Abstract
Objectives
To evaluate 1-year cost-effectiveness of an 8-week supervised education and exercise programme delivered in primary care to patients with symptomatic knee or hip osteoarthritis (OA).
Design
A registry-based pre–post study linking patient-level data from the Good Life with osteoArthritis in Denmark (GLA:D) registry to national registries in Denmark.
Setting and participants
16 255 patients with symptomatic knee or hip OA attending GLA:D.
Intervention
GLA:D is a structured supervised patient education and exercise programme delivered by certified physiotherapists and implemented in Denmark.
Outcome measures
Adjusted healthcare costs per Quality-Adjusted Life Year (QALY) gained from baseline to 1 year (ratio of change in healthcare costs to change in EuroQoL 5-Dimensions 5-Level questionnaire (EQ-5D)). All adjusted measures were estimated using a generalised estimating equation gamma regression model for repeated measures. Missing data on EQ-5D were imputed with Multiple Imputations (3 months: 23%; 1 year: 39 %).
Results
Adjusted change in healthcare cost was 298€ (95% CI: 206 to 419) and 640€ (95% CI: 400 to 1009) and change in EQ-5D was 0.035 (95% CI: 0.033 to 0.037) and 0.028 (95% CI: 0.025 to 0.032) for knee and hip patients, respectively. Hence estimated adjusted healthcare costs per QALY gained was 8497€ (95% CI: 6242 to 11 324) for knee and 22 568€ (95% CI: 16 000 to 31 531) for hip patients. In patients with high compliance, the adjusted healthcare costs per QALY gained was 5438€ (95% CI: 2758 to 9231) for knee and 17 330€ (95% CI: 10 041 to 29 364) for hip patients. Healthcare costs per QALY were below conventional thresholds for willingness-to-pay at 22 804€ (20 000£) and 43 979€ (US$50 000), except the upper limit of the 95% CI for hip patients which was in between the two thresholds.
Conclusions
A structured 8-week supervised education and exercise programme delivered in primary care was cost-effective at 1 year in patients with knee or hip OA supporting large-scale implementation in clinical practice.
Keywords: knee, hip, sports medicine, musculoskeletal disorders, health economics
Strengths and limitations of this study.
The study included a large number of rural and urban patients with knee or hip osteoarthritis treated in primary care across Denmark.
All costs reported are real-life costs retrieved on an individual level from a range of high-quality national registries.
The study is a pre–post study reporting change in healthcare costs against change in generic health-related quality of life (EuroQoL 5-Dimensions 5-Level questionnaire, EQ-5D).
Healthcare costs per Quality-Adjusted Life Year was reported in a 1 year horizon and additional change in healthcare costs were reported in a 3-year horizon.
23% and 39% of the patients did not provide data on EQ-5D immediately following the intervention and at 1 year, respectively, and the missing data were imputed with Multiple Imputations.
Introduction
Knee and hip osteoarthritis (OA) are major contributors to disability and chronic pain worldwide and the implications for both the patients and healthcare systems are severe.1 2 The cost related to OA is estimated to be between 1% and 2.5% of a country’s gross domestic product (GDP) in high-income countries,1 and total annual costs in Europe are estimated to be up to 817 billion € (2013).3 The number of people living with OA has increased over the last years and is expected to increase substantially in the future due to an ageing and more overweight and obese population.4 This will have extensive societal impact, emphasising the need for identifying and implementing cost-effective treatment options that can help relieve the pressure healthcare services are facing around the world.4
Clinical guidelines recommend a stepwise treatment approach, including education and exercise therapy as first-line treatment for knee and hip OA,5–8 with substantial evidence supporting the effects of supervised exercise therapy on pain and physical function.9 10 However, studies of quality of care report that exercise therapy is underutilised, estimated to be provided to less than 40% of patients with OA.11 12 To support the implementation of clinical guidelines into clinical practice, Good Life with osteoArthritis in Denmark (GLA:D) was initiated in 2013 and has been implemented across Denmark. The treatment part of GLA:D is an 8-week supervised patient education and exercise therapy programme delivered in primary care for patients with knee or hip OA and has shown positive results on pain, physical function, quality of life (QOL), intake of painkillers and sick leave.13
Results from previous evaluations of the cost-effectiveness of first-line treatment including exercise therapy and targeting knee or hip OA are heterogeneous, and little is known about the cost-effectiveness of supervised education and exercise therapy implemented in primary care.14 15 Such evaluation is warranted when deciding whether to implement a structured first-line treatment programme, and therefore the aim of the study was to evaluate the cost-effectiveness of GLA:D. We hypothesised that GLA:D would be cost-effective for both knee and hip OA patients.
Method
Study design
This is a registry-based pre–post study evaluating the cost-effectiveness in a healthcare payer perspective of an 8-week supervised education and exercise therapy programme (GLA:D) for patients with symptomatic knee or hip OA by linking patient level data from the GLA:D registry to national registries in Denmark. In the primary analysis, we reported healthcare costs in a healthcare payer perspective per QALY gained in a 1 year horizon calculated as the ratio of change in healthcare costs to change in QOL in the same patients. In addition, as a secondary analysis, mean actual healthcare costs and costs to home care and public transfer payments were reported in a 3-year horizon to assess how costs develop over time in this population of patients with a chronic condition. The study conforms to the consolidated health economic evaluation reporting standards statement for reporting health economic evaluations and recommendations for reporting cost-effectiveness analyses.16 17
Intervention
GLA:D is a structured treatment programme delivered over approximately 8 weeks consisting of two patient education sessions, a session with an expert patient, when available, and of 12 1-hour sessions (delivered twice weekly) of supervised group-based neuromuscular exercise therapy.18 19 Treating therapists are physiotherapists certified to deliver the intervention on a 2-day course at the University of Southern Denmark provided by researchers, clinicians and a former patient. All therapists were instructed in how to diagnose OA and informed about differential diagnosis. Patients are usually referred to the programme by their general practitioner or an orthopaedic surgeon, but they may also refer themselves directly. From 2014 to 2016, the GLA:D programme was delivered in 283 private clinics across the country and in 28 municipal rehabilitation centres of 98 municipalities in Denmark. Most of the patients attending the programme in private physiotherapy clinics would receive public reimbursement of approximately 40% of the fee and most patients attending municipal rehabilitation centres would not be charged. A detailed description of the GLA:D programme has previously been published.13
The GLA:D registry has previously been approved by the Danish Data Protection Agency (no. 10.084) and according to the local ethics committee of the North Denmark Region, ethics approval of GLA:D was not needed. According to the Danish Data Protection Act, patient consent was not required as personal data were processed exclusively for research and statistical purposes.
Population
Patients are eligible for the GLA:D programme if they have a clinical diagnosis of knee and/or hip OA as evaluated by the treating physiotherapist that is, pain or functional limitations associated with knee or hip OA and do not meet any of the following exclusion criteria: (1) another reason for the joint symptoms than OA (eg, tumour, inflammatory joint disease or patellar tendinopathy), (2) other symptoms that are more pronounced than the OA symptoms (eg, chronic generalised pain or fibromyalgia) or (3) do not understand Danish. According to international,20 and Danish,21 guidelines radiographs are not needed for a clinical diagnosis of OA, and therefore not part of the GLA:D eligibility criteria. The current study included patients enrolled between 4 February 2014, when collection of the EuroQoL 5-Dimensions 5-Level questionnaire (EQ-5D) was initiated, and 31 December 2016, allowing for 1-year follow-up since information on all costs was available until the end of 2017. Patients with available baseline information on EQ-5D and information on whether a knee or a hip joint was the most affected joint were included in the study. Reporting mean costs in a 3-year horizon was restricted to patients entering the programme before 31 December 2014, allowing for 3-year follow-up, and reporting costs for public transfer payments were restricted to patients aged 18–63 years both in the preintervention and postintervention period to ensure that they did not turn 65 during the postperiod which was the retirement age in Denmark in 2017. To cover living expenses, public transfer payments are in Denmark provided to adults under the age of retirement who, for example, are unemployed, have low/no ability to work or are enrolled in education. Please find more information about the Danish healthcare system elsewhere.22
Variables
Data in the GLA:D registry are collected at baseline, following the intervention (~3 months as the programme is implemented in primary care and some variation in follow-up time occurs), and at 12 months and includes demographics, a mix of therapist and patient-reported health measures and outcome measures as well as compliance.13 Via the civil registration number, which identifies every citizen in Denmark, the GLA:D registry was linked to national registries from where actual individual level utilisation of somatic healthcare services (including use of primary healthcare services, secondary healthcare services and use of preceptive medication; ie, excluding the use of psychiatric healthcare services), home care and public transfer payments were retrieved.22 In Denmark, home care including practical help and personal care is offered to citizens with low functional level who are unable to manage everyday life on their own. All prices and costs were converted into Euros (€) and reported in present values (2017-level) based on the Danish Consumer Price Index. Costs were given as mean costs per month (1-year horizon) or year (3-year horizon) and public transfer payments were given as full-time weeks (37 hours per week) per month (1-year horizon) or per year (3-year horizon).
Costs related to primary healthcare services, including visits to physiotherapist, chiropractor, general practitioner and others (eg, medical specialist, laboratory work and dentist), were obtained from the Danish National Health Insurance Service Registry. Within the primary healthcare sector in Denmark, physiotherapy is delivered both in private clinics and in municipality settings however, costs for interventions delivered in municipal settings were not available and therefore not included in the analysis. Services and admissions related to secondary healthcare, including total somatic inpatient and outpatient services, were obtained from the Danish National Patient Registry and associated costs were estimated based on the Danish Case Mix System which organise patients with similar diseases and similar expenses into groups that each have annually adjusted tariffs that reflects practice. The Danish National Patient Registry holds information on all inpatient admissions and outpatient activities, including accident and emergency visits in Danish hospitals. Every contact is coded in a classification system incorporating ICD-10 codes and use of resources in contacts where surgery in the knee or hip occurred were reported separately. Costs for prescriptive medications were obtained from the Danish National Prescription Registry holding information on all prescriptions on medications, including date of purchase, number of packages and the reimbursement paid by public funds. All drugs are classified according to the Anatomical Therapeutic Chemical Classification System (ATC) and painkillers (ATC-codes: N02A, N02B, M01A, M02AA) and other medications were reported separately. Individual level information on number and duration of visits for personal care and practical help, respectively, was retrieved from Statistics Denmark and the average care costs per hour (2017) in Denmark was used to calculate costs. Information on nursing care was not available and therefore not included in the analysis. Information on public transfer payments was retrieved from the Registry for Public Transfers, which holds information on type and hours of public transfer payments and was reported as the number of weeks receiving transfer payment (unemployment, sheltered employment, sick leave, rehabilitation, education, disability pension and early retirement).
Outcome was reported as QALYs gained measured with EQ-5D converted into an index score using time-trade-off based weights from the Danish crosswalk value set (−0.624 to 1; worst to best).23 The EQ-5D comprises of five dimensions: mobility, self-care, usual activities, pain discomfort and anxiety/depression each having five levels of response options from ‘no problems’ to ‘severe problems’.24 QALYs combine time lived and QOL into a single index number where ‘1’ corresponds to 1 year of full health and ‘0’ corresponds to being dead.
Information on the covariates age (continuous), sex (male or female), marital status (married/coliving or single), ethnic background (western (countries in EU, associated countries and the four Anglo-Saxon countries) or not western (other countries)), educational level (primary, secondary, vocational, short-term, bachelor, long-term or unknown) and administrative region (Capital, Zealand, Southern Denmark, Central Denmark or North Denmark) were retrieved from the Danish Civil Registration System. Most affected joint (knee or hip) and information on compliance were therapist-reported and high compliance was defined as patients attending at least 10 supervised exercise sessions. Type of clinic (private or municipal) was retrieved from the GLA:D registry and whether the patient died during follow-up was retrieved from the Danish Civil Registration System.
Statistical analyses
Descriptive statistics for baseline characteristics, average actual and adjusted costs from somatic healthcare services and home care and average and adjusted weeks receiving public transfer payments 1 year prior to and one or 3 years after entering the programme, respectively, were reported. To take the potential influence of covariates into account, actual costs and weeks receiving public transfer payments were adjusted using a generalised estimating equation (GEE) gamma regression model for repeated measures. A model for repeated measures was applied as the same patients were included in the preperiod and postperiod. Statistically significant difference between costs in the preintervention and postintervention period was assessed using bootstrap t-test.
We estimated healthcare costs per QALY gained as the ratio of change in actual total healthcare costs to change in QOL. Change in healthcare costs was calculated as the mean cost difference between the year prior to and the year after entering the intervention. QALYs gained was calculated as the mean difference between the EQ-5D score at baseline, calculated as ‘the area under the curve’ taking change over time into account, representing the QOL the year after entering the programme (online supplemental appendix figure S1). Data were not normal distributed and changes in costs and EQ-5D were estimated using a GEE gamma regression model for repeated measures.
bmjopen-2021-049541supp001.pdf (1.3MB, pdf)
In the first step, change in healthcare costs and change in QOL were estimated in two different models, where both raw and adjusted analyses were conducted including gender, age, marital status, ethnicity, educational level and region as covariates. In case of no convergence in the model, selected covariates were omitted. In the second step, the ratio of change in healthcare costs to change in QOL were calculated.
There is no official threshold for willingness-to-pay in Denmark and we compared the healthcare cost per QALY to predefined willingness-to-pay thresholds of a cost-effective treatment defined by the National Institute for Health and Care Excellence at 22 804€ (20 000£) per QALY,25 and the widely used threshold of 43 979€ (US$50 000) per QALY.26 To explore if adherence to the exercise therapy component had an impact on the results, a subanalysis repeating all analyses restricted to patients with high compliance was conducted. All analyses were reported separately for knee and hip patients.
As previously proposed for cost-effectiveness studies and clinical trials in OA,27 28 missing values for the EQ-5D index score at follow-up were imputed using Multiple Imputations (MI) with chained equations under the assumption of data being missing at random.29 Since EQ-5D was not normal distributed, Predictive Mean Matching was applied, and all baseline variables presented in the study and outcome variables of interest were included in the model. In total, 40 data sets were generated, approximately equal to the largest percentages of missing observations for the outcome as recommended.30
Since costs for healthcare services delivered in municipal settings were not available, all analyses were repeated stratified for patients attending GLA:D in private physiotherapy clinics versus in municipal rehabilitation centres. To explore the impact of missing data, a sensitivity analysis repeating all analyses restricted to complete cases was conducted and all analyses were repeated excluding patients who died during follow-up.
The significance level for all statistical analyses was defined a priori at p<0.05. All analyses were performed using the SAS V.9.4 (SAS Institute, North Carolina, USA).
Results
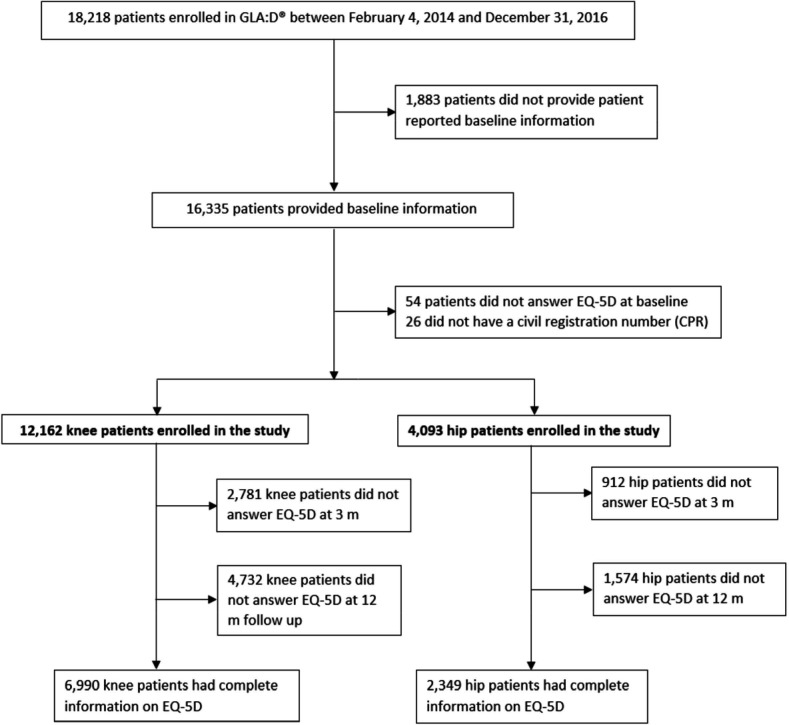
12 162 knee patients and 4093 hip patients were included in the study and follow-up data on EQ-5D were available for 77% immediately after treatment and 61% at 1 year (figure 1). Patients with complete information had slightly better, but most likely not clinically relevant better health status at baseline compared with patients with incomplete information (online supplemental appendix table S1). Baseline characteristics are presented in table 1. Three quarters of the patients were female, median symptom duration was 2 years, almost two-thirds reported use of pain medication and 31% and 4% of knee and hip patients, respectively, reported previous surgery in most affected joint. Seven percent and 17% of knee and hip patients, respectively, reported to have had a joint replacement surgery between start intervention and the 12 months follow-up measurement.
Figure 1.
Flow chart. EQ-5D, EuroQoL 5-Dimensions 5-Level questionnaire; GLA:D, Good Life with osteoArthritis in Denmark.
Table 1.
Baseline characteristics in knee and hip patients attending GLA:D
| Knee | Hip | |
| (n: 12 162) | (n: 4093) | |
| Age (years), mean (SD) | 64.1 (9.8) | 65.7 (9.4) |
| Gender (female), % (n) | 73.1 (8887) | 73.6 (3014) |
| BMI (kg/m2), mean (SD) | 28.6 (5.3) | 26.9 (4.6) |
| Marital status, % (n) | ||
| Married or living with others | 72.4 (8803) | 70.7 (1079) |
| Single | 27.6 (3359) | 29.3 (1200) |
| Ethnic background, % (n) | ||
| Danish | 96.2 (11 701) | 96.8 (3961) |
| Other western | 2.5 (299) | 2.6 (106) |
| Not western | 1.3 (160) | 0.6 (25) |
| Educational level, % (n) | ||
| Primary | 18.7 (2277) | 19.7 (1493) |
| Secondary | 3.0 (367) | 2.7 (112) |
| Vocational | 39.1 (4761) | 36.2 (1481) |
| Short-term | 4.6 (558) | 4.5 (185) |
| Bachelor | 26.2 (3186) | 28.0 (1145) |
| Long-term | 7.2 (873) | 8.0 (329) |
| Unknown | 1.2 (140) | 0.9 (35) |
| Social status, % (n) | ||
| Employed | 43.3 (5264) | 36.5 (1493) |
| Unemployed | 2.1 (256) | 1.5 (61) |
| Sick pay (public funded) | 0.7 (86) | 0.4 (15) |
| Disability pension | 3.7 (444) | 3.7 (152) |
| Early retirement | 6.3 (766) | 7.3 (297) |
| Age pension | 42.8 (5209) | 49.5 (2028) |
| Other | 1.1 (137) | 1.1 (47) |
| Administrative region, % (n) | ||
| Capital Region | 27.7 (3367) | 27.6 (1131) |
| Region Zealand | 13.0 (1578) | 13.1 (535) |
| Region of Southern Denmark | 21.8 (2654) | 25.2 (1030) |
| Central Denmark Region | 25.4 (3085) | 24.9 (1021) |
| North Denmark Region | 12.2 (1478) | 9.2 (376) |
| Number of comorbidities*, % (n) | ||
| 0 | 38.2 (4367) | 39.7 (1533) |
| 1 | 35.7 (4076) | 35.1 (1358) |
| 2 | 17.3 (1979) | 16.8 (649) |
| 3 or more | 8.8 (1.006) | 8.4 (326) |
| Symptom duration (months), median (IQR) | 24 (7–60) | 24 (8–48) |
| Pain intensity (VAS 0–100, best to worst), mean (SD) | 48.6 (22.0) | 47.6 (21.7) |
| Bilateral symptoms, % (n) | 46.3 (5614) | 26.1 (1064) |
| Walk speed† (m/s), mean (SD) | 1.49 (0.33) | 1.49 (0.34) |
| Previous surgery in worst joint‡, % (n) | 30.7 (3725) | 4.0 (161) |
| Use of pain medication§ (yes), % (n) | ||
| Overall | 61.3 (7431) | 64.2 (2629) |
| Paracetamol | 49.9 (6073) | 53.3 (2184) |
| NSAIDs | 35.6 (4325) | 34.6 (1419) |
| Opioids | 7.1 (868) | 9.0 (367) |
| KOOS/HOOS QOL¶ (0–100, best to worst), mean (SD) | 45.2 (14.7) | 47.4 (15.1) |
Missing values: BMI n: 5 (knee), n: 7 (hip); number of comorbidities n: 711 (knee), n: 215 (hip); symptom duration (mainly missing due to technical problems): n: 3.157 (knee), n: 1096 (hip); pain intensity: n: 23 (knee), n: 9 (hip); bilateral symptoms: n: 32 (knee), n: 20 (hip); walk speed: n: 610 (knee), n: 221 (hip); KOOS/HOOS QOL: n: 36 (knee), n: 21 (hip).
*Number of comorbidities calculated from self-report of the following conditions: hypertension, cardiovascular diseases, lung diseases, diabetes, stomach diseases, liver or kidney diseases, blood diseases, cancer, depression, rheumatoid arthritis, neurological disorders, other medical diseases.
†Walking speed was assessed with the 40 m fast-paces walk test under instruction of the GLA:D therapist.
‡Self-reported previous surgery in worst joint.
§Self-reported use of pain medication during last 3 months.
¶KOOS or HOOS Quality of Life sub-scale score.
BMI, body mass index; GLA:D, Good Life with osteoArthritis in Denmark; HOOS, Hip disability and Osteoarthritis Outcome Score; KOOS, Knee injury and Osteoarthritis Outcome Score; NSAIDs, Non-Steoridal Anti-Inflammatory Drugs; QOL, quality of life; VAS, visual analogue scale.
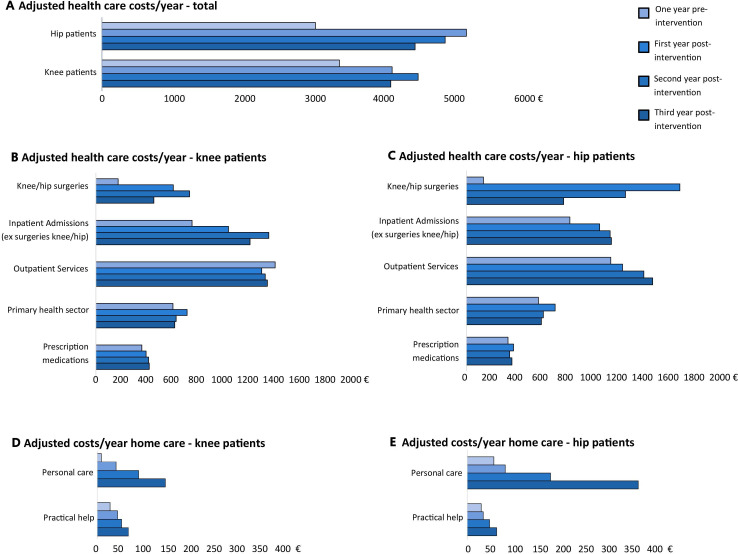
Adjusted healthcare costs and costs for home care 1 year prior to and 3 years after entering the intervention are presented in figure 2A–E, adjusted public transfer payments are presented in table 2 and mean public transfer payments are presented in online supplemental appendix table S2. Additionally, mean and adjusted costs 1 year prior to and 1/3 years after entering the intervention, respectively, are presented in online supplemental appendix tables S3 and S4. To take the potential influence of covariates into account, costs are estimated for average patients, that is, women, 65 years old, married/coliving, ethnic Danish, low educational level and living in the Capital Region. Public transfer payments are estimated for women, 55 years old, married/coliving, ethnic Danish, low educational level and living in the Capital Region since the population was restricted to adults under the age of retirement in this analysis, as public transfer payments target this age group. In the 1-year horizon, monthly adjusted healthcare costs for knee and hip patients were 263€ and 235€ 1 year prior to the intervention, rising to 331€ and 397€ the year after entering the programme (online supplemental appendix table S4). In the 3-year horizon, yearly adjusted healthcare costs 1 year prior to the intervention were 3392€ and 3051€ for knee and hip patients, rising to 4128€ and 4473€ the third year after entering the intervention, observing the highest costs the second-year postintervention for knee patients and the first-year postintervention for hip patients (figure 2A). The increase in mean healthcare costs was mainly due to costs related to surgeries in the knee or hip which the first year after index date in the adjusted analysis accounted for 46€/month of an increase in costs of 68€/month in knee patients and 130.8€/month of an increase in costs of 162.8€/month in hip patients (online supplemental appendix table S4). On average, the raw EQ-5D score increased from 0.711 to 0.756 points for knee patients and from 0.705 to 0.747 for hip patients from baseline to 1-year follow-up (table 3).
Figure 2.
Adjusted healthcare costs and home care costs 1 year prior to and up to 3 years following GLA:D for knee and hip patients. Adjusted health care costs and costs for home care in 3-year horizon for women, 65 years, married/coliving and low education estimated using a generalised estimating equation gamma regression model for repeated measures including sex, age and education. Because of no convergence in the model, age and education were omitted estimating costs for home care. GLA:D, Good Life with osteoArthritis in Denmark.
Table 2.
Adjusted public transfer payments 1 year prior to and 1 or 3 years following GLA:D for knee and hip patients
| One-year horizon | Three-year horizon | |||||||||||||
| Preperiod (1 year) |
Postperiod (months 1–3) |
Postperiod (months 4–12) |
Postperiod (year 1) |
Preperiod (1 year) |
Postperiod (year 1) |
Postperiod (year 2) |
Postperiod (year 3) |
|||||||
| Weeks/ month |
Weeks/ month |
P value | Weeks/ month |
P value | Weeks/ month |
P value | Weeks/ year |
Weeks/ year |
P value | Weeks/ year |
P value | Weeks/ year |
P value | |
| Knee patients in workforce (n: 5586) | Knee patients in workforce (n: 905) | |||||||||||||
| Public transfer payments* | ||||||||||||||
| Unemployed | 0.24 | 0.26 | 0.000 | 0.27 | 0.001 | 0.27 | 0.000 | 3.50 | 3.82 | 0.344 | 3.51 | 0.986 | 3.32 | 0.703 |
| Sheltered employment | 0.10 | 0.10 | 0.959 | 0.10 | 0.967 | 0.10 | 0.982 | 1.61 | 1.59 | 0.907 | 1.69 | 0.671 | 1.78 | 0.422 |
| Sick pay | 0.13 | 0.16 | 0.000 | 0.14 | 0.164 | 0.15 | 0.029 | 1.47 | 1.70 | 0.295 | 1.54 | 0.789 | 1.16 | 0.201 |
| Rehabilitation | 0.01 | 0.01 | 0.254 | 0.01 | 0.494 | 0.01 | 0.407 | 0.09 | 0.03 | 0.017 | 0.09 | 0.960 | 0.06 | 0.731 |
| Education | 0.01 | 0.00 | 0.136 | 0.01 | 0.950 | 0.00 | 0.770 | 0.20 | 0.18 | 0.507 | 0.23 | 0.690 | 0.20 | 0.991 |
| Disability pension | 0.23 | 0.24 | 0.006 | 0.23 | 0.259 | 0.24 | 0.136 | 6.02 | 6.02 | 0.969 | 5.91 | 0.467 | 6.00 | 0.966 |
| Early retirement | 0.37 | 0.46 | 0.000 | 0.46 | 0.000 | 0.46 | 0.000 | 6.06 | 7.35 | 0.000 | 7.02 | 0.071 | 5.44 | 0.354 |
| Hip patients in workforce (n: 1543) | Hip patients in workforce (n: 264) | |||||||||||||
| Public transfer payments† | ||||||||||||||
| Unemployed | 0.19 | 0.19 | 0.540 | 0.20 | 0.309 | 0.20 | 0.325 | 3.36 | 3.56 | 0.730 | 3.20 | 0.865 | 2.90 | 0.672 |
| Sheltered employment | 0.10 | 0.10 | 0.458 | 0.10 | 0.516 | 0.10 | 0.470 | 1.92 | 1.80 | 0.712 | 1.50 | 0.217 | 1.73 | 0.661 |
| Sick pay | 0.10 | 0.13 | 0.082 | 0.16 | 0.001 | 0.15 | 0.003 | 1.38 | 2.00 | 0.327 | 1.41 | 0.967 | 1.32 | 0.903 |
| Rehabilitation | 0.00 | 0.01 | 0.202 | 0.00 | 0.407 | 0.00 | 0.812 | 0.04 | 0.06 | 0.000 | 0.19 | 0.000 | 0.41 | 0.000 |
| Education | 0.01 | 0.01 | 0.321 | 0.01 | 0.659 | 0.01 | 0.557 | 0.27 | 0.20 | 0.593 | 0.20 | 0.598 | 0.12 | 0.183 |
| Disability pension | 0.29 | 0.26 | 0.264 | 0.27 | 0.502 | 0.27 | 0.427 | 3.81 | 3.92 | 0.501 | 3.66 | 0.673 | 3.29 | 0.171 |
| Early retirement | 0.47 | 0.59 | 0.000 | 0.58 | 0.000 | 0.58 | 0.000 | 9.02 | 11.54 | 0.000 | 10.01 | 0.422 | 7.11 | 0.219 |
*Adjusted weeks receiving public transfer payments in 1 year horizon for women, 55 years, married/coliving, Danish ethnicity, low education and living in the Capital Region estimated using a generalised estimating equation gamma regression model for repeated measures including sex, age, marital status, ethnicity, education and region as covariates. Because of no convergence in the model following covariates were omitted: ‘Early retirement’: sex, marital status, ethnicity, education and region; ‘Rehabilitation’: age, marital status, ethnicity and education; ‘Education’: age, marital status, ethnicity and education. Adjusted weeks receiving public transfer payments in 3-year horizon for women, 55 years and low education estimated using a generalised estimating equation gamma regression model for repeated measures including sex, age and education as covariates. Because of no convergence in the model, following covariates were omitted: ‘Sheltered employment’: sex and education; ‘Disability pension’: sex and education; ‘Early retirement’: age.
†Adjusted weeks receiving public transfer payments in 1-year horizon for women, 55 years, married/coliving, Danish ethnicity, low education and living in the Capital Region estimated using a generalised estimating equation gamma regression model for repeated measures including sex, age, marital status, ethnicity, education and region as covariates. Because of no convergence in the model, following covariates were omitted: ‘Early retirement’: sex, marital status, ethnicity, education and region; ‘Rehabilitation’: age, marital status, ethnicity, education and region; ‘Education’: age, marital status, ethnicity, education and region. Adjusted weeks receiving public transfer payments in 3-year horizon for women, 55 years and low education estimated using a generalised estimating equation gamma regression model for repeated measures including sex, age and education as covariates. Because of no convergence in the model, following covariates were omitted: ‘Sheltered employment’: sex and education; ‘Disability pension’: sex and education; ‘Early retirement’: age; ‘Rehabilitation’: sex, age and education.
GLA:D, Good Life with osteoArthritis in Denmark.
Table 3.
Change in health-related quality of life from baseline to 12 months for knee and hip patients attending GLA:D
| Knee (n: 12 162) | Hip (n: 4093) | |||||||
| Preperiod QALY (baseline EQ-5D) |
3 months EQ-5D* |
12 months EQ-5D* |
Composite postperiod QALY† | Preperiod QALY (baseline EQ-5D) |
3 months EQ-5D* |
12 months EQ-5D* |
Composite postperiod QALY† | |
| Mean | 0.711 | 0.752 | 0.756 | 0.748 | 0.705 | 0.733 | 0.747 | 0.735 |
| SD | 0.113 | 0.121 | 0.134 | 0.107 | 0.110 | 0.127 | 0.144 | 0.108 |
*Missing observations for EQ-5D at 3 and 12 months were imputed by Multiple Imputations.
†One-year postperiod QALY was calculated as the area under the curve taking both 3-month and 12-month measurements into account.
EQ-5D, EuroQoL 5-Dimensions 5-Level questionnaire; GLA:D, Good Life with osteoArthritis in Denmark; QALY, Quality-Adjusted Life Year.
Adjusted change in healthcare cost from the year prior to entering GLA:D to the year after entering GLA:D was 298€ (95% CI: 206 to 419) and 640€ (95% CI: 400 to 1009) and QALYs gained were 0.035 (95% CI: 0.033 to 0.037) and 0.028 (95% CI: 0.025 to 0.032) for knee and hip patients, respectively. Hence, 1-year estimated adjusted healthcare costs was 8497€ (95% CI: 6242 to 11 324) for knee patients and 22 568€ (95% CI: 16 000 to 31 531) for hip patients per QALY gained (table 4). Restricting the regression analysis to patients with high compliance, the 1-year adjusted healthcare costs per QALY gained was lower compared with all patients; 5438€ (95% CI: 2758 to 9231) for knee patients and 17 330€ (95% CI: 10 041 to 29 364) for hip patients primarily due to lower change in healthcare costs (table 4). Although the upper limit of the 95% CI for hip patients was in between the two predefined willingness-to-pay thresholds, the estimated healthcare costs per QALY for both knee and hip patients were below both of the two predefined willingness-to-pay thresholds.
Table 4.
Adjusted and raw estimated healthcare costs per QALY from baseline to 12 months for all knee and hip patients attending GLA:D and for knee and hip patients with high compliance
| Knee | Hip | |||||
| Change in healthcare costs (€) (95% CI) |
Change in EQ-5D (QALYs) (95% CI)* |
Euro pr. QALY (95% CI)* |
Change in healthcare costs (€) (95% CI) |
Change in EQ-5D (QALYs) (95% CI)* |
Euro pr. QALY (95% CI)* |
|
| Adjusted† | 298 (206 to 419) |
0.035 (0.033 to 0.037) |
8497 (6242 to 11 324) |
640 (400 to 1009) |
0.028 (0.025 to 0.032) |
22 568 (16 000 to 31 531) |
| Unadjusted | 895 (719 to 1088) |
0.037 | 24 236 | 2162 (1723 to 2671) |
0.030 | 71 478 |
| High compliance†‡ | 197 (91 to 360) |
0.036 (0.033 to 0.039) |
5438 (2758 to 9231) |
492 (241 to 969) |
0.028 (0.024 to 0.033) |
17 330 (10 041 to 29 364) |
*CI not generated from the MI.
†Adjusted for age, gender, marital status, ethnicity, educational level and region.
‡High compliance group defined as patients attending minimum 10 supervised exercise sessions.
EQ-5D, EuroQoL 5-Dimensions 5-Level questionnaire; GLA:D, Good Life with osteoArthritis in Denmark; MI, Multiple Imputations; QALY, Quality-Adjusted Life Year.
Sensitivity analyses showed that knee and hip patients attending GLA:D in a private clinic had similar healthcare costs per QALY but that patients attending GLA:D in a municipal setting had higher costs for knee patients and lower costs for hip patients compared with all patients. This difference was primarily explained by a different change in healthcare costs (online supplemental appendix table S5). The complete case analysis showed lower change in healthcare costs and lower healthcare costs per QALY for knee patients (4829€ (95% CI: 2313 to 8378)) but for hip patients, the ratio was similar to that of all patients (online supplemental appendix table S5). Fifty-three patients died within the 1-year follow-up period and 11 of these within the first 3 months. Repeating all analyses excluding deaths in the regression analyses showed results similar to the main analysis (data not shown).
Discussion
Our study demonstrated that an 8-week supervised patient education and exercise therapy programme for knee or hip OA implemented in primary care is cost-effective in a 1-year horizon with healthcare costs of 8497€ per QALY for knee patients and 22 568€ for hip patients who signed up for the intervention. Despite the physiotherapy visits needed to participate in the GLA:D programme, increased healthcare costs were primarily related to knee or hip surgeries (accounting for 70% and 80% of the increased costs, respectively) and although the mean absolute change in health-related QOL is relatively low (~0.03), the intervention is still considered cost-effective. These results support large-scale implementation of GLA:D in clinical practice.
To the best of our knowledge, this is the first study evaluating the cost-effectiveness of a combined supervised OA education and exercise therapy programme with widespread implementation in primary care. Previous analyses of the GLA:D programme, but with twice the number of supervised neuromuscular exercise sessions, weight loss, insoles and pain medication if needed, have found similar results.15 31 A model-based study suggested that exercise therapy and education was cost-effective as compared with usual care for patients with knee or hip OA in Canada,31 while an analysis of results from a randomised trial comparing supervised exercise therapy, education and other recommended non-surgical interventions to written advice in patients with moderate to severe knee OA found the intervention to be cost-effective with incremental cost effectiveness ratios of 6229 to 20 688€/QALY.15 Even though our study is a pre–post study and therefore not directly comparable, our findings are also in line with other previous studies which have indicated that supervised exercise therapy alone as treatment for OA is cost-effective. Three randomised trials demonstrated that supervised exercise therapy in addition to usual care, supplementary class-based exercise in addition to a home-based programme and supervised exercise therapy compared with general practitioner care alone was likely to be cost-effective in people with knee and/or hip OA.32–34 Also, a model-based study estimated that adding the combination of diet and exercise therapy to usual care for overweight and obese patients with knee OA was cost-effective.35 Our study adds to this body of evidence, that large-scale implementation in clinical practice of a structured combined supervised education and exercise therapy programme seems cost-effective in a 1-year horizon.
In this study, the increased healthcare costs both 1 and 3 years after entering the GLA:D programme were primarily related to surgeries in the knee or hip. According to a stepwise treatment approach, joint replacement surgery is considered to be relevant in patients with end-stage OA once all appropriate non-surgical treatment options such as patient education and supervised exercise therapy of sufficient dose and length, weight loss, walking aids and pain medication have failed to reduce symptoms sufficiently.36 37 Existing evidence indicates that providing supervised exercise therapy can have positive impact on the number of patients having joint replacement surgery,38–40 time to surgery39 40 and outcomes from surgery.41 Ackerman et al conducted a budget impact analysis of implementing a first-line management programme such as GLA:D in Australia and demonstrated that if total knee replacement was avoided in only 1 in 12 GLA:D participants, the programme would generate cost savings in the Australian healthcare system.42 Although the lack of control group in the current study precludes analyses of avoidance of joint replacements, it highlights that regardless of surgery during follow-up, supervised education and exercise therapy is cost-effective.
As a result of similar change in EQ-5D, but lower change in healthcare costs, healthcare costs per QALY were lower in patients compliant to the intervention (ie, attending at least 10 supervised exercise sessions) compared with all patients enrolled in the programme, indicating that the dosage of exercise therapy is important. Although we did not find that higher compliance was associated with greater effects on the EQ-5D, the lower change in healthcare costs in the compliant patients underlines the importance of exercise dosage as suggested by a systematic review and meta-regression analysis of 48 randomised controlled trials in patients with knee OA showing that 12 or more supervised exercise sessions are more effective than fewer supervised sessions,43 and a systematic review and meta-analysis in patients with hip OA showing that supervised exercise therapy with high compliance with dose recommendations compared with uncertain compliance (studies where compliance was not possible to categorise according to recommendations) was more effective.44 Although dosage seems important for the effect and cost-effectiveness, knowledge of optimal exercise dosage in OA is still lacking.9 43 45
As there is no official threshold defining a cost-effective treatment in Denmark, we compared the healthcare costs per QALY to two different internationally widely used willingness-to-pay thresholds. Although the estimated healthcare costs per QALY for both knee and hip patients were below both of the two thresholds, the upper limit of the 95% CI for hip patients was in between the two thresholds, thus we cannot rule out that the true healthcare costs per QALY for hip patients is above the lower willingness-to-pay threshold (22 804€). A threshold value for willingness-to-pay for improvements in health is arbitrary and depending on the context such as budget and other treatment options.26 Country-level threshold value based on GDP per capita has been discussed but remains unsettled.46 When deciding which treatment options to implement and offer, the results from this study can support clinicians and decision-makers in terms of 1-year cost-effectiveness of supervised education and exercise therapy implemented nationwide for patients with knee and hip OA in clinical practice.
Strengths and limitations
The major strength of the study is that all costs reported are real-life costs retrieved on an individual level from a range of high-quality national registries supporting the reliability and validity of the costs.22 47 48 Even though it is likely that a higher level of heterogeneity in treatment protocols occurred compared with in rigorous clinical trials, another major strength is that the study included a large number of rural and urban patients with wide inclusion criteria; joint pain and functional limitations associated with OA, retrieved from a nationwide registry supporting the generalisability of the findings.
The main limitation of the study is that the study is a pre–post study where change in healthcare costs was evaluated against change in EQ-5D. Without a proper control group, it cannot be ruled out that the observed change in EQ-5D is related to other factors than the treatment such as placebo or regression to the mean. In the analysis, EQ-5D measured at baseline represented the QOL the year prior to the intervention, but there is a risk that the change in QOL were overestimated as patients often seek treatment at time of worsening of symptoms. Also, change in costs can potentially have been affected by increasing age, since healthcare costs are expected to increase with increased age and accompanied morbidity.49 As a consequence of lack of model convergence marital status and ethnicity was omitted as covariates in the adjusted model evaluating the costs for home care estimating change in costs per QALY gained in a 1-year horizon. As costs related to home care comprises a rather small proportion of the total costs it is not considered to affect the main result.
In the current study, healthcare costs per QALY was evaluated in a 1-year horizon and additionally change in costs were reported in a 3-year horizon. OA is a long-term chronic condition,36 thus evaluating cost-effectiveness in a 1-year horizon is a relatively short time horizon warranting further long-term cost-effective analyses. However, a recent model-based cost-effectiveness analysis suggested that a physical activity programme for patients with knee OA would lead to favourable long-term clinical and economic benefits.50
Only around 60% of the costs covering the programme for most patients attending GLA:D in private physiotherapy clinics were taken into account in the analyses, that is, patients out-of-pocket costs and costs covering the programme in municipal settings as well as medications bought over the counter were not included. As the increase in costs in the primary healthcare sector and in costs covering medications the first year following index date only constitute a very low proportion of the increased costs in total, this limitation is not considered to substantially affect the overall results.
There was a loss to follow-up in the GLA:D registry and conducting a sensitivity analysis restricted to patients with complete information revealed that they had less mean change in healthcare costs than all included patients, indicating a risk of selective loss to follow-up in the GLA:D registry, however, the evaluation on healthcare costs per QALY included all patients enrolled in GLA:D, imputing the missing outcome values at follow-up. Imputing missing outcome values relied on the assumption that data were missing at random, that is, the missingness was related to variables included in the model. However, there is a risk that loss to follow-up was related to unobserved factors not available for the analysis (eg, good or bad outcome from the GLA:D programme). One-third did not provide information on compliance and there is a risk that lower change in healthcare costs in the subgroup of patients with high compliance is affected by selection bias, that is, that the lower change in healthcare costs could be due to systematically differences in the use of healthcare services between those providing and not providing information about compliance rather than due to the intervention. However, we did not find clinically relevant health status differences at baseline among those not providing information on compliance compared with those who provided this information (data not shown).
The current study is based on real-world outcome data collected in nationwide physiotherapy clinics and actual healthcare costs retrieved from national registries, supporting the generalisability of the results. However, patients attending GLA:D are a preselected group of patients who are commonly referred to physiotherapy for their symptoms with most being able to pay partly for the intervention, which might limit the generalisability.
Conclusions
A structured 8-week supervised education and exercise therapy programme delivered in physiotherapy practice was cost-effective at 1 year in patients with knee and hip OA compared with conventional willingness-to-pay thresholds except the upper limit of the 95% CI for hip patients which was in between two thresholds. Both health-related QOL and healthcare costs increased during the 1 year time horizon, the latter mainly due to knee or hip surgeries. The results support large-scale implementation of a structured supervised evidence-based patient education and exercise therapy programme targeting patients with knee or hip OA and can guide clinicians and decision-makers on what to expect when such programmes are implemented in clinical practice.
Supplementary Material
Acknowledgments
The authors would like to thank the clinicians and patients involved in collecting data for GLA:D.
Footnotes
Twitter: @Dorte_Groenne, @ewa_roos, @KjellbergJakob, @STSkou
Contributors: Study conception and design: DTG, ER, RI, JK and STS. Acquisition of data: DTG, ER and STS. Analysis and interpretation of data: DTG, ER, RI, JK and STS. Drafting the article: DTG and STS. Revising the article critically for important intellectual content: DTG, ER, RI, JK and STS. Final approval of the article: DTG, ER, RI, JK and STS. Obtaining of funding: STS. All authors take responsability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by the Danish Rheumatism Association and the Physiotherapy Practice Foundation. Grant numbers not applicable. The Danish Physiotherapist Association’s fund for research, education and practice development; the Danish Rheumatism Association and the Physiotherapy Practice Foundation have previously supported GLA:D. Dr Skou is currently funded by a grant from Region Zealand (Exercise First) and a grant from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 801790). None of the funding sources had any role in the study other than to provide funding.
Competing interests: Dr Roos is deputy editor of Osteoarthritis and Cartilage, the developer of the Knee injury and Osteoarthritis Outcome Score (KOOS) and several other freely available patient-reported outcome measures and cofounder of Good Life with osteoArthritis in Denmark (GLA:D), a not-for profit initiative hosted at University of Southern Denmark aimed at implementing clinical guidelines for osteoarthritis in clinical practice. Dr Skou is associate editor of the Journal of Orthopaedic & Sports Physical Therapy, has received grants from The Lundbeck Foundation, personal fees from Munksgaard, all of which are outside the submitted work. He is cofounder of GLA:D. RI: None. JK: None. Dr Grønne is employed as data manager in the GLA:D project.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data from the national Danish registries used in this study is available from Statistics Denmark. However, restrictions apply to the availability, as the data was used under license for the current study, and so are not publicly available. Data are however available from the authors ER and STS upon reasonable request and with permission of Statistics Denmark.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
As the study is a registry-based study, according to Danish legislation, ethical approval is not needed and according to the local ethics committee of the North Denmark Region, ethics approval of GLA:D was not needed.
References
- 1.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 2014;10:437–41. 10.1038/nrrheum.2014.44 [DOI] [PubMed] [Google Scholar]
- 2.Kingsbury SR, Gross HJ, Isherwood G, et al. Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology 2014;53:937–47. 10.1093/rheumatology/ket463 [DOI] [PubMed] [Google Scholar]
- 3.Salmon JH, Rat AC, Sellam J, et al. Economic impact of lower-limb osteoarthritis worldwide: a systematic review of cost-of-illness studies. Osteoarthritis Cartilage 2016;24:1500–8. 10.1016/j.joca.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Safiri S, Kolahi A-A, Smith E. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis 2020;79:819–28. 10.1136/annrheumdis-2019-216515 [DOI] [PubMed] [Google Scholar]
- 5.Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 6.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–89. 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 7.Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum 2014;43:701–12. 10.1016/j.semarthrit.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 8.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020;72:220–33. 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med 2015;49:1554–7. 10.1136/bjsports-2015-095424 [DOI] [PubMed] [Google Scholar]
- 10.Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev 2014;21:CD007912. 10.1002/14651858.CD007912.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagen KB, Smedslund G, Østerås N, et al. Quality of community-based osteoarthritis care: a systematic review and meta-analysis. Arthritis Care Res 2016;68:1443–52. 10.1002/acr.22891 [DOI] [PubMed] [Google Scholar]
- 12.Smith T, Collier TS, Smith B, et al. Who seeks physiotherapy or exercise treatment for hip and knee osteoarthritis? A cross-sectional analysis of the English longitudinal study of ageing. Int J Rheum Dis 2019;22:897–904. 10.1111/1756-185X.13480 [DOI] [PubMed] [Google Scholar]
- 13.Skou ST, Roos EM. Good Life with osteoArthritis in Denmark (GLA:D): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord 2017;18:72. 10.1186/s12891-017-1439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzei DR, Ademola A, Abbott JH, et al. Are education, exercise and diet interventions a cost-effective treatment to manage hip and knee osteoarthritis? A systematic review. Osteoarthritis Cartilage 2021;29:456–70. 10.1016/j.joca.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Skou ST, Roos EM, Laursen M, et al. Cost-effectiveness of 12 weeks of supervised treatment compared to written advice in patients with knee osteoarthritis: a secondary analysis of the 2-year outcome from a randomized trial. Osteoarthritis Cartilage 2020;28:907–16. 10.1016/j.joca.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 16.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ 2013;346:f1049. 10.1136/bmj.f1049 [DOI] [PubMed] [Google Scholar]
- 17.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 18.Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord 2010;11:126. 10.1186/1471-2474-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ageberg E, Roos EM. Neuromuscular exercise as treatment of degenerative knee disease. Exerc Sport Sci Rev 2015;43:14–22. 10.1249/JES.0000000000000030 [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis 2010;69:483–9. 10.1136/ard.2009.113100 [DOI] [PubMed] [Google Scholar]
- 21.The Danish Health Authority . Knee osteoarthritis - national clinical guidelines and referral guidelines, 2012. [Google Scholar]
- 22.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–91. 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittrup-Jensen KU, Lauridsen J, Gudex C, et al. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health 2009;37:459–66. 10.1177/1403494809105287 [DOI] [PubMed] [Google Scholar]
- 24.Bilbao A, García-Pérez L, Arenaza JC, et al. Psychometric properties of the EQ-5D-5L in patients with hip or knee osteoarthritis: reliability, validity and responsiveness. Qual Life Res 2018;27:2897–908. 10.1007/s11136-018-1929-x [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence . NICE process and methods guides. guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence, 2013. [Google Scholar]
- 26.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–7. 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 27.Faria R, Gomes M, Epstein D, et al. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157–70. 10.1007/s40273-014-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Losina E, Ranstam J, Collins JE, et al. OARSI clinical trials recommendations: key analytic considerations in design, analysis, and reporting of randomized controlled trials in osteoarthritis. Osteoarthritis Cartilage 2015;23:677–85. 10.1016/j.joca.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Missing data. BMJ 2007;334:424. 10.1136/bmj.38977.682025.2C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 31.Structured education and neuromuscular exercise program for hip and/or knee osteoarthritis: a health technology assessment. Ont Health Technol Assess Ser 2018;18:1–110. [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott JH, Wilson R, Pinto D, et al. Incremental clinical effectiveness and cost effectiveness of providing supervised physiotherapy in addition to usual medical care in patients with osteoarthritis of the hip or knee: 2-year results of the MOA randomised controlled trial. Osteoarthritis Cartilage 2019;27:424–34. 10.1016/j.joca.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 33.Richardson G, Hawkins N, McCarthy CJ, et al. Cost-effectiveness of a supplementary class-based exercise program in the treatment of knee osteoarthritis. Int J Technol Assess Health Care 2006;22:84–9. 10.1017/S0266462306050872 [DOI] [PubMed] [Google Scholar]
- 34.Tan SS, Teirlinck CH, Dekker J, et al. Cost-utility of exercise therapy in patients with hip osteoarthritis in primary care. Osteoarthritis Cartilage 2016;24:581–8. 10.1016/j.joca.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 35.Losina E, Smith KC, Paltiel AD, et al. Cost-effectiveness of diet and exercise for overweight and obese patients with knee osteoarthritis. Arthritis Care Res 2019;71:855–64. 10.1002/acr.23716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 37.Ferket BS, Feldman Z, Zhou J, et al. Impact of total knee replacement practice: cost effectiveness analysis of data from the osteoarthritis initiative. BMJ 2017;356:j1131. 10.1136/bmj.j1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med 2015;373:1597–606. 10.1056/NEJMoa1505467 [DOI] [PubMed] [Google Scholar]
- 39.Svege I, Nordsletten L, Fernandes L, et al. Exercise therapy may postpone total hip replacement surgery in patients with hip osteoarthritis: a long-term follow-up of a randomised trial. Ann Rheum Dis 2015;74:164–9. 10.1136/annrheumdis-2013-203628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skou ST, Roos EM, Laursen MB, et al. Total knee replacement and non-surgical treatment of knee osteoarthritis: 2-year outcome from two parallel randomized controlled trials. Osteoarthritis Cartilage 2018;26:1170–80. 10.1016/j.joca.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 41.Fernandes L, Roos EM, Overgaard S, et al. Supervised neuromuscular exercise prior to hip and knee replacement: 12-month clinical effect and cost-utility analysis alongside a randomised controlled trial. BMC Musculoskelet Disord 2017;18:5. 10.1186/s12891-016-1369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilana Ackerman N N, Roos EM, Barton CJ, et al. Implementing a nationalfirst-line management program formoderate-severe knee osteoarthritis in Australia: a budget impact analysisfocusing on knee replacement avoidance. Osteoarthritis Cartilage Open 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol 2014;66:622–36. 10.1002/art.38290 [DOI] [PubMed] [Google Scholar]
- 44.Moseng T, Dagfinrud H, Smedslund G, et al. The importance of dose in land-based supervised exercise for people with hip osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 2017;25:1563–76. 10.1016/j.joca.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 45.Regnaux J-P, Lefevre-Colau M-M, Trinquart L, et al. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev 2015;26:Cd010203. 10.1002/14651858.CD010203.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods B, Revill P, Sculpher M, et al. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health 2016;19:929–35. 10.1016/j.jval.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish national prescription registry. Int J Epidemiol 2017;46:798–98f. 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet 2009;374:1196–208. 10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva GS, Sullivan JK, Katz JN, et al. Long-term clinical and economic outcomes of a short-term physical activity program in knee osteoarthritis patients. Osteoarthritis Cartilage 2020;28:735–43. 10.1016/j.joca.2020.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-049541supp001.pdf (1.3MB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data from the national Danish registries used in this study is available from Statistics Denmark. However, restrictions apply to the availability, as the data was used under license for the current study, and so are not publicly available. Data are however available from the authors ER and STS upon reasonable request and with permission of Statistics Denmark.