Abstract
Objective
Describe the long-term outcomes of early-start (ES) and delayed-start (DS) glatiramer acetate 40 mg/mL treatment three times weekly (GA40) for up to seven years in the Glatiramer Acetate Low-frequency Administration (GALA) study in patients with relapsing multiple sclerosis (RMS).
Methods
Patients were evaluated every three to six months. The primary efficacy endpoint was annualized relapse rate (ARR); additional endpoints were exploratory or post hoc. For efficacy, data from the entire exposure period were used for the ES and DS cohorts. For safety, exposure only under GA40 was considered.
Results
Of the patients who continued into the open-label extension (OLE), 580/834 (70%) ES and 261/419 (62%) DS completed the OLE. For the entire placebo-controlled and OLE study period, ARR was 0.26 for ES and 0.31 for DS patients (risk ratio = 0.83; 95% confidence interval [CI]: 0.70–0.99). ES prolonged median time to first relapse versus DS (4.9 versus 4.3 years; hazard ratio = 0.82; 95% CI: 0.6–0.96). OLE-only results showed DS patients experienced similar efficacy for relapse and disability outcomes as ES patients. Adverse events were consistent with the well-established GA safety profile.
Conclusions
GA40 treatment conferred clinical benefit up to seven years, resulting in sustained efficacy and was generally well tolerated in RMS patients.
Keywords: multiple sclerosis, open-label extension, glatiramer acetate, disease-modifying therapy, relapse, disability
Introduction
Glatiramer acetate (GA) is a first-line therapy approved for relapsing multiple sclerosis (RMS) treatment, including clinically isolated syndrome, relapsing-remitting and active secondary progressive disease.1,2 Copaxone® (branded GA), administered subcutaneously daily or three times weekly (TIW), has a well-characterized long-term safety profile and established efficacy, with more than 2.8 million patient-years of overall exposure. 2 The Glatiramer Acetate Low-frequency Administration (GALA) study was an international phase 3 clinical trial conducted to investigate the efficacy and safety of GA 40 mg/mL subcutaneous injection administered TIW (GA40) versus placebo over 12 months to patients with RMS. 3 The less-frequent GA40 dosing regimen was shown to have an efficacy and safety profile similar to that of the established 20 mg/mL daily dosing regimen (GA20). 3 Specifically, GALA showed that GA40 dosing significantly reduced the annualized relapse rate (ARR) by 34% and the number of cumulative gadolinium-enhancing (GdE) T1 and new or enlarging T2 lesions versus placebo. 3
Patients completing the 12-month placebo-controlled (PC) phase of the GALA study were eligible to receive GA40 treatment in an ongoing open-label extension (OLE) study. Three-year interim results of the OLE study showed comparable relapse and magnetic resonance imaging (MRI) outcomes between early-start patients (ES; received GA40 treatment throughout both phases of the study) and delayed-start patients (DS; switched from placebo to GA40 in the OLE phase) in Years 2 and 3, suggesting that DS patients also experienced the benefits of GA40 treatment. 4 Adverse events (AEs) in the OLE study were consistent with those of the PC phase. 4
GA40 may offer tolerability and patient satisfaction benefits versus GA20. For example, an open-label, randomized, parallel-group trial observed that the annualized rate of all injection-related AEs (IRAEs) and moderate/severe IRAEs was reduced by 50% (GA40) and 60% (GA20). 5 A recent open-label study demonstrated significantly greater patient satisfaction, better treatment adherence, reduced impact of fatigue on patient activities, and improved patient-reported mental health for patients treated with GA40 versus GA20. 6 These significant differences were maintained over a 12-month period. 7 However, long-term data on the efficacy and safety of GA40 are lacking.
The objective of the current report is to describe the long-term effects of ES and DS GA40 treatment for up to seven years in the GALA PC and OLE study phases.
Materials and methods
Study design
GALA was a randomized, PC, double-blind, parallel-group study carried out at 142 sites in 17 countries. 3 All participating patients provided written informed consent before initiating any aspect of the study; the study protocol was approved by relevant ethical committees and institutional review boards. The patient eligibility criteria have previously been described. 3 Patients were excluded if they previously had treatment with experimental/investigational drugs, immunosuppressive agents or cytotoxic agents within six months prior to screening, treatment with natalizumab or any other monoclonal antibodies, or cladribine within two years prior to screening, treatment with immunomodulators (including interferon β 1a and 1b, and intravenous immunoglobulin) within two months prior to screening, or previous treatment with GA or any other glatiramer.
The study was divided into two phases: 1) a one-year PC phase, in which eligible patients were randomized 2:1 to receive GA40 or placebo, and 2) an OLE phase, in which patients from either PC treatment arm could enroll and receive GA40 until it became commercially available for the treatment of RMS or the study was discontinued by the sponsor. A total of 1404 patients were originally randomized to GA40 (n = 943) or placebo (n = 461), and of these, 1289 (92%) patients completed the PC phase. Of the 1253 patients who completed the PC phase and continued into the OLE, those in the ES cohort (n = 834) received GA40 for the entire duration of the study – defined as the PC and OLE phases. Patients in the DS cohort (n = 419) converted from placebo to GA40 at Month 12, which was the end of the PC phase and the beginning of the OLE phase.
Procedures
Procedures and evaluations performed during the PC phase have been described previously. The OLE phase included scheduled visits every three months for the first 12 months and then scheduled visits every six months thereafter (see Supplemental Figure 1 and Supplemental Text).
Endpoints
The primary efficacy endpoint was ARR, defined as the total number of confirmed relapses adjusted to years of follow-up. Patients with symptoms suggestive of relapse were instructed to contact the study site within 48 h of symptom onset and underwent a complete neurological assessment, performed by the examining neurologist or physician. A relapse was defined as the appearance of one or more new neurological abnormalities, or the reappearance of one or more previously observed neurological abnormalities, lasting at least 48 h and immediately preceded by an improvement in neurological state lasting at least 30 days from the onset of the previous relapse. An event was counted as a relapse only when the patient's symptoms were accompanied by observed objective neurological changes, including an increase of at least 0.5 points in the Expanded Disability Status Scale (EDSS) score compared with the previous evaluation, an increase of one grade in the score of at least 2 of the 7 functional systems (FSs) compared with the previous evaluation or an increase of two grades in the actual score of one functional system compared with the previous evaluation. The patient must not have had any acute metabolic changes, such as fever or other medical abnormality, and a change in bowel or bladder function or cognitive function must not have been entirely responsible for confirmation of a relapse. On confirmation of relapse, follow-up of patients’ neurological condition was performed with scheduled and unscheduled visits. Exploratory endpoints and post hoc analyses are described further in the Supplemental Text.
Safety was assessed by evaluation of the incidence of reported AEs, serious AEs, changes in vital signs (temperature, pulse, and blood pressure), clinical laboratory parameters, and electrocardiogram (ECG) findings over time. Since subcutaneous injections of GA have been associated with injection-site reactions, these were documented, as well as immediate post-injection reactions (IPIRs) involving one or more of the following symptoms: vasodilation, chest pain, dyspnea, palpitation, or tachycardia.
Statistical analyses
For efficacy endpoints, all analyses were based on data from the intent-to-treat (ITT) population, including all randomized patients (N = 1404), regardless of continuation status to the OLE phase. For safety endpoints, exposure only under GA40 was considered.
ARR estimates were derived from a baseline-adjusted exposure-weighted negative binomial regression that modeled the number of confirmed relapses observed during the entire PC and OLE study period. Covariates included baseline EDSS score, log of the number of relapses in the previous two years, baseline volume of T2 lesions, baseline status of GdE T1 activity, and country or geographic region (CGR). All time-to-event analyses were measured from the PC baseline throughout the OLE phase and are presented using Kaplan–Meier methodology. These endpoints were analyzed using the baseline-adjusted Cox proportional hazards model. The analysis model was adjusted for the same covariates as those in the ARR analysis. Proportions estimates of the binary outcome measures were derived from baseline-adjusted logistic regression models (relapse-free and free from six months confirmed disability worsening [6mCDW]; this parameter assessed true clinical disease progression as the computation algorithm used did not permit 6mCDW to be confirmed during a relapse). Covariates were the same as those in the ARR analysis, except for CGR, which was excluded from part of the non-cumulative by-year analyses due to model convergence issues. Analyses done for the OLE period only were adjusted for CGR. Changes from baseline in EDSS were analyzed using the Mixed Model for Repeated Measures (MMRM) using baseline EDSS and CGR as covariates. Safety and tolerability results were reported descriptively.
Results
Patient disposition
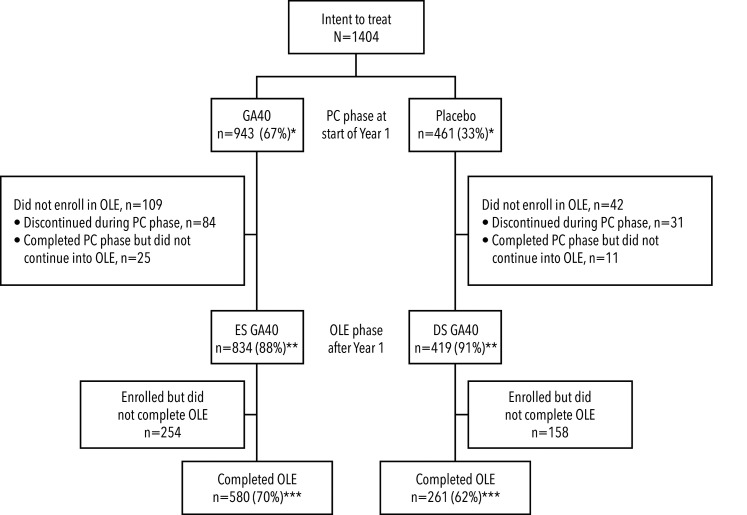
Of the 834 ES patients and 419 DS patients who continued into the OLE phase, 580 (70%) patients in the ES cohort and 261 (62%) patients in the DS cohort completed the entire GALA PC and OLE study (see Figure 1). The most common reasons for discontinuation at any time were withdrawal of consent (21% for ES and 23% for DS, n = 299; main reasons included: personal patient decision; perceived lack of efficacy; no longer wanting injections; relocation; planning a family; relapse/disease progression) and AEs (6% for ES and 7% for DS, n = 93). The median follow-up for the ITT population was 5.5 years. The mean (standard deviation [SD]) age of the patients entering the study was 37.6 (9.3) years, with a mean (SD) time from diagnosis of 3.8 (4.9) years. Baseline demographic information has also been published previously. 3
Figure 1.
Patient disposition
*as percentage of intent-to-treat patients; **as percentage of patients in PC phase at start of Year 1; ***as percentage of patients in OLE phase after Year 1.
DS: delayed start; ES: early start; GA40: glatiramer acetate 40 mg/mL subcutaneous injection administered three times weekly; OLE: open-label extension; PC: placebo-controlled.
Duration of GA exposure
The overall ITT median GA exposure was 4.9 years. A total of 631 (45%) patients were exposed to GA for more than five years (see Supplemental Table 1).
ARR
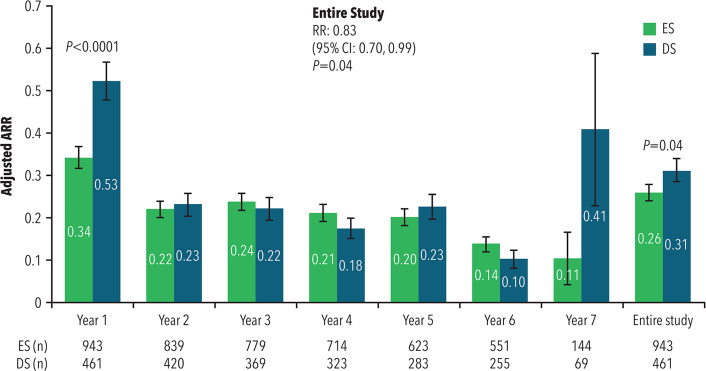
The mean ARR was significantly lower for ES patients (0.34) than for DS (0.53) patients during the first year of PC treatment (risk ratio [RR] = 0.65; 95% confidence interval [CI]: 0.54–0.80; p < 0.0001), as well as during the entire PC and OLE study period (mean ARR of 0.26 for ES versus 0.31 for DS; RR = 0.83; 95% CI: 0.70–0.99; p = 0.04).
As shown in Figure 2, the mean ARRs were low and non-significant between ES and DS for each individual year from Years 2 through 7, with rates of 0.10 (DS) to 0.14 (ES) in Year 6. Since relatively few DS patients were treated for over six years (n = 69), Year 7 mean ARR was more variable in this group. ARR during the OLE period only did not differ by treatment group (RR = 1.06; 95% CI: 0.86–1.31; p = 0.59).
Figure 2.
Annualized relapse rate
ARR: annualized relapse rate; CI: confidence interval; DS: delayed start; ES: early start; RR: risk ratio.
Error bars represent standard error.
Time to first confirmed relapse and percent of relapse-free patients
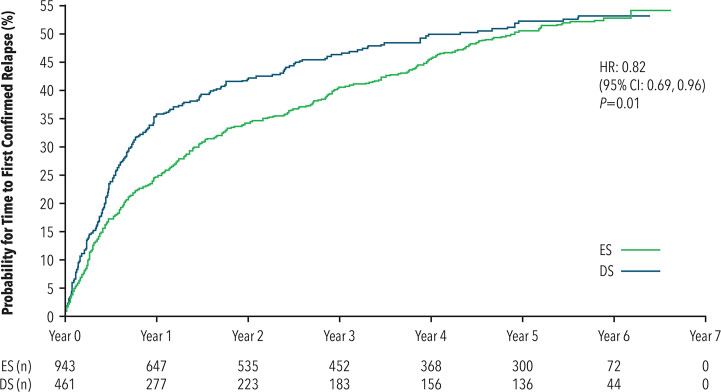
ES treatment prolonged the median time from randomization to first confirmed relapse by approximately six months compared with DS treatment (4.9 years versus 4.3 years). Time-to-event analysis significantly favored ES treatment (hazard ratio [HR] = 0.82; 95% CI: 0.69–0.96; p = 0.01) (see Figure 3). When considering the OLE period only, time to first relapse did not differ by treatment group (HR = 1.10; 95% CI: 0.91–1.34; p = 0.33).
Figure 3.
Time to first confirmed relapse
CI: confidence interval; DS: delayed start; ES: early start; HR: hazard ratio.
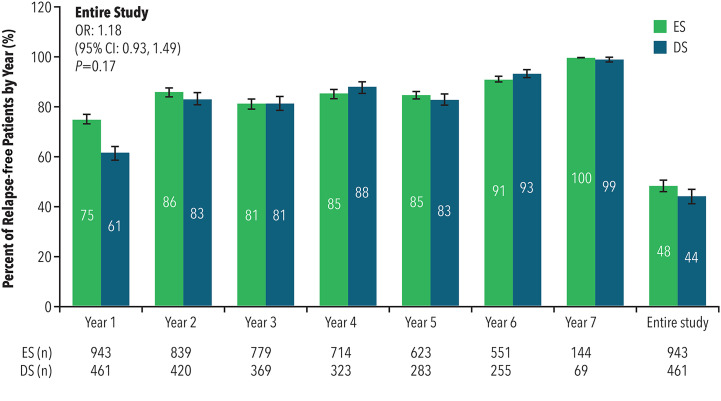
The percent of relapse-free patients was significantly greater for ES patients compared to DS patients during the first year of PC treatment (odds ratio [OR] = 1.91; 95% CI: 1.48–2.47; p < 0.0001), although the ORs were similar for each subsequent year. The percent of patients who remained relapse-free during the entire PC and OLE study period was similar between the ES (48%) and DS (44%) cohorts (OR = 1.18; 95% CI: 0.93–1.49; p = 0.17) (see Figure 4). There was a 21% reduction in the odds of remaining relapse-free for patients in the ES cohort compared to the DS cohort during the OLE period only (OR = 0.79; 95% CI: 0.62–1.02; p = 0.07).
Figure 4.
Percent of relapse-free patients
CI: confidence interval; DS: delayed start; ES: early start; OR: odds ratio.
Time to 6mCDW and percent of patients 6mCDW-free
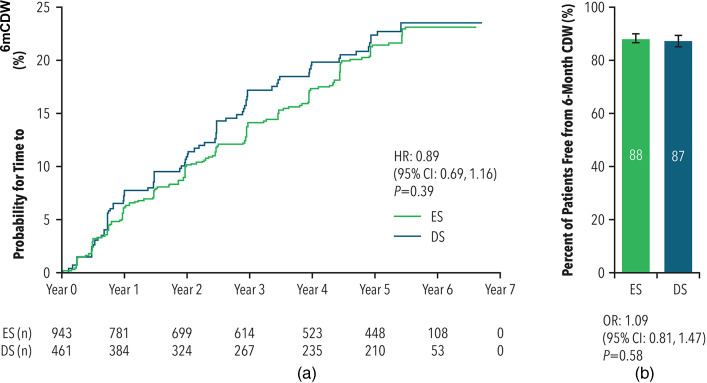
Over the entire PC and OLE study period, patients in the ES cohort had a similar time to 6mCDW compared to the DS cohort (HR = 0.89; 95% CI: 0.69–1.16; p = 0.39) (see Figure 5(a)). Time to 6mCDW during the OLE period only did not differ by treatment group (HR = 1.11; 95% CI: 0.82–1.50; p = 0.49). Median times to 6mCDW could not be calculated due to the high censoring rate.
Figure 5a and b.
Time to 6mCDW (A) and percent of patients free from 6mCDW (B)
CI: confidence interval; CDW: confirmed disease worsening; DS: delayed start; ES: early start; HR: hazard ratio; OR: odds ratio.
The baseline-adjusted percent of patients who remained 6mCDW-free was similar between the cohorts during the entire PC and OLE study period (88% for ES treatment and 87% for DS treatment; OR = 1.09; 95% CI: 0.81–1.47; p = 0.58) (see Figure 5(b)). The percent of patients who experienced 6mCDW during the OLE period only did not differ by treatment group (17% for ES versus 15% for DS; OR = 1.22; 95% CI: 0.88–1.70; p = 0.22).
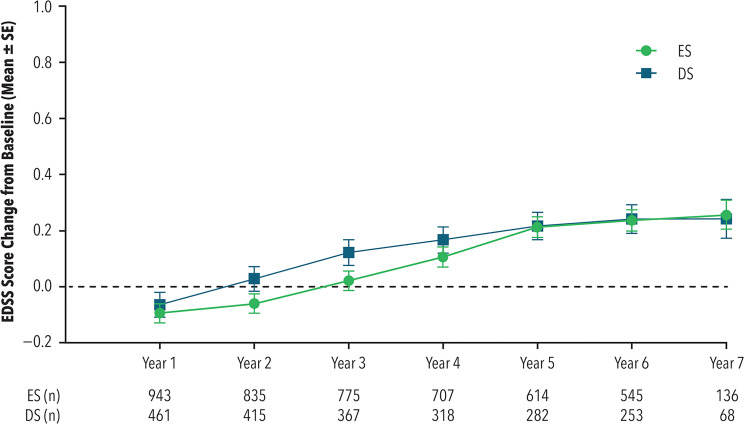
EDSS change from baseline during the entire PC and OLE study period
Mean EDSS values at baseline were 2.81 for ES and 2.75 for DS. During the entire PC and OLE study period, the mean change from baseline was similar for both ES and DS (see Figure 6). At the end of Year 1, the mean (standard error [SE]) change was similar in both the ES group −0.09 (0.03) and the DS group −0.06 (0.04) with mean difference of −0.03 (95% CI: −0.12 to 0.06; p = 0.52). At the end of Year 7, the mean (SE) change was also similar in both the ES group 0.25 (0.05) and the DS group 0.24 (0.07) with difference 0.01 (95% CI: −0.14 to 0.17; p = 0.85).
Figure 6.
Change from baseline in EDSS
DS: delayed start; EDSS: Expanded Disability Status Scale; ES: early start; SE: standard error.
The baseline-adjusted percent of patients with stable or improved EDSS scores was similar between cohorts throughout the entire and OLE-only study period (Supplemental data), as was the percent of patients reaching EDSS = 4 and EDSS = 6 (Supplemental data). Likewise, time to reach an EDSS score of 4 or 6 was not significantly different between the groups (Supplemental data).
Safety profile
GA40 was generally well tolerated, with no new safety signals observed. There were no relevant safety signals with regard to laboratory values, ECG readings, or vital signs. Over 80% of patients exposed to GA40 during the OLE period experienced at least one AE and 11% experienced a serious AE (see Table 1). The most common AEs reported for the entire GA40-treated analysis set – injection-site reactions (40%) and IPIRs (12%) – were generally mild and consistent with the well-established GA20 safety profile. 8 Among those patients with injection-site reactions, injection-site erythema was most commonly reported (27%), with injection-site atrophy occurring least frequently (2%). Dyspnea (4%) and vasodilation (4%) were the most commonly reported IPIRs (Table 2). A total of four deaths occurred in GA-treated patients; each of these deaths was considered not to be related to the study drug by both the investigator and the study sponsor.
Table 1.
Frequency of common AEs in all patients exposed to GA40 during the OLE a .
| ES (n = 943, PY = 4169.7) |
DS (n = 419, PY = 1549.2) |
All (N = 1362, PY = 5718.8) |
|
| Total patients with serious AEs, n (%) | 117 (12) | 36 (9) | 153 (11) |
| Total patients with any AEs, n (%) | 777 (82) | 322 (77) | 1099 (81) |
| Influenza, n (%) | 68 (7) | 25 (6) | 93 (7) |
| Bronchitis, n (%) | 51 (5) | 20 (5) | 71 (5) |
| Nasopharyngitis, n (%) | 167 (18) | 56 (13) | 223 (16) |
| Upper respiratory tract infections, n (%) | 98 (10) | 32 (8) | 130 (10) |
| Urinary tract infection, n (%) | 105 (11) | 36 (9) | 141 (10) |
| Back pain, n (%) | 99 (10) | 33 (8) | 132 (10) |
| Headache, n (%) | 132 (14) | 39 (9) | 171 (13) |
| Total patients with injection-site reactions, n (%) | 378 (40) | 162 (39) | 540 (40) |
| Injection-site erythema, n (%) | 252 (27) | 116 (28) | 368 (27) |
| Injection-site pain, n (%) | 115 (12) | 55 (13) | 170 (12) |
| Injection-site pruritus, n (%) | 68 (7) | 23 (5) | 91 (7) |
| Injection-site swelling, n (%) | 50 (5) | 24 (6) | 74 (5) |
| Injection-site atrophy, n (%) | 20 (2) | 10 (2) | 30 (2) |
AE: adverse event; DS: delayed start; ES: early start; GA40: glatiramer acetate 40 mg/mL subcutaneous injection administered three times weekly; OLE: open-label extension; PY: patient-years.
Individual AEs reported here (Medical Dictionary for Regulatory Activities preferred term) are those occurring in >5% of patients exposed to GA40 at any time during the OLE, with the exception of injection-site atrophy. Injection-site atrophy (categorical term) includes injection-site atrophy, injection-site lipoatrophy, and injection-site lipodystrophy acquired.
Table 2.
Immediate post-injection reactions in all patients exposed to GA40 during the OLE a .
| Total GA40 (N = 1362; PY = 5718.8) | |||
| Number of patients (%) | Number of events | Incidence rate/100 PY | |
| Total | 160 (12) | 326 | 5.7 |
| Dyspnea | 60 (4) | 88 | 1.5 |
| Vasodilation (flushing) | 53 (4) | 85 | 1.5 |
| Tachycardia | 53 (4) | 76 | 1.3 |
| Chest pain | 39 (3) | 52 | 0.9 |
| Palpitations | 19 (1) | 24 | 0.4 |
GA40: glatiramer acetate 40 mg/mL subcutaneous injection administered three times weekly; OLE: open-label extension; PY: patient-years.
IPIRs reported here (Medical Dictionary for Regulatory Activities preferred term) are those occurring in patients exposed to GA40 at any time during the OLE.
Discussion
This study is the first to report treatment outcomes with GA40 over seven years. The study showed sustained clinical benefit of GA40 at levels comparable to those in the PC phase of the GALA study, as well as the three-year open-label analysis.3,4 Overall, 48% and 44% of patients in the ES and DS cohorts, respectively, were free of relapse, whereas a total of 88% (ES) and 87% (DS) did not experience 6mCDW up to seven years. EDSS remained stable in both groups, with mean EDSS score increasing from baseline by a quarter of a point after up to seven years of treatment. Given that mean EDSS values at baseline were 2.81 for ES and 2.75 for DS, EDSS scores of approximately 3 reflect maintenance of walking ability after up to seven years of continuous GA40 treatment.
Early versus delayed start treatment analyses in RMS OLE studies commonly reflect the delayed treatment of two years; in contrast, the PC phase of GALA was only 12 months. After converting from placebo to GA40 at the end of the first study year, when considering the entire duration of the study (PC and OLE phases), ARR and time to first relapse remained lower for ES compared with DS patient cohorts. Observations from Years 2 to 7 (OLE phase only) indicated that DS patients experienced similar efficacy for most outcomes (e.g. ARR, time to first relapse, percent of patients relapse-free, time to 6mCDW, percent of patients 6mCDW-free, percent of patients with stable or improved EDSS scores, time to EDSS = 4/EDSS = 6, and percent of patients reaching EDSS = 4/EDSS = 6) as ES patients. This indicates a treatment benefit for DS patients upon initiation of GA40 comparable to ES patients, which is apparent in year-to-year relapse outcomes. It is interesting that despite differences in ARR during the first year (the PC period) that favored the ES patients, there was no significant difference in disability measures at the end of the OLE period between the two cohorts. This could be due to the patients being reasonably early on in their disease course. Another study investigating intramuscular interferon β 1a 30 µg weekly and GA20 either alone or in combination found no difference across the treatment groups in clinical outcomes at the end of a seven-year OLE, despite differences in MRI changes at three years (unfortunately the ARRs for each group were too low to allow any cross-group comparison throughout the study). 9 Furthermore, long-term disease-modifying treatment (greater than 15 years) in RMS has been shown to prevent neurologic disability, 10 so it is possible that the duration of follow up in the current study was sufficient for the DS patients to benefit commensurably from GA treatment.
GA40 was well tolerated, with a safety profile similar to that of GA20, including a low incidence of serious AEs, and no new or unexpected safety signals being observed in patients receiving GA40 for up to seven years. As expected from prior clinical evaluations of GA40, the incidence of injection-site reactions, including injection-site atrophy, was low for patients exposed to GA40 during the OLE phase. Idiosyncratic and possibly immune-related dose-independent hepatocellular injury with an unpredictable latency ranging from days to years have recently been described following treatment with GA1,11; however, no new safety signals were identified during the OLE phase of the GALA study.
There are potential limitations associated with all long-term, open-labelled extension designs. In addition to not having a control group, long-term extensions can find it difficult to retain patients and may be more likely to retain those patients who respond well to therapy, thus biasing the outcomes observed. 12 This may explain the ARR value seen in Year 7 for the DS patients. At the end of the OLE period, 45% of patients had been exposed to GA40 treatment beyond five years, although this is driven largely by the greater percentage of ES patients exposed beyond five years (59%) compared with DS patients (17%), which is somewhat explained by the nature of the study design. The overall retention rate (60%) is supported by recent research demonstrating high patient satisfaction and adherence with GA40.6,7 Another limitation is the lack of MRI data from three to seven years. The three-year results showed that ES and DS patients exhibited similar numbers of GdE T1 and new or enlarging T2 lesions 4 ; however, ES patients exhibited significantly smaller changes in gray matter volume than DS patients from Year 1 to Year 3 (mean difference, 0.371%; p = 0.02), with a similar trend in whole-brain volume (p = 0.08). 4
In summary, treatment with GA40 conferred clinical benefit up to seven years of the GALA study, resulting in sustained low ARR and rates of disability worsening. Results from this OLE of the GALA study further support the known safety profile and effective long-term use of GA40 in the RMS patient population.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173211061550 for Long-term efficacy and safety of three times weekly dosing regimen of glatiramer acetate in relapsing multiple sclerosis patients: Seven-year results of the Glatiramer Acetate Low-frequency Administration (GALA) open-label extension study by Peter Rieckmann, Robert Zivadinov, Alexey Boyko, Krzysztof Selmaj, Jessica K. Alexander, Shaul Kadosh, Svetlana Rubinchick, Emily Bernstein-Hanlon, Yafit Stark, Natalia Ashtamker, Mat D. Davis and Omar Khan in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
We thank the patients and site personnel involved with this study.
Biography
Jason Allaire, PhD (Generativity Solutions Group with funding from Teva Pharmaceuticals) provided editorial assistance in the preparation of this manuscript. Medical writing support for responding to journal comments was provided by Jackie Phillipson of Ashfield Healthcare, part of UDG Healthcare, and was funded by Teva Pharmaceuticals.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CC BY-NC 4.0. P.R. was the co-PI international for the GALA study. J.K.A. reports personal fees as an employee of Teva Pharmaceuticals. S.K. is a former employee of Teva Pharmaceuticals and reports personal fees for consulting for Teva Pharmaceuticals. S.R. reports personal fees as an employee of Teva Pharmaceuticals. E.B-H. reports personal fees as an employee of Teva Pharmaceuticals. Y.S., M.D., and N.A. are former employees of Teva Pharmaceuticals. R.Z. received personal compensation from EMD Serono, Sanofi, Novartis, Bristol Myers Squibb, Novartis, and Keystone Heart for speaking and consultant fees, as well as financial support for research activities from Sanofi, Novartis, Bristol Myers Squibb, Mapi Pharma, Keystone Heart, V-WAVE Medical, Boston Scientific, and Protembo.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Teva Pharmaceuticals.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Peter Rieckmann, Medical Park, Loipl, Germany.
Robert Zivadinov, Buffalo Neuroimaging Analysis Center, Department of Neurology, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, NY, USA; Center for Biomedical Imaging at the Clinical Translational Science Institute, University at Buffalo, State University of New York, NY, USA.
Alexey Boyko, Federal Centre of Brain Research and Neurotechnology, Moscow, Russia.
Krzysztof Selmaj, Department of Neurology, University of Warmia and Mazury, Olszytn and Center of Neurology, Lodz, Poland.
Jessica K. Alexander, Teva Pharmaceuticals, West Chester, Pennsylvania, USA
Shaul Kadosh, Teva Pharmaceuticals, Netanya, Israel.
Svetlana Rubinchick, Teva Pharmaceuticals, Netanya, Israel.
Emily Bernstein-Hanlon, Teva Pharmaceuticals, Netanya, Israel.
Yafit Stark, Teva Pharmaceuticals, Netanya, Israel.
Natalia Ashtamker, Teva Pharmaceuticals, Netanya, Israel.
Mat D. Davis, Teva Pharmaceuticals, Netanya, Israel
Omar Khan, Wayne State University, Detroit, MI, USA.
References
- 1.Copaxone® (glatiramer acetate injection). Highlights of prescribing information. July 2020. Available at: https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf (Last accessed 29 October 2021).
- 2.Boster AL, Ford CC, Neudorfer Oet al. et al. Glatiramer acetate: long-term safety and efficacy in relapsing-remitting multiple sclerosis. Expert Rev Neurother 2015; 15: 575–586. [DOI] [PubMed] [Google Scholar]
- 3.Khan O, Rieckmann P, Boyko Aet al. et al. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol 2013; 73: 705–713. [DOI] [PubMed] [Google Scholar]
- 4.Khan O, Rieckmann P, Boyko A, et al. Efficacy and safety of a three-times-weekly dosing regimen of glatiramer acetate in relapsing-remitting multiple sclerosis patients: 3-year results of the Glatiramer Acetate Low-Frequency Administration open-label extension study. Mult Scler 2017; 23: 818–829. [DOI] [PubMed] [Google Scholar]
- 5.Wolinsky JS, Borresen TE, Dietrich DW, et al. GLACIER: an open-label, randomized, multicenter study to assess the safety and tolerability of glatiramer acetate 40 mg three-times weekly versus 20 mg daily in patients with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 2015; 4: 370–376. [DOI] [PubMed] [Google Scholar]
- 6.Cutter G, Veneziano A, Grinspan A, et al. Higher satisfaction and adherence with glatiramer acetate 40 mg/mL TIW vs 20 mg/mL QD in RRMS. Mult Scler Relat Disord 2019; 33: 13–21. [DOI] [PubMed] [Google Scholar]
- 7.Cutter G, Veneziano A, Grinspan A, et al. Satisfaction and adherence with glatiramer acetate 40 mg/mL TIW in RRMS after 12 months, and the effect of switching from 20 mg/mL QD. Mult Scler Relat Disord 2020; 40: 101957. [DOI] [PubMed] [Google Scholar]
- 8.Ziemssen T, Ashtamker N, Rubinchick Set al. et al. Long-term safety and tolerability of glatiramer acetate 20 mg/ml in the treatment of relapsing forms of multiple sclerosis. Expert Opin Drug Saf 2017; 16: 247–255. [DOI] [PubMed] [Google Scholar]
- 9.Lublin F, Cofield S, Cutter G, et al. Long-term follow-up of a randomized study of combination interferon and glatiramer acetate in multiple sclerosis: efficacy and safety results up to 7 years. Mult Scler Relat Disord 2017; 18: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalincik T, Diouf I, Sharmin S, et al. Effect of disease-modifying therapy on disability in relapsing-remitting multiple sclerosis over 15 years. Neurology 2021; 96: e783–e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copaxone® (glatiramer acetate injection). EMA summary of product characteristics. August 2021. Available at: https://www.medicines.org.uk/emc/product/7046/smpc#gref (Last accessed 29 October 2021).
- 12.Sormani MP, Bruzzi P. Can we measure long-term treatment effects in multiple sclerosis? Nat Rev Neurol 2015; 11: 176–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173211061550 for Long-term efficacy and safety of three times weekly dosing regimen of glatiramer acetate in relapsing multiple sclerosis patients: Seven-year results of the Glatiramer Acetate Low-frequency Administration (GALA) open-label extension study by Peter Rieckmann, Robert Zivadinov, Alexey Boyko, Krzysztof Selmaj, Jessica K. Alexander, Shaul Kadosh, Svetlana Rubinchick, Emily Bernstein-Hanlon, Yafit Stark, Natalia Ashtamker, Mat D. Davis and Omar Khan in Multiple Sclerosis Journal – Experimental, Translational and Clinical