Abstract
Objective
To examine the prevalence and predictors of patient-reported barriers to care among survivors of head and neck squamous cell carcinoma and the association with health-related quality of life (HRQOL) outcomes.
Study Design
Retrospective cohort study.
Setting
Outpatient oncology clinic at an academic tertiary care center.
Methods
Data were obtained from the UNC Health Registry/Cancer Survivorship Cohort. Barriers to care included self-reported delays in care and inability to obtain needed care due to cost. HRQOL was measured with validated questionnaires: general (PROMIS) and cancer specific (FACT-GP).
Results
The sample included 202 patients with head and neck squamous cell carcinoma with a mean age of 59.6 years (SD, 10.0). Eighty-two percent were male and 87% were White. Sixty-two patients (31%) reported at least 1 barrier to care. Significant predictors of a barrier to care in unadjusted analysis included age ≤60 years (P = .007), female sex (P = .020), being unmarried (P = .016), being uninsured (P = .047), and Medicaid insurance (P = .022). Patients reporting barriers to care had significantly worse physical and mental HRQOL on the PROMIS questionnaires (P < .001 and P = .002, respectively) and lower cancer-specific HRQOL on the FACT-GP questionnaire (P < .001), which persisted across physical, social, emotional, and functional domains. There was no difference in 5-year OS (75.3% vs 84.1%, P = .177) or 5-year CSS (81.6% vs 85.4%, P = .542) in patients with and without barriers to care.
Conclusion
Delay- and affordability-related barriers are common among survivors of head and neck cancer and appear to be associated with significantly worse HRQOL outcomes. Certain sociodemographic groups appear to be more at risk of patient-reported barriers to care.
Keywords: health services accessibility, head and neck neoplasms, patient-reported outcome measures, survivorship, barriers
Head and neck squamous cell carcinoma (HNSCC) contributes to a significant burden of disease in the United States with an estimated 65,410 new cases and 14,620 deaths in 2019.1,2 Despite advancements in treatment, there has been relatively little improvement in oncologic outcomes for patients with HNSCC over the past few decades.3,4 Achieving optimal outcomes for patients with HNSCC relies on timely diagnosis, treatment, and posttreatment surveillance. 5 This can be especially challenging in patients with head and neck cancer because these patients on average have lower socioeconomic status when compared with patients who have other types of cancer 6 and therefore may face many barriers across the continuum of cancer care.
Although there is evidence to suggest that delays in diagnosis, treatment initiation, and time to postoperative radiation are associated with worse survival in HNSCC, there is limited insight into subjective patient-reported barriers to care that may drive these findings. In one study that conducted qualitative interviews of 24 patients with HNSCC, Carroll et al identified several patient-reported barriers, such as transportation, perceived cost, and failure to recognize initial symptoms as a problem. 7 In a study that used patient and provider interviews to assess barriers to timely postoperative radiation therapy in HNSCC, Graboyes et al identified inadequate patient education, postsurgical sequelae, insufficient coordination during care transitions, fragmentation of care across organizations, and patient travel burden as important contributors. 8
A better understanding of patient-reported barriers to care in HNSCC can help identify new targets for intervention aimed at improving oncologic and patient-reported outcomes in this population. To help address this gap in literature, we assessed patient-reported barriers to care among an institutional cohort of patients with HNSCC. We examined the relationship between demographic, social, and clinical variables and reported barriers to care. We also examined associations of barriers to care with health-related quality of life (HRQOL) and survival outcomes.
Materials
This study was approved by the Institutional Review Board at the University of North Carolina (UNC) at Chapel Hill. All subjects provided consent to participate in this study.
Study Design and Sample Selection
We performed a retrospective cross-sectional analysis of patients with head and neck cancer identified through the UNC Health Registry/Cancer Survivorship Cohort (HR/CSC). The HR/CSC is a cohort of patients who presented to UNC oncology outpatient clinics at the North Carolina Cancer Hospital between May 2012 through July 2016. In this sample, 144 (71.3%) cases were incident (enrolled before treatment initiation), and 58 (28.7%) cases were prevalent (enrolled anytime during or after treatment). The mean follow-up was 5.5 years (SD, 2.30) after initial diagnosis. Patients were eligible to participate in the HR/CSC if they were at least 18 years of age and had English or Spanish language proficiency. Patients meeting these eligibility criteria were approached by research staff in the oncology clinic and, upon informed consent, were enrolled in the HR/CSC.
Of the eligible patients with HNSCC approached in clinic for enrollment, 64% consented to participate. When compared with patients who consented to participate, those who were approached but declined to participate were older (mean [SD], 65.2 [11.0] vs 59.2 [12.5] years; P = .007) and more likely to have advanced-stage cancer (III/IV; 81.2% vs 59.1%, P = .002); there was no significant difference in sex (36.1% vs 34.0% female, P = .808) or race (18.5% vs 13.9% Black, P = .525) between those who consented and declined to participate. Patients completed the study questionnaires at a median number of 27 days after enrollment via a computer-assisted telephone interview. Patients from the HR/CSC were included in this analysis if they had a pathologically confirmed diagnosis of HNSCC.
Questionnaires and Data Extraction
Information on demographics, socioeconomic status, medical history, and barriers to care was obtained via a baseline questionnaire. Barriers to care were elicited through 2 questions: “Have you delayed getting care for any of the following reasons in the past year?” and “During the past year, was there any time when you needed any of the following but didn’t get it because you couldn’t afford it?” A full listing of response options is provided in Supplement A (available online). For this analysis, patients were considered to have a barrier to care if they responded “yes” to at least 1 option in question 1 or 2. Questions and response options in Supplement A were derived from the 2010 National Health Interview Survey section on adult access to health care and utilization 9 and the Carolina Head and Neck Cancer Epidemiology Study. 10 In addition to the literature that informed the creation of these 2 surveys, there is evidence supporting the specific patient-reported barriers queried in our study across a variety of cancer types.11-16
Clinical data were extracted from patient medical records, including tumor site, American Joint Committee on Cancer stage (seventh edition), and p16 tumor status. HRQOL outcomes were measured with the PROMIS questionnaire (Patient-Reported Outcomes Measurement Information System) 17 and the FACT-GP questionnaire (Functional Assessment of Cancer Therapy–General Population), 18 which is divided into physical, social, emotional, and functional domains.
Statistical Analysis
We used descriptive statistics to examine the sociodemographic and clinical characteristics of patients with and without barriers to care. Bivariate testing methods included the 2-sided t test, chi-square test, and Fisher’s exact test (for <5 expected observations). We next used univariable and multivariable logistic regression models to estimate significant predictors of having a barrier to care. In addition, we performed a stratified analysis examining delay- and affordability-related barriers separately. Multicollinearity was assessed with variance inflation factor testing. Number of treatment modalities was omitted from the multivariable model due to collinearity with treatment types. We also performed a sensitivity analysis to determine if incident vs prevalent case status (proxy for time between diagnosis and enrollment) had any impact on our primary outcome of patient-reported barriers to care.
We used simple and multiple linear regression models to examine the relationship of barriers to care with HRQOL. For the PROMIS scales, we used T scores normalized to population means. We next constructed Kaplan-Meier curves to assess 5-year overall survival (OS) and cancer-specific survival (CSS) in patients with and without barriers to care. We used the log-rank test to compare the survival curves and obtain P values. We used unadjusted and adjusted Cox proportional hazards models to obtain hazard ratio (HR) and 95% CI estimates for 5-year survival outcomes with respect to barriers to care. The proportional hazards assumption was assessed through Schoenfeld residuals and was met for all variables. Updates on patient vital status were provided monthly up until October 1, 2020, by the North Carolina Department of Health and Human Services. Vital status and cause of death were known for all subjects at 5 years of follow-up from initial diagnosis.
The multivariable logistic regression, multiple linear regression, and adjusted Cox proportional hazards models all adjusted for the following: age, sex, race, marital status, education, insurance status, employment status, distance to hospital, history of tobacco use, history of alcohol use, comorbid depression, comorbid anxiety, tumor site, overall stage, and treatment type. We used a statistical significance criterion of P < .05 for all testing. Adjustment for multiplicity was not performed because this study was strictly exploratory; therefore, any significant results should be interpreted as exploratory and warrant confirmation in additional studies. 19 We used Stata 16.0 (StataCorp LP) for all analyses.
Results
The sample included 202 patients with HNSCC with a mean age of 59.6 years (SD, 10.0). Patients completed the study questionnaires on average 13.8 months (range, 6 days–255 months) after initial diagnosis. Eighty-two percent of patients were male and 87% were White. The majority of patients had private insurance or Medicare (88%), and 70% had advanced-stage cancer (III/IV) at diagnosis. Baseline characteristics are summarized, stratified by patients with and without barriers to care ( Table 1 ).
Table 1.
Baseline Characteristics. a
| Patients reporting barriers to care, No. (%) | |||
|---|---|---|---|
| Yes (n = 62) | No (n = 140) | P value | |
| Age, y, mean (SD) | 57.0 (9.2) | 60.8 (10.2) | .012 |
| Sex | .018 | ||
| Male | 45 (72.6) | 121 (86.4) | |
| Female | 17 (27.4) | 19 (13.6) | |
| Race | .067 | ||
| White | 50 (80.7) | 126 (90.0) | |
| Non-White | 12 (19.3) | 14 (10.0) | |
| Marital status | .015 | ||
| Married | 35 (57.4) | 103 (74.6) | |
| Not married | 26 (42.6) | 35 (25.4) | |
| Education | .091 | ||
| High school or less | 44 (73.3) | 80 (58.0) | |
| College graduate | 11 (18.3) | 33 (23.9) | |
| Postgraduate/ professional degree | 5 (8.3) | 25 (18.1) | |
| Insurance | .022 | ||
| Private | 31 (50.0) | 84 (60.0) | |
| Uninsured | 5 (8.1) | 3 (2.1) | |
| Medicare | 17 (27.4) | 46 (32.9) | |
| Medicaid | 9 (14.5) | 7 (5.0) | |
| Currently work for pay | 27 (44.3) | 68 (49.3) | .514 |
| Distance to hospital, miles, mean (SD) | 72.8 (71.6) | 69.0 (57.6) | .706 |
| History | |||
| Tobacco use | 40 (64.5) | 81 (61.4) | .673 |
| Alcohol use | 32 (51.6) | 67 (47.9) | .622 |
| Comorbid | |||
| Depression | 16 (25.8) | 13 (9.6) | .003 |
| Anxiety | 17 (27.4) | 17 (12.5) | .010 |
| Tumor site | .890 | ||
| Oral cavity | 21 (33.9) | 53 (37.9) | |
| Oropharynx b | 29 (46.8) | 67 (47.9) | |
| Hypopharynx | 2 (3.2) | 4 (2.9) | |
| Larynx | 7 (11.3) | 10 (7.1) | |
| Other | 3 (4.8) | 6 (4.3) | |
| Overall stage: AJCC (7th ed) | .859 | ||
| Early: I/II | 18 (30.5) | 38 (29.2) | |
| Advanced: III/IV | 41 (69.5) | 92 (70.8) | |
| Treatment | .621 | ||
| Surgery alone | 21 (33.9) | 37 (26.6) | |
| Surgery + adjuvant RT | 7 (11.3) | 18 (13.0) | |
| Surgery + adjuvant CRT | 11 (17.7) | 37 (26.6) | |
| RT or chemotherapy alone | 6 (9.7) | 10 (7.2) | |
| CRT | 17 (27.4) | 37 (26.6) | |
| No. of treatment modalities | .235 | ||
| 1 | 27 (43.6) | 45 (32.9) | |
| 2 | 24 (38.7) | 55 (40.1) | |
| 3 | 11 (17.7) | 37 (27.0) | |
Abbreviations: CRT, chemoradiation therapy; RT, radiation therapy.
Bold indicates P < .05.
p16 status was available for 35 patients with oropharyngeal squamous cell carcinoma.
Patient-Reported Barriers to Care
In total, 62 patients (31%) cited at least 1 barrier to care: 33 patients, a delay-related barrier (Supplement A, question 1); 13 patients, an affordability-related barrier (Supplement A, question 2); and 16 patients, a delay- and affordability-related barrier.
Because of a 3-way tie by number of responses (n = 10), the top 3 most frequently reported delay-related barriers actually became the 5 most frequently reported: “You couldn’t get an appointment soon enough” (n = 22), “The clinic or doctor’s office wasn’t open when you got there” (n = 13), “You couldn’t get through on the telephone” (n = 10), “You did not have health insurance” (n = 10), and “You did not have the money you needed to pay expenses” (n = 10). The 3 most frequently reported affordability-related barriers were “dental care, including check-ups” (n = 18), “doctor’s visit” (n = 9), and “over-the-counter medicine” (n = 9).
Predictors of Patient-Reported Barriers to Care
In the univariable analysis, significant predictors of having a barrier to care included age ≤60 years (odds ratio [OR], 2.35 [95% CI, 1.27-4.37]; P = .007), female sex (OR, 2.41 [95% CI, 1.15-5.03]; P = .020), being unmarried (OR, 2.19 [95% CI, 1.16-4.13]; P = .016), being uninsured (OR, 4.52 [95% CI, 1.02-20.03]; P = .047), and having Medicaid (OR, 3.48 [95% CI, 1.19-10.16]; P = .022; Table 2 , Figure 1 ). Comorbid depression and anxiety were also significantly associated with having a barrier to care (P = .004 and P = .012, respectively). In a sensitivity analysis, there was no difference in prevalent/incident case status in patients with and without barriers to care (27.4% vs 29.3% prevalent cases, P = .787).
Table 2.
Univariable Logistic Regression Model for Predictors of Barriers to Care. a
| Any barrier | Delay barrier | Affordability barrier | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age ≤60 vs >60 y | 2.35 (1.27-4.37) | .007 | 2.29 (1.17-4.47) | .015 | 1.77 (0.79-3.98) | .164 |
| Female sex | 2.41 (1.15-5.03) | .020 | 2.04 (0.94-4.43) | .071 | 1.58 (0.62-4.04) | .340 |
| Non-White race vs White | 2.16 (0.93-4.99) | .072 | 2.20 (0.92-5.22) | .075 | 1.10 (0.35-3.46) | .873 |
| Not married vs married | 2.19 (1.16-4.13) | .016 | 1.63 (0.83-3.20) | .157 | 3.14 (1.39-7.09) | .006 |
| Education vs college graduate | ||||||
| High school or less | 1.65 (0.76-3.58) | .205 | 1.23 (0.55-2.77) | .612 | 5.04 (1.14-22.29) | .033 |
| Postgraduate/professional degree | 0.60 (0.18-1.95) | .396 | 0.68 (0.21-2.24) | .526 | 0.72 (0.06-8.36) | .796 |
| Insurance vs private | ||||||
| Uninsured | 4.52 (1.02-20.03) | .047 | 5.71 (1.28-25.48) | .023 | 1.35 (0.15-12.01) | .787 |
| Medicare | 1.00 (0.50-2.00) | .997 | 0.89 (0.42-1.89) | .761 | 1.78 (0.71-4.47) | .217 |
| Medicaid | 3.48 (1.19-10.16) | .022 | 1.56 (0.50-4.88) | .449 | 7.35 (2.30-23.62) | .001 |
| Currently work for pay | 0.82 (0.45-1.50) | .514 | 0.77 (0.40-1.48) | .431 | 0.67 (0.30-1.51) | .336 |
| Distance to hospital >52 miles (median) | 1.05 (0.56-1.97) | .881 | 1.11 (0.56-2.17) | .764 | 0.60 (0.26-1.41) | .241 |
| History | ||||||
| Tobacco use | 1.14 (0.61-2.14) | .673 | 0.94 (0.48-1.82) | .848 | 1.71 (0.71-4.08) | .230 |
| Alcohol use | 1.16 (0.64-2.11) | .622 | 1.38 (0.72-2.63) | .328 | 0.70 (0.31-1.55) | .376 |
| Comorbid | ||||||
| Depression | 3.29 (1.47-7.37) | .004 | 3.00 (1.32-6.81) | .009 | 3.35 (1.34-8.37) | .010 |
| Anxiety | 2.64 (1.24-5.62) | .012 | 3.02 (1.39-6.55) | .005 | 2.10 (0.84-5.23) | .113 |
| Tumor site vs oral cavity | ||||||
| Oropharynx | 1.09 (0.56-2.13) | .795 | 1.06 (0.52-2.16) | .881 | 1.19 (0.50-2.81) | .700 |
| Hypopharynx | 1.26 (0.21-7.42) | .797 | 0.67 (0.07-6.14) | .724 | 1.28 (0.14-12.12) | .830 |
| Larynx | 1.77 (0.59-5.26) | .306 | 1.83 (0.60-5.68) | .296 | 0.85 (0.17-4.31) | .848 |
| Other | 1.26 (0.29-5.12) | .757 | 0.96 (0.18-5.05) | .960 | 0.80 (0.09-7.10) | .841 |
| Advanced stage vs early | 0.94 (0.48-1.84) | .859 | 0.83 (0.41-1.70) | .611 | 0.72 (0.32-1.68) | .446 |
| Treatment vs surgery alone | ||||||
| Surgery + adjuvant RT | 0.69 (0.25-1.91) | .469 | 0.46 (0.14-1.54) | .207 | 0.58 (0.15-2.30) | .441 |
| Surgery + adjuvant CRT | 0.52 (0.22-1.24) | .141 | 0.63 (0.26-1.56) | .321 | 0.39 (0.12-1.31) | .127 |
| RT or chemotherapy alone | 1.06 (0.34-3.32) | .924 | 1.10 (0.33-3.64) | .881 | 0.61 (0.12-3.09) | .550 |
| CRT | 0.81 (0.37-1.78) | .598 | 0.76 (0.33-1.77) | .532 | 0.85 (0.32-2.26) | .751 |
| No. of treatment modalities vs 1 | ||||||
| 2 | 0.73 (0.37-1.43) | .356 | 0.62 (0.30-1.30) | .207 | 0.81 (0.34-1.92) | .636 |
| 3 | 0.50 (0.22-1.13) | .095 | 0.60 (0.25-1.41) | .240 | 0.41 (0.31-1.35) | .144 |
Abbreviations: CRT, chemoradiation therapy; OR, odds ratio; RT, radiation therapy.
Bold indicates P < .05.
Figure 1.
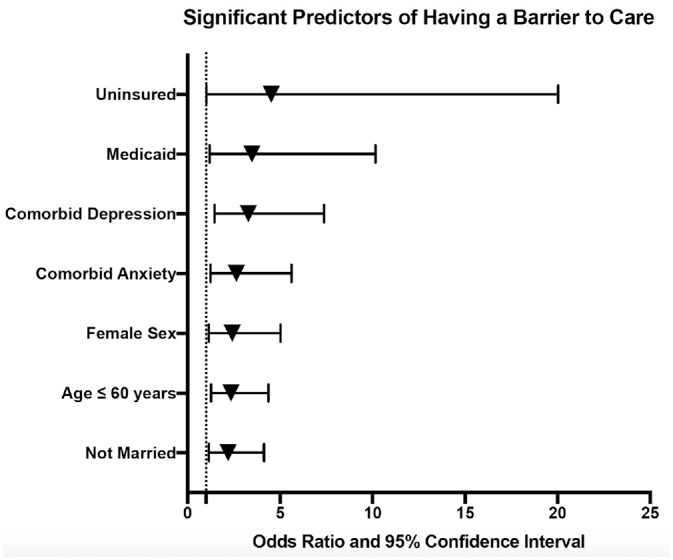
Forest plot displaying significant predictors of having a barrier to care based on the univariable logistic regression models.
In the stratified analysis, the associations for age ≤60 years (P = .015), having no insurance (P = .023), and comorbid anxiety (P = .005) persisted only for delay-related barriers ( Table 2 ). The associations for being unmarried (P = .006) and having Medicaid (P = .001) persisted just for affordability-related barriers. Furthermore, education of high school or less was associated with having an affordability-related barrier (P = .033). A subset analysis was performed to assess the association between p16-positive tumor status and barriers to care among patients with oropharyngeal squamous cell carcinoma (OPSCC). Among patients with available tumor p16 status, there were 26 cases of p16+ OPSCC and 9 cases of p16– OPSCC. There was no significant difference in the rate of p16 positivity in patients with and without barriers to care (75.0% vs 73.9%, respectively; P > .999).
In the multivariable logistic regression model, age ≤60 years (OR, 3.65 [95% CI, 1.28-10.39]; P = .015) and treatment with surgery plus adjuvant chemoradiation (OR, 0.27 [95% CI, 0.08-0.99]; P = .048) remained the only significant associations with having a barrier to care ( Table 3 ).
Table 3.
Multivariable Logistic Regression Model for Predictors of Barriers to Care. a
| Any barrier b | ||
|---|---|---|
| OR (95% CI) | P value | |
| Age ≤60 vs >60 y | 3.65 (1.28-10.39) | .015 |
| Female sex | 2.65 (0.89-7.88) | .079 |
| Non-White race vs White | 1.66 (0.51-5.36) | .397 |
| Not married vs married | 1.40 (0.57-3.44) | .463 |
| Education vs college graduate | ||
| High school or less | 1.37 (0.48-3.90) | .550 |
| Postgraduate/professional degree | 0.67 (0.16-2.77) | .581 |
| Insurance vs private | ||
| Uninsured | 6.09 (0.97-38.38) | .054 |
| Medicare | 1.72 (0.56-5.26) | .345 |
| Medicaid | 1.65 (0.34-8.04) | .537 |
| Currently work for pay | 0.64 (0.25-1.66) | .360 |
| Distance to hospital >52 miles (median) | 1.02 (0.46-2.26) | .965 |
| History | ||
| Tobacco use | 0.98 (0.39-2.44) | .967 |
| Alcohol use | 1.14 (0.50-2.59) | .750 |
| Comorbid | ||
| Depression | 1.77 (0.51-6.23) | .371 |
| Anxiety | 2.62 (0.74-9.28) | .136 |
| Tumor site vs oral cavity | ||
| Oropharynx | 1.41 (0.45-4.40) | .558 |
| Hypopharynx | 1.45 (0.14-14.85) | .755 |
| Larynx | 0.86 (0.16-4.53) | .862 |
| Other | 1.94 (0.28-13.47) | .504 |
| Advanced stage vs early | 1.85 (0.62-5.54) | .272 |
| Treatment vs surgery alone | ||
| Surgery + adjuvant RT | 0.76 (0.17-3.40) | .723 |
| Surgery + adjuvant CRT | 0.27 (0.08-0.99) | .048 |
| RT or chemotherapy alone | 1.69 (0.32-9.05) | .538 |
| CRT | 0.69 (0.20-2.47) | .573 |
Abbreviations: CRT, chemoradiation therapy; OR, odds ratio; RT, radiation therapy.
Bold indicates P < .05.
Adjusted for age, sex, race, marital status, education, insurance status, employment status, distance to hospital, history of tobacco use, history of alcohol use, comorbid depression, comorbid anxiety, tumor site, overall stage, and treatment type.
HRQOL Outcomes
In the unadjusted model, patients citing a barrier to care had significantly worse HRQOL on general (PROMIS) and cancer-specific (FACT-GP) quality-of-life scales ( Table 4 ). Specifically, patients indicating a barrier to care had worse physical HRQOL (mean difference [MD], −4.79 [95% CI, −7.41 to −2.17]; P < .001) and mental HRQOL (MD, −4.14 [95% CI, −6.78 to −1.50]; P = .002) on the PROMIS scales. On the FACT-GP scale, patients who had a barrier to care had significantly worse HRQOL scores: physical (MD, −2.03 [95% CI, −3.52 to −0.55]; P = .008), social (MD, −2.02 [95% CI, −3.68 to −0.35]; P = .018), emotional (MD, −2.18 [95% CI, −3.54 to −0.81]; P = .002), and functional (MD, −2.61 [95% CI, −4.77 to −0.44]; P = .019). Having a barrier to care was not significantly associated with any of the HRQOL scores in the fully adjusted models.
Table 4.
Relationship Between Barriers to Care and Health-Related Quality of Life.
| Patients reporting barriers, mean (SD) | Unadjusted | Adjusted a | ||||
|---|---|---|---|---|---|---|
| Survey: domain | Yes | No | MD (95% CI) | P value | MD (95% CI) | P value |
| FACT-GP | ||||||
| Total | 75.3 (17.7) | 84.1 (15.4) | −8.83 (−13.68 to −3.99) | <.001 | −4.50 (−9.81 to 0.81) | .096 |
| Physical | 21.3 (5.4) | 23.4 (4.7) | −2.03 (−3.52 to −0.55) | .008 | −1.35 (−3.02 to 0.33) | .114 |
| Social | 19.6 (5.8) | 21.6 (5.4) | −2.02 (−3.68 to −0.35) | .018 | −0.67 (−2.53 to 1.19) | .478 |
| Emotional | 18.0 (5.7) | 20.2 (3.9) | −2.18 (−3.54 to −0.81) | .002 | −1.27 (−2.85 to 0.31) | .115 |
| Functional | 16.4 (7.1) | 19.0 (7.2) | −2.61 (−4.77 to −0.44) | .019 | −1.21 (−3.67 to 1.24) | .330 |
| PROMIS | ||||||
| Physical | 44.9 (8.5) | 49.7 (8.8) | −4.79 (−7.41 to −2.17) | <.001 | −2.89 (−5.83 to 0.05) | .054 |
| Mental | 49.6 (9.3) | 53.7 (8.5) | −4.14 (−6.78 to −1.50) | .002 | −1.04 (−3.90 to 1.82) | .472 |
Abbreviations: FACT-GP, Functional Assessment of Cancer Therapy–General Population; MD, mean difference; PROMIS, Patient-Reported Outcomes Measurement Information System.
Adjusted for age, sex, race, marital status, education, insurance status, employment status, distance to hospital, history of tobacco use, history of alcohol use, comorbid depression, comorbid anxiety, tumor site, overall stage, and treatment type.
Survival Outcomes
The mean time from diagnosis to last follow-up was 5.5 years (SD, 2.30), and vital status at 5 years was known for all participants in this study. The 5-year OS rate for the cohort was 81.7%, and the 5-year CSS rate was 84.6%. There was no significant difference in 5-year OS (75.3% vs 84.1%; log-rank P = .177) or CSS (81.6% vs 85.4%; log-rank P = .542) in patients with and without barriers to care, respectively ( Figures 2 and 3 ).
Figure 2.
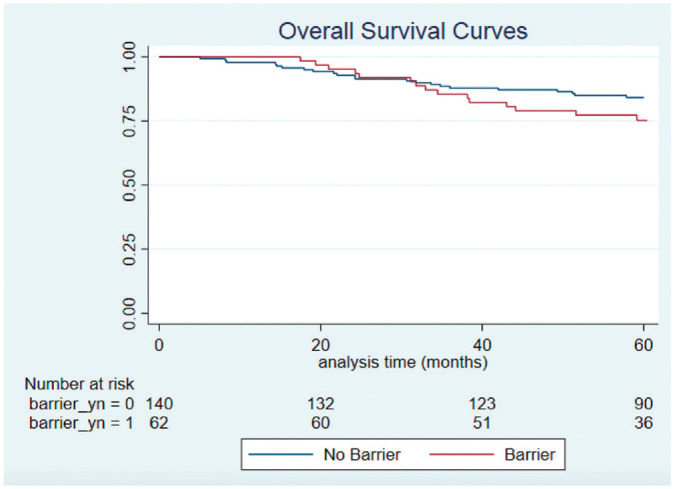
Kaplan-Meier curves for overall survival in patients with and without barriers to care.
Figure 3.
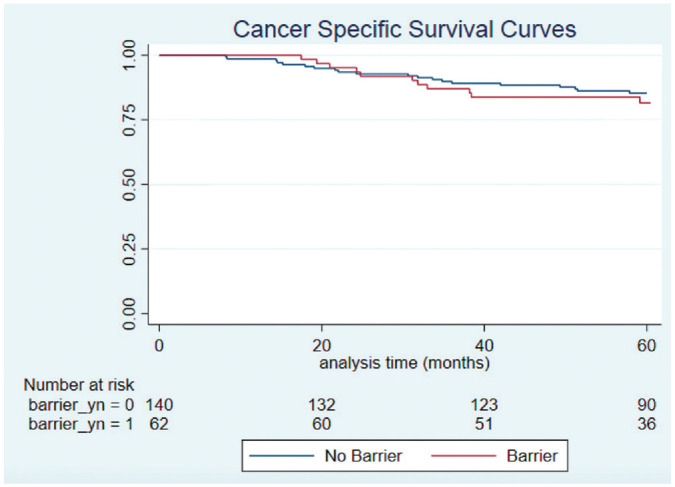
Kaplan-Meier curves for cancer-specific survival in patients with and without barriers to care.
In the unadjusted and adjusted Cox proportional hazard models, there were no significant associations between barriers to care and either 5-year OS or CSS ( Table 5 ). This nonsignificant effect persisted even when stratifying by patients who had a delay- and affordability-related barrier (P = .438 for OS and P = .395 for CSS).
Table 5.
Cox Proportional Hazards Models for 5-Year Mortality With Respect to Patient-Reported Barriers to Care. a
| Unadjusted | Adjusted b | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| 5-y OS | ||||
| Any barrier | 1.57 (0.81-3.02) | .181 | 2.34 (0.92-5.97) | .075 |
| Delay only | 1.59 (0.71-3.59) | .257 | 1.91 (0.74-4.95) | .182 |
| Affordability only | 1.54 (0.46-5.15) | .484 | 2.45 (0.81-7.45) | .114 |
| Delay and affordability | 1.53 (0.53-4.43) | .438 | 1.91 (0.61-6.05) | .269 |
| 5-y CSS | ||||
| Any barrier | 1.26 (0.60-2.62) | .542 | 2.48 (0.88-6.95) | .084 |
| Delay only | 1.53 (0.65-3.62) | .332 | 1.82 (0.60-5.56) | .290 |
| Affordability only | 1.68 (0.50-5.65) | .404 | 1.48 (0.39-5.52) | .563 |
| Delay and affordability | 0.42 (0.06-3.12) | .395 | 3.48 (0.92-13.26) | .067 |
Abbreviations: CSS, cancer-specific survival; HR, hazard ratio; OS, overall survival.
Reference variable is “no barriers” for all models.
Adjusted for age, sex, race, marital status, education, insurance status, employment status, distance to hospital, history of tobacco use, history of alcohol use, comorbid depression, comorbid anxiety, tumor site, overall stage, and treatment type.
Discussion
In this study we used a cross-sectional institutional cohort of HNSCC survivors to retrospectively assess patient-reported barriers to care and their relationship with HRQOL and survival outcomes. The prevalence of patient-reported barriers to care in our sample was 31%, which included delay- and affordability-related barriers. Younger (P = .007), female (P = .020), unmarried (P = .016), uninsured (P = .047), and Medicaid-insured (P = .022) patients were significantly more likely to have a barrier to care in the unadjusted analysis. Patients citing a barrier to care had significantly worse HRQOL on general and cancer-specific questionnaires in the unadjusted analysis, but there was no association with OS or CSS at 5 years. The sociodemographic and HRQOL associations with barriers to care were mostly lost in the adjusted models. Despite this, our exploratory study provides novel insight into which patients may be at most risk for barriers to care and can help inform future research.
Several studies in current literature have reported an association between low socioeconomic status and advanced stage at presentation for HNSCC,20-23 which may be a proxy for delays in cancer diagnosis. In our stratified analysis, indicators of low socioeconomic status, such as having no insurance, Medicaid insurance, and education of high school or less, were all associated with patient-reported barriers to care. Our findings that younger age and unmarried status predict patient-reported barriers to care may be explained by reduced time or financial resources to obtain optimal care. Other studies have found an association between unmarried status and delays in diagnosis of HNSCC.24,25 In a study assessing delays in diagnosis of HPV-positive oropharyngeal cancer, Karp et al noted that retired patients had a trend toward quicker diagnosis (P = .05). 26 Retired individuals may have more time and savings to address important health issues, which may explain our finding that younger age was associated with barriers to care. Finally, female sex was associated with patient-reported barriers to care in our model but did not reach statistical significance in the stratified analyses. This finding could be secondary to known sex-based disparities in income in the United States. 27 More research is warranted to confirm and uncover the drivers of this potential sex disparity.
Interestingly, patient-reported barriers to care were not associated with oncologic outcomes in our study, such as cancer stage or survival. Some studies have found that objective delays in care correlate with worse overall survival in HNSCC, such as time from diagnosis to treatment initiation, time to postoperative radiation therapy, and total treatment package time. 5 This discrepancy may be secondary to our limited sample size or differences in patient characteristics among studies. It is also possible that objective measures of delayed care are more prognostic than subjective patient-reported barriers to care. Despite the lack of association with oncologic outcomes in our study, the high prevalence of patient-reported barriers to care (31%) and association with HRQOL outcomes warrant further consideration. Patient-reported outcomes such as HRQOL are recognized as an important but understudied area of head and neck cancer survivorship. 28 A better understanding of HRQOL in head and neck cancer can be used to guide interventions aimed at improving patient satisfaction and value-based care.29,30
The findings from this study can be used as a foundation for additional research and interventions aimed at addressing barriers to care in head and neck cancer. The at-risk sociodemographic groups identified here may benefit from resources such as social workers and financial navigators. Based on the common delay- and affordability-related barriers indicated by patients, clinics should work to optimize communication with patients about follow-up appointments. This could involve phone call or text reminders, which have been shown to reduce no-show rates.31,32 Finally, systemic interventions that help reduce the number of uninsured and underinsured patients in the United States may help to address some of the underlying issues leading to barriers in care.
Our study has several limitations. It is important to recognize that we did not measure barriers at the provider or system level. These could include issues such as communication among providers, documentation exchange, inadequate knowledge about optimal care, time and resource constraints, workforce shortages, and care coordination among health systems.33,34 On the patient level, we did not measure health literacy, which could plausibly influence barriers to care and outcomes in head and neck cancer. 35 Although our study measured the mean patient distance to the hospital, it failed to capture other geographic barriers, such as rurality and concentration of health care providers in certain areas. Finally, our questionnaire provided patients with several options for barriers to care without the opportunity for free-text elaboration. Ideally, future studies in this area will include mixed qualitative and quantitative components for a more complete understanding.
The potential for selection bias in our study is high because sampling relied on voluntary participation in an outpatient oncology clinic. Patients who declined to participate were disproportionately older (P = .007) and had a more advanced cancer stage (P = .002) than the patients who provided consent. Additionally, it is plausible that eligible patients facing barriers to care may have been less likely to participate given the time and effort involved. By nature of enrolling patients who made it to their clinic appointments, our study systematically fails to include patients facing barriers that prevented them from making it to clinic. Given these collective biases, we expect that the prevalence of barriers to care is an underestimation of the true population statistic. Finally, our sample was from a single large public outpatient clinic setting and may not be generalizable to all patients with HNSCC in the United States. Despite these limitations, our study provides novel insight into patient-reported barriers to care in HNSCC. Further research is needed to confirm these findings and identify ways to address barriers to care in head and neck cancer.
Conclusion
Patient-reported barriers to care affect nearly one-third of patients with head and neck cancer and are associated with significantly worse quality-of-life outcomes. Young age, female sex, unmarried status, no insurance, and Medicaid insurance appear to be the strongest risk factors for patient-reported barriers to care.
Author Contributions
Nicholas R. Lenze, study design, data acquisition, data analysis, interpretation, presentation of research, initial draft, final approval, agreement to be accountable; Jeannette T. Bensen, study design, data acquisition, interpretation, presentation of research, final approval, agreement to be accountable; Laura Farnan, study design, data acquisition, data analysis, interpretation, final approval, agreement to be accountable; Siddharth Sheth, study design, interpretation, final approval, agreement to be accountable; Jose P. Zevallos, study design, interpretation, final approval, agreement to be accountable; Wendell G. Yarbrough, study design, interpretation, final approval, agreement to be accountable; Adam M. Zanation, study design, interpretation, initial draft, final approval, agreement to be accountable
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Supplemental Material
Supplemental material, sj-pdf-1-opn-10.1177_2473974X211065358 for Evaluation of Patient-Reported Delays and Affordability-Related Barriers to Care in Head and Neck Cancer by Nicholas R. Lenze, Jeannette T. Bensen, Laura Farnan, Siddharth Sheth, Jose P. Zevallos, Wendell G. Yarbrough and Adam M. Zanation in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Footnotes
The abstract for this article was presented as a virtual poster at the Cancer Center Survivorship Research Forum; April 15-16, 2021; Minneapolis, Minnesota.
ORCID iD: Nicholas R. Lenze  https://orcid.org/0000-0002-2126-6663
https://orcid.org/0000-0002-2126-6663
Supplemental Material: Additional supporting information is available at http://journals.sagepub.com/doi/suppl/10.1177/2473974X211065358
References
- 1. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. 4th ed. International Agency for Research on Cancer; 2017. [Google Scholar]
- 2. American Cancer Society. Cancer Facts and Figures 2019. American Cancer Society; 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf [Google Scholar]
- 3. Janz TA, Graboyes EM, Nguyen SA, et al. A comparison of the NCDB and SEER database for research involving head and neck cancer. Otolaryngol Head Neck Surg. 2019;160(2):284-294. doi: 10.1177/0194599818792205 [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 5. Graboyes EM, Kompelli AR, Neskey DM, et al. Association of treatment delays with survival for patients with head and neck cancer: a systematic review. JAMA Otolaryngol Head Neck Surg. 2019;145(2):166. doi: 10.1001/jamaoto.2018.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Massa ST, Osazuwa-Peters N, Adjei Boakye E, Walker RJ, Ward GM. Comparison of the financial burden of survivors of head and neck cancer with other cancer survivors. JAMA Otolaryngol Head Neck Surg. 2019;145(3):239-249. doi: 10.1001/jamaoto.2018.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carroll WR, Kohler CL, Carter VL, Hannon L, Skipper JB, Rosenthal EL. Barriers to early detection and treatment of head and neck squamous cell carcinoma in African American men. Head Neck. 2009;31(12):1557-1562. doi: 10.1002/hed.21125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graboyes EM, Halbert CH, Li H, et al. Barriers to the delivery of timely, guideline-adherent adjuvant therapy among patients with head and neck cancer. JCO Oncol Pract. 2020;16(12):e1417-e1432. doi: 10.1200/OP.20.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Center for Health Statistics. 2010 NHIS Questionnaire–Sample Adult. Center for Disease Control and Prevention; 2010. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2010/English/qadult.pdf [Google Scholar]
- 10. Divaris K, Olshan AF, Smith J, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010;21(4):567-575. doi: 10.1007/s10552-009-9486-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barrington WE, DeGroff A, Melillo S, et al. Patient navigator reported patient barriers and delivered activities in two large federally-funded cancer screening programs. Prev Med. 2019;129S:105858. doi: 10.1016/j.ypmed.2019.105858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honein-AbouHaidar GN, Kastner M, Vuong V, et al. Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2016;25(6):907-917. doi: 10.1158/1055-9965.EPI-15-0990 [DOI] [PubMed] [Google Scholar]
- 13. Cassim S, Chepulis L, Keenan R, Kidd J, Firth M, Lawrenson R. Patient and carer perceived barriers to early presentation and diagnosis of lung cancer: a systematic review. BMC Cancer. 2019;19(1):25. doi: 10.1186/s12885-018-5169-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones CE, Maben J, Jack RH, et al. A systematic review of barriers to early presentation and diagnosis with breast cancer among Black women. BMJ Open. 2014;4(2):e004076. doi: 10.1136/bmjopen-2013-004076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ganesan S, Sivagnanganesan S, Thulasingam M, et al. Diagnostic delay for head and neck cancer in South India: a mixed-methods study. Asian Pac J Cancer Prev. 2020;21(6):1673-1678. doi: 10.31557/APJCP.2020.21.6.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sathoo S, Nachiappan DS, Sureshkumar S, Anandhi A. A qualitative exploratory study of delay in the presentation of gastrointestinal cancer. J Family Med Prim Care. 2021;10(1):278-282. doi: 10.4103/jfmpc.jfmpc_1283_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570-579. doi: 10.1200/JCO.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 19. Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54(4):343-349. doi: 10.1016/s0895-4356(00)00314-0 [DOI] [PubMed] [Google Scholar]
- 20. Farquhar DR, Lenze NR, Masood MM, et al. Access to preventive care services and stage at diagnosis in head and neck cancer. Head Neck. Published online July 3, 2020. doi: 10.1002/hed.26326 [DOI] [PubMed] [Google Scholar]
- 21. Shin JY, Yoon JK, Shin AK, Diaz AZ. The influence of insurance status on treatment and outcomes in oral cavity cancer: an analysis on 46,373 patients. Int J Oral Maxillofac Surg. 2018;47(10):1250-1257. doi: 10.1016/j.ijom.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 22. Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, Taioli E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116(2):476-485. doi: 10.1002/cncr.24774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panth N, Simpson MC, Sethi RKV, Varvares MA, Osazuwa-Peters N. Insurance status, stage of presentation, and survival among female patients with head and neck cancer. Laryngoscope. Published online March 22, 2019. doi: 10.1002/lary.27929 [DOI] [PubMed] [Google Scholar]
- 24. Naghavi AO, Echevarria MI, Strom TJ, et al. Treatment delays, race, and outcomes in head and neck cancer. Cancer Epidemiol. 2016;45:18-25. doi: 10.1016/j.canep.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 25. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278. doi: 10.1002/cncr.29171 [DOI] [PubMed] [Google Scholar]
- 26. Karp EE, Yin LX, Moore EJ, et al. Barriers to obtaining a timely diagnosis in human papillomavirus–associated oropharynx cancer. Otolaryngol Head Neck Surg. Published online January 26, 2021. doi: 10.1177/0194599820982662 [DOI] [PubMed] [Google Scholar]
- 27. Graf N, Brown A, Patten E. The Narrowing, but Persistent, Gender Gap in Pay. Pew Research Center; 2019. Accessed March 10, 2021. https://www.pewresearch.org/fact-tank/2019/03/22/gender-pay-gap-facts/ [Google Scholar]
- 28. Miller MC, Shuman AG; for the American Head and Neck Society’s Committee on Survivorship. Survivorship in Head and Neck Cancer: A primer. JAMA Otolaryngol Head Neck Surg. 2016;142(10):1002. doi: 10.1001/jamaoto.2016.1615 [DOI] [PubMed] [Google Scholar]
- 29. Rogers SN, Barber B. Using PROMs to guide patients and practitioners through the head and neck cancer journey. Patient Relat Outcome Meas. 2017;8:133-142. doi: 10.2147/PROM.S129012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takes RP, Halmos GB, Ridge JA, et al. Value and quality of care in head and neck oncology. Curr Oncol Rep. 2020;22(9):92. doi: 10.1007/s11912-020-00952-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lagman RL, Samala RV, LeGrand S, et al. “If you call them, they will come”: a telephone call reminder to decrease the no-show rate in an outpatient palliative medicine clinic. Am J Hosp Palliat Care. 2021;38(5):448-451. doi: 10.1177/1049909120952322 [DOI] [PubMed] [Google Scholar]
- 32. Woods R. The effectiveness of reminder phone calls on reducing no-show rates in ambulatory care. Nurs Econ. 2011;29(5):278-282. [PubMed] [Google Scholar]
- 33. McEntee ML, Cuomo LR, Dennison CR. Patient-, provider-, and system-level barriers to heart failure care. J Cardiovasc Nurs. 2009;24(4):290-298. doi: 10.1097/JCN.0b013e3181a660a0 [DOI] [PubMed] [Google Scholar]
- 34. Jacobs LA, Shulman LN. Follow-up care of cancer survivors: challenges and solutions. Lancet Oncol. 2017;18(1):e19-e29. doi: 10.1016/S1470-2045(16)30386-2 [DOI] [PubMed] [Google Scholar]
- 35. Nilsen ML, Moskovitz J, Lyu L, et al. Health literacy: Impact on quality of life in head and neck cancer survivors. Laryngoscope. 2020;130(10):2354-2359. doi: 10.1002/lary.28360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-opn-10.1177_2473974X211065358 for Evaluation of Patient-Reported Delays and Affordability-Related Barriers to Care in Head and Neck Cancer by Nicholas R. Lenze, Jeannette T. Bensen, Laura Farnan, Siddharth Sheth, Jose P. Zevallos, Wendell G. Yarbrough and Adam M. Zanation in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation


