Abstract
Background and aims
Few data are available regarding the combination of biologics or small molecules in inflammatory bowel disease (IBD) patients. We report safety and efficacy of such combinations through a retrospective multicentre series.
Methods
Combination therapy was defined as the concomitant use of two biologics or one biologic with a small molecule. Patient demographics, disease characteristics and types of combinations were recorded. Safety was evaluated according to the occurrence of serious infection, opportunistic infection, hospitalisation, life‐threatening event, worsening of IBD or immune‐mediated inflammatory diseases (IMID), cancer and death. Efficacy was evaluated as the physician global assessment of the combination and comparison of clinical/endoscopic scores of IBD/IMID activity prior and during combination.
Results
A total of 104 combinations were collected in 98 patients. Concomitant IMID were present in 41 patients. Reasons for starting combination therapy were active IBD (67%), active IMID or extra‐intestinal manifestations (EIM) (22%), both (10%) and unclassified in 1. Median duration of combination was 8 months (interquartile range 5–16). During 122 patient‐years of follow‐up, 42 significant adverse events were observed, mostly related to uncontrolled IBD. There were 10 significant infections, 1 skin cancer and no death. IBD disease activity was clinically improved in 70% and IMID/EIM activity in 81% of the patients. Overall, combination was continued in 55% of the patients.
Conclusions
Combination of biologics and small molecules in patients with IBD and IMID/EIM seems to be a promising therapeutic strategy but is also associated with a risk of opportunistic infections or infections leading to hospitalisation in 10%.
Keywords: biologics, combination therapy, immune mediated inflammatory disease, inflammatory bowel disease, safety, small molecules, treatment
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are inflammatory bowel diseases (IBD) characterised by a widely variable clinical course and heterogeneous presentations including extra‐intestinal manifestations (EIM), mainly rheumatological and dermatological conditions. Their association with other immune‐mediated inflammatory diseases (IMID) is well known. Knowledge and treatments in IBD and other IMIDs are continuously evolving and there is currently a broad range of approved biologics and small molecules that target different inflammatory pathways. 1 Despite the progress in therapeutic armamentarium, it is still common to come across IBD patients with primary or secondary loss of response to numerous medications or in whom EIM are not properly controlled whilst the IBD is in remission.
Key summary.
Summarise the established knowledge on this subject
Questions have arisen regarding potential side effects of combining biologics together or with small molecules.
No new safety signals have been observed in limited case series.
Partial efficacy have been shown in limited case series.
What are the significant and/or new findings of this study?
When the combination was initiated for active inflammatory bowel disease (IBD), clinical and endoscopic improvement is observed in more than half of the patients.
Treatment of immune‐mediated inflammatory disease (IMID)/extra‐intestinal manifestations (EIM) manifestations by combining biologics was successful showing that this strategy is realistic and useful in IBD patients with uncontrolled IMID/EIM.
10% of the patients presented with one opportunistic infection or an infection leading to hospitalisation indicating that infectious complications remain the main concern of this approach.
In this setting, few clinical series reported efficacy and safety outcomes of combining two biologics and/or small molecules in IBD patients. Although no new safety signals have been observed in limited case series, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 questions have naturally arisen regarding potential side effects. One recent meta‐analysis summarised the existing data on safety and efficacy of combining biologics and small molecules. Ahmed et al. 10 identified 279 patients in 30 studies and reported an acceptable safety profile in these patients. Limitations of the pooled analysis were the inconsistent definitions in reporting patient outcomes for efficacy and safety and the significant heterogeneity of the different studies.
The major aim of this study was to determine the safety of combining two biologics or one biologic and one small molecule. The minor aim was to evaluate the therapeutic efficacy of combining these treatments in different IBD and IMID scenarios.
MATERIALS AND METHODS
Study design and patient selection
This was a retrospective multicentre, European observational study. The project received endorsement from the ECCO National Study Group Committee in 2019 and 2020 (ECCO Congresses). Participating investigators were asked to screen their databases for a time window of 3.5 years (starting from 1 November 2016 until 1 June 2020) to identify patients on combination eligible for the study. Inclusion criteria were a diagnosis of CD, UC or IBD‐unclassified; age >18 years and patients willing to sign an informed consent form. Combination was defined as the concomitant use (from 1 day to no limitation in duration) of a minimum of two biologics, or of one biologic combined with one small molecule. Patients on combination including a biologic or a small molecule approved for other IMIDS but not yet for IBD were also included.
Patients were initiated on a second biologic or small molecule (defined hereafter as the combination) for any of the following reasons: (i) active IBD (group A), (ii) both active IBD and associated IMID or EIM (group B) and (iii) active IMID or EIM despite quiescent IBD (group C).
Data collection
Local investigators had access to all source documents and patients were pseudo‐anonymised. An appropriate case report form (CRF) was designed to collect in an homogeneous way disease characteristics (IBD subtype, age on IBD diagnosis, disease duration, disease classification, prior intestinal surgeries, EIM and past and ongoing treatments), patient characteristics (year of birth, significant history and comorbiditiesand associated IMID), characteristics of the combination (type, order, doses and duration), evolution of IBD activity under combination (clinical and endoscopic activity), inflammatory markers (faecal calprotectin and C‐reactive protein [CRP]), clinical evolution of IMID/EIM activity, global physician assessment of the combination, need for surgery and corticosteroid use during combination therapy. We also collected data on significant adverse events (see below) during combination. The following data were also recorded in a standardised way: start date for combination considered as the date when a biologic or a small molecule was added to an existing treatment (biologic or small molecule); indication for combination; dates and reasons for stopping the combination. Treatments used for combination were classified into five categories: anti‐TNF therapies (infliximab, adalimumab, certolizumab, golimumab and etanercept); anti‐integrin therapy (vedolizumab); anti‐interleukin therapies directed against interleukin 4/13, 5, 6, 12/23, 17A and 23 (dupilumab, mepolizumab, tocilizumab, ustekinumab, secukinumab and risankizumab); JAK inhibitor (tofacitinib); and other small molecules (apremilast, ciclosporine, leflunomide and tacrolimus).
Outcomes
The primary outcome measure was safety of the combination according to the occurrence of any significant adverse events and defined as (i) serious infection leading to hospitalisation, (ii) opportunistic infection, (iii) hospitalisation or prolonged hospitalisation for any reason, (iv) life‐threatening event, (v) event resulting in persistent or significant disability/incapacity, (vi) aggravation of IBD or IMID, (vii) recurrent cancer or new incident cancer and (viii) death. Adverse events were graded from 1 to 5 according to the Common Terminology Criteria for Adverse Events: Grade 1: asymptomatic or mild symptoms; clinical or diagnostic observations only; no intervention indicated. Grade 2: moderate, minimal, local or non‐invasive intervention indicated; limiting age‐appropriate instrumental ADL (activities of daily living). Grade 3: severe or medically significant but not immediately life‐threatening; hospitalisation or prolongation of hospitalisation indicated; disabling; limiting self‐care ADL. Grade 4: life‐threatening consequences; urgent intervention indicated. Grade 5: death related to an adverse event.
Secondary outcomes were therapeutic efficacy and persistence of such combinations, evaluated as follows: (i) IBD/IMID disease activity during combination based on physician global assessment using a 5‐point Likert scale: complete improvement, partial improvement, situation stable/unchanged, worsening after initial improvement and worsening; (ii) comparison of simple clinical (for IBD, for IMID or EIM) and endoscopic (for IBD) activity scores (quiescent scores 0 point, mild scores 1, moderate scores 2 and severe scores 3) prior to and during combination. The same scores were used numerically to evaluate the mean clinical and endoscopic activity as well as their evolution under combination therapy. Clinical assessment was done immediately before the start of the combination whilst a time window of maximum 4 months prior to the combination was accepted for endoscopic assessment. There were no predefined time points for clinical and endoscopic evaluation during combination, so the latest information available in the medical file was retrieved; (iii) time on combination therapy (percentage of patients still on combination therapy after 6 and 12 months).
Statistical analysis
All statistical analyses were performed using R‐3.6.0 (The R Foundation for Statistical Computing, Austria, Vienna, 2019). Continuous variables were presented as mean ± standard deviation or median and interquartile range (IQR), as appropriate, whereas categorical variables were presented as numbers and proportions or frequencies. For efficacy analysis, variables before and after intervention were compared using paired t‐test or Wilcoxon signed‐rank test for continuous variables. Statistically significant difference was defined as a two‐tailed p < 0.05 for all tests. Time spent on combination was estimated by the Kaplan–Meier method.
Ethical considerations
Study protocol and materials were approved by the institutional review boards at CHU UCL Namur University Hospital, Yvoir, Belgium, Central Ethic Committee Mont‐Godinne 174/2019 ‘the COMBIO – BIRD2019001’: final acceptance 7 July 2020. The trial was conducted in compliance with the principles of the Declaration of Helsinki, the principles of GCP and in accordance with all applicable regulatory requirements. The protocol and related documents were submitted for review and accepted by Ethics Committees and local regulators from each participating site according to their own legislation. The investigators and the participating sites treated all information and data related to the study disclosed to the participating sites and/or investigators in this study as confidential and did not disclose such information to any third parties or use such information for any purpose other than the performance of the study. The collection, processing and disclosure of personal data, such as patient health and medical information were subject to compliance with applicable personal data protection and the processing of personal data. (General Data Protection Regulation and Belgian Law of 30 July 2018 on the protection of natural persons with regard to the processing of their personal data).
RESULTS
Patients and baseline characteristics
A total of 130 CRF were received. Of these 21 were not analysed because the start date of combination was after the deadline mentioned in the protocol and five due to incomplete data. A total of 104 combinations were identified in 98 IBD patients (6 patients received more than one different combination after failing a first combination). Preliminary data from 27 patients have already been previously reported. 4 , 9 The baseline characteristics of patients are shown in Table 1. The vast majority of patients attended academic centres for their IBD care (88%). The median age at diagnosis was 26 years (IQR 18–37), majority were women (56%) and Caucasians (94%). Median disease duration was 10 years (IQR 6–21) and all patients had failed prior biologics with a median use of three agents (IQR 3–4; Table S1). Concomitant IMID conditions or EIM were present in 41 (42%) and 42 (43%) patients, respectively. Patients were followed up (from the start date of combination until the date of the last consultation) for a median time of 14.5 months (IQR 8–26).
TABLE 1.
Baseline characteristics of patients and diseases
| Patients characteristics | All patients n = 98 | CD n = 58 | UC n = 40 |
|---|---|---|---|
| Male sex (n, %) | 43 (44) | 26 (45) | 17 (43) |
| Age at IBD diagnosis (median, [IQR]) | 26 [18–37] | 27 [17.25–34.75] | 25.5 [19.75–41.25] |
| Montreal classification (n, %) | L1: 10 (17) | ||
| L2: 11 (19) | E1: 3 (8) | ||
| L3: 37 (64) | E2: 13 (33) | ||
| L4: 4 (7) | E3: 24 (60) | ||
| B1: 26 (45) | S1: 2 (5) | ||
| B2: 17 (29) | S2: 9 (23) | ||
| B3: 15 (26) | S3: 26 (65) | ||
| P: 27 (47) | |||
| Tobacco use (n, %) | |||
| Active | 12 (12) | 11 (19) | 1 (3) |
| Never or former | 82 (84) | 46 (79) | 36 (90) |
| Unknown | 4 (4) | 1 (2) | 3 (7) |
| Presence of EIM (n, %) | 42 (43) | 29 (50) | 13 (33) |
| Type of EIM (n, %) | |||
| Osteoarticular | 23 (55) | 17 (59) | 6 (46) |
| Skin | 18 (43) | 12 (41) | 6 (46) |
| Liver | 5 (12) | 3 (10) | 2 (15) |
| AI pancreatitis | 1 (2) | – | 1 (8) |
| Ocular | 10 (24) | 5 (17) | 5 (39) |
| Associated IMID (n, %) | 41 (42) | 26 (45) | 15 (38) |
| Patients with >1 IMID (n, %) | 9 (9) | 6 (10) | 3 (8) |
| Type of IMID (n, %) | |||
| Ankylosing spondylarthritis | 25 (61) | 18 (69) | 7 (47) |
| Psoriasis | 12 (29) | 7 (27) | 5 (33) |
| Psoriatic arthritis | 4 (10) | 2 (8) | 2 (13) |
| Rheumatoid arthritis | 4 (10) | 2 (8) | 2 (13) |
| Others a | 7 (17) | 3 (12) | 4 (27) |
| Patient with prior surgery for IBD (n, %) | 39 (40) | 38 (66) | 1 (3) |
| Number of prior surgeries (median, IQR) | 2 [1–3] | 2 [1–3] | 1 [1–1] |
| Number of prior biologics (median, [IQR]) | 3 [3–4] | 4 [3–5] | 3 [3–4] |
| Number of prior immunomodulators (median, [IQR]) | 2 [1–2] | 2 [1–2] | 1.5 [1–2] |
| History of cancer b (n, %) | 4 (4) | 4 (7) | ‐ |
| History of serious infection c (n, %) | 13 (13) | 9 (16) | 4 (10) |
| Significant co‐morbidities (n, %) | |||
| Cardiopulmonary | 6 (6) | 5 (9) | 1 (3) |
| Others d | 11 (11) | 6 (10) | 5 (13) |
Other IMID: Generalised constitutional eczema and hyperIgE syndrome, haemolytic anaemia, asthma, morphea generalisata, iridocyclitis, lupus and Takayasu’s arteritis.
Breast cancer, hepatocarcinoma, melanoma and squamous cell cancer.
Latent tuberculosis, fistulazing pretibial abcedation (pyoderma gangrenosum), perforated diverticulitis, ophthalmic zona, cutaneous zonas, perineal abscess, sepsis (pulmonary, urinary and Clostridium), staphylococcemia, bronchopneumony, candidiasis, right ulnar osteitis and Clostridium difficile.
Diabetes, interstitial nephritis, cerebrovascular ischaemia, adrenal deficiency, venous thrombosis, liver transplantation, paroxysmal nocturnal haemoglobinuria and prothrombin mutation.
Combination therapies
The type and indications for various combination are shown in Table 2. The reason for combining was mostly identical to the reason for introducing the second treatment but the indication was sometimes double (i.e. partial control of IBD with the first drug and uncontrolled IMID). Details on the type of combination, order, indication and prior use of the second drug are shown in Table S2. The most used combination was anti‐TNF and anti‐integrin followed by anti‐IL23 and an anti‐integrin. Overall, 80% of patients were naïve to the second drug introduced. Data on dose intensification was available for 96% of all combinations: 29% had one drug dose intensified (first or second) and 13% had both drugs dose intensified. On initiation of combination, 14 (13%) patients were receiving concomitant immunomodulators (thiopurines, methotrexate or mycophenolate mofetil) and 34 (33%) were on concomitant systemic corticosteroids.
TABLE 2.
Overview of classes and indications of combinations
| Indications a type of combination | All indications n = 104 a | Active IBD (A) n = 70 | Both active IBD and active IMID/EIM (B) n = 10 | Active IMID/EIM (C) n = 23 |
|---|---|---|---|---|
| Anti‐TNF + VZ | 41 | 31 | 5 | 5 |
| Anti‐TNF + anti‐IL | 11 | 7 | 1 | 3 |
| Anti‐IL + VZ | 21 | 16 | – | 5 |
| Tofacitinib + anti‐TNF | 1 | 1 | – | – |
| Tofacitinib + VZ | 13 | 10 | 2 | 1 |
| Anti‐IL + anti‐IL | 1 | 1 | ‐ | ‐ |
| Combinations with other molecules b | 16 a | 4 | 2 | 9 |
Abbreviations: EIM, extra‐intestinal manifestation; IBD, Inflammatory bowel disease; IL, Interleukin; IMID, immune mediated inflammatory disease; TNF, Tumour Necrosis Factor; VZ, vedolizumab.
One combination was Risankizumab + Tacrolimus for IBD and liver transplantation.
Apremilast, Ciclosporine, Rituximab, Leflunomide and Tacrolimus. Details of all the combinations is given in Supplementary Table S2.
The median age at starting the combinations was 41 years (IQR 30.5–53.5). The median duration of combination was 8 months (IQR 5–16) for a total of 122 patient‐years. At the last follow‐up consultation, 57 (55%) combinations were continuing whilst 47 (45%) had been discontinued.
Safety analysis
Overall, 41 patients (42%) experienced a total of 42 significant adverse events. Events were graded as Grade 1 (n = 2), Grade 2 (n = 8), Grade 3 (n = 17), Grade 4 (n = 3), Grade 5 (n = 0) and not graded (n = 12). Serious infections leading to hospitalisation and/or opportunistic infection were observed in 10 out of 98 patients and are summarised in Table 3. All patients with serious infections had CD, 9 (90%) had an associated IMID/EIM (five ankylosing spondylitis) and 60% were concomitantly treated either with steroids and/or immunomodulators. The median age of patients with serious infections was identical to those without infection (data not shown). Whilst anti‐TNF was used in 57% of all combinations (59/104), all infectious events except 1 (90%) occurred in the TNF‐combined group.
TABLE 3.
Serious and opportunistic infection in patients on combinations
| Serious and opportunistic infections | Combination of treatments | Use of steroids yes/no | Age at event (years) | Grade a | Hospitalisation/ICU | Causality | Status |
|---|---|---|---|---|---|---|---|
| Osteomyelitis after ankle fracture | Adalimumab (SD) + Vedolizumab (SD) | No | 61 | 3 | Yes/No | Possibly related | Resolved |
| Enterocutaneous fistula infection | Vedolizumab (ID) + Infliximab (SD) + Azathioprine | No | 26 | 3 | Yes/No | Possibly related | Resolved |
| Perianal abscess | Ustekinumab (ID) + Etanercept (SD) | No | 44 | 2 | No/No | Possibly related | Resolved |
| Multiple upper respiratory viral infection | Adalimumab (ID) + Vedolizumab (SD) | No | 38 | 2 | No/No | Possibly related | Resolved |
| Campylobacter colitis | Adalimumab (SD) + Vedolizumab (SD) | Yes | 49 | 3 | Yes/No | Possibly related | Resolved |
| Pneumonia | Infliximab (SD) + Vedolizumab (SD) + Methotrexate | No | 40 | 2 | No/No | Possibly related | Resolved |
| Herpetic meningoencephalitis | Certolizumab (SD) + Vedolizumab (SD) + Methotrexate | No | 43 | 3 | Yes/No | Possibly related | Resolved |
| Esophageal candidiasis | Adalimumab (ID) + Ustekinumab (ID) | Yes | 60 | 1 | No/No | Possibly related | Resolved |
| Flu | Ustekinumab (ID) + Etanercept (SD) | No | 43 | 2 | No/No | Possibly related | Resolved |
| Viral upper respiratory infection | Vedolizumab (SD) + Ustekinumab (SD) + Azathioprine | No | 18 | 3 | Yes/No | Possibly related | Resolved |
Abbreviations: ICU: intensive care unit; NB: standard dose (SD) or increased dose (ID).
Events were graded from 1 (asymptomatic or mild symptoms) to 5 (death) according to the Common Terminology Criteria for Adverse Events.
One patient had two hospitalisations considered unrelated to inflammatory disease or combination: the patient was admitted due to atrial fibrillation and later he suffered from angiotensin converting enzyme (ACE) induced angioedema. One patient was hospitalised for flare of primary sclerosing cholangitis resolved without discontinuing combination. Hospitalisation or prolonged hospitalisation for worsening IBD and/or IBD surgery was observed in 11 combinations and were considered unrelated to the combination. Six patients underwent IBD‐related surgery.
Life‐threatening events were observed in two patients (ACE‐induced angioedema and hypersensitivity reaction to infliximab) whereas no event resulting in persistent or significant disability/incapacity was observed. Worsening of IBD and worsening of IMID/EIM without hospitalisation was observed in 13 and 3 combinations, respectively. One patient was diagnosed with a new incident benign skin neoplasia (clear cell acanthoma). Recurrent cancer and death were not observed.
Global evaluation of the combination strategy
Global physician assessment of the combination strategy was available for all patients. In patients receiving the combination for active IBD (n = 80), complete or partial improvement was observed in 21/80 (26%) and 35/80 (44%), respectively. In patients receiving the combination for active EIM or IMID (n = 33), a complete or partial improvement was observed in 12/33 (36%) and 15/33 (45%), respectively (Figure 1).
FIGURE 1.
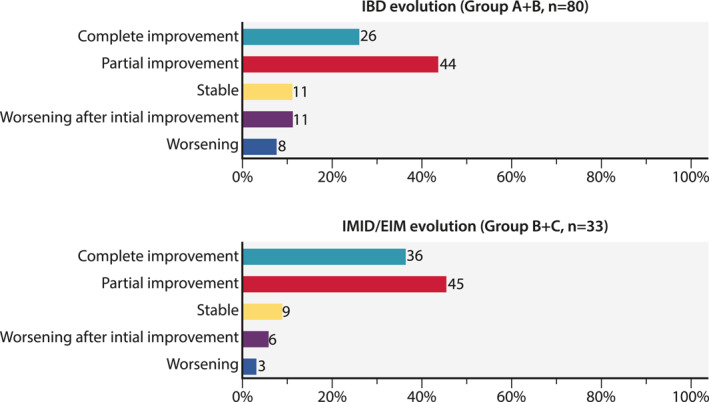
Global evaluation of the combination strategy in all active inflammatory bowel disease and all immune mediated inflammatory disease /extra‐intestinal manifestation patients.
Table 4 gives the evolution of the combinations over time. The median time to discontinuation of combination, estimated by Kaplan–Meier, was 18 months (Figure 2; 95% CI: 10 months – not observed). Among 34 patients receiving corticosteroids at baseline, 58% were able to reduce and/or discontinue steroids by the time of the last follow‐up consultation. Of the 104 combinations, 47 (45%) were discontinued for the following reasons: absence or loss of response for IBD in 25 patients (53%), absence or loss of response for IMID/EIM in 5 (11%), clinical improvement with step‐down strategy in 10 (21%), intolerance in 2 (4%), adverse event in 4 (8%) and patient decision in 1 (2%).
TABLE 4.
Evolution of the combination based on the indication
| Indication a | All | Patients with active IBD | Patients with both active IBD and active IMID/EIM | Patient with active IMID/EIM | |
|---|---|---|---|---|---|
| Group A | Group B | Group C | |||
| N = 104 a | N = 70 | N = 10 | N = 23 | ||
| Total combo duration: 1467.5 months/104 combinations | |||||
| Percentage of patients with ongoing/stopped combination at last visit (n, %) | |||||
| Ongoing | 57 (55) | 41 (59) | 6 (60) | 10 (43) | |
| Stopped | 47 (45) | 29 (41) | 4 (40) | 13 (57) | |
| Percentage of patients on combination at 6 and 12 months among patients with documented follow‐up (n, %) | |||||
| >6 months | 69/91 (76) | 45/60 (75) | 8/9 (89) | 15/21 (71) | |
| >12 months | 36/62 (58) | 24/41 (59) | 4/7 (57) | 8/14 (57) | |
Abbreviations: EIM, extra‐intestinal manifestation; IBD, Inflammatory bowel disease; IMID, immune mediated inflammatory disease.
One combination Risankizumab + Tacrolimus for IBD and liver transplantation.
FIGURE 2.
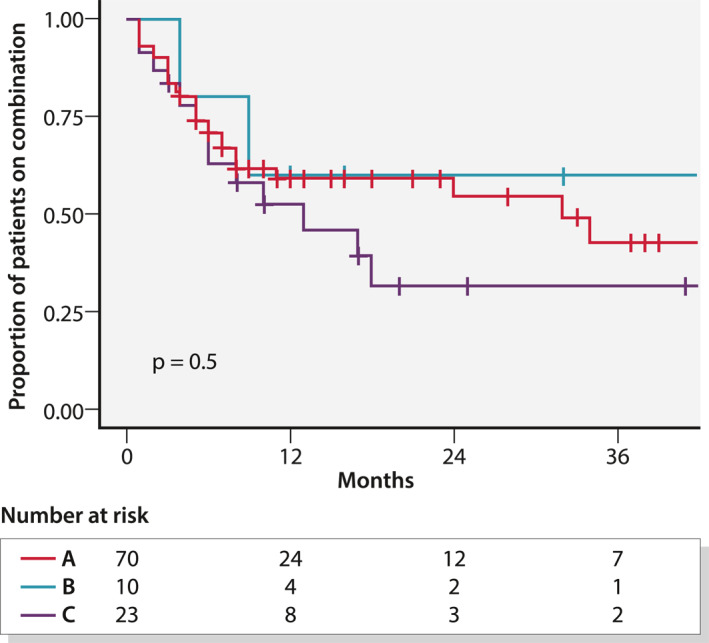
Proportion of patients on combination using Kaplan–Meier curves according to indication of combination. Group A: active inflammatory bowel disease (IBD); Group B: both active IBD and immune mediated inflammatory disease (IMID)/extra‐intestinal manifestation (EIM); Group C: active IMID or EIM
Efficacy of combination therapy for active IBD
In patients with combination initiated for active IBD alone (Group A) and active IBD + active IMID/EIM (Group B), therapeutic efficacy was assessed clinically and endoscopically. The patients in Group A1 were a sub‐group of patients from Group A who were previously exposed to the second drug (Table S2). Table 5 shows the disease activity parameters of the different groups prior to and during combination. Overall, the mean clinical disease activity for IBD (Groups A + B; N = 80) was significantly higher prior to combination than during combination (2.2 ± 0.7 vs. 1.2 ± 1.1; p < 0.0001). Among patients treated for active IBD as a unique indication (Group A), 17 had previously been exposed to the second drug used during combination therapy (Group A1) and had a partial or incomplete response (primary or secondary failure) to that drug in the past. During combination, the reduction of the clinical score did not differ for these patients (Group A‐A1 (n = 53): −1.0 ± 1.0 vs. Group A1 (n = 17): −1.1 ± 1.1; p = 0.94).
TABLE 5.
IBD activity parameters at the start of combination and during combination in patients with active IBD and active IBD + active IMID/EIM
| IBD Disease activity | Patients with active IBD | Patients with active IBD previously exposed to second drug | Patients with both active IBD and active IMID/EIM | |||
|---|---|---|---|---|---|---|
| Group A | Group A1 | Group B | ||||
| N = 70 | N = 17 | N = 10 | ||||
| Start of combination (baseline) | During combination | Start of combination (baseline) | During combination | Start of combination (baseline) | During combination | |
| Clinical assessment (n, %) | ||||||
| Quiescent (n, %) | 2 (3) | 27 (39) | 1 (6) | 6 (35) | – | 3 (30) |
| Mild | 7 (10) | 15 (21) | 2 (12) | 6 (35) | 3 (30) | 2 (20) |
| Moderate | 35 (50) | 17 (24) | 9 (53) | 4 (24) | 5 (50) | 5 (5) |
| Severe | 26 (37) | 11 (16) | 5 (29) | 1 (6) | 2 (20) | – |
| Clinical evolution (n, %) | ||||||
| Worsening (n, %) | 2 (3) | 1 (6) | 1 (10) | |||
| Stable | 21 (30) | 3 (18) | 4 (40) | |||
| Improvement | 47 (67) | 13 (76) | 5 (50) | |||
| p‐value(binominal test) | <0.0001 | 0.002 | 0.22 | |||
| Endoscopic assessment (n, %) | ||||||
| Quiescent (n, %) | 1 (2) | 9 (28) | – | 2 (25) | – | 1 (14) |
| Mild | 12 (20) | 7 (22) | 5 (38.5) | 2 (25) | 1 (12.5) | 2 (29) |
| Moderate | 14 (24) | 6 (19) | 2 (15.5) | 1 (12.5) | 3 (37.5) | 2 (29) |
| Severe | 32 (54) | 10 (31) | 6 (46) | 3 (37.5) | 4 (50) | 2 (29) |
| n = 59 | n = 32 | n = 13 | n = 8 | n = 8 | n = 7 | |
| Endoscopic evolution (n, %) | ||||||
| Worsening (n, %) | 2 (7) | 1 (14) | 0 | |||
| Stable | 12 (40) | 3 (43) | 3 (50) | |||
| Improvement | 16 (53) | 3 (43) | 3 (50) | |||
| p‐value(binominal test) | 0.001 | 0.63 | 0.25 | |||
| n = 30 | n = 7 | n = 6 | ||||
| Steroids use (n, %) | ||||||
| No | 46 (66) | 55 (79) | 9 (53) | 12 (71) | 8 (80) | 9 (90) |
| Yes | 24 (34) | 15 (21) | 8 (47) | 5 (29) | 2 (20) | 1 (10) |
Abbreviations: EIM, extra‐intestinal manifestation; IBD, Inflammatory bowel disease; IMID, immune mediated inflammatory disease.
Endoscopic evaluation was performed in 67 patients at a median time of 1 month (IQR 0.25–3.75) before starting combination and 39 patients received a follow up endoscopy at a median time of 11 months (IQR 6–20) after starting combination (Table 5). In CD, the two most effective combinations (complete or partial improvement obtained) were anti‐integrin + anti‐IL23 in 11/13 (85%) followed by anti‐TNF + anti‐integrin in 16/25 (64%). In UC, chances of success were best observed with anti‐TNF + anti‐integrin in 8/11 (73%) and anti‐integrin + tofacitinib in 8/12 (67%).
Efficacy of combination therapies for active IMID/EIM
The combination was initiated for active IMID/EIM manifestations irrespective of IBD activity in 33 patients and therapeutic efficacy was clinically assessed (Table 6). Mean clinical disease activity for IMID/EIM was significantly higher prior starting combination than during combination (2.3 ± 0.5 vs. 1 ± 0.8; p < 0.0001).
TABLE 6.
IMID/EIM disease activity parameters at the start of combination and during combination in patients with active IMID/EIM ± active inflammatory bowel disease
| IMID/EIM activity | At the start of combination (baseline) | During combination |
|---|---|---|
| Group B + C | ||
| N = 33 | ||
| Clinical assessment a (n, %) | ||
| Quiescent | – | 9 (31) |
| Mild | 1 (3) | 12 (41) |
| Moderate | 19 (66) | 7 (24) |
| Severe | 9 (31) | 1 (3) |
| Clinical score evolution (n, %) | ||
| Worsening | 1 (3) | |
| Stable | 4 (14) | |
| Improvement | 24 (83) | |
| p‐value (binominal test) | p < 0.0001 | |
| Steroids use (n, %) | ||
| No (n, %) | 24 (72.7) | 27 (81.8) |
| Yes (n, %) | 9 (27.3) | 6 (18.2) |
Abbreviations: EIM, extra‐intestinal manifestation; IBD, Inflammatory bowel disease; IMID, immune mediated inflammatory disease.
Clinical assessment was unavailable for four combinations.
DISCUSSION
We report the safety and efficacy of combining biologics and small molecule therapies in a large European multicentre series of IBD patients with concomitant IMID and/or EIM.
The major aim of this study was to evaluate the safety of using two biologics or small molecule simultaneously. During 122 patient‐years of combination, we observed 42 significant adverse events in 41 patients, most of them graded 2 or 3 according to the CTCAE classification. No new safety signals were found and three events were considered unrelated to inflammatory disease or combination. Worsening of IBD/IMID or need for IBD related surgery was expected in this refractory IBD population and should be considered as the absence of response to a treatment more than a medical or surgical complication of the combination. About 10% of our patients presented with one opportunistic infection or an infection leading to hospitalisation indicating a rate of significant infection of 8.2/100 patient‐years. All patients with infections received an anti‐TNF combined with another biologic except for one patient. In line with previous work, 2 we found that a substantial number of these patients were concomitantly on systemic corticosteroids (n = 2) or on immunomodulatory treatment (n = 4). Interestingly, these infectious events mainly occurred in patients with concomitant IMID/EIM. The infectious risk with anti‐TNF combination therapy has also been reported in smaller series. 5 Although the patient populations differed between our study and the work performed by Ford and colleagues, combination of biologics appears to carry a higher risk than that observed in anti‐TNF monotherapy. 11 Some series have not reported any infectious events, 4 but the recent meta‐analysis of patients on biologic combinations reported infectious complications in up to 19% of the patients. 10 However, we found that this number is difficult to interpret owing to the large heterogeneity in the nomenclature of infectious events. Similarly, studies evaluating the combination of biologic therapy in rheumatological diseases have dictated some safety concerns. However, most of these studies carrying a warning message used rituximab, abatacept or biologics that are not used for IBD treatment (i.e. anti‐IL1). The 4% of our population presented a history of cancer prior to combination therapy and no recurrence was observed after a median time of 14.5 months. One patient was diagnosed with new incident benign skin neoplasia. Death was not observed.
In terms of efficacy, we were able to consider the cohort separately according to the indication for combination. Similar to other published series, our IBD patients were young at diagnosis and had received multiple lines of treatments. 3 , 5 The majority of treatments were implemented owing to the persistence of luminal disease activity. Despite the severity of IBD, improvement in clinical and endoscopic activity was observed during combination therapy in a significant proportion of patients. Similar findings have been observed in small cohorts. 3 , 4 , 5 , 9 Like other groups, we have observed a reduction in corticosteroid use. 2 , 4 , 5 Interestingly, a subgroup of our patients (Group A1) had previous exposure to the second drug introduced for combination and overall, this group responded like other patients naïve to the second drug. The same findings were observed in a small group of refractory CD patients treated with combination where prior exposure to the drug did not influence the chance of endoscopic response during combination. 3 Recycling strategies have also been successful for patients who previously experienced loss of response to an anti‐TNF agent. 12 In 33 out of 104 combinations, the indication was the control of EIM or IMID. Treatment of IMID/EIM manifestations by introducing a second drug was very successful in our cohort with an overall efficacy rate of 81% (complete or partial improvement). This information from the largest study available to date confirms that this strategy is realistic and useful in IBD patients with uncontrolled IMID/EIM.
Our study has several strengths. First, our work differs from previously published series by the large number of patients included, particularly the 33 combinations for concomitant IMID/EIM, and the longer follow‐up time. Second, all data, including significant adverse events, were extracted and graded in a standardised way allowing a more robust analysis. Lastly, our study is one of the first studies to analyse in detail the indications, order‐of‐drug combinations and prior exposure to treatment for each single combination. Indeed, a successful combination with a naïve drug can be understood as a simple response to that drug, whatever the context for the combination.
We also acknowledge some weaknesses mostly due to the retrospective nature of the study. We were unable to identify benign infections and mild infections not leading to hospitalisation. Therefore a definitive answer regarding the infectious risk cannot be given. Details of endoscopic scores (SES‐CD, MAYO scores) were lacking for some procedures, and thus, a simplified endoscopic score was applied to homogenise the data. Although the simplified endoscopic score is not ideal, it was done by experts in IBD. Some follow up data on endoscopic activity were missing but these patients were identified as follows: 66% had a clinical response, 34% did not show clinical improvement and 7% underwent surgery. Lastly, although our cohort consisting of IBD patients suffering from active disease was large, there was some heterogeneity in terms of disease characteristics and prior exposure to therapies which limits the accurate appraisal of clinical and endoscopic efficacy. The question of efficacy should be answered through a prospective trial with clinical, endoscopic and biologic evaluation at various time points in a well‐defined IBD population.
CONCLUSION
In conclusion, this multicentre study suggests the efficacy of combining two biologics or one biologic and a small molecule in patients with therapy‐refractory IBD and highlights the favourable efficacy for treating refractory EIM and IMID associated with IBD. Infectious complications remain the main concern in this approach as evidenced by our study. Combining biologics and small molecules should be offered in IBD expert centres to highly selected patients when no other therapeutic options nor access to molecules in development are available. In the future, a better understanding of the rationale for combination is needed to help clinician’s decision in these difficult to treat populations. 13
CONFLICT OF INTEREST
LG has no conflict of interest. JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix, Viela bio; and hold stock options in Intestinal Biotech Development and Genfit. AO has no conflict of interest. MF reports Research grant: Amgen, Biogen, Janssen, Pfizer, Takeda. Consultancy: Abbvie, Boehringer‐Ingelheim, Janssen, MSD, Pfizer, Sandoz, Takeda. Speaker's fee: Abbvie, Amgen, Biogen, Boehringer‐Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, Takeda. JS reports speaker's fees: Abbvie, Falk, and Takeda. Consultancy fees: Janssen. CJ has no conflict of interest. RS has no conflict of interest. LR reports no conflict of interest. AA reports Consultant/Advisory board: AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Biogen, Bristol‐Myers Squibb, Celgene, Celltrion, Ferring, Gilead, Janssen, Lilly, Merck, Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, and Takeda; Speaker fees: AbbVie, Amgen, AstraZeneca, Biogen, Bristol‐Myers Squibb, Ferring, Gilead, Janssen, Merck, Mitsubishi Tanabe Pharma, Nikkiso, Novartis, Pfizer, Roche, Samsung Bioepis, and Takeda; Research funding: Merck, Pfizer, and Takeda. ED reports as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Gilead, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Tillots, Thermofisher. GM reports Speaker TAKEDA, Speaker MSD, speaker Jannsen, paid consultation MSD, paid consultation Genesis. AC reports Consultancy fees: Takeda, Janssen. Speaker fees: Pfizer, Abbvie. JGA has no conflict of interest. TM reports speaker's honoraria from MSD, AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer and Teva. KK reports Speaker fees from Abbvie, Aenorasis, Janssen, MSD, Pfizer and Takeda Consultancy or advisory board member fees from Abbvie, Amgen, Ferring, Galenica, Genesis, Janssen, MSD, Pfizer and Takeda. KG reports KB Gecse has received grants from Pfizer Inc and Celltrion; consultancy fees from AbbVie, Arena Pharmaceuticals, Galapagos, Gilead, ImmunicTherapeutics, Janssen Pharmaceuticals, Novartis, Pfizer Inc., Samsung Bioepis and Takeda and speaker's honoraria from Celltrion, Ferring, Janssen Pharmaceuticals, Novartis, Pfizer Inc, Samsung Bioepis, Takeda and Tillotts. JVO reports speaker fees for Tillots. ML reports speaker and/or principal investigator for: Abbvie, Bristol Myers Squibb, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Janssen‐Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Robarts Clinical Trials, Takeda, Tillotts, Tramedico.He has received research grants from AbbVie, Merck Sharp & Dohme, Dr Falk, Achmea healthcare, Galapagos and ZonMW. KF reports received speaker's honoraria from AbbVie, Janssen, Ferring, Takeda and Goodwill Pharma. RA reports the following paid or unpaid consultancies, business interests, or sources of honoraria payments: AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Celgene, Celltrion Healthcare, Dr Falk Pharma, Ferring, Galapagos, Gilead, GlaxoSmithKline plc, InDex Pharmaceuticals, Janssen‐Cilag, Kiniksa Pharmaceuticals, MSD Sharp & Dohme, Novartis, Pandion Therapeutics, Pfizer, Roche Pharma, Samsung Bioepis, Stelic Institute & Co, Takeda Pharma, Tillotts Pharma AG, Viatris. DGR reports Paid consultancies, lecture fees for the past 2 years: Janssen, Abbvie. CS reports unrestricted research grants from Janssen and, AbbVie, has provided consultancy to Arena, Galapagos, Dr Falk, AbbVie, Takeda, Fresenius Kabi and Janssen, and had speaker arrangements with Celltrion, Dr Falk, AbbVie, Janssen, Pfizer and Takeda. FH reports advisory boards or as speaker for Abbvie, Janssen‐Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr Falk. Funding (Grants/Honoraria): Dr Falk, Janssen‐Cilag, Abbvie, Takeda. Consulting Fees: Celgene, Janssen‐Cilag. BB has no conflict of interest. SS reports Research grants from Takeda, AbbVie, AMGEN, Warner Chilcott, Ferring, MSD, Biohit and Cellgene, serves on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Cellgene, Celltrion, and Tillots Pharma, and has received speaker fees from Abbvie, Jaansen, Merck, Warner Chilcott and Falk Pharma. JFR reports: Speaker fee: Abbvie, MSD, Takeda, Pfizer, Ferring, Falk, Biogen, Amgen, Celltrion. Consultancy: Abbvie, Takeda, Hospira, Mundipharma, MSD, Pfizer, GlaxoSK, Janssen. Research grand: Takeda, Abbvie.
AUTHOR CONTRIBUTIONS
LG, JFC and JFR: Guarantor of the article; LG, JFC, BB, JFR: study concept and design, data acquisition, data analysis and interpretation, statistical analysis and manuscript writing; AO, MF, JS, CJ, RS, LR, SS, AA, ED, GM, AC, JGA, TM, KK, KG, JVO, ML, KF, RA, DGR, CS, FH: manuscript critical revision; AO, MF, JS, CJ, RS, LR, SS, AA, ED, GM, AC, JGA, TM, KK, KG, JVO, ML, KF, RA, DGR, CS, FH, FB, SBH, PB, EBM, MB, CB, CC, GAD, GD, SF, DPG, SG, AK, AL, TLO, JAL, EM, JMC, NN, AO’T, AP, TR, CR, MS, ES, BS, KS, GTV, MT, AV, HY, ASZ: data acquisition. All authors approved the final version of the manuscript.
Supporting information
Table S1
Table S2
ACKNOWLEDGEMENTS
We warmly thank the BIRD office in helping for all legal and ethical considerations, namely Ms Saskia Appelmans, Ms Caroline Saudoyer, Ms Laura Vansteenkiste and Ms Marjan Steppe (BIRD Office). This work was not supported by any financial contribution.
Goessens L, Colombel J‐F, Outtier A, Ferrante M, Sabino J, Judge C, et al. Safety and efficacy of combining biologics or small molecules for inflammatory bowel disease or immune‐mediated inflammatory diseases: a European retrospective observational study. United European Gastroenterol J. 2021;9(10):1136–47. 10.1002/ueg2.12170
Conference presentation: Poster presentation at ECCO Congress February 2020, Vienna, Austria; Oral Abstract presentation at Belgian Week of Gastroenterology March 2020, Antwerp, Belgium.
European COMBIO study group: Filip Baert, Shomron Ben Horin, Peter Bossuyt, Eduard Brunet Mas, Martin Buckley, Clodagh Byron, Carolann Coe, Glen A Doherty, Gabriele Dragoni, Samuel Fernandes, Daniel P. Gaya, Sarah Gleeson, Aine Keogh, Arie Levine, Triana Lobaton Ortega, Alan J. Lobo, Elisabeth Macken, Jane McCarthy, Nurulamin Noor, Aoibhlinn O'Toole, Annelies Posen, Giuseppe Privitera, Daniele Pugliese, Tim Raine, Catherine Reenaers, Tamas Resal, Antonella Scarcelli, Eoin Slattery, Beatrijs Strubbe, Kathleen Sugrue, Maarten Te Groen, Gisela Torres Vicente, Marie Truyens, Anna Viola, Henit Yanai, Syed Akbar Zulquernain.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on a reasonable request to the corresponding author.
REFERENCES
- 1. Sabino J, Verstockt B, Vermeire S, Ferrante M. New biologics and small molecules in inflammatory bowel disease: an update. Therap Adv Gastroenterol. 2019;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glassner K, Oglat A, Duran A, Koduru P, Perry C, Wilhite A, et al. The use of combination biological or small molecule therapy in inflammatory bowel disease: a retrospective cohort study. J Dig Dis. 2020;21:264–71. [DOI] [PubMed] [Google Scholar]
- 3. Yang E, Panaccione N, Whitmire N, Dulai PS, Vande Casteele N, Singh S, et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment Pharmacol Ther. 2020;51:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Privitera G, Onali S, Pugliese D, Renna S, Savarino E, Viola A, et al. Dual targeted therapy: a possible option for the management of refractory inflammatory bowel disease. J Crohns Colitis. 2020. Jul 17;15:jjaa149. 10.1093/ecco-jcc/jjaa149 [DOI] [PubMed] [Google Scholar]
- 5. Kwapisz L, Raffals LE, Bruining DH, Pardi DS, Tremaine WJ, Kane SV, et al. Combination biologic therapy in inflammatory bowel disease: experience from a tertiary care center. Clin Gastroenterol Hepatol. 2021;19:616–17. [DOI] [PubMed] [Google Scholar]
- 6. Yzet C, Dupas JL, Fumery M. Ustekinumab and anti‐TNF combination therapy in patients with inflammatory bowel disease. Am J Gastroenterol. 2016;111:748–9. [DOI] [PubMed] [Google Scholar]
- 7. Bethge J, Meffert S, Ellrichmann M, Conrad C, Nikolaus S. Schreiber S. Combination therapy with vedolizumab and etanercept in a patient with pouchitis and spondylarthritis. BMJ Open Gastroenterol. 2017;4(1):e000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Berre C, Loeuille D, Peyrin‐Biroulet L. Combination therapy with vedolizumab and tofacitinib in a patient with ulcerative colitis and spondyloarthropathy. Clin Gastroenterol Hepatol. 2019;17:794–6. [DOI] [PubMed] [Google Scholar]
- 9. Judge C, Saeidi R, Sugrue K, Rabbitt L, Keogh A, Byron C, et al. Combining therapies in inflammatory bowel disease: end of the line or a new era? Inflamm Bowel Dis. 2021;27:956–9. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed W, Galati J, Kumar A, Christos PJ, Longman R, Lukin DJ, et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2021;S1542–S3565(21):00344‐X. 10.1016/j.cgh.2021.03.034 [DOI] [PubMed] [Google Scholar]
- 11. Ford AC, Peyrin‐Biroulet L. Opportunistic infections with anti‐tumor necrosis factor‐alpha therapy in inflammatory bowel disease: meta‐analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268–76. [DOI] [PubMed] [Google Scholar]
- 12. Gagniere C, Beaugerie L, Pariente B, Seksik P, Amiot A, Abitbol V, et al. Benefit of infliximab reintroduction after successive failure of infliximab and adalimumab in Crohn’s disease. J Crohns Colitis. 2015;9:349–55. [DOI] [PubMed] [Google Scholar]
- 13. Stalgis C, Deepak P, Mehandru S, Colombel J‐F. Rational combination therapy to overcome the plateau of drug efficacy in inflammatory bowel disease. Gastroenterology. 2021;161:394–9. 10.1053/j.gastro.2021.04.068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
The data underlying this article will be shared on a reasonable request to the corresponding author.


