Abstract
Objective:
To explore the effects and mechanism of miR-21 on the osteogenic/adipogenic differentiation of mouse BMSCs.
Methods:
The bilateral ovaries of C57BL/6J mice (n=24) were removed to construct an osteoporosis model. Real-time quantitative polymerase chain reaction (qRT-PCR) was used to detect the expression of miR-21, osteogenic/adipogenic genes, and PTEN. ALP and ARS and ORO staining were used to detect the formation of calcium nodules and lipid droplets in BMSCs. Western blot was used to detect the expression of PTEN.
Results:
miR-21 was significantly down-regulated in osteoporotic mice. The expression of miR-21 was significantly up-regulated after the osteogenic induction of BMSCs, and the expression of miR-21 was significantly down-regulated after the adipogenic induction. Overexpression of miR-21 significantly promoted the osteogenic differentiation of BMSCs and inhibits the adipogenic differentiation of BMSCs.
Conclusion:
MiR-21 can promote osteogenic differentiation of BMSCs and inhibit their adipogenic differentiation by negatively regulating PTEN.
Keywords: Adipose differentiation, BMSCs, miRNA, Osteogenic Differentiation, Osteoporosis
Introduction
Osteoporosis (OP) is a systemic metabolic disorder characterized by reduced bone mass, decreased bone strength as well as increased incidence of fractures[1]. Osteoporosis is increasingly becoming a global health epidemic which negatively affects patients’ health and quality of life[2,3].
In the bone marrow microenvironment, there are a large number of osteoblasts, osteoclasts, hematopoietic stem cells, and BMSCs[4]. Among these cells, BMSCs belong to the class of adult stem cells with the abilities of self-renewal and multi-directional division potential, which makes them able to transform into adipocytes, chondrocytes, osteoblasts, as well as nerve cells under different induction conditions[5,6]. BMSCs are characterized by age-dependent decreased osteoblast differentiation and increased adipocyte differentiation. It ultimately leads to the increase of adipocytes and the decrease of osteoblasts in bone marrow microenvironment, resulting in bone loss and even OP in patients[7-9]. In addition, due to the characteristics of multi-directional differentiation, low immunogenicity, negative immunoregulation and easy amplification in vitro, BMSCs play essential roles in maintaining bone homeostasis and providing important cell sources for bone growth and repair. It is commonly seen in medications for bone regeneration as well as in the seed cells of bone tissue engineering.
MicroRNAs (miRNAs) are small, non-coding, single-stranded RNAs in eukaryotes with length of approximately 18-25 nucleotides, whose hairpin structure is formed by single-stranded RNA precursor processed by Dicer enzyme[10,11]. miRNA binds straight to the 3’-non-coding area in the targeted messenger RNA. It degrades the target RNA and inhibits its genetic translation, and participates in the regulation of post-transcriptional gene expression[12]. Researches revealed that miRNAs are able to manage the osteogenesis and adipogenesis processes in BMSCs either positively or negatively[13-15]. Therefore, it is of great significance to find the key miRNAs that can regulate osteogenic/adipogenic differentiation of BMSCs for the future research on OP[16]. As an important miRNA, miR-21 is involved in the occurrence and progression of osteosarcoma, fracture, multiple myeloma and other bone-related diseases[17-19]. It has been reported that miR-21 can affect the biological function of the pulmonary cancerous cells by regulating its target gene PTEN[20]. In addition, miR-21 inhibits oxidative stress and inflammation by regulating PTEN, and mediates the cardioprotective effect of glycoside on endothelial injury caused by the oxidized low-density lipoprotein[21]. By means of PTEN/AKT signaling regulated by miR-21, Puerarin hinders the epithelial-mesenchymal passage, which further inhibits invasion and metastasis of liver cancer[22]. Therefore, miR-21/PTEN is crucial in regulating cell proliferation, differentiation, invasion, oxidative stress and inflammation.
Our previous experiments showed that the miR-21 expression was remarkably reduced in the bone tissue and BMSCs in mice with OP, so we hypothesized that miR-21 might be closely associated with the occurrence of OP. However, no research has shown if miR-21 can regulate osteogenic/adipogenic divergence of BMSCs. Thus, we explored the regulatory ability of miR-21 in the osteogenic/adipogenic differentiation in BMSCs, along with relevant mechanisms in this study, aiming to present a novel basis for the OP treatment.
Materials and methods
Establishment of OP mouse model
Before modeling, 48 C57BL/6J mice of 8 weeks old and weighed around 18 g (Shanghai Vital River Laboratory Animal Technology Co., Ltd., Animal Approval Number: SYXK (Hei) 2016-001) were divided into simulation group and model group after adaptive feeding for one week, with 24 mice in each group. Before surgery, mice were fasted for 12 h and injected intraperitoneally with 1% pentobarbital. The mice in the model group were performed bilateral ovarian dissection through the dorsal approach. In the sham group, the bilateral ovaries were not removed from the mice, and the other operations were the same as those in the model group. Eight weeks later, mouse bone tissue and BMSCs were isolated for subsequent experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The quantitative PCR enzyme mixture kit (ABI, 4366596, USA) and ABI 7900 (ABI, USA) real-time fluorescence quantitative PCR apparatus were used for detection. qRT-PCR was carried out in the 95°C environment for 30 minutes, with a succession of 40 cycles at the same temperature for 15 seconds and then at 60°C for 30 seconds. The trials were repeated three times, and the relative expression degrees of targeted genes (ALP, Runx2, OPN, OCN, CEBPα, CEBPβ, FABP4, PPARγ) were calculated by 2-∆∆Ct method. All primer sequences are presented in detail in Table 1.
Table 1.
Primer sequence.
| Gene | Primers (5’ to 3’) |
| Runx2 | Upstream: AGAAGGCACAGACAGAAGCTTGA |
| Downstream: AGGAATGCGCCCTAAATCACT | |
| ALP | Upstream: ACAACCTGACTGACCCTTCG |
| Downstream: TCATGATGTCCGTGGTCAAT | |
| OCN | Upstream: TTCTGCTCACTCTGCTGACC |
| Downstream: TTTGTAGGCGGTCTTCAAGC | |
| OPN | Upstream: ACACTTTCACTCCAATCGTCC |
| Downstream: TGCCCTTTCCGTTGTTGTCC | |
| CEBPα | Upstream: GTGTGCACGTCTATGCTAAACCA |
| Downstream: GCCGTTAGTGAAGAGTCTCAGTTTG | |
| CEBPβ | Upstream: TGGACAAGCTGAGCGACGAG |
| Downstream: GAACAAGTTCCGCAGGGTGC | |
| FABP4 | Upstream: TTCCTGTCGTCTGCGGTGATT |
| Downstream: GATGCCTTTGTGGGAACCTGG | |
| PPARγ | Upstream: TCACAAGAGGTGACCCAATG |
| Downstream: CCATCCTTCACAAGCATGAA | |
| β-actin | Upstream: ACTGCCGCATCCTCTTCCT |
| Downstream: TCAACGTCACACTTCATGATGGA |
Isolation and culture of mouse BMSCs
Bilateral femur and tibia of 16-week-old osteoporotic C57BL/6J mice were separated by blunt dissection. Then, 1 mL syringe was used to rinse out the bone marrow, and the same volume of Percoll solution was added to separate the mononuclear cell layer by density-gradient centrifugation. Thereafter, the mononuclear cell layer was rinsed three times with F12-DMEM (Hyclone, USA), which consists of 10% fetal bovine serum (FBS; Invitrogen, USA) and 1% double antibody (Beyotime, China). The cellular suspension was accustomed to the concentration of 2´10[6] cells /mL, which was then injected into a culture bottle of 25 cm2 (NEST, China) and kept in a cell incubator (Thermo, USA) in the 37°C environment of 5% CO2. After a 24-hour period, the fluid was changed, followed by observation of the degree of cell convergence after 72 h. When the cell confluence reached about 90%, pancreatin (Beyotime, China) was applied for digestion, and cells underwent three passages were used for subsequent experiments.
Osteogenic differentiation of BMSCs
Mouse BMSCs after three passages were inoculated into a 6-well plate or a 24-well plate. When a 60% convergence was reached, the F12-DMEM of 10% FBS and 1% double antibody was replaced with the F12-DMEM of 10% FBS, 10-7 mmol/L dexamethasone, 50 µg/mL vitamin-C, and 10 ng/mL β-glycerophosphate. The culture medium was altered every two days. Follow-up experiment was carried out after 14 days.
Adipogenic differentiation of BMSCs
BMSCs were injected onto a 24-well plate with the density of 2.5´10[4] cells/well. When a 70% convergence was reached, the cells were then generated by DMEM consisting of 10% FBS, 10 µmol/L rosiglitazone, and 1 µmol/L dexamethasone. The solution was substituted every three days, and used for subsequent experiments after 20 days.
Cell transfection
The 3rd generation BMSCs grown in logarithmic phase were transfected with miR-21 by lipofection, and divided into four groups of miR-21 mimics, negative control (NC) mimics, miR-21 inhibitors and NC inhibitors. First, the cell culture medium was discarded, and Opti-MEM medium (Hyclone, USA) was added for 2 h of starvation treatment. At the beginning of transfection, the transfection reagent X-treme (Invitrogen, USA) was applied to make the transfection reagent easier to enter the cells. During transfection, the ultimate densities of miR-21 mimics and miR-21 inhibitors were 50 nmol/L and 100 nmol/L, respectively. After 48 h of transfection, the culture medium was substituted with either of the osteogenic and adipogenic induction media for further culture.
RNA extraction and reverse transcription reaction
Bone tissues and cells of mice were collected, of which the total RNA was extricated with Trizol reagent (Thermo, USA). All operations were conducted in strict accordance with reagent instructions. After extraction, RNA concentration and purity were detected by Nanodrop (Thermo, USA). Then based on the guidelines in the reverse transcription kit (Promega, USA), RNA was inversely transcribed into cDNA under the reaction conditions at 37°C for 60 min, and 95°C for 5 min. Thereafter, tissue and cells were stored in a refrigerator (Thermo, USA) -20°C for later use.
Alkaline phosphatase staining
The induction suspension was discarded while the BMSCs got rinsed with PBS for 3 times 14 days of the osteogenic induction. Staining of alkaline phosphatase was achieved based on the kit instructions (Nanjing Jiancheng, China). Finally, the BMSCs were rinsed by PBS, and examined using an inverted microscope (Nikon, Japan) and photographed after PBS removal.
Alizarin red staining
The induction suspension was discarded and BMSCs were rinsed with PBS for 3 times 14 days of the osteogenic induction. Then, the cells were amended with 1 mL of 4% paraformaldehyde (Beyotime, China) and placed at room temperature for 30 minutes. After immobilization, the cells were incubated with 1% Alizarin red dye mixture (Saiye, China) for 20 minutes. Finally, BMSCs were rinsed by PBS, and examined using an inverted microscope (Nikon, Japan) and photographed after PBS removal.
Oil red O staining
Following the removal of lipid induction medium, the cells were cleansed 3 times with PBS, added with 1 mL of 4% paraformaldehyde (Beyotime, China), and left at room temperature for 30 minutes. After fixation, the suspension was discarded and the oil red O working mixture was introduced and kept for a 30-minute-incubation at room temperature. Finally, the staining mixture was discarded, then the cells rinsed with PBS for three times. The formation of lipid droplets was discovered using an inverted microscope, and the field of vision was unmethodically chosen for photos.
Western blot
The treated cells were collected, followed by the extraction of their complete protein. The density of protein was then analyzed by BCA protein quantitative kit (Beyotime, China). Then, 20 µg total protein was introduced into each well, segregated by 10% SDS-PAGE, then moved to a PVDF membrane by wet transfer method. After that, the layer was securely covered with 5% skimmed milk powder, with a supplement of primary antibody target gene PTEN (1:1000, ab32199, Abcam, UK) or internal reference gene β-actin (ab8226, Abcam, UK) respectively for hybridization at 4°C overnight. The gray values of each band were calculated by Image J software.
Bioinformatics prediction
The targeted genes of miR-21 were anticipated by the online bioinformatics website TargetScan (HTTP: //www. targetScan. org), and the functions of these target genes were initially analyzed to keep investigating on the regulatory mechanism of miR-21 in the osteoblastic/adipogenic differentiation of BMSCs.
Statistical methods
All statistics and data were analyzed by SPSS 17. 0 software, with measurement data manifested in the mean±standard deviation (x±s), followed by an inter-group comparison performed by the T test. All trials were performed for at least 3 times. The difference was considered of statistical significance if P<0.05 (*P<0.05, **P<0.01, ***P<0.001).
Results
miR-21 is down-regulated in OP mice
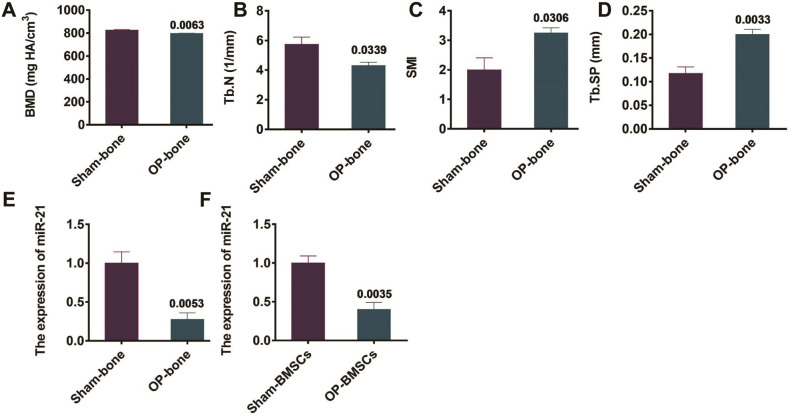
The micro-CT scanning was used to detect the bone microstructure indexes of mice 8 weeks after the establishment of OP-bone mouse model and sham-bone mouse model. The results revealed that the bone density and trabecular number of OP mice significantly decreased (Figure 1A, 1B), in comparison with the control group. In addition, the structural model index and trabecular separation of OP mice significantly increased (Figure 1C, 1D). The above results proved that the OP mouse model was successfully established. Bone tissue and BMSCs of two groups of mice were collected for the determination of miR-21 expression by qRT-PCR. The outcomes revealed that the miR-21expression in bone tissue as well as mouse BMSCs from the OP group was notably down-regulated, in contrast to that from the sham group (P=0.0053 and P=0.0035, Figure 1E, 1F).
Figure 1.
Expression of miR-21 in OP mice. (A-D) Detection of bone-related indexes by microCT, SMI = structure model index; (E) qRT-PCR detection of the expression of miR-21 in bone tissue of OP mice; (F) qRT-PCR detection of the expression of miR-21 in mouse BMSCs in OP group.
Changes of miR-21 expression after osteogenic/adipogenic differentiation in BMSCs
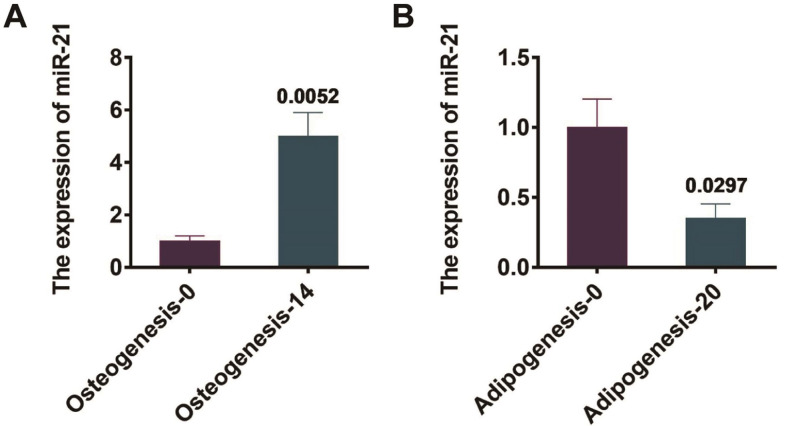
The mouse BMSCs were isolated and cultured, and were induced for osteogenesis for 0 days and 14 days, respectively. The miR-21 expressions before and after osteogenesis of mouse BMSCs were observed by qRT-PCR. Illustrated by Figure 2A, the miR-21 expression was significantly up-regulated on day 14 of BMSCs osteogenesis compared with day 0, and the dissimilarity was statistically remarkable (P=0.0052). In addition, BMSCs were induced into adipogenesis for 0 days and 20 days respectively. And also, the miR-21 expressions in mouse BMSCs before and after adipogenesis were observed by qRT-PCR. Demonstrated in Figure 2B, the miR-21 expression was significantly down-regulated after 20 days of adipogenesis in BMSCs compared with that of day 0, and the dissimilarity was statistically remarkable (P=0.029).
Figure 2.
miR-21 expression changes before and after differentiation in BMSCs of mice. (A) qRT-PCR detection of the expression of miR-21 before and after osteogenesis of mouse BMSCs; (B) qRT-PCR detection of the expression of miR-21 before and after adipogenesis of mouse BMSCs.
miR-21 overexpression promotes the osteogenic differentiation in BMSCs
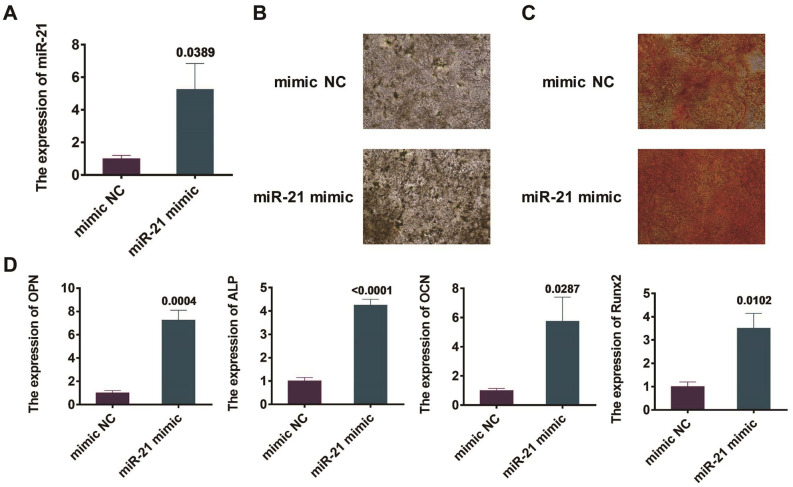
BMSCs were transfected with miR-21 mimics and NC mimics separately. 48 hours into the process, miR-21 expression in BMSCs was observed by qRT-PCR. The outcomes revealed a more remarkable miR-21 expression in the miR-21-transcribed BMSCs than that in those transfected with NC mimics (P=0.0389, Figure 3A).
Figure 3.
Effects of overexpression of miR-21 on osteogenic differentiation of mouse BMSCs. (A) Observation of miR-21 expression in mouse BMSCs by qRT-PCR; (B) Detection of calcium nodule formation in BMSCs by alkaline phosphatase staining; (C) Manifestation of calcium nodule emergence in BMSCs by Alizarin red staining; (D) Detection of the expression degrees of osteogenesis-related genes Runx2, ALP, OCN and OPN in mouse BMSCs by qRT-PCR.
BMSCs were managed by the osteogenic differentiation after the transfections by miR-21 and NC mimics for 48 h. After 14 days, alkaline phosphatase and alizarin red staining methods were utilized to discover the calcium nodules emergence in BMSCs. Compared with those undergone the transfection of NC mimic, the BMSCs transfected by miR-21 mimics showed a remarkable increase in the concentration of calcium nodules (Figure 3B, 3C). In addition, qRT-PCR was exploited to observe the expression of osteogenesis-related genes, including ALP, Runx2, OPN, and OCN, in BMSCs of mice. The results revealed that, in contrast to those undergone the transfection of NC mimic, the BMSCs transfected with miR-21 mimics revealed a remarkable growth in the expression of osteogenic related genes, including Runx2, ALP, OPN, and OCN, with the dissimilarity of statistical signicifance (Figure 3D).
miR-21 overexpression inhibits the adipogenic differentiation in BMSCs
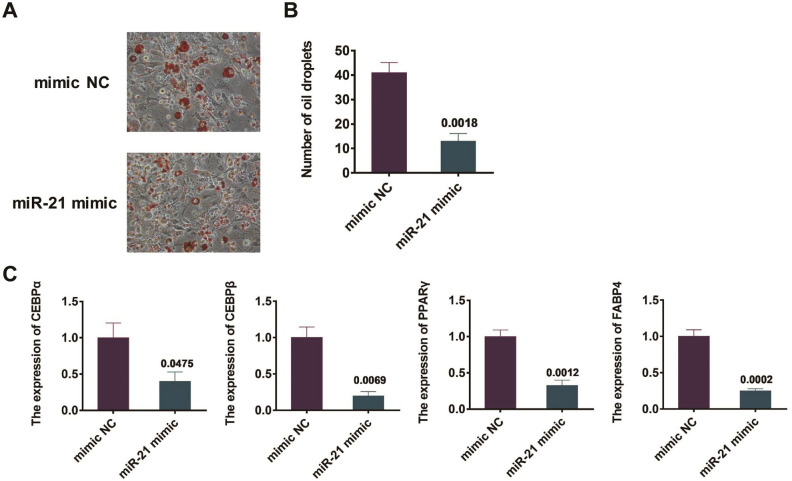
After the transfections with miR-21 mimics and NC mimics for 48 h, BMSCs were processed for adipogenic differentiation. After 20 days, oil red O staining was utilized to illustrate the lipid droplets establishment in BMSCs. The results revealed that, in contrast to those undergone the transfection of NC mimic, the BMSCs transfected with the miR-21 mimics revealed a significant reduction in the lipid droplet establishment (P=0.0018, Figure 4A, 4B). In addition, qRT-PCR was exploited to observe the expression of adipogenesis-related genes, including CEBPα, CEBPβ, PPARγ, and FABP4 in mouse BMSCs. The results revealed that, in contrast to those undergone the transfection of NC mimic, the BMSCs transfected with the miR-21 mimics showed a remarkable decline in the expression degrees of adipogenesis-related genes, including CEBPα, CEBPβ, PPARγ, and FABP4. The dissimilarity between the two groups of BMSCs is of statistical significance (Figure 4C).
Figure 4.
Effect of overexpression of miR-21 on adipogenic differentiation of mouse BMSCs. (A) Detection of the formation of lipid droplets in BMSCs by oil red O staining; (B) Quantitative results of oil red O staining; (C) Detection of the expression levels of adipogenesis related genes CEBPα, CEBPβ, FABP4 and PPARγ in mouse BMSCs by qRT-PCR.
miR-21 targets PTEN to regulate BMSCs differentiation
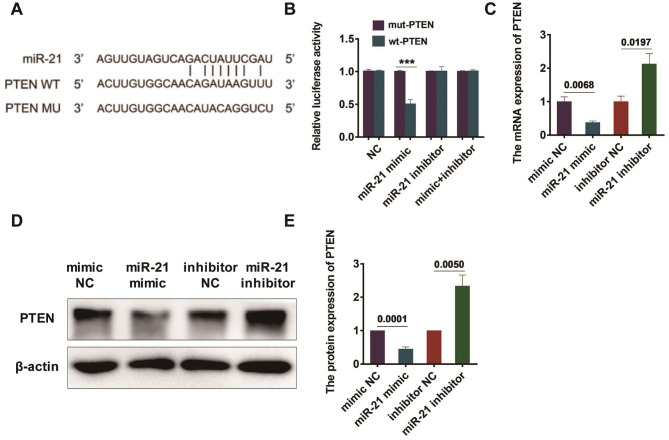
The connection points of miR-21 and PTEN were predicted by bioinformatics website (Figure 5A). The correlation between miR-21 and PTEN was determined by dual luciferase reporter assay, and the outcomes revealed an inversely correlated regulation between the two (Figure 5B). Furthermore, BMSCs were transfected with miR-21 mimics, NC mimics, miR-21 inhibitors and NC inhibitors, followed by the observation of the PTEN expression by qRT-PCR after 48 h. BMSCs transfected by miR-21 mimic showed a remarkable lower expression of PTEN, compared with those transfected by NC mimic. Meanwhile, the PTEN expression was much more notable in BMSCs transfected by miR-21 inhibitors, in comparison with that in BMSCs transfected by NC inhibitors. The dissimilarity was of statistical significance with P=0.0068 and P=0.0197, (Figure 5C). BMSCs were then transfected by miR-21 mimics, NC mimics, miR-21 inhibitors and NC inhibitors, followed by the observation of PTEN’s protein expression in BMSCs, with the aid of Western blot after 48 hours of transfection. The outcomes showed that the PTEN expression was remarkably lower in miR-21 mimic transfected BMSCs, in comparison with that in NC mimic transfected BMSCs. Meanwhile, the PTEN expression was found notably higher in BMSCs transfected with miR-21 inhibitors, in comparison with that in BMSCs transfected by NC inhibitors (P=0.0001 and P=0.005, Figure 5D, 5E).
Figure 5.
miR-21 negatively regulates PTEN expression. (A) The binding site of miR-21 and PTEN; (B) Luciferase reporter assay was used to detect the regulatory association between miR-21 and PTEN; (C) Detection of PTEN mRNA expression by qRT-PCR; (D) Detection of the protein expression of PTEN was observed by western blot; (E) Statistical results of western blot.
Discussion
BMSCs are easy to expand while capable of multiple differentiations. Studies have shown that BMSCs can differentiate into osteoblasts and adipocytes, under certain conditions. If the proportion of these two types of cells in adult bones is unbalanced (decreased osteoblasts and increased adipocytes), a decline in bone mass will occur, which may lead to OP[23,24]. However, there are few studies on BMSCs regarding their potential osteogenic/adipogenic differentiation. Therefore, the regulatory process of BMSCs osteogenic/adipogenic differentiation is a difficult and hot research topic. miRNAs are highly conserved, participating in the regulation of more than 30% of human gene expression. In addition, they are involved in cell growth, differentiation, division, apoptosis regulation of gene expression, humoral regulation, bone formation, wound healing, inflammation, rhythm regulation and endocrine regulation, miRNAs are crucial in the regulation of the development, and progression of diseases[25,26]. The relationship between miRNA and potential targets of protein coding genes, which can be utilized in the treatment and diagnostic process of diseases, may become the focus of future researches on genes and diseases[27]. The purpose of this study was to investigate the regulatory influences of miR-21 on the osteoblastic/adipogenic differentiation in BMSCs, and to further explore its mechanism, so as to provide new basis and targets for the treatment of OP.
The results of this study showed that the miR-21 expression in bone tissue and BMSCs of OP mice was significantly down-regulated, so we speculated that miR-21 is potentially related to the emergence and evolvement of OP. Furthermore, it was demonstrated that the miR-21 expression was remarkably up-regulated 14 days into the BMSCs osteogenesis induction, while it was significantly down-regulated 20 days after adipogenesis induction. Therefore, we hypothesized that miR-21 is potentially associated with the development and progression of OP by regulating osteogenesis and adipogenesis in BMSCs.
In this study, alkaline phosphatase and alizarin red staining were exploited and revealed the miR-21 overexpression could significantly promote the formation of calcium nodules in BMSCs. ALP is considered a relative early marker of osteoblast differentiation and promotes extracellular matrix mineralization of osteoblasts[28]. OPN is a marker of osteoblast metaphase differentiation and a regulatory gene of matrix mineralization, which can promote hydroxyapatite formation, osteoblast mineralization and bone formation[29]. As to OCN, it is a marker of osteoblast formation in the middle and late stage, which promotes bone formation by combining with calcium and hydroxyapatite[30]. Fragmented Runx2 gene has been reported to improve bone mass by promoting BMSCs to differentiate into osteoblasts[31]. The expression levels of osteogenesis-related genes, including ALP, Runx2, OPN, and OCN, were discovered by qRT-PCR. The outcomes revealed that miR-21 overepxression could remarkably increase osteogenesis-related genes manifestations, including those of ALP, Runx2, OPN, and OCN, in BMSCs. The miR-21 overexpression also showed a significant impact on the inhibition of lipid droplet establishment in BMSCs, as manifested by the oil red O staining. PPARγ is the main transcription factor regulating adipogenesis, which can inhibit osteogenic differentiation by promoting ester differentiation[32]. The CCAAT-enhancer binding proteins (C/EBP) are also crucial for the adipocyte production[33,34]. The expressions of adipogenesis-related genes, including those of CEBPα, CEBPβ, PPARγ, and FABP4, were observed by qRT-PCR. The outcomes revealed that miR-21 overexpression could have an impactful role in inhibiting the manifestations of adipogenesis-related genes in BMSCs, including those of CEBPα, CEBPβ, PPARγ, and FABP4.
A group of researchers found that miR-21 enhanced bone formation and accelerated bone remodeling in mice with maxillary defects[35]. Another group revealed that miR-21 could initiate the THE PI3K/Akt pathway to enhance the healing of fractures[36]. Zou et al. claimed that miR-21 expression could remarkably improve health conditions in patients, such as compulsory spondylitis; and miR-21 may be involved in inflammation and bone formation[37]. However, the miR-21’s influences on the osteogenic/adipogenic differentiation in BMSCs and its mechanism have not been demonstrated. In this study, we concluded that miR-21 can target and regulate the expression of its targeted gene PTEN, thus promoting the osteogenic differentiation while hindering the adipogenic differentiation in BMSCs. To sum up, this study confirmed that miR-21 is capable of enhancing the osteogenic differentiation in BMSCs while inhibiting the adipogenic differentiation through the negative regulation of PTEN. As a potential treatment for OP, miR-21 and PTEN provide new potential methods for early diagnosis, prevention, as well as treatment for OP, which brings new hope to OP patients, with favorable clinical application value.
Funding
This study was funded by The Joint Guidance Project of Qiqihar City Science and Technology Plan (LHYD-202058).
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Zhang D, Yu K, Yang J, Xie S, Yang J, Tan L. Senolytic controls bone marrow mesenchymal stem cells fate improving bone formation. Am J Transl Res. 2020;12:3078–3088. [PMC free article] [PubMed] [Google Scholar]
- 2.Guo B, Zhu X, Li X, Yuan CF. The Roles of LncRNAs in Osteogenesis, Adipogenesis and Osteoporosis. Curr Pharm Des. 2021;27(1):91–104. doi: 10.2174/1381612826666200707130246. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Zhou K, Xiao F, Huang Z, Xu J, Chen G, Liu Y, Gu H. Identification of circRNA-associated ceRNA network in BMSCs of OVX models for postmenopausal osteoporosis. Sci Rep. 2020;10:10896. doi: 10.1038/s41598-020-67750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li DJ, Liu GQ, Xu XJ. Silence of lncRNA BCAR4 alleviates the deterioration of osteoporosis. Eur Rev Med Pharmacol Sc. 2020;24:5905–5913. doi: 10.26355/eurrev_202006_21483. [DOI] [PubMed] [Google Scholar]
- 5.Mao X, Li X, Hu W, Hao S, Yuan Y, Guan L, Guo B. Downregulated brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 inhibits osteogenesis of BMSCs through p53 in type 2 diabetes mellitus. Biol Open. 2020;9:bio051482. doi: 10.1242/bio.051482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Gu H, Shi S, Xiao F, Huang Z, Xu J, Chen G, Zhou K, Lu L, Yin X. MiR-1-3p regulates the differentiation of mesenchymal stem cells to prevent osteoporosis by targeting secreted frizzled-related protein 1. Bone. 2020;137:115444. doi: 10.1016/j.bone.2020.115444. [DOI] [PubMed] [Google Scholar]
- 7.Li KC, Chang YH, Hsu MN, Lo SC, Li WH, Hu YC. Baculovirus-Mediated miR-214 Knockdown Shifts Osteoporotic ASCs Differentiation and Improves Osteoporotic Bone Defects Repair. Sci Rep. 2017;7:16225. doi: 10.1038/s41598-017-16547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu C, Xu Y, Zhang S, Guan H, Song S, Wang X, Wang Y, Li Y, Zhao G. miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARgamma and GREM1. Sci Rep. 2016;6:38491. doi: 10.1038/srep38491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125:1509–22. doi: 10.1172/JCI77716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Wang Y, Zhao H, Han X, Zhao T, Qu P, Li G, Wang W. Extracellular vesicle-encapsulated miR-22-3p from bone marrow mesenchymal stem cell promotes osteogenic differentiation via FTO inhibition. Stem Cell Res Ther. 2020;11:227. doi: 10.1186/s13287-020-01707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao G, Meng X, Han X, Li J. Exosomes derived from circRNA Rtn4-modified BMSCs attenuate TNF-alpha-induced cytotoxicity and apoptosis in murine MC3T3-E1 cells by sponging miR-146a. Biosci Rep. 2020:40. doi: 10.1042/BSR20193436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai Z, Chen W, Hu Q, Wang X, Zhao Q, Tuerxunyiming M. High glucose inhibits osteogenic differentiation of bone marrow mesenchymal stem cells via regulating miR-493-5p/ZEB2 signalling. J Biochem. 2020;167:613–621. doi: 10.1093/jb/mvaa011. [DOI] [PubMed] [Google Scholar]
- 13.Xiang S, Li Z, Weng X. Changed cellular functions and aberrantly expressed miRNAs and circRNAs in bone marrow stem cells in osteonecrosis of the femoral head. Int J Mol Med. 2020;45:805–815. doi: 10.3892/ijmm.2020.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu R, Shen X, Si Y, Fu Y, Zhu W, Xiao T, Fu Z, Zhang P, Cheng J, Jiang H. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17:e12794. doi: 10.1111/acel.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–64. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang G, Wang Y, Xu Y, Zhang S, Sun X, Guan H, Zhao X, Wang Y, Li Y, Zhao G. Long non-coding RNA TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of rat bone marrow mesenchymal stem cell by targeting miR-204-5p and miR-125a-3p. J Cell Physiol. 2018;233:6041–6051. doi: 10.1002/jcp.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, Grieb P, Rutkowski P. Molecular Biology of Osteosarcoma. Cancers (Basel) 2020:12. doi: 10.3390/cancers12082130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Li X, Qi H, Hou X, Zhao J, Yuan X, Ma X. MiR-21 nanocapsules promote early bone repair of osteoporotic fractures by stimulating the osteogenic differentiation of bone marrow mesenchymal stem cells. J Orthop Translat. 2020;24:76–87. doi: 10.1016/j.jot.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi M, Altomare E, Botta C, Gallo Cantafio ME, Sarvide S, Caracciolo D, Riillo C, Gaspari M, Taverna D, Conforti F, et al. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma. Leukemia. 2021;35(3):823–834. doi: 10.1038/s41375-020-0947-1. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Dong L, Zhao S, Li Q, Liu D, Zhu X, Ge X, Li R, Wang G. Propofol Affects Non-Small-Cell Lung Cancer Cell Biology By Regulating the miR-21/PTEN/AKT Pathway In Vitro and In Vivo. Anesth Analg. 2020;131:1270–1280. doi: 10.1213/ANE.0000000000004778. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Sun Y, Zhao K, Gao Y, Cui J, Qi L, Huang L. miR21/PTEN pathway mediates the cardioprotection of geniposide against oxidized lowdensity lipoproteininduced endothelial injury via suppressing oxidative stress and inflammatory response. Int J Mol Med. 2020;45:1305–1316. doi: 10.3892/ijmm.2020.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Xue R, Wang J, Ren H. Puerarin inhibits hepatocellular carcinoma invasion and metastasis through miR-21-mediated PTEN/AKT signaling to suppress the epithelial-mesenchymal transition. Braz J Med Biol Res. 2020;53:e8882. doi: 10.1590/1414-431X20198882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Yang F, Gao M, Gong R, Jin M, Liu T, Sun Y, Fu Y, Huang Q, Zhang W, et al. miR-149-3p Regulates the Switch between Adipogenic and Osteogenic Differentiation of BMSCs by Targeting FTO. Mol Ther Nucleic Acids. 2019;17:590–600. doi: 10.1016/j.omtn.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Zheng Y, Jin C, Li X, Jia L, Li W. Long Non-coding RNA H19 Inhibits Adipocyte Differentiation of Bone Marrow Mesenchymal Stem Cells through Epigenetic Modulation of Histone Deacetylases. Sci Rep. 2016;6:28897. doi: 10.1038/srep28897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao L, Liu D, Li CG, Wang YJ. MiR-203 is essential for the shift from osteogenic differentiation to adipogenic differentiation of mesenchymal stem cells in postmenopausal osteoporosis. Eur Rev Med Pharmacol Sci. 2018;22:5804–5814. doi: 10.26355/eurrev_201809_15906. [DOI] [PubMed] [Google Scholar]
- 26.Kolhe R, Mondal AK, Pundkar C, Periyasamy-Thandavan S, Mendhe B, Hunter M, Isales CM, Hill WD, Hamrick MW, Fulzele S. Modulation of miRNAs by Vitamin C in Human Bone Marrow Stromal Cells. Nutrients. 2018;10(2):186. doi: 10.3390/nu10020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B. MicroRNA Regulation in Osteogenic and Adipogenic Differentiation of Bone Mesenchymal Stem Cells and its Application in Bone Regeneration. Curr Stem Cell Res Ther. 2018;13:26–30. doi: 10.2174/1574888X12666170605112727. [DOI] [PubMed] [Google Scholar]
- 28.Abdallah BM, Ali EM. Butein Promotes Lineage Commitment of Bone Marrow-Derived Stem Cells into Osteoblasts via Modulating ERK1/2 Signaling Pathways. Molecules. 2020;25:1885. doi: 10.3390/molecules25081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang H, Yin T, Liu Y, Zhou W, Fan X, Wu L, Song C, Shao J, Yang T. TMEM18 inhibits osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells by inactivating beta-catenin. Exp Cell Res. 2019;383:111491. doi: 10.1016/j.yexcr.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Chen J, Xu D, Zhao S, Song H, Peng Y. Effects of Osteoglycin (OGN) on treating senile osteoporosis by regulating MSCs. BMC Musculoskelet Disord. 2017;18:423. doi: 10.1186/s12891-017-1779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J, Song Y, Du Z, Yu F, Zhang Y, Jiang N, Ge X. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J Mol Histol. 2020;51:455–466. doi: 10.1007/s10735-020-09896-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Wei Z, Li D, Yang Z, Tian M, Kang P. Glucocorticoid Enhanced the Expression of Ski in Osteonecrosis of Femoral Head:The Effect on Adipogenesis of Rabbit BMSCs. Calcif Tissue Int. 2019;105:506–517. doi: 10.1007/s00223-019-00592-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang SH, Gou GH, Wu CC, Shen HC, Lin LC, Pan RY. Increased COUP-TFII Expression Mediates the Differentiation Imbalance of Bone Marrow-Derived Mesenchymal Stem Cells in Femoral Head Osteonecrosis. Biomed Res Int. 2019;2019:9262430. doi: 10.1155/2019/9262430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong BC, Kim TS, Kim HS, Lee SH, Choi Y. Transmembrane protein 64 reciprocally regulates osteoblast and adipocyte differentiation by modulating Wnt/beta-catenin signaling. Bone. 2015;78:165–73. doi: 10.1016/j.bone.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Wang H, Li X, Zhang Z, Zhao X, Wang C, Wei F. MicroRNA-21 promotes bone reconstruction in maxillary bone defects. J Oral Rehabil. 2019;47:4–11. doi: 10.1111/joor.12896. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Liu J, Xia T, Mi BB, Xiong Y, Hu LC, Ruan TY, Zhou W, Liu GH. MiR-21 promotes fracture healing by activating the PI3K/Akt signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:2727–2733. doi: 10.26355/eurrev_201904_17544. [DOI] [PubMed] [Google Scholar]
- 37.Zou YC, Gao YP, Yin HD, Liu G. Serum miR-21 expression correlates with radiographic progression but also low bone mineral density in patients with ankylosing spondylitis:a cross-sectional study. Innate Immun. 2019;25:314–321. doi: 10.1177/1753425919842932. [DOI] [PMC free article] [PubMed] [Google Scholar]