate Cox Regression Analysis for Effectiveness, Safety, and MACE Outcomes.
| Effectiveness Endpoints | Safety Endpoints | MACE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| SPAF strategy | |||||||||
| Warfarin/rivaroxaban | 1.00 | 1.00 | 1.00 | ||||||
| No SPAF | 0.49 | (0.07–3.25) | .463 | 0.19 | (0.02–1.82) | .151 | 0.55 | (0.17–1.74) | .307 |
| Antiplatelet | 0.24 | (0.04–1.56) | .136 | 0.11 | (0.01–1.07) | .058 | 0.34 | (0.11–1.02) | .054 |
| Male | 1.16 | (0.21–6.48) | .866 | 0.40 | (0.05–3.02) | .378 | 2.37 | (0.95–5.92) | .066 |
| Age (years) | 1.09 | (0.98–1.21) | .100 | 1.07 | (0.96–1.20) | .219 | 1.05 | (0.99–1.11) | .126 |
| Pacemaker | 0.26 | (0.03–2.19) | .214 | 1.55 | (0.27–8.85) | .622 | 0.73 | (0.28–1.92) | .527 |
| CHA2DS2-VASc | 0.87 | (0.39–1.92) | .727 | 0.39 | (0.13–1.17) | .092 | 1.23 | (0.83–1.82) | .305 |
| HAS-BLED | 1.83 | (0.50–6.69) | .359 | 2.57 | (0.67–9.84) | .168 | 1.19 | (0.59–2.40) | .624 |
| Mitral regurgitation | 2.84 | (0.32–25.13) | .348 | 0.97 | (0.16–5.74) | .974 | 4.37 | (0.98–19.35) | .052 |
| Thyroid disease | — | — | — | — | — | — | 3.65 | (0.80–16.72) | .095 |
| GFR (mL/min/1.73 m2) | |||||||||
| >60 | 1.00 | 1.00 | 1.00 | ||||||
| ≤60 | 0.78 | (0.16–3.85) | .756 | 0.53 | (0.08–3.44) | .508 | 1.76 | (0.72–4.28) | .214 |
| Period of dronedarone (wk) | |||||||||
| 12–52 | 1.00 | 1.00 | 1.00 | ||||||
| 52.1–104 | 0.56 | (0.10–3.06) | .507 | 0.01 | (0.001–2.12) | .968 | 0.58 | (0.21–1.56) | .279 |
| >104 | 0.10 | (0.01–1.08) | .058 | 0.48 | (0.09–2.52) | .388 | 0.14 | (0.04–0.44) | .001 |
Abbreviations: CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio; MACE, major adverse cardiac event; SPAF, stroke prevention for atrial fibrillation.
Figure 3.
Multivariate Cox regression analysis for effectiveness, safety, and MACE. MACE indicates major adverse cardiac event.
Discussion
The main findings of the study were as follows: (1) physicians tended to neglect anticoagulants while administering dronedarone, (2) concomitant use of dronedarone and rivaroxaban 10 mg was feasible, and (3) the persistence of dronedarone use was independently associated with better outcome.
Restoring and maintaining sinus rhythm is an integral part of AF management, and anticoagulation should be continued indefinitely in patients who are at risk of stroke, regardless of paroxysmal or persistent AF. 5 In the AFFIRM study, the interrupted use of warfarin attenuated rhythm control efficacy. 16 Therefore, current guidelines recommend SPAF be determined only by the CHA2DS2-VASc score, regardless of the application of rhythm control. 5 Despite a lack of data to support aspirin in SPAF, the prevalence of aspirin use was high in many Asian countries before. 3 In a study conducted from 2001 to 2008, the proportion of SPAF with warfarin, aspirin, or no medication in Taiwan was 16%, 62%, and 22%, respectively. 3 In our dronedarone-treated population enrolled between 2009 and 2016, the dilemma remained even though NOAC had been initialized (12.9% of warfarin, 13.5% of rivaroxaban, 48.0% of antiplatelet and 25.6% of no medication). Therefore, we just happened to have the opportunity for this comparative retrospective study into various SPAF strategies on top of routine rhythm control.
A vital interaction mechanism for all NOACs consists of a significant resecretion via a P-glycoprotein (P-gp) transporter. 3 NOAC is a P-gp substrate and can inhibit P-gp and increase drug exposure. 17 Along with the strong effect of P-gp competition and CYP3A4 inhibition via dronedarone, 5 dose reduction of rivaroxaban is essential to keep anticoagulation in the TTR. In a Taiwan cohort, 70% of rivaroxaban recipients took 10 mg while coadministering dronedarone. 13 Our results indicate this combination did not increase the safety endpoints (P = .162). However, the numerically increased rate of minor bleeding (P = .101) also drives the need for proper dose selection. Hemorrhagic stroke occurred only in the group of dronedarone in combination with warfarin. Even though Asians were less intensely anticoagulated with warfarin, the incident rates of intracranial hemorrhage were much higher in Asians than in non-Asians. 3 These data confirmed the finding from the previous report that Asians are prone to bleeding when treated with warfarin. Like previous Asian surveys, the TTR 35% and 21% were similar in our study for INR 1.5 to 2.5 and 2.0 to 3.0, respectively. 3 Both hemorrhagic and ischemic outcomes were worse despite lower TTR, which was also comparable with the Asian phenomenon. When taking strokes and systemic thromboembolism (the effectiveness endpoints) into consideration, there were no major adverse cardiac incidents in the rivaroxaban group.
The annual risk of stroke and systemic thromboembolism was generally higher in Asians than in non-Asians, either on warfarin or on NOAC treatment. 3 However, over 31.5 months of follow-up, on average, the incidence of new ischemic stroke (0.6%, n = 4) was much lower than expected, and the events were only reported among patients using dronedarone alone or concomitant with an antiplatelet. The mean CHA2DS2-VASc score 3.8 ± 1.4 in our study is supposed to have estimated an annual stroke rate of 3.2% to 4.0%, and it would be even higher about 3.9% to 4.6% according to what Chao et al. 4 report in Taiwan. There were only small trends favoring rivaroxaban for the efficacy endpoint (P = .092) and MACE (P = .063) between the 4 SPAF strategies, which did not reach statistical significance. A subanalysis of ROCKET AF trial, which aimed at investigating the outcomes of different AAD in anticoagulated patients, revealed similar findings. 18 Concomitant AADs ([HR] 0.66; 95% [95% CI 0.37‒1.17]) were numerically associated with less mortality and embolism. The stroke rate in the dronedarone alone group was 1.1%, and several published studies have also revealed dronedarone resulted in a reduced stroke rate of 1.6% versus 2.5% for the placebo group.11,19 There are several potential mechanisms by which dronedarone may reduce the risk of stroke. The suppression of AF burden and the restoration of atrial contraction to prevent stroke are logical and appealing. Cox regression is a method for investigating the effect of several variables upon the time a specified event takes to happen. 20 Interestingly, in our model of Cox regression analysis, the period of dronedarone persistence was the strongest predictor for fewer MACE, after adjusting the SPAF strategies and related confounding covariates listed in Table 3. It has been recommended that rhythm control should be used early and persistently.10,21 The finding supports further investigation of causality.
This retrospective study has both strengths and limitations. To our knowledge, the present study is the first in the NOAC era to assess real-world outcomes of rhythm control by dronedarone and its co-administration with 10 mg rivaroxaban. Second, we highlight the changing physician preference in SPAF strategies employed in Taiwan from 2009 to 2016. The analysis offers valuable insights into physician decisions and the related outcomes that are often overlooked in randomized control trials. However, we should only imply the data to high-risk populations due to restricted dronedarone indication in Taiwan. Otherwise, the limitations might come from the various follow-up periods and potential confounding factors even after adjustment analyses. The nonrandomized approach of our study also created imbalances in baseline characteristics which may have further influenced our results.
Conclusion
Physicians tended to neglect the use of OAC while administering dronedarone. However, the dronedarone-treated cohort with high-risk nonpermanent AF presented a low incidence of new strokes, irrespective of the SPAF strategies. The adjusted 10-mg rivaroxaban, in combination with dronedarone, did not increase major safety endpoints. Furthermore, longer persistence of dronedarone use was associated with fewer incidents of MACE. “Our findings warrant further investigations and require confirmation in hypothesis-driven and prospectively-powered, randomized, clinical trials.”
Footnotes
Author Contributions: Conceived and designed the analysis: Lin, Chiou, Su, Lee.
Collected the data: Lin, Liao, Liu, Lee.
Contributed data or analysis tools: Huang, Chen.
Performed the analysis: Lin, Tsai, Kuo, Wu, Lee.
Wrote the paper: Lin, Lee.
Availability of Data and Material: Not applicable.
Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Code Availability: Not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: The Institutional Review Board of MacKay Memorial Hospital approved the study protocol (IRB No. 16MMHIS009), which waived the requirement for informed consent in this retrospective study.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Ying-Hsiang Lee https://orcid.org/0000-0002-1882-9664
References
- 1.Chien KL, Su TC, Hsu HCet al. et al. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. International Journal of Cardiology. 2010;139:173-180. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham study. Archives of Internal Medicine. 1987;147:1561-1564. [PubMed] [Google Scholar]
- 3.Chiang C-E, Wu T-J, Ueng K-Cet al. 2016 guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. Journal of the Formosan Medical Association. 2016;115:893-952. [DOI] [PubMed] [Google Scholar]
- 4.Chao TF, Liu CJ, Wang KLet al. Using the CHA2DS2-VASc score for refining stroke risk stratification in ‘low-risk’ Asian patients with atrial fibrillation. Journal of the American College of Cardiology. 2014;64:1658-1665. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhof P, Benussi S, Kotecha Det al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. [DOI] [PubMed] [Google Scholar]
- 6.Hindricks G, Potpara T, Dagres Net al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;42(5):373-498. [DOI] [PubMed] [Google Scholar]
- 7.Sugihara C, Veasey R, Freemantle N, Podd S, Furniss S, Sulke N. The development of AF over time in patients with permanent pacemakers: objective assessment with pacemaker diagnostics demonstrates distinct patterns of AF. Europace : European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology. 2015;17:864-870. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GYet al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369-2429. [DOI] [PubMed] [Google Scholar]
- 9.Ezekowitz MD, Ellenbogen KA, DiMarco JPet al. et al. A placebo-controlled, double-blind, randomized, multicenter study to assess the effects of dronedarone 400 mg twice daily for 12 weeks on atrial fibrillation burden in subjects with permanent pacemakers. Journal of Interventional Cardiac Electrophysiology: an International Journal of Arrhythmias and Pacing . 2015;42:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin PL, Huang CC, Wu YJet al. Relations between baseline burden, maximum duration, and relative reduction of atrial fibrillation: insights from continuous monitoring in rhythm control. J Cardiovasc Electrophysiol. 2019;30:178-182. [DOI] [PubMed] [Google Scholar]
- 11.Hohnloser SH, Crijns HJ, van Eickels Met al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668-678. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhof P, Camm AJ, Goette Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020; 383:1305-1316. [DOI] [PubMed] [Google Scholar]
- 13.Chiou WR, Huang CC, Lin PLet al. Safety and effectiveness of rivaroxaban in combination with various antiarrhythmic drugs in patients with non-permanent atrial fibrillation. Am J Cardiovasc Drugs. 2020;21(4):459-469. [DOI] [PubMed] [Google Scholar]
- 14.Wann LS, Curtis AB, January CTet al. Members, A.A.T.F. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2011;123:104-123. [DOI] [PubMed] [Google Scholar]
- 15.Jaakkola S, Kiviniemi TO, Nuotio Iet al. Usefulness of the CHA2DS2-VASc and HAS-BLED scores in predicting the risk of stroke versus intracranial bleeding in patients with atrial fibrillation (from the FibStroke study). Am J Cardiol . 2018;121:1182-1186. [DOI] [PubMed] [Google Scholar]
- 16.Wyse DG, Waldo AL, DiMarco JPet al. A comparsion of rate control and rhythm control in patients with atrail fibrillation. N Engl J Med . 2012;347:1825-1833. [DOI] [PubMed] [Google Scholar]
- 17.Mendell J, Zahir H, Matsushima Net al. et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013;13:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg BA, Hellkamp AS, Lokhnygina Yet al. Use and outcomes of antiarrhythmic therapy in patients with atrial fibrillation receiving oral anticoagulation: results from the ROCKET AF trial. Heart Rhythm. 2014;11:925-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christiansen CB, Torp-Pedersen C, Kober L. Impact of dronedarone in atrial fibrillation and flutter on stroke reduction. Clinical Interventions in Aging. 2010;5:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part II: multivariate data analysis--an introduction to concepts and methods. Br J Cancer . 2003;89:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng C-J, Li C-H, Liao Y-Cet al. et al. Rhythm control better prevents stroke and mortality than rate control strategies in patients with atrial fibrillation - A nationwide cohort study. International Journal of Cardiology. 2018;270:154-159. [DOI] [PubMed] [Google Scholar]