Abstract
Normal breathing for healthy humans is taken for granted; it occurs without conscious effort using ambient (1-atmosphere) pressure with 21% oxygen (O2) concentration. The body automatically adjusts for stress, exercise, altitude, and mild disease by increasing the volume and frequency of breathing. Longer term adaptations for exercise and altitude include increases in red blood cell counts and higher concentrations of capillaries in muscle tissue. When more challenging external environmental conditions or pulmonary illnesses exceed the capability for these adaptations, the human system requires technology to maintain sufficient ventilation to preserve life. On the environmental side there are two conditions to be addressed: toxicity of the surrounding atmosphere and changes in external pressure and O2 concentration. On the medical side, mechanisms for assisting breathing include O2 supplementation at ambient pressure, positive pressure/flow without additional O2, or a combination of both. This overview describes the various technologies applied to maintaining a safe breathing environment. Topics for environmental intervention include filter-based and flowing air-supply masks for toxic environments (occupational and laboratory protection), and on-demand gas supply systems for firefighters, self-contained underwater breathing apparatus (SCUBA) divers, and altitude (high performance aircraft, spacecraft) applications. The topics for medical intervention include nasal cannula, continuous positive airway pressure (CPAP), and medical ventilators. The primary purpose of this article is to provide a basic understanding of normal human breathing and the adaptation of breathing in different environments using available technologies.
OVERVIEW
The topic of human breathing mechanics and support has come to the forefront of public awareness due to the current COVID-19 crisis. Wearing hospital masks or improvised facial coverings was broadly implemented to protect against spreading infectious aerosols (Pleil et al, 2020a, Eikenberry et al. 2020). At the outset of the pandemic, the news media reported on the availability and use of medical ventilators as well as oxygen (O2) supplementation via less invasive nasal cannulas or masks to help patients recover from pulmonary disease (Kliff 2020, Ranney et al. 2020). Additionally, breathing protection, supplementation, and therapy appear in the media with respect to firefighters battling wildfires, pilots flying high-performance military aircraft, recreational self-contained underwater breathing apparatus (SCUBA) and professional divers, and medical and laboratory researchers.
This commentary describes normal breathing functionality and strives to put human breathing into context with the modern technologies used to extend the safety envelope beyond sea-level pressure and O2 concentration to support life in situations ranging from extreme pressures in diving, to low pressures in aviation. Herein we discuss all manner of human breathing parameters and how different support systems work. The article describes the current understanding of the physics of breathing and the processes for distributing O2 from the air to the human tissues, organs, and the brain.
The discussions are grouped by the level of intervention; consider that a simple hospital mask only serves to filter particles but does not affect O2 concentration, gas flow, or ambient pressure, whereas a hospital ventilator can take complete control of the human breathing system and dictate timing, flow, volume, and level of oxygen. Between these extremes are applications that address specific parameters. Some common examples are flow-through masks that receive clean air flow from outside to protect the wearer from an indoor contaminated environment, the nasal cannula that provides additional ambient air enriched with O2 for a patient with respiratory distress, the standard respirator that protects the wearer from organic volatile chemicals, and various “on-demand” mask systems that replace ambient air using a separate source.
The mission of this commentary is to provide a basic understanding of human breathing under different scenarios and to present a baseline of breathing parameters that are required to maintain human life. Examples and data are drawn directly from the authors’ research and expertise, as well as being supplemented with the peer-reviewed literature. This article expands on the understanding of the physics parameters of pressure, flow, volume, and timing under different conditions encountered by humans to further the concepts of breath research beyond the chemistry of metabolism (Pleil et al. 2020b).
NORMAL BREATHING
Humans descended from the superfamily Hominoidea (10–20 million years ago); the earliest fossil records for hominids (earliest direct ancestors) date back 4–5 million years (Andrews 1992), and modern homo sapiens date back approximately 200,000 years (Stringer 2016). Over 2.3 billion years ago, early cyanobacteria began emitting O2 via photosynthesis (Kasting and Siefert 2002). Considering that the earth’s atmosphere stabilized at ~21% O2 and ~78% nitrogen (N2) about 550 million years ago (Berner 1999), it is safe to say that modern humans evolved to efficiently use this atmosphere for “normal” breathing (Liem 1988).
Normal breathing is defined here as the movement of air in and out of the lungs in an environment with infinite volume and stable external pressure. Normal breathing is an autonomous function performed without conscious effort, although humans can take conscious control for actions such as breath-holding, talking, singing, swimming, and exercise. The physics of normal breathing are based on differential pressure between the lungs and the ambient atmosphere. As the diaphragm and ribcage expand and contract due to muscle action, air flows in and out to balance pressure. During inhalation, the fresh oxygenated air flows into branching progressively smaller airways to ultimately inflate the alveoli (small membranous air sacs) and exchange O2 for carbon dioxide (CO2), a waste product of cellular energy production. CO2 is expelled upon exhalation and the cycle begins anew. Humans do not absorb all of the inhaled O2 for metabolism; inhaled air contains 21% O2 while exhaled breath contains approximately 16% O2 and 5% CO2.
Oxygen transport
Oxygen transport begins at the mouth/nose, continues into the lungs, consisting of progressively smaller branching airways, ending in alveoli or air sacs where oxygen is transferred to the red blood cells. It is then delivered to the systemic blood flow and ultimately into the various cells by capillaries and diffusion. The first effects of reduced O2 are felt by the brain; in fact, even minor reductions (mild hypoxia) can manifest as disorientation, confusion, and reduced higher executive function. Longer term O2 deprivation can also cause organ damage, especially to the heart and kidneys, but practically, the brain is the organ of primary concern. In a recent National Aeronautics and Space Administration (NASA) Pilot Breathing Assessment (PBA) study of jet fighter pilots, detailed analyses of breathing response, spirometry, and blood oxygenation have shown that there are losses in O2 transport along the way from the face to the organs despite 100% O2 in the breathing gas (NASA PBA Report 2020). A study of environmental contamination has shown that pulmonary function can be lost even when the O2 content and pressure in the air are nominal (sea level) due to pulmonary inflammation (Stiegel et al. 2017). As an example, Figure 1 shows the conceptual model for O2 transport related to jet aircraft life support systems.
Figure 1.
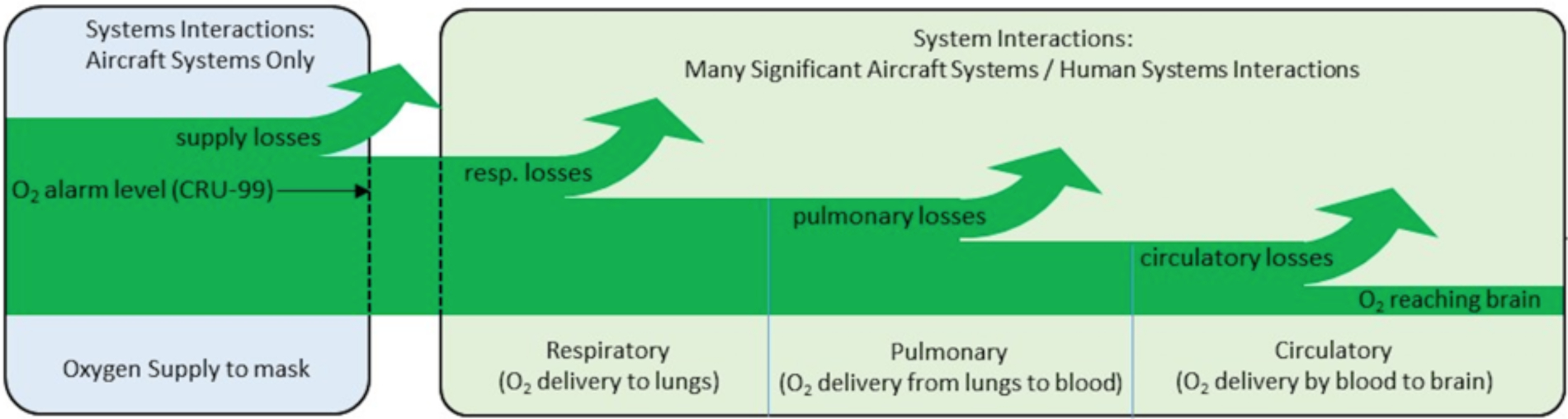
Conceptual diagram of O2 transport from the face to the brain as adapted from NASA PBA study. Despite adequate input air, there are potential losses from various physiological factors including bronchoconstriction, pulmonary inflammation, and impaired circulation (NASA PBA Report 2020).
Breathing frequency, tidal volume, and pulmonary pressure
At complete rest, the typical adult male exchanges approximately 0.5 L (500 mL; 400 mL for female) of air per breath (tidal volume) at a rate of 12 times per minute, resulting in a minute ventilation rate of about 6 L of air per minute. Certainly, there are individual variations due to size, age, gender, and health-state, but these parameters are considered robust default values. Of note is that the first 0.15 L (150 mL) volume of each breath, referred to as the tracheal dead volume, does not participate in O2/CO2 exchange but only fills the trachea and branching airways. In calm, at-rest breathing, the body generates a modest ±3 mmHg pressure swing to create inhalation and exhalation flow. For context, imagine one is blowing up a balloon or drinking a thick milkshake through a straw; there is a maximum amount of positive or negative pressure one can exert, after which the activity fails. According to the literature, the diaphragm and related thoracic muscles can exert maximum exhalation pressures of 44 to 88 mmHg and maximum inhalation pressures of negative 29 to 74 mmHg (Lausted et al. 2006, Evans and Whitelaw 2009). As such, normal at-rest breathing only requires minimal muscle effort compared to the maximum possible exertion.
The human body is highly adaptable and as metabolic need increases for activity, exercise, and warmth, the cardio-pulmonary (heart-lung) system has mechanisms to increase blood flow and O2 perfusion. Heart rate, respiration rate, and tidal volume increase to provide the necessary cellular resources to support energy production. With moderate exercise, normal adults can sustain 30 breaths/minute with 3.5 L/breath, and depending on fitness, age, and other health factors, individuals can adapt to higher needs for O2 perfusion. Pulmonary function tests (PFTs) measure the absolute maximum for a single breath (forced vital capacity, FVC) in the range of 4 to 5.5 L/breath, but such levels are not sustainable, and so this limits human activity. Again, normal at-rest breathing only uses a small amount of the total resources possible.
Table 1 shows the normal breathing parameters for healthy adults. Certainly, individuals can vary greatly depending on cardiovascular fitness, anxiety, inflammatory status, genotype, and phenotype. The values in the table are illustrative and show the potential adaptability of the human system to increased workload. Notably, all measurements of breathing require masks or other devices to measure flows and pressures, and so there is a caveat that these numbers are not exactly “normal breathing.” However, modern measurement technology has evolved to minimize pressure drops and flow restrictions so that such measurements can be taken at face-value. The values in the table are considered “nominal” in that they are generally accepted by the medical community and found in various public websites (e.g., Wikipedia, Mayo Clinic, and Johns Hopkins). Certainly, ranges or slightly different values may also be found, including adjustments for gender or physical size, but the values in Table 1 represent a consensus for normal breathing for healthy adults. More detailed statistical analyses of human breathing have been published by the U.S. Environmental Protection Agency (EPA 2011).
Table 1:
Nominal values for normal human adult breathing at sea-level
| Condition | Rate (Breaths/min) | Tidal Volume (L/breath) | Minute Ventilation (L/min) |
|---|---|---|---|
| Nominal At-Rest | 12 | 0.5 | 6 |
| Normal Activity | 16 | 1 | 16 |
| Moderate Exercise | 20 | 2 | 40 |
| Stress Test | 35 | 3 | 140 |
| Maximum PFT* | NA | 4.5 | NA |
standard pulmonary function test (PFT)
NA: not applicable.
Figure 2 below shows pulmonary function testing with laboratory instrumentation and a handheld device that can be used in the field (US EPA and NASA).
Figure 2:

Examples of pulmonary function testing in the laboratory and in the field drawn from US EPA and NASA studies.
Oxygen saturation
The amount of O2 in the blood, or blood O2 saturation level, is defined as the fraction of O2-saturated hemoglobin in red blood cells compared to the total amount of hemoglobin. Invasive medical tests can be used to measure arterial blood gases (ABG) very accurately; however, standard practice is to estimate peripheral oxygen saturation (SpO2) non-invasively with pulse oximetry using a colorimetric light sensor attached to a finger. SpO2 between 95 and 100% is considered normal; readings between 90 and 95% are considered mildly hypoxic, and a level of 90% or below indicates a serious medical condition called hypoxemia that needs to be treated quickly. The human body can adapt slightly to lower levels of SpO2, but generally this engenders feelings of fatigue and shortness of breath (Hoiland et al. 2016, Hardt et al. 2015).
Altitude adaptation
The default values mentioned above apply for sea-level atmospheric pressure and O2 levels. However, as altitude increases, the percentage (mass fraction) of O2 remains the same but the partial pressure decreases proportionally with total pressure, as such the number of O2 molecules within each breath is proportionally lower. Humans have adapted to elevation as evidenced by major population centers at higher altitudes. In the short term, for visitors to higher altitudes, breathing frequency and tidal volume increase to provide more O2 to the systems biology. Long-term and natural selection adaptations include the increase in red-blood cells, capillary density, and higher efficiencies in hemoglobin transport.
At sea-level, the partial pressure of oxygen (ppO2) is 0.21 atmospheres and normal “at-rest” breathing results with O2 blood perfusion levels of ~98%. At higher elevations, ppO2 decreases; for context, Table 2 shows some examples for major cities, their populations, elevation, and ppO2. For example, one of the biggest cities in the world, Mexico City, Mexico (population ~22 million), has an elevation of 7,350 feet, resulting in ppO2 of 0.16 atm, which represents an O2 density 60% of sea-level. Anecdotally, some newcomers to Mexico City experience some breathing discomfort, headache, mild hypoxia, and elevated respiration; however, they generally adapt within a few days. Likewise, while “altitude training” has served as an intermittent fad in endurance athletes, practical experience has shown that physiological adaptations from training at altitude generally revert to normal within a few days of returning to sea level conditions. As a comparison, Table 2 shows the typical commercial airliner, which cruises at ~36,000 to 40,000 feet altitude with cabin pressurization set to an equivalent altitude of about 8,000 feet (0.16 ppO2 atm) as mandated in the U.S. by Federal Regulation 14 CFR 25.831 (Federal Aviation Administration (FAA) 2002); as such, the typical airliner cabin ppO2 is close to that of Mexico City. The FAA general aviation guidelines further require supplemental O2 supply if cabin pressure drops to 12,500 feet equivalent (0.13 ppO2 atm) for more than 30 minutes and always above 14,000 feet cabin altitude (0.12 ppO2 atm).
Table 2:
Examples of large human populations adapted to breathing normally at different altitudes. As elevation increases, ppO2 decreases and the human adapts with various mechanisms.
| Location | Metropolitan Population (estimate in millions) | Altitude (feet) | Air Pressure (atm) | ppO2 (atm) |
|---|---|---|---|---|
| New York City, NY, USA | 18.83 | 33 | 1.00 | 0.21 |
| Delhi, India | 30.33 | 709 | 0.97 | 0.20 |
| Santiago, Chile | 6.77 | 1615 | 0.94 | 0.20 |
| Denver, CO, USA | 3.21 | 5000 | 0.83 | 0.17 |
| Kabul, Afghanistan | 4.27 | 5602 | 0.81 | 0.17 |
| Mexico City, Mexico | 21.78 | 7350 | 0.76 | 0.16 |
| Commercial Airline Cabin | NA | 8000 | 0.74 | 0.16 |
| El Alto/La Paz, Bolivia | 2.91 | 13600 | 0.60 | 0.13 |
| Denali Summit, AK, USA | NA | 20208 | 0.48 | 0.10 |
| Mount Everest Summit, Nepal | NA | 29000 | 0.31 | 0.07 |
NA: Not applicable
Table 2 also includes two mountains: Denali, Alaska, USA, and Mt. Everest, Nepal for additional context. Climbers of Denali (elevation ~20,000 feet) can adapt to the summit altitude with prior aerobic training and acclimation over a few weeks’ time. Very few climbers of Mt. Everest (summit elevation ~29,000 feet) have ever achieved summiting without supplemental O2. The altitude tipping point for unassisted breathing in very fit individuals (mountain climbers) is around 26,000 feet. The values presented are for illustration only; they are gleaned from consensus of various Wikipedia and other internet sources, as well as published references (e.g., Peacock 1998, West 2000).
Concentration adaptation to CO2 (artificial atmospheres)
Like the adaptations to activity and ambient pressure, the human body also has some leeway in adapting to different concentrations of O2 and CO2. In the previous section, we discussed changes brought about by pressure with constant percentages of constituent gases. Here, we consider only standard pressure, but with different concentrations of CO2 resulting from the buildup from exhalations.
When breathing normally, humans inhale fresh air with 21% O2 along with a negligible amount of ambient CO2 (0.04%), and exhale ~16% O2 and ~5% CO2. Medically, ambient concentrations above 19.5% O2 are considered safe, but there are reported minor adverse effects (e.g., headache, irritability, and confusion) from inhalation of CO2 starting at ~1,000 ppm (0.1%), with increasing adverse effects up to ~20,000 ppm (2%) concentration. Beyond this level, it becomes more difficult for the body to expel metabolic waste CO2, and CO2 concentrations of 100,000 ppm and above can cause loss of consciousness and asphyxiation (National Research Council 2007). Because O2 and CO2 in a confined space represent a zero-sum (add up to 20.95% in dry air), excess CO2 ultimately displaces available O2. Studies have shown that humans can tolerate more CO2 for short periods of time, but from a public health perspective, humans in general are not well-adapted to chronic (indoor) exposures to CO2 beyond 0.1%. Various studies of CO2 inhalation effects are available in the literature (e.g., Shriram et al. 2019, National Research Council 2007, Satish et al.2012), and recently the use of CO2 breath measurements (capnography) has been discussed (Pleil and Christensen 2021).
Consumption of O2 and production of CO2 (artificial atmospheres)
Unlike open air breathing, the act of breathing in confined space environments consumes the available O2 and increases the CO2 concentration. As such, sustaining normal breathing in closed environments requires some form of ventilation to stabilize the gas mixture. Fully sealed spaces such as submarines, spacecraft, underwater habitats, and hyperbaric/hyperoxic chambers, have engineering systems that continually scrub exhaled CO2 and add replacement O2 as needed. More porous environments such as aircraft, cars, large office buildings, crowded auditoriums, and large ships rely on external air exchange via passive or pumped ventilation from the surrounding environment to balance O2 and CO2 levels. In the special cases of aircraft flying at altitude and hyperbaric chambers, the supplemental air must be pressurized as well.
In general, artificial atmospheres cannot cost-effectively maintain a perfect ambient atmosphere with 20.95% O2 and 0.041% CO2. Depending on ventilation, indoor spaces with human occupancy always have lower O2 and higher CO2 than outdoor air. The acceptable American Society of Heating and Air-Conditioning Engineers (ASHRAE) standard for CO2 in offices is 800 ppm (0.08%); however, levels as high as 4,000 to 5,000 ppm (0.4 to 0.5%) have been measured (Zhang et al. 2017). Measurements in cars stopped in traffic using recirculated conditioned air have shown CO2 levels reaching 2,500 ppm (0.25%) in a few minutes and continuing to rise (Angelova et al. 2019). In many cases, the practical tradeoff is temperature control vs. CO2 buildup.
Enclosed spaces such as spacecraft, the International Space Station (ISS) or military submarines always rely on totally artificial atmospheres and maintain a tightly controlled atmospheric gas mixture. Therefore, CO2 levels in these enclosed spaces are higher than the ambient CO2 concentration on earth (0.3 mmHg, 400 ppm, or 0.04% of Earth’s atmosphere). CO2 levels in spacecraft typically range from 2.3 to 5.4 mmHg, with the highest recording of 14.9 mmHg on Apollo 13 (Law et al. 2014). NASA’s spacecraft maximum allowable concentrations (SMACs) for CO2 for 1-h and 24-h exposure were set at 13,000 ppm, but recently the CO2 SMAC was removed and acceptable levels for CO2 in spacecraft are currently under review (National Research Council 2007, NASA 2020).
CO2 levels of more than 10,000 ppm have been reported in nuclear-powered submarines. The U.S. Navy’s emergency and continuous exposure guidance levels for CO2 were evaluated in 2007. The 1-h and 24-h emergency exposure guidance levels of 40,000 ppm were proposed to be lowered to 30,000 ppm and 15,000 ppm, respectively, compared to the National Research Council (NRC) inhalation exposure recommendation of 25,000 ppm for both 1-h and 24-h exposure levels. The 90-day continuous exposure guidance level of 5,000 ppm was proposed to be increased to 7,000 ppm, compared to the 8,000 ppm NRC recommendation (National Research Council 2007).
At complete rest at sea level, a single human consumes approximately 8.6 m3 of air per day of which 5% is exhaled as metabolic CO2, producing approximately 785 gm of CO2. According to the U.S. EPA’s Exposure Factors Handbook (EPA 2011), an adult male of normal weight with moderate activity for 16 hours and rest for 8 hours consumes ~22.8 m3 of air per day with 99th percentile of 23.7 m3. For calculations, this is generally rounded to 24 m3 or 1 m3 per hour as the default assumption and equates to an exhalation of approximately 2.2 kg of CO2 per day.
Using the ISS as an example with ~950 m3 interior volume, a crew of seven astronauts (with moderate activity) is expected to produce a volume of 8.4 m3 of CO2 gas per day. Left uncontrolled, the ISS atmosphere would reach 0.09% CO2 concentration in 24 hours. Empirically, this does not happen in the ISS environment presumably because the astronauts are not working against gravity and produce only about 1 kg CO2 per day. Regardless, the closed environment requires removal of CO2 and supplementation of O2. As such, the environmental control systems onboard the ISS remove approximately 7 kg of CO2 each day to maintain a viable atmosphere and continually replenish the air with O2 that is produced via hydrolysis of water. Figure 3 illustrates space mission environments with the individual space suit, the “shirtsleeve” environment inside the ISS, and an exterior shot of the loneliness of the self-contained human ISS habitat. Within the individual suit, and the whole of the ISS, CO2 is regularly removed and O2 is added to maintain a sustainable atmosphere (photos from NASA archives).
Figure 3:

NASA photos of space mission environments with the individual space suit, the “shirtsleeve” environment inside the International Space Station, and an exterior shot of the loneliness of the self-contained human ISS habitat.
Normal breathing in contaminated air environments
There are many situations where there is sufficient O2 in the air, but contaminants including particulate matter (PM) and dusts, virulent aerosols, gas-phase carcinogens, and adverse odors can make the breathing air unsafe. Typically, these are occupational hazards that require specific respirators or masks; however, now in the era of the COVID-19 pandemic, the general public is also routinely wearing masks to help prevent the spread of the disease (Pleil et al. 2020a).
Respirators:
Breathing protection comes in different forms depending on the expected contaminants. The most common occupational respirators are designed to scrub toxic gases and vapors generally using half-face or full-face masks with adsorbent (chemical) or molecular sieve cartridges. Although there is always some small flow restriction from such cartridges, generally the respirators are very close to normal breathing. Typically, the additional pressure required for inhalation across modern activated carbon filters (ACF) is in the range from 1.5 to 3 mmHg. As such, normal breathing with typical respirators requires ~4.5 to 6 mmHg pulmonary pressure, which is well below the upper limits that are around 40 mmHg (Balanay and Lungu 2016).
Hospital masks:
Many environments need only protection against aerosols and particles; for these scenarios, different levels of physical filter materials are used. Standard medical protection masks like the well-known N-95 mask have a tight fit on the face using a double mesh/cloth layer that forces all air through the filter material. In these masks, the inhalation restriction is ~ 0.025 mmHg, which is negligible compared to normal at rest breathing (Skaria and Smaldone 2014). More conventional hospital (paper/polypropylene) masks are loose-fitting and rely more on aerosol and particle kinetics rather than direct filtration; these masks, like improvised facial coverings (bandanas and scarves), have no measurable pressure drops.
Free-flow air supplies:
In some confined-space scenarios, outside air is pumped directly into a face shield or mask in the breathing zone at a rate high enough to continually displace the microenvironmental air. There is no pressure drop experienced by the worker; in fact, this style of respiratory protection provides a secondary benefit in that the free-flowing external air provides cooling as well. Such air supplies are common in occupational settings, including aircraft systems maintenance and chemical laboratories.
Figure 4 shows some examples of common protective masks that all support safe “normal” breathing. They include simple facial coverings, face shields, as well as half- and full-face respirators.
Figure 4:

Examples of common protective masks used for safe breathing in various settings. (from US Government archives).
Normal breathing with medical O2 enrichment
In some cases, individuals with moderate lung or heart disease require additional O2 to maintain oxygenation; this could be a temporary recovery solution in the hospital, or a longer-term solution for ambulatory outpatient maintenance. This “O2 therapy” can be low- or high-flow. In low-flow O2 therapy, a small tube provides O2 enriched flow to a nasal cannula or mask from a cylinder or a portable O2 generator at up to 15 L per minute (LPM). Of note, low-flow O2 therapy can only deliver up to ~80% fraction of inspired oxygen. In high-flow O2 therapy, flows of more than 15 LPM are delivered to a mask or nasal cannula, typically through larger bore tubing. In low- and high-flow O2 therapy applications, the flow rates and O2 concentrations delivered are tailored to the needs of the patient.
A common current practice uses high-flow nasal cannula (HFNC) to delivery respiratory support. HFNC can deliver up to 60 LPM and range up to 100% O2 (Ward 2013, Helviz and Einav 2018). HFNC is a non-invasive alternative to mask-based O2 therapy and mechanical ventilation; the supplementation remains in the realm of normal breathing in the sense that there is no measured forcing pressure.
Normal breathing in spacesuits and other sealed protective gear
The most esoteric situations for normal breathing involve protective gear that allows independent movement, yet still seals the person completely off from the external environment. The best examples are spacesuits and certain types of HazMat suits. In these configurations, clean air is provided into the suit, especially around the head, without a sealed mask on the face. CO2 is constantly scrubbed and replaced with an equivalent amount of O2 in periodic bursts to maintain a viable atmosphere; the most recent advances in space suit design can be found in the literature (e.g., Goldfarb and Hodgson, 2001, Southern and Moisev, 2019).
Extreme adaptation
There are some people that are adapted or trained for extreme situations focused on breath holding and breathing control. The largest groups are found in the free-diving community and in certain types of meditation. The normal healthy human can hold their breath between 30 to 60 seconds. With some practice and coaching, breath hold times can be quickly improved to about 2 minutes or more. Free divers, without prior oxygen loading, can routinely hold their breath for 10 minutes or more and dive unassisted to 300 feet depth, corresponding to about 10 atmospheres pressure. These extreme activities carry risks for acute injury to tissues and airways, as well as for permanent damage or even death. The book “Deep: Freediving, Renegade Science, and What the Ocean Tells Us about Ourselves” describes extreme adaptation not only for humans but also for creatures in the depths of the ocean (Nestor 2014). The human record for breath holding with O2 loading is 24 minutes 37 seconds achieved by Budimir Šobat in Sisak, Croatia in March 2021; new attempts are ongoing (Guinessworldrecords.com).
ON-DEMAND BREATHING
There are scenarios in human breathing experience that require more than just adaptation or passive filtration, but instead employ non-rebreathing devices to bring clean air directly to the nose/mouth. These systems operate through “on-demand” mechanics; as the person begins to inhale, the slight pressure drop opens a valve, which in turn triggers delivery of an airstream for inhalation. As the person begins to exhale, the pressure increases, shuts off the inhalation valve and flow, and opens the exhalation valve to allow air out. This type of system has the advantage of conserving resources by only providing air when needed. The challenge is to respond properly with the correct flow, concentration, speed, timing, and volume required to sustain life. All on-demand breathing systems work at the ambient pressure (or maybe a tiny bit higher) that surrounds the human.
SCUBA open-circuit breathing
The first practical applications for “on-demand” breathing were for underwater exploration and recreation. Termed self-contained underwater breathing apparatus (SCUBA), this engineering advance replaced the hardhat suits that were tethered to large surface compressors and pumped with air continuously at high flow rates. With SCUBA gear, the diver could now bring along his own breathing gas in a compressed air cylinder and be completely independent of the surface. Although Jacques Cousteau (of underwater filming fame) is credited for developing the Aqua-Lung (twin hose scuba equipment) in France for commercial and recreational diving circa 1945 (Richardson 1999), the original concept of the on-demand regulator and use in a diving suit was developed and patented by Benoît Rouquayrol in 1860 (Siermontowski et al. 2016).
Standard SCUBA uses open-circuit breathing wherein the inlet side from the air supply is pressurized and sealed but the outlet (exhalation) side is expelled directly into the water by a diaphragm valve. The basic configuration has a compressed gas cylinder with a two-stage on-demand regulator system that includes a first stage, which regulates the raw cylinder pressure down to a more practical intermediate pressure proportional to depth, and a second stage (typically held by the mouth) that supplies gas from the reference pressure on-demand to the user. The lips provide the seal; standard SCUBA is mouth breathing only and the nose is sealed into the diving mask airspace to allow the sinus volume to equalize to the breathing pressure. Normal swimming goggles cannot be used as they do not allow pressure equalization of the airspace. A variant is the full-face mask that combines visual and breathing needs. This arrangement seals from the forehead around the face down to the chin which allows both mouth and nose breathing, as well as radio communication.
The most important issue with breathing underwater is the wide range of positive pressures encountered. Each additional 33 feet (10 meters) of seawater depth adds one atmosphere of pressure. Typical recreational (vacation) diving has a maximum of 130 feet (40 meters) and is defined as “no-decompression” diving in that it is possible to ascend to the surface in an emergency without obligatory decompression stops. In general, descents and ascents are done slowly to allow the body, especially sinuses and ear channels, to come to equilibrium with the water pressure, and to allow the dissolved gasses in body tissues time to dissipate. Each breath is delivered at the pressure at that depth; so, when breathing at a depth of 33 feet for example, there are twice as many O2 molecules per L as at the surface. This is not an advantage for conserving volume, however. The body triggers the breathing response based on the build-up of blood CO2, and so the additional O2 is not helpful for metabolism. As such, at 33 feet depth (2 atm), one uses the available air in the cylinder twice as fast as at the surface; at 66 feet depth (3 atm) air is used three times as fast, etc. (Loske 2013).
The standard recreational SCUBA tank is referred to as an “Aluminum 80,” which refers to the material and cubic volume of air in cubic feet when pressurized to 3,000 psi. Standard SCUBA breathing for an individual is defined as a surface air consumption (SAC) rate. This depends mostly on the proficiency of the diver; new divers may have anxiety or difficulty with buoyancy control and breathe up to 1 cubic foot per minute (cfm), whereas experienced divers that are calmer and more efficient underwater may have SAC rates of 0.5 cfm or lower. SAC rate, depth, and tank volume are used to estimate the time available for an individual to remain underwater safely. A new diver with a SAC rate of 1 cfm and an Aluminum 80 can expect no more than ~30 minutes of diving on a reef at 33 feet depth and still maintain a safety margin of ~500 to 800 psi residual volume upon surfacing. Experienced divers generally dive recreational profiles for an hour or more with an Aluminum 80, depending on depth. It is common for recreational SCUBA divers to use O2 enriched mixtures, commonly referred to as “Nitrox”, to increase the partial pressure of O2 and thus lower the partial pressure of N2 in the breathing gas. Common Nitrox mixes range from 28–36% O2. Use of Nitrox generally allows recreational divers to stay down longer with lower accumulated N2 in their tissues, due to the proportionally lower N2 fraction in the breathing gas.
Beyond recreational SCUBA diving there is a complex realm of technical diving using multiple tanks, gas mixtures, long dives, and depths down to 300 feet or more. The most common groups of tech-divers are shipwreck explorers, cave divers, and divers supporting industrial work such as undersea telecommunications, undersea construction, and mining for fossil fuels. Becoming certified in different aspects of technical diving requires years of training, and unlike recreational diving, carries much higher risk. Because human metabolism dissolves N2 into the bloodstream, increased N2 is dissolved into the blood and body tissues at the elevated pressures associated with diving. If the diver were to immediately return to the surface, this dissolved N2 would rapidly come out of solution in the body’s blood and tissues, causing a painful and potentially dangerous condition commonly known as “the bends” or decompression sickness (DCS). DCS is commonly called “the bends” because emerging gas often first manifests as pain and immobility in the body’s joints, leading the sufferer to “bend” themselves in an attempt to alleviate this joint pain. In order to avoid DCS, divers who spend longer times at depth must “decompress” toward the end of the dive, making periodic stops as they ascend to allow the dissolved gasses to dissipate from their tissues at a manageable rate. Because diving at depth for prolonged periods of time requires proportionally longer decompression times, industrial divers will often live “at pressure” continuously for several months at a time, living, eating, and sleeping in a pressurized vessel matched to the working pressure of their diving environment. This so-called “Saturation Diving” allows divers to work effectively at significant depths of 300 feet or more for months at a time. At the end of this period, saturation divers undergo multiple days of decompression at steadily declining pressures before they can return to the ambient surface environment.
While O2 is crucial to the human metabolic process, too much O2 is toxic to humans, and elevated O2 from pressurized breathing at depth carries significant risk of O2 toxicity. Symptoms of O2 toxicity include sudden seizure, convulsion, and sudden loss of consciousness. For obvious reasons, these symptoms, if experienced while diving underwater, can be extremely dangerous or even immediately fatal. Thus, significant care must be taken by divers to account for the elevated pressure of O2 that they are breathing. Pure O2 can become toxic to a human at depths as shallow as 6 meters/19.6 feet, while a typical atmospheric air mixture of 21% O2 can become toxic below 66 meters/216.5 feet.
Tech-divers customize their gear to particular dive scenarios. For long dives above ~120 feet, the typical configuration may be twin back-mounted Steel 120 tanks providing a total of 240 cfm air. Deep dives from 120 to ~300 feet require multiple tanks and specific gas mixtures depending on depth. Typically, these divers use air as a travel gas from the surface down to ~120 feet and then below that level switch to an oxygen-helium-nitrogen (O2-He-N2) “trimix” that has reduced O2 concentration at 10 to 20%, 50% He, and balance N2. For very deep commercial applications (below 330 feet), divers may use a mixture of 79–90% He and 10–21% O2 “heliox,” which eliminates the N2 component entirely. These mixtures are carefully chosen such that the effective ppO2 at depth does not risk becoming toxic to the user. At 330 feet depth, the body experiences ~11 atmospheres of pressure, so each breath has 11 times as many O2 molecules as the same breath on the surface. With a concentration of 10% O2, heliox provides the equivalent of 1.1 ppO2 atm O2. O2 concentration can be adjusted below 10% to accommodate deeper dives without excess risk of O2 toxicity. The deepest commercial dives replace some of the He with hydrogen (H) and may have as little as 2% O2 (Loske 2013). Upon returning to the surface, it is common to switch back to travel gas (air) at 120 feet, ascend very slowly with decompression stops at 90, 60, and 30 feet, and then switch to an O2 enriched mixture (80%) for the final decompression stop at ~20 feet. It is common for technical divers to carry four or more tanks on a single open-water or wreck dive and to stage tanks along the way in caves to use on the return.
The diving industry has many additional mixtures of gases available or custom mixed. The recreational diver may be familiar with “Nitrox” or “EANx” which are O2-N2 blends with 32/68 or 36/64 ratios. These are designed for long-term shallow diving (above 100 feet depth). The lower level of N2 reduces the risk of developing nitrogen narcosis and decompression illness. Nitrox is a popular choice for vacation SCUBA in warm climates.
SCUBA closed-circuit breathing
During open circuit breathing, exhaled breath is wasted by exhaling directly into the water. Consider that humans breathe out most of the O2 they inhale; at the surface they breathe in 21% and exhale 16% O2 and 5% CO2. At depth, this wastage increases proportionally because the mass amount of O2 metabolized into CO2 stays the same. The advent of closed-circuit breathing technology recovers the exhalation volume, scrubs out the metabolic CO2, adds O2 as needed, and recycles this air back into the breathing circuit. This extends the time underwater by a factor of five or more depending on the configuration. An additional advantage is that there are no expended bubbles, which is important in overhead environments (wrecks and caves) where debris could be dislodged and obscure visibility. Rebreathers are also very quiet, which offers obvious advantages for observing wildlife or in military applications where stealth is important. The one caveat to closed-circuit breathing is the high complexity of the system and numerous potential points of failure, including loss of CO2 removal, flooding or poisoning of the scrubber, O2 sensor error, or battery brownout.
Firefighting SCBA gear
The standard turnout gear for firefighters provides protection against heat, abrasion, and airborne toxicity. Breathing gas is provided by a self-contained breathing apparatus (SCBA), which is similar to SCUBA gear in operation. Unlike SCUBA, however, the tanks tend to be smaller and constructed with reinforced carbon fiber wrapped around a thin-walled aluminum shell to reduce weight. As compensation, the tanks are filled to ~4,500 psi and contain 45 cubic feet of air. Another difference is that the regulator/mask assembly is simplified because it works against constant ambient pressure rather than the wide pressure ranges found underwater. However, in contrast to diving in neutral buoyancy, firefighting activity is very strenuous. The protective and breathing gear weigh more than 60 lbs without the advantage of water buoyancy, and the firefighters drag heavy hoses, climb ladders, wield axes, and sometimes carry victims out of buildings. As such, SCBA gear needs to accommodate much higher demands as firefighters generally work near their aerobic threshold. A standard 45 cfm tank is specified for a maximum work cycle of 30 minutes, which is approximately 3-times the “at rest” air demand. For safety, firefighters typically work for 10–20 minute bouts with 5 minute interim periods for recovery to exchange air cylinders (Kesler et al. 2018).
Firefighter masks seal the whole face and use the open-circuit breathing design described above. Rebreather technology (closed circuit design) that requires a small pure O2 cylinder is considered too risky for live-fire environments.
High-performance aircraft (military jetfighters)
Healthy humans can easily and safely breathe ambient air to ~8,000 feet elevation even with moderate exercise. Above that, the reduced ppO2 becomes noticeable, and depending on acclimatization and aerobic demand, the breathing air needs to be enriched with O2 in accordance with altitude. As discussed above, commercial aircraft are pressurized to the equivalent of 8,000 feet elevation for this reason. External air is compressed using jet engine (turbine) bleed air and a constant internal cabin environment is maintained. Passengers breathe normally, although a subset may experience ear pain on ascent and descent.
Cabin pressure
In contrast to commercial aviation, high performance military aircraft generally have only one or two occupants, and so it is more effective to provide breathing air directly with a mask/regulator system much like it is done for divers and firefighters. The main difference from SCUBA or firefighting activity is that the surroundings are at sub-ambient pressures, and aircraft altitude ranges up to ~50,000 feet where the ambient pressure is 0.11 atm, with a ppO2 of 0.023 atm. Although high performance aircraft do not expose the pilot to the actual ambient pressure, in U.S. fighter jets, the cabin pressure is adjusted based on an altitude schedule. From sea level to 8,000 feet, the cabin pressure is ambient, from 8,000 to 23,000 feet the air pressure is held constant, and above 23,000 feet, the cabin pressure is maintained to be 5 psi higher than the ambient pressure. As such, the cabin pressure schedule provides some amount of safety up to 23,000 feet in that the pilot could breathe cabin air if necessary; however, above this altitude O2 supplementation is absolutely required. In general, military regulations always require pilots to wear their breathing gear, regardless of altitude in the event of cabin pressure loss.
Oxygen schedule
There are basically two mask/regulator/supply systems used in high performance aircraft in U.S. jetfighter aircraft, pure O2 “pressure demand” and mixed gas “diluter demand”; other possible systems are beyond the scope of this article. In short, the system either provides pure O2 regardless of altitude or air enriched with some level of O2 adjusted for cabin pressure. From a mechanical perspective, it is simplest to supply pure O2 all the time, but at lower altitudes it is not necessary and may engender oxidative stress in the pilot. In addition, this method of delivery wastes a fair amount of O2, especially at lower altitudes. A more conservative approach is to adjust the breathing air to provide a ppO2 close to sea level and only increase the relative concentration to accommodate lower cabin pressures as altitude increases. Referred to as “diluter demand”, this method is more technically complex, but does a better job at providing only as much O2 as needed by the pilot, and thus also conserves O2 resources. Figure 5 shows representative pictures of respiratory protection/supplementation for scuba, firefighting, aircraft maintenance and jet pilots drawn from the authors’ field studies.
Figure 5:

Examples of respiratory protection/supplementation for scuba, firefighting, aircraft maintenance and jet pilots drawn from the authors’ field studies.
Breathing system disruptions (BSDs)
One of the most difficult challenges to breathing when using on-demand technology occurs with respect to mechanical delays in valve and regulator response. Termed “breathing system disruptions” (BSDs), these have different (but related) indications. The most common is referred to as “hysteresis” wherein the combination of very brief delays between inhalation, the start of gas flow, and delayed shut-off for exhalation create excess pressure demands on the human. As most of the breathing systems previously discussed use mechanically triggered valves, this is a normal characteristic of on-demand systems, and generally, divers, firefighters and pilots adapt quickly, and mild hysteresis is not noticed. When adapting to such delays results in conscious effort, maintenance is required.
A second BSD indication is “pressure no flow”, a more severe condition wherein the regulator and/or mask valves malfunction to the extent that breathing is interrupted noticeably. This is more likely in SCUBA and high-performance jets systems where the system is referenced to varying external pressures. This has been found particularly important in jet fighters where supply pressure, cabin pressure, and mask pressure all interact. A related phenomenon called “free flow” occurs more often in SCUBA diving wherein pressure balance is lost when the second-stage regulator exhalation valve fails and remains open.
The most subtle of the BSDs occur due to high frequency pressure pulses. A recent study of pilot on-demand breathing showed that adaptive pressure controls can “chatter” or “modulate” and at times have a frequency spectrum similar to that of human breathing. Even modest cycling in mask pressure during inhalation (decrease) or during exhalation (increase) may overwhelm the pressures capable of the human system (NASA PBA Report 2020).
Dissolved inert gases
There are additional physics wrinkles in breathing pressurized air based on the thermodynamics of gases dissolved into the body tissues. These are primarily of concern for SCUBA diving where the ambient pressure varies severely, adding one full atmosphere for every 33 feet of depth, but could also affect high-performance aircraft operation, especially during rapid decompression of the cockpit.
Under higher pressures, inert gases (mostly N2) slowly dissolve into the blood and tissues; because these inert gases are not metabolized like O2, they can only leave the system in a reverse process as pressure is reduced. If the ambient pressure drops too quickly, interstitial bubbles can form that may damage the surrounding tissues, leading to DCS. Mild DCS can cause fatigue and malaise while more severe cases can affect the lungs and central nervous system, requiring treatment at a recompression facility (Newton et al. 2001). In SCUBA diving, DCS is avoided by slow ascents, and intermittent decompression stops based on dive tables designed to minimize bubble formation. However, more than 50% of DCS cases occur after no-decompression dives, and factors such as hypothermia, fatigue, dehydration, alcohol, health conditions, and older age can contribute to the risk of developing DCS (Newton et al. 2001).
Inert gas narcosis is another issue that can affect the human system. As inert gases (typically N2) dissolve into cellular membranes, especially those of the central nervous system, they can disrupt the normal function of neurological signaling analogous to anesthesia or alcohol inebriation, leading to symptoms of light-headedness, euphoria, poor judgement, overconfidence, hallucinations, and possibly loss of consciousness. Narcosis typically happens when diving at deeper depths and during rapid descents. This is a physical effect that quickly resolves upon relief of positive pressure (Rocco et al. 2019).
As discussed in the SCUBA section above, He is sometimes used as an inert balance gas to offset nitrogen narcosis and bubble formation. Although less likely to cause DCS or narcosis, He has six times the thermal conductivity of N2 and so has a noticeable systemic cooling effect, removing body heat during exhalation.
POSITIVE PRESSURE VENTILATOR BREATHING
The most invasive level of breathing support technology is used when the pulmonary system cannot sustain adequate ventilation. Medical ventilators literally breathe for the patient. They can be used invasively or non-invasively. Non-invasive ventilation (NIV) is relatively simple as ventilation is provided through a mask that is sealed to the nose and mouth externally. When NIV only provides one continuous pressure setting to a spontaneously breathing patient, this is called continuous positive airway pressure (CPAP); when there is one pressure for inhalation (IPAP) and a lower pressure for exhalation (EPAP) a respiratory rate is provide; this is called bi-level positive airway pressure (BiPAP), since there is a different pressure during inhalation and exhalation. NIV is indicated to help patients who can protect their own airway but need assistance oxygenating and/or ventilating. However, hospital ventilators as used in intensive care units (ICUs) commonly require the patient to be intubated wherein a breathing tube is inserted through the mouth or neck and into the trachea. During this invasive ventilation, the tubing is sealed off and the system periodically inflates the lungs. Breathing frequency and parameters to regulate tidal volume are set specifically for each patient.
Indications for controlled invasive mechanical ventilation
The criteria used to decide to place a spontaneously breathing patient on mechanical ventilation varies, particularly for patients with chronic respiratory diseases, but is typically based on the following five criteria related to breathing stress:
Inability to protect one’s airway
Respiration rate is increasing beyond 30 breaths/minute
O2 saturation rate is no longer maintained above 90% despite supplementation above 60% O2
Blood is trending towards acidic, dropping below pH 7.20
ppCO2 exceeds 50 mmHg
Certainly, there are more immediate conditions that require ventilation, including trauma from chest injuries, collapsed lung, heart failure, head injury/coma, and severe pulmonary infection. Ventilators are also used during surgery to maintain breathing under anesthesia and in postoperative recovery.
During the early stages of the COVID-19 pandemic, ventilators were the primary mechanism to preserve life and maintain oxygenation and ventilation in the setting of acute respiratory distress syndrome while the patient’s immune system geared up to fight the infection. Since then, antiviral treatments and other early interventions such as nasal cannulas and early prone positioning have reduced the need for invasive ventilation. Figure 6 shows two examples of patients on hospital ventilator equipment; the left panel shows a BiPAP system that provides positive pressure when needed and the right panel shows a patient in an ICU.
Figure 6:
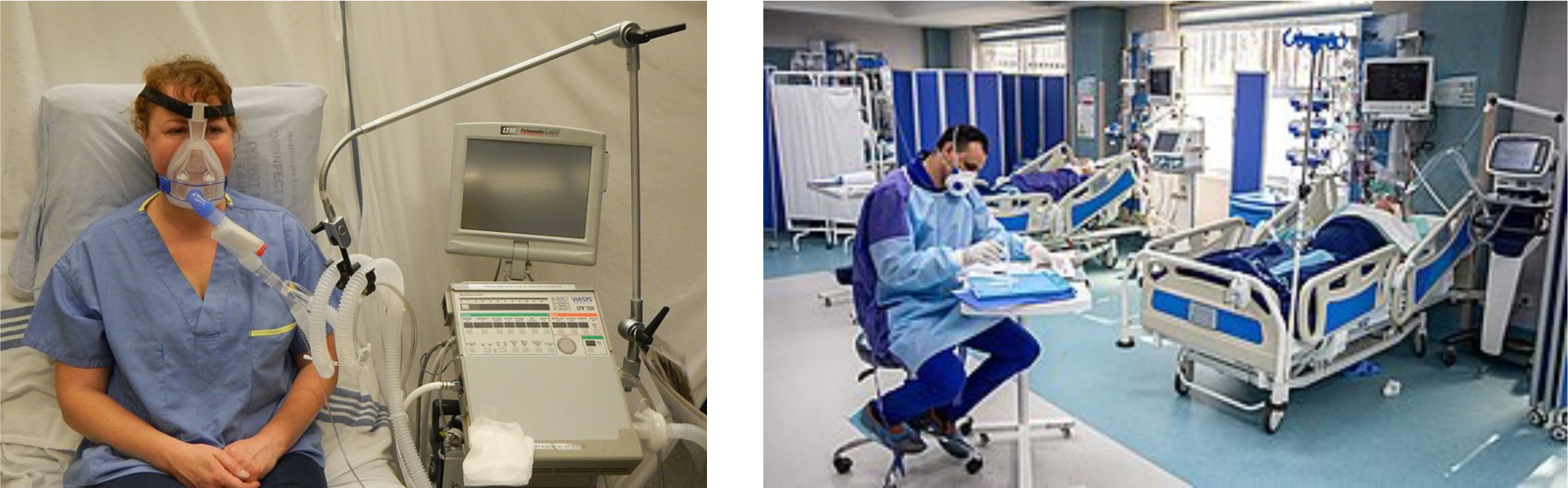
Examples of hospital ventilators that either supplement breathing (left panel), or fully control breathing (right panel) (from https://commons.wikimedia.org/).
Methods for controlled invasive mechanical ventilation
Modern mechanical ventilators are programmable and feedback-regulated to achieve appropriate life support. They have various functions, including volumetric cycling, pressure cycling, flow cycling, and combinations of all three, and are controllable for O2 concentration, frequency of breathing, and inspiratory flow rate. Traditional adult positive pressure ventilators are typically set for 10–30 breaths/minute, but neonatal ventilators are often used with high frequency (300–1200 breaths/min) coupled with low volumes referred to as high-frequency oscillatory ventilation (HFOV) to accommodate complex respiratory diseases of premature infants with small tidal volumes.
Typically, controlled ventilation starts with 60% O2, which may be reduced over time as the patient stabilizes to avoid potential complications from oxidative stress. Modern ventilators can accommodate spontaneous breathing response as the patient improves and ultimately return to an “on-demand” hybrid mode.
Ventilator parameter settings
Adjustable ventilator settings differ with mode and patient demand but generally include the following adjustable parameters and their normal settings (in parentheses):
Respiratory rate (12–20 breaths/min)
Tidal volume (500 mL, or 4–8 mL/kg body mass, depending upon disease pathophysiology)
Trigger sensitivity (adjustable for spontaneous breathing response)
Flow rate (adjustable from 60 to 100 L/min)
Pressure profile - waveform
Inspiratory/expiratory (I/E) time ratio (1/2)
O2 concentration (21–100%)
Positive End Expiratory Pressure (PEEP, 5–10 cmH2O)
These parameters are set based on the patient’s health state, physical size, age, and ventilation needs to approximate “normal breathing” as discussed previously. Based on health state, there are accommodations for restrictions in the airway (lower flow rates), there may be lower tidal volumes with respectively higher frequency used to adapt for trauma or disease, and there may be a decrease in the I/E ratio from 1/2 to 1/4 to allow for longer exhalation in response to compromised pulmonary flexibility. The waveform parameter is a relatively new concept and may be adjusted to adapt to the patient’s particular physiology.
SUMMARY
Human breathing is a complex function that occurs automatically to provide O2 needed by cells and tissues. Although humans have evolved to breathe 1-atmosphere with 21% O2, the human systems biology is adaptable to the immediate environment (altitude, pressure, and CO2). Humans can quickly adapt to small changes in external conditions or O2 demand unconsciously and can also consciously modify breathing for singing, swimming, breath holding, and coughing. However, if something goes awry, lack of pulmonary ventilation becomes life threating in a few minutes.
Technology has been developed to protect humans from inhaling contaminants in the air, to provide adequate air flow and concentrations for flight, travel, and sport activities, and for medical supplementation of O2 within the construct of normal breathing for trauma or disease intervention. Although much of contemporary breathing research involves the chemistry and metabolism of breathing, the physics of breathing described herein is also an important factor in the preservation of human life.
ACKNOWLEDGEMENTS
The authors thank their colleagues in the breath research community, especially those from the International Association of Breath Research (IABR) and the Scientific Board of the Journal of Breath Research (JBR) for inspiration and expertise throughout this project. We especially thank James Dimond and Lauren Carter from JBR for their encouragement and support, John Graf, Marta Shelton, David Alexander and Clinton Cragg from NASA for their expertise in hyperbaric life support, and Kenneth Fent from NIOSH and Gavin Horn from the University of Illinois for insights in firefighter respiration.
REFERENCES
- Andrews P Evolution and environment in the Hominoidea. Nature. 1992;360(6405):641–6. [DOI] [PubMed] [Google Scholar]
- Angelova RA, Markov DG, Simova I, Velichkova R, Stankov P. Accumulation of metabolic carbon dioxide (CO2) in a vehicle cabin. InIOP Conference Series: Materials Science and Engineering. IOP Publishing. 2019;664(1):012010. [Google Scholar]
- Balanay JA, Lungu CT. Determination of pressure drop across activated carbon fiber respirator cartridges. Journal of Occupational and Environmental Hygiene. 2016;13(2):141–7. [DOI] [PubMed] [Google Scholar]
- Berner RA. Atmospheric oxygen over Phanerozoic time. Proceedings of the National Academy of Sciences. 1999;96(20):10955–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenberry SE, Mancuso M, Iboi E, Phan T, Eikenberry K, Kuang Y, Kostelich E, Gumel AB. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infectious Disease Modelling 2020;5:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Exposure Factors Handbook, Edition (Final Report). Chapter 6 “Inhalation Rates” U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F. 2011. [Google Scholar]
- Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respiratory Care. 2009;54(10):1348–1359. [PubMed] [Google Scholar]
- FAA. Federal Regulation 14 CFR 25.831. Federal Aviation Administration, Department of Transportation, Title 14 - Aeronautics and Space. 2002. https://www.faa.gov/documentLibrary/media/Advisory_Circular/AC_25-20.pdf
- Goldfarb JD, Hodgson EW. Advanced, Lightweight, Space Suit Primary Life Support System for Mars Exploration. SAE Technical Paper. 2001. https://ttu-ir.tdl.org/bitstream/handle/2346/84710/ICES-2019-26.pdf?sequence=1
- Guinnessworldrecords.com. https://www.guinnessworldrecords.com/world-records/longest-timebreath-held-voluntarily-(male), accessed 5/03/21.
- Hardt DJ, James RA, Gut CP Jr, McInturf SM, Sweeney LM, Erickson RP, Gargas ML. Evaluation of submarine atmospheres: effects of carbon monoxide, carbon dioxide and oxygen on general toxicology, neurobehavioral performance, reproduction and development in rats. I. Subacute exposures. Inhalation toxicology 2015;27(2):83–99. [DOI] [PubMed] [Google Scholar]
- Helviz Y, Einav S. A systematic review of the high-flow nasal cannula for adult patients. Annual Update in Intensive Care and Emergency Medicine 2018. 2018:177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiland RL, Bain AR, Rieger MG, Bailey DM, Ainslie PN. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2016;310(5):R398–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasting JF, Siefert JL. Life and the evolution of Earth's atmosphere. Science. 2002;296(5570):1066–8. [DOI] [PubMed] [Google Scholar]
- Kesler RM, Deetjen GS, Bradley FF, Angelini MJ, Petrucci MN, Rosengren KS, Horn GP, Hsiao-Wecksler ET. Impact of SCBA size and firefighting work cycle on firefighter functional balance. Applied ergonomics 2018;69:112–119. [DOI] [PubMed] [Google Scholar]
- Kliff S, Satariano A, Silver-Greenberg J, Kulish N. There Aren’t Enough Ventilators to Cope with the Coronavirus The New York Times. 2020;18. [Google Scholar]
- Lausted CG, Johnson AT, Scott WH, Johnson MM, Coyne KM, Coursey DC. Maximum static inspiratory and expiratory pressures with different lung volumes. Biomedical Engineering OnLine. 2006;5(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J, Van Baalen M, Foy M, Mason SS, Mendez C, Wear ML, Meyers VE, Alexander D. Relationship between carbon dioxide levels and reported headaches on the international space station. Journal of occupational and environmental medicine. 2014;56(5):477–83. [DOI] [PubMed] [Google Scholar]
- Liem KF. Form and function of lungs: the evolution of air breathing mechanisms. American Zoologist. 1988;28(2):739–59. [Google Scholar]
- Loske A. Fundamentals of SCUBA-diving physics. International Jounral of Sports Science. 2013;3(2):37–45. [Google Scholar]
- National Research Council. Emergency and continuous exposure guidance levels for selected submarine contaminants: Volume 1, Chapter 3, Carbon Dioxide. National Academies Press. 2007;1(3). [PubMed] [Google Scholar]
- NASA. Spacecraft Maximum Allowable Concentrations for Airborne Contaminants. NASA, Human Health and Performance Directorate. JSC 20584. Revision A. 2020.
- NASA PBA Report. Pilot Breathing Assessment, NESC Document #: NESC-RP-18–01320. 2020. https://www.nasa.gov/sites/default/files/atoms/files/nesc-rp-18-01320_v.1.2_nesc_pba_vol._1_11-19-20_nrb_rp_final-.pdf
- Newton HB. Neurological complications of scuba diving. American family physician. 2001;63(11):2211. [PubMed] [Google Scholar]
- Peacock AJ. Oxygen at high altitude. British Medical Journal. 1998;317(7165):1063–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD, Beauchamp JD, Risby TH, Dweik RA. The scientific rationale for the use of simple masks or improvised facial coverings to trap exhaled aerosols and possibly reduce the breathborne spread of COVID-19. Journal of breath research. 2020a;14(3):030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD, Beauchamp JD, Dweik RA, Risby TH. A special issue: Flow, pressure, volume and time as dependent variables in breath analysis. Journal of breath research. 2020b;15(1):010201. [DOI] [PubMed] [Google Scholar]
- Pleil JD and Christensen LE, 2021. Rationale for developing tunable laser spectroscopy (TLS) technology for high resolution real-time carbon dioxide monitoring (capnography) in human breath, Journal of Breath research (in press) [DOI] [PubMed] [Google Scholar]
- Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. New England Journal of Medicine. 2020;382(18):e41. [DOI] [PubMed] [Google Scholar]
- Richardson D A brief history of scuba diving in the United States. SPUMS Journal. 1999;29:173–176. [Google Scholar]
- Rocco M, Pelaia P, Di Benedetto P, Conte G, Maggi L, Fiorelli S, Mercieri M, Balestra C, De Blasi RA, ROAD Project Investigators.. Inert gas narcosis in scuba diving, different gases different reactions. European journal of applied physiology. 2019:119(1):247–255. [DOI] [PubMed] [Google Scholar]
- Satish U, Mendell MJ, Shekhar K, Hotchi T, Sullivan D, Streufert S, Fisk WJ. Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environmental health perspectives. 2012;120(12):1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriram S, Ramamurthy K, Ramakrishnan S. Effect of occupant-induced indoor CO2 concentration and bioeffluents on human physiology using a spirometric test. Building and Environment. 2019;149:58–67. [Google Scholar]
- Siermontowski P, Zieliński E, Olszańnski R. Hyperbary-the common roots of treatment of people and exploration of sea depths. Archives of Physiotherapy & Global Researches 2016:20(3):13–17. [Google Scholar]
- Skaria SD, Smaldone GC. Respiratory source control using surgical masks with nanofiber media. Annals of occupational hygiene 2014;58(6):771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern TC, Moiseev NA. Commercial EVA Space Suit System Development. International Conferenceon Environmental Systems, ICES 2019;26:1–18. [Google Scholar]
- Stiegel MA and Pleil JD, Sobus JR, Stevens T, Madden MC. Linking physiological parameters to perturbations in the human exposome: environmental exposures modify blood pressure and lung function via inflammatory cytokine pathway. Journal of Toxicology and Environmental Health A. 2017;80(9):485–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C The origin and evolution of Homo sapiens. Philosophical Transactions of the Royal Society B: Biological Sciences 2016;371(1698):20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JJ. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respiratory Care. 2013;58(1):98–122. [DOI] [PubMed] [Google Scholar]
- West JB. Human limits for hypoxia: the physiological challenge of climbing Mt. Everest. Annals of the New York Academy of Sciences. 2000;899(1):15–27. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wargocki P, Lian Z. Physiological responses during exposure to carbon dioxide and bioeffluents at levels typically occurring indoors. Indoor Air. 2017;27(1):65–77. [DOI] [PubMed] [Google Scholar]


