Abstract
Background
Rates of Hepatitis C virus (HCV) testing and diagnosis are variable among people who use drugs (PWUD). In Puglia in 2018, of 871 subjects screened, 38% had HCV antibodies (HCVAb). Despite sustained virologic response at week 12 Sustained virologic response (SVR12) rates >95%, addiction centers in Italy are not allowed to prescribe direct‐acting antivirals (DAA).
Aim
To increase testing and linkage to care a dedicated program including “ad hoc” transportation and fast‐track access to care was offered to PWUD from Puglia.
Methods
Over 12 months, 1,470 individuals seen at 15 Services for Dependence (SERDs) underwent screening. For HCVAb positive, a fast‐track evaluation was offered at our Hepatology Unit. Patients were subsequently taken to their pharmacists to receive the prescribed DAA regimen. Treatment and adherence were supervised by SERDs physicians, SVR12 assessed at our unit. The scalability of the process was based on both, number of patients screened in our region in 2018, and number of PWUD diagnosed and treated at our center during 2018–2019.
Results
Of 1,470 individuals screened, 634 (43.1%) tested HCVAb positive. Overall, 231 were RNA positive, 54% of whom on opioid agonist therapy (OAT) and 32% with cirrhosis. Median interval between RNA assessment and treatment start was 22 days (0–300). Patients received 12‐week sofosbuvir/velpatasvir regimen without Ribavirin; in 220 patients who completed treatment, SVR12 was 98.6%. Among GT3, SVR12 was 98%. No re‐infection was observed. Improvements in screening, and linkage to care were registered.
Conclusions
A PWUD‐tailored service led to HCV care cascade improvement and high SVR12 rates. Despite history of drug addiction, social instability and logistic barriers, micro‐elimination programs providing dedicated care are key drivers of success.
Keywords: antiviral treatment, cirrhosis, HCV, hepatitis C, linkage to care, micro‐elimination, people who use drugs, PWUD, screening, sofosbuvir/velpatasvir
INTRODUCTION
Globally 71 million people live with Hepatitis C virus (HCV) infection, a major cause of end‐stage liver disease. With recent advances in antiviral therapy, HCV has become curable and HCV elimination foreseeable, allowing for major health, societal, and economic benefits. 1 Nevertheless, it has been recently reported that even among high‐income countries, only 11 are on track to eliminate HCV by 2030, five more countries are on track for elimination by 2040, while all the remaining will eliminate HCV by 2050 or later. 2 This is mostly due to an insufficient number of diagnosed patients, low linkage to care and treatment rates across the majority of countries. In addition, the COVID‐19 pandemic is having deep impact on chronic liver diseases management. 3 Screening campaigns appear more difficult to implement, and testing and access to treatment are reduced. 4 It is clear that one size fits all strategy to achieve global elimination would be unsuccessful and that different populations with chronic HCV infection require dedicated programs. 5 Micro‐elimination consisting in achieving elimination in a well‐defined group currently appears more feasible and measurable than macro‐elimination. 6 A key aspect of HCV micro‐elimination is to individualize interventions according to local needs.
Key summary.
Micro‐elimination in a well‐defined group of patients or pathological context is required to achieve global HCV elimination. PWUD represents an underserved group to prioritize. Data on PWUD screened and diagnosed in Puglia remain suboptimal and limited by distance from Services for dependence (SERDs) not allowed to prescribe DAA and prescribing centers. A strategy tailored on the local needs and including a dedicated shuttle service to reduce logistic barriers and a fast‐track baseline assessment to simplify patients pathway was designed to increase the number of PWUD screened, diagnosed and linked to treatment. A strict collaboration between SERD's screening patients and monitoring their treatment, and our center diagnosing HCV infection, prescribing sofosbuvir/velpatasvir combination and assessing SVR12 response was established and resulted helpful in minimizing the effect of the COVID‐19 pandemic.
In comparison to the 9.8% screening rate reported in Puglia in 2018, the 62.9% screening rate registered in our study was a significant improvement. Significantly higher was also the rate of HCVAb positive subjects identified. The rate of PWUD linked to care increased at our center from 83.9% in 2018 to 99% in this study. SVR12 was attained in 96% of patients regardless of cirrhosis or genotype. Patient‐tailored multidisciplinary approaches enhance scalability and lead to many patients benefits including prioritize access to care, rapid start of treatment and high SVR12 rates. After this program, SERDs implemented a shuttle service for their patients.
HCV infection is common among people using substances people who use drugs (PWUD), and PWUD mean young age is a major risk factor for HCV transmission. 7 Although being an underserved and challenging population for several reasons ‐including lack of updated information on undiagnosed cases, low linkage to care rate, difficulties in engaging and completing treatment courses, and risk of re‐infections—PWUD represent a group to prioritize when aiming at achieving global HCV elimination.
In Italy, in 2018 up to 70% prevalence of HCV infection has been reported among PWUD, 8 however, data on HCV screening rate are unclear and variable. Addiction centers are not allowed to prescribe direct‐acting antivirals (DAA) and are often located in areas far from prescription centers. In our geographical region, despite 32 DAA prescribing centers, the number of PWUD screened remains low. Based on a recent estimate, less than 10% of PWUD living in Puglia underwent HCV screening in 2020 over an expected number of substance users of about 9,000. 9 Barriers to treatment include poor venous access, fear of screening and of treatment side effects driven by previous IFN‐based regimens; however, the most impactful appears to be distance from specialist centers. Moreover, lack of direct public transportations linking our prescribing center to the peripheral SERDs, together with complexity and duration of baseline pre‐treatment assessments represent a major obstacle to HCV cure in this population. To increase screening and linkage to care in PWUD, we designed a tailored strategy including educational sessions at 15 different SERDs located in our geographical area, fast‐track local screening activities, and a dedicated transportation service between the SERDs and our prescribing center.
PATIENTS AND METHODS
An outreach program targeting individuals with substance addiction history, followed at 15 SERDs in Puglia was implemented. Subjects underwent HCV screening at each SERD using rapid OraQuick tests. Patients testing HCV antibodies (Ab) positive reached our center by a dedicated shuttle service funded by our Institution. They were offered a fast‐track baseline evaluation within 5 h.
Patients resulting positive for HCVAb were assessed for HCV RNA after a median wait of 1 week and received pre‐treatment liver stiffness (LS) evaluation by transient elastography and abdominal ultrasound. Definition of active intravenous drug use was based on substance use up to 30 days before the study evaluation.
Treatment candidates obtained their treatments without any second appointment by the pharmacists located in proximity to each SERD and were supervised by SERDs physicians taking part in this initiative. After treatment completion, patients were re‐evaluated at our center to assess sustained virologic response at week 12 (SVR12).
Adherence was measured monthly at the local SERDs based on the number of pills taken of those prescribed. Scalability of the process was assessed at the end of the study based on the number of PWUD screened and diagnosed in the entire region and on the number of patients linked to care and treated at our center during the previous year.
The study started on July 1, 2019 and was expected to be completed by June 30, 2020. However, due to the COVID‐19 pandemic lockdown (that in Italy in 2020 was complete and mandatory from March 9 to May 18, and partial for 69 days), the study was put on hold. We consequently extended the study end date, and patients treatment completion was planned by the end of October 2020. However, the second wave of COVID‐19 pandemic once again forced a temporary halt to our project and the end of the study was further extended to January 30, 2021 in order to complete a 12 full months duration. Last study visit was performed on January 29, 2021. Re‐infection was assessed by genotyping when required at the time of SVR12 assessment.
STUDY OUTCOMES
Primary outcome measures include (1) number of hepatitis C antibody testing performed by both OraQuick 10 and of HCV RNA assessments by COBAS TaqMan HCV Test versus 2.0 and (2) number of patients linked to care. Due to the fluctuating number of patients followed at each SERD, the screening test was offered to 100 PWUD per SERD. Patients resulting HCV RNA reactive had genotype assessment and were offered treatment. SVR12 and post‐treatment follow‐up were considered secondary outcomes. SVR12 was assessed for patients starting treatment by effectiveness analysis. Intention to Treat analysis (ITT) was also evaluated.
Scalability
In order to establish scalability, the number of PWUD screened within this project was compared to the number of those screened at regional level published in the annual report of Health Ministry for 2018. 9 The increase in number of patients linked to care and treated was calculated comparing the number of patients linked to care and treated during the 12 months of this program to the number of PWUD linked to care and treated within a real world study conducted in our geographical area between June 2017 and June 2018 by our group, including 218 patients with similar history and baseline characteristics.
RESULTS
At the time of the study commencement, 2,358 PWUD were followed at the 15 participating SERDs. Of them, 1,470 agreed to be tested by OraQuick (62.3%) and 634 HCVAbs carriers were identified (43.1%; Figure 1). Up to 56% of them were aware of their condition but could not produce previous testing result, for this reason they were included in the general analysis. Overall, 42.4% had received the first diagnosis a mean of 15 years earlier, 45.4% had received standard interferon monotherapy or in combination with ribavirin but had not been tested for HCV RNA after treatment. Baseline characteristics of patients testing HCVAb positive are reported in Table 1. The vast majority (90.9%) were male. The median age was relatively high (48.1 years), but in keeping with the mean age of Italian PWUD. 12 Overall, 34.8% were on opioid agonist therapy (OAT), this rate suggests that linking screening to OAT increases patients motivation. All had history of previous substance use and 28.4% were active intravenous drug users. All but five attended the first appointment. Overall, 231 patients of the 629 HCVAb positive who accepted to come to our center to be diagnosed (36.7%) had detectable HCV RNA. Only a minority (2.2%) had human immunodeficiency viruses or (0.5%) Hepatitis B virus surface antigen positivity. An excessive alcohol consumption was reported in the timeframe of 24 weeks before by a relative low proportion of patients and might have increased during the COVID‐19 pandemic. The most common drug used was heroin. Up to 8.8% were treated for mental disorders. Of note, up to 23.1% had LS results compatible with presence of cirrhosis. The proportion of diabetics was 14.7%.
FIGURE 1.
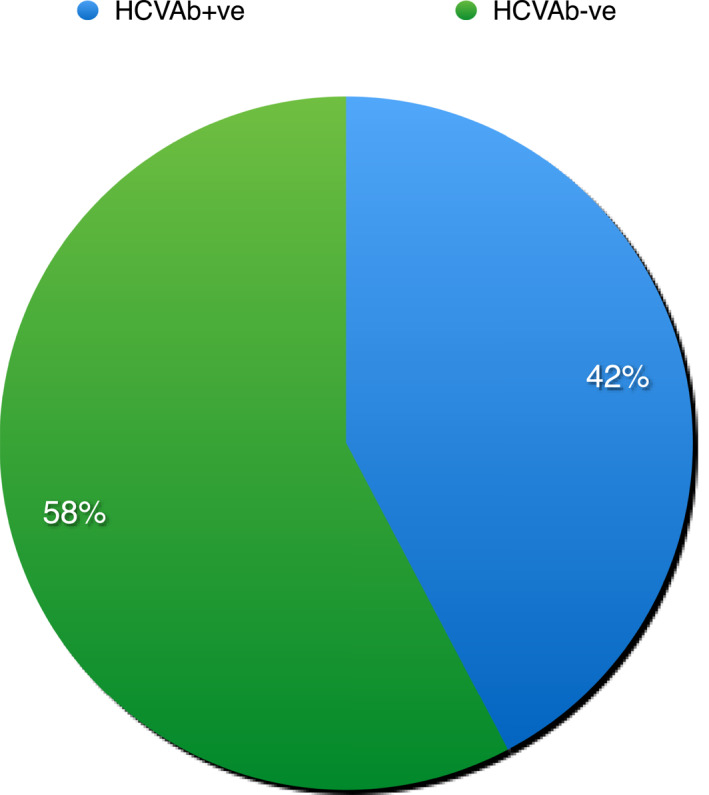
Proportion of patients HCVAb positive of 1470 PWD screened. Abbreviation: HCVAb, HCV antibodies
TABLE 1.
Characteristics of patients HCV Ab+ve
| HCV Ab+ve, N = 634 | |
|---|---|
| Median age (yrs) range | 48.1 (22–71) |
| Male gender n,% | 562 (88.6) |
| Median BMI (range) | 26.6 (14.9–46.4) |
| Diabetes n, (%) | 93 (14.7) |
| Current PWUD | 180 (28.4) |
| Heroin alone | 56 (31.2) |
| Cocaine alone | 23 (12.7) |
| Heroin combined with alcol or others | 101 (56.1) |
| OAT | 221 (34.8) |
| HCV RNA reactive | 231 (36.4) |
| Past HCV treatment | 288 (45.4) |
| HIV positive | 16 (2.5) |
| HBsAg | 3 (0.5) |
| Homeless | 3 (0.5) |
| Mental disorders | 56 (8.8) |
| Alcohol abusers | 158 (24.9) |
| Liver stifness >12.5 Kpa n, (%) | 147 (23.1) |
Abbreviations: BMI, Body Mass Index; GT, genotype; HBsAg, Hepatitis B virus surface antigen; HCV, Hepatitis C virus; HIV, human immunodeficiency viruses; ITT, Intention to Treat; LS, liver stiffness; OAT, opioid agonist therapy; PWUD, people who use drugs; SVR12, sustained virologic response at week 12.
Patients with active HCV infection
Baseline characteristics of patients with active infection are reported in Table 2. Male were 91.1%. The most represented genotype was genotype 1, followed by genotype 3. Of 113 patients with genotype 1 infection, 67% were 1a. When compared to patients who cleared HCV infection spontaneously or after a previous course of treatment, patients on OAT were significantly more frequent among subjects with active HCV infection (46.3% vs. 27.9%; p = 0.0001). Among patients without active HCV infection, subjects previously treated were largely more represented than naive (56.1% vs. 26.8%; p = 0.0001) showing that PWUD are generally unaware of the results of their previous treatment. It can be assumed that the remaining 177 subjects in this group had spontaneously cleared HCV RNA. Among patients with active infection, 26.8% had been treated in the past and did not know their previous treatment course outcome. Very few patients in this group had been treated with first generation protease inhibitors (PI) without response (14%). Remarkably, based on liver stiffness results, a significantly higher number of patients among those with active infection had cirrhosis in comparison to those with inactive (32% vs. 18.6%; p = 0.002). As for co‐morbidities, patients with diabetes were numerically more frequent among patients with active HCV infection.
TABLE 2.
Baseline characteristics of patients candidate to treatment
| Patients who accepted to start treatment, N = 231 | |
|---|---|
| Median age (yrs) range | 48.3 (22–72) |
| Male gender n,% | 199 (90.9) |
| Median BMI (range) | 26.7 (17.0–48.1) |
| Diabetes n, (%) | 38 (18.8) |
| Current PWUD | 157 (74.8) |
| GT1 | 113 (48.9) |
| GT2 | 6 (2.6) |
| GT3 | 100 (43.3) |
|
GT4 |
10 (4.3) |
| mixed | 2 (0.9) |
| GT1a | 74 (65.4) |
| GT1b | 39 (16.8) |
| Patients with mental disorders | 23 (10.5) |
| Alcohol abusers | 42 (18.2) |
| OAT | 119 (54.3) |
| Liver stifness >12.5 Kpa n, (%) | 70 (32.0) |
| Interval between HCV RNA assessment and treatment start | 22.3 (0–300) |
| SVR12 (ITT) | 217 a (94.8) |
| SVR12 (PP) | 217* (98.6) |
Abbreviations: BMI, Body Mass Index; GT, genotype; HCV, Hepatitis C virus; ITT, Intention to Treat; LS, liver stiffness; OAT, opioid agonist therapy; PP, per‐protocol; PWUD, people who use drugs; SVR12, sustained virologic response at week 12.
aOut of 229.
*Out of 220.
Patients linked to care and patients treated
All 231 HCV RNA reactive patients were offered treatment. Patients received sofosbuvir/velpatasvir (SOF/VEL) treatment. As shown in Figure 2, and Table 3, only two did not accept, one due to the simultaneous diagnosis of cancer, the other due to relocation to a different geographical area. All remaining patients accepted to start treatment, however three did not pick up the drug at their local pharmacy and were lost to follow‐up. Of 226 patients who started treatment, six discontinued or were lost to follow‐up before SVR12 assessment. Among them, two patients, both responders, died before SVR12 assessment of reasons not related to treatment (car accident at week 1, and myocardial infarction at week 3 post‐treatment).
FIGURE 2.
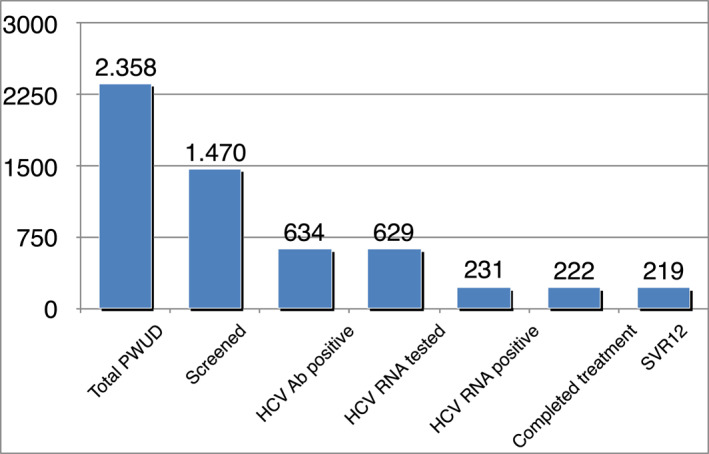
HCV care cascade: total number of subjects per group and rate of positive among the previous group. Abbreviation: HCV, Hepatitis C virus
TABLE 3.
Treatment patient's disposition
| N, (%) | |
|---|---|
| Patients HCV RNA reactive | 231 |
| Patients who accepted to start the treatment | 231 (100) |
| Patients who started treatment | 229 (99.1) |
| Patients who discontinued treatment | 9 (3.9) |
| Patients who completed treatment | 220 (95.6) |
| Patients who experienced virological treatment failure | 3 (1.3) |
Abbreviations: HCV, Hepatitis C virus
Treatment effectiveness
Overall, 220 patients completed the assigned treatment and all but three achieved SVR12 (98.6%).
The three patients who did not achieve SVR12 were also alcohol abusers and they did not return the bottle of pills for the planned monthly drug counts to their SERDs physician. No difference in SVR12 rates between patients with or without cirrhosis was observed (Figure 3). SVR rates were higher than 95%, irrespective of genotype. As shown in Figure 4, SVR12 were 99.1%, 100%, 97.7%, and 100% by genotype. SVR12 among patients with genotype 3 (GT3) infection was slightly lower than for GT1. SVR12 was 98.5% among GT1a and 100% among GT1b. Among 89 patients with GT3 who completed treatment, 30 out of 31 with cirrhosis achieved SVR12, as compared to 57 of 58 patients without cirrhosis. Overall SVR12 for GT3 was 97.7%.
FIGURE 3.
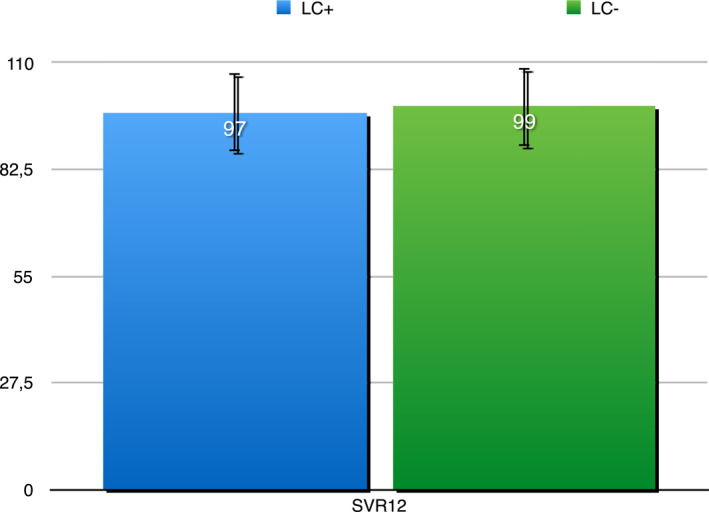
SVR12 by LS results suggesting presence of cirrhosis/bridging fibrosis in 220 PWUD who completed treatment. Abbreviations: LS, liver stiffness; PWUD, people who use drugs; SVR12, sustained virologic response at week 12
FIGURE 4.
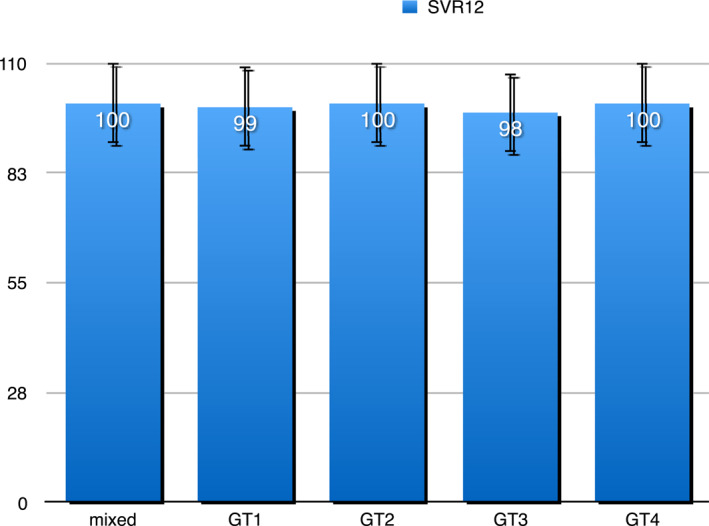
SVR12 by genotype in 220 PWUD who completed treatment. Abbreviations: PWUD, people who use drugs; SVR12, sustained virologic response at week 12
A single re‐infection has been observed at week 12 post‐treatment.
Adherence
In addition to the patients who failed to achieve SVR only two patients did not return empty bottles at week 8 and 12 of treatment, respectively. Both achieved SVR12. Consequently, only 2.27% of patients can be defined treatment non‐adherent. In one case, the number of remaining pills was three for the first month and two for the second. In the second case, three pills per month of treatment were returned.
HCV care cascade
The HCV care cascade among subjects in the study is reported in Figure 2. Of 2,358 PWUD followed at the 15 SERDs upon project commencement, 1,470 accepted to be tested (62.3%).
With the exclusion of 5 of 634 HCVAb positive patients who did not accept to be tested for HCV RNA, all were diagnosed. Of 634 HCVAb positive, 287 were aware of their condition but had no documentation of their own and post‐treatment past virological assessment or had experienced re‐infection. In order to get access to treatment, they asked to be tested again. The rate of HCV RNA positive patients was high, as of 629 HCVAb patients tested, 36.7% had active HCV infection. These evidence suggest that in Italy PWUD still represent an important reservoir of HCV infection. Notably, all the patients with active infection accepted treatment, although two did not start. Consequently, 229 patients (99%) were linked to care (Table 3). Overall, nine patients discontinued treatment, three within week 4, the remaining three after week 4, but before week 8.
Scaling up
In comparison to the 9.8% screening rate reported by the Regional Health Authorities in 2018 in Puglia, 9 our 62.9% rate of screening was significantly higher (p = 0.0001). Out of 1,470 tested in our program, the 43.1% proportion of subjects HCVAb positive was higher than the rate previously reported in our region. Indeed, as previously reported, of 871 evaluated in 2018, 331 (38%) resulted HCVAb positive. 9 Comparison of our 43.1% diagnosis rate to the historical 38% demonstrated a significant improvement (p = 0.02). As a positive result of the strict collaboration between SERDs and our Unit, and of the word of mouth across involved patients, only 5 patients did not accept to be tested for HCV RNA. The number of patients linked to care and treated according to a standard of care strategy used in 2018–2019 represents our own historical control for this patient‐centered treatment strategy initiative. 11 As compared to 218 of 260 (83.8%) PWUD linked to care at our center following a standard strategy in 2018, 11 the 99% rate reported in the present study is a significant increase (p = 0.0001). Moreover, it is important to highlight that ‐countertrend to the impressively low rate of prescription reported by the Agenzia Italiana del Farmaco (AIFA) registry in our country in 2020—the number of patients we were able to treat within this program is higher than the number of patients linked to care and treated during 2019 at our center, outside the context of COVID‐19 pandemic.
Both standard and tailored strategies provided a comparable number of patients treated, number of patients who completed treatment or were loss to follow‐up and number of patients who had a virological failure. Among 229 patients candidate, 220 completed treatment (96.0%) in this study at a similar rate to that of the 210 out of 218 (96.3%) in the past (p = 0.88). The 3.9% rate of discontinuation was comparable to that observed in our previous experience (3.6%). Of 220 subjects who completed treatment in this study, three (1.3%) did not obtain SVR12, in comparison to 5 of 210 (2.3%) of those treated with the standard of care strategy (p = 0.47). Re‐infection rate was an additional outcome evaluated both in this study and in the past. Re‐infection was reported in 2 of 210 cases (0.9%) with standard treatment and in 1 of 220 with the present strategy (p = 0.53).
The implementation of the present patient‐centered strategy did not increase the already optimal treatment response and re‐infection rates attained with standard strategy.
DISCUSSION
PWUD is one of the most appropriate and realistic population to target in HCV micro‐elimination program, however testing and treatment rates have been historically disappointing. Notably, the rate of PWUD screened in Italy in 2018 was 23.8%, and even lower (9.8%) in our region. 9 A national screening program targeting patients born from 1969 to 1989—representing the substance‐abuse‐related second wave of HCV infection in our country—was first launched in Italy in January 2021. 13
Simplified models of care are key aspects in the success of HCV treatment programs in community settings, and enable scaling up. 14 , 15 Among diverse strategies adopted to allow HCV elimination, the most successful appear those based on a personalized intervention. Based on these evidence, we designed and implemented a dedicated micro‐elimination program focusing on PWUD.
Of a total number of 2,358 PWUD followed at 15 SERDs, up to 62.3% were screened. Although the screening acceptance rate in our study remain suboptimal, this rate appears significantly higher as compared to data attained in the entire Puglia in the very recently published 2021 national report. In that report, among 8,819 PWUD followed by SERDs in our region, only 710 had received HCV screening. 9 Reason for the suboptimal acceptance rate might be related to a center effect, as in larger centers the rate of screening was lower than in smaller ones due to the patients burden (data not shown).
Our results appear well in keeping with those by Linnet et al 16 showing a 50% increase in testing using a decentralized model in which hospital infectious disease department were responsible for prescription and monitoring, and other healthcare providers were responsible for testing, dispensing and adherence support. Among subjects screened during 2018, 38% were found HCVAb positive, according to updated Health Ministry reports. 9 Our results show 5% increase in diagnosis despite a current reduction in the number of patients seeking HCV treatment in Italy, proven by the flattered treatment curve in the National AIFA registry. 17 Overall, 231 patients with active HCV infection were identified and linked to care from July 2019 to November 2020 despite COVID‐19 pandemic limiting Hospital access and diagnostic procedures. Although the proportion of patients with active HCV infection in our study is lower than the 71% reported in the private large therapeutic community of San Patrignano—admitting more than 90% of patients determined to recover from substance addition in northern Italy 18 —it might provide an accurate representation of the real situation in this setting in southern Italy. Notably, data from SERDs services for the current year are not yet available.
Previous data on treatment linkage among PWUD in Italy showed that no more than 50% of patients received treatment. 19 In our study of 231 candidates, only two did not start treatment. The availability of pangenotypic regimen associated with comparably high rate of success across the different genotypes 20 , 21 and with minimal monitoring make patient‐centered strategies workable and transferable. 22 Based on this minimal monitoring approach, on the favorable safety drug to drug interaction profile in the presence of anti‐psicotic co‐medications, and on the higher SVR12 expected in patients with genotype 3 and advanced liver disease, we selected SOF/VEL pangenotypic regimen. Our treatment rate appears higher than those reported in other studies from Europe focusing on vulnerable population, 23 but comparable to those reported by Shisha et al using a multidisciplinary same day “test and treat” approach after an awareness campaign for general population in Egypt. 24
There have been recent developments in point of care tests including the rapid oral HCV antibodies test. Incorporation of rapid detection testing (RDT) in the diagnostic pathways can reduce the time from screening to start of treatment. 25 The use of sensitive RDT together with the fast‐track procedure and the dedicated transportation service make different approaches as those based on pharmacist intervention unnecessary 26 and ensure the complete baseline work‐up to be homogeneously performed at the same center. 27 In addition, this strategy entails a sort of positive selection as only patients who accept to come to our center are motivated to continue on their linkage to care pathways. 28
Given the chaotic lifestyle of these patients, treatment discontinuations or lost to follow‐up can be expected. The success of this patient‐tailored approach was due to the close monitoring by physicians and nurse personnel at addiction centers and proven by the high treatment adherence rate and few patients lost of follow‐up despite the recent drug use. Notably, the rate of loss to follow‐up was comparable to that reported in controlled studies. 29 , 30 Of course the very marginal number of homeless among our patients is an undeniable advantage as compared to other similar reports. Moreover, another key of success is the decentralized screening and monitoring. Indeed, in recent reports from Spain where the linkage to treatment was lower than 4%, a centralized approach was used and the rate of patients with mental disorders was higher. 31 , 32
In this study, SVR12 rates were extremely high irrespective of HCV genotypes and of disease severity. SVR12 in GT3 patients with cirrhosis was similar to that reported in previous studies using the same treatment regimen. 33 , 34 Two patients despite decompensated disease achieved SVR12 without the addition of Ribavirin. Interestingly, in comparison to 2018–2019, when 218 patients were treated with the same regimen, we observed similar SVR12 rates in 220 patients able to complete treatment. The use of the same simple pangenotypic regimen in both standard and patient‐tailored strategy studies explain the similar low rate of non virological failures of 3.9% and 3.6%.
No side effects or drug to drug interactions (DDI) were reported. Only two patients taking phenobarbital had to switch drug before starting SOF/VEL. As shown, patients started DAA within no more than 3 weeks from HCV RNA assessment. The benefit of delivering DAA therapy in primary care was clearly shown by the Kirketon Road Clinic in Sydney real life experience study targeting 242 marginalized PWUD. 35 However, general practitioners are not allowed to prescribe DAA in several countries including Italy. Moreover, in that study 79 patients required enhanced support in terms of daily or weekly administration of DAA. In our experience, the strict communication between SERDs and prescribing center increased the adherence to treatment. Our results are well in keeping with those reported by the Toronto Community Hep C program, a community‐based model designed to provide access to HCV treatment for marginalized people. 36 The main difference between that and our own experience was the lack of appropriate provider support in our study. Despite that, adherence and SVR were similarly high.
At variance with reports from other European countries as, for example, Spain, the rate of re‐infection within a short post‐treatment follow‐up appears negligible. 30 , 37 These results are of course due to the patients' motivation to carry out a lifestyle change; they need to be confirmed during a prolonged follow‐up.
Limitation of this study was a spotty contribution by the 15 SERDs as patients’ recruitment was discontinuous during the 12 months period. This was mainly attributable to the COVID‐19 pandemic that led PWUD to switch to other form of drug consumption as heroin vaporization, or crack (free base) smoking, and to less frequent visits to the SERDs. Moreover, it was difficult to persuade patients to undergo the last SVR12 assessment visit and this evidence highlight the usefulness of timely updated European Association for the Study of the Liver guidelines suggesting that SVR12 assessment can be omitted in particularly difficult to manage patients. 15
The strength of this study is represented by the evidence that tailoring micro‐elimination strategies on the local patients needs may be more successful than simply applying national or international guidelines. The strict and direct collaboration (every day phone calls) between the different physicians involved in difficult to treat patients management ensure both decentralization and achievement of pre‐determined objectives despite exceptional and unexpected events as COVID‐19 pandemic. Finally, the project was shown to be self‐sustainable as the shuttle funded by our Institution is from the study end onward funded by some local SERDs.
In conclusion, a tailored approach focused on intensifying screening, fast‐track diagnostic pathways and linkage to care for PWUD who have logistic problems accessing specialized centers is a real example of micro‐elimination. Our innovative patient‐tailored approach lead to increase in screening and linkage to care, prioritizing underserved patients for access to care and rapid start of treatment and providing high SVR12/24 rates.
CONFLICT OF INTEREST
Maria Franca Rina, Antonio Canosa, Valeria Piazzolla, Maria Maddalena Squillante, Ernesto Agostinacchio, Giovanna Cocomazzi, Egidio Visaggi, Nazario Augello, Camilla Iannuzziello, Mattia Falcone, Angelo De Giorgi, Fausto Campanozzi: None. Alessandra Mangia disclose fees for consulting, speakers bureaus and research support from BMS, Gilead, MSD, Intercept.
AUTHOR'S CONTRIBUTION
Mangia A: conceptualization, data analysis, critical revision of the article. Cocomazzi G, Piazzolla V: data managing. Canosa A, Rina, MF, Iannuzziello C, Falcone M, De Giorgi A, Augello N, Agostinacchio E, Visaggi E, Campanozzi F: data collection. Squillante MM, Campanozzi F: drafting the article. Mangia A, Cocomazzi G, Piazzolla V, Canosa A, Rina MF, Iannuzziello C, Falcone M, De Giorgi A, Augello N, Agostinachio E, Visaggi E, Campanozzi F, Squillante MM: final approval.
CLINICAL TRIAL REGISTRATION
Clinical trial.gov NCT 03923595.
ACKNOWLEDGMENTS
This study was funded by Gilead Science IN‐IT‐987‐5481 as part of the LEGA‐C Local Elimination Program leading to Global Action in HCV program.
Mangia A, Rina MF, Canosa A, Piazzolla V, Squillante MM, Agostinacchio E, et al. Increased Hepatitis C virus screening, diagnosis and linkage to care rates among people who use drugs through a patient‐centered program from Italy. United European Gastroenterol J. 2021;9(10):1109–18. 10.1002/ueg2.12156
DATA AVAILABILITY STATEMENT
Data are available at our Institute repository.
REFERENCES
- 1. World Health Organization. Global health sector strategy on viral hepatitis 2016–21. https://www.who.int/hepatitis/strategy2016‐2021/ghss‐hep/en/. Accessed January 2021. [Google Scholar]
- 2. Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high‐income countries. Liver Int. 2020. Mar;40(3):522–9. 10.1111/liv.14324. PMID: 31815353. [DOI] [PubMed] [Google Scholar]
- 3. Wingrove C, Ferrier L, James C, Wang S. The impact of COVID‐19 on hepatitis elimination. Lancet Gastroenterol Hepatol. 2020. Sep;5 (9):792–4. 10.1016/S2468-1253(20)30238-7. PMID: 32730783 PMCID: PMC7384773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. AASLD . Call to action for liver associations to advance progress towards viral hepatitis elimination: a focus on simplified approaches to HCV testing and cure. AASLD; 2019. [Google Scholar]
- 5. Lazarus JV, Wiktor SZ, Colombo M, Thursz M. Microelimination a path to global elimination of hepatitis C. J Hepatol. 2017. Oct;67(4):665–6. 10.1016/j.jhep.2017.06.033. PMID: 28760329. [DOI] [PubMed] [Google Scholar]
- 6. Lazarus JV, Safreed‐Harmon K, Thursz MR, Dillon JF, El‐Sayed MH, Elsharkawy AM, et al. The micro‐elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018. Aug;38(3):181–92. 10.1055/s-0038-1666841. PMID: 29986353. [DOI] [PubMed] [Google Scholar]
- 7. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013. Aug 15;57(2):S56–61. 10.1093/cid/cit271. PMID: 23884067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spada E, Rezza G, Garbuglia AR, Garbuglia AR, Lombardo FL, Zuccaro O, et al. Incidence and risk factors for hepatitis C virus infection among illicit drug users in Italy. J Urban Health. 2018;95:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Presidenza del Consiglio dei Ministri—Dipartimento Politiche Anti‐ droga. Relazione annuale al parlamento sul fenomeno delle tossicodipendenze in Italia 2021. In sullo stato delle tossicodipendenze in Italia. http://www.politicheantidroga.gov.it/it/. Accessed 17 July 2021. [Google Scholar]
- 10. Lee SR, Kardos KW, Schiff E, Berne CA, Mounzer K, Banks AT, et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods. 2011. Mar;172(1‐2):27–31. [DOI] [PubMed] [Google Scholar]
- 11. Mangia A, Piazzolla V, Giannelli A, Visaggi E, Minerva N, Palmieri V, et al. SVR12 rates higher than 99% after sofosbuvir/velpatasvir combination in HCV infected patients with F0‐F1 fibrosis stage: a real world experience. PloS One. 2019. May 15;14(5):e0215783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morisco F, Granata R, Camera S, Ippolito A, Milella M, Conti F, et al. Optimization of direct anti‐viral agent treatment schedule: focus on HCV genotype 3. UEG Journal. 2018;6:225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. http://www.salute.gov.it/portale/news/p3_2_4_1_1.jsp?menu=salastampa&id=5680
- 14. Lazarus JV, Pericàs JM, Picchio C, Cernosa J, Hoekstra M, Luhmann N, et al. We know DAAs work, so now what? Simplifying models of care to enhance the hepatitis C cascade. The Association for the Publication of the J of Intern Med. 2019;286:503–25. [DOI] [PubMed] [Google Scholar]
- 15. Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al. Simplified diagnostic pathways European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: final update to the series. J Hepatol. 2020;73(5):1170–218.32956768 [Google Scholar]
- 16. Linnet M, Peters L, Raben D, Petersen H, Gerstoft J, Lundgren J, et al. Organizational barriers as an explaineation for differences in offer and uptake rates for hepatitis A/B/C and HIV testing in three drug treatment centers in Copenhagen. In Poster presented at HepHIV 2017, Malta. https://hiveurope.eu/Portal/0/Conference2017/Posters/PS1_03.PDF [Google Scholar]
- 17. https://www.aifa.gov.it/documents/20142/847506/Aggiornamento_dati_Registri_AIFA_DAAs‐04‐01‐2021.pdf/3d8c1643‐e0dc‐fd06‐eeb8‐1f6b4d2e6098
- 18. Boschini A, Piselli P, Smacchia C, Poletti R, Begnini M, Ottogalli P, et al. HCV treatment and reinfection risk in injecting drug users in residential treatment setting for drug addiction, San Patrignano HCV‐free. In The liver meeting Digital Experience 2020. [Google Scholar]
- 19. Molinaro S, Resce G, Alberti A, Andreoni M, D’Egidio PPF, Leonardi C, et al. Barriers to effective management of hepatitis C virus in people who inject drugs: Evidence from outpatient clinics. Drug Alcohol Rev. 2019;38:644‐655. [DOI] [PubMed] [Google Scholar]
- 20. Mangia A, Milligan S, Khalili M, Fagiuoli S, Shafran SD, Carrat F, et al. Global real‐world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: analysis of 5552 patients from 12 cohorts liver int. Liver Int. 2020. May 25;40:1841–52. 10.1111/liv.14537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grebely J, Dalgard O, Conwey B, Cunningham EB, Bruggmann P, Hajarizadeh B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY):an open‐label, single‐arm, phase 4, multicentre trial Lancet. Gastroenterol Hepatol. 2018;3:153–61. [DOI] [PubMed] [Google Scholar]
- 22. Solomon S, Wagner‐Cardoso S, Smeaton L, Sowah L, Wimbish C, Robbins G, et al. The keep it simple and safe approach to HCV treatment: primary outcomes from the ACTG A5360 (MINMON) study. AASLD. 2020. Hepatology. [Google Scholar]
- 23. Hashim A, Worthley T, Bremner s, Macken L, Aithal G, Verma S. Hostel‐based models can improve the engagement of homeless individuals with liver services: VALID (vulnerable adults liver disease) study. EASL ILC 2019. 2019. [Google Scholar]
- 24. Shiha G, Elsayed R, Mikhail N, Easterbrook P. Towards HCV elimination: feasibility of complete linkage to care by testing and treatment on the same day of screening: a pilot study. J Hepatol. 2019;70:e41–2. [Google Scholar]
- 25.Simplified HCV treatment algorithm for treatment‐naive adults with compensated cirrhosis from www.hcvguielines.org. Updated August 27, 2020.
- 26. Radley A, de Bruin M, Inglis SK, Donnan PT, Hapca A, Barclay ST, et al. Clinical effectiveness of pharmacist‐led versus conventionally delivered antiviral treatment for hepatitis C virus in patients receiving opioid substitution therapy: a pragmatic, cluster‐randomised trial Lancet. Gastroenterol Hepatol. 2020;5:809–18. [DOI] [PubMed] [Google Scholar]
- 27. Grebely J, Applegate TL, Cunningham P, Feld J. Point‐of‐care diagnostics: in point of care testing in search for a single visit diagnosis. Expert Rev Mol Diagn. 2017;(17):1109–1115. [DOI] [PubMed] [Google Scholar]
- 28. Grebely JG, Robaeyes G, Bruggman P, Aghemo A, Backmund M, Bruneau J. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Pol. 2015;26:893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cunningham EB, Amin J, Feld J, Bruneau J, Dalgard O, Powis J, et al. Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: the SIMPLIFY study. Int J Drug Pol. 2018. Dec;62:14–23. [DOI] [PubMed] [Google Scholar]
- 30. Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct‐acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2018;3(11):754–67. 10.1016/S2468-1253(18)30304-2. PMID: 30245064. [DOI] [PubMed] [Google Scholar]
- 31. Marcos‐Fosch C, Grau‐Lopez L, Daigree C, Palma‐Álvarez RF, Rando Segura A, Llaneras S, et al. Screening and treatment difficulties in hepatitis C virus‐infected patients with substance use disorders or dual diagnoses, despite centralized management in an addiction and dual diagnosis center. ILC. 2020.Thu 344. [Google Scholar]
- 32. Forns X, Colom J, Garcia‐Retortillo J, Quer JC, Lens S, Dominguez‐Hernandez R, et al. Point of care hepatitis C testing and treating strategy in people who inject drugs in harm reduction and addiction centers for hepatitis C elimination. AASLD 2020. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mangia A, Cenderello G, Copetti M, Verucchi G, Piazzolla V, Lorusso C, et al. SVR12 higher than 97% in GT3 cirrhotic patients with evidence of portal hypertension treated with SOF/VEL without Ribavirin: a nation‐wide cohort study. Cells. 2019;8:313. 10.3390/cells8040313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Von Felden J, Vermehren J, Ingiliz P, Mauss S, Lutz T, Simon KG, et al. High efficacy of sofosbuvir/velpatasvir and impact of baseline resistance‐associated substitutions in hepatitis C genotype 3 infection. Aliment Pharmacol Ther. 2018;47:1288–95. [DOI] [PubMed] [Google Scholar]
- 35. Read P, Lothian R, Chronister K, Gilliver R, Kearley J, Dore GJ, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Pol. 2017;47:209–15. [DOI] [PubMed] [Google Scholar]
- 36. Mason K, Dodd Z, Guyton M, Tookey P, Lettner B, Matelski J, et al. Understanding real‐world adherence in the directly acting antiviral era: a prospective evaluation of adherence among people with a history of drug use at a community‐based program in Toronto, Canada. Int J Drug Pol. 2017;47:202–8. [DOI] [PubMed] [Google Scholar]
- 37. Rossi C, Butt ZA, Wong S, Buxton JA, Islam N, Yu A, et al. Hepatitis C virus reinfection after successful treatment with direct‐acting antiviral therapy in a large population‐based cohort. J Hepatol. 2018;69:1007–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at our Institute repository.


