Abstract
Background:
Patients with diabetes mellitus suffer from an additional macrophage dysfunction in the secretion of growth factor, which later decreases transforming growth factor beta 1 (TGF-β1). This condition disrupts proliferation and angiogenesis. Extract of okra fruit (Abelmoschus esculentus) contains flavonoid, an active substance which acts as antioxidant, anti-inflammation, and antidiabetes. The purpose of this study is to analyze the difference in TGF-β1 expression in wound-healing process after tooth extraction of diabetic Wistar rats.
Materials and Methods:
This is a laboratory experimental study using pretest and posttest on 24 Wistar rats which are divided into two groups: control group (treated with streptozotocin induction but without administration of okra fruit extract) and treatment group (treated with streptozotocin induction and oral administration of 250 mg/kg okra fruit extract once a day). Extractions of the rats’ mandibular left incisors were performed using a pair of modified forceps and an elevator. The tooth sockets were then irrigated using saline solution. Four rats in each group were sacrificed on day 3 (KO1, PO1), 5 (KO2, PO2), and 7 (KO3, PO3). The socket tissues from the rats were then immunohistochemically analyzed. Data were analyzed at level significance of 0.05.
Results:
The average level of TGF-β1 expression in the treatment groups was higher compared to the control group: PO1 (11.59 ± 0.58), PO2 (15.15 ± 1.07), and PO3 (18.75 ± 2.73) as compared to KO1 (5.32 ± 1.69), KO2 (8.47 ± 0.60), and KO3 (9.28 ± 1.16) with P = 0.001.
Conclusion:
The administration of okra fruit extract can increase the level of TGF-β1 in wounds after tooth extraction of diabetic Wistar rats.
Keywords: Diabetes mellitus, okra fruit, transforming growth factor beta 1, wound healing, none
INTRODUCTION
Wound healing is a complex biological process involving hemostasis, inflammation, proliferation, and remodeling.[1] On the 3rd day of wound-healing process, there is a transition from inflammatory phase to proliferation phase, in which a transition from macrophage-1 (M1) to macrophage-2 (M2) occurs. The 5th day is the proliferation phase, in which fibroblasts are transferred to the injured area and M2 plays a dominant role as an anti-inflammatory agent. On the 7th day, the angiogenesis phase reaches its peak.[2,3] It is also known that healing process is influenced by systemic factors, one of which is comorbidities, such as diabetes mellitus (DM). DM is known to cause macrophage dysfunction in the patients.[4]
Wound-healing process involves a series of activities of damage repair. Prolonged high-blood glucose level may cause a prolonged inflammatory process and high anti-inflammatory activity.[5] Which results in fibroblast dysfunction occurring in the wound healing process in diabetic patients, resulting in a decrease in the expression level of the tumor growth factor beta 1 (TGF-β1) gene.[6] TGF-β1 expression plays a dominant role in wound recovery among other TGF-β isoforms because TGF-β1 functions to increase proliferation, collagen formation, and differentiation of fibroblasts in the wound proliferation phase. Besides, TGF-β1 also plays a role in forming extracellular matrix (ECM) secretion and those related to morphological proliferation, mononuclear cell differentiation, and osteocytes. TGF-β1 is involved in angiogenesis by increasing the regulation of vascular endothelial growth factor (VEGF). During wound closure process, TGF-β1 increases keratinocyte transfer.[7]
Along with the advancement of science, various treatments have been developed to overcome this problem, one of which uses herbal ingredients. Herbs are in great demand and are used by around 80% of the world's population because of the benefits in terms of safety, effectiveness, cultural acceptance, and less substantial side effects as compared to synthetic chemicals.[8] One herb that can accelerate wound-healing process is the fruit of okra plant (Abelmoschus esculentus).
Okra fruit has antioxidant, anti-inflammatory,[9] and antidiabetic[10] qualities in the process of wound healing. The antioxidant quality of okra is needed in the process of wound healing to eliminate the effects of reactive oxygen species (ROS). Okra fruit's anti-inflammatory feature decreases the production of proinflammatory mediators, such as nitric oxide and ROS, and the production of tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) stimulated by lipopolysaccharide.[9] Flavonoids found in okra fruit also play a role in lowering blood glucose level due to its isoquercetin content, which regulates the level of blood glucose and increases immunoreactivity of pancreatic β-cells. In addition, it also has a role in wound-healing process.[9,10] The objective of this study is to calculate the level of expression of TGF-β1 in posttooth extraction wound-healing process in Wistar rats with DM. In addition, this study also aims to verify that administration of okra fruit extract can increase TGF-β1 expression in posttooth extraction wound-healing process in Wistar rats with DM.
MATERIALS AND METHODS
Research design and animal model
This is a laboratory-based of analytic experimental study, with posttest-only control group design. Wistar rats used as samples in this study were obtained from the Experimental Animal Unit of the Biochemical Laboratory of the Faculty of Medicine, Universitas Airlangga. Ethical clearance of the research was issued by the Health Research Ethical Clearance Commission with a clearance certificate numbered 231/HRECC.FODM/V/2019.
Collection, adjustment, maintenance, and treatment were carried out in the Experimental Animal Unit of the Biochemical Laboratory of the Faculty of Medicine, Universitas Airlangga. Okra fruit was extracted in Materia Medica Batu. Histological preparations were carried out at the Anatomy Pathology Laboratory of the Faculty of Medicine, Universitas Airlangga. Immunohistochemistry dyeing and TGF-β1 expression calculation were carried out at Brawijaya University, Malang.
Okra fruit extract preparation
Fresh okra fruit collected for the study was dried in a drying oven until a constant weight was reached. The dried fruit was then ground into powder. A total of 2 g of powder was extracted with 20 ml of 70% ethanol in a ratio of 1:10 (w/v) during the maceration period (24 h) at room temperature. The mixture of solvent and soaked powder was filtered through filter paper and then concentrated to 1 ml with a rotary evaporator and diluted with 5% dimethyl sulfoxide at a ratio of 1:1 (v/v). The results were then stored at a temperature of − 20°C until further use.[11]
Research procedure
In this study, 24 male Wistar rats aged 2–3 months with a weight of 150–200 g were adapted in the same cage at 25°C ± 2°C. The 24 Wistar rats were divided into two groups (control group and treatment group). The rats were supplied with standard pellet food and distilled water ad libitum for 7 days and for 4 h before being induced with streptozotocin (STZ) (Nacalai Tesque Inc., Japan). The 2% STZ solution was dissolved with 0.1 mol/L citrate buffer solution with pH of 4.4 at a dose of 45 mg/kg and converted to a dose of 6.75 mg/150 g. The solution was then administered to the Wistar rats through intraperitoneal induction.[12] Blood glucose levels were measured on day 3 after STZ induction by taking blood sample from the lateral veins in the rats’ tails. Measurements were performed using a glucometer (Accu Chek® Instant). The Wistar rats were diagnosed with DM if the blood glucose levels ≥200 mg/dl after the STZ induction.[13] The rats’ weight during experiment was not measured.
Wistar rats with DM were then anesthetized through peritoneal injection using 0.1 ml of ketamine per rat. A resting period of 1–1.5 h was given after the injection, after which extractions of the rats’ mandibular left incisors were performed using a pair of modified forceps and an elevator. The tooth sockets were then irrigated with saline solution.[14]
In the control group, the animals did not receive administration of okra fruit extract. Instead, they were only supplied with distilled water before the observation. KO1 was observed on the 3rd day, KO2 was observed on the 5th day, and KO3 was observed on the 7th day. In the treatment group, the rats were given oral administration of okra fruit extract after the tooth extraction with a dose of 250 mg/kg which was converted to a dose of 37.5 mg/150 g once a day during the treatment. PO1 was observed on the 3rd day, PO2 was observed on 5th day, and PO3 was observed on 7th day.
Wistar rats were sacrificed on the 3rd, 5th, and 7th day using lethal injection of intraperitoneal ketamine (no <4 times the anesthetic dose or about 0.4 ml/kg). The mandibular of each rat was taken from the temporomandibular joint. After which, the Wistar rats were buried according to the ethical treatments of experimental animals. The mandibles in the incisor area were cut vertically and treated with paraffin method.
Histopathological specimen preparation
The histological examination procedure was started by putting the tissues into formalin buffer (10% formalin solution in phosphate-buffered saline [PBS] pH 7) to be fixed and then put into paraffin wax. The tissues were cut into slides with a length of 4–6 mm on the glass slide. After being deparaffinized with xylene, the slides were submerged in graded alcohol for dehydration and incubation with EDTA (pH = 8.0) in a microwave oven (750 W) to take TGF-β1 antigens. Slides were incubated for 20 min in 3% H2O2 to inhibit endogenous peroxidase activity and then rinsed three times with PBS for 5 min each. The slides were then incubated with blocking solutions using a superblock (Scy Tek Laboratories Inc., US) and peroxide block (Scy Tek Laboratories Inc., US). Slides were incubated overnight with TGF-β1 antibodies (ab 27969: Abcam, Burlingame, US). After being washed in PBS, the slides were treated with UltraTek antipolyvalent biotinylated antibodies (Scy Tech Laboratories Inc., US) and UltraTek HRP (Scy Tek Laboratories Inc., US). This reaction was visualized by incubating the slides for 7 min in 0.1% 3.3 diaminobenzidine and 0.02% hydrogen peroxide solution. Slides were then countered with Mayer's hematoxylin (Scy Tech Laboratories Inc., US) and covered. Immunohistochemical positive staining was defined as the detection of brown chromogen from DAB Chromogen staining (Scy Tech Laboratories Inc., US) at the edge of the hematoxylin-stained nucleus distributed in the cytoplasm or plasma cell membrane and analyzed under a light microscope with ×1000 at 20 visual fields. TGF-β1 expression would be seen as positive, immunoreactive cells with a yellowish to brown color, while negative cells would correspond to the counterstain coloring agent used.[15]
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 24.0: IBM Corp., USA). Shapiro–Wilk test was used to find out normally distributed data. After the distribution test, Levene's homogeneity test was then performed. Once the distribution was found normal and the data were homogeneous, the analysis was continued with one-way analysis of variance (ANOVA) test and with multiple comparison test using Tukey honestly significant difference (HSD) test.
RESULTS
Based on the laboratory experiment using 24 Wistar rats which were divided into control (KO) and treatment (PO) groups, the researchers have observed the wounds from the extraction of the left maxillary incisors on day 3, 5, and 7 to measure the number of TGF-β1 expression in the wound-healing process after tooth extraction of Wistar rats with DM. The blood glucose level of all rats was above 200 mg/dl after the induction. TGF-β1 examination was carried out under a light microscope with ×1000 at 20 visual fields [Figure 1].
Figure 1.
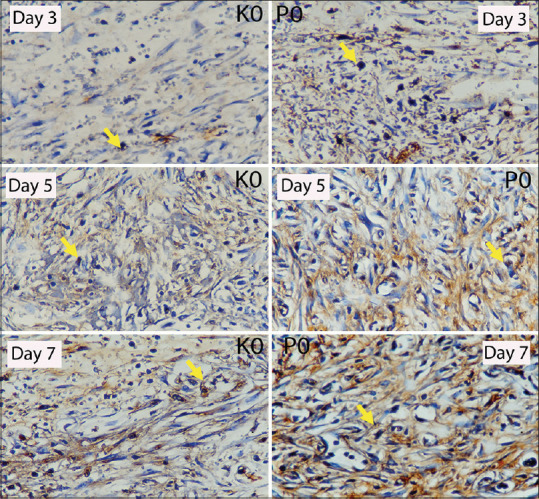
Expression of transforming growth factor beta 1 in day 3, 5, and 7 in socket tissues of Wistar rats with diabetes mellitus in immunohistochemistry examination in microscope with ×400 in control group (KO) and treatment group (PO).
We observed the expression of TGF-β1 both with and without okra fruit extract administration. The ANOVA test showed a significant difference among the groups [Table 1]. Meanwhile, the multiple comparison test result using Tukey HSD showed a significant increase of TGF-β1 expression observed on day 3, 5, and 7 in the control group. Likewise, the treatment group also exhibited the same results for day 3, 5, and 7 [Table 2].
Table 1.
Mean, standard deviation, and normality test of transforming growth factor beta 1 expression (macrophage cells count) in the control and treatment group
| Group | Mean±SD | P |
|---|---|---|
| KO1 | 5.32±1.69 | 0.001 |
| KO2 | 8.47±0.60 | |
| KO3 | 9.28±1.16 | |
| PO1 | 11.59±0.58 | |
| PO2 | 15.15±1.07 | |
| PO3 | 18.75±2.73 |
SD: Standard deviation
Table 2.
Tukey’s multiple comparison test in the control and treatment groups
| Group | KO1 | KO2 | KO3 |
|---|---|---|---|
| KO1 | 0.030* | 0.009* | |
| KO2 | 0.030* | 0.710 | |
|
| |||
| Group | PO1 | PO2 | PO3 |
|
| |||
| PO1 | 0.041* | 0.001* | |
| PO2 | 0.041* | 0.039* | |
*There is a significant difference
TGF-β1 expressions on days 3, 5, and 7 on the prepared Wistar rats’ socket tissues with DM were calculated using a light microscope with ×400 at four visual fields [Figure 1]. TGF-β1 expression appears as gradients of yellow to brown stains pointed with arrows. Based on Figure 2, it can be seen that on the 3rd day, PO1 group showed increasing number of TGF-β1 expression as compared to KO1 group. On the 5th day, PO2 showed increasing number of TGF-β1 expression as compared to KO2 group. On the 7th day, PO3 showed increasing number of TGF-β1 expression as compared to KO3 group.
Figure 2.
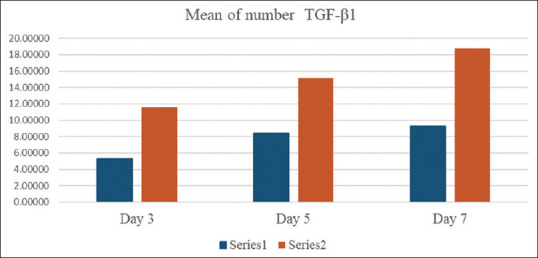
Mean value graph of transforming growth factor beta 1 expression on day 3, 5, and 7.
DISCUSSION
This research aims to prove that okra fruit extract can increase the expression of TGF-β1 in wound-healing process after tooth extraction in Wistar rats with DM. The observations of TGF-β1 expression were carried out on day 3, 5, and 7. Since the 3rd day of the wound-healing process, a transition from inflammatory phase to proliferation phase has taken place. During the same phase, M1 goes under transition to become M2. However, the number of M1 is still above M2. However, on the 5th day, M2 plays a more dominant role than M1 on the wounds. Proliferation phase also takes place during this time in which fibroblasts migrate to the wound area.[3] Then, the peak of the angiogenesis phase starts on the 7th day.[6] M2 acts as an anti-inflammatory in which macrophages release IL-10 and TGF-β, a very strong anti-inflammatory agent that immediately stops the inflammatory process and starts the proliferation phase.[4] This is the reason why we as researchers observed TGF-β1 expression as one of the important growth factors in wound-healing process.
The results of our research confirm the hypothesis that the administration of okra fruit extract can increase TGF-β1 expression in the wound-healing process after tooth extraction of Wistar rats with DM. Observations were done by calculating the amount of TGF-β1 expression in both groups, namely the control group (K) and the treatment group (P). In group K, the TGF-β1 expression from Wistar tooth socket is less than in group P. However, in both K and P groups, we found the highest number of TGF-β1 expression on the 7th day, as compared to on the 3rd and 5th day. This is due to the fact that the healing process that involves fibroblasts cell infiltration to the wound occurs the most on the 7th day; thus, the number of TGF-β1 expression is higher than the 3rd and 5th day.[3,4]
The mean number of TGF-β1 expression in the K group shows lower results than the P group due to the STZ induction. A few days after the STZ induction, damage occurs in pancreatic beta-cells which results in insulin resistance and high-blood glucose level. In addition, the increased oxidative stress due to the formation of Advanced Glycation end products (AGEs) causes disruption of the fibroblast's proliferation, migration, and dysfunction.[6]
Yamano et al. state that at the beginning of tooth extraction, the lowest amount of TGF-β1 expression is obtained compared to the following day.[16] In addition, a study by Hozzein et al. also infers that the administration of intraperitoneal STZ can cause a significant decrease in the regulation of TGF-β1 in wound tissue.[17]
In DM patients, an uncontrolled glycemic control causes a disruption in wound-healing process, which is a disturbance in the angiogenesis activity that causes pathogenesis mechanism.[18] In group K, TGF-β1 expression increased significantly on day 3–5, while on days 5–7, the expression of TGF-β1 also increased, though not as significant. This is due to the fact that DM condition disrupts the innate role and function of immunity cells. High glucose level increases the cellular nuclear factor-κB (NF-κB) activation. If an injury occurs, the prolonged inflammatory phase results in a proinflammatory response that increases the occurrence of chronic inflammation and tissue damage.[6]
High-blood glucose level induces ROS which can be produced both enzymatically and nonenzymatically. Enzymatic production includes nicotinamide adenine dinucleotide phosphate oxidase (oxidase), nitric oxide synthase, cytochrome P-450, cyclooxygenase, lipoxygenase, xanthine oxidase, and myeloperoxidase[19] and results in high proinflammatory cytokines which are released by neutrophils and macrophages as well as an increase in abnormal protease activity, resulting in disruption of growth factor signaling in the wound-healing process. This signaling disruption causes a decrease in growth factor and disrupts in the angiogenesis phase.[13]
On the other hand, the P group had higher average TGF-β1 as compared to K group due to the difference in okra fruit extract administration.[20] Thus, it confirms the hypothesis that okra fruit extract can increase TGF-β1 expression. Okra extract has antioxidant, anti-inflammatory, and antidiabetic qualities in wound-healing process.[8,10] Okra extract contains polyphenols, flavonoids, isoquercetin, and quercetin-3-O-gentiobiose. Flavonoid is useful to repair damaged cells and forms a normal wound-healing process by inducing fibroplasia by TGF-β1.[21] Quercetin plays a role in regulating TGF-β1 expression and decreasing the number of inflammatory cells. Quercetin also decreases the number of TNF-α while increasing fibroblasts proliferation and micro blood vessel density, leading to better reepithelialization and regular collagen deposition.[22] Pang et al. state that low-dose flavonoid alone is able to stimulate TGF-β1 expression which in turn increases TGF-β expression by macrophage stimulation induced by total flavonoids in wounds.[23] TGF-β1, secreted by M2 phenotype macrophages, plays a role in inhibiting the recruitment of inflammatory cells.[24] TGF-β induces the keratinocyte and fibroblasts proliferation, causing the new capillaries formation in the granulation tissue and modulation of ECM deposition resulting in wound healing. TGF-β1 also plays a role in angiogenesis by increasing the regulation of VEGF. During wound closure, TGF-β1 increases keratinocyte migration.[17]
In addition to reducing proinflammatory mediator production, okra fruit plays a role in reducing nitric oxide amount and ROS, as well as in reducing the production of TNF-α.[20] Excessive production of ROS causes activation of the NF-κB signaling pathway. NF-κB is a protein that stimulates cytokines and free radicals. It is also a transcription factor that regulates large numbers of genes involved in various immune and inflammatory response processes and causes vascular complications in DM patients and in inflammatory pathogenic processes that can be inhibited by flavonoids.[21]
Flavonoid contained in okra fruit can reduce blood glucose level,[10] resulting in a decrease in proinflammatory cytokines.[20] High concentration of fiber and polysaccharides in the okra fruit can stabilize blood glucose by limiting the rate of absorption of sugar in the intestine.[25] This complements the flavonoid content of the okra fruit which functions as an inhibitor of α-amylase and α-glucosidase, the enzymes found in the small intestine[21] which act as carbohydrate catalyst by catalyzing oligosaccharides so that glucose absorption may take place. Inhibition of α-glucosidase can cause catalysis of complex carbohydrate diets such as oligosaccharides and polysaccharides to be inhibited by monosaccharides, resulting in decreased blood glucose level.[10,12]
Glycoprotein-A repetitions predominant protein (GARP) is an important regulator in activating latent TGF-β (LTGF-β) and then binding it to LTGF-β. GARP acts as a docking receptor that functions as a carrier of LTGF-β on the cell surface, activating its role.[7] GARP also plays a role in the regulation of T lymphocytes (Tregs) that form complexes with the αVβ8 integrin to release active TGF-β from the cell surface.[17] TGF-β1 stimulates fibroblasts to differentiate into myofibroblasts and then collaborates with these myofibroblasts to produce ECM, as well as collagen and matrix proteins, namely fibronectin.[5] TGF-β1 together with VEGF and fibroblasts stimulate the angiogenesis process.[17] This explains the higher expression of TGF-β1 in P group as compared to K group. Therefore, it can accelerate wound-healing process in the P group.
CONCLUSION
The administration of okra fruit extract can increase the number of TGF-β1 in tooth extraction wounds on Wistar rats with DM.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 2.Benadiba M, Serruya R, Maor Y. Bioaccessibility of Shore Magic® collagen, a low-molecular-weight collagen supplement, in different in vitro barrier models. Heliyon. 2018;4:e00821. doi: 10.1016/j.heliyon.2018.e00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y, Ren P, Wang Q, Jiang SK, Zhang M, Li JY, et al. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J Inflamm (Lond) 2018;15:25. doi: 10.1186/s12950-018-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson K, Hamm RL. Factors that impair wound healing. J Am Coll Clin Wound Spec. 2012;4:84–91. doi: 10.1016/j.jccw.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17:2085. doi: 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kido D, Mizutani K, Takeda K, Mikami R, Matsuura T, Iwasaki K, et al. Impact of diabetes on gingival wound healing via oxidative stress. PLoS One. 2017;12:e0189601. doi: 10.1371/journal.pone.0189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Li Y, Li N, Teng W, Wang M, Zhang Y, et al. TGF-β1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci Rep. 2016;6:1–9. doi: 10.1038/srep32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhowmik D, Sampath Kumar KP, Tripathi P. Traditional herbal medicines: An overview. Arch Appl Sci Res. 2009;1:165–77. [Google Scholar]
- 9.Solomon S. Anti-oxidant and anti-inflammatory activity of Abelmoschus esculentus (Flowers) Indo Am J Pharm Sci. 2016:600–5. [Google Scholar]
- 10.Abbas AY, Muhammad I, AbdulRahman MB, Bilbis LS, Saidu Y, Onu A. Possible antidiabetic mechanism of action of ex-maradi okra fruit variety (Abelmoscus esculentus) on alloxan induced diabetic rats. Niger J Basic Appl Sci. 2018;25:101. [Google Scholar]
- 11.Yeo YL, Chia YY, Lee CH, Sow HS, Yap WS. Effectiveness of maceration periods with different extraction solvents on in-vitro antimicrobial activity from fruit of Momordica charantia L. J Appl Pharm Sci. 2014;4:16–23. [Google Scholar]
- 12.Tian ZH, Miao FT, Zhang X, Wang QH, Lei N, Guo LC. Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin. Asian Pac J Trop Med. 2015;8:1038–42. doi: 10.1016/j.apjtm.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Qinna NA, Badwan AA. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des Devel Ther. 2015;9:2515–25. doi: 10.2147/DDDT.S79885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunawan F, Sularsih S, Soemartono S. The differences effect between low and high molecular weight chitosan on number of lymphocyte cells in the wound healing process of dental extraction. Dent J Kedokt Gigi. 2015;9:113–22. [Google Scholar]
- 15.Sula B, Deveci E, Özevren H, Ekinci C, Elbey B. Immunohistochemical and histopathological changes in the skin of rats after administration of lead acetate. Int J Morphol. 2016;34:918–22. [Google Scholar]
- 16.Yamano S, Kuo WP, Sukotjo C. Downregulated gene expression of TGF-βs in diabetic oral wound healing. J Craniomaxillofac Surg. 2013;41:e42–8. doi: 10.1016/j.jcms.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Hozzein WN, Badr G, Al Ghamdi AA, Sayed A, Al-Waili NS, Garraud O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell Physiol Biochem. 2015;37:940–54. doi: 10.1159/000430221. [DOI] [PubMed] [Google Scholar]
- 18.Raghav A. Crucial role of diabetes mellitus in delayed angiogenesis of wound. J Pharmacol Clin Res. 2018;5:4. [Google Scholar]
- 19.Fakhruddin S, Alanazi W, Jackson KE. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J Diabetes Res. 2017:1–30. doi: 10.1155/2017/8379327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah BN, Seth AK. Anti-inflammatory activity of fruits of Abelmoschus esculantus Linn. Pharmacol Online. 2010;1:208–12. [Google Scholar]
- 21.Roy A, Shrivastava SL, Mandal SM. Functional properties of Okra Abelmoschus esculentus L. (Moench): Traditional claims and scientific evidences. Plant Sci Today. 2014;1:121–30. [Google Scholar]
- 22.Gopalakrishnan A, Ram M, Kumawat S, Tandan S, Kumar D. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF-β1 – PubMed. Indian J Exp Biol. 2016;54:187–95. [PubMed] [Google Scholar]
- 23.Pang Y, Zhang Y, Huang L, Xu L, Wang K, Wang D, et al. Effects and mechanisms of total flavonoids from Blumea balsamifera (L.) DC. on skin wound in rats. Int J Mol Sci. 2017;18:2766. doi: 10.3390/ijms18122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–9. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durazzo A, Lucarini M, Novellino E, Souto EB, Daliu P, Santini A. Abelmoschus esculentus (L.): Bioactive components’ beneficial properties-focused on antidiabetic role-for sustainable health applications. Molecules. 2018;24:38. doi: 10.3390/molecules24010038. [DOI] [PMC free article] [PubMed] [Google Scholar]


