Abstract
Background
Cholangiocarcinoma (CCA) is a cancer arising from the intra‐ and extrahepatic bile ducts. The early stages are often asymptomatic, CCA is frequently diagnosed in an advanced stage and the prognosis of CCA is often dismal.
Objective
Our objective was to estimate the incidence of CCA in Finland and to identify risk factors for CCA, with a special interest in primary sclerosing cholangitis (PSC).
Methods
We identified all CCA cases during 1974–2018 from the Finnish Cancer Registry and calculated age‐standardised incidence rates. Five controls for each case were extracted from the Population Registry, matched by age, gender and municipality of residence at the time of diagnosis. Odds ratios (ORs) for risk factors were estimated with conditional logistic regression and survival estimates with the Kaplan–Meier method.
Results
Incidence of CCA remained stable in both genders; the age‐standardised rate (World Standard) in 2013–2017 in males and females was 1.7 per 100,000 person years and 1.3 per 100,000 person years, respectively. Primary sclerosing cholangitis carried a 30‐fold risk of intrahepatic cholangiocarcinoma (iCCA) and 25‐fold risk of extrahepatic cholangiocarcinoma (eCCA). Diabetes, inflammatory bowel disease and liver cirrhosis were associated with iCCA, whereas cholelithiasis and viral hepatitis C were associated with eCCA. The cumulative 5‐year survival was 4.6%.
Conclusions
The incidence of CCA has been stable. Known risk factors for CCA were confirmed, with PSC having the highest OR. Survival remains poor.
Keywords: cholangiocarcinoma, epidemiology, liver, primary sclerosing cholangitis, risk factors
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumour that originates from the epithelial cells of the bile ducts. It is the second most common hepatobiliary malignancy after hepatocellular carcinoma. 1 CCA is classified into intrahepatic CCA (iCCA), perihilar CCA (pCCA), also known as Klatskin tumours and distal CCA (dCCA). Perihilar CCA and dCCA were previously grouped together as extrahepatic CCA (eCCA). 2 CCA is an aggressive tumour, and the 5‐year survival is under 10%; iCCA has a more dismal prognosis than dCCA. 3 , 4 , 5 The diagnosis is often made in an advanced state, and only one‐third of the patients have a possibility for curative treatment. 6
Key summary.
Established knowledge of this subject
The incidence of cholangiocarcinoma (CCA) has a high geographical variation
Several risk factors have been established in previous studies including choledochal cysts and viral hepatitis
The prognosis is poor with a 5‐year survival of around 9%
Significant or new findings of this study
The incidence of CCA in Finland has not increased over time
Risk factors in Finland were in line with risk factors seen in other studies
The odds ratio for primary sclerosing cholangitis was 30 for intrahepatic CCA and 25 for extrahepatic CCA
There is considerable geographical variation in the age‐adjusted incidence of CCA, with the highest incidence in Asia, which has an incidence for iCCA of up to 2.8 per 100,000 person years compared to Western countries of less than 1.2 per 100,000 person years, and for eCCA 2.2 versus 0.8 per 100 000 person years, respectively. 7 , 8 The incidence of especially iCCA has been shown to increase in many previous studies, even though the results are not consistent. 7 , 9 , 10 , 11 , 12
When studying the long‐term changes in the incidence of CCA, it is important to understand the possibility of its misclassification. It has been reported in many papers that part of the increase in incidence for iCCA could be explained by the World Health Organisation's International Classification of Disease for Oncology (ICD‐O) coding. The ICD‐O‐2 classified pCCA as iCCA instead of eCCA, so it is important to interpret the long‐term trends in both iCCA and eCCA with caution. 12 , 13 , 14
Only a small percentage of CCA cases are associated with known risk factors, while most of the cases are sporadic. 15 Risk factors for CCA include parasitic infections (Clonorchis sinensis, Opisthorchis viverrini), 16 primary sclerosing cholangitis (PSC), biliary duct cysts, hepatolithiasis, hepatitis B or C and toxins. Probable risk factors are cirrhosis, inflammatory bowel disease (IBD), obesity, diabetes, cholelithiasis, alcohol consumption and smoking. Some of the risk factors are associated with intrahepatic and others with eCCA. 8 , 9 , 15 , 17 In line with IBD, the crude incidence of PSC has also increased in most countries, including Finland. 18 , 19
We aimed to determine the incidence of CCA over time and the survival of CCA patients in Finland. We also assessed possible underlying risk determinants for iCCA and eCCA with a special interest in PSC.
MATERIALS AND METHODS
For case‐control analyses on aetiology of CCA, the data on CCA cases diagnosed from 1 January 1971 to 31 December 2014 were retrieved from the population‐based countrywide Finnish Cancer Registry. ICD‐10 codes C22.1, C24.0, C24.8 and C24.9 were used to identify CCA patients. Information on cancer topography, morphology, staging, treatment and municipality was obtained.
For each CCA case, five controls were extracted from the Population Registry of the Digital and Population Data Services Agency, matched by age, gender and municipality at the time of diagnosis. The controls had to be living in Finland on the date of diagnosis of the CCA case (‘index date’). To assess the risk determinants, we linked both the cases and controls to the nationwide patient registry (HILMO) that contains comprehensive healthcare records on inpatients provided by municipal healthcare centres, private healthcare centres and all hospitals. The reports to HILMO include the patient's personal identity code (PIC), admission and discharge dates and discharge diagnoses. The PIC has been given to all residents of Finland since 1967, and it is used as the key in linkages between all main registers in Finland.
As PSC does not have a disease unique ICD‐10 code, PSC diagnoses could not be extracted from HILMO, but we had access to hospital records of all PSC patients in the Helsinki University Hospital (HUS) area from 1990 to 2015. 18 For the current study, we linked those PSC diagnoses done before the index date to all CCA patients and their controls who lived in the HUS area at diagnosis 1990–2014. This region and time‐limited case‐control subset is used in models for assessing the role of PSC as a risk determinant of CCA.
For survival calculations, we used the same cohort of CCA patients from 1971 to 2014 that served as cases in the case‐control study and the HUS subset of the cases. The date of CCA diagnosis was used as the starting point for the survival calculations. The date of death was the outcome event, and the date of emigration or the end of study, 31 December 2014, was used as censoring events. The dates of emigration and death were derived from the Population Registry of the Digital and Population Data Services Agency.
Calculation of the incidences rates of CCA was performed at the end of the study process, when it was possible to use Cancer Registry data until 31 December 2018. Population counts for the incidence calculation were retrieved from Statistics Finland.
Data for the CCA diagnostic code C22.1, C24.0 and C24.9 and morphology codes 8000, 8010, 8020‐8021, 8050, 8070, 8140, 8148, 8160‐8162, 8180, 8260, 8440, 8460 and 8560, were retrieved. The incidence rates were stratified to (1) histologically confirmed CCA, (2) adenocarcinoma and Klatskin (8140 and 8162) and (3) other morphology codes.
Statistical methods
The incidence rates were calculated by sex, year, 5‐year calendar period, 5‐year age group and tumour location. The rates were age standardised to the World Standard Population.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the study variables were estimated with multivariate conditional logistic regression separately for the whole Finland and HUS area. The following variables were available for the whole of Finland: cholelithiasis, diabetes, IBD, liver cirrhosis, viral hepatitis B (HBV) and viral hepatitis C (HCV). Due to high collinearity, IBD and liver cirrhosis were estimated in separate models. Model F1 included cholelithiasis, diabetes, IBD, HBV and HCV. Model F2 included cholelithiasis, diabetes, liver cirrhosis, HBV and HCV.
For the HUS area we also had information on PSC. Due to high collinearity, ORs for IBD, liver cirrhosis and PSC were estimated in separate models. Model H1 included cholelithiasis, diabetes, IBD, HBV and HCV. Model H2 included cholelithiasis, diabetes, liver cirrhosis, HBV and HCV. Model H3 included cholelithiasis, diabetes, PSC, HBV and HCV. Because nobody in the control group had PSC or HBV, unconditional logistic regression was used to estimate ORs for these variables. Cumulative survival was described with Kaplan‐Meier estimates. Statistical analyses were made with SPSS 24 and STATA 16 (StataCorp LP).
RESULTS
Patient characteristics and survival
We identified 6949 CCA cases from 1971 to 2014. The median age at the time of diagnosis was 73 years (range: 24–103 years) for both sexes combined, 70 years (range: 24–96 years) in male (N = 3033) and 76 years (range: 25–103 years) in female (N = 3916). The diagnosis was more common in females (56%).
Data on the treatment of CCA patients were missing in 31% of the patients. The stage of CCA was unknown in 2092 (29%), and the disease was known to be localised in only 1039 (15%) and spread in 3835 (55%) of the cases (Table 1). Of the 6949 CCA patients diagnosed during 1971–2014, 6680 (96%) died before the end of 2014. The median time from diagnosis to death was 2.3 months (Figure 1). Cumulative 5‐year survival was 5.1% for male and 4.3% for female CCA patients.
TABLE 1.
Site, stage at diagnosis and treatment of cholangiocarcinoma in Finland (all regions) 1971–2014, and in HUS area 1990–2014
| All regions 1971–2014 | HUS area 1990–2014 | |||
|---|---|---|---|---|
| N | Percentage | N | Percentage | |
| Site | ||||
| All | 6949 | 100 | 1246 | 100 |
| Intrahepatic | 2572 | 37 | 621 | 50 |
| Extrahepatic | 3555 | 51 | 456 | 37 |
| Unknown origin | 822 | 12 | 165 | 13 |
| Stage | ||||
| Localised carcinoma | 1039 | 15 | 162 | 13 |
| Only regional lymph node metastases | 262 | 4 | 47 | 4 |
| Spread disease | 3573 | 51 | 619 | 50 |
| Unknown | 2075 | 30 | 418 | 34 |
| Treatment | ||||
| Surgery, radical or palliative | 1898 | 27 | 269 | 22 |
| Radical surgery | 562 | 8 | 84 | 8 |
| Chemotherapy | 822 | 12 | 215 | 17 |
| Radiation therapy | 341 | 5 | 69 | 6 |
| Combination of different treatments | 483 | 7 | 333 | 27 |
| No treatment | 2527 | 36 | 365 | 29 |
| No data on treatment | 1333 | 19 | 327 | 27 |
Abbreviation: HUS, Helsinki University Hospital.
FIGURE 1.
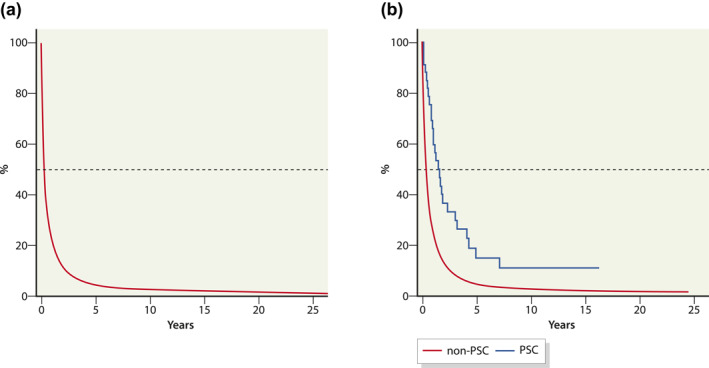
Cumulative survival (%) for all cholangiocarcinoma patients in Finland 1978–2014 (a) and for cholangiocarcinoma patients with and without primary sclerosing cholangitis (PSC) in Helsinki University Hospital area 1990–2014 (b)
In the HUS area, we identified 1246 CCA cases diagnosed in the years 1990–2014. The median age at the time of diagnosis was 71 years (range: 29–100 years) for both sexes combined, 72 years (24–96 years) in male (N = 581) and 74 years (25–103 years) in female (N = 665). Of the 1246 patients, 1193 (96%) died during the study period. The median time from diagnosis to death was 3.2 months. Cumulative 5‐year survival was 5.6% (Figure 1). The study periods for the whole of Finland and the HUS area are different, and therefore, this study does not allow a direct comparison of the results between these two areas.
Incidence
The age‐standardised incidence for CCA, histologically confirmed and unconfirmed, among Finnish males in 1978–2017, 1978–1982 and 2013–2017 was 1.8 per 100,000 person years (95% CI: 1.5–2.1), 1.9 per 100,000 (95% CI: 1.3–2.4) person years and 1.7 per 100,000 (95% CI: 1.4–2.0) person years, respectively (Figure 2). The respective rates in females were 1.5 (95% CI: 1.3–1.7), 1.6 (95% CI: 1.4–1.8) and 1.3 (95% CI: 1.1–1.5). The proportion of histologically confirmed CCA increased over time (Figure 3).
FIGURE 2.
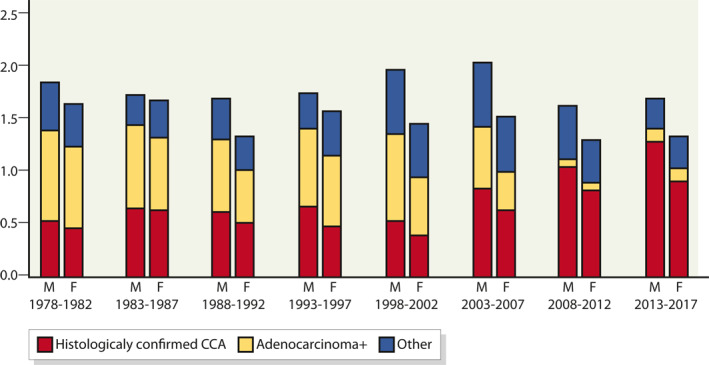
Age‐standardised incidence (World Standard) of cholangiocarcinoma (CCA) per 100,000 person years in Finland, by period and gender
FIGURE 3.
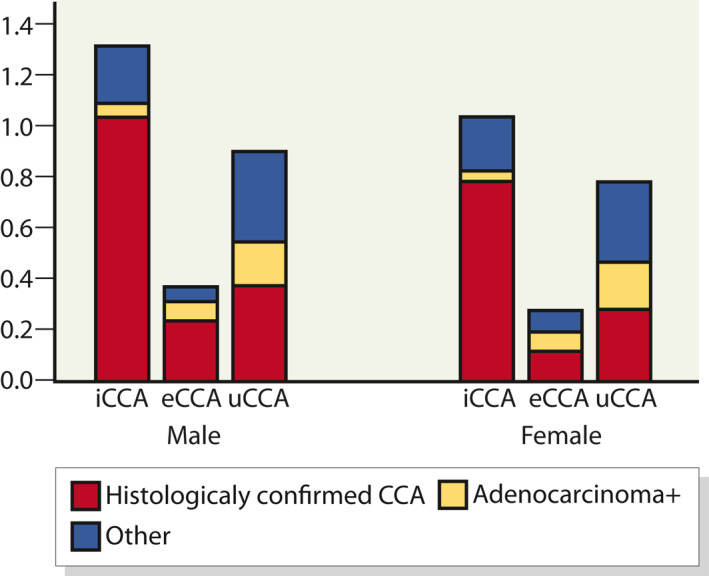
Age‐standardised incidence (World Standard) of cholangiocarcinoma (CCA) 2013–2017 per 100,000 person years, by gender and subclass; intrahepatic CCA (iCCA), extrahepatic CCA (eCCA) and CCA of unknown origin
The incidence of both iCCA and eCCA in 2013–2017 was higher in males than in females (Figure 3). In males, the incidence of iCCA and eCCA was 1.3 (95% CI: 1.1–1.5) per 100,000 person years and 0.4 (95% CI: 0.3–0.5) per 100,000 person years, respectively. The respective rates for females were 1.0 (95% CI: 0.8–1.2) and 0.3 (95% CI: 0.2–0.4). During this period, 35% of CCA cases in male and 36% in females were classified as of unknown origin.
Cholangiocarcinoma is very rarely diagnosed in children or young adults (Figure 4). The incidence rises with age and is highest in the 85+ years category. Males have a higher incidence than females in all age groups except in group 85 years or older.
FIGURE 4.
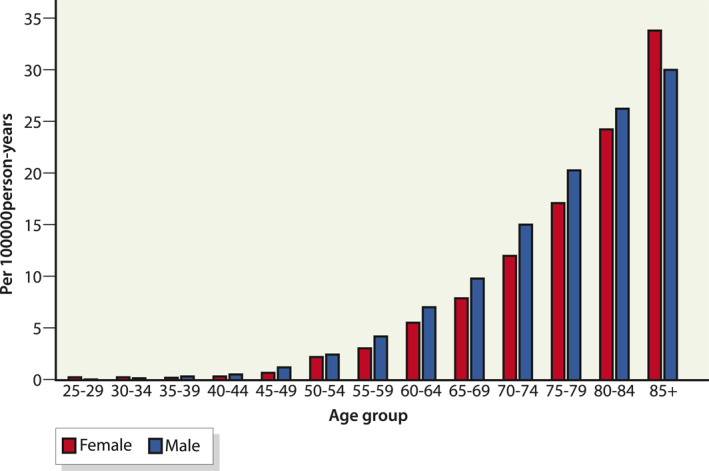
Incidence of cholangiocarcinoma per 100,000 person years in Finland 1971–2018, by sex and age
Risk factors
The ORs for the concomitant medical conditions are listed in Table 2 for the whole of Finland and Table 3 for the HUS area. The OR for IBD in males and in females was 2.7 (95% CI: 2.1–3.5) and 1.3 (95% CI: 0.9–1.7), respectively, in the whole of Finland. In the HUS area, the OR for IBD in males and in females was 7.8 (95% CI: 4.3–14.3) and 2.1 (95% CI: 1.0–4.4), respectively. Primary sclerosing cholangitis is a strong risk factor in both females (OR 36.1, 95% CI: 18.5–70.4) and males (OR: 24.7, 95% CI: 16.3–37.4). For results from the multivariate analysis by sex, see Table S1.
TABLE 2.
OR and 95% CI for preceding comorbidity associated with the risk of CCA and CCA subtypes in Finland (all regions) 1971–2014, derived from multivariate analyses
| Risk determinant | Subtype of CCA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any CCA (N = 6949) | Intrahepatic (N = 2572) | Extrahepatic (N = 3555) | Unknown (N = 822) | |||||||||
| Cases (%) | OR | 95% CI | Cases (%) | OR | 95% CI | Cases (%) | OR | 95% CI | Cases (%) | OR | 95% CI | |
| Cholelithiasis | 1222 (18) | 2.3 | 2.1–2.5 | 348 (14) | 1.8 | 1.5–2.0 | 659 (19) | 2.6 | 2.4–2.9 | 215 (26) | 2.6 | 2.1–3.1 |
| Diabetes | 889 (13) | 1.0 | 0.9–1.1 | 313 (12) | 1.1 | 1.0–1.2 | 404 (11) | 0.8 | 0.7–0.9 | 172 (21) | 1.7 | 1.4–2.1 |
| Inflammatory bowel disease | 336 (5) | 2.0 | 1.6–2.4 | 69 (3) | 2.6 | 1.9–3.4 | 44 (1) | 1.4 | 1.0–2.0 | 18 (2) | 2.0 | 1.2–3.6 |
| Liver cirrhosis | 154 (2) | 2.7 | 2.3–3.1 | 145 (6) | 3.8 | 3.0–4.7 | 78 (2) | 1.6 | 1.2–2.1 | 39 (5) | 3.5 | 2.3–5.3 |
| Hepatitis C | 6 (0.1) | 1.6 | 0.6–4.2 | 4 (0.1) | 2.8 | 0.8–9.5 | 1 (0.03) | 0.7 | 0.1–5.5 | 1 (0.1) | 1.3 | 0.1–20.6 |
| Hepatitis B | 8 (0.1) | 7.0 | 2.2–21.7 | 4 (0.1) | 5.7 | 1.3–25.9 | 3 (0.1) | 16.8 | 1.6–173 | 1 (0.1) | 5.4 | 0.2–119 |
Note: The OR for liver cirrhosis is from model F2 including variables cholelithiasis, diabetes, liver cirrhosis, HBV and HCV. All other ORs are from model F1 (cholelithiasis, diabetes, inflammatory bowel disease, HBV and HCV).
Abbreviations: CCA, cholangiocarcinoma; CI, confidence interval; OR, odds ratio.
TABLE 3.
OR and 95% CI associated with the risk of cholangiocarcinoma and CCA subtypes in HUS area, derived from multivariate analyses
| Risk determinant | Subtype of cholangiocarcinoma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any (N = 1246) | Intrahepatic (N = 621) | Extrahepatic (N = 456) | Unknown (N = 165) | |||||||||
| Cases (%) | OR | 95% CI | Cases (%) | OR | 95% CI | Cases (%) | OR | 95% CI | Cases (%) | OR | 95% CI | |
| Cholelithiasis | 261 (21) | 2.5 | 2.1–2.9 | 108 (17) | 2.0 | 1.6–2.5 | 113 (25) | 3.0 | 2.3–3.8 | 37 (22) | 2.7 | 1.8–4.3 |
| Diabetes | 159 (13) | 1.4 | 1.2–1.7 | 83 (13) | 1.6 | 1.2–2.1 | 51 (11) | 1.1 | 0.8–1.5 | 25 (15) | 1.7 | 1.0–2.8 |
| Inflammatory bowel disease | 38 (3) | 4.5 | 2.9–7.0 | 9 (1) | 5.7 | 3.6–9.9 | 2 (0.4) | 3.1 | 1.3–7.2 | 4 (2) | 2.5 | 0.5–13.7 |
| Liver cirrhosis | 37 (3) | 4.3 | 2.8–6.7 | 21 (4) | 6.0 | 3.2–11.5 | 10 (2) | 2.2 | 1.0–4.7 | 6 (4) | 10.0 | 2.5–40.0 |
| Hepatitis C | 3 (0.2) | 1.2 | 0.2–5.7 | 2 (0.3) | 3.1 | 0.5–18.9 | 0 (0) | a | ‐ | 1 (0.6) | 3.2 | 0.3–35.2 |
| Hepatitis B | 5 (0.4) | 13.8 | 5.7–33.4 | 3 (0.4) | 10.4 | 3.3–32.6 | 1 (0.2) | 35.1 | 4.9–252 | 1 (0.6) | 16.9 | 2.3–122 |
| Primary sclerosing cholangitis | 33 (3) | 29.9 | 21.0–42.4 | 22 (4) | 28.2 | 18.3–43.4 | 9 (2) | 24.2 | 12.4–47.2 | 2 (1) | 18.2 | 4.5–74.0 |
Note: OR for liver cirrhosis from model H2 (cholelithiasis, diabetes, liver cirrhosis, HBV and HCV). OR for primary sclerosing cholangitis from model H3 (cholelithiasis, diabetes, primary sclerosing cholangitis, HBV and HCV). All other ORs are from model H1, including cholelithiasis, diabetes, inflammatory bowel disease, HBV and HCV.
Abbreviations: CCA, cholangiocarcinoma; CI, confidence interval; HUS, Helsinki University Hospital; OR, odds ratio.
There were no cases of hepatitis C in the extrahepatic CCA group.
Of the CAA patients in the HUS area, PSC was identified in 33 (2.6%). The median age at the time of diagnosis of CCA was 51 years (range: 29–79 years). Median survival from the diagnosis of CCA to death was 12.4 months, and the cumulative 5‐year survival was 3.6% (Figure 4). The median time from PSC diagnosis to CCA was 1.6 years (range: 0–18 years). Of the 33 patients with both PSC and CCA, 23 (70%) had concomitant IBD. In all CCA patients in the HUS area, IBD was diagnosed in 83 CCA patients (6.6%).
DISCUSSION
In this population‐based study, the age‐adjusted incidence of CCA did not rise during the study period 1978–2017. The incidence of CCA in the United States rose by 65% from 1973 to 2012, 20 and a much shorter study period in France (2000–2012), the incidence again did not increase. 21
It was not possible to reliably describe long‐term trends in incidence of iCCA and eCCA because of changes in coding over time. Our results for age‐standardised iCCA and eCCA incidence rates in 2013–2017 are comparable with the incidence rates for iCCA and eCCA in other Northern European countries. 7
Risk factors for CCA have not previously been evaluated in Finland. Our ORs for cholelithiasis (2.3) and HCV (1.6) were similar to prior results in other studies. 15 As reported in previous studies, HCV was associated with iCCA. For HBV, the risk increase in our study was present for both iCCA and eCCA, with a higher OR for eCCA, while in previous studies the OR for iCCA has been higher. The prevalence of HBV in Finland is very low, and the absolute number of HBV patients in our study was accordingly very low. 22
Finland has the highest incidence of type 1 diabetes in the world, in children under 14 around 60 per 100,000 person years. 23 About 15% of the prevalent diabetes cases in Finland are of type 1. 24 As our study is based on ICD codes, some type 1 diabetics were wrongly diagnosed as type 2, and vice versa, and many patients had ICD codes for both types. Therefore, we pooled diabetes types 1 and 2 together. In previous studies, type 2 diabetes as well as obesity and metabolic syndrome, all of which can lead to non‐alcoholic fatty liver disease, 25 have been shown to increase the risk for CCA. 26 Non‐alcoholic fatty liver disease can cause steatohepatitis, which may lead to liver cirrhosis and further to an increased risk for CCA. 25
Primary sclerosing cholangitis is associated with many gastrointestinal malignancies, of which the most dreaded is CCA. 27 , 28 , 29 In previous studies, the risk for CCA has been up to 235‐ to 400‐fold. 18 , 19 Because PSC does not have a unique ICD code, it is very challenging to reliably identify PSC patients from ICD‐code‐based registers. In Finland, we have an active search strategy for PSC, and in our previous study, we identified all PSC patients in the HUS area during 1990–2014. 18 This gave us a unique opportunity to study PSC as a risk determinant for CCA in the HUS area, which has not been possible in previous studies.
The median age at the time of diagnosis of CCA in PSC patients was clearly lower than in other CCA patients: 51 versus 73 years. The lifetime risk for CCA in PSC patient is as high as 7%–14%. 13 , 18 The prognosis of CCA in PSC patients was very poor, and the median time from diagnosis to death was only 1.6 years. There are several recommendations for surveillance of CCA in PSC patients, 13 , 30 , 31 , 32 but at present, there is no common consensus for surveillance strategy. It would be of paramount importance to have a working strategy for identifying CCA in PSC at a premalignant stage. Accordingly, it is important to be able to identify the patients at high risk for CCA to better target the biliary dysplasia surveillance. In previous studies, up to half of CCAs are detected already at the time of PSC diagnosis or within 1 year after diagnosing PSC. 33 , 34 In our study, the proportion of CCA cases with PSC was low, most likely due to the fact that if the first presenting diagnosis is CCA, no further evaluation of the underlying predisposing factor is done. Therefore, PSC might be underdiagnosed as a risk factor for CCA.
Survival in all CCA patients after diagnosis is also poor. The survival estimates for the HUS area are higher than in the whole of Finland, but these two are not comparable, as the HUS area only covers the period 1990–2014 and the data for the whole of Finland also include CCA patients diagnosed from 1971 to 1989.
Inflammatory bowel disease also increases the risk of CCA. In earlier studies, 20%–76% of PSC patients had concomitant IBD. 35 , 36 In the current data, 69% of the CCA patients with PSC had concomitant IBD, which is in accordance with the rate of IBD in all PSC patients in the area. 18
Strengths and limitations of the study
The registries in Finland are of high quality and gave us a comprehensive look at malignancy information, risk determinants and vital status at a nationwide level. 37 A limitation is that the discharge register is for hospitals and not for primary care, and some asymptomatic or mild pre‐existing diseases might therefore not be in the register. Another limitation is that the registers do not include information on weight, smoking or alcohol consumption, which are known risk factors for CCA. 38
CONCLUSION
The incidence of CCA in Finland has not increased during the study period. The incidence rates of iCCA and eCCA are comparable to the incidence rates in other Northern European countries. Previously known risk factors were identified in this nationwide analysis, with PSC having the strongest association. The prognosis of CCA is poor.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICS APPROVAL
The local ethics committee of Helsinki University Hospital for Internal Medicine has approved the study protocol, number 278/13/03/01/2009.
AUTHOR CONTRIBUTIONS
Nina Barner‐Rasmussen, Martti Färkkilä and Eero Pukkala: study design and drafting of the manuscript; Nina Barner‐Rasmussen, Martti Färkkilä, Eero Pukkala and Kishor Hadkhale: data analysis. All authors: critical revision of the manuscript and interpretation of the data. All authors have approved the final draft submitted. Guarantor of the article: Martti Färkkilä, martti.farkkila@hus.fi.
INFORMED CONSENT
No informed consent was required in accordance with Finnish regulations for register‐based studies without contact with the study subjects.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This study was supported by a grant from Finska Läkaresällskapet and Svenska Kulturfonden. The funding sources had no involvement in the study.
Barner‐Rasmussen N, Pukkala E, Hadkhale K, Färkkilä M. Risk factors, epidemiology and prognosis of cholangiocarcinoma in Finland. United European Gastroenterol J. 2021;9(10):1128–35. 10.1002/ueg2.12154
DATA AVAILABILITY STATEMENT
Data subject to third party restrictions.
REFERENCES
- 1. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krasinskas AM. Cholangiocarcinoma. Surg Pathol Clin. 2018;11(2):403–29. [DOI] [PubMed] [Google Scholar]
- 3. Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122(5):1500–11. [DOI] [PubMed] [Google Scholar]
- 4. Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69(3):259–70. [DOI] [PubMed] [Google Scholar]
- 5. Waseem D, Tushar P. Intrahepatic, perihilar and distal cholangiocarcinoma: management and outcomes. Ann Hepatol. 2017;16(1):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–69. [DOI] [PubMed] [Google Scholar]
- 7. Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126(11):2666–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(Suppl 1):19–31. [DOI] [PubMed] [Google Scholar]
- 9. Welzel TM, Graubard BI, El‐Serag HB, Shaib YH, Hsing AW, Davila JA, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population‐based case‐control study. Clin Gastroenterol Hepatol. 2007;5(10):1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jepsen P, Vilstrup H, Tarone RE, Friis S, Sorensen HT. Incidence rates of intra‐ and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99(11):895–7. [DOI] [PubMed] [Google Scholar]
- 11. Patel N, Benipal B. Incidence of cholangiocarcinoma in the USA from 2001 to 2015: a US cancer statistics analysis of 50 states. Cureus. 2019;11(1):e3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty‐year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):221–32. [DOI] [PubMed] [Google Scholar]
- 14. Cardinale V, Semeraro R, Torrice A, Gatto M, Napoli C, Bragazzi MC, et al. Intra‐hepatic and extra‐hepatic cholangiocarcinoma: new insight into epidemiology and risk factors. World J Gastrointest Oncol. 2010;2(11):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clements O, Eliahoo J, Kim JU, Taylor‐Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta‐analysis. J Hepatol. 2020;72(1):95–103. [DOI] [PubMed] [Google Scholar]
- 16. Razumilava N, Gores GJ. Cholangiocarcinoma. The Lancet. 2014;383(9935):2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population‐based study in SEER‐Medicare. PloS One. 2017;12(10):e0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barner‐Rasmussen N, Pukkala E, Jussila A, Farkkila M. Epidemiology, risk of malignancy and patient survival in primary sclerosing cholangitis: a population‐based study in Finland. Scand J Gastroenterol. 2020;55(1):74–81. [DOI] [PubMed] [Google Scholar]
- 19. Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Sapanier BW, Poen AC, et al. Population‐based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58(6):2045–55. [DOI] [PubMed] [Google Scholar]
- 20. Mukkamalla SKR, Naseri HM, Kim BM, Katz SC, Armenio VA. Trends in incidence and factors affecting survival of patients with cholangiocarcinoma in the United States. J Natl Compr Canc Netw. 2018;16(4):370–6. [DOI] [PubMed] [Google Scholar]
- 21. Al Mahjoub A, Bouvier V, Menahem B, Bazille C, Fohlen A, Alves A, et al. Epidemiology of intrahepatic, perihilar, and distal cholangiocarcinoma in the French population. Eur J Gastroenterol Hepatol. 2019;31(6):678–84. [DOI] [PubMed] [Google Scholar]
- 22. Karvonen T, Auranen K, Kuusi M, Leino T. Epidemiology of hepatitis B infection in Finland: implications for immunisation policy. Vaccine. 2017;35(3):412–8. [DOI] [PubMed] [Google Scholar]
- 23. Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA. 2013;310(4):427–8. [DOI] [PubMed] [Google Scholar]
- 24. Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777–82. [DOI] [PubMed] [Google Scholar]
- 25. Valenti L, Bugianesi E, Pajvani U, Targher G. Nonalcoholic fatty liver disease: cause or consequence of type 2 diabetes? Liver Int. 2016;36(11):1563–79. [DOI] [PubMed] [Google Scholar]
- 26. Wongjarupong N, Assavapongpaiboon B, Susantitaphong P, Cheungpasitporn W, Treeprasertsuk S, Rerknimitr R, et al. Non‐alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta‐analysis. BMC Gastroenterology. 2017;17(1):149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fevery J, Henckaerts L, Van Oirbeek R, Vermeire S, Rutgeerts P, Nevens F, et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long‐term single‐centre study. Liver Int. 2012;32(2):214–22. [DOI] [PubMed] [Google Scholar]
- 28. Sørensen JO, Nielsen OH, Andersson M, Ainsworth MA, Ytting H, Bélard E, et al. Inflammatory bowel disease with primary sclerosing cholangitis: A Danish population‐based cohort study 1977‐2011. Liver Int. 2018;38(3):532–41. [DOI] [PubMed] [Google Scholar]
- 29. Claessen MMH, Vleggaar FP, Tytgat KMAJ, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50(1):158–64. [DOI] [PubMed] [Google Scholar]
- 30. Aabakken L, Karlsen TH, Albert J, Arvanitakis M, Chazouilleres O, Dumonceau JM, et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. 2017;49(6):588–608. [DOI] [PubMed] [Google Scholar]
- 31. Bowlus CL, Lim JK, Lindor KD. AGA clinical practice update on surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis: expert review. Clin Gastroenterol Hepatol. 2019;17(12):2416–22. [DOI] [PubMed] [Google Scholar]
- 32. Folseraas T, Boberg KM. Cancer risk and surveillance in primary sclerosing cholangitis. Clin Liver Dis. 2016;20(1):79–98. [DOI] [PubMed] [Google Scholar]
- 33. Valle MB, Bjornsson E, Lindkvist B. Mortality and cancer risk related to primary sclerosing cholangitis in a Swedish population‐based cohort. Liver Int. 2011;21:441–8. [DOI] [PubMed] [Google Scholar]
- 34. Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25‐year single‐centre experience. Eur J Gastroenterol Hepatol. 2012;24(9):1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindkvist B, Benito de Valle M, Gullberg B, Bjornsson E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010;52(2):571–7. [DOI] [PubMed] [Google Scholar]
- 36. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–8. [DOI] [PubMed] [Google Scholar]
- 37. Pukkala E, Engholm G, Hojsgaard Schmidt LK, Storm H, Khan S, Lambe M, et al. Nordic Cancer Registries – an overview of their procedures and data comparability. Acta Oncol. 2018;57(4):440–55. [DOI] [PubMed] [Google Scholar]
- 38. Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40(6):505–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data subject to third party restrictions.


