Abstract
Objective:
The objective of the current study is to assess the effect of a seven-week voluntary wheel running intervention on muscles and bones properties in a mouse model mimicking dominant severe osteogenesis imperfecta (OI).
Methods:
Female wild-type (WT) and OI (Col1a1Jrt/+) mice either performed voluntarily wheel-running exercise for 7-weeks or remained sedentary. Running distance and speed, forelimb grip strength, isolated muscle force and fatigability as well as bone morphology and mechanical properties were assessed.
Results:
We demonstrate that female WT and OI mice voluntarily performed exercise, although OI mice exercised less than WT littermates. The exercise regimen increased soleus muscle masses in WT and OI but increased relative grip strength in WT mice only. Specific muscle force and fatigability were similar between WT and OI mice and did not improve with exercise. Furthermore, the exercise regimen did not improve the femoral architectural and biomechanical properties in OI mice.
Conclusion:
Our study suggests that voluntary wheel running is not appropriate to assess the effects of exercise in a mouse model of OI. Findings from exercising OI mice model studies may not necessarily be transferable to humans.
Keywords: Bone, Genetic Animal Model, Osteogenesis Imperfecta, Skeletal Muscle, Wheel Running
Introduction
Osteogenesis imperfecta (OI) is a debilitating, heritable, connective tissue disorder and characterized by bone fragility, long bone fractures, low bone mass, and short stature[1,2]. In about 90% of cases, OI is caused by mutations in the genes encoding for collagen type I alpha chains, COL1A1 and COL1A2, while the remaining cases result from mutations in genes involved in post-translational modification of collagen type I, osteoblast maturation or mineralization[3,4].
Clinically, OI is classified into four types with type I being the mildest form of OI with none or few bone deformities, type II being lethal in the perinatal period, type III being the most severe non-lethal form of OI, with very short stature, limb and spine deformities, and restricted mobility, and type IV being patients with short stature and moderate-to-severe phenotype who do not fit into one of the previously described categories[1].
In addition to the skeletal phenotype, OI patients also experience lower muscle mass and impaired muscle function, which correlates with surrogate measures of bone mass and strength[5-9]. Given the close correlation of muscle force and bone strength[10,11], low muscle mass and muscle weakness may contribute to bone fragility in OI[12], but the cause of the muscle phenotype in OI remains poorly understood[13]. Up to now it was hypothesized that either low physical activity of OI patients[14,15] or different muscle intrinsic properties[5,16] cause the muscle phenotype. Exercise intervention in children with OI types I and IV improved isometric muscle force[17], showing that OI muscles are trainable, but its effect on bone was not assessed. Rodent studies evaluating osseous effects of voluntary wheel running in young or adolescent wild-type or osteopenic animals showed improved bone mineral density, bone microarchitecture and mechanical properties[18-21]. Taken together, human and rodent studies suggest that exercise training benefit muscle function and indirectly improve bone properties in OI.
To provide a better understanding of the relationship between muscle and bone in OI, we examined the effect of a seven-week voluntary wheel running protocol in the Col1a1Jrt/+ mouse, a mouse model mimicking dominant severe form of OI[22]. We assessed whether voluntary wheel running leads to increased muscle mass and muscle strength and indirectly improves bone phenotype in OI. It is hypothesized that voluntary wheel running will induce increase in muscle and bone strength in both the OI and WT phenotypes compared to no voluntary wheel running.
Material & methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Montreal (protocol #14-148). The Col1a1Jrt/+ mouse model on FVB background caries a splice site mutation in exon 9 of the COL1A1 gene leading to an 18-amino acid deletion in the collagen type I α1 chain and are smaller in size, exhibit low bone mass, and increased spontaneous fractures[22], mimicking dominant severe form of OI. The breeding colony was maintained at the Animal Care Facility of the Shriners Hospitals for Children-Canada. Exercise experiments were carried out at the Animal Care Facility of the School of Kinesiology and Physical Activity of the Université de Montréal. At both locations, animals were on a 12-h alternating light and dark cycle and had unrestricted access to water and regular chow.
All experiments were conducted with female mice only. Female mice voluntarily exercise more than their male counterparts[23-25] and have a higher capacity of cardiovascular adaptation to increased workload[26]. Also, a previous study in the Col1a1Jrt/+ mouse model demonstrated that young female mice are more active than their male counterparts[27], suggesting a higher beneficial effect of voluntary wheel running in female Col1a1Jrt/+ mice.
Exercise regimen
Four-week-old wild-type (WT) and Col1a1Jrt/+ mice (OI) were used in the study. After transport and six days of acclimation to the new housing facility, mice were transferred to individual cages equipped with a 12.7 cm diameter exercise wheel with a 5.7 cm wide running surface (Model 80820; Lafayette Instrument, Lafayette, IN). Each wheel cage was equipped with a sensor connected to a computerized data acquisition system enabling continuous sampling of running data from individual mouse as previously described[28]. Mice had access either to a blocked (WT-Ctrl, n=10; OI-Ctrl, n=8) or free running wheel (WR) for the following seven weeks (WT-WR, n=10; OI-WR, n=8). Body mass was measured bi-weekly using a portable scale (Ohaus Corp. Parsippany, NJ, USA). Weekly running distance, average running distance, and maximal speeds (99.5 percentile of the highest single time point each given day) were averaged from daily data computed from wheel data collected at a frequency of one data over 60 sec measuring period. Mice were monitored daily for adverse exercise effects such as injury, weight loss, poor grooming, and change in running behavior. At 12 weeks of age, mice were anesthetized using isoflurane inhalation for muscle and bone dissection. Mice were euthanized by exsanguination.
Grip strength
Forelimb grip strength was assessed using a custom-made grip strength meter consisting of a horizontal mesh pull bar (7.5 x 5.0 cm) mounted to a digital force gauge (DFG55-10; Omega Engineering, Laval, Canada). Measures were performed as described previously[29] at the beginning (5 weeks old) and at the end of the voluntary wheel running protocol (12 weeks old). Briefly, mice forelimbs’ peak forces were assessed by lowering the animal until they grab on the pull bar. Animals were then pulled backward in a straight horizontal line. The highest force displayed by the instrument was recorded. Each mouse was tested five times with approximately five minutes rest between each test. The average of the highest three tests was used for statistical analyses.
Tissue collection
Before euthanasia, free running wheels were blocked for 24 hours in order to examine the effects of long-term running exercise and not the acute effects of the last exercise bout. While under isoflurane anesthesia, soleus (SOL) and extensor digitorum longus (EDL) muscles, liver and gonadal white adipose tissue (gWAT) were isolated, weighed and processed for further analysis. Left SOL and EDL muscles were carefully dissected as described previously[30], transferred to a petri dish containing an oxygenated Tyrode solution, then quickly transferred to a tissue bath system to evaluate mechanical contractile properties. Right SOL and gastrocnemius muscles were quickly dipped in liquid nitrogen and stored at -80oC for gene expression analysis and collagen contents. The right femurs were sampled and stored in phosphate buffered saline-soaked gauze at -20°C until testing or further specimen preparation.
Muscle contractile properties
EDL and SOL muscles were selected for their fast-twitch/slow twitch fiber types and their anatomical characteristics suitable for in vitro incubation; EDL is predominantly composed of fast twitch myofibers whereas SOL is predominantly composed of slow-twitch myofibers. These muscles were mounted and incubated into the Myobath multi-channel tissue bath system (World Precision Instruments, Sarasota, Florida, USA). The changes in muscle tension were digitally recorded by using a custom software application. Each muscle sample was incubated at 25oC in a stimulation chamber filled with approximately 7 mL of oxygenated Tyrode solution (NaCl, 121 mM; KCl, 5 mM; CaCl2, 1.8 mM; NaH2PO4, 0.4 mM; MgCl2, 0.5 mM; NaHCO3, 24 mM; EDTA, 0.1 mM; glucose, 5.5 mM; pH 7.4) by continuously gassing the solution with 95% O2–5% CO2. One muscle tendon was attached to a fixed hook while the other tendon was attached to a force transduction gauge (Fort25, World Precision Instruments, Sarasota, FL). Individual muscles were slowly and carefully stretched to a resting tension of approximately 0.8 g. Muscles were then repeatedly stimulated once every 30 sec (respectively at 100 Hz and 150 Hz frequency and train duration of 3 msec and 5 msec for SOL and EDL) at 60 V intensity as established during preliminary experiments to identify the resting length at which maximal force was produced. A thirty-minute equilibration phase followed to allow muscle to adapt to this environment. During this period, the muscle was stimulated using the same modalities as described above at the periodicity of 1 train/min until at least 5 consecutive tetanic contractions generated identical peak muscle force. Periodicity of the stimulatory trains during this period was 1 min, which equals to a duty cycle of 1.66%, a non-fatiguing stimulation[30].
Force-frequency experiments were performed by stimulating muscles once every 30 sec (train duration: 500 ms, train rate: 0,033 Hz). Tension developed at the frequency tested (SOL: 1, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100 Hz ; and EDL: 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, 180, 200 Hz) was recorded and plotted against one another to generate a force-frequency curve used to assess the maximal (Tmax) and half of maximal (½Tmax) forces and the frequencies at which those forces were achieved. After a 10 min recovery period, a fatigue protocol was initiated at the frequency identified to generate ½Tmax for a duration of 5 min. Five hundred ms electrical stimulation trains were triggered once every 2 sec thereby producing 150 tetani[30]. Muscle resting length and mass were carefully measured at the end of this experiment.
Muscle quantitative real-time-PCR
Total RNA was isolated from snap-frozen SOL using TRIzol™ (ThermoFisher Scientific, Waltham, USA) according to the manufacturer protocol. Reverse transcription of 1 µg RNA was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, ThermoFisher Scientific, Waltham, USA). Real-time PCR was performed with 50 ng of cDNA using a 7500 Applied Biosystems Instrument (ThermoFisher Scientific, Waltham, USA), TaqMan™ Fast Advanced master mix (ThermoFisher Scientific, Waltham, USA) and the following FAM labelled TaqMan® gene expression primers for Myostatin (#Mm01254559) and 18S (#Mm03928990) as endogenous control were used. Gene expression was analyzed according to the delta-delta Ct method.
Muscle collagen content
As a marker of tissue’s collagen content, amount of hydroxyproline was assessed in gastrocnemius muscle as described earlier[31].
Bone micro-computed tomography
Micro-computed tomography scanning of the right femora were performed using Skyscan 1272 (Bruker, MA, US). Scan parameters included voxel size of 5 µm, a 0.40-degree increment angle, 3 frames averaged, and a 62 kV X-ray source with a 0.5-mm Al filter to reduce beam-hardening artefacts. Trabecular bone was analyzed in a region starting at 0.5 mm proximal of the distal femoral growth plate (to avoid primary spongiosa) and scanning a 1.0 mm section of bone in a proximal direction. Trabecular bone was manually selected along the inner cortical surface. Scans were quantified using the system’s analysis software (Skyscan CT Analyser, Version 1.16.1.0). To analyze cortical bone, scanning was performed starting at 44% of the total femur length from the distal end and scanned for 1 mm proximally. The software derives outer bone diameter and the diameter of the bone marrow cavity from cross-sectional areas using a circular bone cross-section model. Cortical thickness was calculated as the difference of these two diameters divided by 2.
Three-point bending test
Following micro-computed tomography scanning, femora were loaded to failure in three-point bending using a Materials Testing System Model 5943 (INSTRON, Norwood, MA, USA). Femora were cleaned of all remaining muscle tissues and soaked overnight in phosphate-buffered saline at room temperature until mechanical testing. The distance between the lower supports was 7 mm with a vertical displacement rate of 50 µm/s. The anterior mid-diaphysis was loaded under tension. Test results were analyzed using the system’s analysis software Bluehill (Illinois Tool Works Inc., Glenview, IL, USA; Version 3.65).
Statistical analyses
Statistical analyses and graphs were performed using GraphPad Prism version 7, (GraphPad Software, La Jolla, CA, USA). Results are expressed as mean ± standard error of the mean (SEM). Running distance was analyzed using a one-way analysis of variance (ANOVA) for repeated measures accounting the genotype (WT vs OI) as source of variance. In addition, two-way ANOVA for single and repeated measures was performed using running activity (WR vs Ctrl) and genotype as source of variance to analyze the following dependent variables: grip force (at 5-week-old and 12-week-old, i.e. pre and post voluntary wheel running protocol), body mass, isolated muscle fatigue and force-frequency experiments, organ weights, muscle hydroxyproline, myostatin gene expression, and bone characteristics. Bonferroni post-hoc tests allowed for multiple comparisons between groups or time intervals or frequencies of stimulation. P values of <0.05 were considered statistically significant and are indicated in tables and figures with “a” representing the main effect of time or wheel running, “b” representing the main effect of genotype, and “c” representing the interaction between time and genotype.
Results
Running distance and speed were significantly lower in OI than WT mice
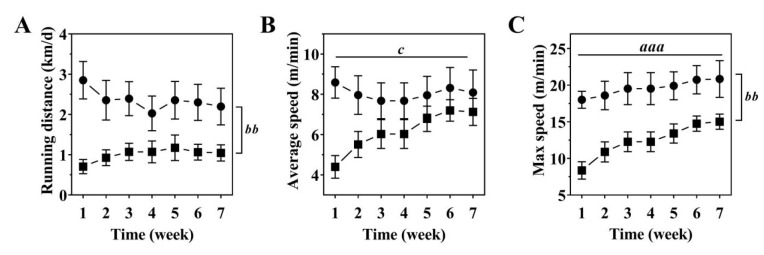
Analysis of the daily distance ran demonstrated that OI mice voluntarily exercised, but a significantly shorter distance than WT mice with an average of 1.00±0.06 km/day (cumulative distance: 47±8 km) compared to 2.35±0.09 km/day (cumulative distance: 114±17 km) (p<0.01; Figure 1A). At week one of exercise, average running speeds were significantly lower in OI than WT mice (p<0.01; Figure 1B) but between-group differences became non-significant over the rest of the exercise period. Maximum running speeds were significantly lower in OI than WT mice (p<0.01) throughout the exercise regimen and maximal speeds increased similarly over time in both groups (p<0.001; Figure 1C). Maximal running speed over the entire seven weeks were on average 12.4±2.3 m/min in OI mice and 19.5±1.0 m/min in WT mice (Figure 1C).
Figure 1.
Running distance (A), average (B) and maximal running speeds (C) of WT (black circle) and OI mice (black square) over a seven-week voluntary wheel running period. Data represent mean ± SEM. n=8-9/group. Significant effects were analyzed using one-way ANOVA (A) and two-way ANOVA (B, C) for repeated measures followed by Bonferroni post-hoc test: Effect of Time: aaap<0.001; Effect of Genotype: bbp<0.01; Time x Genotype interaction: c<0.05.
Voluntary wheel running increased relative grip strength in WT mice only
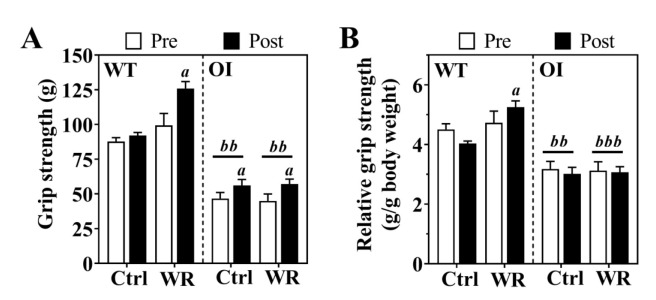
Next, we evaluated forearm grip strength before and after 7-weeks of voluntary wheel running in all mice. Compared to exercise-matched WT mice, OI-Ctrl demonstrated significantly lower absolute and relative grip strength (p<0.001; Figure 2A,B) before and after exercise regimen. Voluntary wheel running increased absolute grip strength in both OI and WT mice (Figure 2A), but grip strength relative to body mass was significantly increased in WT-WR only (Figure 2B).
Figure 2.
Absolute (A) and relative (B) grip strength before (pre) and after (post) voluntary wheel running in WT and OI mice. Data, either expressed as absolute force (A) or relative to body weight (B), represent mean ± SEM. n=8-10/group. Ctrl, control – blocked running wheel; WR, wheel running. Statistical significance was evaluated by two-way ANOVA for repeated measures followed by Bonferroni post-hoc test: Effect of Wheel running: ap<0.05; Effect of Genotype: bbp<0.01, bbbp<0.001.
Voluntary wheel running had no effect on isolated muscle force and fatigue characteristics
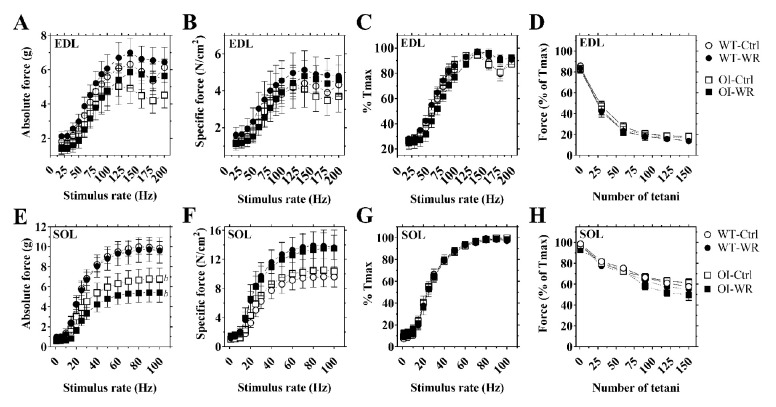
Next, we evaluated muscle force and fatigue characteristics of EDL and SOL isolated muscles in OI and WT mice. Absolute muscle force-frequency relationship revealed no significant difference in EDL muscle force output between OI and WT mice (Figure 3A), while SOL muscle from OI mice generated significantly less force, at several stimulation frequencies, than WT mice (p<0.001; Figure 3E). However, specific muscle force, accounting for individual muscle weight and length, of EDL and SOL showed no genotype-related differences in force generated across the range of frequency stimulations (Figure 3B,F). Evaluation of the time needed to generate maximal force at various stimulation frequencies revealed no genotype-related differences in EDL and SOL muscles (Figure 3C,G). Additionally, fatigue experiments of EDL and SOL revealed similar force reduction over time in both OI-Ctrl and WT-Ctrl mice (Figure 3D,H).
Figure 3.
Ex vivo muscle force and fatigue characteristics of EDL (A-D) and SOL (E-H) from WT and OI mice after seven-week of voluntary wheel running. Data represent mean ± SEM. n=7-10/group. Ctrl, control – blocked running wheel; WR, wheel running. Isolated muscle force-frequency relationship reported in absolute (g of force; panels A and E) and specific (N/cm2; panels B and F). Muscle force-frequency relationship expressed as percentage of each muscle’s maximal force (%Tmax) (C, D, G and H). Force production during the fatigue protocol expressed as percentage of each muscle’s own maximal force (%Tmax) (D and H). Symbols: open circle, WT control – blocked running wheel; black circle, WT wheel running; open square, OI control – blocked running wheel; black square, OI wheel running. Statistical significance was evaluated by two-way ANOVA for repeated measures followed by Bonferroni post-hoc test: Effect of Wheel running: not significant; Effect of Genotype: bp<0.05.
Voluntary wheel running had no significant effect on isolated EDL or SOL muscle force-frequency relationship when reported either as absolute, specific or as a percentage of maximal muscle force in either OI or WT mice (Figure 3A-C and E-G). Similarly, fatigability of EDL and SOL muscles were not affected by voluntary wheel running in either OI or WT mice (Figure 3D and H).
Voluntary wheel running results in SOL muscle mass adaptation in OI and WT mice
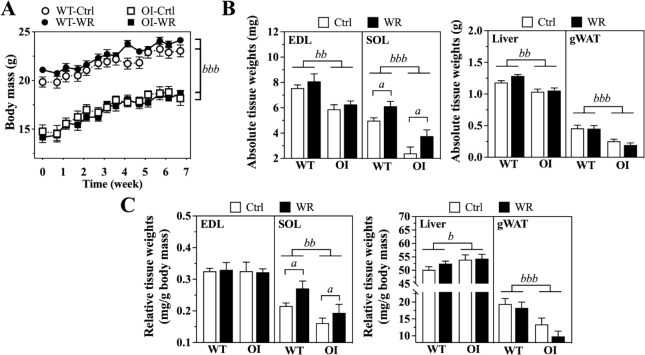
As reported previously[27,32], OI mice demonstrated approximately 20-25% lower body mass than WT mice at the beginning of the exercise regimen (p<0.001; Figure 4A). This difference in body mass persisted during the seven-week training period (Figure 4A). Evaluation of EDL and SOL muscles, liver and gWAT masses at the end of the exercise regimen demonstrated significantly lower tissue masses in OI-Ctrl than WT-Ctrl mice (p<0.01, p<0.001; Figure 4B). Normalization of tissue masses to body mass showed significantly smaller SOL muscles in OI-Ctrl than WT-Ctrl mice (p<0.01), as reported previously[33], while relative EDL muscle masses were not affected. Relative liver and gWAT masses showed significantly higher (p<0.05) and lower (p<0.001) masses in OI-Ctrl mice than in WT-Ctrl mice, respectively (Figure 4C).
Figure 4.
Body mass development (A) and absolute (B) and relative tissue weights (C) of WT and OI mice. Data represent mean ± SEM. n=8-9/group. (B) Absolute tissue weights expressed as mg or g after 7-week exercise regimen. (C) Tissue masses (mg) in relation to body mass (g). Ctrl, control – blocked running wheel; WR, wheel running. Significant effects were analyzed using two-way ANOVA for repeated (A) and single measurements (B, C) with Bonferroni post-hoc test: Effect of Wheel running: ap<0.05; Effect of Genotype: bp<0.05, bbp<0.01, bbbp<0.001.
Voluntary WR did not further improve body mass gain in OI or WT mice over the seven-week exercise regimen (Figure 4A). On tissue level, all found genotype-related differences in absolute and relative tissue masses persisted with WR. However, WR increased absolute and relative SOL masses by about 16% and 19%, respectively, in OI, and by about 33% and 26%, respectively, in WT mice reflecting muscle adaptation to wheel running exercise (p<0.05). Absolute and relative liver and gWAT masses were not affected by WR (Figure 4B,C).
Interestingly, in comparison to WT-Ctrl, myostatin gene expression in SOL muscles of OI-Ctrl mice revealed up to 6-times higher mRNA levels (p=0.014; Table 1). Although WR increased non-significantly myostatin mRNA levels in OI and WT, genotype-related difference in myostatin mRNA levels persisted (p=0.034; Table 1). As SOL masses increased with exercise, we evaluated collagen content in gastrocnemius’s as a marker of muscle protein synthesis[34]. We found no difference in hydroxyproline content in OI-Ctrl compared to WT-Ctrl mice while voluntary wheel running resulted in a by trend lower collagen content in OI mice than WT mice (p=0.07; Table 1).
Table 1.
Myostatin gene expression in SOL muscle and hydroxyproline content of gastrocnemius muscle of WT and OI mice.
| WT | OI | |||
|---|---|---|---|---|
| Ctrl | WR | Ctrl | WR | |
| Myostatin gene expression (fold-change; n=6-10/group) | 1.00 ± 0.23 | 1.91 ± 0.43 | 6.82 ± 2.51 b | 8.88 ± 2.96 b |
| Hydroxyproline (µg/mg; n=7/group) | 0.69±0.06 | 0.78±0.16 | 0.61±0.07 | 0.45±0.09 |
Data are means ± SEM. Myostatin gene expression was normalized to 18S gene expression and represents fold-change of gene expression relative to WT-Ctrl group. Statistical analysis: two-way ANOVA with Bonferroni post-hoc test: Effect of Wheel running: not significant; Effect of Genotype:
p<0.05. WT: Wild type; OI: Osteogenesis Imperfecta; Ctrl: Control; WR: Wheel Running.
Bone properties did not change after 7-week voluntary wheel running in OI and WT mice
Given the close relationship of muscle and bone phenotype, we next evaluated femoral architectural and biomechanical properties. As previously described[32], OI mice demonstrated reduced femoral length, femoral structural and biomechanical properties compared to WT littermates. Seven-week voluntary wheel running had no effect on bone length, BV/TV, trabecular number and thickness in OI and WT mice (Table 2). Additionally, no exercise related effects were found on cortical thickness or diaphyseal diameter at the femoral midshaft or mechanical properties in any of the groups (Table 2).
Table 2.
Structural and biomechanical characteristics of the right femora.
| WT | OI | |||
|---|---|---|---|---|
| Ctrl (n=10) | WR (n=10) | Ctrl (n=6) | WR (n=8) | |
| Bone length (mm) | 14.6 ± 0.1 | 14.9 ± 0.1 | 13.7 ± 0.2 bb | 14.3 ± 0.1 b |
| BV/TV (%) | 12.7 ± 0.4 | 11.5 ± 0.5 | 4.88 ± 0.8 bbb | 4.79 ± 0.3 bbb |
| Tb.N.(1/mm) | 42.7 ± 0.5 | 42.3 ± 0.6 | 33.4 ± 1.5 bbb | 33.7 ± 0.6 bbb |
| Tb.Th.(µm) | 2.9 ± 0.1 | 2.7 ± 0.1 | 1.4 ± 0.2 bbb | 1.4 ± 0.1 bbb |
| Periosteal diameter (mm) | 1.44 ± 0.01 | 1.49 ± 0.02 | 1.29 ± 0.02 bbb | 1.31 ± 0.02 bbb |
| Endosteal diameter (mm) | 1.06 ± 0.01 | 1.12 ± 0.02 | 0.98 ± 0.03 b | 1.00 ± 0.02 bbb |
| Cortical thickness (mm) | 0.19 ± 0.02 | 0.19 ± 0.01 | 0.16 ± 0.01 bbb | 0.16 ± 0.01 bbb |
| Maximum load (N) | 16.4 ± 0.3 | 15.7 ± 0.4 | 7.6 ± 0.4 bbb | 7.8 ± 0.7 bbb |
| Fracture load (N) | 12.1 ± 0.4 | 10.9 ± 0.6 | 6.9 ± 0.7 bbb | 6.7 ± 1.1 bb |
| Energy until fracture (mJ) | 6.8 ± 0.4 | 7.3 ± 0.5 | 0.5 ± 0.2 bbb | 0.4 ± 0.2 bbb |
| Stiffness (N/mm) | 119.2 ± 1.9 | 115.2 ± 3.1 | 80.4 ± 5.3 bbb | 88.6 ± 5.4 bbb |
Data represent mean ± SEM. Statistical analysis: two-way ANOVA for single measurements with Bonferroni post-hoc test: Effect of Wheel running: not significant; Effect of Genotype:
p<0.05,
p<0.01,
p<0.001. WT: Wild type; OI: Osteogenesis Imperfecta; Ctrl: Control; WR: Wheel Running; BV/TV: Bone Volume/Total Volume; Tb.N.: Trabecular Numbers; Tb.Th.; Trabecular Thickness; N: Newtons; mm: millimeters; mJ: micro Joules.
Discussion
In this study, we assessed whether voluntary wheel running would increase muscle mass and improve muscle strength, and consequently result in improved bone properties, in a mouse model of dominant severe OI. We found that WT and OI mice voluntarily performed exercise when provided with a running wheel, although OI mice exercised less than WT littermates. Regarding musculoskeletal adaptation to exercise, we found improved relative grip strength in WT mice only and increased soleus muscle masses in WT and OI mice. Interestingly, assessed specific muscle force and fatigability in EDL and SOL were similar between WT and OI mice and did not improve with exercise. Additionally, voluntary wheel running had no effect on bone parameters in neither WT nor OI mice.
That the forelimbs’ grip strength improved with exercise in WT mice might be due to the higher running exercise volume in WT as compared to OI mice. Indeed, OI mice ran approximately 58% less in regard to cumulative distance and demonstrated approximately 35% lower running speeds than WT littermates. This is in line with documented shorter running distance and lower running speeds of oim/oim mice, a mouse model mimicking recessive severe OI, when provided with a running wheel for voluntary exercise[35]. Interestingly, compared to oim/oim mice, our Col1a1Jrt/+ mice ran less with about 1.0 km/day versus 2.6 km/day in oim/oim. This difference might either be due to the different severity of musculoskeletal impairment caused by the underlying collagen type I mutation (Col1a1Jrt/+ mice: COL1A1; oim/oim mice: COL1A2) or due to the different background strain (Col1a1Jrt/+ mice: FVB; oim/oim mice: C57BL6). However, as shown for the oim/oim mouse model[35], voluntary wheel running failed to improve muscle function and femoral architecture and biomechanics in WT and Col1a1Jrt/+ mice.
A first reason for lack of muscle adaptation in OI mice might be attributed to the muscle-specific fiber type composition, myofiber-type specific mitochondrial content and activity. Previously, we demonstrated that SOL muscles from Col1a1Jrt/+ mice have smaller myofibers and a shifted fiber type composition with a lower proportion of slow-twitch and higher proportions of fast-twitch and slow-fast hybrid fibers compared to WT[33]. Slow-twitch myofibers generally harbor higher mitochondria densities as compared to fast-twitch myofibers[36], suggesting that the shifted fiber type composition in SOL muscle from Col1a1Jrt/+ mice may result in lower mitochondrial density. This in turn might cause reduced mitochondrial energy production, leading to lower muscle-energy. Along these lines, up to 40% lower mitochondrial to nuclear DNA ratios and severe mitochondrial dysfunction have been demonstrated in muscles from oim/oim mice[35]. Notwithstanding these results, we found that the muscle force-frequency relationship was not different between WT and Col1a1Jrt/+ mice suggesting that, despite altered myofiber composition, the contractile machinery and the excitation-contraction processes regulating muscle force development are not affected in Col1a1Jrt/+ mice. Furthermore, the fatigue protocol revealed similar patterns of force reduction over time in both WT and OI, suggesting that Ca2+ release from the sarcoplasmic reticulum[37] is not affected in OI. Thus, in the Col1a1Jrt/+ mouse model other factors seem to compromise muscle adaptation to exercise.
Sex-related differences in skeletal severity in the Col1a1Jrt/+ mouse model exist and depend on the developmental stage. Pre-pubertally, no sex-related skeletal differences can be found[27]. Once animals enter ‘puberty’, female OI and WT mice demonstrate slight increase in femoral BV/TV compared to their male counterparts (internal unpublished data). Nonetheless, compared to female WT mice, female OI mice still demonstrate around 45% less BV/TV at all ages. However, Gremminger et al. investigated voluntary wheel running and swimming-exercise in male and female OI mice with a recessive severe form of OI, the oim/oim mouse model[35]. They demonstrated that male WT and oim/oim mice exhibited decreases in muscle weight and function and slightly compromised bone micro-architecture and biomechanical properties in response to exercise, whereas female mice were minimally affected. In line with our observation, WT mice ran greater-than-average distances compared with their age-matched oim/oim counterparts, regardless of sex. Therefore, sex may not be a major factor explaining why voluntary wheel running did not improve muscle function in the present study.
Another factor might be the high expression of myostatin. Myostatin, a hormone secreted by myocytes and a member of the transforming growth factor β superfamily, has been shown to inhibit muscle differentiation and growth[38,39]. Additionally, myostatin knock-out mice (Mstn-/-) demonstrated, despite muscle hyperplasia and hypertrophy, increased bone mass and biomechanical strength[40,41]. When heterozygous oim mice, a mouse model of mild OI, were made genetically deficient in myostatin, they displayed a partial rescue of muscle mass and bone phenotype[41]. Pharmacological inhibition of myostatin with a neutralizing antibody (ActRIIB-ligand trap) in mild, moderate, and severe OI mouse models resulted in increased muscle mass and OI-severity dependent improvement of the bone phenotype[33,42-44]. Interestingly, muscles of Mstn-/- mice, despite being larger, demonstrated an altered myofiber composition and decreased muscle function[45]. ActRIIB-ligand trap administration in Col1a1Jrt/+ mice resulted in a dose-dependent shift of the myofiber composition towards more slow-fast twitch hybrids[33] but demonstrated in oim/oim mice increased contractile function[44]. Here we found high levels of myostatin in Col1a1Jrt/+ mice which persisted with exercise and may have compromised beneficial muscle adaptation to wheel running. However, given that benefits were also minimal in WT littermates, the effect of increased myostatin on muscle adaptation is probably low in this case.
But, since OI is caused by mutations in the collagen type I genes, the lack of skeletal adaptation to wheel running in Col1a1Jrt/+ mice and other mouse model, such as oim/oim[44], might be related to the collagen type I mutation. Collagen type I is the most abundant type of collagen and a key structural component of the connective tissue of bones, muscles, tendons and skin[46]. In the skeletal muscle extracellular matrix, collagen type I is predominantly found in the perimysium that covers the muscle fascicle connecting muscle and tendon[47], and is instrumental in force transmission during muscle contractions[48]. Tendons contain around 60–80% of collagen type I and are the main structure transmitting the generated muscle force to the bone[49]. The Col1a1Jrt/+ mouse model harbors features of Ehlers-Danlos syndrome with mechanically compromised skin and frayed tendon[22]. Other OI mouse models also demonstrated structurally altered and biomechanically compromised tendons[50-52]. Thus, the abnormal collagen structure may lead to less efficient force transmission from muscle contraction to the bone. Indeed, Berman et al. demonstrated recently that oim/oim mice produce significantly less force on the bone, with on average 1500 µε, than WT mice with about 2500 µε[53], suggesting that oim/oim mice require more force to induce bone formation. Additionally, studies in oim/oim mice observed calcification of the tendons with age[25,53-55] and broken calcaneus[53], which may further compromise muscle-bone force transmission and work load adaptation during voluntary wheel running. However, whether tendon calcification and broken calcaneus compromise muscle-bone interaction in the Col1a1Jrt/+ mouse model remains a question for future studies.
Berman et al. has concluded that oim/oim mice might require more force to induce bone formation[53], and thus, the lack of exercise training effect on bone might be related to the intensity of exercise. Even though there was an early increase in average running speeds in Col1a1Jrt/+ mice which may reflect an improvement in function, Col1a1Jrt/+ mice demonstrated lower maximal running speeds than WT mice. Therefore, the chosen exercise training regimen with continuous low load on the musculoskeletal system probably did not reach the load thresholds necessary to induce bone formation or bone matrix adaptation[56,57]. Additionally, Gremminger et al. pointed out that continuous low load may lead to desensitization of the osteocytes inhibiting adaptation response of the musculoskeletal system in both, WT and OI mice[35]. Furthermore, four weeks of treadmill running training did not increase bone strength in wildtype mice[41] suggesting that running exercise alone may not be sufficient to induce bone morphological and functional adaptations. Thus, perhaps an exercise regimen consisting of endurance and strength training exercises with progressive increments in volume, intensity, duration, and frequency may improve muscle and bone properties in the Col1a1Jrt/+ mouse model. However, given the fragility of the Col1a1Jrt/+ mouse model, forced running treadmill or strength training might increase the risk of spontaneous fractures[22] leading to pain and compromising locomotor function[58]. Additionally, Col1a1Jrt/+ mice display altered lung phenotype[59] and whole-body energy expenditure and homeostasis[27] which may mitigate endurance and strength training exercises effects in this model.
Interestingly, exercise studies in OI patients show a different picture. Indeed, endurance and strength training resulted in increased isometric muscle force in children with mild (type I) and moderate (type IV) OI[17]. This beneficial effect of exercise training in OI patients might be related to the pharmacological treatment of OI with bisphosphonates. Bisphosphonates are mainly administered to increase bone density and reduce fracture rates[1] but have been shown to reduce pain and improve physical functioning in children with OI[60]. Thus, OI patient benefit from medical treatment in combination with exercise. However, depending on type of OI, age and functional level, OI patients need a training program focusing on the individual’s needs to improve musculoskeletal system[61]. Regarding OI mouse models, it remains a question for future studies if bisphosphonate treatment and wheel running exercise will improve musculoskeletal phenotype.
Conclusion
Here we demonstrate that Col1a1Jrt/+ mice, a model of dominant severe OI, well-tolerate voluntary wheel-running and show increasing activity levels over time. Nonetheless, wheel-running benefit was found in the forearm grip strength of WT mice only, while EDL and SOL muscle function was not changed in WT and Col1a1Jrt/+mice, despite increases in SOL mass. These findings suggest that the type of exercise and type of OI are important factors when considering exercise as a treatment option.
Funding
This work was supported by a research grant from the Réseau de Recherche en Santé Buccodentaire et Osseuse (RSBO)-FRQS(#DF 125428).
Authors’ contributions
Conceptualization, FR, LNV and RB; Methodology, FR, LNV, JTT and RB; Investigation, JTT, GHRC, JSM, SA, LNV and RB; Original Draft, RB, LNV; Review & Editing, JTT, LNV and RB; Funding Acquisition, FR and RB; Resources, FR, RB and LNV; Supervision, RB.
Acknowledgement
The authors would like to acknowledge Vincent Samuel-Lafleur, Stéphanie Gravel, and Katja Heinemeier for assistance with hydroxyproline assays.
Footnotes
Dr. Frank Rauch reports personal fees from Novartis, Mereo, and grants from Mesentech, outside the submitted work. The remaining authors have nothing to declare.
Edited by: G. Lyritis
References
- 1.Tauer JT, Robinson ME, Rauch F. Osteogenesis Imperfecta:New Perspectives From Clinical and Translational Research. JBMR Plus. 2019;3:e10174. doi: 10.1002/jbm4.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387:1657–71. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardai G, Moffatt P, Glorieux FH, Rauch F. DNA sequence analysis in 598 individuals with a clinical diagnosis of osteogenesis imperfecta:diagnostic yield and mutation spectrum. Osteoporos Int. 2016;27:3607–13. doi: 10.1007/s00198-016-3709-1. [DOI] [PubMed] [Google Scholar]
- 4.Mileusnic MP, Loeb GE. Mathematical models of proprioceptors II. Structure and function of the Golgi tendon organ. J Neurophysiol. 2006;96:1789–802. doi: 10.1152/jn.00869.2005. [DOI] [PubMed] [Google Scholar]
- 5.Veilleux LN, Lemay M, Pouliot-Laforte A, Cheung MS, Glorieux FH, Rauch F. Muscle anatomy and dynamic muscle function in osteogenesis imperfecta type I. J Clin Endocrinol Metab. 2014;99:E356–62. doi: 10.1210/jc.2013-3209. [DOI] [PubMed] [Google Scholar]
- 6.Brizola E, Staub AL, Felix TM. Muscle strength, joint range of motion, and gait in children and adolescents with osteogenesis imperfecta. Pediatr Phys Ther. 2014;26:245–52. doi: 10.1097/PEP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 7.Caudill A, Flanagan A, Hassani S, et al. Ankle strength and functional limitations in children and adolescents with type I osteogenesis imperfecta. Pediatr Phys Ther. 2010;22:288–95. doi: 10.1097/PEP.0b013e3181ea8b8d. [DOI] [PubMed] [Google Scholar]
- 8.Veilleux LN, Darsaklis VB, Montpetit K, Glorieux FH, Rauch F. Muscle Function in Osteogenesis Imperfecta Type IV. Calcif Tissue Int. 2017;101:362–70. doi: 10.1007/s00223-017-0287-y. [DOI] [PubMed] [Google Scholar]
- 9.Montpetit K, Plotkin H, Rauch F, et al. Rapid increase in grip force after start of pamidronate therapy in children and adolescents with severe osteogenesis imperfecta. Pediatrics. 2003;111:e601–3. doi: 10.1542/peds.111.5.e601. [DOI] [PubMed] [Google Scholar]
- 10.Anliker E, Rawer R, Boutellier U, Toigo M. Maximum ground reaction force in relation to tibial bone mass in children and adults. Med Sci Sports Exerc. 2011;43:2102–9. doi: 10.1249/MSS.0b013e31821c4661. [DOI] [PubMed] [Google Scholar]
- 11.Frost HM. Bone “mass”and the “mechanostat”:a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 12.Veilleux LN, Pouliot-Laforte A, Lemay M, Cheung MS, Glorieux FH, Rauch F. The functional muscle-bone unit in patients with osteogenesis imperfecta type I. Bone. 2015;79:52–7. doi: 10.1016/j.bone.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Veilleux LN, Trejo P, Rauch F. Muscle abnormalities in osteogenesis imperfecta. J Musculoskelet Neuronal Interact. 2017;17:1–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Pouliot-Laforte A, Veilleux LN, Rauch F, Lemay M. Physical activity in youth with osteogenesis imperfecta type I. J Musculoskelet Neuronal Interact. 2015;15:171–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G. A method to assess energy expenditure in children and adults. Am J Clin Nutr. 1983;37:461–7. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]
- 16.Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol. 2010;29:638–44. doi: 10.1016/j.matbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Brusse M, Takken T, Uiterwaal CS, et al. Physical training in children with osteogenesis imperfecta. J Pediatr. 2008;152:111–6. doi: 10.1016/j.jpeds.2007.06.029. 6 e1. [DOI] [PubMed] [Google Scholar]
- 18.Hagihara Y, Nakajima A, Fukuda S, Goto S, Iida H, Yamazaki M. Running exercise for short duration increases bone mineral density of loaded long bones in young growing rats. Tohoku J Exp Med. 2009;219:139–43. doi: 10.1620/tjem.219.139. [DOI] [PubMed] [Google Scholar]
- 19.Isaksson H, Tolvanen V, Finnila MA, et al. Physical exercise improves properties of bone and its collagen network in growing and maturing mice. Calcif Tissue Int. 2009;85:247–56. doi: 10.1007/s00223-009-9273-3. [DOI] [PubMed] [Google Scholar]
- 20.Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS. Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J Appl Physiol (1985) 2003;95:300–7. doi: 10.1152/japplphysiol.01076.2002. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto J, Takeda T, Ichimura S. Effects of exercise on bone mineral density in mature osteopenic rats. J Bone Miner Res. 1998;13:1308–17. doi: 10.1359/jbmr.1998.13.8.1308. [DOI] [PubMed] [Google Scholar]
- 22.Chen F, Guo R, Itoh S, et al. First mouse model for combined osteogenesis imperfecta and Ehlers-Danlos syndrome. J Bone Miner Res. 2014;29:1412–23. doi: 10.1002/jbmr.2177. [DOI] [PubMed] [Google Scholar]
- 23.Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998;28:227–37. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- 24.Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–18. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- 25.De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–34. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- 26.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–76. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boraschi-Diaz I, Tauer JT, El-Rifai O, et al. Metabolic phenotype in the mouse model of osteogenesis imperfecta. J Endocrinol. 2017;234:279–89. doi: 10.1530/JOE-17-0335. [DOI] [PubMed] [Google Scholar]
- 28.Bergeron R, Mentor JS, Cote I, Ngo Sock ET, Rabasa-Lhoret R, Lavoie JM. Loss of ovarian estrogens causes only mild deterioration of glucose homeostasis in female ZDF rats preventable by voluntary running exercise. Horm Metab Res. 2014;46:774–81. doi: 10.1055/s-0034-1381980. [DOI] [PubMed] [Google Scholar]
- 29.Tanase K, Teng Q, Krishnaney AA, Liu JK, Garrity-Moses ME, Boulis NM. Cervical spinal cord delivery of a rabies G protein pseudotyped lentiviral vector in the SOD-1 transgenic mouse. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:128–36. doi: 10.3171/spi.2004.1.1.0128. [DOI] [PubMed] [Google Scholar]
- 30.Park KH, Brotto L, Lehoang O, Brotto M, Ma J, Zhao X. Ex vivo assessment of contractility, fatigability and alternans in isolated skeletal muscles. J Vis Exp. 2012:e4198. doi: 10.3791/4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol (1985) 2006;101:898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roschger A, Roschger P, Keplingter P, et al. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone. 2014;66:182–8. doi: 10.1016/j.bone.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Tauer JT, Rauch F. Novel ActRIIB ligand trap increases muscle mass and improves bone geometry in a mouse model of severe osteogenesis imperfecta. Bone. 2019;128:115036. doi: 10.1016/j.bone.2019.115036. [DOI] [PubMed] [Google Scholar]
- 34.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 35.Gremminger VL, Jeong Y, Cunningham RP, Meers GM, Rector RS, Phillips CL. Compromised Exercise Capacity and Mitochondrial Dysfunction in the Osteogenesis Imperfecta Murine (oim) Mouse Model. J Bone Miner Res. 2019;34:1646–59. doi: 10.1002/jbmr.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 37.Brotto MA, Nosek TM, Kolbeck RC. Influence of ageing on the fatigability of isolated mouse skeletal muscles from mature and aged mice. Exp Physiol. 2002;87:77–82. doi: 10.1113/eph8702224. [DOI] [PubMed] [Google Scholar]
- 38.Latres E, Mastaitis J, Fury W, et al. Activin A more prominently regulates muscle mass in primates than does GDF8. Nat Commun. 2017;8:15153. doi: 10.1038/ncomms15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 40.Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:388–91. doi: 10.1002/ar.a.10044. [DOI] [PubMed] [Google Scholar]
- 41.Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21:477–83. doi: 10.1359/JBMR.051203. [DOI] [PubMed] [Google Scholar]
- 42.Oestreich AK, Carleton SM, Yao X, et al. Myostatin deficiency partially rescues the bone phenotype of osteogenesis imperfecta model mice. Osteoporos Int. 2016;27:161–70. doi: 10.1007/s00198-015-3226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong Y, Daghlas SA, Xie Y, et al. Skeletal Response to Soluble Activin Receptor Type IIB in Mouse Models of Osteogenesis Imperfecta. J Bone Miner Res. 2018;33:1760–72. doi: 10.1002/jbmr.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong Y, Daghlas SA, Kahveci AS, et al. Soluble activin receptor type IIB decoy receptor differentially impacts murine osteogenesis imperfecta muscle function. Muscle Nerve. 2018;57:294–304. doi: 10.1002/mus.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amthor H, Macharia R, Navarrete R, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci U S A. 2007;104:1835–40. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henriksen K, Karsdal MA. Type I collagen. Biochemistry of Collagens, Laminins and Elastin. 2019:1–12. [Google Scholar]
- 47.Csapo R, Gumpenberger M, Wessner B. Skeletal Muscle Extracellular Matrix - What Do We Know About Its Composition, Regulation, and Physiological Roles?A Narrative Review. Front Physiol. 2020;11:253. doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons:involvement in lateral force transmission in skeletal muscle. J Struct Biol. 2007;159:19–28. doi: 10.1016/j.jsb.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 49.Tresoldi I, Oliva F, Benvenuto M, et al. Tendon's ultrastructure. Muscles Ligaments Tendons J. 2013;3:2–6. doi: 10.11138/mltj/2013.3.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sims TJ, Miles CA, Bailey AJ, Camacho NP. Properties of collagen in OIM mouse tissues. Connect Tissue Res. 2003;44(Suppl 1):202–5. [PubMed] [Google Scholar]
- 51.McBride DJ, Jr, Choe V, Shapiro JR, Brodsky B. Altered collagen structure in mouse tail tendon lacking the alpha 2(I) chain. J Mol Biol. 1997;270:275–84. doi: 10.1006/jmbi.1997.1106. [DOI] [PubMed] [Google Scholar]
- 52.Misof K, Landis WJ, Klaushofer K, Fratzl P. Collagen from the osteogenesis imperfecta mouse model (oim) shows reduced resistance against tensile stress. J Clin Invest. 1997;100:40–5. doi: 10.1172/JCI119519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berman AG, Organ JM, Allen MR, Wallace JM. Muscle contraction induces osteogenic levels of cortical bone strain despite muscle weakness in a mouse model of Osteogenesis Imperfecta. Bone. 2020;132:115061. doi: 10.1016/j.bone.2019.115061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landis WJ. Tomographic imaging of collagen-mineral interaction:implications for osteogenesis imperfecta. Connect Tissue Res. 1995;31:287–90. doi: 10.3109/03008209509010825. [DOI] [PubMed] [Google Scholar]
- 55.Grabner B, Landis WJ, Roschger P, et al. Age- and genotype-dependence of bone material properties in the osteogenesis imperfecta murine model (oim) Bone. 2001;29:453–7. doi: 10.1016/s8756-3282(01)00594-4. [DOI] [PubMed] [Google Scholar]
- 56.Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev. 2003;31:45–50. doi: 10.1097/00003677-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Hart NH, Nimphius S, Rantalainen T, Ireland A, Siafarikas A, Newton RU. Mechanical basis of bone strength:influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017;17:114–39. [PMC free article] [PubMed] [Google Scholar]
- 58.Abdelaziz DM, Abdullah S, Magnussen C, et al. Behavioral signs of pain and functional impairment in a mouse model of osteogenesis imperfecta. Bone. 2015;81:400–6. doi: 10.1016/j.bone.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Baglole CJ, Liang F, Traboulsi H, et al. Pulmonary and diaphragmatic pathology in collagen type I alpha1 mutant mice with osteogenesis imperfecta. Pediatr Res. 2018;83:1165–71. doi: 10.1038/pr.2018.36. [DOI] [PubMed] [Google Scholar]
- 60.Garganta MD, Jaser SS, Lazow MA, et al. Cyclic bisphosphonate therapy reduces pain and improves physical functioning in children with osteogenesis imperfecta. BMC Musculoskelet Disord. 2018;19:344. doi: 10.1186/s12891-018-2252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mueller B, Engelbert R, Baratta-Ziska F, et al. Consensus statement on physical rehabilitation in children and adolescents with osteogenesis imperfecta. Orphanet J Rare Dis. 2018;13:158. doi: 10.1186/s13023-018-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]