Abstract
Theories of consciousness using neurobiological data or being influenced by these data have been focused either on states of consciousness or contents of consciousness. These theories have occasionally used evidence from psychophysical phenomena where conscious experience is a dependent experimental variable. However, systematic catalog of many such relevant phenomena has not been offered in terms of these theories. In the perceptual retouch theory of thalamocortical interaction, recently developed to become a blend with the dendritic integration theory, consciousness states and contents of consciousness are explained by the same mechanism. This general-purpose mechanism has modulation of the cortical layer-5 pyramidal neurons that represent contents of consciousness as its core. As a surplus, many experimental psychophysical phenomena of conscious perception can be explained by the workings of this mechanism. Historical origins and current views inherent in this theory are presented and reviewed.
Keywords: consciousness, thalamus, pyramidal neurons, apical dendrite, multicompartment, modulation, perceptual retouch
Introduction
Scientific theories of consciousness are expected to meet several criteria, including the experimental testability for falsifiability. Falsifiability in turn can be based, in addition to logical rigor of discourse, on adequate knowledge about the functioning of brain mechanisms and on the capability to explain experimental phenomena of consciousness. In the pertinent experimental testing, variance in conscious experience is treated as a dependent variable. (In order to avoid unnecessary confounds leading to difficulties in interpretation of experimental data in terms of consciousness mechanisms, it is advisable that independent physical stimulation variables are kept invariant within the experimental design.) The majority of influential scientific consciousness theories have been concerned with explanation of consciousness as a state or based their empirical arguments on a limited set of 1–2 experimental paradigms when explaining how contents of consciousness emerge (e.g. visual masking, perithreshold stimulus discrimination, and binocular rivalry). However, any consciousness theory aspiring for ample explanatory power must be able to explain a multitude of empirical consciousness phenomena, not only one or two. Similarly, any general theory of consciousness must be comfortable with ways to explain both the state and content of consciousness. Ultimately, a sound scientific consciousness theory should show how these variable phenomena converge on the workings of definite neurobiological mechanisms.
With regard to neurobiological foundations of consciousness, the prevailing theoretical and empirical tradition has been concerned with functioning of widespread neural networks and interactions of mutually remote brain areas at the level of large neural populations in bringing about the consciousness state or content (Bogen 1995a,b; Crick and Koch 2003; Sergent and Dehaene 2004; Tononi 2004; Massimini et al. 2005; Lamme 2006; Singer 2006; Alkire et al. 2008; Dehaene and Changeux 2011; Dehaene et al. 2011; Casali et al. 2013; Mashour 2014; Laureys et al. 2015; Tononi and Koch 2015; Tsuchiya et al. 2015; Michel 2017; Northoff and Huang 2017). Single-cell level of analysis in this theoretical and empirical context has been if not ignored, then by and large left to have the role of an ‘orphan’ (Llinás et al. 1998; LaBerge 2006). Yet we cannot ignore the simple fact that all large-scale processes and inter-areal interactions ultimately depend on and are regulated by what is going on at the level of single cell membranes for regulating axonal firing. This firing in turn necessarily forms these large-scale activity patterns.
A few decades ago, a neurobiological theory explaining a multitude of conscious-experience phenomena with a processing model of thalamocortical interaction as mediated by cortical neurons was proposed (perceptual retouch theory—Bachmann 1984, 1994, 1997, 1998, 1999). In that theory, the ‘knowledge about the mechanisms of consciousness states was “borrowed” to explain also emergence of consciousness contents’. Another feature of the theory was a focus on how to ‘explain real-time emergence of conscious perception contents mechanistically at the single-cell and microcircuit level of neuronal processes’ (postulating that the general principle of how single neurons interact when participating in the production of conscious perception sufficiently well mimics the action of larger neural populations in this process). Furthermore, the aim to ‘explain behavioral psychophysically measured phenomena on the basis of neurobiological mechanisms involved in conscious mentation’ can be noted as another defining characteristic of the theory. However, at that time our knowledge of the mechanisms by which different presynaptic signal inflows interact at the single-cell level was insufficient for a more or less convincing kind of the mechanistic model of conscious experience. The model neurons were essentially traditional integrate-and-fire units where content-specific and nonspecific presynaptic afferents jointly regulated postsynaptic membrane potentials and determined firing rate (as a prerequisite for the neuron to broadcast its information to other brain areas). Recent neurobiological research has considerably advanced our knowledge of the integrative function of cortical pyramidal neurons, thus paving the way for the revision and development of the perceptual retouch theory (PRT). (Critical to this advancement, the following features of this recently described mechanism can be outlined: ‘multicompartment functional architecture’ of the cortical neurons allowing both the perceptual content and the context-dependent modulation, ‘bottom-up processing interfaced with top-down processing’, and susceptibility to the effects from both, ‘content processing neural pathways and state regulating neural pathways’—Potez and Larkum 2008; Larkum et al. 2009, 2004, 1999; Murayama and Larkum 2009; Murayama et al. 2009; Palmer et al. 2012; Larkum 2013; Manita et al. 2015; Murphy et al. 2016; Suzuki and Larkum 2020; Takahashi et al. 2020, 2016. The latter one of the above-mentioned features holds a promise to overcome the typical chiasm between theories of consciousness contents and states of consciousness).
Based on the above considerations and the referenced empirical research, in what follows I will describe the original versions and the advanced, new version of the PRT and show how the neurobiological processing architecture implementing the theory mechanistically explains a multitude of experimentally produced and commonly introspected phenomena of conscious experience. Family resemblance of the dendritic integration theory (DIT, Bachmann et al. 2020) and apical amplification (AA) theory (Marvan et al. 2021) with PRT will be outlined. Then the meaning of the typical neural markers of conscious perception [e.g. N200, visual awareness negativity (VAN), P300b, and pre-stimulus electroencephalography (EEG) wave phase] in terms of the PRT/DIT-model-based interpretations of these markers will be presented. The ways the PRT/DIT model is related to the accounts of consciousness such as Tononi’s integrated information theory (IIT), Crick and Koch’s neuroreductionist theory (CK), global neuronal workspace theory (GNW), Lamme’s recurrent processing theory (RP), and Rosenthal’s higher-order thought theory (HOT) will be also briefly noted. Finally, some open questions important to answer in future experimental research will be listed.
The original PRT
The theoretical paper on ‘necessary and sufficient neurobiological mechanisms’ of conscious perception was submitted to Psychological Review in 1982. Based on what was known about thalamocortical interaction and its relation to regulation of the states of consciousness, several psychophysical phenomena of explicit (conscious) perception of stimulus contents were explained by an informal model. Visual masking, selective facilitation of target perception by attention, binocular rivalry, and several other experimental effects were explained by the same mechanism of interaction between the specific afferent system (SP) and nonspecific subcortical modulation system (NSP). The fast-operating specific pathways carry stimulus-specific information, and this information is encoded in cortical-specific neurons (tuned to the perceptual content of this information). For this content to become consciously experienced, content-specific neurons have to be modulated by the slower-operating nonspecific signals from subcortical sources of phasic arousal in order to ‘upgrade’ this content to the level sufficient for its conscious perception. SP is necessary, but insufficient for conscious experience; coordinated operation of SP and NSP is sufficient for conscious experience. The paper was returned, but recommended to be submitted to a more specialized journal, which ultimately resulted in the published article (Bachmann 1984). With a little help of the reviewers the rest was taken out from the paper and it remained focused just on visual masking. (Masking is the gold standard experimental method for depriving an otherwise well-perceived brief stimulus from being consciously experienced.) Later on, explanations of other phenomena besides visual masking returned, but in the other articles and a book (e.g. Bachmann 1994, 1997, 1999). While in Bachmann 1984 the informal model used generic terms like nonspecific thalamic modulation, cortical-specific neurons, etc., in Bachmann 1994 it was developed to a model of conceptual neurons where single cortical pyramidal (specific content-sensitive) neurons were modulated by presynaptic facilitatory input from the nonspecific thalamus (and possibly other related subcortical structures also responsible for phasic arousal). In Fig. 1A the general architecture of the model is depicted and in Fig. 1B the basic element of the model is shown. Figure 1B illustrates the way how presynaptic facilitatory inputs from NSP thalamus gradually depolarize the postsynaptic potential of the specific content neurons (SP), leading to firing and increase in the cumulative firing rate (a prerequisite for wider spread cortical communication and response). (A quantitative model was also developed. It simulated visual backward masking as a result of the mask ‘stealing’ the target-evoked, more slowly operating nonspecific modulation: when the delayed presynaptic modulating input from NSP arrives at the cortical neurons, the fast-reacting cortical SP neuron that represents the mask has a more depolarized excitatory postsynaptic membrane potential (EPSP) compared to the already somewhat decayed EPSP of the SP neuron that represents the preceding target. Therefore, just the mask is experienced in conscious perception instead of the target—Bachmann 1994. The main variables and parameters put in the model and characterizing the stimuli were stimulus onset asynchrony, stimulus intensity, additional facilitation from attention, etc.)
Figure 1.
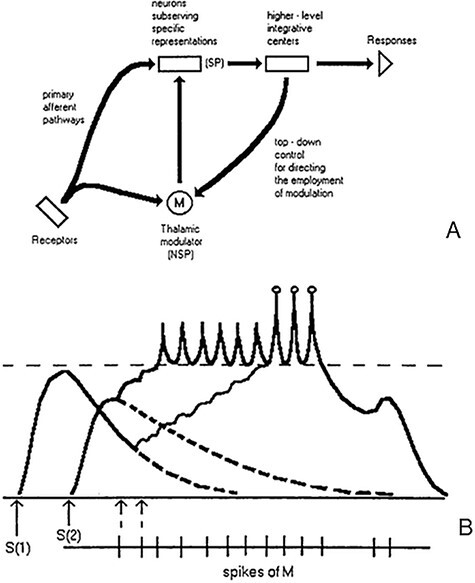
(A) The principal architecture of the neural pathways and functional levels illustrating the system of specific and nonspecific interaction as the core of the PRT. For a cortical SP unit to become active at the level allowing conscious perception of the content it carries, it is necessary that specific afferent activity and nonspecific modulating activity (NSP) from nonspecific thalamus converge on this SP unit. The subcortical NSP unit M can be ignited by collaterals from the receptors running in parallel with the specific afferent pathways and/or by the top-down input from higher-level cortical integrative areas. (B) Illustration of the real-time interaction of presynaptic signals and EPSP in response to two successive stimuli S(1) and S(2) that arrive within less than 100 ms and carry specific sensory/perceptual content. Presynaptic modulating inputs from the NSP (M) alter postsynaptic membrane potentials of the neurons S. Some EPSP change in the direction of the threshold of depolarization (and hence spiking) takes place ‘subliminally’, but after certain number of presynaptic inputs (communicated by axonal spikes of subcortically located neurons M) arriving with sufficiently fast frequency (avoiding return of the EPSP to the previous pre-spike level) the neuron starts firing. As there is a temporal asynchrony between the arrival of stimulus-specific signals to cortical S (expressed as an EPSP) and the arrival of the more delayed input from M, and because evoked EPSPs typically decay fast and exponentially, the specific information represented by S(2) neuron and expressed by higher depolarization amplitude than that for S(1) neuron, S(2) neuron begins firing sooner than S(1) neuron and its cumulative firing rate is also higher. This model property explains (i) backward masking (S2 prevailing over S1 in perception), (ii) facilitation of target perception by pre-cues in selective spatial attention (S1 speeds up and/or enhances subjective contrast of S2 in comparison with control condition without pre-cue), (iii) flash-lag effect (preceding instances of the streamed stimulus input speed up spiking of the neurons representing succeeding instances of the streamed input, which causes faster conscious access to streamed input compared to separately presented input), etc
The PRT basic model (Bachmann 1994) bears several theoretical implications instrumental for explaining empirical data of consciousness studies. (i) Unconscious and preconscious processing is real and substantiated (a) by the dynamics of EPSPs of the content-representing neurons with depolarization level remaining below the spiking threshold or (b) by the substantially lower spiking rate of these neurons compared to the spiking rate of neurons carrying competing information. (For instance, compare spiking rates for S1 neuron and S2 neuron in Fig. 1B simulating how a later arriving stimulus can deprive the preceding stimulus from being consciously perceived. Similar content-specific neurons can interact analogously also in binocular rivalry and some other conscious-perception effects. In sleep, which is an unconscious state, sensory input is processed largely without sufficient nonspecific modulation, which causes these inputs to remain subliminal for perception (although responded to in the cortex, as shown by neuroscience data). (ii) Stimuli that are of different contents compared to the content of an imperative (target-) stimulus can influence target perception because these content-wise different neurons have an effect on the nonspecific modulation applied on the target-content-carrying neurons. As the phasic thalamocortical modulation can easily spread to neurons with different receptive fields, including neurons for different modalities compared to the target-neuron’s modality, perception of target content can be influenced in terms of subjective clarity by spatially non-overlapping, content-wise unrelated, and modally different stimuli. (iii) Sensory stimulus S1 preceding the target stimulus S2 can speed up processing and increase subjective vividness of S2 if it precedes S2 by the time interval optimal for igniting the burst of nonspecific modulation. If the interval is too short, S2 is suppressed when S1 and S2 are sufficiently different and S2 is emphasized when S1 and S2 are identical or naturally transformatively coherent. If the interval is too long, there is either no perceptual effect of S1 on S2 or S2 perception can be suppressed due to the inhibitory after-effect of the nonspecific burst (which leaves S2 insufficiently modulated, although it was a target—e.g. the attentional blink effect). (iv) All substances or invasive interventions that have an effect on the nonspecific subcortical structures such that the normal subcorticocortical interactions are disturbed and/or substantially inhibited (or, alternatively, artificially boosted) have an effect on subjective vividness of perception. Suppression examples: general anesthetics, invasive subcortical activations by microelectrodes or optogenetic methods, and depressants. Enhancement examples: caffeine or amphetamine effects, aminergic facilitation of cell membrane functions, and microelectrode activation of nonspecific subcortical units. (v) For a steady, continuous experience of the environment, continuous activity of the thalamocortical interaction is necessary. In this interaction, subcorticocortical cycles of modulation of the large pool of cortical content-carrying neurons display an ongoing pattern of activity fluctuations characterized by certain optimal frequencies. For this to happen, continuous cycles of bottom-up-top-down-bottom-up! interaction are necessary (see the top-down contour in Fig. 1A). If the regimen of this process changes beyond normal, change in consciousness state occurs, with accompanying change in the currently experienced (not experienced) content. Examples: epileptic attacks, slow-wave sleep, general anesthesia, and alpha-frequency suppression. (The several additional model-based regularities can be found in the references for PRT.) References including supportive experimental evidence for the above five principles of the theoretical model (Bachmann 1994, 1999) are drawn in the second paragraph of Introduction and Consciousness phenomena and their brain-process markers as explained by PRT later on.
By this model, backward masking, facilitation of target by spatial pre-cue in spatial attention, perceptual latency priming, Fröhlich effect, flash-lag effect, binocular rivalry, and some other spatiotemporal effects were explained (Bachmann 1994, 1997, 1999). Additionally, motion-induced blindness can also be interpreted in terms of the retouch model (Bachmann 2013). It is important to point out that the qualifiers ‘specific’ and ‘non-specific’ may easily lead to misunderstanding. While the ‘source’ of modulation belongs to the nonspecific system and the principle how its efferents modulate specific neurons is ‘universal’ (i.e. invariant to what is the specific content of the neuron it modulates), the result of modulation becomes content-specific. This happens as soon as the sufficient share of modulation has been applied to the SP neural unit: the specific content carried by that unit becomes consciously experienced and hence the result is specific with its selective content. By an analogy, when a beam of light will be streamed at some object in dark, the object becomes visible and when the light is switched on then all kinds of different objects in the formerly dark room become visible. When the things the beam enlightens are different or when the room becomes populated with different objects, the ‘same’ kind of light leads to ‘different’ objects being/becoming visible. Conscious experience always includes some specific content, but in order to explicate this initially unconscious content in consciousness, a nonspecific process must be applied.
In the original version of PRT, the subsystem of unspecific modulation was located in the subcortical structures that do not carry the contents of stimulation via primary sensory relays (except the spatial location code with low spatial resolution and code for intensity of input). As noted above, for modulation to have an effect on perceptual experience the time interval between the modulation-igniting input and the task-imperative stimulus has to be optimal for that effect. If the modulation is extremely fast (e.g. the upstate caused by the steady high-level modulation—either by the arousal state or by the psychopharmacological substance), explicit perception can also be exceptionally fast. Conversely, if modulation impulses’ pace is slowed such as with alcohol or depressants, perceptual speed and vividness may be subdued. For example, when structures in the intralaminal parts of the thalamus were artificially stimulated by implanted electrodes in patients with Parkinson’s disease, instead of backward masking the unusually good perception of the first presented stimulus was observed (Bachmann 1994). Anecdotally, any intervention that causes dysfunctioning of the normal membrane processes in the subcortical modulation-sending neurons and/or cortical, the to-be-modulated, content neurons disrupts the subcorticocortical interactions and causes loss of consciousness or illusory sensory effects. (Consider knock-downs in boxing or seeing stars in daylight when falling down, with your head hitting the ground.) In the original PRT the sufficient unspecific modulation was considered as the necessary requirement for conscious experience. In Fig. 1A we see that both feedforward collateral pathways and top-down pathways can participate in regulating the modulation. This leaves open the question whether reentrant processes are necessary or whether bottom-up mode of unspecific modulation impacting cortical content neurons may be sufficient if it is associated with sufficient specific activity of the representational content neurons. PRT has been related to the microgenetic paradigm requesting that conscious experience capacity has evolved along natural evolution, including evolution of the more primordial subcortical systems and more recent neocortical systems, with actual act of perception being a recapitulation of the basic parts of the evolutionary sequence in a momentary microgenetic act (Bachmann 1994, 2000). Although by means of abstract computational models the same input–output regularities can be realized with almost an endless number of algorithm varieties, the real causal ‘structure’ of these evolutionary subsystems poses definite, ‘neurobiologically verifiable constraints’ on these models. One of the constraints is that the known anatomical human brain structure is necessary, but insufficient for consciousness unless it becomes ‘active’ in a neural process of content-specification carried out by nonspecific modulation of these content-carrying structures. The content can come from neuronal memory representations independent of the current sensory input (e.g. dreams, artificial stimulation of cerebral neurons, and hallucinations) as well as from current input driving these representations. The same ‘anatomical brain structure’ with its potential for an endless variety of activation patterns of its subparts can give rise to different conscious experiences depending on which subparts of this structure actually unfold the ‘processes’ pertaining to (1) specific actual content and (2) actual modulations that emphasize this content. Importantly, it is insufficient to relate conscious experience to some ‘abstract principle’ how subsystems interact, but to experimentally verified way how ‘real neurobiological’ subsystems interact when producing this experience. (For example, whether and how in the real brain feedforward and recurrent pathways interact and precisely how the externally ignited and intrinsic brain activities give rise to specific conscious experiences.) I believe that this actual neurobiological precise knowledge puts constraints on the abstract theories about causal structure, which could help deal with the unfolding argument (Doerig et al. 2019) well enough.
One may ask why PRT should be associated with consciousness at all and maybe PRT-like interactions can take place without consciousness as well. First, the nonspecific modulation subsystem has been massively shown to be involved in granting consciousness for human subjects and very likely also to mammals in general (Bogen 1995a,b; Brazier 1977; Singer 2006; Alkire et al. 2008; Mashour 2014; Northoff and Huang 2017). In the present paper in many places pertinent empirical support for this is referred to, including work from neurology, systems neuroscience, and experiments with animal models in vivo. Second, the PRT model shows explicitly how conscious-level vs. unconscious-level processing differs in terms of the relative amount of spiking of the neurons representing the consciously perceived stimuli vs. consciously unperceived stimuli. Third, the spatiotemporal known characteristics of the receptive fields and neural response dynamics of the real neurons are implemented in the interactions as described in the PRT model (Brooks and Jung 1973). The spatiotemporal characteristics of the experimental subjective phenomena of conscious perception quite well fit with these spatiotemporal values implemented in the model.
Given that in an alert subject some content is continuously experienced due to the steady cycles of modulation, how can it be that a newly presented stimulus becomes experienced because of this background modulation? According to PRT it is the phasic, novel stimulus ignited burst of modulation with relatively more intensity than the steady background modulation which causes the newcomer-stimulus to be noticed and experienced on the background of the already earlier present field of perception. (In Bachmann 1994, a concept of ‘miniature orienting reflex’ with a characteristic fast unfolding in time was also briefly considered.)
The variant of retouch theory associated with neuronal synchronization
In a somewhat different version of the PRT, it was assumed that nonspecific modulator may function as a synchronizing device (‘third unit’) sending its oscillating high-frequency signals to feature-sensitive neurons that represent features of certain stimulus objects (Bachmann 2007). It was postulated that SP representations of stimulus objects are formed by synchronizing the oscillating activity of the neurons representing the features of a given object. However, this type of binding (binding features to form objects) is deemed insufficient for explicit perception of the object. Additionally, it is necessary that this synchrony of object-defining feature-neurons becomes modulated and synchronized by the oscillatory activity of the subcortical NSP system—this is in order to bring the object into perceptual conscious experience. Subsequently, quantitative models of such second-order synchronization for consciousness (‘binding the binding’) were developed (Bachmann and Kirt 2013; Kirt and Bachmann 2013). This version of the retouch model successfully simulated psychophysical backward masking (review: Bachmann and Francis 2014), proactive speed-up of target perception by the preceding prime (e.g. Scharlau 2007), and illusory feature misbinding (e.g. Treisman and Schmidt 1982; Whitney 2009). Despite the promising first results of both the early PR model and its oscillatory-activity-based version, the way the modulations of content-carrying neurons at the single-cell and microcircuit level were envisaged was underspecified. New developments in the neurobiology of cortical single-cell functioning suggested a need for revision of the PRT where the traditional integrate-and-fire, leaky integrator type model neurons had been used.
Revision of the PRT informed by the new neurobiological findings
In Introduction the relevant research was already briefly listed, with main emphasis on the studies by Matthew Larkum’s group (Larkum et al. 1999, 2004, 2009; Potez and Larkum 2008; Murayama and Larkum 2009; Murayama et al. 2009; Palmer et al. 2012; Larkum 2013; Manita et al. 2015; Murphy et al. 2016; Takahashi et al. 2016, 2020; Suzuki and Larkum 2020). Under the influence of these new findings, the PRT became to transform gradually into its newer version (Bachmann, 2013; Bachmann and Hudetz 2014; Bachmann 2015) and very similar to the DIT of the state and content of consciousness (Bachmann et al. 2020; Aru et al. 2020b) as well as to the AA theory (Marvan et al. 2021). In Bachmann (2013, 3, emphasis added here) we read: ‘The presynaptic inputs from both, SP-channels (from receptors via the lateral geniculate body up to the cortex) and NSP-channels (from the thalamocortical modulation system) converge on the cortical SP and both types of inputs regulate the excitatory postsynaptic potentials of the SP neurons. When this presynaptic input, combining somatic and dendritic presynaptic effects from direct SP-channels and indirect NSP-channels is strong enough (e.g., as applied onto pyramidal neurons with their characteristic long apical dendrites), the specific neurons begin firing or increase their firing rate.’ (Fig. 2 shows how the SP/NSP interaction became to look like according to the PRT after its revision around 2013–2014, influenced by these new developments in the neurobiology of multicompartment neurons.)
Figure 2.
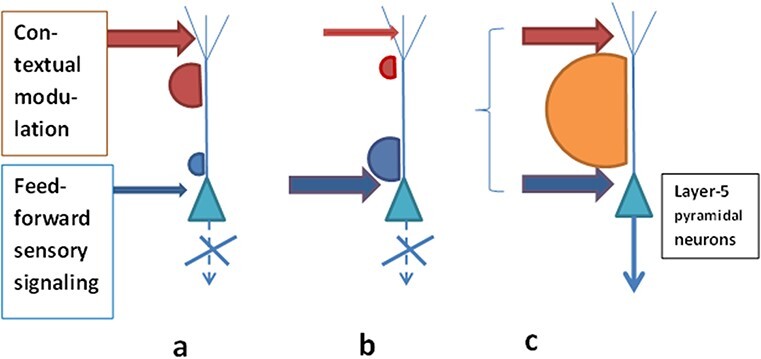
Sufficiently strong and sufficiently synchronized presynaptic inputs from the specific content-carrying feedforward pathways (targeting the perisomatic part of the neuron) and from the modulating pathways (targeting the apical dendrites) are the preconditions for conscious perception of the content to occur (c). When these requirements are not satisfied, neuron’s firing is subdued and longer-range connectivity not available (a, b)—consequently, the perceiver is not conscious of the content represented by the neurons acting in this way
Related to this, a similar theoretical account of how to integrate theories of the contents of consciousness and consciousness states into a coherent single one theory was proposed (Aru et al. 2019).
Dendritic integration theory, interpreted as an up-to-date implementation and revision of the retouch theory
Undoubtedly the strongest impact on the recent development of the PRT and its convergence with the DIT (Bachmann et al. 2020; Aru et al. 2020b) and AA theory (Phillips et al. 2016, 2018) came from the discoveries of BAC mechanism (e.g. Larkum et al. 1999; Larkum 2013). This was conveniently a mechanism allowing to understand how the stimulus-specific information can be selected and/or amplified by the support of the input from brain sources other than the afferent inflows of the stimulus-specific signals. In the PRT the stimulus content-carrying cortical neuron has to be modulated by presynaptic input from another source besides the sensory afference. The two different presynaptic influences are mediated by mutually autonomous presynaptic mechanisms with their effects converging on the same cortical neurons. The mechanism of coupling as featured in BAC suited PRT requirements quite well. Coincidence of the stimulus-specific input closer to the soma of the neuron and contextual input closer to the apical part of the neuron’s dendrite causes a burst of firing predisposing the neuron for long-range communication of its signals. The BAC mechanism is explosive in that a few potential changes in the apical zone are sufficient to cause a plateau-wave-based firing (if the spikes backpropagating from the somatic zone are temporally coincident). For example, the layer-5 pyramidal neurons generate plateau-wave-based spiking when the temporal coincidence of somatic sodium channel-related presynaptic input (i.e. data for the specific content subsystem SP in terms of PRT) and calcium channel-related presynaptic input targeted at the apical compartment of the cell dendrite (i.e. modulation by the subsystem NSP in terms of PRT) occurs. Noteworthy: dendritic calcium spikes are a direct target of anesthetics (Potez and Larkum 2008). Moreover, after release from the anesthetic effects, calcium electrogenesis in layer-5b pyramidal neurons dramatically increases (Murayama and Larkum 2009). These facts support the principal importance of the BAC firing mechanism for consciousness and pave the way for integrating the consciousness state theories and consciousness contents theories because the infragranular pyramidal neurons together with their apical dendrites (extending up to the first cortical layer) are targets to specific afferent input as well as contextual input from remote brain areas. In the PRT it was stated that specific content neurons can be activated below the threshold of conscious perception, but still allowing some subliminal processing (EPSPs below firing threshold or EPSP changes leading to only few spikes). In the BAC-based mechanism, suprathreshold input to the neuron’s body (responsible for the bottom-up inflow of specific sensory signals) produces fewer action potentials of the cell than triggering of the dendritic Ca2+ spikes. This shows that, in addition to the straightforward sensory afference, modulatory brain processes are involved in perception. This kind of mechanism also provides a strong argument for the common effects of biased perception being under the contextual and arousal systems control. While in Fig. 1A we see that the PRT architecture of the earlier version features modulations originating from the phasic arousal system, in the later PRT version (Bachmann 2007) corticocortical contextual top-down effects were added to the model similarly to what is assumed in the BAC-based model of contextual modulation (Larkum 2013). [In the PRT model of conscious perception it was important that the interacting stimuli (in masking, spatial precueing, flash-lag displays, binocular rivalry, etc.) share at least some part of receptive fields in order to allow robust psychophysical phenomena of conscious perception. As spatial location of the sensory input is one of the prime perceptual contexts in the habitat of the organism, it is correct to assume that the mechanisms under consideration here are also highly sensitive to the spatial location context].
Importantly for the PRT, layer-5 ‘content neurons’ receive presynaptic contextual input to dendrites not only from higher-level cortical areas, but also from secondary thalamic nuclei (Murayama and Larkum 2009; Suzuki and Larkum 2020). (This is important also because the subcortical nonspecific modulating effects often acquire their modus operandi as top-down effects. For example, noradrenergic modulation initiated from locus coeruleus and/or cholinergic modulation originating from neurons in the pontine brainstem and its associated higher ‘non-specific’ nuclei flows first in the more rostral areas of cortex and then, from there, top-down to lower-level cortical areas in the temporal and occipital cortex—Descarries et al. 2004; Petzold et al. 2015; Mather et al. 2016; Phillips et al. 2016; Gelbard-Sagiv et al. 2018; Vazey et al. 2018). In their recent work, Larkum’s group showed (Suzuki and Larkum 2020) that the two-compartment dendritic integration model can be updated to a three-compartment model (Aru et al. 2020b). Suzuki and Larkum (2020) discovered a mechanism explaining how nonspecific thalamic nuclei can carry out the interaction between apical and somatic compartments. It has been known for long that disruption of consciousness (e.g. in anesthesia) does not terminate primary feedforward sensory input to cortical content-carrying areas and does not substantially affect the firing properties of cortical neurons in general. How could this be achieved in terms of the underlying mechanisms? In that study the apical compartment of layer-5 pyramidal neurons was optogenetically stimulated and the resulting electrophysiological activity at cell soma was measured. This was done while varying the conscious state of the animal. In the supposedly conscious (awake) state, the apical compartment stimulation caused a substantial effect on the soma together with high-frequency firing of the neurons. However, in the anesthetized state of the animal this effect disappeared—the apical stimulation effect did not propagate to the soma anymore. This is the effect of decoupling: under anesthesia the perisomatic compartment was insensitive to potential influences from apical compartment of the dendrite (these influences potentially generated by top-down signals from brain sources specifying the context). By virtue of this mechanism, the workings of anesthesia manipulations can be understood. To further understand this mechanism, Suzuki and Larkum (2020) attempted to find out what could be the natural source of such an effect in the brain. They found that whether decoupling occurs or not can be gated by the nonspecific thalamic nucleus (the posteromedial nucleus, POm). The authors showed that blocking metabotropic receptor activation arising from nonspecific thalamic input decouples apical and somatic compartments in awake animals. (Additionally, as was shown in awake animals, inactivation of the POm with gamma-Aminobutyric acid (GABA) receptor agonist muscimol caused a breakdown of the signal propagation from the apical compartment to somatic compartment. Hence we know that nonspecific thalamic nuclei can control the interaction between the two compartments of cortical layer-5 pyramidal neurons.) The zone of the dendrite where this gating due to input from NSP can be realized could be a third compartment critical for regulation of consciousness (Aru et al. 2020b). At the same time, this mechanism provides foundation for the integration of corticocentric and thalamocortical theories of consciousness within one coherent theory (Bachmann et al. 2020; Aru et al. 2020b).
However, the above-presented results by Suzuki and Larkum (2020) are somewhat agnostic to subjective and behavioral aspect of the mechanistic effects. In other words, are these results relevant at all for consciousness science? Larkum group research has helped us also with this regard. Capitalizing on a sensory detection task, Takahashi et al. (2016) showed that by manipulation of layer-5 apical dendritic activity it was possible to manipulate the behavioral report of the animal. Rodents learned to detect weak near-threshold whisker stimuli varying in intensity. Psychometric curves expressing stimulation detection were delineated and correlated with neurometric curves. (The activity of the apical tuft dendrites was monitored by performing fast-scanning two-photon Ca2+ imaging.) The Ca2+ signals recorded at apical dendrites significantly correlated with the behavior of the animal and predicted hits and misses of peri-threshold stimuli. When apical dendritic activity was manipulated through pharmacological intervention or optogenetics, detection behavior significantly changed (Takahashi et al. 2016). It is parsimonious to assume that conscious perception of the animal also changed accordingly. (The behavioral effect was evidenced by a shift of the psychometric curve. Interestingly, optogenetic enhancement of apical dendritic activity also caused false alarms—animals responded as if the stimulus was present. I prefer to interpret this as the effect of illusory perception of the stimulus by the animal in the form of hallucination of stimulus presence.)
If layer-5 pyramidal neurons are the site where thalamocortical and corticocortical modulation takes place, are there other neurons with a similar function? We do not know, but some new evidence specifying the types of neurons involved in coupling cortical and thalamocortical interactions has been gathered in other related experiments. The association of dendritic activity with behavior was found to be constrained to the pyramidal neurons in layer 5B of the cortex (Takahashi et al. 2020). This is consistent with the notions accepted in PRT because these cells in layer 5B are known to project to thalamic nuclei (Harris and Shepherd 2015). (As the layer 5A neurons show denser corticocortical projections, they may not be so significantly associated with the interactions intimately related to regulating consciousness, compared to neurons with heavy corticothalamic links.) In a recent study, visual orientation-selective neurons in mouse neocortex were targeted by optogenetic methods (Marshel et al. 2019). Neural discrimination was manipulated by stimulation of different cortical layers. Layer 5 stimulation was more potent than layer 2/3 stimulation at recruiting percept-selective network and causing correct behavior, which supports the importance of layer-5 cells in perceptual functions.
From the preceding descriptions we get that the BAC-based contextual modulation theory (Larkum 2013), DIT (Bachmann, Suzuki, Aru, 2020; Aru et al. 2020a), and PRT (Bachmann 1984, 1994, 1997, 2007) all share several common characteristics and assumptions. (i) In addition to the receiving of afferent information and encoding it, modulation is crucial for the perceiving subject to have arrived at the conscious level of experience. (ii) Specific content providing mechanisms and modulating mechanisms are autonomous and can have independent effects on the different compartments of the neuron’s membrane; only some critical relation of the characteristics of these initially isolated effects can lead to interaction, including interaction necessary for conscious perception. (iii) The neural units that carry perceptual information do not function in the all-or-none mode, but show capacity of gradual change of the activity level; this variability of level can be present also below the level which is necessary for the neuron to fire. Sufficient level of activity at the subliminal level at the unconscious stage of content processing can be regarded as one kind of prerequisites for the neural correlates of consciousness (NCCpr)—Bachmann 2009; Aru et al. 2012) necessary to ignite processes subsequently culminating in conscious experience. This property of the DIT/PRT mechanism substantiates that subliminal processing is really possible. (iv) The modulating input to content-representing neurons can arrive both from subcortical sources and higher-level cortical sources and also from the cortical sources that are ignited by nonspecific subcortical modulation. (v) The level of activity of the pool of cortical layer-5 neurons which is regulated jointly by the modulatory input to (a) apical dendrites and (b) intermediate compartment located between apical and somatic zones of these neurons determines both the state of consciousness and contents of consciousness echoing this particular kind of state.
Consciousness phenomena and their brain-process markers as explained by PRT
The way the PRT/DIT interprets different phenomena where conscious experience is the main dependent variable of interest has been presented in several earlier publications (Bachmann 1994, 1997, 1999, 2000, 2011, 2015; Bachmann and Hudetz 2014; Aru et al. 2019). These sources are recommended for a more broad take of the explanatory repertoire of the theory. Here, I will focus on some more well-known experimental and clinical empirical findings. Of course, each of these facts cannot be regarded as an ultimate proof of the PRT/DIT. Rather they are a converging support where each example in isolation may not be too strong in its explanatory power, but if these different facts are treated in concert and as shown to be related to real, well-researched neural mechanisms, the explanatory resource is hopefully there. It seems useful to compare different theories in terms of how many empirical effects of consciousness they mechanismically explain. It must be emphasized that for many different phenomena there are specific dedicated theories that, independently, purport to explain a specific phenomenon. However, in case of PRT one theory based on the known properties of the real neural interactive mechanism suggests explanations for the multitude of the phenomena. For example, if for masking we have six different theories, each compatible with empirical masking effects, but only one of them is compatible with several other empirical effects of conscious perception then, by parsimony, that one is indirectly supported by the consistency with other phenomena.
Phenomena observed in the general conscious state of the human subject
Perception of stimuli presented with perithreshold magnitude
For example, when a visual, auditory, or tactile brief stimulus is presented with a pre-calibrated individual near-threshold value of magnitude (intensity, contrast, etc.), it is sometimes experienced consciously and sometimes not (Supèr et al. 2001; Koivisto and Grassini 2016; Koivisto et al., 2016, 2018; Rutiku et al. 2016; Tagliabue et al. 2016; Van Vugt et al. 2018; Eklund et al. 2020). The between-trials bistability of perception—conscious vs. not conscious—is typical also for a stimulus presented always with an invariant physical value (intensity, duration, feature contrast, etc.). In terms of PRT this means insufficient net amount of SP-neurons’ activity, given the combined presynaptic input to three compartments of these SP neurons that represent the features of the stimulus. This input (i) comes from afferent pathways signaling actual specific stimulus information and is projected onto somatic part of the neuron; (ii) arrives via top-down pathways from higher cortical areas sending contextual information and is projected onto apical part of the dendrites of the SP neurons; and (iii) comes from NSP sources sending signals to the intermediate compartment of the dendrites of the SP neurons in order to control whether coupling of apical and somatic activity of the cell takes place (and probably the extent to which this coupling occurs). The causes why a stimulus may remain unconsciously processed, but not experienced in conscious perception can be multiple and mutually combined: Type-(i) input is insufficient (fluctuations in adaptation or receptor activity, etc.); Type-(ii) input is insufficient (fluctuations in sustained or top-down attention, change in momentary decision bias, momentary change in working-memory load, etc.); and Type-(iii) input is insufficient (fluctuations in the level of arousal, changes in the state of orienting circuits, etc.). Conversely, when Factors (i)–(iii) are augmented, probability of conscious perception increases. Comparison of the EEG signals from target-conscious and target-unconscious trials shows typically an enhancement of post-target ERP N100, N200 (VAN; auditory awareness negativity, AAN), or N170 (Navajas et al. 2013; Railo et al. 2015; Koivisto and Grassini 2016; Koivisto et al. 2016, 2018; Rutiku et al. 2016; Eklund et al. 2020). Augmentation of the negative-polarity post-target event-related potential (ERP) waves centered around 100–200 ms has been regarded as the marker of early conscious perception in the PRT (Bachmann 1984, 1994). N100-200 can be interpreted as a signature of modulation of the cortical SP neurons by the phasic arousal signals originating from NSP.
Visual backward masking of the target stimulus by a following masking stimulus
As illustrated in Fig. 1B, dominance of the mask (the following stimulus S2) over the preceding target (S1) in consciousness can be explained by the temporal coincidence of the nonspecific long-latency thalamocortical modulation (set in motion by S1 presentation) of the dendrites of S2-related SP neurons and arrival of the fast-latency specific S2-related signals at the soma of this SP neuron. As the depolarization level of the SP neuron that represents S1 (target) content has decayed more than the depolarization level of the S2-related neuron, the information about S2 has higher signal-to-noise ratio and is communicated to other brain areas with higher probability or with more intense burst of firing. The result at the level of subjective experience is conscious perception of the mask instead of the target (or more salient experience of the mask than target). (The quantitative model of masking simulates masking as a function of stimulus onset asynchrony (SOA), relative intensity of S1 and S2, and temporal allocation of attention—Bachmann 1994).
Enhancement of subjective contrast of S2 as a result of priming by the preceding, spatially overlapping or neighboring S1
This experimental effect was described by Bachmann (1988, 1994). PRT explains it similarly to explanation of backward masking, but the roles of mask and target are conventionally reversed: S1 acts as a prime and S2 as a target for which subjective contrast (visibility-) rating is required. In this effect, subjective contrast of S2 appears higher when presented after S1, compared to when S2 is presented as a single stimulus. Supposedly, S1 launches the NSP modulation directed at the intermediate compartment of the dendrite of the S2-representing neuron. If SOA is optimal (optimality defined by the difference between the delays with which S1 signals and S1-evoked NSP signals reach cortex), S2 subjective contrast is enhanced. When S2 is presented alone, the ‘retouching’ modulation from NSP arrives when specific S1-related activity (supposedly the depolarization of the membrane potential) of the S1-representing neuron has decayed already. (The cell is always a leaky integrator.) Interestingly, the same general mechanism explains also how in paracontrast masking (spatially adjacent mask preceding the target) target’s subjective enhancement is obtained and escape of target from metacontrast masking achieved. Paracontrast enhancement, unmasking of metacontrast, and release from object substitution masking have been shown (Breitmeyer et al. 2006; Öğmen et al. 2006; Luiga and Bachmann 2007; Kafaligönül et al. 2009; Agaoglu et al. 2016).
Perceptual latency priming
Similarly to Enhancement of subjective contrast of S2 as a result of priming by the preceding, spatially overlapping or neighboring S1, S1, when preceding the S2, can speed up its conscious perception (Scharlau and Neumann 2003; Scharlau 2007; Spence and Parise 2010). According to the PRT model, S2-treated SP neurons begin firing earlier when preceded by the prime (S1), compared to when S2 is presented alone. (In Fig. 1B we see that firing for S2 starts earlier than for S1.) The fact that speed-up of S2 to consciousness can be caused also by S1 that remains out of consciousness is consistent with PRT: even though S1 is masked by S2, it can launch the NSP process which takes the effect on S2.
The flash-lag effect
In the original flash-lag effect, moving (spatially streamed) and static (locally flashed) stimuli are compared for their relative position at the moment of flash presentation. Typically, the flash appears to lag behind the moving stimulus, although they were aligned when the flash was presented (e.g. Nijhawan 1994). The retouch theory explanation (Bachmann et al. 2003, 2012; Bachmann 2010) contends that because the action of NSP takes more time than SP-encoding and as awareness of the SP-represented content does not emerge before NSP modulation has had its effect, the conscious percept emphasizes features that are or become present in SP somewhat later. In case of the static flashed stimulus its unchanged position (as stored in sensory memory) will be ‘retouched’ for consciousness, but in case of the moving stimulus its advanced spatial position is explicated in consciousness. (The trailing sensory trace of the moving stimulus is erased in SP by a Reichardt type of movement detector; Reichardt 1961.) Thereby the illusion of spatial lag is produced. This explanation is valid also for the flash-initiated conditions (flash presented when motion of the other stimulus begins), the conditions where the post-flash movement directions are unpredictable (contradicting motion-extrapolation theory), and the conditions where the pre-flash stimulation includes contradictory motion direction signals that could nullify or complicate prediction (Khurana and Nijhawan 1995; Whitney and Murakami 1998; Bachmann et al. 2012). A different variety of the flash-lag effect is produced when a spatially localized stimulation stream changes its feature value (e.g. hue or shape of successive items) and is evaluated against a flashed probe or reference stimulus that has an invariant feature value (e.g. Sheth et al. 2000; Bachmann and Põder 2001). In this case, too, the flash-lag effect is produced similarly to what was described for the standard case. When the NSP modulation becomes effective, it helps conscious access for the former feature value of the reference stimulus stored in sensory memory. For the object features within the stream of the changing stimulation, the conscious access is relatively faster because the former stimulation-instances from the earlier moments of stream have ignited the NSP process ahead in time and upon arrival of the new signals from the subsequent input within the stream the corresponding feature values become consciously perceived relatively earlier. The in-stream facilitation effect has been obtained when the preceding in-stream items did not carry any predictive cues about what the next item could be (Bachmann and Põder 2001). Therefore, the mechanism that modulates SP content for access to awareness has to be nonspecific.
The Fröhlich effect
In this phenomenon, the first perceived location of the moving object that moves out from behind the occluder is shifted forward along the motion direction (e.g. Müsseler and Aschersleben 1998). The PRT has explanation for this also. The slower NSP modulation arrives at the active SP representation of the moving stimulus when its position has been advanced during this NSP delay. (The Reichardt detector mechanism works autonomously within the SP system and cancels the trailing edge of the moving stimulus sensory trace before any NSP modulation could have arrived.)
Motion-induced blindness
When a static visual target object is continuously presented on a background of dynamic visual noise, the target periodically disappears from and reappears in awareness (Bonneh et al. 2001). There is evidence that sensory attributes of the stimulus continue to be processed/represented even though the stimulus remains out of awareness because of the motion-induced blindness (MIB) situation (Montaser-Kouhsari et al. 2004). In terms of PRT it is feasible to assume that stimulus features that have faded out of consciousness due to MIB conditions still drive their SP representations in cortex by presynaptic input to the somatic compartment of the SP cells (representing stimulus features). At the same time the apical modulating input from NSP or higher-level cortical nodes is absent or too weak and as a consequence, the stimulus is processed only unconsciously. Any perturbation which causes phasic arousal (e.g. a novel stimulus or change in stimulus value) tends to reinstate the suppressed stimulus in awareness. In one pertinent study, Wu et al. (2009) showed that a flashed stimulus that ‘caused’ reappearance of the target in awareness was perceived ‘after’ the target’s reappearance. (The temporal value of reversal was about 100 ms.) According to PRT, when an object fades from awareness by MIB, its feeding of the somatic compartment of the layer-5 SP neuron will be sustained because cortical-specific signals are constantly present, but dissociated from the more apically directed NSP activity. When the flashed object is presented, the afferent SP process for representation of the flash occurs in parallel with a boost of the NSP process igniting the more apically directed modulation of the target-object SP neuron. This leads to coupling of the already present preconscious perisomatic SP activity of the target object with apical-dendrite activity caused by NSP input. This process is fast, because there is no need for build-up of the content-specific SP representation of the target as it is already there; consequently, its rapid reappearance in consciousness occurs. The flashed object as a newly presented object appears in consciousness not as fast because its corresponding coherent SP representation must be built up, which takes time. The modulation process (directed at apical or intermediate compartment) that services ‘target’ awareness has SP content of the target ready on the ‘waiting list’, but the modulation process for the ‘flashed object’ has to wait as a ‘dummy process’ until the SP content of the flashed object is ready to be modulated.
Facilitation of target perception by a spatial-attention pre-cue
Inspired by seminal works on selective spatial attention by Charles Eriksen and Michael Posner, a large number of studies showed that a pre-cue indicating the expected location of the target facilitates target perception (both in terms of behavioral sensitivity and subjective clarity and measured by reaction time as well as correctness of response) (reviews: Wright and Ward 2008; Chica et al., 2011; Carrasco and Barbot 2019). Exogenous pre-cues are presented at or close to the prospective target location; endogenous pre-cues are presented in a spatially neutral position, symbolically specifying the spatial location (e.g. a small central arrowhead or clockface number). The facilitation effect is influenced by the phasic and sustained alerting systems, including the thalamus (Rafal and Posner 1987; Chica et al. 2016; Engel et al. 2016) and can be brought about crossmodally, thus adding to the modal nonspecificity of the mechanism (e.g. Lee and Spence 2015). In terms of PRT, the exogenous facilitative spatial precueing fully or partly owes to the same mechanism as described in Enhancement of subjective contrast of S2 as a result of priming by the preceding, spatially overlapping or neighboring S1 and Perceptual latency priming. For the endogenous precueing effect PRT postulates a pre-stimulus activation of the higher cortical nodes where spatial locations are symbolically represented and from where the top-down facilitating signals are sent presynaptically at the apical compartment of the SP neurons with receptive fields corresponding to that location. Firing of the neurons for which receptive field of the somatic presynaptic signals (i.e. signals driven by the spatially located target) corresponds to the higher-order receptive field (signaled by the apical input according to the symbolically encoded spatial address) is augmented. This leads to increase in subjective contrast and rate of correct discrimination, and decrease in latency of response, for the target. Importantly, there are many data and a growing consensus that mechanisms of attention and conscious experience are autonomous, although typically interacting (Wyart and Tallon-Baudry 2008; Bachmann 2011; Webb et al. 2016; Maier and Tsuchiya 2020). While in typical cases attention increases veridicality and subjective vividness of target stimuli (supposedly recruiting the consciousness mechanism for facilitative modulation), selective attention can be driven also unconsciously (e.g. Webb et al. 2016) and can even suppress spatially localized sensory experience (Bachmann and Murd 2010; Murd and Bachmann 2011).
Binocular rivalry and continuous flash suppression
When two sufficiently different images are presented to different eyes, they begin to alternate in conscious experience (Levelt 1965; Alais and Blake 2005; Pournaghdali and Schwartz 2020). Despite being explicitly unperceived, the contents of the currently subliminal image are nevertheless represented by the brain processes (Logothetis and Schall 1989; Fang and He 2005; Lin and He 2009; Fahle et al. 2011; Mudrik et al. 2011). The currently unperceived stimulus—either by the content presented to the suppressed eye or by the qualitative content presented from a different modality—can speed up reversals or the speed with which the currently suppressed content becomes conscious (e.g. Zhou et al. 2010; Gayet et al. 2016; Suzuki et al. 2019). From the PRT point of view, the content currently dominant in conscious perception is based on the combined somatic (SP) and apical (NSP) presynaptic input to cortical SP neurons that represent this content. Unconscious processing of the information presented to the currently suppressed eye proceeds exclusively or largely within the SP system without sufficient accompanying amplification/modulation from NSP being directed to the intermediate dendritic compartment of the respective SP neurons. In order to cause reversal in conscious perception, the net outcome of spiking due to the combined presynaptic input to the three compartments (somatic, intermediate, and apical) of the cells representing the suppressed content needs to become higher than that of the cells representing the dominating content. Reversal can be caused e.g. by a sudden appearance of transients to soma of the SP neurons representing suppressed content (e.g. abrupt intensity change or stimulus motion in the suppressed eye). This causes augmented somatic input and a phasic arousal burst modulating the higher compartments of the dendrite of these neurons. As a result, the net outcome of spiking activity will exceed that of the neurons representing the dominant content and conscious contents of perception switchover. It is also known that voluntary, top-down controlled change of what is consciously perceived in rivalry is impossible or only marginally possible; rivalry appears spontaneous. This suggests a relatively low-level mechanism susceptible to sensory adaptation and lateral inhibition (see also Frässle et al. 2014). Perceptual ‘retouch’ by apical amplification finds either one or the other set of neurons that are responsible for alternative representations in a relatively higher upstate, depending on the recent history of adaptation. However, adaptation takes time to take its effect. When rivaling stimuli are presented simultaneously for less than about 100 ms, a fusion occurs and two images are subjectively amalgamated in a blend of contents presented to both eyes (Wolfe 1983). This exceptional case of rivalry can be explained as a result of relative slowness of effective adaptation and setting up low-level rivalry. With very short presentation of the stimuli the process of rivalrous or stochastic adaptation has not had time to take its effect and the SP-level activity of the neurons responsible for content features of both stimuli is at a comparable level when the modulating apical input arrives. As a result, both contents get ‘retouched’ and experienced.
‘Normal’ hallucinations
Even mentally normal people can experience something that is actually not present, which could be called ‘normal hallucinations’ (e.g. Kuhn and Rensink 2016; Powers et al. 2017; Aru et al. 2018; Andermane et al. 2020). Pre-stimulus expectations formed by associative conditioning, biases, and typicality of context are considered among the main causes of hallucinatory experiences (Hohwy 2013; O’Callaghan et al. 2017; De Lange et al. 2018; Corlett et al. 2019). Prior knowledge causes hallucinations typically when the sensory data are degraded (e.g. Powers et al. 2017), ambiguous (Sterzer et al. 2008; Weilnhammer et al. 2018), or out of the focus of attention (Aru et al. 2018) and with subjects being less confident in the correctness of their primary task performance (Vetik et al. 2020). PRT/DIT suggests that an object will be hallucinated, given two main preconditions: (i) higher-level cortical nodes with stored contextual knowledge selectively predispose lower-level expected object-specific SP neurons to firing by applying top-down apical input (depolarizing apical compartment) and (ii) these object-specific neurons have sufficiently high perisomatic activity in order to enable coupling between apical and somatic dendrite activity. It is also likely that by associative conditioning between some Stimulus A and Stimulus B that during learning are present together, in a later occasion when A is presented alone, B becomes nevertheless hallucinated. Due to conditioning the sensitivity of the perisomatic membrane of the B-related SP neurons has been increased and spiking threshold therefore decreased. [The false alarms the animals produced in (Takahashi et al. 2016) in the actual absence of the stimulus can be interpreted also as if the rodents hallucinated about the presence of the stimulus.]
Comparative observation of different consciousness states
Brains of animals, including Homo sapiens, can be in different states depending on many factors such as pathologies, fatigue and exhaustion, neuropsychopharmacological substances, spontaneous transfer between states, physical traumatic effects, etc. The well-known instances of states are (not necessarily fully exclusively) hyper-aroused manic state, alert wakefulness, oneiroid/somnolent state, hypnagogic state, minimally conscious state, rapid eye movement (REM) sleep, non-REM sleep with characteristic EEG slow-wave activity, deep anesthetic state, and coma. The extent to which subjects in these states are conscious varies from the likely total absence of conscious-level mentation (coma and deep anesthesia) to fragmentary/vague conscious experience (minimally conscious state and REM sleep) to hypothetical almost-full consciousness in the locked-in state to the fully conscious state in alert wakefulness (Schiff 2008; Laureys et al. 2015). I will very briefly describe the PRT/DIT account of some of these states in terms of the contribution of multicompartment pyramidal neurons and thalamocortical interactions.
Deep anesthesia and coming out of anesthesia
The central postulate of PRT and DIT (Bachmann 1994, 1997; Aru et al. 2019, 2020b; Bachmann et al. 2020) together with the AA theory (Phillips et al. 2016; Marvan et al. 2021) is that in the anesthetic state specific-content-representing processes by the cortical neurons are decoupled from the processes of modulation targeted at these neurons, with thalamocortical interactions being crucial. More specifically, if input to somatic compartment of the pyramidal neuron (responsible for content processing) is decoupled from intermediate and apical dendritic compartments, consciousness is absent. Inhaled or intravenously applied anesthetic agents disrupt somatic-apical coupling, which leads to loss of consciousness. In this process, disruption of thalamocortical interaction that converges at the cortical layer-5 neurons is common to virtually all known general anesthetics (Alkire et al. 2000; Alkire and Miller 2005; Ward 2011) (despite some smaller specific differences, especially with ketamine anesthesia). Natural or purposely stimulated cases of coming out of anesthesia are associated with reinstatement of thalamocortical dialogue and enhancement of thalamocortical modulation (Schiff 2008, 2020; Långsjö et al. 2012; Honjoh et al. 2018; Redinbaugh et al. 2020). In terms of the multicompartment layer-5b pyramidal neuron model, the state of anesthesia is associated with overall reduction in dendritic excitability in vivo with three different anesthetics (Larkum and Potez 2008); coming out of anesthesia is associated with increased calcium activity in the apical compartment (Murayama and Larkum 2009) and likely controlled by nonspecific thalamocortical input to intermediate compartment of the neuron (Suzuki and Larkum 2020). (Top-down apical modulation as a necessary process in controlling the anesthetic state was stressed also by Meyer 2015, but the role of the thalamus was not emphasized).
Sleep and dreaming
In sleep, similarly to anesthesia, thalamocortical interaction is subdued (this is primarily with regard to the NSP thalamus; specific thalamic relays function virtually as well as in the wakeful state). Thalamic deactivation is observed at sleep onset (Magnin et al. 2010) while thalamocortical activation wakes animal up (Honjoh et al. 2018). Non-REM sleep is associated with widespread frontally reaching suppression of the slow negative ERP potential produced by transcranial magnetic stimulation (TMS) perturbation of caudal cortex (Stamm et al. 2011). As scale-free EEG potentials are associated with consciousness (He and Raichle 2009), causing difference in such slow potential between wakeful and dreamless sleep states by nonspecific perturbation adds to the support of the involvement of nonspecific mechanisms in conscious state. However, immediately before the REM episodes of sleep that are typically accompanied with out-of-control, sporadic manifestations of consciousness (dream imagery), thalamocortical sources send their excitatory signals up to cortex (Steriade et al. 1984). The PRT fully agrees with how Aru and colleagues describe dreaming in sleep in the apical drive theory of dreams (Aru et al. 2020a). In sleep the feedforward input to the somatic compartment of content-representing layer-5 pyramidal cells is turned off or very weak. Thus, the content of dreams has to have internally generated sources largely independently of current sensory input. According to the apical drive hypothesis, the apical integration zone receives contextual information from diverse sources and during dreams the output of internally selected pyramidal neurons is mainly driven by input into this integration zone. Importantly, nonspecific cholinergic and adrenergic arousal systems that are involved in regulating sleep and wakefulness and show different dynamics between these states have dream-driving effects.
Drug-induced and pathological hallucinogenic states
These states are characterized by relative dissociation from reality and experience of more or less bizarre forms of nonveridical experiences (e.g. under the influence of psychedelics such as psilocybin, mescalin, and lysergic acid diethylamide (LSD), with anesthetics such as ketamine, and positive symptoms in psychotic state). Similarly to how PRT/DIT/AA all explain normal hallucinations and REM-stage dreams, hallucinogenic-drug-induced hallucinations can be the result of a relative ‘excess of apical input’ compared to somatic input to content-carrying SP neurons. Subjective experiences become more controlled by intrinsic neurochemical causes leading to unusual presynaptic drive of apical dendrites from sources inconsistent with current sensory input. For example, those patients suffering from Lewy body disease who experience hallucinations place higher relative weighting on prior knowledge in auditory discrimination than non-hallucinating patients and healthy controls (Zarkali et al. 2019). (An opposite effect consisting in atypically weak top-down signals and/or too precise bottom-up signals driving cell soma is hypothesized to be characteristic of autistic traits—Van de Cruys et al. 2014; Coll et al. 2020.) On the other hand, if hallucinogenic substances ‘inhibit sensory inflow’ to the somatic compartment, then relative impact of the apically driven effects could be increased again. What seems to matter most is whether there is normal balance between apical drive and somatic afference. If this balance is disturbed by neurochemical intervention or neuropsychiatric pathology, hallucinations emerge. It is well known that brain serotonergic system, especially as mediated by 5-HT2A receptors, is substantially involved in psychedelic effects (Vollenweider and Preller 2020). Importantly for PRT/DIT, 5-HT2A receptors are highly expressed in the apical dendrites of layer-5 pyramidal cells; they are present also presynaptically on thalamocortical afferents and in subcortical locations; and they are involved in thalamic gating of sensory/cognitive information (Marek 2018). Therefore, serotonergic neuromodulation of the apical dendrites of layer-5 pyramidal neurons is a likely mechanism involved in nonveridical, hallucinatory experiences. However, the picture of the effects of hallucinogens on functional connectivity, spectral power of responses, and cortical spatiotemporal patterns of activity is quite complex and varies between the individual effects of different hallucinogens (Barnett et al. 2020). This tells us that we have to wait for any firm conclusions until future research, combining single-cell-level and network-level approaches, will bring some more pertinent data.
Neural markers of conscious experience
I will limit this part to EEG- and magneto-encephalography (MEG)-based markers (reviews: Rutiku and Bachmann 2017; Förster et al. 2020). There is growing consensus that negative-polarity ERP components emerging relatively early after the external stimulus of interest (latency 100–200 ms depending on modality, stimulus intensity, and experimental task) are the early markers of conscious perception of the stimulus. If recorded from scalp above the more caudal parts of cortex and appropriately referenced, the N100, N200 (VAN), or N170 components show different amplitudes depending on whether the stimulus was consciously perceived or not. This value of the post-stimulus delay is comparable to the time it takes for the phasic NSP modulation to arrive to cortex. If this modulation is a necessary requirement for the contents carried by cortical neurons to become consciously experienced, the interpretation of N100-200 as the conscious-perception marker is quite likely. A couple of relevant evidence is as follows. In the studies that use contrastive analysis (EEG level in target-unconscious trials subtracted from target-conscious trials) it has been repeatedly found that the ERP component called VAN is augmented in response to the stimulus that is consciously perceived (Railo et al. 2015; Koivisto and Grassini 2016; Koivisto et al. 2016, 2018; Rutiku et al. 2016; Schlossmacher et al. 2020). When a graded scale was used for subjective clarity rating of the target awareness, the negative ERP amplitude systematically increased with more high levels of rating (Tagliabue et al. 2016). In neglect patients, visual and auditory N1 (200 or 100 ms delay, respectively) was poorly expressed, but became reinstated after recovery (Hämäläinen et al. 2014). Auditory awareness is marked by auditory awareness negativity at around 200 ms (Eklund and Wiens 2019), and this marker is not confounded by performance or task relevance (Eklund et al. 2020; Schlossmacher et al. 2021). In sleep, later components of ERP are subdued although early components remain unchanged (Uttal and Cook 1962). Hysterical blindness was accompanied with subdued N200, but when patients recovered, the high-amplitude N200 potential was recorded (Schoenfeld et al. 2011). Whether the stimulus is in the attentionally cued or noncued location, the N200 evoked by it has higher amplitude for aware vs. unaware stimuli (Navajas et al. 2017). When conscious perception was assessed in tasks with control over the response stage of processing, late positive ERP components did not correlate with consciousness, but earlier negative-polarity components did (Pitts et al. 2014; Navajas and Kaunitz 2016; Schlossmacher et al. 2020). Supporting the conjecture that P300b is not a marker of early phenomenal conscious perception (Pitts et al. 2014; Schlossmacher et al. 2020), Silverstein and colleagues (2015) recorded this ERP also with consciously not perceived stimuli.
A tentative hypothesis derived from the mechanism of apical modulation of the neurons with thalamocortical and corticocortical converging input assumes the following to be likely. In the apical compartment there is strong Ca2+ activity when the modulating input arrives (possibly reflected in a positive local field potential (LFP)). However, as measured from scalp, this apical activity is reflected in the negative ERP. This negativity is common to markers in different modalities. Indeed there can be common supramodal markers of conscious perception (Sanchez et al. 2020; Dembski et al. 2021). (Let us notice that if presynaptic input arrives mostly to the somatic part of the layer-5 pyramidal neurons without sufficient apical presynaptic input, in which a situation, according to the theory, is characteristic to the lack of conscious perception, ERP negativity has to be lower or absent. If apical dendrites are modulated, ERP negativity emerges—Brazier 1977; Hassler 1978. Let us also bear in mind that because the multicompartment mechanism of L5 pyramidal neurons requires both, some somatic activity and apical activity at the cell membrane, very early positive ERP components can be enhanced also preconsciously for the stimuli that subsequently become consciously experienced. But as 50–100 ms is too fast for visual psychophysical data on the earliest conscious perception delays, very early positive-polarity ERP components—even though possibly enhanced in the stimulus-conscious trials—are unlikely to be the ‘direct’ markers of conscious perception and they are just NCCpr (e.g. Aru et al. 2012; Chen et al. 2017; Schröder et al. 2021).
PRT/DIT and major consciousness theories
Most of the influential scientific theories of consciousness are in one way or another related to the brain—but how this 1.4 kg of tissue can give rise to phenomenal subjective experience? As the PRT/DIT is relatively less known, it would be interesting to see how it relates to the currently better known theories. Due to the lack of space I cannot present a thorough and well-systematized analysis, but in what follows, let me bring out some comparative characterizations.
The characteristic features of CK are sufficiently well presented in Crick and Koch 2003. Many of these characteristics are common to CK and PRT/DIT:
processing of input in a zombie mode (content can be unconscious) which is mainly feedforward;
processing in the conscious mode is typically bidirectional;
specific cells for specific features (necessary, but not sufficient for consciousness of content);
important role of pyramidal layer-5 cells;
driving inputs to pyramidal cells largely contacts the basal dendrites;
mutual support of large pyramidal cells forming coalitions and their projections to the thalamus;
for a feature to reach consciousness, some neural activity for that feature has to cross a threshold;
driving and modulating are two main types of input to feature cells;
in CK it is conceivable (among other candidate mechanisms) that pyramidal cells that project to the front of the brain bear the role of consciousness neurons by maintaining the special activity needed to cross threshold for consciousness (something special about the internal dynamics of the neuron is likely, perhaps involving the accumulation of chemicals such as Ca2+).
Main differences: nonspecific arousal mechanisms as a general enabling factor (not directly participating) in conscious perception in CK, but a direct causal factor in PRT/DIT; modulating inputs from NSP to apical dendrites have diffuse projections in CK, but can project also locally in PRT/DIT; and V1 not involved in the neural correlates of consciousness (NCC) in CT, but may be involved in NCC in PRT/DIT.
Comparison of PRT/DIT and IIT (Tononi 2004):
This comparison cannot be very detailed because the first is focused on the single-cell and microcircuit level while IIT deals with global networks. Main differences: PRT model describes mechanistically how a number of different psychophysical phenomena and phenomenal states may emerge (constrained by spatial and temporal measures and known properties of neural functioning), but IIT is more abstract and information-theoretical. Common features can be nevertheless spotted.
subjective characteristics of conscious experience are the departure point for a brain-based theory (although in PRT/DIT subjectivity comes in the form of the psychophysical phenomena to be explained by the neural theory whereas in IIT some general, abstract axioms of subjectivity are used);
the dynamic core has important role to be played by thalamocortical interactions;
both theories capture both segregation and integration of neural processing in one interactive system (SP/NSP in PRT/DIT, selections out of endless alternatives becoming integrated in IIT);
the mechanism responsible for conscious experience is panmodal (modally universal); and
the mechanistic causal factors for consciousness are intrinsic.
GNW (Dehaene et al. 2011) has also commonalities with PRT/DIT:
a specialized neurophysiological event initiates conscious perception (firing in GNW neurons termed ‘ignition’ and SP/NSP presynaptic interaction leading to somatodendritic coupling in PRT/DIT);
depolarization of pyramidal neurons is one mediating mechanism;
negative cortical potentials may be a marker of conscious perception/working memory (WM); and
content-carrying neurons can be active also in a zombie mode.
Main differences (not necessarily mutually incompatible): PRT/DIT gives thalamocortical and corticocortical processes an equal and doubly necessary role in conscious content perception whereas GNW deals primarily with corticocortical long-range interactions for this purpose; GNW focuses on pyramidal neurons in cortical layer-2/3 while PRT/DIT regards layer-5b neurons as crucial (without denying layer-2/3 importance in preconscious stages); according to GNW, the most consistent earliest correlate of visibility is the relatively late (∼300–500 ms) positive component P3b, but PRT/DIT argues that N100-200 (including VAN) is the earlier marker of conscious perception; GNW concentrates on conscious ‘access’, but PRT/DIT on phenomenal ‘content’. Additionally, for a proponent of PRT/DIT when (s)he would like to delve into finding out to what extent the theories are compatible some GNW basic concepts remain cryptic. What does ‘subjective experience as global broadcasting to many distant areas’ actually mean? If a piece of information A (e.g. in temporal cortex) selected for its salience or relevance is broadcast to neurons B (e.g. in frontal cortex), does B ‘perceive’ A? If so, what will happen to content of A (and hypothetical content of B as well)? Would we just produce noise instead of information in B and A by that kind of coupling the content? Whether content in A will sustain if it has been sent to B? Whether instead of one content A there will be two contents of A in parallel (one remaining in A and the other now in B + A)?
RP (e.g. Lamme 2006) has several affinities with PRT/DIT:
content can be and typically is processed preconsciously;
recorded neural markers of consciousness emerge relatively late (after 100 ms), but content-sensitive processes can be recorded even earlier;
both theories believe that although interacting, consciousness and attention mechanisms are not the same; and
prefrontal processes may not be necessary for phenomenal perception.
RP differs from PRT/DIT in the following ways: while RP regards top-down signals from higher-order nodes back to lower-level nodes as absolutely necessary for conscious perception, PRT/DIT is more flexible by allowing both top-down modulation and/or bottom-up modulation as the sources of input to layer-5 neurons in order to produce conscious perception (the bottom-up variety initiated by input to receptors and coming upstream en route of primary subcortical relays via nonspecific subcortical units that have targets in cortex); RP largely ignores subcortical nonspecific factors as crucial for conscious perception; RP does not deal with state of consciousness.
Similarities of the higher-order thought theory (Rosenthal 2005, 2012) and PRT/DIT appear to be as follows:
both theories accept dual modes of mental processes—unconscious and conscious;
both allow the possibility of misrepresentation in conscious content (PRT by NSP modulation applied to currently nonveridical SP, (HOT) by misinterpretation in higher level of what takes place in lower level);
both are skeptical about the notion that making a content globally available is a defining attribute of conscious processing; and
both believe that although interacting, consciousness and attention mechanisms are not the same.
The differences between HOT and PRT/DIT are also multiple: while in HOT it is accepted that there is no difference between what unconscious and conscious processes can do in terms of practical utility, PRT/DIT assumes an added value of the top-down modulation as this amplificatory sub-process facilitates communication of signals from sensory-perceptual representations to executive network representations; according to HOT a metacognitive activity (‘thinking about experience’) and prefrontal cortex activity are needed for conscious awareness whereas awareness mechanism in PRT/DIT permits that when content-carrying layer-5 neurons in cortical areas more caudal from central sulcus receive somatic and apical input, the corresponding content becomes consciously experienced.
Open questions and limitations
No theory of consciousness is ready and complete until it has described the underlying objective mechanisms in a sufficient detail and until by knowing the workings of these mechanisms it has become possible to causally change the state and contents of consciousness so that they will correspond to what was predicted by the theory as a result of this causal manipulation. Current theories are hypothetical and incomplete and bound with limitations. The PRT/DIT being among them. The list of limitations I will list below is not a complete list.
First, the most convincing evidence supporting PRT/DIT at the cellular level has come from animal research (mainly on rodents). It is somewhat complicated to carry out similar research in humans. So, to what extent the thalamocortical processes are similar between the species we do not know precisely. Second, there is certain variety in the topology and neuroanatomy of layer-5 pyramidal cells potentially usable for falsifying the DIT theory (e.g. Fletcher and Williams 2019; Galloni et al. 2020). Do these cells perform stereotyped computations in all cortical areas as the candidates for representing content? Could the difference in the length of apical dendrites of cells in different cortical areas lead to failure of model predictions (e.g. due to substantial differences in excitability and capability to produce burst firing)? Third, our knowledge of the functional properties of subcortical modulation system cells is limited, especially in their interaction with cortical neurons. Fourth, there are still many uncertainties about the relative roles of different phasic and tonic modulation systems (e.g. aminergic and cholinergic varieties of modulation) in ‘retouching’ the content up to the level of consciousness (e.g. Pal et al. 2018; Marvan et al. 2021). Fifth, although some psychophysical consciousness phenomena such as masking, perithreshold perception, and perceptual hallucinations can be quite well explained by the SP/NSP interaction model, explanations for several others remain too general and insufficiently tested in animal experiments or neurological interventions.
One important open question asks to what extent the top-down modulation from frontal areas to SP neurons in more caudal areas is content-specific already beginning from the frontal source (specific context information) and to what extent it is just a general unspecific modulatory wave originating in the subcortex, traveling to frontal cortex and then down onto posterior cortical parts. This question is related to another important test waiting to be done. For example, Lamme (2006) and (Dehaene et al. 2011) interpret the temporally delayed post-stimulus ignition associated with conscious perception as a result of broadcast of signals from higher-order cortical nodes (already informed about contents) down to lower-order sensory-perceptual nodes. In the PRT/DIT the markers emerging around 100–300 ms after the stimulus may be just a signature of the nonspecific modulation process (with specificity of content emerging at the lower levels after this modulation has arrived).
Most of the experimental perceptual phenomena accounted for by PRT/DIT are short-lived effects on the timescale. But what about some longer lasting effects such as experiences of melodies or motion of a hockey player on ice, suggesting that conscious experience has to involve postdictive effects and relatively long integration epochs for sensory input (e.g. Herzog et al. 2020; Michel and Doerig 2021)? Theoretically, PRT/DIT is prepared to explain these effects by virtue of being a theory of how a dual-process system creates conscious experience. The content system can work and keep sensory effects at the level of subliminal EPSPs for some time after the stimulus itself or its preceding displaced instances have disappeared. The modulating system can push these aftereffects above the spiking threshold of the corresponding neurons also later and perhaps even as accompanied simultaneously by the modulation of the current input signals. Future research must show light on how this can happen and whether the crossing of the threshold and reaching optimal perception is discrete (Herzog et al. 2020) or gradual (Aru and Bachmann 2017). Importantly, e.g. if the temporally successive stimuli have very short durations and inter-stimulus delays then the wave of presynaptic nonspecific modulation of the specific content-carrying neural activity level can act as the mechanism of integration of the preceding and subsequent specific information, which may result in a new quality of subjective perception. This prediction is realistic because the spiking response of the cortical-specific neurons after a stimulus (especially in the unconscious state of the animal) is typically shorter than the cortical response to the modulating afference from nonspecific subcortical sources (Brooks and Jung 1973; Brazier 1977; Hassler 1978). In other words, the one NSP wave of modulation can ‘swallow’ different SP waves of activity from different specific inputs. If this is so, then it remains to be explained how a fused subjective quality different from each of the qualities of separate stimuli (were they individually presented) becomes to be experienced. (I speculate of retouching some higher-level neurons where specific input from lower-level units converges.) Furthermore, if we assume that the SP/NSP interaction proceeds in successive cycles within the ongoing activity of cortical modulation and thereby is a foundation for continuous flow of perception, it must be experimentally demonstrated in neuroscience research with brain imaging being contrasted with subjective, temporally extended experiences. Other outstanding questions are of course also there to be answered (Bachmann et al. 2020).
Conclusion
Many scientific theories of consciousness have conceptualized human consciousness in terms of specific mental state produced by globally interacting brain processes, many in terms of content of consciousness, and several by showing how cellular and sub-cellular level mechanisms in the brain could help to understand states of consciousness. The PRT/DIT account is unique in bringing these perspectives together, and in doing so it is founded on experimentally discovered neurobiological knowledge about functioning of cortical neurons sensitive to specific contents and modulating neurons hypothetically making this content available for conscious experience. This approach has tried to avoid an excess abstractness and isolation from empirically tested experimental phenomena of consciousness, but, instead, has provided mechanismic explanations of these phenomena. It remains for continued research, combining single-cell and network levels of analysis, to test the validity and perhaps universality of this theory.
Acknowledgements
This paper has benefited from recent and earlier discussions with my respected academic friends and colleagues, first of all Jaan Aru, William A. Phillips, Matthew Larkum, Tomáš Marvan, Michál Polak, and Anthony Hudetz.
Data availability
As this paper is a theoretical review, it is not applicable for publical data availability.
Funding
Preparation of this work has not been specifically funded by any grant or contract.
Conflict of interest statement
None declared.
References
- Agaoglu S, Breitmeyer B, Öğmen H. Metacontrast masking and attention do not interact. Atten Percept Psychophys 2016;78:1363–80. [DOI] [PubMed] [Google Scholar]
- Alais D, Blake R (eds). Binocular Rivalry. London: MIT Press, 2005. [Google Scholar]
- Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn 2000;9:370–86. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science 2008;322:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Miller J. General anesthesia and the neural correlates of consciousness. Prog Brain Res 2005;150:229–597. [DOI] [PubMed] [Google Scholar]
- Andermane N, Bosten JM, Seth AK. et al. Individual differences in the tendency to see the expected. Conscious Cogn 2020;85:102989. [DOI] [PubMed] [Google Scholar]
- Aru J, Bachmann T. In and out of consciousness: how does conscious processing (d)evolve over time? Front Psychol 2017;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aru J, Bachmann T, Singer W. et al. Distilling the neural correlates of consciousness. Neurosci Biobehav Rev 2012;36:737–46. [DOI] [PubMed] [Google Scholar]
- Aru J, Siclari F, Phillips WA. et al. Apical drive-A cellular mechanism of dreaming? Neurosci Biobehav Rev 2020a;119:440–55. [DOI] [PubMed] [Google Scholar]
- Aru J, Suzuki M, Larkum ME. Cellular mechanisms of conscious processing. Trends Cogn Sci 2020b;24:814–25. [DOI] [PubMed] [Google Scholar]
- Aru J, Suzuki M, Rutiku R. et al. Coupling the state and contents of consciousness. Front Syst Neurosci 2019;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aru J, Tulver K, Bachmann T. It’s all in your head: expectations create illusory perception in a dual-task setup. Conscious Cogn 2018;65:197–208. [DOI] [PubMed] [Google Scholar]
- Bachmann T. The process of perceptual retouch: nonspecific afferent activation dynamics in explaining visual masking. Percept Psychophys 1984;35:69–84. [DOI] [PubMed] [Google Scholar]
- Bachmann T. Time course of the subjective contrast enhancement for a second stimulus in successively paired above-threshold transient forms: perceptual retouch instead of forward masking. Vision Res 1988;28:1255–61. [DOI] [PubMed] [Google Scholar]
- Bachmann T. Psychophysiology of Visual Masking. The Fine Structure of Conscious Experience. Commack, NY: Nova Science Publishers, 1994, 298. [Google Scholar]
- Bachmann T. Visibility of brief images: the dual-process approach. Conscious Cogn 1997;6:491–518. [DOI] [PubMed] [Google Scholar]
- Bachmann T. Fast dynamics of visibility of brief images: the perceptual-retouch viewpoint. Hameroff SR, Kaszniak AW, Scott AC (eds.), Toward a Science of Consciousness II. The Second Tucson Discussions and Debates. Chapter 31. Cambridge, MA: The MIT Press, 1998, 345–59. [Google Scholar]
- Bachmann T. Twelve spatiotemporal phenomena, and one explanation. Aschersleben G, Bachmann T, Müsseler J (eds.), Cognitive Contributions to the Perception of Spatial and Temporal Events. Amsterdam: Elsevier/North-Holland, 1999, 173–206. (Advances in Psychology, # 129). [Google Scholar]
- Bachmann T. Microgenetic Approach to the Conscious Mind. Amsterdam/ Philadelphia: John Benjamins, 2000. [Google Scholar]
- Bachmann T. Binding binding: departure points for a different version of the perceptual retouch theory. Adv Cogn Psychol 2007;3:41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T. Finding ERP-signatures of target awareness: puzzle persists because of experimental co-variation of the objective and subjective variables. Conscious Cogn 2009;18:804–8. [DOI] [PubMed] [Google Scholar]
- Bachmann T. Priming and retouch in flash-lag and other phenomena of the streaming perceptual input. Nijhawan R, Khurana B (eds.), Space and Time in Perception and Action. Cambridge: Cambridge University Press, 2010, 536–57. [Google Scholar]
- Bachmann T. Attention as a process of selection, perception as a process of representation, and phenomenal experience as the resulting process of perception being modulated by a dedicated consciousness mechanism. Front Psychol 2011;2:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T. Neurobiological mechanisms behind the spatiotemporal illusions of awareness used for advocating prediction or postdiction. Front Psychol 2013;3:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T. How a (sub) cellular coincidence detection mechanism featuring layer-5 pyramidal cells may help produce various visual phenomena. Front Psychol 2015;6:1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T, Francis G. Visual Masking: Studying Perception, Attention, and Consciousness. Oxford: Elsevier/Academic Press, 2014. ISBN: 978-0-12-800250-6. [Google Scholar]
- Bachmann T, Hudetz AG. It is time to combine the two main traditions in the research on the neural correlates of consciousness: C = L × D. Front Psychol 2014;5:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T, Kirt T. Perception of successive brief objects as a function of stimulus onset asynchrony: model experiments based on two-stage synchronization of neuronal oscillators. Cogn Neurodyn 2013;7:465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T, Luiga I, Põder E. et al. Perceptual acceleration of objects in stream: evidence from flash-lag displays. Conscious Cogn 2003;12:279–97. [DOI] [PubMed] [Google Scholar]
- Bachmann T, Murd C. Covert spatial attention in search for the location of a color-afterimage patch speeds up its decay from awareness: introducing a method useful for the study of neural correlates of visual awareness. Vision Res 2010;50:1048–53. [DOI] [PubMed] [Google Scholar]
- Bachmann T, Murd C, Põder E. Flash-lag effect: complicating motion extrapolation of the moving reference-stimulus paradoxically augments the effect. Psychol Res 2012;76:654–66. [DOI] [PubMed] [Google Scholar]
- Bachmann T, Põder E. Change in feature space is not necessary for the flash-lag effect. Vision Res 2001;41:1103–6. [DOI] [PubMed] [Google Scholar]
- Bachmann T, Suzuki M, Aru J. Dendritic integration Theory: a thalamocortical theory of state and content of consciousness. Philos Mind Sci 2020;2:1–24. 1(II: Special Issue on the Neural Correlates of Consciousness). [Google Scholar]
- Barnett L, Muthukumaraswamy SD, Carhart-Harris RL. et al. Decreased directed functional connectivity in the psychedelic state. NeuroImage 2020;209:116462. [DOI] [PubMed] [Google Scholar]
- Bogen JE. On the neurophysiology of consciousness: I. An overview. Conscious Cogn 1995a;4:52–62. [DOI] [PubMed] [Google Scholar]
- Bogen JE. On the neurophysiology of consciousness: part II. Constraining the semantic problem. Conscious Cogn 1995b;4:137–58. [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Cooperman A, Sagi D. Motion-induced blindness in normal observers. Nature 2001;411:798–801. [DOI] [PubMed] [Google Scholar]
- Brazier MAB. Electrical Activity of the Nervous System. London: Pitman, 1977. [Google Scholar]
- Breitmeyer BG, Kafalıgönül H, Öğmen H. et al. Meta-and paracontrast reveal differences between contour-and brightness-processing mechanisms. Vision Res 2006;46:2645–58. [DOI] [PubMed] [Google Scholar]
- Brooks B, Jung R. Neuronal physiology of the visual cortex. Jung R (ed.), Handbook of Sensory Physiology. Central Processing of Visual Information. Part B. Vol. VII/3. New York, NY: Springer-Verlag, 1973, 325–440. [Google Scholar]
- Carrasco M, Barbot A. Spatial attention alters visual appearance. Curr Opin Psychol 2019;29:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M. et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013;5:198ra105. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang X, Yu Y. et al. Dissociable electroencephalograph correlates of visual awareness and feature-based attention. Front Neurosci 2017;11:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB et al. Spatial attention and conscious perception: the role of endogenous and exogenous orienting. Atten Percept Psychophys 2011;73:1065–81. [DOI] [PubMed] [Google Scholar]
- Chica AB, Bayle DJ, Botta F. et al. Interactions between phasic alerting and consciousness in the frontostriatal network. Sci Rep 2016;6:31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll MP, Whelan E, Catmur C. et al. Autistic traits are associated with atypical precision-weighted integration of top-down and bottom-up neural signals. Cognition 2020;199:104236. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Horga G, Fletcher PC. et al. Hallucinations and strong priors. Trends Cogn Sci 2019;23:114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F, Koch C. A framework for consciousness. Nat Neurosci 2003;6:119–26. [DOI] [PubMed] [Google Scholar]
- De Lange FP, Heilbron M, Kok P. How do expectations shape perception? Trends Cogn Sci 2018;22:764–79. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron 2011;70:200–27. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L. The global neuronal workspace model of conscious access: from neuronal architectures to clinical applications. Dehaene S, Christen Y. (eds), Characterizing Consciousness: From Cognition to the Clinic? Berlin, Heidelberg: Springer, 2011, 55–84. [Google Scholar]
- Dembski C, Koch C, Pitts M. Perceptual awareness negativity: a physiological correlate of sensory consciousness. Trends Cogn Sci 2021;25:660–70. [DOI] [PubMed] [Google Scholar]
- Descarries L, Krnjevic K, Steriade M (eds). Acetylcholine in the Cerebral Cortex. Vol. 145. Amsterdam: Elsevier, 2004. [Google Scholar]
- Doerig A, Schurger A, Hess K. et al. The unfolding argument: why IIT and other causal structure theories cannot explain consciousness. Conscious Cogn 2019;72:49–59. [DOI] [PubMed] [Google Scholar]
- Eklund R, Gerdfeldter B, Wiens S. Is auditory awareness negativity confounded by performance? Conscious Cogn 2020;83:102954. [DOI] [PubMed] [Google Scholar]
- Eklund R, Wiens S. Auditory awareness negativity is an electrophysiological correlate of awareness in an auditory threshold task. Conscious Cogn 2019;71:70–8. [DOI] [PubMed] [Google Scholar]
- Engel TA, Steinmetz NA, Gieselmann MA. et al. Selective modulation of cortical state during spatial attention. Science 2016;354:1140–4. [DOI] [PubMed] [Google Scholar]
- Fahle MW, Stemmler T, Spang KM. How much of the “unconscious” is just pre–threshold? Front Hum Neurosci 2011;5:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci 2005;8:1380–5. [DOI] [PubMed] [Google Scholar]
- Fletcher LN, Williams SR. Neocortical topology governs the dendritic integrative capacity of layer 5 pyramidal neurons. Neuron 2019;101:76–90. [DOI] [PubMed] [Google Scholar]
- Förster J, Koivisto M, Revonsuo A. ERP and MEG correlates of visual consciousness: the second decade. Conscious Cogn 2020;80:102917. [DOI] [PubMed] [Google Scholar]
- Frässle S, Sommer J, Jansen A. et al. Binocular rivalry: frontal activity relates to introspection and action but not to perception. J Neurosci 2014;34:1738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloni AR, Laffere A, Rancz E. Apical length governs computational diversity of layer 5 pyramidal neurons. Elife 2020;9:e55761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayet S, Paffen CL, Belopolsky AV. et al. Visual input signaling threat gains preferential access to awareness in a breaking continuous flash suppression paradigm. Cognition 2016;149:77–83. [DOI] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Magidov E, Sharon H. et al. Noradrenaline modulates visual perception and late visually evoked activity. Curr Biol 2018;28:2239–49.e6. [DOI] [PubMed] [Google Scholar]
- Hämäläinen H, Kwon MS, Lindell A. et al. Neglect is a spatial failure of alerting mechanisms required for awareness: an ERP study. J Basic Appl Sci 2014;10:239–56. [Google Scholar]
- Harris KD, Shepherd GM. The neocortical circuit: themes and variations. Nat Neurosci 2015;18:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler R. Interaction of reticular activating system for vigilance and the truncothalamic and pallidal systems for directing awareness and attention under striatal control. Buser PA, Rougeul-Buser A (eds.), Cerebral Correlates of Conscious Experience. Amsterdam: North-Holland, 1978, 111–29. [Google Scholar]
- He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci 2009;13:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog MH, Drissi-Daoudi L, Doerig A. All in good time: long-lasting postdictive effects reveal discrete perception. Trends Cogn Sci 2020;24:826–37. [DOI] [PubMed] [Google Scholar]
- Hohwy J. The Predictive Mind. Oxford: Oxford University Press, 2013. [Google Scholar]
- Honjoh S, Sasai S, Schiereck SS. et al. Regulation of cortical activity and arousal by the matrix cells of the ventromedial thalamic nucleus. Nat Commun 2018;9:2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafaligönül H, Breitmeyer BG, Öğmen H. Effects of contrast polarity in paracontrast masking. Atten Percept Psychophys 2009;71:1576–87. [DOI] [PubMed] [Google Scholar]
- Khurana B, Nijhawan R. Extrapolation or attention shift? Nature 1995;378:566. [DOI] [PubMed] [Google Scholar]
- Kirt T, Bachmann T. Perceptual retouch theory derived modeling of interactions in the processing of successive visual objects for consciousness: two-stage synchronization of neuronal oscillations. Conscious Cogn 2013;22:330–47. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Grassini S. Neural processing around 200 ms after stimulus-onset correlates with subjective visual awareness. Neuropsychologia 2016;84:235–43. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Ruohola M, Vahtera A. et al. The effects of working memory load on visual awareness and its electrophysiological correlates. Neuropsychologia 2018;120:86–96. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Salminen‐Vaparanta N, Grassini S. et al. Subjective visual awareness emerges prior to P3. Eur J Neurosci 2016;43:1601–11. [DOI] [PubMed] [Google Scholar]
- Kuhn G, Rensink RA. The vanishing ball illusion: a new perspective on the perception of dynamic events. Cognition 2016;148:64–70. [DOI] [PubMed] [Google Scholar]
- LaBerge D. Apical dendrite activity in cognition and consciousness. Conscious Cogn 2006;15:235–57. [DOI] [PubMed] [Google Scholar]
- Lamme VA. Towards a true neural stance on consciousness. Trends Cogn Sci 2006;10:494–501. [DOI] [PubMed] [Google Scholar]
- Långsjö JW, Alkire MT, Kaskinoro K. et al. Returning from oblivion: imaging the neural core of consciousness. J Neurosci 2012;32:4935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci 2013;36:141–51. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M. et al. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 2009;325:756–60. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Lüscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex 2004;14:1059–70. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 1999;398:338. [DOI] [PubMed] [Google Scholar]
- Laureys S, Gosseries O, Tononi G (eds). The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. San Diego: Academic Press, 2015. [Google Scholar]
- Lee J, Spence C. Audiovisual crossmodal cuing effects in front and rear space. Front Psychol 2015;6:1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ On binocular rivalry. Doctoral dissertation, Van Gorcum Assen, 1965. [Google Scholar]
- Lin Z, He S. Seeing the invisible: the scope and limits of unconscious processing in binocular rivalry. Prog Neurobiol 2009;87:195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Ribary U, Contreras D. et al. The neuronal basis for consciousness. Philosophical transactions of the Royal Society of London. Ser B: Biol Sci 1998;353:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Schall JD. Neuronal correlates of subjective visual perception. Science 1989;245:761–3. [DOI] [PubMed] [Google Scholar]
- Luiga I, Bachmann T. Different effects of the two types of spatial pre-cueing: what precisely is “attention” in Di Lollo’s and Enns’ substitution masking theory? Psychol Res 2007;71:634–40. [DOI] [PubMed] [Google Scholar]
- Magnin M, Rey M, Bastuji H. et al. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci U S A 2010;107:3829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Tsuchiya N. Growing evidence for separate neural mechanisms for attention and consciousness. Atten Percept Psychophys 2021;83:558–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manita S, Suzuki T, Homma C. et al. A top-down cortical circuit for accurate sensory perception. Neuron 2015;86:1304–16. [DOI] [PubMed] [Google Scholar]
- Marek GJ. Interactions of hallucinogens with the glutamatergic system: permissive network effects mediated through cortical layer V pyramidal neurons. Curr Top Behav Neurosci 2018;36:107–35. [DOI] [PubMed] [Google Scholar]
- Marshel JH, Kim YS, Machado TA. et al. Cortical layer–specific critical dynamics triggering perception. Science 2019;365:eaaw5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvan T, Polak M, Bachmann T. et al. Apical amplification—a cellular mechanism of conscious perception? Neurosci conscious2021;2021:niab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA. Top-down mechanisms of anesthetic-induced unconsciousness. Front Syst Neurosci 2014;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R. et al. Breakdown of cortical effective connectivity during sleep. Science 2005;309:2228–32. [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M. et al. Norepinephrine ignites local hot spots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav Brain Sci 2016;39:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. The role of dendritic signaling in the anesthetic suppression of consciousness. Anesthesiol: J Am Soc Anesthesiol 2015;122:1415–31. [DOI] [PubMed] [Google Scholar]
- Michel M. A role for the anterior insular cortex in the global neuronal workspace model of consciousness. Conscious Cogn 2017;49:333–46. [DOI] [PubMed] [Google Scholar]
- Michel M, Doerig A. A new empirical challenge for local theories of consciousness. Mind Lang 2021. 10.1111/mila.12319. [Google Scholar]
- Montaser-Kouhsari L, Moradi F, Zandvakili A. et al. Orientation-selective adaptation during motion-induced blindness. Perception 2004;33:249–54. [DOI] [PubMed] [Google Scholar]
- Mudrik L, Breska A, Lamy D. et al. Integration without awareness: expanding the limits of unconscious processing. Psychol Sci 2011;22:764–70. [DOI] [PubMed] [Google Scholar]
- Murayama M, Larkum ME. Enhanced dendritic activity in awake rats. Proc Nat Acad Sci 2009;106:20482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M, Pérez-Garci E, Nevian T. et al. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 2009;457:1137–41. [DOI] [PubMed] [Google Scholar]
- Murd C, Bachmann T. Spatially localized motion aftereffect disappears faster from awareness when selectively attended to according to its direction. Vision Res 2011;51:1157–62. [DOI] [PubMed] [Google Scholar]
- Murphy SC, Palmer LM, Nyffeler T. et al. Transcranial magnetic stimulation (TMS) inhibits cortical dendrites. Elife 2016;5:e13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsseler J, Aschersleben G. Localizing the first position of a moving stimulus: the Fröhlich effect and an attention-shifting explanation. Percept Psychophys 1998;60:683–95. [DOI] [PubMed] [Google Scholar]
- Navajas J, Ahmadi M, Quian Quiroga R. Uncovering the mechanisms of conscious face perception: a single-trial study of the N170 responses. J Neurosci 2013;33:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navajas J, Kaunitz LN. Late EEG responses are absent for conscious but task-irrelevant stimuli. J Neurosci 2016;36:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navajas J, Nitka AW, Quian Quiroga R. Dissociation between the neural correlates of conscious face perception and visual attention. Psychophysiology 2017;54:1138–50. [DOI] [PubMed] [Google Scholar]
- Nijhawan R. Motion extrapolation in catching. Nature 1994;370:256–7. [DOI] [PubMed] [Google Scholar]
- Northoff G, Huang Z. How do the brain’s time and space mediate consciousness and its different dimensions? Temporo-spatial theory of consciousness (TTC). Neurosci Biobehav Rev 2017;80:630–45. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C, Kveraga K, Shine JM. et al. Predictions penetrate perception: converging insights from brain, behaviour and disorder. Conscious Cogn 2017;47:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öğmen H, Breitmeyer BG, Todd S. et al. Target recovery in metacontrast: the effect of contrast. Vision Res 2006;46:4726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Dean JG, Liu T. et al. Differential role of prefrontal and parietal cortices in controlling level of consciousness. Curr Biol 2018;28:2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Schulz JM, Murphy SC. et al. The cellular basis of GABAB-mediated interhemispheric inhibition. Science 2012;335:989–93. [DOI] [PubMed] [Google Scholar]
- Petzold A, Valencia M, Pál B. et al. Decoding brain state transitions in the pedunculopontine nucleus: cooperative phasic and tonic mechanisms. Front Neural Circ 2015;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA, Bachmann T, Storm JF. Apical function in neocortical pyramidal cells: a common pathway by which general anesthetics can affect mental state. Front Neural Circ 2018;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA, Larkum ME, Harley CW. et al. The effects of arousal on apical amplification and conscious state. Neurosci Conscious 2016;2016:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MA, Metzler S, Hillyard SA. Isolating neural correlates of conscious perception from neural correlates of reporting one’s perception. Front Psychol 2014;5:1078.doi: 10.1162/jocn_a_00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potez S, Larkum ME. Effect of common anesthetics on dendritic properties in layer 5 neocortical pyramidal neurons. J Neurophysiol 2008;99:1394–407. [DOI] [PubMed] [Google Scholar]
- Pournaghdali A, Schwartz BL. Continuous flash suppression: known and unknowns. Psychon Bull Rev 2020;15:1–33. [DOI] [PubMed] [Google Scholar]
- Powers AR, Mathys C, Corlett PR. Pavlovian conditioning–induced hallucinations result from overweighting of perceptual priors. Science 2017;357:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci U S A 1987;84:7349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railo H, Revonsuo A, Koivisto M. Behavioral and electrophysiological evidence for fast emergence of visual consciousness. Neurosci Conscious 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh MJ, Phillips JM, Kambi NA. et al. Thalamus modulates consciousness via layer-specific control of cortex. Neuron 2020;106:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt W. Autocorrelation, a principle for the evaluation of sensory information by the central nervous system. Rosenblith WA (ed.), Sensory Communication. New York: Wiley, 1961, 303–17. [Google Scholar]
- Rosenthal D. Consciousness and Mind. Oxford: Clarendon Press, 2005. [Google Scholar]
- Rosenthal D. Higher-order awareness, misrepresentation and function. Philos Trans R Soc B: Biol Sci 2012;367:1424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutiku R, Aru J, Bachmann T. General markers of conscious visual perception and their timing. Front Hum Neurosci 2016;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutiku R, Bachmann T. Juxtaposing the real-time unfolding of subjective experience and ERP neuromarker dynamics. Conscious Cogn 2017;54:3–19. [DOI] [PubMed] [Google Scholar]
- Sanchez G, Hartmann T, Fuscà M. et al. Decoding across sensory modalities reveals common supramodal signatures of conscious perception. Proc Nat Acad Sci 2020;117:7437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharlau I. Perceptual latency priming: a measure of attentional facilitation. Psychol Res 2007;71:678–86. [DOI] [PubMed] [Google Scholar]
- Scharlau I, Neumann O. Perceptual latency priming by masked and unmasked stimuli: evidence for an attentional interpretation. Psychol Res 2003;67:184–96. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci 2008;1129:105–18. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central lateral thalamic nucleus stimulation awakens cortex via modulation of cross-regional, laminar-specific activity during general anesthesia. Neuron 2020;106:1–3. [DOI] [PubMed] [Google Scholar]
- Schlossmacher I, Dellert T, Bruchmann M. et al. Dissociating neural correlates of consciousness and task relevance during auditory processing. NeuroImage 2021;228:1–9. [DOI] [PubMed] [Google Scholar]
- Schlossmacher I, Dellert T, Pitts M. et al. Differential effects of awareness and task relevance on early and late ERPs in a no-report visual oddball paradigm. J Neurosci 2020;40:2906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld MA, Hassa T, Hop J-M. et al. Neural correlates of hysterical blindness. Cereb Cortex 2011;21:2394–8. [DOI] [PubMed] [Google Scholar]
- Schröder P, Nierhaus T, Blankenburg F. Dissociating perceptual awareness and postperceptual processing: the P300 is not a reliable marker of somatosensory target detection. J Neurosci 2021;41:4686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Dehaene S. Neural processes underlying conscious perception: experimental findings and a global neuronal workspace framework. J Physiol-Paris 2004;98:374–84. [DOI] [PubMed] [Google Scholar]
- Sheth BR, Nijhawan R, Shimojo S. Changing objects lead briefly flashed ones. Nat Neurosci 2000;3:489–95. [DOI] [PubMed] [Google Scholar]
- Silverstein BH, Snodgrass M, Shevrin H. et al. P3b, consciousness, and complex unconscious processing. Cortex 2015;73:216–27. [DOI] [PubMed] [Google Scholar]
- Singer W. Consciousness and the binding problem. Ann N Y Acad Sci 2006;929:123–46. [DOI] [PubMed] [Google Scholar]
- Spence C, Parise C. Prior-entry: a review. Conscious Cogn 2010;19:364–79. [DOI] [PubMed] [Google Scholar]
- Stamm M, Aru J, Bachmann T. Right-frontal slow negative potentials evoked by occipital TMS are reduced in NREM sleep. Neurosci Lett 2011;493:116–21. [DOI] [PubMed] [Google Scholar]
- Steriade M, Sakai K, Jouvet M. Bulbo-thalamic neurons related to thalamocortical activation processes during paradoxical sleep. Exp Brain Res 1984;54:463–75. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Frith C, Petrovic P. Believing is seeing: expectations alter visual awareness. Curr Biol 2008;18:R697–8. [DOI] [PubMed] [Google Scholar]
- Supèr H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1). Nat Neurosci 2001;4:304–10. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Schwartzman DJ, Augusto R. et al. Sensorimotor contingency modulates breakthrough of virtual 3D objects during a breaking continuous flash suppression paradigm. Cognition 2019;187:95–107. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Larkum ME. General anesthesia decouples cortical pyramidal neurons. Cell 2020;180:666–76. [DOI] [PubMed] [Google Scholar]
- Tagliabue CF, Mazzi C, Bagattini C. et al. Early local activity in temporal areas reflects graded content of visual perception. Front Psychol 2016;7:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Ebner C, Sigl-Glöckner J. et al. Active dendritic currents gate descending cortical outputs in perception. Nat Neurosci 2020;23:1277–85. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Oertner TG, Hegemann P. et al. Active cortical dendrites modulate perception. Science 2016;354:1587–90. [DOI] [PubMed] [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC Neurosci 2004;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Koch C. Consciousness: here, there and everywhere? Philos Trans R Soc B: Biol Sci 2015;370:20140167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A, Schmidt H. Illusory conjunctions in perception of objects. Cogn Psychol 1982;14:107–41. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Wilke M, Frässle S. et al. No-report paradigms: extracting the true neural correlates of consciousness. Trends Cogn Sci 2015;19:757–70. [DOI] [PubMed] [Google Scholar]
- Uttal WR, Cook L. Systematics of the evoked somatosensory cortical potential. IBM J Res Dev 1962;6:179–91. [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R. et al. Precise minds in uncertain worlds: predictive coding in autism. Psychol Rev 2014;121:649–75. [DOI] [PubMed] [Google Scholar]
- Van Vugt B, Dagnino B, Vartak D. et al. The threshold for conscious report: signal loss and response bias in visual and frontal cortex. Science 2018;360:537–42. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Moorman DE, Ashton-Jones G. Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc Nat Acad Sci (PNAS) 2018;115:E9439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetik S, Tulver K, Lints D. et al. Among the two kinds of metacognitive evaluation, only one is predictive of illusory object perception. Perception 2020;49:1043–56. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Preller KH. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci 2020;21:611–24. [DOI] [PubMed] [Google Scholar]
- Ward LM. The thalamic dynamic core theory of conscious experience. Conscious Cogn 2011;20:464–86. [DOI] [PubMed] [Google Scholar]
- Webb TW, Igelström KM, Schurger A. et al. Cortical networks involved in visual awareness independent of visual attention. Proc Nat Acad Sci 2016;113:13923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilnhammer VA, Stuke H, Sterzer P. et al. The neural correlates of hierarchical predictions for perceptual decisions. J Neurosci 2018;38:5008–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D. Neuroscience: toward unbinding the binding problem. Curr Biol 2009;19:R251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D, Murakami I. Latency difference, not spatial extrapolation. Nat Neurosci 1998;1:656–7. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Influence of spatial frequency, luminance, and duration on binocular rivalry and abnormal fusion of briefly presented dichoptic stimuli. Perception 1983;12:447–56. [DOI] [PubMed] [Google Scholar]
- Wright R, Ward L. Orienting of Attention. Oxford: Oxford University Press, 2008. [Google Scholar]
- Wu C-T, Busch NA, Fabre-Thorpe M. et al. The temporal interplay between conscious and unconscious perceptual streams. Curr Biol 2009;19:2003–7. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J Neurosci 2008;28:2667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkali A, Adams RA, Psarras S. et al. Increased weighting on prior knowledge in Lewy body-associated visual hallucinations. Brain Commun 2019;1:fcz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Jiang Y, He S. et al. Olfaction modulates visual perception in binocular rivalry. Curr Biol 2010;20:1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this paper is a theoretical review, it is not applicable for publical data availability.


