Abstract
Background
Hepatitis D virus (HDV) coinfection aggravates the course of hepatitis B virus (HBV). The prevalence of HDV in Austria is unknown.
Objective
This national study aimed at (i) recording the prevalence of HDV‐infection in Austria and (ii) characterizing the “active” HDV cohort in Austria.
Methods
A total of 10 hepatitis treatment centers in Austria participated in this multicenter study and retrospectively collected their HDV patients between Q1/2010 and Q4/2020. Positive anti‐HDV and/or HDV‐RNA‐polymerase chain reaction (PCR) results were retrieved from local database queries. Disease severity was assessed by individual chart review. Viremic HDV patients with clinical visits in/after Q1/2019 were considered as the “active” HDV cohort.
Results
A total of 347 anti‐HDV positive patients were identified. In 202 (58.2%) patients, HDV‐RNA‐PCR test was performed, and 126/202 (62.4%) had confirmed viremia. Hepatocellular carcinoma was diagnosed in 7 (5.6%) patients, 7 (5.6%) patients underwent liver transplantation, and 11 (8.7%) patients died during follow‐up. The “active” Austrian HDV cohort included 74 (58.7%) patients: Evidence for advanced chronic liver disease (ACLD, i.e., histological F3/F4 fibrosis, liver stiffness ≥10 kPa, varices, or hepatic venous pressure gradient ≥6 mmHg) was detected in 38 (51.4%) patients, including 2 (5.3%) with decompensation (ascites/hepatic encephalopathy). About 37 (50.0%) patients of the “active” HDV cohort had previously received interferon treatment. Treatment with the sodium‐taurocholate cotransporting polypeptide inhibitor bulevirtide was initiated in 20 (27.0%) patients.
Conclusion
The number of confirmed HDV viremic cases in Austria is low (<1% of HBV patients) but potentially underestimated. Testing all HBV patients will increase the diagnostic yield. More than half of viremic HDV patients had ACLD. Improved HDV testing and workup strategies will facilitate access to novel antiviral therapies.
Keywords: epidemiology, hepatitis D, viral hepatitis
INTRODUCTION
Hepatitis D virus (HDV) on top of hepatitis B virus (HBV) infection promotes progression to liver cirrhosis (i.e., advanced chronic liver disease, ACLD) and thus, causes considerable morbidity and mortality worldwide. 1 Although vaccination programs against HBV have reduced HDV disease burden in high‐income countries, HDV is still highly endemic in Eastern European, 2 Central Asian, 3 , 4 and Sub‐Saharan countries. 5 Therefore, recent migration dynamics might have influenced the epidemiology of HDV in Central Europe, but updated epidemiological data is largely missing. 6 Importantly, it has been demonstrated that HDV‐RNA viremia is the key driver for disease progression, 7 and thus, identification of patients with active HDV‐RNA replication is of clinical relevance. Unfortunately, most available data on HDV prevalence focuses on anti‐HDV seroprevalence, and the lack of universal “reflex” polymerase chain reaction (PCR) testing for HDV‐RNA contributes to the underestimation of the global “active” HDV prevalence, especially outside Europe. 6
Key summary.
Hepatitis D virus (HDV) coinfection propagates disease progression in patients chronically infected with hepatitis B virus.
No standardized screening strategies for HDV have been implemented in Austria/Central Europe so far.
In our multicenter retrospective study, 347 seropositive and 126 viremic HDV patients were identified, respectively.
Signs of advanced chronic liver disease were detected in more than half of viremic patients and in one out of four HDV‐RNA negative patients, emphasizing the importance of early detection and linkage to care of HDV patients.
Until recently, the only recommended treatment option for HDV coinfection in hepatitis B surface antigen (HBsAg) positive patients had been pegylated interferon alpha (PEG‐IFN) that has limited long‐term efficacy and is frequently associated with unfavorable side effects. 8 , 9 , 10 , 11 In mid‐2020, however, bulevirtide (BLV; Hepcludex®; now Gilead) was approved by the EMA for the treatment of patients with chronic HDV and compensated liver disease. 12 BLV is a novel, potent, and safe pharmacologic blocker of the hepatic sodium‐taurocholate cotransporting polypeptide (NTCP), which allows entry of HDV and HBV into hepatocytes. Other pharmacological agents are currently under investigation. 13
Due to missing recent epidemiological data on HDV viremia in Austria and the availability of novel antiviral therapies against HDV, we aimed to collect current data on the Austrian HDV scenario. Therefore, this national study aimed to (i) record HDV patients in Austria tested in/after Q1/2010 and (ii) characterize the “active” Austrian HDV in continuous care.
METHODS
Patients and study population
Ten specialized Austrian hepatitis treatment centers participated in this retrospective epidemiological study (Medical University of Vienna, Klinik Ottakring, Klinik Favoriten, Medical University of Graz, Medical University of Innsbruck, Paracelsus Medical University, Kepler Universitätsklinikum Linz, Ordensklinikum Linz Barmherzige Schwestern, Klinikum Klagenfurt am Wörthersee, University Hospital St. Pölten). Anti‐HDV positive patients that were diagnosed in one of the participating centers between Q1/2010 and Q4/2020 were identified by a search of the local virology lab records and/or automated inquiries performed by the respective departments for virology or laboratory medicine.
Next, the rate of conducted HDV‐RNA‐PCR tests within seropositive patients was investigated. The number of patients that had never been tested for HDV‐RNA or of those who showed negative test results (i.e., results below the lower limit of quantification (LLQ) of the used HDV‐RNA‐PCR tests) were recorded.
Definition of ACLD and follow‐up
ACLD was defined by a liver stiffness ≥10 kPa detected by vibration controlled transient elastography (VCTE), histological F3/F4‐fibrosis, presence of portal hypertension (i.e., hepatic venous pressure gradient [HVPG] ≥6 mmHg or varices observed during upper gastrointestinal endoscopy) or hepatic decompensation (presence of ascites or hepatic encephalopathy). In patients who did not undergo advanced characterization (n = 18/126), the presence of ACLD was defined by the presence/abscence of (signs of) cirrhosis in cross‐sectional imaging or sonography.
Relevant events during follow‐up, that is, the diagnosis of hepatocellular carcinoma (HCC), liver transplantation, and (liver‐related) death were recorded. Ultimately, patients with at least one clinical visit at the participating centers in or after Q1/2019 were considered as the “active” Austrian HDV cohort to characterize patients that are in continuous care. Information on clinically relevant parameters were collected from all available medical records at the participating centers.
Laboratory tests
Routine laboratory tests were performed by the local departments of laboratory medicine. Commercially available chemiluminescence immunoassays were used for the detection of Anti‐HDV antibodies. HDV‐RNA quantification was conducted at the Austrian reference center for HDV diagnostics, that is, the Center for Virology of the Medical University of Vienna, for 7 out of 10 participating centers, using an in‐house assay with a LLQ of 100 copies/mL developed according to an external reference. 14 Two of the participating centers used an RoboGene® HDV Quantification Kit 2.0 with a specified LLQ of 15 IU/mL. 15 One center referred their samples to a laboratory in Germany that used an in‐house developed assay with a LLQ of 200 copies/mL.
Transient elastography (TE)
TE, that is, the Fibroscan® system (Echosens, Paris) was used to conduct liver stiffness measurements (LSMs) as previously described. 16 The following liver stiffness cut‐offs were used for staging liver fibrosis (F0–F4) 17 : ≤6.0 kPa for F0/F1; ≥6.1 kPa and <10.0 kPa for F2; ≥10.0 kPa and <12.0 kPa for F3; and ≥12.0 kPa for F4. Liver stiffness values ≥10.0 kPa denoted advanced fibrosis/evidence for ACLD. This cut‐off was chosen in accordance with the latest Baveno VI consensus. 18
Statistical analyses
Statistical analyses were performed using GraphPad prism 8 (GraphPad Software) and R 4.0.2 (R Foundation for Statistical Computing).
Categorial variables were reported as the number and percentages of patients with certain characteristics. Continuous parameters were reported as median (interquartile range [IQR]). Patients entered time‐to‐event analyses at the time of their first recorded positive HDV‐RNA‐PCR test and were followed until death, liver transplantation or last clinical contact. Kaplan–Meier analyses were applied to investigate (i) the occurrence of a composite endpoint comprising transplantation, HCC diagnosis and liver‐related death and (ii) overall survival stratified by the presence/absence of ACLD. Moreover, we designed a second model in which patients who were diagnosed with ACLD during follow‐up were classified as non‐ACLD at the start of follow‐up and were censored from the non‐ACLD group upon diagnosis of ACLD, thereupon entering the ACLD models.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Medical University of Vienna (EC Vote No. 1515/2020). All participating centers attained approval by their local institutional review board before initiating the study. No written consent was required for this retrospective analysis.
RESULTS
Seropositivity and HDV‐PCR testing rate
Overall, 347 anti‐HDV positive patients were identified by the participating centers in or after Q1/2010. In 202 (58.2%) anti‐HDV positive patients, at least one HDV‐RNA‐PCR test was performed: 76/202 (37.8%) patients showed no detectable viremia, while 126 (62.2%) patients had detectable viremia (i.e., a positive HDV‐RNA‐PCR test result), as shown in Figure 1. Figure 2 depicts the laboratory “incidence” of newly diagnosed HDV patients over the study period, where no relevant dynamics were observed.
FIGURE 1.

Study cohort flowchart. ACLD, advanced chronic liver disease; HDV, hepatitis D virus; PCR, polymerase chain reaction; RNA, ribonucleic acid. *Data on disease severity available in n = 35/76 non‐viremic patients
FIGURE 2.

Year of HDV diagnosis in the viremic cohort
Patients with negative HDV‐RNA‐PCR results were mostly not fully evaluated regarding liver disease severity, with data on the presence/absence of ACLD being only available in 35/76 (46.1%) of patients. Of note, 8/35 (22.9%) of non‐viremic patients had evidence for ACLD.
Characteristics of viremic HDV patients
The median age at the time of the diagnosis was 46.3 (interquartile range [IQR] 37.9–59.0) years, and 69 (54.8%) of patients were male. Only a small number of patients were Austrian natives (n = 15, 11.9%), while most patients were born outside Austria, the most common regions of origin being Eastern Europe (n = 48, 38.1%), Central Asia (n = 26, 20.6%), and Mediterranean countries (n = 23, 18.3%), as shown in Table 1.
TABLE 1.
Patient characteristics
| Viremic HDV patients | N = 126 |
|---|---|
| Sex, male (%) | 69 (54.8%) |
| Age, years (IQR) | 46.3 (37.9–59.0) |
| Region of origin (%) | |
| Eastern Europe | 48 (38.1%) |
| Central Asia | 26 (20.6%) |
| Mediterranean countries | 23 (18.3%) |
| Austria | 15 (11.9%) |
| African | 8 (6.3%) |
| Middle East | 5 (4.0%) |
| North/South America | 1 (0.8%) |
| HDV‐RNA, copies/mL (IQR) a | 23500 (943–337500) |
| HDV‐RNA, log10 copies/mL (IQR) a | 4.37 (2.97–5.53) |
| HBV‐DNA, IU/mL (IQR) | 50 (20–860) |
| HBeAg positivity, n (%) | 18 (14.3%) |
| HIV coinfection, n (%) | 7 (5.6%) |
| HCV seropositivity, n (%) | 7 (5.6%) |
| Platelets, G/L (IQR) | 143 (98–194) |
| Bilirubin, mg/dL (IQR) | 0.72 (0.50–1.06) |
| Albumin, g/dL (IQR) | 41.60 (31.20–46.20) |
| Creatinine, mg/dL (IQR) | 0.72 (0.62–0.88) |
| Sodium, mmol/L (IQR) | 139 (137–141) |
| AST, U/L (IQR) | 55 (38–87) |
| ALT, U/L (IQR) | 59 (43–94) |
| GGT, U/L (IQR) | 44 (27–83) |
| Alkaline phosphatase, U/L (IQR) | 79 (66–111) |
| INR (IQR) | 1.1 (1.0–1.3) |
| ACLD, n (%) | 69 (54.8%) |
Abbreviations: ACLD, advanced chronic liver disease; ALT, alanine transaminase; AST, aspartate transaminase; DNA, desoxyribonucleic acid; GGT, gamma‐glutaryltransferase; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HEV, hepatitis E virus; HIV, human immunodeficiency virus; INR, International Normalized Ratio; RNA, ribonucleic acid.
HDV‐RNA levels were measured in IU/mL in n = 23 patients.
The median HDV‐RNA level was 4.37 (2.97–5.53) log copies/mL. About 17 (14.3%) patients showed hepatitis B e antigen (HBeAg) positivity at baseline, 7 (5.6%) patients were coinfected with human immunodeficiency virus (HIV), and 7 (5.6%) had a history of hepatitis C virus (HCV) infection. The median alanine transaminase (ALT) level was 59 (43–94) U/L, and 82 (65.1%) patients showed ALT values above the upper limit of normal (ULN, 50 U/mL).
Among all viremic patients, 69 (54.8%) showed evidence for ACLD at baseline (n = 48) or developed ACLD during follow‐up (n = 21), and 24 out of 69 (34.8%) had already developed decompensated disease as evidenced by ascites or hepatic encephalopathy.
Follow‐up data
Viremic patients (n = 126) were followed‐up for a median of 17.9 (4.5–29.4) months. During follow‐up, 7 (5.6%) patients were diagnosed with HCC. Furthermore, 7 (5.6%) patients underwent liver transplantation, 10 (7.9%) and 1 (0.8%) died of liver‐related and non‐liver related causes, respectively, and 11 (8.7%) achieved sustainable PEG‐IFN treatment‐induced viral suppression (n = 6) or spontaneous clearance (n = 5; below the LLQ).
The composite endpoint of HCC diagnosis/liver transplantation/liver‐related mortality occurred in 15 patients who had developed ACLD at baseline versus three patients who were classified as non‐ACLD at baseline (log‐rank test: p < 0.001), as shown in Figure 3. Death due to any cause occurred in nine patients who had developed ACLD at baseline (all liver‐related) versus two patients who did not show ACLD at baseline (one liver‐related and one non‐liver‐related, p < 0.001).
FIGURE 3.

Kaplan–Meier curves depicting (a) the time until the composite endpoint comprising liver‐related death/transplantation/development of hepatocellular carcinoma and (b) overall survival time stratified by the presence/absence of hepatitis D virus‐related advanced chronic liver disease at baseline
Within the (baseline) non‐ACLD cohort, events primarily occurred in patients that progressed to ACLD during follow‐up, as shown in Supplementary Figure 1.
Unfortunately, 23 (18.3%) patients were lost to follow‐up, and thus, 74 (58.7%) patients were finally included in the “active” Austrian HDV cohort, as shown in Figure 1.
The “active” Austrian HDV cohort
Overall, 74 patients with HDV‐viremia were included in the “active” Austrian HDV cohort. About 39 (52.8%) were male, and the median age at last clinical contact was 45.5 (37.1–58.8) years. Again, the proportion of non‐Austrian natives was high (90.6%), as shown in Table 2.
TABLE 2.
Characteristics of the “active” Austrian hepatitis D virus (HDV) cohort
| Viremic HDV patients in “active” care | n = 74 |
|---|---|
| Sex, male (%) | 39 (52.7%) |
| Age, years (IQR) | 45.5 (37.1–58.8) |
| Region of origin (%) | |
| Eastern Europe | 32 (43.2%) |
| Central Asia | 15 (20.3%) |
| Mediterranean countries | 12 (16.2%) |
| Austria | 7 (9.5%) |
| African | 4 (5.4%) |
| Middle East | 3 (4.1%) |
| North/South America | 1 (1.4%) |
| HDV‐RNA, copies/mL (IQR) a | 28306 (410–318858) |
| HDV‐RNA, log10 copies/mL (IQR) a | 4.45 (2.61–5.50) |
| HBV‐DNA, IU/mL (IQR) | 20 (0–34) |
| HBe Ag positivity, n (%) | 6 (8.1%) |
| HIV coinfection, n (%) | 5 (6.8%) |
| HCV seropositivity, n (%) | 4 (5.4%) |
| Platelets, g/L (IQR) | 131 (84–197) |
| Bilirubin, mg/dL (IQR) | 0.60 (0.42–0.88) |
| Albumin, g/L (IQR) | 43.55 (40.30–48.05) |
| Creatinine, mg/dL (IQR) | 0.73 (0.65–0.83) |
| Sodium, mmol/L (IQR) | 139 (138.00–141.00) |
| AST, U/L (IQR) | 46 (33–71) |
| ALT, U/L (IQR) | 48 (34–82) |
| GGT, U/L (IQR) | 39 (22–80) |
| Alkaline phosphatase, U/L (IQR) | 80 (61–96) |
| INR (IQR) | 1.0 (1.0–1.2) |
| Liver stiffness, kPa (IQR) | 10.3 (7.0–21.6) |
| F0/1, n (%) | 11 (14.9%) |
| F2, n (%) | 13 (17.6%) |
| F3, n (%) | 10 (13.5%) |
| F4, n (%) | 26 (35.1%) |
| Missing | 14 (18.9%) |
| IFN treatment, n (%) | |
| Ongoing | 11 (14.9%) |
| Prior | 26 (35.1%) |
| NUC treatment, n (%) | |
| Ongoing | 48 (64.9%) |
| Prior | 3 (4.1%) |
| ACLD, n (%) | 38 (51.4%) |
| Decompensation, n/ACLD (%) | 2/38 (5.3%) |
Abbreviations: ACLD, advanced chronic liver disease; ALT, alanine transaminase; AST, aspartate transaminase; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus; HDV, hepatitis D virus; DNA, desoxyribonucleic acid; HCV, hepatitis C virus; HIV, human immunodeficiency virus; GGT, gamma‐glutamyltransferase; IFN, interferon; INR, International Normalized Ratio; NUC, nucleo(s)tide analog; RNA, ribonucleic acid.
HDV‐RNA levels were measured in IU/mL in n = 19 patients.
The median last baseline (i.e., pretreatment) HDV level was 4.45 (2.61–5.50) log copies/mL. The rates of HBeAg positivity, HIV coinfection, and HCV seropositivity were 8.1%, 6.8%, and 5.4%, respectively. The median ALT level was 48 (34–82) U/mL, and 36 (48.6%) patients had abnormal ALT levels (i.e., ALT > ULN).
LSMs attained via VCTE were available in 60 (81.1%) patients. Advanced fibrosis (F3/4) was present in 36/60 (60.0%) patients, as shown in Figure 4. Overall, 38/74 (51.4%) patients showed evidence for ACLD, and two patients had already progressed to decompensated ACLD.
FIGURE 4.
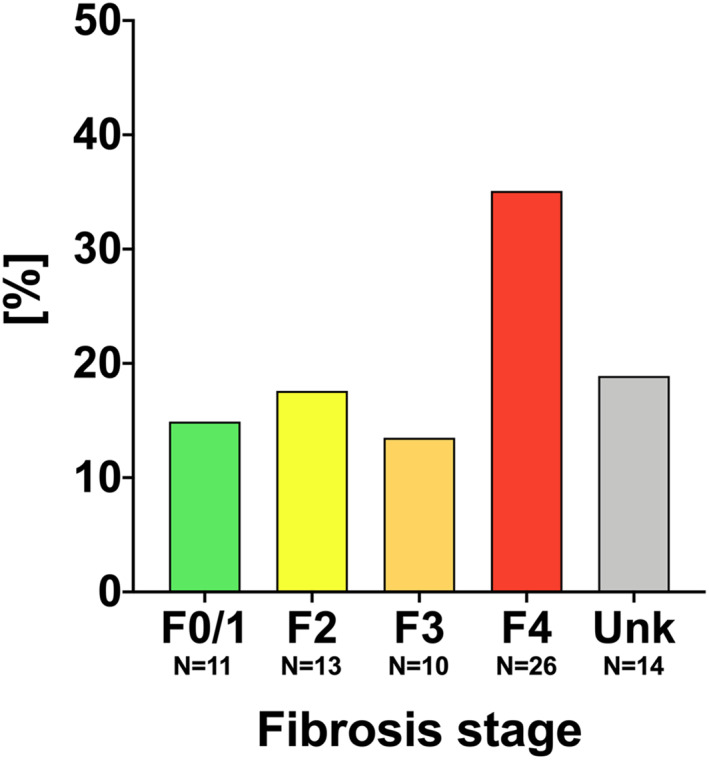
Distribution of fibrosis stages within the “active” Austrian hepatitis D virus cohort
About 50% of patients had previously been treated with IFN without sustained response, and 48 (64.9%) patients were receiving chronic nucleos(t)ide analog (NUC) treatment at the time of data collection. Treatment with the novel NTCP inhibitor BLV had been initiated in 20 (27.0%) patients.
DISCUSSION
In this study, we provide a comprehensive update on the Austrian HDV scenario. By a nationwide effort involving the major national hepatitis treatment centers, we identified 347 Austrian HBsAg positive patients who were positively tested for anti‐HDV antibodies in or after Q1/2010. According to a 2019 report of the Austrian Federal Health Ministry, there are 42,000 HBV patients in Austria. 19 Based on these data and the 347 anti‐HDV patients identified in our study, the HDV‐coinfection rate in HBsAg‐positive individuals is estimated at 0.8% for Austria, which is well within the range of previous reports from other industrialized nations. 6 However, due to a lack of standardized testing for anti‐HDV in HBsAg positive patients in Austria, the 0.8% HDV‐coinfection rate in HBV patients might still underestimate the true burden of HDV infection.
Notably, about 40% of the identified seropositive patients had never been tested for active viremia. Considering the viremia rate of more than 60% in the anti‐HDV positive patients in whom HDV‐RNA‐PCR were conducted, this would indicate that a significant number of Austrian viremic patients has yet to be identified. Importantly, the proportion of patients that did not exhibit active replication within our cohort is similar to previous reports on HDV patients from Switzerland. 20
Previous studies have shown that anti‐HDV positivity has a considerable impact on the prognosis of HBsAg‐positive individuals, and HDV viremia seems to be the key driver of liver disease progression. 7 , 21 In our cohort of viremic HDV patients, more than half of all patients showed significant fibrosis and/or evidence for ACLD. While the presence of ACLD was not investigated by biopsy (i.e., the gold standard) in all our patients and non‐invasive markers for (advanced) fibrosis remain to further validated in HDV patients, our findings corroborate previous reports from other European countries. 7 Furthermore, the deleterious impact of HDV on the individual patient’s morbidity and mortality is highlighted by the considerable number of patients in our cohort that developed HCC, had to undergo liver transplantation, or died because of liver‐related events. Importantly, in our cohort, liver‐related events (decompensation, HCC, transplantation, and liver‐related death) occurred exclusively in patients that had already progressed to ACLD. However, some events were recorded in patients that were originally, that is at baseline, classified as non‐ACLD, and who progressed to ACLD during follow‐up, highlighting the fast disease progression, and its ramifications in HDV. Regarding clinical routine, this would suggest focusing primarily on regular screening for ACLD in chronic HDV in order to identify patients most at risk of developing complications. Still, HCC might theoretically develop earlier, that is in a non‐cirrhotic liver, and thus, we would recommend regular HCC screening in HDV patients at 6 to 12 months intervals depending on the individual risk. Regardless of the patient’s disease severity, we argue that early identification and close clinical follow‐up of all HDV patients and linkage to early treatment of viremic patients is of the utmost clinical and prognostic importance.
Importantly, vaccination programs against HBV have already reduced the burden of HBV/HDV infections in Europe. 22 , 23 In turn, the current Central European HDV population seems to be comprised of both an older, advanced cohort as well as a younger, non‐advanced population of patients mostly immigrating from HDV‐endemic countries. This is reflected by our data, as about one third of patients with ACLD had already developed decompensated disease and developed further clinical events during follow‐up, whilst the active viremic cohort comprises a similar proportion of ACLD patients, but only two patients who have already progressed to decompensated cirrhosis. This decrease of patients with end‐stage liver disease was paralleled by a relative decline of Austrian natives and a simultaneous proportional increase in non‐Austrian natives from HDV‐endemic countries, particularly Eastern Europe and Central Asia. Therefore, it seems most likely that HDV will continue to impose a threat to individual and public health in Austria, even in the era of improved access to HBV vaccinations.
Overall, 50% of the active Austrian HDV cohort had undergone IFN treatment—which was the first available recommended treatment option against HDV 8 —but all without maintained HDV undetectability. Of note, the same proportion of patients showed ALT levels above the ULN during their last clinical visit, indicating ongoing hepatic necroinflammation. Combined, the urgent need for effective and safe antiviral treatment for viremic HDV patients is evident.
Intriguingly, the novel NTCP inhibitor BLV effectively suppressed HDV in a proportion of patients in clinical trials 24 , 25 : A phase 3 study is ongoing, but based on the preliminary results of the phase 2 MYR‐203 study 25 it has received conditional approval by the EMA for the treatment of patients with chronic HDV and compensated liver disease. 12 , 26 Over three years prior approval, Austrian centers received the drug on a compassionate use basis. Selected “active” Austrian HDV cohort patients can now receive BLV by prescription. However, the efficacy of this emerging HDV therapy remains to be established in a real‐life setting outside of clinical trials, although first reports show that BLV is both safe and efficient both as monotherapy and in addition to PEG‐IFN therapy. 27 Importantly, the ideal endpoint of HDV treatment—continuous suppression versus cure—and surrogate markers for therapeutic benefits remain to be further elucidated. 28 Other compounds have also shown promising results in early trials, however, these are not yet approved for HDV treatment. 13 , 29
The natural history of chronic HDV infection is unknown. In a large Swedish study, the overall risk for liver‐related events and HCC was 3.8‐fold and 2.6‐fold higher, respectively, in patients with HDV viremia compared with those without viremia. But the cumulative risk of being free of liver cirrhosis or liver‐related events was 81.9% and 64.0% after 5 and 10 years of follow‐up, respectively. 7 Another large French study has recently confirmed the crucial role of viremia for prognosis in chronic HDV. 21 Accordingly, in the present study, we recorded a significant number of events during follow‐up in viremic patients (HCC, transplantation, and death) that underscore the relevance of HDV and its impact on morbidity and mortality. Interestingly, almost 40% of patients that underwent HCV‐RNA‐PCR testing had negative test results. Despite the lack of detailed evaluation of liver disease severity in those patients, one out of four had clinical evidence for ACLD, suggesting a lower but still considerable impact of past HDV infection on prognosis.
LIMITATIONS
Our study has some limitations: First, the definition of HDV undetectably is test‐dependent: In one third of samples of HDV patients treated in clinical trials that were previously classified as undetectable, HDV‐RNA was detectable using the highly‐sensitive Robogene® assay. 30 In addition, no standardized HDV‐RNA‐PCR test was used across all existing centers, however, the in‐house developed assay that was used in seven out of ten centers has shown excellent sensitivity and specificity. 14 Second, selection and referral bias affected might have affected our results. In particular, the fact that three specialized referral centers that are also transplant centers were included in this study might have led to an overestimation of disease severity, and thus, our findings might not be fully representative of the overall (outpatient) viremic HDV cohort. However, we undertook all reasonable effort to minimize this bias, including the invitation of all other recognized hepatitis treatment centers in Austria to our study, where patients with less severe liver disease are treated. Additionally, patients were identified via automated inquiries into local virological records, including the Center for Virology of the Medical University, which is the national reference center for hepatitis viruses in Austria, further minimizing bias. Finally, this retrospective, epidemiological study focusing on viremic patients does not allow for conclusions regarding the natural history of the overall (seropositive) HDV cohort or the impact of existing or emerging treatment options against HDV owing to its design.
CONCLUSION
In conclusion, HDV seems to be an underdiagnosed disease in Austria. The lack of “reflex” testing (i) for anti‐HDV in HBsAg positive patients, especially in Non‐Austrian natives and (ii) for HDV viremia in anti‐HDV positive patients likely hinders linkage to care and treatment. Given the recent therapeutic developments for HBV and HDV, strategies should be implemented to facilitate identification and antiviral treatment of HDV patients in Austria.
CONFLICTS OF INTERESTS
Caroline Schmidbauer received travel support from Gilead, Abbvie and Gebro; and speaking honoraria from Abbvie. Stephanie Hametner‐Schreil received speaking honoraria and/or advisory board fees from: Gilead, Abbvie, Shionogi, Astellas, Intercept, Roche. Petra Munda received speaking honoraria from AbbVie, Gilead, MSD, Janssen, Roche, Intercept; travel support from AbbVie and Gilead; and consulting/advisory board fees from AbbVie, Gilead, MSD, Janssen, Roche and Intercept. M.M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Gilead, Collective Acumen, and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb, and Gilead. M.P.‐R. received advisory and/or speaker honoraria from AstraZeneca, AbbVie, Amgen, Bayer, Behring, BMS, Eisai, Gilead, Intercept, Ipsen, Lilly, Merz, MSD, Roche, Sanofi, Shionogi, Sobi. M.G. received grants from AbbVie, Gilead, and MSD; speaking honoraria from AbbVie, Gilead, MSD, Janssen, Roche, Intercept; and consulting/advisory board fees from AbbVie, Gilead, MSD, Janssen, Roche and intercept. Peter Ferenci received an unrestricted research grant from Gilead, is on the safety review board of MyrPharma, consulting/advisory board fees from Viravaxx, speaking honoraria from AbbVie, Gilead. T.R. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, MSD, Philips, and W.L. Gore & Associates as well as travel support from Boehringer Ingelheim and Gilead. Mathias Jachs, Teresa Binter, Lukas Hartl, Michael Strasser, Hermann Laferl, Alexander Lindorfer, Kristina Dax, Rudolf E. Stauber, Harald H. Kessler, Sebastian Bernhofer, Andreas Maieron, Lorin Loacker, Simona Bota, Isabel Santonja, and Heidemarie Holzmann declare no conflicts of interest.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENT
This study was supported by a research grant (DEPAU) from MYR GmbH to TR.
Jachs M, Binter T, Schmidbauer C, Hartl L, Strasser M, Laferl H, et al. Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis. United European Gastroenterol J. 2021;9(10):1119–1127. 10.1002/ueg2.12163
Peter Ferenci and Thomas Reiberger share the last authorship position.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon a reasonable request.
REFERENCES
- 1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. [DOI] [PubMed] [Google Scholar]
- 2. Gheorghe L, Csiki IE, Iacob S, Gheorghe C, Trifan A, Grigorescu M, et al. Hepatitis delta virus infection in Romania: prevalence and Risk Factors. J Gastrointestin Liver Dis. 2015;24(4):413–21. [DOI] [PubMed] [Google Scholar]
- 3. Yesmembetov KI, Mukhin NA, Abdurakhmanov DT. 160 hepatitis delta—still of major concern in Russia, due to immigration and intravenous drug usage: view from large single center cohort. J Hepatol. 2012;56:69. [Google Scholar]
- 4. Khodjaeva M, Ibadullaeva N, Khikmatullaeva A, Joldasova E, Ismoilov U, Colombo M, et al. The medical impact of hepatitis D virus infection in Uzbekistan. Liver Int. 2019;39(11):2077–81. [DOI] [PubMed] [Google Scholar]
- 5. Wranke A, Pinheiro Borzacov LM, Parana R, Lobato C, Hamid S, Ceausu E, et al. Clinical and virological heterogeneity of hepatitis delta in different regions world‐wide: the Hepatitis Delta International Network (HDIN). Liver Int. 2018;38(5):842–50. [DOI] [PubMed] [Google Scholar]
- 6. Rizzetto M, Hamid S, Negro F. The changing scenario of hepatitis D. J Hepatol. 2021;74(5):1200–11. [DOI] [PubMed] [Google Scholar]
- 7. Kamal H, Westman G, Falconer K, Duberg AS, Weiland O, Haverinen S, et al. Long‐term study of hepatitis delta virus infection at secondary care centers: the impact of viremia on liver‐related outcomes. Hepatology. 2020;72(4):1177–90. [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370‐98. [DOI] [PubMed] [Google Scholar]
- 9. Abdrakhman A, Ashimkhanova A, Almawi WY. Effectiveness of pegylated interferon monotherapy in the treatment of chronic hepatitis D virus infection: a meta‐analysis. Antivir Res. 2021;185:104995. [DOI] [PubMed] [Google Scholar]
- 10. Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364(4):322–31. [DOI] [PubMed] [Google Scholar]
- 11. Wedemeyer H, Yurdaydin C, Hardtke S, Caruntu FA, Curescu MG, Yalcin K, et al. Peginterferon alfa‐2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT‐II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19(3):275–86. [DOI] [PubMed] [Google Scholar]
- 12. Kang C, Syed YY. Bulevirtide: first approval. Drugs. 2020;80(15):1601–5. [DOI] [PubMed] [Google Scholar]
- 13. Loureiro D, Castelnau C, Tout I, Boyer N, Narguet S, Benazzouz SM, et al. New therapies for hepatitis delta virus infection. Liver Int. 2021;41(Suppl 1):30–7. [DOI] [PubMed] [Google Scholar]
- 14. Le Gal F, Gordien E, Affolabi D, Hanslik T, Alloui C, Dény P, et al. Quantification of hepatitis delta virus RNA in serum by consensus real‐time PCR indicates different patterns of virological response to interferon therapy in chronically infected patients. J Clin Microbiol. 2005;43(5):2363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taranta A, Rogalska‐Taranta M, Gutierrez R, Manns MP, Bock M, Wursthorn K. Rapid hepatitis B and hepatitis delta virus RNA quantification from small‐sized liver tissue samples. J Clin Virol. 2014;61(2):286–8. [DOI] [PubMed] [Google Scholar]
- 16. EASL‐ALEH Clinical Practice Guidelines . Non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64. [DOI] [PubMed] [Google Scholar]
- 17. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16(2):372. [DOI] [PubMed] [Google Scholar]
- 18. De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–52. [DOI] [PubMed] [Google Scholar]
- 19. BMASGK . HIV/AIDS, hepatitis B und C in Österreich. Vienna: Bundesministerium für Arbeit, Soziales, Gesundheit und Konsumentenschutz; 2019. [Google Scholar]
- 20. Béguelin C, Moradpour D, Sahli R, Suter‐Riniker F, Lüthi A, Cavassini M, et al. Hepatitis delta‐associated mortality in HIV/HBV‐coinfected patients. J Hepatol. 2017;66(2):297–303. [DOI] [PubMed] [Google Scholar]
- 21. Roulot D, Brichler S, Layese R, BenAbdesselam Z, Zoulim F, Thibault V, et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J Hepatol. 2020;73(5):1046–62. [DOI] [PubMed] [Google Scholar]
- 22. Brancaccio G, Nardi A, Madonia S, Fasano M, Verucchi G, Massari M, et al. The present profile of chronic hepatitis B virus infection highlights future challenges: an analysis of the Multicenter Italian MASTER‐B cohort. Dig Liver Dis. 2019;51(3):438–42. [DOI] [PubMed] [Google Scholar]
- 23. Stroffolini T, Sagnelli E, Sagnelli C, Sagnelli C, Russello M, De Luca M, et al. Hepatitis delta infection in Italian patients: towards the end of the story? Infection. 2017;45(3):277–81. [DOI] [PubMed] [Google Scholar]
- 24. Wedemeyer H, Bogomolov P, Blank A, Allweiss L, Dandri‐Petersen M, Bremer B, et al. Final results of a multicenter, open‐label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with tenofovir in patients with chronic HBV/HDV co‐infection [abstract no. GS‐005]. J Hepatol. 2018;68(Suppl 1):S3. [Google Scholar]
- 25. Wedemeyer H, Schoneweis K, Bogomolov PO, Voronkova N, Chulanov V, Stepanova T, et al. Final results of a multicenter, open‐label phase 2 clinical trial (MYR203) to assess safety and efficacy of Myrcludex B in with PEG‐interferon alpha 2a in patients with chronic HBV/HDV co‐infection [abstract no. GS‐13]. J Hepatol. 2019;70(1):e81. [Google Scholar]
- 26. Loglio A, Ferenci P, Uceda Renteria SC, Tham CYL, van Bömmel F, Borghi M, et al. Excellent safety and effectiveness of high‐dose myrcludex‐B monotherapy administered for 48 weeks in HDV‐related compensated cirrhosis: a case report of 3 patients. J Hepatol. 2019;71(4):834–9. [DOI] [PubMed] [Google Scholar]
- 27. Asselah T, Loureiro D, Le Gal F, Narguet S, Brichler S, Bouton V, et al. Early virological response in six patients with hepatitis D virus infection and compensated cirrhosis treated with Bulevirtide in real‐life. Liver Int. 2021;41(7):1509–17. [DOI] [PubMed] [Google Scholar]
- 28. Lok AS, Negro F, Asselah T, Farci P, Rizzetto M. Endpoints and new options for treatment of chronic hepatitis D. Hepatology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Z, Urban S. New insights into HDV persistence: the role of interferon response and implications for upcoming novel therapies. J Hepatol. 2021;74(3):686–99. [DOI] [PubMed] [Google Scholar]
- 30. Bremer B, Anastasiou OE, Hardtke S, Caruntu FA, Curescu MG, Yalcin K, et al. Residual low HDV viraemia is associated HDV RNA relapse after PEG‐IFNa‐based antiviral treatment of hepatitis delta: results from the HIDIT‐II study. Liver Int. 2021;41(2):295–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon a reasonable request.


