Abstract
Aim & methods:
We conducted a pilot epigenome-wide association study of women from Tutsi ethnicity exposed to the genocide while pregnant and their resulting offspring, and a comparison group of women who were pregnant at the time of the genocide but living outside of Rwanda.
Results:
Fifty-nine leukocyte-derived DNA samples survived quality control: 33 mothers (20 exposed, 13 unexposed) and 26 offspring (16 exposed, 10 unexposed). Twenty-four significant differentially methylated regions (DMRs) were identified in mothers and 16 in children.
Conclusions:
In utero genocide exposure was associated with CpGs in three of the 24 DMRs: BCOR, PRDM8 and VWDE, with higher DNA methylation in exposed versus unexposed offspring. Of note, BCOR and VWDE show significant correlation between brain and blood DNA methylation within individuals, suggesting these peripherally derived signals of genocide exposure may have relevance to the brain.
Keywords: : differentially methylated region, epigenetics, epigenomic, genocide, intergenerational transmission, maternal stress, methylation, offspring, PTSD, trauma
Lay abstract
The 1994 Rwandan genocide against ethnic Tutsi has been associated with adverse mental health outcomes in survivors decades later, but the molecular mechanisms that contribute to this association remain poorly characterized. Epigenetic mechanisms such as DNA methylation regulate gene function and change in response to life experiences. We identified differentially methylated regions (DMRs) in genocide-exposed versus unexposed mothers and children. In utero genocide exposure was linked with methylation differences in three maternal DMRs, with higher methylation in exposed offspring. Two of three DMRs show correlation between brain and blood methylation within individuals, suggesting that peripherally derived signals of genocide exposure may be relevant to the brain.
Trauma exposure is associated with adverse mental and physical health consequences. Multiple population-based studies have documented prospective associations between trauma exposure and heightened risk for mental and physical disorders, including post-traumatic stress disorder (PTSD), depression and generalized anxiety disorder [1], coronary heart disease [2,3], arthritis [4,5] and gastrointestinal disease and diabetes [5], among others. Despite these well-established associations, the mechanisms mediating the relation between trauma exposure and subsequent adverse health within generations are poorly understood. Moreover, little is known about the effects of exposure to psychological trauma during pregnancy on resulting offspring (i.e., between-generation effects), although a growing body of research supports a link between these two (reviewed in [6,7]).
Epigenetic mechanisms have been a growing focus of investigation at a global level in relation to understanding the role environmental exposures play in mediating later disease susceptibility after prenatal development. Although many human studies have explored the role of epigenetics in long-term consequences of prenatal exposure to environmental conditions, much of this work has focused on toxic environmental exposures (e.g., [8,9] and reviewed in [10]), or maternal stress (e.g., [11,12] and reviewed in [12,13]); investigation of epigenetics in relation to population-level exposure to extreme trauma remain scant. Although a handful of studies have examined the impact of prenatal exposure to extreme trauma – namely, genocide, famine, nutritional deficits, stress and war [14–17] – on subsequent epigenetic profiles of offspring, these have thus far been limited to locus-specific or candidate-gene studies [18–20], leaving the genome-scale impact of genocide exposure unknown, both within and between generations.
Between April and June 1994, almost one million people died in the Rwandan genocide against ethnic Tutsi. More than 25 years later, the long-term impact of the genocide is evident in the prevalence of PTSD in Rwanda – more than 28% among adults [21] and 41% among women survivors [22] – and there is compelling evidence for the transmission of the disorder between exposed individuals and subsequent generations [23,24]. Previously, we examined blood-derived DNA methylation (DNAm) levels in the glucocorticoid receptor gene NR3C1 [23] in mothers and offspring exposed to the Rwandan genocide against ethnic Tutsi, finding that exposure to genocide during pregnancy was associated with higher methylation levels in both mothers and offspring. Also, exposed mothers and their children had lower cortisol levels than non-exposed mothers and their children. Additional work by Vukojevic et al. [25] also supports a link between exposure to the Rwandan genocide and epigenetic modifications in NR3C1 related to the risk of PTSD.
Here, we expand on previous works to conduct a pilot epigenome-wide association study (EWAS) of the impact of genocide exposure on epigenetic modifications across the genome. We focus specifically on genocide as the exposure of interest to gain insight into the impact of exposure to extreme trauma both within and between generations, and to leverage the best-defined phenotype in our sample. Our findings demonstrate that genocide exposure is indeed associated with multiple differentially methylated regions (DMRs) throughout the genome in both mothers and children and that a subset of the maternal DMRs is also found in offspring.
Methods
DNA methylation, pre-processing and quality control
Study participants from case and control groups were both women of Tutsi ethnicity who were pregnant (second and third terms of pregnancy) during the genocide period, and their offspring born after that period [23]. An additional inclusion criterion for participants in the case group, known as the exposed group, was having been exposed to traumatic experiences in the context of Rwanda genocide, whereas for the control group, known as the non-exposed group, inclusion criteria were living abroad at the time of the genocide and therefore not exposed to the traumatic experience. Study participants were recruited, and whole blood samples were collected as previously described [23]. DNAm derived from whole blood was measured using the Illumina Infinium MethylationEPIC BeadChip (Illumina, CA, USA) in 70 samples following the manufacturer’s recommended protocol. Raw DNAm estimates of methylation status ranging from 0 to 1 (i.e., β values) were obtained, and a sex check was performed to remove sex-discordant samples using minfi R packages [26]. Samples and probes with low signal intensity and missing values, including cross-reactive and polymorphic probes, were removed as described by Wani et al. [27]. In total, 3409 CpGs due to low signal intensity or missing values and 43,962 cross-reactive probes were removed, leaving 818,720 probes for subsequent analyses. The data were normalized using single-sample Noob (ssNoob) method implemented in the minfi R package. ComBat adjustment using an empirical Bayesian framework implemented in SVA R package [28,29] was performed to reduce the likelihood of bias due to known chip and positional batch effects, controlling for sex and PTSD, assessed using the PTSD 17-item checklist (PCL-17) to assess the severity of current PTSD [30]. The key traumatic experiences faced by genocide survivors were as follows: potentially being captured or kidnapped, witnessing a massacre, serious injury or attack with a weapon, witnessing the killing of someone, seeing dead and mutilated bodies, sexual abuse or rape and genocide exposure [31]. Cell proportion estimates were computed using the IDOL algorithm [32] implemented in the Epidish R package [33]. Quality control (QC) removed 11 samples, leaving 59 samples (26 children: 16 exposed and 10 unexposed; 33 mothers: 20 exposed and 13 unexposed) for subsequent analyses.
Analytic approach
Because the sample size in this study was modest, we limited our analyses to the top 5% probes (CpGs) with highest variance from the samples that survived QC. To assess the association between methylation levels and genocide exposure (exposed vs unexposed), we performed differential analyses to identify DMRs using the mCSEA R package [34]. This method works by fitting a linear model and ranking the CpGs based on the t-statistics using limma [35]. Once the probes were ranked, enrichment analysis was performed on the CpGs belonging to the same region in the top positions of the ranked list using fgsea package [36]. Finally, the region with CpGs over-represented in the top or bottom of the list was detected as a DMR. Promoter regions were selected as the region of interest in the DMR analyses, and the number of minimum CpGs in the region was set to five which is the default parameter and regions below this threshold were not tested [5]. DMR analyses were performed for mothers and children (exposed vs unexposed) separately to examine the impact of genocide exposure within generations at a genome-wide scale. Results (DMRs) were accepted as significant if the false discovery rate (FDR) corrected p-value was <0.05. The overall workflow of the analytical approach is given in Supplementary Figure 1.
To account for relatedness between mothers and children and assess the impact of genocide exposure between generations, we used penalized regression models [37]. Each DMR had between four and 62 CpGs driving the enrichment score, with an average of 13 CpGs per DMR. Despite this, we found that for most DMRs, the top three CpGs contributed to at least 40% of the enrichment score. For these reasons, we focused on the first three CpGs for our intergenerational analyses. Specifically, the top three CpGs within each DMR that contributed most to the enrichment analysis were used to infer whether in utero genocide exposure was associated with differential DNAm in offspring while controlling for age (of mother), sex (of child), maternal DNAm and leukocyte cell types, similar to prior work examining the intergenerational transmission of telomere length in relation to exposure childhood trauma [38]. Large variance inflation factors (>10) within predictors were found, indicating presence of multicollinearity. To account for this, we conducted our analysis using the glmnet R package to fit a regularized regression model via penalized maximum likelihood [37]. This regression technique allowed us to carry out flexible regularization of coefficients. It is a well-established statistical method and has been successfully applied to handle issues of multicollinearity [39].
We also explored the application of ridge regression, a well-studied variant of penalized regression [37]. This method works by imposing a penalty on coefficient estimates to shrink the coefficients of unimportant predictors and differ in the specific penalty term. Trace plots were produced and examined to evaluate rates of shrinkage for each predictor.
In the ridge regression model, the shrinkage applied to the maternal exposure term was low relative to the penalty imposed on other predictors, which indicates the importance of maternal exposure in accounting for variance in the response. To further validate these findings, stability selection was applied using the c060 R package [40] described by Meinshausen et al. [41]. This method works by sampling from the data without replacement and fitting a penalized regression model to each subsample. Selection probabilities are then associated with each covariate based on their importance across the set of subsamples. The recommended range of selection probability threshold for determining the stable set of covariates is between 0.6 and 0.9 [32]. Stable covariates were selected on the basis of a standard selection probability threshold of 0.6 after adjusting for type 1 error rate (α = 0.05). The CpGs associated with in utero genocide exposure exceeding this selection probability threshold in these models were selected. Estimated marginal means based on the ridge regression were calculated for control and genocide exposure groups for post hoc inference.
Concordance between DNAm levels in blood and brain was evaluated for CpG sites identified as significant (or important if statistical significance was not strictly used) in the intergenerational analysis via the imageCpG database [42]. In addition, we performed an independent Student’s t-test on the top three enriched CpGs in the DMRs that were consistently significant in the within- and between-generation analyses. DNAm levels in exposed versus unexposed groups were evaluated via Student’s t-test (nonpaired and two-tailed) to identify potential differences, and results were accepted if p < 0.05. Finally, we calculated DNAm age and DNAm age difference (AgeAccelerationDiff), the difference between DNAm age and chronological age, to investigate biological age acceleration or deceleration in genocide-exposed compared with unexposed individuals for children and mothers separately using the Horvath age calculator [43]. To evaluate the association between AgeAccelerationDiff and genocide exposure, we used a multiple regression model adjusting for age and estimated cell proportions (CD8T, CD4T, natural killer, B cell and Mono).
Results
Fifty-nine samples passed QC and were included in the analyses. Table 1 presents demographic characteristics of participants included in this analytic sample. After QC, 818,720 CpG sites were retained, and the top 5% CpGs (40,936) based on variance within the available dataset were used in subsequent analyses. Results from analyses investigating the impact of genocide within generations identified 24 DMRs in mothers and 16 in children comparing those exposed versus unexposed to genocide (Tables 2 & 3). ARL5C and PM20D1 were the two top ranked DMRs (p = 1e-10) in mothers, whereas TMEM204 (p = 1e-10) and LDHC (p = 1.6e-08) were the top two in children. Six DMRs – BCOR, FGFR2, HOXA5, PM20D1, VWDE and PRDM8 – were common in mothers and children (Supplementary Table 1). Of these six shared DMRs identified in mothers and children, three PM20D1, VWDE and BCOR showed a significant correlation (rho = 0.87, p = 4.82 × 10-7; rho = 0.81, p = 6.83 × 10-6; and rho = 0.81, p = 9.12 × 10-6) respectively between blood and brain DNAm within individuals in the imageCpG database. Our genome-scale analyses did not identify significant DMRs within the NR3C1 locus that was the focus of earlier candidate gene work.
Table 1. . Demographic characteristics of participants included in the analytic sample, stratified by mothers and children.
| Characteristic | Child | Mother |
|---|---|---|
| Total (n) | 26 | 33 |
| PTSD severity score, median (range) | 37 (12–69) | 46 (17–81) |
| Exposure to genocide (%) | ||
| Yes | 16 (62) | 20 (61) |
| No | 10 (38) | 13 (39) |
| Sex (%) | ||
| Male | 10 (38) | 0 (0) |
| Female | 16 (62) | 33 (100) |
| Age, median (range) DNA methylation beta values, mean (range) |
17 (17–18) 0.60 (0.003–0.99) |
45 (33–57) 0.60 (0.003–0.99) |
PTSD: Post-traumatic stress disorder.
Table 2. . Differentially methylated regions in mothers exposed versus unexposed to genocide.
| DMR | Chr | Pval | Padj | log2err | ES | Size† | leadingEdge | t-value | p-value |
|---|---|---|---|---|---|---|---|---|---|
| ARL5C | 17 | 1E-10 | 3.3E-08 | NA‡ | 0.9985 | 5 | cg07330481, cg16140242, cg00900933, cg06120399, cg09173348 | -4.994 | 3.73E-05 |
| PM20D1 | 1 | 1E-10 | 3.3E-08 | NA‡ | 0.9514 | 11 | cg26354017, cg11965913, cg12898220, cg14893161, cg14159672, cg07533224, cg05841700, cg24503407, cg07157834, cg16334093, cg07167872 | -3.454 | 0.002 |
| BCOR | X | 1.1E-09 | 2.5E-07 | 0.7882 | -0.5858 | 62 | cg15627188, cg02931660, cg02932805, cg18744436, cg15887754, cg01321830, cg20197861, cg11620557, cg21180513, cg05026884, cg04924962, cg24450656, cg20848269, cg15366127, cg23496314, cg01110765, cg05559023, cg15039826, cg22346771, cg18765710, cg19937286, cg10039267, cg07601068, cg12775788, cg13307200, cg00206414, cg12045126, cg11143827, cg27428464, cg21010298, cg14261068, cg03161453, cg02693068, cg06839398 | 2.926 | 0.007 |
| S100A1 | 1 | 3.4E-08 | 4.4E-06 | 0.7195 | -0.8992 | 12 | cg27469660, cg11343894, cg11915664, cg02331910, cg13946767, cg02873163, cg11596404, cg17776284, cg06562291, cg19335413, cg01347250, cg07898899 | 1.767 | 0.091 |
| S100A13 | 1 | 3.4E-08 | 4.4E-06 | 0.7195 | -0.8992 | 12 | cg27469660, cg11343894, cg11915664, cg02331910, cg13946767, cg02873163, cg11596404, cg17776284, cg06562291, cg19335413, cg01347250, cg07898899 | 1.767 | 0.091 |
| HOXA5 | 7 | 2.0E-06 | 0.0002 | 0.6273 | -0.8162 | 13 | cg17432857, cg04863892, cg19759481, cg02005600, cg17569124, cg25307665, cg14014955, cg23936031, cg09207400, cg02646423, cg02916332, cg09549073 | 1.412 | 0.170 |
| FGFR2 | 10 | 1.5E-05 | 0.0014 | 0.5933 | -0.8821 | 8 | cg25052156, cg06791446, cg02210151, cg10379346, cg13437682, cg22633036, cg18566515, cg11430259 | 1.950 | 0.063 |
| TMEM187 | X | 0.0002 | 0.0141 | 0.5188 | -0.6840 | 16 | cg23124111, cg13937627, cg03177323, cg03672915, cg11778030, cg03189022, cg09275137, cg10155960, cg05103731, cg07086565, cg08627233, cg21960840, cg09222696 | 2.003 | 0.054 |
| VWDE | 7 | 0.0002 | 0.0141 | 0.5188 | -0.9254 | 5 | cg03579179, cg06484146, cg20607287, cg19397885 | 3.224 | 0.004 |
| NAA10 | X | 0.0002 | 0.0141 | 0.5188 | -0.8286 | 8 | cg09771319, cg02232536, cg01501311, cg00072288, cg22749239, cg12727431 | 2.298 | 0.028 |
| TMEM232 | 5 | 0.0002 | 0.0141 | 0.5188 | 0.7636 | 12 | cg22429640, cg17946588, cg06414816, cg06429214, cg25259944, cg17248924, cg26583412, cg27037608, cg11641395, cg19526166, cg10597099 | -2.332 | 0.030 |
| OTUD5 | X | 0.0005 | 0.0263 | 0.4773 | 0.6430 | 22 | cg02480419, cg03831206, cg02573613, cg18780401, cg07750402, cg12591117, cg18112782, cg27277239, cg13574945, cg12298823, cg00680673, cg26201401, cg14006678, cg09985072, cg03711046, cg08221357, cg15925199, cg21206285 | -3.371 | 0.002 |
| PRPS2 | X | 0.0005 | 0.0263 | 0.4773 | -0.7422 | 11 | cg09978401, cg26059639, cg01669374, cg27325673, cg07874284, cg26190455, cg21953876, cg02891306 | 2.578 | 0.016 |
| HCFC1 | X | 0.0006 | 0.0274 | 0.4773 | -0.7393 | 12 | cg13937627, cg03177323, cg03672915, cg11778030, cg03189022, cg10155960, cg05103731, cg07086565, cg08627233, cg21960840, cg09222696 | 1.906 | 0.066 |
| CDKL5 | X | 0.0007 | 0.0300 | 0.4773 | -0.6665 | 14 | cg20281601, cg10404653, cg14755341, cg25550082, cg04716051, cg07519908, cg26020914, cg06408185, cg02939364 | 2.160 | 0.040 |
| CACNB2 | 10 | 0.0007 | 0.0300 | 0.4773 | -0.7997 | 8 | cg25327888, cg14094927, cg02635932, cg18959207, cg16108246, cg22858500 | 2.568 | 0.016 |
| AKR7L | 1 | 0.0010 | 0.0368 | 0.4773 | 0.8153 | 7 | cg13935437, cg18202521, cg12798157, cg20677058, cg11376198, cg09990584, cg09045262 | -2.316 | 0.028 |
| FMOD | 1 | 0.0010 | 0.0368 | 0.4551 | -0.7735 | 9 | cg11914824, cg01739509, cg16289210, cg26987645, cg21089380, cg11897689, cg27387030, cg22705746 | 0.975 | 0.338 |
| PCDHB7 | 5 | 0.0011 | 0.0368 | 0.4551 | -0.8631 | 6 | cg25026992, cg03780733, cg16583552, cg03022653, cg19520087 | 3.222 | 0.004 |
| TMEM100 | 17 | 0.0013 | 0.0439 | 0.4551 | 0.8841 | 5 | cg18040354, cg19403377, cg06441396, cg08762247, cg01155092 | -3.590 | 0.002 |
| ATP6AP2 | X | 0.0016 | 0.0486 | 0.4551 | -0.8333 | 7 | cg18105467, cg18679504, cg11014998, cg19200045, cg10347293, cg07381502 | 2.251 | 0.032 |
| PRDM8 | 4 | 0.0017 | 0.0487 | 0.4551 | -0.7567 | 9 | cg18073471, cg05059566, cg06307913, cg22902505, cg00138041 | 2.096 | 0.045 |
| SLC35A2 | X | 0.0017 | 0.0487 | 0.4551 | -0.6370 | 14 | cg06505213, cg02856792, cg13651586, cg10530128, cg14132995, cg01488378, cg23709838, cg27429230, cg07989438 | 2.604 | 0.014 |
| RGAG4 | X | 0.0018 | 0.0487 | 0.4551 | -0.7227 | 10 | cg03679269, cg04210573, cg00374346, cg24569746, cg10482495, cg12905598, cg01997410, cg06796204, cg04072009, cg03056321 | 1.545 | 0.135 |
Number of CpGs associated with the feature (gene).
If p-values were likely overestimated, log2err is set to NA.
The p-value indicates the difference in mean methylation; the t-value indicates the ratio of the difference between the mean methylation of the samples (exposed vs unexposed mothers) of significant CpGs shown in leadingEdge column.
DMRs are sorted by p-values.
Chr: Chromosome; DMR: Differentially methylated region; ES: Enrichment score; leadingEdge: Leading edge CpGs which drive the enrichment for DMR; log2err: Expected error for the standard deviation of the p-value logarithm; NA: Not available; Padj: p-value adjusted by Benjamini-Hochberg method; Pval: Estimated p-value.
Table 3. . Differentially methylated regions in children exposed versus unexposed to genocide.
| DMR | Chr | Pval | Padj | log2err | ES | Size† | leadingEdge | t-value | p-value |
|---|---|---|---|---|---|---|---|---|---|
| TMEM204 | 16 | 1E-10 | 6.6E-08 | NA‡ | -0.9718 | 12 | cg06602086, cg27594616, cg26390081, cg07639376, cg08296037, cg02193187, cg00463982, cg10465839, cg11375102, cg07341220, cg06565913, cg10698762 | 1.900 | 0.082 |
| LDHC | 11 | 1.7E-08 | 5.5E-06 | 0.73376 | 0.9440 | 10 | cg08418111, cg14332815, cg21471707, cg19767548, cg14259717, cg11821245, cg07398106, cg07093428, cg09332484 | -3.851 | 0.001 |
| FGFR2 | 10 | 9.7E-08 | 2.1E-05 | 0.70498 | -0.9543 | 8 | cg02210151, cg25052156, cg18566515, cg22633036, cg13437682, cg11430259, cg06791446, cg10379346 | 2.313 | 0.030 |
| VWDE | 7 | 3.2E-06 | 0.0005 | 0.62726 | -0.9719 | 5 | cg03579179, cg19397885, cg06484146, cg20607287 | 4.330 | 4.47E-04 |
| HOXA4 | 7 | 3.5E-06 | 0.0005 | 0.62726 | -0.9449 | 7 | cg25952581, cg03724423, cg24169822, cg19142026, cg14359292, cg07317062, cg08657492 | 2.112 | 0.046 |
| PRDM8 | 4 | 8.8E-06 | 0.0010 | 0.59333 | -0.9062 | 9 | cg05059566, cg27111250, cg10129063, cg19409579, cg18073471, cg06307913, cg22902505 | 2.550 | 0.021 |
| PM20D1 | 1 | 1.0E-05 | 0.0010 | 0.59333 | 0.8675 | 11 | cg07157834, cg16334093, cg05841700, cg11965913, cg26354017, cg07533224, cg24503407, cg14159672, cg12898220, cg07167872, cg14893161 | -1.372 | 0.191 |
| HOXA5 | 7 | 1.7E-05 | 0.0014 | 0.57561 | -0.8506 | 13 | cg19759481, cg04863892, cg14014955, cg09207400, cg02916332, cg17569124, cg25307665, cg23936031, cg17432857, cg02005600, cg09549073 | 1.823 | 0.089 |
| CPT1B | 22 | 0.0001 | 0.0085 | 0.53843 | 0.8461 | 10 | cg16386697, cg10770023, cg01081346, cg19112186, cg05156901, cg06530441, cg27502912, cg24363820, cg00270625, cg08260245 | -1.540 | 0.137 |
| C21orf56 | 21 | 0.0003 | 0.0181 | 0.49849 | 0.8722 | 8 | cg08742575, cg12016809, cg25545878, cg07747299, cg13012494, cg13732083, cg05896524 | -1.917 | 0.073 |
| ZEB2 | 2 | 0.0003 | 0.0181 | 0.49849 | 0.9071 | 6 | cg05322294, cg00573770, cg24488281, cg00602811, cg16267679 | -2.227 | 0.040 |
| MIR886 | 5 | 0.0003 | 0.0181 | 0.49849 | 0.8946 | 7 | cg08745965, cg18678645, cg18797653, cg00124993, cg26896946, cg25340688, cg06536614 | -1.534 | 0.139 |
| HOXA2 | 7 | 0.0004 | 0.0225 | 0.49849 | -0.8531 | 8 | cg09871315, cg02225599, cg06166490, cg04027736, cg06401979, cg00445443, cg09908750, cg19432993 | 1.494 | 0.151 |
| BCOR | X | 0.0005 | 0.0257 | 0.47727 | -0.4826 | 62 | cg15627188, cg15887754, cg05026884, cg02932805, cg15039826, cg01321830, cg00206414, cg18744436, cg23496314, cg20848269, cg01110765, cg24450656, cg20197861, cg12775788, cg05721877, cg04924962, cg12045126, cg25300435, cg12737514, cg21180513 | 2.519 | 0.019 |
| PIWIL1 | 12 | 0.0006 | 0.0267 | 0.47727 | 0.8839 | 7 | cg24838063, cg13861644, cg11931211, cg18319102, cg24229701, cg27630820, cg19424457 | -1.495 | 0.153 |
| ABAT | 16 | 0.0011 | 0.0432 | 0.45506 | 0.9132 | 5 | cg16586594, cg08834902, cg08241330, cg01881182 | -2.363 | 0.031 |
Number of CpGs associated with the feature (gene).
If p-values were likely overestimated, log2err is set to NA.
The p-value indicates the difference in mean methylation; the t-value indicates the ratio of the difference between the mean methylation of the samples (exposed vs unexposed mothers) of significant CpGs shown in leadingEdge column.
DMRs are sorted by p-values.
Chr: Chromosome; DMR: Differentially methylated region; ES: Enrichment score; leadingEdge: Leading edge CpGs which drive the enrichment for DMR; log2err: Expected error for the standard deviation of the p-value logarithm; NA: Not available; Padj: p-value adjusted by Benjamini-Hochberg method; Pval: Estimated p-value.
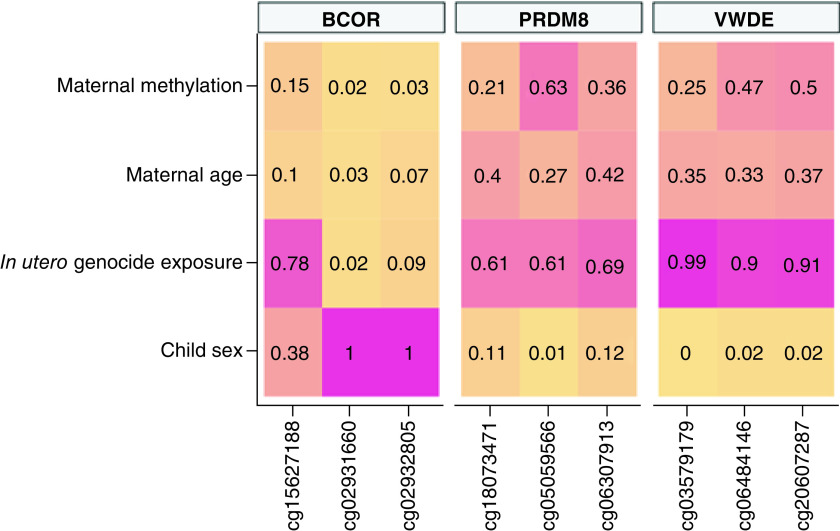
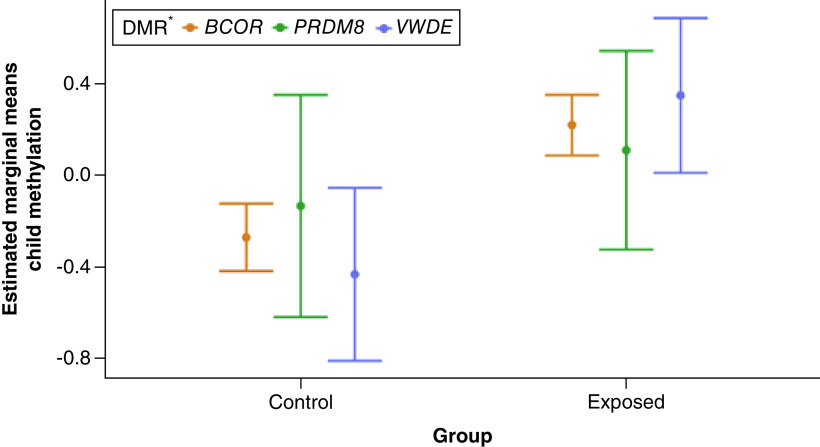
To infer whether in utero genocide exposure was potentially associated with differential methylation in offspring, we examined the top three highly enriched DMR-related CpGs found in mothers using penalized regression models. We found DMRs PRDM8 and VWDE to be consistently associated with in utero genocide exposure; BCOR was also implicated in these analyses. PRDM8 and VWDE CpGs had higher selection probabilities than BCOR for in utero genocide exposure, with PRDM8 and VWDE averaging π = 0.64 and π = 0.93, respectively. BCOR-related CpGs cg02931660 and cg02932805 demonstrated lower selection probabilities (π < 0.10) for in utero genocide exposure, but after evaluating low shrinkage in the ridge regression trace plots and considering BCOR-related CpG cg15627188, which had a high selection probability of π = 0.78, we decided to retain this DMR. Individual selection probabilities for child gender, in utero genocide exposure, age of mother and methylation of mother are shown in Figure 1. Estimated marginal means for post hoc comparison of in utero genocide exposed cases versus controls based on the ridge regression model were calculated for the top three highly enriched CpGs of these DMRs (Figure 2). The figure indicates a pattern of higher child methylation in exposed groups than control groups especially for DMRs, BCOR and VWDE.
Figure 1. . Selection probabilities of child sex, in utero genocide exposure, maternal age and maternal DNA methylation for the top three highly enriched differentially methylated region-related CpGs highly associated with in utero genocide exposure.
The colors represent the strength of the selection probability.
Figure 2. . Post hoc comparison of averaged estimated marginal means of DNA methylation in children for the top three enriched CpG sites of differentially methylated regions associated with in utero genocide exposure by case and control groups.
The post hoc pairwise comparison plot shows that mean child methylation estimates are higher in in utero genocide exposure cases. The standard errors calculated here are for visualization purposes and are only based on an assessment of the variance of the estimates.
*Top three highly enriched CpGs of each DMR: BCOR = cg15627188, cg02931660, cg02932805; PRDM8 = cg18073471, cg05059566, cg06307913; VWDE = cg03579179, cg06484146, cg20607287.
DMR: Differentially methylated region.
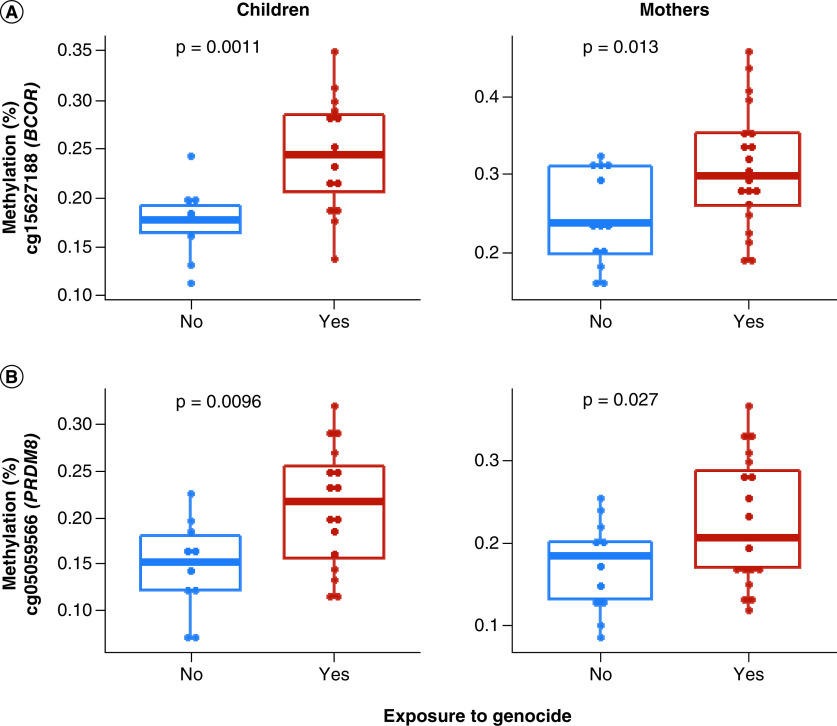
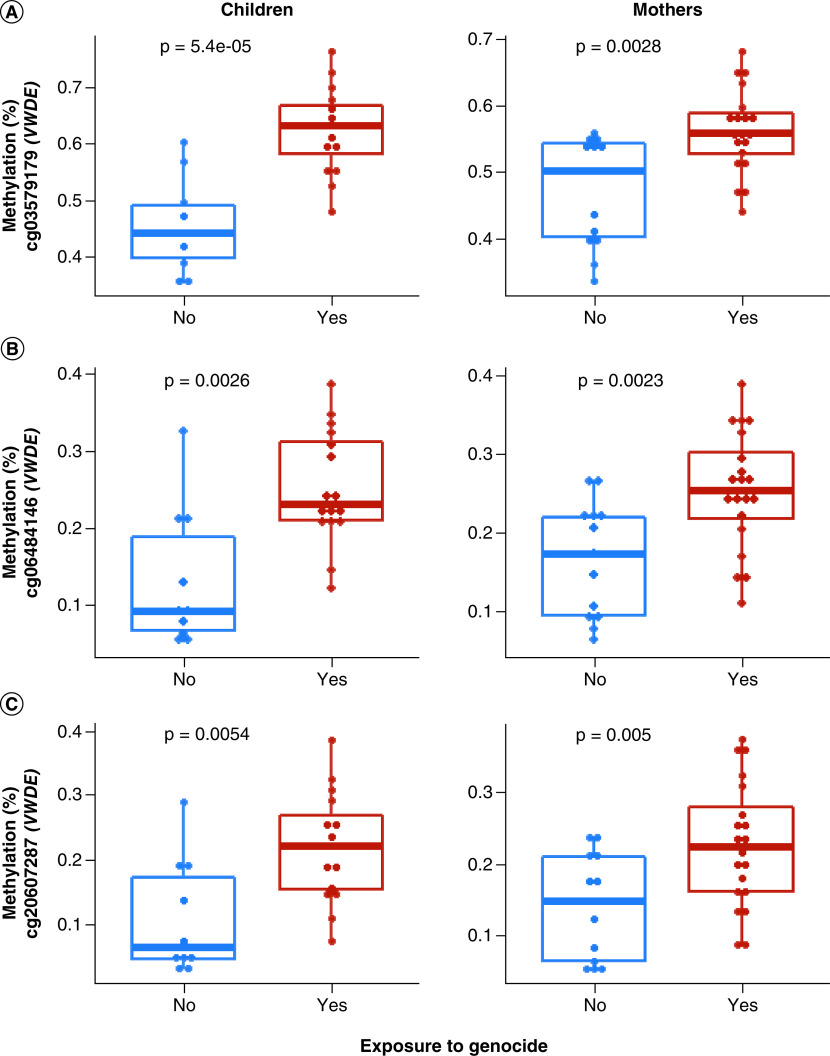
Further, an independent t-test was performed on top three enriched CpGs in the top three DMRs (BCOR, PRDM8 and VWDE) that were consistently significant in DMR analyses for within-generation and penalized regression analyses for analyses implicating effects of genocide exposure between generations (Figure 2). Tests were performed to compare the mean methylation of two groups – exposed versus unexposed to genocide – in both children and mothers separately. There was a significant difference between DNAm levels of CpGs cg15627188 in BCOR and cg05059566 in PRDM8 at p < 0.05 between exposed versus unexposed (Figure 3). For VWDE, all three-top enriched CpGs (cg03579179, cg06484146 and cg20607287) showed a significant difference (p < 0.05) between exposed and unexposed groups (Figure 4). In all the CpGs that were significant via t-test, DNAm levels were higher in those exposed to genocide.
Figure 3. . t-test comparison of methylation (%) means between genocide-exposed versus unexposed children and mothers, respectively.
Blue represents lower mean methylation in unexposed individuals, and red represents higher mean methylation in exposed individuals. Each dot represents an individual sample. Methylation (%) of significant (p < 0.05) CpGs in the top two genes (BCOR, PRDM8) that are consistently significant in the differentially methylated regions and intergenerational analysis is shown. There is a significant difference in methylation levels between exposed versus unexposed subjects in both children and mothers (p < 0.05). Both CpGs (cg15627188, cg05059566) showed higher methylation levels in those exposed to genocide.
Figure 4. . t-test comparison of methylation (%) means between genocide-exposed versus unexposed children and mothers, respectively.
Blue represents lower mean methylation in unexposed individuals, and red represents higher mean methylation in exposed individuals. Each dot represents an individual sample. Methylation (%) of significant CpGs in VWDE is shown. All three-top enriched CpGs (cg03579179, cg06484146, cg20607287) showed higher methylation levels in those exposed to genocide at p < 0.05.
In the previous candidate gene study based on this sample, we performed a targeted analysis of NR3C1 using pyrosequencing to assess DNA methylation levels in genocide exposed versus unexposed participants [37]. To test potential correspondence of findings in this study, we conducted an exploratory targeted DMR analysis of NR3C1 in our current EWAS data. Results showed significant differences (adjusted p = 0.026) in this locus between genocide-exposed compared with unexposed study participants (which were a subset of the original participants in the original targeted pyrosequencing study). However, NR3C1 was not a significant DMR in our epigenome-scale DMR analysis when considering either the top 5% most variable probes as reported in this work or when considering all the probes together. Finally, the association between Age AccelerationDiff and genocide exposure was not statistically significant in children (t = 0.693, p = 0.497) and mothers (t = -0.096, p = 0.9250). In addition, the AgeAccelerationDiff was not significantly associated with genocide exposure in children and mothers together (t = -0.658, p = 0.514).
Discussion
Although previous studies [44–46] have analyzed the impact of the Rwandan genocide against ethnic Tutsi in relation to PTSD and some have shown evidence of transmission of the disorder between generations [24,44], it remains unclear whether exposure to extreme trauma can pass from mothers to offspring prenatally. In particular, understanding of the epigenetic mechanisms that show evidence of intergenerational transmission on a genome-wide scale remains poorly elucidated. This study generated new epigenomic data from a previously published study [23] to begin to address this gap in knowledge. Specifically, we examined blood-derived DNAm from mothers who were exposed to the genocide against the Tutsi in Rwanda during their pregnancy and a control group of pregnant mothers who were not exposed to genocide to characterize within-generation and potential intergenerational transmission of epigenetic effects of genocide exposure. First, we tested for an association between genocide exposure and methylation levels in promoter regions via DMR analyses to understand within-generation impact of genocide exposure and found significant differences between exposed versus unexposed in both mothers (24 DMRs) and children (16 DMRs). We then tested for intergenerational impact of genocide exposure and found that in utero genocide exposure was associated with differential methylation, but not global DNAm, in the offspring in DMRs (BCOR, PRDM8 and VWDE), with evidence for higher methylation levels in those exposed to genocide. Further, no significant association was found between AgeAccelerationDiff and genocide exposure, possibly due to the small sample size.
We performed DMR analyses to identify within-generation epigenomic differences associated with exposure to genocide, focusing on differentially methylated promoter regions. Our within generation analyses identified 16 DMRs in children and 24 in mothers at FDR corrected p < 0.05. ARL5C and PM20D1 in mothers, and TMEM204 and LDHC in children, were the top two DMRs in each within-generation analysis, respectively. ARL5C has been associated with maternal depression during pregnancy [47], which increases the risk of adverse neurodevelopmental outcomes in the offspring [48,49]. A longitudinal study associated changes in DNAm in PM20D1 with traumatic stress and PTSD symptoms [50]. LDHC was previously identified as DMR associated with PTSD [51]. These results suggest that the DMRs identified through our within-generation analyses are relevant to our primary exposure of interest in this study – genocide exposure – and may play a role in shaping mental health after exposure to this extreme traumatic stressor.
There were six DMRs (BCOR, FGFR2, HOXA5, PM20D1, VWDE and PRDM8) common to both mothers and children. BCOR plays a key role in early embryonic development [52] and has been associated with many diseases, including cancer, developmental delay and cutaneous syndrome [53–55]. Dysregulation in FGFR2 has been associated with major depressive disorder (MDD) [56,57], bipolar disorder [58] and schizophrenia [59] and has been shown to be associated with PTSD using gene set enrichment analysis [60]. Similarly, HOXA5 has been associated with MDD [61] and PM20D1 with PTSD [50,62]. Gene VWDE has been associated with intergenerational transmission of DNAm from trauma-exposed fathers to their children [63] and a risk variant (rs10950398) in VWDE has been associated with MDD [64]. PRDM8 plays a significant role in neural development [65] and has been significantly associated with maternal smoking during pregnancy [66]. Among the common DMRs between mothers and children, three DMRs (BCOR, PRDM8 and VWDE) were further implicated in intergenerational analyses and showed higher DNAm in genocide exposed versus unexposed offspring. The CpGs cg02931660 and cg02932805 related to BCOR had the lowest selection probabilities for in utero genocide exposure. For these same CpGs, child sex in the ridge regression models had a high estimated coefficient (e.g., greater than an absolute value of 1) [67] and had a high selection probability (π > 0.90). We assume this considerable effect of sex on the methylation levels of these CpGs is due to BCOR being located on the X chromosome, thus introducing possible methylation differences due to X chromosome inactivation [46]. Our results also align with findings [68] indicating that sex was significant in determining DNAm of X-linked BCOR CpGs and identified cg02931660 to be methylated in a sex-specific manner.
Although our exploratory targeted DMR analysis of NR3C1 showed suggestive methylation differences in genocide exposed versus unexposed participants, we did not identify any DMRs in NR3C1 in our genome-scale analysis. These findings, which contrast somewhat with earlier work, is not unexpected given the divergent approaches used in the prior versus current studies: prior work collected pyrosequencing data on a discrete set of CpGs within NR3C1 using a candidate gene approach, whereas the current work adopted a more agnostic, genome-scale approach, beginning with a survey of CpGs across the genome using microarray technology and then focusing on CpGs showing the highest variance in the current dataset. Nevertheless, as described earlier, the DMRs identified in both mothers and children in the current work are associated with mental disorders, suggesting that genocide exposure has an impact on mental health outcomes not only within generations but also across generations. Moreover, given the consistently higher methylation levels in exposed mothers and children at these intergenerationally implicated CpG sites, it is plausible that some of these effects may be mediated epigenetically.
It is well established that differential DNAm in the brain is associated with many psychiatric disorders (e.g. [69–71]); however, access to brain tissues is not always possible and is limited to postmortem samples. The use of peripheral tissues such as blood and saliva has become common in identifying methylation changes associated with psychiatric disease. A recent study [42] identified CpG sites highly correlated within individuals between peripheral tissues and the brain. Of the three DMRs (BCOR, PRDM8 and VWDE) that showed intergenerational impact of genocide exposure, two (BCOR and VWDE) include CpGs that showed a significant correlation with the brain indicating that these DMRs may have relevance to brain. These genomic sites may potentially serve as indicators of exposure to extreme trauma in utero that also influence offspring early development and help to predict the mental health status of the offspring.
Strengths and limitations of the study
This is the first study showing initial insight into the impact of exposure to the Rwandan genocide against ethnic Tutsi within and between generations with the concept of epigenetics on a genome scale. Findings from this study highlight potential biomarkers of exposure to extreme trauma that have potential intergenerational impact and relevance to brain function, highlighting a plausible link between genocide exposure and subsequent poor mental health, both within and between generations. We recognize the key limitations of the study such as a small sample size, which may limit the interpretation of the findings. In addition, we recognize the gaps of our study concerning the comparability of our study group in relation to possible demographic contexts and differences in the details of participants’ traumatic experiences, given that the findings of this study are based on the previous work that has not been able to collect data on participant’s social behaviors, traumatic experiences and details on demographic situation. However, the ongoing study in which we are currently recruiting more study participants (n = 450) will bridge the reported gaps in this pilot study and hence provide clear evidence on intergenerational transmission of trauma and PTSD and contribution of epigenetic mechanisms.
Conclusion
Our study provides initial insight into the impact of exposure to the Rwandan genocide against ethnic Tutsi within and between generations. Our results show that genocide exposure is associated with epigenetic modifications within generations – that is, in both mothers and in children. In addition, a subset of the epigenetic modifications observed in mothers shows a pattern consistent with potential intergenerational transmission of genocide-exposure-related epigenetic signatures, with maternal genocide exposure during pregnancy associated with increased methylation in offspring. Findings from this study highlight potential biomarkers of exposure to extreme trauma that have potential intergenerational impact and relevance to brain function, highlighting a plausible link between genocide exposure and subsequent poor mental health, both within and between generations.
Future perspective
Our results show that genocide exposure is associated with epigenetic modifications within generations. Our ongoing work will add more value on the role of epigenetics in the transmission of trauma and its effects on memory quality in genocide survivors. A subset of the epigenetic modifications observed in mothers shows a pattern consistent with potential intergenerational transmission of genocide exposure-related epigenetic signatures. These results suggest that the DMRs identified within generations are relevant to our primary exposure of interest in this study – genocide exposure – and may play a role in shaping mental health following exposure to this extreme traumatic stressor.
Summary points.
This is the first study providing genome-scale insights into the impact of exposure to the Rwandan genocide against ethnic Tutsi within and between generations in Rwanda context.
In utero genocide exposure was associated with CpGs in three differently methylated regions DMRs with higher DNA methylation in exposed versus unexposed offspring.
CpGs in two of the three in utero-associated differently methylated regions (DMRs) showed significant correlation between brain and blood methylation within individuals. This suggests peripherally derived signals of genocide exposure may be relevant to the brain.
Results suggest that the DMRs identified within generation are relevant to our primary exposure of interest in this study – genocide exposure – and may play a role in shaping mental health after exposure to this extreme traumatic stressor.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/epi-2021-0310
Financial & competing interests disclosure
This work was funded by the NIH (grant no. U01MH115485). M Uddin was a paid consultant for System Analytic in 2020. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval by the Institutional Review Board (Ref EC/CHUK/025/11) for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement
As part of our funding commitment through the H3Africa funding mechanism, our EWAS data is available to access from the European Genome Archive.
References
- 1.Lowe SR, Joshi S, Galea S et al. Pathways from assaultive violence to post-traumatic stress, depression, and generalized anxiety symptoms through stressful life events: longitudinal mediation models. Psychol. Med. 47(14), 2556–2566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubzansky LD, Koenen KC, Jones C et al. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 28(1), 125–130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubzansky LD, Koenen KC, Spiro A III et al. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch. Gen. Psychiatry 64(1), 109–116 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Keyes KM, McLaughlin KA, Demmer RT et al. Potentially traumatic events and the risk of six physical health conditions in a population-based sample. Depress. Anxiety 30(5), 451–460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husarewycz MN, Gabalawy REI, Logsetty S et al. The association between number and type of traumatic life experiences and physical conditions in a nationally representative sample. Gen. Hosp. Psychiatry 36(1), 26–32 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Jawaid A, Roszkowski M, Mansuy IM et al. Transgenerational epigenetics of traumatic stress. Prog. Mol. Biol. Transl. Sci. 158, 273–298 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Babenko O, Kovalchuk I, Metz GA et al. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 48, 70–91 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Ulloa AC, Gliga A, Love TM et al. Prenatal methylmercury exposure and DNA methylation in seven-year-old children in the Seychelles Child Development Study. Environ. Int. 147, 106321 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Park J, Kim J, Kim E et al. Prenatal lead exposure and cord blood DNA methylation in the Korean Exposome Study. Environ. Res. 195, 110767 (2021). [DOI] [PubMed] [Google Scholar]
- 10.del Blanco B,Barco A. Impact of environmental conditions and chemicals on the neuronal epigenome. Curr. Opin. Chem. Biol. 45, 157–165 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Serpeloni F, Radtke KM, Hecker T et al. Does prenatal stress shape postnatal resilience? – An epigenome-wide study on violence and mental health in humans. Front. Genet. 10, 269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosnowski DW, Booth C, York TP et al. Maternal prenatal stress and infant DNA methylation: a systematic review. Dev. Psychobiol. 60(2), 127–139 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology 41(1), 232–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulligan CJ, D'Errico NC, Stees J et al. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics 7(8), 853–857 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yehuda R,Lehrner A. Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry 17(3), 243–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kertes DA, Kamin HS, Hughes DA et al. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the Democratic Republic of Congo. Child Dev. 87(1), 61–72 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei L, Cao de Rooij SR, King S et al. Prenatal stress and epigenetics. Neurosci. Biobehav. Rev. 117, 198–210 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Yehuda R, Daskalakis NP, Bierer LM et al. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol. Psychiatry 80(5), 372–380 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Bierer LM, Bader HN, Daskalakis NP et al. Intergenerational effects of maternal holocaust exposure on FKBP5 methylation. Am. J. Psychiatry 177(8), 744–753 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Conrad D, Wilker S, Schneider A et al. Integrated genetic, epigenetic, and gene set enrichment analyses identify NOTCH as a potential mediator for PTSD risk after trauma: results from two independent African cohorts. Psychophysiology 57(1), e13288 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munyandamutsa N, Nkubamugisha PM, Fabry MG et al. Mental and physical health in Rwanda 14 years after the genocide. Soc. Psychiatry Psychiatr. Epidemiol. 47(11), 1753–1761 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Schaal S, Dusingizemungu JP, Jacob N et al. Rates of trauma spectrum disorders and risks of posttraumatic stress disorder in a sample of orphaned and widowed genocide survivors. Eur. J. Psychotraumatol. 2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perroud N, Rutembesa E, Giacobino AP et al. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J. Biol. Psychiatry 15(4), 334–345 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Rieder H, Elbert T. Rwanda – lasting imprints of a genocide: trauma, mental health and psychosocial conditions in survivors, former prisoners and their children. Confl. Health 7(1), 6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vukojevic V, Kolassa IT, Fastenrath M et al. Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. J. Neurosci. 34(31), 10274–10284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aryee MJ, Jaffe AE, Corrada-Bravo H et al. Minfi: a flexible and comprehensive bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30(10), 1363–1369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wani AH, Aiello AE, Kim GS et al. The impact of psychopathology, social adversity and stress-relevant DNA methylation on prospective risk for post-traumatic stress: a machine learning approach. J. Affect. Disord. 282, 894–905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leek JT, Johnson WE, Parker HS et al. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics (Oxford, England) 28(6), 882–883 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson WE, Li C, Rabinovic A et al. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8(1), 118–127 (2006). [DOI] [PubMed] [Google Scholar]
- 30.McCutchan PK, Freed MC, Low EC et al. Rescaling the post-traumatic stress disorder checklist for use in primary care. Military Med. 181(9), 1002–1006 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Cook TL, Shannon PJ, Vinson GA et al. War trauma and torture experiences reported during public health screening of newly resettled Karen refugees: a qualitative study. BMC Int. Health Human Rights 15(1), 8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salas LA, Koestler DC, Butler RA et al. An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol. 19(1), 64–64 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teschendorff AE, Breeze CE, Zheng SC et al. A comparison of reference-based algorithms for correcting cell-type heterogeneity in epigenome-wide association studies. BMC Bioinformatics 18(1), 105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martorell-Marugán J, González-Rumayor V, Carmona-Sáez P et al. mCSEA: detecting subtle differentially methylated regions. Bioinformatics 35(18), 3257–3262 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Ritchie ME, Phipson B, Wu Di et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43(7), e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sergushichev AA. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv e060012 (2016). [Google Scholar]
- 37.Friedman JH. Greedy function approximation: a gradient boosting machine. Ann. Statist. 29(5), 1189–1232 (2001). [Google Scholar]
- 38.Etzel L, Hastings WJ, Mattern BC et al. Intergenerational transmission of childhood trauma? Testing cellular aging in mothers exposed to sexual abuse and their children. Psychoneuroendocrinology 120, 104781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herawati N, Nisa K, Nusyirwan S et al. Regularized multiple regression methods to deal with severe multicollinearity. Int. J. Stati. Appl. 8, 167–172 (2018). [Google Scholar]
- 40.Sill M, Hielscher T, Becker N et al. c060: Extended inference with lasso and elastic-net regularized Cox and generalized linear models. J. Stat. Software 62(5), 2 (2014). [Google Scholar]
- 41.Meinshausen N, Bühlmann P. Stability selection. J. Royal Stat. Soc. 72(4), 417–473 (2010). [Google Scholar]
- 42.PR Braun, Han S, Hing B et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl. Psychiatry 9(1), 47–47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 14(10), 3156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perroud N, Rutembesa E, Paoloni-Giacobino et al. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J. Biol. Psychiatry 15(4), 334–345 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Mutuyimana C, Sezibera V, Nsabimana E et al. PTSD prevalence among resident mothers and their offspring in Rwanda 25 years after the 1994 genocide against the Tutsi. BMC Psychol. 7(1), 84 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabakambira JD, Uwera G, Hategeka M et al. Burden of post-traumatic stress disorder acute exacerbations during the commemorations of the genocide against Tutsis in Rwanda: a cross-sectional study. Pan. Afr. Med. J. 30, 216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viuff AC, Sharp GC, Raid D et al. Maternal depression during pregnancy and cord blood DNA methylation: findings from the Avon Longitudinal Study of Parents and Children. Transl. Psychiatry 8(1), 244–244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein A, Pearson RM, Goodman SH et al. Effects of perinatal mental disorders on the fetus and child. Lancet 384(9956), 1800–1819 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Rai D, Lee BK, Dalman C et al. Antidepressants during pregnancy and autism in offspring: population based cohort study. BMJ 358, j2811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutten BPF, Vermetten E, Vinkers CH et al. Longitudinal analyses of the DNA methylome in deployed military servicemen identify susceptibility loci for post-traumatic stress disorder. Mol. Psychiatry 23(5), 1145–1156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krzyzewska IM, Ensink JBM, Nawijn L et al. Genetic variant in CACNA1C is associated with PTSD in traumatized police officers. Eur. J. Hum. Genet. 26(2), 247–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wamstad JA, Corcoran CM, Keating AM et al. Role of the transcriptional corepressor Bcor in embryonic stem cell differentiation and early embryonic development. PLoS ONE 3(7), e2814–e2814 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grossmann V, Tiacci E, Holmes AB et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood 118(23), 6153–6163 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Hilton E, Johnston J, Whalen S et al. BCOR analysis in patients with OFCD and Lenz microphthalmia syndromes, mental retardation with ocular anomalies, and cardiac laterality defects. Eur. J. Hum. Genet. 17(10), 1325–1335 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies HR, Hodgson K, Schwalbe E et al. Epigenetic modifiers DNMT3A and BCOR are recurrently mutated in CYLD cutaneous syndrome. Nat. Comm. 10(1), 4717–4717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans SJ, Choudary PV, Neal CR et al. Dysregulation of the fibroblast growth factor system in major depression. Proc. Natl Acad. Sci. USA 101(43), 15506–15511 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng Z, Deng S, Zhang Mu R et al. Fibroblast growth factors in depression. Front. Pharmacol. 10, 60–60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang T, Zeng Z, Hu Z et al. FGFR2 is associated with bipolar disorder: a large-scale case-control study of three psychiatric disorders in the Chinese Han population. World J. Biol. Psychiatry 13(8), 599–604 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Van Scheltinga AFT, Bakker SC,Kahn RS. Fibroblast growth factors in schizophrenia. Schizophr. Bull. 36(6), 1157–1166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Logue MW, Miller WM, Wolf EJ et al. An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clin. Epigenet. 12(1), 46–46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker RM, Christoforou AN, McCartney DL et al. DNA methylation in a Scottish family multiply affected by bipolar disorder and major depressive disorder. Clinical Epigenetics 8(1), 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ensink JBM, Keding TJ, Henneman P et al. Differential DNA methylation is associated with hippocampal abnormalities in pediatric posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mehta D, Pelzer ES, Bruening D et al. DNA methylation from germline cells in veterans with PTSD. J. Psychiatr. Res. 116, 42–50 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Wray NR, Ripke S, Mattheisen M et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50(5), 668–681 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Komai T, Iwarari H, Mochizuki Y et al. Expression of the mouse PR domain protein Prdm8 in the developing central nervous system. Gene Expr. Patterns 9(7), 503–514 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Joubert BR, Janine F Felix, Yousefi P et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am. J. Human Genet. 98(4), 680–696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan MHR, Bhadra A, Howlader T et al. Stability selection for lasso, ridge and elastic net implemented with AFT models. Stat. Appl. Genet. Mol. Biol. 18(5), 2019). [DOI] [PubMed] [Google Scholar]
- 68.Joo JE, Novakovic B, Cruickshank M et al. Human active X-specific DNA methylation events showing stability across time and tissues. Eur. J. Human Genet. 22(12), 1376–1381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaffe AE, Gao Y, Deep-Soboslay A et al. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat. Neurosci. 19(1), 40–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdolmaleky HM, Yaqubi S, Papageorgis P et al. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr. Res. 129(2-3), 183–190 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Sabunciyan S, Aryee MJ, Irizarry RA et al. Genome-wide DNA methylation scan in major depressive disorder. PLoS ONE 7(4), e34451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.