Abstract
Objectives
To evaluate the effectiveness of the mRNA-1273 vaccine against SARS-CoV-2 variants and assess its effectiveness against the delta variant by time since vaccination.
Design
Test negative case-control study.
Setting
Kaiser Permanente Southern California (KPSC), an integrated healthcare system.
Participants
Adult KPSC members with a SARS-CoV-2 positive test sent for whole genome sequencing or a negative test from 1 March 2021 to 27 July 2021.
Interventions
Two dose or one dose vaccination with mRNA-1273 (Moderna covid-19 vaccine) ≥14 days before specimen collection versus no covid-19 vaccination.
Main outcome measures
Outcomes included infection with SARS-CoV-2 and hospital admission with covid-19. In pre-specified analyses for each variant type, test positive cases were matched 1:5 to test negative controls on age, sex, race/ethnicity, and specimen collection date. Conditional logistic regression was used to compare odds of vaccination among cases versus controls, with adjustment for confounders. Vaccine effectiveness was calculated as (1–odds ratio)×100%.
Results
The study included 8153 cases and their matched controls. Two dose vaccine effectiveness was 86.7% (95% confidence interval 84.3% to 88.7%) against infection with the delta variant, 98.4% (96.9% to 99.1%) against alpha, 90.4% (73.9% to 96.5%) against mu, 96-98% against other identified variants, and 79.9% (76.9% to 82.5%) against unidentified variants (that is, specimens that failed sequencing). Vaccine effectiveness against hospital admission with the delta variant was 97.5% (92.7% to 99.2%). Vaccine effectiveness against infection with the delta variant declined from 94.1% (90.5% to 96.3%) 14-60 days after vaccination to 80.0% (70.2% to 86.6%) 151-180 days after vaccination. Waning was less pronounced for non-delta variants. Vaccine effectiveness against delta infection was lower among people aged ≥65 years (75.2%, 59.6% to 84.8%) than those aged 18-64 years (87.9%, 85.5% to 89.9%). One dose vaccine effectiveness was 77.0% (60.7% to 86.5%) against infection with delta.
Conclusions
Two doses of mRNA-1273 were highly effective against all SARS-CoV-2 variants, especially against hospital admission with covid-19. However, vaccine effectiveness against infection with the delta variant moderately declined with increasing time since vaccination.
Introduction
Vaccines to prevent coronavirus disease 2019 (covid-19) were developed rapidly in response to the covid-19 pandemic. In clinical trials, mRNA based covid-19 vaccines, mRNA-1273 (Moderna Inc, Cambridge, USA; Spikevax) and BNT162b2 (Pfizer Inc, New York, USA; BioNTech Manufacturing GmbH, Mainz, Germany; Comirnaty) were highly efficacious (94% and 95%, respectively) against symptomatic covid-19.1 2 After receiving emergency use authorization in the US, EU, UK, and other countries beginning in December 2020,3 4 5 these vaccines were deployed in phased mass vaccination programs for high risk and general populations.
Subsequently, multiple real world studies conducted before the SARS-CoV-2 delta variant (B.1.617.2 and AY lineage) became predominant reported high effectiveness of mRNA based vaccines against SARS-CoV-2 infection (for example, 82% to 100%6 7 8 9) and admission to hospital with covid-19 (for example, 87-96%).10 11 Few of these studies identified variant specific vaccine effectiveness. In a study in Qatar, vaccine effectiveness of mRNA-1273 against infection with alpha (B.1.1.7) and beta (B.1.351) SARS-CoV-2 variants was 100% and 96.4%, respectively.12 A study in Canada found vaccine effectiveness of mRNA based vaccines against alpha and beta/gamma (P.1) infection to be 90% and 88%, respectively.13
However, as the delta variant became predominant, concerns arose that mRNA based vaccines might be less effective.14 15 The higher transmissibility of delta led to a surge in infections, hospital admissions, and deaths in the US.16 These cases have occurred overwhelmingly among unvaccinated people but have also included breakthrough cases.17 18 19 Although most studies have found sustained effectiveness of mRNA based vaccines against hospital admission with covid-19 during periods overlapping with or during the delta surge,20 21 22 decreased vaccine effectiveness against infection with delta in some studies has been reported (for example, 51-75%).22 23 24 Whether these findings are due to lower protection against delta, waning vaccine immunity over time, or other factors is unclear.
Furthermore, few studies have examined vaccine effectiveness specifically for mRNA-1273 against delta or other SARS-CoV-2 variants. Such studies are critically needed to inform ongoing decisions around booster doses and development of vaccines that may offer broad protection against SARS-CoV-2 variants. Thus, we evaluated the vaccine effectiveness of mRNA-1273 against variants including delta by time since vaccination at Kaiser Permanente Southern California (KPSC).
Methods
Study setting
KPSC is an integrated healthcare system with 15 hospitals and associated medical offices across Southern California. The population of more than 4.6 million health plan members with diverse sociodemographic characteristics is generally representative of the underlying population.25 Members mostly seek care within the KPSC system, and all details of their clinical care (for example, demographics, immunizations, diagnoses, laboratory tests, procedures, and pharmacy records) are recorded in their comprehensive electronic health records, identified through a unique medical record number. Information on care received outside of the KPSC system is captured through claims.
KPSC began administering covid-19 vaccines on 18 December 2020, following state guidelines for vaccine prioritization.26 Covid-19 vaccinations received outside KPSC are imported daily into members’ electronic health records from external sources, including the California Immunization Registry (CAIR),27 CalVax (Cal Poly Pomona mass vaccination site), Care Everywhere (system on the Epic electronic health record platform that allows different healthcare systems to exchange patients’ medical information), claims (for example, retail pharmacies), and self-report by members (with valid documentation).
Laboratory methods
Molecular diagnostic testing for SARS-CoV-2 is widely available and free of charge at KPSC for people with and without symptoms who request testing for any reason or before procedures or hospital admission. Specimens are primarily collected using nasopharyngeal/oropharyngeal swabs or saliva (people without symptoms only) and tested using the RT-PCR TaqPath COVID-19 High-Throughput Combo Kit (Thermo Fisher Scientific, CA, USA). Beginning in March 2021, KPSC began sending all specimens positive for SARS-CoV-2 from people with and without symptoms, regardless of cycle threshold (Ct) values, to a commercial laboratory (Helix, CA, USA) for whole genome sequencing, as described in the supplementary methods.
Study design
The study was conducted as a commitment to multiple regulatory authorities globally, and analyses were pre-specified. To assess vaccine effectiveness of mRNA-1273 against SARS-CoV-2 variants, the study used a test negative design; this design was optimal for using all sequencing data and facilitated analyses of vaccine effectiveness by time since vaccination and vaccine effectiveness of both two doses and one dose of mRNA-1273.
Participants
We included in the study all people with or without symptoms who had a positive test for SARS-CoV-2 sent for whole genome sequencing or a negative test from 1 March to 27 July 2021 if they were aged ≥18 years and had ≥12 months of KPSC membership as of the specimen collection date (needed to ascertain exposure status and covariates accurately). We excluded those who received a covid-19 vaccine other than mRNA-1273, received two doses of mRNA-1273 <24 days apart or <14 days before the specimen collection date, received more than two doses of mRNA-1273 before the specimen collection date, or had a positive SARS-CoV-2 test or covid-19 diagnosis code between 18 December 2020 and 28 February 2021 or ≤90 days before the positive test date (supplementary figure S1).
Test positive cases and test negative controls
We did separate analyses for each SARS-CoV-2 variant, selected on the basis of scientific relevance and prevalence in the KPSC population. These included delta (B.1.617.2, AY.*), alpha (B.1.1.7), epsilon (B.1.427, B.1.429), gamma (P.1, P.1.1, P.1.2), iota (B.1.526, B.1.526.1, B.1.526.2), mu (B.1.621, B.1.621.1), and other (beta, eta, kappa, and any other variants). We defined test positive cases as the first specimen positive for SARS-CoV-2 (that is, any infection, with or without symptoms) identified by whole genome sequencing. We examined cases for which whole genome sequencing failed as a separate category (“unidentified variants”). We defined test positive cases admitted to hospital with covid-19 as a variant with specimen collection date no more than seven days before or during hospital admission with covid-19 confirmed by chart review. We randomly selected test negative controls from eligible people with a negative SARS-CoV-2 test. We matched cases and controls 1:5 on age (18-44 years, 45-64 years, 65-74 years, and ≥75 years), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, and other/unknown), and specimen collection date (±10 days).
Intervention
The intervention studied was receipt of two doses of mRNA-1273 administered ≥24 days apart (fully vaccinated) or one dose of mRNA-1273 (partially vaccinated) ≥14 days before the specimen collection date.
Covariates
We extracted demographic and clinical covariates from electronic health records (supplementary table S1). Variables assessed at the specimen collection date included socioeconomic status (Medicaid, neighborhood median household income), medical center area, pregnancy status, and KPSC physician/employee status. Variables assessed in the two years before the specimen collection date included smoking and body mass index. Variables assessed in the year before the specimen collection date included Charlson comorbidity score, autoimmune conditions, healthcare utilization (virtual, outpatient, emergency department, and inpatient encounters), preventive care (other vaccinations, screenings, and wellness visits), chronic diseases (kidney disease, heart disease, lung disease, liver disease, and diabetes), and frailty index. Other variables included history of SARS-CoV-2 molecular test performed from 1 March 2020 to specimen collection date (irrespective of result), history of covid-19 infection (positive SARS-CoV-2 molecular test or a covid-19 diagnosis code) from 1 March 2020 to specimen collection date, and immunocompromised status.
Statistical analyses
We described the distribution of variants by vaccination status and by calendar time. We described characteristics of cases and controls for each analysis and compared them by using the χ2 test or Fisher’s exact test for categorical variables and the two sample t test or Wilcoxon rank sum test for continuous variables. We used the missing indicator method for covariates with missing data.28 We used conditional logistic regression to estimate the odds ratios and 95% confidence intervals for vaccination, comparing cases and controls. Analyses were adjusted for potential confounders, determined by absolute standardized differences >0.1 and P value <0.1 or by scientific relevance. We calculated vaccine effectiveness (percentage) as (1–adjusted odds ratio)×100. We selected variants with at least 26 cases for analyses according to power calculations. For a 1:5 matched test negative design, assuming a 40% vaccination rate (two doses of mRNA-1273) in test negative controls, we needed 26 cases to detect a vaccine effectiveness of 80% with 80% statistical power.
We analyzed vaccine effectiveness against infection with SARS-CoV-2 variants by time since receipt of second dose of mRNA-1273 (14-60 days, 61-90 days, 91-120 days, 121-150 days, 151-180 days, and >180 days) for delta (overall and by age), non-delta, and unidentified variants. We also assessed one dose vaccine effectiveness against infection with SARS-CoV-2 variants and two dose vaccine effectiveness against hospital admission with SARS-CoV-2 variants. We did post hoc subgroup analyses for two dose vaccine effectiveness against infection with SARS-CoV-2 variants excluding cases and controls with a history of covid-19 infection and excluding cases and controls tested using saliva (people without symptoms). We used SAS software version 9.4 for all analyses.
Patient and public involvement
Although study participants contributed in important ways to this research, no patients were involved in the design, conduct, reporting, or dissemination plans of our research. This regulatory commitment study was discussed in public meetings of the Vaccines and Related Biological Products Advisory Committee and Advisory Committee on Immunization Practices.
Results
The study included 8153 test positive cases that had whole genome sequencing; 7442 (91.3%) of these patients were unvaccinated, 112 (1.4%) were partially vaccinated, and 599 (7.3%) were fully vaccinated (supplementary figure S2). Whole genome sequencing was successful for 5186 (63.6%) cases and failed for 2967 (36.4%) cases. Compared with specimens for which variants were successfully sequenced (table 1), specimens that failed sequencing (unidentified variants) were more often from fully vaccinated cases (326 (11.0%) v 273 (5.3%)), collected via saliva from people without symptoms (275 (9.3%) v 173 (3.3%)), and had Ct values >27 (1974 (66.5%) v 711 (13.7%)) (supplementary table S2). Of identified variants, 2042 (39.4%) were delta, 1436 (27.7%) alpha, 590 (11.4%) epsilon, 357 (6.9%) gamma, 115 (2.2%) iota, 71 (1.4%) mu, and 575 (11.1%) other (table 1; supplementary figures S2 and S3). Fully vaccinated cases primarily had the delta variant (85.0%), and few fully vaccinated cases resulted in admission to hospital (1.8%) or death in hospital with covid-19 (0.0%).
Table 1.
Characteristics of successfully sequenced SARS-CoV-2 specimens, by mRNA-1273 vaccination status. Values are numbers (percentages)
| Characteristics | Vaccinated—1 dose (n=54) | Vaccinated—2 doses (n=273) | Unvaccinated (n=4859) | Total (n=5186) |
|---|---|---|---|---|
| Variants: | ||||
| Alpha | 14 (26) | 13 (5) | 1409 (29.0) | 1436 (27.7) |
| Delta | 15 (28) | 232 (85) | 1795 (36.9) | 2042 (39.4) |
| Epsilon | 7 (13) | 3 (1) | 580 (11.9) | 590 (11.4) |
| Gamma | 8 (15) | 9 (3) | 340 (7.0) | 357 (6.9) |
| Iota | 1 (2) | 3 (1) | 111 (2.3) | 115 (2.2) |
| Mu | 2 (4) | 7 (3) | 62 (1.3) | 71 (1.4) |
| Other* | 7 (13) | 6 (2) | 562 (11.6) | 575 (11.1) |
| Specimen type: | ||||
| Nasopharyngeal/oropharyngeal swab | 53 (98) | 262 (96) | 4698 (96.7) | 5013 (96.7) |
| Saliva (asymptomatic) | 1 (2) | 11 (4) | 161 (3.3) | 173 (3.3) |
| Ct values for N, ORF1ab, and S genes: | ||||
| Any of 3 Ct values ≤27 | 48 (89) | 227 (83) | 4196 (86.4) | 4471 (86.2) |
| All non-missing Ct values >27 | 6 (11) | 46 (17) | 659 (13.6) | 711 (13.7) |
| All 3 Ct values missing | 0 (0) | 0 (0) | 4 (0.1) | 4 (0.1) |
| Ct for N gene: | ||||
| ≤27 | 45 (83) | 216 (79) | 4088 (84.1) | 4349 (83.9) |
| >27 | 9 (17) | 57 (21) | 765 (15.7) | 831 (16.0) |
| Missing | 0 (0) | 0 (0) | 6 (0.1) | 6 (0.1) |
| Ct for ORF1ab gene: | ||||
| ≤27 | 45 (83) | 217 (79) | 4052 (83.4) | 4314 (83.2) |
| >27 | 9 (17) | 56 (21) | 800 (16.5) | 865 (16.7) |
| Missing | 0 (0) | 0 (0) | 7 (0.1) | 7 (0.1) |
| Ct for S gene: | ||||
| ≤27 | 36 (67) | 202 (74) | 2807 (57.8) | 3045 (58.7) |
| >27 | 3 (6) | 57 (21) | 607 (12.5) | 667 (12.9) |
| Missing | 15 (28) | 14 (5) | 1445 (29.7) | 1474 (28.4) |
| Covid-19 hospital admission | 2 (4) | 5 (2) | 309 (6.4) | 316 (6.1) |
| Covid-19 hospital death | 0 (0) | 0 (0) | 30 (0.6) | 30 (0.6) |
Beta, eta, kappa, and other variants.
Among the 2027 cases with the delta variant matched to 10 135 controls (table 2), 66.2% were aged 18-44 years, 55.9% were female, and 42.7% were Hispanic. Cases with the delta variant and controls had similar distributions of lung disease, autoimmune conditions, median neighborhood income, and KPSC physician/employee status. Compared with controls, cases with the delta variant had lower comorbidity and frailty indices and less often had kidney disease, heart disease, liver disease, diabetes, immunocompromised status, pregnancy, and history of covid-19 infection but more often had a history of SARS-CoV-2 molecular testing and Medicaid. Cases with the delta variant also had fewer healthcare visits in the previous year than did controls and less often had preventive care. In addition, we observed some differences by medical center, month of specimen collection, and specimen type. Characteristics of cases and matched controls for non-delta and unidentified variants are described in supplementary tables S3-S9.
Table 2.
Characteristics of SARS-CoV-2 test positive cases for delta variant* and test negative controls (two dose analysis). Values are numbers (percentages) unless stated otherwise
| Characteristics | Test positive (delta) (n=2027) | Test negative (n=10 135) | P value | Absolute standardized difference |
|---|---|---|---|---|
| Mean (SD) age at specimen collection date, years | 39.6 (14.7) | 40.6 (14.6) | 0.001 | 0.07 |
| Age at specimen collection date, years: | NA | NA | ||
| 18-44 | 1342 (66.2) | 6710 (66.2) | ||
| 45-64 | 564 (27.8) | 2820 (27.8) | ||
| 65-74 | 79 (3.9) | 395 (3.9) | ||
| ≥75 | 42 (2.1) | 210 (2.1) | ||
| Sex: | NA | NA | ||
| Female | 1133 (55.9) | 5665 (55.9) | ||
| Male | 894 (44.1) | 4470 (44.1) | ||
| Race/ethnicity: | NA | NA | ||
| Non-Hispanic white | 634 (31.3) | 3170 (31.3) | ||
| Non-Hispanic black | 309 (15.2) | 1545 (15.2) | ||
| Hispanic | 866 (42.7) | 4330 (42.7) | ||
| Non-Hispanic Asian | 75 (3.7) | 375 (3.7) | ||
| Other/unknown | 143 (7.1) | 715 (7.1) | ||
| Body mass index†: | 0.02 | 0.10 | ||
| <18.5 | 17 (0.8) | 118 (1.2) | ||
| 18.5 to <25 | 413 (20.4) | 2147 (21.2) | ||
| 25 to <30 | 568 (28.0) | 2872 (28.3) | ||
| 30 to <35 | 401 (19.8) | 2099 (20.7) | ||
| 35 to <40 | 214 (10.6) | 1071 (10.6) | ||
| 40 to <45 | 92 (4.5) | 505 (5.0) | ||
| ≥45 | 60 (3.0) | 305 (3.0) | ||
| Unknown | 262 (12.9) | 1018 (10.0) | ||
| Smoking†: | <0.001 | 0.10 | ||
| No | 1505 (74.2) | 7653 (75.5) | ||
| Yes | 312 (15.4) | 1717 (16.9) | ||
| Unknown | 210 (10.4) | 765 (7.5) | ||
| Charlson comorbidity score‡: | <0.001 | 0.14 | ||
| 0 | 1695 (83.6) | 7926 (78.2) | ||
| 1 | 192 (9.5) | 1249 (12.3) | ||
| ≥2 | 140 (6.9) | 960 (9.5) | ||
| Frailty index‡: | <0.001 | 0.16 | ||
| Quarter 1 | 536 (26.4) | 2504 (24.7) | ||
| Quarter 2 | 589 (29.1) | 2453 (24.2) | ||
| Quarter 3 | 489 (24.1) | 2551 (25.2) | ||
| Quarter 4 (most frail) | 413 (20.4) | 2627 (25.9) | ||
| Chronic diseases‡: | ||||
| Kidney disease | 37 (1.8) | 262 (2.6) | 0.04 | 0.05 |
| Heart disease | 21 (1.0) | 182 (1.8) | 0.01 | 0.06 |
| Lung disease | 142 (7.0) | 811 (8.0) | 0.13 | 0.04 |
| Liver disease | 25 (1.2) | 275 (2.7) | <0.001 | 0.12 |
| Diabetes | 127 (6.3) | 870 (8.6) | <0.001 | 0.09 |
| Immunocompromised§ | 49 (2.4) | 398 (3.9) | 0.001 | 0.09 |
| Autoimmune conditions‡ ¶ | 46 (2.3) | 252 (2.5) | 0.56 | 0.01 |
| Pregnant at specimen collection date | 51 (2.5) | 521 (5.1) | <0.001 | 0.14 |
| History of SARS-CoV-2 infection** | 16 (0.8) | 758 (7.5) | <0.001 | 0.34 |
| History of SARS-CoV-2 molecular test** | 1063 (52.4) | 4973 (49.1) | 0.006 | 0.07 |
| No of outpatient and virtual visits‡: | <0.001 | 0.28 | ||
| 0 | 235 (11.6) | 689 (6.8) | ||
| 1-4 | 704 (34.7) | 2816 (27.8) | ||
| 5-10 | 555 (27.4) | 2847 (28.1) | ||
| ≥11 | 533 (26.3) | 3783 (37.3) | ||
| No of emergency department visits‡: | 0.001 | 0.09 | ||
| 0 | 1698 (83.8) | 8163 (80.5) | ||
| 1 | 241 (11.9) | 1370 (13.5) | ||
| ≥2 | 88 (4.3) | 602 (5.9) | ||
| No of hospital admissions‡: | 0.07 | 0.06 | ||
| 0 | 1944 (95.9) | 9649 (95.2) | ||
| 1 | 76 (3.7) | 404 (4.0) | ||
| ≥2 | 7 (0.3) | 82 (0.8) | ||
| Preventive care‡ | 1019 (50.3) | 6459 (63.7) | <0.001 | 0.27 |
| Medicaid | 243 (12.0) | 1043 (10.3) | 0.02 | 0.05 |
| Neighborhood median household income: | 0.31 | 0.05 | ||
| <$40 000 | 90 (4.4) | 533 (5.3) | ||
| $40 000-$59 999 | 410 (20.2) | 2186 (21.6) | ||
| $60 000-$79 999 | 530 (26.1) | 2572 (25.4) | ||
| ≥$80 000 | 996 (49.1) | 4837 (47.7) | ||
| Unknown | 1 (0.0) | 7 (0.1) | ||
| KPSC physician/employee | 77 (3.8) | 462 (4.6) | 0.13 | 0.04 |
| Month of specimen collection: | <0.001 | 0.26 | ||
| March 2021 | 0 (0.0) | 0 (0.0) | ||
| April 2021 | 4 (0.2) | 20 (0.2) | ||
| May 2021 | 38 (1.9) | 227 (2.2) | ||
| June 2021 | 339 (16.7) | 2770 (27.3) | ||
| July 2021 | 1646 (81.2) | 7118 (70.2) | ||
| Specimen type: | <0.001 | 0.45 | ||
| Nasopharyngeal/oropharyngeal swab | 1960 (96.7) | 8460 (83.5) | ||
| Saliva (asymptomatic) | 67 (3.3) | 1675 (16.5) |
Medical center area not shown. Distribution of cases and controls across medical center areas differed.
KPSC=Kaiser Permanente Southern California; NA=not applicable.
Delta variant lineages: AY.1, AY.2, AY.3, B.1.617.2.
Defined in two years before specimen collection date.
Defined in one year before specimen collection date.
Defined as HIV/AIDS; leukemia/lymphoma, congenital and other immunodeficiencies, asplenia/hyposplenia; organ transplant; immunosuppressant drugs.
Defined as rheumatoid arthritis, inflammatory bowel disease, psoriasis and psoriatic arthritis, multiple sclerosis, systemic lupus erythematosus.
Defined on basis of all available medical records from 1 March 2020 to specimen collection date.
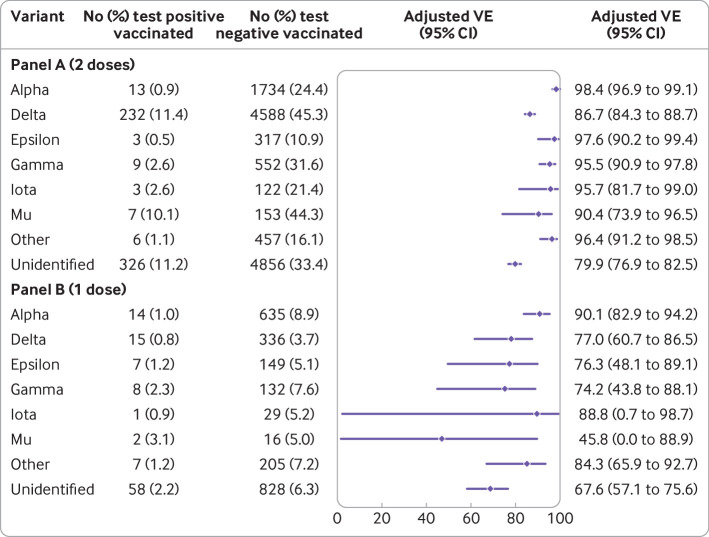
Among cases with the delta variant, 232 (11.4%) were fully vaccinated (fig 1 (panel A) and supplementary table S10). Among controls matched to cases with the delta variant, 4588 (45.3%) were fully vaccinated. By comparison, only 0.9% of cases the alpha variant and 24.4% of matched controls were fully vaccinated. Vaccine effectiveness against infection with the delta variant was 86.7% (95% confidence interval 84.3% to 88.7%), moderately lower than the high vaccine effectiveness against alpha (98.4%, 96.9% to 99.1%). Vaccine effectiveness against mu was 90.4% (73.9% to 96.5%). Vaccine effectiveness against other identified non-delta variants ranged from 95.5% to 97.6%, whereas vaccine effectiveness against unidentified variants was 79.9% (76.9% to 82.5%). Vaccine effectiveness of one dose of mRNA-1273 was lower against all variants, ranging from 45.8% (0.0% to 88.9%) against mu to 90.1% (82.9% to 94.2%) against alpha (Fig 1 (panel B) and supplementary table S11).
Fig 1.
Vaccine effectiveness (VE) of two doses (panel A) and of one dose (panel B) of mRNA-1273 against infection with SARS-CoV-2 variants. “Other” included beta, eta, kappa, and other variants. “Unidentified” were variants for which whole genome sequencing failed
In analyses of vaccine effectiveness against infection with the delta variant by time since receipt of second dose, effectiveness was highest at 14-60 days (94.1% (90.5% to 96.3%)) and declined moderately, with vaccine effectiveness of 80.0% (70.2% to 86.6%) at 151-180 days (fig 2 and supplementary table S12). Vaccine effectiveness against infection with non-delta variants also declined with increasing time since vaccination, although not as sharply as for delta (from 98.6% (97.3% to 99.3%) at 14-60 days to 88.7% (73.2% to 95.2%) at 121-150 days); we could not analyze later time points owing to limited sample size. Vaccine effectiveness against unidentified variants was 83.6% (79.5% to 86.9%) at 14-60 days, declining to 68.5% (51.3% to 79.6%) at 151-180 days.
Fig 2.
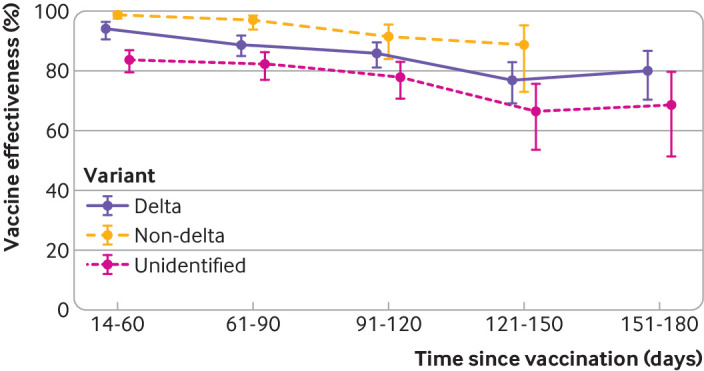
Vaccine effectiveness of two doses of mRNA-1273 against infection with SARS-CoV-2 variants by time since vaccination. Non-delta variants included alpha, epsilon, gamma, iota, mu, and other (beta, eta, kappa, and other variants). Unidentified variants were those for which whole genome sequencing failed
In analyses by age group, vaccine effectiveness of two doses of mRNA-1273 against infection with the delta variant was 87.9% (85.5% to 89.9%) among people aged 18-64 years and 75.2% (59.6% to 84.8%) among those aged ≥65 years (supplementary figure S4). Among people aged 18-64 years, vaccine effectiveness against delta declined from 95.1% (91.8% to 97.1%) at 14-60 days to 79.4% (68.8% to 86.3%) at 151-180 days. Among people aged ≥65 years, confidence intervals were wide owing to fewer cases in this age group, making trends less apparent.
During the study period, we observed robust protection of two doses of mRNA-1273 against hospital admission with the delta variant. Among cases admitted to hospital with delta and controls, five (3.5%) and 365 (51.8%), respectively, were fully vaccinated (table 3). Vaccine effectiveness against admission to hospital with the delta variant was high at 97.5% (92.7% to 99.2%). Similarly, vaccine effectiveness against admission to hospital with unidentified variants was 96.6% (89.5% to 98.9%). We did not estimate vaccine effectiveness against admission to hospital with non-delta variants, as we identified no hospital admissions with non-delta variants among vaccinated people.
Table 3.
Vaccine effectiveness of two doses of mRNA-1273 against admission to hospital with covid-19 due to delta, non-delta, or unidentified variants
| Variant | No | No (%) test positive | No (%) test negative | Odds ratio (95% CI) | Vaccine effectiveness (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Unadjusted | Adjusted* | Unadjusted (%) | Adjusted* (%) | |||||
| Delta | 846 | 5 (4) | 136 (96) | 365 (51.8) | 340 (48.2) | 0.03 (0.01 to 0.07) | 0.03 (0.01 to 0.07) | 97.4 (92.8 to 99.0) | 97.5 (92.7 to 99.2) | |||
| Non-delta† | 1038 | 0 (0) | 173 (100) | 306 (35.4) | 559 (64.6) | NE | NE | NE | NE | |||
| Unidentified‡ | 588 | 7 (7) | 91 (93) | 227 (46.3) | 263 (53.7) | 0.05 (0.02 to 0.13) | 0.03 (0.01 to 0.11) | 94.8 (86.8 to 98.0) | 96.6 (89.5 to 98.9) | |||
This subgroup analysis included test positive cases admitted to hospital and their matched test negative controls.
NE=not estimable.
Model for delta variant adjusted for smoking, Charlson comorbidity score, frailty index, liver disease, pregnancy, history of SARS-CoV-2 infection, number of outpatient and virtual visits, preventive care, medical center area, month of specimen collection, and specimen type; model for unidentified variants adjusted for body mass index, smoking, Charlson comorbidity score, frailty index, pregnancy, history of SARS-CoV-2 infection, number of outpatient and virtual visits, number of emergency department visits, preventive care, medical center area, month of specimen collection, and specimen type.
Non-delta variants included alpha, epsilon, gamma, iota, mu, and other (beta, eta, kappa, and other variants).
Unidentified variants were those for which whole genome sequencing failed.
In post hoc analyses, estimates of two dose vaccine effectiveness against infection with SARS-CoV-2 variants were nearly the same when we excluded cases and controls with a history of covid-19 infection (supplementary table S13) and when we excluded cases and controls tested using saliva (people without symptoms) (supplementary table S14).
Discussion
This real world study provides evidence of high vaccine effectiveness of two doses of mRNA-1273 against multiple SARS-CoV-2 variants, including delta. Although previous studies of BNT162b2 and other covid-19 vaccines have examined vaccine effectiveness against delta,12 13 29 30 31 few studies of mRNA-1273 have reported variant specific vaccine effectiveness.12 30 Our study provides this information, finding that mRNA-1273 was protective against infection with delta and other identified SARS-CoV-2 variants (alpha, epsilon, gamma, iota, mu, and others). Vaccine effectiveness against infection with the delta variant was moderately lower than that against identified non-delta variants (86.7% v 90.4-98.4%), as observed in several other studies.14 32
Comparison with other studies
During the delta phase of the pandemic, breakthrough infections among fully vaccinated people have occurred,17 18 but the effectiveness of covid-19 vaccines against severe disease has remained robust.20 21 33 34 In our study, vaccine effectiveness against admission to hospital with the delta variant was high (97.6%). Only five fully vaccinated people with the delta variant were admitted to hospital, no fully vaccinated people with non-delta variants were admitted, and no deaths in hospital occurred among any fully vaccinated people. This finding is consistent with previous studies suggesting that fully vaccinated people with breakthrough infections tend to have attenuated viral load, fewer symptoms, and shorter duration of illness, although severe outcomes can still occur.8 35 36
In this study, variants were unidentified for the 36% of specimens that failed sequencing, similar to the proportion of specimens that failed sequencing in several other studies (for example, 31% to 47%).37 38 Failed sequencing may be attributed to lower viral loads, timing of specimen collection relative to symptom onset, or poor specimen quality, coupled with the limits of detection of current molecular assays. In our study, some specimens that failed sequencing showed S gene target failure and positive results for the two other gene targets, suggesting that some unidentified variants may belong to the alpha lineage.39 40 41 The higher proportion of failed versus successfully sequenced specimens that were from fully vaccinated people, saliva specimens (used only for asymptomatic testing at KPSC), and specimens with higher Ct values altogether suggest less severe disease, which may help to explain the lower vaccine effectiveness against infection observed for this group (79.9%). Despite the lower vaccine effectiveness against infection, the effectiveness against admission to hospital with unidentified variants remained high (96.6%).
Our study identified moderate waning of mRNA-1273 vaccine effectiveness against infection with the delta variant; effectiveness decreased from 94.1% in the 14-60 days after vaccination to 80.0% in the 151-180 days after vaccination. We observed similar reductions among people aged 18-64 years, but 95% confidence intervals for vaccine effectiveness by time since vaccination were wide among those aged ≥65 years. Other observational studies have found reduced effectiveness of mRNA based vaccines against infection in periods before and after delta became the predominant variant, some of which identified steeper declines than observed in our study.12 22 23 29 For example, a separate KPSC study of BNT162b2 found that vaccine effectiveness against SARS-CoV-2 infection declined from 88% during the first month after full vaccination to 47% after at least five months.31 We also identified a decline in vaccine effectiveness of mRNA-1273 against non-delta infections, but this reduction was less pronounced than for delta or for unidentified variants. Declines in vaccine effectiveness might also be partly due to differences in characteristics and behaviors of people vaccinated earlier versus later in phased vaccine rollout.
Our real world findings complement existing immunogenicity and follow-up data from phase III trials of protection by mRNA-1273 against SARS-CoV-2 variants, including delta. High concentrations of neutralizing antibodies to delta and other variants were elicited following two doses of mRNA-1273.42 These antibodies were found to persist six months after vaccination, albeit at reduced concentrations compared with peak activity. Among phase III trial participants, incidence rates of covid-19 and severe covid-19 during the months when delta was predominant were lower among those who were vaccinated with mRNA-1273 more recently (median eight months after first dose) compared with those vaccinated initially (median 13 months after first dose).43
Implications
The findings of this study have implications for booster doses, which have been authorized in the US and other countries in certain populations. Our findings of reduced vaccine effectiveness of mRNA-1273 against infection with the delta variant, particularly in older people, suggest that booster doses are needed as part of covid-19 vaccination strategies. However, efforts to deploy booster doses must not replace efforts to reach unvaccinated people, who account for most hospital admissions and deaths with covid-19. Booster dose strategies must also prioritize global vaccine production and allocation.
Strengths and limitations of study
Our study had multiple strengths. We systematically collected specimens positive for SARS-CoV-2 across KPSC care settings and sent them for whole genome sequencing regardless of Ct value. We matched test positive cases to test negative controls on demographic factors and calendar time, reducing secular confounding due to differences over time in transmission, vaccination rollout, and testing. We examined mRNA-1273 vaccine effectiveness against multiple variants, including delta and mu. We also evaluated vaccine effectiveness against admission to hospital with the delta variant. With data up to six months after receipt of two doses of mRNA-1273, we stratified analyses of duration of protection by variant type and age group.
Our study also had several limitations. Although test negative designs might reduce bias due to factors associated with care seeking,44 this design was generalizable to people who were tested and was therefore less generalizable to those with mild or no symptoms who did not seek testing. We acknowledge that a variety of reasons for testing may exist; we attempted to account for this by controlling for history of SARS-CoV-2 testing, previous healthcare utilization, and sociodemographic and clinical covariates. However, residual confounding due to unmeasured factors associated with both testing and vaccination could still exist. Vaccine effectiveness against hospital admission was less prone to this potential bias owing to uniform testing procedures in the hospital setting. Misclassification of case/control status could occur owing to false positives or false negatives, although the sensitivity and specificity of PCR testing was high. Misclassification of vaccine exposure was also possible but was unlikely owing to comprehensive KPSC and external covid-19 vaccination records. Covariates were assessed at the specimen collection date instead of at vaccination, as vaccination dates were not available for the unvaccinated group. This could have introduced bias, although such bias was expected to be minimal given that covariates were unlikely to change substantively during the interval between exposure and outcome. Sample size was limited in the subgroup aged ≥65 years for the analysis of vaccine effectiveness against infection with the delta variant by time since vaccination.
Conclusions
This study found high vaccine effectiveness of mRNA-1273 against infection due to SARS-CoV-2 variants, including delta, adding to the limited literature specific for mRNA-1273. Vaccine effectiveness against hospital admission for delta was also high. This study provides reassuring evidence of the effectiveness of two doses of mRNA-1273 in preventing infection with SARS-CoV-2 and hospital admission with covid-19 due to variants including delta. Moderate declines in vaccine effectiveness were observed against infection with the delta variant. Additional research is needed to inform optimal booster dose strategies over time.
What is already known on this topic
Real world studies have found high vaccine effectiveness of mRNA based covid-19 vaccines, but reduced effectiveness against the delta variant and waning protection have been reported
Few studies have examined variant specific vaccine effectiveness of mRNA-1273 (Moderna covid-19 vaccine)
What this study adds
Two doses of mRNA-1273 were highly effective against all SARS-CoV-2 variants
Vaccine effectiveness against infection with the delta variant moderately declined with increasing time since vaccination
Vaccine effectiveness against admission to hospital with the delta variant was 97.5%
Acknowledgments
We acknowledge the following Kaiser Permanente Southern California (KPSC) staff: Donald Kaplan, Daniel Ehrlich, Dale Timothy, Patrick Kerrigan, David Cheng, Kevin Ohara, Joel Christian, Danny Byun, Erin Matsushita, Dennis Curtis, Victoria Hong, and Arthur Librea, for their role in covid-19 vaccine logistics and coordination; Soon Kyu Choi, Jennifer Charter, Joy Gelfond, Radha Bathala, and Lee Childs for their coordination in processing SARS-CoV-2 specimens; and Raul Calderon, Kourtney Kottman, Ana Acevedo, Elmer Ayala, and Jonathan Arguello for their technical and laboratory support in processing SARS-CoV-2 specimens. We acknowledge Helix OpCo, LLC, for whole genome sequencing of SARS-CoV-2 specimens. We also acknowledge the contributions by Moderna staff: Groves Dixon and Julie Vanas. We thank the patients of Kaiser Permanente for their partnership with us to improve their health. Their information, collected through our electronic health record systems, leads to findings that help us to improve care for our members and can be shared with the larger community.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: KJB, LSS, LQ, CAT, and HFT conceptualized the study. KJB, LSS, LQ, BKA, YL, AF, JHK, CAT, and HFT acquired, analyzed, or interpreted the data. KJB and LSS drafted the manuscript. LQ, BKA, YL, GSL, YT, AF, MA, JET, HST, JHK, YDP, CAT, and HFT critically revised the manuscript for important intellectual content. LQ, YL, YT, and JET did the statistical analysis. CAT and HFT obtained funding. LSS, GSL, MA, HST, CAT, and YDP were involved in project administration. CAT and HFT supervised the study. All authors approved the final version of the manuscript. KJB is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by Moderna Inc. Employees of Moderna participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. All authors from KPSC had full access to all the data and can take responsibility for integrity of data and accuracy of data analysis. Assistance in writing and formatting of the manuscript were provided by Srividya Ramachandran and Jared Mackenzie, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, and under the direction of the authors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Moderna for the submitted work; KJB was employed by KPSC during the conduct of the study and is now an adjunct investigator at KPSC; LSS, LQ, BKA, YL, GSL, YT, AF, MA, JET, HST, JHK, and HFT are employees of KPSC, which has been contracted by Moderna to conduct this study; CAT and YDP are employees of and shareholders in Moderna Inc; KJB received funding from GlaxoSmithKline, Dynavax, Pfizer, Gilead, and Seqirus unrelated to this manuscript; LSS received funding from GlaxoSmithKline, Dynavax, and Seqirus unrelated to this manuscript; LQ received funding from GlaxoSmithKline and Dynavax unrelated to this manuscript; BKA received funding from GlaxoSmithKline, Dynavax, Seqirus, and Pfizer unrelated to this manuscript; YL received funding from GlaxoSmithKline, Seqirus, and Pfizer unrelated to this manuscript; GSL received funding from GlaxoSmithKline unrelated to this manuscript; YT received funding from GlaxoSmithKline unrelated to this manuscript; AF received funding from Pfizer, GlaxoSmithKline, and Gilead unrelated to this manuscript; MA received funding from Pfizer unrelated to this manuscript; JET received funding from Pfizer unrelated to this manuscript; HST received funding from GlaxoSmithKline, Pfizer, ALK, and Wellcome unrelated to this manuscript; JHK received funding from GlaxoSmithKline unrelated to this manuscript; HFT received funding from GlaxoSmithKline and Seqirus unrelated to this manuscript and also served on advisory boards for Janssen and Pfizer.
The corresponding author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results of the study will be shared with leaders in the KPSC health system and will be disseminated through news and social media channels. Results have also been posted to a preprint server that is accessible to the general public.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was reviewed and approved by the KPSC Institutional Review Board (IRB #12758). All study staff with access to protected health information were trained in procedures to protect the confidentiality of participants’ data. A waiver of informed consent was obtained, as this is an observational study of authorized and recommended Moderna covid-19 vaccine administered in the course of routine clinical care. To facilitate the conduct of this study, a waiver was obtained for written HIPAA authorization for research involving use of the electronic health records.
Data availability statement
Individual level data reported in this study are not publicly shared. On request, and subject to review, KPSC may provide the de-identified aggregate level data that support the findings of this study. De-identified data (including participants’ data as applicable) may be shared on approval of an analysis proposal and a signed data access agreement.
References
- 1. Baden LR, El Sahly HM, Essink B, et al. COVE Study Group . Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403-16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group . Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Food and Drug Administration. Moderna COVID-19 Vaccine. 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine.
- 4.United States Food and Drug Administration. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- 5.World Health Organization. COVID-19 Vaccine Tracker. 2021. https://covid19.trackvaccines.org/agency/who/.
- 6. Paris C, Perrin S, Hamonic S, et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect 2021;27:1699.e5-8. 10.1016/j.cmi.2021.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrejko KL, Pry J, Myers JF, et al. California COVID-19 Case-Control Study Team . Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clin Infect Dis 2021;ciab640. 10.1093/cid/ciab640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson MG, Burgess JL, Naleway AL, et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med 2021;385:320-9. 10.1056/NEJMoa2107058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butt AA, Omer SB, Yan P, Shaikh OS, Mayr FB. SARS-CoV-2 Vaccine Effectiveness in a High-Risk National Population in a Real-World Setting. Ann Intern Med 2021;174:1404-8. 10.7326/M21-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J Med 2021;385:1355-71. 10.1056/NEJMoa2110362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years - COVID-NET, 13 States, February-April 2021. MMWR Morb Mortal Wkly Rep 2021;70:1088-93. 10.15585/mmwr.mm7032e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021;27:1614-21. 10.1038/s41591-021-01446-y [DOI] [PubMed] [Google Scholar]
- 13. Chung H, He S, Nasreen S, et al. Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators . Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ 2021;374:n1943. 10.1136/bmj.n1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med 2021;385:585-94. 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mallapaty S. COVID vaccines slash viral spread - but Delta is an unknown. Nature 2021;596:17-8. 10.1038/d41586-021-02054-z [DOI] [PubMed] [Google Scholar]
- 16. Scobie HM, Johnson AG, Suthar AB, et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status - 13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1284-90. 10.15585/mmwr.mm7037e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1059-62. 10.15585/mmwr.mm7031e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS-CoV-2 Infection in a Highly Vaccinated Health System Workforce. N Engl J Med 2021;385:1330-2. 10.1056/NEJMc2112981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Twohig KA, Nyberg T, Zaidi A, et al. COVID-19 Genomics UK (COG-UK) consortium . Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis 2021;S1473-3099(21)00475-8. 10.1016/S1473-3099(21)00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tenforde MW, Self WH, Naioti EA, et al. IVY Network Investigators. IVY Network . Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1156-62. 10.15585/mmwr.mm7034e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bajema KL, Dahl RM, Prill MM, et al. SUPERNOVA COVID-19. Surveillance Group. Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group . Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalization - Five Veterans Affairs Medical Centers, United States, February 1-August 6, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1294-9. 10.15585/mmwr.mm7037e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status - New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1306-11. 10.15585/mmwr.mm7037a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K, HEROES-RECOVER Cohorts . Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance - Eight U.S. Locations, December 2020-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1167-9. 10.15585/mmwr.mm7034e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1163-6. 10.15585/mmwr.mm7034e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012;16:37-41. 10.7812/TPP/12-031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.California Department of Public Health. Updated COVID-19 Vaccine Eligibility Guidelines. 2021. https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/VaccineAllocationGuidelines.aspx.
- 27.California Department of Public Health. Reporting Doses Administered: California COVID-19 Vaccination Program. 2021. https://eziz.org/assets/other/IMM-1328.pdf.
- 28. Miettinen OS. Theoretical epidemiology: Principles of Occurrence Research in Medicine. John Wiley & Sons, 1985. [Google Scholar]
- 29. Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021. 10.1038/s41591-021-01548-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrews N, Tessier E, Stowe J, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv 2021:2021.09.15.21263583 10.1101/2021.09.15.21263583 . [DOI]
- 31. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021;398:1407-16. 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nasreen S, Chung H, He S, et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. medRxiv 2021:2021.06.28.21259420. [DOI] [PMC free article] [PubMed]
- 33. Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med 2021. 10.1038/s41591-021-01583-4 [DOI] [PubMed] [Google Scholar]
- 34. Grannis SJ, Rowley EA, Ong TC, et al. VISION Network . Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19-Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance - Nine States, June-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1291-3. 10.15585/mmwr.mm7037e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Havers FP, Pham H, Taylor CA, et al. COVID-19-associated hospitalizations among vaccinated and unvaccinated adults ≥18 years – COVID-NET, 13 states, January 1 – July 24, 2021. medRxiv 2021:2021.08.27.21262356 10.1101/2021.08.27.21262356 . [DOI] [PMC free article] [PubMed]
- 36. Chia PY, Xiang Ong SW, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. Clin Microbiol Infect 2021;S1198-743X(21)00638-8. 10.1016/j.cmi.2021.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Emary KRW, Golubchik T, Aley PK, et al. COVID-19 Genomics UK consortium. AMPHEUS Project. Oxford COVID-19 Vaccine Trial Group . Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 2021;397:1351-62. 10.1016/S0140-6736(21)00628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frampton D, Rampling T, Cross A, et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis 2021;21:1246-56. 10.1016/S1473-3099(21)00170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mishra S, Mindermann S, Sharma M, et al. COVID-19 Genomics UK (COG-UK) Consortium . Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England. EClinicalMedicine 2021;39:101064. 10.1016/j.eclinm.2021.101064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown KA, Gubbay J, Hopkins J, et al. S-Gene Target Failure as a Marker of Variant B.1.1.7 Among SARS-CoV-2 Isolates in the Greater Toronto Area, December 2020 to March 2021. JAMA 2021;325:2115-6. 10.1001/jama.2021.5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Washington NL, Gangavarapu K, Zeller M, et al. Emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States. Cell 2021;184:2587-2594.e7. 10.1016/j.cell.2021.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pegu A, O’Connell SE, Schmidt SD, et al. mRNA-1273 Study Group§ . Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021;373:1372-7. 10.1126/science.abj4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baden LR, El Sahly HM, Essink B, et al. Covid-19 in the Phase 3 Trial of mRNA-1273 During the Delta-variant Surge. medRxiv 2021:2021.09.17.21263624 10.1101/2021.09.17.21263624 . [DOI] [PMC free article] [PubMed]
- 44. Lewnard JA, Patel MM, Jewell NP, et al. Theoretical Framework for Retrospective Studies of the Effectiveness of SARS-CoV-2 Vaccines. Epidemiology 2021;32:508-17. 10.1097/EDE.0000000000001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
Individual level data reported in this study are not publicly shared. On request, and subject to review, KPSC may provide the de-identified aggregate level data that support the findings of this study. De-identified data (including participants’ data as applicable) may be shared on approval of an analysis proposal and a signed data access agreement.