Abstract
Introduction
Childhood and adolescence are the period of rapid physical and cognitive growth and development, requiring adequate nutrition. Malnutrition in the form of undernutrition or micronutrient deficiency or overweight/obesity affects the health, cognition and educational achievement of this age group. The objective of this study is to assess the prevalence of calcium, iron, zinc, selenium, folate, vitamin A, 25 hydroxy vitamin D and vitamin B12 deficiencies in the serum and haemoglobin, ferritin and lead levels and its association with reported dietary intake and cognitive abilities, in urban school going children aged 6–16 years in 10 cities of India.
Methods and analysis
A multicentric cross-sectional study will be conducted to recruit 2400 participants (240 per site) across India. Participants will be selected using random sampling and will be categorised into age groups of 6–11 years and 12–16 years, with equal distribution. Data on socioeconomic status, anthropometric measures and 3-day dietary intake and cognitive performance will be collected. Blood samples will be collected for biochemical analysis of micronutrients. Findings will estimate the prevalence of micronutrient deficiencies and their association with dietary habits and cognitive functioning.
Ethics and dissemination
Study protocol has been reviewed and approved by institutional ethics committee of all 10 participating sites. Results will be shared and published in a peer-reviewed journal, so that the findings will be helpful for the stakeholders in planning nutritional interventions for targeted groups.
Trial registration number
CTRI/2019/02/017783.
Keywords: nutrition & dietetics, community child health, public health
Strengths and limitations of this study.
Multicentric study with sites being representative of different geocultural regions of India.
As a concern of power of study, will enrol 2400 urban school going children.
Use of standardised cognitive assessment tools adapted for Indian children.
Chances of recall bias during self-reported dietary survey.
Introduction
Childhood and adolescence are the period of rapid physical and cognitive growth and development, requiring adequate nutrition. Any change in nutritional status during this age influences health, learning and physical fitness. Nutrients essential for normal growth and functioning of human body are macronutrients like carbohydrate, fat and proteins, required in large quantities and micronutrients like vitamins and minerals required in small quantities. Vitamins, categorised as fat soluble (A, D, E and K) and water soluble (B group and C), are synthesised in human body in quantities lesser than required. Minerals are required for growth, repair and regulation of vital functions of human body. They are major minerals like calcium, phosphorus, sodium, potassium, magnesium and trace elements like iron, iodine, ferritin, fluoride, zinc, copper, cobalt, chromium, manganese, molybdenum, selenium and nickel.
The term malnutrition addresses three broad groups of conditions:
Undernutrition: indicating wasting (low weight-for-height), stunting (low height-for-age) and underweight (low weight-for-age).
Micronutrient-related malnutrition: micronutrient deficiencies or excess.
Overweight and obesity.
Micronutrient deficiencies affect an estimated two billion people or almost one-third of the world’s population.1 Various studies had reported the suboptimal nutritional status of Indian children. Prevalence of anaemia in school children and adolescents is between 19% and 88% across five different cities in India2 and may be attributed to inadequate food intake, poor stores and deficiencies of nutrients.3 Nutrition anaemia is caused by deficiency of iron, folate and vitamin B12.4 The association between nutritional status and health, cognition and educational achievement is established among the school going children.5 Deficiency of micronutrients and nutrients delays cognitive and motor development and is associated with low intelligence quotient (IQ).6–8 There is a consensus on the fact that iron deficiency has a negative impact on cognition, behaviour and motor skills. It has been found that haemoglobin though correlates to cognitive performance, iron supplementation improves cognitive functions regardless of the haemoglobin levels.9 Vitamin B12 deficiency is associated with poor cognitive development, that is, episodic memory, language ability10 and growth in children.11 Zinc, iron, folate, iodine, B12 and protein deficiency can also result in low IQ8 and deficit in attention, learning, memory and neuropsychological behaviour.12 13 Lead, a well-known toxic heavy metal, though widely discontinued in many countries of world, is still a public health problem in developing countries like India. Worldwide, every year, 0.6 million cases of childhood intellectual disabilities are attributed to lead exposure. Iron deficiency, which is common in children, can enhance lead absorption.14
Various studies across the globe had tested the association between nutritional status biomarkers and dietary intake.15–19 Some significant association or role in progression of various malnutrition issues are already established. Plasma concentrations of vitamin B12 and folate are found to be associated with dietary intake provided that gender, age and energy intake are taken into account and this association is independent of physical activity of individual.20 A similar significant positive correlation is found between selenium intake and its blood level.21 Although some authors consider the nutritional assessment as a practical, non-invasive and cost‐effective tool for rapid nutritional evaluation,22 others recommend concurrent collection of biological specimens to estimate levels of dietary biomarkers, so as to overcome possible sources of error, indigenously associated with every method of dietary intake assessment.23
In Indian scenario, there is definitely a need for well-planned, large-scale study using standardised methodologies to estimate the prevalence of micronutrient deficiencies with giving due importance to accurate evaluation of socioeconomic status and representation of the different regions of the country.24
Hence, the present study will be conducted with an aim to assess the prevalence of deficiency of various vitamins (folate, vitamin A, 25 hydroxy vitamin D, vitamin B12) and minerals (calcium, Iion, zinc, selenium) and its association with reported dietary intake and cognitive abilities, in urban school children aged 6–16 years in 10 cities of India.
Methodology
Study design
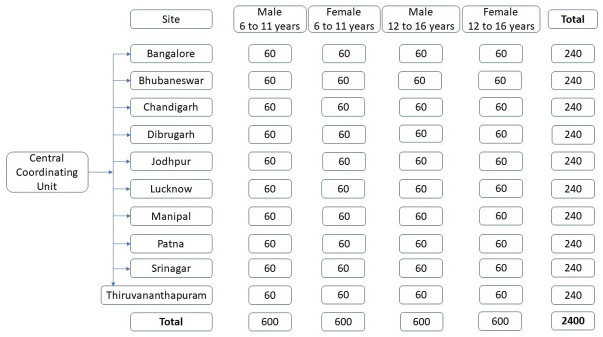
This is a multicentric cross-sectional study. It will be conducted in 10 major cities across India. Each site will recruit 240 participants, having equal proportion of gender and age group (figure 1).
Figure 1.
Distribution of participants based on gender and age group across 10 study sites.
Study setting
Study sites at Bangalore, Bhubaneswar, Chandigarh, Dibrugarh, Jodhpur, Lucknow, Patna, Srinagar, Thiruvananthapuram and Udupi districts are selected as being representative of different geocultural regions of India (figure 2). King George’s Medical University (KGMU), Lucknow will be the central coordinating unit (CCU) for the study.
Figure 2.
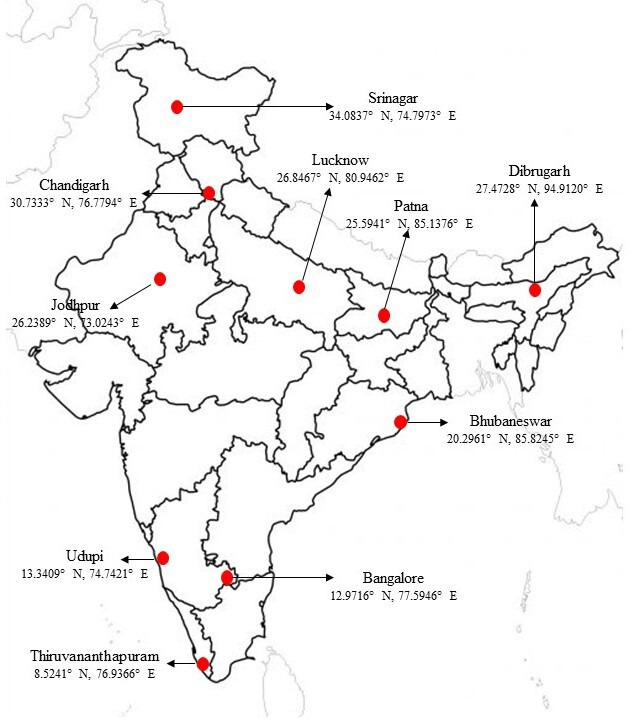
Study sites and their geocoordinates.
These 10 cities have a total population of 24.1 million, which is 2% of country’s total population. Study cohort population in these cities was 7.1 million.25 Demographic characteristics and key anthropometric indicators in urban areas of study sites are shown in table 1.25 26
Table 1.
Demographic characteristics and key anthropometric indicators in urban areas of study sites
| Study site | Urban population of district (in millions)25 | Literacy rate (state)11 | Prevalence rate of (state) (age in years)26 | ||||||
| Severe thinness* | Obesity† | ||||||||
| All ages | 6–16 years | 5–9 | 10–14 | 15–19 | 5–9 | 10–14 | 15–19 | ||
| India | 377.11 | 76.73 | 84.1 | 4.7 | 7.0 | 4.9 | 2.8 | 2.5 | 1.8 |
| M S Ramaiah Medical College & Hospital Bangalore | 8.75 | 1.42 | 85.8 | 5.2 | 3.4 | 4.0 | 2.4 | 1.5 | 8.0 |
| Kalinga Institute of Medical Sciences, Bhubaneswar | 1.08 | 0.20 | 85.7 | 1.5 | 2.5 | 0.4 | 8.9 | 9.1 | 4.5 |
| Post Graduate Institute of Medical Sciences, Chandigarh | 1.03 | 0.20 | 86.2 | – | – | – | – | – | – |
| Assam Medical College, Dibrugarh | 0.24 | 0.043 | 88.5 | 4.3 | 11.9 | 5.7 | 4.3 | 2.2 | 0.0 |
| All India Institute of Medical Sciences, Jodhpur | 1.27 | 0.29 | 79.7 | 4.3 | 7.4 | 5.4 | 1.3 | 1.5 | 1.7 |
| King George’s Medical University, Lucknow | 3.04 | 0.64 | 75.1 | 5.9 | 6.6 | 3.3 | 2.0 | 1.0 | 0.0 |
| All India Institute of Medical Sciences, Patna | 2.52 | 0.62 | 76.9 | 5.5 | 7.6 | 3.2 | 0.8 | 0.2 | 0.2 |
| Sher-i-Kashmir Institute of Medical Sciences, Srinagar | 1.22 | 0.24 | 77.1 | 2.9 | 2.4 | 5.0 | 4.3 | 3.0 | 2.2 |
| Government Medical College, Thiruvananthapuram | 1.77 | 0.28 | 95.1 | 2.9 | 4.1 | 6.4 | 5.4 | 1.8 | 0.8 |
| Kasturba Medical College, Manipal, Udupi | 0.33 | 0.05 | 85.8 | 5.2 | 3.4 | 4.0 | 2.4 | 1.5 | 8.0 |
*BMI for age < –3 SD of the WHO Child Growth Standards median.
†BMI for age >+2 SD of the WHO Child Growth Standards median.
BMI, body mass index.
Objective
The primary objective of the study is to assess the prevalence of calcium, iron, zinc, selenium, folate, vitamin A, 25 hydroxy vitamin D and vitamin B12 deficiencies and blood haemoglobin, ferritin and lead levels in urban school going children aged 6–16 years in 10 cities of India.
Secondary objectives are:
To assess the association of calcium, iron, zinc, selenium, folate, vitamin A, 25 hydroxy vitamin D and vitamin B12 deficiencies and blood haemoglobin, ferritin and lead levels with anthropometric indicators in urban school going children aged 6–16 years in 10 cities of India.
To assess the association of calcium, iron, zinc, selenium, folate, vitamin A, 25 hydroxy vitamin D and vitamin B12 deficiencies and blood haemoglobin, ferritin and lead levels with socioeconomic status in urban school going children aged 6–16 years in 10 cities of India.
-
To assess the association of calcium, iron, zinc, selenium, folate, vitamin A, 25 hydroxy vitamin D and vitamin B12 deficiencies and blood haemoglobin, ferritin and lead levels with cognitive assessments in urban school going children aged 6–16 years in 10 cities of India, by
General intelligence (coloured progressive matrices (CPM), standard progressive matrices (SPM)).
Attention, concentration and visuomotor coordination (coding test).
Working memory (digit span test, arithmetic test).
To assess the association of calcium, iron, zinc, selenium, folate, vitamin A, 25 hydroxy vitamin D and vitamin B12 deficiencies and blood haemoglobin, ferritin and lead levels with 3-day dietary intake assessed by 24 hours recall method in urban school going children aged 6–16 years in 10 cities of India.
Sample size computation
Assuming the prevalence of folate deficiency in India as 30.7%,27 precision of 2% and level of confidence as 0.05, the calculated sample size is 2044 participants. After taking, attrition rate of 10% sample size will inflate to 2400 participants. This sample size will be equally divided into 10 sites, respectively.
Sampling technique
Participants will be selected by using two-stage sampling technique. In the first stage, schools will be selected, and in the second stage, participants will be recruited from the selected schools.
Selection of participants
Each study site will provide a list of schools imparting coeducation to children between 6 and 16 years of age and located within the urban limits of city. From this list, schools will be randomised repeatedly till we get a pool of six schools having at least one to three private schools and rest of the government schools. Principals of recruited schools will be met to obtain written voluntarily informed consent. They will be asked to allocate a coordinating teacher from the school. With the help of coordinating teacher, a gender-wise list of students between 6–11 and 12–16 years of age will be prepared. From each of these lists, 15 students who are apparently healthy and residing within five kilometres of radius from school will be randomly selected. They will be invited to participate into the study. Out of these, first 10 participants whose parents will provide written informed consent will be included into the study. Rest will be kept as back-up in case of exclusions. Participants having body mass index (BMI) below 12.5 will be excluded from the study and their parents will be advised to have a medical evaluation. Written assent will be obtained from all participants who will be 8 years or above of age. Any study-specific assessment will be done after obtaining written informed consent.
Training of study Team
Site team will consist of a social worker, a data entry operator, a nutritionist and a psychologist. Training of nutritionist and psychologist will be conducted at CCU Lucknow by project investigators and co-investigators. These will be oriented to train the site staff on study protocol, procedures, data collection instruments, data entry tools and standard operating procedures.
Data collection
The study is opened for data collection in April 2019 and is still ongoing due to COVID-19 pandemic and is expected to complete by mid of 2021. Since the school calendar of different states of India varies from another owing to their specific geography, culture and climate, time frame of data collection across the sites also varies accordingly.
Demographic and socioeconomic data
Demographic and socioeconomic details of participant will be recorded by interviewing participants and their primary caregiver. Revised Kuppuswamy’s socioeconomic scale28 will be used to assess socioeconomic status.
Anthropometric measurements
Anthropometry will be done by qualified and trained nutritionists. The height will be measured to the nearest 0.1 cm using Seca 213 Mobile Stadiometer (Seca, Hamburg, Deutschland). Participant will be asked to stand barefoot after removing hair barrettes and rubber bands, with the heels, back and head touching the measuring rod. A head board placed above the head perpendicular to the ruler on measuring rod and parallel to the ground will be used to record height measure. Two measurements of height will be taken for each participant. If the difference between the two height measurements is greater than 5 mm, then a second set of two height measurements will be taken to obtain more precise values.
Weight will be measured to the nearest 0.1 km using portable Seca 803 weighing scale (Seca, Hamburg, Deutschland). The unit will be standardised by calibrating it to zero before each measurement. The participants will be weighed barefoot with empty pockets and without any heavy items like woollen blazers or belts.
BMI will be calculated using the standard equation:
For assessing anthropometric indicators, recommendations of the WHO expert committee will be used as shown in table 2.
Table 2.
Recommendations of the WHO expert committee for assessing anthropometric indicators
| Stunting | Height for age < –2 SD of the WHO Child growth standards median |
| Severe thinness | BMI for age < –3 SD of the WHO Child Growth Standards median |
| Thinness | BMI for age < –2 SD of the WHO Child Growth Standards median |
| Overweight | BMI for age >+1 SD of the WHO Child Growth Standards median |
| Obesity | BMI for age >+2 SD of the WHO Child Growth Standards median |
| Severe obesity | BMI for age >+3 SD of the WHO Child Growth Standards median |
BMI, body mass index.
Cognitive assessment
Cognitive assessment will be administered at a mutually convenient time in a separate room to keep relaxed and pleasant environment. The assessment will be conducted in a single individual session. During the assessment session, the psychologist will first make the child comfortable by establishing good rapport. Each participant will be assessed for attention, concentration and visuomotor coordination through coding test,29 general intelligence through CPM30/SPM31 and working memory using arithmetic and digit span test.29
Coding test: Sheet ‘A’ will be used for participants below 8 years of age, rest will use sheet ‘B’ sheet. Participants will be given 120 s to complete the test. One point will be awarded for each correct response, excluding samples.
CPM/SPM: CPM will be administered to participants 6–11 years of age. Participants 12 years or above of age will be given SPM. Each participant will be given about 15–20 min to complete the assessment.
Arithmetic and digit span test: These will be undertaken by all the participants. About 3 min to 5 min will be given to complete digit span test and 10 min will be given for arithmetic test.
For scoring and interpretation of test results, standard tables from respective test’s manual29–31 will be referred.
Dietary assessment
General dietary assessment will include dietary habits, meal frequency and consumption of water, beverages, green leafy vegetables, fruits, animal products. Frequencies of common food items, popular among children, will also be captured.
Data on dietary intake of children will be collected using 24-hour recall method for 2 non-consecutive days and one Sunday, which are not fasting or feasting days. The intake will be recorded by interviewing participant along with his/her mother or primary caregiver, preferably at their home. Previously standardised cups, glass and spoons will be used as an aid to help in recalling the quantity of different foods consumed by the participants in a 24-hour period prior to the investigation.
For calculating the nutritive value of raw ingredients, nutritive value of Indian foods will be used.32 Daily nutrient intake for all the nutrients will be calculated using DIETSOFT Software and will be compared with the recommended dietary allowances.33
Dietary intake will be assessed in terms of nutrient adequacy ratio (NAR).34 The NAR for a given nutrient is the ratio of a participant’s intake to the current recommended allowance for each sex and age category. To estimate the nutrient adequacy of the diet, NAR will be calculated for all the nutrients using the equation:
Participants will be then categorised as having (a) adequate NAR (NAR ≥1.00), (b) fairly adequate NAR (0.66>NAR<1.00) and inadequate NAR (NAR<0.66), for various nutrients.34
Dietary diversity (DD), defined as the number of different foods or food groups consumed in a day, will be measured using Dietary Diversity Score (DDS). DDS will be measured by categorising the food items, consumed in a day, into 14 groups. Simple counting of different types of food groups consumed in a particular day will give individual DDS, which would range from 1 to 14.
Thereafter, participants will be categorised in three classes according to their DDS35
low: ≤8.
moderate: 9.
high: ≥10.
The 14 food groups that we would use are animal meat, cereals and millets, fats and edible oils, meat and poultry, fishes and other sea foods, fruits, pulses and legumes, green leafy vegetables, milk and milk products, nuts and oil seeds, other vegetables, roots and tubers, sugars and miscellaneous foods.
Blood sample collection, processing and storage
Blood sampling will be done at school in presence of parent/s where available, during early school hours, by trained phlebotomists. Venous blood sample of 6 mL (4 mL in clot activator and 2 mL in EDTA) will be collected using vacuum-tube systems using a certified stainless steel hypodermic needle, preferably from cubital vein. Measures to prevent and counter any adverse event like syncope, haematoma or swelling will be adequately employed and recorded.
Blood sample transportation from school to study sites will be done maintaining temperature of 2°C to 8°C. EDTA sample of 1 mL will be sent to a centralised laboratory (National Accreditation Board for Testing and Calibration Laboratories and The College of American Pathologists accredited) for complete blood count (CBC) assessment, through a professional agency having experience in handling and air-transportation of blood samples. The CBC assessment will include estimation of haemoglobin, haematocrit, red blood cell count, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, red cell distribution width - coefficient of variation (RDW-CV), platelet count, total leucocyte count, differential leucocyte count and absolute leucocyte count. Report of CBC assessment will be given to parents with relevant advice by site investigators. Rest of the samples will be processed at site to separate plasma, serum and packed cells, in a trace element-free area. Plasma and serum will be stored in trace element-free cyro tubes, below −20°C and packed cells between 2°C and 8°C, at the study sites, with restricted access. Samples from study sites to CCU will be transported in two batches of 120 each, maintaining required temperatures. Sample transportation will be managed by professional agencies having expertise in handling and shipment of biomedical samples. Samples will be prevented from exposure to light during this whole process.
Biochemical assessment
Biochemical analysis of blood samples will be carried out at Department of Biochemistry, KGMU. Levels of serum calcium, iron, ferritin, folate, vitamin B12, vitamin D, vitamin A, zinc, plasma selenium and lead in whole blood will be assessed.
Inductively coupled plasma-optical emission spectrometry (ICP-OES) (Optima 8000, Perkin Elmer) will be used to assess zinc, selenium and lead. Stock solutions for respective elements will be prepared at a concentration of 1000 mg/L. Working standard will be prepared by diluting stock solution in 2% nitric acid in desired range. Adding 0.5 mL of sample, 1.5 mL nitric acid, 0.5 mL of perchloric acid, 1.0 mL of hydrogen peroxide and 1.0 mL of Mili-Q water will do microwave digestion. A clear microwave digested sample will use to measure analytes using ICP-OES, maintaining operational conditions as shown in table 3.
Table 3.
Operational conditions for inductively coupled plasma-optical emission spectrometry
| Plasma gas flow | 8 L/min |
| Auxiliary gas flow | 0.2 L/min |
| Carrier gas flow | 0.55 L/min |
| RF power | 1300 W |
| View distance | 15 nm |
| Plasma view | Axial |
| Sample flow rate | 1.0 mL/min |
RF, Radio Frequency.
The value of metal (zinc, selenium, lead) will be calculated using the formula:
Folate, vitamin B 12, vitamin D and ferritin levels will be determined using chemiluminescence method using a fully automatic analyser. Folate estimation will be done by using ARCHITECT Folate Reagent Kit (Abbot Diagnostics, Iceland), 1P74-27. The immulite folate is a competitive analogue immunoassay with incubation cycles of 2×30 min. For vitamin B 12 assessment, ARCHITECT B12 Reagent Kit (Abbot Diagnostics, Iceland), 7K61-27 will be used. Immulite 1000 vitamin B12 is a solid-phase, two-site chemiluminescent immunometric assay with incubation cycles of 1×60 min. These estimations depend on chemiluminescence reactions in which part of the chemical energy generated produces excited intermediates that decay to a ground state with the emission of photons. The emitted radiation is measured using a photomultiplier tube and the signal is converted into analyte concentration.
The ARTICHECT 25-OH vitamin D Assay (5P02, G5-6832/R03, Abbot Diagnostics, Iceland) uses chemiluminescent immunoassay technology. Specific antibody to vitamin D is used for coating magnetic particles (solid phase) and vitamin D is linked to an isoluminol derivative. During the incubation, 25-hydroxyvitamin D is dissociated from its binding protein and competes with labelled vitamin D for binding sites on the antibody. After the incubation, the unbound material is removed with a wash cycle. Subsequently, the starter reagents are added and a flash chemiluminescent reaction is initiated. The light signal is measured by a photomultiplier as relative light units and is inversely proportional to the concentration of 25-hydroxyvitamin D present in samples. Quantitative determination of ferritin in serum will be done using ARTICHECT Ferritin 7k59 kit, B7K590 (Abbot Diagnostics, Iceland).
Vitamin A in serum will be analysed by immune-enzymatic assay (ELISA), using commercially available kit (CED051Ge, USCN Wuhan USCN Business), which is principally based on competitive ELISA. This assay employs the competitive inhibition enzyme immunoassay technique. A monoclonal antibody specific to retinol has been precoated onto a microplate. A competitive inhibition reaction is launched between biotin-labelled retinol and unlabeled retinol (standards or samples) with the precoated antibody specific to retinol. After incubation, the unbound conjugate is washed off. Next, avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. The amount of bound HRP conjugate is reverse proportional to the concentration of retinol in the sample. After addition of the substrate solution, the intensity of colour developed is reverse proportional to the concentration of retinol in the sample.
Serum calcium will be measured by Fully Automatic Biochemistry Analyzer by Selectra PRO M, using Calcium Arsenazo III Colorimetric 30160, Lab kit. Calcium with Arsenazo lll (1, 8-Dihydroxy-3, 6-disulpho-2, 7-naphthalene-bis (azo)-dibenzenearsonic acid), at neutral pH, yields a blue-coloured complex. The intensity of the colour formed is proportional to the calcium concentration in the sample.
Serum iron levels will be assessed using ELITech Clinical Systems Selectra Pro Series Analyzers (Iron ferene, FEFE-0203 ELITechGroup empowering IVD, USA.) Iron released from transferrin in acidic pH as ferric ion (Fe3+) is reduced by the ascorbic acid into ferrous ion (Fe2+), which eventually forms a coloured complex with ferene. The 578 nm absorbance of the iron–ferene complex is proportional to the iron concentration of the sample.
Patient and public involvement
Patients and/or the public are not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Quality assurance
Robust mechanisms will be employed to maintain the quality of data collection. Data collection at sites will be done under direct supervision of site investigator/co-investigator. CCU will also monitor data quality by onsite monitoring to observe data collection process and ascertain work in accordance to laid standard operating procedures. Quality of anthropometric measurements will be assured by routinely calibrating the equipment along with the resampling of participants. Scoring sheets for cognitive assessments will also be assessed for scoring and interpretation of results at CCU. Dietary records will be assessed for appropriateness of proportion of ingredients and entry in DIETSOFT software. Retraining will be imparted where lacunae will be identified. Internal quality assurance of bio chemical analysis will be done by using calibrated instruments and analysing test specific standards. For interlaboratory comparison of the test results, 10% of the total samples will be sent to peer laboratory.
Statistical analysis
Data will be entered in MS excel (double data entry), matched electronically and discrepancies will be rectified by referring the source documents. Point estimates and CIs of proportions of different micronutrient deficiencies shall be evaluated. These estimates shall be found for overall proportion as well as citywise proportion. To assess the association of micronutrient deficiencies (continuous variables) with anthropometric measures (height and weight), Pearson’s correlation coefficient shall be used along with their CIs. To assess the association of micronutrient deficiencies (continuous variables) with cognitive assessments (categorical variables), analysis of variance shall be employed. The dietary intake, for each participant, is recorded for 3 days, which will be converted into nutritional value using DIETSOFT software. Using the three observations as repeated measurement for each participant, the appropriate ‘descriptive’ as well as ‘inferential’ analysis shall be done. The hierarchical (nested) linear model shall be used for analysing repeated measures or longitudinal data.
Ethics and dissemination
The study is approved by the Institutional Ethics Committee for MS Ramaiah Medical College and Hospital Bangalore (approval reference number (ARN): MSRMC/EC/AP-02/02–2019), Institutional Ethics Committee for Kalinga Institute of Medical Sciences Bhubaneswar (ARN: KIMS/KIIT/IEC/112/2016), Institutional Ethics Committee for PGIMER Chandigarh (ARN: PGI/IEC/2019/000152), Institutional Ethics Committee (H) Assam Medical College (ARN: AMC/EC/1430), Institutional Ethics Committee for All India Institute of Medical Sciences Jodhpur (ARN: AIIMS/IEC/2017/765), Institutional Ethics Committee for King Georges Medical University (ARN: 9334/Ethics/R. Cell-16), Institutional Ethics Committee for Kasturba Medical College (ARN: IEC:388/2019), Institutional Ethics Committee for All India Institute of Medical Sciences Patna (ARN: IEC/AIIMS/PAT/153/2017), Institutional Ethics Committee for Sher-i-Kashmir Institute of Medical Sciences (ARN: IEC/SKIMS Protocol # RP 175/2018) and Human Ethics Committee Medical College Thiruvananthapuram (ARN: HEC. No.04/34/2019/MCT). The study is registered prospectively with Clinical Trial Registry of India (registration number CTRI/2019/02/017783). Written informed consent will be obtained from parents of all study participants. Findings will be disseminated with stakeholders and will be presented in national and international conferences. Results will be published in a peer-reviewed journal.
Discussion
Micronutrient deficiencies are a major problem in developing countries and India is not an exception. It adversely affects the population health, resulting in decreased national performance and productivity, adding financial burden to the country. Despite the steps taken by government through various food supplementation and food fortification programmes, problem is still deep rooted. Large population of children and adolescents is still bearing the curse of micronutrient deficiencies.26 36–41 Several researchers have investigated the magnitude of problem in India at various time points. These studies were mostly isolated and were confined to anaemia, ferritin, folate, vitamin B12 and vitamin D. In the recent times, much interest has been generated in multiple micronutrient deficiencies, little is known about the magnitude and significance of this problem. The evidence on interactions between micronutrients, however, clearly indicates a need for more work in this area.42 The present study, perhaps first of its kind, will provide the estimates of calcium, iron, zinc, selenium, folate, vitamin A, 25 hydroxy vitamin D and vitamin B12 deficiencies and blood haemoglobin, ferritin and lead levels and its association with anthropometric indicators, socioeconomic status, cognitive abilities and dietary habits along with 3-day dietary intake, at country level.
The dietary intake assessment of population provides important information on the frequency and distribution of diets and nutritional status. This information can be used in designing interventions targeting improvement in dietary habits at community level. A wide variety of methods are available for dietary assessment, each one having its own advantages and disadvantages. The 24-hour dietary recall method is the most widely used method. This is a subjective and retrospective method that requires face-to-face or telephonic interview and consists of precisely recalling, describing and quantifying the intake of foods and beverages consumed in the 24-hour period prior to, or during the day before the interview. Although the most thorough, comprehensive and complete instrument for dietary assessment, 24-hour dietary recall method has extensive dependence on interviewer’s skills and participant’s memory.43 In the current study, since we will be assessing the actual level of micronutrients through biochemical analysis, at the same time, we can correlate the results to that of 24-hour dietary recall.
Cognitive development is a continuous and sequential process from birth through adulthood. Cognition compasses memory, association, concept formation, pattern recognition, language, attention, perception, action, problem solving and mental imagery as a process.44 45 These processes are mandatory and interrelated for any task acquisition. Intelligence varies from person to person because of differences in their environmental and biological components. Biological factors like genes, maternal age and environmental factors like socioeconomic status, malnutrition, etc influence intelligence adversely. Deficiency of micronutrients is associated with impaired neuropsychological development and classroom performance.46 47 Malnutrition during the early part of life (1–5 years) delays physical growth, motor and cognitive development.48–50 Studies have shown that skipping breakfast interferes with cognitive performance of students.
While there are several identified micronutrients, the role of only iron, zinc, iodine and vitamins is studied well in India. The researches clearly indicate that the school-age period is nutritionally significant because this is the prime time to buildup body stores of nutrients in preparation for rapid growth of adolescence.
Hence, the findings of present study may add to the existing body of knowledge not only to the prevalence but also to the association of micronutrient deficiencies with cognitive abilities, dietary pattern anthropometric indicators, socioeconomic status. These findings may be further scaled up to interventions to provide adequate nutrition to targeted groups, so as to promote health and cognitive abilities thus resulting in increased productivity.
Supplementary Material
Acknowledgments
Indian Micronutrient Consortium (in alphabetical order): Abbas Ali Mahdi, King George’s Medical University, Lucknow; Anish TS, Government Medical College, Thiruvananthapuram; B N Mahanta, Assam Medical College, Dibrugarh; Bhavneet Bharti, Post Graduate Institute of Medical Sciences, Chandigarh; C M Singh, All India Institute of Medical Sciences, Patna; Chythra R Rao, Kasturba Medical College, Manipal; Daisy Kheda, All India Institute of Medical Sciences, Jodhpur; Divas Kumar, King George’s Medical University, Lucknow; Girdhar Agarwal, University of Lucknow, Lucknow; Joseph L Mathew, Post Graduate Institute of Medical Sciences, Chandigarh; Karunakara BP, M. S. Ramaiah Institute of Medical Sciences, Bangalore; Kuldeep Singh, All India Institute of Medical Sciences, Jodhpur; Mushtaq A Bhat, Sher-i-Kashmir Institute of Medical Sciences, Srinagar; Shally Awasthi, King George’s Medical University, Lucknow; Shweta Singh, King George’s Medical University, Lucknow; Somashekar AR, M. S. Ramaiah Institute of Medical Sciences, Bangalore; Sonali Kar, Kalinga Institute of Medical Sciences, Bhubaneswar; Suma Nair, Kasturba Medical College, Manipal; Swati Dixit, King George’s Medical University, Lucknow, Tulika Goswami Mahanta, Assam Medical College, Dibrugarh.
Footnotes
Contributors: SA conceived the study and is principal investigator, responsible for finalising study protocol and manuscript. DK contributed to design of the final study protocol, drafted the initial manuscript and coordinated ethical approvals. SS, SD and AAM contributed to the technical design of final study protocol and revised the initial manuscript draft. GA provided biostatistical support. IMC are site investigators and contributed to the design of the final study protocol.
Funding: This work is supported by a grant from Hindustan Unilever Limited (Grant Number: 212332). Funding supports all study-related expenses including manuscripts processing fees. Funding source is not involved in study design, implementation, collection and interpretation of data and in writing of the manuscript.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Thompson B, Amoroso L, eds. Improving Diets and Nutrition: Food-Based Approaches. Rome and Wallingford: FAO and CAB International, 2014. [Google Scholar]
- 2.Srihari G, Eilander A, Muthayya S, et al. Nutritional status of affluent Indian school children: what and how much do we know? Indian Pediatr 2007;44:204–13. [PubMed] [Google Scholar]
- 3.Gomber S, Bhawna , Madan N, et al. Prevalence & etiology of nutritional anaemia among school children of urban slums. Indian J Med Res 2003;118:167–71. [PubMed] [Google Scholar]
- 4.Gonmei Z, Toteja GS. Micronutrient status of Indian population. Indian J Med Res 2018;148:511–21. 10.4103/ijmr.IJMR_1768_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best C, Neufingerl N, van Geel L, et al. The nutritional status of school-aged children: why should we care? Food Nutr Bull 2010;31:400–17. 10.1177/156482651003100303 [DOI] [PubMed] [Google Scholar]
- 6.Black MM. The evidence linking zinc deficiency with children's cognitive and motor functioning. J Nutr 2003;133:1473S–6. 10.1093/jn/133.5.1473S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowri AR, Sangunam HJ. Assessment of mental and motor abilities of school going children with anaemia’. Indian J Nutr Diet 2005;42:99–105. [Google Scholar]
- 8.Schoenthaler SJ, Bier ID, Young K, et al. The effect of vitamin-mineral supplementation on the intelligence of American schoolchildren: a randomized, double-blind placebo-controlled trial. J Altern Complement Med 2000;6:19–29. 10.1089/acm.2000.6.19 [DOI] [PubMed] [Google Scholar]
- 9.Jáuregui-Lobera I. Iron deficiency and cognitive functions. Neuropsychiatr Dis Treat 2014;10:2087–95. 10.2147/NDT.S72491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo-Durán C, Perales CG, Hertrampf ED, et al. Effect of zinc supplementation on development and growth of Chilean infants. J Pediatr 2001;138:229–35. 10.1067/mpd.2001.110530 [DOI] [PubMed] [Google Scholar]
- 11.Strand TA, Taneja S, Ueland PM, et al. Cobalamin and folate status predicts mental development scores in North Indian children 12-18 Mo of age. Am J Clin Nutr 2013;97:310–7. 10.3945/ajcn.111.032268 [DOI] [PubMed] [Google Scholar]
- 12.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol 2006;13:158–65. 10.1016/j.spen.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 13.Lam LF, Lawlis TR. Feeding the brain - The effects of micronutrient interventions on cognitive performance among school-aged children: A systematic review of randomized controlled trials. Clin Nutr 2017;36:1007–14. 10.1016/j.clnu.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 14.Naranjo VI, Hendricks M, Jones KS. Lead toxicity in children: an unremitting public health problem. Pediatr Neurol 2020;113:51–5. 10.1016/j.pediatrneurol.2020.08.005 [DOI] [PubMed] [Google Scholar]
- 15.Kim K-N, Oh S-Y, Hong Y-C. Associations of serum calcium levels and dietary calcium intake with incident type 2 diabetes over 10 years: the Korean Genome and Epidemiology Study (KoGES). Diabetol Metab Syndr 2018;10:50. 10.1186/s13098-018-0349-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim K, Booth A, Szymlek-Gay EA, et al. Associations between dietary iron and zinc intakes, and between biochemical iron and zinc status in women. Nutrients 2015;7:2983–99. 10.3390/nu7042983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds . Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academies Press (US), Selenium, 2000: 7. https://www.ncbi.nlm.nih.gov/books/NBK225470/ [PubMed] [Google Scholar]
- 18.Dimakopoulos I, Magriplis E, Mitsopoulou A-V, et al. Association of serum vitamin D status with dietary intake and sun exposure in adults. Clin Nutr ESPEN 2019;34:23–31. 10.1016/j.clnesp.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 19.Wolffenbuttel BHR, Heiner-Fokkema MR, Green R, et al. Relationship between serum B12 concentrations and mortality: experience in NHANES. BMC Med 2020;18:307. 10.1186/s12916-020-01771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baart AM, Balvers MGJ, de Vries JHM, et al. Relationship between intake and plasma concentrations of vitamin B12 and folate in 873 adults with a physically active lifestyle: a cross-sectional study. J Hum Nutr Diet 2021;34:324–33. 10.1111/jhn.12814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pestitschek M, Sonneck-Koenne C, Zakavi SR, et al. Selenium intake and selenium blood levels: a novel food frequency questionnaire. Wien Klin Wochenschr 2013;125:160–4. 10.1007/s00508-013-0334-2 [DOI] [PubMed] [Google Scholar]
- 22.Vellas B, Guigoz Y, Baumgartner M, et al. Relationships between nutritional markers and the mini-nutritional assessment in 155 older persons. J Am Geriatr Soc 2000;48:1300–9. 10.1111/j.1532-5415.2000.tb02605.x [DOI] [PubMed] [Google Scholar]
- 23.Naska A, Lagiou A, Lagiou P. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res 2017;6:926. 10.12688/f1000research.10703.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srihari G, Eilander A, Muthayya S. Nutritional status of affluent Indian school children: what and how much do we know? Indian Pediatrics 2007;44:199–203. [PubMed] [Google Scholar]
- 25.Chandramouli C, General R. Census of India 2011. provisional population Totals. New Delhi: Government of India, 2011. http://censusindia.gov.in/2011-prov-results/prov_results_paper1_india.html [Google Scholar]
- 26.Ministry of Health and Family Welfare (MoHFW), Government of India, UNICEF and Population Council . Comprehensive national nutrition survey (CNNS) national report. New Delhi, 2019. Available: https://nhm.gov.in/index1.php?lang=1&level=2&sublinkid=1332&lid=713
- 27.Kapil U, Sareen N. Prevalence of ferritin, folate and vitamin B12 deficiencies amongst children in 5-18 years of age in Delhi. Indian J Pediatr 2014;81:312. 10.1007/s12098-013-1091-y [DOI] [PubMed] [Google Scholar]
- 28.Sharma R, Rahul S. Revised Kuppuswamy's socioeconomic status scale: explained and updated. Indian Pediatr 2017;54:867–70. 10.1007/s13312-017-1151-x [DOI] [PubMed] [Google Scholar]
- 29.Malin AJ. Manual for Malin’s intelligence Scale for Indian Children (MISIC). Lucknow: Indian Psychological Corporation, 1969. [Google Scholar]
- 30.Raven J, Raven JC, Court H. Coloured progressive matrices. 1998 edition. USA: Harcourt Assesment, 1998. [Google Scholar]
- 31.Raven J, Raven JC, Court HH. Raven manual: section 3. standard progressive matrices. Oxford: Oxford Psychologists Press, Ltd, 2000. [Google Scholar]
- 32.Gopalan C, Rama Sastri B, Balasubramanian SC. Nutritive value of Indian foods. Hyderabad: National Inst. of Nutrition, 2007. [Google Scholar]
- 33.Kamala K, Bhaskaram P, Bhat RV RT. DIETARY GUIDELINES - A Manual. Natl Inst Nutr [Internet] 2011;3:139 http://ninindia.org/DietaryGuidelinesforNINwebsite.pdf [Google Scholar]
- 34.Rani N, Rani V. Assessment of nutritional status of school going adolescents in Fatehabad district of Haryana research article. Indian J Nutr 2016;3:1–5. [Google Scholar]
- 35.Nithya DJ, Bhavani RV. Dietary diversity and its relationship with nutritional status among adolescents and adults in rural India. J Biosoc Sci 2018;50:397–413. 10.1017/S0021932017000463 [DOI] [PubMed] [Google Scholar]
- 36.Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab 2012;61 Suppl 1:8–17. 10.1159/000345165 [DOI] [PubMed] [Google Scholar]
- 37.Kotecha PV, Lahariya C. Micronutrient supplementation and child survival in India. Indian J Pediatr 2010;77:419–24. 10.1007/s12098-010-0050-0 [DOI] [PubMed] [Google Scholar]
- 38.Kapil U, Bhadoria AS. Prevalence of folate, ferritin and cobalamin deficiencies amongst adolescent in India. J Family Med Prim Care 2014;3:247–9. 10.4103/2249-4863.141619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhardwaj A, Kumar D, Raina SK, et al. Rapid assessment for coexistence of vitamin B12 and iron deficiency anemia among adolescent males and females in northern Himalayan state of India. Anemia 2013;2013:1–5. 10.1155/2013/959605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma S. Incidence of vitamin B12 and folate deficiency amongst adolescents. Int J Contemp MedRes 2017;4:1755–7. [Google Scholar]
- 41.Gupta A, Kapil U, Ramakrishnan L, et al. Prevalence of Vitamin B12 and Folate Deficiency in School Children Residing at High Altitude Regions in India. Indian J Pediatr 2017;84:10.1007/s12098-017-2291-7:289–93. 10.1007/s12098-017-2291-7 [DOI] [PubMed] [Google Scholar]
- 42.Ramakrishnan U. Prevalence of Micronutrient Malnutrition Worldwide; (II)S46–S52, 2002. Available: https://academic.oup.com/nutritionreviews/article-abstract/60/suppl_5/S46/1896146 [DOI] [PubMed]
- 43.Salvador Castell G, Serra-Majem L, Ribas-Barba L. What and how much do we eat? 24-hour dietary recall method. Nutr Hosp 2015;31 Suppl 3:46–8. 10.3305/nh.2015.31.sup3.8750 [DOI] [PubMed] [Google Scholar]
- 44.Best JB. Cognitive psychology. 5th ed, 1999: 15–17. [Google Scholar]
- 45.Coren S, Lawrence MW, James TE. Sensation and perception. 5th ed. Harcourt Brace, 1999: 9. ISBN: 978-0-470-00226-1. [Google Scholar]
- 46.Dubow EF, Boxer P, Huesmann LR. Long-Term effects of parents' education on children's educational and occupational success: mediation by family interactions, child aggression, and teenage aspirations. Merrill Palmer Q 2009;55:224–49. 10.1353/mpq.0.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maurin E. The impact of parental income on early schooling transitions: a re-examination using data over three generations. J Public Econ 2002;85:301–32. [Google Scholar]
- 48.Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ 2006;333:945. 10.1136/bmj.38978.699583.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warsito O, Khomsan A, Hernawati N, et al. Relationship between nutritional status, psychosocial stimulation, and cognitive development in preschool children in Indonesia. Nutr Res Pract 2012;6:10.4162/nrp.2012.6.5.451:451–7. 10.4162/nrp.2012.6.5.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.More S, Shivkumar VB, Gangane N, et al. Effects of iron deficiency on cognitive function in school going adolescent females in rural area of central India. Anemia 2013;2013:1–5. 10.1155/2013/819136 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.