Abstract
In recent decades, hemophilia A treatment has been focused on body weight, without taking pharmacokinetic parameters into account. Previous research has shown that the individual pharmacokinetic response is more effective in predicting the required dose of clotting factor. We want to evaluate the impact on reducing the frequency of bleeding in patients treated with recombinant factor VIII, based on a personalized comprehensive management program. Our aim was to compare the results of a standard comprehensive treatment program (stage I) vs. a personalized pharmacokinetic - based treatment program (stage II) in a cohort of 60 patients with severe hemophilia without inhibitors. The median age was 15.5 years (3-68). The annual bleeding rate (ABR) was 1.03 (62 episodes) in the first stage and 0.58 (35 episodes) in the second one, (p=0.004). By type of bleeding, the impact of the intervention differs significantly in spontaneous bleeding (p=0.007) and a 73% reduction in the first stage. There were no significant differences in traumatic bleeding. The use of pharmacokinetics (PK) for personalized dosing of patients with severe hemophilia A, significantly reduces ABR and spontaneous bleeding, improving the patient’s quality of life and costs for the health system.
Key words: Hemophilia A, Bleeding, Individualized treatment, Recombinant factor VIII, ABR (annual bleeding rate)
Introduction
Severe hemophilia A (FVIII: <1%), exhibits a phenotype of spontaneous bleeding (bleeding for no known reason) and is characterized by recurrent bleeding that affect joints and muscles at an early age, causing severe and progressive musculoskeletal damage, with loss of mobility, pain and inflammation.1-5 10% to 15% of these patients express a milder disease phenotype, with a significantly lower frequency of spontaneous bleeding.2,6
Thus, it is a priority to reduce the frequency of bleeding, since they can cause arthropathy, disability, and a decrease in quality of life.7-10 Until recently, the scheme for prophylaxis has been focused on weight and desired increase in FVIII.7 This dose calculation does not take into account individual pharmacokinetic parameters,7 which can lead to overdosing or underdosing.9,11
The personalized comprehensive management program consists of a patient-specific prophylaxis regimen based on comprehensive care and pharmacokinetics (PK) of FVIII.5,11-14 The pharmacokinetic response is more effective in predicting the required levels of clotting factor,1,12,14,15 given the high rate of variability determined by age, weight, and bleeding pattern, among others.13-16 Comprehensive care consists of a multidisciplinary team that promotes physical and psychosocial health, in order to improve quality of life.5,17,18 Our aim was to compare the results of a standard comprehensive treatment program (stage I) vs a personalized pharmacokinetic-based treatment program (stage II) in a cohort of 60 patients with severe hemophilia.
Materials and Methods
We carried out a descriptive, longitudinal study of a single cohort of 60 patients in a health care institution in Bogotà, Colombia, between 2017 and 2019. The information was collected through medical records. Patients were eligible for the study if they fulfilled the inclusion criteria: diagnosis of severe hemophilia A (FVIII: <1%) without inhibitors, treatment with recombinant factor VIII for at least 24 months, with PK (chromogenic) measures at 3, 24, 48 hours after administration (the same brand of drug was kept for at least 12 months), the data of PK were calculated from the WAPPS-Hemo computer application,19 availability of complete medical records to analyze demographics and clinical evolution (bleeding) of patients during the study time and so study sample size was met.
The variables collected included: state, age, weight (kg), treatment posology (dose expressed in IU - International Units/kg) and frequency of administration, periodicity, pharmacokinetics at 3, 24 and 48 hours, total number of bleeding episodes/patient/ year, number of episodes of spontaneous bleeding/patient/year, number of traumatic bleeding episodes/patient/ year and site of bleeding episodes. We carried out a descriptive analysis of the sociodemographic and clinical variables. The qualitative ones were described from absolute frequencies and percentages, and the quantitative ones, through summary measures. Bivariate analysis was performed using the McNemar non-parametric test in order to establish significant differences between bleeding events for the two stages evaluated first stage: 12 months before pharmacokinetics and second stage: 12 months post pharmacokinetics. Statistical significance was defined with a p value less than 0.05. For the analysis of these data, we use the statistical software SPSS version 26, license of Integral Solutions Research. The selected patients were all those who met the inclusion criteria
Given that the medical records are used, the following biases were considered: information that was controlled by corroborating the data found in the medical record against the reports delivered to the CAC (national database for high-cost diseases). Selection bias where the reference population was properly established with the inclusion criteria, ensuring that the data of the variables are valid and there was a sufficient sample since all the records of the period of interest were analyzed.
The study is classified without risk, according to resolution 8430 of 1993, since retrospective data were used and no intentional modification was generated. The data was delivered anonymously by the IPS Integral Solutions SD to Integral Solutions Research, and at all times the confidentiality of patient data was guaranteed and compliance with good clinical practice standards was ensured.
Results
A total of 60 male patients, diagnosed with severe hemophilia A (FVIII: <1%), aged between 3 and 68 years, with a median of 15.5 years were included. All of the patients received recombinant factor (Table 1). 61.7% of the participants correspond to patients subsidized by the government (vulnerable population).
Eighty percent of the population was distributed nationally in seven cities. Seventy-five percent of the patients had a weight greater than 60 kg for both stages. The most frequent joint bleeding occurred in the knee, with more than 30% for both stages, followed by the ankle (24.4% and 20.9%) and the elbow (11.1% and 9.3%,) respectively (Table 1).
The 50% of patients required 27.5 IU in the first stage vs 25.6 IU in the second, the most frequent administration was 3 times / week (73.3%) vs (71.7%) respectively. The half-life of FVIII was 7.5 hours. At 3 hours, the median FVIII was 34.0 IU, at 12 hours 6.5 IU and 1.7 IU at 48 hours. Some quantitative variables did not have a normal distribution (age and FVIII at 3, 24, 48 hours), which is to be expected (Table 2).
Twenty-nine patients had bleeding events in the first stage and 25 in the second one (p=0.321). For the first stage, 31 patients (51.7%) had no bleeding episodes and the remaining 29 patients (48.3%) had between 1-5 bleeding episodes. In the second stage, 35 patients (58.3%) had no bleeding episodes, while 25 patients (41.7%) had between 1 and 3 bleeding episodes (Figure 1). The total ABR (annual bleeding rate) was 1.03 (62 events) in the first stage and 0.58 (35 events) in the second one, with a reduction in the ABR after the use of PK (p=0.004).
Table 1.
Description of the population (qualitative variables).
| Variable | Category | N | % | |
|---|---|---|---|---|
| State | Bogotà | 12 | 20 | |
| Cartagena | 9 | 15 | ||
| Tolima | 7 | 11,7 | ||
| Bucaramanga | 6 | 10 | ||
| Pasto | 5 | 8.3 | ||
| Tunja | 5 | 8.3 | ||
| Cali | 4 | 6,7 | ||
| Neiva | 3 | 5 | ||
| Armenia | 2 | 3,3 | ||
| Manizales | 2 | 3,3 | ||
| Florencia | 2 | 3,3 | ||
| Popayàn | 2 | 3,3 | ||
| Pereira | 1 | 1,7 | ||
| Affiliation | Subsidized | 37 | 61,7 | |
| Contributory | 23 | 38,3 | ||
| Products | Advate | 22 | 36,7 | |
| Xyntha | 18 | 30 | ||
| Nuwiq | 12 | 20 | ||
| Kogenate | 8 | 13,3 | ||
| First period | Second period | |||
| N | % | N | % | |
| Bleeding site | ||||
| Knee | 14 | 31,10% | 14 | 32,50% |
| Ankle | 11 | 24,40% | 9 | 20,90% |
| Elbow | 5 | 11,10% | 4 | 9,30% |
| Shoulder | 3 | 6,70% | 3 | 7,00% |
| Arms | 2 | 4,40% | 3 | 7,00% |
| Wrist | 2 | 4,40% | 2 | 4,70% |
| Foot | 1 | 2,20% | 2 | 4,70% |
| Leg thigh | 3 | 6,70% | 5 | 11,60% |
| Other | 4 | 8,90% | 1 | 2,30% |
| Periodicity | ||||
| Once a week | 1 | 1,7 | 1 | 1,7 |
| Two times per week | 9 | 15 | 8 | 13,3 |
| Three times per week | 44 | 73,3 | 43 | 71,7 |
| Fout times per week | 6 | 10 | 8 | 13,3 |
Table 2.
Description of the population (quantitative variables).
| Variables | First period | PK | Second period | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Weight | Prescribed | FVIII | FVIII | FVIII | FVIII | Age | Weight | Prescribed | |
| (Years) | (Kg) | Units UI | Half life | 3 hours | 24 hours | 48 hours | (Years) | (Kg) | Units UI | |
| Q1 | 10,2 | 30,0 | 23,5 | 6,0 | 21,8 | 4,5 | 0,8 | 11,2 | 35,2 | 21,8 |
| Median | 15,5 | 49,0 | 27,5 | 7,0 | 34,0 | 6,5 | 1,7 | 17,0 | 53,0 | 25,6 |
| Q3 | 27,0 | 61,7 | 30,4 | 10,0 | 44,1 | 12,4 | 3,6 | 28,0 | 64,7 | 29,9 |
| Media | 19,6 | 47,9 | 26,9 | 7,5 | 35,8 | 8,7 | 0,2 | 20,6 | 51,7 | 25,7 |
| Min | 3 | 14,8 | 15,3 | 2,0 | 8,4 | 0,5 | 14,2 | 4 | 17,0 | 12,5 |
| Max | 67 | 104,0 | 44,2 | 15,0 | 89,9 | 35,0 | 2,7 | 68 | 100,0 | 43,3 |
| p* | 0,00 | 0,29 | 0,37 | 0,48 | 0,00 | 0,00 | 0,00 | 0,00 | 0,38 | 0,23 |
Traumatic bleeding was the most frequent among patients who had hemorrhages episodes (Table 3).
Regarding the type of events, during the first stage, traumatic bleeding occurred in 23 patients (38.3%) and spontaneous bleeding in 15 patients (25.0%). In the second stage, a decrease in spontaneous bleeding was observed in four patients (6.7%) (Table 4). With a reduction in spontaneous bleeding of 73.3% and an increase of 4.1% for traumatic bleeding (Figure 2).
In the bivariate analysis, it was observed that 19 patients had bleeding during the two years, 10 had bleeding events in the first stage but not in the second, 6 did not have bleeding in the first stage, but had them in the second, and 25 who did not bleed in the first stage did not in the second one. There was a decrease of four patients between the first and the second stage. However, no statistical significance was found (p=0.454) (Table 5). When analyzing by type of bleeding, the impact of the intervention (use of pharmacokinetics) differs significantly in the reduction of spontaneous bleeding (p=0.007) (Table 5).
Traumatic bleeding did not show statistically significant differences after the use of PK within a personalized comprehensive management program (p=1.00) (Table 5).
Discussion
In this study, the most affected joints were knees, ankles and elbows, the same finding reported by Chris Barnes et al.,1 Srivastava A et al.,5, Bjorkman S et al.20 and Coppola A et al.21 A decrease in the ABR was observed after incorporating PK in the dose calculation (p=0.004), likewise a significant reduction was observed in spontaneous bleeding (p=0.007). These results are in agreement with the findings of Sun et al.22 in terms of the effectiveness and viability of such intervention. It is important to highlight that after the incorporation of pharmacokinetics within a personalized comprehensive management program, there was an increase in the proportion of patients who did not present any type of bleeding, going from 51.7% to 58.3%. The implementation of a personalized comprehensive management program, based on PK, reduces bleeding and improves the quality of life, which is consistent with the results of a retrospective observational study, carried out by Pasca et al.23 in which evaluated the replacement of standard therapy with a personalized approach based on ABR, PK and lifestyle in a group of children with severe hemophilia A. At the end of the study, the authors demonstrated that the use of PK improved treatment adherence and quality of life, reduced bleeding events and generated direct and indirect cost savings in prophylaxis with PK. Similar data was obtained by Lissitchkov T et al.24
Table 3.
Types of bleeding: standard comprehensive treatment program (first stage) vs personalized pharmacokinetic-based treatment program (second stage).
| Type of bleeding | First Stage | Second Stage | ||
|---|---|---|---|---|
| n | % | n | % | |
| No bleeding | 31 | 51,7 | 35 | 58,3 |
| Traumatic bleeding | 14 | 23,3 | 21 | 35,0 |
| Spontaneous and Traumatic | 9 | 15,0 | 3 | 5,0 |
| Spontaneous bleeding | 6 | 10,0 | 1 | 1,7 |
| Total | 60 | 100 | 60 | 100 |
Table 4.
Events by type of bleeding: standard comprehensive treatment program vs personalized pharmacokinetic-based treatment program.
| Bleeding in the first period | Bleeding in the second period | n | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Positive | 19 | 10 | 60 | 0,454 |
| Negative | 6 | 25 | 60 | 0,454 |
Table 5a.
Bivariate analysis of general bleeding: standard comprehensive treatment program vs personalized pharmacokinetic-based treatment program.
| Firts Stage | Second Stage | |||||||
|---|---|---|---|---|---|---|---|---|
| Traumatic Bleeding | Spontaneous Bleeding | Traumatic Bleeding | Spontaneous Bleeding | |||||
| n | % | n | % | n | % | n | % | |
| Positive | 23 | 38,3 | 15 | 25,0 | 24 | 40,0 | 4 | 6,7 |
| Negative | 37 | 61,7 | 45 | 75,0 | 36 | 60,0 | 56 | 93,3 |
Table 5b.
Bivariate analysis of traumatic bleeding and spontaneous: standard comprehensive treatment program vs personalized pharmacokinetic-based treatment program.
| Traumatic bleeding in the first period | Traumatic bleeding in the second period | n | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Positive | 14 | 9 | ||
| Negative | 10 | 27 | 60 | 1,000 |
| Spontaneous bleeding in the first period | Spontaneous bleeding in the second period | n | P value | |
| Yes | No | |||
| Positive | 2 | 13 | 60 | 0,007 |
| Negative | 2 | 43 | ||
Figure 1.
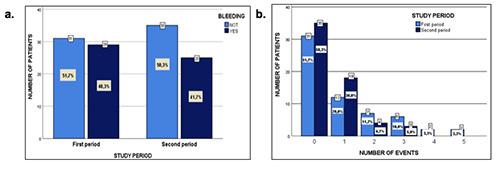
a. Comparative bleeding: Standard comprehensive treatment program vs personalized pharmacokinetic-based treatment program. b. Comparative bleeding events: Standard comprehensive treatment program vs personalized pharmacokinetic-based treatment program.
Figure 2.
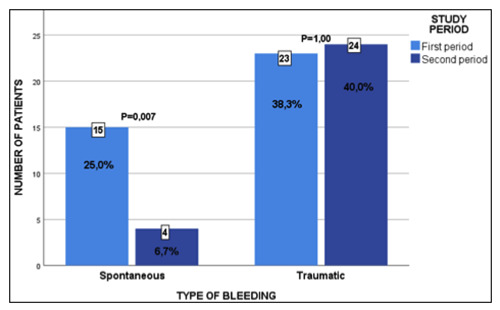
Changes in the type of bleeding: standard comprehensive treatment program vs personalized pharmacokinetic-based treatment program.
Regarding the formulated units, a dose decrease of 7% was observed in the second study stage, after the implementation of PK, (p=0.35), making it necessary to carry out future cost-effectiveness studies to evaluate this result. As demonstrated in other studies where individual PK are seen as a costeffectiveness. 25
Conclusions
The use of PK reduced the ABR, with a p value of 0.004 and significantly decreased the number of spontaneous bleeds (p=0.007), which suggests an improvement in the effectiveness of treatment and quality of life of patients, with cost savings for the health system. Although there was no significant difference in the dose, a factor VIII dose decrease of 7% was observed in the second stage, after the use of the personalized comprehensive management program based on PK. There was no significant difference in traumatic bleeding, which can be explained by the importance of self-care in the prevention of these events, being necessary to promote healthy lifestyle habits regarding the management of the disease.
Limitations
Probably one of the factors that intervened in the reduction of bleeding was the education that was made to the patients and their caregivers, by the interdisciplinary group that occurred after taking the PK, which could not be measured.
Acknowledgements
The authors would like to thank all the scholars who contributed with their knowledge to carry out this study.
Funding Statement
Funding: None.
References
- 1.Barnes C. Importance of pharmacokinetics in the management of hemophilia. Pediatr Blood Cancer 2013;60:S27-9. [DOI] [PubMed] [Google Scholar]
- 2.[Fondo colombiano de enfermedades de alto costo. Situaciòn de la hemofilia en Colombia, 2018.] Available from: https://www.cuentadealtocosto.org/site/images/Publicaciones/Situacion_Hemofilia_2018.pdf. [Google Scholar]
- 3.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor- VIII-deficient haemophiliacs. J Intern Med 1994;236:391-9. [DOI] [PubMed] [Google Scholar]
- 4.Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost 2014;12:1935-9. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava A, Brewer AK, Mauser- Bunschoten EP, et al. Treatment guidelines working group on behalf of The World Federation Of Hemophilia. Haemophilia 2013;19:e1-47.22776238 [Google Scholar]
- 6.Fischer K, Poonnoose P, Dunn A, et al. Choosing outcome assessment tools in haemophilia care and research: a multidisciplinary perspective. Haemophilia 2017;23:11-24. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson IM, Berntorp E, Lofquist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med 1992;232:25-32. [DOI] [PubMed] [Google Scholar]
- 8.Ahlberg A. Haemophilia in Sweden. VII. Incidence, treatment and prophylaxis of arthropathy and other musculoskeletal manifestations of haemophlia A and B. Acta Orthop Scand Suppl 1965;77:3-132. [DOI] [PubMed] [Google Scholar]
- 9.Valentino LA. Considerations in individualizing prophylaxis in patients with haemophilia A. Haemophilia 2014;20:607-15. [DOI] [PubMed] [Google Scholar]
- 10.Blanchette V, O´Mahony B, McJames L, Mahlangu J. Assessment of outcomes. Haemophilia 2014;20:114-20. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K, Berntorp E. Targeting factor replacement therapy in severe hemophilia: which level is important?. Semin Thromb Hemost 2015;41:860-63. [DOI] [PubMed] [Google Scholar]
- 12.Berntorp E. If you know you will also see: population pharmacokinetics is the way to personalize and optimize prophylaxis in hemophilia. J Thromb Haemost 2017;15:1103-05. [DOI] [PubMed] [Google Scholar]
- 13.[FMH (Federación Mundial de Hemofilia). ¿Qué es la profilaxis?, 2014.] Available from: http://www1.wfh.org/publications/files/pdf-1587.pdf. [Google Scholar]
- 14.Fernandes S, Carvalho M, Lopes M, Araujo F. Impact of an individualized prophylaxis approach on young adults with severe hemophilia. Semin Thromb Hemost 2014;40:785-89. [DOI] [PubMed] [Google Scholar]
- 15.Collins PW. Personalized prophylaxis. Haemophilia 2012;18,131–35. [DOI] [PubMed] [Google Scholar]
- 16.McEneny-King A, Lorio A, Foster G, Edginton AN. The use of pharmacokinetics in dose individualization of factor VIII in the treatment of hemophilia A. Expert Opinion on Drug Metabolism & Toxicology 2016;12:1313-21. [DOI] [PubMed] [Google Scholar]
- 17.Carcao M, Lorio A. Individualizing factor replacement therapy in severe hemophilia. Semin Thromb Hemost 2015;41:864-871. [DOI] [PubMed] [Google Scholar]
- 18.[Federación Mundial de Hemofilia (FMH). Guías para el tratamiento de la hemofilia, 2012.] Available from: http://www1.wfh.org/publications/files/pdf-1513.pdf. [Google Scholar]
- 19.Lorio A, Keepanasseril A, Foster G, et al. Development of a web-accessible population pharmacokinetic service – Hemophilia (WAPPS-Hemo): Study Protocol. JMIR Res Protoc 2016; 5:e.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjorkman S, Berntorp E. Pharmacokinetics of coagulation factors: clinical relevance for patients with haemophilia. Clin Pharmacokinet 2001;40:815-32. [DOI] [PubMed] [Google Scholar]
- 21.Coppola A, Tagliaferri A, Di Capua M, Franchini M. Prophylaxis in children with hemophilia: evidence-based achievements, old and new challenges. Semin Thromb Hemost 2012;38:79-94. [DOI] [PubMed] [Google Scholar]
- 22.Sun HL, McIntosh KA, Squire SJ, et al. Patient powered prophylaxis: a 12-month study of individualized prophylaxis in adults with severe haemophilia A. Haemophilia 2017;23:877-883. [DOI] [PubMed] [Google Scholar]
- 23.Pasca S, Milan M, Sarolo L, Zanon E. PK-driven prophylaxis versus standard prophylaxis: when a tailored treatment may be a real and achievable cost-saving approach in children with severe hemophilia A. Thromb Res 2017;157:58-63. [DOI] [PubMed] [Google Scholar]
- 24.Lissitchkov T, Rusen L, Georgiev P, et al. PK-guided personalized prophylaxis with Nuwiq (human-cl rhFVIII) in adults with severe haemophilia A. Haemophilia 2017;23:697-704. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson M, Berntorp E, Björkman S, Lindvall K. Pharmacokinetic dosing in prophylactic treatment of hemophilia A. Eur J Haematol 1993;51:247-252. [DOI] [PubMed] [Google Scholar]


