Abstract
Allergic and related diseases have a substantial epidemiological impact on the pediatric population. Small molecule-based medicines have been traditionally used to manage the diseases. Omalizumab is the first monoclonal antibody-based medicine used in children's allergy and shows great promises. It binds to free IgE and prevents it from binding to IgE receptors, thus interrupting the IgE-dependent allergic inflammatory cascade. Vast amounts of data demonstrate its effectiveness and well tolerance by patients, including the children. However, the drug was only approved to use in allergic asthma and chronic spontaneous urticaria (CSU), though other applications were explored in clinical trials. In this review, we summarized current pediatric applications of omalizumab in allergic diseases, focusing on its usages beyond asthma and CSU, including allergic rhinitis, allergic bronchopulmonary aspergillosis, vernal keratoconjunctivitis, food allergy and atopic dermatitis. In addition, we highlighted the unmet needs and controversial issues of anti-IgE therapy.
Keywords: Allergy treatment, IgE, Pediatrics, Omalizumab
Highlights
-
•
Omalizumab, the first monoclonal antibody-based medicine used in children's allergy, shows great promise.
-
•
Omalizumab is effective in relieving symptoms associated with almost every children's allergic and related diseases beyond asthma and CSU.
-
•
There are unmet needs and controversial issues of anti-IgE therapy in allergic and related diseases.
Introduction
Epidemiological data show a significant increase in the prevalence of childhood allergic and related diseases, such as allergic asthma, chronic urticaria (CU), atopic dermatitis (AD), allergic rhinitis (AR), food allergy, and so on. Prolonged and recurring symptoms not only seriously affect the physical and mental development of children, but also bring an enormous burden to the public medical system in various countries. Immunoglobulin E (IgE) plays a crucial role in mediating allergic reactions. Omalizumab as an IgE-targeting drug opens up a new era of personalized, precise medicine management. Through blocking allergic reactions at their sources, it controls these allergic diseases very effectively. However, different from the nearly 2 decades of successful usage in adult, the data in children are still very limited. This paper will review the efficiency, safety, and unmet need of Omalizumab usage in allergic diseases of the pediatric population.
Mechanisms of IgE-mediated allergy
IgE consists of pairs of heavy and light chains (Fig. 1). Each chain contains 1 variable region and 1 or more constant regions. The variable regions together form the antigenic binding sites. Conformational changes occur upon the binding of IgE to either Fc epsilon RI (FcεRI, high affinity receptor) which is mainly expressed by mast cells, basophils, eosinophils, and antigen presenting cells (APCs), or Fc epsilon RII (FcεRII, low affinity receptor) which is expressed by B cells and other hematopoietic cells. B cells also express membrane-bound IgE (mIgE), which assists in antigen processing and signal transduction.
Fig. 1.
Overview of roles played by IgE in allergic diseases. IgE plays an important role in allergic responses. (Left) Exposures to allergens can result in the binding of IgE to DC and promoting Th2 cell differentiation. Th2 cells release a range of pro-inflammatory mediators such as IL-4 and IL-13, that promote B cell transfer into plasma cells and secrete IgE. In addition, Th2 cell activation further contributes to infiltration and activation of eosinophils, basophils, neutrophils, macrophages and so on. (Right) Upon binding with IgE, mast cells and basophils rapidly release inflammatory mediators, leading to smooth muscle contraction, extracellular matrix accumulation, vasodilation coma mucosal gland secretion, and increasing in vascular permeability and sensory nerve endings. Smooth muscle contraction, activation of eosinophils, vasodilation coma mucosal gland secretion, and increasing in vascular permeability contribute to airway allergic disease. Activation of eosinophils, vasodilation coma mucosal gland secretion, increasing in vascular permeability and sensory nerve endings contribute to disease associated with skin and mucous membranes. And all these factors contribute to food allergy. ABPA, allergic bronchopulmonary aspergillosis; AD, atopic dermatitis; AR, allergic rhinitis; CSU, chronic spontaneous urticaria; DC, dendritic cell; FcεRI, high affinity receptor; FcεRII, low affinity receptor; IL, interleukin; Th, T helpercell; Treg, regulatory T cell; VKC, vernal keratoconjunctivitis
Mast cells and basophils are sensitized by IgE binding. The sensitized mast cells and basophils with pre-bound IgE only require the presence of an allergen to cross-link IgE/FcεRI complexes and to provoke a rapid reaction that involves mast-cell degranulation and releases in inflammatory mediators, such as histamine, tryptase, prostaglandin, and leukotriene. Subsequently, cytokines and chemokines are produced following the aggregation of FcεRI that elicit the recruitment and activation of inflammatory cells at sites sensitive to the allergen. IgE can also bind to dendritic cell (DC) FcεRI and promotes T helper 2 (Th2) cell differentiation, therefore amplifying the cascade reaction.1,2 This process is referred to as the late allergic response. Therefore, IgE is an attractive target for therapeutic intervention of the allergic diseases (Fig. 1).
Mechanism of omalizumab in the treatment of Allergy
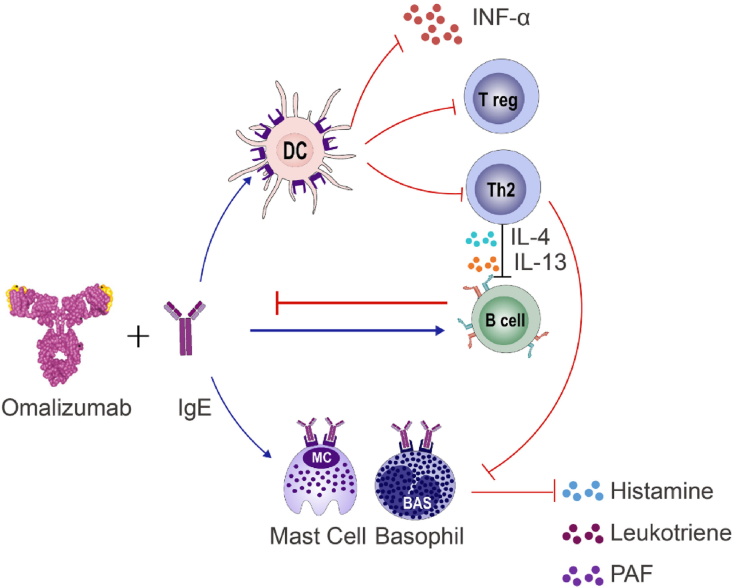
Omalizumab is a medicine based on monoclonal anti-human IgE antibody. In 2003, it was first approved and marketed for asthma patients with ages of 12 years and above. Later in 2009, the permission was extended for its usage in children aged 6 to12 years old with severe persistent asthma. In 2013, it was recommended as add-on treatment in adults and adolescents (≥12 years) with CU. In China, however, its general usage was approved in 2017, and its extended usage for children older than 6 years old was approved by 2018. The bioavailability of subcutaneous omalizumab is 62%, and the serum concentration generally reaches a peak level after 7–8 days with a half-life of 26 days.3 Omalizumab can act on a variety of cells through multiple pathways and mechanisms to block allergic reaction, quickly and effectively controlling allergic symptoms. Putative mechanisms of action of Omalizumab are the following (Fig. 2):
Fig. 2.
Mechanism of action of omalizumab in treatment of pediatric allergic and related diseases. Omalizumab is very effective in treating pediatric allergic diseases. By binding IgE it can block various allergic responses, including decreasing mast cell activation and sensitivity, reducing eosinophil infiltration and activation, leading to a down regulation of inflammatory mediators release. Furthermore, omalizumab improves smooth muscle contraction and proliferation, decreases vasodilation and vascular permeability, reduces mucosal gland secretion and so on. DC, dendritic cell; IFN, interferon; IL, interleukin; PAF, platelet activating factor; Th, T helper cell; Treg, regulatory T cell
Binding to free IgE
Omalizumab can markedly reduce the level of circulating IgE, preventing free IgE from binding to its receptors, FcεRI or FcεRII.4 Within 1 h of initial administration, serum free IgE levels decreased in a dose-dependent manner. At the recommended doses, free IgE levels can be reduced by more than 96%,5 thereby preventing the effect of IgE in the allergic cascade.
Downregulating the number and function of FcεRI
Unbound FcεRI being degraded when they are not stabilized by binding to IgE, leads to rapid reductions of FcεRI in mast cells, basophils, and DCs.6,7 Effects on these cascades can decrease the allergen presentation to T cells and limit the Th2-mediated inflammatory molecular pathway. The significance of this mechanism is indicated by the fact that after giving Omalizumab for 7 days, FcεRI fell by 88%, which may continue to fall by more than 99% if the treatment is extended longer.6,8
Enhancing antiviral immune effects
Recent studies demonstrated that omalizumab can ameliorate the inadequate antiviral response. It can enhance interferon-α (IFN-α) responses in the context of IgE receptor cross-linking, limit cell-to-cell transmission of respiratory viruses, and diminish the severity and duration of infection, viral shedding and the associated level of virus-related illnesses. Toll-like receptor 7 (TLR-7) is an innate signaling receptor that can respond to viruses. It is primarily expressed by plasmacytoid dendritic cells (pDCs), and other DC subsets and myelomonocytic cells, T cells and B cells. A counter-regulatory mechanism was revealed between FcεRI and TLR-7. Activation of TLR-7 or a component of the TLR-7 pathway may inhibit the expression of FcεRI. On the other hand, activation of FcεRI may result in reduced TLR-7-related recognition of, or response to, virus.9, 10, 11
Other possible mechanisms
1) Compound of Omalizumab and IgE can competitively capture free antigens, acting as scavengers for allergens entering the body, thus preventing allergen-mediated mast cell activation;12,13 2) Omalizumab acts on the 2 cross-linked antigen sites of the IgE on the surface of B cells, causing B cells to enter an “inactive” state, affecting transformation to plasma cells and eventually inhibiting synthesis of IgE;14 3) IgE binds to FcεRI on the surface of airway smooth muscle cells, which increases the production of extracellular matrix collagen, thus contributing to the remodeling process. Omalizumab may potentially modify diseases by improving bronchial remodeling;15 4) Regulatory T (Treg) cell inhibits allergic inflammation, while Omalizumab reduces IgE-FcεRI cross-link in pDCs and recoveries generation and function of Treg cell (Fig. 2).16
Application of omalizumab in children’s allergic and related diseases
Allergic asthma
Abnormal activation of immune pathways is the underlying mechanism for airway inflammation. Three major endotypes of airway inflammation (type 2 [Th2], neutrophilic, and paucigranulocytic) have been observed in severe asthma. Th2 inflammation relating to elevated serum IgE and eosinophils is the most prevalent type in children. Omalizumab is recommended as an add-on treatment for children (age≥6 years) with uncontrollable moderate-to-severe asthma. Adequate control of the symptoms after stage 3 criteria by long-acting beta2 agonist (LABA) plus inhaled corticosteroids (ICSs) defines moderate asthma. Symptoms that need higher doses of ICSs and LABA, and other drugs in order to have a complete control or are still uncontrollable by stage 4–5 criteria, are defined as severe asthma.17
Current evidences have demonstrated the efficacy and safety of Omalizumab for asthmatic patients. It is effective in improving asthma symptoms and quality of life, decreasing exacerbations, hospitalizations for acute asthma attacks, with reduced or stopped ICS use. Dosage and frequency of administrations are guided by a nomogram that is based on weight and total serum baseline IgE levels, followed by subcutaneous injections every 2 or 4 weeks. The optimal duration of omalizumab treatment, however, is still unclarified. In consideration of compliance and cost, indefinite treatment is unrealistic. Furthermore, IgE generation decreases over time, indefinite use may not be required.18 Nopp et al19 found that most patients still had a mild, controlled, and stable asthma 3 years after omalizumab treatment. However, other scholars believe that continuous anti-IgE treatment acquires a better control of asthma than those who stopped for 1 year.20
Besides improving symptoms of asthma, omalizumab can also abrogate lung function decreases in theory. Indeed, some clinical trials found that forced expiratory volume in 1-s (FEV1) increased in these who achieved good response to anti-IgE therapy.21,22 However, a meta-analysis including 6 randomized controlled trials (RCT) found only 1 research showing significant FEV1 improvement compared with the control group.23 A two-year follow-up study of 19 children found no significant improvement in FEV1 after any of the 3 period (16, 52, or 104 weeks) usage of omalizumab.24 The disagreements in these observations deserve further study. Factors, such as sample numbers, age of children and poor coordination may all contribute to it.
Enhanced antiviral immunity is a new insight into the benefits of omalizumab. A seasonal peak in asthma exacerbation rates has been consistently observed in the autumn months when children return to school. This has been attributed to both increased allergen and respiratory virus exposure.25 As this autumn epidemic is predictable, and the reason is somewhat understood, it might be preventable. In the Preventive Omalizumab or Step-up therapy for fall Exacerbations (PROSE) study, 513 children with mild/severe asthma were enrolled. Omalizumab was given by injection regularly over 4–6 weeks before returning to school. The result was delightful. Compared to 21% of placebo group, only 11.3% of the omalizumab group had an asthma attack during the first 90 days. Omalizumab improved IFN-α responses to rhinovirus, and within the omalizumab group, greater IFN-α increases were associated with fewer exacerbations. There was no significant difference between omalizumab and ICS boost (8.4% vs 11.1%). In contrast, among participants who experienced an exacerbation during the run-in phase, the addition of omalizumab was more efficacious than stepping up ICS doses to prevent exacerbation later on.9 This suggests that the benefit of omalizumab was more pronounced in children who met the definition of severe asthma. No study provided evidence that this strategy is associated with increased adverse effects other than injection site pain, but it is costly. Even so, there are plenty of questions to be answered, such as how to judge the effectiveness on asthma control, quality of life, asthma-related death, baseline asthma severity, and exacerbation history definitions of exacerbations and standards where possible; The duration of treatment, and the way the treatment ends, with a gradual decrease or a sudden stop, need further study. Furthermore, during the coronavirus pandemic, it is worth noting that there is no evidence indicating that biological therapies in asthma patients should be stopped.26 Omalizumab might even have protective effects over SARS-CoV-2.27 FDA has approved omalizumab for short-term home administration during the COVID-19 pandemic.
Omalizumab may also be considered as a disease-modifying drug in selected children, intervening in the natural history of asthma.28 Putative reasons are the following: 1) Omalizumab disrupts a key element of the allergic inflammatory cascade, so to improve the management of asthma and reduce the development of multiple allergic comorbidities. 2) Airway remodeling is the chronic outcome of inflammation in asthma and a point of intervention between pediatric and adult ages.29 IgE acts on the inflammation and smooth muscle components of the airway, which plays a role in remodeling. It is conceivable that anti-IgE therapy may have the potential to attenuate or reverse remodeling, which may help the patient to escape from the chronicity of asthma. Airway remodeling is a new concern, but there are not a lot of data about it. A Preventing Asthma in High-Risk Kids study is designed to examine if 2 years of omalizumab treatment can reduce the risk of progression to persistent asthma, asthma severity, the numbers of new allergic sensitization, and wheezing episodes in high-risk 2-3-year-olds. This study is still in the phase of actively recruiting and the estimated completion date is around November 27, 2025. 3) Omalizumab add-on contributes to children who can not tolerate specific immunotherapy (SIT), which is considered to be the only potentially causal therapy for allergy diseases. Of note, further relevant studies are needed to confirm the benefits of its early application in pediatric asthma management.
Children who normally do not tolerate SIT treatment are expected to have a successful SIT application after anti-IgE therapy, with significantly lower systemic reaction rates.30,31 Result of a multicenter, randomized double-blind controlled study (n = 248) showed that for patients with moderately persistent allergic asthma that were not adequately controlled, less systemic allergic reactions (13.5%) were observed in omalizumab treated group than those received placebo plus SIT (26.2%). Also, more patients (87.3%) achieved the target maintenance dose than the control group (72.1%).32 Guidelines for discontinuation are not clear at the moment so procedures such as gradual reduction or extension of injection intervals deserve to be further explored.
Effectiveness evaluation is usually carried out at least 16 weeks after the treatment. Global evaluation of treatment effectiveness (GETE) score evaluates multiple aspects to determine the response to omalizumab treatment based on patient interviews, severity of symptoms, usage of medication, and spirometry examinations.33 When GETE score is defined as an excellent or good response, it is suggested to continue to use omalizumab. Moderate responders or these that can not be determined the condition, need to be re-evaluated at 6 months or longer to 12 months. Those who did not respond had their treatment stopped. Recognition of responders to omalizumab is fundamental to improve efficacy, safety, and drug cost-effectiveness. Phenotypic features that predict a favorable clinical response include severe asthma with multiple allergic disease comorbidities. Children with more severe asthma, as measured by: lower lung function (Percent predicted FEV1<90%), previous asthma-related hospitalizations in the past 6 months, 3 or more exacerbations in the previous year, obesity (BMI≥85% of healthy range for age), higher eosinophil counts (≥300 cells/μL) or FENO (≥20 parts per billion) levels, or serum periostin levels (>50 ng/ml), tended to show greater improvement in asthma exacerbation reduction.34,35 Periostin is a downstream molecule of interleukin (IL)-13, a signature of type 2 cytokine. Periostin can be used as a biomarker for 2 aspects in the diagnosis of asthma: type 2 immunity and tissue remodeling. Besides, high levels of total IgE also were suggested as promising predictors of omalizumab efficacy. Tajiri et al35 reported that reduced free serum IgE levels between baseline and 16 or 32 weeks of treatment were found to be associated with reduced exacerbation rate at two years of anti-IgE therapy. While a single measurement may be insufficient, a combination of multiple biomarkers may improve the follow-up results of children treated by omalizumab.
In addition to omalizumab, other drugs, such as lebrikizumab, dupilumab, tezepelumab, mepolizumab, reslizumab, benralizumab, are also used or are studied in both teenagers and adults and could benefit younger children in the near future.36 Nevertheless, a credible conclusion on the clear advantages of omalizumab over other drugs is limited by the relatively little data available. More evidence-based studies are needed to better understand who may benefit more with which drug and with controlled costs.
Chronic spontaneous urticaria
Urticaria is a common disease characterized by recurrent transient and local edema of the skin or mucosa, mostly accompanied by itching. When symptoms last for a period of 6 weeks or longer, they are classified as CU. CU is not frequent in pediatric populations with a prevalence close to 1.8%.37,38 Chronic spontaneous urticaria (CSU) accounts for about 80 to 90% of total CU, resulting from mast-cell activation and subsequent releases of histamine, platelet-activating factor, leukotrienes, and prostaglandins. These factors can further induce vasodilation and increases in vascular permeability. Furthermore, an autoimmune pathogenesis participates in almost half of children with CSU.39 Treatment with second generation H1 antihistamines is the first choice for initial therapy. If a satisfactory improvement does not occur after 2 to 4 weeks or earlier if intolerable symptoms occur with the four-fold normal dosage of antihistamines used, omalizumab should be added.40
Omalizumab was approved by FDA as an add-on treatment for adult and adolescent (≥12 years) CSU. A study spanning a timeframe of 6 years was published in 2020, which included 38 pediatric patients with CU being treated with omalizumab.41 In that study, omalizumab was initiated when children with high disease activity scores (7-day sum of daily Urticaria Activity Scores [UAS7] ≥16) in spite of using four-fold dosage antihistamines. The majority of patients (76.3%) achieved a good or complete response at end of treatment or data lock, whereas 15.8% and 7.9% of patients partially and poorly responded, respectively. The side effects, mainly headache and fatigue, were reported by 12 patients (31.6%). Another study containing 19 children with recalcitrant CSU between ages 6 and 16.9 years found that 16 kids responded to omalizumab. All the responders noted a significant improvement in symptoms within the first days and up to 2 months, although 2 became non-responsive after 6–12 months of application.39 In that study, omalizumab was well-tolerated with no reported side effects, even by the youngest. A series of 13 children (mean age 13, range 4–16y) with cold contact urticaria received off-label prescription of omalizumab after inefficacy or incomplete response, or adverse events with antihistamines. Twelve patients showed clinical improvement and safety was also excellent.42 All of these studies demonstrate high safety and effectiveness of omalizumab therapy in pediatric CU populations, with responding rates similar to the adults.43, 44, 45 In future studies, sufficient number of children with age younger than 12 years should be included to evaluate the efficiency and safety on the younger pediatric populations.
Unlike the dosages used for asthma, a dose of 300 mg for every 4 weeks is recommended for CU, regardless of the patient's serum IgE levels and body weight. In pediatric, doses and intervals are similar to adults. Of note, personalized approach improves clinical benefit in patients with refractory CSU. Initial schedule consisted of 300 mg omalizumab every 4 weeks, with increases to 450 or 600 mg in case of poor treatment response (UAS7>16) after the 6th administration.46 Unfortunately, a largest daily practice cohort study found that the effect did not differ significantly between standard and higher doses.41 Additionally, if satisfactory improvement does not occur after 6 months, or earlier if the symptoms are intolerable after omalizumab has been added, cyclosporine and second-generation H1 antihistamines is recommended.47 But, durations and steps followed the procedures such as dose up-regulation, extension and so on remain unclear and are not discussed by international guidelines.
Allergic rhinitis
Allergic rhinitis (AR) is a disorder of nasal mucus membranes after allergen exposure, strongly co-existing with asthma. Asthma management is affected by AR equally, the intensity and duration of rhinitis is also correlated with the development and remission of asthma. Supporting the “one airway, one disease” hypothesis, both of the symptoms share a common epidemiologic and underlying inflammatory pathophysiology, as well as a common therapeutic route.48 In China, the incidence of pediatric asthma with AR is 35.01% and the incidence of AR with asthma in children is 54.93%.49
Both randomized controlled and observational clinical trials have demonstrated the effectiveness and safety of omalizumab in patients with AR.50, 51, 52, 53 Anti-IgE therapy can improve symptoms, though rescue medications are still needed in patients with AR. In spite of this, it is still used frequently with off-label indication which started almost 2 decades ago. Currently, the benefits of omalizumab in children with AR mostly are expected during the treatment of severe asthma. In a study of 221 children with seasonal allergic rhinitis and total serum IgE levels between 30 and 1300 IU/ml, all of them achieved SIT therapy. The ones treated with omalizumab added had a superior tolerability than those with placebo (0.9% NaCl), demonstrating that omalizumab can significantly enhance efficacy of SIT.54 It points out that omalizumab plus SIT can be beneficial for polysensitized children and adolescents with AR than just with SIT. It is a pity that there are very few trials specifically designed for AR children, particularly for those younger than 12 years old.
Allergic bronchopulmonary aspergillosis
Allergic bronchopulmonary aspergillosis (ABPA) is a severe allergic pulmonary complication characterized by the presence of asthma, recurrent and transient pulmonary, central bronchiectasis, and even pulmonary infiltrates. Global prevalence has been estimated to be as high as 2.5%,55 but very little was seen in children. The underlying pathophysiology is not clearly understood. Repeated inhalation of Aspergillus spores evokes a Th2 type immune response such as increased levels in Aspergillus-specific IgE (>0.35 KAU/L), total IgE levels (>1000 IU/mL), serum precipitins, eosinophilia and proximal bronchiectasis.56 However, these criteria were suggested for adults. There is no pediatric standardized diagnostic criteria.
Corticosteroids and anti-fungal therapy are the mainstay treatment strategies. Omalizumab showed potential benefits for severe ABPA. In 2007, van der Ent et al57 first reported a case about a 12-year-old girl with cystic fibrosis and ABPA in which a dramatic and rapid improvement of respiratory symptoms and lung function was achieved with single dose of omalizumab. Subsequently, uses of omalizumab in ABPA cases have arisen concerns, most of which involved in teenagers and adults. A study in France retrieved 32 patients with cystic fibrosis (11 children and 21 adults) who had received omalizumab for more than 3 months in the context of ABPA. While 5 patients were able to discontinue steroid usages during a followup, 9 patients were capable of reducing their daily doses.58 Up to the present, the youngest child ever reported for omalizumab usage for ABPA is a 4 year-old girl. Because she developed a steroid-induced diabetes, omalizumab was started and oral prednisone was discontinued within 16 weeks.59
Different studies used various dosages ranging from 225 mg to 750 mg according to weight and serum IgE level, from once per week to once per month. The most commonly used dose was 375 mg every two weeks. The ideal treatment duration is still ambiguous. Patients whose serum IgE >1000 IU/ml were more sensitive to anti-IgE treatment than those with IgE<1000IU/ml. The results suggested a greater benefit of omalizumab for patients with higher baseline IgE levels and with subcutaneous administration route.60 Early onset treatment may be particularly favorable since patients with early stage of lung disease seem to benefit the most. Additionally, it is worth noting that the positive culture of Aspergillus is not an unique etiological feature of ABPA; it can also be found in cystic fibrosis children.61
Vernal keratoconjunctivitis
Vernal keratoconjunctivitis (VKC) is a severe allergic ocular disease, commonly co-existing with asthma. Chronic corneo conjunctival and/or limbal inflammation with giant conjunctival tarsal papillaecan result in visual sequelae. The pathogenesis of VKC is still under investigation; immediate and delayed hypersensitivity reactions may play an important role. Conventional therapies including topical antihistamines, mast-cell stabilizers, corticosteroids, and cyclosporin are sometimes insufficient for full symptom control. There were reports showed that omalizumab might be effective in controlling these conventional treatment-resistant cases.
The first VKC case that treated with omalizumab was reported in 2012.62 A 15-year-old girl with VKC had received multiple ophthalmic treatments, immunotherapy, and systemic steroids, but with no clinical response. Then omalizumab was added with doses of 225 mg every 2 weeks. Symptoms of pruritus and photophobia were improved by 6 months, and both papillae and Horner-Trantas dots had disappeared by 2 years. So, the use of ophthalmic drugs was therefore reduced. However, the patient's symptoms recurred, and the papillae reappeared. Interestingly, symptoms remitted upon drug re-initiation.63 Another clinical trial64 showed that the ocular signs and other symptoms of four VKC children (aged 6–11 years) were successfully improved by omalizumab in 6 months. Dosage was defined based on weight and total serum IgE level similar with asthma. Manti S et al65 also reported their successful omalizumab experience with 2 pediatric cases with severe allergic asthma.
To date, omalizumab was shown to be effective in VKC cases in child and adolescent populations, though these findings were mostly from case studies with a very small patient population. Among the studies, only 1 child did not respond to omalizumab and needed oral steroids for his VKC and asthma.66 Interestingly, contrary to the other cases, in this failed case detectable sensitization to any known allergen was not found. Whether the absence of allergy is a risk factor for treatment failure deserves further research. Besides, specific IgE detected in tears may provide a new idea in these negative skin prick test and serum-specific IgE patients. There is an urgent need to start large prospective well-designed trials on the omalizumab use in VKC patients to confirm its effectiveness and to clarify the optimal therapeutic scheme (dose and duration).
Food allergy
Food allergy is a potentially life-threatening health problem that still lacks suitable therapies, apart from strictly dietary allergen avoidance and emergent epinephrine in case of accidental exposures. Most food-induced anaphylaxis involves an IgE-mediated mechanism. Patients with IgE mediated severe food allergy may benefit from anti-IgE therapy. Immunotherapy with allergens is the only treatment that may help the course of allergic diseases.67 However, adverse reactions have been major limitations. In order to improve the safety profile of oral immunotherapy (OIT) schedule and facilitate or maintain its efficacy, omalizumab was recommended as an adjuvant for both adults and children by recent clinical trials.
An observational study evaluated 15 children who were allergic to 37 foods and treated with omalizumab for severe uncontrolled asthma. During the treatment the food allergen threshold increased to 8.6 times of its original value. A total of 70.4% of subjects tolerated the complete challenge dose after 4 months of anti-IgE treatment. Moreover, these foods were reintroduced in daily diet without the needs for any OIT procedures. The quality of life also increased with better asthma controls and reductions in dietary restrictions.68 A case series study involved 5 children ages 7–14 years with severe allergy to cow milk and uncontrolled asthma.69 Milk allergy was diagnosed from the first year of their lives. They all received omalizumab every 4 weeks after failed standard therapies. Four children presented an excellent desensitization, achieving capacity to include milk and other dairy products into their diet. Unfortunately, 2 of them discontinued the procedure after they experienced anaphylaxis again during a viral pharyngitis. It is noted that a 14-year-old boy's milk desensitization failed, although anti-IgE therapy was extended for as long as 48 weeks. However, promising results were also observed in peanut allergic patients who started peanut OIT after an individualized omalizumab treatment.70 All 23 adolescents successfully reached the 2800 mg peanut protein maintenance dose with a median time of 10 weeks. Moderate or systemic allergic reactions were rare while accepting full-dose omalizumab.70 Almost half of the patients (11 of 23) continued with peanut OIT successfully after anti-IgE drug was stopped. In these successful children, median baseline IgE to peanut components and basophil allergen threshold sensitivity tests (CD-sens) were both significantly lower than those in the failed group. Another study that followed the long-term OIT outcomes of 13 children found that 54% patients continued successfully on peanut OIT through 72 months.71
Food sensitization (with or without aeroallergen sensitization) in the first 2 years of life was a stronger predictor of asthma and AR. It supports the role of early life food sensitization in the atopic march and trials to prevent early onset in that may have the potential to reduce the development of allergic airways disease.72 Although there is clear evidence to suggest that omalizumab is effective for food allergy control, there are plenty of questions remaining. For example, what should an optimal duration of treatment be? When should the treatment stop? How should dosage be reduced without sacrificing the effect? What could long-term outcomes be? Baseline IgE and CD-sens Treg cells were both tried as biomarkers to predict the efficiency of omalizumab in multi-food OIT. However, there are no agreed upon answers to these questions so far. Further studies are definitely needed to determine whether the initial benefits provided by omalizumab translate into better, long-term improved care for food allergic patients, including the benefits versus the costs. Finally, it is also worthy to study whether omalizumab can achieve a good response with safety in infants.
Atopic dermatitis
Atopic dermatitis (AD) is a chronic inflammatory dermatoses associated progressively with other allergic conditions such as asthma, rhinitis, and food allergies. Its prevalence is up to 20% in the pediatric population worldwide.73 Impaired skin barrier function and defects in skin innate immunity are the basic characteristics of the disease. Most mild cases can be adequately controlled by topical treatments alone. But severe AD patients who suffer repeated and annoying systemic symptoms, such as itch, pain, and sleep disturbance that could affect quality of life substantially, usually need systemic medical therapies (traditional immunosuppressants, biologics, and small molecule inhibitors).74
Dupilumab (anti-IL-4 and IL-13) is the first FDA-approved biologic therapy for AD, and recently has been approved to be used in children aged 6 to 11 years with moderate to severe AD not adequately controlled with topical therapies. Even though dupilumab is now a major treatment in this indication, there is room for alternatives, especially those with a well-known, very good safety profile as with omalizumab. In 2019, Susan Chan et al75 published a delectable clinical result. The trial enrolled 62 participants of 4 to 19 years old who had severe AD. Patients were treated with subcutaneous omalizumab or placebo for 24 weeks. Dosage was determined by total IgE and body weight. They found omalizumab significantly reduced AD severity and improved quality of life. But other doctors held different views about the trial and believed that it was an overtreatment.76 Compared with the previous studies that yielded negative results, the trial used three-fold doses of omalizumab. Considering the high costs of omalizumab, dupilumab seems a better choice for controlling AD in terms of efficacy and costs.77 In the future, the beneficial management for children with severe AD needs to be carefully evaluated with both the efficacy and costs in mind. With such criteria in mind, the role of omalizumab can not be ignored. Maybe some but not all AD patients, whose characteristics remain to be defined, could benefit from omalizumab.
Safety
A large number of clinical trials demonstrate that omalizumab is well tolerated. The risk of omalizumab-associated anaphylaxis is about 0.1–0.2%, lower than the frequency of other monoclonal antibody-based drugs. A review article covered 3429 children and adults with allergic asthma,78 and a meta-analysis79 involving more than 7500 patients both showed that the risks of severe side-reactions with omalizumab were similar to placebo groups. The main adverse reactions are also similar between adults and children, including pain at the injection site, headache, pharyngitis, upper respiratory tract symptoms, and sinusitis.78,80,81 In pediatric populations, the pain at injection site should probably be more considered than in adults. It should be noted that allergic reactions may occur not only after the first administration, but also during subsequent administrations. In addition, there is no evidence that omalizumab should be stopped during the COVID-19 pandemic.26,82
There are no data to support any link between anti-IgE thrapy and malignancy risk.83 To date, no cases of malignancy have been reported in clinical trials involving omalizumab for children of ages 6 to 12 years old. Anti-drug antibodies against omalizumab were not detected during or after the treatment. With regard to the usage for children younger than 6 years, findings from pregnancy and perinatal studies may provide clues. In order to evaluate perinatal outcomes with omalizumab exposure during pregnancy, a prospective observational study compared 250 women with asthma exposed to omalizumab during pregnancy and 1153 disease-matched cohorts.77 In the omalizumab-treated group, prevalences of major congenital anomalies, premature birth, small for gestational age were identified as 8.1%, 15.0%, 9.7%, respectively, comparable to the values (8.9%, 11.3%, 15.8%) seen in the matched group. Notably, there also was no increased risk of thrombocytopenia in the newborn.84,85 However, considering the fact that the number of safety studies are still scarce, caution is still warranted. Long-term safty evaluation of omalizumab-exposed patients, especially these of younger ages, is urgently needed.
In addition, the high cost of omalizumab must be taken into account. For the base-case analysis, the cost-utility analysis showed that compared with the standard therapy, the addition of omalizumab yielded significantly higher costs and greater gain in quality-adjusted life-years (QALY). Evidence from adults showed conflicting results depending on the country in which the economic analysis was conducted. But in general, currently omalizumab is not a cost-effective strategy for treating children with severe allergic asthma. It is important to select pediatric patients that will benefit most from anti-IgE therapy.
Conclusions
Omalizumab, an IgE antibody-based drug, can block allergic reactions from the source. Since similar pathobiology and host defense mechanisms participate in systemic allergic and immunologic diseases, it is best to find a drug that can address these associated comorbidities at same time. But it should be noted that many problems are far from solved, especially for pediatric patients. Such concerns that require urgent attention include: long-term effectiveness and safety for children younger than 6 year-old, cost-benefit analysis, concerns about relapse after the discontinuation, possibilities for application to other intractable diseases, and so on. Moreover, compared with other biological based medicines, such as anti–IL-5 (mepolizumab, reslizumab, and benralizumab), and anti–IL-4 receptor α (dupilumab) antibodies, does omalizumab have clear advantages? What time and duration should the drug be used to get the most benefits for the pediatric population? Although omalizumab shows great promises for treating children's allergic diseases so far, more evidence and experiences are urgently needed in order to release its full potential for pediatric world.
Abbreviations
ABPA, allergic bronchopulmonary aspergillosis; AD, atopic dermatitis; AR, allergic rhinitis; APCs, eosinophils and antigen presenting cells; CD-sens, basophil allergen threshold sensitivity tests; CSU, chronic spontaneous urticaria; CU, chronic urticaria; DC, dendritic cell; FcεRI, Fc epsilon RI; FcεRII, Fc epsilon RII; FeNO, fractionalexhaled nitric oxide; FEV1, forced expiratory volume in 1-s; GETE, global evaluation of treatment effectiveness; ICSs, inhaled corticosteroids; IFN-α, interferon-α; IgE, Immunoglobulin E; LABA, long-acting beta2 agonist; mIgE, membrane-bound IgE; OIT, oral immunotherapy; pDC, plasmacytoid dendritic cells; PROSE, Preventive Omalizumab or Step-up Therapy for Fall Exacerbations; QALY, quality-adjusted life-years; RCT, randomized controlled trials; SIT, specific immunotherapy; Th2, T helper 2; TLR7, Toll-like receptor 7; Treg, Regulatory T; UAS7, 7-day sum of daily Urticaria Activity Scores; VKC, vernal keratoconjunctivitis.
Authors’ consent for publication
All authors have approved the submission of this manuscript. The results have not been previously published and are not being considered for publication in another journal. The authors declare no conflict of interest.
Author contributions
Lin Yu: Conceptualization, Writing Original draft preparation. Huishan Zhang: Data curation and Investigation, Reviewing. Jianwei Pan: Writing, Drawing figures. Leping Ye: Writing, Reviewing and Editing, Submit manuscript for publication.
Availability of data and materials
This paper is a review, so Availability of data and materials is not applicable.
Ethics approval
This paper is a review, so ethics approval is not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is funded by National Natural Science Foundation of China (NSFC)/number: 81971424 (Leping Ye); Medical Scientific Research Foundation of Zhejiang Province, China/Award number: 2021RC019 (Lin Yu); Science and Technology program of Jinhua Science and Technology Bureau/Award number: 2019-4-132 (Lin Yu).
References
- 1.Blank U., Huang H., Kawakami T. The high affinity IgE receptor: a signaling update. Curr Opin Immunol. 2021;72:51–58. doi: 10.1016/j.coi.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Gasser P., Eggel A. Targeting IgE in allergic disease. Curr Opin Immunol. 2018;54:86–92. doi: 10.1016/j.coi.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Patel T.R., Sur S. IgE and eosinophils as therapeutic targets in asthma. Curr Opin Allergy Clin Immunol. 2017;17(1):42–49. doi: 10.1097/ACI.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami T., Blank U. From IgE to omalizumab. J Immunol. 2016;197(11):4187–4192. doi: 10.4049/jimmunol.1601476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochhaus G., Brookman L., Fox H., et al. Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in thetreatment of allergic asthma. Curr Med Res Opin. 2003;19(6):491–499. doi: 10.1185/030079903125002171. [DOI] [PubMed] [Google Scholar]
- 6.Beck L.A., Marcotte G.V., MacGlashan D., Togias A., Saini S. Omalizumab-induced reductions in mast cell Fce psilon RIexpression and function. J Allergy Clin Immunol. 2004;114(3):527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Prussin C., Griffith D.T., Boesel K.M., et al. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112(6):1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan A.P., Gimenez-Arnau A.M., Saini S.S. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519–533. doi: 10.1111/all.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teach S.J., Gill M.A., Togias A., et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardet J.C., Casale T.B. New insights into the utility of omalizumab. J Allergy Clin Immunol. 2019;143(3):923–926.e1. doi: 10.1016/j.jaci.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill M.A., Bajwa G., George T.A., et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184(11):5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belliveau P.P. Omalizumab: a monoclonal anti-IgE antibody. MedGenMed. 2005;7(1):27. [PMC free article] [PubMed] [Google Scholar]
- 13.Chang T.W. The pharmacological basis of anti-IgE therapy. Nat Biotechnol. 2000;18(2):157–162. doi: 10.1038/72601. [DOI] [PubMed] [Google Scholar]
- 14.Chan M.A., Gigliotti N.M., Dotson A.L., Rosenwasser L.J. Omalizumab may decrease IgE synthesis by targeting membraneIgE+ human B cells. Clin Transl Allergy. 2013;3(1):29. doi: 10.1186/2045-7022-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samitas K., Delimpoura V., Zervas E., Gaga M. Anti-IgE treatment, airway inflammation and remodelling in severe allergic asthma: current knowledge and future perspectives. Eur Respir Rev. 2015;24(138):594–601. doi: 10.1183/16000617.00001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palomares O., Akdis M., Martin-Fontecha M., Akdis C.A. Mechanisms of immune regulation in allergic diseases: the role ofregulatory T and B cells. Immunol Rev. 2017;278(1):219–236. doi: 10.1111/imr.12555. [DOI] [PubMed] [Google Scholar]
- 17.Shrine N., Portelli M.A., John C., et al. Moderate-to-severe asthma in individuals of European ancestry: a genome-wideassociation study. Lancet Respir Med. 2019;7(1):20–34. doi: 10.1016/S2213-2600(18)30389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe P.J., Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPDanalysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72(2):306–320. doi: 10.1111/j.1365-2125.2011.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nopp A., Johansson S.G., Adedoyin J., et al. After 6 years with Xolair; a 3-year withdrawal follow-up. Allergy. 2010;65(1):56–60. doi: 10.1111/j.1398-9995.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 20.Ledford D., Busse W., Trzaskoma B., et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–169.e2. doi: 10.1016/j.jaci.2016.08.054. [DOI] [PubMed] [Google Scholar]
- 21.Paganin F., Mangiapan G., Proust A., et al. Lung function parameters in omalizumab responder patients: an interesting tool? Allergy. 2017;72(12):1953–1961. doi: 10.1111/all.13202. [DOI] [PubMed] [Google Scholar]
- 22.Licari A., Castagnoli R., Denicolo C., et al. Omalizumab in children with severe allergic asthma: the Italian real-life experience. Curr Respir Med Rev. 2017;13(1):36–42. doi: 10.2174/1573398X13666170426094536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai T., Wang S., Xu Z., et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191. doi: 10.1038/srep08191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sztafinska A., Jerzynska J., Stelmach W., Woicka-Kolejwa K., Stelmach I. Quality of life in asthmatic children and their caregivers after two-year treatment with omalizumab, a real-life study. Postepy Dermatol Alergol. 2017;34(5):439–447. doi: 10.5114/ada.2017.71109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston N.W., Johnston S.L., Norman G.R., Dai J., Sears M.R. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117(3):557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Morais-Almeida M., Aguiar R., Martin B., et al. COVID-19, asthma, and biological therapies: what we need to know. World Allergy Organ J. 2020;13(5):100126. doi: 10.1016/j.waojou.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lommatzsch M., Stoll P., Virchow J.C. COVID-19 in a patient with severe asthma treated with Omalizumab. Allergy. 2020;75(10):2705–2708. doi: 10.1111/all.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pajno G.B., Castagnoli R., Arasi S., et al. Pediatric use of omalizumab for allergic asthma. Expet Opin Biol Ther. 2020;20(7):695–703. doi: 10.1080/14712598.2020.1751115. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz O., Yuksel H. Where does current and future pediatric asthma treatment stand? Remodeling and inflammation: Bird's eye view. Pediatr Pulmonol. 2016;51(12):1422–1429. doi: 10.1002/ppul.23488. [DOI] [PubMed] [Google Scholar]
- 30.Stelmach I., Majak P., Jerzynska J., et al. Children with severe asthma can start allergen immunotherapy after controlling asthma with omalizumab: a case series from Poland. Arch Med Sci. 2015;11(4):901–904. doi: 10.5114/aoms.2015.48546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Har D., Lee M.J. Systemic reaction rates with omalizumab, subcutaneous immunotherapy, and combination therapy in children with allergic asthma. Allergy Asthma Proc. 2019;40(1):35–40. doi: 10.2500/aap.2019.40.4173. [DOI] [PubMed] [Google Scholar]
- 32.Massanari M., Nelson H., Casale T., et al. Effect of pretreatment with omalizumab on the tolerability of specificimmunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125(2):383–389. doi: 10.1016/j.jaci.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Bousquet J., Siergiejko Z., Swiebocka E., et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66(5):671–678. doi: 10.1111/j.1398-9995.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 34.Szefler S.J., Casale T.B., Haselkorn T., et al. Treatment benefit with omalizumab in children by indicators of asthma severity. J Allergy Clin Immunol Pract. 2020;8(8):2673–2680.e3. doi: 10.1016/j.jaip.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Tajiri T., Matsumoto H., Gon Y., et al. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy. 2016;71(10):1472–1479. doi: 10.1111/all.12922. [DOI] [PubMed] [Google Scholar]
- 36.Just J., Deschildre A., Lejeune S., Amat F. New perspectives of childhood asthma treatment with biologics. Pediatr Allergy Immunol. 2019;30(2):159–171. doi: 10.1111/pai.13007. [DOI] [PubMed] [Google Scholar]
- 37.Bruske I., Standl M., Weidinger S., et al. Epidemiology of urticaria in infants and young children in Germany--results fromthe German LISAplus and GINIplus Birth Cohort Studies. Pediatr Allergy Immunol. 2014;25(1):36–42. doi: 10.1111/pai.12146. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.J., Ha E.K., Jee H.M., et al. Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy Asthma Immunol Res. 2017;9(3):212–219. doi: 10.4168/aair.2017.9.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ari A., Levy Y., Segal N., et al. Efficacy of omalizumab treatment for pediatric chronic spontaneous urticaria: a multi-center retrospective case series. Pediatr Dermatol. 2020;37(6):1051–1054. doi: 10.1111/pde.14360. [DOI] [PubMed] [Google Scholar]
- 40.Zuberbier T., Abdul L.A., Abuzakouk M., et al. The international EAACI/GA(2)LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2021 Sep 18 doi: 10.1111/all.15090. Online ahead of print. [DOI] [Google Scholar]
- 41.Dekkers C., Alizadeh A.M., de Graaf M., et al. Safety and effectiveness of omalizumab for the treatment of chronic urticaria in pediatric patients. Pediatr Allergy Immunol. 2021;32(4):720–726. doi: 10.1111/pai.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briand C., Tetart F., Soria A., et al. Omalizumab in cold urticaria in children: retrospective case series of 13 patients, review of the literature. Ann Dermatol Venereol. 2021;148(4):269–271. doi: 10.1016/j.annder.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Vadasz Z., Tal Y., Rotem M., et al. Omalizumab for severe chronic spontaneous urticaria: real-life experiences of 280 patients. J Allergy Clin Immunol Pract. 2017;5(6):1743–1745. doi: 10.1016/j.jaip.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 44.Alizadeh A.M., van den Broek F., Rijken F., Knulst A.C., Rockmann H. High-dose omalizumab use in patients with chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2020;8(4):1426–1427.e1. doi: 10.1016/j.jaip.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Song X.T., Chen Y.D., Yu M., et al. Omalizumab in children and adolescents with chronic urticaria: a 16-week real-world study. Allergy. 2021;76(4):1271–1273. doi: 10.1111/all.14686. [DOI] [PubMed] [Google Scholar]
- 46.Niemeyer-van D.K.T., van Maaren M.S., van Doorn M. Personalized omalizumab treatment improves clinical benefit in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;142(6):1992–1994. doi: 10.1016/j.jaci.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 47.Hon K.L., Leung A., Ng W., Loo S.K. Chronic urticaria: an Overview of treatment and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):27–37. doi: 10.2174/1872213X13666190328164931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grossman J. One airway, one disease. Chest. 1997;111(2 Suppl):11S–16S. doi: 10.1378/chest.111.2_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 49.Kou W., Li X., Yao H., Wei P. Meta-analysis of the comorbidity rate of allergic rhinitis and asthma in Chinesechildren. Int J Pediatr Otorhinolaryngol. 2018;107:131–134. doi: 10.1016/j.ijporl.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Tsabouri S., Tseretopoulou X., Priftis K., Ntzani E.E. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2(3):332–340.e1. doi: 10.1016/j.jaip.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Yu C., Wang K., Cui X., et al. Clinical efficacy and safety of omalizumab in the treatment of allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. Am J Rhinol Allergy. 2020;34(2):196–208. doi: 10.1177/1945892419884774. [DOI] [PubMed] [Google Scholar]
- 52.Qiu X., Wang H.T. Safety and efficacy of omalizumab for the treatment of allergic rhinitis:Meta-analysis of randomizedclinical trials. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30(9):694–698. doi: 10.13201/j.issn.1001-1781.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Ma T., Wang H., Wang X. Effectiveness and response predictors of omalizumab in treating patients with seasonal allergic rhinitis: a real-world study. J Asthma Allergy. 2021;14:59–66. doi: 10.2147/JAA.S288952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamin W., Kopp M.V., Erdnuess F., et al. Safety of anti-IgE treatment with omalizumab in children with seasonal allergic rhinitis undergoing specific immunotherapy simultaneously. Pediatr Allergy Immunol. 2010;21(1 Pt 2):e160–165. doi: 10.1111/j.1399-3038.2009.00900.x. [DOI] [PubMed] [Google Scholar]
- 55.Denning D.W., Pleuvry A., Cole D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51(4):361–370. doi: 10.3109/13693786.2012.738312. [DOI] [PubMed] [Google Scholar]
- 56.Rick E.M., Woolnough K., Pashley C.H., Wardlaw A.J. Allergic fungal airway disease. J Investig Allergol Clin Immunol. 2016;26(6):344–354. doi: 10.18176/jiaci.0122. [DOI] [PubMed] [Google Scholar]
- 57.van der Ent C.K., Hoekstra H., Rijkers G.T. Successful treatment of allergic bronchopulmonary aspergillosis with recombinant anti-IgE antibody. Thorax. 2007;62(3):276–277. doi: 10.1136/thx.2004.035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nove-Josserand R., Grard S., Auzou L., et al. Case series of omalizumab for allergic bronchopulmonary aspergillosis in cystic fibrosis patients. Pediatr Pulmonol. 2017;52(2):190–197. doi: 10.1002/ppul.23612. [DOI] [PubMed] [Google Scholar]
- 59.Lehmann S., Pfannenstiel C., Friedrichs F., et al. Omalizumab: a new treatment option for allergic bronchopulmonaryaspergillosis in patients with cystic fibrosis. Ther Adv Respir Dis. 2014;8(5):141–149. doi: 10.1177/1753465814547517. [DOI] [PubMed] [Google Scholar]
- 60.Li J.X., Fan L.C., Li M.H., et al. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: asynthesis review of published literature. Respir Med. 2017;122:33–42. doi: 10.1016/j.rmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 61.Janahi I.A., Rehman A., Al-Naimi A.R. Allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Ann Thorac Med. 2017;12(2):74–82. doi: 10.4103/atm.ATM_231_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez J., Cardona R., Omalizumab An option in vernal keratoconjunctivitis? Allergol Immunopathol (Madr) 2012;40(5):319–320. doi: 10.1016/j.aller.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Santamaria L., Sanchez J. Long-term efficacy of omalizumab in patients with conventional treatment-resistant vernal keratoconjunctivitis. Rev Alerg Mex. 2018;65(2):192–196. doi: 10.29262/ram.v65i2.292. [DOI] [PubMed] [Google Scholar]
- 64.Occasi F., Duse M., Nebbioso M., et al. Vernal keratoconjunctivitis treated with omalizumab: a case series. Pediatr Allergy Immunol. 2017;28(5):503–505. doi: 10.1111/pai.12737. [DOI] [PubMed] [Google Scholar]
- 65.Manti S., Parisi G.F., Papale M., et al. Clinical efficacy and safety of omalizumab in conventional treatment-resistant vernal keratoconjunctivitis: our experience and literature review. Immun Inflamm Dis. 2021;9(1):3–7. doi: 10.1002/iid3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doan S., Amat F., Gabison E., et al. Omalizumab in severe refractory vernal keratoconjunctivitis in children: case series and review of the literature. Ophthalmol Ther. 2017;6(1):195–206. doi: 10.1007/s40123-016-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Costa C., Coimbra A., Vitor A., et al. Food allergy-From food avoidance to active treatment. Scand J Immunol. 2020;91(1) doi: 10.1111/sji.12824. [DOI] [PubMed] [Google Scholar]
- 68.Fiocchi A., Artesani M.C., Riccardi C., et al. Impact of omalizumab on food allergy in patients treated for asthma: a real-life study. J Allergy Clin Immunol Pract. 2019;7(6):1901–1909.e5. doi: 10.1016/j.jaip.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 69.Crisafulli G., Caminiti L., Chiera F., et al. Omalizumab in children with severe allergic disease: a case series. Ital J Pediatr. 2019;45(1):13. doi: 10.1186/s13052-019-0602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brandstrom J., Vetander M., Sundqvist A.C., et al. Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents. Clin Exp Allergy. 2019;49(10):1328–1341. doi: 10.1111/cea.13469. [DOI] [PubMed] [Google Scholar]
- 71.Yee C., Albuhairi S., Noh E., et al. Long-term outcome of peanut oral immunotherapy facilitated initially by omalizumab. J Allergy Clin Immunol Pract. 2019;7(2):451–461.e7. doi: 10.1016/j.jaip.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Alduraywish S.A., Standl M., Lodge C.J., et al. Is there a march from early food sensitization to later childhood allergic airway disease? Results from two prospective birth cohort studies. Pediatr Allergy Immunol. 2017;28(1):30–37. doi: 10.1111/pai.12651. [DOI] [PubMed] [Google Scholar]
- 73.Weidinger S., Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 74.Mancuso J.B., Lee S.S., Paller A.S., Ohya Y., Eichenfield L.F. Management of severe atopic dermatitis in pediatric patients. J Allergy Clin Immunol Pract. 2021;9(4):1462–1471. doi: 10.1016/j.jaip.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 75.Chan S., Cornelius V., Cro S., et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2019;174(1):29–37. doi: 10.1001/jamapediatrics.2019.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu A.C. Omalizumab for atopic dermatitis: overtreatment or lifesaver? JAMA Pediatr. 2020;174(1):15–16. doi: 10.1001/jamapediatrics.2019.4509. [DOI] [PubMed] [Google Scholar]
- 77.Iannelli M., Caminiti L., Vaccaro M., et al. Omalizumab for treatment of refractory severe atopic dermatitis. A pediatric perspective. Dermatol Ther. 2020;33(4) doi: 10.1111/dth.13519. [DOI] [PubMed] [Google Scholar]
- 78.Rodrigo G.J., Neffen H., Castro-Rodriguez J.A. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139(1):28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 79.Corren J., Casale T.B., Lanier B., et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39(6):788–797. doi: 10.1111/j.1365-2222.2009.03214.x. [DOI] [PubMed] [Google Scholar]
- 80.Berger W., Gupta N., McAlary M., Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. 2003;91(2):182–188. doi: 10.1016/S1081-1206(10)62175-8. [DOI] [PubMed] [Google Scholar]
- 81.Adachi M., Kozawa M., Yoshisue H., et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med. 2018;141:56–63. doi: 10.1016/j.rmed.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 82.Kocaturk E., Salman A., Cherrez-Ojeda I., et al. The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy. 2021;76(3):816–830. doi: 10.1111/all.14687. [DOI] [PubMed] [Google Scholar]
- 83.Johnston A., Smith C., Zheng C., et al. Influence of prolonged treatment with omalizumab on the development of solid epithelial cancer in patients with atopic asthma and chronic idiopathic urticaria: a systematic review and meta-analysis. Clin Exp Allergy. 2019;49(10):1291–1305. doi: 10.1111/cea.13457. [DOI] [PubMed] [Google Scholar]
- 84.Namazy J.A., Blais L., Andrews E.B., et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol. 2020;145(2):528–536.e1. doi: 10.1016/j.jaci.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 85.Pfaller B., Jose Y.J., Agache I., et al. Biologicals in atopic disease in pregnancy: an EAACI position paper. Allergy. 2021;76(1):71–89. doi: 10.1111/all.14282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This paper is a review, so Availability of data and materials is not applicable.