Abstract
Calcium signaling is essential for regulating many biological processes. Endoplasmic reticulum inositol trisphosphate receptors (IP3Rs) and the mitochondrial Ca2+ uniporter (MCU) are key proteins that regulate intracellular Ca2+ concentration. Mitochondrial Ca2+ accumulation activates Ca2+-sensitive dehydrogenases of the tricarboxylic acid (TCA) cycle that maintain the biosynthetic and bioenergetic needs of both normal and cancer cells. However, the interplay between calcium signaling and metabolism is not well understood. In this study, we used human cancer cell lines (HEK293 and HeLa) with stable KOs of all three IP3R isoforms (triple KO [TKO]) or MCU to examine metabolic and bioenergetic responses to the chronic loss of cytosolic and/or mitochondrial Ca2+ signaling. Our results show that TKO cells (exhibiting total loss of Ca2+ signaling) are viable, displaying a lower proliferation and oxygen consumption rate, with no significant changes in ATP levels, even when made to rely solely on the TCA cycle for energy production. MCU KO cells also maintained normal ATP levels but showed increased proliferation, oxygen consumption, and metabolism of both glucose and glutamine. However, MCU KO cells were unable to maintain ATP levels and died when relying solely on the TCA cycle for energy. We conclude that constitutive Ca2+ signaling is dispensable for the bioenergetic needs of both IP3R TKO and MCU KO human cancer cells, likely because of adequate basal glycolytic and TCA cycle flux. However, in MCU KO cells, the higher energy expenditure associated with increased proliferation and oxygen consumption makes these cells more prone to bioenergetic failure under conditions of metabolic stress.
Keywords: calcium signaling, IP3 receptor, mitochondrial calcium uniporter, glycolysis, TCA cycle, mitochondrial metabolism, bioenergetics
Abbreviations: AMPK, AMP-dependent kinase; BSA, bovine serum albumin; Cch, carbachol; DMEM, Dulbecco's modified Eagle's medium; ER, endoplasmic reticulum; FCCP, (carbonylcyanide-4-(trifluoromethoxy)phenylhydrazone); FE, fractional enrichment; HEK293, human embryonic kidney 293 cell line; IP3R, inositol trisphosphate receptor; α-KG, α-ketoglutarate; LC3, microtubule-associated protein light chain 3; LDH, lactate dehydrogenase; MCU, mitochondrial Ca2+ uniporter; MEF, mouse embryonic fibroblast; OCR, oxygen consumption rate; PC, pyruvate carboxylase; PCA, perchloric acid; PDH, pyruvate dehydrogenase; p-PDH, phosphorylated PDH; RC, reductive carboxylation; TCA, tricarboxylic acid; TKO, triple KO
Ca2+ signaling plays an important role in regulating many diverse biological processes ranging from cell proliferation, secretion, motility, metabolism, and cell death (1). A key event that initiates Ca2+ signaling is the release of Ca2+ from intracellular stores by the three isoforms of inositol trisphosphate receptor (IP3R) channels (2). The depletion of internal Ca2+ stores secondarily stimulates Ca2+ entry via Orai channels in the plasma membrane. Thus, loss of IP3R-mediated Ca2+ signaling would block both the intracellular and extracellular components of agonist-mediated Ca2+ signaling. An increase of cytosolic Ca2+ in the environment of the mitochondria facilitates Ca2+ uptake into the matrix by a complex of inner membrane proteins of which the mitochondrial Ca2+ uniporter (MCU) is the pore-forming constituent (3). The elevations of Ca2+ in the cytosol and mitochondrial compartments act on multiple effector proteins to alter cell function (1, 4). In the mitochondrial matrix, Ca2+ stimulates the activity of three dehydrogenases of the tricarboxylic acid (TCA) cycle (5), respiratory complexes (6), and ATP synthase (7) to enhance ATP generation. Higher matrix Ca2+ accumulation can open the permeability transition pore and cause cell death (8). Inactivating the function of all three IP3R isoforms or MCU channels would therefore be expected to have wide-ranging effects, including compromising the metabolic and bioenergetic status of the cells.
Global and conditional KO mouse models of individual IP3R isoforms have provided valuable information on the role of Ca2+ signaling in regulating organ function (9, 10, 11, 12, 13, 14). However, functional redundancy and postnatal mortality have complicated these studies (15, 16, 17, 18, 19). Few studies have examined the effects of the conditional deletion of all three IP3R isoforms. In hematopoietic cells, these investigations indicated a critical role for Ca2+ in regulating early developmental checkpoints in T and B lymphocytes (20, 21). In mouse embryonic stem cells, the IP3R TKO cells showed enhanced differentiation into the cardiomyocyte lineage and impaired differentiation into the hematopoietic lineage (22). Inducible KOs of all IP3R isoforms in mouse vascular endothelial cells (14) or smooth muscle cells (13) have been shown to affect basal blood pressure and vascular tone.
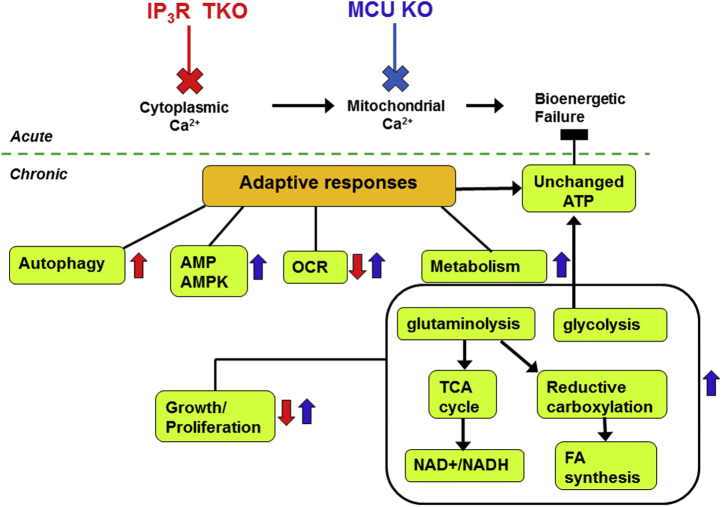
Studying the mechanisms of adaptation to a loss of Ca2+ signaling is much easier in cultured cell systems than in animal KO models. A widely used cell culture model is a chicken DT40 cell line in which all three IP3R isoforms have been disrupted (23). However, studies using DT40 cells have primarily focused on the structure, function, and regulation of IP3Rs (24). A number of reports have noted that DT40 IP3R TKO cells have a baseline increase in autophagy (25, 26, 27). Cardenas et al. (27) proposed that the increased autophagy compensated for the bioenergetic deficit caused by the loss of endoplasmic reticulum (ER)/mitochondrial Ca2+ signaling required to maintain optimal operation of the TCA cycle. Cardenas et al. (28, 29) have extended these studies to compare the effects of acute inhibition of ER/mitochondrial Ca2+ transfer in normal and cancer cells. They concluded that, unlike normal cells, the stimulation of autophagy in cancer cells is not sufficient to compensate for the increased energy demand of uncontrolled growth, with cell death resulting from a “bioenergetic crisis” during mitosis. The advent of new methods of gene disruption has enabled all three IP3R isoforms to be deleted in human embryonic kidney 293 (HEK293) (30, 31) and HeLa (32) human cancer cell lines. While these KO cells have primarily been used as an expression system to study the functional properties of IP3R mutants, they also offer the opportunity to investigate the metabolic and bioenergetic consequences of the chronic effects of a total loss of Ca2+ signaling. This is the primary objective of the present investigation.
Many studies have examined the physiological consequences of disrupting mitochondrial Ca2+ signaling using global and tissue-specific MCU KO mouse models. In most cases, a relatively mild phenotype at baseline has been observed in tissues, such as the heart (33), skeletal muscle (34), exocrine pancreas (35), endocrine pancreas (36), brown adipose tissue (37), and photoreceptors (38) suggesting the existence of robust adaptive mechanisms that have yet to be fully characterized, including upregulation of alternative mechanisms of mitochondrial Ca2+ transport. Where metabolic changes have been investigated in MCU KOs, the results have generally been tissue specific. For example, fatty acid oxidation is increased in cardiac (39) and skeletal muscle (34), and decreased in liver (40) and pulmonary macrophages (41). Relatively few studies have examined the metabolic or bioenergetic consequences of knocking out MCU in cultured cells. Using RNA interference, it was concluded that MCU was dispensable for growth in a breast cancer cell line (42) but stimulated the growth and metastasis of hepatocellular carcinoma (43) and colorectal cancer cells (44). Silencing of MCU in mouse pancreatic β-cells suppressed the increase of ATP/ADP induced by glucose (45). The absence of MCU in vascular smooth muscle cells impaired mitochondrial fusion and entry into the G1/S phase of the cell cycle (46). The conditional deletion of MCU from mouse embryonic fibroblasts (MEFs) enhanced differentiation to myofibroblasts by an epigenetic mechanism that involves enhanced glycolysis and increased accumulation of α-ketoglutarate (α-KG) (47). Overall, it is clear that the metabolic phenotype of the MCU KO cells is dependent on the cell type being investigated. To obtain a clearer picture of the metabolic consequences of disrupting either ER or mitochondrial Ca2+, we have carried out the present study in which IP3Rs or MCU have been knocked out in the same human cancer cell lines. Our studies show that the IP3R TKO cells have adapted to the total loss of Ca2+ signaling with a lower proliferation and oxygen consumption rate (OCR), but no significant changes in adenine nucleotides, even when artificially made to rely entirely on the TCA cycle for their bioenergetic and biosynthetic needs. By contrast, the loss of MCU is associated with an increased proliferation and OCR, increased metabolism of glucose and glutamine, a higher level of basal AMP-dependent kinase (AMPK), and bioenergetic failure when made to rely solely on the TCA cycle. We conclude that baseline rates of aerobic glycolysis and the TCA cycle are sufficient to make constitutive ER/mitochondrial Ca2+ signaling dispensable for the growth and function of both IP3R TKO and MCU KO cancer cells. However, in MCU KO cells, the higher energy expenditure associated with increased proliferation and oxygen consumption make these cells more prone to bioenergetic failure under conditions of metabolic stress.
Results
Cytosolic and mitochondrial Ca2+
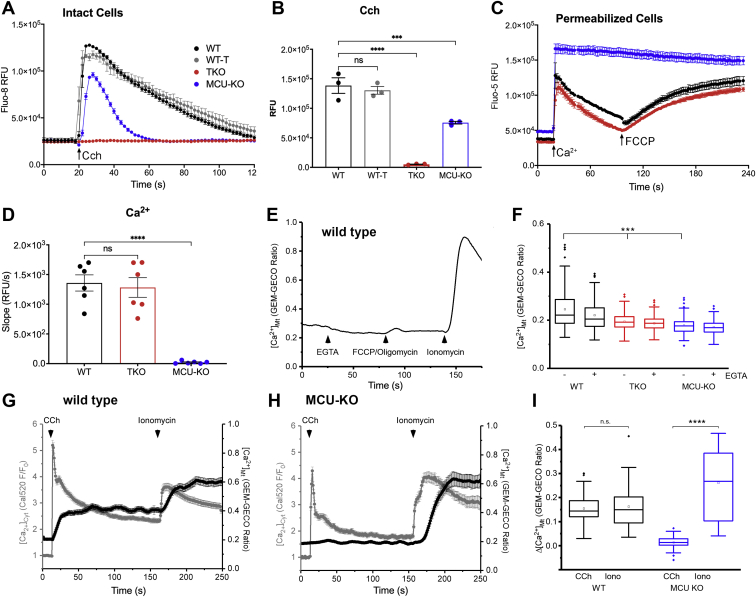
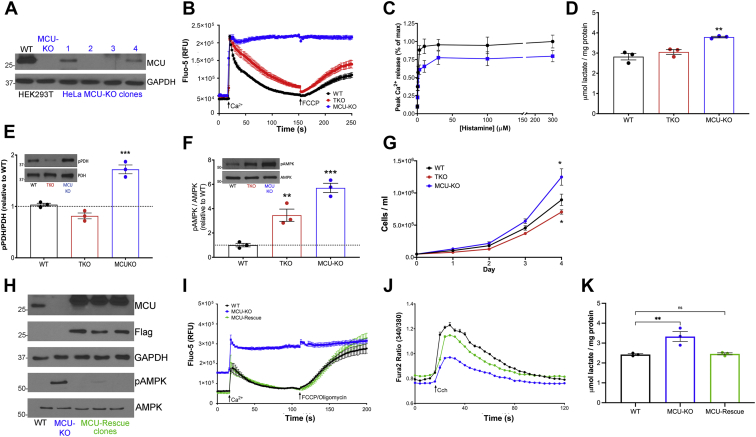
We initially characterized cytosolic Ca2+ signals in the IP3R and MCU KO HEK293 cell models incubated in the absence of extracellular Ca2+. As expected, the loss of all three IP3R isoforms prevented any cytosolic Ca2+ changes induced by carbachol (Cch) stimulation of endogenous muscarinic receptors (Fig. 1, A and B). MCU deletion was carried out in the variant HEK293T cell line (48), and these cells showed a decreased Cch-mediated cytosolic Ca2+ signal when compared with the appropriate control of WT HEK293T cells. The cytosolic Ca2+ responses of WT HEK293 and HEK293T cells were not significantly different (Fig. 1A), and this also proved to be the case for several other parameters measured in this study. This includes cell growth (Fig. 2A), glucose utilization (Fig. S1A), glutamine consumption (Fig. S1B), total NAD+ and NADH (Fig. S1, C and D), lactate accumulation (Fig. S1E), ATP levels (Fig. S1F), phosphorylated AMPK levels (Fig. S1G), and phosphorylated pyruvate dehydrogenase (p-PDH) levels (Fig. S1H). Hence, only the WT HEK293 cells are represented as controls in most of the remaining figures.
Figure 1.
Measurements of cytosolic and mitochondrial Ca2+content in WT, IP3R TKO, and MCU-KO HEK293T cells.A, cytosolic Ca2+ changes in response to 25 μM Cch was measured in the absence of external Ca2+. The data shown are from a representative experiment and are the mean ± SEM of four replicates for each cell type. B, the quantitation of the amplitude of the changes in cytosolic Ca2+. The data are the mean ± SEM of three independent experiments, each carried out in triplicate; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, one-way ANOVA followed by multiple comparison analysis using Dunnett’s test. C, cells were permeabilized with saponin, and the clearance of a 50 μM pulse of Ca2+ added at 18 s was measured as described in the “Experimental procedures” section. A further addition of 1 μM FCCP was made at 100 s. C, from a representative experiment. D, a quantitation in which the initial rate of Ca2+ uptake over a 20 s period was calculated as given in (B) (n = 6). E, representative trace of epifluorescence single-cell recordings of GEM-GECO emission ratio in WT HEK293 showing matrix Ca2+ ([Ca2+]Mt) in response to the removal/chelation of [Ca2+]Ext with EGTA (10 mM) and in response to FCCP (5 μM) and oligomycin (1 μg/ml) was measured. The subsequent addition of ionomycin (10 μM) was used as a uniporter-independent positive control for [Ca2+]Mt. F, the quantification of [Ca2+]Mt in the presence and absence of EGTA as measured by GEM-GECO emission ratio in WT, TKO, and MCU-KO cells. Box plots show mean, median, interquartile range, and outlier markers. ∗∗∗p < 0.001, one-way ANOVA followed by multiple comparison analysis using Dunnett’s test. G and H, simultaneous [Ca2+]Cyt (Cal520) (gray traces) and [Ca2+]Mt (GEM-GECO emission ratio) in response to addition of carbachol (CCh) and ionomycin to WT (G) and MCU-KO (H) HEK cells. The sustained elevation of mitochondrial Ca2+ signal in (G) is probably because of the asynchronous oscillations of [Ca2+]Cyt observed in single cells after the initial rise of Ca2+ (not shown). I, box plot quantification of Δ[Ca2+]Mt from individual cell recordings in (G) and (H). Responses are plotted as [Ca2+]Mt peak (0–150 s) − [Ca2+]Mt baseline for CCh simulation and [Ca2+]Mt peak t > 150 s − [Ca2+]Mt plateau at t = 150 s for ionomycin. ∗∗∗∗p < 0.0001 Wilcoxon signed-rank test. FCCP, (carbonylcyanide-4-(trifluoromethoxy)phenylhydrazone); HEK293T, human embryonic kidney 293T cell line; IP3R, inositol trisphosphate receptor; MCU, mitochondrial Ca2+ uniporter; TKO, triple KO.
Figure 2.
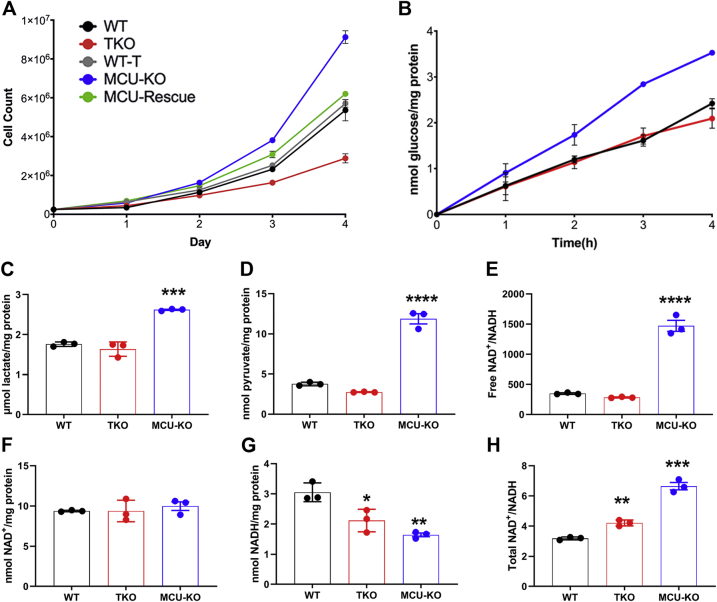
Growth, glycolysis, and pyridine nucleotide levels in WT, IP3R TKO, and MCU-KO HEK293T cells.A, growth curves of the indicated HEK293 cell lines were measured. The data shown are the mean ± SEM of three experiments. B, the media from cells grown for 4 to 5 days were replaced with DMEM containing 5 mM glucose instead of 25 mM glucose. Aliquots of the media sampled at the times indicated were assayed for glucose. The data shown are the mean ± SEM of four experiments. C, cells were deproteinized in 0.6 M perchloric acid, and neutralized lysates were assayed for lactate. D, as in (C), but pyruvate was measured. The cytosolic-free NAD+/NADH ratio was calculated from the lactate/pyruvate ratio (E). Total NAD+ (F) and NADH (G) were measured by an enzymatic cycling procedure. The total NAD+/NADH ratio is quantitated in (H). For (C–H), the data are the mean ± SEM of three independent experiments, each carried out in triplicate; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, one-way ANOVA followed by multiple comparison analysis using Dunnett's test. DMEM, Dulbecco's modified Eagle's medium; HEK293T, human embryonic kidney 293T cell line; IP3R, inositol trisphosphate receptor; MCU, mitochondrial Ca2+ uniporter; TKO, triple KO.
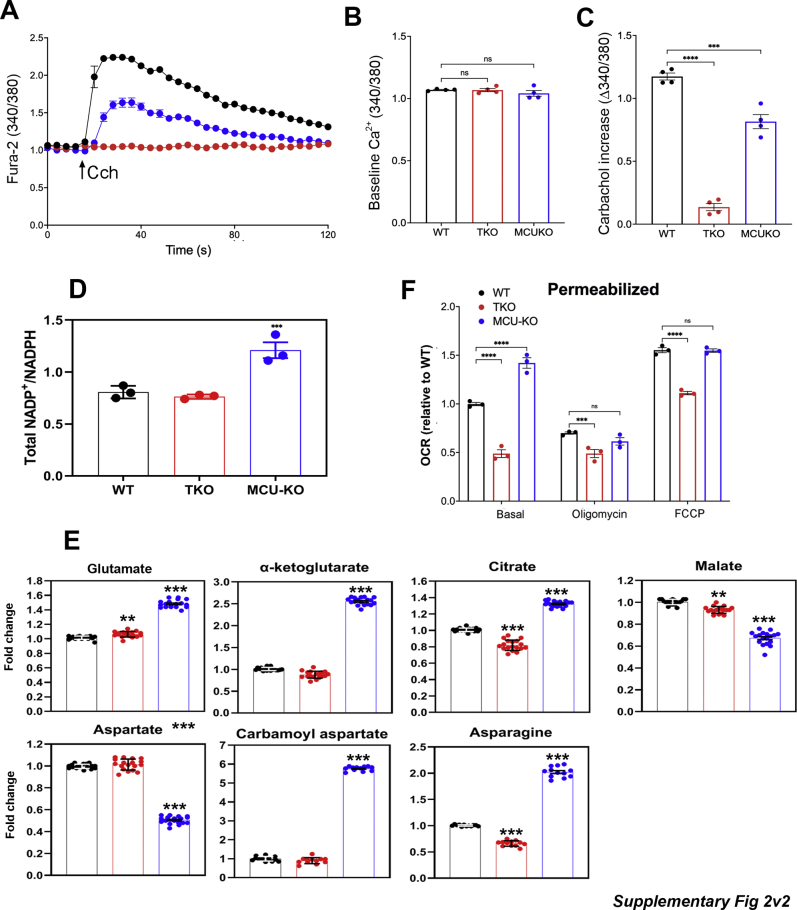
The ability of mitochondria to sequester and release a 50 μM pulse of Ca2+ in saponin-permeabilized cell lines was measured in Figure 1, C and D. Both WT and IP3R TKO cells had comparable rates of mitochondrial uptake and (carbonylcyanide-4-(trifluoromethoxy)phenylhydrazone) (FCCP)-induced release compared with the negligible rates for both processes seen in the MCU KO cells. The MCU KO–permeabilized cells consistently showed an elevated steady state [Ca2+] in the medium prior to Ca2+ pulse addition. This may reflect the failure of the cells to sequester the endogenous contaminating Ca2+ into the mitochondria before the initiation of the experiment. The mitochondrial Ca2+ content in intact cells was estimated using the ratiometric, high-affinity, genetically encoded, fluorescent probe GEM-GECO (Kd ∼340 nM; (49)). The baseline signal in all three cell lines was not significantly decreased by chelation of extracellular Ca2+ or by depolarization with FCCP/oligomycin, indicating that there is very little Ca2+ in the mitochondria under unstimulated conditions (Fig. 1, E and F). The measured basal content was only marginally lower (∼20%) in IP3R TKO and MCU KO cells (Fig. 1F). It should be noted that a variable lowering of mitochondrial Ca2+ content has been reported in mouse MCU KO models (50, 51, 52, 53). The ability of the probe to register increases in mitochondrial Ca2+ was verified by measuring responses to ionomycin and Cch (Fig. 1, E, G, and H). Parallel measurements of cytoplasmic and mitochondrial Ca2+ confirmed that Cch-induced increases in mitochondrial Ca2+ were suppressed in the MCU KO cells (Fig. 1, G–I). Taken together, the results show that IP3R TKO cells have lost agonist-mediated cytosolic Ca2+ signaling but retain the ability to sequester mitochondrial Ca2+, whereas the MCU KO cells have retained cytosolic Ca2+ signaling (albeit at a reduced level) but have lost mitochondrial Ca2+ uptake. The impact of these Ca2+ signaling phenotypes on metabolism and bioenergetics is investigated later.
Cell growth and glucose metabolism
A number of basic growth and metabolic parameters were measured in the HEK293 cell lines (Fig. 2). In the Dulbecco's modified Eagle's medium (DMEM) growth medium (containing glucose [25 mM], glutamine [5 mM], and pyruvate [1 mM]), the IP3R TKO cells grew more slowly, and the MCU KO cells grew more rapidly, than their corresponding WT counterpart (Fig. 2A). The cells were entirely dependent on glucose and glutamine as fuels, since the omission of either substrate prevented growth in all the cell lines (not shown). Glucose consumption was measured at a lower glucose concentration of 5 mM and found to be comparable between WT and IP3R TKO cells (Fig. 2B). However, the MCU KO cells consumed glucose more rapidly (Fig. 2B). Both cellular lactate (Fig. 2C) and pyruvate (Fig. 2D) showed increased levels in the MCU KO cells. The assumption of near equilibrium of the lactate dehydrogenase (LDH) reaction allows calculation of the free cytosolic NAD+/NADH ratio from the pyruvate/lactate ratio (54). This calculation indicated a substantial increase in the NAD+/NADH ratio in MCU KO cells (Fig. 2E). To verify these findings, the total cellular levels of NAD+ and NADH were also measured directly by an enzymatic cycling method (Fig. 2, F and G). Significant changes in NAD+ levels were not observed, but the levels of NADH decreased substantially in MCU KO cells and to a lesser extent in IP3R TKO cells (Fig. 2G). Similar changes were also observed for NADP+/NADPH (Fig. S2A). Quantitation of the total levels of metabolites by mass spectroscopy showed that MCU KO cells have increased levels of glutamate, α-KG, and citrate (Fig. S2B). These metabolite changes are consistent with increased metabolism of glutamine in MCU KO cells. Similar increases in α-KG have been noted in MCU KO fibroblasts (47) and photoreceptors (38). In fibroblasts, the increase in α-KG was attributed to enhanced glutamine metabolism and linked to epigenetic changes altering the methylation of histones. In photoreceptors, enhanced glutamine metabolism was not observed. Both malate and aspartate levels were decreased in the MCU KO HEK cells (Fig. S2B). The fall in aspartate could in part be related to increased utilization, since large increases were observed in carbamoyl aspartate (used in pyrimidine synthesis) and asparagine levels. The fall in malate is expected, since malate is in equilibrium with aspartate through oxaloacetate. The fall in these metabolites may disrupt the malate/aspartate shuttle and may account for the increased reliance on lactate production as a means of regenerating the NAD+ required for glycolysis. Overall, these results indicate that there are marked increases in growth rate, glycolysis, and NAD+/NADH redox state in the MCU KO cells.
Key bioenergetic parameters in WT, IP3R TKO, and MCU KO cells
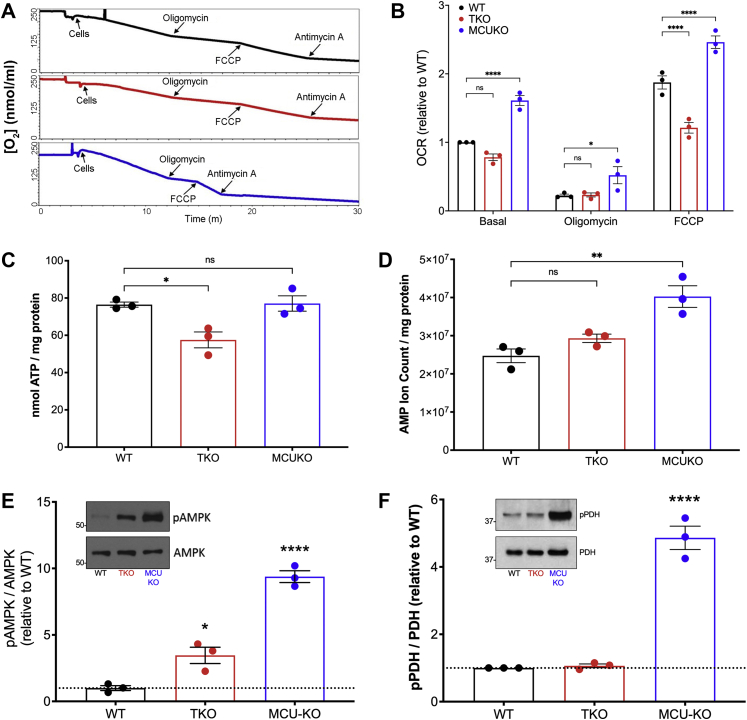
Altered supply of NADH by dehydrogenases and/or utilization by the respiratory chain may underlie the observed changes in NAD+/NADH ratio. This prompted us to examine the OCR of the KO cell lines (Fig. 3, A and B). In intact cells exposed to complete growth medium, the basal OCR of MCU KO cells was increased by ∼60% relative to WT cells. Under the same conditions, the OCR of IP3R TKO cells was decreased by ∼25%. The same trends were observed when respiration was uncoupled with FCCP. Differences in OCR in intact cells could reflect altered availability of substrates to the respiratory chain. However, the decreased OCR in IP3R TKO cells and increased OCR in MCU KO cells was also observed in permeabilized cells supplied with an excess of glutamate/malate and ADP (Fig. S2C). In the case of MCU KO cells, differences of OCR seen in intact cells in the presence of oligomycin or FCCP were not observed in permeabilized cells, suggesting that the differences seen in intact cells under these conditions may reflect enhanced substrate availability. We examined the ability of the cell lines to maintain basal ATP levels and found no statistical differences between the three cell lines (Fig. 3C). However, the basal AMP levels were substantially elevated in the MCU KO cells and to a smaller extent in IP3R TKO cells (Fig. 3D). The different AMP levels correlated with the phosphorylation state of AMPK in the three cell lines (Fig. 3E). PDH is a key mitochondrial phosphoenzyme controlling the flow of glucose carbon into the TCA cycle and would therefore be one of many steps controlling NADH supply. The dephosphorylation and activation of PDH is catalyzed by a Ca2+-sensitive phosphatase (5). In agreement with other MCU KO models (34, 50, 55, 56), the phosphorylation of PDH is substantially increased in MCU KO HEK293T cells. However, PDH phosphorylation was unaltered in IP3R TKO cells (Fig. 3F).
Figure 3.
Some bioenergetic parameters of HEK293 KO cells.A, equal amounts of cells (106) incubated in complete DMEM were added to the chamber of an O2 electrode, and rates of O2 consumption (OCR) were measured under basal conditions and after the sequential additions of oligomycin (1 μg/ml), FCCP (1 μM), and antimycin A (2 μM). B, quantification of OCR is shown normalized to the basal OCR of WT cells. The data are the mean + SEM of three independent experiments, each carried out in triplicate: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, two-way ANOVA followed by multiple comparison analysis using Dunnett's test. C, ATP was measured in neutralized perchloric acid (PCA) lysates with a luciferase assay. D, AMP was measured in lysates processed for LC–MS/MS experiments. E and F, the phosphorylation status of AMPK and PDH measured by immunoblotting with phospho-specific antibodies. Values are normalized to the total levels of the enzymes. Statistical evaluation for (C–F) used one-way ANOVA with symbols as in (B). AMPK, AMP-dependent kinase; DMEM, Dulbecco's modified Eagle's medium; FCCP, (carbonylcyanide-4-(trifluoromethoxy)phenylhydrazone); HEK293, human embryonic kidney 293T cell line; OCR, oxygen consumption rate; PDH, pyruvate dehydrogenase.
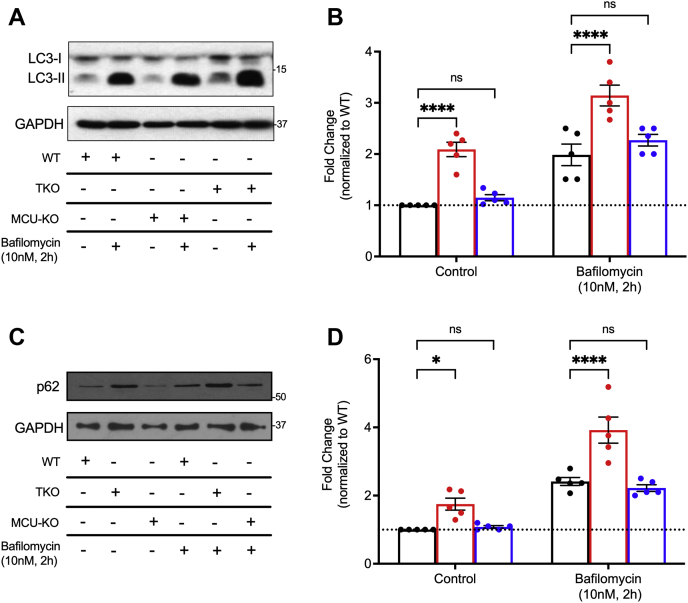
Autophagy is a key cellular process regulated by the energy sensor AMPK (57). We therefore measured autophagy in the three cell lines by quantitating microtubule-associated protein 1 light chain 3 (LC3) conversion and p62 degradation detected by immunoblotting (Fig. 4). The data show increased levels of LC3-II (Fig. 4, A and B) and p62 (Fig. 4, C and D) in the IP3R TKO cells but not in MCU KO cells. In order to distinguish between effects on enhanced autophagosome formation or decreased fusion with lysosomes, we blocked the latter process by preincubation of the cells with bafilomycin (58). The increase in LC3-II/p62 observed in IP3R TKO cells was retained in the bafilomycin-treated cells with no additional changes seen in the MCU KO cells. The results are in line with several studies showing enhanced autophagy in IP3R TKO DT40 lymphocytes that is AMPK independent ((25, 26); but see Ref. (27)). The data also show that the AMPK activation seen in MCU KO cells does not lead to enhanced autophagy.
Figure 4.
Autophagy is enhanced in IP3R KO cells but not in MCU KO cells.A, cells grown for 4 to 5 days in nutrient-replete medium were analyzed by immunoblotting for LC3 in the presence or the absence of bafilomycin treatment (10 nM; 2 h). Quantitation of the LC3-II band is shown in B with data expressed relative to WT, untreated cells. C, lysates were immunoblotted for p62, and the data are quantitated in (D) with data expressed relative to WT, untreated cells. For (B) and (D), each point is an independent experiment performed in duplicate, error bars represent SEM, n = 5; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, two-way ANOVA followed by multiple comparison analysis using Dunnett's test. The data indicated no statistically significant interaction between the effects of cell lines and bafilomycin for either LC3 (F(2,4) = 0.117, p = 0.89) or p62 (F(2,24) = 4.25, p = 0.0263). IP3R, inositol trisphosphate receptor; LC3, microtubule-associated protein light chain 3; MCU, mitochondrial Ca2+ uniporter.
Isotope tracer analysis
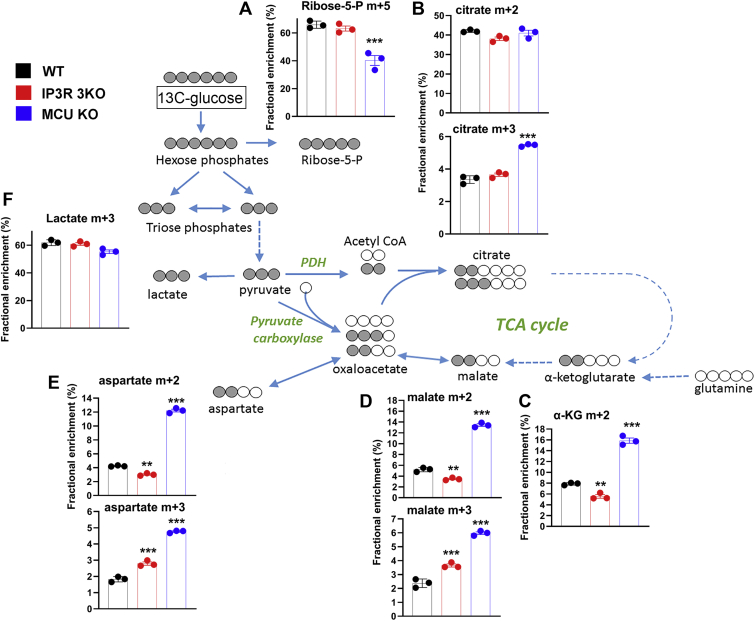
To investigate the metabolism of glucose in more detail, we used U–13C-glucose and mass spectroscopy. For the isotope tracer experiments, the glucose in DMEM was reduced to 5 mM for 30 min and then switched to 5 mM U–13C-glucose for 5 and 60 min. We found the labeling of glycolytic intermediates to be the same at both time points, and therefore, only the 60 min data are shown (Fig. 5). The rapid labeling kinetics precluded any conclusions regarding differences in glycolytic rates based on U–13C-glucose experiments. The fractional enrichment (FE) of m + 5 ribose-5-phosphate was decreased in MCU KO cells suggesting that less glucose carbon may be diverted into the pentose phosphate shunt in these cells (Fig. 5A). This would be compatible with the measured increase in the NADP/NADPH ratio (Fig. S2A). The flow of glucose carbon into the TCA cycle was significantly slower than the labeling of glycolytic intermediates since negligible labeling of TCA cycle intermediates was observed at 5 min (not shown). Despite our observations on the differences in phosphorylation state of PDH, the incorporation of two carbon units into citrate via PDH was not significantly different in the three cell lines (Fig. 5B). Whereas FE of m + 2 citrate reached ∼40%, the FE of m + 2 in α-KG (Fig. 5C), malate (Fig. 5D), or aspartate (a surrogate for labeling in oxaloacetate; Fig. 5E) was much lower in all three cell lines (3–15%). In part, this discrepancy could arise if substantial amounts of citrate are exported from the mitochondria to the cytosol for utilization in the ATP citrate lyase reaction that supplies acetyl CoA for lipid biosynthesis. The formation of m + 2 TCA metabolites was highest in the MCU KO cells (FE, 12–15%) indicating increased forward flux in the TCA cycle, which includes the segments containing Ca2+-sensitive isocitrate and α-KG dehydrogenases.
Figure 5.
[U–13C]-glucose tracer analysis in WT, IP3R TKO, and MCU KO cells. Cells were incubated in glucose-free DMEM for 30 min, and then, the medium was replaced with DMEM containing 5 mM uniformly labeled 13C-glucose for 5 or 60 min. Samples were prepared for LC–MS as described in the “Experimental procedures” section. Only the 60 min data for selected metabolites are shown in (A–F). Data are the fractional enrichment of the indicated isotopolog given as the mean ± SEM of three separate plates for each condition. A simplified cartoon of the flow of labeled carbon (shaded gray) is shown with steps involving multiple enzymes being indicated by dotted arrows. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, one-way ANOVA followed by multiple comparison analysis using Dunnett's test. DMEM, Dulbecco's modified Eagle's medium; IP3R, inositol trisphosphate receptor; MCU, mitochondrial Ca2+ uniporter; TKO, triple KO.
The removal of TCA cycle intermediates for biosynthetic reactions necessitates anaplerotic pathways to replenish these molecules. A major anaplerotic reaction involving glucose metabolism is the formation of oxaloacetate from pyruvate by the pyruvate carboxylase (PC) enzyme. This step acting on m + 3 pyruvate would generate m + 3 oxaloacetate which, in addition to being in equilibrium with m + 3 malate (Fig. 5D) and aspartate (Fig. 5E), can also generate m + 3 citrate (Fig. 5B). The formation of m + 3 citrate has been used to monitor PC flux in U–13C-glucose tracing experiments (59). In WT cells, PC flux was small (FE, ∼3%) relative to the flux of carbon through PDH (FE, ∼40%) (Fig. 5B). However, PC flux was selectively stimulated by ∼70% in MCU KO cells (Fig. 5B).
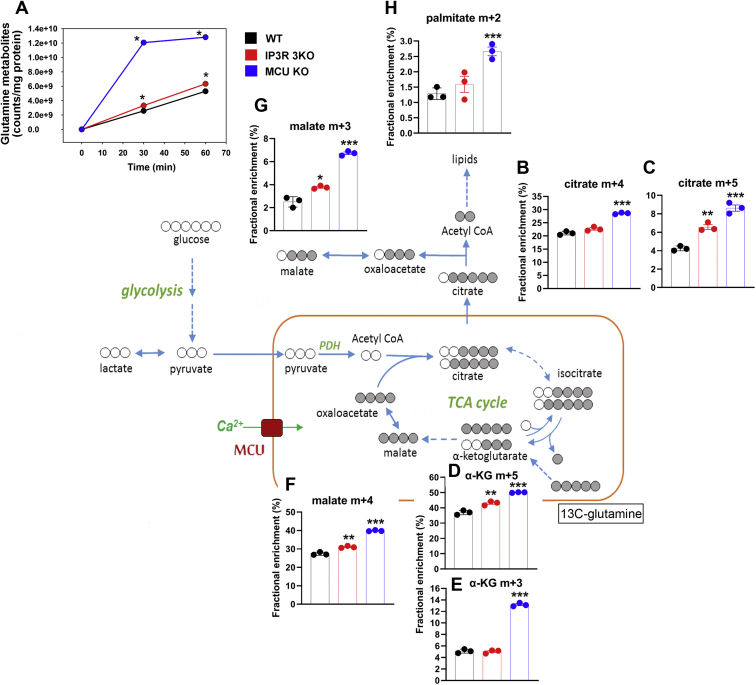
Glutamine is a major substrate feeding carbon into the TCA cycle that can potentially supply both energy and biosynthetic intermediates (60). We therefore analyzed U–13C-glutamine metabolism in the three cell lines using time points of 30 min and 1 h. The sum of label accumulating in several glutamine metabolites (glutamate, α-KG, aspartate, citrate/isocitrate, and cis-aconitate) was taken as a measure of overall glutamine metabolism. This was found to be substantially increased in the MCU KO cells with a much smaller (but significant) increase also noted in the IP3R 3KO cells (Fig. 6A). Metabolism of U–13C-glutamine by the combined actions of glutaminase, glutamate dehydrogenase, and glutamate transaminases would generate m + 5 labeled α-KG. The “forward” direction of the TCA cycle would generate m + 4 oxaloacetate, and condensation with unlabeled acetyl-CoA would produce m + 4 citrate. Any limitation in the supply of acetyl-CoA as a result of a block at PDH should lower labeling of m + 4 citrate, but this was not observed for either of the KO cell lines (Fig. 6B). Indeed, a small increase was seen in the MCU KO cells. The same pattern of changes was also seen for m + 4 labeled malate (Fig. 6F), indicating no suppression of flux at the Ca2+-sensitive α-KG dehydrogenase step. As observed in the glucose labeling data, the oxidative flux through the citrate/α-KG span is increased in the MCU KO cells (Fig. 6E). Carbon entering the TCA cycle as α-KG can also proceed in the “reverse” direction as a result of reductive carboxylation (RC) to generate m + 5 labeled isocitrate, which is in equilibrium with citrate (Fig. 6C). This reverse flux in WT cells was ∼20% of the oxidative flux as judged by the relative FEs of m + 4 and m + 5 citrates (Fig. 6, B and C). In IP3R TKO cells, this ratio of fluxes was increased to ∼30%. In MCU KO cells, there was a twofold increase in RC flux, but since the oxidative flux was also increased, the overall partitioning of the two pathways was also ∼30%. There is evidence that RC of glutamine can supply acetyl-CoA for fatty acid biosynthesis via mitochondrial citrate export and cleavage by the ATP–citrate lyase reaction (61, 62, 63). This reaction acting on m + 5 citrate would generate m + 3 malate (a proxy for m + 3 oxaloacetate). Indeed, the labeling of m + 3 malate in the three cell lines follows the same pattern as m + 5 citrate (Fig. 6G). MCU KO cells showed a significantly increased labeling of m + 2 palmitic acid by acetyl-CoA derived from glutamine metabolism (Fig. 6H).
Figure 6.
[U–13C]-glutamine tracer analysis WT, IP3R TKO, and MCU KO cells. Cells were washed in glutamine-free DMEM and then incubated in a DMEM containing 5 mM uniformly labeled 13C-glutamine for 30 or 60 min. Samples were prepared for LC–MS/MS as described in the “Experimental procedures” section. A, the sum of counts appearing as glutamate, α-ketoglutarate, aspartate, citrate/isocitrate, and cis-aconitate was taken as a measure of glutamine metabolism. The data were normalized to protein. The error bars were smaller than symbol size. Only the 60 min data for selected metabolites are shown in (B–H). Data are the fractional enrichment of the indicated isotopolog given as the mean ± SEM of three separate plates for each condition. A simplified cartoon of the flow of labeled carbon (shaded gray) is shown with steps involving multiple enzymes being indicated by dotted arrows. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, one-way ANOVA followed by multiple comparison analysis using Dunnett’s test. DMEM, Dulbecco's modified Eagle's medium; IP3R, inositol trisphosphate receptor; MCU, mitochondrial Ca2+ uniporter; TKO, triple KO.
KO HeLa cells and MCU rescue of HEK293T cells
To determine if the observed phenotypes of the IP3R TKO and MCU KO are unique to HEK293 cell lines, we examined the effects of these KOs in the HeLa cell line. The IP3R TKO HeLa cells have been used previously and shown to completely lack histamine-induced cytosolic Ca2+ transients (32). CRISPR-mediated gene targeting was used to produce the MCU KO HeLa cells (Fig. 7A). Loss of MCU function in the KO cells was confirmed by the lack of uptake of a pulse of Ca2+ delivered to permeabilized HeLa cells (Fig. 7B). As noted previously (Fig. 1A), the MCU KO HEK293T cells display a smaller cytosolic Ca2+ response to a saturating dose of Cch. The MCU KO HeLa cells also gave a reduced response to histamine (Fig. 7C) when compared with WT HeLa cells, but the differences were smaller than noted in HEK293 cells. As observed in HEK293T cells, lactate levels were also elevated in MCU KO HeLa cells and were unchanged in IP3R TKO cells (Fig. 7D). The smaller effects in HeLa cells may be related to their lesser reliance on glycolysis for ATP production than HEK293 cells (64, 65). The phosphorylation state of PDH (Fig. 7E), AMPK (Fig. 7F), as well as the growth rates of the different HeLa cell lines (Fig. 7G) were all qualitatively similar to the changes observed for the HEK293 cell lines. These data indicate that the observed phenotypes of MCU KO and IP3R TKO cells are not unique to the HEK293 cell line.
Figure 7.
Characteristics of HeLa cells with IP3R and MCU KOs and MCU-rescued HEK293T MCU KO cells.A, MCU immunoblotting confirmed loss of MCU in two of four HeLa clones after CRISPR KO. HEK293T WT and MCU-KO cells were used as positive and negative controls, respectively. GAPDH was the loading control. B, cells were permeabilized with saponin, and the clearance of a 50 μM pulse of Ca2+ added at 18 s was measured using Fluo-5 as described in the “Experimental procedures” section. A further addition of 1 μM FCCP was made as shown. C, HeLa cells were loaded with Fluo-8 AM as described in the “Experimental procedures” section, and changes to cytosolic Ca2+ in response to increasing doses of histamine were measured. D, neutralized PCA lysates of HeLa cells were assayed for lactate. Data are the mean ± SEM, n = 3; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, one-way ANOVA followed by multiple comparison analysis using Dunnett's test. E and F, phosphorylation status of HeLa PDH and AMPK was measured by immunoblotting. Values are normalized to the total levels of each protein with each point representing the mean of an independent experiment performed in duplicate. G, growth curves for WT, TKO, and MCU-KO HeLa cell lines. Mean ± SEM (n = 3). H, immunoblot for MCU and Flag in MCU-rescue in HEK293T clones. GAPDH was used as a loading control. The lower two panels show phosphorylated and total levels of AMPK. I, Ca2+ clearance in permeabilized HEK293T cells was measured as in (B). J, intact HEK293T cells were loaded with Fura-2 as described in the “Experimental procedures” section, and cytosolic Ca2+ changes in response to 25 μM carbachol was measured. K, lactate in HEK293T cells was measured in triplicate for three independent experiments as in (D). Statistical evaluation of data in (E–G) and (K) was as in (D). AMPK, AMP-dependent kinase; FCCP, (carbonylcyanide-4-(trifluoromethoxy)phenylhydrazone); HEK293T, human embryonic kidney 293T cell line; IP3R, inositol trisphosphate receptor; MCU, mitochondrial Ca2+ uniporter; PCA, perchloric acid; PDH, pyruvate dehydrogenase.
Another concern of using KO cells is the possibility that the observed phenotypes are due to off-target effects of the knockdown methodology. To determine if this may be the case in the MCU KO HEK293T cells, we sought to determine if stably reintroducing the MCU reverted some of the observed changes. The rescued expression of MCU was confirmed by immunoblotting (Fig. 7H). The restoration of mitochondrial Ca2+ uptake could be demonstrated in Ca2+ clearance assays using permeabilized cells (Fig. 7I). In intact cells, the Cch-induced cytosolic Ca2+ increase was enhanced in the MCU-rescue cells when compared with the MCU KO cells and was approximately 85% of WT cells (Fig. 7J). The increased lactate levels (Fig. 7K) and increased AMPK phosphorylation (Fig. 7H, lower two panels) observed in MCU KO cells were also diminished toward levels observed in WT cells. We conclude that, at least for the parameters measured, the observed phenotype of MCU KO cells is unlikely to be due to off-target effects.
Forced utilization of the TCA cycle for energy production in the three cell lines
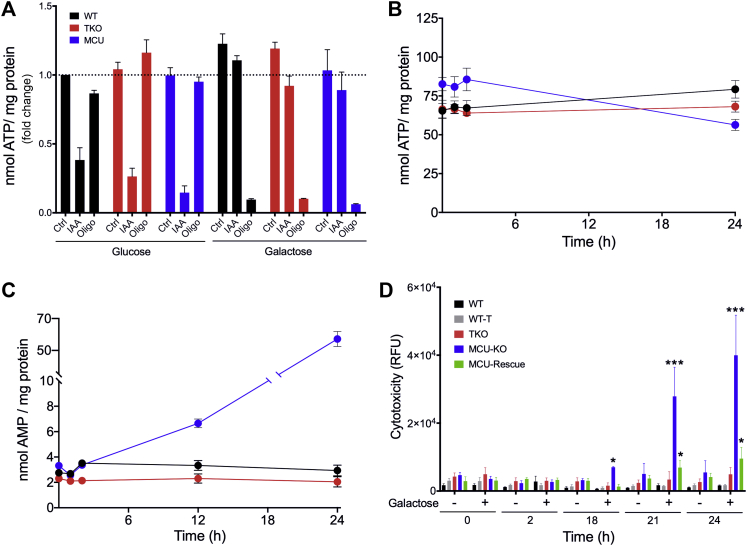
Constitutive Ca2+-stimulated mitochondrial oxidative metabolism has been proposed to be particularly important for maintaining the bioenergetics requirements of cancer cells (27, 28). This hypothesis is difficult to test in our KO models because HEK293 cells rely almost entirely on glycolysis to support their energy requirements (66, 67). Galactose metabolism minimizes the energy yield from glycolysis and makes cells increasingly reliant on the TCA cycle and oxidative phosphorylation to supply their energy needs (68). We therefore removed the glucose in DMEM and replaced it with 10 mM galactose. Oligomycin treatment of WT, IP3R TKO, or MCU KO cells grown in glucose-containing medium had negligible effects on ATP levels, as expected if oxidative phosphorylation makes a minimal contribution to ATP production (Fig. 8A). By contrast, blocking glycolysis with iodoacetate lowered ATP levels in all three cell lines. Oligomycin markedly reduced ATP levels in all three cell lines incubated in galactose medium, indicating the dominant role of oxidative phosphorylation under these conditions. The ability of the cell lines to maintain their levels of ATP and AMP was followed for different periods after the galactose switch. Over a period of 2 h, the cells had no difficulty in maintaining their ATP and AMP (Fig. 8, B and C). However, more prolonged incubation in galactose led to a progressive decrease in ATP and elevation of AMP in the MCU KO cells but not in the WT or IP3R TKO cell lines. When cytotoxicity was measured with a fluorescent dye assay, it was evident that the MCU KO showed evidence of cell death that was significant at 18 h and markedly pronounced at 24 h after galactose incubation (Fig. 8D). There was no evidence of significant cytotoxicity in the WT or IP3R TKO cell lines after prolonged incubation in galactose medium. Interestingly, the cytotoxicity observed in the MCU KO cells was strongly suppressed in the MCU-rescue cell line.
Figure 8.
Forced utilization of the TCA cycle in HEK293 cells.A, 1.0 × 105 cells were seeded on 60 mm plates and grown for 72 h in glucose containing DMEM. Media were aspirated and replaced with glucose-free DMEM containing 10 mM galactose. Cells were treated with either 1 μM iodoacetate (IAA) or 5 μM oligomycin. After 1 h, cells were deproteinized in 0.6 M perchloric acid, neutralized, and ATP was measured with a luciferase assay. B–D, ATP, AMP, and cytotoxicity were measured at various time points following glucose removal and galactose addition. Values shown are the mean ± SEM of three experiments. B, ATP was measured in neutralized PCA lysates using a luciferase assay. C, AMP was measured in neutralized PCA lysates using a Promega AMP-Glo assay. D, cell death was measured in real time using a Promega CellTox Green cytotoxicity assay, n = 3; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, two-way ANOVA followed by multiple comparison analysis using Dunnett’s test. The data indicated a statistically significant interaction between the effects of cell lines and galactose (F(28, 264) = 39.24, p < 0.0001). DMEM, Dulbecco's modified Eagle's medium; HEK293, human embryonic kidney 293 cell line; PCA, perchloric acid; TCA, tricarboxylic acid.
Discussion
The KO cell models used in this study were intended to compare the chronic metabolic and bioenergetic changes resulting from the loss of either cytosolic Ca2+ signaling (IP3R TKO) and/or mitochondrial Ca2+ signaling (MCU KO and IP3R TKO). The changes we measured are summarized in the scheme shown in Figure 9. The most prominent changes observed in the MCU KO HEK293T cells were increased glycolysis, glutamine metabolism, and increased growth rate. Presumably, the enhanced consumption of fuels and the increased oxygen consumption of MCU KO cell are needed to meet the demands of increased growth. Varied results have been reported when examining the effects of MCU loss on growth. Although we found an increased growth rate of MCU KO HEK293T and HeLa cells, Hall et al. (42) found HeLa cell proliferation to be decreased not only when MCU levels were diminished with siRNA but also when MCU activity was increased with MICU1 siRNA. Growth was diminished in MCU KO MEFs (47) but unaltered in siRNA-treated MD-MB-231 breast carcinoma cells (42). In agreement with our findings, knockdown of MCU in primary B lymphocytes enhanced proliferation in response to anti-immunoglobulin M (69).
Figure 9.
Schematic summarizing some of the pathways altered by the chronic loss of IP3Rs and MCU. The acute responses to loss of ER–mitochondrial Ca2+ transport in cancer cells as documented in the literature (29, 30, 94) are shown above the dotted line. The adaptive chronic changes measured in the present study are shown below the dotted line. Upregulated or downregulated parameters are indicated by arrows colored red for IP3R TKO and blue for MCU KO. The absence of an arrow indicates no change or minimal change in that parameter. Unlike acute effects, the chronic loss of ER–mitochondrial Ca2+ signaling maintains ATP levels and avoids bioenergetic failure. ER, endoplasmic reticulum; IP3R, inositol trisphosphate receptor; MCU, mitochondrial Ca2+ uniporter.
A stimulation of glycolysis has been noted in several mouse models of MCU KO (34, 55) and MCU KO MEFs (47). The usual explanation offered for this effect is that loss of mitochondrial Ca2+ increases the phosphorylation state of PDH, and therefore diminishes its activity, which diverts pyruvate into lactate formation instead of entry into the TCA cycle. Although there are exceptions (39, 53), the loss of MCU is consistently associated with increased p-PDH (34, 50, 55, 56), as also seen in our MCU KO cells (Figs. 3F and 7F). However, there are several reasons for believing that lowered mitochondrial Ca2+ and p-PDH changes are not the underlying reason for the increased glycolysis. First, the mitochondrial Ca2+ content of WT and KO cells is already very low and can be estimated to be much lower than the Kd of the GEM-GECO probe (i.e., <300 nM). Since the K0.5 for PDH activation by Ca2+ in isolated mitochondria is in the range of 300 to 500 nM (70), the small changes in baseline Ca2+ content are unlikely to have a major impact on levels of p-PDH. Second, only the MCU KO cells show substantially increased PDH phosphorylation, although both MCU KO and IP3R TKO cells have comparable mitochondrial Ca2+ signals. Previous studies in intact mitochondria have shown that the stimulatory effects of Ca2+ on PDH phosphatase are more evident when the activity of PDH kinase is partially inhibited (71). Pyruvate and NADH are both regulators of PDH kinase and their changes in MCU KO cells are in the direction that would act to partially inhibit PDH kinase (72). Since these metabolites do not change in IP3R TKO cells, it is possible that the sensitivity of PDH has shifted to a lower range of mitochondrial Ca2+ content in MCU KO cells. Finally, the direct measurement of isotope tracer flux of [U–13C] glucose carbon into m + 2 citrate showed no significant differences between the cell lines, indicating that the flow of carbon into the TCA cycle was not diminished despite increased p-PDH. A similar finding was made in a mouse heart MCU KO model using NMR to study the metabolism of [1,2-13C] glucose (73). The increased levels of the PDH substrate pyruvate in the MCU KO cells probably contributes to the maintenance of PDH flux, despite enhanced PDH phosphorylation. If PDH flux is not the critical factor then what else could account for enhanced glycolysis in MCU KO cells? Flux through glycolysis is affected by multiple regulatory mechanisms, but one key factor is the cytosolic NAD+/NADH ratio, which modulates the activity of glyceraldehyde 3-phosphate dehydrogenase. A continuous supply of NAD+ to this enzyme is maintained by NADH utilization by the NADH oxidases of the respiratory chain and enzymes such as LDH and malate dehydrogenase in the cytosol. MCU KO cells have an increased NAD+/NADH ratio that is mainly because of a fall in NADH levels (Fig. 2). Both basal respiration and lactate formation are increased in MCU KO cells. Elevated NAD+/NADH ratios with decreased NADH levels has been shown to enhance glycolytic rate in several experimental systems (74, 75, 76, 77). An additional factor that may contribute to enhanced glycolysis in MCU KO cells is the activation of AMPK (Fig. 3E), which is known to phosphorylate and stimulate phosphofructokinase-2 (78).
Maintaining the higher growth rate of MCU KO cells requires an increased supply of both energy and biosynthetic building blocks. It is well established that glutamine metabolism contributes to both functions (79), and enhanced glutamine metabolism often accompanies increased aerobic glycolysis in cancer cells (60). Stimulated glutamine metabolism has been reported in MCU KO fibroblasts (47). Our experiments with [U–13C] glutamine show that MCU KO HEK293T cells have an increased flow of glutamine carbon into the TCA cycle, and the metabolism of α-KG is enhanced in both the oxidative and reductive directions (Fig. 6). The increased flux in the oxidative direction occurs independently of any increase in mitochondrial Ca2+ and may be related to the increased citrate (Fig. S2B) and lowered NAD(P)H (Figs. 2G and S2A), both of which are allosteric modifiers of mitochondrial isocitrate dehydrogenase-3. Increased flux of α-KG in the oxidative pathway is also required to supply the reducing equivalents required for RC (80). RC is commonly observed in the absence of an adequate supply of acetyl-CoA from PDH, as seen, for example, in hypoxia (81). Since PDH flux is not altered in MCU KO cells, the stimulated RC is more likely to be secondary to increased α-KG availability provided by enhanced glutamine metabolism. Interestingly, increased RC also accompanies stimulated glycolysis (82, 83) or high extracellular lactate (84). Both these conditions prevail in MCU KO cells. A further important conclusion from the 13C-tracer studies is the lack of suppression of basal flux through any of the three Ca2+-sensitive TCA cycle dehydrogenases when MCU or IP3Rs are deleted, implying that any small decreases in basal mitochondrial [Ca2+] occurring in the KO cells are not sufficient to impact flux through these enzymes. This conclusion contrasts with the recent findings of Cardenas et al. (29) that constitutive Ca2+ regulation of α-KG dehydrogenase is required to maintain RC in a mutant osteosarcoma cell line with defective oxidative phosphorylation.
The main changes observed in IP3R TKO HEK293 cells were a reduced growth rate, reduced oxygen consumption, and an increased basal rate of autophagy with no changes in glucose or glutamine consumption. Some of these observations are in agreement with previous studies. A lower rate of proliferation and migration in IP3R TKO HEK293 cells has been reported recently (31). A reduced baseline and FCCP-stimulated oxygen consumption was observed in Seahorse assays (85). Acute inhibition or chronic loss of IP3Rs promotes an increase in basal autophagy in several cell types, but different mechanisms for this effect have been proposed (86, 87). In our studies, it is clear that the effects are independent of AMPK activation. Only minor changes in metabolic rewiring could be observed in the 13C-tracer studies, including an increase in anaplerosis of glucose carbon through PC, and an increase in RC of glutamine and palmitate syntheses. All these effects were significantly smaller than observed with MCU KO cells (Figs. 5 and 6). Overall, the data show that the loss of mitochondrial Ca2+ signaling induced by the stable KO of IP3Rs or MCU produce quite distinct phenotypes. We cannot exclude the possibility that some of the changes seen in MCU KO cells may result from the loss of a scaffolding role of the MCU protein itself, rather than the lack of mitochondrial Ca2+ fluxes. Further investigation of the protein–protein interactions of MCU in the mitochondrial matrix and the role of these complexes in regulating the structure/function of mitochondria are required to test this possibility. Electron microscopy images of MCU KO HEK293T cells provided no indication of differences in gross mitochondrial structure or ER/mitochondrial distances (not shown). Both HEK293T and HeLa cells show a decreased agonist-mediated cytosolic Ca2+ response in the MCU KO cells (Figs. 1, A and H, and 7C). However, an increased agonist-mediated Ca2+ transient has been reported in other MCU KO models including MEFs (47) and vascular smooth muscle cells (46), where the result has been attributed to a lack of mitochondrial Ca2+ buffering. The intracellular store Ca2+ content or the dynamics of IP3-mediated Ca2+ puffs were not altered in MCU KO HEK293 cells (69). Thus, mechanisms acting upstream of IP3 synthesis/degradation, or affecting Ca2+ extrusion from the cytosol via plasma membrane or ER Ca2+ pumps, are more likely to be involved. Increased rates of both ER and plasma membrane Ca2+ pumping have been reported in several MCU KO cell types (69). Further studies are required to establish a definitive mechanism for the decreased cytosolic Ca2+ signaling observed in our MCU KO cells.
A hypothesis that has gained general acceptance is that the baseline bioenergetic needs of both normal and cancer cells requires constitutive ER-to-mitochondrial Ca2+ translocation in order to stimulate mitochondrial Ca2+-dependent dehydrogenases of the TCA cycle (27, 28). It is argued that cancer cells are particularly “addicted” to this process, which, if interrupted, limits ATP supply for cell division and results in cell death (27, 28, 29). The main evidence for this hypothesis has been acquired primarily after acute treatment with the drug Xestospongin-B (which targets the IP3R) and siRNA silencing of MCU or IP3R (27, 28). Our experimental conditions differ because we have examined the long-term effects of MCU and IP3R gene disruption. The studies show that these cells have adapted to maintain their ATP levels, with no requirement for basal mitochondrial or cytosolic Ca2+ signaling. This suggests that glycolysis has adequate excess capacity to maintain ATP requirements and that sufficient mitochondrial Ca2+ is present to operate the TCA cycle and supply biosynthetic precursors. This appears to be true even when glycolysis and glutamine metabolism are accelerated to accommodate the increased growth rate of MCU KO cells. However, differences in behavior are revealed when the cells are switched from glucose to galactose so that they predominantly rely on the TCA cycle for both energy and biosynthetic precursors. Under these conditions, the MCU KO cells show evidence of bioenergetic failure with ATP loss, AMP accumulation, and cell death (Fig. 9). This phenotype is suppressed in the MCU-rescue cells. Surprisingly, the IP3R TKO cells are unaffected by the galactose switch, again underscoring that basal metabolism is relatively insensitive to Ca2+ in these cells. A cell death assay using a 24 h period of incubation of K562 leukemic cells in galactose has been used to screen for genes involved in oxidative phosphorylation (88). Further studies are needed to determine if the behavior of the MCU KO cells in galactose medium simply reflects the inability to meet ATP demand (e.g., for mitosis), or if there are secondary defects in the oxidative phosphorylation machinery.
Calcium is viewed as a universal second messenger regulating a wide variety of biological processes (1). Global and conditional mouse KOs of IP3R isoforms have supported a biologically important role for intracellular Ca2+ signals (15). Yet, it is also apparent that several cancer cells (e.g., HEK293, HeLa, and DT40 lymphocytes) can survive the loss of all three IP3R isoforms. Several evolutionary lineages either lack recognizable IP3Rs (89, 90) or have independently lost IP3Rs while retaining MCU homologs (91, 92). Thus, the phenotype of cells without Ca2+ signaling may utilize ancestral Ca2+-independent signaling mechanisms and/or additional transcriptional rewiring resulting from disruption of IP3R genes. Future RNA-Seq studies could provide useful information on the molecular mechanisms underlying the adaptive responses of growth and metabolism seen after loss of IP3Rs and MCU. Targeting Ca2+ signaling with drugs that block ER–mitochondrial communication is being explored as a strategy for the treatment of various diseases where there is aberrant translocation of Ca2+ into the mitochondria (93, 94, 95). Chronic use of these drugs may induce metabolic and bioenergetic adaptations similar to those seen in the present study. Understanding these mechanisms would aid in optimizing the therapeutic benefits of blocking Ca2+ signaling in disease states.
Experimental procedures
Cell lines and culture
All cells were cultured at 37 °C and 5% CO2 in DMEM supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin, and 0.25 μg/ml amphotericin B. HEK293 IP3R TKO cells and HeLa IP3R TKO cells were made as described previously (30, 32). Most of the experiments in this study used HEK293T MCU KO cells made by TALEN knockdown that were obtained from the laboratory of Dr Vamsi Mootha (48). The appropriate controls for the IP3R TKO and MCU KO cells are WT HEK293 cells and HEK293T cells, respectively. We have compared several parameters investigated in the present study and did not find them to be significantly different in the controls (Figs. 1A and 2A and S1). Therefore, the main figures show only the WT HEK293 cells as the controls for both IP3R TKO and MCU KO cells.
Ca2+ measurements
Trypsin-released cells were seeded on a black, clear-bottom, and 96-well plate at a density of 5 × 105 cells/well and incubated at 37 °C and 5% CO2 for 24 h. Media were aspirated from all wells of the plate and replaced with 190 μl of Ca2+-assay buffer (Hank's balanced salt solution containing MgCl2 [1 mM], CaCl2 [2.3 mM], and 20 mM Hepes [pH 7.2]) supplemented with 5 μM Fluo8-AM, 500 μM Brilliant Black, and 100 μM sulfinpyrazone. The plate was maintained in the incubator for 45 min. Changes in cytosolic Ca2+ were monitored using a FlexStation II plate reader in fluorescence mode at 37 °C. Measurements were made using excitation and emission wavelengths of 485 and 525 nm, respectively. The additions of Cch, FCCP, oligomycin, and ionomycin were made by utilizing the automated fluidics system of the hardware. In some experiments, cells were loaded as aforementioned with 1 μM Fura2-AM, and Ca2+ was measured ratiometrically using excitation and emission wavelengths of 340/380 and 510 nm, respectively. Ca2+ fluxes in permeabilized cells were measured after harvesting cells grown in 100 mm plates with trypsin. The cells were centrifuged (1000g; 2 min),washed in Ca2+/Mg2+-free Hank's balanced salt solution and then resuspended in 600 μl of dye buffer (2 μM Fluo-5N, 120 mM KCl, 1 mM MgCl2, 25 mM Hepes [pH 7.2], 5 mM succinate, 2 mM TrisPi, 4 μM rotenone, and 1 μM thapsigargin), and permeabilized using saponin (10 μg). Permeabilization was verified by trypan blue staining. Aliquots (90 μl) were transferred to wells of a black transparent bottom 96-well plate. The plate was maintained at 37 °C, and measurements were made using excitation and emission wavelengths of 491 and 516 nm, respectively.
For imaging experiments, cells were preincubated in a serum-free extracellular medium (121 mM NaCl, 5 mM NaHCO3, 10 mM Na–Hepes, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2 mM CaCl2, and 10 mM glucose [pH 7.4]) containing 2% bovine serum albumin (BSA). For measurements of [Ca2+]c, cells were loaded with 2 μM Cal520-AM (AAT Bioquest) together with 0.003% pluronic F127 and 100 μM sulfinpyrazone for 30 min at 30 °C. At the end of the preincubation or dye-loading period, the cells were washed into fresh extracellular medium containing 0.25% BSA and transferred to the temperature-regulated stage (37 °C) of the microscope. Fluorescence wide-field imaging of [Ca2+]c, and [Ca2+]m was carried out using a back-illuminated electron multiplying charge-coupled device camera (Photometrics). GEM-GECO was excited with 403/10 nm, whereas emission pairs were monitored using 435/30 nm (Ca2+-free) and 550/30 nm (Ca2+-bound) emission filters. Cal520 was imaged using 490/10 nm excitation filters and a 550 nm long-pass beam splitter, and an image triplet was obtained every 250 ms to maximize kinetic information. Fluorescence is presented as F/F0 or GEM-GECO emission ratios, calculated following background subtraction of the individual wavelengths.
Growth curves
About 2.5 × 105 HEK293, HEK293T, or HeLa cell lines were plated on 100 mm plates. At the indicated times, plates were trypsinized for 4 min and resuspended in 10 ml DMEM. The cell density was counted in triplicate using a hemocytometer.
Metabolite assays
Glucose consumption was measured after a 1 h period in which the cells were incubated in glucose-free medium. About 5 mM glucose was then added, and aliquots of the media (250 μl) were removed for glucose assay at the indicated times. A previously described colorimetric method using glucose oxidase was modified for use on a plate reader as described (96). Lactate was measured in neutralized perchloric acid (PCA) lysates using LDH as previously described (97). Pyruvate was measured with pyruvate oxidase and Amplex red (98). Nicotinamide nucleotides were measured using enzymatic cycling assays. Cells grown on 10 cm2 plates were released with 0.25% trypsin/EDTA and centrifuged (1000g; 5 min). The pellets were resuspended in 1 ml PBS, split into two equal aliquots, and recentrifuged. For NAD+/NADP+ assay, the cell pellet was resuspended with 100 μl 0.6 M PCA. For NADH/NADPH assay, the cell pellet was resuspended in 100 μl of 1 M KOH, heated at 65 °C for 30 min, and briefly sonicated (10 s). All samples were stored at −80 °C prior to assay. NAD+ and NADH were measured by an enzymatic cycling assay using alcohol dehydrogenase (99). NADP+ and NADPH were measured by an enzymatic cycling assay using glucose-6-phosphate dehydrogenase (100). ATP was assayed in neutralized PCA extracts using a luciferase method (101). AMP was assayed using the AMP-glo kit (Promega) following the manufacturer's instructions.
SDS-PAGE/Western blotting
To prepare lysates, cells incubated in DMEM were washed in PBS and lysed in a medium (0.250 ml) containing 1% Triton X-100, 50 mM Tris–HCl (pH 7.8), 150 mM NaCl, 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 20 mM NaF, and a 1× dilution of a complete protease inhibitor mixture (Roche Diagnostics). The lysates were centrifuged at 12,000g for 10 min. The supernatants were denatured in SDS sample buffer. Lysates were boiled at 100 °C for 5 min and then stored at −20 °C until use. Unless otherwise noted, 40 μg of protein were run at 100 V for 90 min on 10% polyacrylamide gels and transferred at 100 V for 60 min onto nitrocellulose membranes. Polyvinylidene difluoride membranes were used in the specific case of LC3 immunoblotting. Membranes were blocked in Tris-buffered saline with Tween-10 supplemented with 5% BSA for 1 h at room temperature. Following 3 × 15 min washes in Tris-buffered saline with Tween-10, the primary antibody was added for 16 h at 4 °C. All the antibodies were used at a dilution of 1:1000 and were obtained from Cell Signaling. Membranes were developed using ECL reagent (Thermo Fisher Scientific). When necessary, membranes were stripped using a buffer containing SDS (2%; w/v), Tris (62.5 mM; pH 6.8), and β-mercaptoethanol (100 mM).
Oxygen consumption
Cells were seeded at a density of 2 × 105 and were grown on 60 mm plates for 5 days. Trypsinized cells (106) resuspended in DMEM were introduced into the chamber of an O2 electrode (Oroboros Instruments GmbH). Baseline rates of O2 consumption and the rates after sequential addition of 5 μM oligomycin, 10 μM FCCP, and 1 μM antimycin A were measured.
Labeled isotope tracing
Cells were seeded on 60 mm plates with 2 × 105 cells in DMEM and grown for 5 days. To label with glutamine, the DMEM, containing all other components except glutamine, was supplemented with 4 mM l-[U–13C] glutamine. The plates were labeled in triplicate with 1 ml of medium for 30 and 60 min. To label with glucose, the DMEM, containing all other components except glucose, was supplemented with 5 mM d-[U–13C] glucose. The plates were labeled in triplicate with 1 ml medium for 5 and 60 min. At the indicated times, the plates were aspirated and rapidly washed in ice-cold PBS. Metabolites were extracted by rocking in 0.5 ml buffer containing 5:3:2 ratios of methanol, acetonitrile, and water for 10 min at 4 °C. The solvent was removed, centrifuged at 12,000g for 10 min, and stored at −80 °C prior to analysis. LC–MS analysis was performed on a Q Exactive HybridQuadrupole-Orbitrap HF-X MS (Thermo Fisher Scientific) equipped with a HESI II probe and coupled to a Vanquish Horizon UHPLC system (Thermo Fisher Scientific). Conditions for chromatography, mass spectroscopy, identification, and quantitation of metabolites were as detailed in the study by Casciano et al. (102).
CRISPR–Cas9 MCU KO and rescue
MCU KO clones of HeLa cells were obtained using Cas9/guide RNA ribonucleoprotein complexes (103). Two CRISPR RNA sequences were used: GAUCGCUUCCUGGCAGAAUUGUUUUAGAGCUAUGCU; UGAACUGACAGCGUUCACGCGUUUUAGAGCUAUGCU. Guide RNA complexes were formed by annealing the CRISPR RNA with transactivating RNA as described by Jacobi et al. (104). The complexes were combined with recombinant Cas9 nuclease and transfected into HeLa cells with Lipofectamine 3000. RNAs and Cas9 were purchased from Integrated DNA Technologies. After 1 week of growth, the cells were subcloned by limiting dilution into 96-well plates. Individual clones were expanded into 35 mm plates and screened for MCU expression by immunoblotting. MCU rescue in MCU KO HEK293T cells was carried out by transfecting a plasmid encoding a C-terminal FLAG-tagged human MCU (Addgene; catalog no.: 50054) using Lipofectamine 3000 and selecting for stable transfectants using hygromycin (20 μg/ml).
Data collection and analysis
Unless otherwise noted, the data are expressed as means ± SEM of three independent experiments, each performed in technical triplicate. GraphPad Prism (GraphPad Software, Inc) was used to generate plots and perform statistical analysis. A complete list of statistical analysis of the figures can be found in Table S1.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Drs Sergio De La Fuente Perez and Mate Katona for helpful advice.
Author contributions
M. P. Y., D. I. Y., K. M., G. H., and S. K. J. conceptualization; D. M. B., D. I. Y., K. M., and S. K. J. methodology; M. P. Y., Z. T. S., D. M. B., D. I. Y., and G. H. formal analysis; D. I. Y., K. M., G. H., and S. K. J. resources; M. P. Y., Z. T. S., D. M. B., D. I. Y., G. H., and S. K. J. data curation; M. P. Y. and Z. T. S. writing–original draft; M. P. Y., D. I. Y., K. M., G. H., and S. K. J. writing–review and editing; M. P. Y. and S. K. J. project administration; M. P. Y., G. H., and S. K. J. funding acquisition.
Funding and additional information
This work was supported by the National Institutes of Health (NIH) RO1 grants GM132611 (to S. K. J.), GM102724 (to G. H.), and DE014756 (to D. I. Y.). Z. T. S. was supported by the NIH/National Cancer Institute CA249950 and CA114046. M. P. Y. and D. M. B. were supported by an NIH National Institute on Alcohol Abuse and Alcoholism training grant T32-AA007463. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Edited by Roger Colbran
Supporting information
Figure S1.
Figure S2.
References
- 1.Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Foskett J.K., White C., Cheung K.H., Mak D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mammucari C., Gherardi G., Rizzuto R. Structure, activity regulation, and role of the mitochondrial calcium uniporter in health and disease. Front. Oncol. 2017;7:139. doi: 10.3389/fonc.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagur R., Hajnoczky G. Intracellular Ca(2+) sensing: Its role in calcium homeostasis and signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Murphy A.N., Kelleher J.K., Fiskum G. Submicromolar Ca2+ regulates phosphorylating respiration by normal rat liver and AS-30D hepatoma mitochondria by different mechanisms. J. Biol. Chem. 1990;265:10527–10534. [PubMed] [Google Scholar]
- 7.Territo P.R., Mootha V.K., French S.A., Balaban R.S. Ca2+ activation of heart mitochondrial oxidative phosphorylation: Role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 8.Giorgio V., Guo L., Bassot C., Petronilli V., Bernardi P. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium. 2018;70:56–63. doi: 10.1016/j.ceca.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M., Nakagawa T., Inoue T., Nagata E., Tanaka K., Takano H., Minowa O., Kuno J., Sakakibara S., Yamada M., Yoneshima H., Miyawaki A., Fukuuchi Y., Furuichi T., Okano H., et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 10.Hisatsune C., Yasumatsu K., Takahashi-Iwanaga H., Ogawa N., Kuroda Y., Yoshida R., Ninomiya Y., Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2007;282:37225–37231. doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- 11.Nakazawa M., Uchida K., Aramaki M., Kodo K., Yamagishi C., Takahashi T., Mikoshiba K., Yamagishi H. Inositol 1,4,5-trisphosphate receptors are essential for the development of the second heart field. J. Mol. Cell. Cardiol. 2011;51:58–66. doi: 10.1016/j.yjmcc.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Jing R., Trexler C., Li Y., Tang H., Pan Z., Zhu S., Zhao B., Fang X., Liu J., Chen J., Ouyang K. Deletion of IP3R1 by Pdgfrb-Cre in mice results in intestinal pseudo-obstruction and lethality. J. Gastroenterol. 2019;54:407–418. doi: 10.1007/s00535-018-1522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Q., Zhao G., Fang X., Peng X., Tang H., Wang H., Jing R., Liu J., Lederer W.J., Chen J., Ouyang K. IP3 receptors regulate vascular smooth muscle contractility and hypertension. JCI Insight. 2016;1 doi: 10.1172/jci.insight.89402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Q., Zhao L., Jing R., Trexler C., Wang H., Li Y., Tang H., Huang F., Zhang F., Fang X., Liu J., Jia N., Chen J., Ouyang K. Inositol 1,4,5-trisphosphate receptors in endothelial cells play an essential role in vasodilation and blood pressure regulation. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikoshiba K. Role of IP(3) receptor in development. Cell Calcium. 2011;49:331–340. doi: 10.1016/j.ceca.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Futatsugi A., Nakamura T., Yamada M.K., Ebisui E., Nakamura K., Uchida K., Kitaguchi T., Takahashi-Iwanaga H., Noda T., Aruga J., Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 17.Uchida K., Aramaki M., Nakazawa M., Yamagishi C., Makino S., Fukuda K., Nakamura T., Takahashi T., Mikoshiba K., Yamagishi H. Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang F., Huang L., Tso A., Wang H., Cui L., Lin L., Wang X., Ren M., Fang X., Liu J., Han Z., Chen J., Ouyang K. Inositol 1,4,5-trisphosphate receptors are essential for fetal-maternal connection and embryo viability. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida K., Nakazawa M., Yamagishi C., Mikoshiba K., Yamagishi H. Type 1 and 3 inositol trisphosphate receptors are required for extra-embryonic vascular development. Developmental Biol. 2016;418:89–97. doi: 10.1016/j.ydbio.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang K., Leandro Gomez-Amaro R., Stachura D.L., Tang H., Peng X., Fang X., Traver D., Evans S.M., Chen J. Loss of IP3R-dependent Ca2+ signalling in thymocytes leads to aberrant development and acute lymphoblastic leukemia. Nat. Commun. 2014;5:4814. doi: 10.1038/ncomms5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang H., Wang H., Lin Q., Fan F., Zhang F., Peng X., Fang X., Liu J., Ouyang K. Loss of IP3 receptor-mediated Ca(2+) release in mouse B cells results in abnormal B cell development and function. J. Immunol. 2017;199:570–580. doi: 10.4049/jimmunol.1700109. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y.J., Huang J., Liu W., Kou X., Tang H., Wang H., Yu X., Gao S., Ouyang K., Yang H.T. IP3R-mediated Ca2+ signals govern hematopoietic and cardiac divergence of Flk1+ cells via the calcineurin-NFATc3-Etv2 pathway. J. Mol. Cell. Biol. 2017;9:274–288. doi: 10.1093/jmcb/mjx014. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara H., Kurosaki M., Takata M., Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor C.W., Rahman T., Tovey S.C., Dedos S.G., Taylor E.J., Velamakanni S. IP3 receptors: Some lessons from DT40 cells. Immunol. Rev. 2009;231:23–44. doi: 10.1111/j.1600-065X.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 25.Wen H., Xu W.J., Jin X., Oh S., Phan C.H., Song J., Lee S.K., Park S. The roles of IP3 receptor in energy metabolic pathways and reactive oxygen species homeostasis revealed by metabolomic and biochemical studies. Biochim. Biophys. Acta. 2015;1853:2937–2944. doi: 10.1016/j.bbamcr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Khan M.T., Joseph S.K. Role of inositol trisphosphate receptors in autophagy in DT40 cells. J. Biol. Chem. 2010;285:16912–16920. doi: 10.1074/jbc.M110.114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardenas C., Miller R.A., Smith I., Bui T., Molgo J., Muller M., Vais H., Cheung K.H., Yang J., Parker I., Thompson C.B., Birnbaum M.J., Hallows K.R., Foskett J.K. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardenas C., Muller M., McNeal A., Lovy A., Jana F., Bustos G., Urra F., Smith N., Molgo J., Diehl J.A., Ridky T.W., Foskett J.K. Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Rep. 2016;14:2313–2324. doi: 10.1016/j.celrep.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardenas C., Lovy A., Silva-Pavez E., Urra F., Mizzoni C., Ahumada-Castro U., Bustos G., Jana F., Cruz P., Farias P., Mendoza E., Huerta H., Murgas P., Hunter M., Rios M., et al. Cancer cells with defective oxidative phosphorylation require endoplasmic reticulum-to-mitochondria Ca(2+) transfer for survival. Sci. Signal. 2020;13 doi: 10.1126/scisignal.aay1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alzayady K.J., Wang L., Chandrasekhar R., Wagner L.E., 2nd, Van Petegem F., Yule D.I. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal. 2016;9 doi: 10.1126/scisignal.aad6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue L., Wang L., Du Y., Zhang W., Hamada K., Matsumoto Y., Jin X., Zhou Y., Mikoshiba K., Gill D.L., Han S., Wang Y. Type 3 inositol 1,4,5-trisphosphate receptor is a crucial regulator of calcium dynamics mediated by endoplasmic reticulum in HEK cells. Cells. 2020;9:275. doi: 10.3390/cells9020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ando H., Hirose M., Mikoshiba K. Aberrant IP3 receptor activities revealed by comprehensive analysis of pathological mutations causing spinocerebellar ataxia 29. Proc. Natl. Acad. Sci. U. S. A. 2018;115:12259–12264. doi: 10.1073/pnas.1811129115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garbincius J.F., Luongo T.S., Elrod J.W. The debate continues - what is the role of MCU and mitochondrial calcium uptake in the heart? J. Mol. Cell. Cardiol. 2020;143:163–174. doi: 10.1016/j.yjmcc.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gherardi G., Nogara L., Ciciliot S., Fadini G.P., Blaauw B., Braghetta P., Bonaldo P., De Stefani D., Rizzuto R., Mammucari C. Loss of mitochondrial calcium uniporter rewires skeletal muscle metabolism and substrate preference. Cell Death Differ. 2018;26:362–381. doi: 10.1038/s41418-018-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chvanov M., Voronina S., Zhang X., Telnova S., Chard R., Ouyang Y., Armstrong J., Tanton H., Awais M., Latawiec D., Sutton R., Criddle D.N., Tepikin A.V. Knockout of the mitochondrial calcium uniporter strongly suppresses stimulus-metabolism coupling in pancreatic acinar cells but does not reduce severity of experimental acute pancreatitis. Cells. 2020;9:1407. doi: 10.3390/cells9061407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgiadou E., Haythorne E., Dickerson M.T., Lopez-Noriega L., Pullen T.J., da Silva Xavier G., Davis S.P.X., Martinez-Sanchez A., Semplici F., Rizzuto R., McGinty J.A., French P.M., Cane M.C., Jacobson D.A., Leclerc I., et al. The pore-forming subunit MCU of the mitochondrial Ca(2+) uniporter is required for normal glucose-stimulated insulin secretion in vitro and in vivo in mice. Diabetologia. 2020;63:1368–1381. doi: 10.1007/s00125-020-05148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flicker D., Sancak Y., Mick E., Goldberger O., Mootha V.K. Exploring the in vivo role of the mitochondrial calcium uniporter in brown fat bioenergetics. Cell Rep. 2019;27:1364–1375.e5. doi: 10.1016/j.celrep.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisbach C.M., Hutto R.A., Poria D., Cleghorn W.M., Abbas F., Vinberg F., Kefalov V.J., Hurley J.B., Brockerhoff S.E. Mitochondrial calcium uniporter (MCU) deficiency reveals an alternate path for Ca(2+) uptake in photoreceptor mitochondria. Sci. Rep. 2020;10:16041. doi: 10.1038/s41598-020-72708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altamimi T.R., Karwi Q.G., Uddin G.M., Fukushima A., Kwong J.Q., Molkentin J.D., Lopaschuk G.D. Cardiac-specific deficiency of the mitochondrial calcium uniporter augments fatty acid oxidation and functional reserve. J. Mol. Cell. Cardiol. 2019;127:223–231. doi: 10.1016/j.yjmcc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Tomar D., Jana F., Dong Z., Quinn W.J., 3rd, Jadiya P., Breves S.L., Daw C.C., Srikantan S., Shanmughapriya S., Nemani N., Carvalho E., Tripathi A., Worth A.M., Zhang X., Razmpour R., et al. Blockade of MCU-mediated Ca(2+) uptake perturbs lipid metabolism via PP4-dependent AMPK dephosphorylation. Cell Rep. 2019;26:3709–3725.e7. doi: 10.1016/j.celrep.2019.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu L., Larson Casey J.L., Andrabi S.A., Lee J.H., Meza-Perez S., Randall T.D., Carter A.B. Mitochondrial calcium uniporter regulates PGC-1alpha expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol. 2019;26:101307. doi: 10.1016/j.redox.2019.101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall D.D., Wu Y., Domann F.E., Spitz D.R., Anderson M.E. Mitochondrial calcium uniporter activity is dispensable for MDA-MB-231 breast carcinoma cell survival. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren T., Zhang H., Wang J., Zhu J., Jin M., Wu Y., Guo X., Ji L., Huang Q., Yang H., Xing J. MCU-dependent mitochondrial Ca(2+) inhibits NAD(+)/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene. 2017;36:5897–5909. doi: 10.1038/onc.2017.167. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Jin M., Wang Y., Zhu J., Tan R., Zhao J., Ji X., Jin C., Jia Y., Ren T., Xing J. MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Signal Transduct. Target Ther. 2020;5:59. doi: 10.1038/s41392-020-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarasov A.I., Semplici F., Ravier M.A., Bellomo E.A., Pullen T.J., Gilon P., Sekler I., Rizzuto R., Rutter G.A. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic beta-cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koval O.M., Nguyen E.K., Santhana V., Fidler T.P., Sebag S.C., Rasmussen T.P., Mittauer D.J., Strack S., Goswami P.C., Abel E.D., Grumbach I.M. Loss of MCU prevents mitochondrial fusion in G1-S phase and blocks cell cycle progression and proliferation. Sci. Signal. 2019;12:eaav1439. doi: 10.1126/scisignal.aav1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardi A.A., Gibb A.A., Arif E., Kolmetzky D.W., Tomar D., Luongo T.S., Jadiya P., Murray E.K., Lorkiewicz P.K., Hajnoczky G., Murphy E., Arany Z.P., Kelly D.P., Margulies K.B., Hill B.G., et al. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat. Commun. 2019;10:4509. doi: 10.1038/s41467-019-12103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sancak Y., Markhard A.L., Kitami T., Kovacs-Bogdan E., Kamer K.J., Udeshi N.D., Carr S.A., Chaudhuri D., Clapham D.E., Li A.A., Calvo S.E., Goldberger O., Mootha V.K. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y., Araki S., Wu J., Teramoto T., Chang Y.F., Nakano M., Abdelfattah A.S., Fujiwara M., Ishihara T., Nagai T., Campbell R.E. An expanded palette of genetically encoded Ca(2)(+) indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan X., Liu J., Nguyen T., Liu C., Sun J., Teng Y., Fergusson M.M., Rovira I.I., Allen M., Springer D.A., Aponte A.M., Gucek M., Balaban R.S., Murphy E., Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmstrom K.M., Pan X., Liu J.C., Menazza S., Liu J., Nguyen T.T., Pan H., Parks R.J., Anderson S., Noguchi A., Springer D., Murphy E., Finkel T. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J. Mol. Cell. Cardiol. 2015;85:178–182. doi: 10.1016/j.yjmcc.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luongo T.S., Lambert J.P., Yuan A., Zhang X., Gross P., Song J., Shanmughapriya S., Gao E., Jain M., Houser S.R., Koch W.J., Cheung J.Y., Madesh M., Elrod J.W. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwong J.Q., Lu X., Correll R.N., Schwanekamp J.A., Vagnozzi R.J., Sargent M.A., York A.J., Zhang J., Bers D.M., Molkentin J.D. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson D.H., Lund P., Krebs H.A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nichols M., Elustondo P.A., Warford J., Thirumaran A., Pavlov E.V., Robertson G.S. Global ablation of the mitochondrial calcium uniporter increases glycolysis in cortical neurons subjected to energetic stressors. J. Cereb. Blood Flow Metab. 2017;37:3027–3041. doi: 10.1177/0271678X16682250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwong J.Q., Huo J., Bround M.J., Boyer J.G., Schwanekamp J.A., Ghazal N., Maxwell J.T., Jang Y.C., Khuchua Z., Shi K., Bers D.M., Davis J., Molkentin J.D. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight. 2018;3 doi: 10.1172/jci.insight.121689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Chen Y. AMPK and autophagy. Adv. Exp. Med. Biol. 2019;1206:85–108. doi: 10.1007/978-981-15-0602-4_4. [DOI] [PubMed] [Google Scholar]
- 58.Yoshii S.R., Mizushima N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017;18:1865. doi: 10.3390/ijms18091865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang C., Chen L., Rabinowitz J.D. Metabolomics and isotope tracing. Cell. 2018;173:822–837. doi: 10.1016/j.cell.2018.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemons J.M., Feng X.J., Bennett B.D., Legesse-Miller A., Johnson E.L., Raitman I., Pollina E.A., Rabitz H.A., Rabinowitz J.D., Coller H.A. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Metallo C.M., Gameiro P.A., Bell E.L., Mattaini K.R., Yang J., Hiller K., Jewell C.M., Johnson Z.R., Irvine D.J., Guarente L., Kelleher J.K., Vander Heiden M.G., Iliopoulos O., Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filipp F.V., Scott D.A., Ronai Z.A., Osterman A.L., Smith J.W. Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res. 2012;25:375–383. doi: 10.1111/j.1755-148X.2012.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reitzer L.J., Wice B.M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 65.Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation. Oncol. Lett. 2012;4:1151–1157. doi: 10.3892/ol.2012.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henry O., Jolicoeur M., Kamen A. Unraveling the metabolism of HEK-293 cells using lactate isotopomer analysis. Bioproc. Biosyst. Eng. 2011;34:263–273. doi: 10.1007/s00449-010-0468-9. [DOI] [PubMed] [Google Scholar]
- 67.Dietmair S., Hodson M.P., Quek L.E., Timmins N.E., Gray P., Nielsen L.K. A multi-omics analysis of recombinant protein production in Hek293 cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguer C., Gambarotta D., Mailloux R.J., Moffat C., Dent R., McPherson R., Harper M.E. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoast R.E., Emrich S.M., Zhang X., Xin P., Arige V., Pathak T., Benson J.C., Johnson M.T., Lakomski N., Hempel N., Han J.M., Dupont G., Yule D.I., Sneyd J., Trebak M. Regulation of interoganellar Ca2+ transfer and NFAT activation by the mitochondrial Ca2+ uniporter. bioRxiv. 2021 doi: 10.1101/438854. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Midgley P.J., Rutter G.A., Thomas A.P., Denton R.M. Effects of Ca2+ and Mg2+ on the activity of pyruvate dehydrogenase phosphate phosphatase within toluene-permeabilized mitochondria. Biochem. J. 1987;241:371–377. doi: 10.1042/bj2410371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCormack J.G. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochem. J. 1985;231:581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugden M.C., Holness M.J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 2003;284:E855–862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 73.Rasmussen T.P., Wu Y., Joiner M.L., Koval O.M., Wilson N.R., Luczak E.D., Wang Q., Chen B., Gao Z., Zhu Z., Wagner B.A., Soto J., McCormick M.L., Kutschke W., Weiss R.M., et al. Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc. Natl. Acad. Sci. U. S. A. 2015;112:9129–9134. doi: 10.1073/pnas.1504705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tilton W.M., Seaman C., Carriero D., Piomelli S. Regulation of glycolysis in the erythrocyte: Role of the lactate/pyruvate and NAD/NADH ratios. J. Lab. Clin. Med. 1991;118:146–152. [PubMed] [Google Scholar]
- 75.Cerdan S., Rodrigues T.B., Sierra A., Benito M., Fonseca L.L., Fonseca C.P., Garcia-Martin M.L. The redox switch/redox coupling hypothesis. Neurochem. Int. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 76.Yellen G. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol. 2018;217:2235–2246. doi: 10.1083/jcb.201803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patgiri A., Skinner O.S., Miyazaki Y., Schleifer G., Marutani E., Shah H., Sharma R., Goodman R.P., To T.L., Robert Bao X., Ichinose F., Zapol W.M., Mootha V.K. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD(+) imbalance. Nat. Biotechnol. 2020;38:309–313. doi: 10.1038/s41587-019-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeon S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016;48 doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J., Pavlova N.N., Thompson C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36:1302–1315. doi: 10.15252/embj.201696151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mullen A.R., Hu Z., Shi X., Jiang L., Boroughs L.K., Kovacs Z., Boriack R., Rakheja D., Sullivan L.B., Linehan W.M., Chandel N.S., DeBerardinis R.J. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7:1679–1690. doi: 10.1016/j.celrep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wise D.R., Ward P.S., Shay J.E., Cross J.R., Gruber J.J., Sachdeva U.M., Platt J.M., DeMatteo R.G., Simon M.C., Thompson C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaude E., Schmidt C., Gammage P.A., Dugourd A., Blacker T., Chew S.P., Saez-Rodriguez J., O'Neill J.S., Szabadkai G., Minczuk M., Frezza C. NADH shuttling couples cytosolic reductive carboxylation of glutamine with glycolysis in cells with mitochondrial dysfunction. Mol. Cell. 2018;69:581–593.e7. doi: 10.1016/j.molcel.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.To T.L., Cuadros A.M., Shah H., Hung W.H.W., Li Y., Kim S.H., Rubin D.H.F., Boe R.H., Rath S., Eaton J.K., Piccioni F., Goodale A., Kalani Z., Doench J.G., Root D.E., et al. A compendium of genetic modifiers of mitochondrial dysfunction reveals intra-organelle buffering. Cell. 2019;179:1222–1238. doi: 10.1016/j.cell.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brodsky A.N., Odenwelder D.C., Harcum S.W. High extracellular lactate causes reductive carboxylation in breast tissue cell lines grown under normoxic conditions. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Filadi R., Leal N.S., Schreiner B., Rossi A., Dentoni G., Pinho C.M., Wiehager B., Cieri D., Cali T., Pizzo P., Ankarcrona M. TOM70 Sustains cell bioenergetics by promoting IP3R3-mediated ER to mitochondria Ca(2+) transfer. Curr. Biol. 2018;28:369–382.e6. doi: 10.1016/j.cub.2017.12.047. [DOI] [PubMed] [Google Scholar]
- 86.Kania E., Roest G., Vervliet T., Parys J.B., Bultynck G. IP3 receptor-mediated calcium signaling and its role in autophagy in cancer. Front. Oncol. 2017;7:140. doi: 10.3389/fonc.2017.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bootman M.D., Chehab T., Bultynck G., Parys J.B., Rietdorf K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium. 2018;70:32–46. doi: 10.1016/j.ceca.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Arroyo J.D., Jourdain A.A., Calvo S.E., Ballarano C.A., Doench J.G., Root D.E., Mootha V.K. A genome-wide CRISPR death screen identifies genes essential for oxidative phosphorylation. Cell Metab. 2016;24:875–885. doi: 10.1016/j.cmet.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mackrill J.J. Ryanodine receptor calcium release channels: An evolutionary perspective. Adv. Exp. Med. Biol. 2012;740:159–182. doi: 10.1007/978-94-007-2888-2_7. [DOI] [PubMed] [Google Scholar]
- 90.Garcia C.R.S., Alves E., Pereira P.H.S., Bartlett P.J., Thomas A.P., Mikoshiba K., Plattner H., Sibley L.D. InsP3 signaling in Apicomplexan parasites. Curr. Top. Med. Chem. 2017;17:2158–2165. doi: 10.2174/1568026617666170130121042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alzayady K.J., Sebe-Pedros A., Chandrasekhar R., Wang L., Ruiz-Trillo I., Yule D.I. Tracing the evolutionary history of inositol, 1, 4, 5-trisphosphate receptor: Insights from analyses of Capsaspora owczarzaki Ca2+ release channel orthologs. Mol. Biol. Evol. 2015;32:2236–2253. doi: 10.1093/molbev/msv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bick A.G., Calvo S.E., Mootha V.K. Evolutionary diversity of the mitochondrial calcium uniporter. Science. 2012;336:886. doi: 10.1126/science.1214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arduino D.M., Wettmarshausen J., Vais H., Navas-Navarro P., Cheng Y., Leimpek A., Ma Z., Delrio-Lorenzo A., Giordano A., Garcia-Perez C., Medard G., Kuster B., Garcia-Sancho J., Mokranjac D., Foskett J.K., et al. Systematic identification of MCU Modulators by orthogonal interspecies chemical screening. Mol. Cell. 2017;67:711–723.e7. doi: 10.1016/j.molcel.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woods J.J., Wilson J.J. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr. Opin. Chem. Biol. 2020;55:9–18. doi: 10.1016/j.cbpa.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Kon N., Murakoshi M., Isobe A., Kagechika K., Miyoshi N., Nagayama T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discov. 2017;3:17045. doi: 10.1038/cddiscovery.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blake D.A., McLean N.V. A colorimetric assay for the measurement of D-glucose consumption by cultured cells. Anal. Biochem. 1989;177:156–160. doi: 10.1016/0003-2697(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 97.Hohorst H.J. 2nd ed. Academic Press; NY: 1965. Methods in Enzymatic Analysis. [Google Scholar]
- 98.Zhu A., Romero R., Petty H.R. A sensitive fluorimetric assay for pyruvate. Anal. Biochem. 2010;396:146–151. doi: 10.1016/j.ab.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davila A., Liu L., Chellappa K., Redpath P., Nakamaru-Ogiso E., Paolella L.M., Zhang Z., Migaud M.E., Rabinowitz J.D., Baur J.A. Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. ELife. 2018;7 doi: 10.7554/eLife.33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu A., Romero R., Petty H.R. An enzymatic fluorimetric assay for glucose-6-phosphate: Application in an in vitro Warburg-like effect. Anal. Biochem. 2009;388:97–101. doi: 10.1016/j.ab.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lust W.D., Feussner G.K., Barbehenn E.K., Passonneau J.V. The enzymatic measurement of adenine nucleotides and P-creatine in picomole amounts. Anal. Biochem. 1981;110:258–266. doi: 10.1016/0003-2697(81)90144-5. [DOI] [PubMed] [Google Scholar]
- 102.Casciano J.C., Perry C., Cohen-Nowak A.J., Miller K.D., Vande Voorde J., Zhang Q., Chalmers S., Sandison M.E., Liu Q., Hedley A., McBryan T., Tang H.Y., Gorman N., Beer T., Speicher D.W., et al. MYC regulates fatty acid metabolism through a multigenic program in claudin-low triple negative breast cancer. Br. J. Cancer. 2020;122:868–884. doi: 10.1038/s41416-019-0711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim S., Kim D., Cho S.W., Kim J., Kim J.S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobi A.M., Rettig G.R., Turk R., Collingwood M.A., Zeiner S.A., Quadros R.M., Harms D.W., Bonthuis P.J., Gregg C., Ohtsuka M., Gurumurthy C.B., Behlke M.A. Simplified CRISPR tools for efficient genome editing and streamlined protocols for their delivery into mammalian cells and mouse zygotes. Methods. 2017;121-122:16–28. doi: 10.1016/j.ymeth.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).