Key Points
Question
Which patients with patent foramen ovale (PFO)–associated stroke might benefit from PFO closure?
Findings
In this individual participant data meta-analysis that included 6 randomized clinical trials with 3740 patients with otherwise cryptogenic stroke and PFO, PFO closure plus medical therapy, compared with medical therapy alone, was associated with varying risk reduction for recurrent stroke among subgroups with different probabilities that the stroke was causally related to the PFO. For patients classified as unlikely (ie, with vascular risk factors and without high-risk PFO features), the hazard ratio (HR) was 1.14 but was not statistically significant; for those classified as possible or probable, the HRs were 0.38 and 0.10, respectively, both statistically significant.
Meaning
Application of a multivariable causal classification system to randomized trial results distinguished subgroups who may benefit from PFO closure from those unlikely to receive benefit.
Abstract
Importance
Patent foramen ovale (PFO)–associated strokes comprise approximately 10% of ischemic strokes in adults aged 18 to 60 years. While device closure decreases stroke recurrence risk overall, the best treatment for any individual is often unclear.
Objective
To evaluate heterogeneity of treatment effect of PFO closure on stroke recurrence based on previously developed scoring systems.
Design, Setting, and Participants
Investigators for the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) Consortium pooled individual patient data from all 6 randomized clinical trials that compared PFO closure plus medical therapy vs medical therapy alone in patients with PFO-associated stroke, and included a total of 3740 participants. The trials were conducted worldwide from 2000 to 2017.
Exposures
PFO closure plus medical therapy vs medical therapy alone. Subgroup analyses used the Risk of Paradoxical Embolism (RoPE) Score (a 10-point scoring system in which higher scores reflect younger age and the absence of vascular risk factors) and the PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System, which combines the RoPE Score with high-risk PFO features (either an atrial septal aneurysm or a large-sized shunt) to classify patients into 3 categories of causal relatedness: unlikely, possible, and probable.
Main Outcomes and Measures
Ischemic stroke.
Results
Over a median follow-up of 57 months (IQR, 24-64), 121 outcomes occurred in 3740 patients. The annualized incidence of stroke with medical therapy was 1.09% (95% CI, 0.88%-1.36%) and with device closure was 0.47% (95% CI, 0.35%-0.65%) (adjusted hazard ratio [HR], 0.41 [95% CI, 0.28-0.60]). The subgroup analyses showed statistically significant interaction effects. Patients with low vs high RoPE Score had HRs of 0.61 (95% CI, 0.37-1.00) and 0.21 (95% CI, 0.11-0.42), respectively (P for interaction = .02). Patients classified as unlikely, possible, and probable using the PASCAL Classification System had HRs of 1.14 (95% CI, 0.53-2.46), 0.38 (95% CI, 0.22-0.65), and 0.10 (95% CI, 0.03-0.35), respectively (P for interaction = .003). The 2-year absolute risk reduction was −0.7% (95% CI, −4.0% to 2.6%), 2.1% (95% CI, 0.6%-3.6%), and 2.1% (95% CI, 0.9%-3.4%) in the unlikely, possible, and probable PASCAL categories, respectively. Device-associated adverse events were generally higher among patients classified as unlikely; the absolute risk increases in atrial fibrillation beyond day 45 after randomization with a device were 4.41% (95% CI, 1.02% to 7.80%), 1.53% (95% CI, 0.33% to 2.72%), and 0.65% (95% CI, −0.41% to 1.71%) in the unlikely, possible, and probable PASCAL categories, respectively.
Conclusions and Relevance
Among patients aged 18 to 60 years with PFO-associated stroke, risk reduction for recurrent stroke with device closure varied across groups classified by their probabilities that the stroke was causally related to the PFO. Application of this classification system has the potential to guide individualized decision-making.
This study evaluates heterogeneity of treatment effect (HTE) of patent foramen ovale (PFO) closure on stroke recurrence based on the Risk of Paradoxical Embolism and PFO-Associated Stroke Causal Likelihood scoring systems using individual patient data pooled from 6 randomized clinical trials (RCTs) that compared PFO closure plus medical therapy vs medical therapy alone in patients with PFO-associated stroke.
Introduction
Patent foramen ovale (PFO)–associated strokes comprise 10% of ischemic strokes in young and middle-aged adults aged 18 to 60 years.1 Therapeutic strategies to prevent recurrent stroke among patients with a PFO-associated ischemic stroke include antithrombotic therapy or percutaneous device closure of the PFO, each of which is endorsed in practice guidelines.2,3,4
Six randomized clinical trials that compared device closure and medical therapy vs medical therapy alone have been completed.5,6,7,8,9,10,11 Study-level meta-analyses1,12 have shown that closure was associated with a lower rate of recurrent ischemic stroke.12 However, absolute risks of stroke recurrence remain very low for some patients with medical therapy, and device closure has associated risks and adverse effects.1 Recent guidelines stressed the importance of informed shared decision-making evaluating the individual probability of benefit and the risks of a lifelong device.2,3,4
However, study-level analysis of randomized clinical trials generally only provide results for the broad reference class of all patients qualifying for a trial. Yet individual patients differ from one another in many ways that can affect the potential for benefit. Conventional, one-variable-at-a-time subgroup analyses explore heterogeneity of treatment effect (HTE) in a limited manner but have well-known issues with both credibility from multiplicity and applicability to individuals because patients vary in many different potentially important ways simultaneously.13,14,15
This individual participant data pooled analysis was undertaken, motivated by new methods proposed for predictive HTE analyses combining many covariates, to narrow the reference class for each individual to more granular, deeply similar, patients.13,14
Methods
Institutional Review Board and Patient Consent
The Tufts Health Sciences institutional review board approved this study. Patient consent was not required for this secondary data analysis.
Study Design and Inclusion Criteria
The study investigators established the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) Consortium to undertake meta-analysis of pooled individual participant data. Study methods adhered to the Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data guidelines and the protocol was registered on PROSPERO (CRD42020186537).16 The collaboration included all randomized phase 3 trials comparing PFO closure vs medical therapy for recurrent stroke prevention published by September 2021. Trial methodology was assessed with the Risk of Bias 2.0 tool (Cochrane Methods). Investigators were contacted and data were collected and harmonized (for details see eAppendix 1 and eTable 1 in the Supplement).
The 3 prespecified study aims were to (1) assess overall efficacy and safety of PFO device closure plus medical therapy vs medical therapy alone for the prevention of recurrent stroke; (2) examine whether the Risk of Paradoxical Embolism (RoPE) Score17 is associated with differential treatment effect; and (3) examine whether the PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System18 is associated with differential treatment effect.
The RoPE Score and the PASCAL Classification System
RoPE is depicted in Table 1 and explicated in eAppendix 2 and eTable 2 in the Supplement. Briefly, the RoPE Score provides an estimate of the probability that a PFO discovered in the setting of an otherwise cryptogenic ischemic stroke is the cause of the stroke rather than an incidental finding, with a higher RoPE Score corresponding to a higher probability. Because PFOs are present in approximately 25% of the general population, patients with PFO can have a stroke either through a PFO-related mechanism (eg, a paradoxical embolism) or through another occult mechanism (eg, paroxysmal atrial fibrillation or minimally stenosing cervicocerebral atherosclerotic plaque). The RoPE Score is based on 2 insights: (1) the prevalence of a PFO among patients with cryptogenic stroke (compared with that in the general population) can be used, via the Bayes theorem, to estimate an average attributable fraction (ie, the proportion of PFOs that are pathogenic rather than incidental) and (2) the presence or absence of a PFO in a patient with a cryptogenic stroke is predictable based on patient characteristics—so that a patient-specific attributable fraction can be estimated based on the probability of discovering a PFO conditional on patient characteristics. Intuitively, a PFO-related stroke is more likely in younger patients, in the absence of vascular risk factors and in the presence of a superficial infarct on neuroimaging. In theory, because closure would prevent only strokes caused by paradoxical embolism, the attributable fraction would be assumed to correspond to the relative risk reduction of closure in preventing a future stroke.19
Table 1. Risk of Paradoxical Embolism (RoPE) Score and PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System.
| Characteristic | Points | |
|---|---|---|
| RoPE Score calculatora | ||
| No history of | ||
| Hypertension | 1 | |
| Diabetes | 1 | |
| Stroke or transient ischemic attack | 1 | |
| Nonsmoker | 1 | |
| Cortical infarct on imaging | 1 | |
| Age, y | ||
| 18-29 | 5 | |
| 30-39 | 4 | |
| 40-49 | 3 | |
| 50-59 | 2 | |
| 60-69 | 1 | |
| >70 | 0 | |
| Total RoPE Score (sum of individual points) = | ||
| PASCAL Classification Systemb | ||
| High RoPE Score (≥7) | High-risk PFO feature (LS and/or ASA) | PFO-related stroke |
| Absent | Absent | Unlikely |
| Absent | Present | Possible |
| Present | Absent | |
| Present | Present | Probable |
Abbreviations: ASA, atrial septal aneurysm; LS, large shunt; PFO, patent foramen ovale.
The RoPE Score assesses the probability that a PFO discovered in the setting of an otherwise cryptogenic stroke was pathogenically related to the stroke rather than an incidental finding. The RoPE Score ranges from 0 to 10, with scores of 0 to 3 indicating a negligible likelihood that the stroke is attributable to the PFO and a score of 9 or 10 indicating an approximately 90% probability that the stroke is attributable to the PFO. See eAppendix 2 and eTable 2 in the Supplement for details.
PASCAL combines the RoPE Score with the presence or absence of high-risk PFO features to determine the likelihood that the PFO was causally related to the index stroke. See eAppendix 3 and eFigure 1 in the Supplement for details. Large shunt size was defined in the database as >20 bubbles in the left atrium on transesophageal echo; ASA was defined as ≥10 mm of excursion from midline.
The RoPE Score has been externally validated to predict the presence of a PFO in the cryptogenic stroke population.20,21 However, the RoPE Score has 2 important limitations: the methods to derive the RoPE Score did not permit inclusion of high-risk features of the PFO and patients with higher RoPE Scores have lower stroke recurrence rates. Thus, the RoPE Score may not provide comprehensive information for patient selection.3,18
The PASCAL Classification System depicted in Table 1 and explicated in eAppendix 3 and eFigure 1 in the Supplement addresses these limitations by integrating the information of the RoPE Score with PFO functional and structural features physiologically expected and epidemiologically confirmed to potentiate PFO stroke risk, namely, shunt size and the presence of an atrial septal aneurysm.22,23,24 Based on these factors, this system algorithmically assigns a likelihood of causal relationship (Table 1).25
Outcomes
The primary efficacy end point was recurrent ischemic stroke: an acute neurologic deficit, presumed to be due to focal ischemia, and either symptoms persisting 24 hours or longer or symptoms persisting less than 24 hours but associated with neuroimaging findings of a new neuroanatomically relevant infarct.26,27
The secondary efficacy outcomes were (1) recurrent PFO-associated ischemic stroke (recurrent ischemic strokes adjudicated as not being attributable to another mechanism by investigators of the individual trials); (2) the composite of recurrent ischemic stroke or early (periprocedural or equivalent medical therapy time frame) all-cause mortality; (3) the composite of recurrent ischemic stroke, early all-cause mortality, or any vascular death; (4) the composite of recurrent ischemic stroke, transient ischemic attack (TIA), or vascular death; (5) disability-worsening recurrent ischemic stroke; and (6) any recurrent stroke (ischemic or hemorrhagic).
Disability-worsening stroke was defined as a new stroke associated with any increase at day 30 or longer after the stroke in the modified Rankin Scale. For subgroup analysis, in addition to the primary end point, only the secondary end point with the greatest number of events was examined: the composite of recurrent ischemic stroke, TIA, or vascular death.
Five safety outcomes were examined, as defined in the primary trials: all serious adverse events; major vascular procedural complication; atrial fibrillation; major bleeding episode; and venous thromboembolism (deep venous thrombosis/pulmonary embolism). Both all atrial fibrillation and atrial fibrillation present any time beyond the first 45 days after randomization (to exclude transient periprocedural events) were analyzed separately.
Statistical Analysis
The primary analyses for efficacy outcomes assessed patients according to the treatment group to which they were randomized. Two additional analyses were conducted in (1) patients according to the treatment they actually received if any crossover occurred (ie, as treated, defined in eAppendix 4 in the Supplement) and (2) patients without major protocol deviations (ie, per protocol, defined in eAppendix 4 in the Supplement). These analyses were adjusted for prerandomization covariates. Safety outcomes were analyzed in the as-treated population only.
The secondary efficacy outcomes were analyzed using sequential gatekeeping28 in the following order: (1) recurrent PFO-associated ischemic stroke; (2) the composite of recurrent ischemic stroke or early all-cause mortality; (3) the composite of recurrent ischemic stroke, early all-cause mortality, or any vascular death; (4) the composite of recurrent ischemic stroke, TIA, or vascular death; (5) disability-worsening recurrent ischemic stroke; and (6) any recurrent stroke (ischemic or hemorrhagic). The sequential gatekeeping strategy tests a prespecified hierarchy of outcomes and stops when an outcome test is not statistically significant to control for multiplicity. Secondary efficacy outcomes that were unavailable uniformly across all trials were to be removed from the gatekeeper hierarchy and examined in exploratory analyses where available.
For all time-to-event outcomes, the equality of the survivor functions was assessed using a stratified (by trial) log-rank test.17,20 Kaplan-Meier estimates were obtained at 6, 12, 24, and 60 months for each treatment group. After confirming no violation of proportional hazards assumptions (eAppendix 1 and eTable 1 in the Supplement), effects were estimated using Cox proportional hazards regression with a study-specific random effect.29 In this 1-stage analysis, a study-specific random effect was used to account for within-study homogeneity in outcomes and a fixed treatment effect.30 The CLOSE Trial, in which some patients were randomized to antiplatelet vs device and others to anticoagulant vs device, was treated as 2 trials. The Breslow method was used for tied survival times.29 Safety analyses were based on comparisons of event proportions between treatment groups, using the Cochran-Mantel-Haenszel test (stratified by trial).31,32 For all hypothesis-testing analyses, a 2-sided P value significance threshold of .05 was used, without adjustments for multiple testing.33 All analyses were conducted using SAS (version 9.4; SAS Institute) and R (version 4.0.2; The R Foundation).
The primary efficacy analysis was adjusted for the following covariates: age, sex, prior myocardial infarction, diabetes, hypertension, hyperlipidemia, prior stroke or TIA, smoking status, index event (stroke vs TIA), atrial septal aneurysm (≥10 mm of excursion from midline, definition in eAppendix 5 and eTables 3 and 4 in the Supplement), PFO shunt size (large vs small, definition in eAppendix 5 and eTables 3 and 4 in the Supplement), and presence vs absence of a visible superficial infarction on neuroimaging. As a stability analysis, the unadjusted effect estimate is also reported. Additional details of the statistical analysis are provided in eAppendix 1 and eTable 1 in the Supplement.
Missing Data
In the primary analysis, patients who exited the trials early were assumed to have outcome events that were noninformative under the missing at random assumption and were censored at last follow-up. Two sensitivity analyses were performed: a multiple imputation analysis (eAppendix 1 and eTable 1 in the Supplement) with covariate adjustment34 and a tipping-point analysis.35,36
For the conditional (adjusted) analysis and for subgroup analyses, multiple imputation to impute missing covariates was used as needed.37
Subgroup and Interaction Analyses
We assessed whether treatment was associated with differential effects across subgroups. A primary HTE analysis was based on the RoPE Score. We tested the significance of effect modification using the RoPE Score as a continuous variable, and dichotomized the RoPE Score into high (≥7) and low (<7) groups for presentation. Because the PC Trial was missing a key RoPE variable (superficial infarct on neuroimaging), multiple imputation was used in the main analysis to include all trials, but we performed 2 stability analyses: (1) using a reduced 9-point RoPE Score, excluding the imaging variable, and (2) a 5-trial analysis excluding the PC Trial. An additional primary HTE analysis was based on the 3 levels of the PASCAL Classification System: unlikely, possible, and probable.
Secondary (exploratory) subgroup analyses were performed across each of the following 9 variables: sex (male vs female); age (≥45 vs <45 years); atrial septal aneurysm (present vs absent; defined as ≥10 mm excursion from midline, depending on study [see eAppendix 5 and eTables 3 and 4 in the Supplement for details]); shunt size (approximately ≥20 bubbles in the left atrium within 3 cardiac cycles, depending on classifications in individual studies [see eAppendix 5 and eTables 3 and 4 in the Supplement for details]); visible superficial infarction on neuroimaging; history of hypertension; history of diabetes; prior stroke or TIA; and current smoking at study entry. Though these analyses are considered exploratory, we report descriptive P values. We assessed effect modification by including appropriate product terms for each variable and randomization assignment in Cox regression models.
Stability Analyses
To assess the robustness of our findings, we performed leave-one-out analyses: the main analyses were repeated after excluding each trial in turn, with particular interest in the analysis omitting the CLOSURE Trial that tested an umbrella-clamshell device class no longer used in clinical practice.
Results
The systematic search identified 6 trials that had enrolled 3740 participants who had been followed up for a median of 57 months (IQR, 24-64) (eAppendix 6 and eFigure 2 in the Supplement). The trials were conducted from 2000 to 2017. Trial details can be found in eAppendix 7 and eTable 5 in the Supplement. All trials had some concerns for risk of bias, generally related to their randomized open-blinded end point (PROBE) design or due to missing outcome data (eAppendix 8 and eTable 6 in the Supplement), but none were rated as high risk of bias. Patient characteristics in the pooled cohort are shown in Table 2 (and for each study in eAppendix 9 and eTables 7-13 in the Supplement).
Table 2. Baseline Patient Characteristics From 6 Pooled Trials of Device Closure vs Medical Therapy for Patent Foramen Ovale–Associated Stroke.
| Variable | No. (%) | |
|---|---|---|
| Device | Medication therapy | |
| No. of total patients | 1889 | 1851 |
| Age, median (IQR), y | 46.2 (39.0-52.7) [n = 1882] | 46.0 (39.0-53.0) [n = 1846] |
| Sex | ||
| Male | 1024 (54.2) | 1034 (55.9) |
| Female | 865 (45.8) | 817 (44.1) |
| Hyperlipidemia | 720 (38.1) | 632 (34.1) |
| Hypertension | 512 (27.1) | 456 (24.6) |
| Tobacco, No./total (%)a | 379/1889 (20.1) | 364/1849 (19.7) |
| Diabetes | 106 (5.6) | 106 (5.7) |
| Index stroke (vs TIA), No./total (%)b | 1766/1888 (93.5) | 1718/1850 (92.9) |
| Presence of a superficial infarct, No./total (%)c | 1003/1420 (70.6) | 971/1432 (67.8) |
| Prior stroke or TIA | 310 (16.4) | 285 (15.4) |
| Prior stroke, No./total (%) | 134/1888 (7.1) | 105/1851 (5.7) |
| Large-sized shunt, No./total (%)d | 767/1787 (42.9) | 815/1743 (46.8) |
| Atrial septal aneurysm, No./total (%)e | 587/1786 (32.9) | 597/1792 (33.3) |
Abbreviation: TIA, transient ischemic attack.
Defined as current smoker in DEFENSE, PC, RESPECT, and CLOSE Trials; current smoker or quit within past 30 days in the CLOSURE Trial; and current smoker or quit less than 12 months ago in the REDUCE Trial.
Defined as symptoms persisting less than 24 hours and not associated with neuroimaging findings of a new neuroanatomically relevant infarct.
Not reported in the PC Trial.
More than 20 bubbles in the left atrium on transesophageal echo for all trials except the CLOSURE (>25) and CLOSE (>30) Trials (see eAppendix 5 and eTables 3 and 4 in the Supplement for details).
See eAppendix 5 and eTables 3 and 4 in the Supplement for details.
Primary and Secondary Efficacy Outcomes
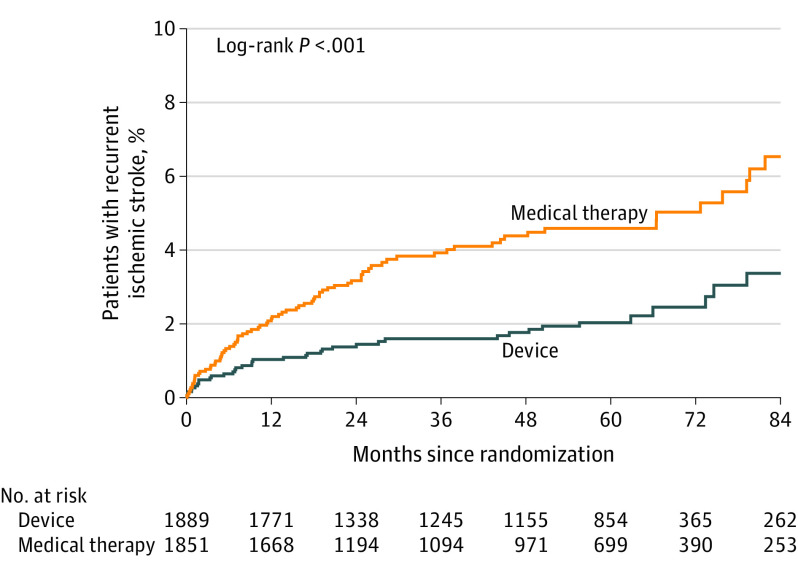
During a median follow-up of 57 months, a total of 121 primary end point ischemic stroke events occurred in the pooled study population. Treatment with PFO closure was associated with reduced incidence of recurrent ischemic stroke (Table 3; Figure 1). The annualized incidence of stroke with medical therapy was 1.09% (95% CI, 0.88%-1.36%) and with device closure was 0.47% (95% CI, 0.35%-0.65%) (adjusted hazard ratio [HR], 0.41 [95% CI, 0.28-0.60]; P < .001). Secondary outcomes showed results that were consistent with the primary outcome (Table 3), with the exception of disability-worsening stroke, which was limited due to missingness.
Table 3. Primary and Secondary Efficacy Outcomes.
| Device | Medical therapy | 2-y Absolute difference, ARR, % (95% CI)a | Unadjusted relative risk, HR (95% CI) | Adjusted relative riskb | ||||
|---|---|---|---|---|---|---|---|---|
| Overall outcome rate events per 100 person-years (95% CI) | Events/No. of patients | Overall outcome rate events per 100 person-years (95% CI) | Events/No. of patients | HR (95% CI) | P value | |||
| Primary efficacy outcome | ||||||||
| Recurrent ischemic stroke | 0.47 (0.35 to 0.65) | 39/1889 | 1.09 (0.88 to 1.36) | 82/1851 | 1.72 (0.73 to 2.72) | 0.42 (0.29 to 0.62) | 0.41 (0.28 to 0.60) | <.001 |
| Secondary efficacy outcomes (in hierarchical order) | ||||||||
| 1. PFO-associated recurrent ischemic strokec | 0.24 (0.15 to 0.40) | 16/1238 | 0.90 (0.69 to 1.18) | 53/1179 | 2.21 (1.08 to 3.34) | 0.25 (0.14 to 0.45) | 0.24 (0.14 to 0.43) | <.001 |
| 2. Recurrent ischemic stroke or early all-cause mortality | 0.47 (0.35 to 0.65) | 39/1889 | 1.09 (0.88 to 1.36) | 82/1851 | 1.72 (0.73 to 2.72) | 0.42 (0.29 to 0.62) | 0.41 (0.28 to 0.60) | <.001 |
| 3. Recurrent ischemic stroke, early all-cause mortality, or vascular death | 0.55 (0.41 to 0.73) | 45/1889 | 1.15 (0.93 to 1.42) | 86/1851 | 1.62 (0.60 to 2.64) | 0.45 (0.32 to 0.65) | 0.44 (0.31 to 0.64) | <.001 |
| 4. Recurrent ischemic stroke, TIA, or vascular death | 1.08 (0.88 to 1.34) | 88/1889 | 1.72 (1.44 to 2.04) | 127/1851 | 1.61(0.27 to 2.96) | 0.61 (0.46 to 0.80) | 0.60 (0.45 to 0.79) | <.001 |
| 5. Disability-worsening recurrent ischemic stroked | 0.16 (0.09 to 0.27) | 13/1685 | 0.27 (0.17 to 0.41) | 20/1641 | 0.18 (−0.39 to 0.75) | 0.62 (0.31 to 1.25) | 0.59 (0.37 to 1.22) | .14 |
Abbreviations: ARR, absolute risk reduction (medical therapy - device); HR, hazard ratio; PFO, patent foramen ovale; TIA, transient ischemic attack.
Absolute difference calculated as differences in Kaplan-Meier event rates at 2 years.
Accounting for age, sex, prior myocardial infarction, diabetes, hypertension, hyperlipidemia, prior stroke or TIA, smoking status, index event (stroke vs TIA), atrial septal aneurysm, PFO shunt size (large vs small, definition in eAppendix 5 and eTables 3 and 4 in the Supplement), and superficial infarction on neuroimaging (present vs absent).
No data for the PC and CLOSURE Trials.
Assume missing outcome is not disabling (no data for PC Trial). Median time to the primary outcome of recurrent ischemic stroke was 13.7 months (IQR, 4.8-29.7; n = 121).
Figure 1. Kaplan-Meier Curve of Recurrent Ischemic Stroke .
Median time to the primary outcome of recurrent ischemic stroke was 13.7 months (IQR, 4.8-29.7; n = 121).
Stability of the Main Results
The main results for the primary outcome were robust to alternative analytic approaches. HRs were similar for the unadjusted (Table 3), per-protocol (HR, 0.37 [95% CI, 0.24-0.57]), and as-treated (HR, 0.40 [95% CI, 0.27-0.59]) analyses. Leave-one-out analyses showed that no single trial was overly influential (eAppendix 10 and eTable 14 in the Supplement); the adjusted HR for closure ranged from 0.32 (95% CI, 0.20-0.51) (without the CLOSURE Trial) to 0.45 (95% CI, 0.30-0.66) (without the CLOSE-A Trial). Early exiting and retained patients were largely similar (eAppendix 11 and eTable 15 in the Supplement), although patients who had early exit from the trials more often had prior stroke, a superficial infarct, and a large shunt. In the multiple imputation analysis with covariate adjustment, the HR for ischemic stroke with PFO closure vs medical therapy was 0.41 (95% CI, 0.26-0.64; P < .001). The tipping-point analysis showed robustness to missing data: imputed outcomes for patients not followed up to the end of each trial would have to be approximately 2-fold higher in the device compared with the medical therapy group to nullify the significance of the main effect (eAppendix 12 and eTable 16 in the Supplement).
Safety Outcomes
Safety analyses are shown in Table 4. Atrial fibrillation was significantly higher in the closure group (adjusted relative risk [RR], 4.54 [95% CI, 2.78-7.39]), but 46% (50/109) of the events were transient, occurring only in the first 45 days after randomization. Beyond this periprocedural period, the rate of atrial fibrillation over a median follow-up of 57 months was 5.0% with device and 1.1% with medical therapy (adjusted RR, 2.60 [95% CI, 1.44-4.70]).
Table 4. Safety Outcomes.
| Safety outcome (as-treated population) | Overall outcome rate, patients with event/No. of patients (%) | Risk (95% CI)a | Mantel-Haenszel test P value | ||
|---|---|---|---|---|---|
| Device | No deviceb | Difference, % | Relative | ||
| Any serious adverse event | 506/1762 (28.7) | 516/1956 (26.4) | 1.97 (−0.89 to 4.82) | 1.07 (0.97 to 1.19) | .18 |
| Atrial fibrillation | |||||
| All events | 88/1762 (5.0) | 21/1956 (1.1) | 3.77 (2.65 to 4.89) | 4.54 (2.78 to 7.39) | <.001 |
| Present beyond 45 d | 43/1762 (2.4) | 16/1956 (0.8) | 1.38 (0.56 to 2.19) | 2.60 (1.44 to 4.70) | .001 |
| Major bleeding episodec | 25/1762 (1.4) | 33/1956 (1.7) | −0.31 (−1.09 to 0.47) | 0.80 (0.47 to 1.40) | .45 |
| Venous thromboembolism | 25/1762 (1.4) | 10/1956 (0.5) | 0.87 (0.22 to 1.51) | 2.59 (1.26 to 5.36) | .007 |
Stratified by study using Cochran-Mantel-Haenszel tests.
Data from 105 patients who were assigned to device but did not receive device were analyzed in the medical therapy group. The median follow-up times from randomization for patients receiving device and not receiving device were 58.7 months (IQR, 23.8-64.0) and 50.0 months (23.8-63.6), respectively.
Major bleeding episode was derived from serious adverse event reporting in each of the individual trials.
RoPE Score, PASCAL Classification, and Treatment Effect Heterogeneity
The RoPE Score–by–treatment interaction was significant (P < .01), using the full 10-point RoPE Score with the neuroimaging variable imputed for the PC Trial. Results were stratified by RoPE Score as 7 or greater vs less than 7 and are shown in Figure 2, indicating a strong interaction (P = .02) on the HR scale (Figure 2A); patients with low vs high RoPE Scores had HRs of 0.61 (95% CI, 0.37 to 1.00) and 0.21 (95% CI, 0.11 to 0.42), respectively. The risks of stroke in the first 2 years for patients with a low RoPE Score were 4.0% (95% CI, 2.5% to 5.5%) and 2.9% (95% CI, 1.6% to 4.2%) (absolute risk reduction [ARR], 1.1% [95% CI, −0.9% to 3.1%]) for the medical therapy and device groups, respectively, and for patients with a high RoPE Score were 2.6% (95% CI, 1.7% to 3.6%) and 0.6% (95% CI, 0.1% to 1.0%) (ARR, 2.1% [95% CI, 1.0% to 3.1%]) for the medical therapy and device groups, respectively (Figure 2B). Results were consistent in stability analyses excluding the PC Trial, or using a 9-point (neuroimaging-free) RoPE Score (eFigure 3 in the Supplement), and with the lead secondary outcome of recurrent ischemic stroke, TIA, or vascular death (eFigure 4 in the Supplement) (eAppendix 13 in the Supplement).
Figure 2. Recurrent Ischemic Stroke Heterogeneity of Treatment Effect (HTE) Analyses for RoPE and PASCAL.
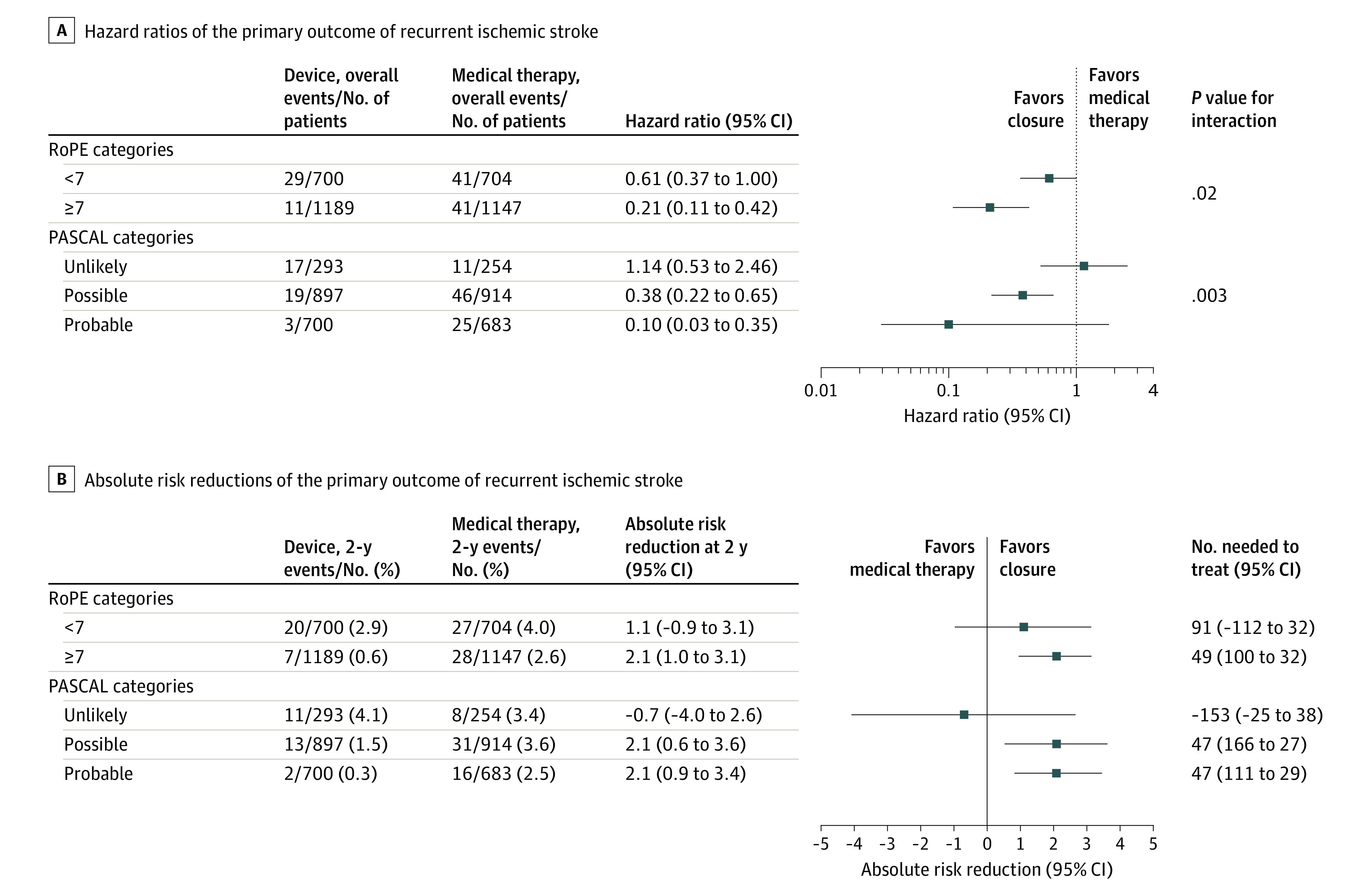
The hazard ratios account for age, sex, prior myocardial infarction, diabetes, hypertension, hyperlipidemia, prior stroke or transient ischemic attack, smoking status, index event (stroke vs transient ischemic attack), atrial septal aneurysm on transesophageal echocardiography (definition in eAppendix 5 and eTables 3 and 4 in the Supplement), patent foramen ovale shunt size (large vs small, definition in eAppendix 5 and eTables 3 and 4 in the Supplement), and superficial infarction on neuroimaging (present vs absent). Two-year absolute risk reductions calculated as differences in Kaplan-Meier event rates at 2 years. Median time to the primary outcome of recurrent ischemic stroke was 13.7 months (IQR, 4.8-29.7; n = 121). RoPE indicates Risk of Paradoxical Embolism (definition provided in Table 1); PASCAL, PFO-Associated Stroke Causal Likelihood.
Subgroup analyses based on PASCAL classification showed strong effect modification across the 3 levels of the PASCAL Classification System on the relative scale; patients with PASCAL classifications of unlikely, possible, and probable had HRs of 1.14 (95% CI, 0.53 to 2.46), 0.38 (95% CI, 0.22 to 0.65), and 0.10 (95% CI, 0.03 to 0.35), respectively (P = .003; Figure 2A), and clinically meaningful differences on the absolute scale at 2 years (Figure 2B). The absolute risk of stroke in the first 2 years for patients with PASCAL classification of unlikely was 3.4% (95% CI, 1.1% to 5.7%) and 4.1% (95% CI, 1.7% to 6.4%) for the medical therapy and device groups, respectively (ARR, −0.7% [95% CI, −4.0% to 2.6%]). For patients with the possible PASCAL classification, the absolute 2-year risk of ischemic stroke was 3.6% (95% CI, 2.4% to 4.9%) and 1.5% (95% CI, 0.7% to 2.3%) for the medical therapy and device groups, respectively (ARR, 2.1% [95% CI, 0.6% to 3.6%]); for patients with the probable PASCAL classification, the 2-year stroke risk was 2.5% (95% CI, 1.3% to 3.7%) and 0.3% (95% CI, −0.1% to 0.8%) for the medical therapy and device groups, respectively (ARR, 2.1% [95% CI, 0.9% to 3.4%]). Again, results were consistent in stability analyses excluding the PC Trial, or using a 9-point (neuroimaging-free) RoPE Score (eFigure 3 in the Supplement), and with the lead secondary outcome of recurrent ischemic stroke, TIA, or vascular death (eFigure 4 in the Supplement).
The difference in the rates of safety outcomes between the device and medical therapy groups was also consistently higher in the unlikely group than in the probable or possible groups of the PASCAL Classification System (eTable 17 in the Supplement). For example, the absolute risk increase of postperiprocedural (occurring >45 days after randomization) atrial fibrillation with a device was 4.41% (95% CI, 1.02% to 7.80%), 1.53% (95% CI, 0.33% to 2.72%), and 0.65% (95% CI, −0.41% to 1.71%) in the unlikely, possible, and probable PASCAL categories, respectively, over the full follow-up period. For comparability with 2-year absolute risk reduction in ischemic stroke, 2-year differences in atrial fibrillation were calculated (eTable 18 in the Supplement) (eAppendix 14 in the Supplement).
Exploratory Subgroup Analysis
Exploratory single-parameter subgroup analyses (eFigure 5 in the Supplement) showed nominally stronger relative risk reductions in strata defined by variables postulated to be associated with PFO-related stroke mechanisms (ie, in the theory-anticipated direction), but evidence of effect modification was generally modest. Exploratory subgroup analyses based on the main secondary outcome showed largely similar patterns (eFigure 6 in the Supplement) (eAppendix 15 in the Supplement).
Discussion
This individual patient data meta-analysis indicates that PFO closure in patients with otherwise cryptogenic stroke was associated with a strong relative reduction in the risk of recurrent stroke. The annualized risk of a future stroke for patients assigned to medical therapy was approximately 1%, which accumulates over time; this risk was reduced by device closure by approximately 60%. The benefits associated with device closure were slightly larger when analysis was confined to trials testing double-disk class closure devices currently used in clinical practice. Overall, PFO closure appeared relatively safe. Atrial fibrillation was somewhat more frequent with device closure. However, most atrial fibrillation was transient and did not cause any permanent harm; postperiprocedural atrial fibrillation was increased by only slightly more than 1% on the absolute scale compared with medical therapy.
While the benefits associated with device closure were robust on average, treatment effects varied substantially across strata classified by the probability that the index stroke was PFO-related. Device closure did not appear to be associated with any benefit for the 15% of patients in the PASCAL unlikely classification who lacked a high-risk PFO (either atrial septal aneurysm or large shunt) and also had vascular risk factors (ie, RoPE Score <7), even with the careful exclusion of patients with defined stroke mechanisms from these trials. Conversely, device closure was associated with approximately a 90% relative risk reduction for patients with a PASCAL probable classification who had both high-risk PFO characteristics and a high RoPE Score. Device closure was associated with intermediate relative effects in the PASCAL possible category. While relative effect estimates differed between the probable and possible PASCAL subgroups, the 2-year absolute risk difference among these patients was approximately 2%, for a 2-year number needed to treat of approximately 50, a comparable effect magnitude to that of the 3 mainstays of medical therapy for secondary stroke prevention: antihypertensive therapy, antiplatelet therapy, and statin therapy.
Moreover, the patients likely to receive greater benefit also appeared to be at lower risk for device-associated adverse events such as atrial fibrillation, making the harm-benefit trade-offs of device closure more clearly favorable in the possible and probable groups. The lower risk of adverse events in the patients with potential high benefit is consonant with prior evidence showing a higher risk of incident atrial fibrillation in patients with lower RoPE Scores,21 who are older and have more vascular risk factors. This increased risk may reflect occult atrial fibrillation being a more likely mechanism for the index stroke in these patients and may also reflect a greater susceptibility to arrhythmogenic effects of device-tissue contact.
The results illustrate several core concepts of HTE analyses. Even in this pooled analysis of 6 clinical trials, most conventional one-variable-at-a-time subgroup analysis did not generally have estimated effects that would be considered clinically or statistically significant, even while estimated effects were consistently in the theorized direction (eg, attenuated effects for each subgroup with a vascular risk factor). Combining variables creates greater contrast in effects between treatment-favorable and -unfavorable patients, and could help personalize decision-making by more comprehensively describing individuals. The clinical reasoning incorporated into the PASCAL Classification System was developed over decades, including the RoPE Score, which was derived on an observational database independent from this study.
These results also underscore the importance of performing HTE analyses on both relative and absolute scales. Clinically important HTE is variation in the absolute risk difference that spans a clinically important decision threshold,13,14,38 such as the difference in the treatment effect observed in the PASCAL unlikely strata (where results were null) vs that observed in other strata. Variation on the relative scale may provide mechanistic information, but even strong and statistically significant interaction effects may be clinically unimportant if all groups benefit (as with the RoPE Score, when used alone).
Individual participant data meta-analysis has several advantages over study-level meta-analysis,39 including the standardization of analyses across studies, better handling of missing data, the ability to estimate both absolute effects at various time points and conditional treatment effects,1,7,11 and the opportunity to assess HTE. This study also used a novel approach to predict patients most likely to respond using multiple variables combined, providing new information to inform decisions about which patients should be treated with PFO closure.
Limitations
This study has several limitations. First, the magnitude of the benefit associated with device closure with respect to preventing disabling stroke remains uncertain, as substantial data were missing with respect to functional outcomes with recurrent stroke. Second, definitions of several key variables, such as large shunt or the presence of an atrial septal aneurysm, were nonidentical across trials. Nevertheless, this variation across trials did not preclude robust results from pooling data across studies. Third, while the analysis validates both the RoPE Score as an indicator of the stroke mechanism (ie, attributable fraction) and the assessed PASCAL system, the original extended PASCAL classification (Appendix 3 and eFigure 1 in the Supplement) could not be evaluated because patients in the uncommon extremely high–risk categories (eg, thrombus straddling PFO; patients with concomitant deep venous thrombosis) were not identified in the study. Fourth, despite the comprehensiveness of this analysis, several important clinical questions remain unaddressed, including the best antithrombotic therapy (eg, anticoagulation vs antiplatelet therapy) with or without closure, the role of new PFO devices, and the role of closure for patients older than 60 years.
Conclusions
Among patients aged 18 to 60 years with PFO-associated stroke, risk reduction for recurrent stroke with device closure varied across groups classified by their probabilities that the stroke was causally related to the PFO. Application of this classification system has the potential to guide individualized decision-making.
eAppendix 1. Analysis Details
eTable 1. Variables of Interest
eAppendix 2. RoPE Score Detail
eTable 2. PFO-Attributable Fraction by RoPE Score
eAppendix 3. PASCAL Score Details
eFigure 1. The Extended PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System
eAppendix 4. Definitions of “Per-Protocol” and “As-Treated” Populations
eAppendix 5. Description of Atrial Septal Aneurysm and Shunt Size Variables
eTable 3. Variable Definition of ASA Class
eTable 4. Variable Definition for Large Shunt Size
eAppendix 6. PRISMA IPD Flow Diagram
eFigure 2. PRISMA IPD Flow Diagram
eAppendix 7. Description of Trials
eTable 5. Features of Patent Foramen Ovale Closure Device Trials
eAppendix 8. Assessment of Risk of Bias and Small Study Effect
eTable 6. Risk of Bias Assessment
eAppendix 9. Patient Characteristics in Each Study
eTable 7. CLOSURE
eTable 8. PC Trial
eTable 9. RESPECT
eTable 10. REDUCE
eTable 11. DEFENSE
eTable 12. CLOSE-A
eTable 13. CLOSE-B
eAppendix 10. Leave-One-Out Stability Analyses
eTable 14. Leave-One-Out Stability Analyses
eAppendix 11. Patient Characteristics of Early Exiting Patients
eTable 15. Patient Characteristics of Early Exiting Patients
eAppendix 12. Tipping Point Analysis
eTable 16. Tipping Point Analysis of Primary Outcome
eAppendix 13. RoPE and PASCAL Analyses
eFigure 3. Recurrent Ischemic Stroke Heterogeneous Treatment Effect Stability Analyses for RoPE and PASCAL
eFigure 4. Secondary Outcome RoPE and PASCAL Heterogeneous Treatment Effect Analyses
eAppendix 14. Safety Outcomes by PASCAL Classification
eTable 17. Safety Outcomes by PASCAL Classification
eTable 18. Safety Outcomes by PASCAL Classification With 2-Year Atrial Fibrillation Rates
eAppendix 15. Outcome Exploratory Subgroup Analyses
eFigure 5. Recurrent Ischemic Stroke Exploratory Subgroup Analyses
eFigure 6. Secondary Outcome Exploratory Subgroup Analyses
eReferences
References
- 1.Saver JL, Mattle HP, Thaler D. Patent foramen ovale closure versus medical therapy for cryptogenic ischemic stroke: a topical review. Stroke. 2018;49(6):1541-1548. doi: 10.1161/STROKEAHA.117.018153 [DOI] [PubMed] [Google Scholar]
- 2.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364-e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 3.Pristipino C, Sievert H, D’Ascenzo F, et al. ; Evidence Synthesis Team; Eapci Scientific Documents and Initiatives Committee; International Experts . European position paper on the management of patients with patent foramen ovale: general approach and left circulation thromboembolism. Eur Heart J. 2019;40(38):3182-3195. doi: 10.1093/eurheartj/ehy649 [DOI] [PubMed] [Google Scholar]
- 4.Messé SR, Gronseth GS, Kent DM, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the guideline subcommittee of the American Academy of Neurology. Neurology. 2020;94(20):876-885. doi: 10.1212/WNL.0000000000009443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll JD, Saver JL, Thaler DE, et al. ; RESPECT Investigators . Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368(12):1092-1100. doi: 10.1056/NEJMoa1301440 [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Carroll JD, Thaler DE, et al. ; RESPECT Investigators . Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-1032. doi: 10.1056/NEJMoa1610057 [DOI] [PubMed] [Google Scholar]
- 7.Søndergaard L, Kasner SE, Rhodes JF, et al. ; Gore REDUCE Clinical Study Investigators . Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-1042. doi: 10.1056/NEJMoa1707404 [DOI] [PubMed] [Google Scholar]
- 8.Meier B, Kalesan B, Mattle HP, et al. ; PC Trial Investigators . Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368(12):1083-1091. doi: 10.1056/NEJMoa1211716 [DOI] [PubMed] [Google Scholar]
- 9.Furlan AJ, Reisman M, Massaro J, et al. ; CLOSURE I Investigators . Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366(11):991-999. doi: 10.1056/NEJMoa1009639 [DOI] [PubMed] [Google Scholar]
- 10.Mas JL, Derumeaux G, Guillon B, et al. ; CLOSE Investigators . Patent foramen ovale closure or anticoagulation vs antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021. doi: 10.1056/NEJMoa1705915 [DOI] [PubMed] [Google Scholar]
- 11.Lee PH, Song JK, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO Trial. J Am Coll Cardiol. 2018;71(20):2335-2342. doi: 10.1016/j.jacc.2018.02.046 [DOI] [PubMed] [Google Scholar]
- 12.Ahmad Y, Howard JP, Arnold A, et al. Patent foramen ovale closure vs medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J. 2018;39(18):1638-1649. doi: 10.1093/eurheartj/ehy121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ. 2018;363:k4245. doi: 10.1136/bmj.k4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent DM, Paulus JK, van Klaveren D, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med. 2020;172(1):35-45. doi: 10.7326/M18-3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahabreh IJ, Hayward R, Kent DM. Using group data to treat individuals: understanding heterogeneous treatment effects in the age of precision medicine and patient-centred evidence. Int J Epidemiol. 2016;45(6):2184-2193. doi: 10.1093/ije/dyw125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart LA, Clarke M, Rovers M, et al. ; PRISMA-IPD Development Group . Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657-1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 17.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81(7):619-625. doi: 10.1212/WNL.0b013e3182a08d59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elgendy AY, Saver JL, Amin Z, et al. Proposal for updated nomenclature and classification of potential causative mechanism in patent foramen ovale-associated stroke. JAMA Neurol. 2020;77(7):878-886. doi: 10.1001/jamaneurol.2020.0458 [DOI] [PubMed] [Google Scholar]
- 19.Kent DM, Saver JL, Ruthazer R, et al. Risk of Paradoxical Embolism (RoPE): estimated attributable fraction correlates with the benefit of patent foramen ovale closure: an analysis of 3 trials. Stroke. 2020;51(10):3119-3123. doi: 10.1161/STROKEAHA.120.029350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prefasi D, Martínez-Sánchez P, Fuentes B, Díez-Tejedor E. The utility of the RoPE score in cryptogenic stroke patients ≤50 years in predicting a stroke-related patent foramen ovale. Int J Stroke. 2016;11(1):NP7-NP8. doi: 10.1177/1747493015607505 [DOI] [PubMed] [Google Scholar]
- 21.Strambo D, Sirimarco G, Nannoni S, et al. Embolic stroke of undetermined source and patent foramen ovale: risk of paradoxical embolism score validation and atrial fibrillation prediction. Stroke. 2021;52(5):1643-1652. doi: 10.1161/STROKEAHA.120.032453 [DOI] [PubMed] [Google Scholar]
- 22.Mas JL, Arquizan C, Lamy C, et al. ; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group . Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345(24):1740-1746. doi: 10.1056/NEJMoa011503 [DOI] [PubMed] [Google Scholar]
- 23.Anzola GP, Zavarize P, Morandi E, Rozzini L, Parrinello G. Transcranial Doppler and risk of recurrence in patients with stroke and patent foramen ovale. Eur J Neurol. 2003;10(2):129-135. doi: 10.1046/j.1468-1331.2003.00561.x [DOI] [PubMed] [Google Scholar]
- 24.Lee BK, Lessler J, Stuart EA. Improving propensity score weighting using machine learning. Stat Med. 2010;29(3):337-346. doi: 10.1002/sim.3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messé SR, Kent DM. Still no closure on the question of PFO closure. N Engl J Med. 2013;368(12):1152-1153. doi: 10.1056/NEJMe1301680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saver JL, Warach S, Janis S, et al. ; National Institute of Neurological Disorders and Stroke (NINDS) Stroke Common Data Element Working Group . Standardizing the structure of stroke clinical and epidemiologic research data: the National Institute of Neurological Disorders and Stroke (NINDS) Stroke Common Data Element (CDE) project. Stroke. 2012;43(4):967-973. doi: 10.1161/STROKEAHA.111.634352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks KA, Mahaffey KW, Mehran R, et al. ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) . 2017 Cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71(9):1021-1034. doi: 10.1016/j.jacc.2017.12.048 [DOI] [PubMed] [Google Scholar]
- 28.Yadav K, Lewis RJ. Gatekeeping strategies for avoiding false-positive results in clinical trials with many comparisons. JAMA. 2017;318(14):1385-1386. doi: 10.1001/jama.2017.13276 [DOI] [PubMed] [Google Scholar]
- 29.Stewart GB, Altman DG, Askie LM, Duley L, Simmonds MC, Stewart LA. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One. 2012;7(10):e46042. doi: 10.1371/journal.pone.0046042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85(2):185-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719-748. [PubMed] [Google Scholar]
- 32.Cochran W. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10(4):417-451. doi: 10.2307/3001616 [DOI] [Google Scholar]
- 33.Cook RJ, Farewell VT. Multiplicity considerations in the design and analysis of clinical trials. J Royal Stat Soc. 1996;159(1):93-110. doi: 10.2307/2983471 [DOI] [Google Scholar]
- 34.Zhao Y, Saville BR, Zhou H, Koch GG. Sensitivity analysis for missing outcomes in time-to-event data with covariate adjustment. J Biopharm Stat. 2016;26(2):269-279. doi: 10.1080/10543406.2014.1000549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liublinska V, Rubin DB. Sensitivity analysis for a partially missing binary outcome in a two-arm randomized clinical trial. Stat Med. 2014;33(24):4170-4185. doi: 10.1002/sim.6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipkovich I, Ratitch B, O’Kelly M. Sensitivity to censored-at-random assumption in the analysis of time-to-event endpoints. Pharm Stat. 2016;15(3):216-229. doi: 10.1002/pst.1738 [DOI] [PubMed] [Google Scholar]
- 37.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 38.Kent DM, van Klaveren D, Paulus JK, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement: explanation and elaboration. Ann Intern Med. 2020;172(1):W1-W25. doi: 10.7326/M18-3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kent DM, Dahabreh IJ, Ruthazer R, et al. Device closure of patent foramen ovale after stroke: pooled analysis of completed randomized trials. J Am Coll Cardiol. 2016;67(8):907-917. doi: 10.1016/j.jacc.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Analysis Details
eTable 1. Variables of Interest
eAppendix 2. RoPE Score Detail
eTable 2. PFO-Attributable Fraction by RoPE Score
eAppendix 3. PASCAL Score Details
eFigure 1. The Extended PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System
eAppendix 4. Definitions of “Per-Protocol” and “As-Treated” Populations
eAppendix 5. Description of Atrial Septal Aneurysm and Shunt Size Variables
eTable 3. Variable Definition of ASA Class
eTable 4. Variable Definition for Large Shunt Size
eAppendix 6. PRISMA IPD Flow Diagram
eFigure 2. PRISMA IPD Flow Diagram
eAppendix 7. Description of Trials
eTable 5. Features of Patent Foramen Ovale Closure Device Trials
eAppendix 8. Assessment of Risk of Bias and Small Study Effect
eTable 6. Risk of Bias Assessment
eAppendix 9. Patient Characteristics in Each Study
eTable 7. CLOSURE
eTable 8. PC Trial
eTable 9. RESPECT
eTable 10. REDUCE
eTable 11. DEFENSE
eTable 12. CLOSE-A
eTable 13. CLOSE-B
eAppendix 10. Leave-One-Out Stability Analyses
eTable 14. Leave-One-Out Stability Analyses
eAppendix 11. Patient Characteristics of Early Exiting Patients
eTable 15. Patient Characteristics of Early Exiting Patients
eAppendix 12. Tipping Point Analysis
eTable 16. Tipping Point Analysis of Primary Outcome
eAppendix 13. RoPE and PASCAL Analyses
eFigure 3. Recurrent Ischemic Stroke Heterogeneous Treatment Effect Stability Analyses for RoPE and PASCAL
eFigure 4. Secondary Outcome RoPE and PASCAL Heterogeneous Treatment Effect Analyses
eAppendix 14. Safety Outcomes by PASCAL Classification
eTable 17. Safety Outcomes by PASCAL Classification
eTable 18. Safety Outcomes by PASCAL Classification With 2-Year Atrial Fibrillation Rates
eAppendix 15. Outcome Exploratory Subgroup Analyses
eFigure 5. Recurrent Ischemic Stroke Exploratory Subgroup Analyses
eFigure 6. Secondary Outcome Exploratory Subgroup Analyses
eReferences