Abstract
In May 2020 and February 2021, capmatinib and tepotinib, respectively were approved by the Food and Drug Administration (FDA) for the treatment of metastatic non‐small cell lung carcinoma harboring mesenchymal‐epithelial transition (MET) exon 14 skipping alterations. Herein, we present a case of intolerable peripheral edema caused by tepotinib, in which MET inhibitor could be continued by switching to capmatinib. Peripheral edema has been identified as one of the most common adverse events in capmatinib and tepotinib; however, there is no unified management for this adverse event. This is the first report that two MET inhibitors have different effects on the development of peripheral edema, and that the MET inhibitors can be continued by switching these drugs.
Keywords: capmatinib, MET ex.14 skipping, peripheral edema, tepotinib
Peripheral edema has been identified as one of the most common adverse events in capmatinib and tepotinib; however, there is no unified management for this side effect. This is the first report that two MET inhibitors have different effects on the development of peripheral edema, and that the MET inhibitors can be continued by switching these drugs.
INTRODUCTION
The mesenchymal–epithelial transition (MET) is a tyrosine kinase receptor that is mostly expressed in epithelial cells. 1 A mutation that results in loss of exon 14 in the MET gene leads to dysregulation and inappropriate signaling that is associated with increased responsiveness to MET tyrosine kinase inhibitors (TKIs). 2 In May 2020 and February 2021, capmatinib and tepotinib, respectively were approved by the Food and Drug Administration (FDA) for the treatment of metastatic non‐small cell lung carcinoma (NSCLC) harboring MET exon 14 skipping alterations. 3 Capmatinib should be administered at 400 mg twice daily under fasting, for a total dose of 800 mg. 4 Moreover, tepotinib should be administered at a dose of 500 mg once daily after meals. 5 Peripheral edema is the characteristic toxicity of these drugs. It frequently occurs in approximately 50% of all grades and 7%–11% of grades 3 or higher. 4 , 5 Herein, we present a case of intolerable peripheral edema caused by tepotinib, in which MET inhibitor could be continued by switching to capmatinib.
CASE REPORT
A 72‐year‐old man who had never smoked with a history of hypertension and diabetes mellitus had undergone left lower lobectomy 4 years previously and was diagnosed with lung adenocarcinoma, pathological T2aN2M0. He received adjuvant chemotherapy. EGFR mutation, ALK, and ROS1 fusions were not detected, and PD‐L1 tumor proportion score (TPS) was 60%. After one and a half years, multiple brain and lung metastases recurred. He was treated with systemic chemotherapy as follows; first‐line pembrolizumab, second‐line combination treatment of cisplatin, pemetrexed and bevacizumab, and third‐line combination treatment of docetaxel and ramucirumab. At this point, MET c.3028 + 2 T > C mutation in the splicing acceptor site was detected in the surgical specimen by FoundationOne®CDx, and the same mutation was confirmed by the ArcherMET assay. Initially, tepotinib was started at 500 mg; however, within 2 weeks, grade 3 based on CTCAE v.5.0 edema developed in the extremities (Figure 1). Despite the administration of diuretics, edema from the thigh to the dorsum of the foot was severe, the range of motion of the ankle joint was severely limited, and gait disturbance also occurred (Figure 2a). After a 2‐week recovery, the edema improved and treatment was resumed at 250 mg; however, grade 3 edema developed again. Onycholysis of fingers was also observed (Figure 2a). Although 250 mg was administered every other day, the edema continued to worsen. He requested that tepotinib was discontinued, resulting in the decision being made to switch him to capmatinib 151 days after tepotinib administration. The dose of capmatinib was reduced and started at 400 mg; however, there was no excerbation of edema. The dose was increased to 600 mg, resulting in a grade 2 increase in serum creatinine. Thereafter, the dose was reduced to 400 mg. He continued capmatinib at 400 mg for approximately 100 days without excerbation of edema (Figure 2b), and his tumor was well controlled (Figure 3).
FIGURE 1.
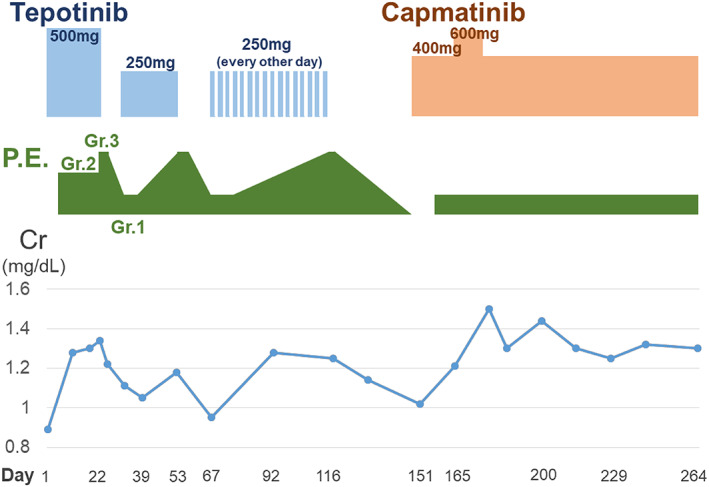
Clinical course of oral administration of tepotinib and capmatinib, the grade of peripheral edema based on CTCAE v.5.0, and changes in serum creatine levels. The number of days after starting oral administration of tepotinib is shown at the bottom. P.E, peripheral edema; Gr, grade; Cr, creatinine. Representative photographs of the extremities of this case
FIGURE 2.
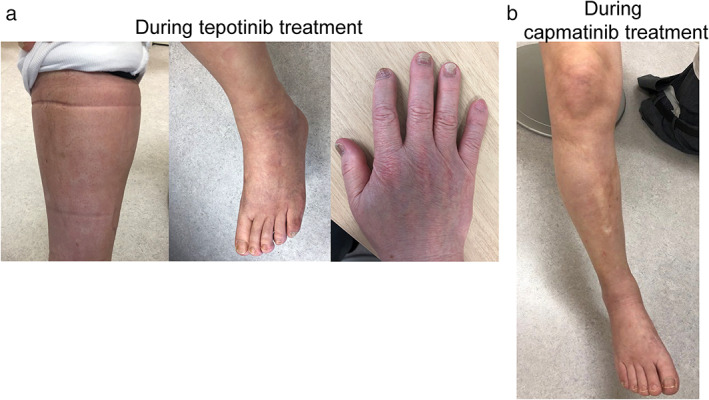
(a) Peripheral edema in the lower legs and strong mark on the socks, edema over the dorsum of the foot and limited ankle range of motion and onycholysis of the fingers associated tepotinib. (b) Peripheral edema in the lower leg during tepotinib treatment was improved during capmatinib treatment
FIGURE 3.
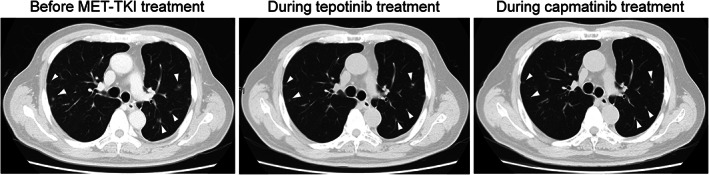
Serial images of chest plain computed tomography showing multiple pulmonary metastases (white arrow heads) before MET TKI introduction, which were reduced by administration of tepotinib, and the reduction was maintained during capmatinib treatment
DISCUSSION
Peripheral edema induced by capmatinib and tepotinib is probably caused by inhibition of the MET signaling pathway. 1 The same adverse events of peripheral edema have been reported with antibody drugs as well as tyrosine kinase inhibitors, which inhibit the MET pathway. 1 , 6 In clinical trials with rilotumumab and onartuzumab, both of which are humanized monoclonal antibodies specific for an epitope in the hepatocyte growth factor (HGF) binding domain of the MET receptor, adverse events of peripheral edema have been frequently observed. 6 Drug‐induced peripheral edema has been described as noninflammatory edema and has four mechanisms: precapillary arteriolar vasodilation (vasodilatory edema), sodium/water retention (renal edema), lymphatic insufficiency (lymphedema) and increased capillary permeability (permeability edema). 7 The etiology of MET inhibitor‐induced edema is still unclear, but may be attributable to an attenuation of HGF‐mediated signaling in the peripheral vascular endothelium. 1 , 6 In physiological conditions, HGF in the vascular endothelium helps to protect against VEGF‐induced endothelial hyperpermeability. Perturbation of HGF/MET signaling could disrupt this balance resulting in endothelial leak. 6 The only treatment for edema is the use of diuretics or the discontinuation of the causative agent, but as shown in this case, the use of diuretics may not be effective. Permeability edema occurs mainly in the lower legs. 7 In the case reported here, the patient was unable to wear shoes due to edema and unable to ride a bicycle due to dorsiflexion. This was a major hindrance to his daily life. Therefore, the MET TKI had to be discontinued.
Capmatinib and tepotinib are novel, ATP‐competitive inhibitors of MET with IC50 of 0.13 and 4 nM, respectively in a cell free assay. In the present case, peripheral edema occurred even at a low dose of tepotinib, suggesting that 50% inhibition concentration of MET kinase activity is not necessarily related to the development of edema. Capmatinib is metabolized mainly by CYP3A, which is not involved in the metabolism of tepotinib. 3 CYP3A polymorphisms specifically involved in the metabolism of capmatinib have not been identified, but it is possible that these differences have produced differences in serum concentration of each drug in individual cases and may have influenced adverse events.
In conclusion, MET TKIs are a promising treatment for NSCLC patients harboring MET ex.14 skipping mutations, but peripheral edema associated with these agents is a common and sometimes difficult to control adverse event. At present, the existence of two MET TKIs available in clinical practice may have a significant aspect in continuing MET TKIs when intolerable edema occurs, as shown in this case.
CONFLICT OF INTEREST
Dr Kunimasa reports grants from the Japan Society for the Promotion of Science (Grant no. JP19K176974), Takeda Science Foundation, The Osaka Medical Research Foundation for Intractable Diseases, and honoraria for lecture from AstraZeneca, Chugai Pharma and Novartis. Dr Tamiya reports grants from Ono Pharmaceutical, Bristol‐Myers Squibb, Boehringer Ingelheim and honoraria for lecture from Taiho Pharmaceutical, Eli Lilly, Asahi Kasei Pharmaceutical, MSD, Boehringer Ingelheim, AstraZeneca, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb; Dr Nishino reports a grant from Nippon Boehringer Ingelheim and honoraria for lecture from Chugai Pharma, AstraZeneca, Nippon Boehringer Ingelheim, Eli Lilly Japan, Roche Diagnostics, Novartis, Pfizer Merk; Dr Kumagai reports grants from Ono Pharmaceutical, MSD K.K., Chugai Pharmaceutical Co. Ltd、AstraZeneca K.K. Takeda Pharmaceutical Companey Limited. Regeneron Pharmaceuticals, Inc. Merck Serono Co., Ltd. Pfizer Japan Inc. Taiho Pharmaceutical Co.,Ltd. Nippon Boehringer lngelheim Co., Ltd. Eli Lilly Japan K.K. Novartis Pharma K.K. AbbVie GK., Delta‐Fly Pharma, Inc. The Osaka Foundation for The Prevention of Cancer and Life‐style related Diseases (Public Interest Incorporated Foundation), and personal fees from Ono Pharmaceutical, AstraZeneca K. K., Taiho Pharmaceutical Co. Ltd., MSD K.K., TEIJIN PHARMA LIMITED, Novartis Pharma K.K. Nippon Boehringer Ingelheim Co., Ltd. Eli Lilly Japan K.K. Pfizer Inc., Chugai Pharceutical Co. Ltd., Bristol‐Myers Squibb K.K.
Kunimasa K, Kawamura T, Tamiya M, Inoue T, Kuhara H, Nishino K, et al. Capmatinib successfully overcomes tepotinib‐induced intolerable peripheral edema. Thorac Cancer. 2021;12:3426–3428. 10.1111/1759-7714.14205
REFERENCES
- 1. Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c‐Met signaling pathway in cancer. Clin Cancer Res. 2009;15(7):2207–14. [DOI] [PubMed] [Google Scholar]
- 2. Reungwetwattana T, Liang Y, Zhu V, Ou SI. The race to target MET exon 14 skipping alterations in non‐small cell lung cancer: the why, the how, the who, the unknown, and the inevitable. Lung Cancer. 2017;103:27–37. 10.1158/1078-0432.ccr-21-1566 [DOI] [PubMed] [Google Scholar]
- 3. Mathieu LN, Larkins E, Akinboro O, Roy P, Amatya AK, Fiero MH, et al. FDA approval summary: capmatinib and tepotinib for the treatment of metastatic NSCLC harboring MET exon 14 skipping mutations or alterations. Clin Cancer Res. 2021. [DOI] [PubMed] [Google Scholar]
- 4. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14‐mutated or MET‐amplified non‐small‐cell lung cancer. N Engl J Med. 2020;383(10):944–57. [DOI] [PubMed] [Google Scholar]
- 5. Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non‐small‐cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hack SP, Bruey JM, Koeppen H. HGF/MET‐directed therapeutics in gastroesophageal cancer: a review of clinical and biomarker development. Oncotarget. 2014;5(10):2866–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Largeau B, Cracowski JL, Lengellé C, Sautenet B, Jonville‐Béra AP. Drug‐induced peripheral oedema: an aetiology‐based review. Br J Clin Pharmacol. 2021;87(8):3043–55. [DOI] [PubMed] [Google Scholar]


