Abstract
One of the most devastating chronic consequences of traumatic brain injury (TBI) is cognitive impairment. One of the possible underlying causes is growth hormone deficiency (GHD) caused by TBI-induced hypopituitarism. Currently, TBI patients are not routinely screened for pituitary function, and there are no standard therapies when GHD is diagnosed. Further, the possible positive effects of GH replacement on cognitive function and quality of life after TBI are not well established. We aimed to assess the current knowledge regarding the effect of GH therapy on cognitive function and quality of life after TBI. We performed a literature search in PubMed, Embase, and Central® databases from inception to October 2019. We extracted data on each term of severity (mild-moderate-severe) of TBI with and without GHD, time since injury, parameters of growth hormone treatment (dosing, length), and cognitive outcomes in terms of verbal and non-verbal memory, and executive, emotional, and motor functions, and performed a meta-analysis on the results of a digit span test assessing working memory. We identified 12 studies (containing two randomized controlled trials) with 264 mild-to-moderate-to-severe TBI patients (Glasgow Coma Score [GCS] varied between 6 and 15) with (n = 255) or without (n = 9) GHD who received GH therapy. GH was administered subcutaneously in gradually increasing doses, monitoring serum insulin-like growth factor-I (IGF-I) level. After TBI, regardless of GCS, 6–12 months of GH therapy, started in the chronic phase post-TBI, induced a moderate improvement in processing speed and memory capacities, decreased the severity of depression, and led to a marked improvement in quality of life. Limitations include the relatively low number of patients involved and the divergent neuropsychological tests used. These results indicate the need for further multi-centric controlled studies to substantiate the use of GH replacement therapy as a potential tool to alleviate TBI-related cognitive impairment and improve quality of life.
Keywords: cognitive impairment, growth hormone, traumatic brain injury
Introduction
Survivors of severe traumatic brain injury (TBI) and patients with mild but repetitive brain trauma are left with significant neuropsychological dysfunction: severe cognitive, behavioral, and communicative disabilities.1,2 First it was demonstrated in 1918 by Cyran that TBI often resulted in pituitary dysfunction. Accordingly, deficiency of one or more anterior pituitary hormones is present in nearly 35% of patients with TBI.1,3–5 Growth hormone deficiency (GHD) has been shown to be the most common pituitary defect following TBI,6 but its incidence is highly variable. Acute-onset GHD (occurring within 1 month of TBI) has been reported to occur in between 2% and 30% or TBI patients7–9 (almost 50% may recover spontaneously),10 and chronic GHD (at least 6 months after trauma) was shown to affect 10–63% of TBI patients.8,11,12 Although GHD seems to be associated with the severity of trauma,13 isolated GHD was reported in 14.7% of patients following repetitive mild head trauma as well.14 The growth hormone-insulin-like growth factor-I (GH-IGF-I) system plays a critical role in regulating adult neurogenesis, brain plasticity, and cell survival.15–17 In addition to developmental processes (maturation of the brain, myelin formation, glial cell differentiation) GH is important for maintaining normal cognitive function.1,18,19 Accordingly, GH enhances both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)- and N-methyl-d-aspartate (NMDA)-receptor-mediated excitatory post-synaptic potentials (EPSPs) in the hippocampal area cornu ammonis (CA)1, which are essential components of long-term potentiation and memory acquisition.1,20,21 In addition to the direct neuronal effects, an intact GH-IGF-I axis plays a central role in the maintenance of the normal architecture of cerebral capillary networks19,22,23 and in mediation of neurovascular coupling, providing nutrients and oxygen for normal neuro-glial function.24 It is logical to posit that GHD plays a central role in TBI-induced chronic cognitive and behavioral deficit, and that GH substitution has a beneficial effect on TBI-related neuropsychological dysfunction. However, only a few studies aimed to test this hypothesis and provided information about the possible routes of GH treatment and the effect of GH substitution on the cognition and psychological function of patients after TBI. In this systematic review and meta-analysis, we summarized literature data of the effect of GH replacement therapy (GHRT) on TBI-related neuropsychological dysfunction and overall quality of life (QoL).
Methods
This systematic review and meta-analysis were reported and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement.25 The protocol was registered at PROSPERO on August 13, 2018, under registration number CRD42018104462.
Deviations from the protocol
We only examined the neuropsychological results, additional outcomes such as metabolic effects were not added in our review and subgroup analysis was not conducted, because we did not find enough studies dealing with these. We decided to use the Joanna Briggs Institute critical appraisal checklist instead of the Newcastle-Ottawa scale for the non-randomized studies risk of bias assessment.
Search
A comprehensive search was conducted by two independent investigators in three databases (PubMed, Embase, and Central®). Our query was based on the Population; Intervention; Comparison; Outcome (PICO) framework. We included studies that examined patients with TBI at all severity levels (P), and assessed them with GH therapy (I) and without replacement of the hormone (C) in terms of cognitive outcome (O) based on neuropsychological tests. We used the following query in all three databases: “{[GH OR (growth AND hormone)] OR [IGF-1 OR (IGF AND 1) OR (insulin-like AND growth AND factor)]} AND [(brain AND injury) OR TBI OR concussion].” We perfomred our search until October 22, 2019.
We limited our search to human studies written in English via the appropriate filters when searching in PubMed and Embase. To expand the search, we performed a recursive hand search on the references of relevant articles. We also searched PROSPERO, an international database for systematic reviews and meta-analyses for previously completed reviews on the subject.
Inclusion and exclusion criteria
We excluded letters, comments, editorials, and reviews. In our review, we included observational studies, interventional trials, and case reports. We imposed no restriction in terms of the etiology of TBI. We included studies with TBI patients who received GH therapy. If there were multiple publications using the same group of patients, the latest was chosen.
Screening and selection
After the initial search, all records were imported into a reference management program (EndNote X7, Clarivate Analytics, Philadelphia, PA, USA). The software was used in the process of removing duplicates by searching for articles with overlapping publication year, author, and/or title. After duplicates were removed, the authors screened the remaining articles against the pre-defined eligibility criteria by title, abstract, and then full text. Two different researchers conducted each step independently. Any disagreements were resolved by consensus.
Data extraction
Data were extracted from studies included by one of the investigators and re-checked by another, and were manually entered in an Excel 2016 sheet (Office 365, Microsoft, Redmond, WA, USA). Data were collected on the first author, year of publication, study design, geographical location, number of patients, basic demographics (age, sex, education), time since injury, GH hormone dose, and the length of the treatment in each group. Finally, data were extracted on the neuropsychological outcomes such as digit span, processing speed index, verbal learning and visuospatial memory, depression, and QoL. Two different researchers conducted each step simultaneously. Any disagreements were resolved by consensus.
Quality assessment and quality of evidence
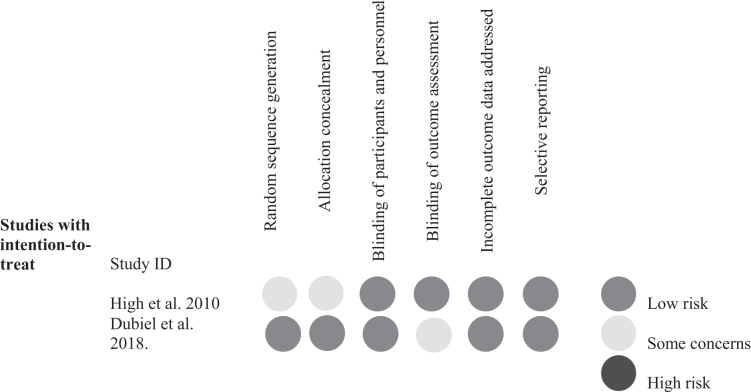
The Joanna Briggs Institute critical appraisal checklist for case reports and case series and for cohort studies was used to assess the quality of the studies included in the analysis26 (Table 1). The Cochrane Risk of Bias Tool was used to assess the quality of the randomized controlled trials (RCTs) included.27 Two different researchers conducted each appraisal checklist independently (Fig. 1). The quality of evidence of this systematic review and meta-analysis was estimated by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system.28 Any disagreements were resolved by consensus.
Table 1.
Results of the Joanna Briggs Institute (JBI) Critical Appraisal Checklist
| JBI Critical Appraisal Checklist for case reports | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | |||
| Springer and Chollet 2001 | Unclear | No | Yes | Yes | No | No | Unclear | Yes | |||
| Tanriverdi et al. 2010 | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | |||
| Bhagia et al. 2010 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | |||
| Devesa et al. 2015 |
No |
No |
Yes |
Yes |
Yes |
Yes |
Unclear |
Yes |
|||
| Q1: Were patient's demographic characteristics clearly described? Q2: Was the patient's history clearly described and presented as a timeline? Q3: Was the current clinical condition of the patient on presentation clearly described? Q4: Were diagnostic tests or assessment methods and the results clearly described? Q5: Was the intervention(s) or treatment procedure(s) clearly described? Q6: Was the post-intervention clinical condition clearly described? Q7: Were adverse events (harms) or unanticipated events identified and described? Q8: Does the case report provide takeaway lessons? | |||||||||||
| JBI Critical Appraisal Checklist for case series | |||||||||||
| Author, year |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Q6 |
Q7 |
Q8 |
Q9 |
Q10 |
|
| Maric et al. 2010 |
Yes |
Yes |
Yes |
Yes |
Yes |
Unclear |
Yes |
Yes |
Yes |
Unclear |
|
| Devesa et al. 2015 |
Yes |
Yes |
Yes |
Yes |
Yes |
Unclear |
Yes |
Yes |
Yes |
Unclear |
|
| Q1: Were there clear criteria for inclusion in the case series? Q2: Was the condition measured in a standard, reliable way for all participants included in the case series? Q3. Were valid methods used for identification the condition for all participants included in the case series? Q4: Did the case series have consecutive inclusion of participants? Q5: Did the case series have complete inclusion of participants? Q6: Was there clear reporting of the demographics of the participants in the study? Q7: Was there clear reporting of clinical information of the participants? Q8: Were the outcomes or follow up results of cases clearly reported? Q9: Was there clear reporting of the presenting site(s)/clinic(s) demographic information? Q10: Was statistical analysis appropriate? | |||||||||||
| JBI Critical Appraisal Checklist for cohort studies | |||||||||||
| Author, year |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Q6 |
Q7 |
Q8 |
Q9 |
Q10 |
Q11 |
| Reimunde et al. 2011 |
Yes |
Yes |
Yes |
Unclear |
Unclear |
Yes |
Yes |
Yes |
Yes |
Unclear |
Yes |
| Moreau et al. 2013 |
Yes |
Yes |
Yes |
Unclear |
Unclear |
Yes |
Yes |
Yes |
Yes |
Unclear |
Yes |
| Mossberg et al. 2017 |
No appl. |
No appl. |
Yes |
Unclear |
Unclear |
Yes |
Yes |
Yes |
Yes |
Unclear |
Yes |
| Q1. Were the two groups similar and recruited from the same population? Q2: Were the exposures measured similarly to assign people to both exposed and unexposed groups? Q3: Was the exposure measured in a valid and reliable way? Q4: Were confounding factors identified? Q5: Were strategies to deal with confounding factors stated? Q6: Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? Q7: Were the outcomes measured in a valid and reliable way? Q8: Was the follow-up time reported and sufficient to be long enough for outcomes to occur? Q9: Was follow-up complete, and if not, were the reasons to loss to follow up described and explored? Q10: Were strategies to address incomplete follow up utilized? Q11: Was appropriate statistical analysis used? | |||||||||||
| The Joanna Briggs Institute critical appraisal checklist for case reports and case series and for cohort studies was used to assess the quality of the studies included in the analysis. | |||||||||||
Fig. 1.
Results of the Cochrane Risk of Bias Tool for the randomized controlled trials (RCTs). The Cochrane Risk of Bias Tool was used to assess the quality of the RCTs included.
Statistical analysis
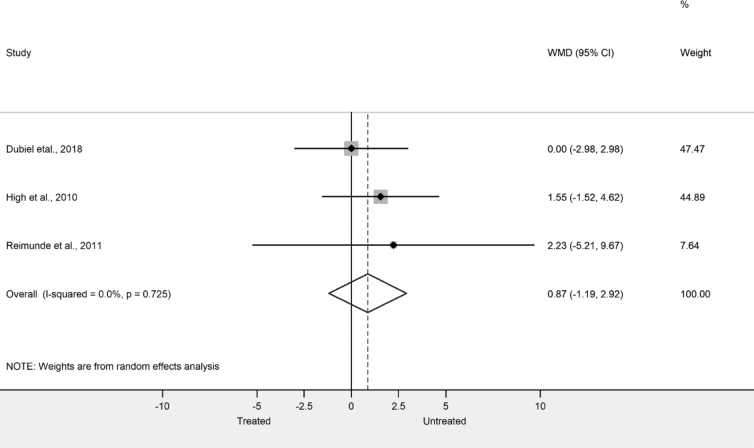
In the meta-analysis, three articles' DigitSpan outcomes scores were pooled: Dubiel and coworkers,29 High and coworkers,30 and Reimunde and coworkers.31 We calculated for the DigitSpan outcome pooled difference in means with 95% confidence intervals. The random effect model was used by DerSimonian and Laird.32 The extent of the heterogeneity by Q test (χ2) and I2 indicator was examined. Results of the meta-analysis were displayed graphically using forest plots. Meta-analytical calculation was performed with Stata Statistical Software: Release 15. (College Station, TX: StataCorp LLC).
Results
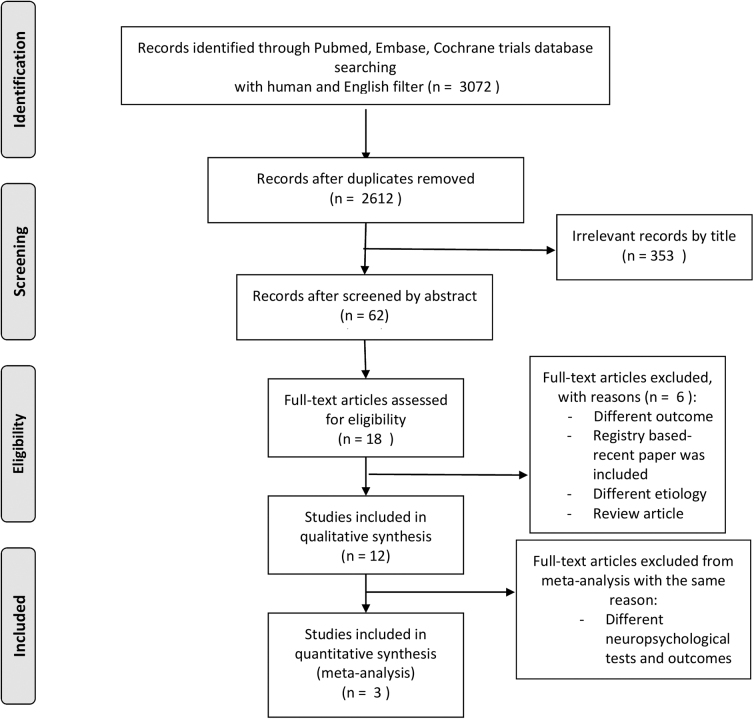
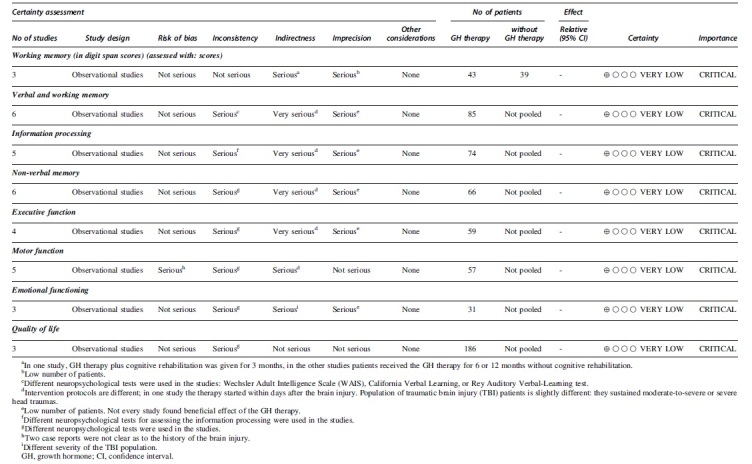
We retrieved 2612 publications and screened them by title and by abstract, finally selecting 18 studies for full review, including two trials (Fig. 2 and Table 2). The results of the risk of bias assessment are detailed in Table 1 and Figure 1. We estimated the quality of evidence of all outcomes of this systematic review and meta-analysis as being very low grade based on the aforementioned GRADE system. Results are shown in Table 3.
Fig. 2.
Flowchart of the study selection procedure.
Table 2.
The Quality of Evidence of All Outcomes of this Study
|
Table 3.
Characteristics of the Studies Included
| Author | Design | Country | (Intervention)No. of patients with treatment | (Comparator)No. of patients without treatment | Time since injury | GHD/non-GHD | GCS (Mean, SD) | Age(Years, mean, SD) |
|---|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | USA | 12 | 11 | WO (5.1 years SD:3.6) W (11.0 years SD:3.2) |
GHD | WO (6.6, SD:3.6) W (5.8, SD:3.4) |
W: 36.1 (10) WO: 39.1 (8.5) |
| Dubiel et al.29 | RCT | USA | 20 | 20 | WO (62.5 days SD:41.4) W (65.7 days SD:30.4) |
GHD and GH sufficient |
GOSE score: Mild, n (%) W: 3 (15) WO: 1 (5) Moderate, n (%) W: 0 (0) WO: 3 (15) Severe, n (%) W: 17 (85) WO: 15 (75) |
W: 32.2 (14.3) WO: 30.1 (13.7) |
| Reimunde et al.31 | Non-RCT: controlled, open-label study | Spain | 11 | 8 | W: 44.55 months (SD: 35.55) WO: 46.6 months, (SD: 28.79) Average: 3.7 years |
GHD and non-GHD | No GCS assessed | W: 53.36 (17.34) WO: 47.12 (14.55) |
| Moreau et al.33 | Non-RCT: uncontrolled, within- subjects | France | 23 | 27 | W: 7.8 years WO: 5.5 years |
WO: non-GHD W: GHD |
W: 8.1(SD:5.1) WO: 9.4(SD:5.1) |
WO: 37.1 (12.4) W: 37.9 (11.7) |
| Mossberg et al.34 | Non-RCT: uncontrolled, within-subjects | USA | 15 | 11.3years (SD:11.2) | Abnormal GH secretion | Moderate to severe TBI | W:34.3 (15.7) | |
| Gardner et al.36 | Cross-sectional registry based | Liverpool, UK | 161 | GHD | No GCS assessed | W: 42.6 (95% CI: 40.8-44.5) | ||
| Maric et al.38 | Case series | Serbia | 4 | 2 | At least 3 years | GHD | 1 out of 4: GCS:8 2 out of 4: 10 1 is unknown |
|
| Devesa et al.42 | Case series | Spain | 13 | 2.5 months to 11 years (average 4.8 years) |
GHD in 5 out of 13 | 4 out of 13: GCS 3 1 out of 13: 4 1 out of 13:8 2 out of 13: 6 |
6-53 | |
| Tanriverdi et al.8 | Case report | Turkey | 2 | 8 and 10 years | GHD | No GCS assessed | 36 and 38 | |
| Devesa et al.35 | Case report | Spain | 1 | 15 months | Non-GHD | 12 then 6 then coma for 6 months | 18 | |
| Bhagia et al.37 | Case report | USA | 1 | 6.8 years | GHD | No GCS assessed | 43 | |
| Springer et al.44 | Case report | USA | 1 | 2 years | GHD | No GCS assessed | 47 |
RCT, randomized controlled trial; W, with; WO, without; SD, standard deviation; GCS, Glasgow Coma Scale; GOSE, Glasgow Outcome Score-Extended; GHD, growth hormone deficiency; CI, confidence interval.
Therapeutic regimen of GH administration after TBI
All 264 patients received GH treatment subcutaneously until IGF-I level reached the normal range (for men the upper limit for those between the ages of 23 and 25 was 346 ng/mL, and for those 46–50 years of age it was 259 ng/mL; for women the upper limit for those between the ages of 23 and 25 was 320 ng/mL, and for those 46–50 years of age it was 227ng/mL). In most cases, the GH dose started at 200 μg for 2 months after which the IGF-I level was checked. GH was then increased up to 600–1000 μg/day and, and then IGF-I was rechecked.29,30,33–35 The follow-up period after treatment was ∼12 months in most of the studies.29,30,33,34,36,37 In one article, patients with GHD received 3 months of hormone treatment plus cognitive therapy.31 In three article the GH therapy lasted 6 or 8 months.35,38,39 In the Devesa case series almost all patients received 1 mg per day of recombinant human growth hormone (rhGH) subcutaneously for 12 months; in two cases the period was 6 or 8 months long.35 Gardner and coworkers gave 370 μg per day of the rhGH therapy. In this cross-sectional study they performed a follow-up for 8 years after treatment.36 The Mossberg group used 500 μg/day because of the onset of symptoms of carpal tunnel syndrome at higher doses34 (Table 4). Possible side effects are carpal tunnel syndrome, type 2 diabetes mellitus, fluid retention, paresthesias, myalgias, and arthralgias.29
Table 4.
Therapeutic Regimen of GH Administration after TBI
| Author | Design | Country | (Intervention)No. of patients with treatment | (Comparator)No. of patients without treatment | Time since injury | rhGh dose | Treatment time (month) |
|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | USA | 12 | 11 | WO (5.1 years SD: 3.6) W (11.0 years SD: 3.2) |
200 μg (increased with 200 μg every month to 600 μg) | 12 |
| Dubiel et al.29 | RCT | USA | 20 | 20 | WO (62.5 days SD: 41.4) W (65.7 days SD: 30.4) |
400 μg /day(increased or decreased in dose until IGF-I reached up to maximum dose of 1000 μg /day) | 6 |
| Reimunde et al.31 | Non-RCT: controlled, open-label study | Spain | 11 | 8 | W: 44.55 months (SD:35.55) WO: 46.6 months, (SD: 28.79) Average: 3.7years |
0.5 mg/day for 20 days, then 1 mg/day for 5 days/week | 3+ cognitive therapy |
| Moreau et al.33 | Non-RCT: uncontrolled, within- subjects | France | 23 | 27 | W: 7.8 years WO: 5.5years |
0.3 mg/day, 0.2-0.6 mg per day | 12 |
| Mossberg et al.34 | Non-RCT: uncontrolled, within-subjects | USA | 15 | 11.3 years (SD: 11.2) | 200 μg (increased with 200 μg every month) |
12 | |
| Gardner et al.36 | Cross-sectional registry based | Liverpool, UK | 161 | Mean dose: 0.37 mg/day | 12 and 8 years | ||
| Maric et al.38 | Case series | Serbia | 4 | 2 | At least 3 years | 0.3 mg/day for male 0.4 mg/day for females |
6 |
| Devesa et al.42 | Case series | Spain | 13 | 2.5 months to 11 years (average 4.8 years) |
0.2 mg/day | 8 | |
| Tanriverdi et al.8 | Case report | Turkey | 2 | 8 and 10 years | Physiological dose of GH | 6 | |
| Devesa et al.35 | Case report | Spain | 1 | 15months | 1 mg/day (specific protocol, including resting time) | 24 and 3 | |
| Bhagia et al.37 | Case report | USA | 1 | 6.8 years | 200 μg /day | 12 | |
| Springer et al.44 | Case report | USA | 1 | 2 years | Not known | Not known |
TBI, traumatic brain injury; rhGh, recombinant human growth hormone; RCT, randomized controlled trial; W, with; WO, without; SD, standard deviation; GCS, Glasgow Coma Scale; GOSE, Glasgow Outcome Score-Extended; GH, growth hormone.
Information processing
High and coworkers,30 starting GH treatment 5 years after the injury, showed significant improvement in processing speed after 12 months of GH treatment. Although no significant improvement was observed in the placebo group in processing speed, differences between the treated and untreated groups were not significant at the end of treatment. Importantly, Dubiel and coworkers,29 treating patients acutely after trauma, did not demonstrate any significant effects of GH treatment on processing speed compared with the placebo group. Moreau and coworkers found a statistically significant difference in attentional functions after 1 year of GH therapy.33 This was similar to the results of High and coworkers in the control group (who did not get the treatment); patients did not show any improvements over time.30 (Table 5).
Table 5.
The Effect of Growth Hormone Therapy on Information Processing of Patients after Traumatic Brain Injury (TBI)
| Author | Design | Severity | No. of patients with treatment | No. of patients without treatment | Treatment beginning and length (months) | WAIS III. PSI | WAIS III. Digit Symbol coding |
Trail Making Test (TMT A and B) | Test for Attentional Performance (TAP) | p value in the treated group from baseline | p value between treated and untreated groups |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | Moderate-to-severe. GCS: 5.8 (SD: 3.4) | 12 | 11 | Chronic, 12 | X | X | <0.05 | n.s. | ||
| Dubiel et al.29 | RCT | All severities GOSE score: 3-17 | 16 | 18 | Acute, 6 | X | X | X | n.s. | ||
| Mossberg et al.34 | Open label study | Moderate-severe | 15 | Chronic, 12 | X | n.s. | |||||
| Moreau et al.33 | Cohort study | All severities GCS: 8.1 (SD: 5.1) | 23 | 27 | Chronic, 12 | X | <0.05 | n.s. | |||
| Maric et al.38 | Case series | Moderate-to severe. GCS: 8-10 | 4 | 2 | Chronic: 6 | X | No improvement |
Speed of information processing was studied by using Wechsler Adult Intelligence Scale (WAIS-III) (PSI: processing speed index),40 the Trail Making Test (TMT),41 and the Test for Attentional Performance (TAP). Acute treatment was started within 1 month after TBI. Statistical significance is indicated at p < 0.05.
RCT, randomized controlled trial; n.s., non-significant; GCS, Glasgow Coma Scale; SD, standard deviation; GOSE, Glasgow Outcome Score-Extended.
Visuospatial ability, non-verbal memory
The effect of GH treatment on visuospatial ability and non-verbal memory was also assessed following TBI. Almost all studies showed a significant improvement in visuospatial ability and non-verbal memory after GH treatment in the chronic phase of TBI-induced GHD. Only the Moreau group33 found a significant difference in these functions between GH-treated and untreated patients with severe TBI (Table 6).
Table 6.
The Effect of Growth Hormone Therapy on Visuospatial Ability and Non-Verbal Memory of Patients after Traumatic Brain Injury (TBI)
| Author | Design | Severity | No. of patients with treatment | No. of patients without treatment | Treatment beginning and length (months) | Rey-Osterrieth Complex Figure Test (ROCFT) | WAIS Perceptual performance, Block design, Matrix reasoning | Visuospatial memory | p value in the treated group from baseline | p value between treated and untreated groups |
|---|---|---|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | Moderate-to-severe. GCS: 5.8 (SD: 3.4) | 12 | 11 | Chronic, 12 | X | X | n.s. | n.s. | |
| Reimunde et al.31 | Cohort study | No GCS assessed | 11 | 8 | Chronic, 3+cognitive therapy | X | 0.011 | |||
| Mossberg et al.34 | Open label study | Moderate-severe | 15 | Chronic, 12 | X | <0.053 | ||||
| Moreau et al.33 | Cohort study | All kind severities GCS: 8.1 (SD: 5.1) | 23 | 27 | Chronic, 12 | X | 0.048 | >0.05 | ||
| Maric et al.38 | Case series | Moderate-to-severe. GCS: 8-10 | 4 | 2 | Chronic, 6 | X | Improvement |
The visuospatial ability and non-verbal memory were assessed in three articles30,33,38 by the Rey-Osterrieth Complex Figure Test (ROCFT) and the Wechsler Adult Intelligence Scale (WAIS) to assess the perceptual organization. Acute treatment was started within 1 month after TBI. Statistical significance is indicated at p < 0.05.
RCT, randomized controlled trial; n.s., non-significant; GCS, Glasgow Coma Scale, SD, standard deviation.
Verbal memory and working memory
Verbal and working memory were demonstrated to significantly improve after GH therapy following TBI, but differences failed to be significant between the treated and untreated groups of patients (Table 7). However, no significant improvement was observed in the untreated groups of patients. Importantly, Dubiel and coworkers, treating the patients acutely following TBI, did not observe any significant effects of GH treatment29 (Table 7). We performed a meta-analysis and quantitative synthesis of the results from the digit span test assessing working memory from Dubiel and coworkers29 and High and coworkers.30 We could not find any differences between the digit span scores of the included studies (Fig. 3).
Table 7.
The Effect of Growth Hormone Therapy on Verbal and Working Memory of Patients after Traumatic Brain Injury (TBI)
| Author | Design | Severity | No. of patients with treatment | No. of patients without treatment | Treatment beginning and length (months) | WAIS III. Verbal comprehension | Digit span | CVLT | Verbal episodic memory | Rey-Auditory verbal learning | p value in the treated group from baseline | p value between treated and untreated groups |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | Moderate-to-severe. GCS: 5.8 (SD: 3.4) | 12 | 11 | Chronic, 12 | X | X | X | In CVLT: <0.05 | |||
| Dubiel et al.29 | RCT | All severities GOSE score: 3-17 | 16 | 18 | Acute, 6 | X | X | n.s. | ||||
| Reimunde et al.31 | Cohort study | No GCS assessed | 11 | 8 | Chronic, 3+cognitive therapy | X | X | In the numbers <0.05 |
n.s. | |||
| Mossberg et al.34 | Open label study | Moderate-severe | 15 | Chronic, 12 | X | n.s. | ||||||
| Moreau et al.33 | Cohort study | All severities GCS: 8.1 (SD: 5.1) | 23 | 27 | Chronic, 12 | X | X | <0.05 | >0.05 | |||
| Maric et al.38 | Case series | Moderate-to-severe. GCS: 8-10 | 4 | 2 | Chronic, 6 | X | X | Improvement |
Verbal and working memory were assessed by the Wechsler Adult Intelligence Scale (WAIS), the California Verbal Learning Test (CVLT), and the Rey Auditory-Verbal Learning test. Acute treatment was started within 1 month after TBI. Statistical significance is indicated at p < 0.05.
RCT, randomized controlled trial; n.s., non-significant; GCS, Glasgow Coma Scale; SD, standard deviation; GOSE, Glasgow Outcome Score-Extended.
Fig. 3.
Forest plot representing weighted mean difference between the digit span scores of three studies.
Executive function
High and coworkers30 found significant improvement in executive function after TBI over time (from baseline to follow-up at 12 months) with rhGH treatment in 12 severe TBI patients. On the contrary, using the same test, Dubiel and coworkers29 did not find any differences in 31 randomized severe TBI patients after treatment for 6 or 12 months with GH. However, in the trial by High and coworkers they started the treatment after years with severe brain injury (mean post-injury time was 11 years), but in their randomized trial, Dubiel and coworkers started the treatment in the acute phase of the TBI in the inpatient rehabilitation unit or in the post-acute transitional rehabilitation setting (mean post-injury time 65.7 days). There was no difference in treatment doses in the two trials (Table 8).
Table 8.
The Effect of Growth Hormone Therapy on Executive Function of Patients after Traumatic Brain Injury (TBI)
| Author | Design | Severity | No. of patients with treatment | No. of patients without treatment | Treatment beginning and length (months) | COWA | Semantic and phonemic fluency | GOAT | WCST | p value in the treated group from baseline | p value between treated and untreated groups |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | Moderate-to-severe. GCS: 5.8 (SD: 3.4) | 12 | 11 | Chronic, 12 | X | X | <0.05 | n.s. | ||
| Dubiel et al.29 | RCT | All severities GOSE score: 3-17 |
16 | 18 | Acute, 6 | X | X | n.s. | |||
| Moreau et al.33 | Cohort study | All severities GCS: 8.1 (SD: 5.1) | 23 | 27 | Chronic, 12 | X | >0.05 | >0.05 | |||
| Maric et al.38 | Case series | Moderate-to-severe. GCS: 8-10 | 4 | 2 | Chronic, 6 | X | Improvement |
Executive function was assessed by the Controlled Oral Word Association test (COWA), the Wisconsin Card Sorting Test (WCST), and the Galveston Orientation Assessment Test (GOAT). Acute treatment was started within 1 month after TBI. Statistical significance is indicated at p < 0.05.
RCT, randomized controlled trial; n.s., non-significant; GCS, Glasgow Coma Scale; SD, standard deviation; GOSE: Glasgow Outcome Score-Extended.
Motor function
Significant improvement was demonstrated in the upper extremity motor speed of the dominant hand by High and coworkers30 from baseline to 1 year of GH therapy in TBI patients. The trial by Dubiel and coworkers29 (using the Functional Independence Measure test [FIM]) demonstrated significantly greater change in motor, cognitive, and total FIM from baseline to 6 month follow-up compared with placebo. The difference remained significant at 12 months of follow-up. These findings were supported by Moreau and coworkers,33 showing that independence in personal and instrumental activities of daily life was significantly improved after 1 year of GH treatment of moderate-to-severe TBI patients with severe GHD. Further confirmation was provided by the case reports of Bhagia and coworkers37 and Devesa and coworkers42 (Table 9).
Table 9.
The Effect of Growth Hormone Therapy on Motor Function of Patients after Traumatic Brain Injury (TBI)
| Author | Design | Severity | No. of patients with treatment | No. of patients without treatment | Treatment beginning and length (months) |
Upper extremity motor speed | FIM | Activities in daily life | p value in the treated group from baseline | p value between treated and untreated groups |
|---|---|---|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | Moderate-to-severe. GCS: 5.8 (SD: 3.4) | 12 | 11 | Chronic, 12 | X | <0.01 | <0.01 | ||
| Dubiel et al.29 | RCT | All severities GOSE score: 3-17 | 16 | 18 | Acute, 6 | X | <0.05 | |||
| Moreau et al.33 | Cohort study | All severities GCS: 8.1 (SD: 5.1) | 23 | 27 | Chronic, 12 | X | 0.001 | n.s. | ||
| Bhagia et al.37 | Case report | No GCS assessed | 1 | Chronic, 12 | X | improvement |
Motor function was assessed by Functional Independence Measure test (FIM), and by quantifying upper extremity motor speed and daily activities. Acute treatment was started within 1 month after TBI. Statistical significance is indicated at p < 0.05.
RCT, randomized controlled trial; n.s., non-significant; GCS, Glasgow Coma Scale; GOSE, Glasgow Outcome Score-Extended.
Emotional functioning, depression
Mossberg and coworkers34 demonstrated that 12 months of rhGH treatment resulted in a significantly lower rate of depression among 15 moderate-to-severe TBI patients with abnormal GH secretion. On the contrary, High and coworkers30 did not find any effects of GH therapy on depression in 12 severe TBI patients who were randomized for GH treatment. Supporting the findings of Mossberg and coworkers34 Maric and coworkers38 showed a significant protective effect of GH therapy in three out of four TBI patients with GHD after 6 months of recombinant human GH (Table 10).
Table 10.
The Effect of Growth Hormone Therapy on Emotional Functioning and Depression of Patients after Traumatic Brain Injury (TBI)
| Author | Design | Severity | No. of patients with treatment | No. of patients without treatment | Treatment beginning and length (months) | BDI | Zhang scale | p value in the treated group from baseline | p value between treated and untreated groups |
|---|---|---|---|---|---|---|---|---|---|
| High et al.30 | RCT | Moderate-to-severe. GCS: 5.8 (SD: 3.4) | 12 | 11 | Chronic, 12 | X | n.s. | n.s. | |
| Mossberg et al.34 | Open label study | Moderate-severe | 15 | Chronic, 12 | X | <0.05 | |||
| Maric et al.38 | Case series | Moderate-to severe. GCS: 8-10 | 4 | 2 | Chronic, 6 | X | Improvement |
To assess emotional functioning and depression the Beck Depression Inventory (BDI, a self-report assessing characteristic attitudes and symptoms of depression43 and the Zhang scale, which measures affective, psychological, and associated somatic symptoms) were used. Acute treatment was started within 1 month after TBI. Statistical significance is indicated at p < 0.05.
RCT, randomized controlled trial; n.s., non-significant; GCS, Glasgow Coma Scale; SD, standard deviation.
QoL
In an interesting case report, Tanriverdi and coworkers39 examined the QoL of two retired 36- and 38-year-old male amateur boxers with GHD, who received rhGH therapy for 6 months after 8 and 10 years of retirement. QoL was assessed by the disease specific Quality of Life Assessment of GH Deficiency in Adults (QoL-AGHDA) questionnaire. Both patients exhibited scores after 1 year of GH therapy that were lower than the initial scores they had received, indicating increased QoL after treatment. Gardner and coworkers36 evaluated the QoL of patients from the Pfizer International Metabolic Database with GHD caused by TBI or by non-functioning pituitary adenomas (NFPA). QoL-AGHDA questionnaire values were compared at baseline before starting the treatment. Patients were followed for a year and in one aspect of the cross-sectional study, 154 patients were followed for 8 years. In the 161 TBI patients, significant improvement was detected in QoL after 12 months of GH treatment, and this improvement was sustained over the 8-year period.36 TBI patients demonstrated normalization of socialization compared with the normal population after 1 year. Self-confidence also returned to the range of the normal population by year 6. However, tiredness and impairment in memory of TBI patients did not return to the level of the normal population. It has to be noted that the overall score showed larger improvement in the NFPA group than in TBI patients (across all QoL-AGHDA subdomains of memory, tiredness, tenseness, socializing, and self-confidence).
Using the Quality of Life after Brain Injury (QOLBI) questionnaire, Moreau and coworkers33 showed, in 23 moderate-to-severe TBI patients with severe GHD, that 12 months of GH therapy resulted in significant improvement in four QOLBI domains (intellectual, psychological, functional, personal) as well as in the total QOLBI score. It has to be noted that no RCTs have been studied QoL in TBI patients after GH therapy (Table 11).
Table 11.
The Effect of Growth Hormone Therapy on Quality of Life of Patients after Traumatic Brain Injury (TBI)
| Author | Design | Severity | No. of patients with treatment | No. of patients without treatment | Treatment beginning and length (months) | QoL-AGHDA | QoLBI | p value in the treated group from baseline | p value between treated and untreated groups |
|---|---|---|---|---|---|---|---|---|---|
| Moreau et al.33 | Cohort study | All severities GCS: 8.1 (SD: 5.1) | 23 | 27 | Chronic, 12 | X | Improvement | ||
| Tanriverdi et al.8 | Case report | No GCS Assessed | 2 | Chronic, 6 | X | Improvement | |||
| Gardener et al.36 | Cross-sectional registry | No GCS assessed | 161 | Chronic, 12 months | X |
Quality of life (QoL) of patients was assessed by Quality of Life Assessment of Growth Hormone Deficiency in Adults (QoL-AGHDA) questionnaire and the Quality of Life after Brain Injury (QOLBI) questionnaire. Statistical significance is indicated at p < 0.05.
GCS, Glasgow Coma Scale.
Discussion
Injury of the pituitary gland is a well-recognized consequence of all forms of TBI, and GHD is the most common pituitary hormone deficiency after TBI.45 GHD has been shown to occur in between 2% and 30% of TBI patients during the 1st month after trauma7–9 (of whom almost 50% may recover spontaneously10), and chronic GHD (at least 6 months after trauma) was demonstrated to affect 10–63% of patients.8,11,12 Although GHD seems to be associated with the severity of trauma,13 isolated GHD was reported in 14.7% of patients following repetitive mild head trauma, as well.14 Chronic hypopituitarism warranting hormone substitution occurs in ∼20% of patients after mild, moderate, and severe TBI.13 However, there is little evidence available on the clinical benefits of GH replacement therapy (rhGH) for TBI patients. Accordingly, in our systematic search we found two randomized, double-blinded, placebo- controlled trials with relatively small sample size (23 and 40 patients, respectively). The large number of records after searching the databases using our query means that (1) hypopituitarism following TBI is well known, but the effect of GH therapy is not frequently studied; and (2) GH therapy is a well-known tool in cases of hypopituitarism of other etiologies (not in TBI) to improve cognitive function and memory, processing speed, and QoL. One of the most important consequences of such lack of evidence is that screening for GHD and GH replacement when deficiency is demonstrated is not routinely performed as part of the rehabilitation of TBI patients.
We found agreement in the studies screened that 1 year of GH replacement until achieving the normal IGF-I level had a positive impact on depression, memory, executive function, and QoL in survivors of TBI with altered GH secretion. It is very important to note that acute GHD following TBI may recover spontaneously. Accordingly, Agha and coworkers demonstrated that almost half of patients having post-traumatic hypopituitarism after moderate-to-severe TBI recovered pituitary function within 6 months after the injury.10 This may explain the negative results of the effect of early GH treatment after TBI on cognitive function as demonstrated by Dubiel et al.,29 and supports the concept that routine pituitary hormonal testing should be performed within 6 months of injury13 and that treatment should be initiated afterward.
Importantly, GH therapy might be beneficial in patients without GHD, as well.42 In this case, patients with the most severe initial dysfunction of different modalities showed the greatest improvement after GH therapy.42 This should be verified by prospective randomized studies.
The most frequent cognitive symptom reported by TBI patients and their relatives is impaired memory.46,47 Accordingly, patients with severe TBI showed a significant impairment on forward and backward digit span tasks, which demonstrates the attenuated ability of working memory.48 Interestingly, as shown by our meta-analysis, GH therapy seems to have no effect on performance of patients with severe TBI on the digit span tasks, indicating that GH replacement may not improve working memory. However, TBI may impair the ability to manipulate information within the working memory rather than working memory capacity itself. TBI also causes deficit in visuospatial and verbal learning memory,49,50 and both showed improvement after GH treatment of TBI patients.30,31,33,38
Slower information processing is one of the conspicuous findings across all post-TBI neuropsychological studies.51,52 High and coworkers observed that severe TBI patients on GH therapy started in the chronic phase after the injury achieved a significant improvement over 12 months' treatment time.30 Processing speed was significantly negatively correlated with injury severity and task complexity.51–53 Difficulty in dealing with two tasks at the same time is a frequent complaint of patients with TBI, and is also frequently reported by patients' relatives.46,52,54 In one article, the attentional function improved significantly over the treatment period.33 It is not known whether the attention deficits are related only to slowed information processing or also to additional impairments of executive control.52
Survivors of severe TBI often show dramatic personality and behavioral changes, which can have major consequences on family and social reintegration and on QoL.55 12 months of rhGH treatment subcutaneously seemed to improve QoL of TBI patients with GHD. However, future studies should extend these results by comparing QoL in patient receiving GH to patients on placebo treatment, as well as investigating the effect of GHT on TBI patients without GHD covering the full spectrum of brain trauma.36
In summary, cognitive function and QoL seem to improve after GH treatment in patients with TBI when treatment is started at least 6 months after the injury and lasts at least 6 months.11,12 Future studies should provide further details comparing treatment protocols and responses of patients with different severity of injury. It has to be noted that improvement in cognitive function after GH treatment in TBI patients was observed in most studies when the results of the applied tests were compared with the baseline; the extent of improvement failed statistical significance in the control groups.29 However, post-treatment results were not statistically different between treated and control patients.29 We think that in future studies, comparison of baseline cognitive and neuropsychological function and QoL of treated and control patients is important, as is the change of these parameters as a function of time within the studied groups of patients.
Possible mechanisms of GH therapy
The underlying mechanisms of the beneficial clinical effect of GH on cognition and behavior after TBI is probably multi-faceted. GH acts via two independent mechanisms: directly via GH receptors (GHR) and by inducing the secretion of IGF-I in the liver. GHR can be found throughout the brain, in high concentrations in the choroid plexus, hippocampus, hypothalamus, and the pituitary gland. In the periphery, GHR is found in other tissues including the liver, muscle, bone, and adipose tissue.56–58 Although the exact mechanism of GH (and IGF-I) on human cognition is not well understood in TBI patients, pre-clinical studies showed that GH and IGF-I modulates expression of the NMDA receptors in the hippocampus, which are essentially involved in long-term potentiation and memory acquisition.20,21,30 This suggests that GH therapy may directly affect the neuronal/synaptic mechanisms of learning and memory in TBI patients. Further, GH treatment modulates metabolism of neurotransmitters. For example, it causes a decrease in the concentration of the dopamine metabolite homovanillic acid (HVA) in the cerebrospinal fluid (CSF),59 similarly to the effect of tricyclic antidepressants and monoamine oxidase inhibitors. This mechanism may explain the significant improvement in the scores of the depression inventory tests of TBI patients after rhGH substitutive treatment, as well.34,38 A decrease in HVA increases the level of aspartate by 30% in the CSF, exerting significant effects on hippocampal long-term potentiation and on attentional functions.31,60,61 The GH/IGF-I axis affects cognitive mechanisms indirectly via exerting a profound effect on the cerebral microvasculature.19,62,63 Normal brain capillary perfusion is essential to maintain intact neuronal function by providing constant nutrients and oxygen supply needed to fuel high metabolic demands of active neurons and glial cells.64 IGF-I has been shown to play a central role in the development and maintenance of normal brain capillary architecture and density.19 Lack of IGF-I, demonstrated in different pre-clinical models, resulted in capillary rarefaction and decreased blood perfusion in the hippocampus, which was associated with decline in learning and memory.23 Importantly, studies using a mouse model with adult-onset IGF-I deficiency after a viral knock down of hepatic production of the hormone showed that in addition to the structural changes, lack of IGF-I leads to cerebrovascular dysfunction, as well.63,65 Namely, low level of IGF-I resulted in dysregulation of astrocytic production of mediators of neurovascular coupling, as well as altered expression of glutamate receptors on astrocytes, leading to impaired neurovascular coupling responses and cognitive decline in these animals.24 Neurovascular uncoupling and cognitive dysfunction are most likely causally linked.66–73 This is suggested by studies showing that pharmacological inhibition of neurovascular hyperemia is associated with impaired performance of mice on neurocognitive tests.24,74 Further, IGF-I deficiency may impair autoregulatory mechanisms of the cerebral circulation, especially if comorbid conditions are present. For example, mice lacking hepatic IGF-I exhibit impaired adaptation of cerebral blood flow autoregulation to hypertension, which contributes to disruption of the blood–brain barrier (BBB) and formation of cerebral microbleeds.63,75 Both BBB disruption and development of cerebral microbleeds are associated with cognitive impairment in these animals.23 Future studies should establish the role of these vascular mechanisms in cognitive decline and behavioral changes associated with TBI-related GH and IGF-I deficiency. It has to be noted that IGF-I declines with normal aging, playing a central role in age-related cognitive dysfunction.76 Future studies should test whether aging exacerbates cognitive decline and behavioral changes associated with GH and IGF-I deficiency following TBI. Pre-clinical studies provided important results showing that administration of IGF-1 improves depression, anxiety-like behavior, motor coordination, and visuospatial and working memory in 24-month-old aged mice.17 Similarly, improved cognitive function was demonstrated after IGF-1 treatment in rats on a high-fat diet.77 Based on these studies, the hypothesis can be formulated that IGF-1 treatment may improve cognitive and neuropsychological function after TBI, as well. This should be tested in the future.
Limitations
Because the population of TBI patients is extremely diverse with varying levels of GHD and GH insufficiency, different treatment strategies should be compared and assessed in a well-controlled manner and an optimal regimen should be worked out specifically for defined subgroups of patients after screening for pituitary hormone profile, especially GH levels. Accordingly, an insufficient number of patients were available for analyzing the differences between GH-insufficient and GH-deficient patients and the effects of GH therapy on cognitive outcome and QoL. A further limitation of this analysis is that most of the available results are obtained in a within-subject design, which did not include healthy control groups or groups of GH-sufficient TBI patients for comparison. Various neuropsychological tests were used in the studies found, making direct comparison difficult. High-quality RCTs should define indication of GH therapy, its timing, exact doses, and routes in TBI patients, as well as the most specific and sensitive neuropsychological tests to follow treatment efficacy. Hope is associated with ongoing trials such as Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER TBI) and Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK TBI) to establish state-of-the-art follow-up instruments setting the stage for such trials.
Funding Information
This work was supported by grants from the National Research, Development and Innovation Office to P.T. (NKFI-FK123798) and A.B. (K-134555), the Hungarian Academy of Sciences Bolyai Research Scholarship to P.T. EFOP-3.6.2.-16-2017-00008, GINOP-2.3.2-15-2016-00048, GINOP-2.2.1-15-2017-00067 to P.T. and A.B., Hungarian Brain Research Program 2.0 Grant No. 2017-1.2.1-NKP-2017-00002 to A.B., the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities –FIKP III to P.T. and A.B., and the National Institute of Health R01-AG055395, R01-NS100782, R01-AT006526 to Z.U.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Nguyen, R., Fiest, K.M., McChesney, J., Kwon, C.S., Jette, N., Frolkis, A.D., Atta, C., Mah, S., Dhaliwal, H., Reid, A., Pringsheim, T., Dykeman, J., and Gallagher, C. (2016). The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can. J. Neurol. Sci. 43, 774–785. [DOI] [PubMed] [Google Scholar]
- 2. van der Naalt, J., van Zomeren, A.H., Sluiter, W.J., and Minderhoud, J.M. (1999). One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complaints and return to work. J. Neurol. Neurosurg. Psychiatry 66, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lieberman, S.A., Oberoi, A.L., Gilkison, C.R., Masel, B.E., and Urban, R.J. (2001). Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J. Clin. Endocrinol. Metab. 86, 2752–2756. [DOI] [PubMed] [Google Scholar]
- 4. Aimaretti, G., Ambrosio, M.R., Di Somma, C., Fusco, A., Cannavo, S., Gasperi, M., Scaroni, C., De Marinis, L., Benvenga, S., degli Uberti, E.C., Lombardi, G., Mantero, F., Martino, E., Giordano, G., and Ghigo, E. (2004). Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin. Endocrinol. 61, 320–326. [DOI] [PubMed] [Google Scholar]
- 5. Popovic, V., Pekic, S., Pavlovic, D., Maric, N., Jasovic-Gasic, M., Djurovic, B., Medic Stojanoska, M., Zivkovic, V., Stojanovic, M., Doknic, M., Milic, N., Djurovic, M., Dieguez, C., and Casanueva, F.F. (2004). Hypopituitarism as a consequence of traumatic brain injury (TBI) and its possible relation with cognitive disabilities and mental distress. J. Endocrinol. Invest. 27, 1048–1054. [DOI] [PubMed] [Google Scholar]
- 6. Popovic, V. (2005). GH deficiency as the most common pituitary defect after TBI: clinical implications. Pituitary 8, 239–243. [DOI] [PubMed] [Google Scholar]
- 7. Olivecrona, Z., Dahlqvist, P., and Koskinen, L.O. (2013). Acute neuro-endocrine profile and prediction of outcome after severe brain injury. Scand. J. Trauma Resusc. Emerg. Med. 21, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanriverdi, F., Senyurek, H., Unluhizarci, K., Selcuklu, A., Casanueva, F.F., and Kelestimur, F. (2006). High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J. Clin. Endocrinol. Metab. 91, 2105–2111. [DOI] [PubMed] [Google Scholar]
- 9. Agha, A., Rogers, B., Mylotte, D., Taleb, F., Tormey, W., Phillips, J., and Thompson, C.J. (2004). Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin. Endocrinol. 60, 584–591. [DOI] [PubMed] [Google Scholar]
- 10. Agha, A., Phillips, J., O'Kelly, P., Tormey, W., and Thompson, C.J. (2005). The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am. J. Med. 118, 1416. [DOI] [PubMed] [Google Scholar]
- 11. Agha, A., Rogers, B., Sherlock, M., O'Kelly, P., Tormey, W., Phillips, J., and Thompson, C.J. (2004). Anterior pituitary dysfunction in survivors of traumatic brain injury. J. Clin. Endocrinol. Metab. 89, 4929–4936. [DOI] [PubMed] [Google Scholar]
- 12. Schneider, H.J., Schneider, M., Saller, B., Petersenn, S., Uhr, M., Husemann, B., von Rosen, F., and Stalla, G.K. (2006). Prevalence of anterior pituitary insufficiency 3 and 12 months after traumatic brain injury. Eur. J. Endocrinol. 154, 259–265. [DOI] [PubMed] [Google Scholar]
- 13. Bavisetty, S., Bavisetty, S., McArthur, D.L., Dusick, J.R., Wang, C., Cohan, P., Boscardin, W.J., Swerdloff, R., Levin, H., Chang, D.J., Muizelaar, J.P., and Kelly, D.F. (2008). Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery 62, 1080–1093. [DOI] [PubMed] [Google Scholar]
- 14. Kelly, D.F., Chaloner, C., Evans, D., Mathews, A., Cohan, P., Wang, C., Swerdloff, R., Sim, M.S., Lee, J., Wright, M.J., Kernan, C., Barkhoudarian, G., Yuen, K.C., and Guskiewicz, K. (2014). Prevalence of pituitary hormone dysfunction, metabolic syndrome, and impaired quality of life in retired professional football players: a prospective study. J. Neurotrauma 31, 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devesa, P., Reimunde, P., Gallego, R., Devesa, J., and Arce, V.M. (2011). Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury. Brain Inj. 25, 503–510. [DOI] [PubMed] [Google Scholar]
- 16. Colon, G., Saccon, T., Schneider, A., Cavalcante, M.B., Huffman, D.M., Berryman, D., List, E., Ikeno, Y., Musi, N., Bartke, A., Kopchick, J., Kirkland, J.L., Tchkonia, T., and Masternak, M.M. (2019). The enigmatic role of growth hormone in age-related diseases, cognition, and longevity. Geroscience 41, 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farias Quipildor, G.E., Mao, K., Hu, Z., Novaj, A., Cui, M.H., Gulinello, M., Branch, C.A., Gubbi, S., Patel, K., Moellering, D.R., Tarantini, S., Kiss, T., Yabluchanskiy, A., Ungvari, Z., Sonntag, W.E., and Huffman, D.M. (2019). Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience 41, 185–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramsey, M.M., Weiner, J.L., Moore, T.P., Carter, C.S., and Sonntag, W.E. (2004). Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience 129, 119–127. [DOI] [PubMed] [Google Scholar]
- 19. Sonntag, W.E., Deak, F., Ashpole, N., Toth, P., Csiszar, A., Freeman, W., and Ungvari, Z. (2013). Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front. Aging Neurosci. 5, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahmoud, G.S., and Grover, L.M. (2006). Growth hormone enhances excitatory synaptic transmission in area CA1 of rat hippocampus. J. Neurophysiol. 95, 2962–2974. [DOI] [PubMed] [Google Scholar]
- 21. Le Greves, M., Zhou, Q., Berg, M., Le Greves, P., Fholenhag, K., Meyerson, B., and Nyberg, F. (2006). Growth hormone replacement in hypophysectomized rats affects spatial performance and hippocampal levels of NMDA receptor subunit and PSD-95 gene transcript levels. Exp. Brain Res. 173, 267–273. [DOI] [PubMed] [Google Scholar]
- 22. Norling, A.M., Gerstenecker, A.T., Buford, T.W., Khan, B., Oparil, S. and Lazar, R.M. (2020). The role of exercise in the reversal of IGF-1 deficiencies in microvascular rarefaction and hypertension. Geroscience 42, 141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarantini, S., Tucsek, Z., Valcarcel-Ares, M.N., Toth, P., Gautam, T., Giles, C.B., Ballabh, P., Wei, J.Y., Wren, J.D., Ashpole, N.M., Sonntag, W.E., Ungvari, Z., and Csiszar, A. (2016). Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age 38, 273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toth, P., Tarantini, S., Ashpole, N.M., Tucsek, Z., Milne, G.L., Valcarcel-Ares, N.M., Menyhart, A., Farkas, E., Sonntag, W.E., Csiszar, A., and Ungvari, Z. (2015). IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14, 1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G., and Group, P. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. [DOI] [PubMed] [Google Scholar]
- 26. Lockwood, C., Munn, Z., and Porritt, K. (2015). Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. Int. J. Evid. Based Healthc. 13, 179–187. [DOI] [PubMed] [Google Scholar]
- 27. Yang, Z.R., Sun, F., and Zhan, S.Y. (2017). Risk on bias assessment: (2) Revised Cochrane risk of bias tool for individually randomized, parallel group trials (RoB2.0)[in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 38, 1285–1291. [DOI] [PubMed] [Google Scholar]
- 28. Murad, M.H., Mustafa, R.A., Schunemann, H.J., Sultan, S. and Santesso, N. (2017). Rating the certainty in evidence in the absence of a single estimate of effect. Evid. Based Med. 22, 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubiel, R., Callender, L., Dunklin, C., Harper, C., Bennett, M., Kreber, L., Auchus, R., and Diaz-Arrastia, R. (2018). Phase 2 randomized, placebo-controlled clinical trial of recombinant human growth hormone (rhGH) during rehabilitation from traumatic brain injury. Front. Endocrinol. 9, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. High, W.M.Jr., Briones-Galang, M., Clark, J.A., Gilkison, C., Mossberg, K.A., Zgaljardic, D.J., Masel, B.E., and Urban, R.J. (2010). Effect of growth hormone replacement therapy on cognition after traumatic brain injury. J. Neurotrauma 27, 1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reimunde, P., Quintana, A., Castanon, B., Casteleiro, N., Vilarnovo, Z., Otero, A., Devesa, A., Otero-Cepeda, X.L., and Devesa, J. (2011). Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj. 25, 65–73. [DOI] [PubMed] [Google Scholar]
- 32. DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clini. Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- 33. Moreau, O.K., Cortet-Rudelli, C., Yollin, E., Merlen, E., Daveluy, W., and Rousseaux, M. (2013). Growth hormone replacement therapy in patients with traumatic brain injury. J. Neurotrauma 30, 998–1006. [DOI] [PubMed] [Google Scholar]
- 34. Mossberg, K.A., Durham, W.J., Zgaljardic, D.J., Gilkison, C.R., Danesi, C.P., Sheffield-Moore, M., Masel, B.E., and Urban, R.J. (2017). Functional changes after recombinant human growth hormone replacement in patients with chronic traumatic brain injury and abnormal growth hormone secretion. J. Neurotrauma 34, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Devesa, J., Reimunde, P., Devesa, P., Barbera, M., and Arce, V. (2013). Growth hormone (GH) and brain trauma. Horm. Behav. 63, 331–344. [DOI] [PubMed] [Google Scholar]
- 36. Gardner, C.J., Mattsson, A.F., Daousi, C., Korbonits, M., Koltowska-Haggstrom, M., and Cuthbertson, D.J. (2015). GH deficiency after traumatic brain injury: improvement in quality of life with GH therapy: analysis of the KIMS database. Eur. J. Endocrinol. 172, 371–381. [DOI] [PubMed] [Google Scholar]
- 37. Bhagia, V., Gilkison, C., Fitts, R.H., Zgaljardic, D.J., High, W.M.Jr., Masel, B.E., Urban, R.J., and Mossberg, K.A. (2010). Effect of recombinant growth hormone replacement in a growth hormone deficient subject recovering from mild traumatic brain injury: a case report. Brain Inj. 24, 560–567. [DOI] [PubMed] [Google Scholar]
- 38. Maric, N.P., Doknic, M., Pavlovic, D., Pekic, S., Stojanovic, M., Jasovic-Gasic, M., and Popovic, V. (2010). Psychiatric and neuropsychological changes in growth hormone-deficient patients after traumatic brain injury in response to growth hormone therapy. J. Endocrinol. Invest. 33, 770–775. [DOI] [PubMed] [Google Scholar]
- 39. Tanriverdi, F., Unluhizarci, K., Karaca, Z., Casanueva, F.F., and Kelestimur, F. (2010). Hypopituitarism due to sports related head trauma and the effects of growth hormone replacement in retired amateur boxers. Pituitary 13, 111–114. [DOI] [PubMed] [Google Scholar]
- 40. Haworth, J., Phillips, M., Newson, M., Rogers, P.J., Torrens-Burton, A., and Tales, A. (2016). Measuring information processing speed in mild cognitive impairment: clinical versus research dichotomy. J. Alzheimers Dis. 51, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tombaugh, T.N. (2004). Trail Making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214. [DOI] [PubMed] [Google Scholar]
- 42. Devesa, J., Diaz-Getino, G., Rey, P., Garcia-Cancela, J., Loures, I., Nogueiras, S., Hurtado de Mendoza, A., Salgado, L., Gonzalez, M., Pablos, T., and Devesa, P. (2015). Brain recovery after a plane crash: treatment with growth hormone (GH) and neurorehabilitation: a case report. Int. J. Mol. Sci. 16, 30470–30482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beck, A.T., Ward, C.H., Mendelson, M., Mock, J., and Erbaugh, J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- 44. Springer, J. and Chollet, A. (2001). A traumatic car crash. Lancet (London, England) 357, 1848. [DOI] [PubMed] [Google Scholar]
- 45. Kgosidialwa, O., Hakami, O., Muhammad Zia-Ul-Hussnain, H., and Agha, A. (2019). Growth hormone deficiency following traumatic brain injury. Int. J. Mol. Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jourdan, C., Bayen, E., Pradat-Diehl, P., Ghout, I., Darnoux, E., Azerad, S., Vallat-Azouvi, C., Charanton, J., Aegerter, P., Ruet, A., and Azouvi, P. (2016). A comprehensive picture of 4-year outcome of severe brain injuries. Results from the PariS-TBI study. Ann. Phys. Rehabil. Med. 59, 100–106. [DOI] [PubMed] [Google Scholar]
- 47. Brooks, D.N., and McKinlay, W. (1983). Personality and behavioural change after severe blunt head injury––a relative's view. J. Neurol. Neurosurg. Psychiatry 46, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vallat-Azouvi, C., Weber, T., Legrand, L., and Azouvi, P. (2007). Working memory after severe traumatic brain injury. J. Int. Neuropsychol. Soc. 13, 770–780. [DOI] [PubMed] [Google Scholar]
- 49. Dobryakova, E., Boukrina, O., and Wylie, G.R. (2015). Investigation of information flow during a novel working memory task in individuals with traumatic brain injury. Brain Connect. 5, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dunning, D.L., Westgate, B., and Adlam, A.R. (2016). A meta-analysis of working memory impairments in survivors of moderate-to-severe traumatic brain injury. Neuropsychology 30, 811–819. [DOI] [PubMed] [Google Scholar]
- 51. Ponsford, J., and Kinsella, G. (1992). Attentional deficits following closed-head injury. J. Clin. Exp. Neuropsychol. 14, 822–838. [DOI] [PubMed] [Google Scholar]
- 52. Azouvi, P., Arnould, A., Dromer, E., and Vallat-Azouvi, C. (2017). Neuropsychology of traumatic brain injury: an expert overview. Rev. Neurol. 173, 461–472. [DOI] [PubMed] [Google Scholar]
- 53. Van Zomeren, A.H., and Deelman, B.G. (1978). Long-term recovery of visual reaction time after closed head injury. J. Neurol. Neurosurg. Psychiatry 41, 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Zomeren, A.H., and van den Burg, W. (1985). Residual complaints of patients two years after severe head injury. J. Neurol. Neurosurg. Psychiatry 48, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ponsford, J., Draper, K., and Schonberger, M. (2008). Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J. Int. Neuropsychol. Soc. 14, 233–242. [DOI] [PubMed] [Google Scholar]
- 56. Lu, M., Flanagan, J.U., Langley, R.J., Hay, M.P., and Perry, J.K. (2019). Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct. Target. Ther. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Halmos, T., and Suba, I. (2019). The physiological role of growth hormone and insulin-like growth factors [in Hungarian]. Orv. Hetil. 160, 1774–1783. [DOI] [PubMed] [Google Scholar]
- 58. Lai, Z.N., Emtner, M., Roos, P., and Nyberg, F. (1991). Characterization of putative growth hormone receptors in human choroid plexus. Brain Res. 546, 222–226. [DOI] [PubMed] [Google Scholar]
- 59. Burman, P., Hetta, J., Wide, L., Mansson, J.E., Ekman, R., and Karlsson, F.A. (1996). Growth hormone treatment affects brain neurotransmitters and thyroxine [see comment]. Clin. Endocrinol. 44, 319–324. [DOI] [PubMed] [Google Scholar]
- 60. van Nieuwpoort, I.C., and Drent, M.L. (2008). Cognition in the adult with childhood-onset GH deficiency. Eur. J. Endocrinol. 159, Suppl. 1, S53–57. [DOI] [PubMed] [Google Scholar]
- 61. Oertel, H., Schneider, H.J., Stalla, G.K., Holsboer, F., and Zihl, J. (2004). The effect of growth hormone substitution on cognitive performance in adult patients with hypopituitarism. Psychoneuroendocrinology 29, 839–850. [DOI] [PubMed] [Google Scholar]
- 62. Tarantini, S., Valcarcel-Ares, N.M., Yabluchanskiy, A., Springo, Z., Fulop, G.A., Ashpole, N., Gautam, T., Giles, C.B., Wren, J.D., Sonntag, W.E., Csiszar, A., and Ungvari, Z. (2017). Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 16, 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Toth, P., Tucsek, Z., Tarantini, S., Sosnowska, D., Gautam, T., Mitschelen, M., Koller, A., Sonntag, W.E., Csiszar, A., and Ungvari, Z. (2014). IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J. Cereb. Blood Flow Metab. 34, 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Attwell, D., Buchan, A.M., Charpak, S., Lauritzen, M., Macvicar, B.A., and Newman, E.A. (2010). Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dong, X., Chang, G., Ji, X.F., Tao, D.B., and Wang, Y.X. (2014). The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PloS One 9, e94845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66. Wiedenhoeft, T., Tarantini, S., Nyul-Toth, A., Yabluchanskiy, A., Csipo, T., Balasubramanian, P., Lipecz, A., Kiss, T., Csiszar, A., Csiszar, A., and Ungvari, Z. (2019). Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience 41, 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tarantini, S., Yabluchanskiy, A., Csipo, T., Fulop, G., Kiss, T., Balasubramanian, P., DelFavero, J., Ahire, C., Ungvari, A., Nyul-Toth, A., Farkas, E., Benyo, Z., Toth, A., Csiszar, A., and Ungvari, Z. (2019). Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience 41, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tarantini, S., Valcarcel-Ares, M.N., Toth, P., Yabluchanskiy, A., Tucsek, Z., Kiss, T., Hertelendy, P., Kinter, M., Ballabh, P., Sule, Z., Farkas, E., Baur, J.A., Sinclair, D.A., Csiszar, A., and Ungvari, Z. (2019). Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lipecz, A., Csipo, T., Tarantini, S., Hand, R.A., Ngo, B.N., Conley, S., Nemeth, G., Tsorbatzoglou, A., Courtney, D.L., Yabluchanska, V., Csiszar, A., Ungvari, Z.I., and Yabluchanskiy, A. (2019). Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience 41, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Csiszar, A., Yabluchanskiy, A., Ungvari, A., Ungvari, Z., and Tarantini, S. (2019). Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience 41, 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Csipo, T., Mukli, P., Lipecz, A., Tarantini, S., Bahadli, D., Abdulhussein, O., Owens, C., Kiss, T., Balasubramanian, P., Nyul-Toth, A., Hand, R.A., Yabluchanska, V., Sorond, F.A., Csiszar, A., Ungvari, Z., and Yabluchanskiy, A. (2019). Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience 41, 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tarantini, S., Valcarcel-Ares, N.M., Yabluchanskiy, A., Fulop, G.A., Hertelendy, P., Gautam, T., Farkas, E., Perz, A., Rabinovitch, P.S., Sonntag, W.E., Csiszar, A., and Ungvari, Z. (2018). Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tarantini, S., Valcarcel-Ares, M.N., Yabluchanskiy, A., Tucsek, Z., Hertelendy, P., Kiss, T., Gautam, T., Zhang, X.A., Sonntag, W.E., de Cabo, R., Farkas, E., Elliott, M.E., Kinter, M.T., Deak, F., Ungvari, Z., and Csiszar, A. (2018). Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol. A Biol. Sci. Med. Sci. 73, 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tarantini, S., Yabluchanksiy, A., Fulop, G.A., Hertelendy, P., Valcarcel-Ares, M.N., Kiss, T., Bagwell, J.M., O'Connor, D., Farkas, E., Sorond, F., Csiszar, A., and Ungvari, Z. (2017). Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience 39, 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ungvari, Z., Tarantini, S., Kirkpatrick, A.C., Csiszar, A. and Prodan, C.I. (2017). Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 312, H1128–H1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Toth, P., Tucsek, Z., Sosnowska, D., Gautam, T., Mitschelen, M., Tarantini, S., Deak, F., Koller, A., Sonntag, W.E., Csiszar, A., and Ungvari, Z. (2013). Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J. Cereb. Blood Flow Metab. 33, 1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang, F., Wang, L., Wang, Y., Li, D., Hu, T., Sun, M., and Lei, P. (2020). Exogenous IGF-1 improves cognitive function in rats with high-fat diet consumption. J. Mol. Endocrinol. 64, 115–123. [DOI] [PubMed] [Google Scholar]