Key Points
Question
Is preserved ratio impaired spirometry (PRISm), defined as the ratio of forced expired volume in the first second to forced vital capacity (FEV1:FVC) greater than or equal to 0.7 with an FEV1 less than 80% predicted, associated with adverse clinical outcomes relative to normal spirometry in a population-based sample of US adults?
Findings
In this retrospective cohort study that included 53 701 participants, the presence of PRISm at baseline, compared with normal spirometry, was significantly associated with increased subsequent risk for all-cause mortality (adjusted hazard ratio [adjusted HR], 1.50), respiratory-related mortality (adjusted HR, 1.95), coronary heart disease (CHD)-related mortality (adjusted HR, 1.55), respiratory-related hospitalizations and mortality (adjusted HR, 1.90), and CHD-related hospitalizations and mortality (adjusted HR, 1.30).
Meaning
PRISm was significantly associated with increased risk for mortality and adverse cardiovascular and respiratory outcomes, although further research is needed to understand whether this association is causal.
Abstract
Importance
Chronic lung diseases are a leading cause of morbidity and mortality. Unlike chronic obstructive pulmonary disease, clinical outcomes associated with proportional reductions in expiratory lung volumes without obstruction, otherwise known as preserved ratio impaired spirometry (PRISm), are poorly understood.
Objective
To examine the prevalence, correlates, and clinical outcomes associated with PRISm in US adults.
Design, Setting, and Participants
The National Heart, Lung, and Blood Institute (NHLBI) Pooled Cohorts Study was a retrospective study with harmonized pooled data from 9 US general population-based cohorts (enrollment, 65 251 participants aged 18 to 102 years of whom 53 701 participants had valid baseline lung function) conducted from 1971-2011 (final follow-up, December 2018).
Exposures
Participants were categorized into mutually exclusive groups by baseline lung function. PRISm was defined as the ratio of forced expiratory volume in the first second to forced vital capacity (FEV1:FVC) greater than or equal to 0.70 and FEV1 less than 80% predicted; obstructive spirometry FEV1:FVC ratio of less than 0.70; and normal spirometry FEV1:FVC ratio greater than or equal to 0.7 and FEV1 greater than or equal to 80% predicted.
Main Outcomes and Measures
Main outcomes were all-cause mortality, respiratory-related mortality, coronary heart disease (CHD)–related mortality, respiratory-related events (hospitalizations and mortality), and CHD-related events (hospitalizations and mortality) classified by adjudication or validated administrative criteria. Absolute risks were adjusted for age and smoking status. Poisson and Cox proportional hazards models comparing PRISm vs normal spirometry were adjusted for age, sex, race and ethnicity, education, body mass index, smoking status, cohort, and comorbidities.
Results
Among all participants (mean [SD] age, 53.2 [15.8] years, 56.4% women, 48.5% never-smokers), 4582 (8.5%) had PRISm. The presence of PRISm relative to normal spirometry was significantly associated with obesity (prevalence, 48.3% vs 31.4%; prevalence ratio [PR], 1.68 [95% CI, 1.55-1.82]), underweight (prevalence, 1.4% vs 1.0%; PR, 2.20 [95% CI, 1.72-2.82]), female sex (prevalence, 60.3% vs 59.0%; PR, 1.07 [95% CI, 1.01-1.13]), and current smoking (prevalence, 25.2% vs 17.5%; PR, 1.33 [95% CI, 1.22-1.45]). PRISm, compared with normal spirometry, was significantly associated with greater all-cause mortality (29.6/1000 person-years vs 18.0/1000 person-years; difference, 11.6/1000 person-years [95% CI, 10.0-13.1]; adjusted hazard ratio [HR], 1.50 [95% CI, 1.42-1.59]), respiratory-related mortality (2.1/1000 person-years vs 1.0/1000 person-years; difference, 1.1/1000 person-years [95% CI, 0.7-1.6]; adjusted HR, 1.95 [95% CI, 1.54-2.48]), CHD-related mortality (5.4/1000 person-years vs 2.6/1000 person-years; difference, 2.7/1000 person-years [95% CI, 2.1-3.4]; adjusted HR, 1.55 [95% CI, 1.36-1.77]), respiratory-related events (12.2/1000 person-years vs 6.0/1000 person-years; difference, 6.2/1000 person-years [95% CI, 4.9-7.5]; adjusted HR, 1.90 [95% CI, 1.69-2.14]), and CHD-related events (11.7/1000 person-years vs 7.0/1000 person-years; difference, 4.7/1000 person-years [95% CI, 3.7-5.8]; adjusted HR, 1.30 [95% CI, 1.18-1.42]).
Conclusions and Relevance
In a large, population-based sample of US adults, baseline PRISm, compared with normal spirometry, was associated with a small but statistically significant increased risk for mortality and adverse cardiovascular and respiratory outcomes. Further research is needed to explore whether this association is causal.
This study of pooled data from 9 US population-based cohorts assessed mortality risk and adverse cardiovascular and respiratory outcomes for chronic lung diseases among adults with normal spirometry vs preserved ratio impaired spirometry.
Introduction
Chronic lung disease is a leading cause of death in the US. The most commonly diagnosed chronic lung disease is chronic obstructive pulmonary disease (COPD), which causes airflow obstruction and is defined as the ratio of forced expired volume in the first second to forced vital capacity (FEV1:FVC) less than 0.70 on postbronchodilator spirometry. Nonobstructive lung function abnormalities, characterized by symmetric reductions in both FEV1 and FVC, have been termed as restrictive, unclassified, nonspecific, low lung function, and more recently, as preserved ratio impaired spirometry (PRISm).1
There are currently no guidelines for the diagnostic evaluation and management of PRISm despite high prevalence rates of PRISm globally, which is estimated at 5% to 20%.1,2,3,4 Compared with individuals with normal spirometry, those with PRISm have increased respiratory symptoms,1,5 reduced functional status,1,5 and greater all-cause6,7,8 mortality. Risk factors for PRISm (identified by prior studies) include advanced age,1 abnormal body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]),5 diabetes,1,4 and smoking.1,5,9
To address knowledge gaps about clinical outcomes associated with PRISm in the US population, data from the National Heart Lung and Blood Institute (NHLBI) Pooled Cohorts Study were analyzed. This pooled data set included a large sample of adults from 9 population-based cohorts and included all major US racial and ethnic groups.10 In addition to determining the prevalence and correlates of PRISm, this study tested the hypothesis that PRISm was associated with a greater risk of cardiovascular-related and respiratory-related hospitalization and mortality compared with normal spirometry.
Methods
Study Population
The NHLBI Pooled Cohorts Study harmonized and pooled data from 9 US cohorts with spirometry assessments recruited from community-dwelling adults10; enrollment periods, enrollment criteria, and censoring year for events in each cohort are shown in eTable 1 (Supplement). All studies were approved by the institutional review boards of the participating institutions; all participants provided written informed consent at enrollment. Specific approvals for secondary analysis of data contained within this manuscript were obtained from the Columbia University institutional review board and from each cohort.10 Individuals with at least 1 valid spirometry examination were retained for analysis; the first spirometry obtained was considered the baseline spirometry.
Spirometry
Lung function was assessed using prebronchodilator spirometry obtained using water seal, dry-rolling seal, or flow-sensing devices according to the American Thoracic Society guidelines at the time of testing (previously described).10 Protocols were standardized and similar across studies, facilitating retrospective quality checks and harmonization of the pooled cohort to the American Thoracic Society/European Respiratory Society 2005 standards. Reference equations derived from the US population using the National Health and Nutrition Examination Survey (NHANES) III data were used to determine percent predicted values based on age, sex, height, race, and ethnicity.11
PRISm was defined as having an FEV1:FVC ratio greater than or equal to 0.7 and an FEV1 less than 80% predicted, obstructive spirometry was defined as an FEV1:FVC ratio less than 0.7, and normal spirometry was defined as an FEV1:FVC ratio greater than or equal to 0.7 and an FEV1 greater than or equal to 80% predicted. In secondary analyses, Global Lung Function Initiative (GLI) reference equations were used to define percent predicted and lower limit of normal (LLN) thresholds for the FEV1:FVC ratio and FEV1, treating the race of all participants as other, which is an alternative approach that may be appropriate for diverse and admixed populations.12,13
Outcomes
Main outcomes were (1) all-cause mortality; (2) respiratory-related mortality; (3) coronary heart disease (CHD)-related mortality; (4) respiratory-related events,14,15 defined as hospitalizations or deaths for which chronic lower respiratory disease was listed as a primary, underlying, or contributing cause; and (5) CHD-related events, defined as hospitalizations or death due to CHD. Follow-up for the outcomes varied by cohort (eFigure 1 in the Supplement). Outcome data from the Hispanic Community Health Study were not available at the time of manuscript preparation. Eight of the 9 cohorts ascertained all-cause mortality and CHD-related hospitalizations and mortality, 6 of the 9 cohorts ascertained respiratory mortality, and 4 of the 9 cohorts assessed respiratory-related hospitalizations. Events were classified by adjudication or administrative criteria following previously validated protocols (eTable 2 in the Supplement).15,16
Covariates
Covariate measurements were collected on the day that baseline spirometry was performed and were harmonized systematically.10 Race and ethnicity, sex, birth year, and education were self-reported according to fixed categories (previously reported).14 Information about race was collected in this study because it was a variable used in the standard lung function prediction equations.11,17 Race and ethnicity were also included in this study to address specific knowledge gaps about members of historically marginalized or underserved populations in research studies about PRISm.1,2,4 Anthropometric measurements were performed using standard methods. Smoking status was self-reported as never or ever, defined as lifetime consumption of at least 100 cigarettes. Among ever-smokers, former or current smoking status (defined as smoking within the past 30 days) was self-reported, with biochemical confirmation using serum or urine cotinine in a subset.18 Pack-years of smoking were calculated as (cigarettes per day/20) multiplied by the number of years of smoking. Comorbidities, including CHD, heart failure, hypertension, diabetes, stroke, and chronic lower respiratory disease, were self-reported. Hypertension was defined as a systolic blood pressure of 140 or higher, diastolic blood pressure of 90 or higher, or use of antihypertensive medications. Diabetes was defined as fasting blood glucose level of 126 mg/dL or greater or use of insulin or hypoglycemic medications. Chronic kidney disease was classified into 3 groups based on estimated glomerular filtration rates (eGFR; <30, 30-59, or ≥60 mL/min/1.73m2).19
Statistical Analysis
Harmonized data from the individual NHLBI cohorts were pooled and analyzed. Multiple imputation (N = 10 data sets) was performed to address missingness in covariate data. The fully conditional specification model that assumes the existence of a joint distribution for all variables, including cohort, was used to impute missing values for the covariates with a relative efficiency of greater than 95%. For descriptive purposes, for the variables with imputed values, the averages were pooled across 10 imputed datasets (Table 1 and eTable 3 in the Supplement). Data on age, sex, race, cohort, spirometry, or outcomes were not imputed. Prospective analyses of the respiratory-related and CHD-related health outcomes were limited to the subsets of cohorts with these data available (eFigure 1 in the Supplement).
Table 1. Baseline Characteristics by Lung Function Categorya.
| Characteristics | PRISm (n=4582)b | Obstructive spirometry (n=9440)b | Normal spirometry (n=39 679)b |
|---|---|---|---|
| Age, mean (SD), y | 54.9 (14.2) | 63.0 (12.0) | 50.7 (15.9) |
| Age group | |||
| <30 y | 349 (7.6) | 170 (1.8) | 6039 (15.2) |
| 30-39 y | 225 (4.9) | 116 (1.2) | 3152 (7.9) |
| 40-49 y | 797 (17.4) | 720 (7.6) | 7082 (17.9) |
| 50-59 y | 1428 (31.2) | 2453 (26.0) | 11 477 (28.9) |
| 60-69 y | 1024 (22.4) | 2875 (30.5) | 6815 (17.2) |
| 70-79 y | 654 (14.3) | 2418 (25.6) | 4239 (10.7) |
| ≥80 y | 105 (2.3) | 688 (7.3) | 875 (2.2) |
| Sex | |||
| Women | 2764 (60.3) | 4126 (43.7) | 23 398 (59.0) |
| Men | 1818 (39.7) | 5314 (56.3) | 16 281 (41.0) |
| Race and ethnicity | |||
| American Indian or Alaska Native | 208 (4.5) | 313 (3.3) | 1258 (3.2) |
| Black | 1115 (24.3) | 1558 (16.5) | 8999 (22.7) |
| East Asian or Pacific Islander | 40 (0.9) | 128 (1.4) | 491 (1.2) |
| Hispanic or Latino | 1515 (33.1) | 1017 (10.8) | 12 065 (30.4) |
| Non-Hispanic White | 1704 (37.2) | 6421 (68.0) | 16 854 (42.5) |
| Otherc | - | 3 (0.03) | 12 (0.03) |
| Educationd | |||
| <High school diploma | 777 (17.0) | 1442 (15.3) | 5637 (14.2) |
| Completed high school | 1250 (27.3) | 2571 (27.2) | 10 918 (27.5) |
| Some college | 702 (15.3) | 1331 (14.1) | 6510 (16.4) |
| ≥College | 1853 (40.4) | 4096 (43.4) | 16 614 (41.9) |
| Smoking statusd | |||
| Ever | 2491 (54.4) | 6959 (54.4) | 18 203 (45.9) |
| Currente | 1153 (46.3) | 2892 (41.6) | 6944 (38.1) |
| Pack-years among ever smokers, median (IQR) | 21.0 (7.0-38.0) | 31.8 (15.0-49.0) | 10.4 (3.0-26.0) |
| Body mass index, mean (SD)d,f | 30.8 (7.2) | 26.6 (5.0) | 28.2 (5.7) |
| FEV1% predicted, mean (SD) | 75.1 (9.4) | 80.6 (20.4) | 103.4 (13.4) |
| FVC% predicted, mean (SD) | 78.1 (11.4) | 100.3 (20.1) | 105.4 (14.4) |
| Preexisting comorbiditiesd | |||
| Hypertensiong | 2531 (55.2) | 5042 (53.4) | 14 955 (37.7) |
| Diabetesh | 1170 (25.5) | 1254 (13.3) | 5312 (13.4) |
| Chronic lower respiratory tract diseasei | 742 (16.2) | 1877 (19.9) | 3440 (8.7) |
| Asthmaj | 627 (13.7) | 1283 (13.6) | 2657 (6.7) |
| Heart failurek | 353 (7.7) | 881 (9.3) | 1899 (4.8) |
| Coronary heart diseasek | 393 (8.6) | 978 (10.4) | 1515 (3.8) |
| Strokek | 147 (3.2) | 282 (3.0) | 516 (1.3) |
| eGFRd,l | |||
| ≥60 | 3805 (83.0) | 7070 (74.9) | 33 794 (85.2) |
| 30-59 | 723 (15.8) | 2275 (24.1) | 5688 (14.3) |
| <30 | 54 (1.2) | 95 (1.0) | 197 (0.5) |
| Cohort | |||
| ARIC | 1031 (6.9) | 3375 (22.6) | 10 538 (70.5) |
| CARDIA | 196 (3.9) | 184 (3.7) | 4624 (92.4) |
| CHS | 404 (8.4) | 2004 (41.6) | 2406 (50) |
| FOC | 310 (8) | 840 (21.5) | 2748 (70.5) |
| HABC | 217 (8.4) | 659 (25.6) | 1702 (66.0) |
| HCHS/SOL | 1406 (10.3) | 868 (6.4) | 11 351 (83.3) |
| JHS | 374 (15) | 116 (4.6) | 2008 (80.4) |
| MESA | 436 (9.5) | 1089 (23.8) | 3060 (66.7) |
| SHS | 208 (11.9) | 305 (17.4) | 1242 (70.8) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities study; CARDIA, Coronary Artery Risk Development in Young Adults study; CHS, Cardiovascular Health Study; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in the first second; FOC, Framingham Offspring Cohort; FVC, forced vital capacity; HABC, Health ABC study; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; JHS, Jackson Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; PRISm, preserved ratio impaired spirometry; SHS, Strong Heart Study.
Values are reported as No. (%) unless otherwise indicated. All P values for comparisons between groups and P values for trend were <.001.
Lung function category definitions: PRISm (FEV1:FVC ratio ≥0.7 and FEV1 <80%), obstructive spirometry (FEV1:FVC ratio <0.7), and normal spirometry (FEV1:FVC ratio ≥0.7 and FEV1 ≥80%).
The race and ethnicity category of Other includes individuals who self-reported as other or as multiracial based on predetermined categories.
Multiple imputation was performed to address missingness. Values indicate averages over 10 imputed data sets.
Ever smokers is the denominator for deriving the percentage of current smokers.
Calculated as weight in kilograms divided by height in meters squared.
Indicates self-reported hypertension, systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mm Hg or greater, or use of antihypertensive medications.
Indicates self-reported diabetes, fasting blood glucose level of 126 mg/dL or greater, or use of oral hypoglycemic agents or insulin.
Indicates self-reported physician diagnosis of chronic obstructive pulmonary disease, chronic bronchitis, or emphysema.
Indicates self-reported physician diagnosis of asthma.
Indicates data on comorbidities at baseline, including self-reported coronary heart disease, congestive heart failure, and stroke. If missing, data was obtained from hospitalization records prior to baseline spirometry. In the JHS, self-reported information on heart failure and hospitalization records prior to baseline spirometry were not available. Hence, JHS participants with heart failure events occurring within 5 years of baseline spirometry were classified as having heart failure at baseline.
For baseline eGFR (measured as mL/min/1.73 m2), measurements obtained at baseline spirometry visit were used. If missing, first available eGFR measurements were used.
Bivariable analyses were performed using the Student t test or Wilcoxon rank-sum test for continuous variables, as appropriate, and the χ2 test for categorical variables. Primary comparisons were between PRISm vs normal spirometry, and comparisons between PRISm vs obstructive spirometry were also performed. Prevalence ratios, adjusted for covariates of age, sex, race and ethnicity, cohort, BMI category, education, smoking status, pack-years of smoking, and for comorbidities (hypertension, diabetes, CHD, heart failure, stroke, and chronic kidney disease) were determined using Poisson regression for baseline data.
Age- and smoking-adjusted absolute incidence density rates and their differences were calculated per 1000 person-years for PRISm, obstructive spirometry, and normal spirometry across all 9 cohorts. Age-adjusted incidence density rates and differences were also computed per stratum of smoking status.
Associations with incident events were tested using Cox proportional hazards models, adjusting for the covariates listed previously. The proportional hazards assumption was confirmed by visual review of residual plots (eFigure 2 in the Supplement). Time to event was treated as age at event, with left truncation at baseline age. For survival models, cohort was treated as a stratum term, allowing for cohort-specific differences in the underlying survival function.18 Adjusted and unadjusted cumulative incidence plots vs time since baseline were compared, and the influence of adjustment for individual covariates was assessed using a leave-one-out strategy. Subgroup analyses were performed comparing PRISm vs normal spirometry, stratified by baseline characteristics, smoking status, comorbid conditions, and cohort; interactions were tested using multiplicative terms in models with main effects included. Secondary analyses were performed separately using GLI-LLN–defined lung function categories including additional adjustment for the FEV1:FVC ratio and limiting to complete case data.
All analyses were completed in SAS statistical software version 9.4. Each analysis was performed separately on each of the imputed data sets. Results were pooled across data sets to arrive at final estimates, standard errors, 95% CIs, and nominal P values. Tests were 2-sided, and P values less than .05 were considered significant. Because of the potential for type I error due to multiple comparisons, study findings should be interpreted as exploratory.
Results
Prevalence and Correlates of PRISm
The study included 53 701 participants (mean [SD] age at baseline, 53.2 [15.8] years, 56.4% women, 48.5% never-smokers). The amount of missing data was less than 2% for all covariates except for stroke (5.4%) and eGFR (20.8%) (eTable 3 in the Supplement). The characteristics of participants at baseline by lung function category are shown in Table 1.
The prevalence of PRISm was 8.5%, obstructive spirometry was 17.6%, and normal spirometry was 73.9%. The unweighted prevalence of PRISm was 11.7% among participants who self-reported as American Indian, 9.6% among Black participants, 6.1% among East Asian participants, 10.4% among Hispanic and Latino participants, and 6.8% among non-Hispanic White participants. However, multivariable-adjusted prevalence ratios comparing PRISm and normal spirometry did not differ significantly by race and ethnicity or educational attainment (Table 2). Adjusted prevalence ratios for PRISm compared with normal spirometry demonstrated significant associations between PRISm and female sex, age older than 45 years, current smoking, more than 15 pack-year history of smoking, abnormal BMI (underweight, overweight, and obese), and comorbidities of hypertension, diabetes, heart failure, CHD, stroke, and eGFR less than 30 mL/min/1.73m2 (Table 2).
Table 2. Prevalence Ratios for Preserved Ratio Impaired Spirometry Relative to Normal Spirometrya.
| Subgroup | PRISm (n = 4582)b | Normal spirometry (n = 39 679)b | PRISm vs normal spirometry | |
|---|---|---|---|---|
| Prevalence ratio (95% CI) | P value | |||
| Men | 1818 | 16 281 | [Reference] | |
| Women | 2764 | 23 398 | 1.07 (1.01-1.13) | .03 |
| Age group | ||||
| <45 y | 827 | 11 336 | [Reference] | |
| 45-64 y | 2663 | 21 294 | 1.16 (1.05-1.27) | .002 |
| ≥65 y | 1092 | 7049 | 1.19 (1.04-1.35) | .01 |
| Race and ethnicity | ||||
| American Indian or Alaska Native | 208 | 1258 | 1.12 (0.97-1.28)c | .12 |
| Black | 1115 | 8999 | 1.02 (0.93-1.11) | .71 |
| East Asian or Pacific Islander | 40 | 491 | 0.78 (0.57-1.08) | .14 |
| Hispanic or Latino | 1515 | 12 065 | 1.03 (0.84-1.27) | .77 |
| Non-Hispanic White | 1704 | 16 854 | [Reference] | |
| Otherd | 0 | 12 | ||
| Smoking status | ||||
| Never | 2091 | 21 472 | [Reference] | |
| Current | 1154 | 6949 | 1.33 (1.22-1.45) | <.001 |
| Former | 1337 | 11 258 | 0.86 (0.79-0.93) | <.001 |
| Smoking, pack-years | ||||
| <15 | 3079 | 32 221 | [Reference] | |
| ≥15 | 1503 | 7458 | 1.74 (1.60-1.89) | <.001 |
| BMIe | ||||
| Normal | 849 | 11 794 | [Reference] | |
| <18.5 | 62 | 403 | 2.20 (1.72-2.82) | <.001 |
| 25-29.9 | 1457 | 15 040 | 1.10 (1.01-1.19) | .03 |
| ≥30 | 2215 | 12 442 | 1.68 (1.55-1.82) | <.001 |
| Education | ||||
| <High school diploma | 774 | 5640 | 1.06 (0.98-1.15) | .16 |
| Completed high school | 1253 | 10 921 | 1.05 (0.98-1.13) | .15 |
| Some college | 704 | 6518 | 1.04 (0.95-1.13) | .40 |
| ≥College | 1851 | 16 600 | [Reference] | |
| Hypertensionf | 2531 | 14 956 | 1.40 (1.31-1.49) | <.001 |
| Diabetesf | 1170 | 5311 | 1.36 (1.28-1.46) | <.001 |
| Congestive heart failuref | 350 | 1900 | 1.37 (1.23-1.53) | <.001 |
| Coronary heart diseasef | 394 | 1515 | 1.31 (1.18-1.46) | <.001 |
| Strokef | 149 | 516 | 1.32 (1.13-1.54) | <.001 |
| eGFRg | ||||
| ≥60 | 3787 | 33 738 | [Reference] | |
| 30-59 | 737 | 5736 | 1.04 (0.93-1.16) | .53 |
| <30 | 59 | 205 | 1.58 (1.23-2.03) | <.001 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; PRISm, preserved ratio impaired spirometry.
Models adjusted for age, sex, race and ethnicity, education, body mass index, smoking status, pack-years, cohort, and medical comorbidities (hypertension, diabetes, coronary heart disease, heart failure, stroke, and chronic kidney disease).
Lung function category definitions: PRISm (FEV1:FVC ratio ≥0.7 and FEV1 <80%) and normal spirometry (FEV1:FVC ratio≥0.7 and FEV1 ≥80%).
Estimated without adjustment for cohort due to the high degree of collinearity in the NHLBI Pooled Cohort Study of American Indian and Alaskan Native race ethnicity and participation in the Strong Heart Study.
The race and ethnicity category of Other includes individuals who self-reported as other or as multiracial based on predetermined categories.
Calculated as weight in kilograms divided by height in meters squared.
Reference group indicates those who did not report the condition or comorbidity.
Calculated as mL/min/1.73 m2.
Mortality and Hospitalizations
Compared with normal spirometry, PRISm was significantly associated with greater absolute risk and relative risk for all study outcomes: all-cause mortality, respiratory-related mortality, CHD-related mortality, respiratory-related events (hospitalizations and mortality) and CHD-related events (hospitalizations and mortality) (Table 3 and Figure 1). Over a median 19.4 years of follow-up, the incidence density rate of all-cause mortality was 29.6 per 1000 person-years in PRISm vs 18.0 per 1000 person-years in the normal spirometry group (difference, 11.6 [95% CI, 10.0-13.1]; adjusted hazard ratio [HR], 1.50 [95% CI, 1.42-1.59]). Compared with normal spirometry, PRISm was also significantly associated with a higher risk of respiratory-related mortality (2.1/1000 person-years vs 1.0/1000 person-years in the normal spirometry group; difference, 1.1 [95% CI, 0.7-1.6]; adjusted HR, 1.95 [95% CI, 1.54-2.48]), CHD-related mortality (5.4/1000 person-years vs 2.6/1000 person-years; difference, 2.7 [95% CI, 2.1-3.4]; adjusted HR, 1.55 [95% CI, 1.36-1.77]), respiratory-related events (12.2/1000 person-years vs 6.0/1000 person-years; difference, 6.2 [95% CI, 4.9-7.5]; adjusted HR, 1.90 [95%CI, 1.69-2.14]), and CHD-related events (11.7/1000 person-years vs 7.0/1000 person-years; difference, 4.7 [95% CI, 3.7-5.8]; adjusted HR, 1.30 [95% CI, 1.18-1.42]).
Table 3. Age- and Smoking-Adjusted Absolute Incidence Density Rates by Lung Function Categorya.
| Outcome | No. of events/No. at risk (cumulative incidence) | Follow-up, median (IQR), y | IDR/1000 person-years (95% CI)b | ||||
|---|---|---|---|---|---|---|---|
| PRISmc | Obstructive spirometryc | Normal spirometryc | Difference, PRISm vs normal spirometry | Difference, PRISm vs obstructive spirometry | |||
| All-cause mortalityd | 15 661/39 835 (39.3) | 19.4 (12.1 to 27.6) | 29.6 (28.1 to 31.1) | 24.5 (23.7 to 25.2) | 18.0 (17.7 to 18.4) | 11.6 (10.0 to 13.1) | 5.1 (3.5 to 6.8) |
| Respiratory-related mortalitye | 1100/31 998 (3.4) | 19.6 (11.5 to 27.8) | 2.1 (1.7 to 2.6) | 3.4 (3.1 to 3.7) | 1.0 (0.9 to 1.1) | 1.1 (0.7 to 1.6) | −1.3 (−1.8 to −0.7) |
| CHD-related mortalityd | 2352/39 628 (5.9) | 19.6 (12.1 to 27.6) | 5.4 (4.7 to 6.0) | 3.7 (3.4 to 4.0) | 2.6 (2.5 to 2.8) | 2.7 (2.1 to 3.4) | 1.7 (1.0 to 2.4) |
| Respiratory-related eventsf,g | 4101/25 080 (16.4) | 14.3 (8.9 to 22.9) | 12.2 (10.9 to 13.5) | 20.3 (19.4 to 21.2) | 6.0 (5.7 to 6.3) | 6.2 (4.9 to 7.5) | −8.1 (−9.6 to −6.5) |
| CHD-related eventsd,g | 5552/38 068 (14.6) | 20.4 (12.3 to 27.7) | 11.7 (10.8 to 12.7) | 9.4 (8.9 to 9.9) | 7.0 (6.8 to 7.3) | 4.7 (3.7 to 5.8) | 2.3 (1.2 to 3.5) |
Abbreviations: CHD, coronary heart disease; IDR, incidence density rate; PRISm, preserved ratio impaired spirometry.
Using the direct method and the observed age distribution across all 9 cohorts, age- and smoking-adjusted absolute incidence density rates, and their differences were calculated per 1000 person-years for each lung function category.
All P values for differences in IDR were less than .001.
Lung function category definitions: PRISm (FEV1:FVC ratio ≥0.7 and FEV1 <80%), obstructive spirometry (FEV1:FVC ratio <0.7), and normal spirometry (FEV1:FVC ratio ≥0.7 and FEV1 ≥80%).
Cohorts included in analysis of all-cause mortality, CHD-related mortality, and CHD-related events: Atherosclerosis Risk in Communities (ARIC), Coronary Artery Risk Development in Young Adults (CARDIA), Cardiovascular Health Study (CHS), Framingham Offspring Cohort (FOC), Health, Aging, and Body Composition (Health ABC), Jackson Heart Study (JHS), Multi-Ethnic Study of Atherosclerosis (MESA), and Strong Heart Study (SHS).
Cohorts included in analysis of respiratory-related mortality: ARIC, CHS, CARDIA, Health ABC, MESA, and SHS.
Cohorts included in analysis of respiratory-related events: ARIC, CHS, Health ABC, and MESA.
The outcomes of respiratory-related events and CHD-related events include hospitalizations and mortality.
Figure 1. Associations Between Lung Function Category, Mortality, and Hospitalizations.
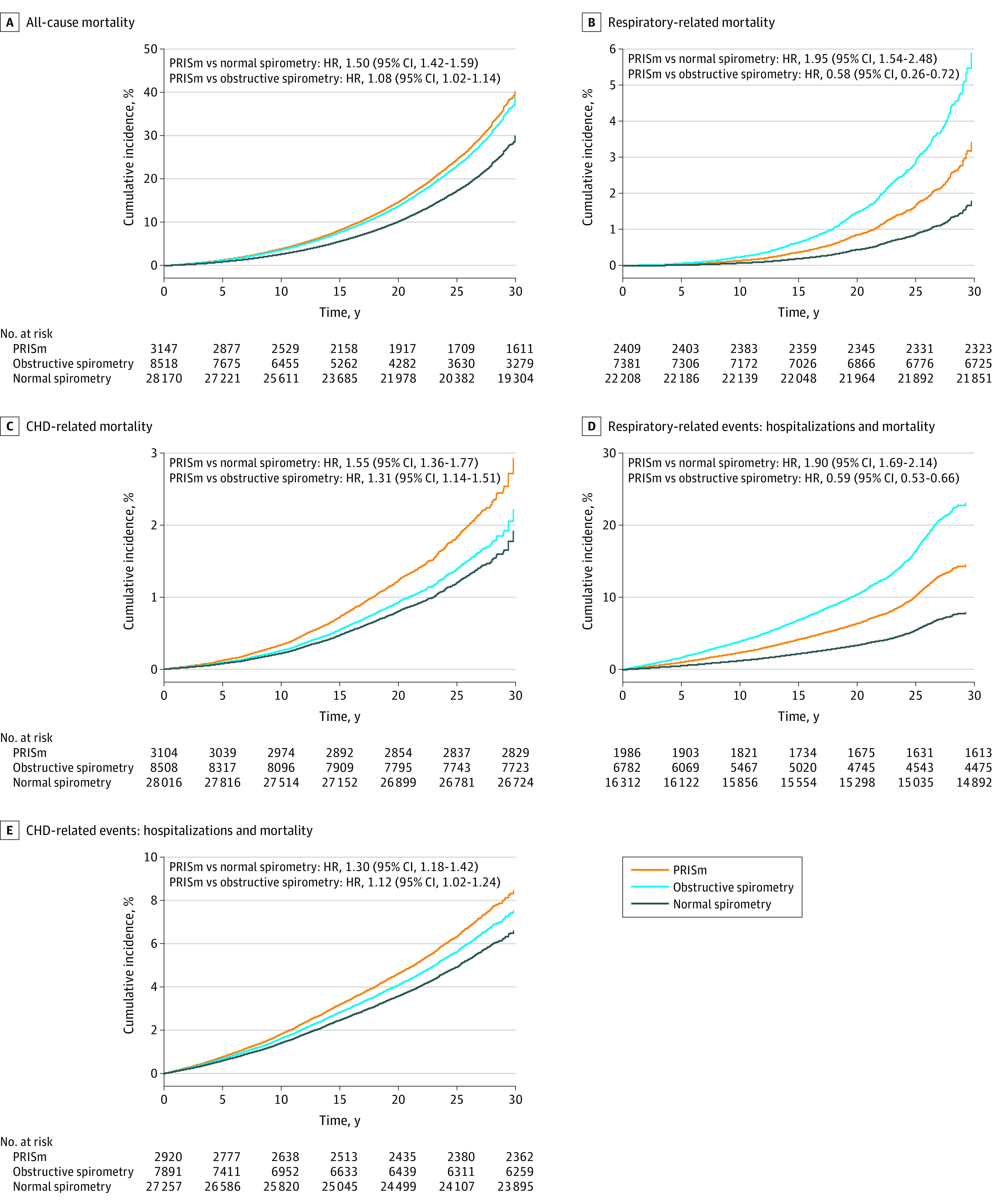
Cox proportional hazards models were adjusted for age, sex, race and ethnicity, education, body mass index, smoking status, and medical comorbidities (hypertension, diabetes, coronary heart disease, heart failure, stroke, and chronic kidney disease); cohort was treated as a stratum variable. Lung function category definitions: preserved ratio impaired spirometry (PRISm) (ratio of forced expired volume in the first second to forced vital capacity [FEV1:FVC] ≥0.7 and FEV1<80%), obstructive spirometry (FEV1:FVC ratio <0.7), and normal spirometry (FEV1:FVC ratio ≥0.7 and FEV1 ≥80%). See footnote a in Table 1 for expanded names of study cohorts. Cohorts included in analysis of all-cause mortality, CHD-related mortality, and CHD-related events (hospitalizations and mortality): ARIC, CARDIA, CHS, FOC, Health ABC, JHS, MESA, and SHS. Cohorts included in analysis of respiratory-related mortality: ARIC, CHS, CARDIA, Health ABC, MESA, and SHS. Cohorts included in analysis of respiratory-related events (hospitalizations and mortality): ARIC, CHS, Health ABC, and MESA. HR indicates hazard ratio.
Compared with obstructive spirometry, PRISm was associated with significantly higher absolute risk and relative risk for all-cause mortality, CHD-related mortality, and CHD-related events (Table 3 and Figure 1 panels A, C, E), but PRISm was associated with significantly lower absolute and relative risk for respiratory-related mortality and respiratory-related events (Figure 1 panels B and D). In contrast, the unadjusted cumulative incidence rates for all study outcomes were highest in the obstructive spirometry group (eFigures 3 and 4 in the Supplement). The influence of adjustment for each covariate on the association of PRISm with all-cause mortality is shown in eFigure 5 in the Supplement, with adjustment for smoking demonstrating the largest attenuation of the point estimates.
Subgroup Analyses
In subgroup analyses, when compared with normal spirometry, PRISm demonstrated higher risk for all study outcomes, although several comparisons did not attain statistical significance. Absolute risks and risk differences for each outcome by smoking status for PRISm, obstructive spirometry, and normal spirometry are shown in Figure 2. Relative to normal spirometry, PRISm was associated with significantly increased absolute risk for all outcomes in all smoking strata except among current smokers for respiratory-related mortality (risk difference, 0.9 [95% CI, −0.7 to 2.5]; adjusted HR, 1.46 [95% CI, 0.93-2.29]; P value for interaction <.001). Among never-smokers, absolute risk was significantly higher for CHD-related mortality in PRISm compared with normal spirometry (risk difference, 1.4 [95% CI, 0.6-2.3]), but relative risk was not significantly different (adjusted HR, 1.23 [95% CI, 0.96-1.58]; P value for interaction, .14). In stratified subgroup analyses of age, sex, race and ethnicity, obesity, and comorbidities (eFigures 6, 7, 8, 9, and 10 in the Supplement), multiplicative interaction terms were nominally significant for several additional covariates. For example, compared with normal spirometry, participants older than 45 years with PRISm had significantly increased risk for all-cause mortality (adjusted HR, 1.95 [95% CI, 1.45-2.62]); P value for interaction <.001) and CHD-related mortality (adjusted HR, 4.66 [95% CI, 1.70-12.78]; P value for interaction, .006). The magnitude of the association between PRISm and all-cause and respiratory-related mortality was similar or greater among healthy participants (non-smokers with normal BMI and no major comorbidities) vs participants who were ever-smokers, overweight, obese, or had any comorbidities, although the CIs were wide due to lower event rates in the healthy group. However, due to multiple testing, all subgroup analyses should be treated as exploratory.
Figure 2. Age-Adjusted Absolute Incidence Density Rates by Lung Function Category.
Data were stratified by smoking status for all-cause mortality, respiratory-related mortality, coronary heart disease (CHD)-related mortality, respiratory-related events (hospitalizations and mortality), and CHD-related events (hospitalizations and mortality). Lung function category definitions: preserved ratio impaired spirometry (PRISm) (ratio of forced expired volume in the first second to forced vital capacity [FEV1:FVC] ≥0.7 and FEV1<80%), obstructive spirometry (FEV1:FVC ratio <0.7), and normal spirometry (FEV1:FVC ratio ≥0.7 and FEV1 ≥80%). Using the direct method and the observed age distribution across all 9 cohorts, age-adjusted absolute incidence density rates and their differences were calculated per 1000 person-years for each combination of baseline smoking status and lung function category.
In cohort-stratified analyses, the magnitude and precision of the estimates varied, yet the direction of the estimates generally suggested PRISm was associated with increased risk for absolute risks (eTables 4, 5, 6, 7, and 8 in the Supplement) and relative risks (eFigures 11, 12, 13, 14, and 15 in the Supplement) for all outcomes compared with normal spirometry in each cohort.
Secondary Analyses
Using GLI-LLN–defined12,13 lung function categories, the prevalence of PRISm-LLN (5.7%) and obstructive spirometry-LLN (14.4%) were lower when compared with the reference equations used in this study’s primary analysis (eTable 9 in the Supplement). However, use of the LLN-defined lung function categories confirmed the significantly increased risk associated with PRISm for each of the study outcomes (eTable 10 in the Supplement). Although the FEV1:FVC ratio is greater than or equal to 0.7 in both PRISm and normal spirometry groups, to assess for potential residual confounding due to occult or early obstruction (not meeting COPD diagnostic criteria), models with additional adjustment for FEV1:FVC ratio were examined; this analysis confirmed a significantly increased risk for all study outcomes with PRISm compared with normal spirometry (eTable 11 in the Supplement). In contrast, after adjustment for baseline FEV1:FVC ratio, the relative risk for respiratory-related mortality and CHD-related mortality in the obstructive spirometry group compared with normal spirometry became nonsignificant. In complete case analyses, PRISm, compared with normal spirometry, was significantly associated with increased relative risk for all outcomes except CHD-related events (adjusted HR, 1.13 [95% CI, 0.99-1.28]; eTable 12 in the Supplement).
Discussion
In this general population-based sample of US adults, a small but notable proportion of participants demonstrated PRISm—a pattern of respiratory impairment distinct from obstructive spirometry and normal spirometry. Compared with normal spirometry, PRISm at baseline was associated with a small but statistically significant increased risk for mortality and adverse cardiovascular and respiratory outcomes.
The magnitude of the increases in absolute risk per 1000 person-years for PRISm relative to normal spirometry for all-cause mortality (11.6) and CHD-related mortality (5.4) were comparable with the risk differences previously reported for diabetes (7.0 for all-cause mortality; 3.5 for CHD-related mortality)20 and atrial fibrillation (3.8 for all-cause mortality; 2.6 for CHD-related mortality).21 Associations between PRISm and clinical outcomes were observed in persons both with and without major medical comorbidities, including obesity, hypertension, diabetes, heart failure, CHD, stroke, and chronic kidney disease. While an association between PRISm and adverse cardiovascular outcomes has been previously reported,22,23 this is the first large study, to our knowledge, to demonstrate an association between PRISm and respiratory-related mortality and hospitalizations.
Prior studies have shown that the prevalence and prognosis of PRISm differ by geography, race, and ethnicity.4,24 Within this US-based cohort, the unadjusted prevalence of PRISm was higher among members of historically marginalized or underserved populations; however, after multivariable adjustment, no significant differences in prevalence by race and ethnicity or educational attainment were observed. The prevalence of PRISm among US-based East Asians in this cohort (6.1%-7.9%) was lower than has been reported in non-US-based East Asian populations globally (19.2%-62.2%).2,4 This discrepancy may be due to differences in health behaviors (smoking and nutrition), environmental determinants (ambient air quality), and comorbid disease, including infectious diseases such as tuberculosis. Future studies examining these associations with PRISm, both in and outside the US, are warranted.
The relationship between PRISm, obesity, and diabetes is also incompletely understood. While increased total and abdominal adiposity have been associated with reduced FEV1 and FVC,25 the absolute difference in lung function is typically less than 100 mL for substantial changes in body mass25,26 and therefore does not account for the severity of the lung function impairment observed with PRISm. Divergent secular trends over the past half century between the stable prevalence of PRISm1,9,27 and the marked increases in the prevalence of overweight and obesity in the US (from 44.9% in 1960 to 71.6% in 2016)28 and diabetes (from 1.8% in 1960 to 9.4% in 2015)29 make a direct causal relationship between obesity, diabetes, and PRISm unlikely. Whether the association of these conditions with PRISm is mediated through shared metabolic pathways or other systemic processes remains to be determined. In addition, the degree to which extrapulmonary processes (eg, chest wall deformity) and other functional abnormalities (eg, diaphragmatic dysfunction) contribute to the prevalence of and adverse respiratory outcomes associated with PRISm has not been comprehensively examined and warrants additional investigation.
While the presence of restrictive and obstructive lung disease has been shown to be a significant predictor of earlier mortality in US population-based cohorts,7 this study focused on the PRISm subgroup and confirmed prior reports of associations between PRISm and increased all-cause mortality and cardiovascular mortality.6,8,22,23 This study also suggested significantly higher absolute risk and relative risk for all-cause mortality, CHD-related mortality, and CHD-related events associated with PRISm when compared with obstructive lung disease. Comorbid diseases that contribute to CHD mortality, such as heart failure and chronic kidney disease, may affect lung function due to cardiomegaly, parenchymal or pleural fluid accumulation, or airway edema. Optimization of fluid status has been shown to improve spirometric values in the short term.30 However, while the prevalence of PRISm among patients with heart failure3 and chronic kidney disease31 is increased compared with individuals without these conditions, the low prevalence of heart failure (2%)32 and stage 4 to 5 chronic kidney disease (0.5%-1.0%)33 in the US makes it unlikely that they are primary contributors to PRISm in this cohort.
PRISm has been associated with interstitial lung disease, in part because its nonobstructive spirometric pattern is often referred to as restrictive.34 While the prevalence of interstitial lung abnormalities has been reported to be 8%,35,36 clinically apparent interstitial lung disease in the US has a prevalence of less than 1%37 and therefore cannot account for the high population prevalence of PRISm. In addition, although the prevalence of interstitial lung abnormalities is enriched among individuals with PRISm (13%), the majority of individuals with PRISm do not have evidence of interstitial lung abnormalities.8,36
Some individuals with PRISm have been misclassified as having COPD. One study38 demonstrated that despite spirometry consistent with PRISm, 7% of patients continued to have an empirical diagnosis of COPD. This misclassification was more common among elderly patients and smokers, and it resulted in persistent inappropriate treatment with inhaled glucocorticoids and bronchodilators in 82% of these individuals.
Limitations
This study has several limitations. First, lack of postbronchodilator spirometry may have resulted in an overestimate of the prevalence of both PRISm and obstructive lung disease. However, while bronchodilator administration has been shown to reduce the prevalence of obstructive lung disease,39 its effect on the prevalence of PRISm is less well-established. Moreover, the prevalence of PRISm in this study using prebronchodilator spirometry was similar to the prevalence of PRISm reported using postbronchodilator spirometry.1
Second, it is possible that some respiratory- and CHD-related hospitalizations were misclassified. However, studies suggest that International Classification of Diseases–based algorithms for these outcomes have reduced sensitivity with good specificity and positive predictive value40; thus the study data may represent an underestimate of cause-specific end points examined.
Third, although this study cohort was racially and ethnically diverse, all participants were from the US so the generalizability of these findings may be limited outside the US.
Fourth, there may be concern about the selection of pulmonary function reference equations and the appropriate approach for self-reported race and ethnicity in the prediction of lung function. However, the study results were consistent when using LLN-defined lung function categories generated using the GLI-other prediction equations for all races.
Fifth, the approach of pooling data from multiple independent cohorts increased the study power but may have introduced additional sources of heterogeneity and confounding.
Sixth, some cohorts were excluded from an analysis due to missing outcome data. However, the consistent findings in both the cohort-adjusted and cohort-stratified analyses support the validity of the results from the pooled analyses.
Seventh, analyses were limited to examination of the relationship between cross-sectional (baseline) PRISm and prospective clinical outcomes and did not address whether distinct longitudinal trajectories within PRISm may be differentially associated with clinical outcomes.8,22
Conclusions
In a large, population-based sample of US adults, baseline PRISm, compared with normal spirometry, was associated with a small but statistically significant increased risk for mortality and adverse cardiovascular and respiratory outcomes. Further research is needed to explore whether this association is causal.
eTable 1. Characteristics of the Cohorts Included in the NHLBI Pooled Cohorts Study
eTable 2. Classification of Coronary Heart Disease (CHD) and Respiratory Mortality and Events
eTable 3. Comparison Between Nonimputed and Imputed Datasets
eTable 4. Age and Smoking-Adjusted Absolute Incidence Rates for All-Cause Mortality, Stratified by Cohort
eTable 5. Age and Smoking-Adjusted Absolute Incidence Rates for Respiratory Mortality, Stratified by Cohort
eTable 6. Age and Smoking-Adjusted Absolute Incidence Rates for Coronary Heart Disease (CHD) Mortality, Stratified by Cohort
eTable 7. Age and Smoking-Adjusted Absolute Incidence Rates for Respiratory-Related Events, Stratified by Cohort
eTable 8. Age and Smoking-Adjusted Absolute Incidence Rates for Coronary Heart Disease (CHD)-Related Events, Stratified by Cohort
eTable 9. Baseline Characteristics of Subjects by Lower Limit of Normal (LLN)-Defined Lung Function Categories
eTable 10. Associations Between Lung Function Category Defined Using GLI Equations for Lower Limit of Normal (LLN), All-Cause, Respiratory, and Coronary Heart Disease (CHD) Mortality, as Well as Respiratory-Related and CHD-Related Hospitalizations and Mortality
eTable 11. Associations Between Lung Function Category, All-Cause, Respiratory, and Coronary Heart Disease (CHD) Mortality, as Well as Respiratory-Related and CHD-Related Hospitalizations and Mortality, Additionally Adjusting for Baseline FEV1:FVC
eTable 12. Associations Between Lung Function Category, Mortality, CHD Events, and Respiratory-Related Events, Complete Case Analysis
eFigure 1. CONSORT
eFigure 2. Residual Plot for Checking the Proportional Hazards Assumption for PRISm and Mortality, CHD Events, and Respiratory-Related Events
eFigure 3. Unadjusted Cumulative Incidence of All-Cause Mortality by Lung Function Category
eFigure 4. Unadjusted Cumulative Incidence of Respiratory- and Coronary Heart Disease (CHD) Mortality, and Respiratory-Related and CHD-Related Hospitalizations and Mortality by Lung Function Category
eFigure 5. Effects of Confounding Variables on Association Between PRISm and All-Cause Mortality
eFigure 6. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for All-Cause Mortality
eFigure 7. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for Respiratory Mortality
eFigure 8. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for CHD Mortality Events
eFigure 9. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for Respiratory-Related Events
eFigure 10. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for CHD-Related Events
eFigure 11. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for All-Cause Mortality
eFigure 12. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for Respiratory Mortality
eFigure 13. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for CHD Mortality
eFigure 14. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for Respiratory-Related Events
eFigure 15. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for CHD-Related Events
References
- 1.Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral AF, Coton S, Kato B, et al. Tuberculosis associates with both airflow obstruction and low lung function. Eur Respir J. 2015;46(4):1104-1112. doi: 10.1183/13993003.02325-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankowich M, Elston B, Liu Q, et al. Restrictive spirometry pattern, cardiac structure and function, and incident heart failure in African Americans: the Jackson Heart Study. Ann Am Thorac Soc. 2018;15(10):1186-1196. doi: 10.1513/AnnalsATS.201803-184OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannino DM, McBurnie MA, Tan W, et al. ; BOLD Collaborative Research Group . Restricted spirometry in the Burden of Lung Disease Study. Int J Tuberc Lung Dis. 2012;16(10):1405-1411. [DOI] [PubMed] [Google Scholar]
- 5.Guerra S, Carsin AE, Keidel D, et al. Health-related quality of life and risk factors associated with spirometric restriction. Eur Respir J. 2017;49(5):1602096. doi: 10.1183/13993003.02096-2016 [DOI] [PubMed] [Google Scholar]
- 6.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(6):499-504. doi: 10.1136/thx.2009.126052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States. Thorax. 2003;58(5):388-393. doi: 10.1136/thorax.58.5.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan ES, Fortis S, Regan EA, et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198(11):1397-1405. doi: 10.1164/rccm.201804-0663OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurth L, Hnizdo E. Change in prevalence of restrictive lung impairment in the US population and associated risk factors. Multidiscip Respir Med. 2015;10(1):7. doi: 10.1186/s40248-015-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oelsner EC, Balte PP, Cassano PA, et al. Harmonization of respiratory data from 9 us population-based cohorts. Am J Epidemiol. 2018;187(11):2265-2278. doi: 10.1093/aje/kwy139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Seibold MA, Aldrich MC, et al. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363(4):321-330. doi: 10.1056/NEJMoa0907897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quanjer PH, Brazzale DJ, Boros PW, Pretto JJ. Implications of adopting the Global Lungs Initiative 2012 all-age reference equations for spirometry. Eur Respir J. 2013;42(4):1046-1054. doi: 10.1183/09031936.00195512 [DOI] [PubMed] [Google Scholar]
- 14.Bhatt SP, Balte PP, Schwartz JE, et al. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA. 2019;321(24):2438-2447. doi: 10.1001/jama.2019.7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oelsner EC, Loehr LR, Henderson AG, et al. Classifying chronic lower respiratory disease events in epidemiologic cohort studies. Ann Am Thorac Soc. 2016;13(7):1057-1066. doi: 10.1513/AnnalsATS.201601-063OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balte PP, Chaves PHM, Couper DJ, et al. Association of nonobstructive chronic bronchitis with respiratory health outcomes in adults. JAMA Intern Med. 2020;180(5):676-686. doi: 10.1001/jamainternmed.2020.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range. Eur Respir J. 2012;40(6):1324-1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oelsner EC, Balte PP, Bhatt SP, et al. Lung function decline in former smokers and low-intensity current smokers. Lancet Respir Med. 2020;8(1):34-44. doi: 10.1016/S2213-2600(19)30276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease. Kidney Int. 2005;67(6):2089-2100. doi: 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 20.Raghavan S, Vassy JL, Ho YL, et al. Diabetes mellitus–related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8(4):e011295. doi: 10.1161/JAHA.118.011295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odutayo A, Wong CX, Hsiao AJ, et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 22.Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Trajectory of preserved ratio impaired spirometry. Am J Respir Crit Care Med. 2021;204(8):910-920. doi: 10.1164/rccm.202102-0517OC [DOI] [PubMed] [Google Scholar]
- 23.Wijnant SRA, De Roos E, Kavousi M, et al. Trajectory and mortality of preserved ratio impaired spirometry. Eur Respir J. 2020;55(1):1901217. doi: 10.1183/13993003.01217-2019 [DOI] [PubMed] [Google Scholar]
- 24.Vaz Fragoso CA, McAvay G, Gill TM, et al. Ethnic differences in respiratory impairment. Thorax. 2014;69(1):55-62. doi: 10.1136/thoraxjnl-2013-203631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130(3):827-833. doi: 10.1378/chest.130.3.827 [DOI] [PubMed] [Google Scholar]
- 26.Fenger RV, Gonzalez-Quintela A, Vidal C, et al. The longitudinal relationship of changes of adiposity to changes in pulmonary function and risk of asthma in a general adult population. BMC Pulm Med. 2014;14:208. doi: 10.1186/1471-2466-14-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford ES, Mannino DM, Wheaton AG, et al. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States. Chest. 2013;143(5):1395-1406. doi: 10.1378/chest.12-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2015-2016. National Center for Health Statistics. October 2018. Accessed October 13, 2021. https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.htm
- 29.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2017. Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. [Google Scholar]
- 30.Chase SC, Fermoyle CC, Wheatley CM, Schaefer JJ, Olson LJ, Johnson BD. The effect of diuresis on extravascular lung water and pulmonary function in acute decompensated heart failure. ESC Heart Fail. 2018;5(2):364-371. doi: 10.1002/ehf2.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navaneethan SD, Mandayam S, Arrigain S, et al. Obstructive and restrictive lung function measures and CKD. Am J Kidney Dis. 2016;68(3):414-421. doi: 10.1053/j.ajkd.2016.03.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers OB, Pankratz VS, Norris KC, Vassalotti JA, Unruh ML, Argyropoulos C. Surveillance of CKD epidemiology in the US—a joint analysis of NHANES and KEEP. Sci Rep. 2018;8(1):15900. doi: 10.1038/s41598-018-34233-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson JD, Theurer WM. A stepwise approach to the interpretation of pulmonary function tests. Am Fam Physician. 2014;89(5):359-366. [PubMed] [Google Scholar]
- 35.Putman RK, Hatabu H, Araki T, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315(7):672-681. doi: 10.1001/jama.2016.0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897-906. doi: 10.1056/NEJMoa1007285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810-816. doi: 10.1164/rccm.200602-163OC [DOI] [PubMed] [Google Scholar]
- 38.Fortis S, Corazalla EO, Jacobs DR Jr, Kim HJ. Persistent empiric COPD diagnosis and treatment after pulmonary function test showed no obstruction. Respir Care. 2016;61(9):1192-1200. doi: 10.4187/respcare.04647 [DOI] [PubMed] [Google Scholar]
- 39.Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B. Estimating the US prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry. Respir Res. 2013;14:103. doi: 10.1186/1465-9921-14-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141(1):87-93. doi: 10.1378/chest.11-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the Cohorts Included in the NHLBI Pooled Cohorts Study
eTable 2. Classification of Coronary Heart Disease (CHD) and Respiratory Mortality and Events
eTable 3. Comparison Between Nonimputed and Imputed Datasets
eTable 4. Age and Smoking-Adjusted Absolute Incidence Rates for All-Cause Mortality, Stratified by Cohort
eTable 5. Age and Smoking-Adjusted Absolute Incidence Rates for Respiratory Mortality, Stratified by Cohort
eTable 6. Age and Smoking-Adjusted Absolute Incidence Rates for Coronary Heart Disease (CHD) Mortality, Stratified by Cohort
eTable 7. Age and Smoking-Adjusted Absolute Incidence Rates for Respiratory-Related Events, Stratified by Cohort
eTable 8. Age and Smoking-Adjusted Absolute Incidence Rates for Coronary Heart Disease (CHD)-Related Events, Stratified by Cohort
eTable 9. Baseline Characteristics of Subjects by Lower Limit of Normal (LLN)-Defined Lung Function Categories
eTable 10. Associations Between Lung Function Category Defined Using GLI Equations for Lower Limit of Normal (LLN), All-Cause, Respiratory, and Coronary Heart Disease (CHD) Mortality, as Well as Respiratory-Related and CHD-Related Hospitalizations and Mortality
eTable 11. Associations Between Lung Function Category, All-Cause, Respiratory, and Coronary Heart Disease (CHD) Mortality, as Well as Respiratory-Related and CHD-Related Hospitalizations and Mortality, Additionally Adjusting for Baseline FEV1:FVC
eTable 12. Associations Between Lung Function Category, Mortality, CHD Events, and Respiratory-Related Events, Complete Case Analysis
eFigure 1. CONSORT
eFigure 2. Residual Plot for Checking the Proportional Hazards Assumption for PRISm and Mortality, CHD Events, and Respiratory-Related Events
eFigure 3. Unadjusted Cumulative Incidence of All-Cause Mortality by Lung Function Category
eFigure 4. Unadjusted Cumulative Incidence of Respiratory- and Coronary Heart Disease (CHD) Mortality, and Respiratory-Related and CHD-Related Hospitalizations and Mortality by Lung Function Category
eFigure 5. Effects of Confounding Variables on Association Between PRISm and All-Cause Mortality
eFigure 6. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for All-Cause Mortality
eFigure 7. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for Respiratory Mortality
eFigure 8. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for CHD Mortality Events
eFigure 9. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for Respiratory-Related Events
eFigure 10. Forest Plot of Stratified Subgroup Analyses (PRISm vs Normal Spirometry) for CHD-Related Events
eFigure 11. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for All-Cause Mortality
eFigure 12. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for Respiratory Mortality
eFigure 13. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for CHD Mortality
eFigure 14. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for Respiratory-Related Events
eFigure 15. Forest Plot of Cohort-Stratified Analyses (PRISm vs Normal Spirometry) for CHD-Related Events



