This cohort study investigates the association of gestational age at birth with suspected developmental coordination disorder in early childhood among children aged 3 to 5 years in China.
Key Points
Question
In addition to preterm birth, are early-term and postterm birth also associated with suspected developmental coordination disorder in early childhood when compared with full-term birth?
Findings
In this cohort study of 152 433 children aged 3 to 5 years in China, early and postterm birth were associated with impaired motor performance compared with completely full-term birth when adjusting for kindergartens (as clusters) and child, family, and maternal health characteristics.
Meaning
These findings suggest that long-term follow-up and rehabilitation interventions should be considered in children born early and post term.
Abstract
Importance
It remains unknown whether children born at different degrees of prematurity, early term, and post term might have a higher risk of developmental coordination disorder (DCD) compared with completely full-term children (39-40 gestational weeks).
Objective
To differentiate between suspected DCD in children with different gestational ages based on a national representative sample in China.
Design, Setting, and Participants
A retrospective cohort study was conducted in China from April 1, 2018, to December 31, 2019. A total of 152 433 children aged 3 to 5 years from 2403 public kindergartens in 551 cities of China were included in the final analysis. A multilevel regression model was developed to determine the strength of association for different gestational ages associated with suspected DCD when considering kindergartens as clusters.
Main Outcomes and Measures
Children’s motor performance was assessed using the Little Developmental Coordination Disorder Questionnaire, completed by their parents. Gestational age was determined according to the mother’s medical records and divided into 7 categories: completely full term (39 to 40 weeks’ gestation), very preterm (<32 weeks), moderately preterm (32-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), late term (41 weeks), and post term (>41 weeks).
Results
A total of 152 433 children aged 3 to 5 years (mean [SD] age, 4.5 [0.8] years), including 80 370 boys (52.7%) and 72 063 girls (47.3%), were included in the study. There were 45 052 children (29.6%) aged 3 years, 59 796 (39.2%) aged 4 years, and 47 585 (31.2%) aged 5 years. Children who were born very preterm (odds ratio [OR], 1.35; 95% CI, 1.23-1.48), moderately preterm (OR, 1.18; 95% CI, 1.02-1.36), late preterm (OR, 1.24; 95% CI, 1.16-1.32), early term (OR, 1.11; 95% CI, 1.06-1.16), and post term (OR, 1.17; 95% CI, 1.07-1.27) were more likely to be classified in the suspected DCD category on the Little Developmental Coordination Disorder Questionnaire than completely full-term children after adjusting for the same characteristics. Additionally, there was no association with suspected DCD in younger (aged 3 years) early-term and postterm children by stratified analyses.
Conclusions and Relevance
In this cohort study, every degree of prematurity at birth, early-term birth, and postterm birth were associated with suspected DCD when compared with full-term birth. These findings have important implications for understanding motor development in children born at different gestational ages. Long-term follow-up and rehabilitation interventions should be considered for children born early and post term.
Introduction
Developmental coordination disorder (DCD) is characterized by marked impairment of motor coordination, which commonly results in persistent and significant difficulties in performing daily activities involving balance or manual skills.1,2,3 The prevalence of DCD in children aged 5 to 11 years is estimated to be 5% to 6% worldwide,4 with an even higher rate of prevalence (8.3%) in China.5,6 However, due to the large variability in normal motor development, a diagnosis of DCD is generally not recommended before the age of 5 years.7
Studies investigating the risk factors for DCD suggest that prenatal and perinatal influences may be associated with the development of later impairments.5 Preterm infants are at a significantly higher risk of suboptimal brain development,8 with the risk of DCD increasing with younger gestational age.9,10 Children born very preterm (<32 weeks) have been found to have a higher risk of developing DCD.11,12,13,14,15,16 Mild and moderate motor impairments occur in almost half of all preterm children (<37 weeks).17 However, although it has been reported that late preterm children (34-36 weeks) have more neonatal morbidities than full-term infants18 and experience neuromotor delay during their first year of life,19 few studies have explored the association between late preterm birth and DCD in preschool or school-aged children beyond the first year of life. There appear to be inconsistencies in the literature, with studies showing that late preterm infants otherwise born healthy seem to have no delay in their cognition, motor performance, behavior, or socioemotional development throughout childhood,20,21 whereas other studies report significant differences in neurodevelopment between late preterm and full-term children.21,22,23,24
Gestational age is a crucial factor in predicting motor development and DCD,25 but less attention has been paid to the relationship between gestational age in weeks and motor development in children born full term (≥37 weeks). Existing data have indicated that longer gestation (within 37-41 weeks) is associated with better cognitive and psychomotor development in children aged 12 months.26 Other studies have reported that children born at 39 weeks or later have fewer neonatal morbidities27 and better cognitive and academic outcomes than those born at 37 to 38 weeks (early term but still full-term birth).26,28,29,30,31 The number of gestational weeks in the full-term range has also been found to be associated with neuromotor and motor development in infants aged 9 to 15 weeks32 and infants aged 12 months.26
Recently, studies33,34,35,36,37 have reported that postterm birth (>41 weeks) is negatively associated with a child’s short-term and long-term health outcomes. A meta-analysis38 found that postterm birth is associated with significant negative effects on cognitive measures compared with full-term birth. Children born post term were more likely to have emotional and behavioral problems at both age 18 and 36 months compared with full-term children.36 Postterm children were also found to manifest increased risk and symptomatology of autism spectrum disorder33,39 and attention-deficit/hyperactivity disorder.40 However, children born post term were also reported to reach the main developmental milestones in their infancy when compared with full-term children.41 Therefore, the literature on the association between postterm birth and later development remains inconclusive.
In this study, we used a retrospective cohort design to examine the association of gestational age with suspected DCD within a sample of urban Chinese children. We hypothesized that children born at every degree of prematurity (<37 weeks), early term (37-38 weeks), and post term (>41 weeks) had an increased risk of suspected DCD compared with children born completely full term (39-40 weeks). The aims of this study were to (1) investigate the associated risk of suspected DCD in children born at different gestational weeks and (2) explore the association between gestational age and the risk of suspected DCD by age and sex.
Methods
Study Population and Study Design
The present study was part of a large national retrospective cohort study in China to explore neurobehavioral development in Chinese children. A stratified cluster sampling plan was used to ensure that the participants included in the current study were representative of the Chinese population. China's 2018 to 2019 National Census data provided the basis for the stratification by geographic region, age, sex, and socioeconomic status. Ethnic information was not collected because more than 99% of the population in the targeted regions were Han according to the National Census. The government-supported maternity and children’s health center in each city was selected to invite their local kindergartens to participate in the study. Class teachers were responsible for distributing the notification to parents to complete the online questionnaire; names and phone numbers of the researchers were provided in case the parents had queries. We used an electronic questionnaire system to enhance the quality of the data by allowing the inclusion of pop-up instructions, error messages, links to further information, and to set conditions to ensure participants could not skip questions. A data coordination center was established to take charge of establishing, managing, and maintaining the database and website, coordinating among health centers.
Only mainstream schools and nurseries were included in the study. Children with severe visual, hearing, or intellectual impairments (according to the examinations before starting kindergarten) or other severe developmental disorders who were required to attend special education schools or nurseries according to the local regulations were excluded. From April 1, 2018, to December 31, 2019, a total of 189 375 preschoolers were recruited from 2403 mainstream kindergartens in 551 cities of China.
It is a normal practice for parents to keep in touch with their children’s nursery via smart devices in China, including all of the kindergartens involved in the current study. It was therefore assumed that all of the parents in the current study had relatively high proficiency in online questionnaire completion. A very small proportion of the parents (n = 561; 0.3%) chose not to participate or left the questionnaire before fully completing it. Children aged 6 years or those with missing covariates were also excluded, leaving a total of 152 433 children for the final analysis (Figure 1).
Figure 1. Flowchart of the Study Population.
DCD indicates developmental coordination disorder; LDCDQ, Little Developmental Coordination Disorder Questionnaire.
The study was approved by the ethics committee of Shanghai First Maternity and Infant Hospital (KS18156). The parents had given online written consent to participate in the study before completing the online questionnaire. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Outcomes, Independent Variables, and Other Covariates
Children’s motor performance was assessed using the Little Developmental Coordination Disorder Questionnaire (LDCDQ). The LDCDQ was developed to screen for motor coordination difficulties in children aged 3 and 4 years,42 and it can also be extended for use with children as old as 5 years.43 The LDCDQ consists of 15 items divided into 3 subcategories: control during movement, fine motor skills, and general coordination. Each category contains 5 items; for each item, parents are asked to compare the performance of their child with that of children of the same age and sex and to rate their child’s performance on a 5-point Likert scale (1 point, not at all relevant to my child, to 5 points, extremely relevant to my child). Each subcategory has a maximum score of 25 points. Scores are summed to give a maximum total score of 75 points, with higher scores indicating a higher level of motor proficiency. A previous study44 reported that the Chinese version of the LDCDQ has high internal consistency and split-half reliability and fair factor construct validity. According to previous recommendations,43,45 we used the age- and sex-specific norms of the LDCDQ, and cutoff scores were provided based on a national sample in China to indicate suspected impairments of motor coordination (suspected DCD was defined as LDCDQ ≤15th percentile; probably not DCD was defined as LDCDQ >15th percentile).
Independent Variables
Gestational age at birth was obtained from the mother’s medical records and was based on ultrasonography examination and date of last menstrual period. Gestational weeks were divided into 7 categories40,46: completely full term was defined as 39 to 40 weeks’ gestation, and the other 6 categories included very preterm (<32 weeks), moderately preterm (32-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), late term (41 weeks), and post term (>41 weeks).
Covariates
We included the child, family, and maternal health characteristics as potential confounders when exploring the association between gestational age and suspected DCD (Table 1). Most of these variables were dichotomized into yes or no; body mass index (calculated as weight in kilograms divided by height in meters squared) was used as an indicator of obesity based on each child’s height and weight.47 Family structures were classified into 3 types: 3-generation (or more) family, nuclear family, and single-parent family. The 3-generation (or more) family refers to a child who lives with their parents and grandparents, which is a traditional family structure in China; a nuclear family refers to a child who lives with only their parents; and single-parent family means that the child lives with 1 of their parents. We divided maternal age into 3 age bands: younger than 30 years, 30 to 34 years, and older than 34 years.48 Maternal complications of pregnancy and delivery were defined according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. The classification is defined as having 1 of the following maternal complications during pregnancy: vaginal bleeding during pregnancy, risk of miscarriage, use of antibiotics, use of fertility drugs, intrauterine distress, or fetal asphyxia.
Table 1. Child, Family, and Maternal Health Characteristics During Pregnancy in the Study Population (N = 152 433).
| Characteristic | Total | No. (%)a | ||||
|---|---|---|---|---|---|---|
| Sex | Age, y | |||||
| Male | Female | 3 | 4 | 5 | ||
| Child characteristics | ||||||
| Children’s age, mean (SD), y | 4.5 (0.8) | 4.5 (0.8) | 4.5 (0.8) | 3.5 (0.3) | 4.4 (0.3) | 5.4 (0.3) |
| BMI, mean (SD) | 15.6 (1.6) | 15.7 (1.6) | 15.4 (1.6) | 15.8 (1.4) | 15.5 (1.6) | 15.5 (1.8) |
| Right-handedness | ||||||
| No | 11 290 (7.4) | 6560 (8.2) | 4730 (6.6) | 4137 (9.2) | 4439 (7.5) | 2714 (5.7) |
| Yes | 141 143 (92.6) | 73 810 (91.8) | 67 333 (93.4) | 40 915 (90.8) | 55 357 (92.5) | 44 871 (94.3) |
| Eyesightb | ||||||
| Normal | 137 378 (90.1) | 72 371 (90.1) | 65 007 (90.2) | 40 862 (90.7) | 53 995 (90.3) | 42 521 (89.4) |
| Abnormal | 15 055 (9.9) | 7999 (9.9) | 7056 (9.8) | 4190 (9.3) | 5801 (9.7) | 5064 (10.6) |
| Birth weight, g | ||||||
| <2500 | 6303 (4.1) | 3068 (3.8) | 3235 (4.5) | 1724 (3.8) | 2659 (4.5) | 1920 (4.0) |
| ≥2500 | 146 130 (95.9) | 77 302 (96.2) | 68 828 (95.5) | 43 328 (96.2) | 57 137 (95.5) | 45 665 (96.0) |
| Gestational age at birth, wk | ||||||
| <32 (very preterm) | 5439 (3.6) | 2930 (3.6) | 2509 (3.5) | 1384 (3.1) | 2100 (3.5) | 1955 (4.1) |
| 32-33 (moderately preterm) | 2322 (1.5) | 1255 (1.6) | 1067 (1.5) | 589 (1.3) | 966 (1.6) | 767 (1.6) |
| 34-36 (late preterm) | 12 915 (8.5) | 7146 (8.9) | 5769 (8.0) | 3463 (7.7) | 5024 (8.4) | 4428 (9.3) |
| 37-38 (early term) | 38 875 (25.5) | 21 484 (26.7) | 17 391 (24.1) | 11 609 (25.8) | 15 069 (25.2) | 12 197 (25.6) |
| 39-40 (completely full term) | 76 501 (50.2) | 39 448 (49.1) | 37 053 (51.4) | 23 180 (51.5) | 30 142 (50.4) | 23 179 (48.7) |
| 41 (late term) | 8923 (5.8) | 4322 (5.4) | 4591 (6.4) | 2843 (6.3) | 3542 (5.9) | 2538 (5.4) |
| >41 (post term) | 7458 (4.9) | 3775 (4.7) | 3683 (5.1) | 1984 (4.4) | 2953 (5.0) | 2521 (5.3) |
| Mode of delivery | ||||||
| Vaginal | 81 718 (53.6) | 42 209 (52.5) | 39 509 (54.8) | 24 766 (55.0) | 32 136 (53.7) | 24 816 (52.2) |
| Cesarean | 70 715 (46.4) | 38 161 (47.4) | 32 554 (45.2) | 20 286 (45.0) | 27 660 (46.3) | 22 769 (47.8) |
| NICU admission | ||||||
| No | 137 063 (89.9) | 71 690 (89.2) | 65 373 (90.7) | 40 137 (89.1) | 53 816 (90.0) | 43 110 (90.6) |
| Yes | 15 370 (10.1) | 8680 (10.8) | 6690 (9.3) | 4915 (10.9) | 5980 (10.0) | 4475 (9.4) |
| Other developmental disordersc | ||||||
| No | 151 474 (99.4) | 79 791 (99.3) | 71 683 (99.5) | 44 802 (99.5) | 59 426 (99.4) | 47 246 (99.3) |
| Yes | 959 (0.6) | 579 (0.7) | 380 (0.5) | 250 (0.5) | 370 (0.6) | 339 (0.7) |
| Psychiatric medication | ||||||
| No | 151 418 (99.3) | 79 778 (99.3) | 71 640 (99.4) | 44 694 (99.2) | 59 409 (99.3) | 47 315 (99.4) |
| Yes | 1015 (0.7) | 592 (0.7) | 423 (0.6) | 358 (0.8) | 387 (0.7) | 270 (0.6) |
| Family characteristics | ||||||
| Higher education of motherd | ||||||
| No | 70 473 (46.2) | 37 710 (46.9) | 32 763 (45.5) | 19 149 (42.5) | 27 165 (45.4) | 23 426 (49.2) |
| Yes | 81 960 (53.8) | 42 660 (53.1) | 39 300 (54.5) | 25 903 (57.5) | 32 631 (54.6) | 24 159 (50.8) |
| Higher education of fatherd | ||||||
| No | 71 493 (46.9) | 38 184 (47.5) | 33 309 (46.2) | 19 816 (44.0) | 27 452 (45.9) | 24 225 (50.9) |
| Yes | 80 940 (53.1) | 42 186 (52.5) | 38 754 (53.8) | 25 236 (56.0) | 32 344 (54.1) | 23 360 (49.1) |
| Mother’s occupation | ||||||
| Employed | 95 328 (62.5) | 50 580 (62.9) | 44 748 (62.1) | 28 643 (63.6) | 37 398 (62.5) | 29 287 (61.5) |
| Unemployed | 57 105 (37.5) | 29 790 (37.1) | 27 315 (37.9) | 16 409 (36.4) | 22 398 (37.5) | 18 298 (38.5) |
| Father’s occupation | ||||||
| Employed | 120 489 (79.0) | 63 548 (79.1) | 56 941 (79.0) | 36 305 (80.6) | 47 258 (79.0) | 36 926 (77.6) |
| Unemployed | 31 944 (21.0) | 16 822 (21.0) | 15 122 (21.0) | 8747 (19.4) | 12 538 (21.0) | 10 659 (22.4) |
| Family annual per-capita income, RMBe | ||||||
| <30 000 | 28 419 (18.6) | 14 829 (18.5) | 13 590 (18.9) | 7839 (17.4) | 11 277 (18.9) | 9303 (19.6) |
| ≥30 000 | 124 014 (81.4) | 65 541 (81.5) | 58 473 (81.1) | 37 213 (82.6) | 48 519 (81.1) | 38 282 (80.5) |
| Family structure | ||||||
| Single-parent families | 3610 (2.4) | 1808 (2.3) | 1802 (2.5) | 899 (20.0) | 1410 (2.4) | 1301 (2.7) |
| Nuclear families | 94 990 (62.3) | 50 269 (62.6) | 44 721 (62.1) | 26 528 (58.9) | 37 390 (62.5) | 31 072 (65.3) |
| Extended families | 53 833 (35.3) | 28 293 (35.2) | 25 540 (35.4) | 17 625 (39.1) | 20 996 (35.1) | 15 212 (32.0) |
| No. of children in the family | ||||||
| 1 | 82 230 (54.0) | 44 355 (55.2) | 37 875 (52.6) | 24 917 (55.3) | 322 595 (54.5) | 24 718 (51.9) |
| ≥2 | 70 203 (46.0) | 36 015 (44.8) | 34 188 (47.4) | 20 135 (44.7) | 27 201 (45.5) | 22 867 (48.1) |
| Maternal health during pregnancy | ||||||
| Maternal age at delivery, y | ||||||
| <30 | 115 739 (75.9) | 61 024 (75.9) | 54 715 (75.9) | 33 061 (73.4) | 45 260 (75.7) | 37 418 (78.6) |
| 30-34 | 27 497 (18.1) | 14 553 (18.1) | 12 944 (18.0) | 8811 (19.5) | 10 957 (18.3) | 7729 (16.3) |
| ≥35 | 9197 (6.0) | 4793 (6.0) | 4404 (6.1) | 3180 (7.1) | 3579 (6.0) | 2438 (5.1) |
| Smoking or passive smoking during pregnancy | ||||||
| No | 111 163 (72.9) | 58 631 (73.0) | 52 532 (72.9) | 32 748 (72.7) | 43 546 (72.8) | 34 869 (73.3) |
| Yes | 41 270 (27.1) | 21 739 (27.0) | 19 531 (27.1) | 12 304 (27.3) | 16 250 (27.2) | 12 716 (26.7) |
| Maternal complications during pregnancyf | ||||||
| No | 145 088 (95.2) | 76 567 (95.3) | 68 521 (95.1) | 42 517 (94.4) | 57 032 (95.4) | 45 539 (95.7) |
| Yes | 7345 (4.8) | 3803 (4.7) | 3542 (4.9) | 2535 (5.6) | 2764 (4.6) | 2046 (4.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NICU, neonatal intensive care unit; RMB, renminbi (Chinese currency).
Units written as No. (%) unless otherwise specified.
Eyesight indicates the power or faculty of seeing.
Other developmental disorders included autism spectrum disorder, attention-deficit/hyperactivity disorder, and learning disorders.
Higher education of mother or father indicates tertiary education leading to an academic degree.
The national average family per-capita income of the year before the survey time. As of November 12, 2021, 1 RMB is equal to 0.16 US dollars.
Indicates having 1 of the following maternal complications during pregnancy: vaginal bleeding during pregnancy, risk of miscarriage, use of antibiotics, use of fertility drugs, intrauterine distress, or fetal asphyxia.
Statistical Analysis
A mixed model using a random intercept (we hypothesized that there was no interaction between kindergartens and total LDCDQ scores) was used to investigate the associations of the different gestational ages (very preterm, moderately preterm, late preterm, early term, late term, and post term) with the total score and subscores of LDCDQ compared with full-term birth. A multilevel logistic regression model was used to determine the strength of association for different gestational ages associated with suspected DCD. In the mixed and logistic regression models, we considered the kindergartens as primary sampling units and other potential confounders (child, family, and maternal health characteristics as shown in Table 1). All P values were 2-sided, and P < .05 was denoted as statistically significant. Statistical analyses were performed using the lmer and Glmer functions in R, version 4.0.1 (R Core Team).
Results
A total of 152 433 children aged 3 to 5 years old (mean [SD] age, 4.5 [0.8] years; 80 370 boys [52.7%]; 72 063 girls [47.3%]) were included in the study. There were 45 052 children (29.6%) aged 3 years, 59 796 (39.2%) aged 4 years, and 47 585 (31.2%) aged 5 years. A total of 5439 births (3.6%) were very preterm, 2322 (1.5%) were moderately preterm, 12 915 (8.5%) were late preterm, 38 875 (25.5%) were early term, 76 501 (50.2%) were full term, 8923 (5.9%) were late term, and 7458 (4.9%) were post term. The child, family, and maternal health during pregnancy characteristics in the study population are shown in Table 1. The LDCDQ scores and rates of suspected DCD by different gestational weeks are shown in eTable 1 in the Supplement and Table 2.
Table 2. Association Between Gestational Age and Suspected DCD in Preschoolers (N = 152 433).
| Gestational age | Probably not DCD, No. (%) (n = 128 106) | Suspected DCD, No. (%) (n = 24 327) | Suspected DCD vs probably not DCD | |||
|---|---|---|---|---|---|---|
| Crude | Adjusteda | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Very preterm (<32 wk) | 4171 (76.7) | 1268 (23.3) | 1.70 (1.59-1.81) | <.001 | 1.52 (1.42-1.63) | <.001 |
| Moderately preterm (32-33 wk) | 1841 (79.3) | 481 (20.7) | 1.49 (1.35-1.66) | 1.349 (1.21-1.50) | ||
| Late preterm (34-36 wk) | 10 338 (80.1) | 2577 (20.0) | 1.41 (1.34-1.48) | 1.29 (1.23-1.36) | ||
| Early term (37-38 wk) | 32 531 (83.7) | 6344 (16.3) | 1.14 (1.10-1.18) | 1.11 (1.07-1.15) | ||
| Completely full term (39-40 wk) | 65 430 (85.5) | 11 071 (14.5) | 1 [Reference] | 1 [Reference] | ||
| Late term (41 wk) | 7658 (85.8) | 1265 (14.2) | 0.98 (0.92-1.04) | 0.98 (0.91-1.04) | ||
| Post term (>41 wk) | 6137 (82.3) | 1321 (17.7) | 1.23 (1.15-1.31) | 1.15 (1.08-1.23) | ||
Abbreviations: DCD, developmental coordination disorder; OR, odds ratio.
Adjusted for kindergartens (as cluster to control the unmeasured factors in kindergarten environment), child, family, and maternal health characteristics during pregnancy.
When children in the suspected DCD and probably not DCD groups for each gestational age category were compared with the completely full-term category, adjusting for kindergarten, child, family, and maternal health characteristics, children with very preterm (odds ratio [OR] 1.52; 95% CI, 1.42-1.63; P < .001), moderately preterm (OR, 1.35; 95% CI, 1.21-1.50; P < .001), late preterm (OR, 1.29; 95% CI, 1.23-1.36; P < .001), early term (OR, 1.11; 95% CI, 1.07-1.15; P < .001), and postterm births (OR, 1.15; 95% CI, 1.08-1.23; P < .001) had increased risk of suspected DCD. Late term birth was not associated with an increased risk of suspected DCD (Table 2). Children who were born very preterm (OR, 1.35; 95% CI, 1.23-1.48), moderately preterm (OR, 1.18; 95% CI, 1.02-1.36), late preterm (OR, 1.24; 95% CI, 1.16-1.32), early term (OR, 1.11; 95% CI, 1.06-1.16), and post term (OR, 1.17; 95% CI, 1.07-1.27) were more likely to be classified in the suspected DCD category on the LDCDQ than completely full-term children after adjusting for the same characteristics (Table 2). Additionally, the total LDCDQ scores and subscores (motor control, writing/fine motor skills, and general coordination) were significantly lower for each gestational age category when compared with the completely full-term category when adjusting for the same covariates (eTable 2 in the Supplement). However, there were no significant differences in the suspected DCD and LDCDQ score between late-term birth and completely full-term birth.
When we divided the children into age years, the results showed that the statistically significant association between gestational age and LDCDQ total score remained in the preterm and early-term children; however, there was no association in postterm children aged 3 years (eFigure in the Supplement). There was no association between gestational age and risk of suspected DCD in early-term and postterm children aged 3 years (Figure 2). The adjusted associations of gestational age (including preterm, early term, and post term) with the LDCDQ total score (eFigure in the Supplement) and with suspected DCD (Figure 2) remained significant in both boys and girls.
Figure 2. Association Between Gestational Age and Risk of Suspected Developmental Coordination Disorder in Preschoolers When Adjusting for Child, Family Characteristic, and Maternal Health by Sex and Age (N = 152 433).
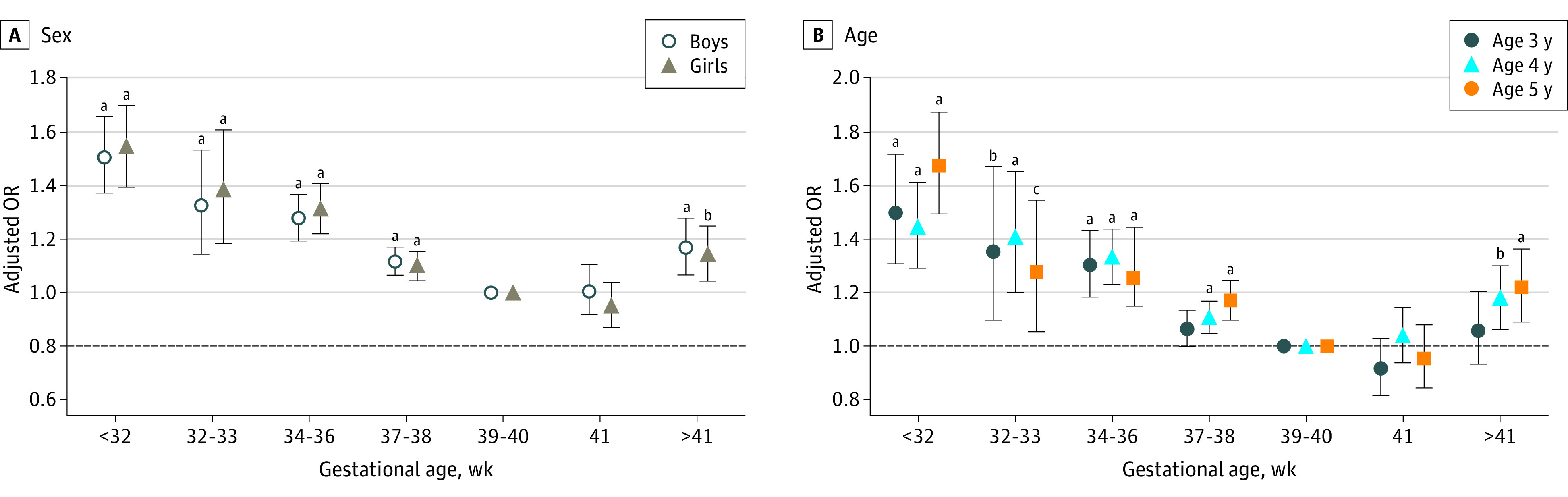
Error bars indicate 95% CIs. OR indicates odds ratio.
aP < .05.
bP < .01.
cP < .001.
Discussion
To our knowledge, this is the first study with a large national representative population sample to explore the association between gestational age in a full range and suspected DCD. We found significant associations in every degree of prematurity with suspected DCD. More importantly, associations between early term (37-38 weeks) and postterm (>41 weeks) birth and suspected DCD were also found when compared with full-term birth. Additionally, we observed that these associations did not exist in younger children (aged 3 years) in stratified analyses.
Our findings were consistent with previous work, which also found that DCD was more likely to occur in children born preterm, especially in those born very preterm11,15,16,49,50 and moderately preterm.9 Infants who were born both moderately preterm and early term showed a higher level of impairment in fine motor skills than infants born full term.51 Infants with moderate and late preterm births displayed a neuromotor delay during the first year of life.19 Previous studies suggested that the brain microstructure is highly correlated with motor impairment in preterm children. For instance, white matter alterations were found in children born preterm with very low birth weight.52 Higher fractional anisotropy in all major white matter tracts in very preterm infants at term-equivalent age was found to be correlated with a superior fine motor performance at age 2 years.53 Additionally, brain development occurs in a very specific order and time frame.29 Preterm infants cared for in neonatal intensive care units (NICUs) face substantial developmental challenges that differ substantially from the natural brain maturation that normally occurs in the uterus. The varying clinical processes in the NICU have different effects on the shape of the brainstem, as is evident in several distinct neurodevelopmental profiles observed in premature newborns.54 Studies have also shown that morphine exposure and exposure to painful procedures were associated with poor cerebellar growth in the neonatal period and neurodevelopmental impairment in very preterm children.55,56 Moreover, the brain structures of early term and full-term infants were found to differ in their concurrent motor, neurologic, and neurobehavioral functions when compared with those of a full-term infant.57
We also found decreased motor performance and increased risk of suspected DCD in early-term children (37-38 weeks) after accounting for a broad range of possible confounders including child, family, and maternal health characteristics. Previous studies emphasized the importance of the last few weeks during gestation, when a large portion of brain development takes place.12 The volume of total gray matter increases by approximately 1.4% per week from 29 to 41 weeks of gestation, along with a 5-fold increase in white matter volume between 35 and 41 weeks of gestation.58,59 The external granular layer expands horizontally to accommodate a more than 30-fold increase in the hemisphere surface area that occurs from 30 to 40 gestational weeks; therefore, the number of cells increases substantially.60 Neuroimaging research indicates that longer gestation is associated with region-specific increases in gray matter density61 and a more efficient neural network.62 Not only preterm birth, but also early term birth, can cause disruption at specific intervals during the brain’s development of neural connections for specific cognitive areas.63 A recent systematic review reported that children born early term are at an increased risk of having cognitive deficits, poorer school performance, and behavioral problems compared with children born full term.64 To our knowledge, our study is the first to report an association between early term birth and an increased risk of suspected DCD, which suggests that children born early term should be monitored more carefully owing to the wide range of developmental effects.
Another finding of our study was the observed association between postterm birth and suspected DCD. It has been previously reported that postterm birth increases the risks of cognitive impairments, severe mental disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and other behavioral and emotional problems in early childhood.33,34,38,39,65,66 Our study results suggest that children born after 41 gestational weeks were also more likely to develop DCD. This may have been due to the complicated conditions related to postterm birth, including prolonged labor, cephalopelvic disproportion, and shoulder dystocia,67 all of which may enhance the risk of perinatal oxygen deficiency. Earlier studies reported that perinatal lack of oxygen is associated with DCD.5 Additionally, postterm birth can increase the risks of fewer nutrients and less oxygen offered to a fetus larger than normal size by a postterm placenta,37 which may be related to atypical motor development. The underlying causes of DCD among children who are born post term should be explored in future research.
Our results also showed that the association between gestational age (children born early term and post term) and suspected DCD did not persist in younger children (aged 3 years). Although there is some debate in the literature about the variability of motor performance,68 it has been reported that typical motor development is characterized by variation and the development of adaptive variability, but atypical motor development is characterized by limitations in variation and variability.69 Therefore, in early term and postterm birth, the motor performance gap between children with typical and atypical development may become evident as children grow older. Early identification of those at risk of later DCD can provide an important opportunity for early intervention.70
Limitations
Our study had several limitations. In our study, the LDCDQ was used to measure motor performance. The LDCDQ was specifically designed to identify preschoolers at risk of DCD. Although previous studies have shown that the LDCDQ has relatively high sensitivity and specificity,42,43,71 there are potential limitations as it is a very short questionnaire. In particular, considering the large variability of motor performance of preschoolers,37 the motor capability measured by LDCDQ may not reflect the complete motor profile of young children. Additionally, because this was a retrospective cohort study, our research results cannot be used to identify causal relationships among variables. Possible recall bias may also exist in a retrospective cohort study. Further research is needed to explain the mechanisms linking early and postterm birth to DCD.
Conclusions
Results of this cohort study suggest that, in addition to children born preterm, children born early term and post term also have an increased risk of DCD. Although the absolute risks of early term and postterm birth were lower than those of preterm birth, children born early term and post term should be monitored more carefully than full-term children; this can be accomplished with long-term follow-up evaluations. Our results may serve to remind clinical professionals, parents, and teachers to not neglect the long-term neurodevelopmental risks of children who are born in the early-term and postterm gestational period. The results of this study may inform clinical professionals when considering the optimal timing of birth during the full-term period. This consideration seems particularly relevant in an era when rates of cesarean delivery are high and induction of labor is common,19,20 and those interventions of elective birth should only be recommended if the risk of continuing the pregnancy (postterm birth) is higher than the risk of delivery.72
eFigure. The Association of Gestational Age With LDCDQ Scores in Preschoolers When Adjusting for Child, Family, and Maternal Health Characteristics by (A) Sex and (B) Age
eTable 1. The LDCDQ Scores by Different Gestational Weeks
eTable 2. The Association Between Gestational Age and LDCDQ Scores in Preschoolers
References
- 1.Poulsen AA, Ziviani JM. Can I play too? Physical activity engagement of children with developmental coordination disorders. Can J Occup Ther. 2004;71(2):100-107. doi: 10.1177/000841740407100205 [DOI] [PubMed] [Google Scholar]
- 2.Wright KE, Furzer BJ, Licari MK, et al. Physiological characteristics, self-perceptions, and parental support of physical activity in children with, or at risk of, developmental coordination disorder. Res Dev Disabil. 2019;84:66-74. doi: 10.1016/j.ridd.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 3.Cermak SA, Katz N, Weintraub N, et al. Participation in physical activity, fitness, and risk for obesity in children with developmental coordination disorder: a cross-cultural study. Occup Ther Int. 2015;22(4):163-173. doi: 10.1002/oti.1393 [DOI] [PubMed] [Google Scholar]
- 4.Kirby A, Sugden DA. Children with developmental coordination disorders. J R Soc Med. 2007;100(4):182-186. doi: 10.1177/014107680710011414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hua J, Gu G, Jiang P, Zhang L, Zhu L, Meng W. The prenatal, perinatal and neonatal risk factors for children’s developmental coordination disorder: a population study in mainland China. Res Dev Disabil. 2014;35(3):619-625. doi: 10.1016/j.ridd.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 6.Hua J, Jin H, Gu G, Liu M, Zhang L, Wu Z. The influence of Chinese one-child family status on developmental coordination disorder status. Res Dev Disabil. 2014;35(11):3089-3095. doi: 10.1016/j.ridd.2014.07.044 [DOI] [PubMed] [Google Scholar]
- 7.Blank R, Barnett AL, Cairney J, et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev Med Child Neurol. 2019;61(3):242-285. doi: 10.1111/dmcn.14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 2014;4(1):57-67. doi: 10.2217/pmt.13.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faebo Larsen R, Hvas Mortensen L, Martinussen T, Nybo Andersen AM. Determinants of developmental coordination disorder in 7-year-old children: a study of children in the Danish National Birth Cohort. Dev Med Child Neurol. 2013;55(11):1016-1022. doi: 10.1111/dmcn.12223 [DOI] [PubMed] [Google Scholar]
- 10.Du W, Ke L, Wang Y, Hua J, Duan W, Barnett AL. The prenatal, postnatal, neonatal, and family environmental risk factors for developmental coordination disorder: a study with a national representative sample. Res Dev Disabil. 2020;104:103699. doi: 10.1016/j.ridd.2020.103699 [DOI] [PubMed] [Google Scholar]
- 11.Edwards J, Berube M, Erlandson K, et al. Developmental coordination disorder in school-aged children born very preterm and/or at very low birth weight: a systematic review. J Dev Behav Pediatr. 2011;32(9):678-687. doi: 10.1097/DBP.0b013e31822a396a [DOI] [PubMed] [Google Scholar]
- 12.Bolk J, Farooqi A, Hafström M, Åden U, Serenius F. Developmental coordination disorder and its association with developmental comorbidities at 6.5 years in apparently healthy children born extremely preterm. JAMA Pediatr. 2018;172(8):765-774. doi: 10.1001/jamapediatrics.2018.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok C, Mackay M, Agnew JA, Synnes A, Zwicker JG. Does the movement assessment battery for children-2 at 3 years of age predict developmental coordination disorder at 4.5 years of age in children born very preterm? Res Dev Disabil. 2019;84:36-42. doi: 10.1016/j.ridd.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Wocadlo C, Rieger I. Motor impairment and low achievement in very preterm children at eight years of age. Early Hum Dev. 2008;84(11):769-776. doi: 10.1016/j.earlhumdev.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 15.Goyen TA, Lui K. Developmental coordination disorder in “apparently normal” schoolchildren born extremely preterm. Arch Dis Child. 2009;94(4):298-302. doi: 10.1136/adc.2007.134692 [DOI] [PubMed] [Google Scholar]
- 16.Roberts G, Anderson PJ, Davis N, De Luca C, Cheong J, Doyle LW; Victorian Infant Collaborative Study Group . Developmental coordination disorder in geographic cohorts of 8-year-old children born extremely preterm or extremely low birthweight in the 1990s. Dev Med Child Neurol. 2011;53(1):55-60. doi: 10.1111/j.1469-8749.2010.03779.x [DOI] [PubMed] [Google Scholar]
- 17.Spittle AJ, Orton J. Cerebral palsy and developmental coordination disorder in children born preterm. Semin Fetal Neonatal Med. 2014;19(2):84-89. doi: 10.1016/j.siny.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 18.Mitha A, Chen R, Altman M, Johansson S, Stephansson O, Bolk J. Neonatal morbidities in infants born late preterm at 35-36 weeks of gestation: a Swedish nationwide population-based study. J Pediatr. 2021;233:43-50.e5. doi: 10.1016/j.jpeds.2021.02.066 [DOI] [PubMed] [Google Scholar]
- 19.Chatziioannidis I, Kyriakidou M, Exadaktylou S, Antoniou E, Zafeiriou D, Nikolaidis N. Neurological outcome at 6 and 12 months corrected age in hospitalised late preterm infants -a prospective study. Eur J Paediatr Neurol. 2018;22(4):602-609. doi: 10.1016/j.ejpn.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 20.Gurka MJ, LoCasale-Crouch J, Blackman JA. Long-term cognition, achievement, socioemotional, and behavioral development of healthy late-preterm infants. Arch Pediatr Adolesc Med. 2010;164(6):525-532. doi: 10.1001/archpediatrics.2010.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benzies KM, Magill-Evans J, Ballantyne M, Kurilova J. Longitudinal patterns of early development in Canadian late preterm infants: A prospective cohort study. J Child Health Care. 2017;21(1):85-93. doi: 10.1177/1367493516689167 [DOI] [PubMed] [Google Scholar]
- 22.Cheong JLY, Thompson DK, Olsen JE, Spittle AJ. Late preterm births: new insights from neonatal neuroimaging and neurobehaviour. Semin Fetal Neonatal Med. 2019;24(1):60-65. doi: 10.1016/j.siny.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 23.McGowan JE, Alderdice FA, Holmes VA, Johnston L. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127(6):1111-1124. doi: 10.1542/peds.2010-2257 [DOI] [PubMed] [Google Scholar]
- 24.Cheong JL, Doyle LW, Burnett AC, et al. Association between moderate and late preterm birth and neurodevelopment and social-emotional development at age 2 years. JAMA Pediatr. 2017;171(4):e164805. doi: 10.1001/jamapediatrics.2016.4805 [DOI] [PubMed] [Google Scholar]
- 25.Zhu JL, Olsen J, Olesen AW. Risk for developmental coordination disorder correlates with gestational age at birth. Paediatr Perinat Epidemiol. 2012;26(6):572-577. doi: 10.1111/j.1365-3016.2012.01316.x [DOI] [PubMed] [Google Scholar]
- 26.Rose O, Blanco E, Martinez SM, et al. Developmental scores at 1 year with increasing gestational age, 37-41 weeks. Pediatrics. 2013;131(5):e1475-e1481. doi: 10.1542/peds.2012-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACOG Committee . ACOG Committee opinion No. 765: avoidance of nonmedically indicated early-term deliveries and associated neonatal morbidities. Obstet Gynecol. 2019;133(2):e156-e163. doi: 10.1097/AOG.0000000000003076 [DOI] [PubMed] [Google Scholar]
- 28.Richards JL, Drews-Botsch C, Sales JM, Flanders WD, Kramer MR. Describing the shape of the relationship between gestational age at birth and cognitive development in a nationally representative U.S. birth cohort. Paediatr Perinat Epidemiol. 2016;30(6):571-582. doi: 10.1111/ppe.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan E, Leong P, Malouf R, Quigley MA. Long-term cognitive and school outcomes of late-preterm and early-term births: a systematic review. Child Care Health Dev. 2016;42(3):297-312. doi: 10.1111/cch.12320 [DOI] [PubMed] [Google Scholar]
- 30.Noble KG, Fifer WP, Rauh VA, Nomura Y, Andrews HF. Academic achievement varies with gestational age among children born at term. Pediatrics. 2012;130(2):e257-e264. doi: 10.1542/peds.2011-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta S, Carrion V, Shelton J, et al. Adverse neonatal outcomes associated with early-term birth. JAMA Pediatr. 2013;167(11):1053-1059. doi: 10.1001/jamapediatrics.2013.2581 [DOI] [PubMed] [Google Scholar]
- 32.van Batenburg-Eddes T, de Groot L, Arends L, et al. Does gestational duration within the normal range predict infant neuromotor development? Early Hum Dev. 2008;84(10):659-665. doi: 10.1016/j.earlhumdev.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 33.Persson M, Opdahl S, Risnes K, et al. Gestational age and the risk of autism spectrum disorder in Sweden, Finland, and Norway: a cohort study. PLoS Med. 2020;17(9):e1003207. doi: 10.1371/journal.pmed.1003207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahti M, Eriksson JG, Heinonen K, et al. Late preterm birth, post-term birth, and abnormal fetal growth as risk factors for severe mental disorders from early to late adulthood. Psychol Med. 2015;45(5):985-999. doi: 10.1017/S0033291714001998 [DOI] [PubMed] [Google Scholar]
- 35.Odd D, Glover Williams A, Winter C, Draycott T. Associations between early term and late/post term infants and development of epilepsy: a cohort study. PLoS One. 2018;13(12):e0210181. doi: 10.1371/journal.pone.0210181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Marroun H, Zeegers M, Steegers EA, et al. Post-term birth and the risk of behavioural and emotional problems in early childhood. Int J Epidemiol. 2012;41(3):773-781. doi: 10.1093/ije/dys043 [DOI] [PubMed] [Google Scholar]
- 37.Caughey AB, Snegovskikh VV, Norwitz ER. Postterm pregnancy: how can we improve outcomes? Obstet Gynecol Surv. 2008;63(11):715-724. doi: 10.1097/OGX.0b013e318186a9c7 [DOI] [PubMed] [Google Scholar]
- 38.Glover Williams A, Odd D. Investigating the association between post-term birth and long term cognitive, developmental and educational impacts: a systematic review and Meta-analysis. J Matern Fetal Neonatal Med. 2020;33(7):1253-1265. doi: 10.1080/14767058.2018.1514379 [DOI] [PubMed] [Google Scholar]
- 39.Movsas TZ, Paneth N. The effect of gestational age on symptom severity in children with autism spectrum disorder. J Autism Dev Disord. 2012;42(11):2431-2439. doi: 10.1007/s10803-012-1501-4 [DOI] [PubMed] [Google Scholar]
- 40.Ask H, Gustavson K, Ystrom E, et al. Association of gestational age at birth with symptoms of attention-deficit/hyperactivity disorder in children. JAMA Pediatr. 2018;172(8):749-756. doi: 10.1001/jamapediatrics.2018.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olesen AW, Olsen J, Zhu JL. Developmental milestones in children born post-term in the Danish national birth cohort: a main research article. BJOG. 2015;122(10):1331-1339. doi: 10.1111/1471-0528.13237 [DOI] [PubMed] [Google Scholar]
- 42.Rihtman T, Wilson BN, Parush S. Development of the little developmental coordination disorder questionnaire for preschoolers and preliminary evidence of its psychometric properties in Israel. Res Dev Disabil. 2011;32(4):1378-1387. doi: 10.1016/j.ridd.2010.12.040 [DOI] [PubMed] [Google Scholar]
- 43.Cantell M, Houwen S, Schoemaker M. Age-related validity and reliability of the Dutch little developmental coordination disorder questionnaire (LDCDQ-NL). Res Dev Disabil. 2019;84:28-35. doi: 10.1016/j.ridd.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 44.Geng S, Dai X, Wang T, Cui W, Hua J. The preliminary study on the reliability and validity of the Chinese version of the little developmental coordination disorder questionnaire. Article in Chinese. [J Clin Pediatr]. 2020;38(12):921-924. doi: 10.3969/j.issn.1000-3606.2020.12.010 [DOI] [Google Scholar]
- 45.Wilson BN, Creighton D, Crawford SG, et al. Psychometric properties of the Canadian little developmental coordination disorder questionnaire for preschool children. Phys Occup Ther Pediatr. 2015;35(2):116-131. doi: 10.3109/01942638.2014.980928 [DOI] [PubMed] [Google Scholar]
- 46.Zambrana IM, Vollrath ME, Sengpiel V, Jacobsson B, Ystrom E. Preterm delivery and risk for early language delays: a sibling-control cohort study. Int J Epidemiol. 2016;45(1):151-159. doi: 10.1093/ije/dyv329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Consultation WHOE; WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 48.Teng X, Shane MI, Pan S. The changing situation about maternal age, risk factors and pregnancy outcomes after the two-child policy: a retrospective cohort study. Ann Palliat Med. 2020;9(3):824-834. doi: 10.21037/apm.2020.04.27 [DOI] [PubMed] [Google Scholar]
- 49.Davis NM, Ford GW, Anderson PJ, Doyle LW; Victorian Infant Collaborative Study Group . Developmental coordination disorder at 8 years of age in a regional cohort of extremely-low-birthweight or very preterm infants. Dev Med Child Neurol. 2007;49(5):325-330. doi: 10.1111/j.1469-8749.2007.00325.x [DOI] [PubMed] [Google Scholar]
- 50.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA. 2009;302(20):2235-2242. doi: 10.1001/jama.2009.1708 [DOI] [PubMed] [Google Scholar]
- 51.Kerstjens JM, de Winter AF, Bocca-Tjeertes IF, ten Vergert EM, Reijneveld SA, Bos AF. Developmental delay in moderately preterm-born children at school entry. J Pediatr. 2011;159(1):92-98. doi: 10.1016/j.jpeds.2010.12.041 [DOI] [PubMed] [Google Scholar]
- 52.Hollund IMH, Olsen A, Skranes J, et al. White matter alterations and their associations with motor function in young adults born preterm with very low birth weight. Neuroimage Clin. 2017;17:241-250. doi: 10.1016/j.nicl.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Kooij BJ, de Vries LS, Ball G, et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol. 2012;33(1):188-194. doi: 10.3174/ajnr.A2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lo M, Zubiaurre-Elorza L, Wild C, et al. Brainstem shape is affected by clinical course in the neonatal intensive care unit. Neuroimage Clin. 2017;15:62-70. doi: 10.1016/j.nicl.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwicker JG, Miller SP, Grunau RE, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. 2016;172:81-87.e2. doi: 10.1016/j.jpeds.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ranger M, Zwicker JG, Chau CM, et al. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J Pediatr. 2015;167(2):292-8.e1. doi: 10.1016/j.jpeds.2015.04.055 [DOI] [PubMed] [Google Scholar]
- 57.George JM, Fiori S, Fripp J, et al. Relationship between very early brain structure and neuromotor, neurological and neurobehavioral function in infants born <31 weeks gestational age. Early Hum Dev. 2018;117:74-82. doi: 10.1016/j.earlhumdev.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 58.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81-88. doi: 10.1053/j.semperi.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 59.Quigley MA, Poulsen G, Boyle E, et al. Early term and late preterm birth are associated with poorer school performance at age 5 years: a cohort study. Arch Dis Child Fetal Neonatal Ed. 2012;97(3):F167-F173. doi: 10.1136/archdischild-2011-300888 [DOI] [PubMed] [Google Scholar]
- 60.Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24(9):1085-1104. doi: 10.1177/0883073809338067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis EP, Buss C, Muftuler LT, et al. Children’s brain development benefits from longer gestation. Front Psychol. 2011;2:1. doi: 10.3389/fpsyg.2011.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim DJ, Davis EP, Sandman CA, et al. Longer gestation is associated with more efficient brain networks in preadolescent children. Neuroimage. 2014;100:619-627. doi: 10.1016/j.neuroimage.2014.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877-888. doi: 10.1038/nrn1787 [DOI] [PubMed] [Google Scholar]
- 64.Nielsen TM, Pedersen MV, Milidou I, Glavind J, Henriksen TB. Long-term cognition and behavior in children born at early term gestation: a systematic review. Acta Obstet Gynecol Scand. 2019;98(10):1227-1234. doi: 10.1111/aogs.13644 [DOI] [PubMed] [Google Scholar]
- 65.Simon L, Nusinovici S, Flamant C, et al. Post-term growth and cognitive development at 5 years of age in preterm children: Evidence from a prospective population-based cohort. PLoS One. 2017;12(3):e0174645. doi: 10.1371/journal.pone.0174645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell MK, Ostbye T, Irgens LM. Post-term birth: risk factors and outcomes in a 10-year cohort of Norwegian births. Obstet Gynecol. 1997;89(4):543-548. doi: 10.1016/S0029-7844(97)00049-5 [DOI] [PubMed] [Google Scholar]
- 67.Norwitz ER, Snegovskikh VV, Caughey AB. Prolonged pregnancy: when should we intervene? Clin Obstet Gynecol. 2007;50(2):547-557. doi: 10.1097/GRF.0b013e31804c9b11 [DOI] [PubMed] [Google Scholar]
- 68.Wade MG, Kazeck M. Developmental coordination disorder and its cause: the road less travelled. Hum Mov Sci. 2018;57:489-500. doi: 10.1016/j.humov.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 69.Hadders-Algra M. Variation and variability: key words in human motor development. Phys Ther. 2010;90(12):1823-1837. doi: 10.2522/ptj.20100006 [DOI] [PubMed] [Google Scholar]
- 70.Zwicker JG, Lee EJ. Early intervention for children with/at risk of developmental coordination disorder: a scoping review. Dev Med Child Neurol. 2021;63(6):659-667. doi: 10.1111/dmcn.14804 [DOI] [PubMed] [Google Scholar]
- 71.Hua J, Du W, Dai X, et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder—Chinese (Mandarin) translation. Dev Med Child Neurol. 2020. doi: 10.1111/dmcn.14693 [DOI] [PubMed] [Google Scholar]
- 72.Molina G, Weiser TG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314(21):2263-2270. doi: 10.1001/jama.2015.15553 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. The Association of Gestational Age With LDCDQ Scores in Preschoolers When Adjusting for Child, Family, and Maternal Health Characteristics by (A) Sex and (B) Age
eTable 1. The LDCDQ Scores by Different Gestational Weeks
eTable 2. The Association Between Gestational Age and LDCDQ Scores in Preschoolers



