Summary
Malnutrition is common in AH, and almost all patients with severe AH have some component of malnutrition. The classic phenotype of malnutrition in AH is sarcopenia, but this has become more difficult to discern clinically as patients have become more obese. Patients with AH are often drinking 10–15 standard drinks/day. This substantial alcohol consumption becomes a major source of calories, but these should be considered “empty” calories that contain little nutritional value. Malnutrition is associated with liver complications such as hepatic encephalopathy and worse liver outcomes. Nutrition support, including interventions such as late-night snacks, can improve nutrition status and reduce complications.
Keywords: Nutrition, Malnutrition, Alcohol-associated hepatitis (AH), Micronutrients, Standard drink
Bulleted Key Points: Malnutrition is common in AH, Malnutrition increases with severity of AH and is associated with worse outcomes, Nighttime snacks help prevent muscle loss
Introduction
The role of alcohol in liver injury is complex and influenced by multiple factors including dose of alcohol consumed, duration and pattern of drinking (e.g., binge drinking), and, as reviewed in this chapter, potential interactions with nutrition. From a nutrition perspective, alcohol is an important source of calories, but these should be considered “empty” calories – that is, they contain little or no macronutrients such as protein or fat, and basically no micronutrients such as vitamins and minerals1. Regular alcohol intake can be a major source of unwanted calories; for example, beer has approximately 150 kcal per 12-ounce can and a mixed drink has approximately 125 kcal per drink. The 2020–2025 Dietary Guidelines for Americans highlighted the concept of the standard drink (14 grams alcohol) and the fact that if alcohol is consumed, it should be in moderation (i.e., up to 1 drink per day for women and 2 drinks per day for men).1 If subjects have underlying liver disease, no alcohol intake is safe or acceptable. Moreover, alcohol intake may have more adverse outcomes if subjects are overweight or obese. We know that many patients with alcohol-associated hepatitis (AH) are drinking approximately 15 drinks/day, which amounts to about 2,000 empty calories/day1 (Figure 1). Patients with AH often have inadequate protein intake and may have nutritionally imbalanced fat intake, with excess omega-6 and low omega-3 intake.1 Diets are also often deficient in micronutrients such as zinc.1 Thus, alcohol and altered nutrition intersect at many levels to cause AH and its complications. This chapter will review: (1) definition of malnutrition and diagnostic tests; (2) prevalence; (3) gut-liver axis and AH; (4) inpatient management including impact on prognosis; (5) outpatient management; and (6) selected vitamins and trace metals in AH.
Figure 1.
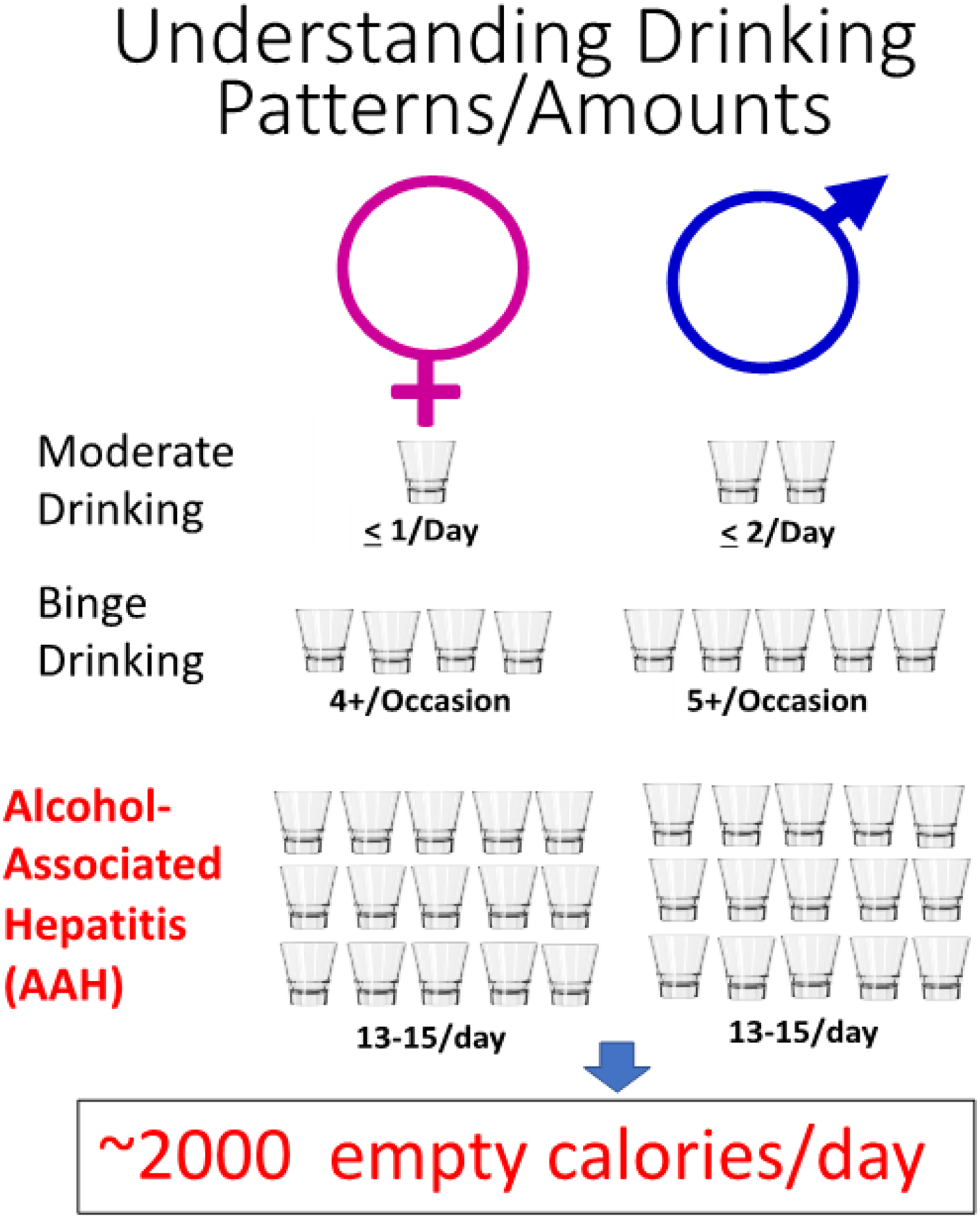
Drinking levels and their consequences. In the United States, drinking levels are expressed in terms of standard drinks consumed—that is, the number of alcoholic beverages drunk, each containing about 0.6 fluid ounce or 14 grams of pure alcohol. The Dietary Guidelines for Americans 2015–2020 defined moderate drinking as consuming up to 2 drinks/day for men and up to 1 drink/day for women. The Substance Abuse and Mental Health Services Administration defines binge drinking as consuming 5 or more (for men) or 4 or more (for women) alcoholic drinks on the same occasion on at least 1 day in the past 30 days. Patients hospitalized for severe AH in the NIAAA-funded DASH study were drinking approximately 13–15 drinks/day. (Data from http://www.rethinkingdrinking.niaaa.nih.gov)
DISCUSSION:
1. Definition of Malnutrition and Diagnostic Tests
Malnutrition is classically defined as a disorder of inadequate nutrition intake or uptake that leads to a decrease in body cell mass. Sarcopenia has generally been the clinical phenotype of malnutrition in AH. However, as obesity has increased in the United States, sarcopenia is often more difficult to discern clinically. Indeed, unpublished data from the NIAAA-sponsored DASH consortium showed that the average BMI of patients with severe AH was about 30. Patients may also be malnourished due to isolated micronutrient deficiency, such as zinc deficiency. The most extensive studies of malnutrition in liver disease are in alcohol associated liver disease (ALD), mainly in patients with AH. Patients with severe AH and cirrhosis have the most severe nutritional deficiencies, indicating that advanced disease is associated with more severe malnutrition.
There are several diagnostic tests that can be used to diagnose malnutrition in AH, although many of them have accuracy limitations in liver disease (Box 1). Anthropometry, which includes body mass index (BMI), change in body weight, and triceps skin fold (TSF), is widely used to evaluate nutritional status. However, if patients have fluid retention with edema or ascites, this can overestimate BMI or underestimate weight loss. Biological parameters, such as visceral proteins, are commonly used to indicate prognosis and nutritional status. These visceral proteins most commonly include albumin, pre-albumin, and retinol-binding protein. All of these proteins are produced in the liver, which makes them inaccurate markers of malnutrition, and they correlate better with the severity of underlying liver disease than with malnutrition.
Box 1. Selected Methods for assessing malnutrition in AH.
Selected Methods for assessing malnutrition in AH
Anthropometry (BMI, weight change, triceps skinfold)
Biological parameters (visceral proteins)
Dual-energy x-ray absorptiometry, CT
24-h urinary creatinine height index
Assessment of muscle strength
Subjective global assessment
Bioelectrical impedance
Energy balance
Metabolomics
Various imaging modalities are being used to assess muscle mass and body composition. A single cross-sectional computed tomography (CT) slice has been validated as an accurate method to measure whole-body skeletal muscle and fat mass. Psoas thickness on CT has been used to predict muscle mass, and has been shown to be predictive of mortality in cirrhotic patients.2 Dual-energy X-ray absorption (DEXA) testing can assess whole-body and regional distribution of lean tissue and fat. DEXA is more often used in the outpatient setting.
24-hour creatinine height index is another method of assessing muscle mass. It is the ratio of measured 24-hour urine creatinine excretion in a patient compared to expected excretion in a normal individual of the same sex and height. A study by Pirlich and coworkers showed a strong correlation between muscle cell mass as assessed by creatinine and body cell mass as assessed by total body potassium count.3 Liver dysfunction did not alter urinary creatinine, but renal dysfunction did. Measurement of handgrip strength using a handgrip dynamometer is a quick and easy method to quantify muscle strength. It has been correlated with other markers of malnutrition in liver disease and is an indicator of functional status.
Subjective global assessment (SGA) is a regularly used, simple method, which includes patient history regarding weight loss, usual dietary intake, gastrointestinal symptoms, and evidence of malnutrition of physical exam, such as muscle wasting or presence of edema.4 Bioelectrical impedance (BIA) is increasingly used for body composition analysis. The theory behind it is that each body tissue has a specific electrical conductivity which is directly related to the water and electrolyte content of that tissue. Pirlich and coworkers showed a strong correlation between BIA and the gold standard of total body potassium for assessing malnutrition. Advances have made this method more accurate, even in patient with ascites, which was previously a limiting factor.3 This technology is simple to use and provides not only information on muscle mass, but also extracellular water.
The assessment of energy intake/balance and nitrogen balance can be useful in determining if patients are meeting energy and protein targets. These come in the form of calorie counts in the inpatient setting, and food diaries in the outpatient setting. Air-displacement plethysmography calculates the density of the body using mass and body volume to assess body composition. Lastly, metabolomics is a quantitative analysis of all metabolites, which can be endogenous or from multiple outside sources, including food and the environment. Metabolomic assays provide an opportunity not only for identifying patients with malnutrition, but also identifying metabolic pathways that lead to malnutrition.
2. Prevalence
Studies have examined the prevalence of malnutrition in ALD, and although there were multiple methods of assessment, all consistently found high prevalence of malnutrition in patients along the whole spectrum of ALD. Prevalence in AH reaches 100%, which was shown by two large studies from the Veterans Administration. The first study showed that over 280 patients, every patient had some evidence for malnutrition. Patients were divided in groups with mild, moderate, or severe alcohol-associated hepatitis based on clinical and biochemical markers. The mean alcohol consumption among these patients was 228g/day, meaning approximately 50% of energy intake was from alcohol. The severity of malnutrition correlated closely with complications of liver disease, including encephalopathy, ascites, hepatorenal syndrome, and overall mortality.5,6 Similar data were observed in a follow-up VA study.5–9 Over time, these result trends have remained consistent. Singal and colleagues found that among 261 patients with alcohol-associated cirrhosis undergoing transplant, 84% had malnutrition when assessed with the SGA.10
3. Gut-Liver Axis
Bacteria, fungi and other microorganisms make up the human gut microbiome, with the bacterial microbiome being the best studied. Intestinal bacterial dysbiosis is defined as an imbalance among the different microbial entities in the intestine with a disruption of symbiosis.11 There are three major types of dysbiosis including pathobiont expansion, reduced diversity, and loss of beneficial microbes, and they are not mutually exclusive. Early animal studies documented that the gut flora/bacterial dysbiosis and gut-derived toxins play a critical role in the development of both liver disease and its complications. Indeed, over a half a century ago, it was shown that germ-free rodents or rodents treated with antibiotics to “sterilize the gut” were resistant to nutritional and toxin-induced liver injury. Elegant studies by Broitman and co-workers showed that rats fed a choline deficient diet developed cirrhosis which could be prevented by oral neomycin.12 However, when endotoxin was added to the water supply, neomycin could no longer prevent the development of liver injury and fibrosis.12 Most recent studies use some variation of the Lieber DeCarli liquid diet alcohol feeding mouse model. Using this alcohol feeding paradigm, we demonstrated a decline in the abundance of both Bacteroidetes and Firmicutes phyla, with a proportional increase in the Gram-negative Proteobacteria and Gram-positive Actinobacteria phyla.13 Bacterial genera analysis showed the greatest expansion in Gram-negative alkaline tolerant Alcaligenes and Gram-positive Corynebacterium. These alterations were accompanied by an increase in colonic pH and liver steatosis and injury.13 Treatment with the probiotic, Lactobacillus rhamnosus GG, attenuated dysbiosis, gut barrier dysfunction, and liver injury.13–15 We also analyzed polar metabolites in the feces from mice fed with or without alcohol. Taurine, nicotinic acid, and several major short chain fatty acids (SCFAs), were significantly decreased in mice fed alcohol.16 Thus, fecal metabolites are also a likely target for AH treatment. Indeed, studies from Schabl’s laboratory demonstrated the importance of cytolysin, an exotoxin secreted by Enterococcus faecalis, in the pathogenesis of hepatocyte death in AH, and the potential for bacteriophage therapy.17
Alcohol abuse was reported to cause small intestinal bacterial overgrowth in humans over three decades ago using culture techniques.18 Development of sequencing techniques greatly advanced the gut bacterial studies. In a study of 244 patients with alcohol-associated cirrhosis, investigators found that intestinal dysbiosis was more severe in patients with decompensated cirrhosis compared to compensated cirrhosis.19 Studies from the NIAAA-funded TREAT consortium documented that there are distinct changes in the fecal microbiome associated with the development, but not severity of AH. There also were clear changes in fecal metabolites, such as decreased SCFAs and altered tryptophan metabolites, similar to findings described above in alcohol fed mice.20
Restoration of gut eubiosis is the major aim of gut-microbiota-based therapies in AH. Several approaches including diet modulation, probiotics/prebiotics/antibiotics intervention, fecal bacteria transplantation, bacteriophage therapy, and engineered bacteria therapy have proven to be effective in alleviating experimental alcohol-induced liver injury through positively modifying gut microbiota, and pilot studies are ongoing in human AH.11,13,17,21–24
4. Inpatient management
Patients with AH are frequently malnourished at baseline and are at risk of worsening malnutrition during hospitalization, especially when in intensive care unit. Thus, hospitalized patients should be evaluated for nutritional status on a regular basis. Nutritional therapy in the inpatient with alcohol-associated liver disease is challenging. Most of these patients are admitted due to acute hepatic decompensation (hepatic encephalopathy, ascites, etc.) or due to severe AH. The mechanisms for malnutrition are multiple and listed in Box 2. The barriers to nutritional therapy include pre-existing nutritional deficiencies, high risk of refeeding syndrome, poor appetite, altered taste, nausea, early satiety, development of alcohol withdrawal syndrome, lack of understanding of the severity of the illness, and desire to keep some control during hospitalization, at least over one’s own diet.
Box 2. Major Causes of Malnutrition.
Major Causes of Malnutrition
Anorexia/altered taste/smell
Nausea/vomiting/delayed gastric emptying
Diarrhea/malabsorption/bacterial overgrowth
Poor food availability/quality/unpalatable diets (Na, protein)
Hormones/cytokine effects
Complications of liver disease (hepatic encephalopathy, ascites)
Fasting for procedures/interruption in feeding
It is important to gain early patient cooperation concerning the need for nutrition as a critical component of their treatment plan (Box 3). Is useful to educate the most severely ill patients about their mortality risk, and in severe AH this can be achieved by calculating their prognosis using the “Mayo Mortality Risk from Alcoholic Hepatitis Calculator” https://www.mayoclinic.org/medical-professionals/transplant-medicine/calculators/meld-score-and-90-day-mortality-rate-for-alcoholic-hepatitis/itt-20434719, and sharing the information with them to increase their willingness to accept a feeding tube if they do not achieve their nutrition goals. The refeeding syndrome risk is also a potential problem because it forces an initial conservative approach in ramping-up caloric and protein intake.
Box 3. Nutrition support goals for hospitalized patients with AH.
| Nutrition support goals for hospitalized patients with AH |
|---|
|
It is imperative to rapidly diagnose metabolic disturbances including electrolyte disorders, and to monitor food consumption and utilize oral nutritional supplements, including a nighttime snack, in patients to ensure optimal protein/energy requirements by the oral route. The use of a probiotic yogurt twice a day can help in maintaining stable intestinal flora and decreasing the risk of overt hepatic encephalopathy.25 A 24-hour calorie count or estimate of caloric intake helps to rapidly identify the need for a feeding tube for enteral nutrition. In patients with inadequate oral intake, early enteral nutrition (EN) support is especially important because it has the potential to reduce complications and length of stay, and to positively impact patient outcomes. Two randomized clinical trials of enteral nutrition therapy provide important insight into in hospital nutrition support in AH. The first was a multicenter randomized study from Spain of enteral nutrition versus corticosteroids in patients with AH. These patients had their enteral feeding supplements delivered by feeding tube. Results indicated a similar overall short-term mortality (one month survival - primary endpoint).26 Importantly, those receiving enteral nutrition (rich in BCAAs) had a better long-term outcome. In the most recent multi-center trial, patients with biopsy-documented severe AH were treated with either intensive EN plus methylprednisolone or conventional nutrition plus methylprednisolone.27 In the intensive EN group, EN was given via feeding tube for 14 days. While the authors concluded in the title that intensive EN was ineffective, the six-month mortality (primary endpoint) was numerically lower in the EN group (44.4%) compared with the control group (52.1%). Importantly, patients from either group receiving <21.5 kcal/kg/day had significantly lower survival rates, as did those receiving <77.6 gm of protein per day. There were frequent tube feeding adverse events, highlighting the importance of experience with this technology and use of oral supplements if possible.27 Importantly, EN is favored over parenteral nutrition (PN) because of risk of infections, cost, and maintenance of the gut barrier function. If PN is used, we recommend return to the enteral route as soon as the small bowel shows evidence of recovered function. It is important to remember that in patients taking lactulose, the radiologist will often diagnose “ileus” due to air-fluid levels seen in the bowel. If the patient has bowel movements they do not have a true ileus, and enteral feeding should be given.
EN support should be initiated within 24–48 hours following hospitalization in patients unable to maintain oral nutritional intake, with the aim to provide >80% of estimated or calculated goal energy and protein within 48–72 hrs. Whole protein formulae are generally recommended. More concentrated formulae are preferable in patients with ascites to avoid positive fluid balance. BCCA-enriched formulae could be considered in patients with encephalopathy arising during EN and without any other explanation,28 but this will be rare.
When using EN by feeding tube, it is useful to specify both the total volume of formula to be given in a day and the rate to be delivered. Is a good practice to calculate the feeding rate as a 20-hour course, allowing 4 hours for feeding interruption due to the multiple diagnostic and therapeutic interventions that the patient may need. The tube should be flushed forcefully with 30 mL of water each time the feeding is interrupted, to prevent “tube clogging”.
Protein intake is usually recommended at 1.2–1.5 g/Kg body weight per day (g/kgBW/d). A caloric target of 35–40Kcal/Kg body weight per day (cal/kgBW/d) is recommended in hospitalized nonobese patients.28 Interrupting feeding for diagnostic tests or nurse procedures should be minimized. Obese patients with AH represent a nutrition challenge. Current guidelines recommend hypocaloric, high protein nutrition therapy in an attempt to preserve lean body mass, to mobilize fat stores, and to minimize overfeeding complications in these at-risk obese patients with liver disease.29 Energy targets are low in patients with BMI of 30–50, usually 65–70% of requirements as measured by indirect calorimetry or approximately 11–14k cal/kg BW/d. However, protein requirements are high, usually projected at 2.0–2.5 g/kg of ideal body weight per day.29
It is important to assess for electrolyte disturbances on admission, not only for increased risk of refeeding syndrome related to chronic alcoholism, but also because patients with AH usually have abnormalities with electrolytes. Guidelines recommend assessing the risk of refeeding syndrome on admission (Box 4) and screening should be repeated weekly (National Institute for Health and Clinical Excellence -NICE 2006).30 The correction of electrolytes abnormalities must occur before starting of feeding (table mineral and electrolyte replacement). For example, in a 70 Kg person with normal renal function, the amount of oral minerals he/she will be receiving at time of starting feeding (unless pre-feeding levels were high) will be:
Mg Oxide 400 mg: 1 tablets 3-times a day (will give 60 mEq a day)
K-Phos Original 500 mg: 2 tab 4-times a day (will give 60 mEq of phosphate + 30 mEq of K)
KCl 750 mg: 3 tablets TID or QID (will give 90 or 120 mEq of K as KCl plus 30 mEq from the K-Phos Original = 120 or 150 mEq per day, respectively)
Box 4. Risk for refeeding syndrome.
| Risk of re-feeding syndrome |
|---|
Extremely High risk
|
High risk (NICE criteria for refeeding syndrome)
|
In patients with high risk for refeeding syndrome, EN should be started slowly (percentage of estimated target energy and protein needs depends of severity of risk) and it should be built up to meet full needs over some period of time (2–7 days). Is also important restoring circulatory volume and monitoring fluid balance closely (Box 4, NICE 2006).30
5. Outpatient management
The cornerstones of treatment of malnutrition in patients with AH in the outpatient setting are nutritional intervention and alcohol cessation. Outpatient management of malnutrition, specifically in AH, is not well studied but can be extrapolated from data on malnutrition in patients with alcohol-associated cirrhosis. Studies show improved outcomes with nutritional support. In Hirsch et al.,31 outpatients who supplemented their diet with an enteral product containing 1000 kcal (4.2 MJ/d) and 34 grams protein, had significantly improved protein intake and fewer hospitalizations, as well as improved immunity.32 The early VA studies indicate that 85 grams of protein per day or more is required to maintain nitrogen balance in AH, but both inpatients and outpatients were eating 20 gm/day less than this.7 A study in Japan evaluated over 200 patients with cirrhosis, covering multiple etiologies including alcohol, who received either standard-of-care or nutritional counseling from a dietician following a nutritional assessment. After five years, the patients in the nutritional counselling arm had improved survival. ESPEN Nutritional Guidelines recommend an calorie intake of 35–40 kcal/kgBW/d and a protein intake of 1.2–1.5 g/kgBW/d.28 If patients are overweight, caloric goals should be reduced.
An important aspect in the dietary management of AH, as well as other forms of ALD, is to avoid long periods of time without food intake. During these periods, patients with advanced liver disease can enter a “starvation mode” with decreased glucose oxidation and increased protein and fat catabolism. A study by Owen and coworkers outlined the reasoning behind an important intervention. Patients with cirrhosis (it can be inferred that patients with AH will react similarly) develop a “starvation” metabolic state overnight, whereas a healthy person takes 2–3 days of fasting to develop “starvation”.33 A late evening snack can block the overnight catabolic state of patients with AH, as well as other forms of liver disease, and improve body protein stores/muscle mass. Optimally, these patients should have three meals, three snacks, and that bedtime snack. Breakfast early in the morning can improve cognitive function in subclinical hepatic encephalopathy.34 Plank et al. documented improvement in muscle mass with nighttime supplements by randomizing 103 cirrhotic patients to receive two cans of Ensure Plus (710 kcal with 26 g protein) or two cans of Diabetic Resource (500 kcal with 30 g protein), either during the day or at bedtime for one year. The addition of the nighttime snack was associated with a gain of muscle mass and improvement in quality of life over that year, which was not shown with the daytime snack.35 This highlights the importance of supplementation with a high-nutrient bedtime snack in patients with AH.
A diet high in omega-6 unsaturated fat is a risk factor for the development and progression of experimental ALD and is correlated with severity. Alternatively, omega-3 unsaturated fat enrichment and dietary DHA and EPA (specific type of omega-3 fat) supplementation have been shown to alleviate alcohol induced liver injury.36,37 This suggests that eating foods with omega-3s may be beneficial in patients with AH, but human studies are needed.38 Data have increasingly suggested beneficial effects of prebiotics (non-digestible food substances to promote growth of beneficial bacteria) and probiotics (live microorganisms that are favorable to the host) in ALD. As discussed earlier, gut microbiota plays an etiologic role in AH, suggesting that pre-and probiotics may be effective therapy.39
In patients with AH, meeting caloric needs is frequently difficult with ongoing alcohol intake. Abstinent patients have a higher caloric intake compared with those with ongoing alcohol use. Abstinence may increase the beneficial effects of nutritional support on cell mediated immunity.32 Developing more effective treatment options for alcohol cessation would have important effects on malnutrition in AH.
6. Vitamins and Trace Metals
Vitamin A
In ALD, vitamin A storage and release are impaired. The liver is the major storage site for vitamin A (retinol) most found in stellate cells. Venu et al. found the majority of patients evaluated for liver transplantation in a single center had vitamin A and D deficiency without documentation of night blindness.40 Abbott-Johnson et al. found impaired dark adaptation in almost fifty percent of patients with liver diseases, and also showed patients with ALD had the greatest impairment of dark adaptation. Most patients with impairment were asymptomatic.41 It is important to monitor levels of Vitamin A when a patient with AH is undergoing Vitamin A supplementation because of the risk of Vitamin A-induced liver toxicity.
Vitamin D
The primary function of vitamin D is regulation of intestinal calcium absorption. Vitamin D undergoes 25-hydroxylation in the liver, yielding the 25-hydroxyvitamin D peptide before it undergoes hydroxylation in the kidneys. Patients with liver diseases frequently suffer from vitamin D deficiency and are at high risk for osteoporosis. The prevalence of osteopenia in ALD is between 34% and 48% and the prevalence of osteoporosis is between 11 and 36%.42 Vitamin D also plays an important role in immune function and may help maintain gut barrier function in ALD.
Vitamin B12/Folate
The liver plays an important role in the storage and transport of vitamin B12. Elevated serum levels of B12 are often found in patients with AH, cirrhosis and hepatocellular carcinoma. This is due to the release of stored cobalamin through hepatocyte degradation.43 Folate participates in DNA methylation and replication. It is stored in the liver. Chronic alcoholics are often folate deficient due to reduced dietary intake, intestinal malabsorption, reduced liver uptake and storage, and increased urinary excretion.44
Vitamin E
Vitamin E is a potent lipid-soluble antioxidant that prevents the propagation of free radicals.45 Multiple studies in experimental ALD documented a clear link between oxidative stress and liver damage, and antioxidants including Vitamin E have been protective in experimental ALD. Vitamin E has been shown to reduce hepatic fat and inflammation in human non-alcoholic steatohepatitis. However, Mezey et al. tested the effects of vitamin E (1000 IU) on laboratory parameters of liver function in patients with mild to moderate AH and found no beneficial effect.46
Zinc
Zinc deficiency is found in approximately 83% of patients with cirrhosis, correlating with disease severity and with decrease transplant-free survival.47 Zinc deficiency may present in multiple diverse fashions including skin lesions, impaired wound healing altered mental status or altered immune function.48 The underlying mechanism are multiple and include increased urinary zinc excretion, decreased absorption in the intestine, and decreased dietary intake due to alcohol “empty calories”. Zinc deficiency plays a major role in gut barrier dysfunction and dysbiosis in experimental ALD.49 The dose of zinc used for treatment of AH is usually 50 mg of elemental zinc per day.
Magnesium
Magnesium is an intracellular cation essential for multiple enzymatic reactions. Hypo-magnesemia frequently occurs in alcoholics due to vomiting, diarrhea, excessive urinary loss and an inadequate diet. Prior studies showed that magnesium treatment may decrease levels of AST/ALT and could potentially increase handgrip muscle strength.50,51 Magnesium deficiency is often associated with muscle cramps, a frequent complaint in patients with AH. Muscle cramps may be treated with 400mg of magnesium oxide.
SUMMARY
Malnutrition is a common complication of AH and it correlates directly with degree of liver disease and mortality. Studies have shown that virtually 100% of patients with AH have some degree of malnutrition. Malnutrition is generally defined by a state of inadequate protein and/or calorie intake which results in sarcopenia. The contributing factors to this are numerous and include anorexia/nausea, high intake of empty calories, complications of severe liver disease, and unpalatable or incorrectly recommended diets. Adequate nutrition can be argued as the most critical form of treatment for AH other than abstinence. A prompt evaluation of nutrition risk should be performed in the inpatient setting and in clinic. Enteral nutrition is always preferable, and tube feedings should be considered when oral intake is inadequate. In both, the inpatient and outpatient setting, the bedtime snack helps to prevent overnight starvation and muscle breakdown. Patients with AH are at increased risk to develop deficiency of multiple vitamins and minerals. Identification and replacement of these can be critical. Importantly, new observations concerning the gut-liver axis and dysbiosis should lead to exciting potential therapies in the form of prebiotics/probiotics, among others. Overall, nutritional support improves nutritional status and may improve liver function and decrease the risk for liver-related complications and mortality.
Clinics Care Point.
Alcohol is a major source of empty calories.
Patients with AH often consume a diet inadequate in protein, and loss of muscle mass is common in AH.
Nighttime snacks should be prescribed to help prevent muscle loss.
Deficiencies in micronutrients such as zinc are common in AH, and deficiencies should be replaced with supplementation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barve S, Chen SY, Kirpich I, Watson WH, McClain C. Development, Prevention, and Treatment of Alcohol-Induced Organ Injury: The Role of Nutrition. Alcohol Res. 2017;38(2):289–302. [PMC free article] [PubMed] [Google Scholar]
- 2.Durand F, Buyse S, Francoz C, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60(6):1151–1157. [DOI] [PubMed] [Google Scholar]
- 3.Pirlich M, Schütz T, Spachos T, et al. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology. 2000;32(6):1208–1215. [DOI] [PubMed] [Google Scholar]
- 4.Makhija S, Baker J. The Subjective Global Assessment: a review of its use in clinical practice. Nutr Clin Pract. 2008;23(4):405–409. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. 1984;76(2):211–222. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall CL, Tosch T, Weesner RE, et al. VA cooperative study on alcoholic hepatitis. II: Prognostic significance of protein-calorie malnutrition. Am J Clin Nutr. 1986;43(2):213–218. [DOI] [PubMed] [Google Scholar]
- 7.Mendenhall CL, Moritz TE, Roselle GA, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology. 1993;17(4):564–576. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall CL, Moritz TE, Roselle GA, et al. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. The VA Cooperative Study Group #275. JPEN J Parenter Enteral Nutr. 1995;19(4):258–265. [DOI] [PubMed] [Google Scholar]
- 9.Mendenhall C, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res. 1995;19(3):635–641. [DOI] [PubMed] [Google Scholar]
- 10.Singal AK, Kamath PS, Francisco Ziller N, et al. Nutritional status of patients with alcoholic cirrhosis undergoing liver transplantation: time trends and impact on survival. Transpl Int. 2013;26(8):788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, McClain CJ, Feng W. Microbiome dysbiosis and alcoholic liver disease. Liver Research. 2019;3(3):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broitman SA, Gottlieb LS, Zamcheck N. Influence of Neomycin and Ingested Endotoxin in the Pathogenesis of Choline Deficiency Cirrhosis in the Adult Rat. J Exp Med. 1964;119:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bull-Otterson L, Feng WK, Kirpich I, et al. Metagenomic Analyses of Alcohol Induced Pathogenic Alterations in the Intestinal Microbiome and the Effect of Lactobacillus rhamnosus GG Treatment. Plos One. 2013;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Kirpich I, Liu Y, et al. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179(6):2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao T, Zhao C, Li F, et al. Intestinal HIF-1alpha deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction. J Hepatol. 2018;69(4):886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L, Li F, Yin X, et al. Profiling of Polar Metabolites in Mouse Feces Using Four Analytical Platforms to Study the Effects Of Cathelicidin-Related Antimicrobial Peptide in Alcoholic Liver Disease. J Proteome Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575(7783):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bode JC, Bode C, Heidelbach R, Durr HK, Martini GA. Jejunal Microflora in Patients with Chronic Alcohol-Abuse. Hepato-Gastroenterol. 1984;31(1):30–34. [PubMed] [Google Scholar]
- 19.Bajaj JS, Hylemon P, Heuman DM, Sanyal AJ, Gillevet PM. The Cirrhosis Dysbiosis Ratio Provides Insight into Gut Microbiome Changes Across the Spectrum of Cirrhosis: A Prospective Study of 250 Patients. Hepatology. 2013;58:274a–274a. [Google Scholar]
- 20.Smirnova E, Puri P, Muthiah MD, et al. Fecal Microbiome Distinguishes Alcohol Consumption From Alcoholic Hepatitis But Does Not Discriminate Disease Severity. Hepatology. 2020;72(1):271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–224. [DOI] [PubMed] [Google Scholar]
- 22.Keshavarzian A, Choudhary S, Holmes EW, et al. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299(2):442–448. [PubMed] [Google Scholar]
- 23.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205(3):243–247. [DOI] [PubMed] [Google Scholar]
- 24.Sehrawat TS, Liu M, Shah VH. The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol Hepatol. 2020;5(5):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajaj JS, Saeian K, Christensen KM, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103(7):1707–1715. [DOI] [PubMed] [Google Scholar]
- 26.Cabre E, Rodriguez-Iglesias P, Caballeria J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32(1):36–42. [DOI] [PubMed] [Google Scholar]
- 27.Moreno C, Deltenre P, Senterre C, et al. Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology. 2016;150(4):903–910 e908. [DOI] [PubMed] [Google Scholar]
- 28.Plauth M, Cabre E, Riggio O, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25(2):285–294. [DOI] [PubMed] [Google Scholar]
- 29.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. [DOI] [PubMed] [Google Scholar]
- 30.Excellence NIfHaC. Nutrition support in adults Clinical guideline CG32. 2006. www.nice.org.uk/page.aspx?o=cg032. Published 2006. Accessed 11/23/20, 2020.
- 31.Hirsch S, Bunout D, de la Maza P, et al. Controlled trial on nutrition supplementation in outpatients with symptomatic alcoholic cirrhosis. JPEN J Parenter Enteral Nutr. 1993;17(2):119–124. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch S, de la Maza MP, Gattas V, et al. Nutritional support in alcoholic cirrhotic patients improves host defenses. J Am Coll Nutr. 1999;18(5):434–441. [DOI] [PubMed] [Google Scholar]
- 33.Owen OE, Trapp VE, Reichard GA Jr., et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72(5):1821–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaisman N, Katzman H, Carmiel-Haggai M, Lusthaus M, Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr. 2010;92(1):137–140. [DOI] [PubMed] [Google Scholar]
- 35.Plank LD, Gane EJ, Peng S, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48(2):557–566. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Zhang X, Ma LJ, et al. Omega-3 polyunsaturated fatty acids ameliorate ethanol-induced adipose hyperlipolysis: A mechanism for hepatoprotective effect against alcoholic liver disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863(12):3190–3201. [DOI] [PubMed] [Google Scholar]
- 37.Huang W, Wang B, Li X, Kang JX. Endogenously elevated n-3 polyunsaturated fatty acids alleviate acute ethanol-induced liver steatosis. Biofactors. 2015;41(6):453–462. [DOI] [PubMed] [Google Scholar]
- 38.Kirpich IA, Miller ME, Cave MC, Joshi-Barve S, McClain CJ. Alcoholic Liver Disease: Update on the Role of Dietary Fat. Biomolecules. 2016;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65(5):830–839. [DOI] [PubMed] [Google Scholar]
- 40.Venu M, Martin E, Saeian K, Gawrieh S. High prevalence of vitamin A deficiency and vitamin D deficiency in patients evaluated for liver transplantation. Liver Transpl. 2013;19(6):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbott-Johnson WJ, Kerlin P, Abiad G, Clague AE, Cuneo RC. Dark adaptation in vitamin A-deficient adults awaiting liver transplantation: improvement with intramuscular vitamin A treatment. British Journal of Ophthalmology. 2011;95:544–548. [DOI] [PubMed] [Google Scholar]
- 42.Kizilgul M, Ozcelik O, Delibasi T. Bone health and vitamin D status in alcoholic liver disease. Indian J Gastroenterol. 2016;35(4):253–259. [DOI] [PubMed] [Google Scholar]
- 43.Ermens AA, Vlasveld LT, Lindemans J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin Biochem. 2003;36(8):585–590. [DOI] [PubMed] [Google Scholar]
- 44.Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beier JI, Arteel GE, McClain CJ. Advances in alcoholic liver disease. Curr Gastroenterol Rep. 2011;13(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40(1):40–46. [DOI] [PubMed] [Google Scholar]
- 47.Sengupta S, Wroblewski K, Aronsohn A, et al. Screening for Zinc Deficiency in Patients with Cirrhosis: When Should We Start? Dig Dis Sci. 2015;60(10):3130–3135. [DOI] [PubMed] [Google Scholar]
- 48.Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27(1):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong W, Wei X, Hao L, et al. Paneth Cell Dysfunction Mediates Alcohol-related Steatohepatitis Through Promoting Bacterial Translocation in Mice: Role of Zinc Deficiency. Hepatology. 2020;71(5):1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poikolainen K, Alho H. Magnesium treatment in alcoholics: a randomized clinical trial. Subst Abuse Treat Prev Policy. 2008;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gullestad L, Dolva LO, Soyland E, Manger AT, Falch D, Kjekshus J. Oral magnesium supplementation improves metabolic variables and muscle strength in alcoholics. Alcohol Clin Exp Res. 1992;16(5):986–990. [DOI] [PubMed] [Google Scholar]


