Abstract
Pain is common among adults with traumatic brain injury (TBI), yet little data exist regarding prevalence of opioid use in this population. The objective of this retrospective cohort study was to evaluate the association between lifetime TBI exposure, opioid use, and pain in a nationally representative sample of 1022 adults aged 50+ who participated in the Health and Retirement Study (HRS). Our primary exposure was lifetime TBI history measured via the Ohio State University TBI Identification Method. We evaluated three alternate TBI exposures (years since most recent TBI, age at first TBI, and number of lifetime TBIs) in sensitivity analyses. We evaluated two outcomes: recent opioid medication use, and moderate-to-severe pain measured over two HRS waves. We classified three pain groups (persistent, intermittent, and no pain). Prevalences of opioid use among individuals with and without TBI were 19.7% and 13.6%, respectively. After adjustment for age, sex, and race, individuals with TBI had a 52% increased risk for opioid use compared with individuals without TBI (relative risk = 1.52, 95% confidence interval: 1.11, 2.04). Individuals with recent TBI (1–10 years ago), first TBI after age 40+, and 2+ lifetime TBIs had greatest risk for opioid use. Compared with individuals without TBI, individuals with TBI had 4.9-times increased odds for persistent versus no pain, and 1.9-times increased odds of intermittent versus no pain. Persistent pain among adults with lifetime TBI is elevated compared with the general population, which may contribute to increased opioid use among persons with TBI, particularly those with recent injuries or multiple lifetime TBIs.
Keywords: pain, prescription opioid use, traumatic brain injury
Introduction
The consequences of long-term opioid use are well-documented, including risk for unintentional overdose, misuse, and dependence.1,2 Data from the National Survey on Drug Use and Health in 2015 found more than one in three noninstitutionalized adults in the United States used prescription opioids in the last year.3 Among opioid users, the most commonly reported motivation for opioid misuse was to relieve physical pain (63.4%), compared with much smaller proportions of adults motivated to get high (11.6%) or relax (10.9%).3,4 Prevention and treatment of pain is a top priority of the National Academy of Medicine5; therefore, recognizing subgroups at high risk for pain is critical to identifying individuals in the general population who may be at greater risk for prescription opioid misuse.
Chronic pain is present in more than 50% of individuals living with traumatic brain injury (TBI).6 Research concerning veterans has shown that moderate-to-severe TBI is independently associated with an increased risk for pain,7 and among veterans with a chronic pain diagnosis, history of moderate-to-severe TBI is associated with increased likelihood of short- and long-term opioid use.8 Results from a recent post-deployment military sample documented rates of opioid receipt ranging from 8.9–36.8%, with greater rates among individuals with comorbid chronic pain and post-traumatic stress disorder.9 Data on pain and pain treatment in civilian populations with TBI is more sparse; a recent single-center retrospective study found 30% of adults hospitalized with TBI were using prescription opioids 12 months after injury.10
Many individuals with a history of TBI live with long-term cognitive and behavioral impairments that may increase vulnerability to opioid misuse.11 Research has shown individuals with TBI have an elevated risk for high risk substance use12,13 and mood disorders.14 In particular, TBI exposure during early life is related to later adolescent and adult substance use problems.15,16 There is increasing concern that individuals with TBI using opioids may be susceptible to opioid misuse.17,18 Multiple studies have shown that individuals with a history of TBI are more likely to die from accidental poisonings compared with the general population,19–21 particularly middle aged and older adults with a history of TBI.19
There is a paucity of population-based estimates of prescription opioid use prevalence among individuals with a lifetime history of TBI. Previously documented high rates of pain in TBI populations may be one possible contributor to elevated prescription opioid use relative to the general population, but these relationships have not been investigated thoroughly. To this end, the objectives of the present study were to evaluate the association between lifetime history of TBI exposure and (1) recent prescription opioid use and (2) persistent or intermittent moderate-to-severe pain in a nationally representative US sample of adults over age 50. We hypothesized individuals with a history of TBI would have higher risk of recent prescription opioid use and higher odds of persistent or intermittent moderate-to-severe pain.
Methods
Study design
This was a retrospective cohort study using data from a subsample of the Health and Retirement Study (HRS), a longitudinal panel survey of adults over age 50 in the United States. The survey uses a national probability sample to capture a representative sample of adults living in households in the contiguous United States.22 For the present study, we combined data from two HRS waves (2014 and 2016). We gathered lifetime TBI exposure information from the 2014 wave and information on opioid pain medication use in the 2016 wave, the first HRS wave where opioid use data were available. We obtained information on presence of pain and pain severity in the 2014 and 2016 HRS waves. We excluded individuals who were deceased or lost between the 2014 and 2016 waves.
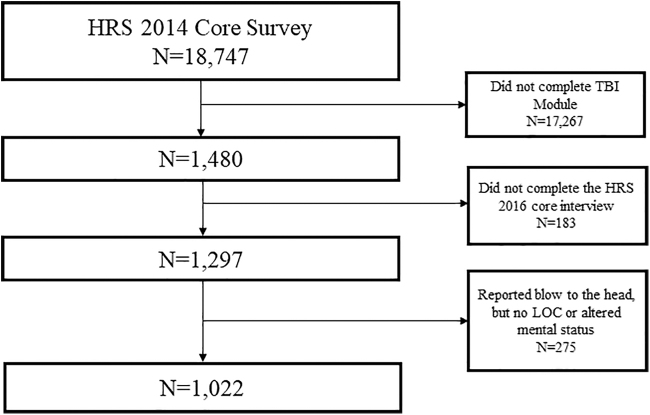
The flow diagram for the analytic cohort is provided in Figure 1. The dataset analyzed in the current study is publically available on the HRS website.23 This secondary data analysis study was considered nonhuman subjects research by our Institutional Review Board.
FIG. 1.
Flow diagram for derivation of the analytic cohort. The inception cohort for this study were Health and Retirement Study (HRS) participants who were assigned randomly and completed the 2014 HRS Traumatic Brain Injury (TBI) Module. These participants were then followed up at the 2016 HRS core interview. Individuals who were lost from 2014 to 2016 and those who reported a blow to the head but no loss of consciousness (LOC) or altered mental status were excluded. Of note, N = 15 individuals were omitted in the analytic sample because they had a survey weight of 0.
Lifetime TBI exposure
The HRS implemented a TBI module in 2014 on a random subsample (N = 1480) of the core HRS sample. The HRS collected history of TBI exposure through a modified version of the Ohio State University TBI Identification Method (OSU TBI-ID), a structured interview that queries participants on whether they have sustained injuries to the head or neck in their lifetimes. The OSU TBI-ID has high validity and interrater reliability24 and is used widely for retrospective TBI ascertainment, including within HRS studies.25–27 For each reported injury, HRS data collectors asked follow-up questions on age of each injury, and whether participants reported loss of consciousness (LOC), were dazed or experienced loss of memory, and whether they were hospitalized. For the present study, our primary exposure was TBI that resulted in LOC or a period of being dazed/loss of memory. Consistent with previous HRS studies,25–27 we excluded individuals who reported a past blow to the head that did not result in LOC or being dazed/having loss of memory, because this subgroup would not meet standard definitions for TBI.28–31
The additional information in the OSU TBI-ID on timing and severity of injury prompted examination of three additional TBI exposure classifications: (1) years since most recent TBI, (2) age at first TBI, and (3) number of lifetime TBIs. To characterize years since most recent TBI, we subtracted the age of reported TBI from the age at interview of the 2016 HRS wave.
Opioid pain medication use
In 2016, HRS participants were queried on their opioid use in the last three months. Data collectors specifically asked participants whether they have used a “…class of pain medications, called “opioids,” [which] includes such things as Vicodin, OxyContin, codeine, morphine, or similar medications. In the past three months, have you taken any opioid pain medications?”
The HRS also collected data on over-the-counter (OTC) pain medication use, which we included in the present study as a comparator to prescription opioids. Participants were asked whether “In the past three months have you taken any OTC pain medications?” Data collectors specified to participants that OTC pain medications include “…such things as Advil, Aleve, Tylenol, aspirin or similar medications.”
Moderate-to-severe pain
Participants were asked two pain-related questions in the HRS 2014 and 2016 waves. The first question was, “Are you often troubled by pain?” If participants responded yes, then they were asked the follow-up question, “How bad is the pain most of the time: mild, moderate, or severe?” We classified three pain groups over the study period based on reporting of moderate or severe pain. Individuals reporting moderate-to-severe pain in both 2014 and 2016 were considered to have persistent pain; individuals reporting moderate-to-severe pain in 2014 or 2016, but not both, comprised the intermittent pain group; and those not reporting moderate-to-severe pain at either time point were considered to be in the no pain group. Our classification of persistent pain is consistent with a previous HRS study.32
Covariates
All models adjusted for the following covariates (measured at 2016 HRS wave): age, sex, race (white, Black, other). Unlike other potential demographic covariates, these characteristics are not affected by TBI exposure.33 We descriptively characterized additional covariates by TBI exposure, including education, living situation, active military service history, health insurance, and presence of selected chronic conditions.
Statistical analysis
Analyses were performed using SAS 9.4 (Cary, NC)34 survey procedures to account for the HRS complex multi-stage sample design involving stratification, clustering, and survey weights. We used 2016 HRS survey weights for analyses. We descriptively characterized the cohort by TBI exposure, opioid use, and pain groups. Next, we conducted a series of multi-variable logistic regression models evaluating the association between lifetime history of TBI and recent prescription opioid use, and OTC pain medication use, adjusted for age, sex, and race.
In pre-planned sensitivity analyses, we evaluated three alternate TBI exposure classification metrics (time since last TBI, age at first TBI, and number of lifetime TBIs) and recent use of opioid pain medications and OTC pain medications. We converted adjusted odds ratios (ORs) from all multi-variable logistic regression models to adjusted relative risks (RRs) using a published formula to quantify risk.35 We then evaluated the association between history of TBI and pain group (none, intermittent, persistent) using adjusted multi-nomial logistic regression, with the no pain group as the referent outcome. As a post hoc exploratory analysis, we characterized pain groups among individuals recently using opioid pain medications by TBI exposure to provide descriptive information on overlap between pain and opioid use by lifetime TBI exposure.
Results
Characteristics of the participants
We reported demographic characteristics by lifetime TBI exposure status in Table 1. There were 360 (38.3%) individuals who reported a history of TBI and 647 (61.7%) individuals who reported no history of TBI. Individuals with a history of TBI were on average younger, more often male, unmarried, had an active military service history, and had greater rates of selected medical and psychiatric conditions compared with individuals without a history of TBI. Participants lost to follow-up were slightly older, more often lived alone, and had higher burden of select medical chronic conditions, but were otherwise similar to the analytic cohort including similar rates of TBI exposure and moderate-to-severe pain (see Supplemental Table S1).
Table 1.
Demographic Characteristics┼ of Adults 50+ by Traumatic Brain Injury Status
| Total | No TBI | History of TBI | |
|---|---|---|---|
| N € , weighted row % | 1007 | 647 (61.7) | 360 (38.3) |
| Age, weighted mean (SE) | 67.4 (0.3) | 68.1 (0.5) | 66.2 (0.4) |
| N, weighted column % | |||
| Sex (male) | 415 | 219 (35.2) | 196 (59.5) |
| Race/ethnicity | |||
| White | 716 | 459 (82.1) | 257 (83.5) |
| Black | 197 | 130 (10.1) | 67 (10.1) |
| Other | 92 | 57 (7.8) | 35 (6.4) |
| Education | |||
| Less than High School | 165 | 116 (12.0) | 49 (9.6) |
| High School or greater | 842 | 531 (88.0) | 311 (90.4) |
| Living situation | |||
| Married, living with spouse | 582 | 366 (62.6) | 216 (58.0) |
| Unmarried, living with others | 166 | 109 (10.9) | 57 (13.3) |
| Unmarried, living alone | 250 | 166 (26.5) | 84 (28.7) |
| Marital status | |||
| Married | 533 | 340 (59.3) | 193 (53.9) |
| Divorced/widowed/separated | 399 | 262 (31.0) | 137 (35.5) |
| Never married | 72 | 43 (9.7) | 29 (10.6) |
| Active military service history (yes) | 136 | 67 (9.4) | 69 (18.8) |
| Health insurance coverage | |||
| Medicare only | 329 | 221 (30.6) | 108 (24.2) |
| Medicaid only | 43 | 23 (1.4) | 20 (3.4) |
| Medicare and Medicaid | 79 | 41 (4.4) | 38 (8.9) |
| Private insurance (with or without public insurance) | 486 | 317 (58.5) | 169 (57.4) |
| Uninsured | 70 | 45 (5.2) | 25 (6.0) |
| Presence of chronic conditions | |||
| High blood pressure | 640 | 393 (55.5) | 247 (63.4) |
| Diabetes | 274 | 173 (24.4) | 101 (26.3) |
| Any cancer | 174 | 114 (16.9) | 60 (14.0) |
| Chronic lung disease | 100 | 48 (8.0) | 52 (12.9) |
| Heart condition | 242 | 130 (18.5) | 112 (27.5) |
| Stroke | 84 | 48 (7.2) | 36 (7.9) |
| Psychiatric condition§ | 217 | 104 (17.3) | 113 (28.4) |
TBI, traumatic brain injury; SE, standard error.
All variables adjusted for 2016 HRS complex survey design weights to be representative of the United States; column percentages reported; missing data exists for some variables wherein categories do not add up to column total.
N = 15 individuals had a survey weight of 0 and were dropped from weighted analysis.
Defined by single Health and Retirement Study question: “Have you ever had or has a doctor ever told you that you have any emotional, nervous, or psychiatric problems?”
We have also reported characteristics associated with opioid use (Supplemental Table S2) and pain groups (Supplemental Table S3). We observed that persons reporting opioid use had significantly younger age, less education, and higher rates of selected chronic conditions (any cancer, chronic lung disease, stroke, and psychiatric conditions). We also observed persons with persistent pain tended to be less educated, more often unmarried (living alone), have Medicaid insurance, and greater rates of selected health conditions (high blood pressure, chronic lung disease, heart condition, and psychiatric conditions).
Association between history of TBI and recent opioid pain medication use
The prevalence of prescription opioid use in the past three months among individuals with a history of TBI was 19.6% compared with 13.6% among individuals without TBI. In the multi-variable logistic model (see Table 2), individuals with TBI were at 52% increased risk for recent opioid use in later life compared with individuals without TBI (RR = 1.52, 95% confidence interval [CI]: 1.11, 2.04). Sustaining a TBI in the last 10 years (RR = 3.01, 95% CI: 1.57, 4.70), first TBI at age 40+ (RR = 1.98, 95% CI: 1.18, 3.04), and 2+ lifetime TBIs (RR = 2.00, 95% CI: 1.30, 2.90) were associated with significantly increased risk of opioid use, compared with those without TBI.
Table 2.
Multi-Variable Logistic Regression Models for Prescription Opioid Medication Use among Adults Age 50+*
| |
|
|
|
Prescription opioid use in last 3 months |
|
|---|---|---|---|---|---|
| Models | TBI exposure classification | N, Weighted %† | RR (95% CI) | ||
| Model 1 | History of TBI | No history of TBI | 76 (13.6) | Reference | |
| History of TBI | 76 (19.7) | 1.52 (1.11, 2.04) | |||
| Sensitivity analyses | Model 2‡ | Years since most recent TBI | No history of TBI | 76 (13.6) | Reference |
| 1–10 | 20 (38.1) | 3.01 (1.57, 4.70) | |||
| 11–39 | 26 (19.6) | 1.40 (0.91, 2.08) | |||
| 40+ | 28 (16.1) | 1.29 (0.83, 1.92) | |||
| Model 3‡ | Age at first TBI | No history of TBI | 76 (13.6) | Reference | |
| <25 | 42 (18.9) | 1.46 (0.99, 2.07) | |||
| 25–39 | 12 (18.1) | 1.34 (0.66, 2.47) | |||
| 40+ | 20 (24.2) | 1.98 (1.18, 3.04) | |||
| Model 4 | Number of lifetime TBIs | No history of TBI | 76 (13.6) | Reference | |
| 1 | 38 (16.0) | 1.25 (0.83, 1.82) | |||
| 2+ | 38 (25.5) | 2.00 (1.30, 2.90) | |||
TBI, traumatic brain injury; RR, relative risk; CI, confidence interval.
Multivariable Logistic Regression Model adjusted for age, sex, and race; All models adjusted 2016 Health and Retirement Study (HRS) complex survey design weights to be representative of the contiguous United States; N = 15 individuals had a survey weight of 0 and were dropped from weighted analysis.
Percentages are adjusted for HRS complex survey design to be representative of the contiguous United States; row percentages reported.
n = 12 individuals reported history of traumatic brain injury but did not remember the age of injury and were excluded from Models 2 and 3.
As a comparator to prescription opioid use, we also evaluated the association between history of TBI and OTC pain medication use. History of TBI was not associated with risk of recent OTC pain medication use (see Supplemental Table S4). Years since most recent TBI, age at first TBI, and number of lifetime TBIs were also not associated with OTC pain medication use.
Associations between history of TBI and pain group
Rates of intermittent and persistent moderate-to-severe pain among individuals with a history of TBI were 22.5% and 29.4%, respectively, compared with 18.6% and 10.7% among individuals without TBI. In the multi-nomial model (see Table 3), compared with individuals with no history of TBI, individuals with TBI had 1.89 times increased odds of intermittent pain versus the no pain group (OR = 1.89, 95% CI: 1.33, 2.68), and 4.93 times increased odds of persistent pain versus the no pain group (OR = 4.93, 95% CI: 3.43, 7.08).
Table 3.
Multi-Nomial Regression Model for Moderate-Severe Pain in 2014 (T1) and 2016 (T2) Among Adults 50+*
| |
No pain (T1 and T2) |
Intermittent pain (T1 or T2) |
Persistent pain (T1 and T2) |
|---|---|---|---|
| N (%†) | N (%†) | N (%†) | |
| No history of TBI | 434 (70.7) | 127 (18.6) | 79 (10.7) |
| History of TBI | 164 (48.1) | 80 (22.5) | 114 (29.4) |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| History of TBI vs. no history of TBI | Reference | 1.89 (1.33, 2.68) | 4.93 (3.43, 7.08) |
TBI, traumatic brain injury; OR, odds ratio; CI, confidence interval.
Multinomial Regression Model adjusted for age, sex, and race.
Percentages adjusted for 2016 Health and Retirement Study complex survey design weights to be representative of the United States; row percentages reported; N = 15 individuals had a survey weight of 0 and were dropped from weighted analysis; some individuals (N = 9) did not have pain data available in 2014 and 2016 and were not included in this analysis.
Characterization of pain groups by opioid use and TBI exposure
In Figure 2, we descriptively characterized pain groups by opioid use and TBI exposure. Among recent opioid users with TBI, the percentage of each pain group was: 11.2% (no pain), 30.1% (intermittent pain), and 58.7% (persistent pain). Among recent opioid users without TBI, the percentage of each pain group was: 33.1% (no pain), 31.3% (intermittent pain), and 35.6% (persistent pain).
FIG. 2.
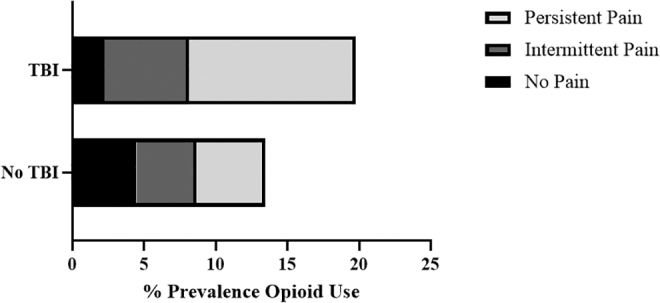
Pain groups among opioid users by traumatic brain injury (TBI) exposure. All percentages are adjusted for 2016 Health and Retirement (HRS) complex survey design weights. Pain groups (no pain, intermittent pain, and persistent pain) were based on reporting of moderate-severe pain across 2014 and 2016 HRS waves. Opioid use was based on self-report in the 2016 HRS wave.
Discussion
We found approximately 20% of adults in the United States over age 50 living with a lifetime history of TBI have used prescription opioids in the past three months. Opioid use was highest among individuals with a TBI in the past decade and those with multiple lifetime TBIs. After adjustment for covariates, those with TBI were more than 50% more likely to use opioids than those without TBI. The most commonly cited reason for prescription opioid initiation in the general population is to cope with physical pain.36 Our data indicated individuals with TBI had nearly five times greater odds of persistent moderate-to-severe pain over the study period compared with their counterparts in the population without TBI.
Our data align with evidence gathered from military and veteran samples that have suggested individuals with a history of TBI exposures are at increased risk for opioid medication use, particularly among individuals with concurrent chronic pain diagnoses.7–9 Current Department of Defense/Veterans Administration clinical guidelines37 recommend against opioid therapies in favor of nonopioid therapies for individuals with TBI. This suggests in military/veteran samples there may be some notable discrepancy between clinical guidelines and prescribing practices for pain management among individuals with TBI. Our findings reinforce existing evidence6,7 that individuals with a history of TBI are at higher likelihood of persistent pain compared with the general population.
While we did not have information on indication for opioid medication use, post hoc descriptive analyses indicated 88.8% of individuals with TBI (compared with 67.1% of individuals without TBI) who reported recent opioid use endorsed persistent or intermittent moderate-to-severe pain during the study period. The observed higher rates of opioid use by TBI exposure in this study may be explained in part by significantly greater rates of moderate-to-severe pain experienced among individuals with TBI. Of note, we did not find evidence in our study of an association between history of TBI and OTC pain medication use.
Our sensitivity analyses indicated timing and number of lifetime TBIs was associated with risk of prescription opioid use in later life. Specifically, we showed individuals with more recent injuries (1–10 years ago) and first TBI age 40+ had the greatest relative risk of opioid use, compared with those without TBI. The current evidence on opioid use patterns after TBI is based on military and veteran samples,8,9 wherein TBI exposures more commonly occur at younger ages during military service, which may not be generalizable to TBI exposures in the general population. For example, one veteran study found that veterans who sustained TBI in earlier life (18–24 years old) were at greatest risk for short- and long-term opioid use compared with later life TBI.8
In our study of adults over age 50, individuals with first TBI at age 40+ were at greatest risk of opioid use in later life compared with those without TBI. Individuals who sustained their first TBI before age 25 also had an elevated risk of opioid use (RR = 1.46, 95% CI: 0.99, 2.07) compared with those without TBI, although this CI included one in this study. These findings suggest more life-course research is warranted to elucidate the influence of age of TBI on risk of pain and opioid use in later life.
We also documented that individuals in this cohort with two or more lifetime TBIs had double the risk for opioid use compared with those without TBI. There has been considerable interest in evaluating the long-term consequences of repetitive TBI, and past studies have indicated individuals with repetitive mild TBI may be at increased risk for substance use disorders.38–40 The current study warrants future investigation into the relationship between repetitive TBI and opioid use disorders.
The consequences of opioid use in TBI populations could be substantial. A TBI can cause chronic cognitive and behavioral impairments that increase vulnerability for substance use disorders.38 Dopamine reward circuitry, which has been postulated to mediate addictive behaviors,41,42 has been recognized to be compromised after TBI.43,44 There is increasing concern that persons with TBI exposure may be uniquely susceptible to opioid misuse and its negative consequences.17,18 Of note, a recent study found that two-thirds of unintentional poisoning deaths after TBI involved narcotic drugs.20 Future studies would benefit from evaluating mediators of opioid use and misuse in TBI populations, including presence of psychiatric conditions that we observed in the present study were associated with history of TBI, opioid use, and pain.
There are limitations in the current study. We retrospectively evaluated lifetime TBI exposure through the OSU-TBI ID. Although this instrument has been found to be sensitive to behavioral, structural and proteomic differences,45,46 it is possible that individuals did not recall past exposures or the extent of their past TBI effects such as LOC, particularly in early life. We also did not distinguish the cause or severity of TBI in our study. We relied on self-report for pain medication utilization and did not have information on drug type, dosage, or adherence.
Our results focus on any opioid use and may therefore not be applicable to the association between TBI and opioid misuse. Future studies would benefit from more detailed measurement of opioid misuse. We did not have any information available in this study on prescription nonopioid pharmacological treatments or nonpharmacological treatments for pain (e.g., physical therapy, meditation, or psychotherapy). We also did not have any information on types of pain (e.g., neuropathic, psychogenic, musculoskeletal, trigeminal). Future studies measuring specific pain types experienced by persons with TBI may provide insight into use of opioid versus other medications for treatment of pain.
Pain and opioid pain medication use were ascertained after TBI exposure assessment in this sample; however, because HRS did not collect opioid use data before 2016, we cannot rule out that individuals with TBI used opioid pain medication before their injuries. It is also possible that opioid use or pain may increase risk for TBI; however, we were unable to test for this specifically in this study.
In our sensitivity analysis, we observed only persons with multiple lifetime TBIs had a significantly elevated risk of opioid use compared with persons without TBI. We did observe the suggestion of elevated risk among persons with a single TBI compared with those without TBI, but this was not statistically significant. It is possible we were underpowered in the current study to observe an effect, and future larger studies should further investigate opioid use among persons with history of a single lifetime TBI. HRS does not have a direct measure of chronic pain, but our classification of persistent pain, which may reflect chronic pain, is consistent with a previous HRS study.32
Finally, some individuals were lost to follow-up or deceased between their 2014 and 2016 interviews and were excluded from this study. Those lost to follow-up had similar rates of TBI exposure and moderate-to-severe pain; therefore, selection bias would be less likely to markedly affect the study findings.
Despite these limitations, this study has several strengths. We address a major gap in research by identifying the prevalence of prescription opioid use among a representative sample of middle-aged and older adults living with TBI. Previous studies evaluating opioid use in TBI populations have relied on convenience hospital-based samples or exclusively military or veteran samples, and have crucially lacked a population-based comparison group without TBI. Our study also highlights novel associations concerning the timing and number of lifetime TBI exposures in the context of risk for opioid use, wherein individuals with first TBI after age 40, recent TBI in the last 10 years, and multiple lifetime TBIs have the greatest risk for opioid use in later life.
Conclusion
In this nationally representative sample of adults over age 50 in the United States, persons with a lifetime history of TBI reported more than 50% greater rates of recent prescription opioid use than those without TBI. High burden of persistent pain in TBI populations may be a contributing factor to greater opioid use in this population. Future research is urgently needed to evaluate prevalence and risk factors for opioid misuse disorders in populations with TBI.
Supplementary Material
Authors' Contributions
All authors contributed to the design of the study and the analysis plan. Dr. Kumar conducted the literature search and acquired the data. Dr. Kumar led the analysis, while Dr. Dams-O'Connor and Dr. Ornstein had statistical oversight over the analysis. All authors helped to interpret the data and provided a critical revision of the article for important intellectual content.
Funding Information
Dr. Adams and Dr. Corrigan's effort was supported in part by a grant from the National Institute on Disability Independent Living and Rehabilitation Research (NIDILRR) to Ohio State University (Dr. Corrigan: Grant Number 90DPTB0001-01-00, Dr. Corrigan and Dr. Adams: Grant Number 90DPGE0007). Dr. Dams-O'Connor and Dr. Kumar's effort were supported in part by a grant from NIDLRR to the Icahn School of Medicine at Mount Sinai (90DP0038 and 90DPTB0009). The contents of this publication do not necessarily represent the policy of NIDILRR, the Administration on Community Living, nor the U.S. Department of Health and Human Services, and endorsement by the federal government should not be assumed.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Compton, W.M., and Volkow, N.D. (2006). Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 81, 103–107. [DOI] [PubMed] [Google Scholar]
- 2. Han, B., Compton, W.M., Jones, C.M., and Cai, R. (2015). Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA 314, 1468–1478. [DOI] [PubMed] [Google Scholar]
- 3. Han, B., Compton, W.M., Blanco, C., Crane, E., Lee, J., and Jones, C.M. (2017). Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann. Intern. Med. 167, 293–301. [DOI] [PubMed] [Google Scholar]
- 4. Han, B., Compton, W.M., Blanco, C., and Jones, C.M. (2018). Correlates of prescription opioid use, misuse, use disorders, and motivations for misuse among US adults. J. Clin. Psychiatry 79, 17m11973. [DOI] [PubMed] [Google Scholar]
- 5. National Academies of Sciences, Engineering, and Medicine (2020). Framing Opioid Prescribing Guidelines for Acute Pain: Developing the Evidence. Washingtn, D.C.: National Academies Press. [PubMed] [Google Scholar]
- 6. Nampiaparampil, D.E. (2008). Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA 300, 711–719. [DOI] [PubMed] [Google Scholar]
- 7. Seal, K.H., Bertenthal, D., Barnes, D.E., Byers, A.L., Strigo, I., Yaffe, K., and Chronic Effects of Neurotrauma Consortium Study Group. (2017). Association of traumatic brain injury with chronic pain in Iraq and Afghanistan veterans: effect of comorbid mental health conditions. Arch. Phys. Med. Rehabil. 98, 1636–1645. [DOI] [PubMed] [Google Scholar]
- 8. Seal, K.H., Bertenthal, D., Barnes, D.E., Byers, A.L., Gibson, C.J., Rife, T.L., Yaffe, K., and Chronic Effects of Neurotrauma Consortium Study Group. (2018). Traumatic brain injury and receipt of prescription opioid therapy for chronic pain in Iraq and Afghanistan veterans: do clinical practice guidelines matter? J. Pain 19, 931–941. [DOI] [PubMed] [Google Scholar]
- 9. Adams, R.S., Larson, M.J., Meerwijk, E.L., Williams, T.V., and Harris, A.H. (2019). Postdeployment polytrauma diagnoses among soldiers and veterans using the Veterans Health Affairs polytrauma system of care and receipt of opioids, nonpharmacologic, and mental health treatments. J. Head Trauma Rehabil. 34, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunn, L.K., Taylor, D.G., Smith, S.J., Skojec, A.J., Wang, T.R., Chung, J., Hanak, M.F., Lacomis, C.D., Palmer, J.D., Ruminski, C., Fang, S., Tsang, S., Spangler, S.N., Durieux, M.E., and Naik, B.I. (2019). Persistent post-discharge opioid prescribing after traumatic brain injury requiring intensive care unit admission: a cross-sectional study with longitudinal outcome. PloS One 14, e0225787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashman, T.A., Gordon, W.A., Cantor, J.B., and Hibbard, M.R. (2006). Neurobehavioral consequences of traumatic brain injury. Mt. Sinai J. Med. 73, 999–1005. [PubMed] [Google Scholar]
- 12. Beaulieu-Bonneau, S., St-Onge, F., Blackburn, M.C., Banville, A., Paradis-Giroux, A.A., and Ouellet, M.C. (2018). Alcohol and drug use before and during the first year after traumatic brain injury. J. Head Trauma Rehabil. 33, E51–E60. [DOI] [PubMed] [Google Scholar]
- 13. Ponsford, J., Whelan-Goodinson, R., and Bahar-Fuchs, A. (2007). Alcohol and drug use following traumatic brain injury: a prospective study. Brain Inj. 21, 1385–1392. [DOI] [PubMed] [Google Scholar]
- 14. Alway, Y., Gould, K., Johnston, L., McKenzie, D., and Ponsford, J. (2016). A prospective examination of Axis I psychiatric disorders in the first 5 years following moderate to severe traumatic brain injury. Psychol. Med. 46, 1331–1341. [DOI] [PubMed] [Google Scholar]
- 15. Adams, R.S., Campbell-Sills, L., Stein, M.B., Sun, X., Larson, M.J., Kessler, R.C., Ursano, R.J., Jain, S., and Corrigan, J.D. (2020). The association of lifetime and deployment-acquired traumatic brain injury with post-deployment binge and heavy drinking. J. Head Trauma Rehabil. 35, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy, E., Heron, J., and Munafò, M. (2017). Substance use, criminal behaviour and psychiatric symptoms following childhood traumatic brain injury: findings from the ALSPAC cohort. Eur. Child Adolesc. Psychiatry 26, 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams, R.S., Corrigan, J.D., and Dams-O'Connor, K. (2020). Opioid use among individuals with traumatic brain injury: a perfect storm? J. Neurotrauma 37, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corrigan, J.D., and Adams, R.S. (2019). The intersection of lifetime history of traumatic brain injury and the opioid epidemic. Addict. Behav. 90, 143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Byers, A.L., Li, Y., Barnes, D.E., Seal, K.H., Boscardin, W.J., and Yaffe, K. (2020). A national study of TBI and risk of suicide and unintended death by overdose and firearms. Brain Inj. 34, 328–334. [DOI] [PubMed] [Google Scholar]
- 20. Hammond, F., Ketchum, J., Dams-O'Connor, K., Corrigan, J.D., Miller, A.C., Haarbauer-Krupa, J., Faul, M., Trexler, L.E., and Harrison-Felix, C.L. (2020). Mortality secondary to unintentional poisoning after inpatient rehabilitation among individuals with moderate to severe traumatic brain injury. J. Neurotrauma 37. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrison-Felix, C., Pretz, C., Hammond, F.M., Cuthbert, J.P., Bell, J., Corrigan, J., Miller, A.C., and Haarbauer-Krupa, J. (2015). Life expectancy after inpatient rehabilitation for traumatic brain injury in the United States. J. Neurotrauma 32, 1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heeringa, S.G,. and Connor, J.H. (1995). Technical description of the Health and Retirement Survey sample design. Ann Arbor: University of Michigan. [Google Scholar]
- 23. University of Michigan Health and Retirement Study. Available at hrsonline.isr.umich.edu. Last accessed February 18, 2021.
- 24. Corrigan, J.D., and Bogner, J. (2007). Initial reliability and validity of the Ohio State University TBI Mdentification method. J. Head Trauma Rehabil. 22, 318–329. [DOI] [PubMed] [Google Scholar]
- 25. Gardner, R.C., Langa, K.M., and Yaffe, K. (2017). Subjective and objective cognitive function among older adults with a history of traumatic brain injury: a population-based cohort study. PLoS Med. 14, e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kornblith, E.S., Langa, K.M., Yaffe, K., and Gardner, R.C. (2020). Physical and functional impairment among older adults with a history of traumatic brain injury. J. Head Trauma Rehabil. 35, E320–E329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar, R.G., Ornstein, K.A., Bollens-Lund, E., Watson, E.M., Ankuda, C.K., Kelley, A.S., and Dams-O'Connor, K. (2020). Lifetime history of traumatic brain injury is associated with increased loneliness in adults: a US nationally representative study. Int. J. Geriatr. Psychiatry 35, 553–563. [DOI] [PubMed] [Google Scholar]
- 28. Cifu, D., Bowles, A., Hurley, R., Cooper, D., Peterson, M., Drake, A., and Barth, J. (2009). Management of Concussion/Mild Traumatic Brain Injury. US Department of Veterans Affairs and US Department of Defense. Washington, DC: The Management of Concussion/mTBI Working Group. [Google Scholar]
- 29. Centers for Disease Control and Prevention (2003). Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention 45. [Google Scholar]
- 30. Holm, L., Cassidy, J.D., Carroll, L., and Borg, J. (2005). Summary of the WHO Collaborating Centre for Neurotrauma Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 37, 137–141. [DOI] [PubMed] [Google Scholar]
- 31. American Congress of Rehabilitation Medicine (1993). Definition of mild traumatic brain injury. J Head Trauma Rehabil. 8, 86–87. [Google Scholar]
- 32. Whitlock, E.L., Diaz-Ramirez, L.G., Glymour, M.M., Boscardin, W.J., Covinsky, K.E., and Smith, A.K. (2017). Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern. Med. 177, 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corrigan, J.D., Yang, J., Singichetti, B., Manchester, K., and Bogner, J. (2018). Lifetime prevalence of traumatic brain injury with loss of consciousness. Inj. Prev. 24, 396–404. [DOI] [PubMed] [Google Scholar]
- 34. Inc, S.I. (2013). SAS 9.4 [Computer software]. Cary, NC. [Google Scholar]
- 35. Zhang, J., and Yu, K.F. (1998). What's the relative risk?: A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280, 1690–1691. [DOI] [PubMed] [Google Scholar]
- 36. Weiss, R.D., Potter, J.S., Griffin, M.L., McHugh, R.K., Haller, D., Jacobs, P., Gardin II, J., Fischer, D., and Rosen, K.D. (2014). Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. J. Subst. Abuse Treat. 47, 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenberg, J.M., Bilka, B.M., Wilson, S.M., and Spevak, C. (2018). Opioid therapy for chronic pain: overview of the 2017 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guideline. Pain Med. 19, 928–941. [DOI] [PubMed] [Google Scholar]
- 38. Corrigan, J.D., Bogner, J., and Holloman, C. (2012). Lifetime history of traumatic brain injury among persons with substance use disorders. Brain Inj. 26, 139–150. [DOI] [PubMed] [Google Scholar]
- 39. Cottler, L.B., Abdallah, A.B., Cummings, S.M., Barr, J., Banks, R., and Forchheimer, R. (2011). Injury, pain, and prescription opioid use among former National Football League (NFL) players. Drug Alcohol Depend. 116, 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dams-O'Connor, K., Cantor, J.B., Brown, M., Dijkers, M.P., Spielman, L.A., and Gordon, W.A. (2014). Screening for traumatic brain injury: findings and public health implications. J. Head Trauma Rehabil. 29, 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonci, A., Bernardi, G., Grillner, P., and Mercuri, N.B. (2003). The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol. Sci. 24, 172–177. [DOI] [PubMed] [Google Scholar]
- 42. Sell, L., Morris, J., Bearn, J., Frackowiak, R., Friston, K., and Dolan, R.J. (1999). Activation of reward circuitry in human opiate addicts. Eur. J. Neurosci. 11, 1042–1048. [DOI] [PubMed] [Google Scholar]
- 43. Bales, J.W., Wagner, A.K., Kline, A.E., and Dixon, C.E. (2009). Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci. Biobehav. Rev. 33, 981–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wagner, A., Sokoloski, J., Ren, D., Chen, X., Khan, A., Zafonte, R., Michael, A., and Dixon, C. (2005). Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 95, 457–465. [DOI] [PubMed] [Google Scholar]
- 45. Corrigan, J., and Bogner, JA. (2018). The Ohio State University Traumatic Brain Injury Identification Method. New York, NY: Springer International Publishing. [Google Scholar]
- 46. Peltz, C.B., Kenney, K., Gill, J., Diaz-Arrastia, R., Gardner, R.C. and Yaffe, K. (2020). Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology 95, e1126–e1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.