Highlights
-
•
PD management is complex and evolves as the disease progresses.
-
•
Therapy dose escalations, switches, discontinuations, and add-ons are common.
-
•
Relapses and adverse events are the most common reasons for therapy changes.
-
•
Switches and dose escalations often occur within 6 months of initiation.
-
•
Treatment in older patients is more cautious than in younger patients.
Keywords: Parkinson’s disease, Levodopa, Monoamine oxidase B, Dopaminergic agonists, Disease progression, Patterns, Discontinuation, Switch
Abstract
Background
Parkinson’s disease (PD) management seeks to balance the benefits and harms of current medications and evolves as the disease progresses. The natural history of PD and associated patterns of treatment change were analyzed to identify unmet needs in treatment of PD symptoms.
Methods
Medical charts of patients from clinics across the US diagnosed on or before June 30th, 2014 were retrospectively reviewed. Index date was the first clinic visit, and the post-index period was through study end (June 30th, 2019). Outcomes included the frequency of therapy changes in the post-index period, reasons for therapy change, and adverse events (AE).
Results
Patients (n = 203) at index were receiving levodopa-peripheral dopa decarboxylase inhibitor (PDDI) monotherapy (47%), dopaminergic agonist (DA) monotherapy (15%), monoamine oxidase B inhibitor (MAOBI) monotherapy (14%), or combination therapies. The percentage of patients in Hoehn-Yahr disease Stage 1–2 was 52% at index and 20% by the end of the study. Frequencies of motor, non-motor, and neuropsychiatric symptoms increased during the enrollment. Levodopa-PDDI monotherapy and levodopa-PDDI + MAOBI had the lowest rates of therapy changes. Symptom relapse was the most common reason for dose escalation, add-on, and dose reduction, whereas AEs were the most common reason for discontinuation and switching. Dose escalation, add-on, and forward switch were most likely to occur in the first 6 months of treatment.
Conclusions
Therapy changes during the study period reflected the challenging and evolving management of PD as the disease progresses. New or add-on symptomatic treatments are needed that are well-tolerated and able to control PD symptoms.
1. Introduction
Parkinson’s disease (PD) is a movement disorder characterized by gait disturbance, bradykinesia, tremor at rest, muscle rigidity, and postural instability[1]. Non-motor symptoms are also common and may include constipation, sleep disturbances, orthostatic hypotension, erectile dysfunction, constipation, and urinary incontinence[2]. Patients also commonly experience neuropsychiatric symptoms such as hallucinations, dementia, cognitive impairment, depression, and anxiety[2]. The motor symptoms of PD are primarily attributed to low dopamine levels in the brain as a consequence of the progressive loss of dopaminergic neurons[3]. Current therapies seek to improve motor symptoms by increasing dopamine levels[4]. The dopamine precursor levodopa is the most potent therapy for PD and is the gold standard for the treatment of motor symptoms,[4], [5] but chronic administration can itself lead to motor and autonomic symptoms, particularly dyskinesia[6], [7]. Dopaminergic agonists (DA) are another class of first-line PD treatments, however, DA can be associated with non-motor side effects such as hallucinations, impulse control disorders, sudden daytime sleepiness, and acute orthostasis[8]. Inhibitors of monoamine oxidase B (MAOB), the enzyme that breaks down dopamine in the brain, are less effective than levodopa or DA, but are better tolerated[9], [10].
There are currently no approved treatments that prevent the progression of PD and there are no widely accepted treatment guidelines[10]. Treatment is tailored to the individual and becomes more complex over time as the disease progresses[9], [10]. Management of symptoms is a balancing act between the benefits and harms of medications used not only to treat the motor symptoms of PD (anti-PD medications), but also supportive medications used to treat the non-motor and neuropsychiatric symptoms[9], [11]. Physicians and patients may change regimens and doses as symptoms wax and wane and as drug-related adverse events occur. Add-on treatments may help to reach this balance by delaying increases in doses of anti-PD medications or delaying switches to anti-PD medications that are more effective but have poorer tolerability[10].
The nature of PD management necessitates changes in therapy as the disease progresses and in response to tolerability and efficacy issues. The objective of this analysis was to describe the natural history of PD and associated patterns of treatment change in a cohort of patients in the United States, in order to identify unique challenges and unmet needs in PD.
2. Methods
2.1. Study design
This was a retrospective medical chart review of patients with PD managed in 18 neurology, neuro-psychiatric, and geriatric clinics across the United States. Potential study sites were identified based on recommendations from the study sponsor as well as previously vetted clinics identified during prior chart review studies conducted by the study investigators. In order to obtain a representative mix of study sites, specific clinics were recruited based on the type of specialty practice (i.e., general neurology, specialty neurology, neuro-psychiatric, and geriatric practices), the size of practice, practice designation (single or multi-specialty), geographic location, affiliations, and the study investigators’ prior experience of site willingness for recruitment and collaboration.
Duration of patients’ charts spanned at least 5 years, starting on June 30th, 2014 or before, up to June 30th, 2019. The earliest date of the first eligible clinic visit was designated as the index visit. The post-index period was defined as any time during the study period after the first 31 days following the index visit.
A central Institutional Review Board reviewed the study protocol and approved a waiver of patient informed consent. All abstracted chart data was completely de-identified of any protected patient health information.
2.2. Chart abstraction
A detailed electronic chart abstraction form (eCAF) was developed for the study. Chart abstracters were clinically trained healthcare professionals with extensive previous experience in chart abstraction. All chart abstracters were given a chart abstraction manual, detailed instructions, key variable definitions, and a trouble-shooting guide.
Data on demographics, clinic site characteristics, patient clinical characteristics (e.g., disease stage and PD symptoms), comorbidities, details of anti-PD medication use to manage primary motor symptoms (i.e., levodopa-peripheral dopa decarboxylase inhibitor [PDDI], DA [ropinirole, pramipexole, rotigotine], catechol-O-methyltransferase inhibitors [entacapone, tolcapone], MAOB inhibitors [rasagiline, selegiline, safinamide], anticholinergics [trihexyphenidyl, benztropine], amantadine) and details of supportive PD medication use to manage non-motor symptoms (i.e., medications for nausea, depression, rapid eye movement sleep behavior disorder, orthostatic hypotension, sialorrhea, hallucinations, Parkinson’s disease dementia, Parkinson’s disease mild cognitive impairment) were abstracted from the patient charts into the eCAF. Disease stage (Stages 1–5) was based on Hoehn-Yahr (H-Y) scores;[12] however, H-Y scores were not readily available within all patient charts. Therefore, a symptom-based algorithm was developed to mirror the H-Y score based on laterality and documented impairments (Supplemental Table E1). Patients may have had additional staging information derived from the Unified Parkinson’s Disease Rating Scale or from information captured by the Mini Mental scores for psychiatric PD manifestations.
The abstracted data were uploaded into an abstraction database. A quality control assessment of the abstraction database was conducted in tandem with the chart abstraction process.
2.3. Patient selection
Patients eligible for the study were required to have a diagnosis of PD indicated by an International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9 CM) code of 332.XX or International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10 CM) code of G20.XX or G21.XX on or before June 30th, 2014. Eligible patients also had to have initiated treatment with a PD medication within 30 days after diagnosis, had at least 2 clinic visits for PD between the index visit and the end of the study (June 30th, 2019), and had a record of a medication order for a PD medication within 6 months of the end of the study. Patients excluded from the study were those with a deep brain stimulation surgical procedure during the study period, those with a concomitant diagnosis of dementia at the index visit (e.g., ICD-9 CM code of 290.XX, 331.XX, 292.XX, 291.2X, 294.1X or ICD-10 code of F01.XX, F03.XX, G31.XX, F02.XX, F10.27, F10.97, F18.97, F18.17, F18.27, F13.27, F13.97, F19.97, F19.17, or F19.27), and those with missing age or sex information.
2.4. Outcomes
Outcomes for the study were the frequency of therapy change (e.g., dose escalation, dose reduction, discontinuation, switching, and add-on therapy) per patient per year (PPPY) in the post-index period, reasons for therapy change, adverse events (AEs) associated with medication (drug-related AEs), and the consequence or intervention because of drug-related AEs.
Therapy changes could be initiated by the physician or patient-initiated with approval of the treating physician. Multiple therapy change situations may have occurred with the same patient, but each therapy change event was counted and reported separately. Dose escalation and dose reduction were defined as a record in the chart indicating that the maintenance daily dose of the index anti-PD medication increased or decreased, respectively, by at least 25% during the post-index period. Discontinuation was defined as a record in the chart indicating discontinuation of the index anti-PD medication with no further reported use of the drug during the post-index period. Forward switching was defined as a record in the chart of discontinuation of the index anti-PD medication and initiation of a different anti-PD medication within at least 30 days and its use continued over the remainder of the post-index period. Add-on was defined as a record in the chart indicating that one or more anti-PD medications were added to the index anti-PD medication within at least 30 days after a record of index drug use and use of the add-on medication continued over the remainder of the post-index period.
Reasons for therapy change were tracked post-index in a binary fashion (yes/no) using the following reasons: 1) patient reported an AE that required a therapy change; 2) patient/physician perceived lack of efficacy (based on subjective or objective evidence); 3) patient had an acute exacerbation event (i.e. relapse of symptoms); 4) patient complained of ‘wearing off’ effect /’on-off’ phenomenon; 5) patient was not adherent or complained about adherence; 6) physical exam or other tests indicated progressive disease; 7) excessive medication monitoring was required; 8) potential drug-drug interactions; 9) potential drug-disease interaction (interaction with current comorbidities); 10) excessive cost to the patient; 11) medication not covered on insurance with forced switch to covered formulary product; or 12) other reason.
For drug-related AE reporting, the nature and severity of the AE, any indicators of clinical consequence that occurred based on the AE, and whether or not a therapy change occurred because of the AE, were captured for the post-index period. Drug-related AEs captured during the post-index period included physical AEs (e.g., nausea/vomiting, dry mouth, constipation, blurred vision, dizziness, fatigue, dry skin/eyes, urinary retention, sexual dysfunction, headache, orthostatic hypotension, sialorrhea) and neuropsychiatric AEs (e.g., depression, anxiety, hallucinations, excessive sedation, impulsive and compulsive behaviors, cognitive impairment/mental confusion). Severity of AEs was based on the accepted National Cancer Institute’s AE Severity Grading Score or the Common Terminology Criteria for Adverse Events AE Grading system, with Grade 1 (mild) defined as asymptomatic or mild symptoms with clinical or diagnostic observations only and no intervention indicated, Grade 2 (moderate) defined as minimal, local or noninvasive intervention indicated and limiting age-appropriate instrumental activities of daily living (ADL), Grade 3 (severe or medically significant but not immediately life-threatening) defined as hospitalization or prolongation of hospitalization indicated, disabling, and limiting of self-care ADL, Grade 4 defined as having life-threatening consequences with urgent intervention indicated, and Grade 5, defined as death related to the AE. Consequences or interventions (e.g., no intervention or therapy change) because of the AE, and the date of the consequence or intervention, were captured for the post-index period.
2.5. Statistical analysis
The number of therapy changes was normalized to PPPY given the variable observation time windows for each patient in the study. All continuous variables were analyzed for mean, median, standard deviation, and inter-quartile ranges, as appropriate. All categorical variables were analyzed as percentages or frequencies. Statistical comparisons among groups for the analyzed outcomes were precluded in many comparisons by the small case count. All analyses were conducted using SAS version 9.4 statistical software (Cary, NC).
Post hoc analyses of outcomes by age of disease onset (<68 y vs ≥ 68 y), duration of disease (<median duration vs ≥ median duration), and index disease stage (Stage 0–2 vs Stage 3–5) were completed, since these have been identified in the literature as potentially important drivers of therapy change and/or patterns of PD treatment. The specific strata used were arbitrary and subjective.
3. Results
3.1. Patient demographics
Data were collected from 203 eligible patient charts from 18 clinics across the United States. The mean time from PD diagnosis to end of data collection was 6.9 years. Most (88%) of the patients were managed in a general neurology clinic. At the index visit, the majority (63%) of patients were on levodopa-PDDI, either as monotherapy or in combination with other anti-PD medications. The largest proportion of patients were receiving levodopa-PDDI monotherapy (47%), followed by DA monotherapy (15%), MAOBI monotherapy (14%), levodopa-PDDI + MAOBI (7%), and levodopa-PDDI + DA (5%).
The majority of patients were male (59%) with a mean age of 75 years (Table 1). Information on race was only available for 44% of patients; of these patients, 83% were white. Patients receiving levodopa-PDDI monotherapy at the index visit had the highest mean age (78 years) and had a higher age of onset (71 years) compared with patients receiving DA or MAOBI monotherapy (Table 1). In contrast, the patients receiving DA or MAOBI monotherapy at the index visit had a significantly longer duration of PD compared with those receiving levodopa-PDDI monotherapy.
Table 1.
Patient characteristics at index overall and by index medication.
| Index Medication | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total Population (N = 203) |
Levodopa-PDDI, no DA, no MAOBI (n = 95) |
Levodopa-PDDI + DA, no MAOBI (n = 10) |
Levodopa-PDDI + MAOBI, no DA (n = 15) |
DA monotherapy (n = 30) |
MAOBI monotherapy (n = 29) |
Other combinations (n = 24) |
| Age, mean y (SD) | 75 (9.6) | 78 (9.7) | 74 (8.8) | 75 (5.8) | 71 (8.3) | 73 (9.1) | 71 (8.8) |
| Male, n (%) | 120 (59) | 48 (51) | 8 (80) | 9 (60) | 15 (50) | 22 (76) | 18 (75) |
| Age at PD onset, mean y (SD) | 68 (9.8) | 71 (10.0) | 65 (10.7) | 68 (5.7) | 63 (9.3) | 65 (8.7) | 63 (8.8) |
| Duration of PD, mean y (SD) | 7.4 (2.8) | 7.0 (2.4) | 9.2 (4.7) | 6.7 (1.3) | 8.0 (3.3) | 7.6 (2.1) | 8.0 (3.6) |
| Referral status, n (%) | |||||||
| New patient | 13 (6.4) | 8 (8.4) | 0 | 0 | 1 (3.3) | 3 (10.3) | 1 (4.2) |
| Not documented | 54 (26.6) | 26 (27.4) | 4 (40) | 4 (26.7) | 8 (26.7) | 8 (27.6) | 4 (16.7) |
| Referred patient/established | 136 (67.0) | 61 (64.2) | 6 (60) | 11 (73.3) | 21 (70.0) | 18 (62.1) | 19 (79.2) |
| Comorbidities, n (%) | |||||||
| Depression | 28 (13.8) | 15 (15.8) | 2 (20) | 1 (6.7) | 4 (13.3) | 4 (13.8) | 2 (8.3) |
| Alzheimer’s disease | 36 (17.7) | 17 (17.9) | 2 (20) | 2 (13.3) | 5 (16.7) | 4 (13.8) | 6 (25.0) |
| Arthralgias | 6 (3.0) | 0 | 0 | 2 (13.3) | 3 (10.0) | 1 (3.5) | 0 |
| Hypertension | 72 (35.5) | 37 (39.0) | 3 (30) | 2 (13.3) | 20 (33.3) | 11 (37.9) | 9 (37.5) |
| Heart failure | 2 (1.0) | 0 | 0 | 0 | 1 (3.3) | 1 (3.5) | 0 |
| Stroke | 4 (2.0) | 3 (3.2) | 1 (10) | 0 | 0 | 0 | 0 |
| Neoplastic disease | 20 (9.9) | 9 (9.5) | 0 | 3 (20) | 3 (10.0) | 3 (10.3) | 2 (8.3) |
| COPD | 1 (0.5) | 0 | 0 | 0 | 1 (3.3) | 0 | 0 |
| Asthma | 6 (3.0) | 4 (4.2) | 0 | 0 | 1 (3.3) | 1 (3.5) | 0 |
| Diabetes | 21 (10.3) | 10 (10.5) | 1 (10) | 1 (6.7) | 4 (13.3) | 2 (7.0) | 3 (12.5) |
| Multiple sclerosis | 1 (0.5) | 0 | 1 (10) | 0 | 0 | 0 | 0 |
| Traumatic brain injury | 2 (1.0) | 1 (1.0) | 1 (10) | 0 | 0 | 0 | 0 |
| Psychotic disorder | 2 (1.0) | 1 (1.0) | 0 | 0 | 1 (3.3) | 0 | 0 |
COPD, chronic obstructive pulmonary disorder; DA, dopaminergic agonist; MAOBI, monoamine oxidase B inhibitor; PD, Parkinson’s disease; PDDI, peripheral dopa decarboxylase inhibitor; SD, standard deviation.
3.2. Patient clinical characteristics at index and post-index
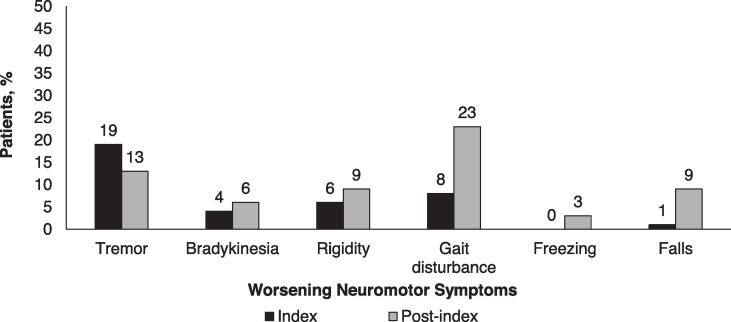
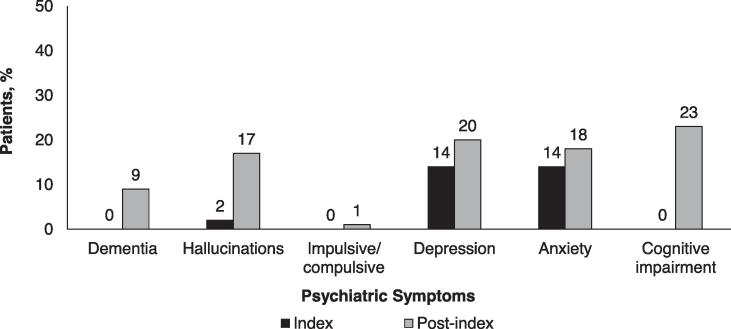
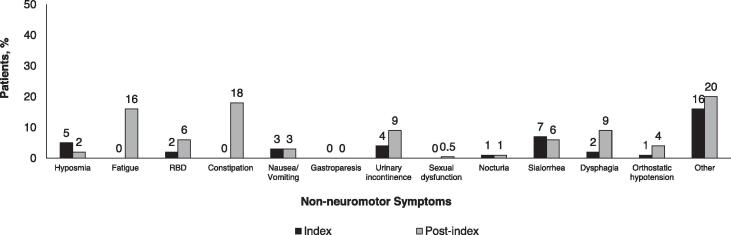
At the index visit, the majority (52%) of patients were in Stage 1 or 2 of disease, but by the end of the study only 20% were in Stage 1 or 2 (Supplemental Figure E1). The most common worsening motor symptom at the index visit was tremor, documented in 38% of patients (Fig. 1)A. From the index visit through the post-index period, there was a substantial increase in the percentage of patients experiencing worsening gait disturbance and falls (Fig. 1)A. There were also increases in the percentage of patients reporting neuropsychiatric symptoms, with the largest rate increases documented for cognitive impairment, hallucinations, and dementia (Fig. 1)B. Increases in the percentage of patients reporting non-motor symptoms were documented, with the largest rate increases observed for constipation, fatigue, and dysphagia (Fig. 1)C.
Fig. 1.
Percentage of patients with A) worsening neuromotor, B) psychiatric, and C) non-neuromotor symptoms at index and post-index.
At the index visit, the most common medications used to treat motor symptoms were carbidopa/levodopa, the MAOBI, rasagiline, and the DA, pramipexole (Table 2). The percentage of patients using carbidopa/levodopa increased from the index visit through the post-index period whereas there was a small decrease in the percentage of patients using rasagiline and pramipexole (Table 2). There was a substantial increase in the use of supportive medications used to treat neuropsychiatric and non-motor PD symptoms, in particular anti-depressants, anti-psychotics, cholinesterase inhibitors, and benzodiazapines (Table 2). Nausea/vomiting was the most frequent drug-related AE and was documented for 16% of patients (Supplemental Table E2). The next most common drug-related AEs were dizziness and fatigue, which were documented for 5% and 4% of patients, respectively (Supplemental Table E2). The most common drug-related psychiatric AE was hallucination and was documented for 12% of patients (Supplemental Table E2). Excessive sedation and cognitive impairment/mental confusion were each documented for 4% of patients.
Table 2.
Percentage of patients using medications to treat motor symptoms and supportive medications to treat psychiatric and non-motor symptoms of PD at the index visit and post-index.
| Medication, n (%) | Index | Post-index |
|---|---|---|
| For motor symptoms | ||
| Carbidopa/levodopa | 127 (63) | 180 (89) |
| Rasagiline | 60 (30) | 56 (28) |
| Pramipexole | 28 (14) | 27 (13) |
| Ropinirole | 20 (10) | 28 (14) |
| Carbidopa/levodopa/entacapone | 14 (7) | 20 (10) |
| Propranolol | 5 (2) | 6 (3) |
| Trihexyphenidyl | 4 (2) | 5 (2) |
| Entacapone | 0 | 17 (8) |
| Supportive medications | ||
| Antidepressant | 11 (5) | 34 (17) |
| Benzodiazepine | 9 (4) | 18 (9) |
| Antipsychotic | 5 (2) | 25 (12) |
| Cholinesterase inhibitor | 2 (1) | 25 (12) |
| Muscarinic antagonist | 1 (0.5) | 6 (3) |
| Antihypotensive | 1 (0.5) | 1 (0.5) |
| Dopamine antagonist | 0 | 1 (0.5) |
| Corticosteroid | 0 | 3 (1) |
| NSAID | 0 | 0 |
| Alpha2-adrenoceptor antagonist | 0 | 4 (2) |
NSAID, non-steroidal anti-inflammatory drug.
3.3. Therapy changes
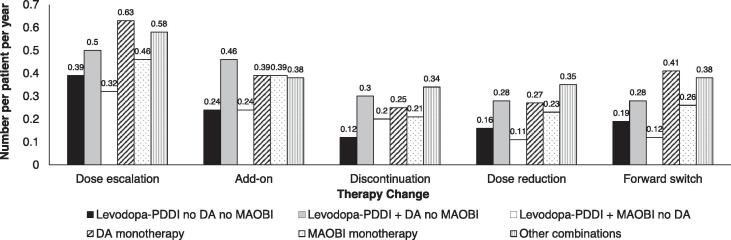
In all, 96% (n = 195) of patients had at least one therapy change; 52% (n = 105) discontinued index treatment, 86% (n = 175) had a dose escalation, 56% (n = 114) had a dose reduction, 71% (n = 145) had an add-on therapy, and 51% (n = 104) had a forward switch. Dose escalation was the most common documented therapy change (Fig. 2). Patients receiving DA monotherapy at the index visit had the highest rates of dose escalation and forward switch, whereas patients receiving levodopa-PDDI + DA had the highest rates of add-on, discontinuation, and dose reduction (Fig. 2). Patients receiving levodopa-PDDI monotherapy or levodopa-PDDI + MAOBI had the lowest rates of all therapy changes. Patients with older age of PD onset were less inclined to have add-ons and more inclined to have dose escalations than younger onset patients (Supplemental Table E3). Patients with higher index disease stages were more inclined to discontinue therapy than lower index disease stage patients (Supplemental Table E3).
Fig. 2.
Number of therapy changes per patient per year. DA, dopaminergic agonist; MAOBI, monoamine oxidase B inhibitor; PDDI, peripheral dopa decarboxylase inhibitor.
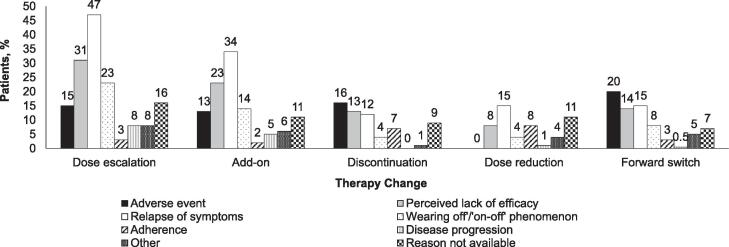
Among patients with a therapy change, the most common reason for dose escalation, add-on, and dose reduction was that the patient had a relapse of symptoms, whereas AEs were the most common reason for discontinuation and forward switch (Fig. 3). The greatest percentage who changed therapy because of AEs (besides “other combinations”) were those receiving levodopa-PDDI + MAOBI and DA monotherapy (both 53%) and the lowest percentage were those receiving levodopa-PDDI monotherapy (28%; Supplemental Figure E2. Patients with older age of PD onset were less inclined to add-on because of AEs than younger onset patients and patients with higher index disease stages were more inclined to discontinue therapy because of AEs than lower index disease stage patients (Supplemental Table E3).
Fig. 3.
Percentage of patients reporting various reasons for therapy change among those who had a therapy change. Totals can add up to more than 100%.
Among patients with a therapy change, dose escalation, add-on, and forward switch were most likely to occur in the first 6 months after the index visit (Supplemental Figure E3). Discontinuation tended to occur later after treatment initiation. Patients with older age of PD onset tended to delay switching medications while escalating doses sooner than those with younger age of PD onset (Supplemental Figure E4A). Patients with greater duration of disease tended to delay dose escalation and switching but tended to reduce dosage and add-on treatment sooner than patients with a shorter duration of disease (Supplemental Figure E4B). Patients with lower index disease stages tended to delay dose escalation but were quicker to add-on or discontinue therapy compared with patients with higher index disease stages (Supplemental Figure E4C).
4. Discussion
The lack of a treatment that prevents disease progression is a well-known unmet need in PD. The results of the current analysis are consistent with the known progression trajectory of PD, with rates of motor, non-motor, and neuropsychiatric symptoms increasing over the course of the study period. Accordingly, the rates of supportive medications including anti-depressants, anti-psychotics, cholinesterase inhibitors and benzodiazepines were much higher in the post-index when compared with the index visit. As patients and physicians struggle to seek the balance between managing symptoms and tolerability, a high rate of therapy changes were documented, highlighting the unstable, changing dynamic nature of PD therapy. This suggests there is a need for well-tolerated treatments to manage symptoms, possibly through add-on treatments that delay dose escalations or treatment switches.
Symptom relapse was the most common reported reason for dose escalation, add-on, and dose reduction, suggesting that treatments may not have been adequate by themselves or were inadequately titrated for maximal effect. A prospective, international, observational real-world study of PD therapies in 2195 patients followed for 33 months found that DA monotherapy decreased over time whereas adding-on of DA to levodopa increased over time, supporting the well-known observation that most patients eventually require combination treatment to manage symptoms[13]. Adverse events were the most common reason for discontinuations and for forward switching in the current analysis. The lowest rate of discontinuation was in patients receiving levodopa-PDDI monotherapy, indicating the importance of this ‘gold standard’ treatment in managing PD and PD symptoms. Similarly, a retrospective claims database analysis of patients with PD in the United States by Houghton et al,[14] found that a smaller percentage of patients (30.4%) initiated on levodopa (with or without PDDI) monotherapy switched or added-on therapies compared with DA monotherapy (60.6%) or MAOBI monotherapy (72.8%).
Analysis of the time to therapy changes indicated that switching, dose escalation, and add-on occurred most frequently in the first 6 months after the index visit. These changes are likely as physicians and patients adjusted to the medications and experimented with what worked best for them. More detailed analyses of therapy changes indicated a cautious approach to treatment for patients with an older age of PD onset, with delays in switching and earlier dose escalations compared with younger age of onset, possibly in attempts to remain on each medication for as long as possible. The retrospective database analysis by Houghton et al,[14] also found that switching or add-on was delayed in older patients compared with younger patients. In contrast, the current analysis found that patients in early disease stages at index delayed dose escalations and were quicker to add-on therapies. Add-on therapies may be an approach that keeps patients on treatments longer without increasing the dose, saving the need for dose escalation until the disease becomes more severe. Recently, the approach of initiating low-dose levodopa monotherapy early in disease has been advocated, primarily based on results from the PD MED and Levodopa in Early Parkinson’s Disease studies.[15], [16], [17] A follow-up analysis of the current study is planned to evaluate therapy changes over time specifically in patients initiating levodopa monotherapy. In addition, more modeling analyses are ongoing to develop predictive models of reasons for therapy change and time to therapy change. Modeling of therapy changes in newly defined PD subtypes that are influenced by disease duration and severity may also be valuable.[18]
Limitations of the analysis include those inherent to retrospective chart reviews, such as data entry errors into either the chart or the eCAF and missing or incomplete information. Another limitation of the analysis is that while the chart record indicated medication utilization, the patient may not have filled the prescription or taken the medication as prescribed. Furthermore, samples of medication given to the patient may not have been captured in the chart. Some of the therapy changes may have been driven by insurance formulary changes, rather than for clinical reasons and may not have been recorded in the chart. The analyses may also be subject to selection bias since the charts were not randomly selected, and the recruited sites may not be truly reflective of general PD treatment patterns. Generalizability of the analysis may be impacted by the small sample size and potential selection bias.
5. Conclusions
Changes to symptomatic PD therapy were common over the study period, reflecting the challenging and evolving management of PD as the disease progresses. New or add-on symptomatic treatments are needed that are well-tolerated and able to control PD symptoms.
6. Data availability
All data supporting the conclusions of the study are reported within the manuscript.
7. Funding statement and role of the funding source
Funding for this study was provided by Cerevel Therapeutics, Cambridge, MA. The funder was involved in the manuscript writing, editing, approval, and decision to publish.
Conflicts of interest
P. Navaratnam and H. Friedman are employees of DataMed Solutions, LLC, which provides contracted services to Cerevel Therapeutics. S. Arcona, M. Leoni, and R. Sasane are employees of Cerevel Therapeutics. S. Shaik an employee of Indegene, Inc., which provided contracted services to Cerevel Therapeutics.
CRediT authorship contribution statement
Prakash Navaratnam: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing – review & editing. Steve Arcona: Conceptualization, Methodology, Funding acquisition, Writing – review & editing. Howard S. Friedman: Methodology, Data curation, Formal analysis, Software, Visualization, Writing – review & editing. Matthew Leoni: Conceptualization, Methodology, Visualization, Writing – review & editing. Shajahan Shaik: Data curation, Software, Writing – review & editing. Rahul Sasane: Conceptualization, Methodology, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding for this study was provided by Cerevel Therapeutics. Medical writing and editorial assistance were provided by Erin P. Scott, PhD, of Scott Medical Communications, LLC. This assistance was funded by Cerevel Therapeutics.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2021.100125.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Weintraub D., Comella C.L., Horn S. Parkinson's disease–Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care. 2008;14:S40–S48. [PubMed] [Google Scholar]
- 2.Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J Neurochem. 2016;139(Suppl 1):318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 3.Meder D., Herz D.M., Rowe J.B., Lehéricy S., Siebner H.R. The role of dopamine in the brain - lessons learned from Parkinson's disease. Neuroimage. 2019;190:79–93. doi: 10.1016/j.neuroimage.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Troncoso-Escudero P., Sepulveda D., Pérez-Arancibia R., et al. On the Right Track to Treat Movement Disorders: Promising Therapeutic Approaches for Parkinson's and Huntington's Disease. Front Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.571185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: a review. Jama. 2014;311(16):1670. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 6.Turcano P., Mielke M.M., Bower J.H., et al. Levodopa-induced dyskinesia in Parkinson disease: A population-based cohort study. Neurology. 2018;91:e2238–e2243. doi: 10.1212/WNL.0000000000006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faulkner M.A. Safety overview of FDA-approved medications for the treatment of the motor symptoms of Parkinson's disease. Expert Opin Drug Saf. 2014;13:1055–1069. doi: 10.1517/14740338.2014.931369. [DOI] [PubMed] [Google Scholar]
- 8.Borovac J.A. Side effects of a dopamine agonist therapy for Parkinson's disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89:37–47. [PMC free article] [PubMed] [Google Scholar]
- 9.Parkinson's disease in adults.: National Institute for Health and Care Excellence (NICE); 2017 [cited 2021 September 30]. Available from: https://www.guidelinecentral.com/summaries/parkinsons-disease-in-adults/#section-442.
- 10.Fox S.H., Katzenschlager R., Lim S.Y., et al. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2018;33:1248–1266. doi: 10.1002/mds.27372. [DOI] [PubMed] [Google Scholar]
- 11.Seppi K., Weintraub D., Coelho M., et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S42–S80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkinson's Disease Diagnosis-Rating Scales.: Health Union, LLC; 2017 [cited 2021 September 30]. Available from: https://parkinsonsdisease.net/diagnosis/rating-scales-staging/.
- 13.Muller T., Tolosa E., Badea L., et al. An observational study of rotigotine transdermal patch and other currently prescribed therapies in patients with Parkinson's disease. J Neural Transm (Vienna). 2018;125:953–963. doi: 10.1007/s00702-018-1860-x. [DOI] [PubMed] [Google Scholar]
- 14.Houghton R., Boess F., Verselis L., et al. Treatment Patterns in Patients with Incident Parkinson's Disease in the United States. J Parkinsons Dis. 2019;9:749–759. doi: 10.3233/JPD-191636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bie R.M.A., Clarke C.E., Espay A.J., Fox S.H., Lang A.E. Initiation of pharmacological therapy in Parkinson's disease: when, why, and how. Lancet Neurol. 2020;19(5):452–461. doi: 10.1016/S1474-4422(20)30036-3. [DOI] [PubMed] [Google Scholar]
- 16.Verschuur C.V.M., Suwijn S.R., Boel J.A., et al. Randomized Delayed-Start Trial of Levodopa in Parkinson's Disease. N Engl J Med. 2019;380:315–324. doi: 10.1056/NEJMoa1809983. [DOI] [PubMed] [Google Scholar]
- 17.Gray R., Ives N., Rick C., et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384:1196–1205. doi: 10.1016/S0140-6736(14)60683-8. [DOI] [PubMed] [Google Scholar]
- 18.Erro R., Picillo M., Scannapieco S., Cuoco S., Pellecchia M.T., Barone P. The role of disease duration and severity on novel clinical subtypes of Parkinson disease. Parkinsonism Relat Disord. 2020;73:31–34. doi: 10.1016/j.parkreldis.2020.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the conclusions of the study are reported within the manuscript.