Abstract
Objective
To describe adherence, persistence, discontinuation, restarting, switching, dosing, and health care costs among patients with psoriatic arthritis (PsA) treated with ixekizumab (IXE).
Methods
MarketScan administrative claims databases were used to select adults (≥18 years) initiating IXE between January 1, 2016, and June 30, 2019, for this retrospective study (earliest IXE claim = index). Eligible patients had one or more PsA diagnoses during the 12 months preceding the index and had 12 months of follow‐up time after the index. Adherence (measured by proportion of days covered [PDC]) persistence (<60‐day gap), discontinuation (≥90‐day gap), switching, and dosing patterns were reported. Health care costs were reported per patient per month (PPPM) during follow‐up and were assessed after adjusting PsA treatment costs for discount rates reported by the Institute for Clinical and Economic Review (ICER).
Results
A total of 496 patients met the selection criteria (mean age, 51.1 years; 50.4% female). Over the 12‐month follow‐up, 52.8% remained persistent, 38.7% discontinued, 13.5% had no other treatment, 4.6% restarted, and 20.6% switched to a new biologic/traditional synthetic disease‐modifying antirheumatic drug. 70.6%of patients were identified as highly adherent (i.e. PDC > 0.80)to IXE prior to discontinuation. Dose values were consistent with prescribing information for patients with and without comorbid psoriasis. Although IXE costs ($5233 [SD = $2497]) accounted for 85.6% of PsA‐related health care costs, only 3.5% of IXE costs were patient out‐of‐pocket expenses. Adjusting for the ICER discounts decreased all‐cause and PsA‐related costs by $2509 PPPM.
Conclusion
Results from this real‐world analysis suggest that treatment patterns and costs among patients with PsA initiating IXE are consistent with prior literature for other biologics.
Significance & Innovations.
To our knowledge, this is the first retrospective claims analysis study detailing health care costs and utilization for patients with psoriatic arthritis treated with ixekizumab (IXE) in the United States.
Adjustments for Institute for Clinical and Economic Review–published discount factors, which account for cost offsets such as discounts and rebates not captured in claims, provide a possibly more realistic estimate of real‐world costs for IXE.
INTRODUCTION
Psoriatic arthritis (PsA) is a progressive chronic inflammatory form of arthritis that, when untreated, can lead to permanent joint damage and reduced quality of life (1, 2, 3). Because of the lack of a proven biomarker, clinical diagnosis often relies on a combination of clinical judgment and the application of a tool such as the Classification Criteria for Psoriatic Arthritis criteria, which was developed for classifying patients in clinical trials (4, 5). PsA is estimated to affect 0.06% to 0.25% of the overall population and 6% to 41% of those with psoriasis (PsO) in the United States (3). The prognosis can vary from a relatively mild form to a severe and destructive polyarticular form with progressive joint deformities (2). The economic burden of PsA was estimated from a review of 49 studies from 1998 to 2009 to be as high as $1.9 billion in the United States, with indirect costs associated with work loss and disability accounting for 52% to 72% of the total. Costs are higher for patients with decreased physical function and more active disease (6).
Treatment for PsA has evolved over the past decade, and selection is impacted by disease severity and the presence of conditions commonly associated with PsA such as metabolic disease, cardiovascular conditions, obesity, osteoporosis, axial spondyloarthritis, and mental health conditions (eg, depression and anxiety) (3, 7, 8). Pharmacologic treatment for PsA includes symptomatic therapy (eg, nonsteroidal anti‐inflammatory drugs and corticosteroids) and disease‐modifying antirheumatic drugs (DMARDs). Recommended DMARDs include conventional synthetics (eg, methotrexate), biologics (eg, interleukin‐17 inhibitors [IL‐17is] and tumor necrosis factor inhibitors [TNFis]), and targeted synthetics (eg, tofacitinib and apremilast) (9, 10). Ixekizumab (IXE), a high‐affinity IL‐17i antagonist, was approved for PsA in 2017 (11) after demonstrating improvements in disease activity and physical function for both biologic‐naive and TNFi‐experienced patients with active PsA and inhibition of structural damage progression among biologic‐naive patients compared with placebo (12).
Real‐world studies among patients with PsA or PsO treated with biologic therapy suggest that, during the first year of treatment, 36% to 56% discontinue treatment (13, 14, 15, 16), 32% to 61% are persistent on therapy (16, 17), 18% to 53% remain adherent to the initial therapy (14, 15), and 2% to 28% switch therapies (13, 14, 16, 17). Evidence suggests that continuous therapy is an important factor in maintaining treatment effectiveness (18, 19) and that patients who switch therapy have higher pharmacy costs than those who remain on their initial therapy (20).
Research describing treatment patterns and costs is important for understanding how new therapies are used and prescribed. Although there are substantial real‐world treatment pattern and cost data on older biologics, IXE was approved for the treatment of PsA in 2017, and there are limited published data on this population. This retrospective cohort study uses real‐world data to describe adherence, persistence, discontinuation, restarting, switching, dosing patterns, and health care costs among patients with PsA treated with IXE.
PATIENTS AND METHODS
Study design and data source
A retrospective design was used to describe treatment patterns among patients with PsA initiating IXE. Analysis was performed using the IBM MarketScan Commercial Claims and Encounters Database, the MarketScan Medicare Supplemental and Coordination of Benefits database, and the MarketScan Early View administrative claims database from January 1, 2015, to July 31, 2019. All study data were obtained using codes from the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD‐9 and ICD‐10); the Current Procedural Terminology, Fourth Edition; the Healthcare Common Procedure Coding System; and the National Drug Codes. All data analyses were conducted using WPS version 4.1 (World Programming).
The commercial database captures the inpatient medical, outpatient medical, and outpatient prescription drug data for more than 155‐million employees and their dependents, including 26.8‐million individuals in 2016, covered under a variety of fee‐for‐service and managed care health plans. The Medicare database contains the same data for approximately 10.6‐million Medicare‐eligible retirees with employer‐sponsored Medicare supplemental plans, including two‐million lives in 2016. The MarketScan Monthly Early View Database includes components found in the standard MarketScan Commercial and Medicare Supplemental Databases and captures health care services incurred as late as approximately 45 days before data release.
All database records are statistically deidentified and certified to be fully compliant with United States patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this study used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from institutional review board approval.
Patient selection
Initial selection included patients with at least one medical or pharmacy claim between January 1, 2016, and June 30, 2019, (selection window) for biologic or targeted synthetic DMARDS (tsDMARDs). To identify a cohort of patients initiating IXE, the index date was assigned as the date of the first claim for IXE during the selection window (the index date was not stratified by specialist/provider). Patients were required to be at least 18 years old and continuously enrolled in MarketScan, with medical and pharmacy benefits for 12 months before (baseline period) and 12 months after (follow‐up period) the index date. All patients had to index by July 31, 2018, in order to meet the 12 months of follow‐up requirement. Patients were required to have at least one nondiagnostic inpatient or outpatient claim with a PsA diagnosis (ICD‐9 696.0x or ICD‐10 L405.0‐L405.9) during baseline. Diagnostic claims were defined as those for laboratory or radiology services or procedures used to rule out a condition and therefore not sufficient to ensure an accurate diagnosis of PsA and not used in patient identification. Patients newly initiating IXE were targeted by excluding those with a claim for IXE during the baseline period.
Treatment pattern outcomes
Treatment patterns, including persistence, discontinuation, switching, restarting, adherence, and dosing of IXE, were measured over the 12‐month follow‐up period. Patients were defined as persistent if they did not experience a gap of at least 60 days between claims. The percentage of patients who were persistent through the end of the entire post‐period and the duration of persistence was calculated. A sensitivity analysis was performed using 45‐ and 90‐day treatment gaps. Kaplan‐Meier curves were created to display the time to nonpersistence.
Treatment discontinuation was defined as having a gap of 90 or more days without IXE drug supply from prior prescription(s). The date of discontinuation was the date of the last day of drug supply before the 90‐day gap. The percentage of patients who discontinued and the number of days from the index date to discontinuation were reported. Patients who restarted IXE and those who switched to a different biologic or tsDMARD were identified. Restarting was defined as having at least one claim of IXE after discontinuation and was calculated as the percentage of patients who restarted and the number of days from discontinuation to restart. Switching was defined as having a claim for a new biologic or tsDMARD that is different from IXE as a monotherapy for at least 30 days following the discontinuation of IXE. If the new treatment had less than 30 days overlapping with the IXE, the switching date was set as the date of the first claim of the new therapy. If the overlapping period with IXE was 30 days or more, the switching date was set as the first drug supply date after the overlapping period. The percentages of patients who switched and the drugs to which they switched were calculated. Likewise, the time from index date to switch date was estimated.
The proportion of days covered (PDC), the primary measure of adherence, was defined as the number of days covered by days' supply divided by the time period of interest. PDC was calculated for the following two time periods: 1) the 12‐month follow‐up period and 2) the time on treatment. The number of days covered by days' supply truncated any overlapping days' supply between consecutive fills. The secondary measure of adherence medication possession ratio (MPR) was defined as sum of days’ supply divided by the period of interest for both time periods. The sum of days' supply appended overlapping days' supply between consecutive fills.
IXE dosing was calculated by multiplying the drug strength by the quantity and dividing by the days of supply for each claim. Dosing was calculated separately for the starting and the maintenance periods. The starting period began on the index claim and ended at the date of the last claim within the window defined according to prescribing information (ie, all claims during first 12 weeks for patients with comorbid PsO [defined by having at least one nondiagnostic medical claim with ICD‐9 696.1 or ICD‐10 L40.0‐L40.9 during baseline] and all claims during first 4 weeks for patients without comorbid PsO). The maintenance period started on the date of the first claim outside of the starting window and ended at the date of discontinuation or the end of 12 months of follow‐up, whichever came first. Patients were required to have at least one claim in both periods for inclusion in the dosing analysis. Doses were reported separately for patients with and without comorbid PsO because of differences in the prescribing recommendations (11).
Cost outcomes
All‐cause and PsA‐related health care costs were estimated over the 120month follow‐up period, reported per patient per month (PPPM), and inflated to 2019 dollars using the Medical Care Component of the Consumer Price Index (21). Costs were reported overall and by type of service, including inpatient, outpatient medical, and outpatient pharmacy. Costs were based on paid amounts of adjudicated claims, including insurer and health plan payments as well as the patient‐paid portion (ie, copayment, deductible, and coinsurance). PsA‐related costs were defined as inpatient claims with a diagnosis for PsA in the primary position, outpatient medical claims with a diagnosis for PsA in any position, and claims (medical and pharmacy) for PsA‐related treatment (inclusive of biologics, tsDMARDs, azathioprine, methotrexate, leflunomide, cyclosporine, and sulfasalazine). To account for discounts (such as rebates, patient assistance programs, and commission to wholesalers) not consistently captured in claims, Institute for Clinical and Economic Review (ICER) discount rates (based on net price divided by wholesale acquisition costs) were applied to IXE costs and other biologic and tsDMARDS patients may have switched to during follow‐up. Because there is no ICER report specific for PsA, the reference for PsO was used (22). ICER‐adjusted IXE costs, ICER‐adjusted all‐cause health care costs, and ICER‐adjusted PsA‐related health care costs were reported.
Patient characteristics
Patient demographic characteristics, including age, sex, payer, and index year, were measured at index. The Deyo‐Charlson comorbidity score was calculated using health care claims in the 12‐month baseline period and was used as a measure of overall health status (23). The number of patients with a diagnosis in the baseline period of select comorbid conditions (PsO, anxiety, cardiovascular disease, depression, diabetes, hypertension, hyperlipidemia, obesity, other autoimmune disorders, osteoarthritis, and sleep apnea) was calculated.
The following PsA‐related treatment parameters were assessed during baseline: 1) whether patients were biologic or tsDMARD naive (ie, no claims for a biologic or tsDMARD in the 12‐month baseline period) or experienced, 2) the number of unique biologics or tsDMARDs used in the baseline period, 3) the number of patients who used specific biologics or tsDMARDs during the baseline period; and 4) the number of patients who used systemic corticosteroids (oral or injectable) or conventional synthetic DMARDs (azathioprine, cyclosporine, leflunomide, methotrexate, and sulfasalazine) during the baseline period. Also captured was the number of patients who used systemic corticosteroids or conventional synthetic DMARDs concomitant with IXE.
RESULTS
Patient characteristics
On the basis of the patient selection criteria, the study included 496 patients with PsA newly initiating IXE (Figure 1). Patients had a mean (SD) age of 51.1 (9.8) years and were split evenly by sex (Table 1). The majority were covered by commercial insurance (94.0%), and 78.8% indexed in 2017 or 2018. The mean (SD) preperiod Deyo‐Charlson comorbidity score was 0.7 (1.4), and 93.4% of patients had comorbid PsO. Among the other conditions examined, the most common comorbidities were hypertension, hyperlipidemia, obesity, and osteoarthritis (46.6%, 35.9%, 31.7%, and 23.6%, respectively).
Figure 1.

Patient selection. PsA, psoriatic arthritis; tsDMARD, targeted synthetic disease‐modifying antirheumatic drug. *Ixekizumab, certolizumab pegol, secukinumab, etanercept, adalimumab, abatacept (subcutaneous and intravenous), infliximab, golimumab (subcutaneous and intravenous), ustekinumab (subcutaneous), apremilast, and tofacitinib. †International Classification of Diseases, Ninth Revision, diagnosis code 696.1x or International Classification of Diseases, Tenth Revision, diagnosis codes L40.50 to L40.59.
Table 1.
Patient characteristics and PsA‐related treatment during the 12 months prior to initiating IXE
| IXE (N = 496) | |
|---|---|
| Age, mean (SD), yr | 51.1 (9.8) |
| Male sex, n (%) | 246 (49.6) |
| Payer commercial, n (%) | 466 (94.0) |
| Deyo‐Charlson comorbidity score, mean (SD) | 0.7 (1.4) |
| Comorbid conditions, n (%) | |
| Psoriasis | 463 (93.4) |
| Hypertension | 231 (46.6%) |
| Hyperlipidemia | 178 (35.9) |
| Obesity | 157 (31.7) |
| Osteoarthritis | 117 (23.6) |
| Diabetes | 102 (20.6) |
| Sleep apnea | 95 (19.2) |
| Other autoimmune disorders | 28 (5.7) |
| Depression | 77 (15.5) |
| Anxiety | 76 (15.3) |
| Cardiovascular diseases | 48 (9.7) |
| Uveitis | 6 (1.2) |
| Ulcerative colitis | 4 (0.8) |
| Crohn’s disease | 3 (0.6) |
| Biologic‐ or tsDMARD‐experienced, a n (%) | 456 (91.9) |
| Number of different biologic or tsDMARDs used, mean (SD) | 1.4 (0.6) |
| Type of biologic or tsDMARDs used in the 12 months prior to initiating IXE, n (%) | |
| Secukinumab | 173 (34.9) |
| Adalimumab | 143 (28.8) |
| Ustekinumab | 106 (21.4) |
| Apremilast | 95 (19.2) |
| Etanercept | 58 (11.7) |
| Certolizumab | 22 (4.4) |
| Subcutaneous golimumab | 13 (2.6) |
| Infliximab | 11 (2.2) |
| Subcutaneous abatacept | 7 (1.4) |
| Intravenous abatacept | 5 (1.0) |
| Tofacitinib | 5 (1.0) |
| Intravenous golimumab | 1 (0.2) |
| Other PsA treatments used in the 12 months prior to initiating IXE, n (%) | |
| Corticosteroids (oral or injectable) | 293 (59.1) |
| Methotrexate | 117 (23.6) |
| Leflunomide | 23 (4.6) |
| Sulfasalazine | 20 (4.0) |
| Cyclosporine | 11 (2.2) |
| Azathioprine | 2 (0.4) |
Abbreviations: IXE, ixekizumab; PsA, psoriatic arthritis; tsDMARD, targeted synthetic disease‐modifying antirheumatic drug.
Certolizumab, secukinumab, etanercept, adalimumab, abatacept, infliximab, golimumab, ustekinumab, apremilast, or tofacitinib.
Prior to initiating IXE, 91.9% of patients had recent experience with a different biologic or tsDMARD, and the mean (SD) number of different biologics or tsDMARDs used by experienced patients was 1.4 (0.6) (Table 1). The most common biologics or tsDMARDs used during the baseline period were secukinumab (34.9%), adalimumab (28.8%), and ustekinumab (21.4%). Prior to the initiation of IXE, 59.1% of patients were treated with corticosteroids, and 23.6% were treated with methotrexate.
Ixekizumab treatment patterns
Using a 60‐day allowable gap, 52.8% (n = 262) of patients remained persistent on IXE for the 12‐month follow‐up period (Table 2). The mean (SD) duration of persistence using a 60‐day gap was 8.7 (4.1) months (Figure 2). The sensitivity analyses with 45‐ and 90‐day allowable gaps resulted in 47.0% and 61.3%, respectively, of patients remaining persistent on IXE.
Table 2.
Treatment patterns over 12 months of follow‐up
| Ixekizumab (N = 496) | |
|---|---|
| Persistence (60‐day gap) | |
| Patients persistent on therapy, n (%) | 262 (52.8) |
| Days persistent on therapy, mean (SD) | 262 (123) |
| Discontinuation | |
| Number of patients who discontinue treatment, n (%) | 192 (38.7) |
| Days from index to discontinuation, mean (SD) | 132 (73) |
| Restarting | |
| Proportion of restarters among all patients, n (%) | 23 (4.6) |
| Proportion of restarters among those with discontinuation, n (%) | 23 (12.0) |
| Days from discontinuation to restart, mean (SD) | 146 (57) |
| Switching | |
| Proportion of switchers among all patients, n (%) | 102 (20.6) |
| Proportion of switchers among those with discontinuation, n (%) | 102 (53.1) |
| Days to first switch, mean (SD) | 161 (85) |
| Adherence | |
| PDC entire 12‐month follow‐up, mean (SD) | 0.63 (0.28) |
| PDC ≥80%, n (%) | 190 (38.3) |
| PDC prior to discontinuation, mean (SD) | 0.85 (0.12) |
| PDC ≥80%, n (%) | 350 (70.6) |
| MPR entire 12‐month follow‐up, mean (SD) | 0.66 (0.29) |
| MPR ≥80%, n (%) | 216 (43.6) |
| MPR prior to discontinuation, mean (SD) | 0.88 (0.12) |
| MPR ≥80%, n (%) | 375 (75.6) |
| Concomitant medication use, n (%) | |
| Corticosteroids (oral and injectable) | 260 (52.4) |
| Methotrexate | 94 (19.0) |
| Leflunomide | 20 (4.0) |
| Sulfasalazine | 14 (2.8) |
| Cyclosporine | 7 (1.4) |
| Azathioprine | 2 (0.4) |
Abbreviations: MPR, medication possession ratio; PDC, proportion of days covered.
Figure 2.
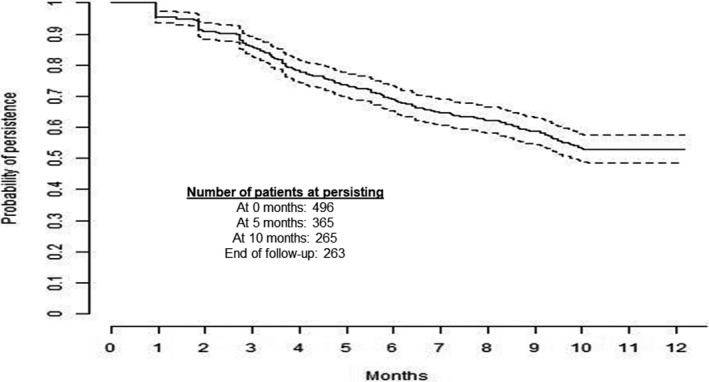
Kaplan‐Meier curve persistence over 12 months of follow‐up using 60‐day gap.
Over 12 months of follow‐up, 192 (38.7%) patients discontinued IXE. The mean (SD) time from IXE initiation to discontinuation was 4.4 (2.4) months. Of all 496 patients, 4.6% restarted IXE and 20.6% switched to a new biologic or a tsDMARD (Table 2). Among those that discontinued (n = 192), 102 (53.1%) switched to a new biologic or tsDMARD, 23 (12.0%) restarted IXE, and 67 (34.9%) had no further biologic or tsDMARD therapy within the 12 months (Table 2). For switchers, the mean (SD) time from discontinuation to switch was 5.4 (2.8) months, whereas, for restarters, the mean (SD) time from discontinuation to restart was 4.9 (1.9) months. The most common drugs switched to were secukinumab (n = 22; 21.6%), apremilast (n = 17; 16.7%), ustekinumab (n = 16; 15.7%), and tofacitinib (n = 16; 15.7%).
Adherence to IXE measured prior to discontinuation was high, with a mean (SD) PDC of 0.85 (0.12) (Table 2). Overall, 70.6% of patients were identified as highly adherent (ie, PDC > 0.80) to therapy prior to discontinuation. The mean (SD) PDC over the full 12 months of follow‐up was 0.63 (0.28), and the percentage of patients with PDC of 80% or more was 38.3%. These trends were supported by the MPR analysis.
IXE dosing
There were 434 patients with PsA initiating IXE who were eligible for the dosing analysis (n = 404 with comorbid PsO and n = 30 without comorbid PsO). For patients with PsA and comorbid PsO (defined by at least one nondiagnostic claim with an diagnosis code for PsO during the 12 month preindex period; patients were not required to have active disease), dose was reported biweekly during a starting period of 12 weeks and then monthly during maintenance starting at Week 14 and continuing until discontinuation or the end of the 12‐month follow‐up period. These time periods were used to compare the calculated dose in claims with the recommended dose based on United States Food and Drug Administration (FDA)–approved prescribing information (defined as 160 mg at Week 0 and 80 mg at Weeks 2, 4, 6, 8, 10, and 12 [ie, total or 640 mg/14 weeks = 91 mg biweekly during induction] followed by 80 mg every 4 weeks thereafter) for patients with moderate to severe PsO. For patients without comorbid PsO, the starting dose was reported separately and then dose was reported monthly until discontinuation or the end of the 12‐month follow‐up period. This time period was used to compare the calculated dose in claims with the recommended dose based on FDA‐approved prescribing information (defined as a starting dose of 160 mg at Week 0, followed by 80 mg every 4 weeks after) for patients with active PsA. Dose values during the IXE starting and maintenance periods were consistent with prescribing information for patients with and without comorbid PsO (Figure 3).
Figure 3.

IXE dose* observed in real‐world setting versus recommended (based on prescribing information for United States Food and Drug Administration [FDA]–approved dose). For patients with comorbid psoriasis (PsO), the dose was measured during an induction period of 12 weeks and then during maintenance starting at Week 14 and continuing until discontinuation or the end of the 12‐month follow‐up period. The recommended dose based on prescribing information for the FDA‐approved dose was defined as 160 mg at Week 0 followed by 80 mg at Weeks 2,4,5,8,10, and 12 (equivalent to a dose of 91 mg biweekly over the entire induction period).
Health care costs
Of the 496 eligible patients, the IXE monthly drug costs were $5233 (SD = $2497). After applying ICER discounts, the adjusted mean monthly IXE drug costs were $2930 (SD = $1399). IXE costs accounted for 85.6% of all PsA‐related health care costs and 68.5% of total all‐cause health care costs, although only 3.5% of IXE costs were patient out‐of‐pocket expenses (96.5% were health plan paid). In total, the all‐cause and PsA‐related health care costs measured PPPM over a 12 month period after applying ICER discounts were $5129 (SD = $5052) and $3604 (SD = $1486), which were reduced by $2509 (from the value prior to ICER adjustment) (Table 3).
Table 3.
Health care costs measured PPPM during the 12 months of follow‐up
| Ixekizumab (N = 496) | |
|---|---|
| All‐cause health care costs, mean (SD) | |
| Total (unadjusted) | $7638 ($5370) |
| Inpatient admissions | $396 ($4271) |
| Outpatient services | $811 ($1570) |
| All outpatient pharmacy | $6431 ($2498) |
| Total (ICER a adjusted) | $5129 ($5052) |
| PsA‐related health care costs, mean (SD) | |
| Total (unadjusted) | $6113 ($2431) |
| Inpatient admissions | $0 ($0) |
| Outpatient services | $151 ($524) |
| All outpatient pharmacy | $5962 ($2394) |
| Total (ICER a adjusted) | $3604 ($1486) |
| Ixekizumab costs, mean (SD) | |
| Total (unadjusted) | $5233 ($2497) |
| Out of pocket | $182 ($319) |
| Plan paid | $5050 ($2472) |
| Total (ICER a adjusted) | $2930 ($1399) |
Abbreviations: ICER, Institute for Clinical and Economic Review; PsA, psoriatic arthritis.
ICER discount factors are as follows: adalimumab discount factor = 31%, apremilast discount factor = 22%, certolizumab discount factor = 36%, etanercept discount factor = 31%, infliximab discount factor = 22%, ixekizumab discount factor = 44%, secukinumab discount factor = 38%, and ustekinumab discount factor = 27%. Drugs approved for PsA but not psoriasis (abatacept, golimumab, and tofacitinib) had no available discount factor and could not be adjusted.
DISCUSSION
The current analysis is among the first to examine real‐world treatment patterns and health care costs of patients with PsA initiating IXE. Of 496 patients with PsA initiating IXE, 93.4% had comorbid PsO and 91.9% were biologic or tsDMARD experienced. On average, patients remained persistent on therapy for 8.7 months, and 38.7% discontinued IXE within 12 months of initiation. Prior studies have shown that bioexperienced patients are associated with higher discontinuation; the observed discontinuation rate was relatively low despite the cohort being predominantly bioexperienced (25). Of those who discontinued, 53.1% moved on to a new biologic or tsDMARD, whereas, 12.0% restarted IXE after a 90‐day or longer treatment gap. The remaining 34.9% did not have any additional biologic or tsDMARD therapy by the end of the study follow‐up period. The mean adherence (PDC) during the 12 months of follow‐up was 0.63, and 38.3% were highly adherent (PDC ≥ 80%) to IXE for the full 12 months. Average adherence (PDC) was much higher when measured prior to discontinuation (mean, 0.85). Dosing for both patients with and without comorbid PsO was consistent with dosing per the prescribing recommendations for IXE (11), which is important, as prior literature suggests dose increases are associated with higher costs among TNFis (24).
Understanding health care costs of patients with PsA initiating IXE is important when evaluating how this treatment fits with other options. In this study, mean IXE costs were $5233 per month for patients with PsA. In claims‐based analyses, drug discount programs are not reliably captured. To account for this, we applied discount estimates published in the 2018 ICER report (22). Mean monthly IXE costs after adjustment were $2930. The total all‐cause and PsA‐related health care costs after adjusting for ICER discounts were reduced by 32.8% and 41.0%, respectively. A prior study of biologic‐naive patients with PsA initiating other biologics (certolizumab, etanercept, golimumab, infliximab, secukinumab, or ustekinumab) reported health care costs over a 12‐month period to be $54 443 ($4536 per month) in 2016 dollars (17). Biologic pharmacy costs represented 71.9% of the all‐cause total costs. In the current study, IXE comprised 68.5% of the mean monthly total all‐cause health care costs ($7638). It is worth noting that comparison of the current study results with those of the previous study may be limited because the current study comprised mostly of biologic‐ or tsDMARD‐experienced patients. A previous study of mostly biologic‐experienced patients with PsA using adalimumab, etanercept, or golimumab between 2011 and 2015 examined costs among patients with different levels of treatment dose (little to no above‐label dose and above‐label dose for ≥30 days) (25). Among those with little to no above‐label dose use, costs for biologics ranged from $1901 to $2152 per month depending on the biologic used (in 2014 dollars) and comprised 69.4% to 74.5% of total health care costs during a 12‐month period. Although raw numbers are not comparable because of inflation (from 2014 or 2016 to 2019) the proportion of total costs for IXE is consistent with those of other biologics in prior published literature (17). Management of PsA with comorbid PsO is often multidisciplinary, with both dermatologists and rheumatologists involved in the most effective approach to treat both skin and musculoskeletal involvement (26). Prior studies have found low persistence and adherence among patients with PsA who are using biologics (15, 16, 17, 27, 28). In one retrospective study of treatment patterns among patients with PsA using adalimumab, certolizumab, etanercept, golimumab, or secukinumab between January 15, 2016, and July 31, 2017, 70.7% were biologic naive and 29.3% were found to be biologic experienced (15). The percentage of discontinuations (90‐day gap) in the biologic‐experienced cohort ranged from 38.7% to 50.0%, and the mean duration of ranged from 8.0 to 9.4 months depending on the biologic used. The mean PDC ranged from 0.54 to 0.66, and the proportion of patients highly adherent (PDC ≥0.80) to their index biologic was 39.0% in the biologic‐experienced cohort. Results from this and other prior studies of other biologic therapies are consistent with the current analysis of IXE over 1 year of follow‐up (38.7% discontinued, 52.8% persistent, duration of persistence = 8.7 months, PDC = 0.63, and 38.3% with high adherence).
Because of its recent approval date for PsA, there is limited prior evidence of treatment patterns of patients receiving IXE. In the clinical trial setting, 63.3% of biologic‐naive patients with PsA achieved and maintained at least a 20% improvement from baseline in PsA symptoms as measured by the American College of Rheumatology index for 1 year following IXE initiation, and 49.5% achieved and maintained at least a 50% improvement in symptoms (29). In addition, patients with PsA and with prior TNFi treatment experienced improved signs and symptoms of disease following treatment with IXE compared with placebo (30). In the real‐world setting, a study of patients with PsO found that patients treated with IXE had longer persistence, had higher adherence, and were less likely to discontinue treatment when compared with patients treated with adalimumab (19). The current study provides some preliminary insight into treatment patterns using real‐world data on patients with PsA receiving ixekizumab. Results show similar patterns of discontinuation, persistence, and adherence measures with other treatment options. Future research is needed to compare IXE with other currently available treatments.
The primary limitation is that, although the majority of patients indexed in 2017 or later, this study included a subset of patients who initiated therapy in 2016, which was prior to the FDA approval of IXE for the treatment of PsA. These patients were likely prescribed IXE by a dermatologist with the intention to treat comorbid PsO. Because comorbid PsO is not a requirement to use IXE, these data may differ from actual clinical practice and could be associated with higher costs of IXE in a PsA population because of the long starting dose period recommended for PsO treatment (vs PsA only). Additionally, the data set was not filtered by prescribing physician (dermatologists or rheumatologists), so we do not know how treatment patterns may have varied by specialty. A second point of consideration is, given that this the patient cohort is likely enriched with patients with more recalcitrant disease and longer disease duration, the results may not be generalizable to all patient populations. Prior analysis has shown that newly approved drugs are subject to channeling bias, as patients with uncontrolled disease and those who experienced intolerable side effects from prior medications are more likely to try a newly approved medication (31).
This study is also subject to the limitations common to all claims‐based studies, including that data collected for administrative purposes may not be validated with the same rigor as data collected for research purposes; therefore, these data sets are subject to miscoding, undercoding, and coding limitations, which may introduce bias or measurement error. In addition, the study was limited to only those individuals with commercial health coverage or private Medicare supplemental coverage. Consequently, the results of this analysis may not be generalizable to patients with PsA who are without health insurance coverage. And finally, treatment pattern analyses were built on the assumption that patients took medications as prescribed.
This study was among the first to examine treatment patterns and health care costs in PsA patients initiating IXE in the real‐world. Persistence and adherence to IXE as well as discontinuation and switch rates are consistent with results in prior literature with patients with PsA receiving other biologics and tsDMARDs. Dosing on IXE measured using real‐world data was consistent with prescribing recommendations. IXE costs comprised the majority of total health care costs, which is consistent with prior literature for other biologics. Patient out‐of‐pocket costs are only 3.5% of the total IXE costs. Further research on a biologic‐ or tsDMARD‐naive population and patients without comorbid PsO is needed.
ACKNOWLEDGEMENTS
Medical writing services were provided by Jessamine Winer‐Jones of IBM Watson Health. Programming services were provided by Caroline Henriques of IBM Watson Health. These services were paid for by Eli Lilly and Company.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. The corresponding author (Nicole Princic) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Mwangi J. Murage, William Malatestinic, Baojin Zhu, Bilal Atiya, Scott A. Kern, Keri B. Stenger, Aubrey Trevelin Sprabery, Alexis Ogdie.
Acquisition of data
Nicole Princic and Julie Park.
Analysis and interpretation of data
Mwangi J. Murage, William Malatestinic, Baojin Zhu, Bilal Atiya, Scott A. Kern, Keri B. Stenger, Aubrey Trevelin Sprabery, Alexis Ogdie, Nicole Princic and Julie Park.
ROLE OF THE STUDY SPONSOR
Eli Lilly and Company funded this project and employees Mwangi J. Murage, William Malatestinic, Baojin Zhu, Bilal Atiya, Scott A. Kern, Keri B. Stenger, Aubrey Trevelin Sprabery are authors on this paper. All Eli Lilly authors had a role in the study design and interpretation of the data analysis. Eli Lilly authors in collaboration with Nicole Princic, Julie Park, and Alexis Ogdie made a decision to submit the manuscript for publication. Publication of this article was contingent upon approval by Eli Lilly and Company authors.
This study was funded by Eli Lilly and Company.
Drs. Murage, Malatestinic, Zhu, Atiya, Stenger, and Sprabery and Mr. Kern are employees and stockowners at Eli Lilly and Company. Ms. Princic and Ms. Park are employed by IBM Watson Health, which received funding from Eli Lilly and Company to conduct this study. Dr. Ogdie has received consulting fees from Amgen, AbbVie, Bristol‐Myers Squibb, Celgene, Corrona, Janssen, Lilly, Novartis, and Pfizer. Dr. Ogdie has also received grant support from Pfizer, Novartis, and Amgen. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Belasco J, Wei N. Psoriatic arthritis: what is happening at the joint? [Original Article]. Rheumatol Ther 2019;6:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64 Suppl 2:14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 5. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 6. Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T 2010;35:680–9. [PMC free article] [PubMed] [Google Scholar]
- 7. Haddad A, Zisman D. Comorbidities in patients with psoriatic arthritis. Rambam Maimonides Med J 2017;8:e0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaine J, Song X, Kim G, Hur P, Palmer JB. Higher incidence rates of comorbidities in patients with psoriatic arthritis compared with the general population using U.S. administrative claims data. J Manag Care Spec Pharm 2019;25:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the treatment of psoriatic arthritis. Arthritis Care Res 2019;71:2–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology (Oxford) 2020;59:i37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taltz [package insert]. Indianapolis (IN): Eli Lilly and Company. 2020. [Google Scholar]
- 12. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin‐17A specific monoclonal antibody, for the treatment of biologic‐naive patients with active psoriatic arthritis: results from the 24‐week randomised, double‐blind, placebo‐controlled and active (adalimumab)‐controlled period of the phase III trial SPIRIT‐P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang HF, Gauthier G, Hiscock R, Curtis JR. Treatment patterns in psoriatic arthritis patients newly initiated on oral nonbiologic or biologic disease‐modifying antirheumatic drugs. Arthritis Res Ther 2014;16:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doshi JA, Takeshita J, Pinto L, Li P, Yu X, Rao P, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol 2016;74:1057–65.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oelke KR, Chambenoit O, Majjhoo AQ, Gray S, Higgins K, Hur P. Persistence and adherence of biologics in US patients with psoriatic arthritis: analyses from a claims database. J Comp Eff Res 2019;8:607–21. [DOI] [PubMed] [Google Scholar]
- 16. Walsh JA, Adejoro O, Chastek B, Palmer JB, Hur P. Treatment patterns among patients with psoriatic arthritis treated with a biologic in the United States: descriptive analyses from an administrative claims database. J Manag Care Spec Pharm 2018;24:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feldman SR, Pelletier CL, Wilson KL, Mehta RK, Brouillette MA, Smith D, et al. Treatment patterns and costs among biologic‐naive patients initiating apremilast or biologics for psoriatic arthritis. J Comp Eff Res 2019;8:699–709. [DOI] [PubMed] [Google Scholar]
- 18. Brezinski EA, Armstrong AW. Off‐label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PloS One 2012;7:e33486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blauvelt A, Shi N, Burge R, Malatestinic WN, Lin CY, Lew CR, et al. Comparison of real‐world treatment patterns among psoriasis patients treated with ixekizumab or adalimumab. Patient Prefer Adherence 2020;14:517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palmer JB, Li Y, Herrera V, Liao M, Tran M, Ozturk ZE. Treatment patterns and costs for anti‐TNFα biologic therapy in patients with psoriatic arthritis. BMC Musculoskeletal Disord 2016;17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Bureau of Labor Statistics . Consumer price index: measuring price change in the cpi: medical care. 2020. URL: https://www.bls.gov/cpi/factsheets/medical‐care.htm#A1.
- 22. Institute for Clinical and Economic Review . Targeted immunomodulators for the treatment of moderate‐to‐severe plaque psoriasis: condition update. Final Evidence Report. 2018. URL: https://icer‐revieworg/wp‐content/uploads/2017/11/ICER_Psoriasis_Update_Final_Evidence_Report_080118pdf.
- 23. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 24. Ollendorf DA, Klingman D, Hazard E, Ray S. Differences in annual medication costs and rates of dosage increase between tumor necrosis factor‐antagonist therapies for rheumatoid arthritis in a managed care population. Clin Ther 2009;31:825–35. [DOI] [PubMed] [Google Scholar]
- 25. Schwartzman S, Li Y, Zhou H, Palmer JB. Economic impact of biologic utilization patterns in patients with psoriatic arthritis. Clin Rheumatol 2017;36:1579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cunha JS, Qureshi AA, Reginato AM. Management of psoriasis and psoriatic arthritis in a multidisciplinary rheumatology/dermatology clinic. Fed Pract 2015;32:14S–20S. [PMC free article] [PubMed] [Google Scholar]
- 27. Murage MJ, Tongbram V, Feldman SR, Malatestinic WN, Larmore CJ, Muram TM, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 2018;12:1483–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. George MD, Baker JF, Ogdie A. Comparative persistence of methotrexate and tumor necrosis factor inhibitors in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2020;47:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van der Heijde D, Gladman DD, Kishimoto M, Okada M, Rathmann SS, Moriarty SR, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52‐week results from a phase iii study (SPIRIT‐P1). J Rheumatol 2018;45:367–77. [DOI] [PubMed] [Google Scholar]
- 30. Genovese MC, Combe B, Kremer JM, Tsai TF, Behrens F, Adams DH, et al. Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: week 52 results from SPIRIT‐P2. Rheumatology (Oxford) 2018;57:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther 2011;90:777–90. [DOI] [PubMed] [Google Scholar]


