Abstract
We examined the relationship between CSF immune cells and neurocognition and neuronal damage in HIV+ individuals before and after initiating antiretroviral therapy. Multivariate analysis at baseline indicated that greater CD4+ T cell abundance was associated with better cognition (p = .017), while higher CSF HIV RNA was associated with increased neuronal damage (p = .014). Following 24 weeks of antiretroviral therapy, CD8+ T cells, HLA-DR expressing CD4+ and CD8+ T cells, B cells, NK cells, and non-classical monocyte percentage decreased in CSF. Female gender was negatively associated with cognitive performance over time, as was higher percentage of HLA-DR expressing CD8+ T cells at baseline.
Keywords: HIV, AIDS, Cognition, Cerebrospinal fluid, Flow cytometry
1. Introduction
Even in the era of combined antiretroviral therapy (ART), HIV-associated neurocognitive disorder (HAND) remains prevalent and is associated with an increased risk of morbidity and mortality (Heaton et al., 2010; Vivithanaporn et al., 2010). Additionally, studies using both soluble and imaging biomarkers have shown that neuronal damage also persists during HIV despite ART (Harezlak et al., 2011; Yilmaz et al., 2017). A possible explanation for these findings is the steadfast inflammatory state of the central nervous system (CNS) of infected individuals (Ulfhammer et al., 2018). Much like the peripheral blood, where a significant association has been found between inflammation and adverse outcomes during HIV (Sandler et al., 2011), CNS inflammation may have a role in the development of HIV-associated neurocognitive impairment and neuronal damage, even during suppressive ART (Eden et al., 2016).
The traditional “Trojan horse” model of HIV neuropathogenesis involves the trafficking of HIV-infected monocytes across the blood brain barrier (BBB) with resulting neuroinflammation (Thompson et al., 2011; Wiley et al., 1986). However, lymphoid homing T cells surveilling the CNS in the setting of HIV infection may also promote local inflammation. (Ellwardt et al., 2016; Louveau et al., 2015). Cases of CD8+ T-cell encephalitis support the concept that certain lymphoid subsets in the CNS could be detrimental during HIV (Lescure et al., 2013).
The ability of flow cytometry to characterize samples with low cellular abundance has provided a means to study cerebrospinal fluid (CSF) cells from HIV-infected individuals. The discovery of resident immune cells in the CSF with flow cytometry has led to further investigation of the role of these cells in immune surveillance (Masopust and Soerens, 2019; Spudich, 2016). Previous studies have found that there is a relative predominance of CD8+ T cells from the CSF T cell pool among HIV-infected individuals and a lower CD4+ to CD8+ ratio in the CSF compared to HIV seronegatives (Ho et al., 2013; Margolick et al., 1988). However, few studies have examined the relationship between CSF immune cells and cognition in the ART era, and there is a dearth of evidence on CSF cytometry change over time after ART initiation. Evidence is also needed to better understand the potential relationship between CSF immune cells and neuronal damage.
In this study, our group utilized flow cytometry to examine the effect of CSF immune surveilling cell populations on cognition and neuronal damage in the absence of virologic control as well as change in cognition and neuronal damage over time after initiation of ART. We hypothesized that CSF immune characteristics would be associated with cognition as well as neuronal damage independently of CSF HIV RNA levels.
2. Materials and methods
2.1. Study participants
HIV-infected (HIV+) and HIV-seronegative (HIV-negative) individuals were enrolled prospectively from 2014 to 2017 at the Emory University Center for AIDS Research (CFAR) clinical core site. HIV+ individuals were eligible for inclusion if: 1) Age at least 18 years old and 2) ART-naïve or ART-experienced but off treatment for at least six months. HIV-negative individuals were eligible if at least 18 years old and confirmed to be HIV-negative at baseline with the commercially available fourth generation combined HIV antigen/antibody test. Exclusion criteria for both participant groups were: 1) history of any neurologic disease that is known to affect memory (including stroke, malignancy involving the brain, traumatic brain injury, and AIDS-related opportunistic infection of the central nervous system); 2) active substance use (marijuana use in the last 7 days OR cocaine, heroin, methamphetamine, or other non-marijuana illicit drug use in the last 30 days); 3) heavy alcohol consumption in the last 30 days (defined as > 7 drinks per week for women and > 14 drinks per week for men); or 4) serious mental illness including schizophrenia and bipolar disorder (depression was not excluded if participants were well controlled on treatment). Lastly, participants were excluded if cognitive symptoms had rapidly progressed in the past 30 days so that medical evaluation could be undertaken. The study was approved by the Institutional Review Board and written consent was obtained from all participants. The HIV+ participants were invited for a second visit after starting on ART and continuing for at least 24 weeks. Only participants who achieved plasma HIV RNA decrease of at least 1log10 copies/ml were analyzed at the second visit. Gender was defined by participant report and review of medical record.
2.2. Neuropsychological assessment
A comprehensive neuropsychological (NP) testing battery was administered to the HIV+ participants at both visits that included 14 tests used commonly in studies of cognition and HIV infection (Robertson and Yosief, 2014): 1) Trailmaking Part A; 2) Trailmaking Part B; 3) Hopkins Verbal Learning Test total learning; 4) Hopkins Verbal Learning Test delayed recall; 5) Brief Visuospatial Learning Test total learning; 6) Brief Visuospatial Learning Test recall; 7) Grooved Pegboard (dominant) 8) Grooved Pegboard (non-dominant); 9) Finger board tapping test (right hand) 10) Stroop Color Naming, 11) Stroop Color-Word interference; 12) Digit Vigilance test (total time); 13) Digit Vigilance test (errors), and 14) Letter Fluency (Controlled Oral Word Association Test). These tests were selected in order to examine at least five domains as recommended in the most recent nosology of HAND criteria (Antinori et al., 2007). Scores were adjusted for demographic characteristics (including age, gender, race, and education) using published norms (Heaton et al., 2004). A composite global mean T score (NPT-14) was then calculated by average of individual T scores. Score adjustment for practice effects was made for longitudinal visits by published methods (Cysique et al., 2011). Global Deficit Score (GDS), a validated measure of neurocognitive impairment in HIV based on T scores was also calculated (Carey et al., 2004), with GDS ≥ 0.5 being consistent with overall impairment.
2.3. Laboratory procedures
Blood and CSF were obtained at the same study visit. HIV RNA was measured from plasma and CSF at the Emory Center for AIDS Research Virology Core using the Abbott Laboratories m2000 Real Time HIV-1 assay system (reverse transcriptase polymerase chain reaction, lowest limit of detection of 40 copies/ml). Neurofilament light chain (NFL) was quantitated with a standard Enzyme Linked Immunosorbent Assay (ELISA) that has been used commonly in studies of HIV and other neurological diseases (UMAN diagnostics) (Peterson et al., 2014). All flow cytometry was performed in real time on the day of sample collection. Paired whole blood and CSF were stained in BD TruCOUNT ™ tubes (BD Biosciences, San Jose, CA) for absolute cell quantification using a “no wash” procedure. For whole blood staining, antibodies were combined with 50 μl of whole blood and incubated for 20 min at room temperature in the dark. Red blood cells were then lysed with 450 μl of 1× FACS Lysing Solution (BD Biosciences). After recording total volume of CSF, the fluid was centrifuged at 300 RCF for 15 min. The cell pellet was then re-suspended in this residual volume before transfer to tubes for staining. CSF cells were stained in the dark for 20 min then re-suspended in 350 μl of 0.5% formaldehyde. Antibodies used for FACS staining of whole blood and CSF samples included anti-CD45 (fluorochrome PerCP-Cy5.5; clone 2D1), anti-CD3 (fluorochrome Alexa 700; clone UCHT1), anti-CD4 (fluorochrome APC-Cy7; clone RPA-T4), anti-CD8 (fluorochrome V500; clone SK1), anti-CD19 (fluorochrome FITC; clone HIB19), anti-CD14 (fluorochrome Pac Blue; clone M5E2), anti-CD16 (fluorochrome PE; clone 3G8), anti-CD56 (fluorochrome PE-Cy7; clone HCD56), and anti-HLA-DR (fluorochrome APC; clone G46–6). All antibodies were obtained from BD Biosciences, except anti-CD56, which was obtained from Biolegend.
Data were collected using an LSRII flow cytometer (BD Biosciences). For whole blood, approximately 20,000 bead events were acquired, and for CSF samples, the entire cell suspension was acquired. Analysis and compensation of data was performed using FlowJo (Tree Star, Inc., Ashland, OR). The gating strategy used to define each population (Fig. 1) was determined using blood analysis (top panel) and then applied to CSF (bottom panel) with some adjustments for CSF gating as described below. After single cell selection by Forward Scatter Height vs Area (FSC-H vs FSC-A) and Side Scatter (SSC) Height vs Area (SSC-H vs SSC-A), CD45 was plotted against SSC-A and total WBC and beads gates were drawn separately to exclude debris. From the total WBC, lymphocytes (Part C) were gated in a CD45 vs SSC plot. CD3+ and CD19+ populations were identified as T and B cells separately from the lymphocyte gate. The CD3+ population was then further gated for CD4+ and CD8+ T cells (part D and E). HLA-DR expression was used to identify activated CD4 and CD8 T cells (part H and I). From the total WBC, another gate was drawn to include both lymphocytes and monocytes (part J). After T (CD3+) and B (CD19+) cell populations were excluded, CD56 + cells were identified as NK cells (part K). Monocytes were identified within CD3−CD19−CD56− HLA-DR+ population and gated for the CD14 + CD16−, CD14 + CD16+, and CD14dimCD16++ subsets (part L, M, and N). TruCOUNT beads were used to determine the absolute number of events per microliter of blood or per milliliter of CSF. Absolute counts were calculated by dividing the cell population event count by the TruCOUNT bead event count, then multiplying by the ratio of known bead input divided by the original blood (50 μl for all sample) or specific CSF volume (typically 15–20 ml) for each sample.
Fig. 1.
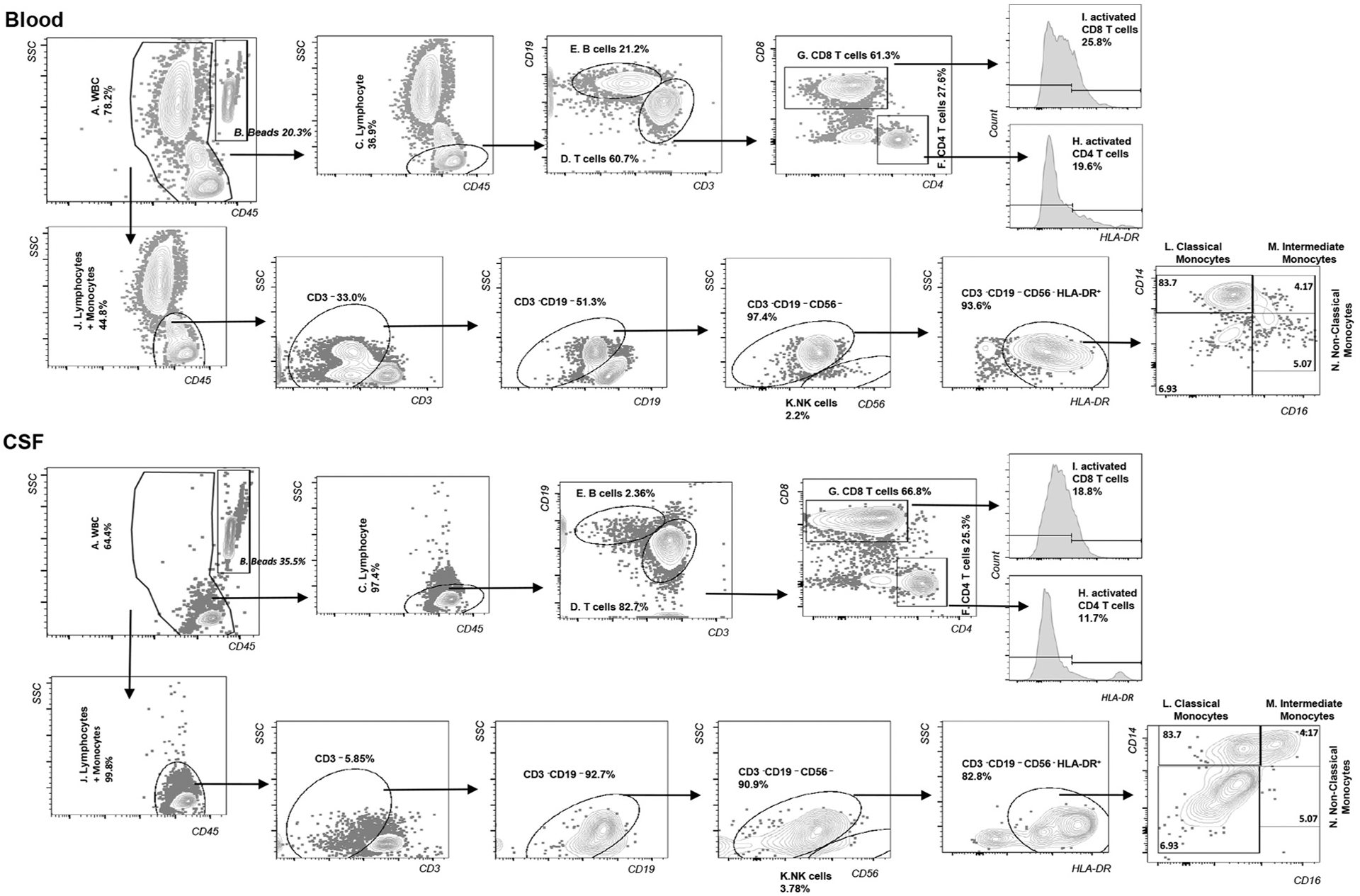
Gating Strategy.
2.4. Statistical analyses
For the univariate analysis, continuous variables were first assessed for normality with the Shapiro-Wilk test. Because conditions for normality were not met in most cases (including the cytometry results), the Wilcoxon rank sum test was used to test the differences in continuous variables. Fisher’s exact test was used to compare categorical variables. For correlation between immune cells from blood and CSF compartments, spearman’s rho was generated. Linear regression was used to account for differences in HIV+ and negative groups for CSF cytometry results (specifically accounting for CSF protein, a marker of blood brain barrier permeability which was different between the two groups). For cellular changes over time, Wilcoxon signed rank test was used. For NP change over time, paired t-test was used given normal distribution. For the analysis of NPT-14 and NFL as dependent variables at baseline, we incorporated age, gender, CSF HIV RNA (log10 transformed), CSF CD4+ T cells, and CSF CD8+ T cells as variables in univariate and multivariate linear regression analysis. Given pre-existing literature, we also incorporated percentage of CSF CD8+ expressing the immune activation-associated molecule HLA-DR as well as the percentage of CSF monocytes expressing a non-classical phenotype (Fischer-Smith et al., 2001; Grauer et al., 2015). We also performed an exploratory analysis of the relationship between NPT-14 and the same blood cells and plasma HIV RNA. For the analysis of change in NPT-14 and NFL over time, we examined these same CSF cell subsets at baseline as well as CSF HIV RNA, age, and sex all as independent variables. Alpha level for statistical significance was set at < 0.05.
3. Results
3.1. Baseline evaluation
44 HIV+ participants and 13 HIV-negative participants were enrolled. As seen in Table 1, the two groups were statistically similar in terms of age, race, gender, and medical comorbidities. There were no trans-gender participants in the study. Nine HIV+ participants were ART-naïve (20.5%), while the rest were ART-experienced with a median time off treatment of 12 months. As mentioned in the methods, there were no AIDS-defining diseases of the central nervous system. However, seven participants had a history of thrush or esophageal candidiasis, three had a history of pneumocystis pneumonia, one had a history of Kaposi’s Sarcoma, and one had a history of non-meningeal cryptococcosis. Mean composite global T score on the comprehensive neuropsychologic testing battery (NPT-14) for the HIV+ group was 48.3, with standard deviation (SD) = 7.6. 13 (29.5%) met criteria for neuropsychologic (NP) impairment by Global Deficit Score (GDS). The mean volume of CSF dedicated for cytometry was between 8.0 and 9.0 ml from both the first and second visits among HIV+ participants as well as among HIV-negative participant visits. CSF red blood cell count was not significantly different between the two groups, but CSF total protein level was slightly higher in the HIV+ group (median concentration 36 versus 30 mg/deciliter, p = .03). Median CSF neurofilament light chain (NFL) concentration in the HIV+ group was 814.4 picograms/ml (interquartile range [IQR] = 682.6–1501.8). Among HIV + participants, median CSF cell yield at visit one was 1767 cells/ml (1608 cells/ml were lymphocytes), and median CSF cell yield at visit two was 1539 cells/ml (1328 cells/ml were lymphocytes). Among HIVnegative participants, median CSF cell yield was 951 cells/ml (779 cells/ml were lymphocytes).
Table 1.
Baseline characteristics by HIV status. Second row of CSF values are adjusted for CSF protein difference between HIV+ and HIV-negatives. Blood cells are per microliter and CSF cells are per milliliter.
| Variable | HIV+ (n = 44) | HIV-neg (n = 13) | P value |
|---|---|---|---|
| Age in years | 40.1 (32.2–47.8) | 35.0 (31.6–43.8) | 0.4 |
| Male sex | 36 (81.8%) | 11 (84.6%) | 1.0 |
| African American race | 39 (88.6%) | 11 (84.6%) | 0.7 |
| Medical Comorbidities: | |||
| Hypertension | 3 (6.8%) | 0 (0%) | 1.0 |
| Diabetes | 1 (2.3%) | 0 (0%) | 1.0 |
| Hepatitis C | 3 (6.8%) | 0 (0%) | 1.0 |
| Months since HIV diagnosis | 90 (30–199) | – | |
| Nadir CD4+ | 107 (27–231) | – | |
| Plasma HIV RNA (log10) | 4.7 (4.2–5.3) | – | |
| CSF HIV RNA (log 10) | 3.2 (2.3–3.8) | – | |
| CSF Protein (mg/dl) | 36 (29–48) | 30 (24–38) | 0.03* |
| CSF RBC (per microliter) | 0 (0–1) | 0 (0−10) | 0.58 |
| CSF NFL (pg/ml) | 814 (683–1502) | – | |
| Flow Cytometry (blood): | |||
| CD4+ T cells | 200 (59–395) | 1048 (846–1427) | < 0.0001* |
| CD8+ T cells | 737 (444–1622) | 456 (353–655) | 0.02* |
| CD4+/CD8+ | 0.23 (0.11–0.45) | 2.36 (1.94–3.36) | < 0.0001* |
| % CD4 HLADR+ | 14.6 (10.5–26.0) | 2.6 (1.6–3.1) | < 0.0001* |
| %CD8 HLADR+ | 16.0 (10.5–25.2) | 1.7 (1.2–2.5) | < 0.0001* |
| CD19+ B cells | 176 (99–312) | 319 (263–467) | 0.006* |
| NK cells | 93 (63–133) | 235 (174–386) | 0.003* |
| Monocytes (total) | 332 (212–564) | 383 (306–455) | 0.51 |
| Monocytes (%classical) | 75% (68–84%) | 78% (76–86%) | 0.3 |
| Monocytes (%intermediate) | 9% (7–15%) | 6% (5–7%) | 0.002* |
| Monocytes (%non-classical) | 13% (7–18%) | 16% (9–17%) | 0.38 |
| Flow Cytometry (CSF): | |||
| CD4+ T cells | 326 (53–1223), 296 (28–1160) | 360 (164–930), 316 (142–777) | 0.52, 0.52 |
| CD8+ T cells | 889 (360–4352) 860 (317–4236) | 155 (91–264) 132 (70–154) | < 0.0001*, < 0.0001 |
| CD4+/CD8+ | 0.31 (0.10–0.47) 0.19 (0.04–0.43) | 2.48 (1.54–4.10) 3.44 (1.94–4.45) | < 0.0001*, < 0.0001 |
| % CD4 HLADR+ | 13% (7–24%), 20% (15–25%) | 4% (1–6%), 4% (1–7%) | < 0.0001*, 0.007* |
| % CD8 HLADR+ | 14% (8–26%), 17% (14–21%) | 3% (1–5%), 4% (1–7%) | < 0.0001*, 0.0007* |
| CD19+ B cells | 54 (27–125), 144 (63–255) | 31 (14–41), 164 (11–316) | 0.01*, 0.82 |
| NK cells | 24 (14–73), 59 (31–86) | 25 (10–37), 72 (20–124) | 0.32, 0.66 |
| Monocytes (total) | 25 (13–53), 41 (24–57) | 71 (15–93), 75 (44–105) | 0.17, 0.06 |
| Monocytes (%classical) | 33% (25–42%), 33% (29–37%) | 24% (16–28%), 23% (15–30%) | 0.008*, 0.023* |
| Monocytes (% intermediate) | 52% (42–62%), 52% (47–57%) | 61% (44–68%), 57% (48–66%) | 0.25, 0.35 |
| Monocytes (%non-classical) | 14% (7–18%), 15% (11–19%) | 16% (7–33%), 20% (13–27%) | 0.37, 0.23 |
P values < .05 are denoted by asterisk.
Similar to blood, CSF CD8+ T cells as well as the percentage of CD4+ and CD8+ T cell subsets expressing HLA-DR (activated CD4+ and activated CD8+ respectively) were higher in HIV+ compared to HIV-negative. This difference remained significant in the analysis incorporating difference in CSF protein between groups (as shown in the second line of each CSF flow cytometry row in Table 1). In contrast to blood, CSF CD4+ levels were not significantly different between HIV+ and HIV-negatives. See Fig. 2 for box and whisker plots showing differences in cell abundance between HIV+ and HIV-negative at visit 1. While blood B cell concentrations were lower in HIV+, there was no difference between CSF B cell concentrations between the two participant groups when adjusting for CSF protein concentration. Lastly, while percentage of monocytes that were intermediate was higher in the blood for HIV+ participants, the difference in CSF monocyte populations between participant groups was related to classical monocyte phenotypes (CD14++CD16neg) from the HIV+ participant samples. See supplementary text file for absolute concentrations of HLADR+ lymphocyte subsets and monocyte subsets.
Fig. 2.
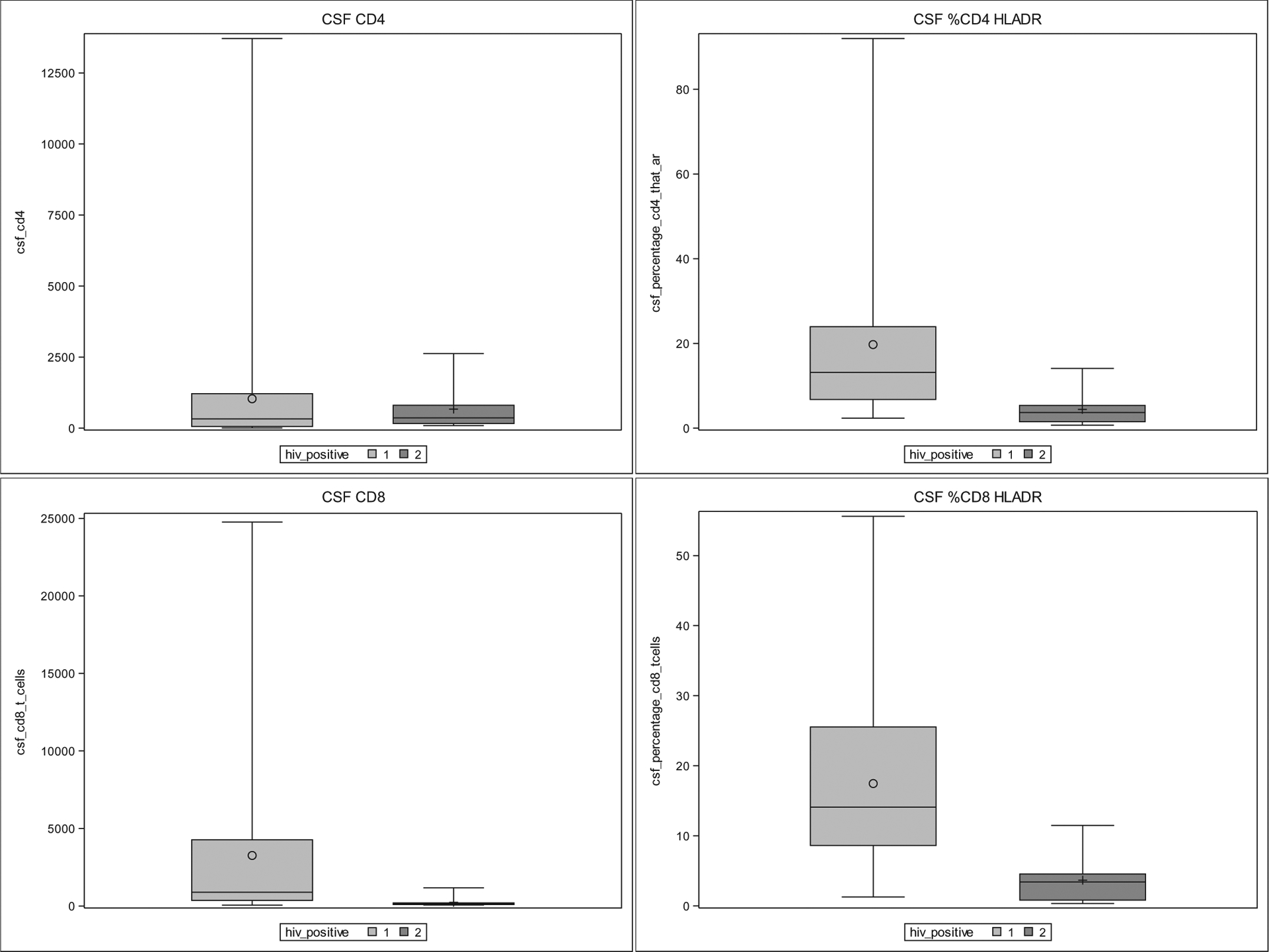
CSF cytometry results by box and whisker plots comparing HIV+ at first visit (1) and HIVnegative (2). Box represents 25–75% range with median (horizontal line) and mean (O or +). Whiskers represent minimum and maximum. P < .05 for %CD4 + HLA-DR+, CD8+ and %CD8 + HLA-DR.
As shown in Table 2, there was a positive correlation between blood and CSF concentrations of CD4+ cells, CD8+ cells, and monocytes. In the univariate analysis of NPT-14 at baseline, only CSF CD4+ T cell concentration (Beta = 0.0015, p = .003) and CSF CD8+ T cell concentration (Beta = 0.00046, p = .044) were significantly associated with higher NPT-14. All other variables were not significantly associated (all p > .2). In the multivariate model that incorporated all covariables (Table 5, part 1), only higher CSF CD4+ concentration remained significantly associated with higher NPT-14 (Beta = 0.002, p = .017). An exploratory multivariate model evaluating the same cell subsets from blood as independent variables and NPT-14 as the dependent variable yielded the following coefficients: age = 0.01879, p = .8810; female sex = −0.3113, p = .9154; CD4 + = −0.00092, p = .7983; CD8 + = 0.003675, p = .0247; %CD8 HLA-DR + = −0.00467, p = .9627; %non-classical monocytes (CD14dimCD16+ +) = 20.0934, p = .1499, plasma log10 HIV RNA = −1.3099, p = .3516. As seen in Table 2, multiple cell types correlated between blood and CSF (including CD4+ and CD8+). Therefore due to collinearity, blood cell results were not incorporated into the final model of NPT-14 at baseline. In the univariate analysis of NFL at baseline, only higher CSF HIV RNA (Beta = 3663, p = .039) was associated with higher NFL. All other variables were not significantly associated (all p > .2) with NFL. In the multivariate model incorporating covariables (Table 5, part 2), higher CSF HIV RNA remained significantly associated (Beta = 5756, p = .014) with increasing NFL.
Table 2.
Correlations between blood and CSF cell types at baseline.
| Cell type | Spearman’s rho coefficient (p value) | |
|---|---|---|
| Total group at baseline (n = 57) | HIV+ group only (n = 44) | |
| CD4+ T cells | 0.44 (0.0005)* | 0.57 (< 0.0001)* |
| CD8+ T cells | 0.36 (0.006)* | 0.33 (0.029)* |
| CD19+ B cells | −0.10 (0.46) | 0.08 (0.60) |
| NK cells | 0.02 (0.89) | 0.13 (0.40) |
| Monocytes (total) | 0.28 (0.034)* | 0.22 (0.15) |
< 0.05
Table 5.
Multivariate results – association of covariates with NPT-14 baseline, NFL baseline, NPT change over time, and NFL change over time.
| Variable | Beta estimate | P value | 95% CI (lower, upper) |
|---|---|---|---|
| Outcome = NPT-14 baseline | |||
| Age | 0.000525 | 0.9969 | −0.2739, 0.2750 |
| Female sex | −3.2748 | 0.2562 | −9.0318, 2.4822 |
| CSF CD4+ | 0.002396 | 0.0173* | 0.000449, 0.004344 |
| CSF CD8+ | −0.00029 | 0.5289 | −0.00121, 0.000632 |
| CSF percentage HLA-DR+ CD8+ T cells | 0.07469 | 0.4514 | −0.1243, 0.2737 |
| CSF percentage non-classical monocytes | 5.1778 | 0.5724 | −13.2536, 23.6092 |
| log CSF HIV RNA | −1.5744 | 0.2511 | −4.3115, 1.1627 |
| Outcome = NFL baseline | |||
| Age | 234.30 | 0.2999 | −217.49, 686.09 |
| Female sex | −4168.45 | 0.3783 | −13,646, 5309.30 |
| CSF CD4+ | 0.1884 | 0.9058 | −3.0178, 3.3946 |
| CSF CD8+ | −0.9800 | 0.1977 | −2.4946, 0.5345 |
| CSF percentage HLA-DR+ CD8+ T cells | −198.33 | 0.2274 | −525.90, 129.23 |
| CSF percentage non-classical monocytes | −1858.89 | 0.9018 | −32,202, 28,485 |
| log CSF HIV RNA | 5755.84 | 0.0137* | 1249.69, 10,262 |
| Outcome = NPT-14 change | |||
| Age | 0.1762 | 0.1700 | −0.08325, 0.4357 |
| Female sex | −6.4043 | 0.0134* | −11.3011, −1.5075 |
| CSF CD4+ baseline | −0.00085 | 0.1578 | −0.00207, 0.000364 |
| CSF CD8+ baseline | 0.000226 | 0.4158 | −0.00035, 0.000799 |
| CSF % HLA-DR+ CD8+ T cells baseline | −0.2511 | 0.0056* | −0.4181, −0.08417 |
| CSF % non-classical monocytes baseline | −2.7496 | 0.7314 | −19.3755, 13.8764 |
| log CSF HIV RNA baseline | −0.4862 | 0.6630 | −2.7990, 1.8266 |
| Outcome = NFL change | |||
| Age | −192.91 | 0.6912 | −1199.98, 814.17 |
| Female sex | 6695.85 | 0.4674 | −12,308, 25,700 |
| CSF CD4+ baseline | 0.4785 | 0.8329 | −4.2347, 5.1917 |
| CSF CD8+ baseline | 0.9218 | 0.3938 | −1.3009, 3.1444 |
| CSF % HLA-DR+ CD8+ T cells baseline | 339.36 | 0.2845 | −308.54, 987.27 |
| CSF % non-classical monocytes baseline | 4890.66 | 0.8748 | −59,632, 69,414 |
| log CSF HIV RNA baseline | −8559.65 | 0.0603 | −17,535, 415.86 |
P values < .05 are denoted by asterisk.
3.2. Longitudinal evaluation
25 HIV+ participants who were maintained in follow up and who had a decline in plasma HIV of at least 1log10 copies/ml at the second visit were analyzed (median time between visits = 29 weeks, IQR = 27.5–33). 23 of these participants (92%) had suppressed plasma HIV RNA to < 200 copies/ml. NPT-14 increased significantly (p = .007) between the two visits from a mean of 47.7 (SD = 7.8) to a mean of 50.5 (SD = 7.4), while CSF NFL decreased significantly (p < .001) from a median of 824.8 pg/ml (IQR 656.6–1823.9) to a median of 686.3 pg/ml (IQR = 496.4–975.0). See Fig. 3 for box and whisker plots showing differences in cell abundance between HIV+ and HIV-negative at visit 2. In both blood and CSF, the percentage of CD4+ and CD8+ T cells that were activated decreased by approximately 50% (Table 3). Although ART use led to an increase in blood CD4+ T cells, blood CD8+ T cells remained stable over time. CSF CD4+ T cell abundance did not significantly change over time, while CSF CD8+ T cells significantly decreased. B cell changes were inverted between the two compartments (increasing in blood and decreasing in CSF) over time. NK cells decreased in the CSF compartment, while intermediate monocyte percentage in blood and non-classical monocyte percentage in CSF respectively decreased. Compared to the HIV-negative group, the subset of HIV+ participants at visit two (see Table 4) was again statistically similar in terms of age, gender, race, and medical comorbidities. CSF total protein was again higher in the HIV+ group (p = .027). CD8+ T-cell concentration, percent of CD4+ that were HLA DR+, and percent of monocytes that were non-classical remained significantly different in CSF when comparing HIV+ to HIV-negative, even when accounting for differences in CSF protein.
Fig. 3.
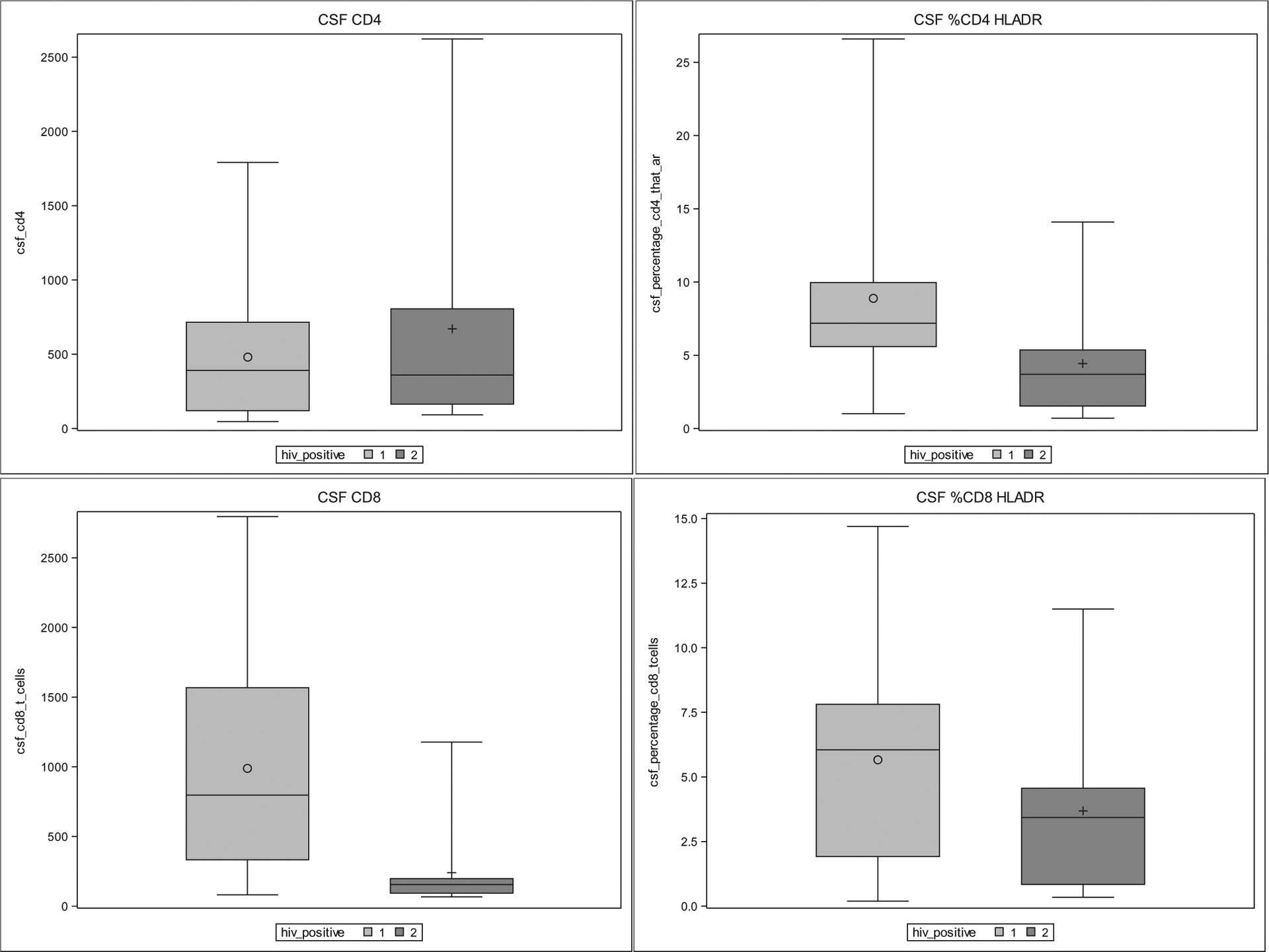
CSF cytometry results by box and whisker plots comparing HIV+ at second visit (1) and HIVnegative (2). Box represents 25–75% range with median (horizontal line) and mean (O or +). Whiskers represent minimum and maximum. P < .05 for CD8+ and %CD4 + HLA-DR+.
Table 3.
Cytometry changes in HIV+ participants at 24 week visit. Blood cells are per microliter and CSF cells are per milliliter.
| Visit 1 | Visit 2 | P value | |
|---|---|---|---|
| Blood cell type | |||
| CD4+ | 189 (68–523) | 358 (165–479) | 0.0002* |
| %CD4+ that are HLADR+ | 15 (12–25) | 8 (5–13) | 0.007* |
| CD8+ | 672 (457–1484) | 772 (603–1089) | 0.80 |
| %CD8+ that are HLADR+ | 15 (7–25) | 7 (2–9) | 0.001* |
| CD4/CD8 | 0.19 (0.13–0.34) | 0.41 (0.22–0.65) | 0.021* |
| CD19+ | 163 (94–296) | 300 (182–464) | 0.007* |
| NK | 91 (64–154) | 118 (69–154) | 0.99 |
| Monocytes total | 325 (227–436) | 312 (195–415) | 0.31 |
| Monocytes (%classical) | 75% (68%–85%) | 81% (72%–86%) | 0.25 |
| Monocytes (%intermediate) | 11% (6%–15%) | 5% (3%–8%) | 0.01* |
| Monocytes (%non-classical) | 12% (6%–18%) | 12% (10%–17%) | 0.55 |
| CSF cell type | |||
| CD4 | 593 (56–1472) | 391 (116–731) | 0.09 |
| %CD4+ that are HLADR+ | 13 (9–24) | 7 (6–10) | 0.003* |
| CD8 | 1696 (316–5800) | 797 (329–1579) | 0.006* |
| %CD8+ that are HLADR+ | 16 (6–25) | 6 (2–8) | 0.0003* |
| CD4/CD8 | 0.34 (0.11–0.55) | 0.44 (0.35–0.64) | 0.02* |
| CD19 | 94 (31–270) | 42 (21–57) | 0.002* |
| NK | 53 (16–89) | 16 (10–39) | 0.027* |
| Monocytes total | 39 (15–55) | 23 (10–62) | 0.39 |
| Monocytes (%classical) | 30% (25%–36%) | 37% (25%–43%) | 0.42 |
| Monocytes (%intermediate) | 54% (43%–63%) | 57% (49%–67%) | 0.45 |
| Monocytes (%non-classical) | 13% (9%–19%) | 8% (6%–14%) | 0.03* |
P values < .05 are denoted by asterisk.
Table 4.
Second visit for HIV+ versus HIV negative at baseline. Second row of CSF values are adjusted for CSF protein difference between HIV+ and HIV-negatives. Blood cells are per microliter and CSF cells are per milliliter.
| Variable | HIV+ (n = 25) | HIV-neg (n = 13) | P value |
|---|---|---|---|
| Age in years | 43.3 (34.5–48.7) | 35.0 (31.6–43.8) | 0.12 |
| Male sex | 20 (80%) | 11 (84.6%) | 1.0 |
| African American race | 21 (84%) | 11 (84.6%) | 1.0 |
| Medical Comorbidities: | |||
| Hypertension | 1 (4%) | 0 (0%) | 1.0 |
| Diabetes | 0 (0%) | 0 (0%) | 1.0 |
| Hepatitis C | 0 (0%) | 0 (0%) | 1.0 |
| Nadir CD4+ | 106.5 (32–227.5) | – | |
| Plasma HIV RNA (log10) | < 1.6 (< 1.6–1.87) | – | |
| CSF HIV RNA (log 10) | < 1.6 (< 1.6–1.6) | – | |
| CSF Protein (mg/dl) | 38 (31–44) | 30 (24–38) | 0.027* |
| CSF RBC (per microliter) | 0 (0–5) | 0 (0–10) | 0.64 |
| CSF NFL (pg/ml) | 686 (496–975) | – | |
| Flow Cytometry (blood): | |||
| CD4+ T cells | 358 (165–479) | 1048 (846–1427) | < 0.0001* |
| CD8+ T cells | 772 (603–1089) | 456 (353–655) | 0.0006* |
| CD4+/CD8+ | 0.41 (0.22–0.65) | 2.36 (1.94–3.36) | < 0.0001* |
| %CD4 HLADR+ | 8% (5%–13%) | 2.6% (1.6%–3.1%) | < 0.0001* |
| %CD8 HLADR+ | 7% (2%–9%) | 1.7% (1.2%–2.5%) | 0.002* |
| CD19+ B cells | 300 (182–464) | 319 (263–467) | 0.6 |
| NK cells | 118 (69–154) | 235 (174–386) | 0.0003* |
| Monocytes (total) | 312 (195–415) | 383 (306–455) | 0.23 |
| Monocytes (classical) | 81% (72%–86%) | 78% (76%–86%) | 0.81 |
| Monocytes (intermediate) | 5% (3%–8%) | 6% (5%–7%) | 0.48 |
| Monocytes (non-classical) | 12% (10%–17%) | 16% (9%–17%) | 0.69 |
| Flow Cytometry (CSF): | |||
| CD4+ T cells | 391 (116–731), 467 (228–706) | 360 (164–930), 665 (333–997) | 0.5, 0.35 |
| CD8+ T cells | 797 (329–1579), 978 (682–1274) | 155 (91–264), 213 (91–335) | 0.0003*, 0.005* |
| CD4+/CD8+ | 0.44 (0.35–0.64), 0.52 (0.09–0.96) | 2.48 (1.54–4.10), 2.92 (2.32–3.52) | < 0.0001*, < 0.0001* |
| %CD4 HLADR+ | 7% (6%–10%), 9% (7%–12%) | 4% (1%–6%), 4% (1%–8%) | 0.007*, 0.02* |
| % CD8 HLADR+ | 6% (2%–8%), 6% (4%–8%) | 3% (1%–5%), 4% (1%–6%) | 0.2, 0.12 |
| CD19+ B cells | 42 (21–57), 52 (34–70) | 31 (14–41), 31 (6–56) | 0.18, 0.19 |
| NK cells | 16 (10–39), 29 (17–41) | 25 (10–37), 26 (9–43) | 0.58, 0.76 |
| Monocytes (total) | 23 (10–62), 42 (24–61) | 71 (15–93), 54 (28–80) | 0.23, 0.5 |
| Monocytes (classical) | 37% (25%–43%), 33% (28%–38%) | 24% (16%–28%), 24% (17%–31%) | 0.02*, 0.04* |
| Monocytes (% intermediate) | 57% (49%–67%), 57% (51%–63%) | 61% (44%–68%), 57% (49%–66%) | 0.98, 0.99 |
| Monocytes (%non-classical) | 8% (6%–14%), 10% (6%–14%) | 16% (7%–33%), 19% (13%–25%) | 0.046*, 0.02* |
P values < .05 are denoted by asterisk.
In the univariate analysis of NPT-14 change over time using baseline cytometry values, only increasing percentage of CD8+ that were activated was associated with NPT-14 decrease over time (Beta = −0.1905, p = .03). However, in the multivariate analysis of NPT-14 change over time using baseline cytometry values, both female gender (Beta = −6.4043, p = .013) and increasing percentage of CD8+ that were activated at baseline (Beta = −0.2511, p = .006) were associated with NPT-14 decrease (Table 5 part 3). No other baseline cytometry variables were associated with NPT-14 decrease over time. No variables were significantly associated with NFL over time in univariate or multivariate analysis (Table 5 part 4), though there was a trend between higher CSF HIV RNA at baseline and decrease in NFL (p = .06).
4. Discussion
While the CNS has long been considered a mostly immune privileged site, the identification of the “glymphatic” system has stimulated new interest in the role of migratory immune surveillance function by cells in the CNS (Ellwardt et al., 2016; Louveau et al., 2015). With the high prevalence of neurocognitive impairment among HIV+ individuals on ART as well as the persistence of neuroinflammation and neuronal injury in this population (Heaton et al., 2010; Ulfhammer et al., 2018; Yilmaz et al., 2017), a better understanding of the immune system in the CNS is needed. Our study utilized flow cytometry to characterize and quantify key immune populations from CSF of HIV-infected subjects before and after initiation of ART, with a specific focus on lymphocyte subtypes and non-classical monocytes associated with cognition in previously published evidence. While many studies have analyzed CSF flow cytometry in HIV+ individuals, few have examined the relationship between resident CSF immune populations and cognitive function (Grauer et al., 2015; Margolick et al., 1988; Sadagopal et al., 2008; Schrier et al., 2015). In this study, we determined that cognitive function at baseline was most strongly associated with CSF CD4+ T-cell concentration among individuals off ART as compared with CSF HIV RNA or other immune cell types. Studies from the pre-ART era as well as the ART era have suggested that lower CSF CD4+ concentrations are associated with NP impairment (Margolick et al., 1988; Schrier et al., 2015). However, these findings were based on either unadjusted analysis or became non-significant when adjusted analyses were performed using CSF HIV RNA as a covariate. Here we show that lower CSF CD4+ T cell abundance is more closely associated with worse NP performance than CSF HIV RNA. This suggests that T-cell mediated immunity in the CNS could affect cognitive function in the context of chronic HIV. A higher CSF CD4 count may be suggestive of a more robust cell-mediated immune response to CNS inflammation in HIV. However, the nature of the relationship needs further investigation. Future studies are warranted to evaluate if this association extends to people with virologic suppression on ART, as well as more in depth analysis of the potential relationship of specific CSF CD4+ T cell characteristics/function with cognitive performance.
In contrast, we found that higher CSF NFL, a marker of direct axonal damage (Yilmaz et al., 2017), was significantly associated with higher CSF HIV RNA concentrations among individuals not on ART. Another study that used CSF flow cytometry showed no relationship between CSF CD8+ T cell activation and CSF NFL among individuals with acute infection (Kessing et al., 2017). However, that study did show a relationship between higher CSF CD8+ activation and higher CSF NFL in chronically infected individuals (in unadjusted analysis). Activation in that study was defined by CD38+/CD127− (as opposed to our study which utilized T cell expression of the HLA-DR molecule to define activation). With these differences in activation definition in mind, our study suggests that magnitude of CNS virus is more important than T cell activation in the neuronal damage that occurs in untreated HIV.
In the longitudinal component of the current study, CSF CD4+ did not significantly change over time. In contrast, CD8+ T cells, percentage CD4+ that expressed HLA DR, percentage CD8+ that expressed HLA DR, B cells, and NK cells all decreased significantly in the CSF longitudinally with ART. These findings are congruent with studies showing decrease in extracellular markers of inflammation in CSF after ART initiation (Mellgren et al., 2007), and support the hypothesis that ART attenuates inflammation in the CNS over time. In contrast, longitudinal evidence from HIV+ individuals who do not start ART shows an increase in percentage of CD4+ T cells and CD8+ T cells with activation markers over time (Suh et al., 2014). The one study that to our knowledge examined CSF cytometry changes with ART initiation was limited to a very small subset of participants (n = 6), and specific changes in this subset were not reported (Schrier et al., 2015).
Although CSF NFL decreased significantly between the two visits after ART initiation in the current study, we did not find a significant relationship between baseline lymphocyte cytometry and decrease in NFL, though there was a trend in association between higher CSF HIV RNA and NFL decrease. However, the number of participants who met criteria for analysis at the second visit was relatively small. Therefore, our power to detect associations in the NFL change analysis may have been limited.
In contrast, NP change over time was related to both gender and T cell properties. Specifically, female gender was significantly associated with decreasing NP performance after ART initiation. The only two participants who had a decrease in plasma HIV RNA of at least one log 10 but did not achieve HIV RNA < 200 copies/ml were male. There was also no difference between male and female participants in terms of proportion who had CSF HIV RNA < 50 copies/ml at the second visit (90% versus 80%, p = .5). Therefore, this NP change difference between genders does not appear to be from differences in HIV suppression. Previous studies of NP change with ART initiation have not focused on gender as a predictive variable (Marra et al., 2003; Robertson et al., 2004). With female gender being a risk factor for late HIV presentation (Hachfeld et al., 2015; Wilton et al., 2019), more work will be needed to optimize neurocognitive outcomes in women who are initiating ART. We also found that increasing percentage of CSF CD8+ that were HLA DR+ at baseline was significantly associated with NPT-14 decrease over time. This suggests that CD8+ T cell activation may play a role in preventing cognitive recovery in individuals starting ART. To put this into context, a study from Germany found an inverse relationship between CSF CD8+ HLADR+ and NP performance among HIV+ individuals, the majority of whom were on ART. While that was a cross-sectional study with unadjusted analysis (Grauer et al., 2015), the findings support the hypothesis that CD8+ activation is detrimental to cognition during HIV. Interestingly, a study that examined other CSF CD8+ T-cell markers found that some CD8+ T cell subtypes were associated with worse NP performance while others were associated with better NP performance during HIV. Specifically, CSF CD8+ T cells with constitutive expression of interferon gamma (IFNγ) were associated with worse NP performance, while CSF CD8+ T cells with CD107a expression (a cytolytic molecule) were associated with better NP performance (Schrier et al., 2015). However, the longitudinal part of that study involving individuals initiating ART was very limited (n = 6), and the relationship between cytometry and NP change was not reported in this subset of participants. More research will be required to better define the relationship between CD8+ activation and cognition change in the setting of HIV.
Other findings from the current study build on previously published studies. In the current study at baseline, there was a correlation between lymphocytes from the CSF and blood compartments in the total cohort as well as the HIV+ group only. However, there was only a weak correlation between total monocytes of the two compartments and no correlation between B cells or NK cells between compartment. These findings are similar to a previous HIV study by Ho et al. and suggest that these non-T cell subset populations in the CNS are tissue resident and restricted from peripheral circulation (Ho et al., 2013). While we found a significant correlation between CSF and blood compartments in terms of CD4+ and CD8+ T cells, the correlation coefficients were relatively low. Therefore, these T cell subsets in CSF may not be completely dependent on T cell subsets in the peripheral blood. Specifically, these cells may exhibit compartmental restriction and may be trafficking into the CNS due to chemokine signaling or other pro-inflammatory stimuli. This contrasts with evidence from the pre-ART era that showed a very high correlation between blood and CSF lymphocyte cell subsets (Margolick et al., 1988). It is unclear why the magnitude of correlation between compartments was particularly high in pre-ART era work, but it may have been driven by individuals who had very advanced AIDS with a highly compromised blood brain barrier. The fact that CSF CD4+ concentrations were not significantly different in the HIV+ and negative groups despite significant differences in blood concentrations has also been shown in other studies (Ho et al., 2013). This also supports the hypothesis that CSF CD4+ T-cells may not be exclusively related to blood CD4+ T-cells.
We acknowledge the limitations of our study, including its limited sample size. The small number of participants and the loss of several participants throughout the duration of the study may have reduced study power. While 44 participants were initially enrolled, only 25 were analyzed at the second visit. This was due to a number of factors, including attendance at study visit appointments and adherence to ART. Larger studies will be necessary to fully elucidate changes in CSF lymphocyte profile and the implication of these changes on neuronal damage and cognitive performance after initiation of ART. The cellular characteristics of CSF was the focus of this study. Our group and others have identified CSF cytokines that are associated with worse neurocognition in the setting of HIV. These include interferon alpha, neopterin, and others (Anderson et al., 2017; Eden et al., 2016). However, the aim of the current study was to evaluate the immune cells in the CNS as opposed to cytokines. Immune cells are actually the source of cytokines in most instances. In a relatively small study, it would be very challenging to ascertain the relative contribution of cytokines and the actual immune cells, particularly because the cells and the cytokines covary with each other. We acknowledge that this is a limitation of the study.
We acknowledge that the flow cytometry panel was limited in the number of cellular markers. While we chose to focus on HLA DR, other markers of cellular activation could be useful to better understand the potential relationship between CSF immune activation and neuronal damage and cognition. Additionally, further defining which T cells were memory versus effector may have provided additional insight. Several studies have used novel techniques such as direct tetramer staining, ex vivo cellular expansion, and other methods to study specific characteristics of CSF lymphocytes during HIV infection (Ganesh et al., 2016; Kessing et al., 2017; Neuenburg et al., 2005; Sadagopal et al., 2008; Shacklett et al., 2004; von Geldern et al., 2007). Such approaches may be promising to better understand the relationship between CNS immune cells and outcomes such as cognition and neuronal damage in future studies of HIV+ individuals on ART.
Supplementary Material
Funding
NIH K23 MH095679, R21 MH118092, R01 AG062387.
The study was also supported by the Emory Center for AIDS Research (NIH P30AI050409).
Financial disclosures
BA none.
AMA none.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention or the Department of Health and Human Services.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jneuroim.2020.577246.
References
- Anderson AM, Lennox JL, Mulligan MM, Loring DW, Zetterberg H, Blennow K, et al. , 2017. Cerebrospinal fluid interferon alpha levels correlate with neurocognitive impairment in ambulatory HIV-infected individuals. J. Neurovirol 23, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. , 2007. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. , 2004. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J. Clin. Exp. Neuropsychol 26, 307–319. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Franklin D Jr., Abramson I, Ellis RJ, Letendre S, Collier A, et al. , 2011. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J. Clin. Exp. Neuropsychol 33, 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden A, Marcotte TD, Heaton RK, Nilsson S, Zetterberg H, Fuchs D, et al. , 2016. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 11, e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwardt E, Walsh JT, Kipnis J, Zipp F, 2016. Understanding the role of T cells in CNS homeostasis. Trends Immunol 37, 154–165. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, et al. , 2001. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J. Neurovirol 7, 528–541. [DOI] [PubMed] [Google Scholar]
- Ganesh A, Lemongello D, Lee E, Peterson J, McLaughlin BE, Ferre AL, et al. , 2016. Immune activation and HIV-specific CD8(+) T cells in cerebrospinal fluid of HIV controllers and noncontrollers. AIDS Res. Hum. Retrovir 32, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Geldern G, Cepok S, Nolting T, Du Y, Grummel V, Adams O, et al. , 2007. CD8 T-cell subsets and viral load in the cerebrospinal fluid of therapy-naive HIV-infected individuals. Aids 21, 250–253. [DOI] [PubMed] [Google Scholar]
- Grauer OM, Reichelt D, Gruneberg U, Lohmann H, Schneider-Hohendorf T, Schulte-Mecklenbeck A, et al. , 2015. Neurocognitive decline in HIV patients is associated with ongoing T-cell activation in the cerebrospinal fluid. Ann Clin Transl Neurol 2, 906–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachfeld A, Ledergerber B, Darling K, Weber R, Calmy A, Battegay M, et al. , 2015. Reasons for late presentation to HIV care in Switzerland. J. Int. AIDS Soc 18, 20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. , 2011. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids 25, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I, 2004. Revised Comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults scoring program 2004.
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. , 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho EL, Ronquillo R, Altmeppen H, Spudich SS, Price RW, Sinclair E, 2013. Cellular composition of cerebrospinal fluid in HIV-1 infected and uninfected subjects. PLoS One 8, e66188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing CF, Spudich S, Valcour V, Cartwright P, Chalermchai T, Fletcher JL, et al. , 2017. High number of activated CD8+ T cells targeting HIV antigens are present in cerebrospinal fluid in acute HIV infection. J. Acquir. Immune Defic. Syndr 75, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure FX, Moulignier A, Savatovsky J, Amiel C, Carcelain G, Molina JM, et al. , 2013. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 57, 101–108. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. , 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolick JB, McArthur JC, Scott ER, McArthur JH, Cohn S, Farzadegan H, et al. , 1988. Flow cytometric quantitation of T cell phenotypes in cerebrospinal fluid and peripheral blood of homosexual men with and without antibodies to human immunodeficiency virus, type I. J. Neuroimmunol 20, 73–81. [DOI] [PubMed] [Google Scholar]
- Marra CM, Lockhart D, Zunt JR, Perrin M, Coombs RW, Collier AC, 2003. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology 60, 1388–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Soerens AG, 2019. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol 37, 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M, 2007. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology 69, 1536–1541. [DOI] [PubMed] [Google Scholar]
- Neuenburg JK, Cho TA, Nilsson A, Bredt BM, Hebert SJ, Grant RM, et al. , 2005. T-cell activation and memory phenotypes in cerebrospinal fluid during HIV infection. J. Acquir. Immune Defic. Syndr 39, 16–22. [DOI] [PubMed] [Google Scholar]
- Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, et al. , 2014. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 9, e116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Yosief S, 2014. Neurocognitive assessment in the diagnosis of HIV-associated neurocognitive disorders. Semin. Neurol 34, 21–26. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Robertson WT, Ford S, Watson D, Fiscus S, Harp AG, et al. , 2004. Highly active antiretroviral therapy improves neurocognitive functioning. J. Acquir. Immune Defic. Syndr 36, 562–566. [DOI] [PubMed] [Google Scholar]
- Sadagopal S, Lorey SL, Barnett L, Basham R, Lebo L, Erdem H, et al. , 2008. Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J. Virol 82, 10418–10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. , 2011. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis 203, 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, et al. , 2015. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 10, e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacklett BL, Cox CA, Wilkens DT, Karl Karlsson R, Nilsson A, Nixon DF, et al. , 2004. Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J. Infect. Dis 189, 2202–2212. [DOI] [PubMed] [Google Scholar]
- Spudich SS, 2016. Immune activation in the central nervous system throughout the course of HIV infection. Curr. Opin. HIV AIDS 11, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Sinclair E, Peterson J, Lee E, Kyriakides TC, Li FY, et al. , 2014. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J. Neuroinflammation 11, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KA, Cherry CL, Bell JE, McLean CA, 2011. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am. J. Pathol 179, 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfhammer G, Eden A, Mellgren A, Fuchs D, Zetterberg H, Hagberg L, et al. , 2018. Persistent central nervous system immune activation following more than 10 years of effective HIV antiretroviral treatment. Aids 32, 2171–2178. [DOI] [PubMed] [Google Scholar]
- Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, et al. , 2010. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology 75, 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB, 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci 83, 7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J, Light L, Gardner S, Rachlis B, Conway T, Cooper C, et al. , 2019. Late diagnosis, delayed presentation and late presentation among persons enrolled in a clinical HIV cohort in Ontario, Canada (1999–2013). HIV medicine 20, 110–120. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Blennow K, Hagberg L, Nilsson S, Price RW, Schouten J, et al. , 2017. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert. Rev. Mol. Diagn 17, 761–770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


