Abstract
Background and Objectives
To determine whether patients with Parkinson disease (PD) eligible for subthalamic nucleus deep brain stimulation (STN-DBS) with probable REM sleep behavior disorder (RBD) preoperatively could be more at risk of poorer motor, nonmotor, and quality of life outcomes 12 months after surgery compared to those without RBD.
Methods
We analyzed the preoperative clinical profile of 448 patients with PD from a French multicentric prospective study (PREDISTIM) according to the presence or absence of probable RBD based on the RBD Single Question and RBD Screening Questionnaire. Among the 215 patients with PD with 12 months of follow-up after STN-DBS, we compared motor, cognitive, psycho-behavioral profile, and quality of life outcomes in patients with (pre-opRBD+) or without (pre-opRBD–) probable RBD preoperatively.
Results
At preoperative evaluation, pre-opRBD+ patients were older (61 ± 7.2 vs 59.5 ± 7.7 years; p = 0.02), had less motor impairment (Movement Disorder Society–sponsored version of the Unified Parkinson’s Disease Rating Scale [MDS-UPDRS] III “off”: 38.7 ± 16.2 vs 43.4 ± 7.1; p = 0.03) but more nonmotor symptoms on daily living activities (MDS-UPDRS I: 12.6 ± 5.5 vs 10.7 ± 5.3; p < 0.001), had more psychobehavioral manifestations (Ardouin Scale of Behavior in Parkinson's Disease total: 7.7 ± 5.1 vs 5.1 ± 0.4; p = 0.003), and had worse quality of life (Parkinson's Disease Questionnaire–39: 33 ± 12 vs 29 ± 12; p = 0.03), as compared to pre-opRBD– patients. Both pre-opRBD+ and pre-opRBD– patients had significant MDS-UPDRS IV score decrease (−37% and −33%, respectively), MDS-UPDRS III “med ‘off’/stim ‘on’” score decrease (−52% and −54%), and dopaminergic treatment decrease (−52% and −49%) after surgery, with no between-group difference. There was no between-group difference for cognitive and global quality of life outcomes.
Conclusions
In patients with PD eligible for STN-DBS, the presence of probable RBD preoperatively is not associated with a different clinical outcome 1 year after neurosurgery.
Trial Registration Information
Classification of Evidence
This study provides Class II evidence that in patients with PD eligible for STN-DBS, the presence of probable RBD preoperatively is not associated with poorer outcomes 1 year post surgery.
Recent evidence suggests that patients with Parkinson disease (PD) who have REM sleep behavior disorder (RBD) might exhibit a more severe phenotype,1 including greater motor disabilities and axial symptoms,1,2 dysautonomia,3 cognitive impairment,4,5 visual hallucinations,6 and impulse control disorders.7,8 Subthalamic nucleus deep brain stimulation (STN-DBS) is a well-documented treatment for severe PD with motor fluctuations and dyskinesias.9-11 However, some patients may demonstrate worsening of other symptoms following STN-DBS, such as apathy,10 emergence of impulsive behaviors,10 and axial symptoms including freezing of gait, postural instability, and dysarthria.12-14 Neuroimaging indicators have been proposed for guiding surgical decisions for DBS, such as connectivity profile,15 lower boundaries of T2 relaxometry in the STN reported to predict motor outcome,16 or accumbens nucleus volume reported as a marker of risk of cognitive decline after STN-DBS.17 Those tools are difficult to use in daily practice and identifying patients at risk of negative outcomes after STN-DBS remains a challenge because there are few clinical indicators to determine patients most likely to benefit from this treatment.18,19 Until recently, only 2 monocentric studies (conducted in 41 and 50 patients, respectively) have assessed outcomes of patients with PD with RBD undergoing STN-DBS, and reported conflicting results.20,21
The aim of this study was to investigate whether the presence of RBD before STN-DBS could affect motor, cognitive, psycho-behavioral, and quality of life outcomes 12 months after STN-DBS, using a large French prospective cohort of patients with PD, and to characterize the preoperative profile of patients with PD with and without probable RBD.
Methods
Patients
This study was an ancillary analysis using data collected from the PREDISTIM cohort. PREDISTIM is an ongoing prospective multicentric cohort (Protocol 2013-A00193-42) sponsored by the University Hospital of Lille, conducted in 17 PD expert centers from the French clinical research network (NS-Park/F-CRIN). The primary objective was to study predictive factors of the therapeutic response to STN-DBS on long-term quality of life. Briefly, patients undergoing STN-DBS in each of the participating centers were consecutively included into the study between November 2013 and September 2019. The inclusion criteria were a diagnosis of PD according to the UK Parkinson's Disease Brain Bank, disease duration ≥5 years, age between 18 and 75 years, and indication for STN-DBS. Exclusion criteria included atypical parkinsonism, severe cognitive impairment, severe psychiatric disorders, acute levodopa motor response <30%, and contraindications to surgery. Clinical data were collected at baseline and then at 1 year postsurgery.
Among 795 patients enrolled in the PREDISTIM cohort and awaiting STN-DBS at the time of the analysis (February 2019), 56% (n = 448) completed RBD Screening Questionnaire (RBD–SQ) or RBD Single Question (RBD–1Q) preoperatively and were included in our ancillary study. RBD questionnaires were not initially part of the PREDISTIM study and this ancillary study began in April 2015. The clinical and demographic characteristics of patients with PD with probable RBD preoperatively (pre-opRBD+) and without probable RBD preoperatively (pre-opRBD−) were described in the whole population (n = 448). Then we investigated the outcomes 12 months after STN-DBS, in terms of variations of motor, nonmotor, and quality of life scores compared to baseline, in a subset of patients (n = 215) who had both pre- and postoperative evaluations (eFigure 1, links.lww.com/WNL/B589). Data from patients with preoperative screening of probable RBD were included into the analysis.
The primary research question of this ancillary study was whether patients with PD with probable RBD preoperatively could be more at risk of poorer motor, nonmotor, and quality of life outcomes 12 months after STN-DBS (Level of evidence: Class II).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by CPP (Comité de Protection des Personnes) Nord Ouest-IV Ethical Committee and registered in the ClinicalTrials.gov website (NCT02360683). Patients gave written informed consent, the study was conducted according to good clinical practice and local regulations, and data collection was compliant with General Data Protection Regulation (GDPR) rules.
Study Design and Clinical Scores
The diagnosis of probable RBD was assessed preoperatively using both RBD–1Q and RBD–SQ scores, which are validated as sensitive and specific tools for detecting RBD in the general population22,23 and in PD.24,25 Probable preoperative RBD (pre-opRBD+) was defined by a positive response on RBD–1Q or a score ≥6 on RBD–SQ at preoperative evaluation.22,25 Clinical assessments at each visit included collection of demographic data, parkinsonism, neuropsychological testing, quality of life questionnaires, and medical and treatment history. Parkinsonism evaluation was performed using the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS),26 which assesses nonmotor and motor experiences of daily living (MDS-UPDRS I, II), motor examination (MDS-UPDRS III), and motor complications (MDS-UPDRS IV). MDS-UPDRS parts I–IV were performed in “off” and “on” state during a standardized acute levodopa challenge.
At baseline, motor assessment was performed using MDS-UPDRS Part III in the “off” state after 12-hour withdrawal of antiparkinsonian medication (med “off”) and in the “on” state after taking 1.5 times the usual morning levodopa dose using dispersible levodopa (med “on”). Twelve months after surgery, the efficacy of STN-DBS and of levodopa was assessed in the morning after at least 12 hours of withdrawal of antiparkinsonian medication using MDS-UPDRS Part III in 3 conditions: medication off–stimulation off (med-off/stim-off), medication off–stimulation on (med-off/stim-on), and medication on–stimulation on (med-on/stim-on). Comparison between baseline and 12 months after surgery was performed for MDS-UPDRS III “off,” defined by the conditions med-off preoperatively vs med-off/stim-off postoperatively, and for MDS-UPDRS III “on,” defined by the conditions med-on preoperatively vs med-on/stim-on postoperatively. The effect of chronic STN-DBS was also evaluated comparing MDS-UPDRS III in med-off condition preoperatively vs med-off/stim-on postoperatively. The axial score was defined as the sum of the following motor subscores: speech, gait, posture, and postural stability (items 18, 28, 29, and 30 on the MDS-UPDRS Part III).27
Cognitive functions were assessed using 11 validated tests in PD, according to the French consensus for the evaluation of cognitive functions in PD,28 which included the following: Montreal Cognitive Assessment (MoCA) for global efficiency29; Symbol Digit Modalities Test (SDMT) for attention and working memory; Digit Span Forward and Backward, subtests 16-item Free/Cued Recall Test and 10/36 Spatial Recall Test for episodic memory; Oral Letter–Number Sequencing task and D-KEFS Color–Word Interference Test for executive function; Benton Judgement of Line Orientation and CLOX clock-drawing test for visuospatial function; and Boston Naming Test and Animal fluency test for language. Regarding psycho-behavioral symptoms, we used the French version of the Ardouin Scale of Behavior in Parkinson's Disease (ASBPD) to assess hypo- and hyperdopaminergic disorders, nonmotor fluctuations, and impulse control disorder (ICD) (compulsive eating, hobbyism, punding, compulsive shopping, pathologic gambling, hypersexuality, and dopaminergic addiction).30 For this scale, higher scores indicate worse mood and behavioral disturbances. The Parkinson's Disease Questionnaire–39 (PDQ-39) scale was used to assess quality of life. The PDQ-39 summary index (PDQ-39-SI) ranges from 0 to 100; higher score indicates worse quality of life.31
The levodopa equivalent daily dose (LEDD) was provided for each patient taking account of levodopa treatments, dopamine agonists, and other antiparkinsonian drugs such as MAO-B inhibitors, COMT inhibitors, and amantadine.32
Statistical Analysis
Continuous data were expressed as mean and SD or median and interquartile range according to statistical distribution. The assumption of normality was assessed by the Shapiro-Wilk test. Regarding nonrepeated measures (such as comparison of baseline features between pre-opRBD+ and pre-opRBD–), continuous variables were compared between groups by Student t test or Mann-Whitney test when the assumptions of t test were not met. Homoscedasticity was analyzed using the Fisher-Snedecor test. Categorical measures were compared between groups using χ2 or Fisher exact tests. To compare baseline and 1 year after STN-DBS variations between pre-opRBD+ and preopRBD−, random-effects models were performed to measure time and group (pre-opRBD+ and pre-opRBD−) effects and their interaction time × group, taking into account between- and within-patient variability (subject as random effect). This interaction corresponds to the difference of variation (absolute change between baseline and evaluation at M12) between pre-opRBD+ and pre-opRBD−. The normality of residuals from these models was studied using the Shapiro-Wilk test. When appropriate, a logarithmic transformation has been proposed to achieve the normality of dependent outcomes. Then, multivariate analyses were performed using adjustment on covariates fixed according to the univariate results and to the clinical relevance: age, sex, preoperative LEDD, preoperative MDS-UPDRS III “off” scores, and baseline values of studied dependent outcomes. Random-effects models were also carried out to analyze intragroup variation for pre-opRBD+ and pre-opRBD− patients. A sensitivity analysis was conducted to evaluate the statistical nature of missing data and their potential effect on results. More precisely, patients with and without follow-up at 1 year were compared for their baseline characteristics. As data were missing completely at random regarding each clinical variable, no imputation of data was applied.
Statistical analyses were performed using Stata software, version 15 (StataCorp). The tests were 2-sided, with a type I error set at 5%. As analyses were exploratory, the individual p values have been reported without applying systematically mathematical correction according to several works reported in the literature, but specific attention was given to the magnitude of differences and to clinical relevance.33,34 Indeed, it has been previously suggested that the choice and the number of tested hypotheses may be data dependent, which means that multiple significance tests can be used for descriptive purposes but not for decision making, regardless of whether multiplicity corrections are performed or not. Significant results based upon exploratory analyses should be clearly labeled as exploratory results. Conversely, the lack of multiple correction of the type I error cannot affect nonsignificant results.35
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Patient Characteristics
Among 795 patients with PD enrolled in the PREDISTIM cohort and awaiting STN-DBS, 448 completed RBD-SQ or RBD-1Q preoperatively. Out of them, 330 patients underwent surgery at the time of our study, and 215 had a postoperative evaluation 12 months after STN-DBS (eFigure 1, links.lww.com/WNL/B589).
Demographic and Clinical Profile of PD Candidates for STN-DBS at Preoperative Evaluation According to the Presence of Probable RBD Preoperatively
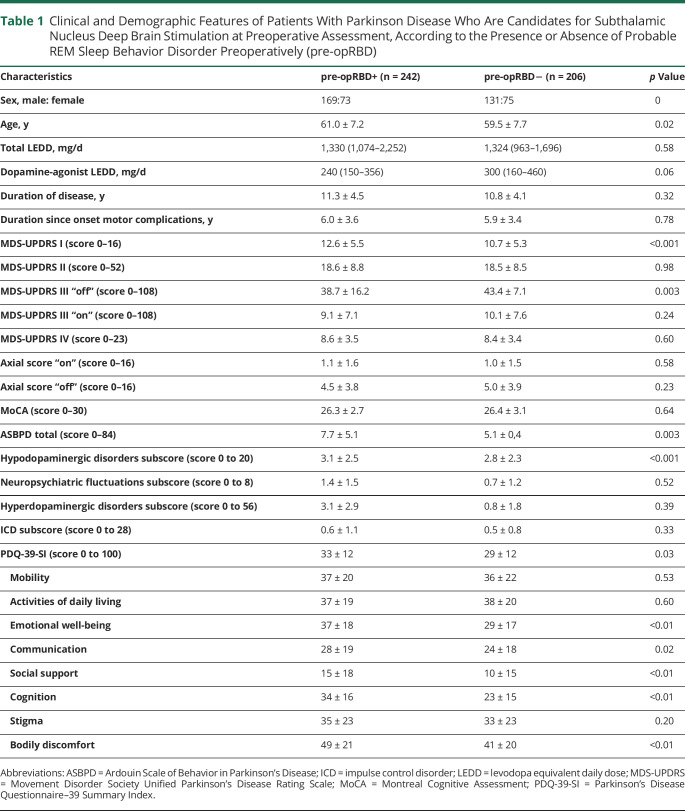
We characterized the demographic and clinical profile of RBD+ and RBD– preoperatively in the whole group (n = 448) (300 male; mean age 63.3 ± 7.4 years; mean disease duration 11.0 ± 4.3 years). Among 448 patients with PD at baseline, 242 (54%) were pre-opRBD+, while 206 were pre-opRBD–. Motor symptoms, nonmotor symptoms, and quality of life of pre-opRBD+ and pre-opRBD– patients before STN-DBS are presented in Table 1.
Table 1.
Clinical and Demographic Features of Patients With Parkinson Disease Who Are Candidates for Subthalamic Nucleus Deep Brain Stimulation at Preoperative Assessment, According to the Presence or Absence of Probable REM Sleep Behavior Disorder Preoperatively (pre-opRBD)
Pre-opRBD+ patients were older (p = 0.02), with a higher MDS-UPDRS I score (p < 0.001) and lower MDS-UPDRS III score assessed in “med-off” condition (p = 0.003) compared to pre-opRBD− patients.
Regarding psycho-behavioral characteristics, ASBPD total score was significantly higher in pre-opRBD+ compared to pre-opRBD− (p = 0.03). When considering each dimension of the scale, we observed higher scores in pre-opRBD+ for hypodopaminergic symptoms (p < 0.001). In contrast, nonmotor fluctuations and hyperdopaminergic behaviors (including ICD subscores) did not differ between groups.
PDQ-39-SI score was significantly higher in pre-opRBD+ compared to pre-opRBD− (p = 0.03), specifically for emotional well-being (p < 0.01), communication (p = 0.02), social support (p < 0.01), cognition (p < 0.01), and bodily discomfort (p < 0.01) subscores.
Clinical Outcomes of Patients With PD After STN-DBS According to the Presence of Probable RBD Preoperatively
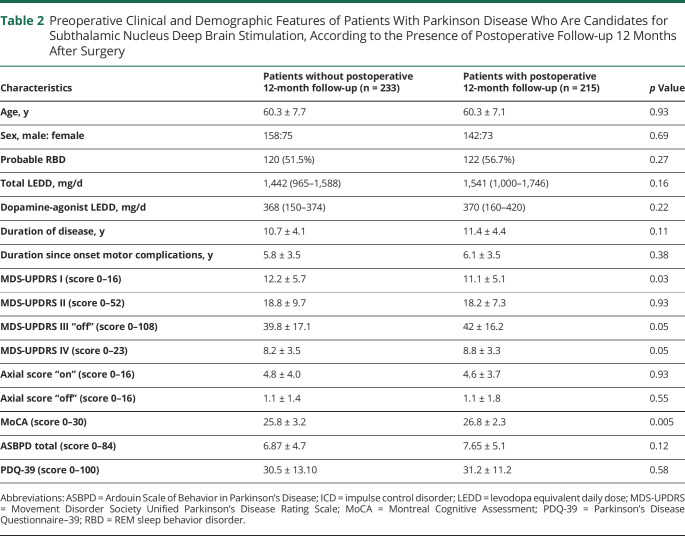
Analyses of clinical scores at 12 months and variation of scores between baseline and 12 months were performed on 215 patients (142 male; mean age: 60.3 ± 7.1 years; mean disease duration: 11.4 ± 4.4 years). To rule out different profile of this sample compared to the whole group, we compared demographic and clinical characteristics of both groups (Table 2). The groups with and without follow-up at 12 months were comparable for all clinical data except for MDS-UPDRS I, which was lower, and MoCA, which was greater, in the group with a postoperative assessment (respectively 11.1 ± 5.1 vs 12.2 ± 5.7; p = 0.003 for MDS-UPDRS I and 26.8 ± 2.3 vs 25.8 ± 3.2; p < 0.001 for MoCA), although nonsignificant clinical size effect.
Table 2.
Preoperative Clinical and Demographic Features of Patients With Parkinson Disease Who Are Candidates for Subthalamic Nucleus Deep Brain Stimulation, According to the Presence of Postoperative Follow-up 12 Months After Surgery
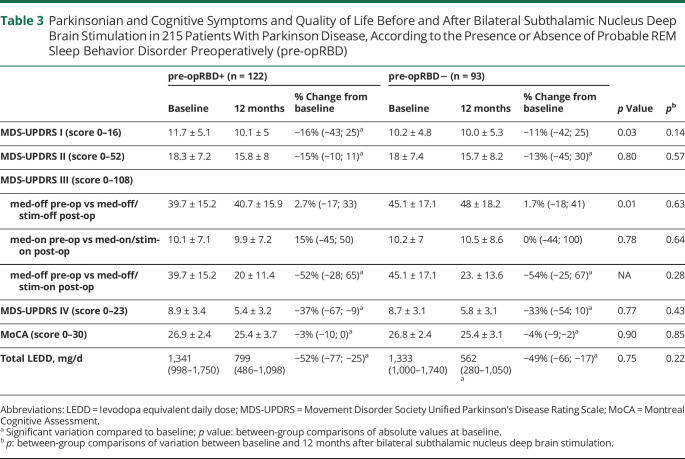
Among 215 patients with PD who were assessed both preoperatively and 12 months after STN-DBS, 122 (57%) were pre-opRBD+, while 93 (43%) were pre-opRBD−. Parkinsonian, cognitive, psycho-behavioral symptoms, treatment doses, and quality of life score variations between baseline and postoperative evaluation at 12 months depending on the presence of pre-opRBD are presented in Tables 3–6.
Table 3.
Parkinsonian and Cognitive Symptoms and Quality of Life Before and After Bilateral Subthalamic Nucleus Deep Brain Stimulation in 215 Patients With Parkinson Disease, According to the Presence or Absence of Probable REM Sleep Behavior Disorder Preoperatively (pre-opRBD)
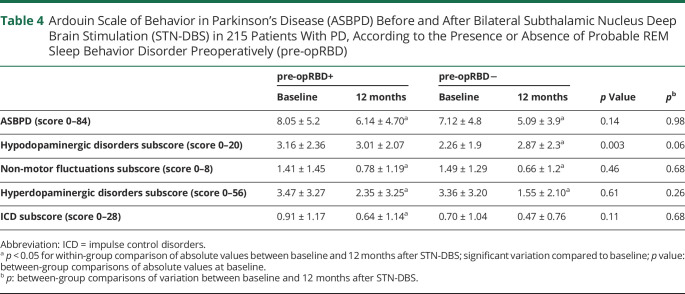
Table 4.
Ardouin Scale of Behavior in Parkinson’s Disease (ASBPD) Before and After Bilateral Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) in 215 Patients With PD, According to the Presence or Absence of Probable REM Sleep Behavior Disorder Preoperatively (pre-opRBD)
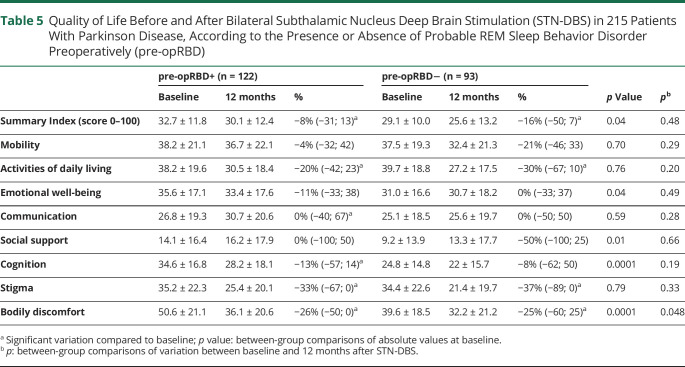
Table 5.
Quality of Life Before and After Bilateral Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) in 215 Patients With Parkinson Disease, According to the Presence or Absence of Probable REM Sleep Behavior Disorder Preoperatively (pre-opRBD)
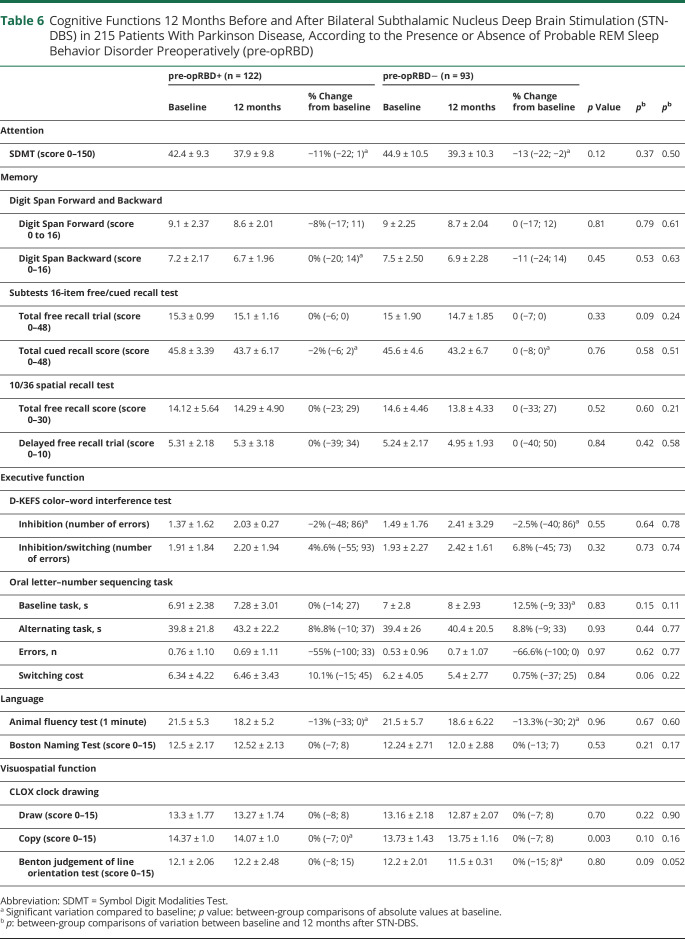
Table 6.
Cognitive Functions 12 Months Before and After Bilateral Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) in 215 Patients With Parkinson Disease, According to the Presence or Absence of Probable REM Sleep Behavior Disorder Preoperatively (pre-opRBD)
STN-DBS resulted in a mean decrease of MDS-UPDRS III med-off scores compared to baseline (defined as the chronic effect of stimulation), by 52% in pre-opRBD+ (p < 0.01) and by 54% in pre-opRBD− (p < 0.001), with no between-group difference (Table 3). MDS-UPDRS IV score significantly decreased by 37% (p < 0.01) in pre-opRBD+ and by 33% (p < 0.01) in pre-opRBD– with no between-group difference. STN-DBS also led to a LEDD total reduction compared to baseline in both groups (pre-opRBD+ −52%, p < 0.01; pre-opRBD− −49%, p < 0.01), with no between-group difference.
MDS-UPDRS I and MDS-UPDRS II significantly decreased in both groups, yet no between-group difference of variation was observed. MoCA score decreased both in pre-opRBD− (p < 0.05) and in pre-opRBD+ (p < 0.05) after STN-DBS, but without any significant group differences.
Regarding psycho-behavioral features, hyperdopaminergic total subscores significantly decreased in both groups, with no between-group difference. There was no postoperative variation of hypodopaminergic total subscore in the pre-opRBD+ group, while it significantly worsened in pre-opRBD− (p = 0.03), but there was no significant between-group difference. ASBPD total score and nonmotor fluctuation subscores significantly decreased in both groups (Table 4).
PDQ-39-SI scores significantly decreased in pre-opRBD− (−16%, p < 0.01) and in pre-opRBD+ (−8%, p < 0.01), with no between-group difference. Activities of daily living and stigma subscores significantly decreased in both groups, whereas cognition and communication subscores only decreased in pre-opRBD+ (p < 0.01), although none of these variations significantly differed between groups. Bodily discomfort was the only quality of life subscore that, while significantly decreasing in both groups, decreased more significantly in the pre-opRBD+ group (p = 0.04) (Table 5).
At baseline, there was no difference between pre-opRBD+ and pre-opRBD– for any cognitive assessment except for CLOX copy, for which pre-opRBD+ patients presented statistically better scores compared to pre-opRBD− patients (p = 0.003), although this difference was not clinically significant because scores were in the normal range for both groups. There was no significant between-group difference for postoperative variation of attentional, instrumental, and memory functions assessed by the 11 cognitive tests, nor was there any between-group difference at postoperative evaluation for these tests (Table 6).
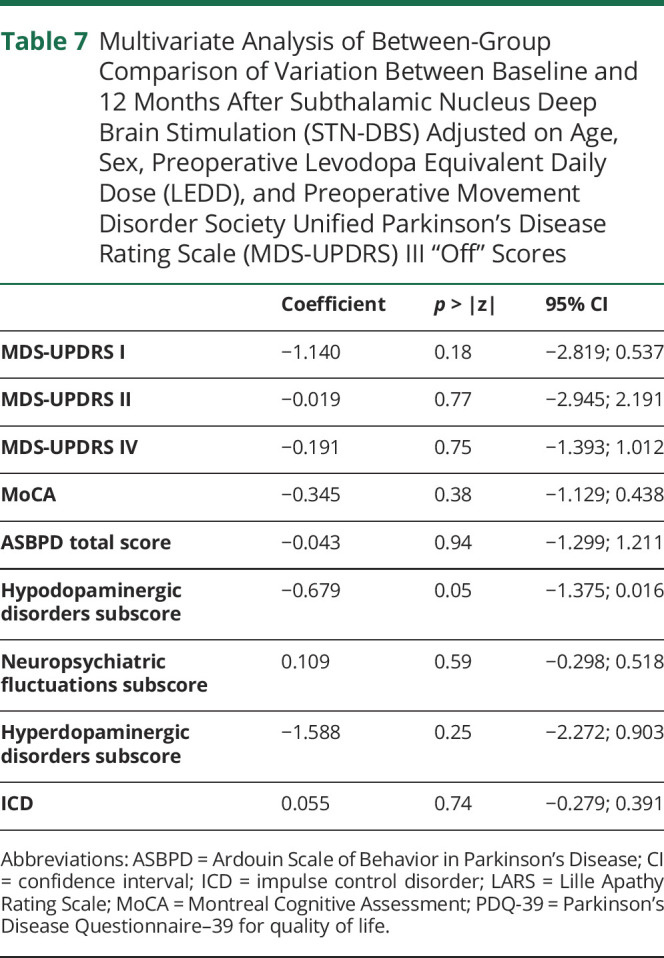
Multivariate analysis taking into account the effect of age, sex, LEDD, and MDS-UPDRS III “off” scores at preoperative evaluation confirmed that there was no difference of variation 12 months after STN-DBS between pre-opRBD+ and pre-opRBD– for MDS-UPDRS II and IV, MoCA, and ASBPD total and subtotal scores (Table 7).
Table 7.
Multivariate Analysis of Between-Group Comparison of Variation Between Baseline and 12 Months After Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) Adjusted on Age, Sex, Preoperative Levodopa Equivalent Daily Dose (LEDD), and Preoperative Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) III “Off” Scores
Discussion
Among patients with PD who are candidates for STN-DBS, pre-opRBD+ patients were older, with milder motor symptoms, but with poorer nonmotor aspects of daily living, more hypodopaminergic psycho-behavioral symptoms, and decreased quality of life compared to pre-opRBD– patients at preoperative evaluation. Twelve months after STN-DBS, both pre-opRBD+ and pre-opRBD– patients had MDS-UPDRS IV score decrease (−37% and −33%, respectively), MDS-UPDRS III med-off decrease (−52% and −54%), and total LEDD decrease (−52% and −49%) after surgery, with no between-group difference. There was no between-group difference for cognitive, psycho-behavioral, or global quality of life outcomes.
To our knowledge, this is the first study to assess motor and nonmotor outcomes of STN-DBS depending on the presence of probable preoperative RBD in a large multicentric cohort of patients with PD. So far, 2 prospective studies assessed the outcome of patients with PD with and without pre-opRBD and reported conflicting results. The first was conducted on 41 patients with PD screened for probable preoperative RBD (12% of patients) using a semi-structured clinical interview.20 The second was conducted on 50 patients with PD, and RBD diagnosis was confirmed preoperatively by night polysomnography (48% of patients).21
These studies did not report any significant difference between patients with PD with and without pre-opRBD at baseline for motor symptoms assessed with UPDRS III “off,” whereas in our study pre-opRBD+ patients had better motor performance than pre-opRBD− patients. These findings are in contrast with the concept that patients with PD with RDB usually have greater motor disabilities and more axial impairment.36,37 Because we observed no difference at baseline for composite axial signs between pre-opRBD+ and pre-opRBD−, it is possible that we selected a subgroup of patients with PD with RBD as good candidates for surgery, because those with axial signs such as freezing of gait or postural instability were not meeting STN-DBS criteria, thereby excluding a range of patients with PD with associated RBD. Conversely, Zibetti et al.20 suggested that more severe axial symptoms preoperatively in pre-opRBD patients with PD could account for the lower percentages of improvement of motor symptoms after STN stimulation that they reported in this group at 1 and 3 years.
The second study compared UPDRS II and UPDRS III absolute scores at baseline (med-on condition) and postoperatively (med-on/stim-on condition 12 months after STN-DBS) in patients with PD with and without polysomnography-confirmed RBD.21 Aligned with our results, they report a significant improvement of UPDRS III “on” score in both groups, without between-group difference. Moreover, UPDRS II “on” score significantly improved in pre-opRBD+, whereas it remained stable in pre-opRBD–.21
Global psycho-behavioral outcomes after STN-DBS did not differ between groups and total ASBPD score was improved in both groups. These results are in line with the Bargiotas et al.22 study showing that patients with PD with RBD have greater apathy and depression symptoms than those without RBD at baseline, whereas no difference remained 1 year after STN-DBS. In the whole population of PD, the effect of STN-DBS on psycho-behavioral hypodopaminergic symptoms is debated, with some studies reporting improvement13,38 and others describing worsening.14,39-41
Previous studies did not assess hyperdopaminergic outcomes after STN-DBS in patients with PD with and without preopRBD. The lack of association with ICD preoperatively in our study in the RBD+ group goes against the described association between RBD and ICDs in PD, and could be due to the selection of a nonrepresentative “less severe” subgroup of patients with PD with RBD as candidates for STN-DBS.42,43 Both groups improved after surgery for total hyperdopaminergic ASBPD and for ICD subscores.
No between-group difference was found for cognitive outcomes 12 months after surgery. Previous studies assessing STN-DBS outcome of PD with RBD did not investigate detailed cognitive functions, but only cognitive dimension of PDQ-39. In the study by Bargiotas et al.,22 no between-group difference was observed after STN-DBS for the cognitive dimension of PDQ-39. These findings probably reflect the selection of a specific subgroup of RBD+ patients fulfilling criteria for STN-DBS, with better cognitive profile compared to what is generally reported in RBD. Indeed, cognitive impairment has been reported in patients with PD with RBD compared to patients with PD without RBD and with controls,44 and more particularly visuo-perceptive alterations seem to be specifically associated with RBD either idiopathic or secondary to PD.4 Moreover, pre-opRBD+ and preopRBD− patients did not differ for attentional tasks at baseline or at postoperative evaluation, but both groups presented a significant postoperative decrease compared to baseline for SDMT, with no between-group difference for variation. Our results are in line with attention/concentration impairment previously reported after STN-DBS45 and suggest that pre-opRBD+ patients fulfilling STN-DBS criteria do not have a greater risk for increased attentional impairment 1 year after surgery compared to pre-opRBD− patients.
In both groups, global quality of life improves after surgery with no between-group differences. However, while pre-opRBD+ patients have worse global quality of life preoperatively, it does not differ from pre-opRBD− patients 12 months after STN-DBS.
A number of limitations of this study must be noted. First, the lack of polysomnography-confirmed diagnosis of RBD could lead to some patients with PD having been misclassified. Yet, in order to increase the sensitivity of the clinical diagnosis, 2 different screening tools were combined to detect the presence of symptoms of RBD: the RBD-1Q and the RBD-SQ. The latter showed a moderate to high sensitivity and specificity (0.842 and 0.962, respectively) using a cutoff ≥6.25 Furthermore, the limited access to video-polysomnography in clinical practice limited this examination on such an important multicentric cohort. Another limitation is that RBD status of patients was not controlled after STN-DBS, though STN-DBS has been reported to have an effect on RBD symptoms and could modify their prevalence postoperatively.46,47 Indeed, RBD symptoms may fluctuate along the evolution of PD,36 but in spite of these clinical variations, the loss of muscle atonia during REM sleep, which is the polysomnographic feature of RBD, appears to be a long-lasting marker in PD.48 Thus, a specific long-term trait seems to be associated with RBD, possibly in relation with a specific pattern of neurodegeneration, leading us to assess RBD as a predictor for STN-DBS outcome in that study. The lack of data regarding the location of electrodes and stimulation measures should also be acknowledged and could interfere with postoperative results. However, we noted in our whole population an improvement of 52% for motor MDS-UPDRS scores in the “off” medication state, a reduction of 36% for dyskinesia, and a reduction of antiparkinsonian drugs by approximately 36% after STN-DBS. Those results are close to the classically observed motor improvements of STN-DBS treatment, justifying the correct location of electrodes in this population.9 It should also be acknowledged that, while we report the largest cohort available in a study focusing on the predictive role of probable RBD before STN-DBS, there are limits as to the inferences that this study will support given the limited sample of baseline patients with 12 months follow-up. Moreover, these results are based on an early analysis 1 year after STN-DBS, and the follow-up of the whole cohort 3 and 5 years after surgery will bring more light on the question of the long-term motor and nonmotor prognosis associated with the presence of preoperative probable RBD.
Altogether, our results suggest that the presence of probable RBD preoperatively is not associated with different motor, cognitive, or global quality of life outcomes 1 year after STN-DBS. Total psycho-behavioral symptoms were also similarly improved in both groups. STN-DBS improves quality of life in patients with PD regardless of the presence of pre-opRBD. Thus, although the presence of RBD has been previously reported to be associated with a more severe phenotype of PD, it does not seem to constitute a marker of risk of poor outcome after surgery in patients with PD eligible for STN-DBS. Further studies, assessing long-term outcomes associated with the presence of RBD preoperatively 3 years and 5 years after surgery, will improve our comprehension of the specific prognosis associated with the presence of RBD in patients with PD who are candidates for STN-DBS.
Study Funding
The study was funded by the France Parkinson charity and French Ministry of Health (PHRC National 2012) and promoted by CHU Lille (coordinated by Pr Devos and Pr Corvol) with the support of the French network NS-PARK and supported by NS-PARK/F-CRIN and the Fédération de la Recherche Clinique du CHU de Lille.
Acknowledgment
The authors thank the participants and their families for cooperation and Natasha Louise Taylor (Brain and Mind Center, Parkinson's Disease Research Clinic, University of Sydney) for English language editing.
Glossary
- ASBPD
Ardouin Scale of Behavior in Parkinson's Disease; ICD
- impulse control disorder; LEDD
levodopa equivalent daily dose; MDS-UPDRS
- Movement Disorder Society Unified Parkinson's Disease Rating Scale; MoCA
Montreal Cognitive Assessment; PD
- Parkinson disease; PDQ-39
Parkinson's Disease Questionnaire–39; PDQ-39-SI
- Parkinson's Disease Questionnaire–39 Summary Index; pre-opRBD+
with probable REM sleep behavior disorder preoperatively; pre-opRBD−
- without probable REM sleep behavior disorder preoperatively; RBD
REM sleep behavior disorder; RBD-1Q
- RBD Single Question; RBD-SQ
RBD Screening Questionnaire; SDMT
- Symbol Digit Modalities Test; STN-DBS
subthalamic nucleus deep brain stimulation
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Disclosure
E. Besse, B. Pereira, F. Durif, M. Livia Fantini, E. Durand, B. Debilly, P. Derost, and C. Moreau report no disclosures relevant to the manuscript. E. Hainque served on scientific advisory boards for Medtronic and Boston Scientific and received speech honorarium from Medtronic and Boston Scientific. T. Rouaud, A. Eusebio, I. Benatru, S. Drapier, D. Guehl, O. Rascol, D. Maltete, O. Lagha-Boukbiza, C. Giordana, and M. Tir report no disclosures relevant to the manuscript. S. Thobois served as a consultant for Boston Scientific. L. Hopes, C. Hubsch, B. Jarraya, A.-S. Rolland, J.-C. Corvol, D. Devos, and A. Marques report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Duarte Folle A, Paul KC, Bronstein JM, Keener AM, Ritz B. Clinical progression in Parkinson's disease with features of REM sleep behavior disorder: a population-based longitudinal study. Parkinsonism Relat Disord. 2019;62:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Videnovic A, Marlin C, Alibiglou L, Planetta PJ, Vaillancourt DE, Mackinnon CD. Increased REM sleep without atonia in Parkinson disease with freezing of gait. Neurology. 2013;81(12):1030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JS, Park HE, Oh YS, et al. Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J Neurol Sci. 2016;362:59-63. [DOI] [PubMed] [Google Scholar]
- 4.Marques A, Dujardin K, Boucart M, et al. . REM sleep behaviour disorder and visuoperceptive dysfunction: a disorder of the ventral visual stream? J Neurol. 2010;257(3):383-391. [DOI] [PubMed] [Google Scholar]
- 5.Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson's disease. Sleep. 2017;40(8):zsx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenka A, Hegde S, Jhunjhunwala KR, Pal PK. Interactions of visual hallucinations, rapid eye movement sleep behavior disorder and cognitive impairment in Parkinson's disease: a review. Parkinsonism Relat Disord. 2016;22:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Fantini ML, Macedo L, Zibetti M, et al. Increased risk of impulse control symptoms in Parkinson's disease with REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2015;86(2):174-179. [DOI] [PubMed] [Google Scholar]
- 8.Lu H-T, Shen Q-Y, Zhao Q-Z, et al. . Association between REM sleep behavior disorder and impulsive-compulsive behaviors in Parkinson's disease: a systematic review and meta-analysis of observational studies. J Neurol. 2020;267(2):331-340. [DOI] [PubMed] [Google Scholar]
- 9.Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368(7):610-622. [DOI] [PubMed] [Google Scholar]
- 10.Abbes M, Lhommée E, Thobois S, et al. Subthalamic stimulation and neuropsychiatric symptoms in Parkinson's disease: results from a long-term follow-up cohort study. J Neurol Neurosurg Psychiatry. 2018;89(8):836-843. [DOI] [PubMed] [Google Scholar]
- 11.Azulay JP, Witjas T, Eusebio A. Effect of subthalamic deep brain stimulation on non-motor fluctuations in Parkinson's disease. J Neural Transm. 2013;120(4):655-657. [DOI] [PubMed] [Google Scholar]
- 12.Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349(20):1925-1934. [DOI] [PubMed] [Google Scholar]
- 13.Lhommée E, Wojtecki L, Czernecki V, et al. Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson's disease with early motor complications (EARLYSTIM trial): secondary analysis of an open-label randomised trial. Lancet Neurol. 2018;17(3):223-231. [DOI] [PubMed] [Google Scholar]
- 14.Thobois S, Ardouin C, Lhommée E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson's disease: predictors and underlying mesolimbic denervation. Brain. 2010;133(Pt 4):1111-1127 [DOI] [PubMed] [Google Scholar]
- 15.Horn A, Reich M, Vorwerk J, et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82(1):67-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lönnfors-Weitzel T, Weitzel T, Slotboom J, et al. . T2-relaxometry predicts outcome of DBS in idiopathic Parkinson's disease. Neuroimage Clin. 2016;12:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Planche V, Munsch F, Pereira B, et al. . Anatomical predictors of cognitive decline after subthalamic stimulation in Parkinson's disease. Brain Struct Funct. 2018;223(7):3063-3072. [DOI] [PubMed] [Google Scholar]
- 18.Witt K, Granert O, Daniels C, et al. Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson's disease: results from a randomized trial. Brain. 2013;136(Pt 7):2109-2119. [DOI] [PubMed] [Google Scholar]
- 19.Schuepbach WMM, Tonder L, Schnitzler A, et al. Quality of life predicts outcome of deep brain stimulation in early Parkinson disease. Neurology. 2019;92(10):e1109-e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zibetti M, Rizzi L, Colloca L, et al. Probable REM sleep behaviour disorder and STN-DBS outcome in Parkinson's disease. Parkinsonism Relat Disord. 2010;16(4):265-269. [DOI] [PubMed] [Google Scholar]
- 21.Bargiotas P, Debove I, Bargiotas I, et al. . Effects of bilateral stimulation of the subthalamic nucleus in Parkinson's disease with and without REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2019;90(12):1310-1316. [DOI] [PubMed] [Google Scholar]
- 22.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire: a new diagnostic instrument. Mov Disord. 2007;22(16):2386-2393. [DOI] [PubMed] [Google Scholar]
- 24.Figorilli M, Marques AR, Meloni M, et al. . Diagnosing REM sleep behavior disorder in Parkinson's disease without a gold standard: a latent-class model study. Sleep. 2020;43(7):zsz323. [DOI] [PubMed] [Google Scholar]
- 25.Nomura T, Inoue Y, Kagimura T, Uemura Y, Nakashima K. Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson's disease patients. Sleep Med. 2011;12(7):711-713. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41-47. [DOI] [PubMed] [Google Scholar]
- 27.Welter ML, Houeto JL, Tezenas du Montcel S, et al. Clinical predictive factors of subthalamic stimulation in Parkinson's disease. Brain. 2002;125(3):575-583. [DOI] [PubMed] [Google Scholar]
- 28.Dujardin K, Auzou N, Lhommée E, et al. French consensus procedure for assessing cognitive function in Parkinson's disease. Rev Neurol. 2016;172(11):696-702. [DOI] [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 30.Rieu I, Martinez-Martin P, Pereira B, et al. . International validation of a behavioral scale in Parkinson's disease without dementia: neuropsychiatric assessment in PD. Mov Disord. 2015;30(5):705-713. [DOI] [PubMed] [Google Scholar]
- 31.Auquier P, Sapin C, Ziegler M, et al. . Validation of the French language version of the Parkinson's Disease Questionnaire: PDQ-39. Rev Neurol. 2002;158(1):41-50. [PubMed] [Google Scholar]
- 32.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease: systematic review of LED reporting in PD. Mov Disord. 2010;25(15):2649-2653. [DOI] [PubMed] [Google Scholar]
- 33.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. [PubMed] [Google Scholar]
- 35.Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol. 2001;54(4):343-349. [DOI] [PubMed] [Google Scholar]
- 36.Bugalho P, Viana-Baptista M. REM sleep behavior disorder and motor dysfunction in Parkinson's disease: a longitudinal study. Parkinsonism Relat Disord. 2013;19(12):1084-1087. [DOI] [PubMed] [Google Scholar]
- 37.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79(10):1117-1121. [DOI] [PubMed] [Google Scholar]
- 38.Czernecki V, Pillon B, Houeto JL, et al. . Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson's disease? J Neurol Neurosurg Psychiatry. 2005;76(6):775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limousin P, Foltynie T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol. 2019;15(4):234-242. [DOI] [PubMed] [Google Scholar]
- 40.Saint-Cyr JA, Trépanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2000;123(Pt 10):2091-2108. [DOI] [PubMed] [Google Scholar]
- 41.Drapier D, Drapier S, Sauleau P, et al. . Does subthalamic nucleus stimulation induce apathy in Parkinson's disease? J Neurol. 2006;253(8):1083-1091. [DOI] [PubMed] [Google Scholar]
- 42.Molde H, Moussavi Y, Kopperud ST, Erga AH, Hansen AL, Pallesen S. Impulse-control disorders in Parkinson's disease: a meta-analysis and review of case-control studies. Front Neurol. 2018;9:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fantini ML, Durif F, Marques A. Impulse control disorders in REM sleep behavior disorder. Curr Treat Options Neurol. 2019;21(5):23. [DOI] [PubMed] [Google Scholar]
- 44.Vendette M, Gagnon JF, Décary A, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. 2007;69(19):1843-1849. [DOI] [PubMed] [Google Scholar]
- 45.Mehanna R, Bajwa JA, Fernandez H, Wagle Shukla AA. Cognitive impact of deep brain stimulation on Parkinson's disease patients. Parkinson Dis. 2017;2017:3085140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monaca C, Ozsancak C, Jacquesson JM, et al. . Effects of bilateral subthalamic stimulation on sleep in Parkinson’s disease. J Neurol. 2004;251(2):214-218. [DOI] [PubMed] [Google Scholar]
- 47.Arnulf I, Bejjani BP, Garma L, et al. Improvement of sleep architecture in PD with subthalamic nucleus stimulation. Neurology. 2000;55(11):1732-1734. [DOI] [PubMed] [Google Scholar]
- 48.Figorilli M, Marques AR, Vidal T, et al. . Does REM sleep behavior disorder change in the progression of Parkinson's disease? Sleep Med. 2020;68:190-198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.