Abstract
Background
A heterogeneous radiological response is frequently observed in cancer patients and could reflect tumor heterogeneity. We investigated the prognostic impact of heterogeneous radiological responses in patients with advanced non‐small‐cell lung cancer (NSCLC) who received platinum‐based chemotherapy.
Methods
The treatment response according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria was evaluated in 212 patients with advanced NSCLC who received platinum‐based chemotherapy. Patients with partial response (PR) or stable disease (SD) were classified into “PR homo,” “PR hetero,” “SD homo,” and “SD hetero” by the presence of a heterogeneous radiological response, and survival was compared between groups. We also compared survival based on the presence of metabolic responses in lesions with heterogeneous radiological responses.
Results
Fifty‐two patients (24.5%) were classified as PR, 112 patients (52.8%) as SD, and 48 patients (22.7%) as progressive disease (PD). There was no significant difference in progression‐free survival (PFS) and overall survival (OS) between the PR homo and PR hetero groups. The SD homo group had a longer PFS and OS than the SD hetero group. In the SD hetero group, patients with increased maximum standardized uptake value (SUVmax) in lesions with heterogeneous radiological responses had a shorter PFS than those with a stable SUVmax.
Conclusions
The presence of lesions with radiological heterogeneity was associated with disease progression and poor prognosis in the SD group. Patients with heterogeneous radiological responses require careful monitoring.
Keywords: metabolic response, non‐small‐cell lung cancer, radiological heterogeneity, RECIST, survival
Patients with heterogeneous radiological responses in the SD group had a shorter PFS and OS. In particular, patients with increased SUVmax in lesions with heterogeneous radiological responses had a shorter PFS than patients with a stable SUVmax. The presence of lesions with radiological heterogeneity was associated with disease progression and poor prognosis in the SD group.
INTRODUCTION
Non‐small‐cell lung cancer (NSCLC) accounts for approximately (~85%) of primary lung cancer and approximately (~60%) of NSCLC patients are diagnosed in the advanced stage. 1 , 2 For decades, platinum‐based chemotherapy has been used as the standard treatment for advanced NSCLC patients who were not eligible for targeted therapies or immunotherapies. 3 However, 5‐year survival is only 4% in the advanced stage. 1 Some lung cancers are initially resistant to chemotherapy, and acquired resistance develops rapidly even in lung cancers that initially respond to chemotherapy. 4 A comprehensive evaluation of the treatment response is important to determine the treatment regimen when disease progression is suspected.
Response Evaluation Criteria in Solid Tumors (RECIST) criteria have limitations because they do not reflect tumor heterogeneity and do not correlate with survival. 5 Tumor heterogeneity is associated with tumor progression, treatment failure, and relapse. 6 Heterogeneous radiological responses are frequently observed in cancer patients treated with chemotherapy or radiotherapy. 7 , 8 Therefore, it is important to identify heterogeneous radiological responses because they reflect tumor heterogeneity. 7 , 9 , 10 Although a few studies have investigated the association of radiological heterogeneity and prognosis in colorectal cancer and renal cell carcinoma, the prognostic impact on aggressive malignancies, such as lung cancer, have not yet been investigated. 7 , 11 , 12 , 13 Positron emission tomography (PET) is expected to provide additional information on metabolic responses for the evaluation of tumor responses, based on anatomic changes evaluated with RECIST. 14 However, further evidence is still needed for the use of PET in the evaluation of tumor responses. 15
The aim of this study was to investigate the prognostic impact of heterogeneous radiological responses to platinum‐based chemotherapy. In addition, we investigated the additional information that PET could provide in the evaluation of treatment responses.
METHODS
Study population
A total of 1242 patients histologically diagnosed with advanced NSCLC and treated with platinum doublet as first‐line therapy from July 2006 to June 2014 at Inha University Hospital were initially considered for this study. Among them, patients who did not undergo response evaluation (n = 431) or who were not treated with 18F‐fluorodeoxyglucose positron emission tomography/computed tomography (FDG‐PET/CT) during response evaluation (n = 458) were excluded. Patients with brain metastasis at diagnosis (n = 97) or who received chemoradiation therapy (n = 44) were also excluded. In total, 212 patients were included in this study. Information about age, sex, smoking habits, Eastern Cooperative Oncology Group performance status (ECOG PS), histology, epidermal growth factor receptor (EGFR) mutation, staging, and chemotherapeutic agents were analyzed. The stage of all patients was estimated according to the 8th edition of the TNM classification system. 16 All information was collected prospectively from the records of the Lung Cancer Cohort of Inha University Hospital. 17 This study was approved by the Institutional Review Board of Inha University Hospital, and the requirement of obtaining written informed consent from patients was waived.
Response evaluation
Chest CT, PET/CT, and brain magnetic resonance imaging were conducted at the time of diagnosis and every 8–12 weeks using standard protocols. Responses were evaluated using RECIST 1.1. 5 Radiological heterogeneity was assessed at the first reevaluation in patients who showed a partial response (PR) or stable disease (SD). Heterogeneous radiological response was defined as when the longest diameter of any measurable target lesion or the shortest diameter of any measurable target lymph node changed by more than 20%. Patients with PR or SD were classified as “PR homo,” “PR hetero,” “SD homo,” and “SD hetero” by the presence of lesions with heterogeneous radiological responses. We compared survival by the presence of heterogeneous radiological responses.
In addition, metabolic response was evaluated by changes in the maximum standardized uptake value (SUVmax) using PET/CT. The presence of abnormal fluorodeoxyglucose (FDG) uptake was indicated when accumulation of the radiotracer moderately to markedly increased relative to the expected uptake in normal structures or surrounding tissues, with the exclusion of physiological bowel and urinary activities. We compared survival by the presence of metabolic responses in lesions with heterogeneous radiological responses.
Statistical analysis
To assess the association between treatment responses and clinical variables, Pearson's χ2 test was conducted. Progression‐free survival (PFS) and overall survival (OS) were calculated according to the Kaplan–Meier method and log‐rank test. To assess the effect of radiological heterogeneity, we performed univariate and multivariate analyses using the Cox proportional hazards model. Variables that were found to have a value of p ≤ 0.1 in univariate analysis were included in a multivariate Cox proportional hazards model. Statistical significance was considered as two‐sided p values of < 0.05. All statistical analyses were performed by using a statistical software package (SPSS, version 19.0).
RESULTS
Patient characteristics
According to RECIST 1.1, 52 patients (24.5%) were classified as PR, 112 patients (52.8%) as SD, and 48 patients (22.7%) as progressive disease (PD). Table 1 summarizes the baseline characteristics of the study population. The median age of the patients was 66 years (range, 37–84 years), and 155 (73.1%) patients were male. No statistically significant differences were found in the baseline variables between the three groups, except for the ECOG PS and EGFR mutation status. All patients received a combination of platinum‐based chemotherapy, and more than half received irinotecan/cisplatin (60.8%).
TABLE 1.
Characteristics of non‐small cell lung cancer patients treated with platinum‐based chemotherapy
| Variables | All patients (n = 212) | Treatment response | |||
|---|---|---|---|---|---|
| PR (n = 52) | SD (n = 112) | PD (n = 48) | p value | ||
| Age, yr | |||||
| <66 | 101 (47.6) | 21 (40.4) | 61 (54.5) | 19 (39.6) | 0.109 |
| ≥66 | 111 (52.4) | 31 (59.6) | 51 (45.5) | 29 (60.4) | |
| Sex | |||||
| Male | 155 (73.1) | 40 (77.0) | 85 (75.9) | 30 (62.5) | 0.167 |
| Female | 57 (26.9) | 12 (23.0) | 27 (24.1) | 18 (37.5) | |
| Smoking habit | |||||
| Never | 43 (20.3) | 8 (15.3) | 21 (18.8) | 14 (29.2) | 0.194 |
| Current + former | 169 (79.7) | 44 (84.7) | 91 (81.2) | 34 (70.8) | |
| ECOG PS | |||||
| 0–1 | 161 (75.9) | 32 (61.5) | 93 (83.0) | 36 (75.0) | 0.011 |
| ≥2 | 51 (24.1) | 20 (38.5) | 19 (17.0) | 12 (25.0) | |
| Histology | |||||
| SQC | 78 (36.8) | 23 (44.2) | 39 (34.8) | 16 (33.3) | 0.454 |
| ADC | 114 (53.8) | 23 (44.2) | 65 (58.1) | 26 (54.2) | |
| Others | 20 (9.4) | 6 (11.6) | 8 (7.1) | 6 (12.5) | |
| EGFR mutation | |||||
| No | 105 (49.5) | 20 (38.5) | 64 (57.1) | 21 (43.8) | 0.040 |
| Yes | 38 (18) | 8 (15.4) | 22 (19.7) | 8 (16.7) | |
| Not tested | 69 (32.5) | 24 (46.1) | 26 (23.2) | 19 (39.5) | |
| Stage | |||||
| III | 65 (30.7) | 16 (30.8) | 33 (29.5) | 16 (33.4) | 0.888 |
| IV | 147 (69.3) | 36 (69.2) | 79 (70.5) | 32 (66.6) | |
| Chemotherapy agent | |||||
| Irinotecan/cisplatin | 129 (60.8) | 32 (61.5) | 62 (55.3) | 35 (72.9) | 0.354 |
| Gemcitabine/cisplatin | 32 (15.2) | 7 (13.5) | 19 (17.0) | 6 (12.5) | |
| Pemetrexed/cisplatin | 31 (14.6) | 6 (11.5) | 21 (18.8) | 4 (8.3) | |
| Others | 20 (9.4) | 7 (13.5) | 10 (8.9) | 3 (6.3) | |
Abbreviations: ADC, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; PD, progressive disease; PR, partial response; SD, stable disease; SQC, squamous cell carcinoma.
Survival by treatment response
The median PFS and OS times were 5.7 months (95% confidence interval [CI] = 5.2–6.3 months) and 15 months (95% CI = 13.1–16.9 months), respectively. The PD group had shorter PFS than the SD and PR groups (median PFS [95% CI]; 3.1 months [2.7–3.6 months] in the PD group, 5.7 months [4.9–6.5 months] in the SD group, and 8.0 months [6.8–9.2 months] in the PR group, p < 0.001, Figure 1(a)). However, there was no significant difference in PFS between the SD and PR groups. The PD group had a shorter OS than the SD and PR groups (median OS [95% CI]; 10.3 months [7.4–13.2 months] in the PD group, 17.0 [14.3–19.8 months] in the SD group, and 17.2 [11.5–22.9 months] in the PR group, p < 0.001, Figure 1(b)). However, there was no significant difference in the OS between the SD and PR groups.
FIGURE 1.
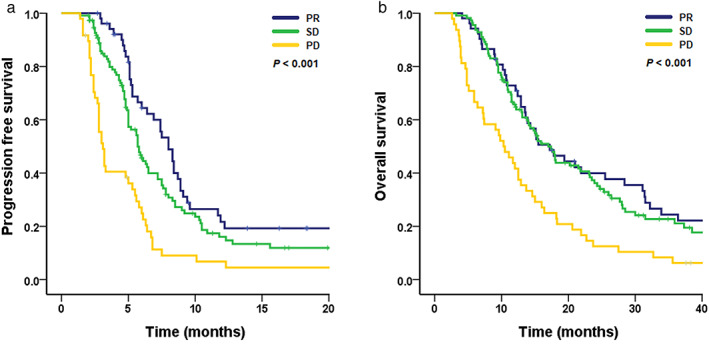
Kaplan–Meier curve of (a) progression‐free survival and (b) overall survival by treatment response
The effect of radiological heterogeneity on survival
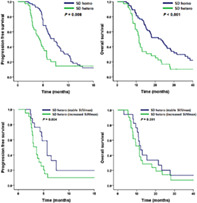
Of the 52 patients in the PR group, 46 patients (88.5%) had a homogeneous response (PR homo group) and 6 patients (11.5%) had a heterogeneous response (PR hetero group). There was no significant difference in PFS (median PFS [95% CI]; 8.3 months [7.2–9.4 months] in the PR homo group and 5.2 months [5.0–5.4 months] in the PR hetero group, p = 0.271) and OS (median OS [95% CI]; 17.2 months [11.7–22.7 months] in the PR homo group and 13.9 months [0.0–34.9 months in the PR hetero group, p = 0.530) between the PR homo and PR hetero groups. Of the 112 patients in the SD group, there were 74 patients (66.1%) in the SD homo group and 38 patients (33.9%) in the SD hetero group. The SD homo group had a longer PFS (median PFS [95% CI]; 7.2 months [5.7–8.7 months] vs. 4.3 [3.1–5.5 months], p = 0.008) and OS (23.1 months [16.2–30.0 months] vs. 11.5 [10.7–12.3 months], p < 0.001) than the SD hetero group (Figure 2). Two representative examples of how the size of the lesion showing radiological heterogeneity increased, resulting PD in the patients of SD hetero group were highlighted in Figure S1.
FIGURE 2.
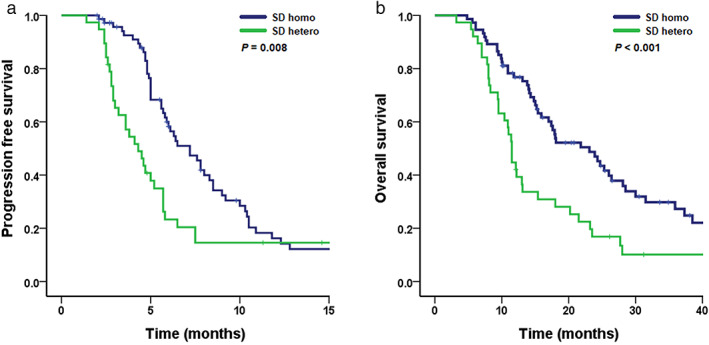
Kaplan–Meier curve of (a) progression‐free survival and (b) overall survival by radiological heterogeneity in the stable disease group
Using multivariate analysis, smoking history (hazard ratio [HR] = 2.50 and 95% CI = 1.34–4.67, p = 0.004), stage (HR = 1.76 and 95% CI = 1.02–3.01, p = 0.041), and radiological heterogeneity (HR = 1.77 and 95% CI = 1.12–2.78, p = 0.014) were associated with PFS, whereas only radiological heterogeneity (HR = 2.27 and 95% CI = 1.42–3.61, p = 0.001) was associated with OS (Table 2).
TABLE 2.
Effect of radiological heterogeneity on progression‐free survival and overall survival in non‐small cell lung cancer patients: univariate and multivariate analyses
| Variables | Progression‐free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, yr | 0.756 | 0.169 | ||||||
| <66 | Reference | Reference | ||||||
| ≥66 | 0.93 (0.60–1.44) | 1.35 (0.88–2.06) | ||||||
| Sex | 0.127 | <0.001 | 0.820 | |||||
| Male | Reference | Reference | Reference | |||||
| Female | 0.67 (0.40–1.12) | 0.33 (0.18–0.58) | 0.89 (0.33–2.39) | |||||
| Smoking habit | 0.038 | 0.004 | <0.001 | 0.052 | ||||
| Never | Reference | Reference | Reference | Reference | ||||
| Ever | 1.86 (1.03–3.34) | 2.50 (1.34–4.67) | 3.92 (1.96–7.85) | 2.98 (0.99–8.95) | ||||
| ECOG PS | 0.211 | 0.179 | ||||||
| 0–1 | Reference | Reference | ||||||
| ≥2 | 1.45 (0.81–2.58) | 1.45 (0.84–2.51) | ||||||
| Histology | 0.965 | 0.006 | 0.289 | |||||
| SQC | Reference | Reference | Reference | |||||
| ADC | 0.95 (0.59–1.50) | 0.48 (0.31–0.76) | 0.74 (0.44–1.25) | |||||
| Others | 0.91 (0.35–2.37) | 0.81 (0.34–1.94) | 1.34 (0.54–3.45) | |||||
| EGFR mutation | 0.704 | 0.031 | 0.317 | |||||
| No | Reference | Reference | Reference | |||||
| Yes | 0.83 (0.46–1.48) | 0.49 (0.27–0.89) | 0.67 (0.34–1.31) | |||||
| Not tested | 0.83 (0.49–1.41) | 1.15 (0.69–1.91) | 0.73 (0.41–1.27) | |||||
| Stage | 0.095 | 0.041 | 0.893 | |||||
| III | Reference | Reference | Reference | |||||
| IV | 1.51 (0.93–2.45) | 1.76 (1.02–3.01) | 1.03 (0.65–1.64) | |||||
| Chemotherapy agent | 0.054 | 0.099 | 0.804 | |||||
| Irinotecan/cisplatin | Reference | Reference | Reference | |||||
| Gemcitabine/cisplatin | 1.80 (1.00–3.23) | 1.91 (1.06–3.46) | 1.15 (0.67–2.00) | |||||
| Pemetrexed/cisplatin | 2.21 (1.15–4.23) | 1.90 (0.96–3.77) | 0.95 (0.48–1.91) | |||||
| Others | 1.22 (0.57–2.58) | 1.22 (0.57–2.60) | 0.74 (0.34–1.64) | |||||
| Radiological heterogeneity | 0.010 | 0.014 | 0.001 | 0.001 | ||||
| SD homogeneous | Reference | Reference | Reference | Reference | ||||
| SD heterogeneous | 1.81 (1.15–2.83) | 1.77 (1.12–2.78) | 2.17 (1.39–3.38) | 2.27 (1.42–3.61) | ||||
Abbreviations: ADC, adenocarcinoma; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease; SQC, squamous cell carcinoma.
Association between metabolic response and survival in the SD hetero group
Among the SD hetero group (n = 38), lesions with heterogeneous radiological responses in 21 patients (55.3%) showed an increased SUVmax. In addition, lesions with heterogeneous radiological responses in 17 patients (44.7%) showed a stable SUVmax. Patients with an increased SUVmax in lesions with heterogeneous radiological responses had a shorter PFS than patients with a stable SUVmax (3.6 months vs. 5.7 months, p = 0.034) (Figure 3(a)). However, there was no significant difference in OS between patients with increased SUVmax and those with stable SUVmax (11.4 months vs. 12.1 months, p = 0.391) (Figure 3(b)).
FIGURE 3.
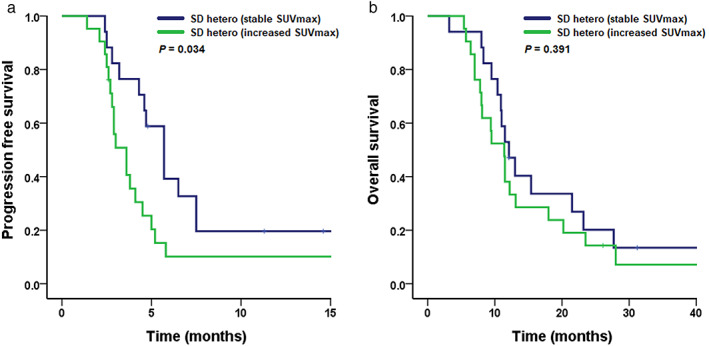
Kaplan–Meier curve of (a) progression‐free survival and (b) overall survival by metabolic response in the stable disease hetero group
DISCUSSION
In this study, patients with heterogeneous radiological responses in the SD group had shorter PFS and OS. This result suggested that radiological heterogeneity was associated with poor prognosis in NSCLC patients who received platinum‐based chemotherapy. The presence of tumor cell variants with varying sensitivities to chemotherapy leads to the emergence of drug resistance. 18 , 19 In addition, differences in blood supply within tumors are associated with poor delivery of chemotherapeutic agents to areas of low vascularity, resulting in impaired treatment response. 20 Therefore, we speculate that radiological heterogeneity represents tumor heterogeneity and the presence of treatment‐resistant clones.
In this study, 11.5% of patients in the PR group and 44.7% of patients in the SD group showed radiological heterogeneity. This incidence is comparable to those of radiological heterogeneity in other kinds of cancer in previous studies. 12 , 13 We defined radiological heterogeneity as more than 20% increase in the size of particular lesion in patients with PR or SD, considering the criteria in RECIST. Various criteria have been applied to the definition of radiological heterogeneity in previous studies, but it is necessary to establish clear definition. In addition, whereas previous studies limited the study subjects to patients with specific metastatic organs or mutations, we showed that the prognostic impact of radiological heterogeneity is applicable even in the broad patient population.
This study also showed that metabolic response was a predictive factor for disease progression in the SD hetero group. A PET/CT parameter, such as SUVmax, can reflect glucose metabolism of the tumor. 21 , 22 , 23 Increased FDG uptake is associated with tumor cell proliferation because of enhanced glycolysis and overexpression of glucose transport in malignant cells. 24 , 25 , 26 This potential mechanism could explain why patients with increased SUVmax in lesions with heterogeneous radiological responses had a significantly shorter PFS.
There were several limitations in this study. First, this study was conducted at a single center, so external confirmatory studies should be conducted in the future. However, all clinical information was obtained prospectively from the Inha Lung Cancer Cohort. Second, this study population included patients with EGFR mutations who received platinum‐based chemotherapy as a first‐line treatment. This was because some patients were included before EGFR tyrosine kinase inhibitors were officially approved as a first‐line therapy for metastatic NSCLC in the Republic of Korea. However, we adjusted for the effect of EGFR mutations on survival by using multivariate analyses. Third, in the PR group, radiological heterogeneity did not show a prognostic impact on survival, which could result from the small number of patients in the PR hetero group, so further research is needed.
In conclusion, the presence of lesions with radiological heterogeneity is associated with disease progression and poor prognosis in the SD group. We suggest that patients with heterogeneous radiological responses should be carefully monitored with frequent imaging tests. In particular, we suggest that clinicians can predict disease progression by the presence of metabolic responses in lesions with heterogeneous radiological responses.
CONFLICT OF INTEREST
The authors declare that they have no competing interests to disclose.
Supporting information
Figure S1. Two representative cases of the SD hetero group. (a) Patient 89 showed the decrease in the size of the primary lung tumor (red arrow), whereas the increase in the size of the metastatic bone lesion (arrowhead) at the first response evaluation. Patients were classified as stable disease by RECIST criteria and treatment with initial chemotherapeutic agents were continued. However, in the second response evaluation, this patient classified as progressive disease with the further increase in the size of the metastatic bone lesions. (b) Patient 161 showed the decrease in the size of the primary lung tumor (red arrow) and metastatic hepatic lesion (yellow arrow), whereas the increase the size of the metastatic bone lesion (arrowhead) at the first response evaluation. However, in the subsequent response evaluation, the sizes of the primary lung tumor, metastatic bone lesion and metastatic hepatic lesion increased, resulting PD. RE, reevaluation
ACKNOWLEDGMENTS
This work was supported by an Inha University Research Grant and Inha University Hospital Research Grant.
Ryu WK, Kim JS, Park MH, Lee M, Kim H‐J, Ryu J‐S, et al. Heterogeneous radiological response to chemotherapy is associated with poor prognosis in advanced non‐small‐cell lung cancer. Thorac Cancer. 2021;12:3333–3339. 10.1111/1759-7714.14207
Woo Kyung Ryu and Jung Soo Kim contributed equally to this work.
Funding information Inha University; Inha University Hospital
Contributor Information
Jeong‐Seon Ryu, Email: jsryu@inha.ac.kr.
Jun Hyeok Lim, Email: jhl@inha.ac.kr.
REFERENCES
- 1. Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2016; based on November 2018 SEER data submission. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 2. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. [DOI] [PubMed] [Google Scholar]
- 3. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non–small‐cell lung cancer. J Clin Oncol. 2009;27:6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wangari‐Talbot J, Hopper‐Borge E. Drug resistance mechanisms in non‐small cell lung carcinoma. J Can Res Updates. 2013;2:265–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 6. Jamal‐Hanjani M, Quezada SA, Larkin J, Swanton C. Translational implications of tumor heterogeneity. Clin Cancer Res. 2015;21:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Kessel C, Samim M, Koopman M, Van Den Bosch M, Rinkes IB, Punt C, et al. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur J Cancer. 2013;49:2486–93. [DOI] [PubMed] [Google Scholar]
- 8. Win T, Miles KA, Janes SM, Ganeshan B, Shastry M, Endozo R, et al. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non–small cell lung cancer. Clin Cancer Res. 2013;19:3591–9. [DOI] [PubMed] [Google Scholar]
- 9. Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion‐specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong ZY, Zhai HR, Hou QY, Su J, Liu SY, Yan HH, et al. Mixed responses to systemic therapy revealed potential genetic heterogeneity and poor survival in patients with non‐small cell lung cancer. Oncologist. 2017;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crusz SM, Tang YZ, Sarker S‐J, Prevoo W, Kiyani I, Beltran L, et al. Heterogeneous response and progression patterns reveal phenotypic heterogeneity of tyrosine kinase inhibitor response in metastatic renal cell carcinoma. BMC Med. 2016;14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brunsell TH, Cengija V, Sveen A, Bjørnbeth BA, Røsok BI, Brudvik KW, et al. Heterogeneous radiological response to neoadjuvant therapy is associated with poor prognosis after resection of colorectal liver metastases. Eur J Surg Oncol. 2019;45:2340–6. [DOI] [PubMed] [Google Scholar]
- 13. Hall PE, Shepherd ST, Brown J, Larkin J, Jones R, Ralph C, et al. Radiological response heterogeneity is of prognostic significance in metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted therapy. Eur Urol Focus. 2020;6:999–1005. [DOI] [PubMed] [Google Scholar]
- 14. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinker K, Riedl C, Weber WA. Evaluating tumor response with FDG PET: updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur J Nucl Med Mol Imaging. 2017;44:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 17. Ryu J‐S, Ryu HJ, Lee S‐N, Memon A, Lee S‐K, Nam H‐S, et al. Prognostic impact of minimal pleural effusion in non–small‐cell lung cancer. J Clin Oncol. 2014;32:960–7. [DOI] [PubMed] [Google Scholar]
- 18. Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, et al. Tumour evolution inferred by single‐cell sequencing. Nature. 2011;472:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruiz C, Lenkiewicz E, Evers L, Holley T, Robeson A, Kiefer J, et al. Advancing a clinically relevant perspective of the clonal nature of cancer. Proc Natl Acad Sci U S A. 2011;108:12054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non‐small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol. 2012;22:796–802. [DOI] [PubMed] [Google Scholar]
- 21. Berghmans T, Dusart M, Paesmans M, Hossein‐Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG‐PET) is of prognostic value for survival in non‐small cell lung cancer (NSCLC): a systematic review and meta‐analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. [DOI] [PubMed] [Google Scholar]
- 22. Lee DH, Kim S‐K, Lee H‐Y, Lee SY, Park SH, Kim HY, et al. Early prediction of response to first‐line therapy using integrated 18F‐FDG PET/CT for patients with advanced/metastatic non‐small cell lung cancer. J Thorac Oncol. 2009;4:816–21. [DOI] [PubMed] [Google Scholar]
- 23. Kohutek ZA, Wu AJ, Zhang Z, Foster A, Din SU, Yorke ED, et al. FDG‐PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non‐small cell lung cancer. Lung Cancer. 2015;89:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown RS, Leung JY, Kison PV, Zasadny KR, Flint A, Wahl RL. Glucose transporters and FDG uptake in untreated primary human non‐small cell lung cancer. J Nucl Med. 1999;40:556–65. [PubMed] [Google Scholar]
- 25. de Geus‐Oei L‐F, van Krieken JHJ, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of FDG uptake in non‐small cell lung cancer. Lung Cancer. 2007;55:79–87. [DOI] [PubMed] [Google Scholar]
- 26. Guo D, Jin F, Jing W, Li M, Chen D, Zou B, et al. Incorporation of the SUVmax measured from FDG PET and neutrophil‐to‐lymphocyte ratio improves prediction of clinical outcomes in patients with locally advanced non–small‐cell lung cancer. Clin Lung Cancer. 2019;20:412–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Two representative cases of the SD hetero group. (a) Patient 89 showed the decrease in the size of the primary lung tumor (red arrow), whereas the increase in the size of the metastatic bone lesion (arrowhead) at the first response evaluation. Patients were classified as stable disease by RECIST criteria and treatment with initial chemotherapeutic agents were continued. However, in the second response evaluation, this patient classified as progressive disease with the further increase in the size of the metastatic bone lesions. (b) Patient 161 showed the decrease in the size of the primary lung tumor (red arrow) and metastatic hepatic lesion (yellow arrow), whereas the increase the size of the metastatic bone lesion (arrowhead) at the first response evaluation. However, in the subsequent response evaluation, the sizes of the primary lung tumor, metastatic bone lesion and metastatic hepatic lesion increased, resulting PD. RE, reevaluation


