Key Points
Question
What is the percentage of asymptomatic individuals with positive test results for SARS-CoV-2 among tested individuals and those with confirmed COVID-19 diagnosis?
Findings
In this systematic review and meta-analysis of 95 unique studies with 29 776 306 individuals undergoing testing, the pooled percentage of asymptomatic infections was 0.25% among the tested population and 40.50% among the population with confirmed COVID-19.
Meaning
The high percentage of asymptomatic infections from this study highlights the potential transmission risk of asymptomatic infections in communities.
This systematic review and meta-analysis evaluated the percentage of asymptomatic COVID-19 infections among individuals undergoing testing and those with confirmed infections.
Abstract
Importance
Asymptomatic infections are potential sources of transmission for COVID-19.
Objective
To evaluate the percentage of asymptomatic infections among individuals undergoing testing (tested population) and those with confirmed COVID-19 (confirmed population).
Data Sources
PubMed, EMBASE, and ScienceDirect were searched on February 4, 2021.
Study Selection
Cross-sectional studies, cohort studies, case series studies, and case series on transmission reporting the number of asymptomatic infections among the tested and confirmed COVID-19 populations that were published in Chinese or English were included.
Data Extraction and Synthesis
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Random-effects models were used to estimate the pooled percentage and its 95% CI. Three researchers performed the data extraction independently.
Main Outcomes and Measures
The percentage of asymptomatic infections among the tested and confirmed populations.
Results
Ninety-five unique eligible studies were included, covering 29 776 306 individuals undergoing testing. The pooled percentage of asymptomatic infections among the tested population was 0.25% (95% CI, 0.23%-0.27%), which was higher in nursing home residents or staff (4.52% [95% CI, 4.15%-4.89%]), air or cruise travelers (2.02% [95% CI, 1.66%-2.38%]), and pregnant women (2.34% [95% CI, 1.89%-2.78%]). The pooled percentage of asymptomatic infections among the confirmed population was 40.50% (95% CI, 33.50%-47.50%), which was higher in pregnant women (54.11% [95% CI, 39.16%-69.05%]), air or cruise travelers (52.91% [95% CI, 36.08%-69.73%]), and nursing home residents or staff (47.53% [95% CI, 36.36%-58.70%]).
Conclusions and Relevance
In this meta-analysis of the percentage of asymptomatic SARS-CoV-2 infections among populations tested for and with confirmed COVID-19, the pooled percentage of asymptomatic infections was 0.25% among the tested population and 40.50% among the confirmed population. The high percentage of asymptomatic infections highlights the potential transmission risk of asymptomatic infections in communities.
Introduction
COVID-19, the disease caused by SARS-CoV-2, was first reported in December 2019.1 Globally, as of January 28, 2021, there have been 100 455 529 confirmed cases, including 2 166 440 deaths.2 The disease course of COVID-19 ranges from asymptomatic to mild respiratory infections to pneumonia and even to acute respiratory distress syndrome.3 Patients with no symptoms at screening point were defined as having asymptomatic infections, which included infected people who have not yet developed symptoms but go on to develop symptoms later (presymptomatic infections), and those who are infected but never develop any symptoms (true asymptomatic or covert infections).4,5 Owing to the absence of symptoms, these patients would not seek medical care and could not be detected by temperature screening. Presymptomatic transmission will also make temperature screening less effective.6 Only extensive testing and close contact tracing could lead to identification of more asymptomatic infections.7
Unlike SARS, which had little known transmission from asymptomatic patients, evidence showed that asymptomatic patients were a potential source of transmission of COVID-19.3,6 A previous study8 showed that the upper respiratory viral loads in asymptomatic patients were comparable to those in symptomatic patients. Meanwhile, the highest viral load in throat swabs at the time of symptom onset indicated that infectiousness peaked on or before symptom onset.9 Moreover, studies showed that asymptomatic infections might have contributed to transmission among households, nursing facilities, and clusters.10,11,12,13 As the pandemic has been contained in many countries and regions, travel restrictions have been lifted and public places have reopened. Asymptomatic infections should be considered a source of COVID-19 infections that play an important role in the spread of the virus within community as public life gradually returns to normal. The management of asymptomatic carriers was essential for preventing cluster outbreaks and transmission within a community.
However, comprehensive evaluation of the percentage of asymptomatic infections among the tested population and the population with confirmed COVID-19 (confirmed population) is limited. Current results from different studies3,5,7,8,10,11 varied considerably owing to different study design and study population. Thus, we conducted a meta-analysis to better understand the global percentage of asymptomatic infections among the tested and confirmed COVID-19 populations. Our results could be useful for strategies to reduce transmission by asymptomatic infections.
Methods
Search Strategy
We conducted the meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. This review was not registered. Three researchers (Q.L., L.K., and R.L.) searched the published studies on February 4, 2021, through PubMed, EMBASE, and ScienceDirect without language restriction. The search terms used included COVID-19, coronavirus, SARS-CoV-2, asymptomatic transmission, asymptomatic infection, asymptomatic proportion, asymptomatic case, asymptomatic cases, asymptomatic contact, asymptomatic ratio, asymptomatic people, asymptomatic patients, and asymptomatic patient. The detailed search strategies are shown in eMethods 1 in the Supplement. Three researchers (Q.L., L.K., and R.L.) reviewed the titles, abstracts, and full texts of articles independently and identified additional studies from the reference lists. Disagreements were resolved by 2 other reviewers (W.J. and Y.W.).
Selection Criteria
Asymptomatic individuals with positive test results for SARS-CoV-2 (asymptomatic infections) were defined as those who did not present any symptoms at the time of SARS-CoV-2 testing or diagnosis.14 Individuals with a confirmed COVID-19 diagnosis were defined as those who had a throat swab or other specimen with positive results for SARS-CoV-2 using a real-time reverse-transcription polymerase chain reaction assay. Inclusion criteria consisted of (1) studies reporting the number of asymptomatic infections, tested population, and confirmed population and (2) cross-sectional studies, cohort studies, case series studies, and case series on transmission. Exclusion criteria consisted of (1) reviews, systematic reviews, and meta-analysis; (2) duplicate publications; (3) preprints; (4) multiple studies reporting on overlapping participants (the study with more information was included); (5) articles with ambiguous definition of asymptomatic infections; and (6) articles not written in English or Chinese.
Data Extraction and Quality Assessment
Three researchers (Q.L., L.K., and R.L.) performed the data extraction independently. Data were extracted for the first author, date of publication, study location, number of tested individuals, number of individuals with confirmed COVID-19, and number of asymptomatic infections. The ratio of male to female individuals (MFR) and mean age of study participants were gathered if available. The quality of studies included in the meta-analysis was assessed using the Joanna Briggs Institute Prevalence Critical Appraisal Tool15 for cross-sectional studies and the Newcastle-Ottawa scale16 for cohort studies (eMethods 2 in the Supplement). Case series on transmission were assessed using the quality assessment tool developed by Yanes-Lane et al.17 Two researchers (Q.L. and L.K.) performed the quality assessment independently. Disagreements were resolved by 2 other reviewers (W.J. and Y.W.). Outcomes of interest included the percentages of asymptomatic infections among the tested and the confirmed populations.
Statistical Analysis
We performed a meta-analysis to estimate the pooled percentage of asymptomatic infections among the tested and confirmed populations. Untransformed percentages and DerSimonian and Laird random-effects models18 were used to calculate the pooled percentage and its 95% CI. The heterogeneity among studies was assessed using I2 values.19 We performed subgroup analyses by study location (Africa, Asia, Europe, North America, and South America), countries’ development level (developed vs developing), study population (air or cruise travelers, close contact, community residents, health care workers or in-hospital patients, nursing home residents or staff, and pregnant women), publication period (June 2020 and earlier vs July 2020 and later), sample size for the tested population (1-99, 100-999, 1000-9999, and ≥10 000), sample size for the confirmed population (1-99, 100-499, and ≥500), study design (case series, case series on transmission, cohort studies, and cross-sectional studies), study quality (low, moderate, and high), MFR (0 to <0.5, 0.5 to <1.0, 1.0 to <1.5, and ≥1.5), and mean age (<20, 20-39, 40-59, and ≥60 years). Publication bias was assessed by funnel plot and the Egger regression test.20 We performed 3 sensitivity analyses to test the robustness of our results, by using the Knapp-Hartung adjustments21 to calculate the 95% CIs around the pooled effects, by excluding 3 studies with a tested population more than 200 000 and studies with low quality. Two-sided P < .05 indicated statistical significance. All analyses were performed using R, version 4.0.0 (R Project for Statistical Computing).
Results
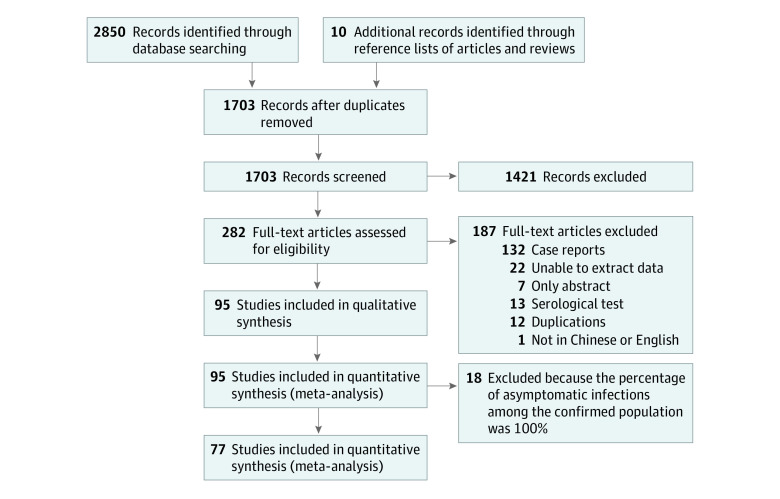
We identified 2860 studies through database search and the reference lists of articles and reviews. Of these, 282 studies underwent full-text review. Ninety-five studies with information concerning the percentage of asymptomatic infections among the tested and confirmed populations were included in the final analysis12,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115 (Figure 1).
Figure 1. Flow Diagram of Study Selection.
Among these studies, 44 (46.32%) were cross-sectional studies, 41 (43.16%) were cohort studies, 7 (7.37%) were case series, and 3 (3.16%) were case series on transmission studies. Thirty-five studies (36.84%) were conducted in Europe; 32 (33.68%), in North America; and 25 (26.32%), in Asia. Seventy-four studies (77.89%) were conducted in developed countries. Thirty-seven studies (38.95%) were conducted among health care workers or in-hospital patients; 17 (17.89%), among nursing home residents or staff; 14 (14.74%), among community residents; 13 (13.68%), among pregnant women; 8 (8.42%), among air or cruise travelers; and 6 (6.32%), among close contacts. Twenty-one studies (22.11%) were published in June or before; 74 (77.89%), in July and after. Forty-nine studies (51.58%) had sample size of 100 to 1000. Fifty-three studies (55.79%) were assessed as low quality; 17 (17.89%), high quality; and 25 (26.32%), moderate quality (Table). For cross-sectional studies, low-quality studies were mostly those without random sampling or with 2 or more biases (selection bias, reporting bias, or detection bias). For cohort studies, low-quality studies were mostly those with 1 or more biases.
Table. Characteristics of the Studies Included for Meta-analysis.
| Source | Country | Study design | Time of publication | Population group | No. tested individuals | No. confirmed individuals | No. asymptomatic infections | Quality |
|---|---|---|---|---|---|---|---|---|
| Abdelmoniem et al22 | Egypt | Cross-sectional | January 2020 | Health care workers or in-hospital patients | 203 | 29 | 29 | Low |
| Abeysuriya et al23 | UK | Cross-sectional | September 2020 | Pregnant women | 180 | 7 | 6 | Low |
| Akbarialiabad et al24 | Iran | Cross-sectional | September 2020 | Health care workers or in-hospital patients | 1805 | 86 | 19 | Low |
| Al-Qahtani et al25 | Kingdom of Bahrain | Cohort | November 2020 | Air or cruise travelers | 2714 | 188 | 116 | High |
| Al-Shamsi et al26 | United Arab Emirates | Cohort | November 2020 | Health care workers or in-hospital patients | 109 | 32 | 6 | Low |
| Arnold et al27 | US | Cross-sectional | January 2021 | Health care workers or/in-hospital patients | 2882 | 103 | 38 | Moderate |
| Arons et al12 | US | Cross-sectional | April 2020 | Nursing home residents or staff | 76 | 48 | 27 | Moderate |
| Aslam et al28 | US | Cohort | January 2020 | Health care workers or in-hospital patients | 11 622 | 69 | 42 | Low |
| Bayle et al29 | France | Cross-sectional | January 2021 | Nursing home residents or staff | 241 | 32 | 24 | Moderate |
| Bender et al30 | US | Cohort | September 2020 | Pregnant women | 318 | 8 | 8 | Moderate |
| Bianco et al31 | US | Cross-sectional | May 2020 | Pregnant women | 155 | 24 | 24 | Low |
| Blain et al32 | US | Case series | July 2020 | Nursing home residents or staff | 113 | 44 | 8 | Moderate |
| Blitz et al33 | US | Cohort | August 2020 | Pregnant women | 382 | 71 | 45 | Low |
| Blumberg et al34 | US | Cohort | October 2020 | Health care workers or in-hospital patients | 1198 | 7 | 6 | Low |
| Bosworth et al35 | UK | Cross-sectional | July 2020 | Health care workers or in-hospital patients | 1282 | 53 | 16 | Moderate |
| Cao et al36 | China | Cross-sectional | November 2020 | Community residents | 9 865 404 | 300 | 300 | High |
| Carroll et al37 | Ireland | Cohort | October 2020 | Close contact | 4586 | 310 | 209 | Moderate |
| Cattelan et al38 | Italy | Cohort | August 2020 | Health care workers or in-hospital patients | 7595 | 395 | 109 | Low |
| Cloutier et al39 | Canada | Cross-sectional | August 2020 | Community residents | 330 | 6 | 6 | Low |
| Corcorran et al40 | US | Cohort | August 2020 | Health care workers or in-hospital patients | 25 | 10 | 4 | Low |
| Deng et al41 | China | Case series on transmission | October 2020 | Close contact | 347 | 27 | 1 | High |
| Dora et al42 | US | Cross-sectional | May 2020 | Nursing home residents or staff | 235 | 27 | 18 | Low |
| Duan et al43 | China | Cross-sectional | September 2020 | Health care workers or/in-hospital patients | 4729 | 4 | 4 | Moderate |
| Figueiredo et al44 | Portugal | Cohort | October 2020 | Pregnant women | 184 | 11 | 9 | Low |
| Goldfarb et al45 | US | Cross-sectional | May 2020 | Pregnant women | 757 | 20 | 9 | Moderate |
| Graham et al46 | UK | Cross-sectional | September 2020 | Nursing home residents or staff | 464 | 129 | 54 | Moderate |
| Grechukhina et al47 | US | Cohort | November 2020 | Pregnant women | 1567 | 141 | 44 | High |
| Gruskay et al48 | US | Cohort | June 2020 | Health care workers or in-hospital patients | 99 | 12 | 7 | Low |
| Han et al49 | China | Cross-sectional | June 2020 | Community residents | 29 299 | 18 | 18 | Low |
| Harada et al50 | Japan | Cohort | December 2020 | Health care workers or in-hospital patients | 1259 | 79 | 33 | Low |
| Hcini et al51 | France | Cohort | February 2020 | Pregnant women | 507 | 137 | 103 | Low |
| Hoxha et al52 | Belgium | Cross-sectional | July 2020 | Nursing home residents or staff | 280 427 | 8325 | 6244 | Moderate |
| Hung et al53 | China | Case series | September 2020 | Air or cruise travelers | 215 | 9 | 6 | High |
| Ibrahim et al54 | Indonesia | Case series | August 2020 | Health care workers or in-hospital patients | 4617 | 582 | 55 | Low |
| Kennelly et al55 | Ireland | Cohort | September 2020 | Nursing home residents or staff | 2968 | 1105 | 290 | Low |
| Kessler et al56 | Germany | Cross-sectional | December 2020 | Health care workers or in-hospital patients | 689 | 1 | 1 | Moderate |
| Kimball et al57 | US | Cross-sectional | April 2020 | Nursing home residents or staff | 76 | 23 | 13 | Moderate |
| Kirshblum et al58 | US | Cohort | July 2020 | Health care workers or in-hospital patients | 103 | 12 | 12 | Low |
| Krüger et al59 | Germany | Cohort | January 2021 | Health care workers or in-hospital patients | 6940 | 27 | 7 | Low |
| Kwon et al60 | South Korea | Cross-sectional | July 2020 | Health care workers or in-hospital patients | 2087 | 42 | 6 | Low |
| LaCourse et al61 | US | Cohort | May 2020 | Pregnant women | 230 | 13 | 1 | Low |
| Ladhani et al62 | UK | Cohort | September 2020 | Nursing home residents or staff | 518 | 158 | 97 | High |
| Lan et al63 | US | Cross-sectional | November 2020 | Community residents | 104 | 21 | 16 | Moderate |
| Lavezzo et al64 | Italy | Cross-sectional | July 2020 | Community residents | 2812 | 73 | 29 | Moderate |
| Livingston et al65 | UK | Cohort | October 2020 | Health care workers or in-hospital patients | 344 | 131 | 16 | Moderate |
| Lombardi et al66 | Italy | Cohort | June 2020 | Health care workers or in-hospital patients | 1573 | 139 | 28 | Low |
| Ly et al67 | France | Cross-sectional | November 2020 | Nursing home residents or staff | 1691 | 226 | 46 | Moderate |
| Lytras et al68 | Greece | Cross-sectional | April 2020 | Air or cruise travelers | 783 | 40 | 35 | Low |
| Maechler et al69 | Germany | Cross-sectional | December 2020 | Community residents | 4333 | 333 | 14 | High |
| Marossy et al70 | UK | Cross-sectional | September 2020 | Nursing home residents or staff | 2455 | 160 | 115 | Moderate |
| Marschner et al71 | Germany | Cross-sectional | July 2020 | Health care workers or in-hospital patients | 139 | 1 | 1 | Low |
| Martinez-Fierro et al72 | Mexico | Cross-sectional | October 2020 | Close contact | 81 | 34 | 5 | Low |
| Massarotti et al73 | Italy | Cross-sectional | August 2020 | Pregnant women | 333 | 7 | 6 | Low |
| Mattar et al74 | Caribbean | Cross-sectional | December 2020 | Close contact | 686 | 35 | 18 | Low |
| Menting et al75 | Germany | Cross-sectional | January 2020 | Health care workers or in-hospital patients | 1185 | 11 | 2 | Low |
| Migueres et al76 | France | Cross-sectional | September 2020 | Health care workers or in-hospital patients | 123 | 44 | 17 | Low |
| Milani et al77 | Italy | Cross-sectional | June 2020 | Community residents | 197 | 21 | 21 | Moderate |
| Nishiura et al78 | Japan | Cross-sectional | May 2020 | Air or cruise travelers | 565 | 13 | 4 | Low |
| Ochiai et al79 | Japan | Cross-sectional | June 2020 | Pregnant women | 52 | 2 | 2 | Low |
| Olalla et al80 | Spain | Cross-sectional | August 2020 | Health care workers or in-hospital patients | 498 | 2 | 2 | Low |
| Olmos et al81 | Chile | Cross-sectional | January 2021 | Health care workers or in-hospital patients | 413 | 14 | 14 | Low |
| Park et al82 | South Korea | Cross-sectional | April 2020 | Community residents | 1143 | 97 | 8 | High |
| Park et al83 | Korea | Cohort | December 2020 | Air or cruise travelers | 39 | 30 | 4 | Low |
| Patel et al84 | United States | Cohort | June 2020 | Nursing home residents or staff | 126 | 35 | 14 | Low |
| Pavli et al85 | Greece | Case series on transmission | September 2020 | Air or cruise travelers | 891 | 5 | 2 | High |
| Petersen et al86 | United Kingdom | Cross-sectional | October 2020 | Community residents | 36 061 | 115 | 88 | Moderate |
| Puckett et al87 | United States | Cohort | December 2020 | Health care workers or in-hospital patients | 227 | 2 | 2 | Low |
| Ralli et al88 | Italy | Cohort | December 2020 | Community residents | 298 | 12 | 9 | Low |
| Rashid-Abdi et al89 | Sweden | Cohort | November 2020 | Health care workers or in-hospital patients | 131 | 21 | 1 | Low |
| Ren et al90 | China | Cohort | February 2021 | Air or cruise travelers | 19 398 384 | 3103 | 1749 | High |
| Rincón et al91 | Spain | Cohort | September 2020 | Health care workers or in-hospital patients | 192 | 36 | 14 | Low |
| Roxby et al92 | United States | Cohort | May 2020 | Nursing home residents or staff | 80 | 3 | 2 | Low |
| Sacco et al93 | France | Cohort | November 2020 | Nursing home residents or staff | 179 | 63 | 12 | Low |
| Santos et al94 | Portugal | Cross-sectional | December 2020 | Health care workers or in-hospital patients | 8037 | 211 | 47 | Low |
| Scheier et al95 | Switzerland | Cross-sectional | February 2021 | Health care workers or in-hospital patients | 2807 | 68 | 8 | High |
| Shah et al96 | US | Case series | July 2020 | Health care workers or in-hospital patients | 625 | 1 | 1 | Low |
| Shi et al97 | US | Cohort | October 2020 | Nursing home residents or staff | 389 | 146 | 66 | Moderate |
| Singer et al98 | US | Case series | October 2020 | Health care workers or in-hospital patients | 4751 | 18 | 10 | High |
| Tang et al99 | China | Cross-sectional | July 2020 | Health care workers or in-hospital patients | 1027 | 52 | 13 | High |
| Tang et al100 | US | Cohort | November 2020 | Nursing home residents or staff | 1970 | 752 | 424 | High |
| Temkin et al101 | Israel | Cross-sectional | October 2020 | Health care workers or in-hospital patients | 522 | 1 | 1 | Low |
| Trahan et al102 | Canada | Cohort | November 2020 | Pregnant women | 803 | 41 | 11 | Low |
| Tsou et al103 | China | Case series | November 2020 | Community residents | 17 935 | 100 | 10 | Moderate |
| van Buul et al104 | The Netherlands | Cohort | Decem ber 2020 | Nursing home residents or staff | 839 | 25 | 6 | High |
| Varnell et al105 | US | Cohort | January 2021 | Health care workers or in-hospital patients | 281 | 24 | 9 | Moderate |
| Wadhwa et al106 | US | Cohort | December 2020 | Community residents | 172 | 19 | 12 | Moderate |
| Wi et al107 | South Korea | Case series | July 2020 | Community residents | 17 400 | 111 | 25 | High |
| Wood et al108 | Indiana | Cross-sectional | August 2020 | Community residents | 511 | 1 | 1 | Low |
| Yamahata et al109 | Japan | Cross-sectional | May 2020 | Air or cruise travelers | 3711 | 696 | 410 | Moderate |
| Yassa et al110 | Turkey | Cohort | July 2020 | Pregnant women | 296 | 23 | 12 | Low |
| Yau et al111 | Canada | Cohort | July 2020 | Health care workers or in-hospital patients | 330 | 22 | 12 | Low |
| Yousaf et al112 | US | Cohort | July 2020 | Close contact | 195 | 47 | 6 | Low |
| Zhang et al113 | China | Case series on transmission | April 2020 | Close contact | 8437 | 25 | 3 | High |
| Zhang et al114 | China | Cohort | September 2020 | Health care workers or in-hospital patients | 8553 | 235 | 21 | Low |
| Zhao et al115 | China | Cohort | August 2020 | Health care workers or in-hospital patients | 1060 | 160 | 38 | Low |
Percentage of Asymptomatic Infections Among the Tested Population
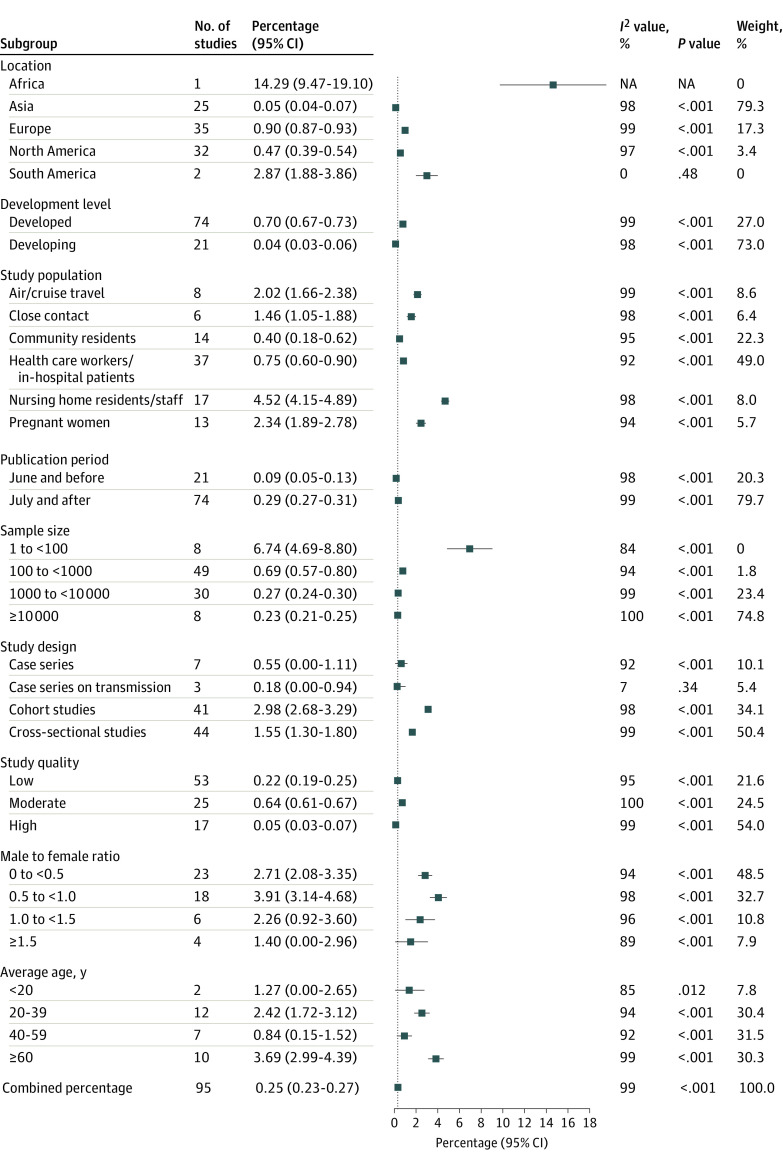
Ninety-five studies were included in the meta-analysis for the percentage of asymptomatic infections among the tested population, covering 29 776 306 tested individuals, among whom 11 516 had asymptomatic infections. The pooled percentage of asymptomatic infections among the tested population was 0.25% (95% CI, 0.23%-0.27%), with high heterogeneity among studies (I2 = 99%; P < .001) (eFigure 1 in the Supplement).
Among tested individuals in different study populations, the pooled percentage of asymptomatic infections was 4.52% (95% CI, 4.15%-4.89%) in nursing home residents or staff, 2.02% (95% CI, 1.66%-2.38%) in air or cruise travelers, 2.34% (95% CI, 1.89%-2.78%) in pregnant women, 1.46% (95% CI, 1.05%-1.88%) in close contacts, 0.75% (95% CI, 0.60%-0.90%) in health care workers or in-hospital patients, and 0.40% (95% CI, 0.18%-0.62%) in community residents. The pooled percentage of asymptomatic infections was 0.90% (95% CI, 0.87%-0.93%) in Europe, 0.47% (95% CI, 0.39%-0.54%) in North America, and 0.05% (95% CI, 0.04%-0.07%) in Asia. The pooled percentage was higher in developed countries (0.70% [95% CI, 0.67%-0.73%]), studies published in July or later (0.29% [95% CI, 0.27%-0.31%]), studies with a sample size of less than 100 (6.74% [95% CI, 4.69%-8.80%]), and cohort studies (2.98% [95% CI, 2.68%-3.29%]). In studies with MFR of 0.5 to less than 1.0, the pooled percentage was higher (3.91%; [95% CI, 3.14%-4.68%]). The pooled percentage was higher when the mean age of the study population was 60 years or older (3.69% [95% CI, 2.99%-4.39%]) (Figure 2).
Figure 2. Percentage of Asymptomatic Infections Among the Tested Population by Subgroups.
Includes 29 776 306 tested individuals, among whom 11 516 had asymptomatic infections.
Percentage of Asymptomatic Infections Among the Confirmed Population
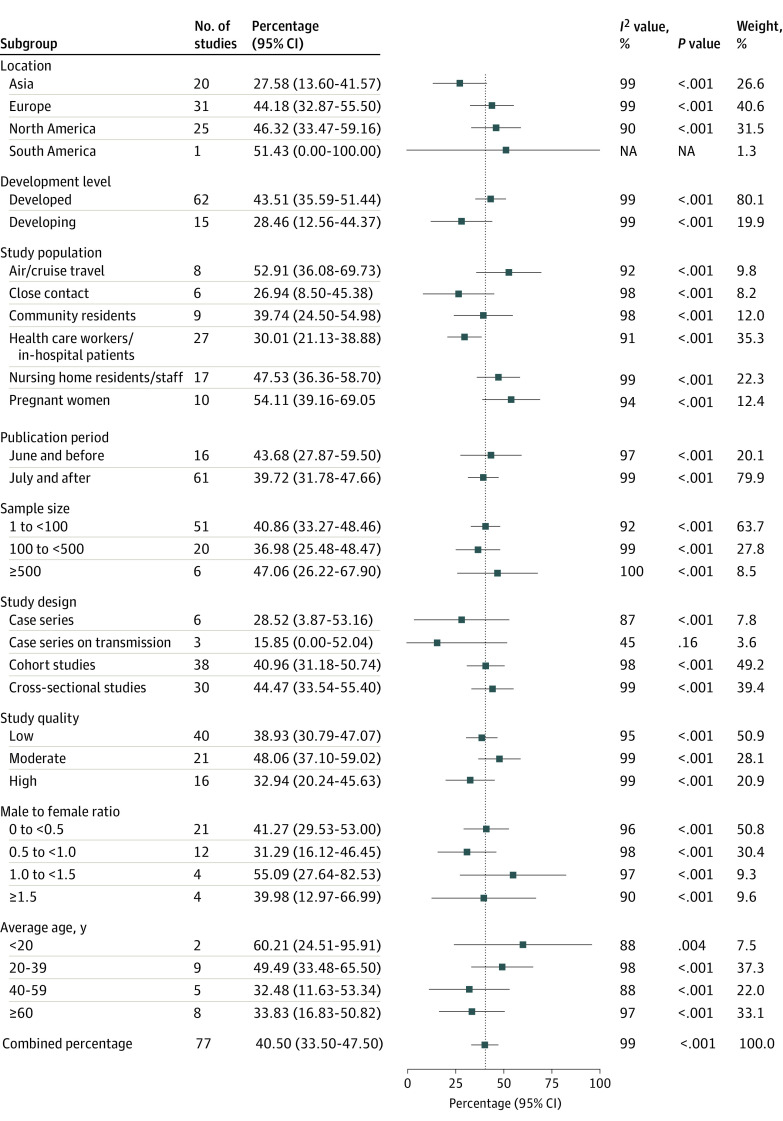
Among 95 studies, 18 were excluded because that the percentage of asymptomatic infections among the confirmed population was 100%.22,30,31,36,39,43,49,56,58,71,77,79,80,81,87,96,101,108 The remaining 77 studies were included in the meta-analysis for the percentage of asymptomatic infections among the confirmed population,12,23,24,25,26,27,28,29,32,33,34,35,37,38,40,41,42,44,45,46,47,48,50,51,52,53,54,55,57,59,60,61,62,63,64,65,66,67,68,69,70,72,73,74,75,76,78,82,83,84,85,86,88,89,90,91,92,93,94,95,97,98,99,100,102,103,104,105,106,107,109,110,111,112,113,114,115 covering 19 884 individuals with confirmed COVID-19, among whom 11 069 had asymptomatic infections. The pooled percentage of asymptomatic infections among the confirmed population was 40.50% (95% CI, 33.50%-47.50%), with high heterogeneity among studies (I2 = 99%; P < .001) (eFigure 2 in the Supplement).
Among the confirmed population, the pooled percentage of asymptomatic infections was 54.11% (95% CI, 39.16%-69.05%) in pregnant women, 52.91% (95% CI, 36.08%-69.73%) in air or cruise travelers, 47.53% (95% CI, 36.36%-58.70%) in nursing home residents or staff, 39.74% (95% CI, 24.50%-54.98%) in community residents, 30.01% (95% CI, 21.13%-38.88%) in health care workers or in-hospital patients, and 26.94% (95% CI, 8.50%-45.38%) in close contacts. The pooled percentage of asymptomatic infections was 46.32% (95% CI, 33.47%-59.16%) in North America, 44.18% (95% CI, 32.87%-55.50%) in Europe, and 27.58% (95% CI, 13.60%-41.57%) in Asia. The pooled percentage was higher in developed countries (43.51% [95% CI, 35.59%-51.44%]), studies published in June or earlier (43.68% [95% CI, 27.87%-59.50%]), studies with sample size of 500 or greater (47.06% [95% CI, 26.22%-67.90%]), and cross-sectional studies (44.47% [95% CI, 33.54%-55.40%]). The pooled percentage was slightly lower for cohort studies (40.96% [95% CI, 31.18%-50.74%]). Among studies with MFR of 1.0 to less than 1.5, the pooled percentage was higher (55.09% [95% CI, 27.64%-82.53%]). The pooled percentage was higher when the mean age of the study population was younger than 20 years (60.21% [95% CI, 24.51%-95.91%]) or 20 to 39 years (49.49% [95% CI, 33.48%-65.50%]) (Figure 3).
Figure 3. Percentage of Asymptomatic Infections Among the Confirmed Population by Subgroups.
Includes 19 884 individuals with confirmed COVID-19, among whom 11 069 had asymptomatic infections.
Sensitivity Analysis and Publication Bias
After using the Knapp-Hartung adjustments, the pooled percentage of asymptomatic infections among the tested population was 0.25% (95% CI, 0.11%-0.39%), and the 95% CI of the pooled percentage became slightly larger (eFigure 3 in the Supplement). The percentage of asymptomatic infections among the confirmed population was 40.50% (95% CI, 34.94%-46.07%), and the 95% CI of the pooled percentage became slightly narrower (eFigure 4 in the Supplement).
After excluding 3 studies with tested populations of more than 200 000,36,52,90 the pooled percentage of asymptomatic infections among the tested population was 1.61% (95% CI, 1.47%-1.76%), which was higher than the original results. The percentage of asymptomatic infections among the confirmed population was 39.37% (95% CI, 33.86%-44.87%), which was slightly lower than the original results. After excluding 53 low-quality studies, the pooled percentage of asymptomatic infections among the tested population was 0.24% (95% CI, 0.23%-0.26%), and the percentage of asymptomatic infections among the confirmed population was 41.71% (95% CI, 31.89%-51.53%). Both percentages were similar to the original results.
Funnel plots are shown in Figure 4. Egger regression tests for the percentage of asymptomatic infections among the tested population (z = 43.1725; P < .001) and for the percentage of asymptomatic infections among the confirmed population (z = 2.3846; P = .02) indicated that there might be publication bias.
Figure 4. Funnel Plots Based on the Percentage of Asymptomatic Infections.
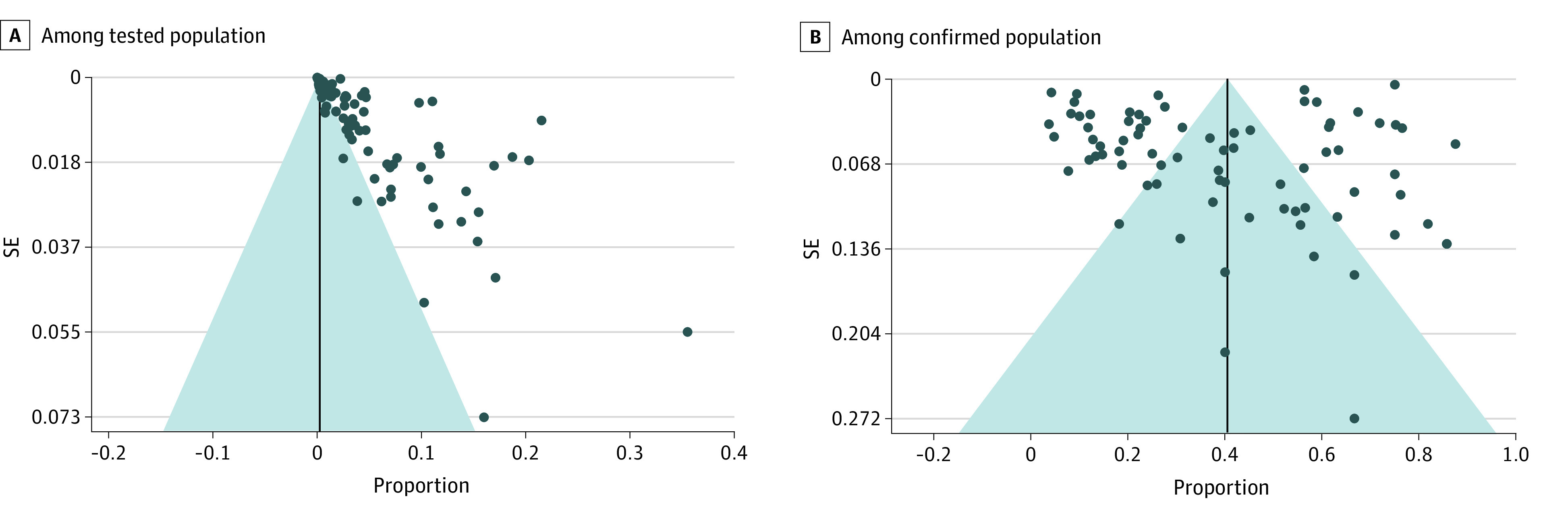
Includes 29 776 306 tested individuals, among whom 11 516 had asymptomatic infections and 19 884 individuals with confirmed COVID-19, among whom 11 069 had asymptomatic infections. Funnel plot asymmetry indicated possible publication bias.
Discussion
In this meta-analysis, we found that the pooled percentage of asymptomatic infections among the tested population was 0.25% (95% CI, 0.23%-0.27%), and the pooled percentage of asymptomatic infections among the confirmed population was 40.50% (95% CI, 33.50%-47.50%). At present, there are only a few meta-analyses for the percentage of asymptomatic infections among the tested population. We found that the percentage of asymptomatic infections was highest among the tested population in nursing homes and lowest among community residents. Because the percentage of asymptomatic individuals varies as a function of community prevalence, it was not available in all studies. This might be a potential driver of heterogeneity across studies. Furthermore, the percentages of asymptomatic infections among the tested population were different between studies conducted in different locations. Studies in Asia had the lowest percentage, whereas studies in other locations had higher percentages. This lower percentage in Asia might be related to the large city-wide SARS-CoV-2 nucleic acid screening program in China.36 In the sensitivity analyses, we found that the pooled percentage of asymptomatic infections among the tested population was higher than the original results after excluding studies with large sample sizes. This indicated that studies with different sample sizes were very heterogeneous. Owing to severe outcomes among older patients with COVID-19, more studies were conducted among nursing home residents or staff. Thus, asymptomatic individuals were more likely to be tested among this population. As more and more countries conducted expanded screening, studies concerning the percentage of asymptomatic infections among the general population would increase in the future.
In this study, the pooled percentage of asymptomatic infections among the confirmed population was 40.50%. The pooled percentage of asymptomatic infections was 40.96% among cohort studies, which was slightly lower than that among cross-sectional studies (44.47%). The patients who developed symptoms later were mistakenly classified as having asymptomatic infection in cross-sectional studies because the observation time was not long enough.14 Thus, the percentage of asymptomatic infections was lower in cohort studies, because some patients with presymptomatic findings were identified during follow-up. There were limited case series of great interest in the first months of the pandemic; however, these studies mostly traced and tested limited contacts, which contributed limited value to the evidence of the percentage of asymptomatic infections.17 Several meta-analyses concerned the percentage of asymptomatic infections among the confirmed population. Chen et al5 conducted a meta-analysis that included 104 published studies and preprints before May 13, 2020. They found that the percentage of asymptomatic individuals among those with COVID-19 was 13.34% (95% CI, 10.86%-16.29%). Unlike our study, Chen et al5 searched a Chinese database. Thus, the percentage of Chinese studies was higher in their study than in the present study. He et al14 searched PubMed and Embase before May 20, 2020, and included 41 published studies. More than 50% of the studies were from China, and the pooled percentage of asymptomatic infection was 15.6% (95% CI, 10.1%-23.0%). In our study, we only included published studies. The percentage of countries excluding China was higher than the previous meta-analysis.14 This might be the reason for the higher percentage of asymptomatic infections found in our study compared with studies conducted by Chen et al5 and He et al.14 Another meta-analysis conducted by Yanes-Lane et al17 included published studies and preprints before June 22, 2020. After quality assessment, 28 studies were of high or moderate quality and were included in the meta-analysis. The percentage of asymptomatic infection among persons with confirmed COVID-19 varied among different study populations, with the highest observed in obstetric patients (95% [95% CI, 45%-100%]).
In our study, the percentage of asymptomatic infections among the confirmed population was 54.11% in pregnant women and 52.91% in air or cruise travelers. The percentage was 47.53% in nursing home residents or staff. This finding of a high percentage of asymptomatic infections among air or cruise travelers suggests that screening and quarantine on airport arrival is important for reducing community transmissions, especially in countries without local transmission.3,25 In addition, we found that the percentage of asymptomatic infections among the tested population was relatively low among community residents. However, the percentage of asymptomatic infection among confirmed individuals was 39.74% in communities. These findings suggest that asymptomatic infections might contribute to the transmission of SARS-CoV-2 within the community. To prevent further transmission in communities, asymptomatic individuals among the general population should be tested. If resources are limited, workers in specific industries such as air transportation should be routinely tested. In addition, we found that approximately one-third of individuals with confirmed COVID-19 were asymptomatic among health care workers or in-hospital patients. Because asymptomatic health care workers might contribute to disease spread in and out of hospitals, surveillance of asymptomatic individuals is important for infection control and transmission reduction in health care settings and community.116,117 Meanwhile, hand hygiene and personal protective equipment were necessary for hospital visitors.117 A previous study showed that most asymptomatic patients belong to younger groups,3 which was consistent with the findings of our study. The percentage of asymptomatic infections was higher among groups younger than 39 years than in other age groups, possibly because the young adults were more likely to show only mild or moderate clinical symptoms.5 This indicated that young adults who often presented mild or no symptoms were a potential source of transmission in the community.
In the meta-analysis, we included studies published before February 3, 2021, providing the most updated pooled percentage of asymptomatic infections among tested and confirmed populations. We included countries in Africa, Asia, Europe, North America, and South America and estimated the percentage of asymptomatic infections for different populations. Our results could raise awareness among the public and policy makers and provide evidence for prevention strategies.
Limitations
This study has several limitations. First, we did not include preprints and therefore may have missed some relevant studies; however, we thought that the results of published studies were more reliable. Second, some relevant articles written in Chinese may not be included because we did not search Chinese literature databases such as China National Knowledge Infrastructure. Third, most studies did not follow up to identify presymptomatic and covert infections. Future studies should evaluate the percentage of these 2 types of asymptomatic infection among the confirmed population. Fourth, most studies were conducted in a specific population; thus, our findings might not be generalizable to the general population. Fifth, the heterogeneity between studies was high, which might be related to different study location, period, population, and sample size. Sixth, the Egger regression test suggested potential publication bias in this study. Because studies that did not detect asymptomatic infections were less likely to be published, our pooled percentage of asymptomatic infections might be overestimated.
Conclusions
In this systematic review and meta-analysis, we found that the pooled percentage of asymptomatic SARS-CoV-2 infections among the tested population was 0.25%. Among the confirmed population, 40.50% of individuals had asymptomatic infections. The high percentage of asymptomatic infections highlights the potential transmission risk of asymptomatic infections in communities. Screening for asymptomatic infection is required, especially for countries and regions that have successfully controlled SARS-CoV-2. Asymptomatic infections should be under management similar to that for confirmed infections, including isolating and contact tracing.
eMethods 1. Search Strategies
eMethods 2. Study Quality Assessments
eFigure 1. Percentage of Asymptomatic Cases Among the Tested Population
eFigure 2. Percentage of Asymptomatic Cases Among the Confirmed Population
eFigure 3. Percentage of Asymptomatic Cases Among the Tested Population by Subgroups, Using the Knapp-Hartung Adjustments
eFigure 4. Percentage of Asymptomatic Cases Among the Confirmed Population by Subgroups, Using the Knapp-Hartung Adjustments
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed January 28, 2021. https://covid19.who.int/
- 3.Kronbichler A, Kresse D, Yoon S, Lee KH, Effenberger M, Shin JI. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180-186. doi: 10.1016/j.ijid.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Coronavirus disease (COVID-19): how is it transmitted? December 13, 2020. Accessed January 22, 2021. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted
- 5.Chen C, Zhu C, Yan D, et al. The epidemiological and radiographical characteristics of asymptomatic infections with the novel coronavirus (COVID-19): a systematic review and meta-analysis. Int J Infect Dis. 2021;104:458-464. doi: 10.1016/j.ijid.2021.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020;20(5):e102-e107. doi: 10.1016/S1473-3099(20)30129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnasamy N, Natarajan M, Ramachandran A, et al. Clinical outcomes among asymptomatic or mildly symptomatic COVID-19 patients in an isolation facility in Chennai, India. Am J Trop Med Hyg. 2021;104(1):85-90. doi: 10.4269/ajtmh.20-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ra SH, Lim JS, Kim GU, Kim MJ, Jung J, Kim SH. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. 2021;76(1):61-63. doi: 10.1136/thoraxjnl-2020-215042 [DOI] [PubMed] [Google Scholar]
- 9.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 10.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. doi: 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong ZD, Tang A, Li KF, et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26(5):1052-1054. doi: 10.3201/eid2605.200198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arons MM, Hatfield KM, Reddy SC, et al. ; Public Health–Seattle and King County and CDC COVID-19 Investigation Team . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081-2090. doi: 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Muscatello D, Tian Y, et al. Role of presymptomatic transmission of COVID-19: evidence from Beijing, China. J Epidemiol Community Health. 2021;75(1):84-87. doi: 10.1136/jech-2020-214635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2021;93(2):820-830. doi: 10.1002/jmv.26326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020. Accessed September 5, 2021. https://jbi-global-wiki.refined.site/space/MANUAL
- 16.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa Hospital Research Institute; 2011. Accessed September 5, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 17.Yanes-Lane M, Winters N, Fregonese F, et al. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: a systematic review and meta-analysis. PLoS One. 2020;15(11):e0241536. doi: 10.1371/journal.pone.0241536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139-145. doi: 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693-2710. doi: 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 22.Abdelmoniem R, Fouad R, Shawky S, et al. SARS-CoV-2 infection among asymptomatic healthcare workers of the emergency department in a tertiary care facility. J Clin Virol. 2021;134:104710. doi: 10.1016/j.jcv.2020.104710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abeysuriya S, Wasif S, Counihan C, et al. Universal screening for SARS-CoV-2 in pregnant women at term admitted to an East London maternity unit. Eur J Obstet Gynecol Reprod Biol. 2020;252:444-446. doi: 10.1016/j.ejogrb.2020.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbarialiabad H, Abdolrahimzadeh Fard H, Abbasi HR, et al. Our experience of trauma management during novel coronovirus 2019 (COVID-19) pandemic in a busy trauma center in southern Iran. Bull Emerg Trauma. 2020;8(3):199-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Qahtani M, AlAli S, AbdulRahman A, Salman Alsayyad A, Otoom S, Atkin SL. The prevalence of asymptomatic and symptomatic COVID-19 in a cohort of quarantined subjects. Int J Infect Dis. 2021;102:285-288. doi: 10.1016/j.ijid.2020.10.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Shamsi HO, Coomes EA, Aldhaheri K, Alrawi S. Serial screening for COVID-19 in asymptomatic patients receiving anticancer therapy in the United Arab Emirates. JAMA Oncol. 2021;7(1):129-131. doi: 10.1001/jamaoncol.2020.5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold FW, Bishop S, Oppy L, Scott L, Stevenson G. Surveillance testing reveals a significant proportion of hospitalized patients with SARS-CoV-2 are asymptomatic. Am J Infect Control. 2021;49(3):281-285. doi: 10.1016/j.ajic.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslam A, Singh J, Robilotti E, et al. SARS CoV-2 surveillance and exposure in the perioperative setting with universal testing and personal protective equipment (PPE) policies. Clin Infect Dis. Published online October 22, 2020. doi: 10.1093/cid/ciaa1607 [DOI] [PMC free article] [PubMed]
- 29.Bayle C, Cantin D, Vidal JS, et al. ; APHP COVID 19 research collaboration . Asymptomatic SARS COV-2 carriers among nursing home staff: A source of contamination for residents? Infect Dis Now. 2021;51(2):197-200. doi: 10.1016/j.idnow.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bender WR, Hirshberg A, Coutifaris P, Acker AL, Srinivas SK. Universal testing for severe acute respiratory syndrome coronavirus 2 in 2 Philadelphia hospitals: carrier prevalence and symptom development over 2 weeks. Am J Obstet Gynecol MFM. 2020;2(4):100226. doi: 10.1016/j.ajogmf.2020.100226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bianco A, Buckley AB, Overbey J, et al. Testing of patients and support persons for coronavirus disease 2019 (COVID-19) infection before scheduled deliveries. Obstet Gynecol. 2020;136(2):283-287. doi: 10.1097/AOG.0000000000003985 [DOI] [PubMed] [Google Scholar]
- 32.Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test-retest strategy in residents and health care personnel of a nursing home facing a COVID-19 outbreak. J Am Med Dir Assoc. 2020;21(7):933-936. doi: 10.1016/j.jamda.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blitz MJ, Rochelson B, Rausch AC, et al. Universal testing for coronavirus disease 2019 in pregnant women admitted for delivery: prevalence of peripartum infection and rate of asymptomatic carriers at four New York hospitals within an integrated healthcare system. Am J Obstet Gynecol MFM. 2020;2(3):100169. doi: 10.1016/j.ajogmf.2020.100169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumberg TJ, Adler AC, Lin EE, et al. Universal screening for COVID-19 in children undergoing orthopaedic surgery: a multicenter report. J Pediatr Orthop. 2020;40(10):e990-e993. doi: 10.1097/BPO.0000000000001657 [DOI] [PubMed] [Google Scholar]
- 35.Bosworth A, Whalley C, Poxon C, et al. Rapid implementation and validation of a cold-chain free SARS-CoV-2 diagnostic testing workflow to support surge capacity. J Clin Virol. 2020;128:104469. doi: 10.1016/j.jcv.2020.104469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao S, Gan Y, Wang C, et al. Post-lockdown SARS-CoV-2 nucleic acid screening in nearly ten million residents of Wuhan, China. Nat Commun. 2020;11(1):5917. doi: 10.1038/s41467-020-19802-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll C, Conway R, O’Donnell D, et al. Routine testing of close contacts of confirmed COVID-19 cases: national COVID-19 contact management programme, Ireland, May to August 2020. Public Health. 2021;190:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattelan AM, Sasset L, Di Meco E, et al. An integrated strategy for the prevention of SARS-CoV-2 infection in healthcare workers: a prospective observational study. Int J Environ Res Public Health. 2020;17(16):E5785. doi: 10.3390/ijerph17165785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cloutier L, Merindol N, Pépin G, et al. Asymptomatic carriers of COVID-19 in a confined adult community population in Quebec: a cross-sectional study. Am J Infect Control. 2021;49(1):120-122. doi: 10.1016/j.ajic.2020.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corcorran MA, Olin S, Rani G, et al. Prolonged persistence of PCR-detectable virus during an outbreak of SARS-CoV-2 in an inpatient geriatric psychiatry unit in King County, Washington. Am J Infect Control. 2021;49(3):293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng ZQ, Xia W, Fan YB, et al. Analysis on transmission chain of a cluster epidemic of COVID-19, Nanchang [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(9):1420-1423. [DOI] [PubMed] [Google Scholar]
- 42.Dora AV, Winnett A, Jatt LP, et al. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans: Los Angeles, California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(21):651-655. doi: 10.15585/mmwr.mm6921e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan P, Deng ZQ, Pan ZY, Wang YP. Safety considerations during return to work in the context of stable COVID-19 epidemic control: an analysis of health screening results of all returned staff from a hospital. Epidemiol Infect. 2020;148:e214. doi: 10.1017/S0950268820002150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figueiredo R, Tavares S, Moucho M, Ramalho C. Systematic screening for SARS-CoV-2 in pregnant women admitted for delivery in a Portuguese maternity. J Perinat Med. 2020;48(9):977-980. doi: 10.1515/jpm-2020-0387 [DOI] [PubMed] [Google Scholar]
- 45.Goldfarb IT, Diouf K, Barth WH, et al. Universal SARS-CoV-2 testing on admission to the labor and delivery unit: low prevalence among asymptomatic obstetric patients. Infect Control Hosp Epidemiol. 2020;41(9):1095-1096. doi: 10.1017/ice.2020.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham NSN, Junghans C, Downes R, et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020;81(3):411-419. doi: 10.1016/j.jinf.2020.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grechukhina O, Greenberg V, Lundsberg LS, et al. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am J Obstet Gynecol MFM. 2020;2(4)(suppl):100246. doi: 10.1016/j.ajogmf.2020.100246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruskay JA, Dvorzhinskiy A, Konnaris MA, et al. Universal testing for COVID-19 in essential orthopaedic surgery reveals a high percentage of asymptomatic infections. J Bone Joint Surg Am. 2020;102(16):1379-1388. doi: 10.2106/JBJS.20.01053 [DOI] [PubMed] [Google Scholar]
- 49.Han X, Wei X, Alwalid O, et al. Severe acute respiratory syndrome coronavirus 2 among asymptomatic workers screened for work resumption, China. Emerg Infect Dis. 2020;26(9):2265-2267. doi: 10.3201/eid2609.201848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada S, Uno S, Ando T, et al. Control of a nosocomial outbreak of COVID-19 in a university hospital. Open Forum Infect Dis. 2020;7(12):a512. doi: 10.1093/ofid/ofaa512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hcini N, Maamri F, Picone O, et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2021;257:11-18. doi: 10.1016/j.ejogrb.2020.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoxha A, Wyndham-Thomas C, Klamer S, et al. Asymptomatic SARS-CoV-2 infection in Belgian long-term care facilities. Lancet Infect Dis. 2021;21(4):e67. doi: 10.1016/S1473-3099(20)30560-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung IFN, Cheng VCC, Li X, et al. SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: a case series. Lancet Infect Dis. 2020;20(9):1051-1060. doi: 10.1016/S1473-3099(20)30364-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ibrahim F, Natasha A, Saharman YR, Sudarmono P. Preliminary report of COVID-19 testing: experience of the clinical microbiology laboratory Universitas Indonesia, Jakarta, Indonesia. New Microbes New Infect. 2020;37:100733. doi: 10.1016/j.nmni.2020.100733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennelly SP, Dyer AH, Noonan C, et al. Asymptomatic carriage rates and case-fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing. 2021;50(1):49-54. doi: 10.1093/ageing/afaa220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kessler T, Wiebe J, Graf T, Schunkert H, Kastrati A, Sager HB. SARS-CoV-2 infection in asymptomatic patients hospitalized for cardiac emergencies: implications for patient management. Front Cardiovasc Med. 2020;7:599299. doi: 10.3389/fcvm.2020.599299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimball A, Hatfield KM, Arons M, et al. ; Public Health–Seattle & King County; CDC COVID-19 Investigation Team . Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility: King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381. doi: 10.15585/mmwr.mm6913e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirshblum SC, DeLauter G, Lopreiato MC, et al. Screening testing for SARS-CoV-2 upon admission to rehabilitation hospitals in a high COVID-19 prevalence community. PM R. 2020;12(10):1009-1014. doi: 10.1002/pmrj.12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krüger S, Leskien M, Schuller P, et al. Performance and feasibility of universal PCR admission screening for SARS-CoV-2 in a German tertiary care hospital. J Med Virol. 2021;93(5):2890-2898. doi: 10.1002/jmv.26770 [DOI] [PubMed] [Google Scholar]
- 60.Kwon YS, Park SH, Kim HJ, et al. Screening clinic for coronavirus disease 2019 to prevent intrahospital spread in Daegu, Korea: a single-center report. J Korean Med Sci. 2020;35(26):e246. doi: 10.3346/jkms.2020.35.e246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LaCourse SM, Kachikis A, Blain M, et al. Low prevalence of SARS-CoV-2 among pregnant and postpartum patients with universal screening in Seattle, Washington. Clin Infect Dis. 2021;72(5):869-872. doi: 10.1093/cid/ciaa675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladhani SN, Chow JY, Janarthanan R, et al. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine. 2020;26:100533. doi: 10.1016/j.eclinm.2020.100533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lan FY, Suharlim C, Kales SN, Yang J. Association between SARS-CoV-2 infection, exposure risk and mental health among a cohort of essential retail workers in the USA. Occup Environ Med. 2021;78(4):237-243. [DOI] [PubMed] [Google Scholar]
- 64.Lavezzo E, Franchin E, Ciavarella C, et al. ; Imperial College COVID-19 Response Team; Imperial College COVID-19 Response Team . Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584(7821):425-429. doi: 10.1038/s41586-020-2488-1 [DOI] [PubMed] [Google Scholar]
- 65.Livingston G, Rostamipour H, Gallagher P, et al. Prevalence, management, and outcomes of SARS-CoV-2 infections in older people and those with dementia in mental health wards in London, UK: a retrospective observational study. Lancet Psychiatry. 2020;7(12):1054-1063. doi: 10.1016/S2215-0366(20)30434-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lombardi A, Consonni D, Carugno M, et al. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin Microbiol Infect. 2020;26(10):1413.e9-1413.e13. doi: 10.1016/j.cmi.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ly TDA, Zanini D, Laforge V, et al. Pattern of SARS-CoV-2 infection among dependant elderly residents living in long-term care facilities in Marseille, France, March-June 2020. Int J Antimicrob Agents. 2020;56(6):106219. doi: 10.1016/j.ijantimicag.2020.106219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lytras T, Dellis G, Flountzi A, et al. High prevalence of SARS-CoV-2 infection in repatriation flights to Greece from three European countries. J Travel Med. 2020;27(3):taaa054. doi: 10.1093/jtm/taaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maechler F, Gertler M, Hermes J, et al. Epidemiological and clinical characteristics of SARS-CoV-2 infections at a testing site in Berlin, Germany, March and April 2020-a cross-sectional study. Clin Microbiol Infect. 2020;26(12):1685.e7-1685.e12. doi: 10.1016/j.cmi.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marossy A, Rakowicz S, Bhan A, et al. A study of universal severe acute respiratory syndrome coronavirus 2 RNA testing among residents and staff in a large group of care homes in South London. J Infect Dis. 2021;223(3):381-388. doi: 10.1093/infdis/jiaa565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marschner S, Corradini S, Rauch J, et al. SARS-CoV-2 prevalence in an asymptomatic cancer cohort: results and consequences for clinical routine. Radiat Oncol. 2020;15(1):165. doi: 10.1186/s13014-020-01609-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez-Fierro ML, Ríos-Jasso J, Garza-Veloz I, et al. The role of close contacts of COVID-19 patients in the SARS-CoV-2 transmission: an emphasis on the percentage of nonevaluated positivity in Mexico. Am J Infect Control. 2021;49(1):15-20. doi: 10.1016/j.ajic.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Massarotti C, Adriano M, Cagnacci A, et al. Asymptomatic SARS-CoV-2 infections in pregnant patients in an Italian city during the complete lockdown. J Med Virol. 2021;93(3):1758-1760. doi: 10.1002/jmv.26458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mattar S, Martinez-Bravo C, Rivero R, et al. Epidemiological and viral features of a cohort of SARS-CoV-2 symptomatic and asymptomatic individuals in an area of the Colombian Caribbean. Ann Clin Microbiol Antimicrob. 2020;19(1):58. doi: 10.1186/s12941-020-00397-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menting T, Krause K, Benz-Tettey F, et al. Low-threshold SARS-CoV-2 testing facility for hospital staff: prevention of COVID-19 outbreaks? Int J Hyg Environ Health. 2021;231:113653. doi: 10.1016/j.ijheh.2020.113653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Migueres M, Mengelle C, Dimeglio C, et al. Saliva sampling for diagnosing SARS-CoV-2 infections in symptomatic patients and asymptomatic carriers. J Clin Virol. 2020;130:104580. doi: 10.1016/j.jcv.2020.104580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milani GP, Montomoli E, Bollati V, et al. ; UNICORN Consortium investigators . SARS-CoV-2 infection among asymptomatic homebound subjects in Milan, Italy. Eur J Intern Med. 2020;78:161-163. doi: 10.1016/j.ejim.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis. 2020;94:154-155. doi: 10.1016/j.ijid.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ochiai D, Kasuga Y, Iida M, Ikenoue S, Tanaka M. Universal screening for SARS-CoV-2 in asymptomatic obstetric patients in Tokyo, Japan. Int J Gynaecol Obstet. 2020;150(2):268-269. doi: 10.1002/ijgo.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olalla J, Correa AM, Martín-Escalante MD, et al. ; ROBLE group . Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: a Spanish experience. QJM. 2020;hcaa238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olmos C, Campaña G, Monreal V, et al. SARS-CoV-2 infection in asymptomatic healthcare workers at a clinic in Chile. PLoS One. 2021;16(1):e0245913. doi: 10.1371/journal.pone.0245913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park SY, Kim YM, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666-1670. doi: 10.3201/eid2608.201274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park JH, Jang JH, Lee K, Yoo SJ, Shin H. COVID-19 outbreak and presymptomatic transmission in pilgrim travelers who returned to Korea from Israel. J Korean Med Sci. 2020;35(48):e424. doi: 10.3346/jkms.2020.35.e424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel MC, Chaisson LH, Borgetti S, et al. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020;71(11):2920-2926. doi: 10.1093/cid/ciaa763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pavli A, Smeti P, Hadjianastasiou S, et al. In-flight transmission of COVID-19 on flights to Greece: an epidemiological analysis. Travel Med Infect Dis. 2020;38:101882. doi: 10.1016/j.tmaid.2020.101882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petersen I, Phillips A. Three quarters of people with SARS-CoV-2 infection are asymptomatic: analysis of English household survey data. Clin Epidemiol. 2020;12:1039-1043. doi: 10.2147/CLEP.S276825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puckett Y, Wilke L, Weber S, Parkes A, LoConte NK. Low rate of SARS-CoV-2 infection in adults with active cancer diagnosis in a nonendemic region in the United States. WMJ. 2020;119(4):286-288. [PubMed] [Google Scholar]
- 88.Ralli M, Morrone A, Arcangeli A, Ercoli L. Asymptomatic patients as a source of transmission of COVID-19 in homeless shelters. Int J Infect Dis. 2021;103:243-245. doi: 10.1016/j.ijid.2020.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rashid-Abdi M, Krifors A, Sälléber A, Eriksson J, Månsson E. Low rate of COVID-19 seroconversion in health-care workers at a Department of Infectious Diseases in Sweden during the later phase of the first wave: a prospective longitudinal seroepidemiological study. Infect Dis (Lond). 2021;53(3):169-175. doi: 10.1080/23744235.2020.1849787 [DOI] [PubMed] [Google Scholar]
- 90.Ren R, Zhang Y, Li Q, et al. Asymptomatic SARS-CoV-2 infections among persons entering China from April 16 to October 12, 2020. JAMA. 2021;325(5):489-492. doi: 10.1001/jama.2020.23942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rincón A, Moreso F, López-Herradón A, et al. The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin Kidney J. 2020;13(4):542-549. doi: 10.1093/ckj/sfaa119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roxby AC, Greninger AL, Hatfield KM, et al. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med. 2020;180(8):1101-1105. doi: 10.1001/jamainternmed.2020.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sacco G, Foucault G, Briere O, Annweiler C. COVID-19 in seniors: findings and lessons from mass screening in a nursing home. Maturitas. 2020;141:46-52. doi: 10.1016/j.maturitas.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Santos EJF, Ferreira RJO, Batista R, et al. Health care workers not in the frontline are more frequently carriers of coronavirus disease 2019: the experience of a tertiary Portuguese hospital. Infect Prev Pract. 2020;2(4):100099. doi: 10.1016/j.infpip.2020.100099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scheier T, Schibli A, Eich G, et al. Universal admission screening for SARS-CoV-2 infections among hospitalized patients, Switzerland, 2020. Emerg Infect Dis. 2021;27(2):404-410. doi: 10.3201/eid2702.202318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shah AS, Walkoff LA, Kuzo RS, et al. The utility of chest computed tomography (CT) and RT-PCR screening of asymptomatic patients for SARS-CoV-2 prior to semiurgent or urgent hospital procedures. Infect Control Hosp Epidemiol. 2020;41(12):1375-1377. doi: 10.1017/ice.2020.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi SM, Bakaev I, Chen H, Travison TG, Berry SD. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21(10):1378-1383.e1. doi: 10.1016/j.jamda.2020.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singer JS, Cheng EM, Murad DA, et al. Low prevalence (0.13%) of COVID-19 infection in asymptomatic pre-operative/pre-procedure patients at a large, academic medical center informs approaches to perioperative care. Surgery. 2020;168(6):980-986. doi: 10.1016/j.surg.2020.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang H, Tian JB, Dong JW, et al. Serologic detection of SARS-CoV-2 infections in hemodialysis centers: a multicenter retrospective study in Wuhan, China. Am J Kidney Dis. 2020;76(4):490-499.e1. doi: 10.1053/j.ajkd.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang O, Bigelow BF, Sheikh F, et al. Outcomes of nursing home COVID-19 patients by initial symptoms and comorbidity: results of universal testing of 1970 residents. J Am Med Dir Assoc. 2020;21(12):1767-1773.e1. doi: 10.1016/j.jamda.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Temkin E; Healthcare Worker COVID-19 Surveillance Working Group . Extremely low prevalence of asymptomatic COVID-19 among healthcare workers caring for COVID-19 patients in Israeli hospitals: a cross-sectional study. Clin Microbiol Infect. 2021;27(1):130.e1-130.e4. doi: 10.1016/j.cmi.2020.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trahan MJ, Mitric C, Malhamé I, Abenhaim HA. Screening and testing pregnant patients for SARS-CoV-2: first-wave experience of a designated COVID-19 hospitalization centre in Montreal. J Obstet Gynaecol Canada. 2021;43(5):571-575. doi: 10.1016/j.jogc.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsou TP, Chen WC, Huang ASE, Chang SC; Taiwan COVID-19 Outbreak Investigation Team . Epidemiology of the first 100 cases of COVID-19 in Taiwan and its implications on outbreak control. J Formos Med Assoc. 2020;119(11):1601-1607. doi: 10.1016/j.jfma.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Buul LW, van den Besselaar JH, Koene FMHPH, Buurman BM, Hertogh CMPM; COVID-19 NH-Study Group; COVID-19 NH-Study Group . Asymptomatic cases and limited transmission of SARS-CoV-2 in residents and healthcare workers in three Dutch nursing homes. Gerontol Geriatr Med. 2020;6:2333721420982800. doi: 10.1177/2333721420982800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varnell C Jr, Harshman LA, Smith L, et al. COVID-19 in pediatric kidney transplantation: the Improving Renal Outcomes Collaborative. Am J Transplant. 2021;21(8):2740-2748. doi: 10.1111/ajt.16501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wadhwa A, Fisher KA, Silver R, et al. Identification of presymptomatic and asymptomatic cases using cohort-based testing approaches at a large correctional facility: Chicago, Illinois, USA, May 2020. Clin Infect Dis. 2021;72(5):e128-e135. doi: 10.1093/cid/ciaa1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wi YM, Lim SJ, Kim SH, et al. Response system for and epidemiological features of COVID-19 in Gyeongsangnam-do Province in South Korea. Clin Infect Dis. 2021;72(4):661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wood J, Datta D, Hudson BL, et al. Prevalence of asymptomatic SARS-CoV-2 infection in children and adults in Marion County, Indiana. Cureus. 2020;12(8):e9794. doi: 10.7759/cureus.9794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamahata Y, Shibata A. Preparation for quarantine on the cruise ship Diamond Princess in Japan due to COVID-19. JMIR Public Health Surveill. 2020;6(2):e18821. doi: 10.2196/18821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yassa M, Yirmibes C, Cavusoglu G, et al. Outcomes of universal SARS-CoV-2 testing program in pregnant women admitted to hospital and the adjuvant role of lung ultrasound in screening: a prospective cohort study. J Matern Fetal Neonatal Med. 2020;33(22):3820-3826. doi: 10.1080/14767058.2020.1798398 [DOI] [PubMed] [Google Scholar]
- 111.Yau K, Muller MP, Lin M, et al. COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis. 2020;76(5):690-695.e1. doi: 10.1053/j.ajkd.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yousaf AR, Duca LM, Chu V, et al. A prospective cohort study in nonhospitalized household contacts with severe acute respiratory syndrome coronavirus 2 infection: symptom profiles and symptom change over time. Clin Infect Dis. 2021;73(7):e1841-e1849. doi: 10.1093/cid/ciaa1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang T, Wu HT, Wang LH, Yang WZ. Scenario-based study of medical resource requirement rapid assessment under the COVID-19 pandemic [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(0):E059. doi: 10.3760/cma.j.cn112338-20200401-00488 [DOI] [PubMed] [Google Scholar]
- 114.Zhang S, Guo M, Wu F, et al. Factors associated with asymptomatic infection in health-care workers with severe acute respiratory syndrome coronavirus 2 infection in Wuhan, China: a multicentre retrospective cohort study. Clin Microbiol Infect. 2020;26(12):1670-1675. doi: 10.1016/j.cmi.2020.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao D, Wang M, Wang M, et al. Asymptomatic infection by SARS-CoV-2 in healthcare workers: a study in a large teaching hospital in Wuhan, China. Int J Infect Dis. 2020;99:219-225. doi: 10.1016/j.ijid.2020.07.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. doi: 10.1016/S0140-6736(20)30917-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Passarelli VC, Faico-Filho K, Moreira LVL, et al. Asymptomatic COVID-19 in hospital visitors: the underestimated potential of viral shedding. Int J Infect Dis. 2021;102:412-414. doi: 10.1016/j.ijid.2020.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search Strategies
eMethods 2. Study Quality Assessments
eFigure 1. Percentage of Asymptomatic Cases Among the Tested Population
eFigure 2. Percentage of Asymptomatic Cases Among the Confirmed Population
eFigure 3. Percentage of Asymptomatic Cases Among the Tested Population by Subgroups, Using the Knapp-Hartung Adjustments
eFigure 4. Percentage of Asymptomatic Cases Among the Confirmed Population by Subgroups, Using the Knapp-Hartung Adjustments