Abstract
Background
Diabetic constipation is traditionally attributed to slow colonic transit, despite limited evidence. More than half of patients find treatment unsatisfactory. To improve treatment, there is a need for better diagnostic understanding of the condition.
Objective
In this wireless motility capsule study, we aimed to investigate gastrointestinal transit and contractility in diabetes patients with and without constipation, and in healthy controls.
Methods
We prospectively included type 1 or type 2 diabetes patients with gastrointestinal symptoms. Based on the Gastrointestinal Symptom Rating Scale we distinguished into two groups: with constipation and without constipation. Non‐diabetic controls were asymptomatic. All were examined with wireless motility capsule, determining transit times and contractility parameters.
Results
57 patients (42 women, 46 with type 1 diabetes) and 26 healthy controls (14 women) were included. We found no difference in transit times between diabetes patients with and without constipation. Compared to healthy controls (35:55, h:min), whole‐gut transit was slower in both diabetes patients with constipation (66:15, p = 0.03) and without constipation (71:16, p < 0.001). Small bowel motility index correlated r s = −0.32 (p = 0.01) with constipation symptoms.
Conclusions
Diabetes patients with constipation had similar transit times as those without constipation. Both groups had slower whole‐gut transit than healthy controls. Constipation was associated with reduced small bowel, but not colonic contractility. Our results imply that other mechanisms than slow colonic transit may be more important in the pathogenesis of diabetic constipation.
Keywords: constipation, diabetes mellitus, gastroenteropathy, motility, transit, wireless motility capsule
Key Summary.
Summarise the established knowledge on this subject
Constipation is very frequent in diabetes and often has a large impact on quality of life. Half of all patients find treatment unsatisfactory.
Diabetic constipation has traditionally been attributed to slow colonic transit, but this knowledge is based on a small number of decades‐old studies.
What are the significant and/or new findings of this study?
Using wireless motility capsule, we investigated gastrointestinal transit times and contractility in diabetes patients with and without constipation, and in healthy controls.
We found no difference in transit between diabetes patients with and without constipation, but both diabetes groups had slower whole‐gut transit than healthy controls. We also found an association between constipation and reduced small bowel contractility.
Our results may indicate that slow colonic transit is less important in the pathogenesis of diabetic constipation than previously believed. When evaluating these patients, clinicians should consider other disease mechanisms.
INTRODUCTION
Gastrointestinal symptoms are common in diabetes, and constipation is especially frequent. 1 , 2 In tertiary centres, up to 60% report constipation, while community studies have found a prevalence of 10%–17%. 1 , 2 Constipation leads to reduced quality of life in half of the patients, and a similar proportion find the treatment unsatisfactory. 3 Causes may be multifactorial, including dietary factors, medications and comorbid conditions, but is often due to diabetic gastroenteropathy, a dysmotility disorder potentially affecting the entire gastrointestinal tract. 4
Constipation has traditionally been defined as less than three weekly bowel movements, but recent Rome criteria have also included symptoms of straining, incomplete evacuation, anorectal obstruction, hard faeces or the need of manual stimulation to facilitate defecation. 5 Constipation can be categorised into normal‐transit constipation, slow‐transit constipation and rectal evacuation disorders. 6 Diabetic constipation has traditionally been associated with slow colonic transit, but this knowledge is based on a limited number of studies, often including few patients suffering from constipation. 7 , 8 , 9 Other studies have been retrospective, registry‐based, designed to investigate different hypotheses or contained a mixed constipation cohort, where diabetes patients constituted a minority. 10 , 11
The two most established methods for measuring colonic transit are radiopaque markers and colonic scintigraphy, but both have disadvantages, such as radiation exposure, poor standardisation and only providing motility results from one single gastrointestinal segment. 12 The wireless motility capsule, however, is not depending on radiological examinations and measures transit through all gut regions in one test. 13 It also has the added advantage of measuring contractility parameters, such as contractions per minute and the motility index. 14 These measurements might provide valuable information about intestinal motility, but their utility in diabetic constipation is so far undefined.
Consequently, in this study, we hypothesised that diabetes patients with constipation had delayed colonic transit and reduced intestinal contractility compared to diabetes patients without constipation, and healthy controls.
MATERIALS AND METHODS
Study population
The study was a cross‐sectional case–control observational study with consecutive inclusion. It was performed at a tertiary centre at Haukeland University Hospital, Bergen, Norway between 2014 and 2018. Two groups were included: diabetes patients and healthy controls. Exclusion criteria for both groups were age < 18 years, breastfeeding or pregnancy, previous major intra‐abdominal surgery or inability to adhere to the study protocol.
Diabetes patients
In the patient group, inclusion criteria were type 1 diabetes or type 2 diabetes, chronic gastrointestinal symptoms (minimum duration >6 months), and a normal upper endoscopy during the last 2 years. Patients were referred from all of Norway for diagnostic evaluation at Haukeland University Hospital. They were admitted for the first 3 days of the study period and were outpatients for the last five. While at hospital, they were evaluated by a physician, delivered blood‐, urine‐ and stool samples (Table 1), and underwent tests of gastrointestinal motility. Questionnaires were distributed in advance and collected at admittance. During fast and examinations, patients received glucose‐insulin infusion (target glucose level 4–10 mmol/L).
TABLE 1.
Clinical characteristics of diabetes patients with constipation, without constipation and healthy controls
| Variables | Diabetes | Healthy controls n = 26 | p‐value | ||
|---|---|---|---|---|---|
| Constipation n = 15 | No constipation n = 42 | p‐value | |||
| General demographics | |||||
| Women, n (%) | 14 (93%) | 28 (67%) | 0.08 | 14 (54%) | 0.03 a |
| Age, years, mean (SD) | 51 (9) | 47 (13) | 0.19 | 42 (15) | 0.07 |
| BMI, kg/m2, mean (SD) | 28.2 (5.8) | 26.2 (5.8) | 0.29 | 24.0 (2.2) | 0.12 |
| Current smokers, n (%) | 2 (13%) | 15 (36%) | 0.08 | ‐ | ‐ |
| Diabetes status | |||||
| Type 1 diabetes, n (%) | 14 (93%) | 32 (76%) | 0.26 | ‐ | ‐ |
| Diabetes duration, years, mean (SD) | 34 (10) | 24 (13) | 0.009 | ‐ | ‐ |
| Late complications, n (%) | 11 (73%) | 29 (69%) | 1.0 | ‐ | ‐ |
| Retinopathy, n (%) | 9 (60%) | 23 (55%) | 0.77 | ‐ | ‐ |
| Nephropathy, n (%) | 3 (20%) | 12 (29%) | 0.74 | ‐ | ‐ |
| Peripheral neuropathy, n (%) | 6 (40%) | 19 (45%) | 0.77 | ‐ | ‐ |
| Diabetic wounds, n (%) | 3 (20%) | 4 (10%) | 0.37 | ‐ | ‐ |
| Cardiovascular disease, n (%) | 2 (13%) | 3 (7%) | 0.60 | ‐ | ‐ |
| Biochemistry | |||||
| B‐HbA1c, mmol/mol | 63 (9) | 67 (31) | 0.23 | ‐ | ‐ |
| S‐TSH, mIE/L | 1.3 (1.2) | 1.5 (1.0) | 0.33 | ‐ | ‐ |
| P‐fT4, pmol/L | 16.8 (5.8) | 15.6 (3.9) | 0.52 | ‐ | ‐ |
| U‐ACR, mg/mmol | 0.7 (10.9) | 2.0 (4.7) | 0.17 | ‐ | ‐ |
| F‐calprotectin, mg/kg | 34 (24) | 15 (34) | 0.73 | ‐ | ‐ |
| F‐elastase‐1, mg/g | 473 (149) | 500 (233) | 0.62 | ‐ | ‐ |
Note: Results are given as median (IQR) unless otherwise indicated. Frequencies are given as n (%), where percentages are calculated from the total n in each column. Biochemical reference values as used at Haukeland University Hospital: B‐HbA1c, 20–42 mmol/mol; S‐TSH, 0.40–4.50 mIE/L; P‐fT4, 8.0–21.0 pmol/L; U‐ACR, 0–2.5 mg/mmol; F‐calprotectin, <50 mg/kg; and F‐elastase‐1, <200 mg/g.
Abbreviations: ACR, albumin to creatinine ratio; B, whole blood; F, faecal; FT4, free thyroxine; HbA1c, glycosylated haemoglobin; IQR, interquartile range; P, plasma; S, serum; SD, standard deviation; TSH, thyroid stimulating hormone; U, urinary.
Sub‐group analyses: higher percentage of women in the group with constipation compared with healthy controls (p = 0.01), not compared to the group without constipation (n = 0.32).
Healthy controls
As part of a collaborative study, healthy volunteers were examined with wireless motility capsule. 15 All were screened for gastrointestinal symptoms by modified Rome III questionnaires and interviewed by a clinical investigator (physician or study nurse) to rule out pre‐existing conditions or use of drugs potentially affecting gastrointestinal motility.
Motility capsule testing
The wireless motility capsule (SmartPill®, Medtronic) measures pH, temperature and pressure throughout the gastrointestinal tract (Figure 1). After an overnight fast, the capsule was swallowed together with a 260‐kcal nutrient bar (SmartBar®, Medtronic) and 120 mL of water. To achieve simultaneous examination with gastric emptying scintigraphy, diabetes patients also ingested a radiolabelled boiled egg (90 kcal). Prior to the investigation and during the study, participants had to pause medications possibly altering gastrointestinal motility. Full details are presented in a previous article. 16 For data analyses, we used MotiliGI® software version 3.0 (Medtronic).
FIGURE 1.
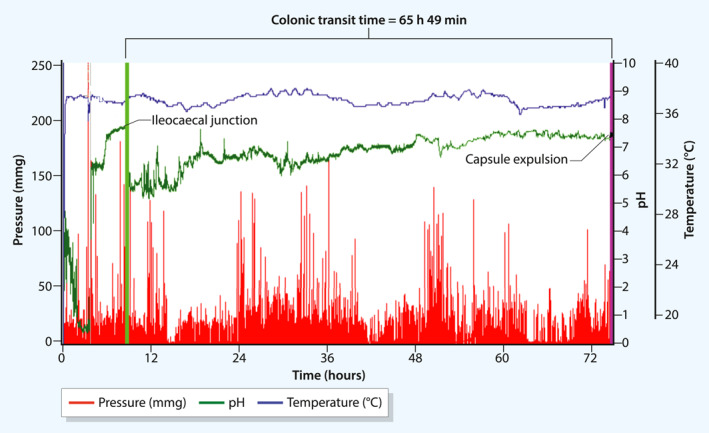
Illustration of a wireless motility capsule recording in a diabetes patient with constipation. The recording shows temperature (°C, top blue curve), pH (middle green curve) and pressure (mmHg, bottom red curve). Colonic transit time is measured from the ileocaecal junction to capsule expulsion, as marked by arrows. In this patient, colonic transit was 65 h 49 min (normal: <5:00–50:30, h:min), indicating slow‐transit constipation
We measured transit times using standardised definitions: gastric emptying time (capsule ingestion–pylorus), small bowel transit time (pylorus–ileocaecal junction) and colonic transit time (ileocaecal junction–capsule expulsion). 13 Normative cut‐off values for colonic transit: rapid (<5:00, h:min), normal (<5:00–50:30) and delayed (>50:30). 13 We also measured the motility index and contractions per minute in the small bowel and colon, and sub‐segments: duodenum (first 60 min after the pylorus), ileum (last 60 min before the ileocaecal junction), caecum (first 60 min after the ileocaecal junction) and rectum (last 60 min before capsule expulsion). 14
Questionnaires
We assessed constipation symptoms using the Gastrointestinal Symptom Rating Scale (GSRS). GSRS can be split into five syndromes, where constipation is a mean of scores on the individual symptoms: (1) decreased passage of stools, (2) hard stools and (3) feeling of incomplete evacuation. 17 Based on prior studies, we chose a cut‐off value ≥ 4 to define constipation. 18 We also performed a psychometric evaluation using the Hospital Anxiety and Depression Scale, where cases of anxiety and depression were defined by a sum score ≥ 11 on the respective subscales. 19
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki. The investigation of diabetes patients was approved by The Western Norway Regional Medical Ethics Committee (2015/58), while the study of healthy participants was approved by The South‐Eastern Norway Regional Medical Ethics Committee (2014/2222 and 2019/28472). All participants submitted oral and written informed consent.
Statistical analysis
Normality was assessed by examination of skewness, kurtosis, Q–Q plots and Shapiro Wilk’s test. In cases of normality, continuous variables were stated as mean (standard deviation, SD). Differences between two groups were examined with the independent samples t‐test. Differences between multiple groups were analysed with one‐way independent analysis of variance corrected by Welch's F and using Games–Howell post hoc test. In cases of non‐normality, continuous variables were stated as median (interquartile range, IQR). We used the Mann–Whitney U test to compare two, and the Kruskal–Wallis test to compare multiple continuous variables, performing sub‐group analyses using Mann–Whitney U test with Bonferroni correction. We used Pearson’s product‐moment correlation (r) and Spearman's rank order correlation (r s) to examine associations between normally and non‐normally distributed continuous variables, respectively. Categorical variables were stated as n (%), and differences between them were assessed using Fisher’s exact test. Statistical significance was defined as p ≤ 0.05. Analyses were performed using IBM SPSS Statistics (Ver. 27, IBM Corporation).
RESULTS
A total of 72 diabetes patients and 26 healthy participants were included in the study. Of these 68 diabetes patients and all healthy participants were examined with wireless motility capsule. We could not identify the ileocaecal junction in three patients, preventing the determination of small bowel and colonic transit times. Another 8 patients had missing data on the GSRS, leaving 57 available for all comparisons. An inclusion flowchart is shown in Figure 2.
FIGURE 2.
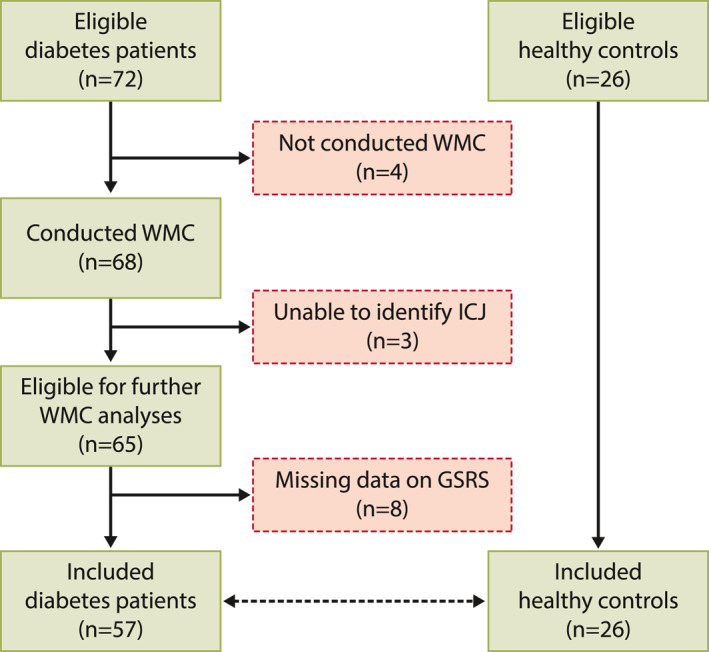
Inclusion flow chart
Clinical characteristics
Clinical characteristics are presented in Table 1. Fifteen diabetes patients (26%) had constipation. Mean constipation score in all patients were 2.6 (SD = 1.5). Women (2.9, SD = 1.5) had more constipation than men (2.0, SD = 1.2), p = 0.046. We found no difference between type 1 diabetes (2.6, SD = 1.6) and type 2 diabetes (2.6, SD = 1.2), p = 0.95. Patients with constipation had longer diabetes duration than those without constipation (p = 0.009). Diabetes duration also correlated with the constipation score (r = 0.38, p = 0.04), but we found no association with age (p = 0.19). Patients with late complications of their diabetes had a constipation score of 2.7 (SD = 1.5); those without complications 2.4 (SD = 1.4), p = 0.40.
Patients with anxiety had more constipation (p = 0.01; Table S1). We found no difference in constipation symptoms when comparing other comorbid conditions. Neither did we find any difference in biochemical parameters (Table 1), nor when looking at medications where constipation is a known side‐effect (Table S2).
Transit times
Table 2 and Figure 3 show transit times in all groups. We found no difference in gastric emptying (p = 0.99), small bowel transit (p = 0.28), colonic transit (p = 0.96) or whole‐gut transit (p = 0.69) when comparing diabetes patients with and without constipation. Neither did we find any associations between transit time parameters and the constipation score (all p > 0.27).
TABLE 2.
Wireless motility capsule measurements of gastrointestinal transit times and contractility parameters: A comparison between diabetes patients with constipation, without constipation and healthy controls
| Variable, unit | Diabetes | Healthy controls | p‐value | Correlation | |||
|---|---|---|---|---|---|---|---|
| Constipation | No constipation | p‐value | r s | p‐value | |||
| Transit times, h:min | |||||||
| Gastric emptying | 4:17 (15:52) | 4:30 (24:51) | 0.99 | 2:58 (1:24) | <0.001 a | −0.12 | 0.38 |
| Small bowel | 5:08 (1:51) | 4:18 (2:46) | 0.28 | 4:13 (1:37) | 0.16 | 0.15 | 0.27 |
| Colon, mean (SD) | 47:48 (38:00) | 45:59 (33:23) | 0.96 | 28:27 (16:21) | 0.01 b | 0.11 r | 0.42 |
| Whole gut, mean (SD) | 66:15 (38:23) | 71:16 (36:33) | 0.69 | 35:55 (16:54) | <0.001 c | −0.05 r | 0.70 |
| Motility index, mmHg × s/min | |||||||
| Small bowel (total) | 129.6 (120.4) | 143.4 (154.2) | 0.50 | 111.0 (49.5) | 0.29 | −0.32 | 0.01 |
| Duodenum | 85.3 (72.2) | 86.3 (123.5) | 0.82 | 63.9 (56.5) | 0.33 | −0.25 | 0.06 |
| Ileum | 146.0 (144.8) | 193.3 (306.8) | 0.61 | 182.0 (166.3) | 0.88 | −0.21 | 0.13 |
| Colon (total) | 132.7 (119.7) | 163.3 (173.2) | 0.51 | 160.9 (151.5) | 0.71 | −0.14 | 0.29 |
| Caecum | 104.4 (135.6) | 92.9 (106.5) | 0.90 | 92.1 (159.0) | 0.98 | −0.11 | 0.42 |
| Rectum | 364.0 (435.0) | 246.1 (302.7) | 0.36 | 336.5 (403.9) | 0.34 | 0.13 | 0.35 |
| Contractions per minute, number | |||||||
| Small bowel (total) | 3.8 (2.8) | 3.9 (3.2) | 0.99 | 3.2 (1.1) | 0.77 | −0.19 | 0.17 |
| Duodenum | 2.9 (1.6) | 2.9 (3.2) | 0.80 | 2.2 (1.9) | 0.83 | −0.27 | 0.047 |
| Ileum, mean (SD) | 4.9 (2.2) | 4.4 (2.5) | 0.47 | 4.7 (1.9) | 0.70 | 0.002 r | 0.99 |
| Colon (total) | 1.5 (1.0) | 1.3 (1.0) | 0.57 | 1.8 (0.7) | 0.21 | −0.08 | 0.55 |
| Caecum | 2.5 (2.6) | 2.5 (2.2) | 0.93 | 3.5 (3.0) | 0.22 | −0.13 | 0.33 |
| Rectum, mean (SD) | 2.4 (1.2) | 1.9 (1.0) | 0.15 | 2.5 (1.1) | 0.08 | 0.15 r | 0.27 |
Note: Results are given as median (IQR) unless otherwise indicated. Correlations are examined between the continuous GSRS constipation score and each wireless motility capsule variable. Correlation coefficients are given as Spearman's r s unless marked by r, indicating Pearson's product‐moment correlation (r). Sub‐group analyses are corrected for multiple comparisons.
Abbreviations: GSRS, Gastrointestinal Symptom Rating Scale; IQR, interquartile range; SD, standard deviation.
Faster gastric emptying time in healthy controls than in diabetes patients with constipation (p = 0.003) and without constipation (p < 0.001).
Faster colonic transit time in healthy controls than in diabetes patients without constipation (p = 0.01), but not compared with patients with constipation (p = 0.18).
Faster whole‐gut transit time in healthy controls than in diabetes patients with constipation (p = 0.03) and without constipation (p < 0.001).
FIGURE 3.
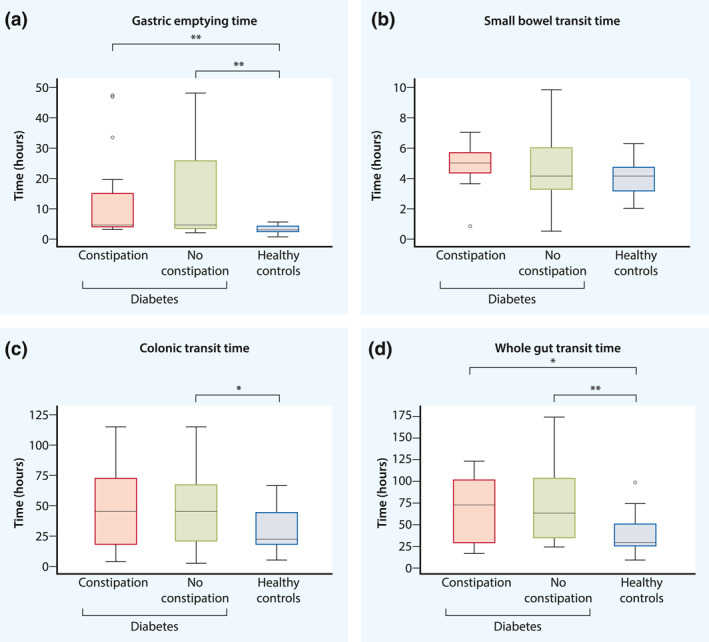
Box‐plots showing comparisons of (a) gastric emptying time, (b) small bowel transit time, (c) colonic transit time and (d) whole‐gut transit time between diabetes patients with constipation, without constipation and healthy controls. Statistical significance of p ≤ 0.05 are marked by * and p < 0.01 by **. Full results are presented in Table 2. To summarise, we found faster gastric emptying (a) and whole gut transit (d) in healthy controls than in both diabetes groups. We also found faster colonic transit (c) in healthy controls than in diabetes patients without constipation, but found no difference in small bowel transit (b). Neither did we find any difference when comparing transit times between diabetes patients with and without constipation
Healthy controls had faster gastric emptying than both diabetes groups: with constipation (p = 0.003) and without constipation (p < 0.001). Healthy controls also had faster colonic transit than diabetes patients without constipation (p = 0.01), but not compared with patients with constipation (p = 0.18). Whole‐gut transit was faster in healthy controls than in diabetes patients with constipation (p = 0.03) and without constipation (p < 0.001).
In Figure 4, we have presented proportions with delayed, normal and rapid transit in diabetes patients with and without constipation, and in healthy controls. Seven (47%) patients with constipation had delayed colonic transit, while 17 (41%) without constipation had delayed colonic transit, p = 0.75. In comparison, 2 (9%) healthy controls had delayed colonic transit, p = 0.01.
FIGURE 4.
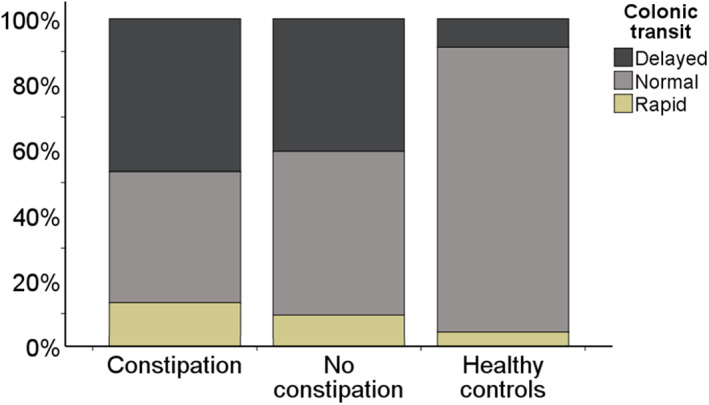
Proportions with delayed, normal and rapid colonic transit in diabetes patients with constipation, without constipation and in healthy controls. Frequencies are given as n (%), where percentages are calculated from the total n in each group. Constipation (n = 15): 7 (47%) delayed; 6 (40%) normal; 2 (13%) rapid. Without constipation (n = 42): 17 (41%) delayed; 21 (50%) normal; 4 (10%) rapid. Healthy controls (n = 23): 2 (9%) delayed; 20 (87%) normal; 1 (4%) rapid. Proportions were equally distributed in the diabetes groups (p = 0.75), both differing from the distribution in healthy controls, p = 0.01
Contractility parameters
Results from contractility measurements are presented in Table 2. We found that small bowel motility index correlated r s = −0.32 (p = 0.01) with the constipation score. When comparing the three groups, we found no difference in any of the contractility parameters.
DISCUSSION
In this study, we investigated gastrointestinal transit and contractility in diabetes patients with and without constipation, and in a group of healthy controls. Contrary to our hypothesis, we found no difference in transit times when comparing diabetes patients with and without constipation. We did, however, find an association between reduced small bowel contractility and constipation symptoms. Compared with healthy controls, both diabetes groups had slower whole‐gut transit.
The lack of association between constipation symptoms and transit times found in our study, may have several causes. Firstly, gastrointestinal symptoms are regularly proven to be unspecific markers of organ dysfunction. 20 Exemplifying this, we have previously shown that patients with familial GUCY2C diarrhoea syndrome had increased colonic transit time, despite having four loose stools per day. 15 Constipation is particularly problematic, as the original definition based on stool frequency, correlates poorly with patients’ complaints. 21 Instead, patients perceive constipation as a multi‐symptom disorder, where straining, hard stool, abdominal discomfort, bloating and the feeling of incomplete evacuation are all equated with infrequent bowel movements. 3 Symptoms like abdominal discomfort and bloating are even more unspecific, also being frequent in gastroparesis and small bowel dysmotility. 4
Furthermore, there is an overlap in symptoms between rectal evacuation disorders, normal‐transit constipation and slow‐transit constipation. 3 In primary constipation, rectal evacuation disorders are seen more frequently than slow‐transit constipation. 6 The prevalence in diabetes is unknown, but a 1998 pilot‐study identified rectal evacuation disorders in 3 out of 10 patients. 22 A recent study supports these findings, demonstrating that constipated diabetes patients had reduced maximal squeeze pressures and recto‐anal pressure gradients, and impaired rectal sensitivity. 23 Intact rectal sensitivity is an essential mechanism in the process of defecation, as gradual rectal filling of faeces elicits an urge to defecate. 24 Without sensing this stimulation, the urge to defecate may be attenuated, leading to accumulation of faeces. 21 In patients with refractory functional constipation, 25% had rectal hyposensitivity. 25 Given the potential of diabetic neuropathy for disrupting anorectal sensory pathways, we consider it likely that rectal hyposensitivity is a main mechanism also in diabetic constipation.
On the other hand, there are also findings of visceral hypersensitivity in diabetes. 26 Visceral hypersensitivity is traditionally associated with functional gastrointestinal disorders, but the borderline between diabetic gastroenteropathy and functional disorders may be blurred. 27 In this study, we did not perform tests of visceral sensitivity, meaning that our patient cohort may have been a mix of patients with reduced and increased intestinal sensation. This may result in a different perception of symptoms, and possibly explain the lack of difference in colonic transit between patients with and without constipation.
Another potential explanation, is that the constipation is not caused by diabetes‐induced dysmotility but common psychiatric comorbidities, such as anxiety and depression. 28 We have previously demonstrated that mood disorders are prevalent in diabetic gastroparesis, and in this study we found that patients with anxiety had more constipation. 29 Our results are consistent with previous studies. 30 However, the relationship between mental status and gastrointestinal symptoms is likely bidirectional: whereas mood disorders may lead to hypervigilance and altered interpretation of symptoms, they may also be a consequence of the disease burden. 31
Finally, while not finding any difference in transit times comparing diabetes patients with constipation and without constipation, we found that both groups had slower gastric emptying and whole‐gut transit than healthy controls. An interpretation of these results may be that diabetic constipation is a manifestation of a global gastrointestinal dysfunction secondary to diabetic gastroenteropathy. Our results are supported by other transit studies and by histomorphological findings, showing similar alterations in both the stomach and colon, most notably loss of Interstitial Cells of Cajal and enteric neurons. 4 , 32 , 33 In addition, hyperglycaemia in itself have been shown to induce dysmotility in the whole gastrointestinal tract. 34
As a secondary aim, we wanted to examine intestinal contractility using the wireless motility capsule’s pressure measurements. Unfortunately, research on intestinal contractility is scarce in diabetic constipation, but a wireless motility capsule study on diabetic gastroparesis patients found blunted colonic contractions compared with healthy controls. 32 Interestingly, we found a moderate correlation between decreased small bowel contractility and constipation symptoms, but no association with colonic dysmotility. The interpretation of this finding is uncertain but may lend support to the theory that constipation in diabetes is not caused by isolated colonic dysfunction. The lack of difference in colonic contractility between patients with and without constipation strengthens this argument. However, another explanation may be that the wireless motility capsule has insufficient sensitivity to detect clinically relevant contractility disturbances. Unlike manometry catheters, the capsule floats freely in the lumen and has only one pressure sensor, which complicates the differentiation between different contractility patterns. 32 , 35 Nevertheless, a study comparing patients with functional constipation, irritable bowel syndrome and healthy controls, was able to identify altered colonic contractility in constipated patients. 36 Considering that the wireless motility capsule has advantages over manometry in availability, ease of use and increased patient comfort, we support head‐to‐head validation studies to determine its future role in colonic contractility assessments.
Our study was cross‐sectional and exploratory and thus not designed to investigate causality. Despite this, our findings may have clinical significance. When so many patients with diabetic constipation experience inadequate treatment, this may indicate that the diagnostics have not identified the causative mechanism behind the symptoms. Slow transit has for long been considered the main mechanism behind diabetic constipation, but other possible explanations have been sparsely investigated. In this paper, we have attempted to discuss some of these potential causes. Of these, evacuation disorders caused by diabetes‐induced damage to the neural regulation may be the most likely and merits further investigation. In addition, we have shown that diabetes patients with constipation had higher anxiety levels. Chronic anxiety may further contribute to the development of rectal evacuation disorders. 37 Most likely, diabetic constipation is a heterogeneous disorder. We therefore emphasise the need for a thorough investigation before initiating treatment, which should be individualised based on diagnostic findings. Prokinetic agents may still have a place in treatment but other causes like rectal evacuation disorders and psychiatric comorbidities should be ruled out first, as these require an entirely different approach to treatment than slow‐transit constipation. 25 , 30 When performing gastrointestinal motility testing, our findings also underline the relevance of evaluating more than just colonic transit, as diabetes patients regularly show concurrent affection of multiple gastrointestinal segments. 33 Hopefully, a broader diagnostic approach to patients with diabetic constipation will lead to improved clinical outcomes in these patients.
Our study had some additional limitations. It was conducted at a tertiary centre and most of the patients had type 1 diabetes. Findings may therefore not be representative for diabetes patients in the general population. The sample size of the constipation group was also small, increasing the risk for type II errors. When performing multiple comparisons, as in our study, there is a risk of type I errors. To control for this, we used the Games‐Howell and Bonferroni post hoc tests when calculating results from normally and non‐normally distributed parameters, respectively. As comorbidities associated with constipation are frequent in diabetes patients, excluding these would potentially introduce a selection bias. Of ethical reasons, we also advised patients to continue their regular medications, except those discouraged by the wireless motility capsule protocol. Controlling for the effect of comorbidities and medications, we found no difference in constipation symptoms. Neither did we find any difference in thyroid function tests, faecal calprotectin and faecal elastase‐1. Due to the simultaneous investigation with scintigraphy, diabetes patients received a meal with 90 kcal higher caloric content than healthy controls. Although we cannot exclude a minor influence on gastric emptying, we find it unlikely that colonic and whole‐gut transit results are affected. The lack of a predefined cut‐off value is a limitation of the GSRS questionnaire. To control for this, we performed correlation analyses, without finding any association between constipation symptoms and transit times. Healthy controls were recruited as part of a collaborating study and included a lower proportion of women compared to diabetes patients with constipation. Healthy controls also trended towards a lower mean age. This may have introduced a bias due to gender differences in transit times. 13 Finally, healthy controls did not answer the GSRS but were screened prior to inclusion using modified Rome III questionnaires and clinical interview.
CONCLUSION
In conclusion, we found that colonic transit did not differ between diabetes patients with and without constipation. Compared to healthy controls, we found delayed whole‐gut transit in both diabetes groups, regardless of constipation symptoms. We also found an association between constipation symptoms and decreased small bowel, but not colonic contractility. Overall, our results may imply that diabetes patients with constipation need a more comprehensive diagnostic investigation than transit time studies, and that other factors may be more important in generating constipation symptoms in these patients.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Dag A. Sangnes, Odd Helge Gilja, Georg Dimcevski and Eirik Søfteland designed the study. Dag A. Sangnes, Eirik Søfteland, Katarina Lundervold, Mattis Bekkelund and Hilde L. von Volkmann performed and analysed the tests and contributed to data entry. Dag A. Sangnes and Katarina Lundervold performed the statistical analysis. Dag A. Sangnes, Katarina Lundervold and Birgitte Berentsen drafted the manuscript with contributions from Odd Helge Gilja, Georg Dimcevski, Eirik Søfteland, Mattis Bekkelund and Hilde L. von Volkmann. All authors approved the final version of the manuscript.
ETHICS APPROVAL
The study was conducted in accordance with the Declaration of Helsinki. The investigation of diabetes patients was approved by The Western Norway Regional Medical Ethics Committee (2015/58), while the study of healthy participants was approved by The South‐Eastern Norway Regional Medical Ethics Committee (2014/2222 and 2019/28472).
INFORMED CONSENT
All participants submitted oral and written informed consent.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
The authors would like to thank the Department of Medicine for providing research facilities, and all hospital personnel assisting us during the study. We also thank all participating patients. Dag A. Sangnes has received a PhD Scholarship grant from the Western Norway Regional Health Authority. Katarina Lundervold and Mattis Bekkelund have received grants from the Norwegian Competence Centre for Functional Gastrointestinal Disorders. Katarina Lundervold received funding from the Norwegian Competence Centre for Ultrasound in Gastroenterology and the NeuroSysMed Centre, Haukeland University Hospital. Mattis Bekkelund received a grant from the Norwegian Research Council through the medical student research programme at University of Oslo. The study has otherwise been funded by Haukeland University Hospital.
Sangnes DA, Lundervold K, Bekkelund M, von Volkmann HL, Berentsen B, Gilja OH, et al. Gastrointestinal transit and contractility in diabetic constipation: a wireless motility capsule study on diabetes patients and healthy controls. United European Gastroenterol J. 2021;9(10):1168–77. 10.1002/ueg2.12169
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the current study are available from the corresponding author on a reasonable request.
REFERENCES
- 1. Du YT, Rayner CK, Jones KL, Talley NJ, Horowitz M. Gastrointestinal symptoms in diabetes: prevalence, assessment, pathogenesis, and management. Diabetes Care. 2018;41(3):627–37. [DOI] [PubMed] [Google Scholar]
- 2. Sommers T, Mitsuhashi S, Singh P, Hirsch W, Katon J, Ballou S, et al. Prevalence of chronic constipation and chronic diarrhea in diabetic individuals in the United States. Am J Gastroenterol. 2019;114(1):135–42. [DOI] [PubMed] [Google Scholar]
- 3. Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25(5):599–608. [DOI] [PubMed] [Google Scholar]
- 4. Meldgaard T, Keller J, Olesen AE, Olesen SS, Krogh K, Borre M, et al. Pathophysiology and management of diabetic gastroenteropathy. Therap Adv Gastroenterol. 2019;12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krogh K, Chiarioni G, Whitehead W. Management of chronic constipation in adults. United Eur Gastroenterol J. 2017;5(4):465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandler J, Camilleri M. Pretest and post‐test probabilities of diagnoses of rectal evacuation disorders based on symptoms, rectal exam, and basic tests: a systematic review. Clin Gastroenterol Hepatol. 2020;18(11):2479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iber FL, Parveen S, Vandrunen M, Sood KB, Reza F, Serlovsky R, et al. Relation of symptoms to impaired stomach, small bowel, and colon motility in long‐standing diabetes. Dig Dis Sci. 1993;38(1):45–50. [DOI] [PubMed] [Google Scholar]
- 8. Wegener M, Börsch G, Schaffstein J, Luerweg C, Leverkus F. Gastrointestinal transit disorders in patients with insulin‐treated diabetes mellitus. Dig Dis. 1990;8(1):23–36. [DOI] [PubMed] [Google Scholar]
- 9. Jung HK, Kim DY, Moon IH, Hong YS, Colonic transit time in diabetic patients – comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J. 2003;44:265–72. [DOI] [PubMed] [Google Scholar]
- 10. Zikos TA, Kamal AN, Neshatian L, Triadafilopoulos G, Clarke JO, Nandwani M, et al. High prevalence of slow transit constipation in patients with gastroparesis. J Neurogastroenterol Motil. 2019;25(2):267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parkman HP, Sharkey E, McCallum RW, Hasler WL, Koch KL, Sarosiek I, et al. Constipation in patients with symptoms of gastroparesis: analysis of symptoms and gastrointestinal transit. Clin Gastroenterol Hepatol. 2020. 10.1016/j.cgh.2020.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rao SSC, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23(1):8–23. [DOI] [PubMed] [Google Scholar]
- 13. Wang YT, Mohammed SD, Farmer AD, Wang D, Zarate N, Hobson AR, et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther. 2015;42(6):761–72. [DOI] [PubMed] [Google Scholar]
- 14. Farmer AD, Wegeberg AML, Brock B, Hobson AR, Mohammed SD, Scott SM, et al. Regional gastrointestinal contractility parameters using the wireless motility capsule: inter‐observer reproducibility and influence of age, gender and study country. Aliment Pharmacol Ther. 2018;47(3):391–400. [DOI] [PubMed] [Google Scholar]
- 15. von Volkmann HL, Brønstad I, Gilja OH, Tronstad R R, Sangnes DA, Nortvedt R, et al. Prolonged intestinal transit and diarrhea in patients with an activating GUCY2C mutation. PLoS One. 2017;12(9): e0185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sangnes DA, Søfteland E, Bekkelund M, Frey J, Biermann M, Gilja OH, et al. Wireless motility capsule compared with scintigraphy in the assessment of diabetic gastroparesis. Neuro Gastroenterol Motil. 2020;32(4):e13771. [DOI] [PubMed] [Google Scholar]
- 17. Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I. Well‐being and gastrointestinal symptoms among patients referred to endos owing to suspected duodenal ulcer. Scand J Gastroenterol. 1995;30(11):1046–52. [DOI] [PubMed] [Google Scholar]
- 18. Sangnes DA, Dimcevski G, Frey J, Søfteland E. Diabetic diarrhoea: a study on gastrointestinal motility, pH levels and autonomic function. J Intern Med. 2021. 10.1111/joim.13340 [DOI] [PubMed] [Google Scholar]
- 19. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 20. Arora Z, Parungao JM, Lopez R, Heinlein C, Santisi J, Birgisson S. Clinical utility of wireless motility capsule in patients with suspected multiregional gastrointestinal dysmotility. Dig Dis Sci. 2014;60(5):1350–7. [DOI] [PubMed] [Google Scholar]
- 21. Lembo AJ. Constipation. In: Feldman M, Friedman L, Brandt L, editors. Sleisenger and Fordtran's gastrointestinal and liver disease. 10th ed. Philadelphia: Elsevier Saunders; 2016. p. 270–96. [Google Scholar]
- 22. Maleki D, Camilleri M, Burton DD, Rath‐Harvey DM, Oenning L, Pemberton JH, et al. Pilot study of pathophysiology of constipation among community diabetics. Dig Dis Sci. 1998;43(11):2373–8. [DOI] [PubMed] [Google Scholar]
- 23. Reszczyńska M, Kempiński R. The prevalence of enteropathy symptoms from the lower gastrointestinal tract and the evaluation of anorectal function in diabetes mellitus patients. J Clin Med. 2021;10(3):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Dig Dis Sci. 2012;57(6):1445–64. [DOI] [PubMed] [Google Scholar]
- 25. Vollebregt PF, Burgell RE, Hooper RL, Knowles CH, Scott SM. Clinical impact of rectal hyposensitivity: a cross‐sectional study of 2,876 patients with refractory functional constipation. Am J Gastroenterol. 2021;116(4):758–68. [DOI] [PubMed] [Google Scholar]
- 26. Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SSC. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. 2008;20(6):635–42. [DOI] [PubMed] [Google Scholar]
- 27. Pasricha PJ, Grover M, Yates KP, Abell TL, Bernard CE, Koch KL, et al. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Gastroenterology. 2021;160(6):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Kort S, Kruimel JW, Sels JP, Arts ICWW, Schaper NC, Masclee AAMM. Gastrointestinal symptoms in diabetes mellitus, and their relation to anxiety and depression. Diabetes Res Clin Pract. 2012;96(2):248–55. [DOI] [PubMed] [Google Scholar]
- 29. Teigland T, Iversen MM, Sangnes DA, Dimcevski G, Søfteland E. A longitudinal study on patients with diabetes and symptoms of gastroparesis – associations with impaired quality of life and increased depressive and anxiety symptoms. J Diabetes Complications. 2018;32(1):89–94. [DOI] [PubMed] [Google Scholar]
- 30. Yamada E, Namiki Y, Takano Y, Takamine H, Inazumi K, Sasaki H, et al. Clinical factors associated with the symptoms of constipation in patients with diabetes mellitus: a multicenter study. J Gastroenterol Hepatol. 2018;33(4):863–8. [DOI] [PubMed] [Google Scholar]
- 31. Van Oudenhove L, Levy RL, Crowell MD, Drossman DA, Halpert AD, Keefer L, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coleski R, Wilding GE, Semler JR, Hasler WL. Blunting of colon contractions in diabetics with gastroparesis quantified by wireless motility capsule methods. PLoS One. 2015;10(10):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rouphael C, Arora Z, Thota PN, Lopez R, Santisi J, Funk C, et al. Role of wireless motility capsule in the assessment and management of gastrointestinal dysmotility in patients with diabetes mellitus. Neurogastroenterol Motil. 2017;29(9):1–7. [DOI] [PubMed] [Google Scholar]
- 34. Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin‐dependent diabetes mellitus. Gastroenterology. 1997;113(1):60–6. [DOI] [PubMed] [Google Scholar]
- 35. Camilleri M, Bharucha AE, Di Lorenzo C, Hasler WL, Prather CM, Rao SS, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20(12):1269–82. [DOI] [PubMed] [Google Scholar]
- 36. Hasler WL, Saad RJ, Rao SS, Wilding GE, Parkman HP, Koch KL, et al. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):1107–14. [DOI] [PubMed] [Google Scholar]
- 37. Rao SSC, Bharucha AE, Chiarioni G, Felt‐Bersma R, Knowles C, Malcolm A, et al. Anorectal disorders. Gastroenterology. 2016;150(6):1430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on a reasonable request.


