Abstract
Root caries is a growing problem for the worldwide aging population. Silver diamine fluoride (SDF) contains high concentrations of silver and fluoride ions, which prevents and arrests root caries, as well as dentin caries in the primary teeth of young children. Unlike other fluoride products that mainly reduce the formation of new carious lesions, 38% SDF is an effective agent that can efficiently arrest the carious process, remineralize the decayed dental tissues, and protect the tooth structure against the formation of new caries lesions. The use of SDF can result in more caries-resistant tooth structures. Despite these merits, its clinical disadvantages are the deep penetration of silver ions and sequential formation of silver compounds, which cause esthetic concern due to the discoloration and impaired efficacy of dentin bonding after using SDF. Thus, this narrative review, by addressing the primary experimental results and clinical applications of SDF on root caries, proposes management methods for root caries in conjunction with the application of SDF. We propose a two-visit treatment protocol to take advantage of the SDF application for root surface caries and utilize the discoloration caused by SDF.
Keywords: Prevention, Remineralization, Silver diamine fluoride, SDF, Adhesion, Caries detection
1. Introduction
The tooth retention has increased in the growing aging population [1]. Despite increasing tooth retention, chronic dental diseases such as caries and periodontal disease are not sufficiently managed [2,3]. As for caries lesions, the root surface tends to be exposed in elderly people with advanced periodontal disease and gingival recession, and root caries is especially prevalent.
In general, the development of a carious lesion comprises a dynamic biological process in which dental plaque bacteria ferment dietary sugars into acids that cause the demineralization of dental hard tissues [4,5]. The progression of the disease process, accompanied by further loss of tooth minerals, leads to a cavitated lesion. Arresting cavitated lesions is more complex, as the loss of the tooth structure creates niches for the biofilm, which are not easily accessible. However, preserving tooth structure and pulpal health is also a guiding principle for managing cavitated carious lesions [6]. Therefore, early detection, diagnosis, and the use of effective nonrestorative treatments are crucial for the management of non-cavitated carious lesions. Hence, the management of dentine caries has traditionally focused on treatment via the excision of diseased tissues and the subsequent restoration of the defect. It should be noted that mechanical tooth preparation is a destructive and irreversible procedure in which natural dental tissues are removed [7].
The contemporary caries management philosophy has moved from the traditional surgical approach, that is, by conventional rotary burs, to the atraumatic restorative technique (ART), which is a medical model. The disease itself needs to be treated: to alter the bacteria flora, strengthen the tooth, enhance the saliva, and decrease sugar consumption. Pharmacological treatment of caries should be accomplished with antimicrobials, remineralizing agents, salivary stimulation, and dietary behavior modification [8].
This approach can also be indicated for root caries management. The aging population presents various diseases, which are complicated by medical and social circumstances. As it is essential to provide affordable, accessible, and acceptable care to an aging population [9,10], ART, especially the use of different therapeutic methods, is considered a suitable method [[11], [12], [13]].
Many chemotherapeutic agents have been used to prevent the development of root caries or arrest root caries lesions, including sodium fluoride (NaF), silver diamine fluoride (SDF), chlorhexidine (CHX), and casein phosphopeptide‐amorphous calcium phosphate (CPP‐ACP) [14]. These agents appear to be quite effective in reducing the incidence of root caries in older adults. Especially, SDF regimes have been suggested with considerable success in high‐risk elders [15]. In a randomized clinical trial with a 30‐month observation period by Li et al., a single professional annual 38% SDF application reduced the burden of care in vulnerable, dependent patients [14]. An annual application of SDF varnish arrested approximately 90% of the active root carious lesions [14]. Thus, professionally applied SDF seems to be efficacious for decreasing the progression and initiation of root caries [10]. On the other hand, disadvantages of SDF application have been suggested, including staining/discoloration [[16], [17], [18]], inflammatory influence on dentin-pulp complex, and the impairment of the bond strength [19]. Root caries treatment does not necessarily raise aesthetic concerns unless it is applied to the root surface of the anterior teeth. However, extra care should be taken to treat root caries developed close to the dentin-pulp complex, given the extremely high concentration of silver and fluoride ions and the alkaline solution [20]. Although the aforementioned disadvantages of SDF should not negate its benefits, we have to apply SDF with caution in clinical cases. Precautions are provided by Japanese manufacturer (BEE BRAND MEDICO DENTAL.CO.,LTD., Osaka, Japan) if the SDF accidentally contacts the gingiva or oral mucosa. SDF may cause corrosion if applied near the gingiva, so a rubber dam should be used to prevent contact with the gingiva, or if this is not possible, Vaseline or cocoa butter should be applied to the gingiva in advance. In case of accidental contact, rinse immediately with water, saline solution, or diluted hydrogen peroxide solution.
When a severe caries lesion has developed, there is a limitation in managing root caries with plaque control and simply applying fluoride agents [13,21]. In this case, where decay and defects in the dentin are advanced, restorative materials must be used as a management method. Although SDF application can arrest the development of caries in the teeth, the placement of dental restorations may be needed to fill cavities. Stained cavities caused by the application of SDF should be repaired with tooth-colored restorative materials such as resin composites, glass ionomer cements (GIC), resin-modified glass ionomer, or polyacid-modified composite resins (compomers). This restorative approach is considered an atraumatic method that can provide patients with esthetic satisfaction [22]. Hence, the placement of restorative materials in conjunction with SDF application has attracted attention as an appropriate approach for managing advanced root caries lesions [21].
However, it is difficult to determine the appropriate restorative materials because bonding to SDF-applied dentin is challenging [21,23,24]. GIC is used alternatively with composite resin, despite its inadequate mechanical strength [[25], [26], [27], [28]]. The benefits of SDF application have been extensively examined, but little effort has been made to discuss how to restore root caries after SDF is applied. This narrative review summarizes the clinical and scientific evidence for SDF and highlights techniques for restoring SDF-applied dentin.
2. Silver in dentistry
Silver was first used in dentistry as early as the 1840s in the form of “nitrate of silver” (known today as silver nitrate, AgNO3). This salt is extremely caustic. Early dentists used it to instantaneously cauterize carious lesions to achieve an effect analogous to the hard, dark crust observed on teeth whose untreated decay had been arrested naturally over time [29]. Silver nitrate continued to be a popular dental medicament through the era of G.V. Black and his modernization of operative dentistry. In 1917, an ammoniacal silver nitrate solution (AgNH3NO3) was developed and marketed as an antimicrobial product that supposedly could penetrate even deeper into the dentin. Until the 1950s, this “Howe’s solution” was used to sterilize lesions after preparation and was even advocated as a disinfectant in root canal therapy. Silver has a [Kr]4d105s1 electron configuration. As the 4d shell does not effectively shield the 5s1 electrons, they are strongly attracted to the nucleus, and a relatively high standard reduction potential is observed (E0(Ag+/Ag) = +0.799 V).
On the other hand, silver has the lowest first ionization potential in Group 11, which cconsistes consisting of copper, silver and gold (Au). Thus, Ag+ is the most stable species in aqueous solutions, including physiological fluids and solids. Due to the filled 4d orbital, Ag+ compounds present a significant covalent character, and they tend to form organometallic compounds [30]. Silver ions form bonds preferably with sulfur but also with nitrogen and oxygen. Thus, Ag+ can bind to enzymes with S-pending groups as well as N atoms from nucleic acids [31]. Besides formulations containing Ag+, other silver-based compounds have also been successfully applied in dentistry. For instance, the linear complex structure of the SDF [Ag(NH3)2+]F has been used in Japan since approval over 80 years ago [8,32]. More recently, SDF was cleared for reducing tooth sensitivity by U.S. Food and Drug Administration.
3. SDF
3.1. History of SDF
SDF was first introduced by Yamaga et al. at Osaka University in Japan in the late 1960s [33]. The idea was to combine the powerful antimicrobial properties of silver with the benefits of a high dose of fluoride. This formulation also resulted in a precipitate that occluded dentinal tubules and reduced hypersensitivity [34]. Soon after, the use of “diamine silver fluoride” as a cariostatic agent was approved by the Central Pharmaceutical Council of the Ministry of Health and Welfare of Japan and marketed under the name Saforide (Toyo Seiyaku Kasei Co. Ltd., Osaka, Japan). Studies reported that the topical application of SDF could be considered a cost-effective, simple, and non-invasive technique for caries management [17,35,36]. A literature review said that SDF is regarded as a safe and effective caries preventive agent that seems to meet the criteria of the WHO millennium goals and the FDA, a US institute [32].
3.2. The composition of SDF and its interactions
Chemically, SDF comprises the diamine silver ion complex [Ag (NH3)2]+ and fluoride ion (F−). The diamine-silver ion complex is less oxidizing and more stable than silver ion, as metal ammine complexes are more stable than their corresponding aqueous complexes [37].
Recently, a 38% SDF product equivalent to Saforide (named Advantage Arrest) has been launched in the US market, while lower concentrations of 12% and 30% SDF products are marketed in Brazil [38]. The SDF at 38% seems effective in arresting dentine caries [[38], [39], [40]]. The 38% SDF solution contains high concentrations of silver (253,900 ppm) and fluoride (44,800 ppm F) with the pH being between 10 and 12. When SDF is applied to the tooth structure, it penetrates enamel [33] and dentin deeply [41]. In the case of dentin, the silver compound penetrated approximately 1200 μm within the underlying dentin and interacted with the dentin through the silver and fluorine components [41].
3.3. The interactions of silver
Silver reacts with hydroxyapatite, forming silver phosphate (Ag3PO4) and silver oxide (Ag2O). Both Ag3PO4 and Ag2O compounds are unstable and thus are later replaced by silver chloride, as silver chloride has a lower solubility product [42]. A small portion of these compounds is occasionally reduced to form metallic silver [43] with hexagonal- or square-shaped crystals of a sub-micrometer size [44,45]. Furthermore, Chen et al. mentioned that silver could be incorporated into the crystal structure of hydroxyapatite to produce silver-containing hydroxyapatite [46]. These silver-containing products could be described as Ca10-xAgx(PO4)6(OH)2 with 0.0 ≤ x ≤ 0.5 [47] through an ion exchange reaction [48]. Regarding its interactions with collagen, silver can be reduced by proteins (collagen), resulting in the metallic silver attached to the protein (silver-protein complex). This complex indirectly protects dentin collagen by inhibiting dentin collagenases such as matrix metalloproteinases (MMPs) and cathepsins [35,49].
The formed silver compounds release silver ions by being ionized in the presence of water and biologic fluids [50]. The released ions express a significant antibacterial effect through various mechanisms. Silver ions can interact with the thiol group of enzymes, consequently deactivating enzymes and resulting in bacterial cell death [51]. They can interact with the DNA of bacterial cells, causing DNA mutation and bacterial cell death. Further, silver ions bind to bacterial cells by interacting with their cell membranes or cell walls; they “electrostatically bind to the anionic portions of the membranes,” resulting in leakage or rupture of the membrane and subsequent bacterial death. Silver can also bind with amino acids, forming a protein-metallic complex. As this complex breaks down, silver ions accumulate inside the bacterial cell, causing the inactivation of bacterial DNA and RNA [52].
3.4. Fluoride interactions
The released fluoride ions can react with tooth minerals (hydroxyapatite) to produce fluorapatite (FAp) through ion exchange [53] and calcium fluoride (CaF2) through apatite dissolution [54]. Both FAp and CaF2 play an essential role in the remineralization and protection capabilities of SDF against carious lesions. Fluoride ions produce an antibacterial effect through the direct inhibition of cellular enzymes or by enhancing the proton permeability of cell membranes in the form of hydrogen fluoride [55,56].
4. SDF’s effectiveness against carious lesions
4.1. In vitro studies
Numerous in-vitro studies have reported the high efficiency of SDF. Due to the various antibacterial mechanisms of silver, it is difficult for bacteria to develop resistance against it. Suzuki et al. in 1976 found that SDF inhibited both the sucrose-induced and dextran-induced agglutination of S. mutans through the inhibition of glucosyl- and fructose-transferase activities, which are related to the synthesis of polysaccharides [33]. In 2015, Savas et al. reported that SDF inhibited the growth of the S. mutans biofilm [57]. Other studies revealed similar results for the strong antibacterial effect of SDF in inhibiting the growth of bacterial biofilm with regard to the mono-species of S. mutans or Actinomyces naeslundii [56,58,59], the dual-species of S. mutans and Lactobacillus acidophilus [42], and even a multi-species biofilm composed of S. mutans, Streptococcus sobrinus, L. acidophilus, Lactobacillus rhamnosus, and A. naeslundii [60].
Moreover, SDF promotes the remineralization of existing carious lesions and prevents the development of new lesions, as SDF reduces lesion depth and mineral loss [61] and increases microhardness after its application to the enamel [62,63] and dentin [64,65]. This remineralization ability also protects dental tissues against mechanical and chemical abrasion [66,67].
In addition, SDF protects the dentinal collagen against chemical degradation by inhibiting collagenase enzymes’ catalytic action, especially that of MMPs, including MMP-2, MMP-8, and MMP-9, compared with sodium fluoride and silver nitrate [35,68]. Additionally, SDF could inhibit other collagenases such as cathepsins (B and K) [49,69]. However, it may cause alterations in collagen morphology due to its high alkalinity and high silver content [56].
4.2. Clinical application
The clinical success of SDF is well-documented in more than 20 clinical studies performed worldwide. A 2017 review concluded that SDF is very effective for preventing and arresting caries in pediatric, adolescents (with mixed dentations), and the elderly population (age >60 y) [70]. Moreover, SDF, being a non-invasive option, is suitable for patients who cannot handle traditional surgical techniques [39].
Compared with other fluoride materials, SDF can arrest carious lesions with 91% at 6 m, while fluoride percentage with the annual application was 70%, and glass ionomer arrested 82% [71]. A randomized controlled clinical trial demonstrated that SDF effectively prevented root caries in elders by utilizing three randomized groups, which received only oral hygiene instructions (OHIs), OHIs + SDF, or OHIs/SDF with an annual oral health education lecture [72]. Regarding the economic side, SDF was proved to be cost-effective for the prevention of caries, compared to fluoride rinse and chlorohexidine [6].
5. Root caries
The aging world population has been confronted with the growing occurrence of root caries because of the increase in retained teeth, coupled with inadequate oral care and the prevalence of gingival recessions [10]. Root caries is defined as a non-cavitated or cavitated lesion below the cemento-enamel junction (CEJ) that does not include the adjacent enamel [73]. A swept-source optical coherence tomographic observation was attempted on extracted human teeth to examine the prevalence of the exposed root surface [74]. This non-invasive method revealed that the occurrence of exposed dentin with 36.5% of the examined teeth but a higher percentage of buccal and lingual surfaces than proximal surfaces. This finding has spurred clinicians into taking measures to prevent the development of root caries.
Dentin and cementum have higher solubility compared with enamel [75]. In terms of critical pH, the pH value required to initiate mineral loss ranges between pH 6 and 6.8 in root dentine, compared with pH 5.4 in enamel [76]. After hydroxyapatite is dissolved by acid from bacteria, the demineralized dentin matrix, mainly type-I collagen, is degraded by internal enzymes. As the collagen matrix works as a scaffold for mineral deposition, its loss causes further mineral loss [77]. Hence, the incidence and progress of root caries can be explained as follows:
-
i
The influence of cementum loss causes root dentin to be exposed to the oral cavity environment.
-
ii
Dentin is prone to demineralize due to low critical pH.
-
iii
The organic matrix degradation is responsible for the development of root caries.
6. Guidelines for root caries treatment
In the initial stages of root caries, non-invasive treatments using fluorides and SDF are adequate to prevent further progress. The daily use of mouth rinse and toothpaste containing fluoride has been recommended in promoting the remineralization of carious lesions without removing the tooth structure [10,11,78]. These therapeutic attempts are cost-effective, less stressful to elderly patients, and applicable for people who face difficulties in ambulation. Suggested restorative options for decayed lesions on root caries include fillings with resin composites or glass-ionomer cements [13]. As dentin adhesive systems have been innovated to achieve a clinically high success rate [79], composite resin restoration is feasible when cavity isolation is ensured during restoration. Alternatively, GIC restorations may be recommended when the isolation is difficult, especially for posterior teeth. After one year, the survival rate of root restorations was reported to be 90%, but it decreased to 65% in two years [11,80,81]. This poor prognosis could be explained by multiple factors, including the dislodgment force with the lack of mechanical retention, low salivary flow and buffering capacity, and difficulties in bonding to radicular dentin [13]. However, there have been no controlled studies comparing the clinical outcomes of GIC restorations, resin composite restorations, and topical fluoride therapy in patients with root surface caries.
7. GIC restoration in conjunction with the application of SDF
Regarding SDF and GIC, there is some evidence that GIC can enhance the effectiveness of SDF. In 2021, Eslami et al. proved that one or two applications of SDF followed by GIC coverage could remineralize advanced dentin caries in the presence of artificial saliva [82]. Other studies stated that SDF did not affect the shear bond strength of GIC with sound dentin [83] or carious dentin [84] and could improve the resistance of GIC to secondary caries [83]. A systematic review and meta-analysis concluded that a prior application of SDF did not weaken the bonding of GIC to dentin [85].
8. Resin restoration in conjunction with the application of SDF
Seto et al. stated that the applied SDF indeed blocks the dentinal tubules [86], which may hinder the penetration of the bonding agent into dentin, resulting in the lower bonding strength of resin restorations and, consequently, reducing the clinical success and longevity of resin restorations. While multiple studies reported a decrease in bond strength with SDF-treated dentin [[87], [88], [89]], other studies reported no degradation in the bond strength if the SDF was rinsed after application [23,90,91]. SDF may potentially alter dentin surface property because the SDF solution is ammoniated with high heavy metal concentrations. We expect that bond strengths would be restored when SDF-altered dentin is removed chemically or mechanically. Knight et al. reported that the decrease in bond strength with auto-cure glass ionomer could be prevented by adequately rinsing the precipitates after SDF application. However, the bond strength after SDF application may need further investigation [88].
9. For limitations and precautions
Although SDF is a clear solution, the most prominent SDF limitation is its dark discolorations. The previously mentioned silver compounds and metallic silver produced from SDF reactions are responsible for these dark stains. SDF stains demineralized dental tissues at a higher rate than sound ones [92], and the discoloration depth increases over time [41]. Thus, it was suggested that SDF be used as a detecting dye for caries tissues [93]. In addition, SDF immediately turns black if exposed to a strong light source such as a light-curing device [93].
Various attempts have been made to solve the discoloration problem. Knight et al. suggested applying a saturated potassium-iodide solution immediately after SDF to reduce the discoloration [94]. The iodide ions react with the excess silver ions within the media, leading to silver iodide crystals (yellowish-white precipitates) that decrease the dark stains. However, a 30-month clinical trial reported that the application of potassium-iodide did not have a long-term effect on improving the staining problem, and eventually, the discoloration returned [95]. Other approaches were suggested to overcome the staining problem, such as adding glutathione to SDF, which significantly decreased the discoloration, but slight discoloration remains [45]. Other attempts were made using nano-silver, which has an effective antimicrobial power but does not cause discoloration [56,96] However, additional studies need to be performed to determine the proper concentration of nano-silver particles.
Pulpal response is another concern regarding SDF. Although SDF has an acceptable safety record [97,98], a study found an increase in pulpal cell death when the remaining dentin thickness between the pulp and the applied SDF was considerably thin [39]. In a toxicity test using rat pulpal cells, cytotoxicity of diluted 38% SDF solutions (10-5fold dilution) was reported [20]. Meanwhile, another ex-vivo study reported that SDF caused minimal adverse effects on the pulpal tissue [99].
Some precautions should be considered when SDF is used, especially in carious lesions extending subgingivally, as SDF may cause transient gingivitis upon contact with soft tissues. Therefore, the application of rubber dams and cotton rolls or coating the gingival tissues with petroleum jelly to protect the gingiva is advised [100].
10. Potential use of SDF in the detection of carious dentin
Commercially available caries-detector dyes are presumed to distinguish infected dentin and sound dentin. However, it has not been established whether the dye is specific to infected dentin [101]. These non-specific protein dyes are stainable for the organic matrix of less mineralized dentin. However, dye staining and bacterial penetration occur independently [102], which significantly limits the usefulness of these dyes for diagnostic purposes. Especially, caries-detector dyes are not practical tools for assessing root caries lesions, which exhibit discoloration, becoming light brown, yellow, dark brown, and black if advanced. This discolored lesion is not distinguished by caries-detector dyes.
Notably, SDF stains demineralized dental tissues more specifically than sound ones, and the depth of discoloration increases over time [26,41]. It has been suggested that SDF be used as a detecting dye for carious tissues to guide caries excavation. This technique using SDF can provide several advantages, such as arresting caries chemically and biologically over the currently used caries-detecting dyes [93].
11. A two-visit treatment protocol for root caries treatment
A two-visit treatment protocol can be introduced to to take advantage of discoloration caused by SDF. In this technique, SDF is applied on the carious lesion and left for a few days or a week until the next visit. Fig. 1 shows in vitro cross-sectional views to illustrate the function of the SDF in this protocol. On thee first visit, when SDF was applied on the root lesion, it was difficult to distinguish between caries-affected dentin and sound dentin, especially root caries lesions. Cross-sectional images demonstrate that caries lesions were not easily identifiable immediately after application of SDF as shown in Fig. 1a. Then, this sample was stored at 100% RH and 36.7 °C for one week to simulate the second visit. The SDF-applied cavity became dark-colored (Fig. 1b) and more easily detectable, which might prevent the removal of excess tissue.
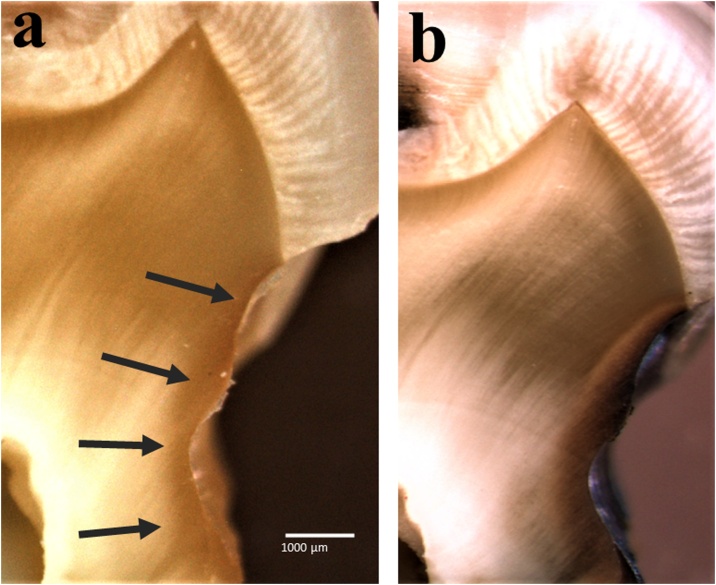
Fig. 1.
Optical microscopic cross-sectional images under net magnification of 4.0× [(5×) × (0.8×)] to simulate the two-visit protocol. (a) Non-stained lesion by the application of SDF-treated lesion, which is shown by the arrows. (b) Simulated appearance at the second visit, which was observed after a week of storage at 100% RH and 36.7 °C. Note: The stained lesion was not detectable when SDF was applied on the first visit (a), while the SDF-applied lesion became stained on the second visit and was easily detectable (b).
For advanced caries lesions in the dentin (Fig. 2), the two-visit treatment protocol can be supported by the use of caries-detecting dye. In vitro cross-sectional images show a heavily discolored carious layer (Fig. 2a). This superficial, heavily discolored carious layer was removed using a low-speed handpiece or a hand excavator, exposing the underlying stained soft infected dentin. Then, the SDF was applied to the cavity. The heavy stain was not detectable (Fig. 2b), because the severe caries lesion had already been removed. This sample was then stored at 100% RH and 36.7 °C for one week to simulate the condition at the second visit. A week later, SDF unambiguously discriminated caries from a sound lesion, turning the carious tissue into darkly stained (Fig. 2c). This stained tissue was easily detectable at this time. In addition, the application of SDF rendered the dentin lesion harder due to the silver compounds that were generally formed in the dentin lesion until the second visit. These outcomes lowered the risk of excess cavity preparation.
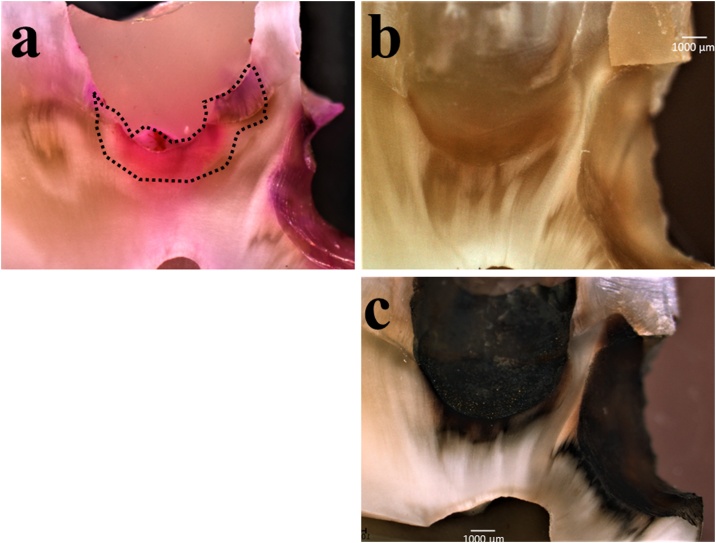
Fig. 2.
Optical microscopic cross-sectional images for advanced caries lesions in the dentin under net magnification of 4.0× [(5×) × (0.8×)] to simulate the two-visit protocol. (a) Cavity preparation using the caries-detecting dye. (b) After the severe caries lesion was removed, as shown in a dotted line in (a), the SDF was applied to the cavity for 1 min (using a micro-brush) and washed for 30 s. The SDF application stains the cavity slightly. This time, a heavy stain was not detectable, because the severe caries lesion had already been removed. (c) The SDF-applied cavity became dark-colored after a week of storage at 100% RH and 36.7 °C to simulate the second visit. This stained tissue was more easily detectable, which prevented the removal of excess tissue.
Fig. 3 shows the clinical steps in this treatment protocol for root caries. On the first visit, SDF was applied to the lesion (Fig. 3a). The lesion exhibited slight discoloration after SDF application (Fig. 3b). On the second visit, the SDF-applied lesion became discolored and turned dark brown, identifying the caries lesion from the sound lesion (Fig. 3c). Cavity preparation was considered complete when this area was hard to a sharp probe and stain-free (Fig. 3d), followed by resin restoration (Fig. 3e). Thus, the two-visit treatment protocol ensures that the conventional tactile and optical criteria are sufficient to assess the caries status during cavity preparation on the second visit. The clinical steps of the two-visit treatment protocol are shown in the flowchart (Fig. 4).
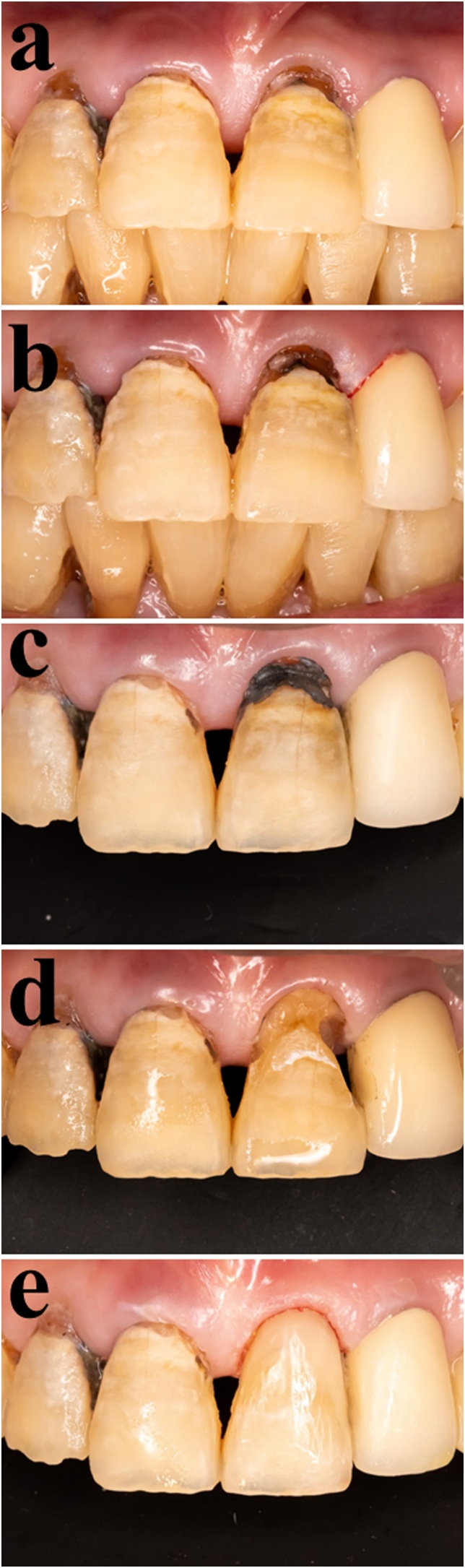
Fig. 3.
Clinical images of two-visit treatment protocol using SDF for root caries. (a) On the first visit, a caries lesion was detected on the root surface. This area was found to be soft when assessed by the conventional tactile method. (b) SDF was applied to the lesion, making the dentin slightly brown-colored. (c) On the second visit, one week later, the SDF-applied lesion turned dark brown, which was easily detectable. Note: Undoubtedly, the stained area became harder than what it was on the first visit. (d) Cavity preparation was carried out and considered complete when this area was hard to a sharp probe and stain-free. (e) The cavity was restored directly with resin composites.
Fig. 4.
Clinical steps of two-visit treatment protocol.
12. Conclusion
The use of non-invasive approaches to control root caries is recommended. Especially, the SDF application to exposed root surfaces is effective for initiation and progression of root caries, although the SDF causes staining/discoloration on dentin tissue. In case of advanced root caries lesion, the cavity should be restored using glass ionomer or resin-based materials. Silver compound is formed in the dentin tissue after application, which has the effect of eliminating the activity and survival of pathogenic bacteria and hardening the dentin tissue of the affected areas. The two-visit treatment protocol is proposed to promote the beneficial properties of SDF, such as disinfection and remineralization. More importantly, staining and discoloration, which are drawbacks of SDF, are also effectively taken into account in this protocol. On the first visit, SDF is applied to a root caries lesion, making the dentin slightly brown-colored. On the second visit, the SDF-applied lesion turns darker in color and harder than on the first visit, which facilitates cavity preparation followed by resin composite restorations.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was partially supported by the Japan Society for the Promotion of Sciences (21K09924).
References
- 1.Tan H., Peres K.G., Peres M.A. Retention of teeth and oral health-related quality of life. J Dent Res. 2016;95(12):1350–1357. doi: 10.1177/0022034516657992. [DOI] [PubMed] [Google Scholar]
- 2.Hayes M., Burke F., Allen P.F. Root caries: from prevalence to therapy. Karger Publishers; 2017. Etiology, risk factors and groups of risk; pp. 9–14. [Google Scholar]
- 3.Hayes M., Burke F., Allen P.F. Root caries: from prevalence to therapy. S. Karger AG; Basel, Switzerland: 2017. Incidence, prevalence and global distribution of root caries; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 4.Featherstone J. Dental caries: a dynamic disease process. Aust Dent J. 2008;53(3):286–291. doi: 10.1111/j.1834-7819.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 5.Slayton R.L. Clinical decision-making for caries management in children: an update. Pediatr Dent. 2015;37(2):106–110. [PubMed] [Google Scholar]
- 6.Schwendicke F., Gostemeyer G. Cost-effectiveness of root caries preventive treatments. J Dent. 2017;56(January):58–64. doi: 10.1016/j.jdent.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Tsang P., Qi F., Shi W. Medical approach to dental caries: fight the disease, not the lesion. Pediatr Dent. 2006;28(2):188–191. [PubMed] [Google Scholar]
- 8.Horst J.A., Ellenikiotis H., Milgrom P.L. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications and consent. J Calif Dent Assoc. 2016;44(January (1)):16–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Allen F. Pragmatic care for an aging compromised dentition. Aust Dent J. 2019;64:S63–S70. doi: 10.1111/adj.12670. [DOI] [PubMed] [Google Scholar]
- 10.Wierichs R.J., Meyer-Lueckel H. Systematic review on noninvasive treatment of root caries lesions. J Dent Res. 2015;94(2):261–271. doi: 10.1177/0022034514557330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo E., Luo Y., Tan H.P., Dyson J.E., Corbet E.F. ART and conventional root restorations in elders after 12 months. J Dent Res. 2006;85(10):929–932. doi: 10.1177/154405910608501011. [DOI] [PubMed] [Google Scholar]
- 12.Cruz Gonzalez A.C., Marin Zuluaga D.J. Clinical outcome of root caries restorations using ART and rotary techniques in institutionalized elders. Braz Oral Res. 2016;30(1) doi: 10.1590/1807-3107BOR-2016.vol30.0063. [DOI] [PubMed] [Google Scholar]
- 13.AlQranei M.S., Balhaddad A.A., Melo M.A. The burden of root caries: updated perspectives and advances on management strategies. Gerodontology. 2020 doi: 10.1111/ger.12511. [DOI] [PubMed] [Google Scholar]
- 14.Li R., Lo E.C., Liu B.Y., Wong M.C., Chu C.H. Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J Dent. 2016;51(August):15–20. doi: 10.1016/j.jdent.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Tan H.P., Lo E., Dyson J.E., Luo Y., Corbet E.F. A randomized trial on root caries prevention in elders. J Dent Res. 2010;89(10):1086–1090. doi: 10.1177/0022034510375825. [DOI] [PubMed] [Google Scholar]
- 16.Duangthip D., Chu C.H., Lo E. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides—18 month results. J Dent. 2016;44:57–63. doi: 10.1016/j.jdent.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Llodra J.C., Rodriguez A., Ferrer B., Menardia V., Ramos T., Morato M. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84(August (8)):721–724. doi: 10.1177/154405910508400807. [DOI] [PubMed] [Google Scholar]
- 18.Chu C.H., Lo E., Lin H.C. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81(11):767–770. doi: 10.1177/0810767. [DOI] [PubMed] [Google Scholar]
- 19.Greenwall-Cohen J., Greenwall L., Barry S. Silver diamine fluoride-an overview of the literature and current clinical techniques. Br Dent J. 2020;228(11):831–838. doi: 10.1038/s41415-020-1641-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim S., Nassar M., Tamura Y., Hiraishi N., Jamleh A., Nikaido T., et al. The effect of reduced glutathione on the toxicity of silver diamine fluoride in rat pulpal cells. J Appl Oral Sci. 2021;29 doi: 10.1590/1678-7757-2020-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang M., Mei M.L., Wong M.C.M., Chu C.H., Lo E.C.M. Effect of silver diamine fluoride solution application on the bond strength of dentine to adhesives and to glass ionomer cements: a systematic review. BMC Oral Health. 2020;20(1):40. doi: 10.1186/s12903-020-1030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang M., Wong M.C.M., Chu C.H., Dai L., Lo E.C.M. Effects of restoring SDF-treated and untreated dentine caries lesions on parental satisfaction and oral health related quality of life of preschool children. J Dent. 2019;88 doi: 10.1016/j.jdent.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Van Duker M., Hayashi J., Chan D.C., Tagami J., Sadr A. Effect of silver diamine fluoride and potassium iodide on bonding to demineralized dentin. Am J Dent. 2019;32(3):143–146. [PubMed] [Google Scholar]
- 24.Ko A.K., Matsui N., Nakamoto A., Ikeda M., Nikaido T., Burrow M.F., et al. Effect of silver diammine fluoride application on dentin bonding performance. Dent Mater J. 2020:2019–2057. doi: 10.4012/dmj.2019-057. [DOI] [PubMed] [Google Scholar]
- 25.Zhao I.S., Mei M.L., Burrow M.F., Lo E.C., Chu C. Prevention of secondary caries using silver diamine fluoride treatment and casein phosphopeptide-amorphous calcium phosphate modified glass-ionomer cement. J Dent. 2017;57:38–44. doi: 10.1016/j.jdent.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Lueckel H., Machiulskiene V., Giacaman R.A. How to intervene in the root caries process? Systematic review and meta-analyses. Caries Res. 2019;53(6):599–608. doi: 10.1159/000501588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregory D., Hyde S. Root caries in older adults. J Calif Dent Assoc. 2015;43(8):439–445. [PubMed] [Google Scholar]
- 28.Deutsch A. An alternate technique of care using silver fluoride followed by stannous fluoride in the management of root caries in aged care. Spec Care Dent. 2016;36(2):85–92. doi: 10.1111/scd.12153. [DOI] [PubMed] [Google Scholar]
- 29.Stebbins E.A. What value has argenti nitras as a therapeutic agent in dentistry? Int Dent J. 1891;12:661–670. [PubMed] [Google Scholar]
- 30.Greenwood N.N., Earnshaw A. Elsevier; 2012. Chemistry of the elements. [Google Scholar]
- 31.Bovenkamp G.L., Zanzen U., Krishna K.S., Hormes J., Prange A. X-ray absorption near-edge structure (XANES) spectroscopy study of the interaction of silver ions with Staphylococcus aureus, Listeria monocytogenes, and Escherichia coli. Appl Environ Microbiol. 2013;79(October (20)):6385–6390. doi: 10.1128/AEM.01688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenblatt A., Stamford T.C., Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet”. J Dent Res. 2009;88(February (2)):116–125. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T., Nishida M., Sobue S., Moriwaki Y. Effects of diammine silver fluoride on tooth enamel. J Osaka Univ Dent Sch. 1974;14(September):61–72. [PubMed] [Google Scholar]
- 34.Yamaga R. Diamine silver fluoride and its clinical application. J Osaka Univ Dent Sch. 1972;12:1–20. [PubMed] [Google Scholar]
- 35.Mei M.L., Li Q.L., Chu C.H., Yiu C.K., Lo E.C. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent Mater. 2012;28(August (8)):903–908. doi: 10.1016/j.dental.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Chu C.H., Lo E.C. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent. 2008;36(June (6)):387–391. doi: 10.1016/j.jdent.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson K.B., Persson I., Kessler V.G. Coordination chemistry of the solvated AgI and AuI ions in liquid and aqueous ammonia, trialkyl and triphenyl phosphite, and tri-n-butylphosphine solutions. Inorg Chem. 2006;45(17):6912–6921. doi: 10.1021/ic060175v. [DOI] [PubMed] [Google Scholar]
- 38.Soares-Yoshikawa A.L., Cury J.A., Tabchoury C.P.M. Fluoride concentration in SDF commercial products and their bioavailability with demineralized dentine. Braz Dent J. 2020;31(3):257–263. doi: 10.1590/0103-6440202003669. [DOI] [PubMed] [Google Scholar]
- 39.Burgess J.O., Vaghela P.M. Silver diamine fluoride: a successful anticarious solution with limits. Adv Dent Res. 2018;29(1):131–134. doi: 10.1177/0022034517740123. [DOI] [PubMed] [Google Scholar]
- 40.Fung M., Duangthip D., Wong M., Lo E., Chu C.H. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J Dent Res. 2018;97(2):171–178. doi: 10.1177/0022034517728496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayed M., Matsui N., Uo M., Nikaido T., Oikawa M., Burrow M.F., et al. Morphological and elemental analysis of silver penetration into sound/demineralized dentin after SDF application. Dent Mater. 2019;35(September):1718–1727. doi: 10.1016/j.dental.2019.08.111. [DOI] [PubMed] [Google Scholar]
- 42.Mei M.L., Chu C.H., Low K.H., Che C.M., Lo E.C. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 2013;18(November (6)):824. doi: 10.4317/medoral.18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou Y.L., Botelho M.G., Darvell B.W. Reaction of silver diamine [corrected] fluoride with hydroxyapatite and protein. J Dent. 2011;39(September (9)):612–618. doi: 10.1016/j.jdent.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Hou W.C., Stuart B., Howes R., Zepp R.G. Sunlight-driven reduction of silver ions by natural organic matter: formation and transformation of silver nanoparticles. Environ Sci Technol. 2013;47(July (14)):7713–7721. doi: 10.1021/es400802w. [DOI] [PubMed] [Google Scholar]
- 45.Sayed M., Matsui N., Hiraishi N., Nikaido T., Burrow M.F., Tagami J. Effect of glutathione bio-molecule on tooth discoloration associated with silver diammine fluoride. Int J Mol Sci. 2018;19(April (5)):1322–1334. doi: 10.3390/ijms19051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W., Liu Y., Courtney H.S., Bettenga M., Agrawal C.M., Bumgardner J.D., et al. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials. 2006;27(November (32)):5512–5517. doi: 10.1016/j.biomaterials.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Singh B., Dubey A.K., Kumar S., Saha N., Basu B., Gupta R. In vitro biocompatibility and antimicrobial activity of wet chemically prepared Ca10−xAgx(PO4)6(OH)2 (0.0≤x≤0.5) hydroxyapatites. Mater Sci Eng C. 2011;31(October (7)):1320–1329. [Google Scholar]
- 48.Ling Feng Q., Nam Kim T., Wu J., Seo Park E., Ock Kim J., Young Lim D., et al. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Films. 1998;335(November (1–2)):214–219. [Google Scholar]
- 49.Mei M.L., Ito L., Cao Y., Lo E.C., Li Q.L., Chu C.H. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J Dent. 2014;42(April (4)):395–402. doi: 10.1016/j.jdent.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Marx D.E., Barillo D.J. Silver in medicine: the basic science. Burns. 2014;40:S9–S18. doi: 10.1016/j.burns.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Russell A.D., Hugo W.B. Progress in medicinal chemistry. Elsevier; 1994. 7 antimicrobial activity and action of silver; pp. 351–370. [DOI] [PubMed] [Google Scholar]
- 52.Lansdown A. Silver I: its antibacterial properties and mechanism of action. J Wound Care. 2002;11(4):125–130. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 53.Øgaard B., Seppä L., Rolla G. Professional topical fluoride applications—clinical efficacy and mechanism of action. Adv Dent Res. 1994;8(2):190–201. doi: 10.1177/08959374940080021001. [DOI] [PubMed] [Google Scholar]
- 54.Rošin-Grget K., Peroš K., Šutej I. The cariostatic mechanisms of fluoride. Acta Med Acad. 2013;42(2):179. doi: 10.5644/ama2006-124.85. [DOI] [PubMed] [Google Scholar]
- 55.Koo H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res. 2008;20(1):17–21. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]
- 56.Sayed M., Hiraishi N., Matin K., Abdou A., Burrow M.F., Tagami J. Effect of silver-containing agents on the ultra-structural morphology of dentinal collagen. Dent Mater. 2020;36(7):936–944. doi: 10.1016/j.dental.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 57.Savas S., Kucukyilmaz E., Celik E.U., Ates M. Effects of different antibacterial agents on enamel in a biofilm caries model. J Oral Sci. 2015;57(4):367–372. doi: 10.2334/josnusd.57.367. [DOI] [PubMed] [Google Scholar]
- 58.Chu C.H., Mei L., Seneviratne C.J., Lo E.C. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22(January (1)):2–10. doi: 10.1111/j.1365-263X.2011.01149.x. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi M., Matin K., Matsui N., Shimizu M., Tsuda Y., Uchinuma S., et al. Effects of silver diamine fluoride preparations on biofilm formation of Streptococcus mutans. Dent Mater J. 2021 doi: 10.4012/dmj.2020-341. [DOI] [PubMed] [Google Scholar]
- 60.Mei M.L., Li Q., Chu C., Lo E.C., Samaranayake L.P. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 2013;12(February (1)):4. doi: 10.1186/1476-0711-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimizu M., Matsui N., Sayed M., Hamba H., Obayashi S., Takahashi M., et al. Micro-CT assessment of the effect of silver diammine fluoride on inhibition of root dentin demineralization. Dent Mater J. 2021:2020–2290. doi: 10.4012/dmj.2020-290. [DOI] [PubMed] [Google Scholar]
- 62.Klein U., Kanellis M.J., Drake D. Effects of four anticaries agents on lesion depth progression in an in vitro caries model. Pediatr Dent. 1999;21(3):176–180. [PubMed] [Google Scholar]
- 63.Rosas S.G.P., Téllez M.ÁA., Espinoza E.V. In vitro efficiency of fluoride-containing compounds on remineralization of carious enamel lesions under cyclic pH conditions. Rev Odontol Mex. 2014;18(2):96–104. [Google Scholar]
- 64.Knight G.M., McIntyre J.M., Craig G.G., Mulyani Zilm P.S., Gully N.J. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int. 2009;40(February (2)):155–161. [PubMed] [Google Scholar]
- 65.Thanatvarakorn O., Islam M.S., Nakashima S., Sadr A., Nikaido T., Tagami J. Effects of zinc fluoride on inhibiting dentin demineralization and collagen degradation in vitro: a comparison of various topical fluoride agents. Dent Mater J. 2016;35(October (5)):769–775. doi: 10.4012/dmj.2015-388. [DOI] [PubMed] [Google Scholar]
- 66.Ainoosah S.E., Levon J., Eckert G.J., Hara A.T., Lippert F. Effect of silver diamine fluoride on the prevention of erosive tooth wear in vitro. J Dent. 2020;103 doi: 10.1016/j.jjodo.2020.100015. [DOI] [PubMed] [Google Scholar]
- 67.Sayed M., Tsuda Y., Matin K., Abdou A., Martin K., Burrow M.F., et al. Effects of mechanical abrasion challenge on sound and demineralized dentin surfaces treated with SDF. Sci Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-77035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tjäderhane L., Carrilho M.R., Breschi L., Tay F.R., Pashley D.H. Dentin basic structure and composition—an overview. Endod Topics. 2009;20(1):3–29. [Google Scholar]
- 69.Altinci P., Mutluay M., Tjäderhane L., Tezvergil-Mutluay A. Inhibition of dentin matrix-bound cysteine cathepsins by potassium fluoride. Eur J Oral Sci. 2019;127(1):1–9. doi: 10.1111/eos.12581. [DOI] [PubMed] [Google Scholar]
- 70.Yeung S.S., Argáez C. Canadian Agency for Drugs and Technologies in Health; 2017. Summary of evidence. Silver diamine fluoride for the prevention and arresting of dental caries or hypersensitivity: a review of clinical effectiveness, cost-effectiveness and guidelines [Internet] [PubMed] [Google Scholar]
- 71.Zhi Q.H., Lo E.C., Kwok A.C. An in vitro study of silver and fluoride ions on remineralization of demineralized enamel and dentine. Aust Dent J. 2013;58(March (1)):50–56. doi: 10.1111/adj.12033. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W., McGrath C., Lo E., Li J.Y. Silver diamine fluoride and education to prevent and arrest root caries among community-dwelling elders. Caries Res. 2013;47(4):284–290. doi: 10.1159/000346620. [DOI] [PubMed] [Google Scholar]
- 73.Cai J., Palamara J., Manton D.J., Burrow M.F. Status and progress of treatment methods for root caries in the last decade: a literature review. Aust Dent J. 2018;63(1):34–54. doi: 10.1111/adj.12550. [DOI] [PubMed] [Google Scholar]
- 74.Kumar Araveti S., Hiraishi N., Kominami N., Otsuki M., Sumi Y., Yiu C.K., et al. Swept-source optical coherence tomographic observation on prevalence and variations of cemento-enamel junction morphology. Lasers Med Sci. 2020;35(1):213–219. doi: 10.1007/s10103-019-02847-9. [DOI] [PubMed] [Google Scholar]
- 75.Driessens F. Mineral aspects of dentistry. Monogr Oral Sci. 1982;10 [PubMed] [Google Scholar]
- 76.Hoppenbrouwers P., Driessens F., Borggreven J. The mineral solubility of human tooth roots. Arch Oral Biol. 1987;32(5):319–322. doi: 10.1016/0003-9969(87)90085-9. [DOI] [PubMed] [Google Scholar]
- 77.Uemura R., Miura J., Ishimoto T., Yagi K., Matsuda Y., Shimizu M., et al. UVA-activated riboflavin promotes collagen crosslinking to prevent root caries. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-018-38137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allen P.F., Da Mata C., Hayes M. Minimal intervention dentistry for partially dentate older adults. Gerodontology. 2019;36(2):92–98. doi: 10.1111/ger.12389. [DOI] [PubMed] [Google Scholar]
- 79.Akimoto N., Takamizu M., Momoi Y. 10-year clinical evaluation of a self-etching adhesive system. Oper Dent. 2007;32(1):3–10. doi: 10.2341/06-46. [DOI] [PubMed] [Google Scholar]
- 80.Hu J.Y., Chen X.C., Li Y.Q., Smales R.J., Yip K.H. Radiation-induced root surface caries restored with glass-ionomer cement placed in conventional and ART cavity preparations: results at two years. Aust Dent J. 2005;50(September (3)):186–190. doi: 10.1111/j.1834-7819.2005.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 81.Gil-Montoya J., Gil-Montoya J., Mateos-Palacios R., Mateos-Palacios R., Bravo M., Bravo M., et al. Atraumatic restorative treatment and Carisolv use for root caries in the elderly: 2-year follow-up randomized clinical trial. Clin Oral Invest. 2014;18(May (4)):1089–1095. doi: 10.1007/s00784-013-1087-z. [DOI] [PubMed] [Google Scholar]
- 82.Panahpour Eslami N., Chan D.C.N., Sadr A. Effect of silver diammine fluoride and glass ionomer on remineralisation of natural dentine caries. J Dent. 2021;106(March):103578. doi: 10.1016/j.jdent.2020.103578. [DOI] [PubMed] [Google Scholar]
- 83.François P., Greenwall-Cohen J., Goff S.L., Ruscassier N., Attal J., Dursun E. Shear bond strength and interfacial analysis of high-viscosity glass ionomer cement bonded to dentin with protocols including silver diammine fluoride. J Oral Sci. 2020;62(4):444–448. doi: 10.2334/josnusd.20-0065. [DOI] [PubMed] [Google Scholar]
- 84.Puwanawiroj A., Trairatvorakul C., Dasanayake A.P., Auychai P. Microtensile bond strength between glass ionomer cement and silver diamine fluoride-treated carious primary dentin. Pediatr Dent. 2018;40(July (4)):291–295. [PubMed] [Google Scholar]
- 85.Fröhlich T.T., Rocha R.d.O., Botton G. Does previous application of silver diammine fluoride influence the bond strength of glass ionomer cement and adhesive systems to dentin? Systematic review and meta-analysis. Int J Paediatr Dent. 2020;30(January (1)):85–95. doi: 10.1111/ipd.12571. [DOI] [PubMed] [Google Scholar]
- 86.Seto J., Horst J.A., Parkinson D.Y., Frachella J.C., Derisi J.L. Enhanced tooth structure via silver microwires following treatment with 38 percent wilver diamine fluoride. Pediatr Dent. 2020;42(May):226–231. [PMC free article] [PubMed] [Google Scholar]
- 87.Soeno K., Taira Y., Matsumura H., Atsuta M. Effect of desensitizers on bond strength of adhesive luting agents to dentin. J Oral Rehabil. 2001;28(December (12)):1122–1128. doi: 10.1046/j.1365-2842.2001.00756.x. [DOI] [PubMed] [Google Scholar]
- 88.Knight G., McIntyre J., Mulyani The effect of silver fluoride and potassium iodide on the bond strength of auto cure glass ionomer cement to dentine. Aust Dent J. 2006;51(March (1)):42–45. doi: 10.1111/j.1834-7819.2006.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 89.Ko A.K., Matsui N., Nakamoto A., Ikeda M., Nikaido T., Burrow M.F., et al. Effect of silver diammine fluoride application on dentin bonding performance. Dent Mater J. 2020:2019–2057. doi: 10.4012/dmj.2019-057. [DOI] [PubMed] [Google Scholar]
- 90.Selvaraj K., Sampath V., Sujatha V., Mahalaxmi S. Evaluation of microshear bond strength and nanoleakage of etch-and-rinse and self-etch adhesives to dentin pretreated with silver diamine fluoride/potassium iodide: an in vitro study. Indian J Dent Res. 2016;27(October (4)):421–425. doi: 10.4103/0970-9290.191893. [DOI] [PubMed] [Google Scholar]
- 91.Wu D.I., Velamakanni S., Denisson J., Yaman P., Boynton J.R., Papagerakis P. Effect of silver diamine fluoride (SDF) application on microtensile bonding strength of dentin in primary teeth. Pediatr Dent. 2016;38(March (2)):148–153. [PubMed] [Google Scholar]
- 92.Sayed M., Matsui N., Hiraishi N., Inoue G., Nikaido T., Burrow M.F., et al. Evaluation of discoloration of sound/demineralized root dentin with silver diamine fluoride: in-vitro study. Dent Mater J. 2018;38(November):143–149. doi: 10.4012/dmj.2018-008. [DOI] [PubMed] [Google Scholar]
- 93.Sayed M., Nikaido T., Abdou A., Burrow M.F., Tagami J. Potential use of silver diammine fluoride in detection of carious dentin. Dent Mater J. 2021 doi: 10.4012/dmj.2020-308. [DOI] [PubMed] [Google Scholar]
- 94.Knight G.M., McIntyre J.M., Craig G.G., Mulyani, Zilm P.S., Gully N.J. Differences between normal and demineralized dentine pretreated with silver fluoride and potassium iodide after an in vitro challenge by Streptococcus mutans. Aust Dent J. 2007;52(March (1)):16–21. doi: 10.1111/j.1834-7819.2007.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 95.Li R., Lo E., Liu B.Y., Wong M., Chu C.H. Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J Dent. 2016;51:15–20. doi: 10.1016/j.jdent.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Schwass D.R., Lyons K.M., Love R., Tompkins G.R., Meledandri C.J. Antimicrobial activity of a colloidal AgNP suspension demonstrated in vitro against monoculture biofilms: toward a novel tooth disinfectant for treating dental caries. Adv Dent Res. 2018;29(February (1)):117–123. doi: 10.1177/0022034517736495. [DOI] [PubMed] [Google Scholar]
- 97.Gotjamanos T. Safety issues related to the use of silver fluoride in paediatric dentistry. Aust Dent J. 1997;42(June (3)):166–168. doi: 10.1111/j.1834-7819.1997.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 98.Gotjamanos T., Orton V. Fluorideion concentration in 40 per cent silver fluoride solutions determined by ion selective electrode and ion chromatography techniques. Aust Dent J. 1998;43(February (1)):55–56. doi: 10.1111/j.1834-7819.1998.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 99.Rossi G., Squassi A., Mandalunis P., Kaplan A. Effect of silver diamine fluoride (SDF) on the dentin-pulp complex: ex vivo histological analysis on human primary teeth and rat molars. Acta Odontol Latinoam. 2017;30(April (1)):5–12. [PubMed] [Google Scholar]
- 100.Mei M.L., Lo E.C., Chu C. Clinical use of silver diamine fluoride in dental treatment. Compend Contin Educ Dent (Jamesburg, N.J.: 1995) 2016;37(February (2)):93–98. quiz100. [PubMed] [Google Scholar]
- 101.McComb D. Caries-detector dyes—how accurate and useful are they? J Can Dent Assoc. 2000;66(April (4)):195–198. [PubMed] [Google Scholar]
- 102.Boston D.W., Graver H.T. Histobacteriological analysis of acid red dye-stainable dentin found beneath intact amalgam restorations. Oper Dent. 1994;19(March (2)):65–69. [PubMed] [Google Scholar]